Abstract
Yield losses due to nutrient deficiency are estimated as the primary cause of the yield gap worldwide. Understanding how plant roots perceive external nutrient status and elaborate morphological adaptations in response to it is necessary to develop reliable strategies to increase crop yield. In the last decade, reactive oxygen species (ROS) were shown to be key players of the mechanisms underlying root responses to nutrient limitation. ROS contribute in multiple ways to shape the root system in response to nutritional cues, both as direct effectors acting on cell wall architecture and as second messengers in signalling pathways. Here, we review the mutual interconnections existing between perception and signalling of the most common forms of the major macronutrients (nitrogen, phosphorus and potassium), and ROS in shaping plant root system architecture. We discuss recent advances in dissecting the integration of these elements and their impact on morphological traits of the root system, highlighting the functional ductility of ROS and enzymes implied in ROS metabolism, such as class III peroxidases.
Keywords: class III peroxidase, nitrate, phosphate, potassium, root development, ROS
Summary statement
Reactive oxygen species (ROS) are major regulators of root development and root plasticity in response to stress, by acting both as direct effectors by affecting the cell wall and as signalling molecules involved in signalling pathways. Class III peroxidases, as well as their upstream activator (e.g., UPBEAT1) or enzymes producing its substrates (e.g., NADPH oxidases), play a major role in controlling ROS pattern and thus their functionality in either cell wall stiffening or loosening. Recent advances are uncovering the importance of such functions in the case of nutrient stress, and attribute to ROS a pivotal role in determining root phenotype.
1. INTRODUCTION
The root system is often overlooked as the front line in the battle to increase crop yield or resistance to stress. Drought, heavy metal, salinity, waterlogging and soil‐borne pests are a few examples of extremely relevant challenges in both agriculture and ecology contexts. Nutrient limitation, however, is among the most important challenges, particularly as arable land is increasingly scarce, resulting in a need to expand the environmental competency of crops beyond their present limitations. One of the major levers to improve nutrient acquisition is the plasticity of root system architecture. Reactive oxygen species (ROS) and reduction/oxidation (redox) processes are acknowledged as important enablers of root system plasticity, however, their interactions with nutrient signalling in this context have received little attention. Another major influence on nutrient acquisition, the capacity of nutrient transporters, has been recently reviewed (Yadav et al., 2021) and will not be considered here.
Following germination, root growth through apical cell division and elongation is driven by primary tropic cues, notably gravity, water availability and physical pressure or touch (Muthert et al., 2020). As the root system develops, it encounters a patchy nutrient distribution, influenced by organic input and microbial decomposition (Hodge, 2004; Morris et al., 2017). Plants have therefore evolved nutrient sensing (Gojon et al., 2009; Nath & Tuteja, 2016) to enable root function to adapt to heterogeneity by modifying the direction of growth, branching, surface area and branch angle. Other cues, however, are also heterogenous in space and time, including water, oxygen and physical pressure (Considine et al., 2017; Dietrich, 2018). ROS and redox signalling are a common feature of plant responses to these cues, and this enables environmental complexity to be integrated effectively, resulting in an optimal root system architecture (Eljebbawi et al., 2021). The role of phytohormones in this calibration of form is also considerable, but has previously received attention (Sharma et al., 2021; Takahashi et al., 2009), and will not be considered in detail here.
This review highlights the interactions between ROS and nutrient perception in shaping root architecture. We focus on the three most well‐studied macronutrients, namely nitrogen (N), phosphorus (P), and potassium (K), also known as the NPK triad, by focusing on their most assimilated forms by roots: nitrate (NO3 −), inorganic phosphate (Pi), and potassium cation (K+). We attempt to describe a framework based on recent research advances, where root response to nutrient scarcity is mediated by changes in ROS signalling and metabolism. For detailed overviews of the discrete roles of ROS and nutrients in root plasticity, the reader is referred to other reviews (Eljebbawi et al., 2021; Huang & Zhang, 2020; Mase & Tsukagoshi, 2021; Shahzad & Amtmann, 2017; Sustr et al., 2019; Undurraga et al., 2017).
2. ROS METABOLISM AND SIGNALLING PLAY MULTIPLE ROLES IN ROOT DEVELOPMENT
ROS have long been viewed as toxic compounds, generated as byproducts of metabolism; their overaccumulation leads to oxidative damage and cell disfunction (Desikan et al., 2005; Farooq et al., 2019). Antioxidants such as glutathione and ascorbate, which are the most abundant soluble antioxidants, together with the operation of redox metabolism, enables recycling and the precise control of ROS abundance in space and time. Therefore, ROS are increasingly becoming recognised as having important physiological roles in plant development and stress response, notably when they are produced as primary products in apoplast and cell wall (Mittler, 2017). For example, ROS gradients were shown to control root and root hair elongation (Chu et al., 2021; Foreman et al., 2003; Liszkay et al., 2004; Mangano et al., 2017; Tognetti et al., 2017; Trevisan et al., 2019; Tsukagoshi et al., 2010). Three main ROS produced in the apoplast, superoxide anion radical (O2∙‐), hydroxyl radical (∙OH), and hydrogen peroxide (H2O2), participate in these processes. In this review, we consider ROS functions in root development as functioning in one of two primary roles: direct effects and signalling (indirect effects) (Figure 1).
Figure 1.
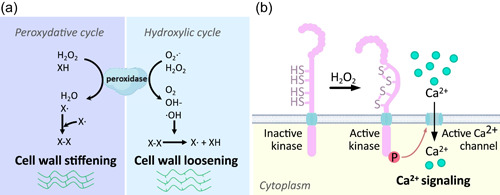
Reactive oxygen species (ROS) can affect root development in direct and indirect ways. (a) Direct mode of action: class III peroxidases can directly act on cell wall polymers (X) through their peroxidative and hydroxylic cycles. In the first case, electron transfer from H2O2 to X favours polymer crosslinking and thus cell wall stiffening, while in the second case hydroxyl radicals (∙OH) can break cell wall polymer crosslinks and result in cell wall loosening. (b) Indirect mode of action: the case of the HPCA1 receptor illustrates the role of ROS as signalling molecules. In the presence of H2O2, two pairs of Cys located on the extracellular domain of HPCA1 are oxidised, triggering a conformational change that activates the intracellular kinase domain. This leads to the phosphorylation of adjacent Ca2+ channels and a rapid increase of [Ca2+]cytosol, resulting in the activation of Ca2+‐dependent signalling pathways. [Color figure can be viewed at wileyonlinelibrary.com]
2.1. Enzymes involved in root ROS metabolism in the apoplast
In the apoplast, ROS are produced and/or processed principally by NADPH oxidases, superoxide dismutases (SODs) and class III peroxidases (PODs). NADPH oxidases form a small family of plasma membrane proteins found in plants (10 members in Arabidopsis) which reduce O2 to O2∙‐ in the apoplast using cytosolic NADPH as an electron donor (Chapman et al., 2019; Sagi & Fluhr, 2001). They are homologues of the respiratory burst oxidases found in animals and for this reason, they are also named respiratory burst oxidase homologues (RBOHs). Such enzymes are known to cover important roles in the physiology of all the parts of the root system, especially in lateral roots and root hairs. Early studies in the Arabidopsis mutant rhd2 (root hair‐defective mutant 2), inactive for the RBOH isoform C (RBOHC), showed that RBOHC activity is needed for root hair development where it controls the activation of Ca2+ channels (Foreman et al., 2003). RBOH activity is also responsible for the formation of ROS gradients that drive directional growth in root hairs (E.‐J. Kim et al., 2019; Nestler et al., 2014; Takeda et al., 2008). In lateral roots, ROS generated by RBOHs are required in the emergence stage, where they remodel the tissue layers lying above the lateral root primordia dome (Arthikala & Quinto, 2018; Orman‐Ligeza et al., 2016). In the primary root of Medicago truncatula, RBOH‐dependent ROS increase was shown to trigger cell elongation, while in Arabidopsis primary roots ROS generated through AtRBOHD/F are known to be required for ABA‐dependent growth inhibition (Jiao et al., 2013; Kwak et al., 2003; Zhang et al., 2014). RBOH expression in a cell can be induced by RBOH activity in neighbouring cells, resulting in the propagation of the ROS signal to act as a systemic response. This phenomenon has been denominated the ‘ROS wave’ (Mittler et al., 2011).
The SOD family comprises a small number of isoforms, divided into Mn‐SOD, Fe‐SOD, and Cu/Zn‐SOD based on the ion used as cofactor. SOD catalyzes the dismutation of the majority of apoplastic O2∙‐ to H2O2; a minority of dismutation occurs spontaneously (Podgórska et al., 2017). Recently, MSD2, a protein considered to belong to the Mn‐SOD family, was confirmed to act as an Mn‐SOD but unlike most Mn‐SOD which have mitochondrial localisation, it accumulates in the apoplast of roots of Arabidopsis seedlings (H. Chen et al., 2022). The roots of Arabidopsis seedlings lacking MSD2 showed an altered skotomorphogenesis, such that the onset of root hair growth was delayed, and their location was closer to the root tip than wild type. To date, however, this is the only report of the function of apoplastic SOD in root development; other studies have documented functions for mitochondrial and plastid SOD (Dvořák et al., 2021; Morgan et al., 2008).
Class III PODs form a large family of isoenzymes comprising 73 members in Arabidopsis (Lüthje & Martinez‐Cortes, 2018). They are only present in plants and are in large part addressed to the plasma membrane or cell wall. They can perform different activities such as an oxidative activity producing O2∙‐ at the expense of NADH, a hydroxylic activity producing ∙OH from O2∙‐ and H2O2, and a peroxidative activity eliminating H2O2 using substrates such as cell wall phenolic compounds or auxin (S. Chen & Schopfer, 1999; Passardi et al., 2005; Šukalović et al., 2005; Veljović Jovanović et al., 2018). Although class III PODs can catalyse all these reactions in vitro (Passardi et al., 2004), their activities in planta might be specialised, depending on spatio‐temporal expression of the genes, specificity of the enzymes for the substrates, availability of substrates and regulation by factors such as pH alkalinization, calcium binding, glycosylation or phosphorylation (Cosio & Dunand, 2009; Felle, 2001; Francoz et al., 2015; Mangano et al., 2016; Shigeto & Tsutsumi, 2016). This versatility might be the reason for maintaining such a large family of functional genes, which can be used in multiple ways in a plethora of different stress responses and developmental processes (Eljebbawi et al., 2021; Passardi et al., 2005). POD isoforms present in the root tip were shown to play a crucial role in the control of root ROS patterns and its gradients in the root tip, which in turn determine the transition from a zone of proliferation to a zone of elongation and differentiation (Tsukagoshi et al., 2010). These PODs are under the control of the helix‐loop‐helix transcription factor UPBEAT1, which is mostly expressed in the transition and elongation zones (Trevisan et al., 2019; Tsukagoshi et al., 2010). Likewise, specific PODs (Arabidopsis PRX01, PRX44, and PRX73) were suggested to be involved in the ROS‐mediated assembly of extension networks, which are important for root hair development in Arabidopsis (Marzol et al., 2022).
2.2. ROS‐dependent control of root growth through direct modification of the cell wall
ROS‐dependent control of root growth is exerted through a direct modification of the cell wall. ROS accumulation in primary root tips is a critical feature to determine growth rates (Dunand et al., 2007; Liszkay et al., 2004; Trevisan et al., 2019; Tsukagoshi et al., 2010; Zang et al., 2020). ROS form concentration gradients to either promote growth through cell division in the meristem zone (O2∙‐) and cell elongation in the elongation zone (∙OH) through polymer breaking and cell wall loosening or, in contrast, restrict growth in the differentiation zone (H2O2) through polymer cross‐linking and cell wall stiffening (Dunand et al., 2007; Liszkay et al., 2004; Tsukagoshi et al., 2010) (Figure 2). Other processes that seem to require direct ROS action include mature tissue differentiation mechanisms such as xylem differentiation and Casparian strip formation (Fernández‐Marcos et al., 2017; Hoffmann et al., 2020; Lee et al., 2013; Mangano et al., 2017).
Figure 2.
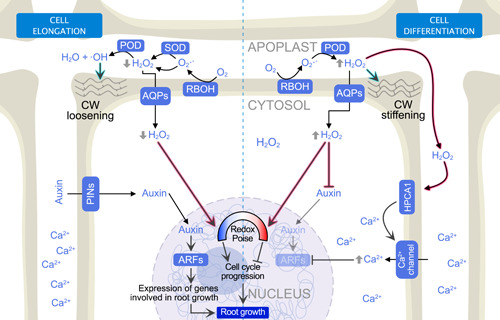
Involvement of reactive oxygen species (ROS) metabolism in root cell elongation and differentiation. The left‐hand side of the figure shows the how ROS metabolism is regulated and coordinated with other signals to promote root cell elongation, whereas the right‐side of the figure shows the involvement of these molecules in root cell differentiation. Arrows with cyan outer glow indicate a direct function of ROS in cell wall modification, whereas arrows with a magenta outer glow indicate their signalling role through oxidation of specific protein residues to modulate enzymatic functions. AQPs, Aquaporins; ARFs, Auxin response factors; HPCA1, Hydrogen‐peroxide‐induced Ca2+ Increases (a H2O2 plasma membrane receptor); POD, Peroxidases; R, Respiratory burst oxidase homologue; SOD, Superoxide dismutase. [Color figure can be viewed at wileyonlinelibrary.com]
2.3. ROS can act as messengers in signalling pathways involved in root development
One can imagine that when ROS accumulate above a given threshold in the apoplast of the root tip, they can enter the adjacent cells and affect the redox poise of the cytoplasm, oxidising the soluble antioxidants glutathione and ascorbate. H2O2 is a good candidate for this role because it is relatively stable, with a half‐life of ~1 ms by comparison with 2–4 μs for O2∙‐ and ∙OH (Smirnoff & Arnaud, 2019). Moreover, its small size facilitates passive transport across membranes and through plasmodesmata and aquaporins (Dynowski et al., 2008; Wu et al., 2020). Finally, H2O2 functions in systemic ROS signalling – the so‐called ROS wave mentioned above – where H2O2 synthesis is propagated from cell to cell, functioning synergistically with Ca2+ signalling to trigger acclimation responses (Mittler et al., 2022). The recently identified HYDROGEN‐PEROXIDE‐INDUCED CA2+ INCREASES (HPCA1) protein establishes the functional link between these two small signal molecules (Wu et al., 2020).
Within the cell, catalase is a key enzyme in processing H2O2. The function of CAT2 is most well‐established in photorespiration (Yang et al., 2019), although it is also required to enable orderly β‐oxidation during post‐germinative growth (Liu et al., 2017). Moreover, CAT2 plays a role in alleviating stress under prolonged iron deficiency. Catalase is Fe‐dependent, and under prolonged Fe deficiency, cellular H2O2 levels are elevated, and root elongation is enhanced (von der Mark et al., 2021). The cat2‐1 mutants show impaired iron homoeostasis, although the underlying mechanisms require full elucidation.
The studies of mutants deficient in ascorbate and glutathione have been formative for our understanding of ROS functions in development and abiotic stress (Considine & Foyer, 2014). Mutants severely deficient in glutathione display a defective primary root (Vernoux et al., 2000) and reduced number of lateral roots (Hoang et al., 2021) as well as elevated sensitivity to biotic and abiotic stress (Hoang et al., 2021). Mutants for ascorbate production (vtc1, vitamin C defective 1) also have shorter primary root but develop more lateral roots than WT plants. Curiously, other vitamin C defective mutants (vtc2‐4 and vtc5‐2) did not display differences in terms of primary root but exhibited a reduced number of secondary roots in control conditions. However, under different abiotic stress conditions the vitamin C defective mutants (vtc2‐4 and vtc5‐2) develop much more secondary roots than WT plants (Hoang et al., 2021). Together, these data suggest that both ascorbate and glutathione participate in the plasticity of the root architecture system, both in control and stress conditions. Moreover, in two independent studies, greater oxidation of glutathione and ascorbate was shown to induce G1 arrest in cells of the quiescent centre in the root apical meristem (Considine & Foyer, 2014; Velappan et al., 2017), suggesting that glutathione‐ and ascorbate‐dependent regulation of cytosolic ROS levels is required for a correct root system development (De Simone et al., 2017).
Recent research in Arabidopsis showed that not only H2O2 but also O2∙‐ have a critical role in root apical meristem maintenance as they act as second messengers downstream of the signalling initiated by the perception of the peptide hormone Root Meristem Growth factor 1 (RGF1). This signalling pathway modulates the O2∙‐/H2O2 balance across the root tip, and increased O2∙‐ levels were shown to increase the stability of PLETHORA2 proteins, a master regulators of root development (Yamada et al., 2020).
2.4. ROS signal transduction through protein oxidation
ROS accumulated inside the cells not only oxidise glutathione and ascorbate but also macromolecules such as proteins. The sulphurs present in exposed Cys and Met residues of many proteins are susceptible to nucleophilic attack, resulting in posttranslational redox modifications crucial for various cellular functions (Mittler et al., 2022; Mock & Dietz, 2016). Such modification can lead to loss or gain of protein function and has been proposed to play a role in the transduction of H2O2 signals. This was first demonstrated in yeast with a glutathione peroxidase (GPX) involved in the activation of a transcription factor that results in protection of the yeast from oxidative damage (Delaunay et al., 2002). GPXs are a large family of non‐heme peroxidases with different subcellular localisations that use either the thiol group of thioredoxins or glutathione as an electron acceptor to reduce H2O2 and organic peroxides (Bela et al., 2022). In plants, GPXs playing a role in lateral root development were identified (Passaia et al., 2014). Whether their role is linked to transduction of an H2O2 signal remains to be determined. H2O2 signalling through protein redox modification was nevertheless demonstrated in plants for the HPCA1 sensor, a leucine rich repeat receptor kinase (Wu et al., 2020). Once its reactive Cys residues are oxidised by apoplastic H2O2, it induces a Ca2+ influx, providing the molecular link between observations reporting a concomitant apoplastic ROS production and Ca2+‐mediated responses (Gilroy et al., 2016; M. J. Kim et al., 2010; Mori & Schroeder, 2004; Wu et al., 2020). Given the capacity of Ca2+ to modulate several signalling pathways, the discovery of HPCA1 provides direct evidence of how apoplastic H2O2 and redox status can determine the fate of diverse biological processes, connecting RBOH‐ and POD‐dependent apoplastic ROS accumulation to intracellular downstream responses (Figure 2).
Protein oxidation may be reversible following reduction back to the native state through the action of redoxins, proteins susceptible to redox posttranslational modifications thanks to the highly reactive thiol groups present in exposed Cys residues (Meyer et al., 2008). Based on the cofactor used as reducing agent, redoxins can be classified as thioredoxins (TRXs) (NADPH as reducing agent), glutaredoxins (GRXs) (glutathione as reducing agent), and peroxiredoxins (PRXs, as they lack a cofactor they require an external electron donor, such as TRXs or GRXs, for regeneration after each catalytic cycle). The redoxin family is well‐known in plants (Montrichard et al., 2009), but available information about the isoforms involved in root development is limited. However, four GRXs (S3, S4, S5 and S8) involved in the control of primary root growth were identified in Arabidopsis in a transcriptomic analysis of shoot response to N nutrition (Patterson et al., 2016; see Section 3.1). In this study, the inactivation by RNAi silencing of all four corresponding genes, which are similar and in tandem, led to mutant plants with increased primary root length. Later, other GRXs (of the ROXY subclass) involved in root hair elongation were identified (Jung et al., 2018). Regarding the roles of TRXs in root development, not much is known to date, although two TRX isoforms of Arabidopsis were suggested to be required for correct activity of the root apical meristem (Reichheld et al., 2007).
The roles of ROS in root development have been recently reviewed (Eljebbawi et al., 2021; Mase & Tsukagoshi, 2021; Zhou et al., 2020). In the following sections, we focus specifically on their roles in regulating the root system architecture in response to nutrient stress.
3. ROS AND NUTRIENT STRESS
3.1. NO3 − deficiency and ROS homoeostasis: An intricate relation
N deficiency was first shown to induce ROS accumulation in a localised manner in the root hair extremities of Arabidopsis (Shin et al., 2005). Changes in ROS accumulation linked to NO3 − availability were later observed in the primary root tips of maize (Zea mays) and M. truncatula. In maize, NO3 − deficiency was shown to activate a signalling pathway that stimulates primary root elongation by inducing POD‐dependent H2O2 accumulation, which promotes cell elongation and differentiation (Trevisan et al., 2019). On the other hand, an excess of NO3 − reduced H2O2 levels but increased O2 .‐ levels. Thus, it seems that the balance between these molecules might be key in regulating root growth in response to NO3 − (Trevisan et al., 2019). The authors found a maize orthologue of AtUPBEAT1 transcription factor to be responsible for regulating ROS levels by repressing the expression of a POD gene (Zm00001d024119) in a similar fashion to what was described in Arabidopsis, suggesting the existence of an evolutionarily conserved signalling module common to both monocots and eudicots (Trevisan et al., 2019; Tsukagoshi et al., 2010). Accordingly, in M. truncatula, the orthologue of AtUPBEAT1, MtUPBEAT1, was recently found to be downregulated in response to 5 mM NO3 − treatment (Zang et al., 2022).
In M. truncatula, the NO3 − transporter MtNPF1.7 controls ROS homoeostasis in the primary root tip and promote cell elongation through an increased RBOH activity (Zhang et al., 2014). A link between ROS accumulation and NO3 − availability was proposed although conclusive evidence was lacking. The link was made later by showing that NO3 − deficiency increases ROS accumulation in the primary root tip of M. truncatula seedlings, following changes in POD activities levels (Figure 3) (Zang et al., 2020). In the presence of 5 mM NO3 −, a net decrease in H2O2 and ∙OH was notably correlated with an increase in POD peroxidative activity (which eliminates H2O2) and a decrease in POD hydroxylic activity, which produces ∙OH. Interestingly, no change in ROS accumulation or POD activity was observed in the primary root tip of the mutant npf6.8 deficient in the NO3 − transporter MtNPF6.8 that has lost the sensitivity to NO3 − (Zang et al., 2022). It is noteworthy that such a mechanism does not occur in lateral roots, in which growth rate is enhanced in the presence of NO3 −. In Arabidopsis, enzymes of the GPX family were found to be required for the correct development of lateral root primordia (Passaia et al., 2014). Thus, it remains possible that the GPX‐dependent control of [H2O2]cytosol during lateral root development has to be more stringent compared to the control of [H2O2]apoplast, which on the other hand appears to be mostly delegated to PODs and is a major factor in primary root development.
Figure 3.
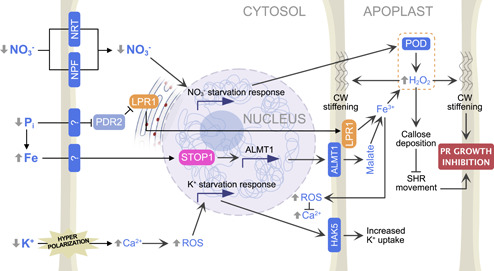
Signalling effect of nitrate, phosphate, and potassium on primary root growth. NO3 − and Pi deficit induce primary root growth inhibition, through apoplastic H2O2 accumulation and callose deposition. Moreover, Ca2+ and reactive oxygen species (ROS) signalling participates in the increase of potassium uptake under potassium deficit conditions. ALMT1, Aluminium‐activated malate transporter 1; HAK5, High affinity K+ transporter; LPR1, Low phosphate root1; NPF, NRT1/PTR family; NRT, Nitrate transporter; PDR2, Phosphate deficiency response 2; PHR, Phosphate starvation response; PR, primary root; SHR, Short root; STOP1, Sensitive to proton rhizotoxicity 1. For further discussion, see text. [Color figure can be viewed at wileyonlinelibrary.com]
The comparison of the results obtained in maize and M. truncatula highlights some differences and indicates that the relation ROS‐NO3 − may be rather species‐specific (Figure 4). However, it reveals a central role played by H2O2 in the control of the root growth by NO3 − that was further supported by experiments in which H2O2 concentration was manipulated during M. truncatula root growth. In this work, exogenous H2O2 was shown to completely abolish the NO3 − effect on both the primary root growth and the lateral roots while KI, an H2O2 scavenger, mimicked NO3 − effects (Zang et al., 2020).
Figure 4.
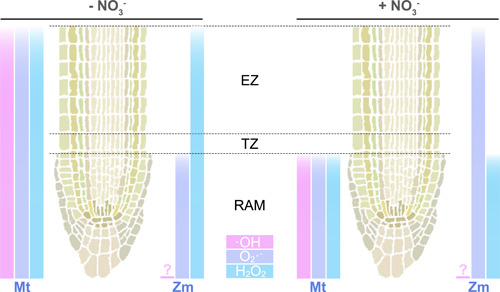
Effects of NO3 − supply on reactive oxygen species (ROS) accumulation in root tips of Medicago truncatula and maize. The left panel presents an absence of NO3 − supply to roots, while the right panel shows a NO3 ‐‐sufficient condition. The right half of each root represents data from maize (Zm), left part data from M. truncatula. Bars indicate the extent of accumulation of each ROS in the root in relation to species and NO3 ‐ status. Note that data for ∙OH quantification are not available for maize. Data elaborated from Trevisan et al. (2019), Zang et al. (2020). EZ, elongation zone; RAM, Root apical meristem; TZ, transition zone. [Color figure can be viewed at wileyonlinelibrary.com]
The GRX S3, S4, S5 and S8 genes identified in Arabidopsis are induced in the shoot by a NO3 − signalling pathway mediated by cytokinins to inhibit primary root growth (Patterson et al., 2016). To explain this long‐distance effect, the authors proposed a model where the products of the genes were transported in the phloem to the primary root to both reduce the growth and induce an expression of the NO3 − transporter gene AtNRT2.1 when NO3 − is present. Such a hypothesis was latter supported by the work of the Matsubayashi group (Ohkubo et al., 2017; Ota et al., 2020). The GRX genes identified in these studies were overexpressed in the shoot in response to CEP (C‐terminally encoded peptide), a root‐to shoot peptide originating from the N‐starved roots, and as a result were named C‐terminally encoded peptide downstream 1 (CEPD1) (Ota et al., 2020).
Two ROXY GRX genes were shown to be oppositely regulated, in a manner that functionally influenced the growth response to NO3 − availability. AtROXY9 was upregulated while AtROXY15 was downregulated in response to NO3 − starvation in Arabidopsis seedlings. Overexpression of AtROXY9 enhanced the length of root hairs as compared to the wild type, while seedlings over‐expressing AtROXY15 showed shorter root hairs. This suggests that the regulation of GRX expression is crucial to modulate root hair growth in both NO3 −‐deficient and sufficient conditions (Jung et al., 2018).
More recent research from Arabidopsis showed that NO3 − starvation represses the expression of several GARP (Golden2, ARR‐B, Psr1) family transcription factor genes, which are responsible for keeping intracellular ROS levels low by positively regulating the expression of different redoxin genes and repressing RBOHC expression (Safi et al., 2021). The resulting ROS accumulation is required to regulate a large subset of genes involved in the NO3 − starvation response, including several high‐affinity NO3 − transporters (Safi et al., 2021). Besides GARP transcription factors, the HOMOLOGUE OF BRASSINOSTEROID ENHANCED EXPRESSION2 INTERACTING WITH IBH1 (HBI1) transcription factor was recently highlighted as another key regulator of ROS levels in response to NO3 − availability, in both roots and shoots of Arabidopsis (Chu et al., 2021). Here, the authors showed that high environmental NO3 − levels induce HBI1 expression, which in turn promotes the expression of several PODs genes and CAT2 (a catalase gene), resulting in low ROS cellular levels (Chu et al., 2021). The combination of these findings with the previously mentioned research on the roles of POD in primary root development points to a scenario where ROS act both as signal and effectors in response to NO3 − availability depending on spatio‐temporal variables.
3.2. ROS and Fe availability are determinant in Pi deficiency responses
Pi availability is one of the most limiting nutritional cues for plant growth. Therefore, plants have developed sophisticated sensing and signalling mechanisms to optimise Pi uptake as a function of its external concentrations (Chien et al., 2018). ROS have been known to be involved in Pi signalling pathways for almost two decades, when Pi deficiency was shown to induce ROS production in the cortex of primary roots but not in the tips of Arabidopsis, leading to the hypothesis that ROS are involved in Pi deficiency signalling (Shin et al., 2005). Further research showed that such ROS accumulation has a negative effect on primary root growth and stimulates lateral root growth, according to a model of the root ideotype optimised for Pi acquisition from the topsoil (Tyburski et al., 2009, 2010; York et al., 2013).
Although the initial step in Pi sensing is yet to be uncovered, it is known that one of the earliest signalling steps is the loss of the interaction between the nuclear proteins SPX (SYG/PHO81/XPR1 domain protein) and PHR (phosphate starvation) transcription factor, which is then released and activated (Puga et al., 2014). This pathway is known to govern Pi starvation responses at the systemic level. At the local level, studies on Arabidopsis roots indicated that two pathways appear to control Pi deficiency‐dependent primary root growth inhibition: the PDR2‐LPR1/2 and the STOP1‐ALMT1 modules (Crombez et al., 2019). In the first case, low extracellular Pi levels triggers the relocation of the ER localised ferroxidase low phosphate root1 (LPR1) to the cell wall where it triggers Fe accumulation and promotes H2O2 accumulation and callose deposition (Figure 3, Müller et al., 2015). Not much is known about the elements upstream of LPR1, besides that it is under negative genetic control of the ER‐resident ATPase PDR2 (phosphate deficiency response 2) (Figure 3, Ticconi et al., 2009).
In parallel with this pathway, a high extracellular Fe/Pi ratio, typical of low Pi conditions, post‐transcriptionally activates the transcription factor STOP1 (sensitive to proton toxicity1) which triggers an increase in apoplastic malate concentration by upregulating the gene expression of the ALMT1 transporter (aluminium‐activated malate transporter 1) (Figure 3). Such malate accumulation was shown to further increase apoplastic Fe2+ availability, which ultimately triggers apoplastic ROS accumulation and callose deposition (Balzergue et al., 2017; Godon et al., 2019). While callose deposition around plasmodesmata limits the movement of the SHORTROOT transcription factor, a master regulator of primary root development (Salvi et al., 2018), increased apoplastic H2O2 sustain the peroxidative cycle of PODs, resulting in increased cell wall stiffening (Balzergue et al., 2017; Müller et al., 2015). Both events ultimately inhibit primary root elongation (Figure 3). Thus, it appears that POD activity is a one of the major determinants of Pi deficiency‐induced inhibition of primary root growth. Accordingly, proteomic and transcriptomic data from Arabidopsis primary root tips revealed that Pi starvation modulates the expression of about 30 PODs (Hoehenwarter et al., 2016). Taken together with the data discussed in the previous paragraph, a scenario is emerging whereby PODs act as central actors in root system architecture plasticity in response to both Pi and NO3 − starvation. This might not come as a surprise given that several points of convergence between Pi and NO3 − signalling are already known (Hu et al., 2019; Medici et al., 2019; Pueyo et al., 2021).
In addition to the modulation of apoplastic ROS, intracellular ROS accumulation was shown to take place as a consequence of increased Fe2+ availability following Pi starvation in the Arabidopsis primary root (Matthus et al., 2019), although the biological significance of this event remains to be elucidated. It would be interesting to test whether GPXs are involved in this process, as is the case during NO3 − starvation.
Although modulation of primary and lateral root growth is required to increase Pi acquisition from the topsoil, possibly the most influential evolutionary adaptation of roots to cope with Pi deficiency was the increase in root hair length and density (López‐Bucio et al., 2002; York et al., 2013). Such an adaptation allows a more efficient soil acidification and thus increased Pi solubilisation. In this context, research in Arabidopsis showed that Proline‐rich Extensin‐like Receptor Kinase 13 (PERK13) is involved in Pi sensing and that loss‐of‐function mutants of PERK13 have altered root hair development in response to Pi deficiency. Both gain‐of‐function and loss‐of‐function PERK13 mutant lines showed increased ROS accumulation in root hairs under Pi deficiency compared to WT lines. These observations suggest that PERK13 controls ROS levels, and thus growth rate, in root hairs in response to Pi deficiency (Xue et al., 2021). It remains to be determined which ROS‐producing enzymes are the downstream targets of this kinase.
3.3. ROS functions in K+ deficiency responses
Compared to NO3 − and Pi, our current knowledge about root responses to K+ deficiency is relatively limited. Recent research efforts are aiming to fill this gap (Sustr et al., 2019; Wang et al., 2021). Nevertheless, an important role for ROS in root system architecture response to K+ deficiency has been known for some time. The activity of RBOHs are known to be required to induce the expression of genes involved in the K+ deficiency response, such as genes encoding high‐affinity K+ transporters in the primary root of Arabidopsis (Shin & Schachtman, 2004). When RBOH activity was inhibited by DPI, exogenous application of H2O2 was able to restore the expression levels of genes coding for high‐affinity K+ transporters (Shin & Schachtman, 2004), highlighting the importance of extracellular ROS production in early response to K+ deficiency. Recent research in Nicotiana tabacum roots subjected to K+ deficiency showed that such ROS accumulation is downstream of Ca2+ signalling (Wang et al., 2021), although it remains to be determined how this increase in cytosolic Ca2+ triggers ROS accumulation.
The POD AtRCI3 was also previously shown to be involved in ROS production upon K+ deficiency and to positively regulate the expression of the AtHAK5 high‐affinity transporter gene in Arabidopsis, a master gene of the K+ deficiency response (M. J. Kim et al., 2010). Interestingly, overexpression of AtRCI3 correlates with increased ROS levels in the primary root, suggesting that the main activity of this POD is linked to ROS generation rather than detoxification.
4. CONCLUSIONS AND PERSPECTIVES
To date, research into the regulators of root system plasticity in response to nutrient availability has mostly focused on hormonal influences. Studies discussed here have highlighted the crucial role of ROS and redox processes in signalling pathways that facilitate or interact with nutrient sensing (Figure 3). ROS and redox signalling act on cellular processes such as cell division and expansion in ways that are dependent on, and independent of, hormone functions. ROS can directly influence hormonal dynamics, as shown in the case of attenuation of auxin signalling through oxidation (Peer et al., 2013) (Figure 5). Conversely, several hormones, such as abscisic acid, auxins, cytokinins, and ethylene are known to influence ROS accumulation in roots. Thus, integrating research on ROS homoeostasis and hormonal signalling in response to nutrient stress is a necessary step to achieve a more comprehensive understanding of root system plasticity, as already suggested by earlier studies (Mangano et al., 2017; Zhang et al., 2014). We suggest that the complex and coordinated interplay between hormones and ROS is the main determinant that constitutes the backbone for signal transduction that allows the root system architecture to respond to nutritional cues, ultimately resulting in modifications of root morphology (Figure 5).
Figure 5.
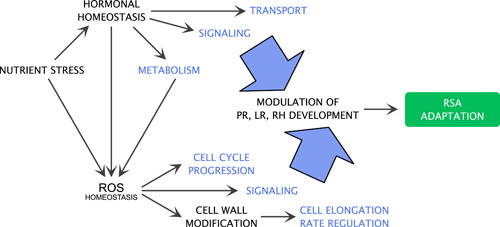
Schematic representation of the main pathways downstream of nutrient stress that modulate root system architecture. Nutrient stress induces changes in the hormonal homoeostasis, including their metabolism, transport, and signalling which in turn modulate primary root, lateral root and root hair development. Both nutrient stress and hormonal metabolism modulate reactive oxygen species (ROS) homoeostasis which affects cell cycle progression and cell wall composition. These events together with ROS signalling also modulate the development of the primary root, lateral roots, and root hairs. [Color figure can be viewed at wileyonlinelibrary.com]
The identification of master regulators situated at the interface of ROS and nutrient signalling pathways will help us achieve a more detailed description of how molecular inputs are converted to morphological outputs in plant roots, potentially revealing powerful targets for crop amelioration. By combining studies on the impact of nutrient deficiency with investigations into spatial and temporal changes in ROS dynamics, important mechanistic details can be added to our current knowledge of root responses to nutrient stress. A next step in this process will be to integrate such knowledge with the effect of root microbiota on nutrient acquisition and consider the concerted effect of multiple nutrient stresses. A full consideration of such a multitude of factors will be crucial to generate knowledge that can be applied to crops growing in field conditions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We would like to thank the editors and the reviewers that contributed to improve the quality of this manuscript with their comments and suggestions. Santiago Signorelli is an active member of the Uruguayan System of Researchers (SNI, Uruguay). Łukasz P. Tarkowski is funded by the National Institute of Research for the Agriculture, the Alimentation and the Environment (INRAE, France). Michael J. Considine is an Australian Research Council Future Fellow (ARC, Australia, FT180100409).
Tarkowski, Ł. P. , Signorelli, S. , Considine, M. J. & Montrichard, F. (2023) Integration of reactive oxygen species and nutrient signalling to shape root system architecture. Plant, Cell & Environment, 46, 379–390. 10.1111/pce.14504
REFERENCES
- Arthikala, M.K. & Quinto, C. (2018) RbohA coordinates lateral root emergence in common bean. Communicative & Integrative Biology, 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzergue, C. , Dartevelle, T. , Godon, C. , Laugier, E. , Meisrimler, C. , Teulon, J.M. et al. (2017) Low phosphate activates STOP1‐ALMT1 to rapidly inhibit root cell elongation. Nature Communications, 8, 15300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bela, K. , Riyazuddin, R. & Csiszár, J. (2022) Plant glutathione peroxidases: non‐heme peroxidases with large functional flexibility as a core component of ROS‐processing mechanisms and signalling. Antioxidants, 11, 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, J.M. , Muhlemann, J.K. , Gayomba, S.R. & Muday, G.K. (2019) RBOH‐Dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chemical Research in Toxicology, 32, 370–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Lee, J. , Lee, J.M. , Han, M. , Emonet, A. , Lee, J. et al. (2022) MSD2, an apoplastic Mn‐SOD, contributes to root skotomorphogenic growth by modulating ROS distribution in Arabidopsis. Plant Science, 317, 111192. [DOI] [PubMed] [Google Scholar]
- Chen, S. & Schopfer, P. (1999) Hydroxyl‐radical production in physiological reactions. A novel function of peroxidase. European Journal of Biochemistry, 260, 726–735. [DOI] [PubMed] [Google Scholar]
- Chien, P.S. , Chiang, C.P. , Leong, S.J. & Chiou, T.J. (2018) Sensing and signaling of phosphate starvation: from local to long distance. Plant and Cell Physiology, 59, 1714–1722. [DOI] [PubMed] [Google Scholar]
- Chu, X. , Wang, J.G. , Li, M. , Zhang, S. , Gao, Y. , Fan, M. et al. (2021) HBI transcription factor‐mediated ROS homeostasis regulates nitrate signal transduction. The Plant Cell, 33, 3004–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine, M.J. , Diaz‐Vivancos, P. , Kerchev, P. , Signorelli, S. , Agudelo‐Romero, P. , Gibbs, D.J. et al. (2017) Learning to breathe: developmental phase transitions in oxygen status. Trends in Plant Science, 22, 140–153. [DOI] [PubMed] [Google Scholar]
- Considine, M.J. & Foyer, C.H. (2014) Redox regulation of plant development. Antioxidants & Redox Signaling, 21, 1305–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio, C. & Dunand, C. (2009) Specific functions of individual class III peroxidase genes. Journal of Experimental Botany, 60, 391–408. [DOI] [PubMed] [Google Scholar]
- Crombez, H. , Motte, H. & Beeckman, T. (2019) Tackling plant phosphate starvation by the roots. Developmental Cell, 48, 599–615. [DOI] [PubMed] [Google Scholar]
- Delaunay, A. , Pflieger, D. , Barrault, M.B. , Vinh, J. & Toledano, M.B. (2002) A thiol peroxidase is an H2O2 receptor and redox‐transducer in gene activation. Cell, 111, 471–481. [DOI] [PubMed] [Google Scholar]
- Desikan, R. , Hancock, J.T. , Bright, J. , Harrison, J. , Weir, I. , Hooley, R. et al. (2005) A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiology, 137, 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, D. (2018) Hydrotropism: how roots search for water. Journal of Experimental Botany, 69, 2759–2771. [DOI] [PubMed] [Google Scholar]
- Dunand, C. , Crèvecoeur, M. & Penel, C. (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist, 174, 332–341. [DOI] [PubMed] [Google Scholar]
- Dvořák, P. , Krasylenko, Y. , Ovečka, M. , Basheer, J. , Zapletalová, V. , Šamaj, J. et al. (2021) In vivo light‐sheet microscopy resolves localisation patterns of FSD1, a superoxide dismutase with function in root development and osmoprotection. Plant, Cell & Environment, 44, 68–87. [DOI] [PubMed] [Google Scholar]
- Dynowski, M. , Schaaf, G. , Loque, D. , Moran, O. & Ludewig, U. (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2 . Biochemical Journal, 414, 53–61. [DOI] [PubMed] [Google Scholar]
- Eljebbawi, A. , Guerrero, Y.C.R. , Dunand, C. & Estevez, J.M. (2021) Highlighting reactive oxygen species as multitaskers in root development. iScience, 24, 101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq, M.A. , Niazi, A.K. , Akhtar, J. , Saifullah, Farooq, M. , Souri, Z. et al. (2019) Acquiring control: the evolution of ROS‐Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiology and Biochemistry, 141, 353–369. [DOI] [PubMed] [Google Scholar]
- Felle, H.H. (2001) pH: signal and messenger in plant cells. Plant Biology, 3, 577–591. [Google Scholar]
- Fernández‐Marcos, M. , Desvoyes, B. , Manzano, C. , Liberman, L.M. , Benfey, P.N. , Pozo, J.C. et al. (2017) Control of Arabidopsis lateral root primordium boundaries by MYB36. New Phytologist, 213, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman, J. , Demidchik, V. , Bothwell, J.H.F. , Mylona, P. , Miedema, H. , Torres, M.A. et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature, 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Francoz, E. , Ranocha, P. , Nguyen‐Kim, H. , Jamet, E. , Burlat, V. & Dunand, C. (2015) Roles of cell wall peroxidases in plant development. Phytochemistry, 112, 15–21. [DOI] [PubMed] [Google Scholar]
- Gilroy, S. , Białasek, M. , Suzuki, N. , Górecka, M. , Devireddy, A.R. , Karpiński, S. & Mittler, R. (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiology, 171, 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon, C. , Mercier, C. , Wang, X. , David, P. , Richaud, P. , Nussaume, L. et al. (2019) Under phosphate starvation conditions, Fe and Al trigger accumulation of the transcription factor STOP1 in the nucleus of Arabidopsis root cells. The Plant Journal, 99, 937–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojon, A. , Nacry, P. & Davidian, J.C. (2009) Root uptake regulation: a central process for NPS homeostasis in plants. Current Opinion in Plant Biology, 12, 328–338. [DOI] [PubMed] [Google Scholar]
- Hoang, M.T.T. , Doan, M.T.A. , Nguyen, T. , Tra, D.‐P. , Chu, T.N. , Dang, T.P.T. et al. (2021) Phenotypic characterization of Arabidopsis ascorbate and glutathione deficient mutants under abiotic stresses. Agronomy, 11, 764. [Google Scholar]
- Hodge, A. (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist, 162, 9–24. [Google Scholar]
- Hoehenwarter, W. , Mönchgesang, S. , Neumann, S. , Majovsky, P. , Abel, S. & Müller, J. (2016) Comparative expression profiling reveals a role of the root apoplast in local phosphate response. BMC Plant Biology, 16, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, N. , Benske, A. , Betz, H. , Schuetz, M. & Samuels, A.L. (2020) Laccases and peroxidases co‐localize in lignified secondary cell walls throughout stem development. Plant Physiology, 184, 806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, B. , Jiang, Z. , Wang, W. , Qiu, Y. , Zhang, Z. , Liu, Y. et al. (2019) Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nature Plants, 5, 401–413. [DOI] [PubMed] [Google Scholar]
- Huang, G. & Zhang, D. (2020) The plasticity of root systems in response to external phosphate. International Journal of Molecular Sciences, 21, 5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, Y. , Sun, L. , Song, Y. , Wang, L. , Liu, L. , Zhang, L. et al. (2013) AtrbohD and AtrbohF positively regulate abscisic acid‐inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. Journal of Experimental Botany, 64, 4183–4192. [DOI] [PubMed] [Google Scholar]
- Jung, J.Y. , Ahn, J.H. & Schachtman, D.P. (2018) CC‐type glutaredoxins mediate plant response and signaling under nitrate starvation in Arabidopsis. BMC Plant Biology, 18, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E.‐J. , Kim, Y.‐J. , Hong, W.‐J. , Lee, C. , Jeon, J.‐S. & Jung, K.‐H. (2019) Genome‐wide analysis of root hair preferred RBOH genes suggests that three RBOH genes are associated with auxin‐mediated root hair development in rice. Journal of Plant Biology, 62, 229–238. [Google Scholar]
- Kim, M.J. , Ciani, S. & Schachtman, D.P. (2010) A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Molecular Plant, 3, 420–427. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS‐dependent ABA signaling in arabidopsis. The EMBO Journal, 22, 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. , Rubio, M.C. , Alassimone, J. & Geldner, N. (2013) A mechanism for localized lignin deposition in the endodermis. Cell, 153, 402–412. [DOI] [PubMed] [Google Scholar]
- Liszkay, A. , van der Zalm, E. & Schopfer, P. (2004) Production of reactive oxygen intermediates (O2•‐, H2O2, and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology, 136, 3114–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.‐C. , Han, T.‐T. , Yuan, H.‐M. , Yu, Z.‐D. , Zhang, L.‐Y. , Zhang, B.‐L. et al. (2017) CATALASE2 functions for seedling postgerminative growth by scavenging H(2) O(2) and stimulating ACX2/3 activity in Arabidopsis. Plant, Cell & Environment, 40, 2720–2728. [DOI] [PubMed] [Google Scholar]
- López‐Bucio, J. , Hernández‐Abreu, E. , Sánchez‐Calderón, L. , Nieto‐Jacobo, F. , Simpson, J. & Herrera‐Estrella, L. (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology, 129, 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthje, S. & Martinez‐Cortes, T. (2018) Membrane‐bound class III peroxidases: unexpected enzymes with exciting functions. International Journal of Molecular Sciences, 19, 2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano, S. , Denita‐Juarez, S.P. , Choi, H.S. , Marzol, E. , Hwang, Y. , Ranocha, P. et al. (2017) Molecular link between auxin and ROS‐mediated polar growth. Proceedings of the National Academy of Sciences, 114, 5289–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano, S. , Juárez, S.P.D. & Estevez, J.M. (2016) ROS regulation of polar growth in plant cells. Plant Physiology, 171, 1593–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark, C. , Ivanov, R. , Eutebach, M. , Maurino, V.G. , Bauer, P. & Brumbarova, T. (2021) Reactive oxygen species coordinate the transcriptional responses to iron availability in Arabidopsis. Journal of Experimental Botany, 72, 2181–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mase, K. & Tsukagoshi, H. (2021) Reactive oxygen species link gene regulatory networks during Arabidopsis root development. Frontiers in Plant Science, 12, 660274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthus, E. , Wilkins, K.A. , Swarbreck, S.M. , Doddrell, N.H. , Doccula, F.G. , Costa, A. et al. (2019) Phosphate starvation alters abiotic‐stress‐induced cytosolic free calcium increases in roots. Plant Physiology, 179, 1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici, A. , Szponarski, W. , Dangeville, P. , Safi, A. , Dissanayake, I.M. , Saenchai, C. et al. (2019) Identification of molecular integrators shows that nitrogen actively controls the phosphate starvation response in plants. The Plant Cell, 31, 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, Y. , Siala, W. , Bashandy, T. , Riondet, C. , Vignols, F. & Reichheld, J.P. (2008) Glutaredoxins and thioredoxins in plants. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1783, 589–600. [DOI] [PubMed] [Google Scholar]
- Mittler, R. (2017) ROS are good. Trends in Plant Science, 22, 11–19. [DOI] [PubMed] [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Suzuki, N. , Miller, G. , Tognetti, V.B. , Vandepoele, K. et al. (2011) ROS signaling: the new wave? Trends in Plant Science, 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Mittler, R. , Zandalinas, S.I. , Fichman, Y. & Van Breusegem, F. (2022) Reactive oxygen species signalling in plant stress responses. Nature Reviews Molecular Cell Biology, 23, 663–6679. [DOI] [PubMed] [Google Scholar]
- Mock, H.P. & Dietz, K.J. (2016) Redox proteomics for the assessment of redox‐related posttranslational regulation in plants. Biochimica et Biophysica Acta, 1864, 967–973. [DOI] [PubMed] [Google Scholar]
- Montrichard, F. , Alkhalfioui, F. , Yano, H. , Vensel, W.H. , Hurkman, W.J. & Buchanan, B.B. (2009) Thioredoxin targets in plants: the first 30 years. Journal of Proteomics, 72, 452–474. [DOI] [PubMed] [Google Scholar]
- Morgan, M.J. , Lehmann, M. , Schwarzländer, M. , Baxter, C.J. , Sienkiewicz‐Porzucek, A. , Williams, T.C.R. et al. (2008) Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiology, 147, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, I.C. & Schroeder, J.I. (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology, 135, 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, E.C. , Griffiths, M. , Golebiowska, A. , Mairhofer, S. , Burr‐Hersey, J. , Goh, T. et al. (2017) Shaping 3D root system architecture. Current Biology, 27, R919–R930. [DOI] [PubMed] [Google Scholar]
- Müller, J. , Toev, T. , Heisters, M. , Teller, J. , Moore, K.L. , Hause, G. et al. (2015) Iron‐dependent callose deposition adjusts root meristem maintenance to phosphate availability. Developmental Cell, 33, 216–230. [DOI] [PubMed] [Google Scholar]
- Muthert, L.W.F. , Izzo, L.G. , van Zanten, M. & Aronne, G. (2020) Root tropisms: investigations on earth and in space to unravel plant growth direction. Frontiers in Plant Science, 10, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath, M. & Tuteja, N. (2016) NPKS uptake, sensing, and signaling and miRNAs in plant nutrient stress. Protoplasma, 253, 767–786. [DOI] [PubMed] [Google Scholar]
- Nestler, J. , Liu, S. , Wen, T.J. , Paschold, A. , Marcon, C. , Tang, H.M. et al. (2014) Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot‐specific NADPH oxidase. The Plant Journal, 79, 729–740. [DOI] [PubMed] [Google Scholar]
- Ohkubo, Y. , Tanaka, M. , Tabata, R. , Ogawa‐Ohnishi, M. & Matsubayashi, Y. (2017) Shoot‐to‐root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nature Plants, 3, 17029. [DOI] [PubMed] [Google Scholar]
- Orman‐Ligeza, B. , Parizot, B. , de Rycke, R. , Fernandez, A. , Himschoot, E. , van Breusegem, F. et al. (2016) RBOH‐mediated ROS production facilitates lateral root emergence in Arabidopsis. Dev, 143, 3328–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota, R. , Ohkubo, Y. , Yamashita, Y. , Ogawa‐Ohnishi, M. & Matsubayashi, Y. (2020) Shoot‐to‐root mobile CEPD‐like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nature Communications, 11, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaia, G. , Queval, G. , Bai, J. , Margis‐Pinheiro, M. & Foyer, C.H. (2014) The effects of redox controls mediated by glutathione peroxidases on root architecture in Arabidopsi. Journal of Experimental Botany, 65, 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi, F. , Cosio, C. , Penel, C. & Dunand, C. (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, 24, 255–265. [DOI] [PubMed] [Google Scholar]
- Passardi, F. , Penel, C. & Dunand, C. (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends in Plant Science, 9, 534–540. [DOI] [PubMed] [Google Scholar]
- Patterson, K. , Walters, L.A. , Cooper, A.M. , Olvera, J.G. , Rosas, M.A. , Rasmusson, A.G. et al. (2016) Nitrate‐regulated glutaredoxins control arabidopsis primary root growth. Plant Physiology, 170, 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer, W.A. , Cheng, Y. & Murphy, A.S. (2013) Evidence of oxidative attenuation of auxin signalling. Journal of Experimental Botany, 64, 2629–2639. [DOI] [PubMed] [Google Scholar]
- Podgórska, A. , Burian, M. & Szal, B. (2017) Extra‐cellular but extra‐ordinarily important for cells: apoplastic reactive oxygen species metabolism. Frontiers in Plant Science, 8, 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo, J.J. , Quiñones, M.A. , Coba de la Peña, T. , Fedorova, E.E. & Lucas, M.M. (2021) Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Frontiers in Plant Science, 12, 644218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga, M.I. , Mateos, I. , Charukesi, R. , Wang, Z. , Franco‐Zorrilla, J.M. , de Lorenzo, L . et al. (2014) SPX1 is a phosphate‐dependent inhibitor of phosphate starvation response 1 in arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 111, 14947–14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld, J.‐P. , Khafif, M. , Riondet, C. , Droux, M. , Bonnard, G. & Meyer, Y. (2007) Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. The Plant Cell, 19, 1851–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi, A. , Medici, A. , Szponarski, W. , Martin, F. , Clément‐Vidal, A. , Marshall‐Colon, A. et al. (2021) GARP transcription factors repress Arabidopsis nitrogen starvation response via ROS‐dependent and ‐independent pathways. Journal of Experimental Botany, 72, 3881–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi, M. & Fluhr, R. (2001) Superoxide production by plant homologues of the gp91 phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology, 126, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi, E. , Di Mambro, R. , Pacifici, E. , Dello Ioio, R. , Costantino, P. , Moubayidin, L. et al. (2018) SCARECROW and SHORTROOT control the auxin/cytokinin balance necessary for embryonic stem cell niche specification. Plant Signaling & Behavior, 13, 1507402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad, Z. & Amtmann, A. (2017) Food for thought: how nutrients regulate root system architecture. Current Opinion in Plant Biology, 39, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M. , Singh, D. , Saksena, H.B. , Sharma, M. , Tiwari, A. , Awasthi, P. et al. (2021) Understanding the intricate web of phytohormone signalling in modulating root system architecture. International Journal of Molecular Sciences, 22, 5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeto, J. & Tsutsumi, Y. (2016) Diverse functions and reactions of class III peroxidases. New Phytologist, 209, 1395–1402. [DOI] [PubMed] [Google Scholar]
- Shin, R. , Berg, R.H. & Schachtman, D.P. (2005) Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant and Cell Physiology, 46, 1350–1357. [DOI] [PubMed] [Google Scholar]
- Shin, R. & Schachtman, D.P. (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proceedings of the National Academy of Sciences, 101, 8827–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone, A. , Hubbard, R. , De La Torre, N.V. , Velappan, Y. , Wilson, M. , Considine, M.J. et al. (2017) Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis thaliana . Antioxidants & Redox Signaling, 27, 1505–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff, N. & Arnaud, D. (2019) Hydrogen peroxide metabolism and functions in plants. New Phytologist, 221, 1197–1214. [DOI] [PubMed] [Google Scholar]
- Šukalović, V.H.T. , Vuletić, M. & Vučinić, Ž. (2005) The role of p‐coumaric acid in oxidative and peroxidative cycle of the ionically bound peroxidase of the maize root cell wall. Plant Science, 168, 931–938. [Google Scholar]
- Sustr, M. , Soukup, A. & Tylova, E. (2019) Potassium in root growth and development. Plants, 8, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Miyazawa, Y. & Fujii, N. (2009) Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Molecular Biology, 69, 489–502. [DOI] [PubMed] [Google Scholar]
- Takeda, S. , Gapper, C. , Kaya, H. , Bell, E. , Kuchitsu, K. & Dolan, L. (2008) Local positive feedback regulation determines cell shape in root hair cells. Science, 319, 1241–1244. [DOI] [PubMed] [Google Scholar]
- Ticconi, C.A. , Lucero, R.D. , Sakhonwasee, S. , Adamson, A.W. , Creff, A. , Nussaume, L. et al. (2009) ER‐resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proceedings of the National Academy of Sciences, 106, 14174–14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti, V.B. , Bielach, A. & Hrtyan, M. (2017) Redox regulation at the site of primary growth: auxin, cytokinin and ROS crosstalk. Plant, Cell & Environment, 40, 2586–2605. [DOI] [PubMed] [Google Scholar]
- Trevisan, S. , Trentin, A.R. , Ghisi, R. , Masi, A. & Quaggiotti, S. (2019) Nitrate affects transcriptional regulation of UPBEAT1 and ROS localisation in roots of Zea mays L. Physiologia Plantarum, 166, 794–811. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi, H. , Busch, W. & Benfey, P.N. (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell, 143, 606–616. [DOI] [PubMed] [Google Scholar]
- Tyburski, J. , Dunajska, K. & Tretyn, A. (2009) Reactive oxygen species localization in roots of Arabidopsis thaliana seedlings grown under phosphate deficiency. Plant Growth Regulation, 59, 27–36. [Google Scholar]
- Tyburski, J. , Dunajska, K. & Tretyn, A. (2010) A role for redox factors in shaping root architecture under phosphorus deficiency. Plant Signaling & Behavior, 5, 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undurraga, S.F. , Ibarra‐Henríquez, C. , Fredes, I. , Álvarez, J.M. & Gutiérrez, R.A. (2017) Nitrate signaling and early responses in Arabidopsis roots. Journal of Experimental Botany, 68, 2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velappan, Y. , Signorelli, S. & Considine, M.J. (2017) Cell cycle arrest in plants: what distinguishes quiescence, dormancy and differentiated G1? Annals of Botany, 120, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljović Jovanović, S. , Kukavica, B. , Vidović, M. , Morina, F. & Menckhoff, L. (2018) Class III Peroxidases: Functions, Localization and Redox Regulation of Isoenzymes. In: Gupta, D.K. , Palma, J.M. & Corpas, F.J. (Eds.) Antioxidants Enzyme High Plants. Cham: Springer International Publishing, pp. 269–300. [Google Scholar]
- Vernoux, T. , Wilson, R.C. , Seeley, K.A. , Reichheld, J.P. , Muroy, S. , Brown, S. et al. (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione‐dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. The Plant Cell, 12, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Dai, X. , Xu, G. , Dai, Z. , Chen, P. , Zhang, T. et al. (2021) The Ca2+‐CaM signaling pathway mediates potassium uptake by regulating reactive oxygen species homeostasis in tobacco roots under Low‐K+ stress. Frontiers in Plant Science, 12, 658609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, F. , Chi, Y. , Jiang, Z. , Xu, Y. , Xie, L. , Huang, F. et al. (2020) Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature, 578, 577–581. [DOI] [PubMed] [Google Scholar]
- Xue, C. , Li, W. , Shen, R. & Lan, P. (2021) PERK13 modulates phosphate deficiency‐induced root hair elongation in Arabidopsis. Plant Science, 312, 111060. [DOI] [PubMed] [Google Scholar]
- Yadav, B. , Jogawat, A. , Lalnitrate, S.K. , Lakra, N. , Mehta, S. , Shabek, N. et al. (2021) Plant mineral transport systems and the potential for crop improvement. Planta, 253, 45. [DOI] [PubMed] [Google Scholar]
- Yamada, M. , Han, X. & Benfey, P.N. (2020) RGF1 controls root meristem size through ROS signalling. Nature, 577, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Mhamdi, A. & Noctor, G. (2019) Analysis of catalase mutants underscores the essential role of CATALASE2 for plant growth and day length‐dependent oxidative signalling. Plant, Cell & Environment, 42, 688–700. [DOI] [PubMed] [Google Scholar]
- York, L.M. , Nord, E.A. & Lynch, J.P. (2013) Integration of root phenes for soil resource acquisition. Frontiers in Plant Science, 4, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, L. , Morère‐Le Paven, M.C. , Clochard, T. , Porcher, A. , Satour, P. , Mojović, M. et al. (2020) Nitrate inhibits primary root growth by reducing accumulation of reactive oxygen species in the root tip in Medicago truncatula . Plant Physiology and Biochemistry, 146, 363–373. [DOI] [PubMed] [Google Scholar]
- Zang, L. , Tarkowski, Ł.P. , Morère‐le Paven, M.C. , Zivy, M. , Balliau, T. , Clochard, T. et al. (2022) The nitrate transporter MtNPF6.8 is a master sensor of nitrate signal in the primary root tip of Medicago truncatula . Frontiers in Plant Science, 13, 832246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Bousquet, A. & Harris, J.M. (2014) Abscisic acid and lateral root organ defective/numerous infections and polyphenolics modulate root elongation via reactive oxygen species in Medicago truncatula . Plant Physiology, 166, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Xiang, Y. , Li, C. & Yu, G. (2020) Modulatory role of reactive oxygen species in root development in model plant of Arabidopsis thaliana . Frontiers in Plant Science, 11, 485932. [DOI] [PMC free article] [PubMed] [Google Scholar]


