Abstract
Objective
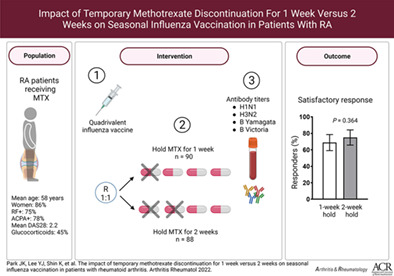
This clinical trial was conducted to investigate whether discontinuing methotrexate (MTX) for 1 week after seasonal influenza vaccination is noninferior to discontinuing for 2 weeks after vaccination in patients with rheumatoid arthritis (RA).
Methods
In this multicenter, prospective, randomized, parallel‐group noninferiority trial, RA patients receiving a stable dose of MTX were randomly assigned at a ratio of 1:1 to discontinue MTX for 1 week or for 2 weeks after they received the quadrivalent 2021–2022 seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains. The primary outcome measure was the proportion of patients with a satisfactory vaccine response, which was defined as ≥4‐fold increase in antibody titers, as determined with the hemagglutination inhibition assay, against ≥2 of the 4 vaccine strains at 4 weeks after vaccination.
Results
The modified intent‐to‐treat population included 90 patients in the 1‐week MTX hold group and 88 patients in the 2‐week MTX hold group. The mean ± SD MTX doses were 12.6 ± 3.4 mg/week in the 1‐week MTX hold group and 12.9 ± 3.3 mg/week in the 2‐week MTX hold group. The proportion of satisfactory vaccine responses did not differ between the groups (68.9% versus 75.0%; P = 0.364). The rate of seroprotection and the fold increase in antibody titers for each of the 4 influenza antigens were similar between the groups.
Conclusion
A temporary discontinuation of MTX for 1 week after vaccination was noninferior to a discontinuation of MTX for 2 weeks after vaccination, regarding induction of a satisfactory vaccine response to a seasonal influenza vaccine in patients with RA receiving a stable dose of MTX.

INTRODUCTION
Vaccinations against preventable diseases, including seasonal influenza, are strongly recommended for patients with rheumatoid arthritis (RA), who are at increased risk of infections as a result of underlying immune dysfunction and treatment‐induced immunosuppression (1, 2).
Methotrexate (MTX) remains the first‐line disease‐modifying antirheumatic drug (DMARD), which patients are prescribed either as monotherapy or in combination with other synthetic and biologic DMARDs, for the treatment of RA because of its high efficacy rate and favorable safety profile (3, 4). However, MTX significantly decreases the vaccine response to pneumococcal and seasonal influenza vaccines, particularly the response to antigens from novel strains (5).
We previously showed that temporarily discontinuing MTX for 2 weeks in RA patients who had been receiving a stable dose of MTX significantly increased immunogenicity without significantly increasing disease activity levels, whereas a 4‐week discontinuation was associated with a transient increase in the risk of RA flare, defined as an increase in the Disease Activity Score in 28 joints (DAS28) (6, 7, 8, 9). However, it is not clear whether discontinuing MTX for 1 week improves the efficacy of influenza vaccines (or other vaccines) to the same extent as discontinuing MTX for 2 weeks. Therefore, we compared the effects of MTX discontinuation for 1 week versus 2 weeks after vaccination on the response to seasonal influenza vaccination in RA patients in a randomized, controlled, noninferiority trial.
METHODS
Study design
In this 16‐week multicenter, prospective, randomized, investigator‐blinded, parallel‐group noninferiority intervention trial, we investigated whether discontinuing MTX for 1 week versus 2 weeks after vaccination with the 2021–2022 seasonal quadrivalent influenza vaccine resulted in any differences in a satisfactory vaccine response in RA patients receiving a stable dose of MTX. Control participants without autoimmune diseases were enrolled as a reference group to give information on the satisfactory vaccine response in people without autoimmune disease and who were not using MTX.
All enrolled participants received a quadrivalent influenza vaccine (GC Flu; Green Cross) in a 0.5‐ml prefilled syringe as a single intramuscular injection. Antibody titers against influenza antigens were measured the day before vaccination (prevaccination titer) and at 4 weeks and 16 weeks after vaccination (Supplementary Figure 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42318).
This study reported our primary outcome measure, i.e., a satisfactory vaccine response 4 weeks after vaccination. The study was approved by the Institutional Review Board of the Seoul National University Hospital (2109‐020‐1252) and was conducted in accordance with the principles of the Declaration of Helsinki and in accordance with good clinical practice guidelines. Written informed consent was obtained from all participants before enrollment in the trial.
Participants
Patients with RA who were ages ≥19 years and had received the same dose of MTX for ≥6 weeks were eligible for inclusion. RA was defined according to the 2010 American College of Rheumatology/EULAR classification criteria for rheumatoid arthritis (10). The exclusion criteria were as follows: pregnant or lactating women, patients with a previous anaphylactic response to vaccine components or to an egg component, evidence of an acute infection with a temperature >38°C at the time of vaccination, and history of Guillain‐Barré syndrome or demyelinating syndromes. Patients with any other additional rheumatic disease, except secondary Sjögren's disease, were also excluded.
Patient randomization and masking
The Medical Research Collaborating Center at Seoul National University Hospital generated a randomization table that was stratified by the participating centers. The eligible patients were randomly assigned to discontinue MTX for 1 week or for 2 weeks after vaccination by a central interactive web response system at a ratio of 1:1 according to the randomization table. Information on the intervention was concealed from the investigators who enrolled or assessed the study patients. Investigators who performed the hemagglutination inhibition assay (HIA) to determine antibody titers were blinded to the allocated treatment. Patients were not blinded to the intervention. To measure patient adherence to the study protocol, study patients were required to record their MTX administration in a diary.
Intervention
The 2021–2022 seasonal quadrivalent influenza vaccine contained 4 antigens: 15 μg of A/Victoria/2570/2019 IVR‐215 (H1N1), 15 μg of A/Cambodia/e0826360/2020 IVR‐224 (H3N2), 15 μg of B/Washington/02/2019, and 15 μg of B/Phuket/3073/2013 in a 0.5‐ml prefilled syringe. The vaccine was delivered as a single intramuscular injection in the deltoid muscle by health care providers.
After vaccination, the patients discontinued MTX for either 1 week or 2 weeks according to their assigned group and then resumed MTX at the previous dose. Adding or changing DMARDs was not allowed until serum samples were collected after vaccination. During the MTX discontinuation period, acetaminophen (650 mg up to 3 times per day), nonsteroidal antiinflammatory drugs (at standard dosing levels), and/or prednisolone (or its equivalent) up to 10 mg per day were allowed to treat RA flares. Medications for other comorbid conditions were also allowed.
Detection of influenza antibodies
The serum of the patients was collected before vaccination (week 0) and at 4 and 16 weeks after vaccination. The HIAs for measurement of antibody titers against each of the 4 influenza strains in the vaccine were conducted by an independent laboratory (the Vaccine Bio Research Institute of the Catholic University, Seoul, Republic of Korea).
Outcomes
The primary outcome measure was the frequency of a satisfactory vaccine response to influenza antigens 4 weeks after vaccination. A satisfactory vaccine response was a priori defined as a ≥4‐fold increase in HIA antibody titer at 4 weeks after vaccination relative to the baseline level in ≥2 of 4 influenza vaccine antigens. The secondary outcome measures included seroconversion (defined as a ≥4‐fold increase in HIA antibody titer at 4 weeks after vaccination relative to the baseline level); a positive response (i.e., seroconversion) to ≥1 antigens, ≥3 antigens, or 4 antigens; the frequency of seroprotection (defined as HIA titers of ≥1:40); and the fold change after vaccination in HIA antibody titers against each vaccine antigen relative to the baseline. The exploratory end points included the rate of a satisfactory vaccine response in controls and the incidence of influenza infection or influenza‐like illness during the 2021–2022 influenza season (11). Influenza‐like illness was defined clinically as the presence of a fever >38°C and cough within 48 hours of symptom onset (12). Patients were interviewed using a structured questionnaire, with patients interviewed during their follow‐up visit to the clinic or by telephone. Adverse events that were associated with vaccination were captured from the patients at each visit or telephone interview. DAS28 using the C‐reactive protein level (DAS28‐CRP) was determined at week 0 and at week 4 after vaccination. An RA flare was defined as an increase in the DAS28 of >1.2 (or >0.6 if the baseline DAS28 was ≥3.2) (13).
Statistical analysis
We conducted all analyses according to a predefined protocol. The analysis population was the modified intent‐to‐treat population, which included all study patients who received the influenza vaccine and had both prevaccination and postvaccination HIA titers at 4 weeks after vaccination available. A per‐protocol analysis was conducted as a sensitivity analysis. The safety profile was summarized for all participants who received the vaccination.
In our previous study, the satisfactory response to a quadrivalent seasonal influenza vaccine in RA patients continuing MTX and patients discontinuing MTX treatment for 2 weeks was 54% and 75%, respectively (6). To calculate the sample size, we specified a noninferiority margin of −20% points for the difference in satisfactory vaccine response. Assuming a 1‐sided alpha level of 0.025, a power of 0.80, and a dropout rate of 20%, we calculated that 92 patients per group (184 in total) were required for the study. The study was not powered to test the equivalence or superiority of RA patients versus control subjects.
We compared continuous variables using a t‐test or the Mann‐Whitney U test, as appropriate. We analyzed the binary secondary efficacy variables (the frequency of a satisfactory vaccine response and the frequency of RA flares) using chi‐square tests or the Fisher's exact test, as appropriate. A P value of less than 0.05 was considered statistically significant. All analyses were performed using SPSS version 20.
RESULTS
Baseline characteristics
Study patients were recruited by their primary rheumatologists in outpatient clinics at 3 tertiary medical centers in the Republic of Korea. The first patient was recruited on October 6, 2021, and the last patient was recruited on December 12, 2021. We enrolled 184 patients with RA (92 patients assigned to the 1‐week hold group and 92 patients assigned to the 2‐week hold group). Because 2 patients withdrew their consent after randomization, 182 patients received the vaccination. The antibody titer could not be measured in 1 patient in the 1‐week hold group and in 3 patients in the 2‐week hold group. Accordingly, 90 patients in the 1‐week hold group and 88 patients in the 2‐week hold group were included in the modified intent‐to‐treat population (Supplementary Figure 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42318).
The 2 groups did not differ at baseline in terms of demographic or disease characteristics, including age, sex, seropositivity for rheumatoid factor or anti–cyclic citrullinated peptide antibody, or DAS28‐CRP score. The 2 groups were also comparable in terms of their treatment regimen at baseline, including their use of systemic glucocorticoids and MTX dose (Table 1). The mean ± SD MTX doses in the 1‐week hold group and the 2‐week hold group were 12.6 ± 3.4 mg/week and 12.9 ± 3.3 mg/week, respectively. Of note, 91.6% of patients received a COVID‐19 vaccination, but none of the patients received COVID‐19 vaccinations between visits 1 and 2 (data not shown). Of the planned 92 healthy controls without autoimmune disease and not taking MTX, 62 (67.4%) were enrolled in the reference group.
Table 1.
Baseline characteristics of the modified intent‐to‐treat population*
| 1‐week MTX hold group (n = 90) | 2‐week MTX hold group (n = 88) | Healthy control group (n = 62) | |
|---|---|---|---|
| Female | 80 (88.9) | 73 (83.0) | 50 (80.6) |
| Age, mean ± SD years | 57.9 ± 12.5 | 58.7 ± 10.8 | 54.1 ± 13.3 |
| Duration of RA, mean ± SD years | 11.1 ± 8.1 | 9.9 ± 6.9 | – |
| Body mass index, mean ± SD kg/m2 | 23.2 ± 3.6 | 23.2 ± 3.6 | 24.6 ± 4.0 |
| Diabetes mellitus | 5 (5.6) | 7 (8.0) | 8 (12.9) |
| Hypertension | 33 (36.7) | 21 (23.9) | 15 (24.2) |
| Smoking, ever smoked | 16 (17.8) | 13 (14.8) | 14 (22.6) |
| RF positivity | 71/90 (78.9) | 64/88 (72.7) | – |
| Anti‐CCP positivity | 73/88 (83.0) | 61/84 (72.6) | – |
| DAS28‐CRP score, mean ± SD | 2.2 ± 1.1 | 2.3 ± 1.0 | – |
| Treatment | |||
| GC | 39 (43.3) | 42 (47.7) | – |
| GC dose, mean ± SD mg/day | 3.9 ± 1.8 | 4.3 ± 1.9 | – |
| MTX | 90 (100) | 88 (100) | – |
| MTX dose, mean ± SD mg/week | 12.6 ± 3.4 | 12.9 ± 3.3 | – |
| Sulfasalazine | 5 (5.6) | 7 (8.0) | – |
| Hydroxychloroquine | 23 (25.6) | 25 (28.4) | – |
| Leflunomide | 17 (18.9) | 18 (20.5) | – |
| Tacrolimus | 1 (1.1) | 3 (3.4) | – |
| Biologic DMARDs | |||
| TNF inhibitor | 12 (13.3) | 4 (4.5) | – |
| Abatacept | 3 (3.3) | 1 (1.1) | – |
| Tocilizumab | 1 (1.1) | 4 (4.5) | – |
| Rituximab | 0 | 1 (1.1) | – |
| JAK inhibitor | 3 (3.3) | 5 (5.7) | – |
Except where indicated otherwise, values are the number (%) of people/group. MTX = methotrexate; RA = rheumatoid arthritis; RF = rheumatoid factor; anti‐CCP = anti–cyclic citrullinated peptide; DAS28‐CRP = Disease Activity Score in 28 joints using C‐reactive protein; GC = glucocorticoid; DMARDs = disease‐modifying antirheumatic drugs; TNF = tumor necrosis factor.
Effects of MTX discontinuation on vaccine response
A similar proportion of patients in the 1‐week hold group and in the 2‐week hold group achieved a satisfactory vaccine response (68.9% versus 75.0%; P = 0.364; difference 6.1% [95% confidence interval (95% CI) −7.1, 19.3]). Similarly, the proportion achieving a positive vaccine response (i.e., ≥4‐fold increase in HIA antibody titer) to ≥1 antigens (92.2% versus 93.2%; P = 0.806; difference 0.96% [95% CI −6.7, 8.6]), ≥3 antigens (46.7% versus 40.9%; P = 0.439; difference 5.8% [95% CI −8.8, 20.3]), and 4 antigens (25.6% versus 27.3%; P = 0.795; difference 1.7% [95% CI −11.2, 14.7]) did not differ between the 1‐week hold group and the 2‐week hold group (Figure 1). The proportion of healthy controls achieving a positive vaccine response to ≥1 antigens was 88.7% (95% CI 80.6, 96.8), to ≥2 antigens was 62.9% (95% CI 50.5, 75.3), to ≥3 antigens was 41.9% (95% CI 29.3, 54.6), and to 4 antigens was 25.8% (95% CI 14.6, 37.0).
Figure 1.
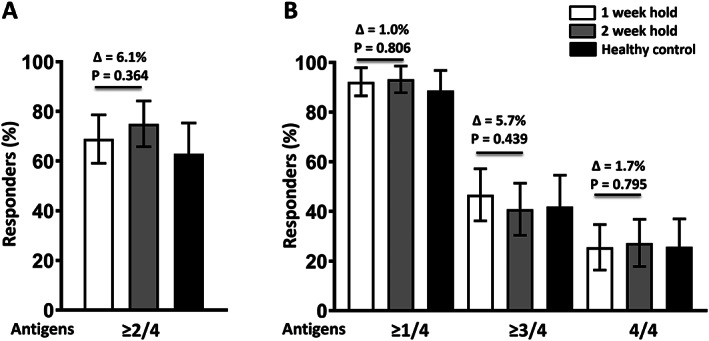
Frequency of vaccination responses to the influenza antigens in patients with rheumatoid arthritis who withheld methotrexate for 1 week or 2 weeks and in healthy controls. Graphs show the percentage of participants in each group achieving a satisfactory vaccine response, defined as a ≥4‐fold increase in antibody titers, as determined with the hemagglutination inhibition assay, at 4 weeks postvaccination against ≥2 vaccine strains (A) and against ≥1, ≥3, and 4 vaccine strains (B). Bars show the mean ± 95% confidence interval. The 1‐week and the 2‐week methotrexate hold groups were compared by a chi‐square test.
In terms of the responses to the individual vaccine antigens, the 1‐week hold group and the 2‐week hold group showed a similar frequency of a satisfactory vaccine response and a similar fold increase in antibody titers and frequency of seroprotection to each of the 4 influenza antigens (Table 2). Of note, the vaccine response did not differ between the 1‐week hold group and the 2‐week hold group according to the different baseline MTX dose ranges (Supplementary Figure 2, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42318).
Table 2.
Immunogenicity of influenza vaccination, showing antibody titers, fold changes in antibody titers, vaccine response, and SP rates before and after vaccination according to individual strains in the vaccine*
| 1‐week MTX hold group (n = 90) | 2‐week MTX hold group (n = 88) | Healthy control group† (n = 62) | P ‡ | |
|---|---|---|---|---|
| H1N1 | ||||
| Prevaccination titer, GMT (95% CI) | 16.8 (13.9–20.2) | 15.9 (12.9–19.7) | 15.6 (12–20.5) | 0.592 |
| Postvaccination titer, GMT (95% CI) | 165 (129.1–210.9) | 185.8 (146.6–235.6) | 154.7 (117.5–203.7) | 0.389 |
| Fold increase, GMT (95% CI) | 9.8 (7.6–12.7) | 11.7 (8.8–15.5) | 9.9 (7.1–13.7) | 0.247 |
| Satisfactory vaccine response | 78 (86.7) | 73 (83.0) | 51 (82.3) | 0.490 |
| Prevaccination SP | 28 (31.1) | 25 (28.4) | 17 (27.4) | 0.693 |
| Postvaccination SP | 83 (92.2) | 84 (95.5) | 58 (93.5) | 0.371 |
| H3N2 | ||||
| Prevaccination titer, GMT (95% CI) | 29 (23.8–35.2) | 24.4 (20.1–29.5) | 23.4 (18.2–30.2) | 0.276 |
| Postvaccination titer, GMT (95% CI) | 138.2 (109.3–174.8) | 128.3 (102.2–161.1) | 75.7 (57.9–98.8) | 0.396 |
| Fold increase, GMT (95% CI) | 4.8 (3.7–6.2) | 5.3 (4–6.9) | 3.2 (2.4–4.4) | 0.712 |
| Satisfactory vaccine response | 53 (58.9) | 56 (63.6) | 31 (50.0) | 0.452 |
| Prevaccination SP | 47 (52.2) | 41 (46.6) | 28 (45.2) | 0.452 |
| Postvaccination SP | 83 (92.2) | 85 (96.6) | 53 (85.5) | 0.330 |
| B/Yamagata lineage | ||||
| Prevaccination titer, GMT (95% CI) | 34 (28.2–41.1) | 24.6 (19.4–31.1) | 12 (9.5–15.1) | 0.025 |
| Postvaccination titer, GMT (95% CI) | 86.4 (71.5–104.4) | 76.3 (60.7–96) | 33.8 (26.4–43.3) | 0.526 |
| Fold increase, GMT (95% CI) | 2.5 (2.1–3.1) | 3.1 (2.5–3.9) | 2.8 (2.2–3.6) | 0.132 |
| Satisfactory vaccine response | 32 (35.6) | 38 (43.2) | 28 (45.2) | 0.298 |
| Prevaccination SP | 53 (58.9) | 41 (46.6) | 27 (43.5) | 0.100 |
| Postvaccination SP | 84 (93.3) | 74 (84.1) | 50 (80.6) | 0.051 |
| B/Victoria lineage | ||||
| Prevaccination titer, GMT (95% CI) | 17.1 (14.4–20.4) | 16.4 (13.7–19.7) | 27.1 (20.6–35.5) | 0.537 |
| Postvaccination titer, GMT (95% CI) | 61.6 (49.7–76.3) | 57.5 (45.8–72) | 69.2 (53.5–89.5) | 0.888 |
| Fold increase, GMT (95% CI) | 3.6 (2.9–4.5) | 3.5 (2.7–4.5) | 2.6 (2–3.2) | 0.680 |
| Satisfactory vaccine response | 47 (52.2) | 41 (46.6) | 26 (41.9) | 0.452 |
| Prevaccination SP | 27 (30.0) | 21 (23.9) | 14 (22.6) | 0.356 |
| Postvaccination SP | 71 (78.9) | 68 (77.3) | 40 (64.5) | 0.794 |
Except where indicated otherwise, values are the number (%) of people/group. Antibody titers and fold increase in titers before and after vaccination are expressed as the geometric mean titer (GMT). A satisfactory vaccine response (i.e., seroconversion) was defined as a ≥4‐fold improvement in titers relative to baseline. Seroprotection (SP) was defined as titers of ≥1:40. 95% CI = 95% confidence interval.
The control group served as a reference group. The study was not powered to test the equivalence or superiority of the methotrexate (MTX) hold group versus controls.
Comparison between the 1‐week MTX and the 2‐week MTX hold groups. P values were generated by independent t‐tests for continuous variables and chi‐square tests for categorical variables.
After vaccination, 6 patients in the 1‐week hold group and 9 patients in the 2‐week hold group deviated from the MTX discontinuation protocol. The primary and secondary outcome measurements in all of the patients who did not deviate from the protocol (i.e., in the per‐protocol population) did not differ between the 1‐week hold group and the 2‐week hold group, confirming the results of the main analysis (Supplementary Figure 3 and Supplementary Table 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42318).
Disease activity
Regarding RA disease activity, the change in the DAS28‐CRP score was only 0.1 ‐point higher (from baseline) in the 2‐week hold group than in the 1‐week hold group (0.0 ± 0.7 for the 1‐week hold group versus 0.1 ± 0.8 for the 2‐week hold group; P = 0.365). However, 4.5% of patients in the 1‐week hold group and 12.9% of patients in the 2‐week hold group experienced an RA flare over the 4 weeks after vaccination (P = 0.05) (Supplementary Figure 4, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42318). Similar proportions of patients in both groups required rescue medications for increased joint pain (3.4% versus 8.0%; P = 0.187).
Safety
The vaccine was well tolerated. No serious adverse events related to the vaccine were reported during the follow‐up period (Supplementary Table 2, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42318).
DISCUSSION
Our study demonstrated that, among RA patients who were receiving a stable dose of MTX, discontinuing MTX for 1 week after vaccination was noninferior to discontinuing MTX for 2 weeks regarding satisfactory vaccine responses in RA patients. A similar proportion of patients in each group developed seroprotection to each component of the seasonal quadrivalent influenza vaccine. Coupled with the results of our previous study that showed that discontinuing MTX for 2 weeks produced better immunogenicity than MTX continuation, our current study suggests that withholding MTX after influenza vaccination is an efficient strategy for patients who are receiving MTX.
Because RA patients are more susceptible to infections due to immune dysfunction associated with the underlying autoimmunity and immunosuppressive treatments than the general population, RA patients should be vaccinated for preventable infectious diseases (1, 2). MTX significantly reduces the immunogenicity of various vaccines, including seasonal influenza vaccine and COVID‐19 vaccines (14); thus, a novel vaccination strategy is required to restore a robust vaccine response in RA patients. Our previous randomized controlled trials showed that temporarily discontinuing MTX for 2 weeks or 4 weeks after vaccination significantly improves the immunogenicity of seasonal influenza vaccination in RA patients receiving a stable dose of MTX (6, 7).
In the present study, a 1‐week MTX discontinuation was as effective as a 2‐week MTX discontinuation in inducing a satisfactory vaccine response in RA patients. A satisfactory vaccine response, the fold increase in postvaccination antibody titers, and seroprotection rates did not differ between the 1‐week and 2‐week MTX hold groups. Of note, the vaccine response in RA patients was comparable to that in the healthy controls (reference level) without autoimmune disease who did not take immunosuppressant medications, suggesting that withholding MTX might restore the suppressed vaccine response of RA patients to a level similar to the response in healthy controls (7).
Although our study patients had no significant change in the mean DAS28‐CRP score after vaccination, 4.5% of patients in the 1‐week MTX hold group and 12.9% of patients in the 2‐week MTX hold group experienced an RA flare during the 4 weeks after vaccination (P = 0.05) (Supplementary Figure 4, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42318). In our prior studies, the RA flare rate in patients who continued MTX during the follow‐up period after vaccination was 5.8%, whereas patients in the 2‐week and 4‐week MTX discontinuation groups had RA flare rates of 10.8% and 20.5%, respectively. Therefore, patients who had 1 week of MTX discontinuation had no increase in the risk of an RA flare above the background flare rate seen in patients who continued MTX treatment (8). We observed a slight increase in immunogenicity in patients who discontinued low‐dose MTX for 2 weeks versus that shown at 1 week, although RA flare rates and RA disease activity were similar between the 2 groups. These data may be incorporated into future guidance on the optimal duration of withholding MTX, balancing tradeoffs of increased immunogenicity and possible changes in RA disease activity.
A 1‐week MTX discontinuation to restore the humoral immune response has several potential clinical implications. First, both patients and physicians would not be hesitant to withhold MTX for 1 week to restore a vaccine response, since most patients who skip MTX once do not experience an RA flare in routine clinical practice. Second, minimizing the MTX discontinuation period is critical when a vaccination protocol, such as those for COVID‐19 and hepatitis, requires multiple doses; repeated MTX discontinuation for 2 weeks or longer during a vaccination series of 2 doses given 3–4 weeks apart would cumulatively increase the risk of an RA flare. Third, pausing MTX for 1 week after vaccination might be applicable to other immunosuppressive drugs, such as mycophenolate mofetil in patients with other autoimmune diseases and organ transplant recipients.
Because this is an interim analysis, we did not present data on the antibody titers at 16 weeks after vaccination and the incidence of flu‐like illness. These results will be presented later after conclusion of the study.
This study has several limitations. This study did not include a group of RA patients who continued MTX after vaccination as an active comparator. The effect of MTX discontinuation on long‐term immune protection after vaccination and waning immunity needs further investigation. The mean dose of MTX in the study was ~13 mg/week, which is lower than the recommended maximum dose of 25 mg/week. In the future, it could be necessary to reconfirm our results in patients receiving a higher dose of MTX (7). Finally, further studies are needed to determine whether this novel strategy can be applied to other vaccinations of different mechanisms, such as messenger RNA or vector‐based vaccines.
In conclusion, temporary discontinuation of MTX for 1 week is noninferior to MTX discontinuation for 2 weeks after influenza vaccination to induce a satisfactory vaccine response to a seasonal influenza vaccine in patients with RA who receive a stable dose of MTX.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting and revising the article for important intellectual content, and all authors approved the final version to be published. Dr. E. Lee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Jin Park, Winthrop, E. Lee.
Acquisition of data
Y. Lee, Shin, Kang, Ha, Jun Park, Min Kim, Mi Kim, Choi, Y. Jung, J. Lee, J. Jung, J. Kim.
Analysis and interpretation of data
Y. Lee, Shin, Kang, Ha, Jun Park, Min Kim, Mi Kim, Choi, Y. Jung, J. Lee, J. Jung, J. Kim.
Supporting information
Disclosure Form
Supplementary figure S1 Study design (A) and patient flow (B).
Supplementary figure S2. Impact of baseline methotrexate (MTX) dose on vaccination responses to the influenza antigens.
Supplementary figure S3. Per‐protocol analysis of vaccination responses to the influenza antigens.
Supplementary figure S4. The change in disease activity and the flare rate of rheumatoid arthritis.
Supplementary table S1. Immunogenicity of the influenza vaccine (per‐protocol analysis).
Supplementary table S2. Adverse events.
ACKNOWLEDGMENTS
We thank all of the study participants for their support of the study.
A graphical abstract can be found in the online article at https://onlinelibrary.wiley.com/doi/10.1002/art.42318/abs
ClinicalTrials.gov identifier: NCT05069714.
Supported by a National Research Foundation of Korea grant funded by the government of the Republic of Korea (Ministry of Science and ICT) (grant 2021R1A2C2004874).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42318&file=art42318‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Van Assen S, Agmon‐Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 3. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Landewe RB, Bijlsma JW, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 5. Friedman MA, Curtis JR, Winthrop KL. Impact of disease‐modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2017;76:1559–65. [DOI] [PubMed] [Google Scholar]
- 7. Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JK, Kim MJ, Choi Y, et al. Effect of short‐term methotrexate discontinuation on rheumatoid arthritis disease activity: post‐hoc analysis of two randomized trials. Clin Rheumatol 2020;39:375–9. [DOI] [PubMed] [Google Scholar]
- 9. Prevoo ML, van't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 10. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 11. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004;103:133–8. [DOI] [PubMed] [Google Scholar]
- 12. Monto AS, Gravenstein S, Elliott M, et al. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000;160:3243–7. [DOI] [PubMed] [Google Scholar]
- 13. Van der Maas A, Lie E, Christensen R, et al. Construct and criterion validity of several proposed DAS28‐based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis 2013;72:1800–5. [DOI] [PubMed] [Google Scholar]
- 14. Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID–19 vaccine in immune‐mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary figure S1 Study design (A) and patient flow (B).
Supplementary figure S2. Impact of baseline methotrexate (MTX) dose on vaccination responses to the influenza antigens.
Supplementary figure S3. Per‐protocol analysis of vaccination responses to the influenza antigens.
Supplementary figure S4. The change in disease activity and the flare rate of rheumatoid arthritis.
Supplementary table S1. Immunogenicity of the influenza vaccine (per‐protocol analysis).
Supplementary table S2. Adverse events.


