Abstract
The literature on mixed-species flocks references a wide variety of bird associations. These studies, however, have used an array of unstructured characteristics to describe flocks, ranging from the temporal occurrence of flocking to the identity and behavioural features of constituent members, with little consensus on which key traits define and characterize a mixed-species flock. Moreover, although most studies report species-specific roles, there is no clear consensus about what these roles signify nor how to define them. This lack of consistency limits our ability to compare flocks from different habitats, regions and species pools. To unify this sizable body of literature, we reviewed and synthesized 538 studies on mixed-species flocks. We propose 13 categories to classify mixed-species flocks using behavioural and physical traits at the flock and participant level, as well as the habitat where the flock occurs. Lastly, we discuss the historical terminology for different species roles and propose definitions to clarify and distinguish among nuclear, leader, sentinel, and flock-following species. We envision that these guidelines will provide a universal language for mixed-species flock research, paving the way for future comparisons and new insight between different regions and systems.
This article is part of the theme issue ‘Mixed-species groups and aggregations: shaping ecological and behavioural patterns and processes’.
Keywords: multi-species groups, foraging groups, flocking behaviour
1. Introduction
One of the goals of community ecology is to unravel the processes that structure natural communities. Although communities are often thought to be structured by negative interactions (e.g. competition, predation and parasitism), positive interactions among species, such as sociality, can also influence community structure [1]. Sociality emerges as a result of co-occurring positive interactions that allow species to expand into new ecological niches and persist in these environments, which might otherwise be unfavourable to less social species [2–4]. As such, sociality has evolved in numerous taxa, and coordinated behaviour among individuals within a social group exists in both monospecific and multi-species groups [5–8]. The greater prevalence of monospecific groups is expected because all individuals share the same ecological requirements. Although they may compete for resources, the benefits of sociality in such situations outweigh the costs and allow for the evolution of intricate patterns of kin and non-kin relationships. One example of kin-based sociality is cooperative breeding, where strong social bonds between individuals are maintained by direct and indirect benefits linked to kinship and group augmentation [9]. Alternatively, multi-species groups harbour individuals with different ecological requirements where, for example, different species eavesdrop on one another to gain information about risks [10,11] and/or resources [12]. One key example of heterospecific attraction is the formation of avian multi-species groups known as mixed-species flocks.
Mixed-species flocks of birds (hereafter, mixed flocks) have been known and described for more than 150 years. Although researchers have long noticed similarities and differences among flock systems across the globe [13–19], there is no clear operational definition of these social systems. Nevertheless, the hallmark of a mixed flock is that, while different species of birds are travelling together, benefits that accrue to an individual depend upon the behaviour of the other flock members [20–23]. Participants of mixed flocks have been shown to increase their foraging efficiency by observing other nearby, successful foragers, either by copying their behaviour or through kleptoparasitism [24–28]. Additionally, participants of mixed flocks diminish their predation risk through a combination of group vigilance, where species have different abilities to detect predators, predator confusion, and the dilution effect [10,26,29,30]. Thus, regardless of the acting mechanism, flocking behaviour likely results in higher individual survival [10,29–31]. However, participation in flocks would only be favoured when the benefits of associating with heterospecifics outweigh the cost of interspecific competition for resources [32]. Multi-species flocks have been hypothesized to form when the constituent species have complementary foraging niches and predator-detection skills or when resources cannot be monopolized by individual flock members [33–35]. Throughout the mixed flock literature, different organizational systems have been described, but unravelling these differences will be the key to further understanding the forces that lead to interspecific sociality.
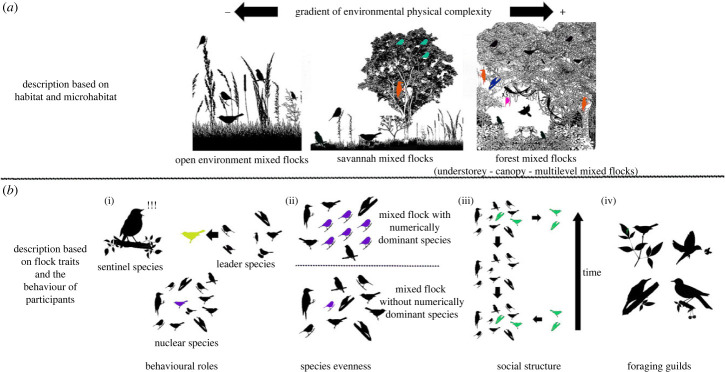
Mixed flocks have been described using an array of unstructured characteristics, ranging from the temporal occurrence of flocking to the identity and behavioural features of constituent members. In the literature, mixed flocks have been characterized as either seasonal or stable throughout the year [21,36], and, in some cases, as territorial when participants share and defend a common territory [22,37,38]. Mixed flocks have also been described based on foraging height, body size, plumage colour, taxonomic relationships, foraging behaviour and the habitat preferences of the participants [39–42]. For instance, some studies report distinct understory and canopy mixed flocks [37,43–45], whereas others report colour-based flocks [46,47], taxon-specific flocks (such as those formed by woodpeckers and parrots [48–50]), and multilevel mixed flocks that span multiple categories [42,51]. Finally, some authors define flocks according to habitat types, such as open-environment flocks [52–54] or lowland mixed flocks [55,56]. Yet rather than following any standardized terminology, most of these descriptions depend on specific definitions that researchers apply to their own study system. For this reason, it is a great challenge to describe and classify the many different mixed flock systems that exist around the world. Ultimately, this lack of consistency limits our ability to compare flocks from different habitats, regions and species pools.
One shared aspect of mixed flocks is that participating species are believed to have different behavioural roles, leading to certain species being more important for the formation and cohesion of flocks [20,57,58]. Such roles have been hypothesized to reflect coevolutionary processes and therefore have the potential to be a major structuring force in bird communities [59–61]; alternatively, it has been suggested that species may fulfil these roles inadvertently, a simple byproduct of having traits that influence flock participation, thereby leading to benefits for other flock members [31]. Despite the potential significance of these roles for mixed flocks and community organization, the terminology has yet to be standardized. Species roles have been categorized based on the frequency of participation, the effect that one species may have on the participation of another species [57], the amount of time a species remains with a mixed flock [20,57], centrality measurements based on network analysis [62], or specific behaviours that each species performs within the flock [20,58]. For example, ‘nuclear’ species have been defined as those that contribute to a flocks' formation and/or cohesion, whereas ‘attendant’ species contribute little to flocks other than their presence [57]. On the other hand, ‘regular’ species have been defined as those staying with the flock for prolonged periods, seldom being detected away from flocks, whereas ‘occasional’ participants associate only temporarily with the flock when it passes through their territories [22,63,64]. The term ‘leader’ is used to denote a species that is usually at the head of flocks—the first to move in a particular direction—but also more generally as a species that plays an essential role within the flock [20,31]. Finally, the term ‘sentinel’ has been used to describe species that first emit alarm calls when a predator is detected but also species that lead and attract other members [22]. In addition to all these examples, many other terms appear in the literature, sometimes as synonyms, leading to even more confusion [31].
The likelihood of highly co-evolved species roles increases in more highly stable systems, where the selection of such behaviours is strong and constant [61]. As such, it has been proposed that mixed flocks reach their highest diversity and complexity within tropical forest environments, where most of these potential co-evolved interactions have been described [26,36,38,65]. Indeed, these coevolutionary interactions have been hypothesized to increase the diversity of species that can coexist within tropical forests, where flocks are characterized by a sentinel-alarm system, a core set of species (that nearly always occur in flocks), permanent territories, and the maintenance of interspecific bonds through time [21,22,37]. However, most studies of flocks, in both tropical and temperate zones, lack comparable data on flock structure, and there has been little consistency in the characterization of flock behavioural roles. To further blur the lines, ‘nuclear species’ and ‘leader species’ can change from location to location [66] and year to year [67]. Further, in some areas, it has been shown that roles are not fixed but rather depend on the composition of the flock and its intrinsic features, such as flock size [53,68]. Therefore, the universality of the nuclear/sentinel/leader behavioural roles remains uncertain [69,70]. Consequently, confusion arises when concepts are used inconsistently. This adds to the unavoidable challenges linked to the study and description of complex social systems. For this reason, clear definitions and operational metrics are pivotal to better understand and compare the patterns and processes that lead to the formation of mixed flocks in different parts of the world.
In this work, we reviewed the published literature on terrestrial mixed flocks (n = 538 papers). We first summarized the various ways different authors have historically described both mixed flock systems and species roles. Based on this review, we developed a classification scheme for mixed flocks. Next, we proposed a set of standard flock traits to describe and measure the features of mixed flocks, which will facilitate comparisons among studies. Lastly, we recommend a series of best practices to help further standardize these descriptions and conclude by discussing the future of mixed-flock research.
2. Methods
To review and discuss the body of literature on mixed flocks, we organized and coordinated a team of experienced researchers who work with mixed flocks across the globe. To identify relevant literature, we employed a preexisting online bibliography on terrestrial mixed flocks, which has been curated for more than 10 years and is regularly updated [71], with the last update occurring on 16 September 2021, before the start of this review. This database included 564 published papers, book chapters and ‘grey’ literature (e.g. conference proceedings and short reports). We complemented this database with missing literature (n = 21), especially those from regional publications not in English and Rapid Assessment Program (RAP) reports that mention mixed flocks, achieving a final compilation of 585 studies. However, of these, we reviewed 538 studies (Appendix 1): 18 were discarded because they were not peer-reviewed (i.e. opinion pieces or congress presentations in lecture format), and we were unable to locate an additional 29, either because the official source was unavailable or was only available in paper copy, which we could not access. We then selected key variables used to describe mixed flocks and the potential coevolved interactions among participant species, specifically if studies detailed nuclear, leader and sentinel species or terminology associated with these roles. We compiled a summary table for the reviewed literature using this set of variables. Each study represented a unique row within the summary table, except for those studies that presented results separately for two or more localities or when authors reported results from distinctly different kinds of mixed flocks. For each publication, we recorded (1) author(s), (2) year of publication, (3) title, and (4) journal or place of publication. We then extracted the following variables for each study, according to the degree of detail provided: (5) country, (6) locality, (7) environment as described by the author(s), (8) GPS coordinates, (9) presence/absence of sentinel species, (10) presence/absence of nuclear species, (11) presence/absence of leader species, (12) seasonality of occurrence, and (13) whether the flocks were territorial. Further, if the author(s) described any distinctive feature of the mixed flock system (14), we characterized the types of flocks defined by the authors (15), the traits used to classify those flocks (16), whether flock composition is available, at least in part (17), and whether the mean number of species (18) and individuals per species (19) were reported. Lastly (20), we described any additional relevant information reported by the author(s).
Because this information was absent or incomplete from some studies, we established a protocol for data extraction. For studies that provided a GPS coordinate (n = 360), we extracted the underlying biogeographic realm and country name based on the ecoregion's GIS layer [72] and the UN Environment Programme's World Conservation Monitoring Centre (UNEP-WCMC). For studies that provided a locality but not a GPS coordinate, we extracted country names, continents and biogeographic realms using the most accurate locality described (n = 150). For all remaining articles (e.g. reviews, theoretical manuscripts), we labelled these columns as ‘not available’ (n = 28). We only included particular species roles (i.e. sentinel, leader or nuclear) when the author(s) either specifically used this terminology or applied associated language, such as ‘lead the movement of the flock’ (reported as leader) and ‘emitted alarm calls’ (regarded as sentinel).
In some cases, authors used different words or categories for the same behaviour within a single study (i.e. referring to the same species as both leader and nuclear in successive paragraphs); in these instances, we characterized the information as ‘ambiguous’. Whenever a study was conducted within a single season, we determined that seasonality could not be evaluated, and we correspondingly labelled this information as ‘not available’. Similarly, when the authors explicitly mentioned or assumed territoriality, we reiterated whatever designation the authors supplied. However, when the information was missing, we also recorded this as ‘not available’. We considered that flock composition was recorded when the author(s) reported some information about participant species, either in the main text or within the supplementary material. Lastly, details about the average number of species and individuals proved challenging to extract from most papers in our search. Therefore, we recorded this information at the minimum level provided and described these metrics as either ‘reported’ or ‘not reported’.
Of the 538 studies we reviewed, 60 presented information on distinctly different mixed flocks, either because the author(s) sampled disparate study areas or reported results separately from different kinds of flocks within the same study area (min = 2, max = 13 different kinds of flocks per study). Within a study, we therefore considered each distinct flock as the sample unit, totalling 682 sample units. We set up several contingency tables to evaluate the relationship between biogeographic realms and the terminology used. Because the expected frequencies were less than 5 in some cases, we used Fisher's exact test to explore how seasonality and territoriality were reported as well as how different terminologies for species roles were employed between different biogeographic realms.
3. Results
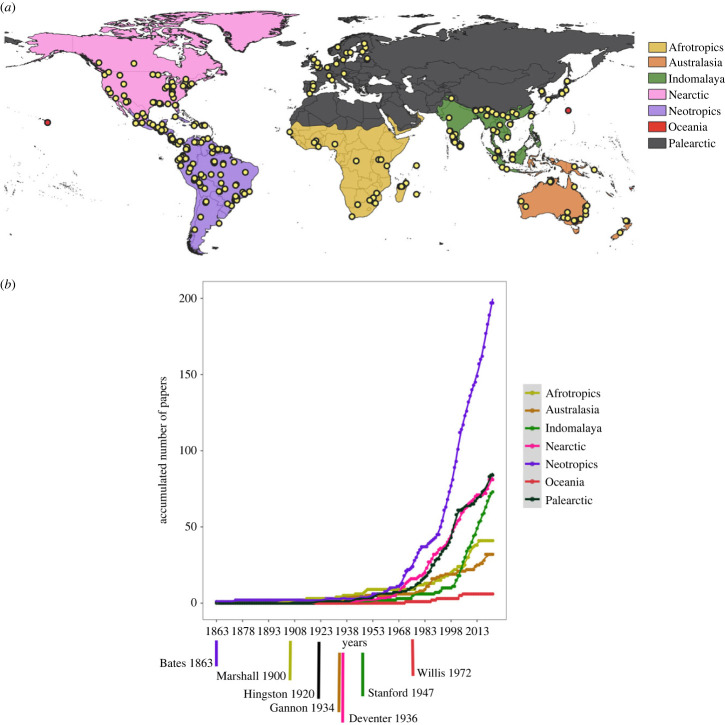
We reviewed 538 studies encompassing 682 flock sample units (Appendix 1). These studies were conducted in 71 countries, 159 ecoregions, and all major biogeographic realms except Antarctica (figure 1a). Most studies took place in the Neotropics (198 studies), followed by the Palearctic (84), Nearctic (81), Indomalaya (72), Afrotropics (41), Australasia (33) and Oceania (6; figure 1b). Studies on mixed flocks were published sporadically from 1863 until 1950, after which there has been a consistent increase in publications, regardless of the biogeographic realm, despite the Neotropics comprising the plurality of all published studies (36% of the 538 reviewed studies, figure 1b).
Figure 1.
(a) Map partitioning the reviewed flock studies according to major biogeographic realms and (b) the global accumulation of studies published each year, with the timeline denoting the first published study within each biogeographic realm.
Our review identified considerable variation in flock descriptions over the last 158 years (1863–2021), with many studies only disclosing location and habitat information. Mixed flock traits such as joint territorial defence, seasonal occurrence and species roles were often excluded or only reported when the authors' objective was to evaluate them explicitly, while flock composition and quantifiable metrics were seldom specified. For instance, of the 538 studies, only half (49%; n = 265) mentioned at least some of the participant species, either in the main text or the electronic supplementary material. Even fewer reported the average number of species (26%, n = 139) and individuals (22%, n = 117).
(a) . Mixed flock traits in the literature
Of the 538 studies analysed, 46% (n = 245) used a particular trait to characterize their study flocks. These traits ranged from the taxonomic composition of participants (species, genera, order) to their foraging niche, foraging strata and behaviour, as well as the flocks’ seasonal occurrence or territorial behaviour. We identified and selected 12 traits that authors consistently used to describe their flocking systems (table 1). Broadly, these traits can be organized under three major headings: (i) habitat—the environment and particularities of where the study was conducted, usually related to climatic seasonality or whether a specific type of vegetation dominated the environment; (ii) behavioural and physical traits at the flock level—peculiarities of the flock, such as its size, the presence of co-evolved traits (nuclear, leader or sentinel species), taxonomic composition, seasonality of occurrence, territoriality or how close in space participants move; and (iii) behavioural and physical traits at the participant level—usually related to a specific behaviour or intrinsic feature of participants, with particular emphasis on how species and individuals remain connected over time, their plumage coloration or the foraging guild of participating species (table 1).
Table 1.
Compiled traits used to describe mixed flock systems. Traits are organized hierarchically under three major traits with subcategories specified.
| major trait | specific trait | usage in the literature | types used |
|---|---|---|---|
| habitat feature | structure | used to describe mixed flock systems according to their habitat configuration | the number of vegetation strata; environmental humidity; elevation; vegetation composition |
| stability of climatic conditions | usually related to the seasonality of the environment but also the stability of temperature and humidity throughout the year and the possibility of flooding | seasonal and aseasonal; flood probability |
|
| behavioural and physical traits at the flock level | composition | based on the taxonomic positions or foraging guilds of the mixed flock participants | taxonomy at the order, genus or species level; foraging guilds |
| seasonality | the seasonal occurrence and/or seasonal changes in composition and flocking propensity of species in mixed flocks | seasonal; year-round |
|
| territoriality | whether participants consistently use the same area and jointly defend that territory | territorial; not territorial |
|
| cohesiveness | the duration and spatial proximity of participants | cohesive; not cohesive |
|
| social structure | at the individual level: a. the temporal consistency of individual participants within mixed flocks, b. related to the content, quality, and patterning of social relationships emerging from repeated interactions between individuals; at the species level: if network metrics are used, network strength is measured as the consistency of species co-occurrence in flocks |
fission-fusion process; repeated observations over time of tagged individuals; node- and network-level metrics; node-level metrics: social bond strength and weighted degree; network-level metrics: homophily, modularity, and clustering |
|
| size | the number of species and individuals that a mixed flock supports | small: two to five species; large: not standardized |
|
| potential presence of co-evolved traits | the different roles that participating species have within a mixed flock | sentinel; leader; nuclear; follower |
|
| behavioural and physical traits at the level of participants | foraging height | the vegetation strata used by participants | terrestrial; understory; midstory; canopy |
| foraging guild | the primary foraging guild of participants | insectivorous; frugivorous; granivorous; omnivorous |
|
| morphology | body size: the average size of participant species | small; large |
|
| plumage colour: the overarching plumage colour for the majority of flock participants | brown black-and-white; colourful |
(b) . Territoriality of mixed flocks
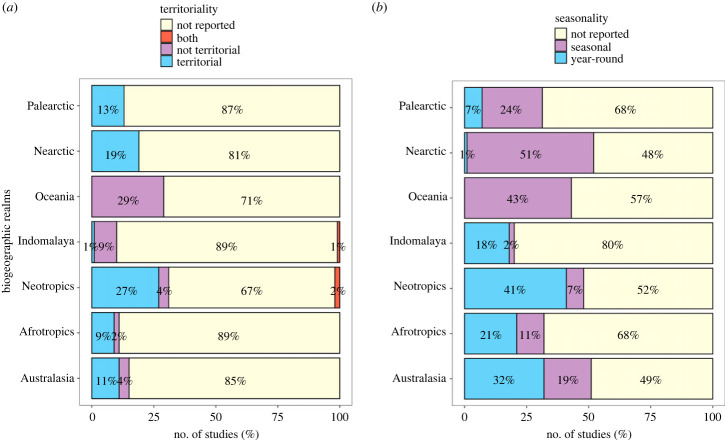
Most studies did not report territoriality (79%, n = 539 of 682 sample units). Of the 143 that did (21%), most (82%, n = 117) reported that flocks were territorial. The remaining 26 flocks were either reported as not territorial (13%, n = 18) or that territoriality depended on the location or type of mixed flock being studied (6%, n = 8). Between the seven biogeographic realms, we found significant differences in how studies report territoriality (p-value < 0.001; figure 2a). From studies with biogeographic data, we found that most territorial flocks (n = 116 of 682) were described from the Neotropics (66%, n = 77), followed by the Nearctic (15%, n = 17), Palearctic (10%, n = 12), Australasian, Afrotropical and Indomalayan regions (4%, 3% and 1% respectively). Non-territorial mixed flocks (n = 23 of 682) were also most often reported from the Neotropics (43%, n = 10), followed by the Indomalayan region (35%, n = 8), Oceania, Australasia and the Afrotropics (9%, 9% and 4% respectively); no flocks from the Nearctic or Palearctic were reported specifically as non-territorial. Within each biogeographical realm, our results indicate that the number of studies not reporting territoriality ranges from 67% to 89% (figure 2a).
Figure 2.
Percentage of mixed flock studies in each biogeographic realm that reported (a) territoriality and (b) seasonality.
(c) . Seasonality of mixed flocks
Although fewer studies omitted seasonality compared with territoriality, most studies were either conducted during a single season or did not report whether flocks formed seasonally or year round (62% of 538 studies). For studies that reported seasonality (n = 274), 40% (n = 109) mentioned that flocking was seasonal. By contrast, 60% (n = 165) reported that flocks could be found year round, even though some variation in size, composition and behaviour were reported between seasons. Again, we found significant differences in the reporting of seasonality among the seven biogeographic realms (p-value < 0.001, figure 2b). Most year-round flocks were reported from the Neotropics (68%, n = 116), followed by Indomalaya (12%, n = 20), Australasia, the Afrotropics, Nearctic and Palearctic (9%, 6%, 1% and <1% respectively). By contrast, most studies that reported seasonal flocks were in the Nearctic (42%, n = 45), followed by the Palearctic (21%, n = 23), Neotropics (19%, n = 20), Australasia, Afrotropics, Oceania and Indomalayan regions (8%, 5%, 3% and 2%, respectively). Within each biogeographic realm, our results illustrate that in most regions, the majority of studies did not report seasonality (48–80%; figure 2b).
(d) . Participant roles in mixed flocks
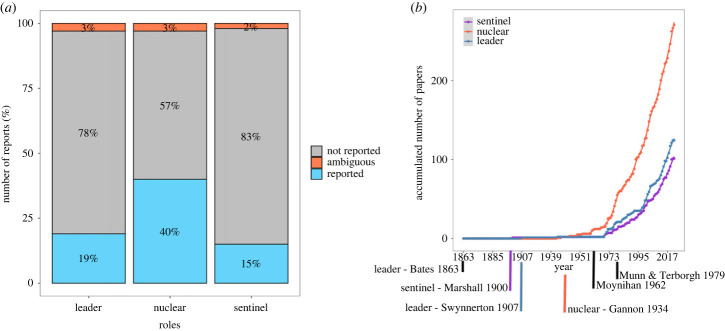
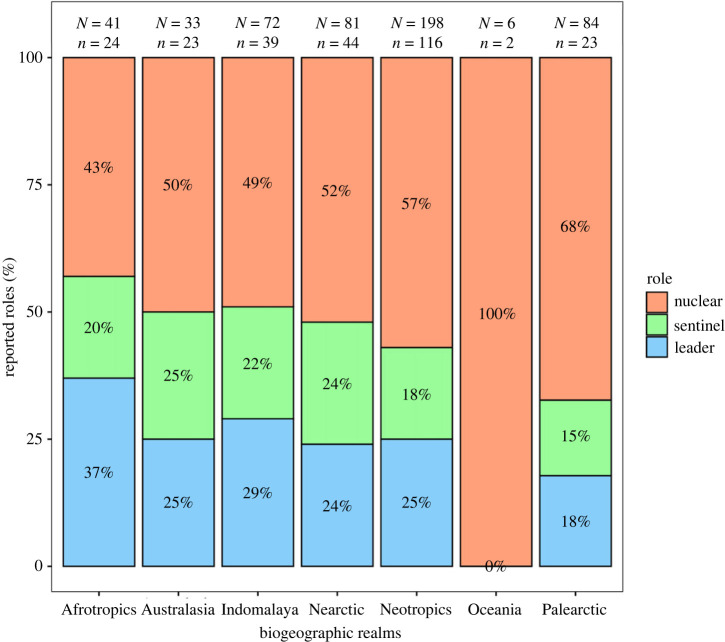
Our review revealed that 53% of studies (n = 284 of 538) discussed flocks containing one or more species that behave as leaders, sentinels or nuclear species. Of the 682 sample units, leader species were mentioned for 22% (n = 149); of this subset, 14% (n = 22) used other terms as synonyms within the same work (e.g. a species first mentioned as leader and then later as nuclear). Nearly double that number, 43% (n = 292), mentioned nuclear species, with 7% (n = 21) using apparent synonyms for the same concept within the same study. Sentinel species, however, were referenced the least (17%, n = 118), and 13% of these (n = 15) were reported ambiguously, either by indirectly describing sentinel species or noting that the behaviour was only observed once, such that no further conclusions could be drawn (figure 3a). Species roles have been mentioned in the literature since 1863, with alarm call emission (i.e. sentinel species) the first to be reported in field observation, followed by the concept of leadership (1907) and, lastly, the idea of a nuclear species capable of attracting others (1934). These species roles, however, started to be described more frequently after 1960 (figure 3b). We did not find region-specific differences in how studies reported species roles (p-value = 0.66; figure 4).
Figure 3.
(a) Summary of the terminology used to report notable species roles in mixed flocks. (b) The accumulation of studies that reported these roles over the past 150+ years. The timeline in (b) is annotated with the first published manuscript to mention either sentinel, leader or nuclear behaviour. Note that ‘leader’ is labelled twice here. Even though Bates [13] was the first to mention this behaviour, the local native communities were the ones who communicated this to him, which Bates largely dismissed as a myth. Two additional important works are denoted on the far right, after which species roles have been reported more frequently.
Figure 4.
Summary of studies reporting species roles across each of the seven biogeographic realms. Across the top, capital ‘N’ represents the total number of published studies within each biogeographic realm, whereas the lowercase ‘n’ refers to the number of studies that reported participant roles, which were used to calculate the percentages shown.
(e) . Mixed flocks described in the literature
Descriptions of mixed flock types can be found in any section of the studies we reviewed (introduction, methods, results or discussion), depending on the main objective of the research. Consequently, a quantitative analysis could be misleading. Thus, based on how the author(s) described their own mixed flocks, the terminology and combination of traits employed, and the availability of descriptions in the literature, we were able to collapse all studied flocks into one of 13 different mixed flock systems (table 2). For those studies that provided sufficient detail (n = 255), we were able to quantify the prevalence of these 13 categories. The two most frequently used descriptors were understorey and canopy flocks (n = 104 and n = 83, respectively), followed by flocks of large-sized birds (n = 39), chickadee-tit (Paridae) flocks (n = 29), open environment (n = 27) and multilevel flocks (n = 25), flocks across an elevational gradient (n = 16), minor flocks (n = 15), flocks with numerically dominant nuclear species (n = 13), and finally transient (n = 12), unstructured (n = 11), brown-and-black (n = 8) and antwren flocks (n = 7). Some of these flock types have only been reported from certain biogeographic regions (i.e. antwren flocks in the Neotropics and mixed flocks with numerically dominant nuclear species in the Indomalayan and Afrotropical regions; table 3). By contrast, other types of mixed flocks were reported from more than three biogeographic regions (i.e. open environment flocks, multilevel flocks, understorey flocks, canopy flocks and mixed flocks of large-sized birds; table 3).
Table 2.
Mixed flock systems described to date in the literature. A brief summary is presented based on the original description of the author(s), along with references to studies of similar mixed flock systems and some of the other names used to refer to that flock type.
| name of the mixed flock system | brief description | other names used in the literature |
|---|---|---|
| wandering interspecific flocks | terrestrial mixed flocks in the broad sense, using older terminologies. A group of different bird species with reduced competition and different foraging guilds [13,17,39,63,73–76]. | bird parties; mixed bird parties; compact troops of insectivorous birds; columna caminante de aves; bandadas heterogéneas |
| open environment mixed flocks | flocks composed of open-environment species from grasslands to savannahs. Mainly described as a group of birds feeding on the ground or at low heights, including arboreal species in savannah environments. In some cases, these flocks are described as not being attracted to clumped food sources, while in others, as moving together among scattered patches of food resources [52–54,77–92]. | sparrow flocks in the desert; sparrow, starlings, chaffinches; Fringillidae and Emberizidae mixed flocks; pasture mixed flocks with Corvidae, Sturnidae and Icteridae; soil-mixed flocks; monte desert mixed flocks; junco mixed flocks; savannah mixed flocks |
| transient mixed flocks | a complex mixture of resident and transient species, either elevational migrants or latitudinal migrants, especially for migratory passages, ecotone and stopover areas where these flocks are usually described as being larger in edges or transitional areas [93–96]. | mangrove mixed flocks; transient mixed flocks; warbler mixed flocks |
| mixed flocks of large-sized birds | primarily composed of ‘large-sized’ species. Depending on the author, this could range from parrots to jays to toucans, woodpeckers, or caciques. Usually, these flocks represent omnivorous species that forage among all vegetation strata. Described mainly for savannah and forest environments. These flocks are also described as ‘waves' of birds, usually feeding in one area and moving to another; although other species may join, a core set of species remain together [39,40,45,48,50,65,97–108]. | woodpecker mixed flocks; parrot and parakeet mixed flocks; jay mixed flocks; jay and flicker mixed flocks; jays and caciques |
| antwren mixed flocks | forest antwrens and antshrikes that forage and travel together. Participants defend joint territories, incorporate fledglings into the flocks and roost (or gather) together. Other species besides antwrens may also join the flocks and roost with them [22,39,109–112]. | antwren mixed flocks; antwren alliance |
| mixed flocks with numerically dominant nuclear species | the numerical dominance of nuclear species characterizes these flocks. Usually described from Sri Lanka and Taiwan, with drongos and babblers as the dominant species, but also mentioned for Neotropical areas [68,74,113–115]. | |
| chickadee-tit (Paridae) mixed flocks | parid flocks with many other species. Described especially from north temperate forests in the boreal winter. Other non-parid species include woodpeckers, gnatcatchers, kinglets, nuthatches and warblers. Complex system of hierarchy within species. Some species may be attuned to the alarm calls of the parids [116–131]. | warbler mixed flocks; mixed-species flocks of tits |
| mixed flocks across an elevational gradient | usually described in a comparative manner across an elevation gradient, from the lowlands to the highlands. Mixed flocks are defined by the lower and upper elevational range of different forest types [55,56,73,96,107,132–136]. In particular, lowland mixed flocks are described from Neotropical lowlands such as the Amazon rainforest (floodplain and terra firme), as well as the lowlands along the foothills of mountainous areas in the Indomalayan region. They are usually characterized by particular nuclear species and having smaller home ranges than mixed flocks at higher elevations. Also, strong cohesiveness and stable bonds between individuals, with communal territoriality and roosting sites. Montane mixed flocks are described from montane forests, occupying the understorey and canopy strata. High species richness and dynamic fission-fusion processes characterize these flocks. Home range boundaries are well-defined, and the home ranges are larger than those of lowland mixed flocks. Evidence for well-defined nuclear or core species varies between studies, and the roles of sentinel and leader can change between species. Lastly, highland mixed flocks are described from very high elevations at the tree line (for example, Polylepis forest and paramo in the Andean highlands). They are characterized by smaller home ranges than montane flocks. Well-defined nuclear species are usually absent but vary among study areas, and the role of sentinel and leader can change between different species. |
lowland mixed flocks; montane mixed flocks; highland mixed flocks; mixed flocks in lowland and sub-montane forests |
| brown-and-black mixed flocks | these mixed flocks emanate from different environments and ecoregions. Participants are characterized by having brown or dull coloration. Eventually, this mixed flock system can join a ‘colourful’ one. Described for thornbills in Australia, babbler/pitohui alliances in New Guinea, Furnariidae in Patagonian forest, and mainly Furnariidae and Dendrocolaptidae in the Atlantic Forest [46,47,137,138]. | brown bird mixed flocks; thornbill mixed flocks |
| unstructured mixed flocks | flocks contain numerous weak interactions. Flock composition is dynamic and unstructured. Several redundant nuclear species are present, with varied importance based on ecological conditions [39,62,136,139,140]. | loosely attached mixed flocks |
| understorey mixed flocks | a compact unit of species that forage in the understorey (approx. 1–4 m or 1–9 m, depending on the forest type). Different types of understorey mixed flocks have been described based on the identity of the nuclear and/or leader species (e.g. Habia rubica in the Atlantic Forest). Usually described as the most complex or ‘evolved’ flocks since they are generally led by one species that also assumes the sentinel role. In some areas, these flocks are described as having permanent territories that are stable across years, but in other areas, territoriality is unclear [22,37,39,41,95,103,107,134,141–145]. | permanent mixed flocks; cohesive mixed flocks |
| canopy mixed flocks | usually described as a compact set of species moving at high speed through the canopy. In some cases, a particular species is described as the nuclear species, such as greenlets in the Amazon rainforest. In some systems, these flocks have the same multi-species territoriality as understory mixed flocks and antwren flocks [37,39,41,95,107,108,134,144,146]. | small and colourful insectivores; blue and green tanager and honeycreeper clusters |
| minor mixed flocks | a smaller version of other flock systems. Flocks described as being small are typically composed of two to five species with a minimum of three individuals. One distinctive feature is that participants are usually quiet and inconspicuous while moving through the environment [108,147–149]. | fairywren mixed flocks; simple mixed flocks |
| multilevel mixed flocks | mixed flocks that emerge when different flocks temporarily combine (i.e. understorey and canopy flocks). Species roles are maintained within each sub-unit of the merged flocks. Participants of these flocks encompass all vegetation strata available, represent species with different body sizes, and sometimes even include small mammals [37,39,41,42,46,73,96,102,108,134,138,139,144,150–153]. | mega mixed flocks; meta mixed flocks; absolute mixed flocks |
Table 3.
Biogeographic realms where the different mixed flock types have been reported. MSF, mixed-species flock.
| MSF / biogeographic realms | open environment MSF | transient MSF | MSF of large-sized birds | antwren MSF | MSF with numerically dominant nuclear species | chickadee-tit (Paridae) MSF | MSF across elevation | brown-and-black MSF | unstructured MSF | understorey MSF | canopy MSF | minor MSF | multilevel MSF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palearctic | X | X | X | X | |||||||||
| Nearctic | X | X | X | ||||||||||
| Oceania | X | X | X | X | X | ||||||||
| Indomalaya | X | X | X | X | X | X | X | X | X | ||||
| Australasia | X | X | X | X | X | ||||||||
| Afrotropics | X | X | X | X | |||||||||
| Neotropics | X | X | X | X | X | X | X | X | X | X | X | X | X |
4. Discussion
Around the world, studies of mixed flocks have documented and emphasized many different types of flocks. Yet, in spite of their differences, these mixed flocks are still considered part of the general ‘mixed-species flock’ phenomenon. Thus, all described mixed flocks can be regarded as subsets under the title of mixed flocks. This approach provides a powerful tool to encode all types of mixed flocks and better understand these multispecies group foraging societies. However, it may also preclude us from further understanding patterns of species interactions and their interspecific dependence [154]. As a result, the mixed flock literature has been historically dominated by descriptive studies or those testing the hypothesized benefits of flocking: namely, reduced predation and increased foraging efficiency. More recently, the literature has expanded to include coevolutionary interactions among flocking species and the importance of mixed flocks for community structure. However, due to the diversity and complexity of mixed flocks, our ability to generalize and tease apart the various mechanisms influencing foraging efficiency, predation risk and coevolved interactions are still limited. To move beyond singular, insular descriptions of mixed flocks, we reviewed all terrestrial flock systems that have been described to date. This comprehensive review led us to propose a classification scheme, which develops criteria to distinguish among different types of mixed flocks, and a standardized way to describe these varied and complex social interactions.
(a) . What is a mixed flock of birds?
Mixed flocks have been studied for more than a century, and, accordingly, different definitions have emerged for what constitutes a flock. Some works identify mixed flocks based on the presence of one key species (e.g. Thamnomanes caesius, T. schistogynus [66,155]), others assume that all birds within a predefined area are part of a single flock [156], while still others identify a mixed flock based on the collective behaviour of the birds [56–58,68]. These three definitions encompass both advantages and disadvantages. For example, although identifying a mixed flock system based on the presence of one key species facilitates locating flocks (by following vocalizations of a single species), it may also exclude flock systems that are not formed around that particular species (see, for example, understorey flocks with or without Habia rubica in the Atlantic Forest [41]). Moreover, assuming that all individuals within a predefined area are part of a single flock could result in unwanted ‘bycatch’—that is, including species that are just present by chance and are not properly interacting with the flock (e.g. including a raptor as a flock participant). However, this methodology might help to define an objective upper limit for a particular studied flock. Lastly, defining a mixed flock based on collective behaviour can lead researchers to subjective bias, even though subjective impressions of behaviour have proven valuable for enhancing scientific knowledge [57,157]. Here we propose an operational solution that encompasses multiple former definitions, allowing researchers with different scopes to identify a mixed flock.
A mixed flock is a roving group of three or more individuals from at least two species, moving in concert and behaving cohesively while foraging over a non-aggregated food resource. Social interactions such as calling, responding to potential threats and coordinated behaviour all provide additional evidence that these birds belong to the same flock. The threshold for delimiting a mixed flock, either by the amount of time participants spend together, interindividual distances, or the joint distance covered, will vary from place to place and depend on the kind of mixed flock studied; therefore, this threshold needs to be flexible and determined locally. Nevertheless, a consensus can be reached that flocking individuals should remain within 25 m of each other and move together in the same direction for at least 5 min, as has been used previously and successfully in the literature [20,21,56,66,68,158]. Thus, this definition excludes groups of birds foraging on emerging insects, foraging stationary at fruiting trees or aggregations of birds consuming prey flushed by army ants. By following this definition, researchers can identify a mixed flock in the field, which can then be studied and understood using the descriptions proposed in table 5 and figure 5 (and organized in table 2).
Table 5.
Guideline for future research on mixed flocks. The left-hand column contains two major items related to basic preparation before collecting data in the field (A) and to habitat descriptions (B), and three major items related to mixed flocks information (C-E; descriptive information, standardized metrics and desirable variables). The nature of a particular study will determine whether a descriptive or quantitative approach is most applicable. We acknowledge that desirable variables might not always be feasible to collect and may exceed the minimum requirements to unify further understanding of mixed flocks across the globe. MSF, mixed-species flocks.
| major items | sub-items | description |
|---|---|---|
| (A) basic preparation | gain experience | train over a sufficient period of time until recognition and identification of bird species are as accurate as possible. Results will depend on how well and fast species can be identified. |
| (B) descriptive information of study area | habitat structure | describe the environment where the study is conducted using specialized local literature; always present the GPS coordinates from the primary study location(s); include the physical complexity of the environment, whether it is an open environment dominated by one physiognomic type of vegetation, an ecotone as part of an environmental gradient, or a forest environment |
| habitat seasonality | describe the seasonality of the study area based on climatic conditions (e.g. wet and dry seasons) and reproductive seasons for birds. For instance, the reproductive season of birds may be limited by weather seasonality or be protracted, extending throughout much of the year. | |
| (C) descriptive information of studied flocks | identify the type(s) of mixed flocks | specify whether the studied MSF matches any previously published categories (table 2); if not, state how it differs. Additionally, identify if one or more mixed flock systems co-occur in the study area and define what distinguishes them (e.g. forest stratum). |
| seasonality of MSF | identify the seasonal occurrence of mixed flocks in the area, whether they occur in certain seasons or can be found year-round, and, if possible, determine how flocks vary between seasons. If performing year-round surveys deviates from the scope of the study, clarify that seasonal occurrence was not evaluated and avoid making assumptions about the seasonality of mixed flocks for that area. | |
| territoriality of MSF | identify if mixed flocks are territorial or have fixed home ranges using repeat surveys at the same site. However, if performing repeat surveys is impossible or deviates from the study's main objective, clarify that territoriality was not evaluated and avoid making assumptions about the territoriality of the studied flocks. | |
| species roles | whenever possible, try to identify and quantify the potential roles played by different species, such as leader, nuclear and sentinel behaviour (see definitions in table 4), through careful observation, natural experiments and playback experiments | |
| cohesiveness of MSF | describe whether the studied flocks move together as a compact unit with < 10 m between participants or, rather, flock cohesion is more dispersed, with > 10 m between individuals | |
| (D) standardized metrics to quantify the studied flocks | sample size (N) of studied flocks | depending on the space use of the study system, count and report the number of independent flocks sampled. If flocks are territorial, the flock within the territorial boundary would be a single, independent unit. On the other hand, if flocks are not territorial, consider waiting at least 24 h to sample a second flock within that same survey unit (plot, transect, or point). And always explain how and why flocks were considered independent samples. |
| MSF size | quantify the average size of the flocks () by species and individuals within at least a consistent 10 min time frame, ensuring that all participating individuals are counted. If the terrain is very steep, a 5 min time frame might be more practical. | |
| MSF species evenness | using MSF size, calculate the species evenness of the studied flocks | |
| foraging guild | present the guild breakdown of flocks as an average of the participant foraging guilds. If performing foraging observations deviates too much from the objective of the study, guild assignments can be extracted from local specialized literature. | |
| composition | describe the taxonomic composition of your flock (the raw number of orders, families and species). In the supplementary material, provide a cumulative list of species that participated in flocks alongside the total sampling effort. Reporting the cumulative presence/absence of species across flocks would be desirable (the number of times a particular species was found in mixed flocks over the total number of mixed flocks recorded). | |
| (E) desirable descriptions and metrics | social structure | when feasible, describe the social structure of the studied flocks. This description can be achieved either through network metrics or through behavioural observations of each flock (by colour-banding individuals within flocks and observing whether flocks go through fission–fusion processes over time). If fission–fusion processes exist, report if it is atomistic or multilevel fission–fusion. In atomistic fission–fusion flocks, the process is individualistic, and subunits vary widely in composition [172]. This is the case when individuals often move between flocks that constantly vary in size and composition. On the contrary, flocks following multilevel fission–fusion dynamics merge and split in predictable patterns, with individuals maintaining their close social sub-group while splitting and merging [172,173]. |
| flocking propensity | flocking propensity can be measured as (i) community-level flocking propensity (the number of species that join mixed flocks over the total number of available species in the studied area), and (ii) species-level flocking propensity (the number of times a particular species is recorded within mixed flocks over the total number of times this species was recorded inside and outside of flocks). At both levels, flocking propensity requires additional standardized point counts or transect surveys. | |
| moving speed | while following a flock, measurements of distance covered and elapsed time can quantify speed of movement | |
| participants' foraging behaviour | describe the foraging niches of the constituent species, with special emphasis on foraging height, search behaviour (myopic versus scanning at a distance from an open perch), and substrates from which prey are caught (e.g. live versus dead leaves, air, bark), all of which can influence which species can flock together and how fast the flock moves | |
| participants' morphology | describe the average size of participant species. If more than 70% of participants are larger than 20 cm, they can be considered ‘large body size’ species. Additionally, describe whether there is a predominant colour plumage of flock participants. | |
| behavioural records | describe anecdotal behaviours, such as kleptoparasitism, false alarm calls, communal bathing and flocking while feeding chicks or fledglings. When researchers witness predation or the presence of a nearby predator, report the identity of the predator, the success of the attack, and which species emits an alarm call, eliciting a freezing or flushing response from other participants (plausible sentinel behaviour). When possible, record the alarm call to enable quantification of such behaviour through playback experiments. |
Figure 5.
Standardized traits to report when conducting studies on mixed flocks. (a) Suggested traits related to habitat, such as the structure and configuration of the environment. Note that, as the physical complexity of the environment increases, clarification is needed about which forest stratum is used by the flock. Different bird species are exemplified with different silhouettes and/or colours. (b) Traits related to behaviour and the physical traits of participants, comprising four main sub-categories: (i) behavioural roles—whether the studied flock contains sentinel, nuclear or leader species. Simply put, sentinel species produce alarm calls, leaders (denoted in yellow) are the first to move in a particular direction, and nuclear species (denoted in violet) contribute to the formation and/or cohesion of flocks. (ii) Species evenness evaluates the numerical dominance of flock participants, with special attention to the numerical dominance of nuclear species (denoted in violet); (iii) social structure of the flock considers whether participants join and leave the flock over time, where the birds coloured in green represent the same individuals joining and leaving the same mixed flock at different times; and (iv) foraging guilds illustrate the need to supply descriptions about foraging techniques and the primary foraging guild.
(b) . Factors that impact flocking behaviour
From our review, nearly half of all studies (46%, see Results) described mixed flocks using one or a combination of three major traits: (1) habitat and (2) behavioural and physical traits at either the flock or (3) participant level. Of these, territoriality and seasonality stand out as particularly influential for flocking behaviour. Although flock complexity has been proposed to depend upon the presence of a stable territory [38,141], most studies (79%, see Results) did not provide this information. As expected, territoriality has received the most attention in the Neotropics, where most studies were located and where most territorial flocks have been reported. However, territorial flocks have also been described from all other biogeographic realms (except Oceania, which contains few published studies [n = 6]). Further, even though individual flock members are replaced over time, the flock territory and gathering sites can remain stable [61]. Thus, some areas are more critical for flock formation than others, even within the same habitat type [38]. However, the incomplete information about flock territoriality—together with the lack of proper assessment of this trait (e.g. long-term studies, behavioural observations, individual tagging)—make it challenging to identify these important areas for flock formation, perform further comparisons between different regions and make conservation-based decisions. Thus, assessing the territoriality of mixed flocks becomes a key topic for future research.
Similarly, flocking behaviour has been demonstrated to vary seasonally, even in environments that lack strong seasonality, such as the Amazon Rainforest [159,160]. In fact, seasonal differences within flocks can exceed habitat-specific differences between flocks, illustrating that habitat effects could be misleading if seasonality is ignored [160]. Yet, 62% of studies (see Results) were only conducted in one season or did not report whether the studied flocks formed seasonally or year round. As expected, more seasonal flocks were reported from biogeographic realms with well-known climatic seasonality (e.g. Palearctic and Nearctic). However, some studies conducted throughout the year in these biogeographic realms reported year-round mixed flocks, highlighting the importance of performing yearlong studies of flocking behaviour, even in environments with strong climatic seasonality.
(c) . Terminology used to define species roles
More than half of all studies (53%, see Results) discussed flocking species that fulfilled a specific role, either as leaders, nuclear species or sentinels. However, despite these roles being important to study the social complexity of flocks, inconsistent terminology raises unnecessary confusion. In part, this confusion could result from applying terminology developed from one kind of flock system to another, which may be organized differently, but it also reflects a need for more precise definitions of these terms. Thus, to define and classify these species roles, it is necessary to understand how these roles have been used in the literature historically.
Among the earliest descriptions of mixed flocks, Henry Walter Bates (1863; [13, chapter XII]) reported that locals mentioned to him that Amazonian flocks were ‘led by a little grey bird, called the Uirá-pará’. Even though Bates did not fully embrace this idea himself, he nonetheless regarded the observation with some semblance of truth. Almost a half-century later, Swynnerton (1907; [161, p. 34]) seems to have been the first to mention some species leading other flock participants, describing ‘various species herding together under the leadership of the Drongos mentioned by Marshall (1900)’. However, even in the early stage of this conceptual framing, there seems to be some confusion about that previous interpretation, as Marshall [15, p. 222] appears to be introducing the idea of a sentinel instead: ‘smaller birds find it advisable to associate as a means of protection, … the Drongos acting as a sort of body-guard’. Bertoni [17, p. 398] adds to the idea of leadership in the Neotropics: ‘what is most striking is that some species seem to lead the expedition and others invariably follow them in perfect harmony’. A few years later, Gannon [137], in Australasia, described some species as being more important than others for flock formation. Following this trend, Winterbottom [74] seems to have been the first to use the terminology of ‘nucleus’ species in Zambia, where nuclear species are intraspecifically gregarious and can also be found outside of flocks. This concept, developed in the Afrotropics, was later applied to mixed flocks in the Atlantic Forest of Brazil [63], where only two species meet the criteria for being intraspecifically gregarious.
In 1949, Winterbottom presented a formal, if somewhat confusing, description of nuclear species: ‘species apparently much more important in the African savannah and Burmese forest than in South America’ [75, p. 439]. During subsequent years, the debate about whether nuclear species are intraspecifically gregarious permeated the Neotropical literature [153,162,163]. Later, Moynihan [57] provided a different approach, which resembled that proposed by Gannon [137], describing a nuclear species as one that ‘contributes appreciably to stimulate the formation and/or maintain the cohesion’ of flocks [57, p. 135]. In the Nearctic, Morse [20] returned to the definitions of leader and nuclear species, concluding that using them was not feasible in his study system, so he developed a new classification based on the ratio of the number of species led versus followed, combined with the ratio of time that a species led versus followed. Thus, there may not have been a distinctive nuclear species in these flocks. However, at least one species he studied (Baeolophus bicolor) has been proposed to be a nuclear species, on which other flock members eavesdrop [164].
Similarly, the definition of sentinel behaviour has a long and convoluted history. As mentioned above, Marshall [15] seems to have been the first to introduce the idea of sentinel species, but Winterbottom [74] was the first to specifically discuss the communication of fear between flock participants (even though neither stated a clear sentinel role). Later, Munn & Terborgh [22] and Munn [37] reported that Thamnomanes ardesiacus, T. schistogynus and Lanio versicolor (in Amazonian Peru) perform unambiguous alarm calls, which other flock participants interpret as an alarm, a result confirmed by playback experiments [11,165,166]. Several subsequent studies, in the tropical forests of South America, have identified Thamnomanes antshrikes as either nuclear, leader or sentinel species [11,21,37,38,66,103,158]. Outside the Neotropics, other studies have reported species that produce alarm calls, especially Cyanistes caeruleus, Parus major and Periparus ater for Parid mixed flocks in the temperate forests of the Palearctic [70,167], Dicrurus paradiseus in Sri Lanka and Nepal [65,168], and Turdoides bicolor in semiarid regions of South Africa [148]. However, confusion arises when some works define nuclear species as those that not only initiate and lead flock movements but also produce alarm calls after predator detection [22,158], in contrast to works that refer to two different types of nuclear species—those that function as sentinels and those that are intraspecifically gregarious [169].
Lastly, another role has received even less attention, where participants are described as circumference species [74,162], attendant species [53,57,114] or followers [20,26]. To some extent, this last concept is less problematic because the various author(s) agree that these participants do not play a role in flock formation but rather accompany either leader, nuclear or sentinel species. However, considering the wide variety of species and situations in which this role has been defined, some potential features may still be obscured under such a categorization. Thus, even after more than 100 years have passed since the first studies introduced these specialized flock roles, the four concepts continue to cause confusion. Therefore, following the glossary we present in table 4, we recommend maintaining distinct concepts for leader, nuclear and sentinel species, as well as flock followers, while simultaneously encouraging further clarification when a single species meets more than one of these roles.
Table 4.
Glossary with proposed definitions for species roles within mixed flocks.
| roles | proposed usage |
|---|---|
| nuclear | we propose that this term should continue to be used in the sense of Moynihan [57]: namely, as a species that contributes to the formation and/or cohesion of flocks. By ‘formation’, we mean both the initiation and growth of flocks by accumulating new species. We use ‘cohesion’ in the sense of the flock being held together once formed. Based on our definition, species that provide adaptive benefits and serve as cues or flock markers can be considered nuclear. This definition does not require that a nuclear species is always present nor that it is the most frequent or abundant participant in a flock. In that way, our definition continues to follow Moynihan's [57] emphasis by describing nuclearity purely in behavioural terms. While there is no straightforward method to identify or quantify a nuclear species in the field, we believe that testing attractiveness of species to others through playback experiments, natural experiments (e.g. comparison of flocks with and without a potential nuclear species), and actual observation of flock formation (by identifying flock roosting sites and observing assembly patterns in the morning) are approaches that can shed light on this topic. Although we acknowledge that context-dependent interpretations and modifications of the nuclear species concept may be important for developing new hypotheses, holding to an operational definition of nuclear species is crucial to compare different mixed flock systems. |
| leader | within the flock literature, the term ‘leader’ has been used both as a general term for functionally important species and to indicate a relative spatial position within the flock. As we advance, we suggest that the term ‘leader’ be reserved exclusively for the latter meaning—only species at the head or forefront of a moving flock are called ‘leaders'. It is important to note that leadership is typically ‘inadvertent’, a natural consequence of how often a species is followed compared to how often it follows other species. We hypothesize that a species is more likely to follow another species if it plays an outsized role in providing direct benefits. Thus, a leader species can be identified when the observer remains stationary, checking the order of arriving and departing species. Therefore, the first flock species to move in a particular direction, steering flock movement as a whole, would be the leader. We suggest that leaders be defined as those that initiate more movements than any other flock participant (e.g. the first flock species to cross a gap or consistently move at a new tree). |
| sentinel | sentinel species broadcast alarm calls, evoking a clear fear response from other participants, which then either flush and/or remain silent for an extended period. This behavioural role will likely be the most difficult to identify because there are few opportunities to witness the detection of a predator, the emission of an alarm call, and the flock participants' reaction to that alarm. Nevertheless, verifying this behaviour by experimental playback of alarm calls [11,58,164–166] would allow further opportunities to test convergent evolution in communities with different evolutionary histories. |
| follower | follower species trail after the leader when a mixed flock is on the move. In some cases, species considered followers might assume other roles in different areas or even different flocks within the same area. We suggest paying particular attention to followers as other potential roles may be obscured within this group and have yet to be explored. |
(d) . For the future: best practices and recommendations
Our findings demonstrate that the basic natural history characteristics of mixed flocks require more consistent attention. The theoretical knowledge of mixed flocks has its foundations in the natural history of the birds themselves, as expressed by Miller [170, p. 125] a century ago: ‘There are no overseers, yet there are no shirkers; necessity sees to that … No humanly devised Utopia has ever approached this in practicability’. However, in recent years, this important information has been neglected or limited to brief sentences, as natural history itself has fallen out of favour [171], and space limits in most modern journals do not allow for lengthy descriptions. To bridge this gap, we encourage future authors to categorize their study flocks using the names provided in table 2, apply the standardized trait terminology of habitat, behaviour and physical traits (summarized in table 1 and figure 5), and adhere to clear, consistent definitions of species roles (table 4). Where such details are too lengthy for inclusion in the main text, we urge authors to report them systematically within the online supplementary material. Further, to facilitate and expand comparative studies across flocks, and based on the results of this study, we outline best practices and recommendations for all future studies of mixed flocks (table 5). Lastly, we provide a blank template with an example of how to gather this information in a standardized manner (electronic supplementary material, table S1 and S2), which can be downloaded, edited and then uploaded separately to enable further comparisons across the globe.
5. Conclusions
Despite the historical value placed on negative interactions, there is a growing consensus that positive interactions can dominate community structure in highly seasonal or high predator-pressure environments [1,174]. Even though competition may occur between flock participants [32], mixed flocks arise through positive interactions, and the prevalence of flocks across all continents (except Antarctica) can be considered sufficient evidence for their adaptive value [175]. Researchers once argued that small insectivorous birds gathered because it gave them pleasure and satisfied the inherent gregarious instinct of the species [137,142]. But today, the heterospecific attraction hypothesis suggests that species obtain benefits from other species and cues about high-quality sites [1]. Decades of research have provided supporting evidence that mixed flocks work as a facilitation mechanism to increase species' foraging efficiency and/or diminish their predation risk [26,28,32,123,176]. However, in the quest to understand these associations, researchers have been tempted to describe their study systems idiosyncratically, leading to a conspicuous lack of consensus among authors and, thus, highlighting the need for a common language. We acknowledge that classification schemes are always under debate, and this dynamism pushes research forward. Likewise, we also recognize that mixed flocks are socially complex systems and that behaviour is not fixed or stereotyped. Thus, the classification of social complexity is likely to remain under constant change with advances in the field. Nevertheless, we expect that our proposed classification scheme will help current and future researchers to better define their study system, ultimately increasing our ability to compare flocks across different habitats, regions and species pools, as well as to understand and communicate about mixed flocks more holistically. This knowledge may eventually lead to an understanding of where and how flocking behaviour and coevolutionary interactions among flocking species emerged and whether different patterns of species evenness might provide any insight [74]. Therefore, studying the evolutionary origin of flock species in conjunction with standardized behavioural traits may reveal how this complex social behaviour developed and shed more light on what ultimately led to the formation of mixed flocks [177].
Acknowledgements
The authors would like to thank each of the organizers and participants of the 2021 symposium for Mixed-species Animal Groups, who encouraged the creation of enthusiastic mixed-researcher working groups to help advance the field. We also thank two anonymous reviewers and the editors of this themed issue, especially Nora Carlson, whose insightful comments greatly elevated the caliber of our work. Further, the authors are indebted to those in charge of maintaining the terrestrial mixed-species flock database, and we are especially grateful to Priti Bangal, who assisted us during the formative stages of this manuscript. We encourage future researchers to share their publications with the database administrators so that we can unravel the mysteries of mixed flocks together. Lastly, the authors thank Facundo Gandoy for his editorial support and for extracting the relevant information from satellite imagery.
Data accessibility
The raw compiled table for this review is available from the Zenodo repository: https://doi.org/10.5281/zenodo.7712927 [178].
A Spanish abstract is available in the electronic supplementary materials [179].
Authors' contributions
G.G.M.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing; C.L.R.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing; H.S.: conceptualization, data curation, investigation, methodology, project administration, validation, visualization, writing—review and editing; G.B.: conceptualization, data curation, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing; J.M.: conceptualization, data curation, investigation, methodology, validation, visualization, writing—review and editing; S.K.R.: conceptualization, data curation, investigation, methodology, validation, visualization, writing—review and editing; F.M.-C.: conceptualization, investigation, methodology, visualization, writing—review and editing; A.Z.: conceptualization, data curation, investigation, methodology, visualization, writing—review and editing; M.E.F.: conceptualization, data curation, investigation, methodology, visualization, writing—review and editing; G.F.-A.: conceptualization, data curation, investigation, methodology, project administration, visualization, writing—review and editing; S.X.: conceptualization, validation, visualization, writing—review and editing; E.C.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We have no competing interests to declare.
Funding
The work was supported by a postdoctoral scholarship from the National Council for Science and Technology (CONICET) of Argentina and a François Vuilleumier grant from the Neotropical Ornithological Society.
References
- 1.Mönkkönen M, Helle P, Niemi GJ, Montgomery K. 1997. Heterospecific attraction affects community structure and migrant abundances in northern breeding bird communities. Can. J. Zool. 75, 2077-2083. ( 10.1139/z97-842) [DOI] [Google Scholar]
- 2.Cornwallis CK, Botero CA, Rubenstein DR, Downing PA, West SA, Griffin AS. 2017. Cooperation facilitates the colonization of harsh environments. Nat. Ecol. Evol. 1, 1-10. ( 10.1038/s41559-016-0057) [DOI] [PubMed] [Google Scholar]
- 3.Lin YH, Chan SF, Rubenstein DR, Liu M, Shen SF. 2019. Resolving the paradox of environmental quality and sociality: the ecological causes and consequences of cooperative breeding in two lineages of birds. Am. Nat. 194, 207-216. ( 10.1086/704090) [DOI] [PubMed] [Google Scholar]
- 4.Downing AL, Jackson C, Plunkett C, Ackerman Lockhart J, Schlater SM, Leibold MA. 2020. Temporal stability vs. community matrix measures of stability and the role of weak interactions. Ecol. Lett. 23, 1468-1478. ( 10.1111/ele.13538) [DOI] [PubMed] [Google Scholar]
- 5.Goodale E, et al. 2020. Mixed company: a framework for understanding the composition and organization of mixed-species animal groups. Biol. Rev. 95, 889-910. ( 10.1111/brv.12591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller GB. 2009. The deer and the tiger: study of wild life in India. Chicago, IL: University of Chicago Press. [Google Scholar]
- 7.Caraco T, Wolf LL. 1975. Ecological determinants of group sizes of foraging lions. Am. Nat. 109, 343-352. ( 10.1086/283001) [DOI] [Google Scholar]
- 8.Powell GVN. 1974. Experimental analysis of the social value of flocking by starlings (Sturnus vulgaris) in relation to predation and foraging. Anim. Behav. 22, 501-505. ( 10.1016/S0003-3472(74)80049-7) [DOI] [Google Scholar]
- 9.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245. ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez AE, Parra E, Collado LF, Vredenburg VT. 2017. Deconstructing the landscape of fear in stable multi-species societies. Ecology 98, 2447-2455. ( 10.1002/ecy.1935) [DOI] [PubMed] [Google Scholar]
- 11.Camerlenghi E, Tellaroli P, Griggio M, Martínez AE. 2019. Information about predators varies across an Amazonian Rain Forest as a result of sentinel species distribution. Am. Nat. 194, E134-E139. ( 10.1086/705242) [DOI] [PubMed] [Google Scholar]
- 12.Farine DR, Aplin LM, Sheldon BC, Hoppitt W. 2015. Interspecific social networks promote information transmission in wild songbirds. Proc. R. Soc. B 282, 20142804. ( 10.1098/rspb.2014.2804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates HW. 1863. A naturalist on the river Amazon, Vols 1/2. London, UK: John Murray. [Google Scholar]
- 14.Belt T. 1874. The naturalist in Nicaragua: a narrative of a residence at the gold mines of Chontales; journeys in the savannahs and forests. With observations on animals and plants in reference to the theory of evolution of living forms. London, England: John Murray. [Google Scholar]
- 15.Marshall GA. 1900. Notes on Mashonaland birds. Ibis 42, 221-270. [Google Scholar]
- 16.Swynnerton CFM. 1915. Mixed bird parties. Ibis 67, 346-354. [Google Scholar]
- 17.Bertoni W. 1926. Apuntes ornitológicos. El Hornero 3, 396-401. [Google Scholar]
- 18.Allee WC. 1931. Co-operation among animals. Am. J. Soc. 37, 386-398. [Google Scholar]
- 19.Hartley PHT. 1953. An ecological study of the feeding habits of the English titmice. J. Anim. Ecol. 22, 261-288. ( 10.2307/1817) [DOI] [Google Scholar]
- 20.Morse DH. 1970. Ecological aspects of some mixed-species foraging flocks of birds. Ecol. Monogr. 40, 119-168. ( 10.2307/1942443) [DOI] [Google Scholar]
- 21.Powell GVN. 1985. Sociobiology and adaptive significance of interspecific foraging flocks in the Neotropics. Ornith. Monogr. 36, 713-732. ( 10.2307/40168313) [DOI] [Google Scholar]
- 22.Munn CA, Terborgh JW. 1979. Multi-species territoriality in Neotropical foraging flocks. Condor 81, 338-347. ( 10.2307/1366956) [DOI] [Google Scholar]
- 23.Greenberg R. 2000. Birds of many feathers: the formation and structure of mixed-species flocks of forest birds. In On the move: how and why animals travel in groups (eds Boinski S, Garber PA), pp. 521-558. Chicago, IL: University of Chicago Press. [Google Scholar]
- 24.Krebs JR. 1973. Social learning and the significance of mixed-species flocks of chickadees (Parus spp.). Can. J. Zool. 51, 1275-1288. ( 10.1139/z73-181) [DOI] [Google Scholar]
- 25.Hino T. 2000. Intraspecific differences in benefits from feeding in mixed-species flocks. J. Avian Biol. 31, 441-446. ( 10.1034/j.1600-048X.2000.310402.x) [DOI] [Google Scholar]
- 26.Sridhar H, Beauchamp G, Shanker K. 2009. Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim. Behav. 78, 337-347. ( 10.1016/j.anbehav.2009.05.008) [DOI] [Google Scholar]
- 27.Muñoz ZJ, Colorado ZGJ. 2021. Importance of tropical mixed-species flocks for migratory birds in shade-grown coffee: implications of foraging together. J. Field Ornith. 92, 212-230. ( 10.1111/jofo.12379) [DOI] [Google Scholar]
- 28.Mangini GG, Mokross K, Gandoy F, Areta JI. 2022. Mixed-species flocking is associated with low arthropod detectability and increased foraging efficiency by Yungas forest birds in Argentina. Ornithology 139, ukab087. ( 10.1093/ornithology/ukab087) [DOI] [Google Scholar]
- 29.Jullien M, Clobert J. 2000. The survival value of flocking in neotropical birds: reality or fiction? Ecology 81, 3416-3430. [Google Scholar]
- 30.Freeberg TM, Eppert SK, Sieving KE, Lucas JR. 2017. Diversity in mixed species groups improves success in a novel feeder test in a wild songbird community. Sci. Rep. 7, 1-9. ( 10.1038/srep43014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodale E, Beauchamp G, Ruxton G. 2017. Mixed-species groups of animals: behavior, community structure, and conservation. London, UK: Academic Press. [Google Scholar]
- 32.Chen CC, Liao CC, Walther BA. 2022. Interspecific competition and facilitation coexist in mixed-species bird flocks of montane coniferous forests in Taiwan. J. Avian Biol. 2022, e02947. ( 10.1111/jav.02947) [DOI] [Google Scholar]
- 33.Buskirk WH, Powell GV, Wittenberger JF, Buskirk RE, Powell TU. 1972. Interspecific bird flocks in tropical highland Panama. Auk 89, 612-624. [Google Scholar]
- 34.Buskirk WH. 1976. Social systems in a tropical forest avifauna. Am. Nat. 110, 293-310. ( 10.1086/283065) [DOI] [Google Scholar]
- 35.Darrah AJ, Smith KG. 2014. Ecological and behavioral correlates of individual flocking propensity of a tropical songbird. Behav. Ecol. 25, 1064-1072. ( 10.1093/beheco/aru086) [DOI] [Google Scholar]
- 36.Greenberg R, Gonzales CE, Bichier P, Reitsma R. 2001. Nonbreeding habitat selection and foraging behavior of the black-throated green warbler complex in southeastern México. Condor 103, 31-37. ( 10.1093/condor/103.1.31) [DOI] [Google Scholar]
- 37.Munn CA. 1985. Permanent canopy and understory flocks in Amazonia: species composition and population density. Ornith. Monogr. 36, 683-712. (https://www.jstor.org/stable/40168312) [Google Scholar]
- 38.Jullien M, Thiollay JM. 1998. Multi-species territoriality and dynamic of neotropical forest understorey bird flocks. J. Anim. Ecol. 67, 227-252. ( 10.1046/j.1365-2656.1998.00171.x) [DOI] [Google Scholar]
- 39.Willis EO. 1972. The behavior of spotted antbirds. Ornith. Monogr. 10, 1-158. [Google Scholar]
- 40.Bell HL. 1986. Sexual differences in the behavior of wintering golden whistlers Pachycephala pectoralis at Wollomombi, NSW. Emu 86, 2-11. ( 10.1071/MU9860002) [DOI] [Google Scholar]
- 41.Maldonado-Coelho M, Marini MA. 2003. Composição de bandos mistos de aves em fragmentos de Mata Atlântica no sudeste do Brasil. Papéis Avulsos Zool. 43, 31-54. ( 10.1590/S0031-10492003000300001) [DOI] [Google Scholar]
- 42.Liao CC, Ding TS, Chen CC. 2022. The formation of ‘mega-flocks’ depends on vegetation structure in montane coniferous forests of Taiwan. Ecol. Evol. 12, e8608. ( 10.1002/ece3.8608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodale E, et al. 2009. Regional variation in the composition and structure of mixed-species bird flocks in the Western Ghats and Sri Lanka. Curr. Sci. 97, 648-663. [Google Scholar]
- 44.Srinivasan U. 2008. Structure, composition and heterospecific associations in mixed-species flocks of birds in a lowland tropical rainforest in northeastern India. Ph.D Thesis, Manipal University, Manipal. [Google Scholar]
- 45.Srinivasan U, Raza RH, Quader S. 2010. The nuclear question: rethinking species importance in multi-species animal groups. J. Anim. Ecol. 79, 948-954. ( 10.1111/j.1365-2656.2010.01707.x) [DOI] [PubMed] [Google Scholar]
- 46.Diamond J. 1987. Flocks of brown and black New Guinean bird: a bicolored mixed-species foraging association. Emu 87, 201-211. ( 10.1071/MU9870201) [DOI] [Google Scholar]
- 47.Bell HL. 1983. A bird community of lowland rainforest in New Guinea. 5. Mixed-species feeding flocks. Emu 82, 256-275. ( 10.1071/MU9820256s) [DOI] [Google Scholar]
- 48.Short LL. 1969. Foraging association of green-barred flickers and Campo flickers in Argentina. Wilson Bull. 81, 468-470. [Google Scholar]
- 49.McLean IG, Wells MS, Brown R, Creswell P, Mckenzie J, Musgrove R. 1987. Mixed-species flocking of forest birds on Little Barrier Island. N.Z. J. Zool .14, 143-147. ( 10.1080/03014223.1987.10422985) [DOI] [Google Scholar]
- 50.Lammertink M. 2004. Grouping and cooperative breeding in the great slaty woodpecker. Condor 106, 309-319. ( 10.1093/condor/106.2.309) [DOI] [Google Scholar]
- 51.Camerlenghi E, McQueen A, Delhey K, Cook CN, Kingma SA, Farine DR, Peters A. 2022. Cooperative breeding and the emergence of multilevel societies in birds. Ecol. Lett. 25, 766-777. ( 10.1111/ele.13950) [DOI] [PubMed] [Google Scholar]
- 52.Eichinger J, Moriarty DJ. 1985. Movement of Mojave Desert sparrow flocks. Wilson Bull. 97, 511-516. [Google Scholar]
- 53.Ragusa-Netto J. 2002. Vigilance towards raptors by nuclear species in bird mixed flocks in a Brazilian savannah. Stud. Neotrop. Fauna Environ. 37, 219-226. ( 10.1076/snfe.37.3.219.8573) [DOI] [Google Scholar]
- 54.Höglund J. 1985. Foraging success of rooks Corvus frugilegus in mixed-species flocks of different sizes. Ornis Fennica 62, 19-22. [Google Scholar]
- 55.McClure HE. 1967. The composition of mixed species flocks in lowland and sub-montane forests of Malaya. Wilson Bull. 79, 131-154. [Google Scholar]
- 56.Hutto RL. 1987. A description of mixed-species insectivorous bird flocks in western Mexico. Condor 89, 282-292. ( 10.2307/1368481) [DOI] [Google Scholar]
- 57.Moynihan M. 1962. The organization and probable evolution of some mixed species flocks of neotropical birds. Smithson. Misc. Collect. 143, 1-140. [Google Scholar]
- 58.Zhou L, Peabotuwage I, Luo K, Quan RC, Goodale E. 2021. Using playback to test leadership in mixed-species flocks and compare flocking with mobbing. Anim. Behav. 180, 151-166. ( 10.1016/j.anbehav.2021.08.012) [DOI] [Google Scholar]
- 59.Barash DP. 1974. An advantage of winter flocking in the black-capped chickadee, Parus atricapillus. Ecology 55, 674-676. ( 10.2307/1935161) [DOI] [Google Scholar]
- 60.Goodale E, Kotagama SW. 2005. Testing the roles of species in mixed-species bird flocks of a Sri Lankan rain forest. J. Trop. Ecol. 21, 669-676. ( 10.1017/S0266467405002609) [DOI] [Google Scholar]
- 61.Martínez AE, Gomez JP. 2013. Are mixed-species bird flocks stable through two decades? Am. Nat. 181, E53-E59. ( 10.1086/669152) [DOI] [PubMed] [Google Scholar]
- 62.Jones HH, Robinson SK. 2020. Patch size and vegetation structure drive changes to mixed-species flock diversity and composition across a gradient of fragment sizes in the Western Andes of Colombia. Condor 122, 1-26. ( 10.1093/condor/duaa006) [DOI] [Google Scholar]
- 63.Davis DE. 1946. A seasonal analysis of mixed flocks of birds in Brazil. Ecology 27, 168-181. ( 10.2307/1932511) [DOI] [Google Scholar]
- 64.King DI, Rappole JH. 2001. Mixed-species bird flocks in dipterocarp forest of north-central Burma (Myanmar). Ibis 143, 380-390. ( 10.1111/j.1474-919X.2001.tb04939.x) [DOI] [Google Scholar]
- 65.Gosai KR, Goodale E. 2021. The composition of mixed-species flocks of birds in and around Chitwan National Park. Nepal. Avian Res. 12, 1-9. ( 10.1186/s40657-021-00292-3) [DOI] [Google Scholar]
- 66.Stotz DF. 1993. Geographic variation in species composition of mixed species flocks in lowland humid forests in Brazil. Papeis Avulsos Zool. 38, 61-75. [Google Scholar]
- 67.Sridhar H, Jordan F, Shanker K. 2013. Species importance in a heterospecific foraging association network. Oikos 122, 1325-1334. ( 10.1111/j.1600-0706.2013.00101.x) [DOI] [Google Scholar]
- 68.Chen CC, Hsieh F. 2002. Composition and foraging behaviour of mixed-species flocks led by the grey-cheeked fulvetta in Fushan Experimental Forest, Taiwan. Ibis 144, 317-330. ( 10.1046/j.1474-919X.2002.00020.x) [DOI] [Google Scholar]
- 69.Matthysen E. 1993. Nonbreeding social organization in migratory and resident birds. Curr. Ornithol. 11, 94-141. ( 10.1007/978-1-4757-9912-5_3) [DOI] [Google Scholar]
- 70.Moore BA, Doppler M, Young JE, Fernández-Juricic E. 2013. Interspecific differences in the visual system and scanning behavior of three forest passerines that form heterospecific flocks. J. Comp. Physiol. A 199, 263-277. ( 10.1007/s00359-012-0790-6) [DOI] [PubMed] [Google Scholar]
- 71.Goodale E, Sridhar H, Bangal P2021. Mixed-species bird flocks: a bibliography. See https://www.researchgate.net/publication/271999666_Mixed-species_bird_flocks_a_bibliography_Copyright_2010-2015_by_Eben_Goodale_and_Hari_Sridhar .
- 72.Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GV, Underwood EC, Kassem KR. 2001. Terrestrial Ecoregions of the World: a new map of life on earth; a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51, 933-938. ( 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) [DOI] [Google Scholar]
- 73.Hingston RWG. 1921. A naturalist in Himalaya. London, UK: H. F. & G. Witherby. [Google Scholar]
- 74.Winterbottom JM. 1943. On woodland bird parties in northern Rhodesia. Ibis 85, 437-442. ( 10.1111/j.1474-919X.1943.tb03857.x) [DOI] [Google Scholar]
- 75.Winterbottom JM. 1949. Mixed bird parties in the tropics, with special reference to Northern Rhodesia. Auk 66, 258-263. ( 10.2307/4080356) [DOI] [Google Scholar]
- 76.Neunteufel A. 1953. Un ejemplo de simbiosis temporal de aves silvestres. Hornero 10, 074-077. [Google Scholar]
- 77.Cody ML. 1970. Chilean bird distribution. Ecology 51, 455-464. ( 10.2307/1935380) [DOI] [Google Scholar]
- 78.Cody ML. 1971. Finch flocks in the Mohave Desert. Theor. Popul. Biol. 2, 142-158. [DOI] [PubMed] [Google Scholar]
- 79.Rubenstein DI, Barnett RJ, Ridgely RS, Klopfer PH. 1977. Adaptive advantages of mixed-species feeding flocks among seed-eating finches in Costa Rica. Ibis 119, 10-21. ( 10.1111/j.1474-919X.1977.tb02040.x) [DOI] [Google Scholar]
- 80.Greig-Smith PW. 1978. The formation, structure and function of in West African savanna woodland. Ibis 120, 284-297. ( 10.1111/j.1474-919X.1978.tb06789.x) [DOI] [Google Scholar]
- 81.Balph DF, Balph MH. 1979. Behavioral flexibility of pine siskins in mixed species foraging groups. Condor 81, 211-212. [Google Scholar]
- 82.Millikan GC, Gaddis P, Pulliam HR. 1985. Interspecific dominance and the foraging behaviour of juncos. Anim. Behav. 33, 428-435. ( 10.1016/S0003-3472(85)80067-1) [DOI] [Google Scholar]
- 83.Beveridge FM, Deag JM. 1987. The effects of sex, temperature and companions on looking-up and feeding in single and mixed species flocks of house sparrows (Passer domesticus), chaffinches (Fringilla coelebs), and starlings (Sturnus vulgaris). Behaviour 100, 303-319. ( 10.1163/156853987X00170) [DOI] [Google Scholar]
- 84.Clergeau P. 1990. Mixed flocks feeding with starlings: an experimental field study in Western Europe. Bird Behav. 8, 95-100. ( 10.3727/015613890791784308) [DOI] [Google Scholar]
- 85.Lima SL, Zollner PA. 1996. Anti-predatory vigilance and the limits to collective detection: visual and spatial separation between foragers. Behav. Ecol. Sociobiol. 38, 355-363. ( 10.1007/s002650050252) [DOI] [Google Scholar]
- 86.Ragusa-Netto J. 1999. Sócio-ecologia dos bandos mistos de aves em campo-cerrado (Brotas, SP). PhD Thesis, Universidade Estadual Paulista, Rio Claro. [Google Scholar]
- 87.Dhanasarnpaiboon S, Round PD. 2004. Foraging of greater racket-tailed drongo (Dicrurus paradiseus) and lesser racket-tailed drongo (D. remifer) in mixed species bird flocks at Khao Yai National Park. Nat. Hist. Bull. Siam Soc. 52, 115-117. [Google Scholar]
- 88.Jolles JW, King AJ, Manica A, Thornton A. 2013. Heterogeneous structure in mixed-species corvid flocks in flight. Anim. Behav. 85, 743-750. ( 10.1016/j.anbehav.2013.01.015) [DOI] [Google Scholar]
- 89.Zarco A, Cueto VR. 2017. Winter flock structure in the central Monte Desert, Argentina. Ardea 105, 89-97. ( 10.5253/arde.v105i2.a6) [DOI] [Google Scholar]
- 90.Zarco A, Cueto VR, Sagario MC, Marone L. 2019. Effects of livestock grazing on flocks of seed-eating birds in the central Monte Desert, Argentina. Can. J. Zool. 97, 606-611. ( 10.1139/cjz-2018-0223) [DOI] [Google Scholar]
- 91.Ferrari A, Motta-Junior JC. 2018. Mixed flocks in Gubernetes yetapa (Passeriformes: Tyrannidae) and Pseudoleistes guirahuro (Passeriformes: Icteridae) in grasslands of a Cerrado preserve, southeast Brazil. Atual. Ornitol. 201, 4-5. [Google Scholar]
- 92.Delgardo MP, Sanza MA, Morales MB, Traba J, Rivera D. 2013. Habitat selection and coexistence in wintering passerine steppe birds. J. Ornithol. 154, 469-479. ( 10.1007/s10336-012-0914-3) [DOI] [Google Scholar]
- 93.Lefebvre G, Poulin B, McNeil R. 1994. Spatial and social behaviour of Nearctic warblers wintering in Venezuelan mangroves. Can. J. Zool. 72, 757-764. ( 10.1139/z94-102) [DOI] [Google Scholar]
- 94.Rodewald PG, Brittingham MC. 2002. Habitat use and behavior of mixed species landbird flocks during fall migration. Wilson Bull. 114, 87-98. ( 10.1676/0043-5643(2002)114[0087:HUABOM]2.0.CO;2) [DOI] [Google Scholar]
- 95.Robin VV, Davidar P. 2002. The vertical stratification of birds in mixed species flocks at Parambikulam, South India: a comparison between two habitats. J. Bombay Nat. Hist. Soc. 99, 389-399. [Google Scholar]
- 96.Merkord CL. 2010. Seasonality and elevational migration in an Andean bird community. PhD thesis, University of Missouri-Columbia. [Google Scholar]
- 97.Westcott PW. 1969. Relationships among three species of jays wintering in southeastern Arizona. Condor 71, 353-359. ( 10.2307/1365734) [DOI] [Google Scholar]
- 98.Balda RP, Bateman GC, Foster GF. 1972. Flocking associates of the Piñon jay. Wilson Bull. 84, 60-76. [Google Scholar]
- 99.Cannon CE. 1984. Flock size of feeding Eastern and pale-headed rosellas (Aves: Psittaciformes). Wildl. Res. 11, 349-355. ( 10.1071/WR9840349) [DOI] [Google Scholar]
- 100.Read AF. 1987. The breeding and flocking behaviour of yellowheads at Arthur's Pass National Park. Notornis 34, 11-18. [Google Scholar]
- 101.Garnett S, Crowley G. 1995. Feeding ecology of hooded parrots Psephotus dissimilis during the early wet season. Emu 95, 54-61. ( 10.1071/MU9950054) [DOI] [Google Scholar]
- 102.Davis WE, Recher HF. 2002. Winter mixed-species foraging flocks in Acacia woodlands of Western Australia. Corella 26, 74-78. [Google Scholar]
- 103.Martínez O. 2003. Species composition and substrate use by mixed-species bird flocks in an Andean cloud forest of Bolivia. Ecol. Bolivia 38, 99-120. [Google Scholar]
- 104.Jones ZF, Bock CE. 2003. Relationships between Mexican jays (Aphelocoma ultramarina) and northern flickers (Colaptes auratus) in an Arizona oak savanna. Auk 120, 429-432. (https://academic.oup.com/auk/article/120/2/429/5562029) [Google Scholar]
- 105.Schaefer RR, Rudolph DC, Conner RN, Saenz D. 2004. Composition of mixed-species foraging flocks associated with red-cockaded woodpeckers. In Red-cockaded woodpecker: road to recovery (eds Costa Ralph, Daniels Susan J), pp. 672-677. Blaine, WA: Hancock House Publishers. [Google Scholar]
- 106.Della-Flora F. 2014. Richness and composition of avian mixed flocks in savannas and forests of southwestern Brazil. PhD Thesis, Universidade federal de Santa María, Brazil. [Google Scholar]
- 107.Srinivasan U, Raza RH, Quader S. 2012. Patterns of species participation across multiple mixed-species flock types in a tropical forest in northeastern India. J. Nat. Hist. 46, 2749-2762. ( 10.1080/00222933.2012.717644) [DOI] [Google Scholar]
- 108.Mangini GG. 2018. Las bandadas mixtas de aves como estrategia: comportamiento y estacionalidad en la Selva Pedemontana de las Yungas Australes. PhD Thesis, Universidad Nacional de Tucumán, Argentina. [Google Scholar]
- 109.Wiley RH. 1980. Multispecies antbird societies in lowland forests of Surinam and Ecuador: stable membership and foraging differences. J. Zool. 191, 127-145. ( 10.1111/j.1469-7998.1980.tb01453.x) [DOI] [Google Scholar]
- 110.Gradwohl J, Greenberg R. 1980. The formation of antwren flocks on Barro Colorado Island, Panama. Auk 97, 385-395. ( 10.1093/auk/97.2.385) [DOI] [Google Scholar]
- 111.Powell GVN. 1989. On the possible contribution of mixed species flocks to species richness in Neotropical avifaunas. Behav. Ecol. Sociobiol. 24, 387-393. ( 10.1007/BF00293266) [DOI] [Google Scholar]
- 112.Sigel BJ, Robinson WD, Sherry TW. 2010. Comparing bird community responses to forest fragmentation in two lowland Central American reserves. Biol. Conserv. 143, 340-350. ( 10.1016/j.biocon.2009.10.020) [DOI] [Google Scholar]
- 113.Kotagama SW, Goodale E. 2004. The composition and spatial organization of mixed-species flocks in a Sri Lankan rainforest. Forktail 20, 63-70. [Google Scholar]
- 114.Hsieh F, Chen CC. 2011. Does niche-overlap facilitate mixed-species flocking in birds? J. Ornithol. 152, 955-963. ( 10.1007/s10336-011-0678-1) [DOI] [Google Scholar]
- 115.Slud P. 1960. The birds of Finca ‘La Selva’, Costa Rica: a tropical wet forest locality. Bull. Am. Mus. Nat. Hist. 121, 49-148. [Google Scholar]
- 116.Ogasawara K. 1965. The analysis of the mixed flock of the family Paridae in the Botanical Garden of the Tohoku University, Sendai. I. Seasonal change of the flock formation. Sci. Rep. Tôhuku Univ. Series IV 31, 167-180. [Google Scholar]
- 117.Morse DH. 1978. Structure and foraging patterns of flocks of tits and associated species in an English woodland during the winter. Ibis 120, 298-312. ( 10.1111/j.1474-919X.1978.tb06790.x) [DOI] [Google Scholar]
- 118.Ficken MS, Ficken RW. 1974. Is the golden-winged warbler a social mimic of the black-capped chickadee? Wilson Bull. 86, 468-471. [Google Scholar]
- 119.MacDonald DW, Henderson DG. 1977. Aspects of the behaviour and ecology of mixed-species bird flocks in Kashmir. Ibis 119, 481-493. ( 10.1111/j.1474-919X.1977.tb02055.x) [DOI] [Google Scholar]
- 120.Hogstad O. 1978. Differentiation of foraging niche among tits, Parus spp., in Norway during winter. Ibis 120, 139-146. ( 10.1111/j.1474-919X.1978.tb06770.x) [DOI] [Google Scholar]
- 121.Gaddis P. 1980. Mixed flocks, accipiters, and antipredator behavior. Condor 82, 348-349. [Google Scholar]
- 122.Sullivan KA. 1984. Information exploitation by downy woodpeckers in mixed-species flocks. Behaviour 91, 294-311. ( 10.1163/156853984X00128) [DOI] [Google Scholar]
- 123.Dolby AS, Grubb TC Jr. 1998. Benefits to satellite members in mixed-species foraging groups: an experimental analysis. Anim. Behav. 56, 501-509. ( 10.1006/anbe.1998.0808) [DOI] [PubMed] [Google Scholar]
- 124.Brennan LA, Morrison ML, Dahlsten DL. 1999. Winter flocking by chestnut-backed and mountain chickadees in the western Sierra Nevada. Northwestern Nat. 80, 84-89. ( 10.2307/3536654) [DOI] [Google Scholar]
- 125.Dolby AS, Grubb TC. 1999. Functional roles in mixed-species foraging flocks: a field manipulation. Auk 116, 557-559. ( 10.2307/4089392) [DOI] [Google Scholar]
- 126.Suhonen J. 1993. Risk of predation and foraging sites of individuals in mixed-species tit flocks. Anim. Behav. 45, 1193-1198. ( 10.1006/anbe.1993.1141) [DOI] [Google Scholar]
- 127.Suhonen J, Alatalo RV, Gustafsson L. 1994. Evolution of foraging ecology in Fennoscandian tits (Parus spp.). Proc. R. Soc. Lond. B 258, 127-131. ( 10.1098/rspb.1994.0152) [DOI] [Google Scholar]
- 128.Cimprich DA, Grubb TC Jr. 1994. Consequences for Carolina chickadees of foraging with tufted titmice in winter. Ecology 75, 1615-1625. ( 10.2307/1939622) [DOI] [Google Scholar]
- 129.Walther B, Gosler A. 2001. The effects of food availability and distance to protective cover on the winter foraging behaviour of tits (Aves: Parus). Oecologia 129, 312-320. ( 10.1007/s004420100713) [DOI] [PubMed] [Google Scholar]
- 130.Seki SI, Sato T. 2002. The effect of a typhoon on the flocking and foraging behavior of tits. Ornithol. Sci. 1, 53-61. ( 10.2326/osj.1.53) [DOI] [Google Scholar]
- 131.Post W. 1978. Social and foraging behavior of warblers wintering in Puerto Rican coastal scrub. Wilson Bull. 90, 197-214. [Google Scholar]
- 132.Powell GV. 1979. Structure and dynamics of interspecific flocks in a Neotropical mid-elevation forest. Auk 96, 375-390. [Google Scholar]
- 133.Herzog SK, Soria R, Troncoso A, Matthysen E. 2002. Composition and spatial structure of avian mixed-species flocks in a high-Andean Polylepis forest in Bolivia. Ecotropica 8, 133-143. [Google Scholar]
- 134.Shermila WGDDM, Wikramasinghe S. 2013. Composition of mix species foraging flocks of birds in Riverstan of Montane Region, Sri Lanka. J. Trop. For. Environ. 3, 55-66. ( 10.31357/jtfe.v3i1.1123) [DOI] [Google Scholar]
- 135.Muñoz J. 2016. The role of facilitation in the structure of tropical bird communities: a case study of mixed-species flocks. MSci Dissertation, University of British Columbia. [Google Scholar]
- 136.Montaño-Centellas FA. 2020. Interaction networks of avian mixed-species flocks along elevation in the tropical Andes. Ecography 43, 930-942. ( 10.1111/ecog.05135) [DOI] [Google Scholar]
- 137.Gannon GR. 1934. Associations of small insectivorous birds. Emu 34, 122-129. ( 10.1071/MU934122) [DOI] [Google Scholar]
- 138.Aleixo A. 2013. Composition of mixed-species birds flocks and abundance of flocking species in a semideciduous forest of southeastern Brazil. Rev. Bras. Ornit. 5, 18. [Google Scholar]
- 139.Moynihan M. 1979. Geographic variation in social behavior and in adaptations to competition among Andean birds. Nuttall Ornithol. 18, 1-162. [Google Scholar]
- 140.Fanjul ME. 2016. Bandadas mixtas de aves en un gradiente latitudinal en las selvas montanas de las Yungas de Argentina. PhD Thesis, Universidad Nacional de Córdoba, Argentina. [Google Scholar]
- 141.Thiollay JM. 2003. Comparative foraging behavior between solitary and flocking insectivores in a Neotropical forest: does vulnerability matter. Ornitol. Neotrop. 14, 47-65. [Google Scholar]
- 142.Hindwood KA. 1937. The flocking of birds with particular reference to the association of small insectivorous birds. Emu 36, 254-261. ( 10.1071/MU936254) [DOI] [Google Scholar]
- 143.Willis EO. 1973. Local distribution of mixed flocks in Puerto Rico. Wilson Bull. 85, 75-77. [Google Scholar]
- 144.Claessens O. 2002. Diversity and guild structure of the Petit Saut bird community. Rev. Ecol. Terre Vie 8, 77-102. [Google Scholar]
- 145.Kirkconnell A. 2002. Aspectos ecológicos de las bijiritas migratorias en Cuba. Cotinga 17, 23-32. [Google Scholar]
- 146.Hino T. 2002. Breeding bird community and mixed-species flocking in a deciduous broad-leaved forest in western Madagascar. Ornithol. Sci. 1, 111-116. ( 10.2326/osj.1.111) [DOI] [Google Scholar]
- 147.Ridley AR, Raihani NJ. 2007. Facultative response to a kleptoparasite by the cooperatively breeding pied babbler. Behav. Ecol. 18, 324-330. ( 10.1093/beheco/arl092) [DOI] [Google Scholar]
- 148.Ridley AR, Raihani NJ, Bell MB. 2010. Experimental evidence that sentinel behaviour is affected by risk. Biol. Lett. 6, 445-448. ( 10.1098/rsbl.2010.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnson AE, Masco C, Pruett-Jones S. 2018. Song recognition and heterospecific associations between two fairy-wren species (Maluridae). Behav. Ecol. 29, 821-832. ( 10.1093/beheco/ary071) [DOI] [Google Scholar]
- 150.Partridge L, Ashcroft R. 1976. Mixed-species flocks of birds in hill forest in Ceylon. Condor 78, 449-453. ( 10.2307/1367093) [DOI] [Google Scholar]
- 151.Jabłoński PG, Lee SD. 2002. Foraging niche shifts in mixed-species flocks of tits in Korea. J. Field Ornith. 73, 246-252. ( 10.1648/0273-8570-73.3.246) [DOI] [Google Scholar]
- 152.Guevara EA, Valarezo JC, Onofa A, Cupuerán F. 2011. Mixed-species flock composition in a northwestern Ecuadorian cloud forest. Ornitol. Neotrop. 22, 379-386. [Google Scholar]
- 153.Pizo MA, Tonetti VR. 2020. Living in a fragmented world: birds in the Atlantic Forest. Condor 122, duaa023. ( 10.1093/condor/duaa023) [DOI] [Google Scholar]
- 154.Pearman PB. 2002. The scale of community structure: habitat variation and avian guilds in tropical forest understory. Ecol. Monogr. 72, 19-39. ( 10.1890/0012-9615(2002)072[0019:TSOCSH]2.0.CO;2) [DOI] [Google Scholar]
- 155.Williams SM, Lindell CA. 2021. Context-specific behavior serves as a mechanism of interspecific cohesion in mixed-species flocks. Ornithology 138, ukab046. ( 10.1093/ornithology/ukab046) [DOI] [Google Scholar]
- 156.Hamel PB, Kirkconnell A. 2005. Composition of mixed-species flocks of migrant and resident birds in Cuba. Cotinga 24, 28-34. [Google Scholar]
- 157.Darwin C. 1859. The origin of species by means of natural selection, Or, The preservation of favoured races in the struggle for life. London, UK: Incorporated, Pub. [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou L, et al. 2019. The response of mixed-species bird flocks to anthropogenic disturbance and elevational variation in southwest China. Condor 121, duz028. ( 10.1093/condor/duz028) [DOI] [Google Scholar]
- 159.Mangini GG, Areta JI. 2018. Bird mixed-species flock formation is driven by low temperatures between and within seasons in a Subtropical Andean-foothill forest. Biotropica 50, 816-825. ( 10.1111/btp.12551) [DOI] [Google Scholar]
- 160.Rutt CL, Stouffer PC. 2021. Seasonal dynamics of flock interaction networks across a human-modified landscape in lowland Amazonian rain forest. Ecol. Appl. 31, e02235. ( 10.1002/eap.2235) [DOI] [PubMed] [Google Scholar]
- 161.Swynnerton CFM. 1907. On the birds of Gazaland, Southern Rhodesia. Ibis 49, 30-74. [Google Scholar]
- 162.Eaton SE. 1953. Wood warblers wintering in Cuba. Wilson Bull. 65, 169-174. [Google Scholar]
- 163.Fanjul ME, Echevarria A. 2015. Composición, estructura y rol social de las bandadas mixtas de aves de la selva montana de Yungas, provincia de Tucumán, Argentina. Acta Zool. Lillo. 59, 141-154. [Google Scholar]
- 164.Contreras TA, Sieving KE. 2011. Leadership of winter mixed-species flocks by tufted titmice (Baeolophus bicolor): are titmice passive nuclear species? Int. J. Zool. 2011, 670548. ( 10.1155/2011/670548) [DOI] [Google Scholar]
- 165.Munn CA. 1986. Birds that ‘cry wolf’. Nature 319, 143-145. [Google Scholar]
- 166.Martínez AE, Parra E, Gomez JP, Vredenburg VT. 2021. Shared predators between primate groups and mixed species bird flocks: the potential for forest-wide eavesdropping networks. Oikos 2022, e08274. ( 10.1111/oik.08274) [DOI] [Google Scholar]
- 167.Carlson NV, Healy SD, Templeton CN. 2020. What makes a ‘community informant’? Reliability and anti-predator signal eavesdropping across mixed-species flocks of tits. Anim. Behav. Cogn. 7, 214-246. ( 10.26451/abc.07.02.13.2020) [DOI] [Google Scholar]
- 168.Satischandra SHK, Kodituwakku P, Kotagama SW, Goodale E. 2010. Assessing ‘false’ alarm calls by a drongo (Dicrurus paradiseus) in mixed-species bird flocks. Behav. Ecol. 21, 396-403. ( 10.1093/beheco/arp203) [DOI] [Google Scholar]
- 169.Cordeiro NJ, Borghesio L, Joho MP, Monoski TJ, Mkongewa VJ, Dampf CJ. 2014. Forest fragmentation in an African biodiversity hotspot impacts mixed-species bird flocks. Biol. Conserv. 188, 61-71. ( 10.1016/j.biocon.2014.09.050) [DOI] [Google Scholar]
- 170.Miller RC. 1922. The significance of the gregarious habit. Ecology 3, 122-126. ( 10.2307/1929145) [DOI] [Google Scholar]
- 171.Soares L, et al. 2022. Neotropical Ornithology: Reckoning with historical assumptions, removing systemic barriers, and reimagining the future. ecoevorxiv preprint. ( 10.32942/osf.io/yu2fx) [DOI]
- 172.Kappeler PM. 2019. A framework for studying social complexity. Behav. Ecol. Sociobiol. 73, 13. ( 10.1007/s00265-018-2601-8) [DOI] [Google Scholar]
- 173.Grueter CC, et al. 2020. Multilevel organisation of animal sociality. Trends Ecol. Evol. 35, 834-847. ( 10.1016/j.tree.2020.05.003) [DOI] [PubMed] [Google Scholar]
- 174.Dodds WK. 1988. Community structure and selection for positive or negative species interactions. Oikos 53, 387-390. ( 10.2307/3565540) [DOI] [Google Scholar]
- 175.Zou F, Jones H, Jiang D, Lee TM, Martínez A, Sieving K, Zhang M, Zhang Q, Goodale E. 2018. The conservation implications of mixed-species flocking in terrestrial birds, a globally-distributed species interaction network. Biol. Conserv. 224, 267-276. ( 10.1016/j.biocon.2018.06.004) [DOI] [Google Scholar]
- 176.Mangini GG, Gandoy FA, Areta JI, Blendinger PG. 2022. Benefits of foraging in mixed-species flocks depend on species role and foraging strategy. Ibis 165, 629-646. ( 10.1111/ibi.13162) [DOI] [Google Scholar]
- 177.Ippi S, Trejo A. 2003. Dinámica y estructura de bandadas mixtas de aves en un bosque de lenga (Nothofagus pumilio) del Noroeste de la Patagonia argentina. Ornitol. Neotrop. 14, 353-362. [Google Scholar]
- 178.Mangini GG, et al. 2023. A classification scheme for mixed-species bird flocks. Zenodo. ( 10.5281/zenodo.7712927) [DOI] [PMC free article] [PubMed]
- 179.Mangini GG, et al. 2023. A classification scheme for mixed-species bird flocks. Figshare. ( 10.6084/m9.figshare.c.6461110) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mangini GG, et al. 2023. A classification scheme for mixed-species bird flocks. Zenodo. ( 10.5281/zenodo.7712927) [DOI] [PMC free article] [PubMed]
- Mangini GG, et al. 2023. A classification scheme for mixed-species bird flocks. Figshare. ( 10.6084/m9.figshare.c.6461110) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The raw compiled table for this review is available from the Zenodo repository: https://doi.org/10.5281/zenodo.7712927 [178].
A Spanish abstract is available in the electronic supplementary materials [179].