Abstract
Though immunotherapy has to some extent improved the prognosis of patients with advanced non‐small cell lung cancer (NSCLC), only a few patients benefit. Furthermore, immunotherapy efficacy is affected by inflammatory and nutritional status of patients. To investigate whether dynamics of inflammatory and nutritional indexes were associated with prognosis, 223 patients were analysed retrospectively. The inflammatory indexes of interest were neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and systemic immune‐inflammation index (SII) while prognostic nutritional index (PNI) and the haemoglobin, albumin, lymphocyte and platelet (HALP) score were considered as nutritional indexes. Patients were divided into high and low groups or into ‘increase’ and ‘decrease’ groups based on pre‐treatment cut‐off values and index dynamics after 6‐week follow‐up respectively. High pre‐treatment PLR (OR = 2.612) and increase in NLR during follow‐up (OR = 2.516) were significantly associated with lower objective response rates. Using multivariable analysis, high pre‐treatment PLR (HR, 2.319) and increase in SII (HR, 1.731) predicted shorter progression‐free survival, while high pre‐treatment NLR (HR, 1.635), increase in NLR (HR, 1.663) and PLR (HR, 1.691) and decrease in PNI (HR, 0.611) predicted worse overall survival. The nomogram's C‐index in inside validation was 0.718 (95% CI: 0.670–0.766). Our results indicated both nutritional and inflammatory indexes are associated with survival outcomes. Inflammatory indexes were additionally linked to treatment response. Index dynamics are better predictors than baseline values in predicting survival in advanced NSCLC patients receiving PD‐1 inhibitor combined with chemotherapy as first‐line.
Keywords: dynamic, immunotherapy, inflammatory indexes, non‐small cell lung cancer (NSCLC), nutritional indexes
1. INTRODUCTION
Immune checkpoint inhibitors (ICIs) treatment, represented by programmed cell death protein‐1 (PD‐1) inhibitors, has greatly improved the prognosis of patients with advanced non‐small cell lung cancer (NSCLC) compared to traditional chemotherapy, 1 leading to major reformation of treatment patterns. ICIs, combined with chemotherapy therapy, have become the standard first‐line treatment for advanced NSCLC with negative driver gene. However, only about 10%–40% patients benefit from durable responses from immunotherapy, with some even going through hyper‐progression or fatal toxicity. 2 , 3 , 4 , 5 To date, tumour tissue PD‐L1 expression and tumour mutation burden (TMB) have been the most widely accepted predicting biomarkers approved by the Food and Drug Administration (FDA). Nevertheless, considerable inconsistency exists in many different cases. 6 , 7 As for other reported biomarker candidates, including tumour neoantigen burden (TNB), deficient mismatch repair (dMMR), high microsatellite instability (MSI‐high), T‐cell receptor clonality, tumour‐infiltrating lymphocytes (TIL), DNA damage and repair genes (DDR), effector T‐cell gene signature and intestinal microbiota, 8 , 9 , 10 the invasive nature and high cost would be the additional concerns besides the unacceptable inaccuracy in identifying patients who may benefit from immunotherapy. Therefore, finding an inexpensive prognostic biomarker of clinical response to immunotherapy remains urgently required.
Patient conditions, including inflammatory and nutritional status, is likely to impact immune responses and yet this is not fully understood. Recently, peripheral blood indexes representing inflammation or nutrition have been increasingly studied in predicting treatment responses to immunotherapy among patients with NSCLC. 11 , 12 , 13 The majority of the studies have focused on single haematological indexes, such as white blood cell (WBC), neutrophils, lymphocytes, platelets, haemoglobin and albumin, while the prognostic value of a single index is relatively low. Although there were studies exploring combined peripheral blood indexes, the patients recruited were mostly heterogeneous or only baseline indicators were evaluated. 14 , 15 , 16 , 17
In this study, we carried out a retrospective analysis to examine the capacity of combined peripheral blood indexes in predicting the response and survival of patients in a dynamic pattern. The combined peripheral blood indexes were divided into inflammatory markers, including neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and systemic immune‐inflammation index (SII); and nutritional indicators, including prognostic nutritional index (PNI) and the haemoglobin, albumin, lymphocyte and platelet (HALP) score. The present study was initiated to investigate whether the two categories of indexes are associated with clinically prognostic values and a new nomogram with relatively high accuracy was developed for assessment.
2. RESULTS
2.1. Clinical characteristics of patients
A total of 223 patients were included in this study. Their characteristics are summarized in Table 1. The average age at the time of diagnosis was 60.4 years. Most patients were male (84.8%, n = 189). Every subject had an Eastern Cooperative Oncology Group Performance Status (ECOG‐PS) score of 0 or 1. Lung adenocarcinoma accounted for most cases (59.6%; n = 133), while lung squamous cell carcinoma was observed in 40.4% (n = 90) of patients. About 87.4% of the cases (n = 195) were in Stage IV. The number of patients with Kirsten rat sarcoma viral oncogene homologue (KRAS) mutation, v‐RAF murine sarcoma viral oncogene homologue B1 (BRAF) mutation, human epidermal growth factor receptor‐2 (HER2) mutation and epidermal growth factor receptor gene exon 20 insertion (EGFR‐20ins) mutation were 9, 2, 2 and 3 respectively. The number of patients with positive and negative PD‐L1 expression were 76 and 43 respectively, and there were 104 patients for whom the PD‐L1expression was unknown.
TABLE 1.
Baseline characteristics of the patients
| Characteristics | No. of patients | % |
|---|---|---|
| Total | 223 | 100.0 |
| Age | ||
| ≤60 | 109 | 48.9 |
| >60 | 114 | 51.1 |
| Sex | ||
| Male | 189 | 84.8 |
| Female | 34 | 15.2 |
| ECOG‐PS | ||
| 0 score | 113 | 50.7 |
| 1 score | 110 | 49.3 |
| Histology | ||
| LUAD | 133 | 59.6 |
| LUSC | 90 | 40.4 |
| Stage | ||
| IIIB/IIIC | 28 | 12.6 |
| IV | 195 | 87.4 |
| Metastatic sites | ||
| Lung | 59 | 26.5 |
| Pleura | 51 | 22.9 |
| Liver | 11 | 4.9 |
| Bone | 87 | 39.0 |
| CNS | 18 | 8.1 |
| Adrenal gland | 8 | 3.6 |
| Distant lymph node metastasis | 22 | 9.9 |
| PD‐L1 expression | ||
| Negative | 45 | 20.2 |
| Positive | 73 | 32.7 |
| Unknown | 105 | 47.1 |
| Baseline index | ||
| NLR, M (IQR) | 3.18 | (2.33–4.32) |
| PLR, M (IQR) | 144.37 | (106.32–196.27) |
| SII, M (IQR) | 792.07 | (570.01–1247.30) |
| PNI, M (IQR) | 50.50 | (47.25–54.60) |
| HALP, M (IQR) | 39.33 | (26.54–53.27) |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HALP, haemoglobin, albumin, lymphocyte and platelet; IQR, interquartile range; LUAD, Lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; NLR, neutrophil‐to‐lymphocyte ratio; PD‐L1, programmed cell death ligand 1; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutrition index; PS, performance status; SII, systemic immune‐inflammation index.
All subjects received anti‐PD1 therapy combined with chemotherapy as first‐line treatment with a median follow‐up of 20.4 months (95% confidence interval [CI]: 14.5–26.3). The median progression‐free survival (PFS) and overall survival (OS) for all patients were 12 months (95% CI, 10.674–13.326) and 20 months (95% CI, 18.536–21.464), respectively. The cut‐off values for NLR, PLR, SII, PNI and HALP were 3.18, 144.37, 792.07, 50.50 and 39.33, respectively.
2.2. Correlation of treatment response with pre‐treatment values and dynamics of inflammatory and nutritional indexes
Better objective response rate (ORR) was observed in patients with lower levels of pre‐treatment PLR (66.1%) and SII (61.6%) and higher levels of pre‐treatment PNI (43.8%) and HALP (42.0%), compared with respective counterpart groups (39.6% for PLR, 44.1% for SII, 62.2% for PNI and 64.0% for HALP), with the p value of p < 0.001, 0.009, 0.006 and 0.001 respectively. It is also the case for patients with an ECOG‐PS score of 0 in contrast to those with an ECOG‐PS score of 1 (61.1% and 44.5% respectively, p = 0.013).
Patients with positive PD‐L1 expression has higher ORR (64.4%) than those with negative PD‐L1 expression (40.0%) (p = 0.010). There was no difference between patients with unknown PD‐L1 expression and those with negative or positive PD‐L1 expression (p = 0.239 or 0.066, respectively). Age, sex, histology, stage and pre‐treatment NLR levels were not associated with ORR (Table 2, Figure 1A).
TABLE 2.
Relationships between indexes and objective response rate (ORR)
| PR group (N) | Non‐PR group (N) | ORR (%) | χ2 | p | |
|---|---|---|---|---|---|
| Total | 118 | 105 | |||
| Clinical indexes | |||||
| Age | |||||
| ≤60 | 62 | 47 | 56.9 | 1.346 | 0.246 |
| >60 | 56 | 58 | 49.1 | ||
| Sex | |||||
| male | 98 | 91 | 51.9 | 0.562 | 0.453 |
| female | 20 | 14 | 58.8 | ||
| ECOG‐PS | |||||
| 0 score | 69 | 44 | 61.1 | 6.103 | 0.013 |
| 1 score | 49 | 61 | 44.5 | ||
| Histology | |||||
| LUAD | 76 | 57 | 57.1 | 2.364 | 0.124 |
| LUSC | 42 | 48 | 46.7 | ||
| Stage | |||||
| IIIB/IIIC | 18 | 10 | 64.3 | 1.662 | 0.197 |
| IV | 100 | 95 | 51.3 | ||
| PD‐L1 expression | |||||
| Negative | 18 | 27 | 40.0 | 125.348 | <0.001 |
| Positive | 47 | 26 | 64.4 | ||
| Unknown | 53 | 52 | 50.5 | ||
| Pre‐treatment indexes | |||||
| NLR | |||||
| Low | 67 | 46 | 59.3 | 3.379 | 0.053 |
| High | 51 | 59 | 46.4 | ||
| PLR | |||||
| Low | 74 | 38 | 66.1 | 15.632 | <0.001 |
| High | 44 | 67 | 39.6 | ||
| SII | |||||
| Low | 69 | 43 | 61.6 | 6.824 | 0.009 |
| High | 49 | 62 | 44.1 | ||
| PNI | |||||
| Low | 49 | 63 | 43.8 | 7.585 | 0.006 |
| High | 69 | 42 | 62.2 | ||
| HALP | |||||
| Low | 47 | 65 | 42.0 | 10.829 | 0.001 |
| High | 71 | 40 | 64.0 | ||
| Variance indexes | |||||
| NLR | |||||
| Decrease | 76 | 49 | 60.8 | 7.098 | 0.008 |
| Increase | 42 | 56 | 42.9 | ||
| PLR | |||||
| Decrease | 58 | 56 | 50.9 | 0.389 | 0.533 |
| Increase | 60 | 49 | 55.0 | ||
| SII | |||||
| Decrease | 64 | 52 | 55.2 | 0.495 | 0.482 |
| Increase | 54 | 53 | 50.5 | ||
| PNI | |||||
| Decrease | 59 | 53 | 52.7 | 0.005 | 0.943 |
| Increase | 59 | 52 | 53.2 | ||
| HALP | |||||
| Decrease | 75 | 56 | 57.3 | 2.397 | 0.122 |
| Increase | 43 | 49 | 46.7 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HALP, haemoglobin, albumin, lymphocyte and platelet; LUAD, Lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; NLR, neutrophil‐to‐lymphocyte ratio; PD‐L1, programmed cell death ligand 1; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutrition index; PR, partial response; PS, performance status; SII, systemic immune‐inflammation index.
FIGURE 1.
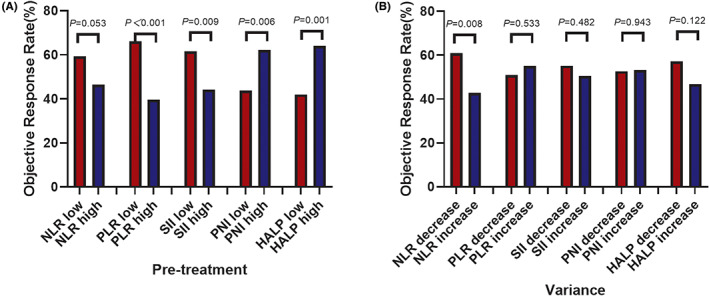
Different tumour responses of patients with advanced non‐small cell lung cancer (NSCLC) patients who received death protein‐1 (PD‐1) inhibitor combined with chemotherapy as first‐line according to inflammatory/nutritional indexes. (A) Pre‐treatment, (B) variance. HALP, haemoglobin, albumin, lymphocyte and platelet; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune‐inflammation index.
Higher ORR could be observed in subjects demonstrating a decline in NLR (76/125, 60.8%) compared with those in NLR‐increase group (42/98; 42.9%, p = 0.008). But it was not the case for other indexes (Figure 1B).
In the multivariate logistic regression analysis, high pre‐treatment PLR (OR = 3.557, 95% CI: 1.993–6.350, p = 0.000) and dynamic increase in NLR (OR = 2.626, 95% CI: 1.466–4.703, p = 0.003) were significantly associated with inferior ORR.
2.3. Relationships of patient outcomes with pre‐treatment values and dynamics of inflammatory and nutritional indexes
The median PFS values of patients with higher pre‐treatment levels of NLR (low group, 14 months vs. high group, 9 months; hazard ratio [HR], 1.571; [95% CI, 1.151–2.144]; p = 0.004), PLR (low group, 14 months vs. high group, 10 months; HR, 1.716; [95% CI, 1.264–2.329]; p = 0.001), SII (low group, 13 months vs. high group, 10 months; HR, 1.371; [95% CI, 1.012–1.857]; p = 0.042) were shorter than those at the lower pre‐treatment level. Low ECOG‐PS score (0 score, 13 months vs. 1 score, 9 months; HR, 1.619; [95% CI, 1.193–2.198]; p = 0.002) was linked to longer PFS (Table 3). No associations were found between PFS with baseline levels of the two nutritional indexes, PNI and HALP (Figure 2). Our data only support a role for the pre‐treatment levels of the NLR score (low group, 21 months vs. high group, 19 months; HR, 1.587; [95% CI, 1.110–2.268]; p = 0.011) (Figure 2), and the ECOG‐PS scores (0 score, 21 months vs. 1 score, 17 months; HR, 1.639; [95% CI, 1.162–2.312]; p = 0.005) in predicting the OS of patients. PD‐L1 expression was not associated with PFS and OS (Table 3).
TABLE 3.
Univariate Cox regression analysis of PFS and OS
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| median | HR | (95% CI) | p | median | HR | (95% CI) | p | |
| m | m | |||||||
| Clinical indexes | ||||||||
| Age(y) | ||||||||
| ≤60 | 13 | 20 | ||||||
| >60 | 11 | 1.096 | 0.809–1.484 | 0.555 | 19 | 1.057 | 0.751–1.486 | 0.752 |
| Sex | ||||||||
| Female | 13 | 26 | ||||||
| Male | 11 | 1.296 | 0.859–1.955 | 0.217 | 19 | 1.575 | 0.997–2.489 | 0.051 |
| ECOG‐PS | ||||||||
| 0 score | 13 | 21 | ||||||
| 1 score | 9 | 1.619 | 1.193–2.198 | 0.002 | 17 | 1.639 | 1.162–2.312 | 0.005 |
| Histology | ||||||||
| LUSC | 12 | 20 | ||||||
| LUAD | 11 | 0.799 | 0.586–1.089 | 0.155 | 20 | 0.832 | 0.589–1.176 | 0.298 |
| Stage | ||||||||
| IIIB/IIIC | 14 | 22 | ||||||
| IV | 12 | 1.477 | 0.868–2.514 | 0.151 | 19 | 0.948 | 0.551–1.630 | 0.847 |
| PD‐L1 expression | ||||||||
| Negative | 10 | 19 | ||||||
| Positive | 11 | 0.830 | 0.536–1.286 | 0.405 | 21 | 0.682 | 0.426–1.093 | 0.111 |
| Unknown | 13 | 0.827 | 0.546–1.251 | 0.369 | 19 | 0.827 | 0.533–1.283 | 0.395 |
| Pre‐treatment indexes | ||||||||
| NLR | ||||||||
| Low | 14 | 21 | ||||||
| High | 9 | 1.571 | 1.151–2.144 | 0.004 | 19 | 1.587 | 1.110–2.268 | 0.011 |
| PLR | ||||||||
| Low | 14 | 20 | ||||||
| High | 10 | 1.716 | 1.264–2.329 | 0.001 | 19 | 1.382 | 0.978–1.951 | 0.066 |
| SII | ||||||||
| Low | 13 | 20 | ||||||
| High | 10 | 1.371 | 1.012–1.857 | 0.042 | 19 | 1.140 | 0.808–1.610 | 0.455 |
| PNI | ||||||||
| Low | 10 | 20 | ||||||
| High | 13 | 0.898 | 0.661–1.221 | 0.492 | 19 | 0.959 | 0.680–1.353 | 0.813 |
| HALP | ||||||||
| Low | 10 | 20 | ||||||
| High | 13 | 0.771 | 0.569–1.045 | 0.093 | 19 | 0.996 | 0.709–1.401 | 0.493 |
| Variance indexes | ||||||||
| NLR | ||||||||
| Decrease | 14 | 23 | ||||||
| Increase | 9 | 2.000 | 1.463–2.735 | <0.001 | 17 | 2.261 | 1.586–3.223 | <0.001 |
| PLR | ||||||||
| Decrease | 13 | 22 | ||||||
| Increase | 11 | 1.384 | 1.022–1.875 | 0.036 | 19 | 1.539 | 1.092–2.169 | 0.014 |
| SII | ||||||||
| Decrease | 14 | 21 | ||||||
| Increase | 10 | 1.667 | 1.230–2.260 | 0.001 | 18 | 1.607 | 1.142–2.262 | 0.007 |
| PNI | ||||||||
| Decrease | 11 | 17 | ||||||
| Increase | 13 | 0.642 | 0.472–0.873 | 0.005 | 26 | 0.449 | 0.315–0.640 | <0.001 |
| HALP | ||||||||
| Decrease | 11 | 19 | ||||||
| Increase | 13 | 0.863 | 0.635–1.174 | 0.348 | 21 | 0.736 | 0.519–1.045 | 0.087 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HALP, haemoglobin, albumin, lymphocyte and platelet; HR, hazard ratio; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OS, overall survival; NLR, neutrophil‐to‐lymphocyte ratio; PFS, progression‐free survival; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutrition index; PS, performance status; SII, systemic immune‐inflammation index.
FIGURE 2.
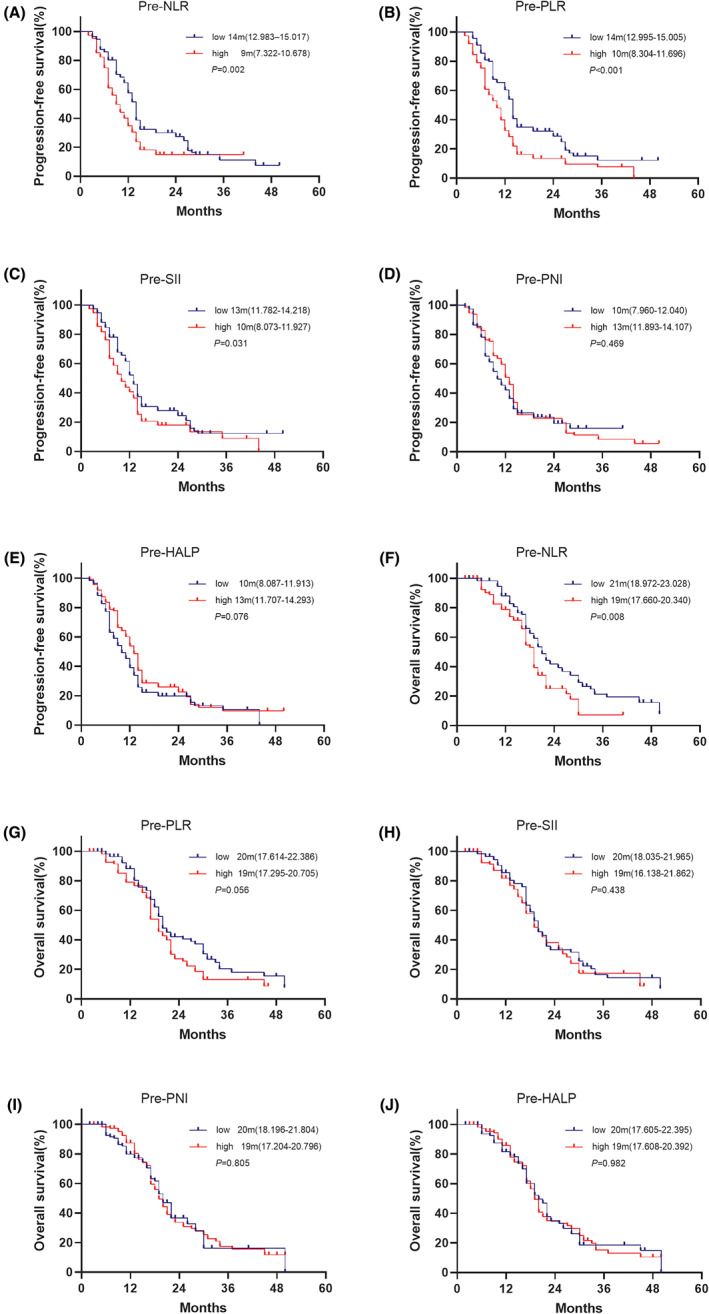
Kaplan–Meier curves for progression‐free survival (PFS) and overall survival (OS) comparing patients with advanced non‐small cell lung cancer (NSCLC) patients who received death protein‐1 (PD‐1) inhibitor combined with chemotherapy as first‐line according to pre‐treatment inflammatory/nutritional indexes. (A, F) PFS and OS stratified by the baseline neutrophil‐to‐lymphocyte ratio (NLR) index, (B, G) PFS and OS stratified by the baseline platelet‐to‐lymphocyte ratio (PLR) index, (C, H) PFS and OS stratified by the baseline systemic immune‐inflammation index (SII) index, (D, I) PFS and OS stratified by the baseline prognostic nutritional index (PNI) index, (E, J) PFS and OS stratified by the baseline haemoglobin, albumin, lymphocyte and platelet (HALP) index.
As shown in Figure 3 and Table 3, patients demonstrating an increase in NLR (decrease group, 14 months vs. increase group = 9 months, HR, 2.000, [95% CI, 1.463–2.735], p < 0.001), PLR (decrease group = 13 months vs. increase group = 11 months, HR, 1.384, [95% CI, 1.022–1.875], p = 0.036) and SII (decrease group = 14 months vs. increase group = 10 months, HR, 1.667, [95% CI, 1.230–2.260], p < 0.001) suffered from shorter PFS than those showing a decline pattern. In comparison, patients demonstrating an increase in PNI (decrease group = 11 months vs. increase group = 13 months, HR, 0.642, [95% CI, 0.472–0.873], p = 0.005) were associated with longer PFS. No significant difference was observed between the decrease and the increase group for HALP (11 months vs. 13 months respectively, HR, 0.863, [95% CI, 0.635–1.174], p = 0.348).
FIGURE 3.

Kaplan–Meier curves for progression‐free survival (PFS) and overall survival (OS) comparing patients with advanced non‐small cell lung cancer (NSCLC) patients who received death protein‐1 (PD‐1) inhibitor combined with chemotherapy as first‐line according to variances of inflammatory/nutritional indexes. (A, F) PFS and OS stratified by the baseline neutrophil‐to‐lymphocyte ratio (NLR) index, (B, G) PFS and OS stratified by the baseline platelet‐to‐lymphocyte ratio (PLR) index, (C, H) PFS and OS stratified by the baseline systemic immune‐inflammation index (SII) index, (D, I) PFS and OS stratified by the baseline prognostic nutritional index (PNI) index, (E, J) PFS and OS stratified by the baseline haemoglobin, albumin, lymphocyte and platelet (HALP) index.
The dynamics of NLR (decrease group, 23 months vs. increase group, 17 months; HR, 2.261; [95% CI, 1.586–3.223]; p < 0.001), PLR (decrease group , 22 months vs. increase group, 19 months; HR, 1.539; [95% CI, 1.092–2.169]; p = 0.014), SII (decrease group, 21 months vs. increase group, 18 months; HR, 1.607; [95% CI, 1.142–2.262]; p = 0.007) and PNI (decrease group, 17 months vs. increase group, 26 months; HR, 0.449; [95% CI, 0.315–0.640]; p < 0.001) were closely associated with OS. The median OS tended to be longer in patients showing an increasing pattern for HALP compared to those demonstrating a decline pattern (decrease group, 19 months vs. increase group, 21 months; HR, 0.736; [95% CI, 0.519–1.045]; p = 0.087).
Results from multivariate Cox regression analysis showed that ECOG‐PS score (HR, 1.460; 95% CI, 1.064–2.005; p = 0.019), pre‐treatment PLR (HR, 2.319; 95% CI, 1.496–3.595; p < 0.001) and dynamics of SII (HR, 1.731; 95% CI, 1.063–2.819; p = 0.027) were independently associated with PFS. As for OS, the ECOG‐PS score (HR, 1.442; 95% CI, 1.014–2.052; p = 0.042), pre‐treatment NLR (HR, 1.635; 95% CI, 1.121–2.386; p = 0.011), as well as dynamics of NLR (HR, 1.663; 95% CI, 1.064–2.599; p = 0.026), PLR (HR, 1.691; 95% CI, 1.068–2.677; p = 0.025) and PNI (HR, 0.611; 95% CI, 0.415–0.900; p = 0.013) were found to be the independent predicting factors (Table 4).
TABLE 4.
Multivariate Cox regression analysis of PFS and OS
| PFS | OS | ||||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p | HR | (95% CI) | p | ||
| ECOG‐PS | ECOG‐PS | ||||||
| 0 score | 0 score | ||||||
| 1 score | 1.460 | 1.064–2.005 | 0.019 | 1 score | 1.442 | 1.014–2.052 | 0.042 |
| Pre‐treatment indexes | Pre‐treatment indexes | ||||||
| NLR | NLR | ||||||
| Low | Low | ||||||
| High | 1.326 | 0.893–1.970 | 0.162 | High | 1.635 | 1.121–2.386 | 0.011 |
| PLR | |||||||
| Low | |||||||
| High | 2.319 | 1.496–3.595 | 0.000 | ||||
| SII | |||||||
| Low | |||||||
| High | 0.964 | 0.606–1.532 | 0.876 | ||||
| Variance indexes | Variance indexes | ||||||
| NLR | NLR | ||||||
| Decrease | Decrease | ||||||
| Increase | 1.421 | 0.956–2.110 | 0.082 | Increase | 1.663 | 1.064–2.599 | 0.026 |
| PLR | PLR | ||||||
| Decrease | Decrease | ||||||
| Increase | 1.397 | 0.900–2.167 | 0.136 | Increase | 1.691 | 1.068–2.677 | 0.025 |
| SII | SII | ||||||
| Decrease | Decrease | ||||||
| Increase | 1.731 | 1.063–2.819 | 0.027 | Increase | 0.936 | 0.579–1.513 | 0.787 |
| PNI | PNI | ||||||
| Decrease | Decrease | ||||||
| Increase | 0.904 | 0.641–1.275 | 0.566 | Increase | 0.611 | 0.415–0.900 | 0.013 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; NLR, neutrophil‐to‐lymphocyte ratio; PFS, progression‐free survival; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutrition index; PS, performance status; SII, systemic immune‐inflammation index.
2.4. Nomogram and predictive models using R
The five independent risk factors (i.e., ECOG‐PS score, pre‐treatment NLR as well as dynamics of NLR, PLR and PNI) determined with the multivariate Cox regression analysis were used in developing a predictive nomogram for NSCLC patients treated with received PD‐1 inhibitor combined with chemotherapy as first‐line treatment. The resulting nomogram (see Figure 4) yielded an internal verification C‐index of 0.718 (95% CI, 0.670–0.766), indicating that the model has excellent prediction accuracy.
FIGURE 4.
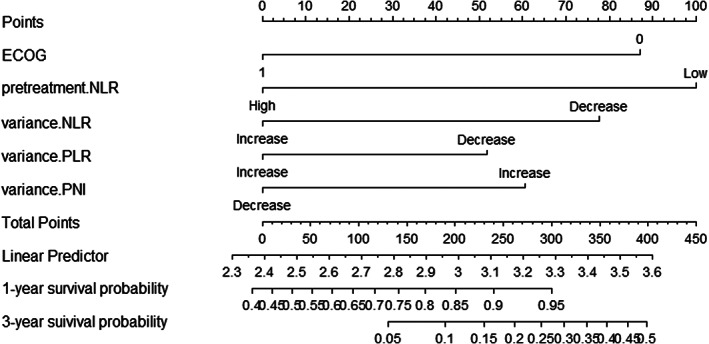
Nomogram for predicting advanced non‐small cell lung cancer (NSCLC) patients who received death protein‐1 (PD‐1) inhibitor combined with chemotherapy as first‐line. ECOG, Eastern Cooperative Oncology Group; NLR, neutrophil‐to‐lymphocyte ratio; PNI, prognostic nutritional index
3. DISCUSSION
In this study, we found that both pre‐treatment values and dynamics of inflammatory indexes were related to ORR, PFS and OS, while only the dynamics of nutritional indexes correlated with OS. Our study also showed dynamics of these clinical indexes were much stronger than pre‐treatment values in predicting survival of patients. To our knowledge, we are among the first to comprehensively explore the prognostic value of dynamic variations in a series of inflammatory and nutritional indexes in NSCLC treated with chemo immunotherapy as first‐line treatment and develop a nomogram to help clinicians identify potential unfavourable factors in order to adopt appropriate intervention measures.
Inflammation is crucial in all stages of tumour development and progression. It also impacts the tumour immune microenvironment and treatment response. 18 , 19 High neutrophil count is related to the release of tumour‐promoting substances (e.g., reactive oxygen species, arginase, inflammatory cytokines, tumour or vascular growth factors, metalloproteinases) and may lead to cancer progression and spread. 20 The mouse model of subcutaneous mesothelioma demonstrates that neutrophils promote the growth of tumour cells by inhibiting CD8+ T cells. 21 Low lymphocyte counts are associated with aggrieved anti‐tumour response, CD8+ T‐cell cytotoxicity and CD4+ helper T‐cell functions. 22 Platelets play an important and multifaceted role in cancer progression, releasing many cytokines, such as platelet‐derived growth factor and platelet‐reactive protein, which may promote haematogenous spread and invasion. 23 In our study, patients in the NLR‐decrease group (60.8%) had a higher ORR than in the NLR‐increase group (42.9%), and high pre‐treatment values of and a dynamic increase in NLR were independent risk factors predicting a shorter OS. Multiple retrospective studies and meta‐analyses have suggested that low pre‐treatment PLR and SII may be a potential prognostic biomarker favouring the survival of cancer patients. 12 , 24 , 25 , 26 , 27 , 28 , 29 These were consistent with our results.
Given an important role for nutritional status in tumour immunotherapy, accumulating studies have explored the link between nutritional status‐related factors and prognosis. 30 , 31 The scarcity of essential nutrients, such as amino acids, glucose and fatty acids, can induce altered metabolic reprogramming and functions in immune cells. 32 Some immune cells may lose their anti‐tumour functions, while others may differentiate or polarize into immunosuppressive phenotypes. Dysfunctional metabolism can also affect immune responses and lead to an immunosuppressive tumour microenvironment (TME). 33 As a sign of malnutrition, low levels of albumin and haemoglobin were associated with unfavourable clinical outcomes, but they were not predictors for disease control. 34 , 35 , 36 , 37 The HALP score has been shown to be a prognostic indicator 38 , 39 for NSCLC patients who have undergone surgery 14 and adjuvant chemotherapy. 40 However, there are no recent studies looking into the relationship of the HALP score with prognoses of NSCLC patients treated with immunotherapy. In this study, we did not find the association between HALP and patient survival. Previous studies suggested that a low pre‐treatment PNI score is an independent risk factor for the survival of advanced NSCLC patients receiving PD‐1 inhibitor treatment, 41 , 42 which is confirmed by a recent meta‐analysis. 43 Our results demonstrated significant improvement in the survival of patients showing a dynamic increase in PNI but without association with treatment efficacy.
Patients with advanced cancer are prone to malnutrition, which, in turn, leads to various complications, severe toxicity and shortened survival. Patients with good nutritional status may have better tolerance to following treatments, after the failure of first‐line treatment. Nutritional status may be more relevant to long‐term survival. Nutritional interventions should thus be considered for malnourished patients.
However, the prognostic value of pre‐treatment indexes was questionable. For instance, Suh et al. found no difference in PFS between patients with NLR < 5 and NLR ≥ 5 at baseline among NSCLC patients treated with anti‐PD‐1 antibody. 44 These conflicting results may derive from differences in characters of recruited patients, such as age, sex and race. Maybe it is not proper to set a fixed cut‐off value for NLR. Some research found reduced NLR after 6 or 12 weeks of treatment was connected to higher survival rates in two reports. 12 , 13 And in another study, NLR levels of non‐responders increased by 6.6 compared to responders after two courses of nivolumab treatment. 45 NLR was also found to decrease by 0.09 every month among patients who had response to complete response/partial response (CR/PR) when receiving PL‐(L)1 inhibitors. 46 Consistently, our study found decreased NLR was associated with higher ORR, while no correlation was found between pre‐treatment NLR and ORR. In addition, dynamic changes in PLR, SII and PNI were found to be related to PFS and the OS, while it was not the case for baseline scores. It thus appears that dynamics rather than baseline levels of the clinical parameters could accurately predict the survival outcomes.
In this study, we used a nomogram to estimate the 1 and 3‐year survival rates of advanced NSCLC patients who received PD‐1 inhibitor combined with chemotherapy. We then conducted internal verification to check the nomogram and found the C‐index to be 0.718 (95% CI, 0.670–0.766), indicating that the accuracy of the prediction model is high.
PD‐L1 expression and tumour mutational burden (TMB) are approved by the FDA as indicators for predicting the efficacy of immunotherapy in NSCLC. However, the failure of Checkmate 026 indicates PD‐L1 expression may not predict prognosis accurately in all patients. 6 The ORRs of NSCLC patients with positive PD‐L1 expression were 38.3%–46.1% when receiving first‐line monotherapy with PD‐1/PD‐L1 inhibitors. 2 , 47 , 48 Estimation of TPS by pathologists may lead to intra/inter‐observer bias. Heterogeneity may exist in different regions of the tumour tissues, and the dynamic changes in the expression of PD‐L1 in tumours have been documented. 49 In our study, although PD‐L1 positive patients tended to be associated with better PFS and OS, the differences failed to reach statistical significance, which may arise from missing values. For TMB, the ORR in NSCLC patients with high TMB treated with nivolumab plus ipilimumab was only around 33%–48%. 7 , 50 Lack in standard test methods and high cost limit application of TMB in clinical settings. In 2017, FDA approved Pembrolizumab for the treatment of all patients with MSI‐H/dMMR, regardless of tumour type. But it may not be the case in lung cancer patients, because of low incidence. 51 It is thus indispensable to build a multidimensional predicting model for individual immunotherapy, not only due to complex interactions between tumour cells and TME, but also nutritional and inflammatory state of hosts.
Our study has several limitations that should be considered when evaluating the research results. First, the study was a single‐centre retrospective study with a limited sample size. Multi‐centre prospective studies are needed to confirm our results. Second, single chemotherapy may cause changes in peripheral blood indexes, suggesting only patients receiving chemotherapy should be included in the positive control group. Unfortunately, in our study we could not recruit these patients for the positive control group because chemotherapy alone as first‐line treatment is rare in current clinical practice. Third, the standard cut‐off values for these indexes have not been confirmed. While some authors selected the median values, others only chose the values previously reported by previous studies. Finally, data were missing from a large portion of patients, since the detection of PD‐L1 expression is not necessary before initiation of first‐line chemotherapy combined with immunotherapy. 52
While inflammatory and nutritional indexes could serve as independent predictors of long‐term survival, inflammatory indexes are additionally linked to treatment response. Dynamics of the explored clinical parameters were stronger than baseline values in predicting survival in advanced NSCLC patients receiving PD‐1 inhibitor combined with chemotherapy as first‐line.
4. METHODS
4.1. Patients
We performed a retrospective analysis of consecutive patients with advanced NSCLC at the Shanghai Pulmonary Hospital from March 2017 to March 2019. Patients were eligible for inclusion in this study if they had received anti‐PD‐1 antibody combined with chemotherapy treatment as first‐line treatment. Combination chemotherapies were based on platinum doublet chemotherapies, including paclitaxel/nab‐paclitaxel, and pemetrexed in accordance with the tumour histology. All study participants had to meet the criterion: (i) at least 18 years old; (ii) histologically or cytologically confirmed unresectable NSCLC (based on the International Association for the Study of Lung Cancer guidelines, 8th edition); (iii) driver‐genes including epidermal growth factor receptor (EGFR)/ROS proto‐oncogene 1 (ROS1)/anaplastic lymphoma kinase (ALK) wildtype; (iv) ECOG‐PS <3; and (v) exhibited disease progression after receiving therapy once every 3 weeks for at least two courses.
Patient clinical information was obtained from the patients' electronic medical records and the following were included: age at diagnosis, sex, baseline ECOG‐PS score, pathology type, stage, sites of distant metastases, types of driver mutations, treatment regimen, best response to treatment, date of progression, date of death, and date of the last follow‐up. Routine blood test results and the blood biochemical index scores at baseline and 6 weeks after treatment (0 weeks, 6 weeks) were also collected. Data of PD‐L1 expression of the tumour sample at diagnosis detected by immunohistochemistry according to standard practice (clone 22C3; DAKO) was collected. Considering the percentage of viable tumour cells with partial or complete membrane staining, the tumour proportion score (TPS) of ≥1% was defined as PD‐L1 positive. Patients were accordingly divided into three groups according to the PD‐L1 expression: positive, negative and unknown. This study was approved by the Institutional Ethical Review Board of the Shanghai Pulmonary Hospital and permitted waiver of the written informed consents because of the retrospective and anonymous study design.
4.2. Definition of inflammatory and nutritional indexes
The inflammatory markers are defined as follows: NLR = the ratio of neutrophil count to lymphocyte count(109/L); PLR = the ratio of platelet count(109/L) to lymphocyte count(109/L); SII = platelet count(109/L) × NLR; The PNI = sum of albumin value (g/L) and five times lymphocyte count (109/L); HALP = Haemoglobin (g/L) × Albumin (g/L) × Lymphocytes(109/L)/Platelets (109/L). For all indexes, the median value was calculated and used as the cut‐off value and handled as binary variables in the analysis. Based on the difference between the baseline scores and the score at 6 weeks (after two treatment cycles) collected before anti‐PD‐1 antibody treatment, patients were clustered into two groups (increase and decrease).
4.3. Assessment
According to the immune response evaluation criteria in solid tumours (iRECIST) guidelines, the ORR is defined as the percentage of patients with the best overall response of CR or PR. PFS is defined as the time from PD‐1 inhibitor treatment until disease progression or death. OS is defined as the time from the disease onset until death from any cause or the last follow‐up, whichever came first.
4.4. Statistical analysis
Categorical variables were summarized as numbers and percentages and were statistically measured using the chi‐square or Fisher's exact test. Continuous variables with non‐normal distribution were shown as median and interquartile range (IQR). The relationship between the dependent variable and multiple independent variables was explored using binary logistic regression. The Kaplan–Meier method was used to draw the survival curves to estimate the PFS and OS probabilities. The Cox regression analysis was used to analyse the prognostic factors. Lastly, the nomogram and the prediction model were generated using the R programming language. All tests were two‐sided, and the statistical significance was set at p < 0.05. IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA), R (version x64 4.2.0) and GraphPad Prism 9.0.0 were used for the statistical analyses and display.
AUTHOR CONTRIBUTIONS
Conceptualization: Qiyu Fang, Jie Zhang and Caicun Zhou; methodology: Qiyu Fang, Wei Li, Jia Yu and Bin Chen; Formal analysis and investigation: Wei Li, Jia Yu, Jie Luo, Qinfang Deng; Writing—original draft preparation, Qiyu Fang; Writing—review and editing, Qiyu Fang, Yayi He and Jie Zhang; Resources: Jie Zhang; Supervision Jie Zhang and Caicun Zhou; All authors read and approved the final manuscript.
FUNDING INFORMATION
This study was supported in part by grants from the Key Project of National Natural Science Foundation of China (No. 82141101), Shanghai Shenkang Three‐year Action Plan (No. SHDC2020CR1036B), Shanghai Shenkang database support plan (No. SHDC2020CR5001‐02), Collaborative innovation cluster plan of Shanghai Municipal Commission of Health and Family Planning (No. 2020CXJQ02), Science and Technology Innovation Action Plan ‘Experimental Animal Research Project’ of Science and Technology Commission of Shanghai Municipality (STCSM) (No. 201409003600) and Clinical Science and Technology Innovation Project of Shanghai Shenkang Hospital Development Center (No. SHDC12019X29).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Fang Q, Yu J, Li W, et al. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD‐1 inhibitor therapy. Clin Exp Pharmacol Physiol. 2023;50(2):178‐190. doi: 10.1111/1440-1681.13740
Funding information Key Project of National Natural Science Foundation of China, Grant/Award Number: 82141101; Shanghai Shenkang Three‐year Action Plan, Grant/Award Number: SHDC2020CR1036B; Shanghai Shenkang database support plan, Grant/Award Number: SHDC2020CR5001‐02; Collaborative innovation cluster plan of Shanghai Municipal Commission of Health and Family Planning, Grant/Award Number: 2020CXJQ02; Science and Technology Commission of Shanghai Municipality, Grant/Award Number: 201409003600; Shanghai Shenkang Hospital Development Center, Grant/Award Number: SHDC12019X29
Contributor Information
Jie Zhang, Email: zhangjie2172@163.com.
Caicun Zhou, Email: caicunzhoudr@163.com.
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
REFERENCES
- 1. Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver‐negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18:625‐644. [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 3. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819‐1830. [DOI] [PubMed] [Google Scholar]
- 4. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078‐2092. [DOI] [PubMed] [Google Scholar]
- 5. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature. 2017;541:321‐330. [DOI] [PubMed] [Google Scholar]
- 6. Killock D. Lung cancer: frontline nivolumab ‐ CheckMate026 ends in stalemate. Nat Rev Clin Oncol. 2017;14(8):458‐459. [DOI] [PubMed] [Google Scholar]
- 7. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093‐2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olivares‐Hernández A, Del Barco ME, Parra Pérez C, et al. Influence of DNA mismatch repair (MMR) system in survival and response to immune checkpoint inhibitors (ICIs) in non‐small cell lung cancer (NSCLC): retrospective analysis. Biomedicine. 2022;10(2):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non‐small cell lung cancer (NSCLC). Cancer. 2020;126:260‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonaggio A, Elaidi R, Fournier L, et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non‐small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol Immunother. 2020;69:2513‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Wen S, Xia J, et al. Association of dynamic changes in peripheral blood indexes with response to PD‐1 inhibitor‐based combination therapy and survival among patients with advanced non‐small cell lung cancer. Front Immunol. 2021;12:672271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiong Q, Huang Z, Xin L, et al. Post‐treatment neutrophil‐to‐lymphocyte ratio (NLR) predicts response to anti‐PD‐1/PD‐L1 antibody in SCLC patients at early phase. Cancer Immunol Immunother. 2021;70:713‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhai B, Chen J, Wu J, et al. Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte‐to‐monocyte ratio (LMR) in patients with non‐small cell lung cancer after radical lung cancer surgery. Ann Transl Med. 2021;9:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrova MP, Donev IS, Radanova MA, et al. Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second‐line pembrolizumab in patients with non‐small‐cell lung cancer. Clin Exp Immunol. 2020;202:353‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banna GL, Signorelli D, Metro G, et al. Neutrophil‐to‐lymphocyte ratio in combination with PD‐L1 or lactate dehydrogenase as biomarkers for high PD‐L1 non‐small cell lung cancer treated with first‐line pembrolizumab. Transl Lung Cancer Res. 2020;9:1533‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang M, Peng W, Pu X, et al. Peripheral blood biomarkers associated with outcome in non‐small cell lung cancer patients treated with nivolumab and durvalumab monotherapy. Front Oncol. 2020;10:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, Yu R, Cai T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88:106939. [DOI] [PubMed] [Google Scholar]
- 19. Rosenbaum SR, Wilski NA, Aplin AE. Fueling the fire: inflammatory forms of cell death and implications for cancer immunotherapy. Cancer Discov. 2021;11:266‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukui T, Okuma Y, Nakahara Y, et al. Activity of nivolumab and utility of neutrophil‐to‐lymphocyte ratio as a predictive biomarker for advanced non‐small‐cell lung cancer: a prospective observational study. Clin Lung Cancer. 2019;20:208‐214.e202. [DOI] [PubMed] [Google Scholar]
- 21. Kargl J, Busch SE, Yang GH, et al. Neutrophils dominate the immune cell composition in non‐small cell lung cancer. Nat Commun. 2017;8:14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu B, Yang XR, Xu Y, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212‐6222. [DOI] [PubMed] [Google Scholar]
- 23. Haemmerle M, Stone RL, Menter DG, Afshar‐Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33:965‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Li Y, Chen P, Xu W, Wu Y, Che G. Prognostic value of the pretreatment systemic immune‐inflammation index (SII) in patients with non‐small cell lung cancer: a meta‐analysis. Ann Transl Med. 2019;7:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio in non‐small cell lung cancer patients treated with immune checkpoint inhibitors: a meta‐analysis. Int Immunopharmacol. 2020;85:106677. [DOI] [PubMed] [Google Scholar]
- 26. Bilen MA, Martini DJ, Liu Y, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced‐stage cancer treated with immunotherapy. Cancer. 2019;125:127‐134. [DOI] [PubMed] [Google Scholar]
- 27. Russo A, Russano M, Franchina T, et al. Neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non‐small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv Ther. 2020;37:1145‐1155. [DOI] [PubMed] [Google Scholar]
- 28. Terasaki F, Sugiura T, Okamura Y, et al. Systemic immune‐inflammation index as a prognostic marker for distal cholangiocarcinoma. Surg Today. 2021;51:1602‐1609. [DOI] [PubMed] [Google Scholar]
- 29. Nøst TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. 2021;36:841‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonçalves AC, Richiardone E, Jorge J, et al. Impact of cancer metabolism on therapy resistance ‐ clinical implications. Drug Resist Updat. 2021;59:100797. [DOI] [PubMed] [Google Scholar]
- 31. Chapek MA, Martindale RG. Nutrition in cancer therapy: overview for the cancer patient. JPEN J Parenter Enteral Nutr. 2021;45:33‐40. [DOI] [PubMed] [Google Scholar]
- 32. Weng CY, Kao CX, Chang TS, Huang YH. Immuno‐metabolism: The role of cancer niche in immune checkpoint inhibitor resistance. Int J Mol Sci. 2021;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guerra L, Bonetti L, Brenner D. Metabolic modulation of immunity: a new concept in cancer immunotherapy. Cell Rep. 2020;32:107848. [DOI] [PubMed] [Google Scholar]
- 34. Kinoshita F, Tagawa T, Yamashita T, et al. Prognostic value of postoperative decrease in serum albumin on surgically resected early‐stage non‐small cell lung carcinoma: a multicenter retrospective study. PLoS One. 2021;16:e0256894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takada K, Takamori S, Yoneshima Y, et al. Serum markers associated with treatment response and survival in non‐small cell lung cancer patients treated with anti‐PD‐1 therapy. Lung Cancer. 2020;145:18‐26. [DOI] [PubMed] [Google Scholar]
- 36. Wallace K, Li H, Brazeal JG, et al. Platelet and hemoglobin count at diagnosis are associated with survival in African American and Caucasian patients with colorectal cancer. Cancer Epidemiol. 2020;67:101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214‐2221. [PubMed] [Google Scholar]
- 38. Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir. 2021;92:283‐292. [PubMed] [Google Scholar]
- 39. Wang X, He Q, Liang H, et al. A novel robust nomogram based on preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) for predicting lymph node metastasis of gastric cancer. J Gastrointest Oncol. 2021;12:2706‐2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei S, Shao J, Wang J, Wang G. The preoperative hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for non‐small cell lung cancer patients undergoing adjuvant chemotherapy: a retrospective study. Ann Transl Med. 2022;10:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu N, Jiang A, Zheng X, et al. Prognostic nutritional index identifies risk of early progression and survival outcomes in advanced non‐small cell lung cancer patients treated with PD‐1 inhibitors. J Cancer. 2021;12:2960‐2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsubara T, Takamori S, Haratake N, et al. The impact of immune‐inflammation‐nutritional parameters on the prognosis of non‐small cell lung cancer patients treated with atezolizumab. J Thorac Dis. 2020;12:1520‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li D, Yuan X, Liu J, Li C, Li W. Prognostic value of prognostic nutritional index in lung cancer: a meta‐analysis. J Thorac Dis. 2018;10:5298‐5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suh KJ, Kim SH, Kim YJ, et al. Post‐treatment neutrophil‐to‐lymphocyte ratio at week 6 is prognostic in patients with advanced non‐small cell lung cancers treated with anti‐PD‐1 antibody. Cancer Immunol Immunother. 2018;67:459‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khunger M, Patil PD, Khunger A, et al. Post‐treatment changes in hematological parameters predict response to nivolumab monotherapy in non‐small cell lung cancer patients. PLoS One. 2018;13:e0197743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ameratunga M, Chénard‐Poirier M, Moreno Candilejo I, et al. Neutrophil‐lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD‐1/PD‐L1 inhibitors. Eur J Cancer. 2018;89:56‐63. [DOI] [PubMed] [Google Scholar]
- 47. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first‐line treatment of PD‐L1‐selected patients with NSCLC. N Engl J Med. 2020;383(14):1328‐1339. [DOI] [PubMed] [Google Scholar]
- 48. Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD‐L1‐positive locally advanced or metastatic non‐small‐cell lung cancer: KEYNOTE‐042 China study. Int J Cancer. 2021;148(9):2313‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pan Y, Fu Y, Zeng Y, et al. The key to immunotherapy: how to choose better therapeutic biomarkers for patients with non‐small cell lung cancer. Biomark Res. 2022;10((1)):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ready N, Hellmann MD, Awad MM, et al. First‐line nivolumab plus ipilimumab in advanced non‐small‐cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luo W, Wang Z, Zhang T, et al. Immunotherapy in non‐small cell lung cancer: rationale, recent advances and future perspectives. Precis Clin Med. 2021;4(4):258‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ettinger DS, Wood DE, Aisner DL, et al. Non‐smallcelllung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497‐530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.


