Abstract
Background and Objectives
Platelet transfusions are used across multiple patient populations to prevent and correct bleeding. This scoping review aimed to map the currently available systematic reviews (SRs) and evidence‐based guidelines in the field of platelet transfusion.
Materials and Methods
A systematic literature search was conducted in seven databases for SRs on effectiveness (including dose and timing, transfusion trigger and ratio to other blood products), production modalities and decision support related to platelet transfusion. The following data were charted: methodological features of the SR, population, concept and context features, outcomes reported, study design and number of studies included. Results were synthesized in interactive evidence maps.
Results
We identified 110 SRs. The majority focused on clinical effectiveness, including prophylactic or therapeutic transfusions compared to no platelet transfusion (34 SRs), prophylactic compared to therapeutic‐only transfusion (8 SRs), dose, timing (11 SRs) and threshold for platelet transfusion (15 SRs) and the ratio of platelet transfusion to other blood products in massive transfusion (14 SRs). Furthermore, we included 34 SRs on decision support, of which 26 evaluated viscoelastic testing. Finally, we identified 22 SRs on platelet production modalities, including derivation (4 SRs), pathogen inactivation (6 SRs), leucodepletion (4 SRs) and ABO/human leucocyte antigen matching (5 SRs). The SRs were mapped according to concept and clinical context.
Conclusion
An interactive evidence map of SRs and evidence‐based guidelines in the field of platelet transfusion has been developed and identified multiple reviews. This work serves as a tool for researchers looking for evidence gaps, thereby both supporting research and avoiding unnecessary duplication.
Keywords: decision support, haemostasis, platelet apheresis, platelet transfusion, scoping review, thrombocytopenia, viscoelastic
Highlights.
This scoping review identified a total of 110 systematic reviews (SRs) and mapped them in interactive evidence maps according to clinical context and concept.
Several areas, for example, platelet transfusion in intracerebral haemorrhage and the ratio of blood products in massive transfusion and viscoelastic testing to guide platelet transfusion, are served by multiple overlapping SRs.
Scoping reviews can be a tool to avoid research waste. This work provides a comprehensive overview of the available research in the field of platelet transfusion.
INTRODUCTION
Platelets are the second most commonly transfused cellular blood component. Platelets are involved in haemostasis, but also have other roles [1]. Thrombocytopenia, or disorders of platelet function, may result in bleeding, which can be life‐threatening [2]. Thrombocytopenia is often defined by a platelet count <100 to 150 × 109/L, whereas severe thrombocytopenia is defined by a platelet count <50 × 109/L [2, 3]. Thrombocytopenia can occur due to increased use (e.g. in severe bleeding), decreased production (e.g., haematological disorders) or immune‐mediated destruction of platelets (e.g., neonatal alloimmune thrombocytopenia). Therefore, platelets are widely used across multiple clinical settings in hospitalized patients [4, 5], and can be administered either therapeutically, to stop bleeding, or prophylactically, to prevent bleeding [6]. Platelet transfusions are most often used in patients with haematological malignancies, undergoing cardiac surgery or before procedures in intensive care settings [7]. The increased demand and limited supply of platelet products show that judicious use of platelet transfusions is crucial.
Several methods for preparing platelets, platelet dosing, platelet transfusion threshold and platelet product specifications have been investigated in studies [8, 9, 10, 11, 12, 13, 14]. This demonstrates the importance of considering the best available evidence on the effectiveness and cost‐effectiveness of procedures, collected in systematic reviews (SRs), to guide the development of evidence‐based guidelines for clinical practice [15]. A current overview of existing SRs and topics for which no SR is as yet published is not available. Therefore, the aim of this scoping review is to develop an evidence map informing future SRs in the field of platelet transfusion.
MATERIALS AND METHODS
A completed (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses [PRISMA])‐Scoping Reviews reporting checklist can be found in Appendix S1. A concise methods section is presented below, and a full version can be found in Appendix S2.
Selection criteria
We included patients of any age eligible for platelet transfusion. We included any SR of controlled research, with an explicit methods section, in the field of platelet transfusion, in any clinical context, without language restrictions. We included SRs comparing the effectiveness of platelet transfusion to no platelet transfusion, prophylactic to therapeutic platelet transfusion, different doses and timings of platelet transfusion, different ratios of platelets to other blood products in major transfusion and different production modalities for platelet transfusions, decision support systems for platelet transfusion and the impact of platelets on refractoriness or alloimmunization.
Search strategy and study selection
We searched for SRs on 5 May 2021, without applying date limits, in PubMed, Embase, Web of Science, Cumulated Index to Nursing and Allied Health Literature, Cochrane Database of Systematic Reviews, Transfusion Evidence Library and International Prospective Register of Systematic Reviews (PROSPERO).
Studies were assessed for eligibility by two independent reviewers at the title and abstract and full‐text levels. Discrepancies were resolved by discussion or consulting a third reviewer. We screened the reference lists and the first 20 ‘similar articles’ in PubMed of included studies for additional eligible studies.
Data charting, synthesis and presentation
Data charting from eligible SRs was done by two independent reviewers. We analysed data by population, concept, outcome type, methodological features, patient age and outdatedness. Data are presented in interactive evidence maps using EPPI‐Mapper v2.1.0 [16].
RESULTS
Search results
A total of 9199 records were identified from the database searches, leading to a total of 5583 unique records that were reviewed. Following exclusions, 169 records, reporting on 110 unique SRs, were included, 13 of which were identified through screening of reference lists and ‘similar articles’ in PubMed of included records (Appendix S3). Multiple records reporting on the same topic area were noted, including previous versions of the SRs, protocols and PROSPERO registrations and conference abstracts. The eligibility of another 48 records, reporting on 40 SRs, could not be ascertained due to a lack of information (mainly review protocols and conference abstracts of ongoing SRs). These records have an ‘awaiting classification’ status and may be assessed for eligibility again in future updates of this scoping review (Appendix S4). An overview of studies not meeting eligibility criteria can be found in Appendix S5.
Characteristics of the included SRs
An interactive evidence map describing the characteristics of the included SRs can be accessed online through this link: https://www.cebap.org/storage/cebap/20220110-eppimap-studydesign.html. A static overview is included in this article as Figure 1. A detailed overview of charted characteristics can be found in Appendix S6.
FIGURE 1.
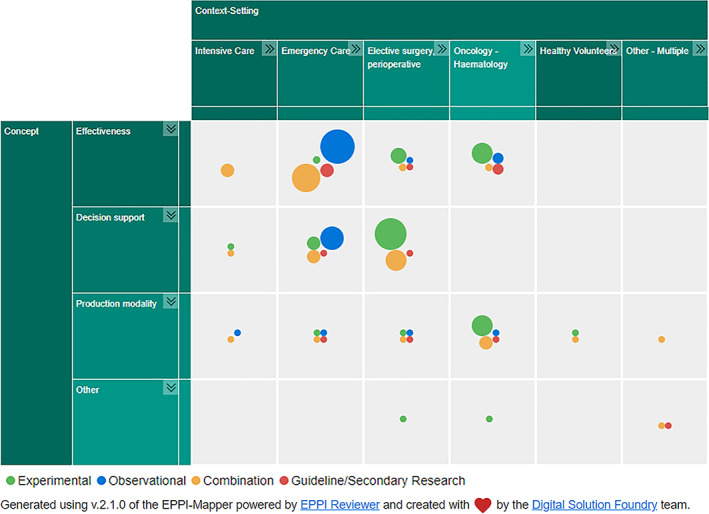
Evidence map illustrating the number of identified systematic reviews, mapped by clinical context and concept, segmented by study design included
Of the 110 included SRs, 11 were SRs embedded in evidence‐based guideline projects [9, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26], and three were overviews of reviews concerning the management of traumatic brain injury [27, 28] or trauma‐induced coagulopathy [29]. The remainder were SRs of primary research, either experimental studies, observational studies or both. The majority of SRs were conducted in the United Kingdom (20%), Canada (19%) and the United States (18%).
Populations studied
Nine SRs included studies on intensive care unit (ICU) patients, of which one specifically included dengue patients. Fifty‐three SRs focused on emergency care patients, including spontaneous or traumatic brain injury patients or patients defined as ‘trauma’ or ‘massive bleeding’ patients. Furthermore, 31 SRs included studies conducted in a haematological or oncological setting. Eighteen SRs studied general oncology/haematology patients, five SRs focused on haematopoietic stem cell transplant patients, nine SRs on hypoproliferative bone marrow disorders, three SRs on thrombotic or immune thrombocytopenia purpura, one SR on disseminated intravascular coagulation, two SRs on heparin‐induced thrombocytopenia and five SRs on foetal/neonatal alloimmune thrombocytopenia. Thirty‐seven SRs concerned surgery patients, 19 of which concerned cardiac surgery patients, eight concerned liver surgery patients, five concerned patients undergoing minor procedures and 16 concerned other or unspecified elective surgery patients. Finally, three SRs included studies with healthy volunteers, and the context of studies included in four SRs was classified as ‘other’.
Four SRs specifically focused on the paediatric population, and a further five investigated platelet transfusion in foetal/neonatal alloimmune thrombocytopenia. The remainder either did not specify or specifically included studies in an adult population. The interactive evidence map, segmented by population age, can be accessed through this link https://www.cebap.org/storage/cebap/20220110-eppimap-age.html; a static overview can be found in Figure 2.
FIGURE 2.
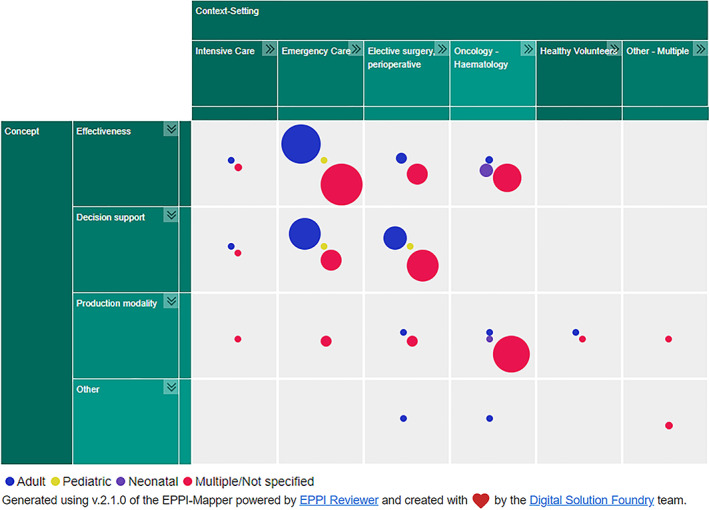
Evidence map illustrating the number of identified systematic reviews, mapped by clinical context and concept, segmented by age category
Concept studied
Fifty‐eight SRs addressed the clinical effectiveness of platelet transfusions. Thirty‐four SRs investigated the effectiveness of platelet transfusions compared to no platelet transfusions to prevent or treat bleeding, while eight compared prophylactic to therapeutic‐only platelet transfusions. Eleven SRs focused on the dose and timing of platelet transfusions, and 15 included studies on platelet transfusion thresholds in prophylactic platelet transfusion. Finally, 14 SRs included studies comparing the ratio of platelets to other blood products in massive transfusion. Thirty‐four SRs looked at decision support systems to guide platelet transfusions. Twenty‐six SRs focused on point‐of‐care viscoelastic testing (thromboelastography and/or rotational thromboelastometry), while eight looked at other decision supports, mainly the use of formal transfusion protocols. Twenty‐two SRs were identified that included information on platelet production modalities. Seven SRs included studies on the impact of platelet storage duration, while two searched for studies on the storage temperature of platelets. Four SRs included studies on derivation methods of platelets (whole blood derived vs. apheresis derived), six SRs concentrated on pathogen inactivation of platelets and four SRs assessed leucodepletion. Finally, five SRs investigated the clinical impact of platelet matching by ABO type or human leucocyte antigen cross‐matching.
Outcomes reported
Regarding outcome types included, 76 SRs reported a death‐related outcome (e.g. 30‐day mortality), 69 SRs reported morbidity (e.g., bleeding, re‐operation due to bleeding), 62 SRs reported outcomes related to transfusion (e.g., number of blood products used, transfusion requirement, transfusion interval), 33 SRs reported a haematological outcome (e.g., platelet count increment), 37 SRs reported length of stay outcomes (e.g., length of hospital stay or ICU stay), 48 SRs reported at least one adverse event (e.g., acute transfusion reactions), 7 SRs reported an economic outcome (e.g., costs associated) and 13 SRs reported an outcome that did not fit in any of the prior categories (e.g., quality of life).
Methodological features
The included SRs differed from each other in terms of methodological features. Only 66 SRs had a clearly defined research question, including elements of a population, intervention, control and outcomes question. The vast majority searched more than one database, but 15 SRs searched only in PubMed/Medline. A formal quality appraisal of included studies was conducted in 80 SRs, and 33 also assessed the certainty of the body of evidence, all but one, using the grading of recommendations, assessment, development and evaluation methodology [15]. Fifty‐two of the included SRs were published before 2017, while 58 were published in 2017 or later. For the SRs supporting an evidence‐based clinical practice guideline, eight were published in 2017 or later, and only three were from before 2017.
Regarding the outdatedness of the searches, 36 SRs had a search date less than 5 years old, while 69 SRs had a search date older than 5 years old, and therefore, considered outdated. The search data of five SRs were not sufficiently reported to assess outdatedness. An interactive evidence map segmenting the SRs by outdatedness can be accessed via https://www.cebap.org/storage/cebap/20220714-eppimap-outdatedness.html; a static version is shown in Figure 3.
FIGURE 3.
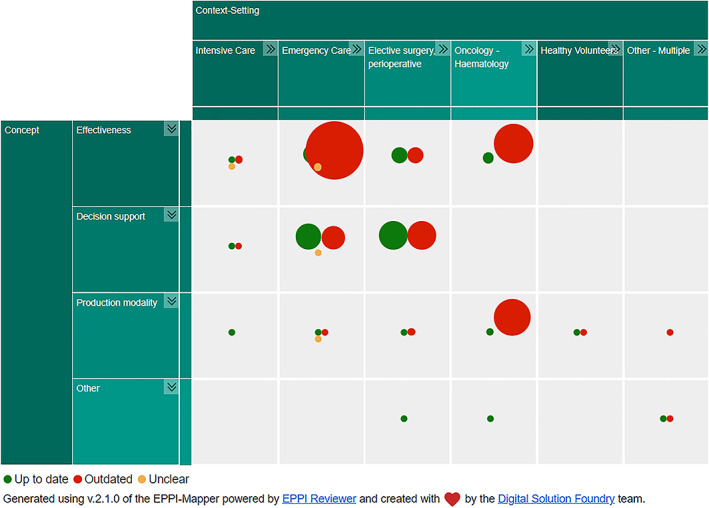
Evidence map illustrating the number of identified systematic reviews, mapped by clinical context and concept, segmented by outdatedness
DISCUSSION
This scoping review mapped the currently available SRs in the field of platelet transfusion. It not only provides an overview of the evidence but is also an important reference for clinician education. Moreover, it promotes new research by identifying research gaps and redundancy. We have identified 110 SRs. The high number of reviews, many published in the last few years, raises important questions as to which reviews should be accessed by busy clinicians. We labelled 40 SRs as ‘awaiting’ classification, which demonstrates that this is clearly an active and ongoing field of research.
About a third of the identified SRs had a search date less than 5 years old, which has been suggested to be a relevant cut‐off for considering a SR as up‐to‐date [30]. If resources allow, the interactive versions of our evidence maps that are online available will be updated regularly. Future updates of the evidence map resulting from this project will likely have an important impact on the completeness of this overview.
It is clear that some areas of research are better served by SRs than others. This may correspond to the amount of underlying primary research available, the need to combine studies in meta‐analyses when sample sizes are small or when outcomes are infrequent, or in areas of controversy. Several indications, for example, the use of viscoelastic testing to guide transfusion in cardiac surgery or the effectiveness of therapeutic platelet transfusion in traumatic brain injury, are covered by several overlapping SRs. This demonstrates the added value of a scoping review, which can serve as a tool to minimize future redundancy. Chalmers and Glasziou highlighted that many trials are conducted and reported without reference to existing literature, thereby potentially leading to studies answering already solved research questions [31]. Our scoping review identified a similar concept for SRs. In addition, several authors demonstrated that the number of published SRs and meta‐analyses had grown steadily over the years [32, 33]. In order to avoid overlap and duplication in evidence syntheses, an initial consideration of whether a new SR is actually needed should be the first step in the set‐up of a potential new SR protocol. Scoping reviews and evidence gap maps might be useful tools to answer this need [34, 35], especially if they include a search in the international prospective register for SRs, PROSPERO, for ongoing SRs [36].
Our approach has several advantages. First, we used elaborate search methods and a rigorous methodology [37], thereby aiming to obtain as complete an overview of the state‐of‐the‐art in platelet transfusion as possible. Furthermore, our approach to visually display the identified SRs in interactive maps has the advantage of usability, whereby users can quickly scan the existing evidence for their topic of interest using the different filtering options available. Finally, our work directly demonstrates the research gaps in this field and can thereby inform future reviewers and researchers as to where useful work may be undertaken. When looking at the outcomes reported in the SRs, it is clear that few existing SRs pay attention to economic aspects related to platelet transfusion and production modalities or quality of life. Five of the seven SRs, including an economic outcome, focus on decision support systems [38, 39, 40, 41, 42], and two on platelet dose [9, 43]. The few SRs that defined quality of life as an outcome of interest actually did not identify any primary research study reporting this outcome [2, 6, 12, 39, 43, 44, 45, 46, 47, 48]. Although a minority of SRs report at least one adverse event, no SR was identified with platelet transfusion‐related adverse events as the main focus, despite the fact that platelets are regularly associated with adverse events and carry a higher risk of bacterial contamination than other blood products [49, 50]. Other potential research gaps may include prophylactic versus therapeutic‐only platelet transfusion in the paediatric patient population [51], cardiac surgery or critical illness.
Limitations include an initial scope that included alternatives to platelet transfusion (e.g., tranexamic acid). Second, our scoping review is secondary research. It does not show whether there are primary research studies available in a given field of work. Finally, given that we have conducted a scoping review, the purpose of this exercise is to map the existing evidence and not a formal critical appraisal and evaluation of effectiveness.
In conclusion, we have mapped the currently available secondary research in the field of platelet transfusion using a rigorous scoping review methodology. This work serves both clinicians, researchers and guideline developers in search for a quick and clear overview regarding the state‐of‐the‐art in themes related to platelet transfusion, from clinical effectiveness to production modalities and decision support.
CONFLICT OF INTEREST
Relevant financial conflicts of interest directly related to this review: Bert Avau, Dorien O, Koen Veys, Hans Van Remoortel, Jørgen Georgsen, Nadine Shehata, Simon J. Stanworth, Emmy De Buck, Veerle Compernolle and Philippe Vandekerckhove declared not having any relevant direct financial conflict of interest. Susan Nahirniak declared to have received travel reimbursements from the Canadian Blood Services for travel to Scientific Research Advisory Committee, which occasionally discusses International Collaboration for Transfusion Medicine Guidelines (ICTMG) projects.
Relevant financial conflicts of interest not directly related to this review: Bert Avau, Dorien O, Koen Veys, Hans Van Remoortel, Emmy De Buck, Veerle Compernolle and Philippe Vandekerckhove are employees of Belgian Red Cross‐Flanders, which is responsible for supplying adequate quantities of safe blood products to hospitals in Flanders and Brussels on a continuous basis and is funded by the Ministry of Social Affairs. Belgian Red Cross‐Flanders received a grant from the European Blood Alliance to conduct this review. Nadine Shehata reported having received personal fees from the ICTMG for the development of the ICTMG platelet guideline. Simon J. Stanworth declared being an employee of the NHSBT, a blood service supplier for England, which manufactures platelets and having received grants for the conduct of trials of platelets in neonates and platelets and tranexamic acid in haematological cancers. Jørgen Georgsen and Susan Nahirniak declared not having any other relevant financial conflicts of interest.
Relevant intellectual conflicts of interest: Bert Avau, Dorien O, Koen Veys, Hans Van Remoortel, Jørgen Georgsen, Emmy De Buck, Veerle Compernolle and Philippe Vandekerckhove declared not having any intellectual conflict of interest. Simon J. Stanworth declared having authored multiple systematic reviews (SRs) on the use of platelets and tranexamic acid in haematology—none within the last three years and being involved in a new guideline on platelets and plasma in critically ill children. Simon J. Stanworth and Susan Nahirniak declared being the co‐chair of the platelet guideline revision working group of the ICTMG. Nadine Shehata declared having been a co‐author on previous SRs and a guideline on platelet transfusion.
Supporting information
Appendix S1‐S6. Supporting information.
ACKNOWLEDGEMENTS
Funding for this project has been provided partly through an Agreement with the European Blood Alliance (EBA) and partly by the Foundation for Scientific Research of the Belgian Red Cross.
B.A., K.V., D.O. and H.V. performed the research, B.A. wrote the first draft of the manuscript, B.A., K.V., D.O. and H.V. analysed the data, H.V, E.D., V.C. and P.V conceptualized the research, H.V., E.D., V.C., P.V., J.G., N.S., S.N. and S.S. supervised the research, K.V., D.O., H.V., E.D., V.C., P.V., J.G., N.S., S.N. and S.S. reviewed and edited the manuscript. Funding for this project has been provided partly through an Agreement with the EBA and partly by the Foundation for Scientific Research of the Belgian Red Cross. The contents of this document do not necessarily reflect the views and policies of the EBA, nor does the mentioning of trade names or commercial products constitute endorsement or recommendation of use.
Avau B, O D, Veys K, Georgsen J, Nahirniak S, Shehata N, et al. Systematic reviews on platelet transfusions: Is there unnecessary duplication of effort? A scoping review. Vox Sang. 2023;118:16–23.
Funding information European Blood Alliance, Grant/Award Number: 2021‐01; Foundation for Scientific Research of the Belgian Red Cross, Grant/Award Number: Structural Funding
REFERENCES
- 1. Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–9. [DOI] [PubMed] [Google Scholar]
- 2. Estcourt LJ, Malouf R, Hopewell S, Doree C, Van Veen J. Use of platelet transfusions prior to lumbar punctures or epidural anaesthesia for the prevention of complications in people with thrombocytopenia. Cochrane Database Syst Rev. 2018;4:CD011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA, et al. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207. [DOI] [PubMed] [Google Scholar]
- 4. Freireich EJ. Origins of platelet transfusion therapy. Transfus Med Rev. 2011;25:252–6. [DOI] [PubMed] [Google Scholar]
- 5. Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–8. [DOI] [PubMed] [Google Scholar]
- 6. Crighton GL, Estcourt LJ, Wood EM, Trivella M, Doree C, Stanworth S. A therapeutic‐only versus prophylactic platelet transfusion strategy for preventing bleeding in patients with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev. 2015;9:CD010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Estcourt LJ. Why has demand for platelet components increased? A review. Transfus Med. 2014;24:260–8. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Shahi Salman R, Law ZK, Bath PM, Steiner T, Sprigg N. Haemostatic therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev. 2018;4:CD005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nahirniak S, Slichter SJ, Tanael S, Rebulla P, Pavenski K, Vassallo R, et al. Guidance on platelet transfusion for patients with hypoproliferative thrombocytopenia. Transfus Med Rev. 2015;29:3–13. [DOI] [PubMed] [Google Scholar]
- 10. Cid J, Lozano M. Lower or higher doses for prophylactic platelet transfusions: results of a meta‐analysis of randomized controlled trials. Transfusion. 2007;47:464–70. [DOI] [PubMed] [Google Scholar]
- 11. Kreuger AL, Caram‐Deelder C, Jacobse J, Kerkhoffs JL, van der Bom JG, Middelburg RA. Effect of storage time of platelet products on clinical outcomes after transfusion: a systematic review and meta‐analyses. Vox Sang. 2017;112:291–300. [DOI] [PubMed] [Google Scholar]
- 12. Estcourt LJ, Malouf R, Doree C, Trivella M, Hopewell S, Birchall J. Prophylactic platelet transfusions prior to surgery for people with a low platelet count. Cochrane Database Syst Rev. 2018;9:CD012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vamvakas EC. Meta‐analysis of the studies of bleeding complications of platelets pathogen‐reduced with the intercept system. Vox Sang. 2012;102:302–16. [DOI] [PubMed] [Google Scholar]
- 14. Vogt KN, Van Koughnett JA, Dubois L, Gray DK, Parry NG. The use of trauma transfusion pathways for blood component transfusion in the civilian population: a systematic review and meta‐analysis. Transfus Med. 2012;22:156–66. [DOI] [PubMed] [Google Scholar]
- 15. Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Digital Solution Foundry and EPPI‐Centre . EPPI‐mapper, version 1.2.2. London, UK: EPPI‐Centre, UCL Social Research Institute, University College London; 2021. [Google Scholar]
- 17. Lieberman L, Greinacher A, Murphy MF, Bussel J, Bakchoul T, Corke S, et al. Fetal and neonatal alloimmune thrombocytopenia: recommendations for evidence‐based practice, an international approach. Br J Haematol. 2019;185:549–62. [DOI] [PubMed] [Google Scholar]
- 18. Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2016;176:365–94. [DOI] [PubMed] [Google Scholar]
- 19. Kozek‐Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017;34:332–95. [DOI] [PubMed] [Google Scholar]
- 20. Kumar A, Mhaskar R, Grossman BJ, Kaufman RM, Tobian AA, Kleinman S, et al. Platelet transfusion: a systematic review of the clinical evidence. Transfusion. 2015;55:1116–27; quiz 5. [DOI] [PubMed] [Google Scholar]
- 21. Schiffer CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36:283–99. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Riyami AZ, Jug R, La Rocca U, Keshavarz H, Landry D, Shehata N, et al. Quality of evidence‐based guidelines for platelet transfusion and use: a systematic review. Transfusion. 2021;61:948–58. [DOI] [PubMed] [Google Scholar]
- 23. Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curry N, Davenport R, Pavord S, Mallett S, Kitchen DP, Klein AA, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guideline. Br J Haematol. 2018;182:789–806. [DOI] [PubMed] [Google Scholar]
- 25. Bugaev N, Como JJ, Golani G, Freeman JJ, Sawhney JS, Vatsaas CJ, et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;89:999–1017. [DOI] [PubMed] [Google Scholar]
- 26. Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:605–17. [DOI] [PubMed] [Google Scholar]
- 27. Moore L, Tardif PA, Lauzier F, Bérubé M, Archambault P, Lamontagne F, et al. Low‐value clinical practices in adult traumatic brain injury: an umbrella review. J Neurotrauma. 2020;37:2605–15. [DOI] [PubMed] [Google Scholar]
- 28. Synnot A, Bragge P, Lunny C, Menon D, Clavisi O, Pattuwage L, et al. The currency, completeness and quality of systematic reviews of acute management of moderate to severe traumatic brain injury: a comprehensive evidence map. PLoS One. 2018;13:e0198676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curry N, Stanworth S, Hopewell S, Doree C, Brohi K, Hyde C. Trauma‐induced coagulopathy—a review of the systematic reviews: is there sufficient evidence to guide clinical transfusion practice? Transfus Med Rev. 2011;25:217–231.e2. [DOI] [PubMed] [Google Scholar]
- 30. Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007;147:224–33. [DOI] [PubMed] [Google Scholar]
- 31. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374:86–9. [DOI] [PubMed] [Google Scholar]
- 32. Hoffmann F, Allers K, Rombey T, Helbach J, Hoffmann A, Mathes T, et al. Nearly 80 systematic reviews were published each day: observational study on trends in epidemiology and reporting over the years 2000‐2019. J Clin Epidemiol. 2021;138:1–11. [DOI] [PubMed] [Google Scholar]
- 33. Siontis KC, Ioannidis JPA. Replication, duplication, and waste in a quarter million systematic reviews and meta‐analyses. Circ Cardiovasc Qual Outcomes. 2018;11:e005212. [DOI] [PubMed] [Google Scholar]
- 34. Snilstveit B, Vojtkova M, Bhavsar A, Gaarder M. Evidence gap maps? A tool for promoting evidence‐informed policy and prioritizing future research. Washington, DC: The World Bank; 2013. [Google Scholar]
- 35. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Adelaide, Australia: JBI; 2020. [Google Scholar]
- 38. Mitra B, O'Reilly G, Cameron PA, Zatta A, Gruen RL. Effectiveness of massive transfusion protocols on mortality in trauma: a systematic review and meta‐analysis. ANZ J Surg. 2013;83:918–23. [DOI] [PubMed] [Google Scholar]
- 39. Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;2018:CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kovalic AJ, Khan MA, Malaver D, Whitson MJ, Teperman LW, Bernstein DE, et al. Thromboelastography versus standard coagulation testing in the assessment and reversal of coagulopathy among cirrhotics: a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol. 2020;32:291–302. [DOI] [PubMed] [Google Scholar]
- 41. Bianchi P, Beccaris C, Norbert M, Dunlop B, Ranucci M. Use of coagulation point‐of‐care tests in the Management of Anticoagulation and Bleeding in Pediatric Cardiac Surgery: a systematic review. Anesth Analg. 2020;130:1594–604. [DOI] [PubMed] [Google Scholar]
- 42. Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point‐of‐care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost‐effectiveness analysis. Health Technol Assess. 2015;19:v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McQuilten ZK, Crighton G, Brunskill S, Morison JK, Richter TH, Waters N, et al. Optimal dose, timing and ratio of blood products in massive transfusion: results from a systematic review. Transfus Med Rev. 2018;32:6–15. [DOI] [PubMed] [Google Scholar]
- 44. Estcourt LJ, Desborough M, Hopewell S, Doree C, Stanworth SJ. Comparison of different platelet transfusion thresholds prior to insertion of central lines in patients with thrombocytopenia. Cochrane Database Syst Rev. 2015;12:CD011771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Estcourt LJ, Stanworth S, Doree C, Trivella M, Hopewell S, Blanco P, et al. Different doses of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev. 2015;10:CD010984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Estcourt LJ, Stanworth SJ, Doree C, Hopewell S, Trivella M, Murphy MF. Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev. 2015;11:Cd010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Estcourt LJ, Malouf R, Hopewell S, Trivella M, Doree C, Stanworth SJ, et al. Pathogen‐reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev. 2017;7:CD009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011;12:CD009052. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009052.pub2/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Narayan S (Ed) D. Poles et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2020 Annual SHOT Report. Available from: https://www.shotuk.org/wp‐content/uploads/myimages/Interactive_SHOT‐REPORT‐2020_V2.1.pdf.
- 50. Levy JH, Neal MD, Herman JH. Bacterial contamination of platelets for transfusion: strategies for prevention. Crit Care. 2018;22:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saini A, West AN, Harrell C, Jones TL, Nellis ME, Joshi AD, et al. Platelet transfusions in the PICU: does disease severity matter? Pediatr Crit Care Med. 2018;19:e472–e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1‐S6. Supporting information.


