Abstract
Background
The objective of this study was to evaluate the safety and efficacy of nab‐paclitaxel, trastuzumab, and pertuzumab as neoadjuvant therapy (NAT) in patients with human epidermal growth factor receptor 2 HER2+ breast cancer (HER2+ BC) to determine pathologic complete response (pCR), invasive disease‐free survival (iDFS), and overall survival.
Methods
Forty‐five patients with HER2+ BC Stages II–III were to be enrolled from 2013 to 2017. Patients were treated with weekly nab‐paclitaxel (100 mg/m2 intravenously), weekly trastuzumab (4 mg/kg loading dose, then 2 mg/kg), and six cycles of pertuzumab (840 mg loading dose, then 420 mg intravenously day 1 every 21 days).
Results
Median follow‐up was 60 months (95% CI, 32.3–55.6) and pCR was 29/45 (64%). The 5‐year iDFS for patients who achieved pCR (N = 29) was 96.3% (95% CI, 76.5–99.5) and non‐pCR patients (N = 16) was 74.3% (95% CI, 39.1–91.0). The 5‐year overall survival (N = 45) was 94.1% (95% CI, 77.6–98.5). Based on hormonal status, the 5‐year iDFS for HR+ pCR patients (N = 14) was 92.3% (95% CI, 56.6–98.9) and for HR− (N = 15) was 100% (p = .3).
Conclusions
This anthracycline/carboplatin‐free regimen with nab‐paclitaxel achieved a pCR rate of 64% in patients with HER2+ BC. The 5‐year iDFS in patients with and without pCR was 96.3% and 74.3%, respectively. The pCR rate is comparable with docetaxel, carboplatin, trastuzumab, and pertuzumab therapy in the NAT setting, but with fewer treatment‐associated toxicities. This finding suggests the possibility of safe avoidance of anthracyclines and carboplatin as components of NAT in patients with HER2+ BC.
Keywords: HER2‐positive, nab‐paclitaxel, neoadjuvant, pertuzumab, primary and locally‐advanced breast cancer, trastuzumab
Short abstract
This anthracycline/carboplatin‐free regimen with nab‐paclitaxel achieved a pathologic complete response rate of 64% in patients with human epidermal growth factor receptor 2 breast cancer (HER2+ BC). This finding suggests the possibility of safe avoidance of anthracyclines and carboplatin as components of neoadjuvant therapy in HER2+ BC patients.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) overexpression occurs in 20% to 25% of patients with breast cancer (BC) and is associated with poor prognosis. 1 Nab‐paclitaxel (100 mg/m2 weekly dose) demonstrated acceptable safety profile and better response rate and progression‐free survival (PFS), particularly in subsets of patients with visceral metastasis when compared with docetaxel in Phase 2 studies. In addition, a Phase 3 study showed improved PFS with nab‐paclitaxel every 3 weeks compared with paclitaxel every 3 weeks as first‐line treatment of patients with metastatic BC, with less neurotoxicity and hypersensitivity. 2 , 3 Furthermore, the ION 04‐012 randomized phase 2 study compared nab‐paclitaxel with or without trastuzumab in first‐line treatment of patients with metastatic breast cancer (MBC) and demonstrated an overall response rate of 52.4% in HER2‐overexpressing patients with median PFS of 14.5 months, which includes HER2 nonoverexpressing patients. 4
The addition of trastuzumab to chemotherapy significantly improved disease‐free survival (DFS) and overall survival (OS) in the adjuvant setting. 5 Pertuzumab in combination with trastuzumab showed synergistic inhibition of BC cells overexpressing HER2. 6 , 7 , 8 The Phase 3 CLEOPATRA study established the addition of pertuzumab to docetaxel and trastuzumab as first‐line therapy in HER2‐positive MBC (HER2+ MBC). 9 , 10 , 11 , 12 The combination of pertuzumab, trastuzumab, and docetaxel had increased PFS at 18.4 months compared with trastuzumab and docetaxel at 12.4 months, with no increase in cardiotoxicity when given as first‐line treatment for MBC. 11 , 12 Long‐term follow‐up revealed significant improvement in OS with first‐line treatment of pertuzumab, trastuzumab, and docetaxel for patients with HER2+ MBC, with a 16.3‐month median improvement (40.8 vs. 57.1 months). 13 In addition, the activity and safety when pertuzumab and trastuzumab were combined with weekly paclitaxel have also been reported, supporting such combination for patients with HER2+ MBC. 14
A series of trials tested the feasibility and efficacy (as defined by pathologic complete response [pCR] rate at surgery) of dual targeting neoadjuvant therapy (NAT) in patients with HER2+ locally advanced BC. Based on the positive outcome of such trials including the Neosphere trial (pCR rate of 45.8%), the Food and Drug Administration approved the use of neoadjuvant dual targeting therapy with trastuzumab, pertuzumab, and a docetaxel. 15 Five‐year PFS, DFS, and safety was reported and suggested that pCR could predict the long‐term outcome in patients with early‐stage HER2+ BC. 16
Because docetaxel requires premedication with dexamethasone to decrease third‐space fluid retention, we initiated a clinical trial of nab‐paclitaxel with trastuzumab and pertuzumab as an alternative to docetaxel in patients with locally advanced HER2+ BC. In addition, we planned to deescalate NAT by avoiding the use of anthracycline and other chemotherapeutic agents. The objectives of this study were to evaluate the safety and efficacy of nab‐paclitaxel, trastuzumab, and pertuzumab in patients with HER2+ BC to determine the pCR, invasive DFS (iDFS), and OS including estrogen receptor–positive (ER+) and progesterone receptor–positive (PR+) patients.
MATERIALS AND METHODS
Patients
The study eligibility included patients with HER2+ BC, aged ≥18 years, Eastern Cooperative Oncology Group performance status 0–2, institutional normal cardiac ejection fraction, and confirmation of HER2 positivity defined as 3+ on immunohistochemistry or gene amplification on fluorescence in situ hybridization (>2.0). The City of Hope institutional review board approved the study and all patients provided written voluntary consent to participate (NCT01730833). A total of 45 patients with biopsy‐confirmed primary HER2+ BC were enrolled from 2013 to 2017. Supportive care was in line with standard practice, and American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines were followed.
Study design and treatment
Patients were recruited from the medical oncology clinics at City of Hope National Medical Center. Before initiation of therapy, workup included a computed tomography scan of the chest, abdomen and pelvis, and bone. Each 21‐day cycle of treatment consisted of nab‐paclitaxel 100 mg/m2 intravenously weekly, with trastuzumab 4 mg/kg loading dose, then 2 mg/kg intravenously weekly, and pertuzumab 840 mg loading dose followed by 420 mg intravenously every 21 days until disease progression, unmanageable toxicity, physician or patient request to discontinue therapy, or study termination by sponsors (Figure 1). Toxicity assessment was performed before each cycle. Patients received six cycles of treatment before surgery. Response to therapy was assessed on the pathologic specimen obtained at definitive surgery, with pCR defined as no residual invasive disease in breast and lymph nodes with or without residual in situ carcinoma (ypTis). iDFS was defined as occurrence of any ipsilateral or contralateral invasive breast cancer, regional or distant recurrence of breast cancer.
FIGURE 1.
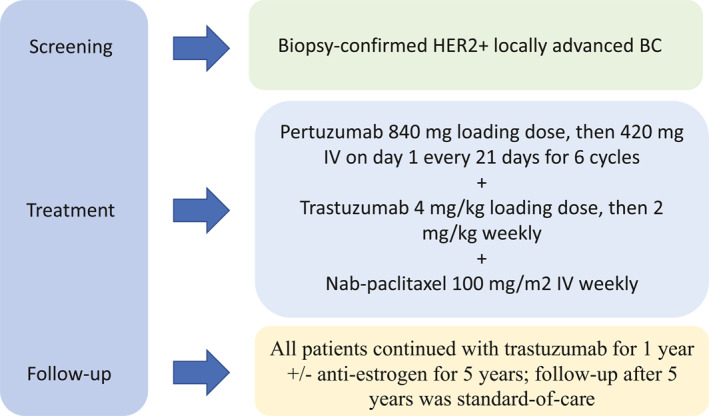
Study summary including patient enrollment, protocol therapy, and patient follow‐up.
Statistical methods
The original protocol included a separate cohort for patients with metastatic disease; however, accrual to that cohort was limited (n = 18) and here we report only the neoadjuvant cohort. In the neoadjuvant setting, a pCR rate of 39.3% was observed with the pertuzumab, trastuzumab, and docetaxel. 15 This study was designed to use nab‐paclitaxel on the proposed dose and schedule because of its lower toxicity. With 40 neoadjuvant cases, the probability of a true discouraging neoadjuvant pCR rate of 29.3% exceeding the benchmark of 39.3% (16 or more) is 10% (type I error), and the probability of a true neoadjuvant pCR rate of 49.3% resulting in an observation below 39.3% (15 or less) is less than 10% (>90% power). This design is equivalent to testing the null hypothesis H0: pCR rate is 29.3% against an alternative H1: pCR rate is 49.3%, with a type I error (one‐sided) of 10% and a type II error less than 10% (at least 90% power). Because of encouraging results on the neoadjuvant arm (exceeding the required pCR number with acceptable toxicity), and poor accrual for the metastatic arm, accrual to the neoadjuvant arm was increased beyond 40 patients with sponsor approval to expand on the neoadjuvant experience.
RESULTS
Patients
Patient characteristics are summarized in Table 1 and study summary is shown in Figure 1. Median age was 56 (31–78) years. One of 45 patients (2%) was stage I, 16/45 (36%) were stage IIA, 14/45 (31%) were stage 2B, 10/45 (22%) were stage IIIA, 1/45 (2%) were stage 3B, and 3/45 (7%) were stage 3C. The patients received a median of six cycles of treatment (1–6), and pCR was documented in 29/45 (64%) patients, with pCR of 15/20 (75%) hormone receptor negative (HR−) and 14/25 (56%) hormone receptor positive (ER+ and/or PR+, HR+) (Table 2). One non‐pCR patient received doxorubicin and cyclophosphamide chemotherapy in the adjuvant setting. One patient developed metastatic disease and received trastuzumab deruxtecan (Enhertu), and one patient received neratinib but developed toxicity and stopped after 1 month. The median follow‐up was 60 months (95% CI, 32.3–55.6). The 5‐year iDFS for patients who achieved pCR (n = 29) was 96.3% (95% CI, 76.5–99.5) and for non‐pCR patients (n = 16) was 74.3% (95% CI, 39.1–91.0) (p = .10) (Figure 2A). The 5‐year OS for pCR patients (n = 29) was 94.1% (95% CI, 65.0–99.1) and for non‐pCR patients (n = 16) was 93.3% (95% CI, 61.3–99.0) (p = .68) (Figure 2B). Based on hormonal status, the 5‐year iDFS for HR+ pCR patients (n = 14) was 92.3% (95% CI, 56.6–98.9) and for HR− pCR patients (n = 15) was 100% (p = .3) (Figure 3A). The 5‐year iDFS for HR+ non‐pCR patients (n = 11) was 76.2% (95% CI, 33.2–93.5) and for HR− non‐pCR patients (n = 5) was 66.7% (95% CI, 5.4–94.5) (p = 1.0) (Figure 3B). The 5‐year iDFS for ER+ and PR+ pCR patients (n = 8) was 100.0%; ER+/PR− pCR patients (n = 6) was 83.3% (95% CI, 27.3–97.5); and ER−/PR− pCR patients (n = 15) was 100% (p = .2) (Figure 4A). The 5‐year iDFS for ER+/PR+ non‐pCR patients (n = 8) was 80.0% (95% CI, 20.4–96.9); ER+/PR− non‐pCR patients (n = 3) was 66.7% (95% CI, 5.4–94.5); and ER−/PR− non‐pCR patients (n = 5) was 66.7% (95% CI, 5.4–94.5) (p = .9) (Figure 4B).
TABLE 1.
Patient and disease characteristics
| Patients (N = 45) | |
|---|---|
| Age, median (range), years | 56 (31–78) |
| Race/ethnicity, N (%) | |
| Hispanic | 19 (42) |
| Non‐Hispanic White | 17 (38) |
| Asian | 7 (16) |
| African American | 1 (2) |
| Unknown | 1 (2) |
| ECOG performance status, N (%) | |
| 0 | 28 (62) |
| 1 | 17 (38) |
| Menopausal status, N (%) | |
| Postmenopause | 24 (53) |
| Premenopause | 19 (42) |
| Perimenopause | 2 (4) |
| Histology type, N (%) | |
| Infiltrating ductal carcinoma | 45 (100) |
| Tumor stage at initial diagnosis, N (%) a | |
| I | 1 (2) b |
| IIA | 16 (36) |
| IIB | 14 (31) |
| IIIA | 10 (22) |
| IIIB | 1 (2) |
| IIIC | 3 (7) |
| Receptor status at initial diagnosis, N (%) | |
| HR− HER2+ | 20 (44) |
| HR+ HER2+ | 25 (56) |
| Prior radiation, N (%) | |
| No | 45 (100) |
Abbreviations: AJCC, American Joint Commission on Cancer; ECOG, Eastern Cooperative Oncology Group; HER2+, human epidermal growth factor receptor 2 positive; HR–, hormone receptor negative; HR+, hormone receptor positive.
AJCC 7th edition.
Treating physician choice.
TABLE 2.
Median follow‐up, dose delay, and dose reduction
| Patients (N = 45) | |
|---|---|
| Median follow‐up (95% CI), months | 50.0 (32.3–55.6) |
| Median treatment cycles completed (range) | 6 (1–6) |
| Median treatment cycles delayed (range) | 0 (0–2) |
| Patients with >1 cycle delayed | 4 (9%) |
| Median treatment cycles reduced (range) | 3 (0–6) |
| Patients with >1 cycle reduced | 32 (71%) |
| pCR | 29/45 (64%) |
| HR− HER2+ | 15/20 (75%) |
| HR+ HER2+ | 14/25 (56%) |
Abbreviations: HER2+, human epidermal growth factor receptor 2 positive; HR–, hormone receptor negative; pCR, pathologic complete response
FIGURE 2.
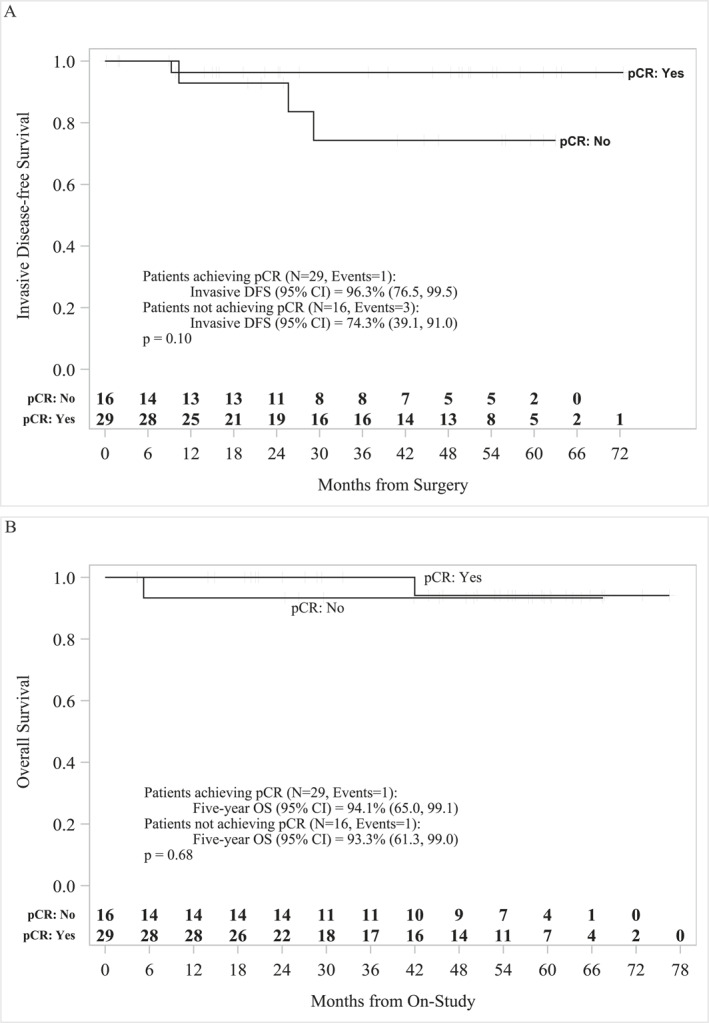
Kaplan–Meier survival analysis for pCR vs. non‐pCR patients. (A) The 5‐year iDFS for pCR patients (N = 29) was 96.3% (95% CI, 76.5–99.5) and for non‐pCR patients (N = 16) was 74.3% (95% CI, 39.1–91.0). (B) The 5‐year OS for pCR patients (N = 29) was 94.1% (95% CI, 65.0–99.1) and for non‐pCR (N = 16) patients was 93.3% (95% CI, 61.3–99.0). iDFS indicates invasive disease‐free survival; OS, overall survival; pCR, pathologic complete response.
FIGURE 3.
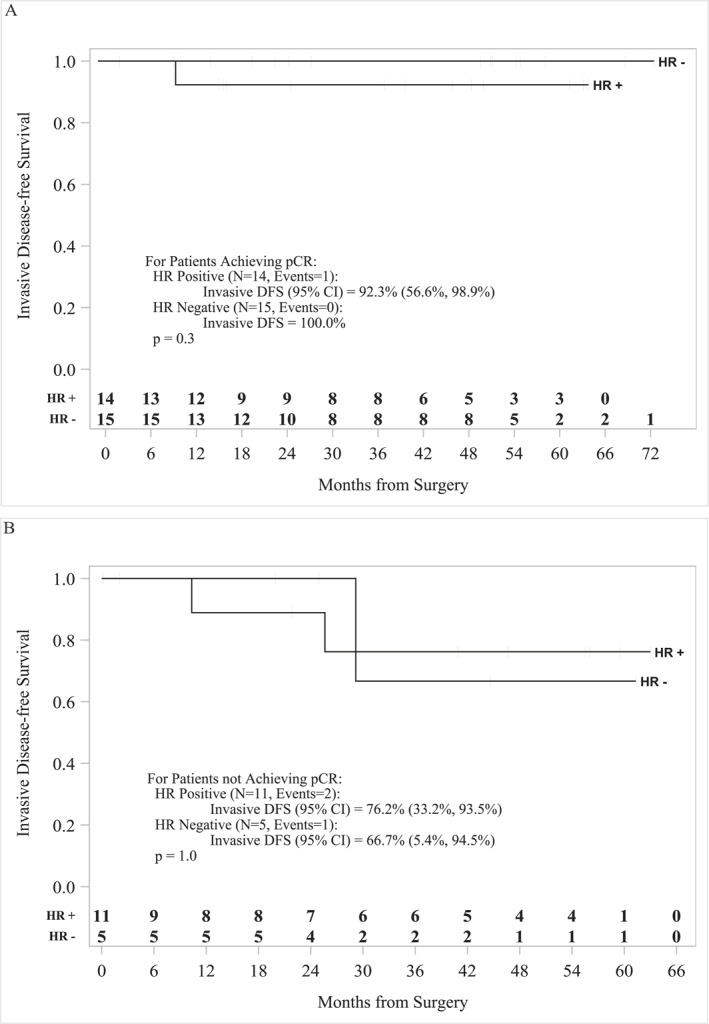
Kaplan–Meier survival analysis for HR+ and HR− patients. (A) The 5‐year iDFS for HR+ patients achieving pCR (N = 14) was 92.3% (95% CI, 56.6–98.9) and for HR− patients (N = 15) was 100% (p = .3). (B) The 5‐year iDFS for HR+ non‐pCR patients (N = 11) was 76.2% (95% CI, 33.2–93.5) and for HR− non‐pCR patients (N = 5) was 66.7% (95% CI, 5.4–94.5) (p = 1.0). HR– indicates hormone receptor negative; HR+, hormone receptor positive; iDFS, invasive disease‐free survival; pCR, pathologic complete response.
FIGURE 4.
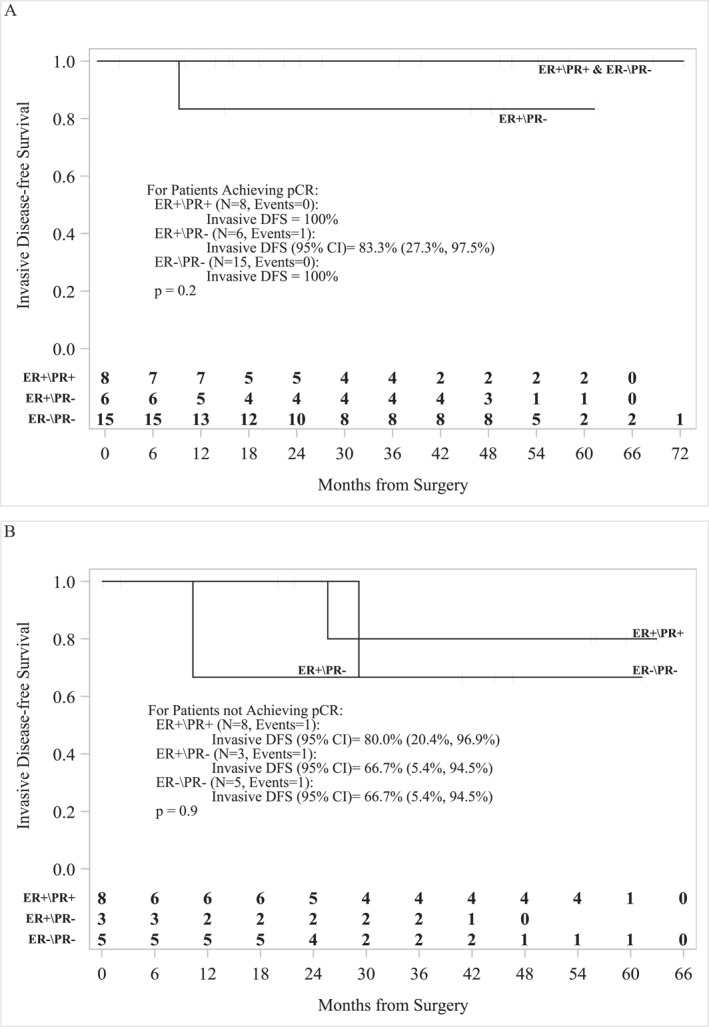
Kaplan–Meier survival analysis for patients stratified by receptor status. (A) The 5‐year iDFS for ER+/PR+ pCR patients (N = 8) was 100.0%; ER+/PR− pCR patients (N = 6) was 83.3% (95% CI, 27.3–97.5); and ER−/PR− pCR patients (N = 15) was 100% (p = .2). (B) The 5‐year iDFS for ER+/PR+ non‐pCR patients (N = 8) was 80.0% (95% CI, 20.4–96.9); ER+/PR− non‐pCR patients (N = 3) was 66.7% (95% CI, 5.4–94.5); and ER−/PR− non‐pCR patients (N = 5) was 66.7% (95% CI, 5.4–94.5) (p = .9). ER– indicates estrogen receptor negative; ER+, estrogen receptor positive; iDFS, invasive disease‐free survival; pCR, pathologic complete response.
Adverse events
A total of 4/45 (9%) patients had >1 cycle delayed and 32/45 (71%) patients had >1 cycle reduced (Table 2). Grade (G) 3 AEs (>2 patients) included 7/45 (16%) of patients with hypertension; 4/45 (9%) with anemia; and 2/45 (4%) with diarrhea, elevated liver enzymes, fatigue, or rash. G4 AEs included 1/45 (2%) dyspnea (Table 3). AEs of interest included one (2%) G3 cardiac disorder and 26 (58%) G1 and eight (18%) G2 chemotherapy‐induced peripheral neuropathy. No febrile neutropenia was reported.
TABLE 3.
All treatment‐related adverse events (with at least one grade 2 or above)
| Patients (N = 45) | ||||
|---|---|---|---|---|
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Bone marrow | ||||
| Anemia | 25 (56%) | 11 (24%) | 4 (9%) | |
| Leukopenia | 22 (49%) | 4 (9%) | 1 (2%) | |
| Neutropenia | 7 (16%) | 5 (11%) | 1 (2%) | |
| Lymphopenia | 2 (4%) | 2 (4%) | ||
| Gastrointestinal | ||||
| Nausea/vomiting | 29 (64%) | 2 (4%) | ||
| Dyspepsia | 3 (7%) | |||
| Diarrhea | 18 (40%) | 7 (16%) | 2 (4%) | |
| Constipation | 6 (13%) | 1 (2%) | ||
| Mucositis oral | 6 (13%) | 1 (2%) | ||
| Electrolytes | ||||
| Hyponatremia | 11 (24%) | 1 (2%) | ||
| Hypokalemia | 5 (11%) | 2 (4%) | ||
| Hypophosphatemia | 4 (9%) | 1 (2%) | ||
| Hypoalbuminemia | 1 (2%) | 2 (4%) | ||
| Hypomagnesemia | 1 (2%) | |||
| Cardiovascular | ||||
| Hypertension | 6 (13%) | 10 (22%) | 7 (16%) | |
| Cardiac disorders | 1 (2%) | |||
| Atrial fibrillation | 1 (2%) | |||
| Infection | ||||
| Urinary tract infection | 2 (4%) | |||
| Nail infection | 3 (7%) | |||
| Tooth infection | 1 (2%) | |||
| Pain | ||||
| Back pain | 2 (4%) | 1 (2%) | ||
| Respiratory | ||||
| Dyspnea | 1 (2%) | 1 (2%) | ||
| General | ||||
| Peripheral sensory neuropathy | 26 (58%) | 8 (18%) | ||
| Fatigue | 26 (58%) | 10 (22%) | 2 (4%) | |
| Rash acneiform | 19 (42%) | 3 (7%) | 1 (2%) | |
| Alanine transaminase | 16 (36%) | 3 (7%) | 2 (4%) | |
| Edema of the limbs | 14 (31%) | 1 (2%) | ||
| Skin disorder | 12 (27%) | 3 (7%) | ||
| Alopecia | 8 (18%) | 5 (11%) | ||
| Rash maculopapular | 7 (16%) | 1 (2%) | 1 (2%) | |
| Irregular menstruation | 1 (2%) | 1 (2%) | ||
| Metabolism and nutrition disorder | 1 (2%) | 1 (2%) | ||
| Infusion‐related reaction | 1 (2%) | 6 (13%) | ||
| Paronychia | 1 (2%) | 3 (7%) | ||
| Nail loss | 1 (2%) | 2 (4%) | ||
| Hemorrhoids | 2 (4%) | |||
DISCUSSION
In this study, adding nab‐paclitaxel to trastuzumab and pertuzumab in the neoadjuvant setting achieved a pCR rate of 64.4%, with pCR defined as no residual invasive disease in breast and lymph nodes with or without in situ residual ypTis. This pCR rate favorably compared with the pCR rate of TRYPHANEA study, which was reported as 66.2% (ypTis included). The TRYPHANEA study evaluated docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) regimen versus anthracycline‐based regimen of 5‐fluorouracil, epirubicin, and cyclophosphamide/trastuzumab plus pertuzumab, followed by docetaxel, trastuzumab, and pertuzumab. 17 , 18 Our study's pCR was higher than the pCR of 45.8% reported in the NeoSphere study. 15
A deescalation approach in the treatment of patients with HER2+ BC, both in the neoadjuvant and adjuvant settings, has been studied in clinical trials. Nitz et al. randomized 132 patients (2:1) to NAT trastuzumab and pertuzumab +/− weekly paclitaxel at 80 mg/m2 in a phase 2 study (WSG‐ADAPT) conducted in HR– HER2+ patients. The pCR rate was 78.6% (excluding ypTis) in the trastuzumab and pertuzumab plus paclitaxel treated arm versus 24.4% in the trastuzumab and pertuzumab arm. 19
Neoadjuvant trastuzumab and pertuzumab and chemotherapy (docetaxel + carboplatin) versus trastuzumab emtansine (T‐DM1) + pertuzumab was studied in a phase 3 study by Hurvitz et al. (Kristine Study). 20 The pCR was achieved in 44.4% of patients who received T‐DM1 plus pertuzumab versus 55.7 of patients who received TCHP. Three‐year iDFS was significantly different in favor of pCR, regardless of the neoadjuvant regimens (97.2% vs. 87.4%; hazard ratio, 0.24; 0.09–0.60). We report a longer follow‐up period of 5 years with an iDFS of 96.3% for patients who achieved pCR and 74.3% for patients who did not achieve pCR. The lack of statistical significance in favor of pCR achieved with our deescalated regimen is possibly from the small sample size.
Within the context of the adaptively randomized trials conducted by the I‐SPY consortium, cohorts of patients with HER2+ stage 2 and 3 received either T‐DM1 and pertuzumab (n = 52; pCR rate of 55%), or trastuzumab, pertuzumab, and paclitaxel (n = 45; pCR rate of 56%) in comparison to the control of trastuzumab and paclitaxel (n = 31; pCR rate of 25%) followed by doxorubicin and cyclophosphamide as part of NAT. Three‐year event‐free survivals were 88%, 92%, and 87%, respectively, for the three groups. 21 Although cross‐study comparisons are problematic, the patients in our study presented with somewhat more advanced/higher risk primaries than participants in the earlier studies, based on the intent to enroll predominantly node positive and locally advanced cases. However, the results compare favorably to the outcome of the I‐SPY and the other trials mentioned, and we achieved these results without the inclusion of anthracyclines in the neoadjuvant or adjuvant setting. Our patient population received adjuvant trastuzumab every 3 weeks for a total duration of 52 weeks, and those with HR+ disease were prescribed antiestrogen therapies in line with then‐current practice guidelines. The 5‐year iDFS was numerically lower in patients without pCR (74.3%) compared with patients with pCR (96.3%), but OS was not noticeably different. When this study was conducted, adjuvant TDM‐1 was not a part of the standard of care in patients who did not achieve pCR after NAT. The current practice is to offer patients adjuvant T‐DM1 based on the KATHERINE study results (iDFS 77% with trastuzumab and 88.3% with T‐DM1). 22 Our single‐institutional study was limited by small sample size and nonrandom design. However, the results suggest that patients with pCR had improved iDFS regardless of hormonal status. HR– patients showed a trend toward improved iDFS. We also identified a trend toward worse outcomes in patients with ER+ PR−, especially in those without pCR possibly because of the small numbers of patients. Our prospective single institutional study compared favorably with similar trials.
CONCLUSION
This anthracycline/carboplatin‐free regimen, which included nab‐paclitaxel, trastuzumab, and pertuzumab, achieved a pCR rate of 64% in patients with HER2+ BC with fewer treatment‐related toxicities in comparison to both anthracycline‐ and docetaxel/carboplatin–containing regimens. Our patients achieved a pCR rate comparable to TCHP therapy in the NAT setting, and those with pCR achieved an iDFS of 96.3% versus patients without pCR 74.3%. These results also compare favorably with the outcome following T‐DM1 and paclitaxel, or pertuzumab, trastuzumab, and paclitaxel followed by anthracycline and cyclophosphamide. Our finding suggests that we may be able to safely avoid anthracyclines and carboplatin as components of NAT for patients with HER2+ BC.
AUTHOR CONTRIBUTIONS
Sayeh M. Lavasani: Formal analysis, investigation, methodology, project administration, resources, writing – original draft, and writing – review and editing. George Somlo: Conception, supervision, data curation, funding acquisition, and writing – review and editing. Susan E. Yost: Data curation, writing – original draft, and writing – review and editing. Paul H. Frankel: Software, formal analysis, validation, and visualization. Christopher Ruel: Software, formal analysis, validation, and visualization. Yujie Cui: Software, formal analysis, validation, and visualization. Mireya Murga: Project administration and supervision. Aileen Tang: Project administration and supervision. Norma Martinez: Project administration and supervision. Laura Kruper: Project administration and supervision. Lusine Tumyan: Project administration and supervision. Daniel Schmolze: Project administration and supervision. Christina Yeon: Project administration and supervision. Yuan Yuan: Project administration and supervision. James R. Waisman: Project administration and supervision. Joanne Mortimer: Formal analysis, investigation, methodology, project administration, resources, writing – original draft, and writing – review and editing.
CONFLICTS OF INTEREST
Yuan Yuan has contracted research sponsored by Merck, Eisai, Novartis, Puma, Genentech, Celgene, and Pfizer; is a consultant for Puma, Pfizer, and Immunomedics; and is on the Speakers Bureau for Eisai, Novartis, Genentech, AstraZeneca, Daiichi Sankyo, and Immunomedics. The other authors made no disclosures.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The protocol and amendments were approved by City of Hope’s institutional review board–approved protocol 12147. All procedures were performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. Privacy rights were observed and written informed consent was obtained from all participants of this study under institutional review board 12147 and ClinicalTrials.gov (NCT01730833).
ACKNOWLEDGMENTS
The authors gratefully acknowledge Celgene/BMS and Genentech for sponsoring the study and Celgene/BMS for providing study drug. Research reported in this publication includes work performed at the Pathology and Biostatistics Core of City of Hope National Cancer Center, supported by the National Cancer Institute (NCI) under award number P30CA033572.
Lavasani SM, Somlo G, Yost SE, et al. Phase 2 prospective open label study of neoadjuvant nab‐paclitaxel, trastuzumab, and pertuzumab in patients with HER2‐positive primary breast cancer. Cancer. 2023;129(5):740‐749. doi: 10.1002/cncr.34589
REFERENCES
- 1. Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2‐positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9(1):16‐32. doi: 10.1038/nrclinonc.2011.177 [DOI] [PubMed] [Google Scholar]
- 2. O'Shaughnessy J, Gradishar WJ, Bhar P, Iglesias J. Nab‐paclitaxel for first‐line treatment of patients with metastatic breast cancer and poor prognostic factors: a retrospective analysis. Breast Cancer Res Treat. 2013;138(3):829‐837. doi: 10.1007/s10549-013-2447-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression‐free survival with nab‐paclitaxel compared with docetaxel as first‐line therapy for metastatic breast cancer. J Clin Oncol. 2009;27(22):3611‐3619. doi: 10.1200/jco.2008.18.5397 [DOI] [PubMed] [Google Scholar]
- 4. Mirtsching B, Cosgriff T, Harker G, Keaton M, Chidiac T, Min M. A phase II study of weekly nanoparticle albumin‐bound paclitaxel with or without trastuzumab in metastatic breast cancer. Clin Breast Cancer. 2011;11(2):121‐128. doi: 10.1016/j.clbc.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 5. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2‐positive breast cancer. N Engl J Med. 2011;365(14):1273‐1283. doi: 10.1056/nejmoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nahta R, Hung MC, Esteva FJ. The HER‐2‐targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64(7):2343‐2346. doi: 10.1158/0008-5472.can-03-3856 [DOI] [PubMed] [Google Scholar]
- 7. Fuentes G, Scaltriti M, Baselga J, Verma CS. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res. 2011;13(3):R54. doi: 10.1186/bcr2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28(7):1138‐1144. doi: 10.1200/jco.2009.24.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2‐positive metastatic breast cancer. N Engl J Med. 2015;372(8):724‐734. doi: 10.1056/nejmoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2‐positive metastatic breast cancer (CLEOPATRA): end‐of‐study results from a double‐blind, randomised, placebo‐controlled, phase 3 study. Lancet Oncol. 2020;21(4):519‐530. [DOI] [PubMed] [Google Scholar]
- 11. Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2‐positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol. 2013;14(6):461‐471. doi: 10.1016/s1470-2045(13)70130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109‐119. doi: 10.1056/nejmoa1113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swain S, Kim S, Cortes J, et al. 350O_PR ‐ final overall survival (Os) analysis from the Cleopatra Study of first‐line (1L) pertuzumab (Ptz), trastuzumab (T), and docetaxel (D) in patients (Pts) with Her2‐positive metastatic breast cancer (Mbc). Ann Oncol. 2014;25:v1. doi: 10.1093/annonc/mdu438.7 [DOI] [Google Scholar]
- 14. Datko FM, D'Andrea G, Dickler MN, et al. Phase II study of pertuzumab, trastuzumab, and weekly paclitaxel in patients with HER2‐overexpressing metastatic breast cancer (MBC). J Clin Oncol. 2013;31(15):1. doi: 10.1200/jco.2013.31.15_suppl.606 23129739 [DOI] [Google Scholar]
- 15. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2‐positive breast cancer (NeoSphere): a randomised multicentre, open‐label, phase 2 trial. Lancet Oncol. 2012;13(1):25‐32. doi: 10.1016/s1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 16. Gianni L, Pienkowski T, Im YH, et al. 5‐year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early‐stage HER2‐positive breast cancer (NeoSphere): a multicentre, open‐label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791‐800. doi: 10.1016/s1470-2045(16)00163-7 [DOI] [PubMed] [Google Scholar]
- 17. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline‐containing and anthracycline‐free chemotherapy regimens in patients with HER2‐positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278‐2284. doi: 10.1093/annonc/mdt182 [DOI] [PubMed] [Google Scholar]
- 18. Schneeweiss A, Chia S, Hickish T, et al. Long‐term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline‐containing and anthracycline‐free chemotherapy regimens in patients with HER2‐positive early breast cancer. Eur J Cancer (Oxford, England: 1990). 2018;89:27‐35. doi: 10.1016/j.ejca.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 19. Nitz UA, Gluz O, Christgen M, et al. De‐escalation strategies in HER2‐positive early breast cancer (EBC): final analysis of the WSG‐ADAPT HER2+/HR− phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann Oncol. 2017;28(11):2768‐2772. doi: 10.1093/annonc/mdx494 [DOI] [PubMed] [Google Scholar]
- 20. Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2‐positive breast cancer: three‐year outcomes from the Phase III KRISTINE Study. J Clin Oncol. 2019;37(25):2206‐2216. doi: 10.1200/jco.19.00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clark AS, Yau C, Wolf DM, et al. Neoadjuvant T‐DM1/pertuzumab and paclitaxel/trastuzumab/pertuzumab for HER2(+) breast cancer in the adaptively randomized I‐SPY2 trial. Nat Commun. 2021;12(1):6428. doi: 10.1038/s41467-021-26019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geyer C Jr, Huang C, Mano M, et al. Phase III study of trastuzumab emtansine (T‐DM1) vs trastuzumab as adjuvant therapy in patients with HER2‐positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2‐targeted therapy including trastuzumab: primary results from KATHERINE. Paper presented at: Cancer Research2019.


