Abstract
Objective
Alprazolam administered via the Staccato® breath‐actuated device is delivered into the deep lung for rapid systemic exposure and is a potential therapy for rapid epileptic seizure termination (REST). We conducted an inpatient study (ENGAGE‐E‐001 [NCT03478982]) in patients with stereotypic seizure episodes with prolonged or repetitive seizures to determine whether Staccato alprazolam rapidly terminates seizures in a small observed population after administration under direct supervision.
Methods
Adult patients with established diagnosis of focal and/or generalized epilepsy with a documented history of seizure episodes with a predictable pattern were enrolled. They were randomized 1:1:1 to double‐blind treatment of a single seizure event with one dose of Staccato alprazolam 1.0 mg or 2.0 mg, or Staccato placebo in an inpatient unit. The primary end point of the study was the proportion of responders in each treatment group achieving seizure activity cessation within 2 min after administration of study drug and no recurrence of seizure activity within 2 h.
Results
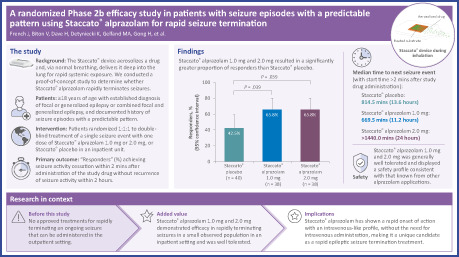
A total of 273 patients were screened, and 116 randomized patients received treatment with the study drug in the double‐blind part. The proportion of treated patients who were responders was 65.8% for each of Staccato alprazolam 1.0 mg (n = 38; p = .0392) and 2.0 mg (n = 38; p = .0392), compared with 42.5% for Staccato placebo (n = 40). Staccato alprazolam was well tolerated when administered as a single dose of 1.0 or 2.0 mg: cough and somnolence were the most common adverse events (AEs) (both 14.5%), followed by dysgeusia (13.2%). AEs were mostly mild or moderate in intensity; there were no treatment‐related serious AEs.
Significance
Both 1.0 mg and 2.0 mg doses of Staccato alprazolam demonstrated efficacy in rapidly terminating seizures in an inpatient setting and were well tolerated. The next step is a Phase 3 confirmatory study to demonstrate efficacy and safety of Staccato alprazolam for rapid cessation of seizures in an outpatient setting.
Keywords: acute treatment, alprazolam, clinical trial, deep lung delivery, epilepsy, rapid onset, seizure
Key points.
The Staccato device aerosolizes a drug and, via normal breathing, delivers it deep into the lung for rapid systemic exposure.
We conducted a study in patients with prolonged or repetitive seizures to determine whether Staccato alprazolam rapidly terminates seizures.
Results showed that Staccato alprazolam 1.0 and 2.0 mg resulted in a significantly greater proportion of responders than Staccato placebo.
The responder rate was the same with 1.0 and 2.0 mg doses of Staccato alprazolam, but seizure recurrence rate was lower with the 2.0 mg dose.
The adverse event profile of Staccato alprazolam was similar to what has been reported for alprazolam for other indications.
1. INTRODUCTION
There are currently no approved treatments for rapidly terminating an ongoing seizure that can be administered in the outpatient setting. Therapies currently approved in the United States for seizure emergencies in this setting, including rectally administered diazepam gel, 1 , 2 intranasal midazolam, 3 and intranasal diazepam, 4 are intended to prevent or delay subsequent seizures in a cluster episode. In general, benzodiazepines can terminate most seizures in the acute setting, and the time to treatment effect is related directly to the route of administration. The time to onset of action is related mainly to the time required for the drug to be absorbed from the site of administration to reach clinically relevant plasma and brain levels. Clinical data from these nonintravenous formulations (nasal and rectal) showed onset of action several minutes after drug administration, 5 which suggests that clinically relevant plasma concentration may not be achieved in time to rapidly terminate an ongoing seizure. Nasal and buccal preparations are rapidly replacing rectal preparations in older children and adults due to ease of administration, but these preparations would likely not work rapidly enough, for example, to prevent a tonic‐clonic seizure preceded by a prolonged aura. We hope that further clinical trials of inhaled alprazolam will identify whether an inhaled and rapidly absorbed preparation will provide benefit in scenarios such as this.
The development of treatment options with a rapid onset of action that can be easily administered in the outpatient setting is important to limit adverse consequences of seizures including injury, social harm, prolonged recovery period, or progression to seizure emergencies such as status epilepticus. Alprazolam administered via the Staccato® breath‐actuated device is an investigational therapy being evaluated for the rapid treatment of evolving seizure activity. In order for Staccato alprazolam to be suitable to rapidly treat acute seizure activity, both the drug and the delivery system must be appropriate for this use.
Alprazolam is a highly characterized benzodiazepine that acts as a positive allosteric modulator of multiple γ‐aminobutyric acid type A (GABAA)–receptor subtypes and is used commonly for the treatment of anxiety disorders. 6 , 7 , 8 The antiseizure properties of benzodiazepines are mediated through the inhibitory effects on neuronal activity, and indeed alprazolam is potent and efficacious in various animal models of antiseizure activity (including DBA/2 and DBA/2J mice, which are genetically susceptible to sound‐induced seizures). 8 , 9 , 10 , 11 In a comparator study, alprazolam was more potent than either clonazepam or diazepam in reducing audiogenic seizures. 10 Therefore, like other benzodiazepines, alprazolam has the potential to treat acute seizures.
The Staccato device is a novel delivery mechanism in epilepsy that, when placed into the user's mouth, actuates the aerosolization of a drug via a normal breath, allowing it to be delivered deep into the lung for rapid absorption and systemic exposure. A Phase 2a proof‐of‐concept study in patients with photosensitive epilepsy showed that clinically relevant plasma concentrations of alprazolam were achieved within 2 min following Staccato alprazolam administration. 7 The three dose levels of Staccato alprazolam (0.5, 1.0, and 2.0 mg) rapidly suppressed epileptiform activity within 2 min, with a favorable adverse event (AE) profile. 7 The effect was sustained for 4–6 h. 7
We conducted a proof‐of‐concept study of Staccato alprazolam for rapid epileptic seizure termination (REST) 12 in patients with stereotypic seizure episodes with a consistent pattern supporting an early identification as prolonged or repetitive seizures—a concept referred to as seizures with a predictable pattern. Although a REST treatment would ultimately be most valuable in the outpatient setting, for the purpose of this proof‐of‐concept study, Staccato alprazolam was administered under direct supervision, in an inpatient setting.
2. MATERIALS AND METHODS
2.1. Study design
ENGAGE‐E‐001 (NCT03478982) was a Phase 2b, double‐blind, randomized, placebo‐controlled, inpatient, dose‐ranging efficacy study of Staccato alprazolam in patients with epilepsy with seizure episodes that have a predictable pattern. The study design is provided in Figure S1.
This was an inpatient study, preceded by outpatient screening and ending with a single follow‐up visit for study participation. Patients with frequent prolonged or recurrent seizures with a predictable onset pattern were admitted to a clinical research unit (CRU) or epilepsy monitoring unit (EMU) for 2–8 days. For each patient, a single stereotypic seizure event following the predictable onset was treated with the study drug. The duration and timing of the seizure event and occurrence of subsequent seizures were assessed by the staff through clinical observation.
This was a novel study design in a new type of patient population. Therefore, study conduct feasibility was first evaluated open label in eight patients receiving a 1.0 mg dose of Staccato alprazolam. After completion of the initial open‐label part, the study progressed to a double‐blind, randomized, placebo‐controlled part. The feasibility data (with special emphasis on the drug administration and clinical assessment procedures) from these eight patients were analyzed and reviewed by the sponsor and study team before starting the double‐blind part of the study.
As part of the screening process, the patient's seizure events were confirmed as acceptable for inclusion in the study by the Epilepsy Study Consortium (ESC) Review Board. An attempt was made to identify onset patterns that would predict a prolonged or cluster event at least 80% of the time.
Qualified patients were admitted to the CRU/EMU for randomization and treatment. The “seizure episode with a predictable pattern” was identified and described in detail so that the CRU/EMU staff could identify it once the patient was admitted for the treatment visit. The ESC form also identified which of the patient's seizure episodes were not typically prolonged or clustered and therefore should not be treated.
Following the feasibility assessment, enrollment into the double‐blind part was initiated and patients were randomized 1:1:1 by an interactive web response system to double‐blind treatment with one dose of Staccato alprazolam 1.0 mg or 2.0 mg, or Staccato placebo (Figure S1). Patients were stratified by the use of inducing vs noninducing antiseizure medication and the use of chronic daily benzodiazepines (yes or no). Chronic benzodiazepine use was defined as an average of four or more administrations per week prior to admission to the inpatient unit. The double‐blind part was quadruple masked (patient, care provider, investigator, outcomes assessor). In both the open‐label and double‐blind parts, a single seizure episode with a predictable pattern was treated for each patient. Study treatment was self‐administered, if feasible, or administered by a staff member immediately upon identification of the seizure episode with a predictable pattern, although some sites did not allow patients to self‐administer a benzodiazepine due to institutional policies. Patients were observed by a staff member and monitored on video electroencephalography throughout their stay in the CRU/EMU and were discharged ~24–32 h after treatment (or when they discontinued from the study).
2.2. Patients
Patients eligible to enroll were male or female, ≥18 years of age, with an established diagnosis of focal or generalized epilepsy or combined focal and generalized epilepsy with a documented history of seizure episodes with a predictable pattern that included at least one of the following:
Generalized seizure episodes starting with a flurry of absence seizures or myoclonic seizures with a minimum duration of 5 min; or
Episodes of a prolonged focal seizure with a minimum duration of 3 min; or
Episodes of multiple (two or more) seizures within a 2‐h period.
Eligible patients must have experienced four or more seizure episodes with a predictable pattern during the past 4 weeks (qualification period) and no more than 1 week without a seizure episode with a predictable pattern before entry into the inpatient unit (CRU/EMU). Cytochrome P450 (CYP) 3A inducers including carbamazepine, eslicarbazepine acetate, and oxcarbazepine were among the concomitant antiseizure medications that were permitted (see the Supporting Information for exclusions).
Patients were excluded if they had any of the following: a history or diagnosis of nonepileptic seizures; a history of status epilepticus in the 6 months before screening; a progressive neurological disorder such as brain tumor, demyelinating disease, or degenerative central nervous system disease that was likely to progress in the next 3 months; use of strong CYP3A4 inhibitors; and use of medications to treat airways disease such as asthma or chronic obstructive pulmonary disorder or any acute respiratory signs/symptoms (e.g., wheezing). Full patient inclusion and exclusion criteria are provided in the Supporting Information.
An institutional review board or independent ethics committee reviewed and approved the patient informed consent, trial protocol, and trial amendments. Patients provided informed consent or had a legally authorized representative sign the informed consent on their behalf before completing any study related procedures.
2.3. Objectives, end points, and outcome measures
The overall objectives of the study were to assess the efficacy and safety of a single administration of Staccato alprazolam in patients with epilepsy with seizures that have a predictable pattern. The primary objectives were to assess the efficacy of Staccato alprazolam (1.0 mg and 2.0 mg) compared with placebo in treating a seizure episode, to assess the clinical feasibility and safety of the inhalation of Staccato alprazolam (1.0 mg and 2.0 mg) compared with placebo in patients during a seizure episode, and to assess the sedation associated with administration of Staccato alprazolam (1.0 mg and 2.0 mg) compared with placebo.
The primary end point of the study was the proportion of “responders” in each treatment group as defined by achieving seizure activity cessation within 2 min after the administration of the study drug without recurrence of seizure activity within 2 h. Secondary end points included assessment of seizure episode severity by the patient and/or staff member, use of rescue medication, and assessment of secondary generalization of the seizure (evolution to a complex partial seizure and/or a generalized tonic‐clonic seizure). Seizure episode severity was an assessment of the severity of the treated seizure episode in relation to typical seizures experienced by the patient, measured 6‐h post‐treatment (classified as “much worse than,” “worse than,” “same as,” “better than,” and “much better than”).
Exploratory end points included assessment of number of seizures during the 4‐, 6‐, and 12‐h periods after study drug administration, and evaluation of time to next seizure event with start time >2 min after study drug administration.
Safety end points included assessment of the safety and tolerability of Staccato alprazolam by evaluating AEs, vital signs, clinical laboratory information, concomitant medications, electrocardiography (ECG) results, physical examinations, and assessment of sedation using a patient's visual analog scale (VAS) (Supporting Information).
Pharmacokinetic end points included measurements of plasma alprazolam concentration pre‐dose and at 10, 30, and 60 min, and 2 and 6 h after dosing of the study drug.
2.4. Statistical analyses
2.4.1. Sample size
There are no studies in the literature that provide reliable estimates of active treatment or placebo response rates. Assuming a 10% rate for drop‐out or protocol violation, ~115 patients were to be enrolled in the double‐blind part of the study to provide ~35 evaluable patients per treatment arm (105 patients with ~30% of the overall study population randomized in the study being patients with chronic daily benzodiazepines as part of their epilepsy management). Power calculations assume a two‐sided test and significance level of 0.05 with 90% power and are based on the assumption that the proportion of responders with Staccato alprazolam is 57% (best active), whereas the assumed placebo responder rate is 20%. Per protocol, eight patients were to be enrolled into the open‐label part.
2.4.2. Efficacy analyses
The efficacy population (intent‐to‐treat; ITT) included all patients who had a seizure event and received the study drug during the double‐blind treatment period. The modified ITT (mITT) population consisted of all patients in the ITT that had at least one evaluation after study drug administration. Data were summarized by active treatment by dose level vs patients administered placebo (i.e., by treatment group). Continuous measures were summarized descriptively (mean, standard deviation, median, minimum value, and maximum value), and categorical measures were presented as number and percentage.
All statistical tests were two‐sided with a significance value of .05. There were no adjustments for multiple comparisons. The primary efficacy analysis was conducted following completion of the double‐blind treatment period for the last patient.
Post hoc analyses were conducted to assess the efficacy (proportion of responders) of Staccato alprazolam (1.0 mg and 2.0 mg) and both doses combined compared with placebo in treating a seizure episode within the subgroups of study drug self‐administration (self‐administered vs administered by a staff member), and use of chronic benzodiazepines (yes or no).
2.4.3. Pharmacokinetic analyses
The pharmacokinetic population included all patients who received the study drug and had at least one pharmacokinetic sample drawn and analyzed. Plasma concentrations were summarized by descriptive statistics as appropriate and listed for each patient.
2.4.4. Safety analyses
The safety population included all patients who received the study drug in the treatment phase from both the open‐label feasibility part and the double‐blind part. AEs, vital sign measurements, physical examination findings, ECG, clinical laboratory information, and concomitant medications were tabulated and summarized by treatment and dose level. Separate tabulations were produced for all treatment‐emergent AEs (TEAEs), drug‐related TEAEs, serious AEs (SAEs), discontinuations due to AEs or TEAEs, and severe AEs (Grade 3 or higher severity). Listings were provided for each patient for any deaths, SAEs, and AEs leading to discontinuation of treatment.
3. RESULTS
3.1. Patients
The study was conducted between March 2018 and January 2020 in Australia, Jamaica, and the United States. A total of 273 patients were screened and 156 patients at 48 sites were randomized or assigned the open‐label study drug. Per protocol, the patients were admitted on day 1 and remained as inpatients until they had a treatable seizure or until 7 days had passed, whichever occurred first. If the patient went 7 days without a treatable seizure, the patient exited the study after completing exit procedures. Patients who were admitted on day 1 and went 7 days without a treatable seizure, were considered completed without treatment and excluded from the analysis populations. A total of 124 patients received treatment with the study drug.
Of the 156 enrolled patients, the ITT population and safety population consisted of 124 patients, including eight patients in the open‐label part and 116 randomized patients in the double‐blind part. Of these, all were included in the mITT population.
3.2. Open‐label feasibility part
Eight patients were enrolled in the open‐label feasibility part of the study; seven of eight patients had focal epilepsy and one had generalized epilepsy (Table 1). The results demonstrated successful enrollment in each eligibility category of seizures with a predictable pattern, and feasibility of study procedures for administration and dosing of 1.0 mg Staccato alprazolam. In the open‐label part, six of eight patients did not self‐administer Staccato alprazolam. The treatment was well tolerated; no SAEs were reported (Table 2). Treatment with 1.0 mg of Staccato alprazolam appeared efficacious, with a 62.5% seizure response rate (five of eight patients) with the predefined responder criteria (seizure cessation in 2 min of Staccato alprazolam administration and no recurrence in 2 h).
TABLE 1.
Patient baseline demographics and characteristics (mITT/safety population) a
| Open‐label | Double‐blind | |||
|---|---|---|---|---|
| 1.0 mg Staccato alprazolam (n = 8) | Staccato alprazolam | |||
| Staccato placebo (n = 40) | 1.0 mg (n = 38) | 2.0 mg (n = 38) | ||
| Demographics | ||||
| Mean age (range), years | 48.1 (24–69) | 33.1 (21–58) | 34.8 (18–62) | 33.5 (18–66) |
| Female, n (%) | 8 (100.0) | 20 (50.0) | 21 (55.3) | 26 (68.4) |
| Mean duration of epilepsy (range), years | 32.4 (6.9–63.1) | 23.1 (1.4–52.7) | 20.1 (1.0–52.3) | 23.1 (2.9–50.4) |
| Type of seizure with a predictable pattern b , n (%) | ||||
| Clusters (multiple seizures) | 3 (37.5) | 22 (55.0) | 27 (71.1) | 28 (73.7) |
| Prolonged focal seizures | 4 (50.0) | 18 (45.0) | 10 (26.3) | 9 (23.7) |
| Flurries of generalized seizures | 1 (12.5) | 4 (10.0) | 4 (10.5) | 4 (10.5) |
| Concomitant ASMs taken by ≥20% of patients in any group, n (%) | ||||
| Lamotrigine | 2 (25.0) | 17 (42.5) | 15 (39.5) | 14 (36.8) |
| Levetiracetam | 3 (37.5) | 16 (40.0) | 13 (34.2) | 15 (39.5) |
| Clonazepam | 2 (25.0) | 12 (30.0) | 10 (26.3) | 13 (34.2) |
| Lacosamide | 2 (25.0) | 9 (22.5) | 13 (34.2) | 13 (34.2) |
| Lorazepam | 4 (50.0) | 8 (20.0) | 12 (31.6) | 8 (21.1) |
| Topiramate | 0 | 5 (12.5) | 9 (23.7) | 8 (21.1) |
| Cannabidiol | 0 | 9 (22.5) | 5 (13.2) | 6 (15.8) |
| Zonisamide | 4 (50.0) | 4 (10.0) | 3 (7.9) | 4 (10.5) |
Abbreviations: ASM, antiseizure medication; mITT, modified intent‐to‐treat population.
All 124 patients in the safety population were included in the mITT.
Patients could have had more than one type of seizure with a predictable pattern and were not stratified by seizure type.
TABLE 2.
Safety summary (safety population)
| n (%) | Open‐label | Double‐blind | |||
|---|---|---|---|---|---|
| 1.0 mg Staccato alprazolam (n = 8) | Staccato alprazolam | ||||
| Staccato placebo (n = 40) | 1.0 mg (n = 38) | 2.0 mg (n = 38) | Combined active group (n = 76) | ||
| Total number of AEs | 5 | 20 | 35 | 39 | 74 |
| Patients with at least one AE | 3 (37.5) | 12 (30.0) | 14 (36.8) | 19 (50.0) | 33 (43.4) |
| Serious AEs | 0 | 2 (5.0) | 1 (2.6) | 0 | 1 (1.3) |
| Severe AEs | 1 (12.5) | 3 (7.5) | 1 (2.6) | 0 | 1 (1.3) |
| Drug‐related | 1 (12.5) | 3 (7.5) | 8 (21.1) | 15 (39.5) | 23 (30.3) |
| Resulting in using concomitant medication | 1 (12.5) | 4 (10.0) | 3 (7.9) | 2 (5.3) | 5 (6.6) |
| Discontinuation due to AEs | 0 | 0 | 0 | 0 | 0 |
| AEs occurring in ≥5% of patients in any treatment group | |||||
| Cough | 1 (12.5) | 0 | 4 (10.5) | 7 (18.4) | 11 (14.5) |
| Somnolence | 0 | 1 (2.5) | 7 (18.4) | 4 (10.5) | 11 (14.5) |
| Dysgeusia | 0 | 1 (2.5) | 6 (15.8) | 4 (10.5) | 10 (13.2) |
| Dizziness | 0 | 2 (5.0) | 2 (5.3) | 2 (5.3) | 4 (5.3) |
| Nausea | 0 | 1 (2.5) | 1 (2.6) | 2 (5.3) | 3 (3.9) |
| Sedation | 0 | 0 | 0 | 2 (5.3) | 2 (2.6) |
| Throat irritation | 1 (12.5) | 0 | 0 | 2 (5.3) | 2 (2.6) |
| Headache | 0 | 2 (5.0) | 1 (2.6) | 0 | 1 (1.3) |
| Fatigue | 0 | 2 (5.0) | 0 | 0 | 0 |
| Musculoskeletal chest pain | 1 (12.5) | 0 | 0 | 0 | 0 |
| Gastroenteritis viral | 1 (12.5) | 0 | 0 | 0 | 0 |
| Dysmenorrhea | 1 (12.5) | 0 | 0 | 0 | 0 |
Abbreviation: AE, adverse event.
3.3. Double‐blind part
3.3.1. Demographics, disposition, and baseline characteristics
The baseline characteristics and demographics of the patients are summarized in Table 1. In the double‐blind part, patients were randomized to placebo (n = 40), 1.0 mg Staccato alprazolam (n = 38) or 2.0 mg Staccato alprazolam (n = 38). Most patients had focal epilepsy (placebo: 30 [75.0%]; 1.0 mg: 30 [78.9%]; 2.0 mg: 34 [89.5%]). Generalized epilepsy was reported by 20 (50.0%), 16 (42.1%), and 17 (44.7%) patients in the placebo, 1.0 mg, and 2.0 mg groups, respectively. One patient (2.5%) in the placebo group and two patients (5.3%) in the 1.0 mg group had unclassified epilepsy. There were 53 patients with generalized tonic–clonic seizures or focal to bilateral tonic–clonic seizures described as an element in one or more of their historical treatable episodes. Most patients did not self‐administer the study drug (placebo: 33 [82.5%]; 1.0 mg: 26 [68.4%]; 2.0 mg: 28 [73.7%]).
3.3.2. Efficacy
Of those patients who received Staccato alprazolam, 65.8% were treatment responders for both the 1.0 mg and 2.0 mg treatment groups compared with 42.5% of patients who were administered placebo (Figure 1A). The differences between placebo and both doses of Staccato alprazolam were statistically significant (p = .0392). There was no difference observed between the two doses of Staccato alprazolam (p = 1.000).
FIGURE 1.
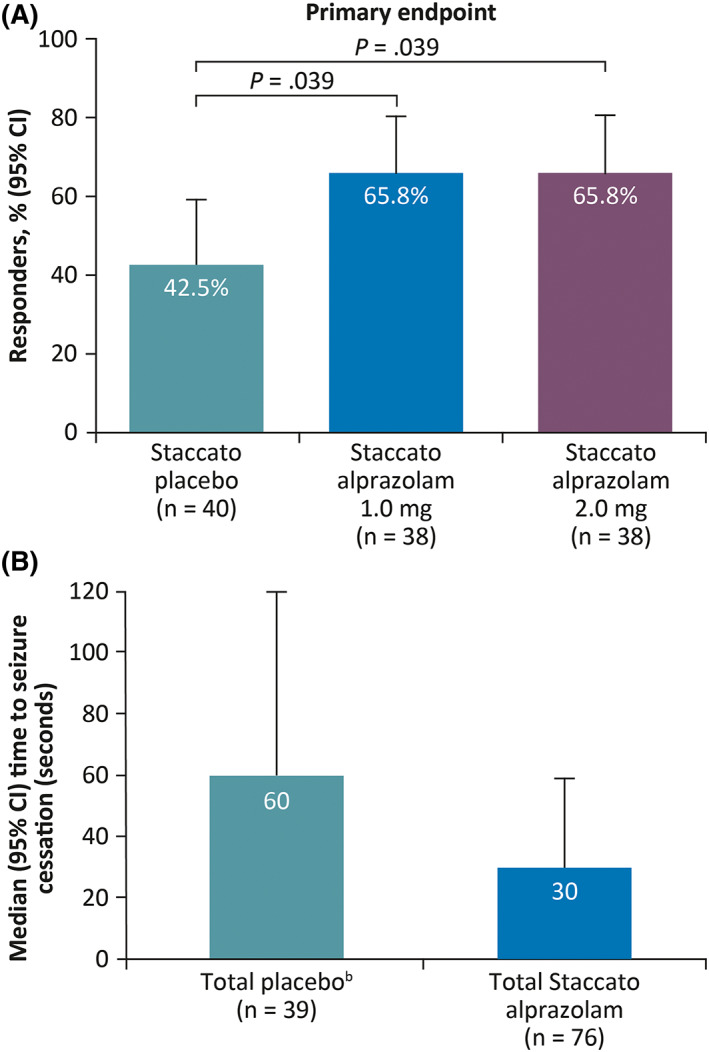
Proportion of respondersa (A) and median time to seizure cessation (B) (double‐blind part; mITT). aPatients who achieved seizure activity cessation within 2 min of treatment and no recurrence within 2 h; bdata missing for one patient. Abbreviations: CI, confidence interval; mITT, modified intent‐to‐treat; SD, standard deviation.
In a post hoc analysis, the mean time to seizure cessation in all patients who received Staccato alprazolam (1.0 mg and 2.0 mg) was approximately half the duration observed in patients who received placebo (145.2 vs 277.4 s); the median (95% confidence interval) time to seizure cessation was 30 (19, 59) s in patients who received Staccato alprazolam vs 60 (30, 120) s on placebo (Figure 1B). The median (95% confidence interval) time to seizure cessation in patients with prolonged focal seizures who received Staccato alprazolam (n = 19) vs placebo (n = 17) was 60 (42, 93) s vs 120 (60, 360) s, respectively.
There was no differentiation in seizure severity between placebo and Staccato alprazolam at either dose in the mITT population, and also between the two doses of Staccato alprazolam (Table S1). Rescue medication use was low and was similar between treatment groups (Table S2). No patients in the mITT population in any treatment group had seizures that evolved from a focal aware to a focal impaired aware and/or a bilateral tonic‐clonic seizure during the double‐blind part.
The number of seizures during the 0 to 6‐h time period tended to be lower (p = .0496) in the 2.0 mg Staccato alprazolam treatment group compared with placebo. The median time to next seizure event (that occurred >2 min after study drug administration) in the 2.0 mg dose group was >1440 min (24 h) and the median (interquartile range) time to next seizure event in the group administered placebo was 814.5 (25th percentile: 89.5; 75th percentile: 1458) min, suggesting a slight tendency to an increase in time to next seizure event with Staccato alprazolam treatment (hazard ratio 0.571; p = .0787) (Figure 2).
FIGURE 2.
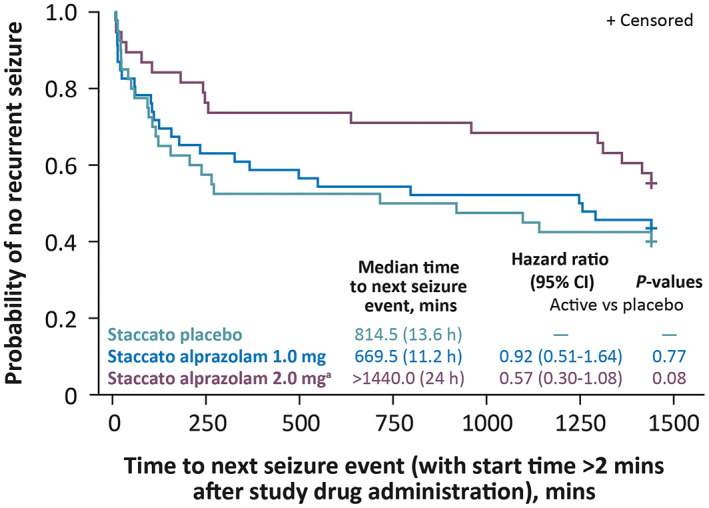
Kaplan‐Meier plot of time to next seizure event (double‐blind part; mITT). The time to next seizure is considered as censored at 24 h (1440 min) if there was no post treatment seizure observed in the inpatient period. aThe median time to next seizure event in the Staccato alprazolam 2.0 mg dose group was above the point of censoring at 24 h. Abbreviations: CI, confidence interval; mITT, modified intent‐to‐treat.
A generally similar proportion of responders were observed in all patients receiving Staccato alprazolam whether the patients did (63.6%) or did not (66.7%) self‐administer the study drug (Figure S2). In all patients receiving Staccato alprazolam, the proportion of responders was 69.4% of patients who were not taking chronic benzodiazepines compared with 59.3% in patients who were taking chronic benzodiazepines (Figure S3).
3.3.3. Safety
In general, patients in this study experienced an AE profile consistent with the patients' underlying disease (Table 2). This is consistent with the great majority of events in the groups treated with Staccato alprazolam assessed as unrelated to treatment by the investigators. Staccato alprazolam was well tolerated when administered as a single dose of 1.0 mg or 2.0 mg. In patients treated with Staccato alprazolam, cough and somnolence were the most common AEs (both 14.5%), followed by dysgeusia (13.2%). AEs were mostly mild or moderate in intensity with no treatment‐related SAEs. There were no deaths and no discontinuations because of TEAEs. No clinically relevant changes in laboratory parameters and vital signs were observed. Among patients on Staccato alprazolam, the median oxygen saturation was 97.0% or above at all assessed time points (up to 6 h post dose).
In general, patients treated with Staccato alprazolam had lower scores in the Sedation and Alert VAS (Figure S4) and Sleepy and Awake Scale VAS than patients administered placebo up to 6 h post dose, with the lowest scores at 10 min post dose, indicating an initial modest increase in sedation relative to placebo with equalization by 6 h.
Overall, the safety data suggest that Staccato alprazolam can be safely administered at doses up to 2.0 mg in patients with epilepsy with episodes of prolonged or cluster seizures with a predictable pattern.
3.3.4. Pharmacokinetics
Pharmacokinetic analysis showed that the active drug was successfully dosed in patients (Figure 3). Alprazolam was detected in the bloodstream by the first pharmacokinetic time point of 10 min, indicating feasible dosing and adequate absorption. Plasma alprazolam concentrations were shown to be approximately two‐fold higher following a 2.0 mg dose compared with 1.0 mg. Alprazolam concentrations were similarly achieved in patients across different seizure types, including patients with focal seizures with impaired awareness (Figure 4).
FIGURE 3.
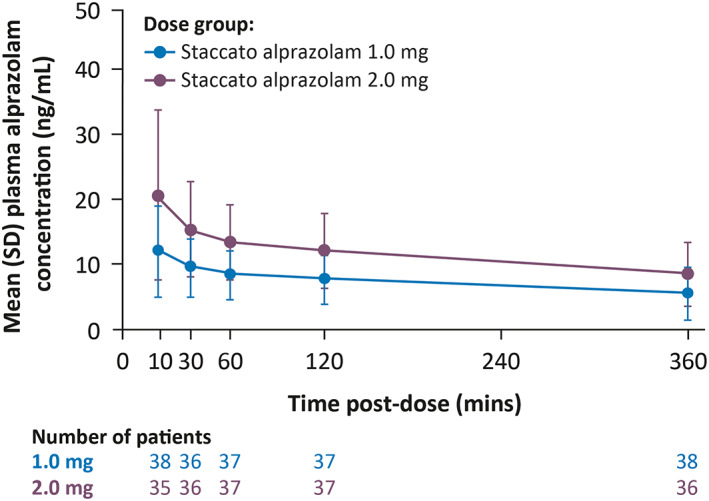
Mean (SD) plasma concentration of alprazolam (double‐blind part; pharmacokinetic population). Abbreviation: SD, standard deviation.
FIGURE 4.
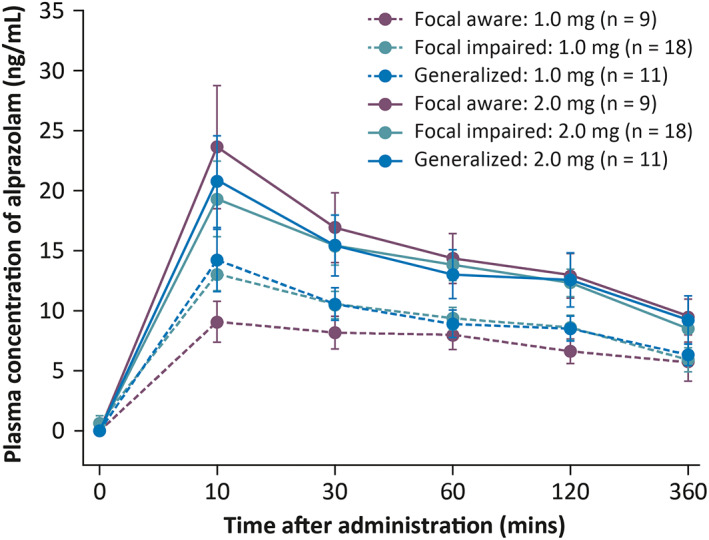
Mean (SD) plasma concentration of alprazolam across different seizure types and doses (double‐blind part; pharmacokinetic population). Dashed and solid lines indicate Staccato alprazolam 1.0 mg and 2.0 mg, respectively. Abbreviation: SD, standard deviation.
4. DISCUSSION
This is the first study evaluating the efficacy and safety of administration of Staccato alprazolam during an acute seizure episode. Data from this study showed that treatment with Staccato alprazolam was efficacious, resulting in a significantly greater proportion of responders than Staccato placebo. The responder rate was the same with 1.0 mg and 2.0 mg doses, but the number of seizures during the 6 h time period following administration of the study drug was lower with the 2.0 mg dose. However, it is important to note that evaluation of seizure recurrence applies only to patients who have multiple seizures per day; patients with a prolonged seizure may not have another seizure during the day.
Both doses of Staccato alprazolam demonstrated similar REST activity based on the analysis of the responder rate as defined in the primary end point. Generally similar responder rates were observed whether Staccato alprazolam was self‐administered by the patient or administered by a staff member. Use of chronic benzodiazepines had minimal effects on efficacy (responder rates) in all patients receiving Staccato alprazolam. However, these data are exploratory and should be interpreted with caution based on the small sample sizes.
Median time to seizure cessation (30 s in patients who received active treatment vs 60 s for placebo) is consistent with the rapid onset of clinical activity of Staccato alprazolam. In patients with prolonged focal seizures, the time to seizure cessation was increased in comparison with the overall population while maintaining the treatment effect at the same level. The breath‐actuated Staccato device heats the inert metal substrate, which rapidly aerosolizes the film of alprazolam and provides rapid absorption and systemic delivery of alprazolam. Rectally and intranasally administered commercially available benzodiazepines may not be suitable candidates for REST treatment because of their inability to reach sufficiently high concentrations during the window of opportunity for proactive seizure cessation. 12 A study in healthy volunteers demonstrated that pharmacodynamic effects of intranasal midazolam were less pronounced and occurred later compared with intravenous midazolam, with a significantly longer median time to onset of electroencephalography effects. 13 These findings highlight the slower onset of action with intranasal compared with intravenous administration.
Benzodiazepines delivered by the intravenous route have an onset of anticonvulsive action in 1–3 min but are not practical options for outpatient REST therapy because of the requirement for parenteral administration by medically trained professionals. Staccato alprazolam has shown a rapid onset of action with an intravenous‐like profile, without the need for intravenous administration, making it a unique candidate for clinical use as an REST treatment.
In the groups treated with Staccato alprazolam, the most common AEs were cough, somnolence (both 14.5%), and dysgeusia (13.2%). Staccato alprazolam was generally well tolerated and displayed a safety profile consistent with that known. No new safety signals have been observed to date, with AEs mostly mild or moderate in intensity and no treatment‐related serious or severe AEs. This is consistent with prior trial experience. There was no difference in tolerability between the two doses. Among patients on Staccato alprazolam in the double‐blind part, the median oxygen saturation of 97.0% or above at all assessed time points supports the favorable respiratory safety profile of Staccato alprazolam during seizures.
There is a theoretical concern that patients with seizures with impaired awareness may not be able to properly use the Staccato device. Pharmacokinetic analysis showed that Staccato alprazolam was dosed successfully during the seizure in patients who received active drug. Alprazolam plasma concentrations were shown to be similar across different seizure types, including in patients with focal seizures with impaired awareness, indicating no impact of patient awareness status on the dosing of Staccato alprazolam. This demonstrates that Staccato alprazolam is a passive breath‐actuated device, and active inhalation is not necessary. The dosing may need to be assisted by a caregiver to correctly place the device in the mouth of patients whose awareness is impaired during the seizure. Although our study recruited 53 patients who historically had generalized tonic‐clonic or focal to bilateral tonic‐clonic seizures as an element in one or more of their treatable episodes, none occurred during the double‐blind part of the study. Ideally Staccato alprazolam should be used before the tonic‐clonic phase, or once breathing resumes. Further evaluation in this seizure population is warranted.
This was a novel study design in a new type of patient population. Although less important for clinical use, for study purposes, predictability at onset was important in order to identify the appropriate prolonged or cluster seizure for treatment intervention that would not terminate without therapy, and thus reduce the occurrence of “placebo response.” As part of the screening process, the patient's seizures and events leading up to them were reviewed by the ESC Review Board, in an effort to enroll patients whose seizure activity would remit without intervention less than 20% of the time. As a consequence of significant variability in seizure episode durations, even among patients carefully selected for consistent seizure patterns, some episodes would be expected to resolve without treatment. This inevitably results in a “placebo effect” in some patients. Therefore, the relatively high seizure cessation response of 42.5% in the placebo group in this study is not unexpected and likely not usually reflective of a psychological placebo effect.
To summarize, this proof‐of‐concept study showed that both 1.0 mg and 2.0 mg doses of Staccato alprazolam demonstrated efficacy in rapidly terminating seizures in a small observed population in an inpatient setting and were well tolerated. The next step will be a larger Phase 3 outpatient confirmatory study intended to demonstrate the efficacy and safety of Staccato alprazolam for rapid cessation of seizures in an outpatient setting among patients with stereotypical prolonged seizures who are 12 years of age and older. 14
AUTHOR CONTRIBUTIONS
Jacqueline French: conceptualization (supporting); methodology (equal); formal analysis (supporting); investigation (equal); writing – review and editing (equal). Victor Biton: investigation (equal); writing – review and editing (equal). Hina Dave: investigation (equal); writing – review and editing (equal). Kamil Detyniecki: investigation (equal); writing – review and editing (equal). Michael A. Gelfand: investigation (equal); writing – review and editing (equal). Hui Gong: investigation (equal); writing – review and editing (equal). Kore Liow: investigation (equal); writing – review and editing (equal). Terence J. O'Brien: investigation (equal); writing – review and editing (equal). Ahmed Sadek: investigation (equal); writing – review and editing (equal). Bree DiVentura: methodology (equal); formal analysis (supporting); writing – review and editing (equal). Brittany Reich: methodology (equal); formal analysis (equal); writing – original draft preparation (equal); writing – review and editing (equal). Jouko Isojarvi: methodology (equal); formal analysis (equal); writing – original draft preparation (equal); writing – review and editing (equal).
FUNDING INFORMATION
This study was funded by Engage Therapeutics, Inc., which was also responsible for the study design and collection and analysis of the data. Engage Therapeutics, Inc., is now a wholly owned subsidiary of UCB Pharma. The authors, some of whom are employees of Engage Therapeutics, Inc., or UCB Pharma, were responsible for data interpretation, revising the manuscript for intellectual content, and approving of the manuscript for submission.
CONFLICT OF INTEREST
Staccato® is a registered trademark of Alexza Pharmaceuticals, Inc. and is used by UCB Pharma under license. Jacqueline French receives salary support from the Epilepsy Foundation and for consulting work and/or attending scientific advisory boards on behalf of The Epilepsy Study Consortium for Adamas, Aeonian/Aeovian, Alterity Therapeutics Limited, Anavex, Arkin Holdings, Arvelle Therapeutics, Inc., Athenen Therapeutics/Carnot Pharma, Baergic Bio, Biogen, BioMarin Pharmaceutical Inc., BioXcel Therapeutics, BridgeBio Pharma Inc., Cavion, Cerebral Therapeutics, Cerevel, Clinical Education Alliance, Corlieve Therapeutics, Crossject, CuroNZ, Eisai, Eliem Therapeutics, Encoded Therapeutics, Engage Therapeutics, Inc., Engrail, Epalex, Epihunter, Epiminder, Equilibre BioPharmaceuticals, Fortress Biotech, Greenwich Biosciences, GW Pharmaceuticals, Janssen Pharmaceuticals, Knopp Biosciences, LivaNova, Longboard Pharmaceuticals, Lundbeck, Marinus, Mend Neuroscience, Merck, NeuCyte Inc., Neumirna Therapeutics, Neurocrine, Neuropace, NxGen Medicine Inc., Otsuka Pharmaceutical Development, Ovid Therapeutics Inc., Passage Bio, Pfizer, Praxis, PureTech LTY Inc., Redpin, Sage, SK Life Science, Sofinnova, Stoke, Supernus, Synergia Medical, Takeda, UCB Pharma, West Therapeutic Development, Xenon Pharmaceuticals, Xeris, Zogenix, and Zynerba; has received research support from the Epilepsy Study Consortium (funded by Andrews Foundation, Eisai, Engage Therapeutics, Inc., Lundbeck, Pfizer, SK Life Science, Sunovion, UCB Pharma, and Vogelstein Foundation), Epilepsy Study Consortium/Epilepsy Foundation (funded by UCB Pharma), GW Pharmaceuticals/Finding A Cure for Epilepsy and Seizures (FACES), and National Institute of Neurological Disorders and Stroke; is on the editorial board of Lancet Neurology and Neurology Today; is chief medical/innovation officer for the Epilepsy Foundation; and has received travel reimbursement related to research, advisory meetings, or presentation of results at scientific meetings from the Epilepsy Study Consortium, the Epilepsy Foundation, Arvelle Therapeutics, Inc., Biogen, Cerevel, Clinical Education Alliance, Engage Therapeutics, Inc., Lundbeck, NeuCyte, Inc., Otsuka Pharmaceutical Development, Sage, UCB Pharma, Xenon Pharmaceuticals, Inc., and Zogenix. Hina Dave served on the advisory board to Engage Therapeutics, Inc., but did not receive any consultation fees. Kamil Detyniecki has served as an advisory board member for Greenwich Pharma; and has received consultation fees from/served on advisory boards for Aquestive Therapeutics, Neurelis, and UCB Pharma. Michael A. Gelfand has received research funding from Aquestive Therapeutics, Cerevel, LivaNova, Otsuka, SK Pharma, UCB Pharma, and Xenon Pharmaceuticals, Inc. Terence J. O'Brien has received support from and/or has served as a paid consultant for Eisai, National Health and Medical Research Council, National Institute of Neurological Disorders and Stroke, Praxis Precision Medicines, Royal Melbourne Hospital Neuroscience Foundation, UCB Pharma, and Zynerba Pharmaceuticals. Ahmed Sadek has served as advisory board member for GW Pharmaceuticals and SK Life Science; speaker for Sunovion; and has received research support from Sunovion and UCB Pharma. Bree DiVentura is an employee of The Epilepsy Study Consortium. The Consortium consulted with Alexza Pharmaceuticals, Inc., Engage Therapeutics, Inc., and UCB Pharma and was paid for these services. Within the past year the Epilepsy Consortium has received funding for research support, meeting support, and services provided from the following companies: Alterity Therapeutics Limited, Amzell, Anavex, Aquestive Therapeutics, Arvelle Therapeutics, Inc., Autifony Therapeutics Limited, Biogen, BioMarin Pharmaceutical Inc., Bloom Science, BridgeBio Pharma Inc., Cerebral Therapeutics, Cerevel, Clinical Education Alliance, Corlieve Therapeutics, Eisai Medical Research, Eliem Therapeutics, Encoded Therapeutics, Engrail, Epalex, Epihunter, Epiminder, Equilibre BioPharmaceuticals, Greenwich Biosciences, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Knopp Biosciences, LivaNova, Longboard Pharmaceuticals, Lundbeck, Marinus, Medtronic, Neumirna Therapeutics, Neurelis, Neurocrine, Neuropace, NxGen Medicine Inc., Ono Pharmaceutical Co., Ltd, Otsuka Pharmaceutical Development, Ovid Therapeutics Inc., Passage Bio, Praxis, Prevail Therapeutics, PureTech LYT Inc., Rafa Laboratories Ltd., SK Life Science, Stoke, Supernus, Takeda, UCB Pharma, Ultragenyx Pharmaceutical Inc., Ventus Therapeutics, Xenon Pharmaceuticals, and Zogenix. Brittany Reich and Jouko Isojarvi were salaried employees of Engage Therapeutics, Inc., and received stock or stock options from their employment at the time this study was conducted. The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This article is dedicated to the memory of our friend and colleague Dr. Jouko Isojarvi. Jouko was passionate about epilepsy research and drug development and will be remembered as a clinician and scientist who made a difference in the lives of people living with epilepsy. The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to this study (see Appendix S1, Investigator appendix, in the Supporting Information). The authors developed the first draft of the manuscript and approved the content of the final version. The authors would like to acknowledge Ciara Duffy, PhD, CMPP (Evidence Scientific Solutions, Sydney, Australia) and Leonard Wills, BSc(Hons) (Evidence Scientific Solutions, London, UK), who were involved in helping with the creation of the manuscript from Draft 2 onwards. Their role included coordinating the author review process; incorporation of comments provided by the authors; editing and formatting the text; production of original figures; formatting of tables and figures; verifying the accuracy of the data; verifying the accuracy of references; collecting author contribution and conflict of interest statements; and assisting with the online submission process by uploading files, which was funded by UCB Pharma. Publication coordination was provided by Tom Grant, PhD (UCB Pharma, Slough, UK). Brittany Reich was an employee of Engage Therapeutics, Inc., at the time that this study was conducted and is currently affiliated with UCB Pharma. Jouko Isojarvi was an employee of Engage Therapeutics, Inc., at the time this study was conducted, and was contracted by UCB Pharma and affiliated with ASD Consulting LLC during the article's development.
French J, Biton V, Dave H, Detyniecki K, Gelfand MA, Gong H, et al. A randomized phase 2b efficacy study in patients with seizure episodes with a predictable pattern using Staccato® alprazolam for rapid seizure termination. Epilepsia. 2023;64:374–385. 10.1111/epi.17441
DATA AVAILABILITY STATEMENT
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents which may include: analysis‐ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Before use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.
REFERENCES
- 1. Valeant Pharmaceuticals North America LLC . Diastat C‐IV (diazepam rectal gel) rectal delivery system prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020648s014lbl.pdf (2016). Accessed 2 Mar 2022.
- 2. Tatum WO 4th. Adult patient perceptions of emergency rectal medications for refractory seizures. Epilepsy Behav. 2002;3:535–8. [DOI] [PubMed] [Google Scholar]
- 3. Berg AK, Myrvik MJ, Van Ess PJ. Pharmacokinetics, pharmacodynamics, and tolerability of USL261, midazolam nasal spray: randomized study in healthy geriatric and non‐geriatric adults. Epilepsy Behav. 2017;71:51–9. [DOI] [PubMed] [Google Scholar]
- 4. Sperling MR, Haas KF, Krauss G, Seif Eddeine H, Henney HR 3rd, Rabinowicz AL, et al. Dosing feasibility and tolerability of intranasal diazepam in adults with epilepsy. Epilepsia. 2014;55:1544–50. [DOI] [PubMed] [Google Scholar]
- 5. Almohaish S, Sandler M, Brophy GM. Time is brain: acute control of repetitive seizures and status epilepticus using alternative routes of Administration of Benzodiazepines. J Clin Med. 2021;10:1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pfizer . XANAX® alprazolam tablets, USP, prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018276s045lbl.pdf (2011). Accessed 2 Mar 2022.
- 7. French JA, Wechsler R, Gelfand MA, Pollard JR, Vazquez B, Friedman D, et al. Inhaled alprazolam rapidly suppresses epileptic activity in photosensitive participants. Epilepsia. 2019;60:1602–9. [DOI] [PubMed] [Google Scholar]
- 8. Jenck F, Moreau JL, Bonetti EP, Martin JR, Haefely WE. Ro 19‐8022, a nonbenzodiazepine partial agonist at benzodiazepine receptors: neuropharmacological profile of a potential anxiolytic. J Pharmacol Exp Ther. 1992;262:1121–7. [PubMed] [Google Scholar]
- 9. Herink J. Effect of alprazolam and ketamine on seizures induced by two different convulsants. Acta Medica (Hradec Kralove). 1997;40:9–11. [PubMed] [Google Scholar]
- 10. De Sarro G, Gitto R, Rizzo M, Zappia M, De Sarro A. 1,4‐benzodiazepine derivatives as anticonvulsant agents in DBA/2 mice. Gen Pharmacol. 1996;27:935–41. [DOI] [PubMed] [Google Scholar]
- 11. Ueki S, Watanabe S, Yamamoto T, Kataoka Y, Tazoe N, Shibata S, et al. Behavioral and electroencephalographic effects of alprazolam and its metabolites. Nihon Yakurigaku Zasshi. 1981;77:483–509. [PubMed] [Google Scholar]
- 12. Asnis‐Alibozek A, Detyniecki K. The unmet need for rapid epileptic seizure termination (REST). Epilepsy Behav Rep. 2020;15:100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hardmeier M, Zimmermann R, Ruegg S, Pfluger M, Deuster S, Suter K, et al. Intranasal midazolam: pharmacokinetics and pharmacodynamics assessed by quantitative EEG in healthy volunteers. Clin Pharmacol Ther. 2012;91:856–62. [DOI] [PubMed] [Google Scholar]
- 14. ClinicalTrials.gov . A study to test the efficacy and safety of Staccato alprazolam in study participants 12 years of age and older with stereotypical prolonged seizures (NCT05077904). https://clinicaltrials.gov/ct2/show/NCT05077904. Accessed 2 Mar 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents which may include: analysis‐ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Before use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.


