Abstract
Hyperosmolar Hyperglycaemic State (HHS) is a medical emergency associated with high mortality. It occurs less frequently than diabetic ketoacidosis (DKA), affects those with pre‐existing/new type 2 diabetes mellitus and increasingly affecting children/younger adults. Mixed DKA/HHS may occur. The JBDS HHS care pathway consists of 3 themes (clinical assessment and monitoring, interventions, assessments and prevention of harm) and 5 phases of therapy (0–60 min, 1–6, 6–12, 12–24 and 24–72 h). Clinical features of HHS include marked hypovolaemia, osmolality ≥320 mOsm/kg using [(2×Na+) + glucose+urea], marked hyperglycaemia ≥30 mmol/L, without significant ketonaemia (≤3.0 mmol/L), without significant acidosis (pH >7.3) and bicarbonate ≥15 mmol/L. Aims of the therapy are to improve clinical status/replace fluid losses by 24 h, gradual decline in osmolality (3.0–8.0 mOsm/kg/h to minimise the risk of neurological complications), blood glucose 10–15 mmol/L in the first 24 h, prevent hypoglycaemia/hypokalaemia and prevent harm (VTE, osmotic demyelination, fluid overload, foot ulceration). Underlying precipitants must be identified and treated. Interventions include: (1) intravenous (IV) 0.9% sodium chloride to restore circulating volume (fluid losses 100–220 ml/kg, caution in elderly), (2) fixed rate intravenous insulin infusion (FRIII) should be commenced once osmolality stops falling with fluid replacement unless there is ketonaemia (FRIII should be commenced at the same time as IV fluids). (3) glucose infusion (5% or 10%) should be started once glucose <14 mmol/L and (4) potassium replacement according to potassium levels. HHS resolution criteria are: osmolality <300 mOsm/kg, hypovolaemia corrected (urine output ≥0.5 ml/kg/h), cognitive status returned to pre‐morbid state and blood glucose <15 mmol/L.
Keywords: emergency, HHS, hyperosmolar hyperglycaemic state, inpatient
What's new?
A new summarised care pathway and algorithm for osmolality/glucose changes.
Updated bedside monitoring chart.
We introduced a formal definition of resolution of HHS and audit standards.
1. INTRODUCTION
Until the publication of the first edition of this document, national guidelines on the management of hyperosmolar hyperglycaemic state (HHS) in adults had been uncommon. As is now well recognised, HHS is different to diabetic ketoacidosis (DKA), and treatment requires a different approach. Although typically occurring in those aged over 45, 1 , 2 HHS can present in children and younger adults, 1 , 3 , 4 often as the initial presentation of type 2 diabetes mellitus (T2DM). 1 , 5
HHS is uncommon. In the United States, it accounts for only 13% of hyperglycaemia‐related emergency admissions 2 but has a higher mortality than DKA. 6 , 7 , 8 , 9 There are no recent publications from the United Kingdom on mortality in HHS, but reported series suggest mortality may have improved though remains high at between 15% and 20%. 10 , 11 , 12 , 13 , 14
HHS often develops over many days, and consequently, the dehydration and metabolic disturbances are usually more extreme than with DKA. Many people with diabetes have severe but transient elevations of blood glucose—the difference between this and HHS, being the duration of hyperglycaemia and the accompanying dehydration.
As with many serious but rare metabolic emergencies, the evidence for treatment is based more on clinical experience and consensus than randomised controlled trials. What is clear is that the greater mortality and morbidity in HHS is only in part related to age and co‐morbidities. There has been controversy around the speed and type of fluid replacement and when insulin should be introduced. However, the use of the previous edition of this guideline has standardised practice across many parts of the United Kingdom, with most teams feeling they were of good quality and safe. 15
These guidelines are evidence based as far as that evidence exists, otherwise, they reflect a consensus derived from an analysis of the published literature in English and the views of specialist diabetes clinicians in the United Kingdom. 7 The emphasis throughout is on ensuring that biochemical evaluation must go hand in hand with clinical evaluation. Correction of the biochemistry alone does not guarantee a good outcome. They are intended for use by any healthcare professional that manages HHS in adults.
Management of those <18 years old managed by the paediatric team should follow the following guidelines http://www.a‐c‐d‐c.org/wp‐content/uploads/2012/08/Practical‐Management‐of‐Hyperglycaemic‐Hyperosmolar‐State‐HHS‐in‐children‐8.pdf 16
The care pathway for the management of HHS is divided into timeline phases to emphasise the importance of regular monitoring and updates in treatments and initiate escalations where appropriate (Figures 1 and 2).
FIGURE 1.
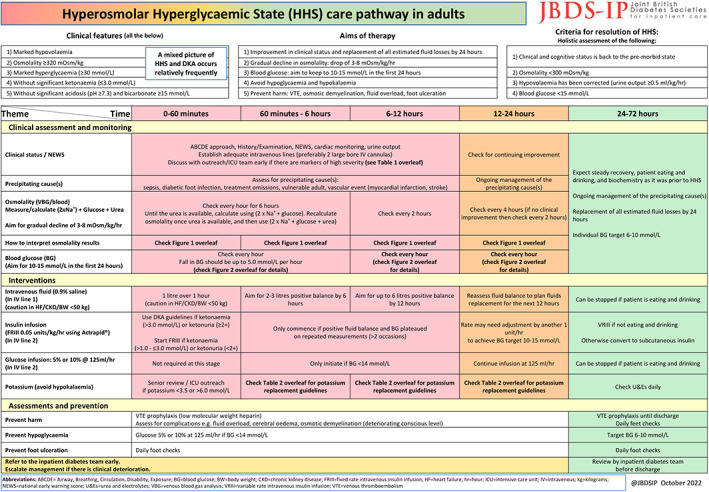
Page 1 of the HHS care pathway. This side includes time‐based thematic division of the care pathway by clinical assessment and monitoring, interventions, assessments and preventions and referral to the inpatient diabetes team.
FIGURE 2.
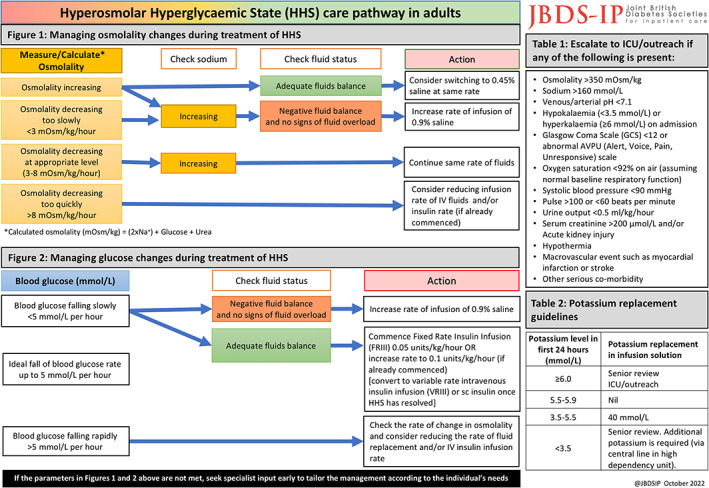
Page 2 of the HHS care pathway. This side includes algorithms for the management of osmolality, glucose and potassium during the management of HHS. Also included are the criteria of when escalate care.
2. DEFINITION AND DIAGNOSIS
International guidelines vary as to the precise definition of HHS, 7 but there are characteristic features that differentiate it from other hyperglycaemic states such as DKA. 17 Defining HHS by osmolality alone is inappropriate without considering other clinical features (Figure 3).
FIGURE 3.
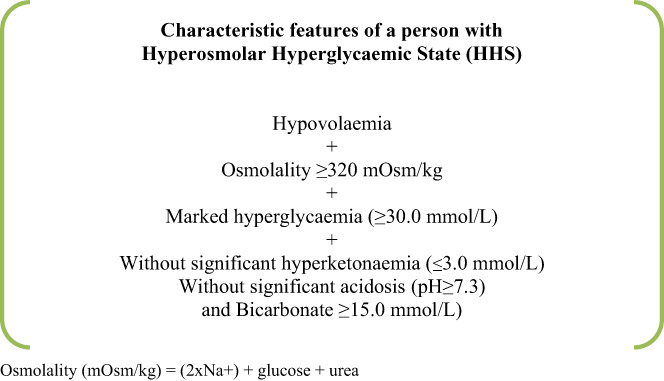
Definition and characteristic features of a person with HHS.
Previously called HyperOsmolar Non‐Ketotic (HONK) coma, it was apparent that most of these people were not comatosed but were extremely ill. Changing the name to Hyperosmolar Hyperglycaemic State (HHS) allows for the fact that some people with severely raised blood glucose may also be mildly ketotic and acidotic. The reasons why these people do not develop ketoacidosis are not fully understood. 18 In HHS, insulin levels are just adequate to prevent ketogenesis but fail to counteract hyperglycaemia. Therefore, the presentation is mainly driven by extreme levels of hyperglycaemia and consequent osmotic diuresis. 19
People with HHS are generally older than those with DKA, but increasingly, as the diabetes pandemic crosses generational boundaries, it may be seen in young adults and even children as the first presentation of newly diagnosed diabetes. 1 , 3 , 4 HHS has a slower onset than DKA. This is important because the brain tissue of those who develop HHS, particularly in those who are older, is at higher risk of injury due to rapid shifts in sodium, water and glucose. Therefore, to prevent significant neurological damage, HHS requires less aggressive fluid resuscitation and slower glucose‐lowering than DKA.
2.1. Clinical presentation
The most common precipitating factors for HHS include: infections, discontinuation/omission of antidiabetic medications, cardiovascular events, pancreatitis and drugs (corticosteroids, thiazides, sympathomimetic agents and conventional antipsychotics).
Clinical evaluation of HHS should follow the ABCDE approach. 20 The constellation of sunken eyes, longitudinal furrows on the tongue and extremity weakness correlates well with raised blood urea. 21 , 22 Severe hypovolaemia may manifest as tachycardia (pulse > 100 bpm) and/or hypotension (systolic blood pressure < 100 mm Hg). 6 , 23 , 24 People will usually be identified as being at high risk by use of a validated triage system, for example, NEWS. 20 However, despite these severe electrolyte losses and total body volume depletion, often the person with HHS may not look as dehydrated as they are, because the hypertonicity leads to preservation of intravascular volume, (causing movement of water from intracellular to extracellular spaces). 4 , 25 , 26 , 27 Typical fluid losses in HHS are detailed in Table 1.
TABLE 1.
Typical fluid and electrolyte losses in HHS
| For a 60 kg individual | For a 100 kg individual | ||
|---|---|---|---|
| Water | 100–220 ml/kg | 6–13 L | 10–22 L |
| Na+ | 5–13 mmol/kg | 300–780 mmol | 500–1300 mmol |
| Cl− | 5–15 mmol/kg | 300–900 mmol | 500–1500 mmol |
| K+ | 4–6 mmol/kg | 240–360 mmol | 400–600 mmol |
Note: Na+ = Sodium, Cl− = Chloride, K+ = Potassium.Adapted from Kitabchi & Nyenwe. 11
Acute cognitive impairment may not be necessarily present, may be associated with dehydration but is not specific to the condition. Alterations in cognitive status are more common when the osmolality rises >330 mOsm/kg. HHS can have marked effects on cerebral function and be associated with transient changes in cognitive performance and also with longer‐term effects. This may be due to a number of things including, but not limited to: cerebral oedema in severe cases, the presence of significant electrolyte disturbances, acute changes in osmolality, dehydration, infection/sepsis, hypoglycaemia during treatment or kidney injury. A daily assessment of cognition during admission with a comparison to the pre‐morbid state should accompany the full history, physical examination and review of drug therapy on admission.
2.2. Biochemical abnormalities
HHS should not be diagnosed using biochemical parameters alone. However, the blood glucose is markedly raised (usually ≥30 mmol/L), as is the osmolality (usually ≥320 mOsm/kg). Osmolality is useful as an indicator of severity and for monitoring the rate of change with treatment. Serum osmolality is often provided in biochemistry reports, either calculated or measured, but can be calculated using the formula [(2×Na+) + glucose + urea]. This formula gives the best approximation to measured osmolality, although a more accurate formula has been derived. 27 For the sake of clarity, calculated osmolarity and measured osmolality is referred to as osmolality in this guideline. Urea is not an effective osmolyte but including it in the calculation is important in the hyperosmolar state, as it is one of the indicators of severe dehydration. The laboratory calculation may use a different formula and that laboratory measurement is a batched procedure, that is, may run once a day, so not usually available for rapid repeat measurements unless it is discussed with the laboratory. An increasing number of emergency departments and critical care units use blood gases machines that provide point of care measurement of osmolality.
3. MIXED DKA/HHS
In HHS there is usually no significant ketosis/ketonaemia (blood ketones ≤3.0 mmol/L), although a mild acidosis (pH <7.3, bicarbonate >15.0 mmol/L) may accompany those affected by acute kidney injury or severe sepsis. Some people have severe hypertonicity and ketosis and acidosis (mixed DKA and HHS). 8 This situation is likely to reflect a relative insulin deficiency due to beta cell exhaustion as a result of temporary glucotoxicity and excess counter‐regulatory hormone production. These individuals may require a modification of this treatment guideline to take into account which aspect predominates. If a predominant diagnosis is unclear (HHS vs. mixed HHS/DKA), early specialist input should be sought to help tailor management according to the individual's need. However, if ketone concentrations are high (blood ketones >3.0 mmol/L), then the DKA protocol may be appropriate (Figure 4).
FIGURE 4.
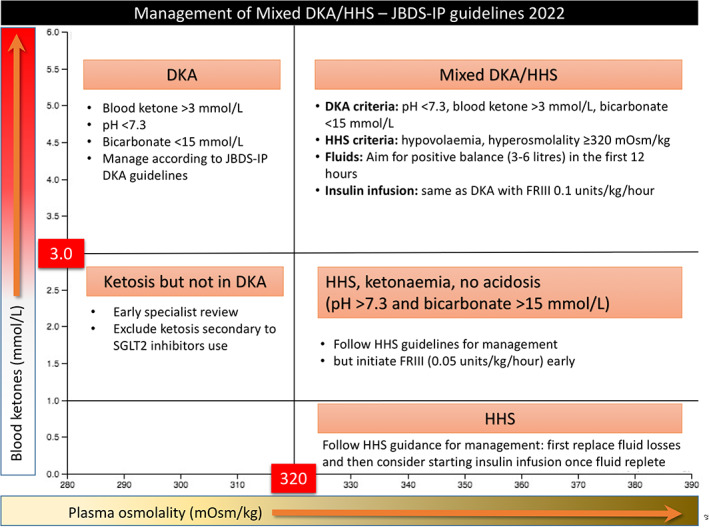
The overlap between diabetic ketoacidosis (DKA) and hyperosmolar hyperglycaemic state (HHS) and how to approach management.
Treat as mixed DKA/HHS if all the following criteria are met: marked hypovolaemia, marked hyperosmolality (osmolality ≥320 mOsm/kg), pH <7.3, bicarbonate <15 mmol/L and blood ketones >3.0 mmol/L. Start a Fixed Rate Intravenous Insulin Infusion (FRIII) and IV fluids immediately and treat according to DKA pathway using 0.1 units/kg/h. IV fluid replacement should aim to achieve a positive balance of 3–6 L during the first 12 h and the remaining replacement of estimated fluid loss during the following 12 h, although complete normalisation of biochemistry may take up to 72 h.
4. MANAGEMENT
4.1. Goals of therapy
The goals of treatment of HHS are to address the underlying cause(s) and to achieve:
Gradual normalisation of the osmolality
Safe replacement of fluid and electrolyte losses
Safe and gradual normalisation of blood glucose
Prevention of arterial or venous thrombosis
Prevention of potential complications, for example, cerebral oedema, osmotic demyelination syndrome (ODS)
Prevention of foot ulceration
4.2. General treatment principles
Early senior review by a clinician familiar with the management of HHS is essential to confirm the treatment plan and review progress. The diabetes inpatient specialist team should be involved in the care as soon as possible.
4.3. Osmolality, sodium and glucose
The key parameter in HHS is osmolality. Sodium and glucose are the main contributors to this, and too rapid changes are dangerous because large fluid shifts can lead to neurological complications, in particular cerebral oedema and osmotic demyelination. Because these parameters are interrelated, we advise that they are plotted on a graph or tabulated to permit appreciation of the rate of change (See Appendix S1 ).
Total body water is divided between the intra and extracellular fluid spaces, and its distribution is determined by the presence of osmotically effective substances on either side of the cell membrane. Intracellular osmotic pressure is exerted principally by potassium, chloride and phosphate ions while extracellular osmotic pressure is primarily dependent upon sodium, chloride and bicarbonate ions. These osmoles play a critical role in the movement of free water across the cell membrane since they are themselves unable to pass freely between the intra and extracellular compartments. Glucose, lipids and proteins also exert an osmotic pressure, being largely confined to the extracellular space, while urea and ethanol are termed ineffective osmoles, recognising that because they are able to freely move across cell membranes they play no role in the distribution of free water. 26 , 28
Serum sodium, a close approximation to osmolality in the absence of hyperglycaemia, may be reassuringly normal or even low in the presence of hyperglycaemia. The addition of glucose to the extracellular space causes an osmotic shift of free water into the extracellular fluid and a resultant dilution of serum sodium.
There are many different formulae to calculate osmolality, for example [(2×Na+) + glucose + urea], [2(Na++K+) + glucose], and [2×Na+ + glucose]. The best approximation to measured osmolality can be calculated using the formula [(2×Na+) + glucose + urea], though a more accurate formula has been derived. 29 However, as urea is an ineffective osmolyte, it can be omitted from the equation to allow calculation of tonicity (or effective osmolality). This is of the greatest importance when someone is hyponatraemic since tonicity indicates risk of cerebral oedema, that is, hypo‐osmolality.
Thus, an individual with a Na+ 122 mmol/L, glucose 13 mmol/L, urea 23 mmol/L has a calculated osmolality of 280 mOsm/kg and an effective osmolality of 257 mOsm/kg, whereas a person with a Na+ 122 mmol/L, glucose 30 mmol/L, urea 4 mmol/L has a calculated osmolality of 278 mOsm/kg and an effective osmolality of 274 mOsm/kg. So the person with the raised urea has a much lower effective osmolality and is therefore at a greater risk of osmotic demyelination should correction of the hyponatraemia be too fast. The hyponatraemia in an individual with a blood glucose of 30 mmol/L is largely dilutional and will correct as the glucose falls (corrected Na+ = 122 + (2.4 × 4) = 131.6). 30 In the hyperosmolar state, osmolality is useful as an indicator of severity and for monitoring the rate of change with treatment. If frequent measurement of osmolality is not practical, osmolality should be calculated using the formula [(2×Na+) + glucose + urea]. 29 This is the formula used throughout this guideline.
Use of the previous version of this guideline 31 has confirmed that fluid replacement alone will lower glucose concentrations. An FRIII should not be started as part of the initial treatment unless significant ketonaemia is present (>3.0 mmol/L or urine ketones >2+). In all other circumstances, intravenous fluids should be administered first, and an FRIII only started once the glucose has stopped falling. The risk of adding insulin at the start of treatment will lead to larger osmotic shifts leading to neurological complications. In addition, adding IV insulin too early will also potentially lead to circulatory collapse 4 (see Appendix S2 ).
If the IV fluids and FRIII are managed appropriately, the fall in serum osmolality should be within the target range of 3.0–8.0 mOsm/kg/h. If the rate is faster than this, it increases the risk of neurological complications such as cerebral oedema and osmotic demyelination.
4.4. Fluid replacement
The aim of treatment should be to replace approximately 50% of estimated fluid loss within the first 12 h and the remainder in the following 12 h. However, this will in part be determined by the initial severity, degree of renal impairment and co‐morbidities such as heart failure, which may limit the speed of correction. An initial target glucose of between 10 and 15 mmol/L is a reasonable goal until the person is eating and drinking normally, and then an individual target glucose (if appropriate 6–10 mmol/L) should be set by the diabetes specialist team and the person with diabetes. Ideally, people will recover quickly enough to replace the water deficit themselves by taking fluids orally.
The goal of the initial therapy is expansion of the intravascular and extravascular volume and to restore peripheral perfusion. There are almost no data on the benefits or risks of particular fluid replacement regimens in HHS. Controversies persist around the speed and type of fluid replacement, and a systematic review is being undertaken. 32 However, a Cochrane review recommended the use of crystalloid fluids rather than colloid in critically ill individuals because the use of crystalloids is associated with less need for further interventions. 33 As the majority of electrolyte losses are sodium, chloride and potassium, the initial fluid replacement of choice should be 0.9% sodium chloride solution with potassium added as required. 34
Rapid changes in osmolality may be harmful. 0.9% sodium chloride solution should be used as the principal fluid to restore circulating volume and reverse dehydration because it is relatively hypotonic compared to the serum in someone with HHS. Check osmolality every hour initially and the rate of fluid replacement adjusted accordingly to ensure a positive fluid balance sufficient to promote a gradual decline in osmolality. Fluid replacement alone (without insulin) will lower glucose concentrations which will lower serum osmolality by causing a shift of water into the intracellular space. This inevitably results in a rise in serum sodium (a fall in blood glucose of 5.5 mmol/L will result in a 2.4 mmol/L rise in sodium). This is not necessarily an indication to give hypotonic solutions. A rising sodium is only a concern if the osmolality is not declining concurrently. If the inevitable rise in serum sodium is much greater than 2.4 mmol/L for each 5.5 mmol/L fall in blood glucose this would suggest insufficient fluid replacement. 30
Overall, the rate of fall of serum sodium should not exceed 10 mmol/L in 24 h. 35 A safe rate of fall of plasma glucose should not be more than 5 mmol/h. However, if the osmolality is no longer declining despite adequate fluid replacement with 0.9% sodium chloride solution and an adequate rate of fall of plasma glucose is not being achieved, then 0.45% sodium chloride solution should be substituted. There are no data to justify using fluids that are less concentrated than 0.45% sodium chloride solution.
Complete normalisation of electrolytes and osmolality may take up to 72 h.
4.5. Potassium replacement
Hypokalaemia in HHS is multifactorial. Osmotic diuresis and consequent hypertonicity from hyperglycaemia lead to fluids shifts from the intracellular space into the extracellular space. This leads to intracellular potassium loss. In addition, hypoperfusion leads to the activation of aldosterone secretion, further exacerbating the potassium loss. 4 , 19 Vomiting, if present, worsens hypokalaemia. 18 Lack of insulin in DKA, accumulation of ketone bodies leading to ketoacidosis and drop in bicarbonate ion concentration leads to a further loss of potassium from the intracellular to the extracellular space. In both cases (DKA and/or HHS) there is a total body loss of potassium. Treatment with insulin will lead to a shift of potassium back into the intracellular space which unravels hypokalaemia.
Those on diuretics may be profoundly hypokalaemic to start with. It is also worth noting that if acute kidney injury is present, hyperkalaemia may be present owing to reduced clearance and therefore closer monitoring is required.
Potassium level assessment and replacement is crucial in management of HHS and if mixed with ketosis/DKA. For guidance on potassium replacement see Table 2.
TABLE 2.
Suggested potassium replacement regimen in HHS
| Potassium level in first 24 h (mmol/L) | Potassium replacement in infusion solution |
|---|---|
| ≥6.0 | Senior review ICU/outreach |
| 5.5–5.9 | Nil |
| 3.5–5.5 | 40 mmol/L |
| <3.5 | Senior review as additional potassium is required (via central line in high dependency unit) |
4.6. Insulin dose and timing
Fluid replacement alone with 0.9% sodium chloride solution will result in a falling blood glucose. Lowering glucose level by early insulin use in HHS will lower osmolality precipitously and risks osmotic fluid shifts leading to circulatory collapse. There is also higher risk of hypokalaemia and hypoglycaemia. To prevent the serum osmolality falling too quickly, the plasma glucose should ideally fall by no more than 5 mmol/L/h. Insulin treatment prior to adequate fluid replacement may result in cardiovascular collapse because water will move out of the intravascular space, resulting in a reduction in intravascular volume (a consequence of insulin‐mediated glucose uptake and a diuresis from urinary glucose excretion). 4
Once the glucose has ceased to fall following initial fluid resuscitation, reassessment of fluid intake and evaluation of renal function must be undertaken. Insulin may be started at this point. As with DKA, an FRIII is preferred, though generally lower doses are required. The recommended initial insulin dose is an FRIII given at 0.05 units/kg/h. If an FRIII is already in place, the infusion rate can be increased by 1.0 unit/h.
Glucose infusion (5% or 10%) should be started once blood glucose is <14 mmol/L and run alongside FRIII and other fluid replacement. Choice of the glucose fluid concentration (5% or 10%) depends on the individual patient factors (e.g., risk of fluid overloads) and/or institutional factors (e.g. cost and availability).
In cases of HHS associated with raised ketones levels, earlier initiation of insulin is required to address the state of ketosis (see Mixed DKA/HHS section).
4.7. Clinical scenarios and interpretation of serum sodium, osmolality and glucose concentrations
During the phases of management of HHS, it is important to observe changes in osmolality, serum sodium and glucose. The flow diagrams in the care pathway illustrate the options (Figure 2).
4.8. Antibiotic therapy
As with all acutely ill people, sepsis may not be accompanied by pyrexia. An infective source should be sought on clinical history and examination. Antibiotics should be given when there are clinical signs, and/or laboratory or radiological evidence of infection. Follow the local infection and sepsis guidelines.
4.9. Anticoagulation
Whilst thrombotic complications such as myocardial infarction, stroke or peripheral arterial thrombosis occur more frequently in HHS, it is not known whether or not these can be prevented by prophylaxis with low dose LMWH or anti‐platelet therapy, or if a full therapeutic dose should be used. 36 , 37
Having diabetes is associated with an increased risk of developing venous thromboembolic disease (VTE). 38 People with HHS have an increased risk of arterial and VTE. 39 , 40 A study of hyperglycaemia (not specifically with HHS) during COVID‐19 admissions suggested that the risk of arterial and VTE was three times higher than those without hyperglycaemia. 41 Other work has estimated that people with diabetes and hyperosmolality have a risk of VTE similar, or only marginally above those with acute renal failure, acute sepsis or acute connective tissue disease. 42 , 43 The risk of venous thromboembolism is greater than in diabetic ketoacidosis. 39 , 44 , 45 Other factors, such as hypernatraemia and increasing vasopressin concentrations can promote thrombogenesis by producing changes in haemostatic function consistent with a hypercoagulable state. 46 Everyone with HHS should receive prophylactic low molecular weight heparin (LMWH) for the full duration of admission unless contraindicated. There are no data to recommend that this advice be extended to therapeutic anticoagulation. Full, therapeutic anticoagulation should only be considered in those with suspected thrombosis or acute coronary syndrome.
4.10. Other electrolyte imbalances and complications associated with HHS
Hypophosphataemia and hypomagnesaemia are common in HHS. There are no data looking at the use of replacement of either of these in HHS. It is likely that this represents an epiphenomenon, particularly for magnesium where protein binding is affected by the change in the extracellular milieu and that therefore tissue status is likely normal. Many studies in ICU settings demonstrate no evidence of tissue deficiency nor any benefit from magnesium replacement in acutely ill patients with hypomagnesaemia. If low concentrations persist beyond the acute phase of treatment and the patient is apparently symptomatic with good risk factors for long‐term magnesium deficiency, oral replacement may be considered (IV is associated with high urinary excretion therefore very little of the infusion remains in the circulation so should only be considered if severely deplete and symptomatic, for example, ECG changes or neurological manifestations); see local magnesium replacement guidelines for further advice. Proton pump inhibitor use is common in the same population who develop HHS and the use of these drugs has been associated with hypomagnesaemia.
4.11. Foot protection
People with HHS are at high risk of pressure‐related foot ulceration. An initial foot assessment should be undertaken on admission and daily during admission. 47 Heel protectors and an appropriate mattress should be provided for those with immobility, neuropathy, peripheral vascular disease or lower limb deformity. If the individuals are too confused or drowsy to cooperate with the assessment of sensation assume they are at high risk.
4.12. Point of care versus laboratory testing
Blood gas machines are readily available in almost all UK emergency departments. These can produce reliable measurements of pH, electrolytes, glucose etc. and should be used to frequently monitor progress and calculation of osmolality. Increasingly in some units, blood gas machines can offer measurements of osmolality. Unless it is necessary to also measure oxygen saturation, venous rather than arterial samples are sufficient. Local facilities will determine which mechanism is the most safe and efficient.
Serum lactate and ketones must also be checked, usually using venous blood gases (VBG) and point of care testing. The former can indicate lactic acidosis related to sepsis, for example and the latter will exclude significant ketonaemia (β‐hydroxybutyrate <1.0 mmol/L).
5. ESCALATION OF MANAGEMENT
Care for people with HHS can be complex, they often have multiple co‐morbidities and may require intensive monitoring. The presence of one or more of the following should prompt discussion about the need for admission to a High‐Dependency Unit/Level 2 environment. Immediate senior review by a clinician skilled in the management of HHS should be considered:
Measured or calculated Osmolality >350 mOsm/kg
Sodium >160 mmol/L
Venous/arterial pH <7.1
Hypokalaemia (<3.5 mmol/L) or hyperkalaemia (≥6 mmol/L) on admission
Glasgow Coma Scale (GCS) <12 or abnormal AVPU (Alert, Voice, Pain, Unresponsive) scale
Oxygen saturation <92% on air (assuming normal baseline respiratory function)
Systolic blood pressure < 90 mm Hg
Pulse >100 or <60 bpm
Urine output <0.5 ml/kg/h
Serum creatinine >200 μmol/L and/or acute kidney injury
Hypothermia
Macrovascular event such as myocardial infarction or stroke
Other serious co‐morbidity
6. DEFINITION OF RESOLUTION OF HHS
Because the precise definition of HHS has not been agreed, it is difficult to give a precise definition of when HHS has resolved. Different authors have used different criteria for resolution, with some using osmolality as the criteria, others using volume status or cognitive status. It is important to remember that a normal glucose or sodium concentration in isolation is not sufficient to say that the episode has resolved. It can also be difficult to gauge the degree of dehydration at the bedside. However, we propose that a holistic approach be used.
Resolution of HHS can be defined as outlined in Table 3.
TABLE 3.
Criteria for resolution of HHS
| Criteria for resolution of HHS: Holistic assessment of the following: |
| 1) Clinical and cognitive status is back to the pre‐morbid state |
| 2) Osmolality <300 mOsm/kg |
| 3) Hypovolaemia has been corrected (urine output ≥0.5 ml/kg/h) |
| 4) Blood glucose <15 mmol/L |
7. RECOVERY PHASE
Complete correction of electrolyte and osmolality abnormalities may take up to 72 h. Because many of these individuals are elderly with multiple co‐morbidities, recovery will largely be determined by their previous functional status and the underlying precipitant(s) of HHS. Early mobilisation is essential, as is the need for good nutrition. IV insulin can usually be discontinued once they are eating and drinking but IV fluids may be required for longer if intake is inadequate. Most people should be transferred to subcutaneous insulin (the regimen is determined by their circumstances). For those with previously undiagnosed diabetes or who were well‐controlled on oral agents, switching from insulin to the appropriate oral or subcutaneous glucose‐lowering drugs should be considered after a period of stability. This may take weeks or months. Everyone with HHS will require diabetes specialist input (including the offer of education) to reduce the risk of recurrence, prevent long‐term complications and review of other factors that may have led to episodes of HHS (e.g., psychosocial factors in vulnerable adults). Where applicable, holistic multiple cardiovascular risk factor optimisation should also occur.
8. COMPLICATIONS
HHS has high mortality because it may be due to, or complicated by, vascular events such as myocardial infarction, stroke or peripheral arterial and venous thrombosis. 8 , 48 Neurological complications, such as cerebral oedema and ODS are uncommon but can be seen as a complication of the rapid changes in osmolality during treatment of HHS. 49 , 50
9. HHS DEVELOPING INPATIENT STAYS
HHS may develop in the inpatient setting due to a number of factors including sepsis, initiation of new therapies (e.g., corticosteroids and antipsychotics), inadequate antidiabetic treatments/insulin, omissions/interruptions to care pathways, surgery, new/ongoing parenteral/enteric feeding. National audit data from England suggests HHS in hospital is particularly likely to develop in those admitted with or treated for stroke. 51
The occurrence of HHS during inpatient stays, as is DKA, should be reported and investigated as an adverse incident through the governance pathways. Serious inpatient harms data (severe hypoglycaemia, Diabetic ketoacidosis (DKA)/hyperosmolar hyperglycaemic state (HHS), and new foot ulcer) are included in the data collection for National Diabetes Inpatient Safety Audit (NDISA) (previously NaDIA‐Harms).
10. CARE PATHWAY
The management timelines of HHS are divided into five phases as below.
0–60 min
1–6 h
6–12 h
12–24 h
Beyond 24 h
T = 0 is the time when the intravenous fluids are commenced. It is important to establish adequate intravenous access (preferably 2 large‐bore intravenous cannulas). Line 1 can be dedicated to intravenous fluids replacement and line 2 for insulin (when initiated) and intravenous glucose (later in the pathway). If there is a problem with intravenous access critical care support should be requested immediately.
The pathway is divided into 3 main sections: (1) Clinical assessment and monitoring, (2) Interventions and (3) Assessments and prevention.
All cases of HHS must be referred to the inpatient diabetes team for review and care planning. For full details of the care pathway see Figures 1 and 2.
11. IMPLEMENTATION AND AUDIT STANDARDS
Since the publication of the first version of the guidelines, it has been implemented and adapted widely. The guidelines are reviewed regularly for updates in line with the emerging evidence. This document should be regarded as a ‘live document’ and we welcome feedback.
This version includes audit standards for implementation and evaluation derived from institutional and national diabetes standards. 52 , 53 See Appendix S3 .
AUTHOR CONTRIBUTION
Omar G. Mustafa, Masud Haq, Umesh Dashora, Erwin Castro, Ketan K. Dhatariya are the writing group of the JBDS HHS guidelines (2022). Omar G. Mustafa and Ketan K. Dhatariya drafted the manuscript. All authors reviewed and approved the manuscript.
CONFLICT OF INTEREST
OM has received honoraria, travel and personal fees from Sanofi Diabetes, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. MH has received honoraria, travel and personal fees from Eli Lilly, Astra Zeneca, Novo Nordisk, NAPP, Sanofi and Boehringer Ingelheim. UD has received honoraria and speaker fee from Sanofi Diabetes, Eli Lillly, Astra Zeneca, Boehringer Ingelheim and Novo Nordisk. KD is the chair of the Joint British Diabetes Societies for Inpatient Care and has received honoraria, travel and personal fees from Sanofi Diabetes, Eli Lilly, AstraZeneca, Boehringer Ingelheim and Novo Nordisk.
Supporting information
Appendix S1.
Appendix S2.
Appendix S3.
Appendix S4.
ACKNOWLEDGEMENT
We wish to thank Christine Jones (Norfolk & Norwich University Hospitals NHS trust) for her support and dedication to seeing this guideline completed. We would like to thank the support of the following organisation which made the work of JBDS possible: Diabetes UK, Association of British Clinical Diabetologists (ABCD), and Diabetes Inpatient Specialist Nurse (DISN) UK Group.
Mustafa OG, Haq M, Dashora U, Castro E, Dhatariya KK, the Joint British Diabetes Societies (JBDS) for Inpatient Care Group . Management of Hyperosmolar Hyperglycaemic State (HHS) in Adults: An updated guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Diabet Med. 2023;40:e15005. doi: 10.1111/dme.15005
DATA AVAILABILITY STATEMENT
There is no data in our document. It is guidelines and a DUK position statement. So this section is irrelevant.
REFERENCES
- 1. Zubkiewicz‐Kucharska A, Chrzanowska J, Noczyńska A. Hyperosmolar hyperglycaemic state (HHS) as the first manifestation of type 2 diabetes in a child. Pediatr Endocrinol Diabetes Metab. 2019;25(2):85‐89. doi: 10.5114/pedm.2019.85819 [DOI] [PubMed] [Google Scholar]
- 2. Benoit SR, Hora I, Pasquel FJ, Gregg EW, Albright AL, Imperatore G. Trends in emergency department visits and inpatient admissions for hyperglycemic crises in adults with diabetes in the U.S., 2006–2015. Diabetes Care. 2020;43(5):1057‐1064. http://care.diabetesjournals.org/content/43/5/1057.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenbloom AL. Hyperglycemic hyperosmolar state: an emerging pediatric problem. J Pediatr. 2010;156(2):180‐184. [DOI] [PubMed] [Google Scholar]
- 4. Zeitler P, Haqq A, Rosenbloom A, Glaser N. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9‐14. doi: 10.1016/j.jpeds.2010.09.048 [DOI] [PubMed] [Google Scholar]
- 5. Ekpebegh CO, Longo‐Mbenza B, Akinrinmade A, Blanco‐Blanco E, Bardi M, Levitt NS. Hyperglycaemic crisis in the eastern cape province of South Africa: high mortality and association of hyperosmolar ketoacidosis with a new diagnosis of diabetes. S Afr Med J. 2010;100(12):822‐826. [DOI] [PubMed] [Google Scholar]
- 6. Delaney MF, Zisman A, Kettyle WM. Diabetic ketoacidosis and hyperosmolar nonketotic syndrome. Endocrinol Metab Clin N Am. 2000;39(4):683‐705. [DOI] [PubMed] [Google Scholar]
- 7. Dhatariya KK, Vellanki P. Treatment of diabetic ketoacidosis (DKA) /hyperglycemic hyperosmolar state (HHS): novel advances in the management of hyperglycemic crises (UK versus US). Curr Diab Rep. 2017;17(5):33‐39. doi: 10.1007/s11892-017-0857-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasquel FJ, Tsegka K, Wang H, et al. Clinical outcomes in patients with isolated or combined diabetic ketoacidosis and hyperosmolar hyperglycemic state: a retrospective, hospital‐based cohort study. Diabetes Care. 2020;43(2):349‐357. http://care.diabetesjournals.org/content/early/2019/11/07/dc19‐1168.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kruljac I, Ćaćić M, Ćaćić P, et al. The impact of hyperosmolarity on long‐term outcome in patients presenting with severe hyperglycemic crisis: a population based study. Exp Clin Endocrinol Diabetes. 2018;126(9):564‐569. [DOI] [PubMed] [Google Scholar]
- 10. Pinies JA, Cairo G, Gaztambide S, Vazquez JA. Course and prognosis of 132 patients with diabetic non ketotic hyperosmolar state. Diabete Metab. 1994;20(1):43‐48. [PubMed] [Google Scholar]
- 11. Kitabchi AE, Nyenwe E. Hyperglycaemic crises in adult patients with diabetes mellitus. In: Wass JH, Stewart PM, Amiel SA, Davies MJ, eds. Oxford Textbook of Endocrinology and Diabetes. 2nd ed. Oxford University Press; 2011. doi: 10.1093/med/9780199235292.001.1 [DOI] [Google Scholar]
- 12. Macisaac RJ, Lee LY, McNeil KJ, Tsalamandris C, Jerums G. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J. 2002;32(8):379‐385. [DOI] [PubMed] [Google Scholar]
- 13. Chung ST, Perue GG, Johnson A, et al. Predictors of hyperglycaemic crises and their associated mortality in Jamaica. Diabetes Res Clin Pract. 2006;73(2):184‐190. http://www.sciencedirect.com/science/article/pii/S0168822706000064 [DOI] [PubMed] [Google Scholar]
- 14. Tittel SR, Sondern KM, Weyer M, et al. Multicentre analysis of hyperglycaemic hyperosmolar state and diabetic ketoacidosis in type 1 and type 2 diabetes. Acta Diabetol. 2020;57(10):1245‐1253. doi: 10.1007/s00592-020-01538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sampson M, Jones C. Joint British diabetes societies for inpatient care: clinical guidelines and improving inpatient diabetes care. Diabet Med. 2018;35(8):988‐991. doi: 10.1111/dme.13672 [DOI] [PubMed] [Google Scholar]
- 16. Ng SM, Edge JA, Timmis AE. Practical Management of Hyperglycaemic Hyperosmolar State (HHS) in Children: Association of Children's Diabetes Clinicians Guideline. Association of Children's Diabetes Clinicians. 2021. http://www.a‐c‐d‐c.org/wp‐content/uploads/2012/08/Practical‐Management‐of‐Hyperglycaemic‐Hyperosmolar‐State‐HHS‐in‐children‐8.pdf [Google Scholar]
- 17. Dhatariya KK, Joint British Diabetes Societies for Inpatient Care . The management of diabetic ketoacidosis in adults‐an updated guideline from the joint British diabetes Society for Inpatient Care. Diabet Med. 2022;39(6):e14788. [DOI] [PubMed] [Google Scholar]
- 18. English P, Williams G. Hyperglycaemic crises and lactic acidosis in diabetes mellitus. Postgrad Med J. 2004;80(943):253‐261. http://pmj.bmjjournals.com/cgi/content/abstract/80/943/253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. doi: 10.1038/s41572-020-0165-1 [DOI] [PubMed] [Google Scholar]
- 20. Royal College of Physicians . National Early Warning Score (NEWS) 2: Standardising the assessment of acute‐illness severity in the NHS. Updated report of a working party. RCP. Published 2017. https://www.rcplondon.ac.uk/projects/outputs/national‐early‐warning‐score‐news‐2 [Google Scholar]
- 21. Sinert R, Spektor M. Clinical assessment of hypovolemia. Ann Emerg Med. 2005;45(3):327‐329. doi: 10.1016/j.annemergmed.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 22. Gross CR, Lindquist RD, Wolley AC, Granieri R, Allard K, Webster B. Clinical indicators of dehydration severity in elderly patients. J Emerg Med. 1992;10(3):267‐274. [DOI] [PubMed] [Google Scholar]
- 23. Lapides J, Bourne RB, Maclean LR. Clinical signs of dehydration and extracellular fluid loss. JAMA. 1965;191(5):413‐415. [DOI] [PubMed] [Google Scholar]
- 24. Kavouras SA. Assessing hydration status. Curr Opin Clin Nutr Metab Care. 2002;5(5):519‐524. [DOI] [PubMed] [Google Scholar]
- 25. Coller FA, Maddock WG. A study of dehyrdation in humans. Ann Surg. 1935;102(5):947‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mange K, Matsuura D, Cizman B, et al. Language guiding therapy: the case of dehydration versus volume depletion. Ann Intern Med. 1997;127(9):848‐853. [DOI] [PubMed] [Google Scholar]
- 27. Bartoli E, Bergamasco L, Castello L, Sainaghi PP. Methods for the quantitative assessment of electrolyte disturbances in hyperglycaemia. Nutr Metab Cardiovasc Dis. 2009;19(1):67‐74. doi: 10.1016/j.numecd.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 28. Bhave G, Neilson EG. Volume depletion versus dehydration: how understanding the difference can guide therapy. Am J Kidney Dis. 2011;58(2):302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhagat CI, Garcia‐Webb P, Fletcher E, Beilby JP. Calculated vs measured plasma osmolalities revisited. Clin Chem. 1984;30(10):1703‐1705. doi: 10.1093/clinchem/30.10.1703 [DOI] [PubMed] [Google Scholar]
- 30. Katz MA. Hyperglycemia‐induced hyponatremia ‐ calculation of expected serum sodium depression. N Engl J Med. 1973;16(289):843‐844. [DOI] [PubMed] [Google Scholar]
- 31. Scott AR, The Joint British Diabetes Societies (JBDS) for Inpatient Care Group . Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med. 2015;32(6):714‐724. doi: 10.1111/dme.12757 [DOI] [PubMed] [Google Scholar]
- 32. Gershkovich B, English SW, Doyle MA, Menon K, McIntyre L. Choice of crystalloid fluid in the treatment of hyperglycemic emergencies: a systematic review protocol. Syst Rev. 2019;8(1):228. doi: 10.1186/s13643-019-1130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8(8):Cd000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Patient Safety Agency . Potassium solutions: risks to patients from errors occurring during intravenous administration. NPSA. 2002. Published 2002. [Google Scholar]
- 35. Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342(20):1493‐1499. [DOI] [PubMed] [Google Scholar]
- 36. Wordsworth G, Robinson AH, Ward A, Atkin M. HHS—full or prophylactic anticoagulation? British Journal of Diabetes. 2014;14(2):64‐66. [Google Scholar]
- 37. Dhatariya K, Weston P. The argument against everyone with hyperosmolar hyperglycaemic syndrome being given prophylactic treatment dose anticoagulation. Br J Diabetes. 2021;21(2):282‐283. [Google Scholar]
- 38. Bryk‐Wiązania AH, Undas A. Hypofibrinolysis in type 2 diabetes and its clinical implications: from mechanisms to pharmacological modulation. Cardiovasc Diabetol. 2021;20(1):191. doi: 10.1186/s12933-021-01372-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tripodi A, Branchi A, Chantarangkul V, et al. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis. 2011;31(2):165‐172. doi: 10.1007/s11239-010-0506-0 [DOI] [PubMed] [Google Scholar]
- 40. Whelton MJ, Walde D, Havard CW. Hyperosmolar non‐ketotic diabetic coma: with particular reference to vascular complications. Br Med J. 1971;1(5740):85‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calvisi SL, Ramirez GA, Scavini M, et al. Thromboembolism risk among patients with diabetes/stress hyperglycemia and COVID‐19. Metab Clin Exp. 2021;123:123. doi: 10.1016/j.metabol.2021.154845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paton RC. Haemostatic changes in diabetic coma. Diabetologia. 1981;21(3):172‐177. doi: 10.1007/BF00252650 [DOI] [PubMed] [Google Scholar]
- 43. Keenan CR, Murin S, White RH. High risk for venous thromboembolism in diabetics with hyperosmolar state: comparison with other acute medical illnesses. J Thromb Haemost. 2007;5(6):1185‐1190. [DOI] [PubMed] [Google Scholar]
- 44. Halmos PB, Nelson JK, Lowry RC. Hyperosmolar non‐ketoacidotic coma in diabetes. Lancet. 1966;287(7439):675‐679. doi: 10.1016/S0140-6736(66)91626-6 [DOI] [PubMed] [Google Scholar]
- 45. Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48(5):1017‐1021. doi: 10.1007/s00125-005-1715-5 [DOI] [PubMed] [Google Scholar]
- 46. Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complicat. 2001;15(1):44‐54. https://www.sciencedirect.com/science/article/pii/S105687270000132X [DOI] [PubMed] [Google Scholar]
- 47. NHS Digital . National Diabetes Inpatient Audit ‐ Harms, 2019. Published 2020. https://digital.nhs.uk/data‐and‐information/publications/statistical/national‐diabetes‐inpatient‐audit—harms/national‐diabetes‐inpatient‐audit—harms‐2019/2019
- 48. Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care. 2014;37(11):3124‐3131. http://care.diabetesjournals.org/content/37/11/3124.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kusumoto K, Koriyama N, Kojima N, Ikeda M, Nishio Y. Central pontine myelinolysis during treatment of hyperglycemic hyperosmolar syndrome: a case report. Clinical Diabetes and Endocrinology. 2020;6(1):23. doi: 10.1186/s40842-020-00111-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Varela D, Held N, Linas S. Overview of cerebral edema during correction of hyperglycemic crises. Am J Case Rep. 2018;19:562‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. NHS Digital . National Diabetes Inpatient Audit (NaDIA) Harms ‐ England annual report (2020). 8/7/2021. Accessed May 31, 2022. https://digital.nhs.uk/data‐and‐information/publications/statistical/national‐diabetes‐inpatient‐audit‐‐‐harms/national‐diabetes‐inpatient‐audit‐‐‐harms‐2020
- 52. Lamont T, Cousins D, Hillson R, Bischler A, Terblanche M. Safer administration of insulin: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c5269. doi: 10.1136/bmj.c5269 [DOI] [PubMed] [Google Scholar]
- 53. NHS Improvement . Never Events list 2018. Published 2018. Accessed May 31, 2022. https://improvement.nhs.uk/documents/2899/Never_Events_list_2018_FINAL_v7.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Appendix S2.
Appendix S3.
Appendix S4.
Data Availability Statement
There is no data in our document. It is guidelines and a DUK position statement. So this section is irrelevant.


