Abstract
Dietary supplementation with l-arginine has been reported to reduce white fat mass in diet-induced obese rats and in obese humans. This study was conducted to test the hypothesis that the arginine treatment regulates glucose and fatty acid metabolism in insulin-sensitive tissues. Male Sprague–Dawley rats (4-week-old) were fed either low- or high-fat diets for 15 weeks (n = 16/diet). Thereafter, lean or obese rats were fed their respective diets and received drinking water containing either 1.51% l-arginine-HCl or 2.55% alanine (isonitrogenous control) (n = 8/treatment group). After 12 weeks of treatment, rats were euthanized and tissue samples were collected for biochemical assays. High-fat feeding increased the size of adipocytes isolated from retroperitoneal (RP) adipose tissue, while arginine treatment reduced their size. The total number of adipocytes in the adipose tissue did not differ among the four groups of rats. Glucose oxidation in extensor digitorum longus (EDL) muscle, soleus muscle, and RP adipose tissue were reduced in response to high-fat feeding. On the contrary, oleic acid oxidation in RP adipose tissue was enhanced in rats fed the high-fat diet. Arginine treatment stimulated both glucose and oleic acid oxidation in EDL and soleus muscles, while having no effect on glucose oxidation, oleic acid oxidation, or basal lipolysis per 106 adipocytes in RP adipose tissue. Collectively, these results indicate that oral supplementation with arginine to diet-induced obese rats promoted the oxidation of energy substrates in skeletal muscle, thereby reducing white fat in the body.
Keywords: Amino acids, adipose tissue, metabolism, nutrition, obesity, skeletal muscle
Impact Statement
Obesity is a major health issue worldwide, increasing risks for type 2 diabetes, certain types of cancer, and cardiovascular diseases, as well as high rates of premature mortality and high economic costs. l-Arginine supplementation has been reported to reduce white fat mass in diet-induced obese rats and in obese humans, while improving whole-body insulin sensitivity. However, the underlying mechanisms are largely unknown. This study determined the oxidation of glucose and oleic acid, as well as the release of lactate and glycerol (indicators of glycolysis and lipolysis, respectively) by both white adipose tissue and skeletal muscle from rats fed a low- or high-fat diet supplemented with or without l-arginine. Our results indicate that l-arginine supplementation has physiological roles in regulating the tissue-specific metabolism of energy substrates in diet-induced obese rats and provide a metabolic basis for the use of arginine to drive the loss of white fat in obese individuals.
Introduction
l-Arginine is a physiological precursor of nitric oxide (NO), a free radical that is synthesized in almost all cell types by NO synthase (NOS). 1 There are three isoforms of NOS: neuronal (nNOS or NOS1), endothelial (eNOS or NOS3), and inducible (iNOS or NOS2). Through cGMP-dependent and independent pathways, NO plays an important role in regulating vascular tone, neurotransmission, host immunity, and whole-body homeostasis.1,2 There is growing evidence that the arginine-NO pathway is also involved in regulating energy-substrate metabolism, such as glycolysis, glucose transport and oxidation, gluconeogenesis, lipolysis, fatty acid synthesis, and fatty acid oxidation.3–5 The underlying mechanisms may include an increase in the expression and phosphorylation of AMP-activated protein kinase, hormone-sensitive lipase, and perilipins; a decrease in the intracellular concentration of malonyl-CoA due to the inhibition of acetyl-CoA carboxylase and the activation of malonyl-CoA decarboxylase; reduced expression of key genes related to lipogenesis and gluconeogenesis; enhanced expression and activity of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) to promote the oxidation of glucose and fatty acids in the mitochondria; augmentation of the mass and function of brown adipose tissue; and enhanced flow of blood to insulin-sensitive tissues for the uptake of glucose and fatty acids. In our previous study, we found that dietary arginine supplementation reduced body fat mass, increased muscle weight, and improved whole-body insulin sensitivity in both lean and diet-induced obese (DIO) rats.6,7 This antiobesity effect of arginine has also been reported for Zucker diabetic fatty (ZDF) rats 8 and obese humans with type 2 diabetes. 9 In addition, short-term administration of arginine improved insulin sensitivity in obese humans with type 2 diabetes mellitus, 10 whereas long-term (3-month) oral arginine maintained lean-tissue mass in obese individuals fed a weight-reducing diet. 11
Based on the previous findings that implicate an important role for NO in regulating the metabolism of energy substrates, 3 we hypothesized that dietary arginine supplementation to DIO rats reduced fat mass through increasing the oxidation of glucose and fatty acids in insulin-sensitive tissues. This hypothesis was tested with the use of 14C-labeled glucose and oleic acid, as well as the measurement of the release of lactate and glycerol (indicators of glycolysis and lipolysis, respectively) from adipose tissue and skeletal muscle.
Materials and methods
Chemicals
D-[U-14C]glucose and [1-14C]oleic acid were purchased from American Radiolabeled Chemicals (St Louis, MO). Collagenase (type I) was obtained from Worthington Biochemical Corporation (Lakewood, NJ). Bovine serum albumin (BSA) was procured from Intergen Company (Purchase, NY). High-performance liquid chromatography (HPLC)-grade methanol and water were obtained from Fisher Scientific (Houston, TX). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO).
Animals
This study was approved by The Institutional Animal Care and Use Committee of Texas A&M University.
Male Sprague–Dawley rats from Harlan Labs were used in this study. The experimental design (including the diets) was detailed in our published work. 6 Briefly, 4-week-old male Sprague–Dawley rats were fed either a low- or high-fat diet for 15 weeks (n = 16/diet). Rats fed the high-fat diet became obese. 6 Beginning at 19 weeks of age, rats were fed for 12 weeks their same respective diets and received drinking water containing either 1.51% l-arginine-HCl or 2.55% l-alanine (isonitrogenous control) (n = 8/treatment group). The rationale for the use of alanine as the isonitrogenous control in animals has been provided in our previous publications.6–8 In essence, alanine is not a substrate of arginine synthesis, but is extensively degraded in rats and other animals (e.g. pigs) and, at the dose used, has no effect on blood glucose concentrations.6–8
In support of the foregoing notion, we found that adding 2.55% l-alanine to drinking did not affect the concentrations of glucose, fatty acids, cholesterol, tissue (e.g. skeletal muscle and white adipose tissue) weight, and body weight in rats between 19 and 32 weeks of age. 7 Similar results were reported by Araujo et al. 12 for mice that received drinking water containing 2.55% l-alanine between 30 and 90 days of age, as compared with the absence of l-alanine in drinking water. By contrast, Cunningham et al. 13 demonstrated that the addition of 10 mM l-alanine to culture medium increased the expression of genes for anti-inflammatory cytokines and antiapoptotic proteins in a clonal rat pancreatic beta-cell line, compared with the absence of l-alanine (0 mM). However, the concentration of l-alanine used for the in vitro study (10 mM) 13 was about 13–25 times the concentrations of l-alanine (0.4 and 0.75 mM) in the plasma of rats supplemented without or with l-alanine, respectively. 6 Note that the plasma of mammals always contains l-alanine (0.20–0.75 mM depending on species), 14 and l-alanine is required for metabolic pathways such as protein and peptide syntheses. Therefore, the use of 0 mM l-alanine as the control for 10 mM l-alanine, as in the work of Cunningham et al., 13 would not indicate nutritional or physiological effects of this amino acid (AA) in animals.
After 12 weeks of treatment with l-arginine or l-alanine, rats were euthanized, and tissue samples were collected for biochemical assays. 6 This experiment was part of our previous study. 6 Data on the food intake, water consumption, plasma concentrations of AAs, tissue (e.g. skeletal muscle and white adipose tissue) weights, and body-weight gain of rats in all the treatment groups were reported in our previous paper. 6
Determination of NO synthesis in freshly isolated tissues
Retroperitoneal (RP) adipose tissue and gastrocnemius muscle (~100 mg) were minced and rinsed with oxygenated (95%O2/5%CO2) Krebs-Henseleit bicarbonate buffer (pH 7.4) containing 5 mM of D-glucose and 4% BSA (KHB). Tissues were then incubated with gentle shaking (70 oscillations/min) at 37°C in 1 mL of fresh KHB containing 1 mM arginine. At the end of a 3 h incubation period, incubation medium was collected and analyzed for nitrite (a major stable end product of NO oxidation) by an HPLC method. 15 The production of nitrite was used as an indicator of NO synthesis by tissues. In all experiments, the incubation medium without tissues was included as blanks. We did not analyze nitrate (another major stable end product of NO oxidation) in the present study.
Determination of lipolysis and lactate release from RP adipose tissue
Minced RP adipose tissue (~100 mg) was rinsed with KHB, as described by Fried et al. 16 and was then incubated at 37°C in KHB containing 0 or 10 μM norepinephrine (NE) to determine basal and NE-stimulated lipolysis, respectively. After a 2-h incubation period, all tubes were placed on ice immediately, and the incubation media were rapidly transferred into clean tubes for determination of glycerol 17 and lactate. 18
Isolation of adipocytes and determination of RP adipose tissue cellularity
Adipocytes from RP adipose tissue were isolated according to the method described by Rodbell. 19 Briefly, RP adipose tissue (~1 g) was minced and rinsed with KHB, and then incubated with KHB containing 1 mg/mL collagenase for 45 min at 37°C with gentle shaking. After incubation, the cell suspensions were filtered through a 250 μm nylon grid, washed three times with KHB, and suspended in KHB at 25°C for 3 min. The floating layer of adipocytes was collected into clean tubes and diluted with KHB to obtain a cell concentration of 10% (v/v). The number of cells was counted using a hemocytometer (Fisher Scientific, Waltham, MA). An aliquot of the cell suspension was spotted onto a slide and the diameter of adipocytes was determined under a microscope with a ruler. For each slide, at least 120 cells were measured for their diameter, and the mean volume of adipocytes was calculated according to the method described by Goldrick 20 : V = π/6 × d (d2 + 3σ2), where d is the mean diameter and σ is the variance of the diameter of cells. The lipid content in RP adipose tissue on per gram basis was determined using the Folch method. 21 Based on the assumption that adipocytes are primarily triolein droplets and the density of adipocytes is 0.915, the lipid content per adipocyte and the number of adipocytes in the whole RP adipose tissue pad was calculated. 22
Determination of glucose and fatty acid oxidation
RP adipose tissue, extensor digitorum longus (EDL) muscle, and soleus muscle were minced and rinsed with KHB. The tissues were then placed in polystyrene tubes and incubated at 37°C for 2 h in 1 mL oxygenated KHB containing 3 nM insulin and either 0.5 μCi D-[U-14C]glucose or 0.2 mM of oleic acid plus [1-14C]oleic acid (0.5 μCi). The tubes were filled with 95%O2/5%CO2, sealed with rubber stoppers and fitted with hanging center-wells. At the end of a 2-h incubation period, 0.2 mL NCS-II (Amersham, Piscataway, NJ) was added through the rubber stopper into a 500-μL microcentrifuge tube placed in the center-well, followed by addition of 0.2 mL of 1.5 M HClO4 to the incubation medium. After an additional 1-h incubation period, the microcentrifuge tubes were carefully transferred to scintillation vials. Fifteen milliliters of counting cocktail was added into the vials, and 14C radioactivity was determined using a liquid scintillation counter (Packard Instrument Company, Downers Grove, IL). 23 The specific activities of D-[U-14C]glucose and [1-14C]oleic acid in the incubation media were used to calculate the rate of 14CO2 production.
Data analysis
Results were given as means ± SEM. Data for muscle tissues are expressed per gram tissue weight, and data for adipose tissue are expressed on the basis of both gram tissue and 106 adipocytes. The Levene’s test was used to assess the normality of data distribution. 24 Statistical analysis was performed using two-way analysis of variance (ANOVA) with the Tukey multiple comparison to determine the effects of diet, AA, and the interaction between diet and AA (diet × AA). 24 Probability values ⩽ 0.05 were taken to indicate statistical significance.
Results
The size and number of adipocytes in RP adipose tissue
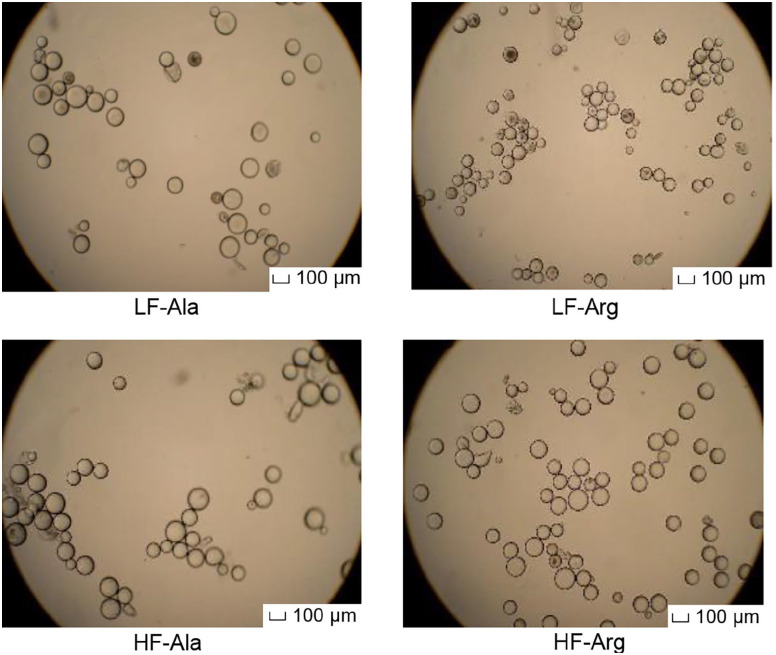
The high-fat diet enhanced (P < 0.005) the size of adipocytes isolated from RP adipose tissue by 11% and 20% in alanine- and arginine-supplemented rats, respectively (Table 1). Adipocytes isolated from arginine-supplemented rats fed the low-fat diet exhibited the smallest size (mean diameter 77 ± 3 µm), when compared to other three groups of rats (mean diameter > 90 µm) (Figure 1). Dietary arginine supplementation reduced (P < 0.005) the mean diameter of adipocytes by 15% and 7% in rats fed the low- and high-fat diets, respectively (Table 1). Arginine treatment resulted in a shift of adipocyte size toward a smaller diameter, compared with alanine supplementation (Figure 2). The total numbers of adipocytes in the entire RP adipose tissue did not differ among the four groups of rats. However, high-fat feeding reduced (P < 0.005) the density of adipocytes per gram of RP adipose tissue due to the increased lipid filling, compared with rats fed the low-fat diet (Table 1). In contrast, dietary arginine supplementation increased (P < 0.005) the density of adipocytes, in comparison with alanine-supplemented rats (Table 1).
Table 1.
Cellularity of retroperitoneal (RP) adipose tissue in rats fed a low- or high-fat diet supplemented with or without l-arginine.
| Variables | Low fat | High fat | P value | ||||
|---|---|---|---|---|---|---|---|
| Alanine | Arginine | Alanine | Arginine | Diet | AA | Diet × AA | |
| Adipocyte size (µm) | 90.4 ± 1.9 | 77.1 ± 3.1 | 99.9 ± 2.8 | 92.7 ± 2.5 | 0.001 | 0.001 | 0.26 |
| Total adipocytes/fat pad (106 cells) | 15.6 ± 1.6 | 14.4 ± 0.7 | 17.2 ± 0.9 | 15.1 ± 1.1 | 0.33 | 0.14 | 0.70 |
| Adipocyte density (106 cells/g tissue) |
2.13 ± 0.16 | 3.27 ± 0.27 | 1.62 ± 0.11 | 2.05 ± 0.14 | 0.001 | 0.001 | 0.22 |
Between 4 and 19 weeks of age, rats were fed low- and high-fat diets, respectively. Thereafter, rats in each dietary group received drinking water containing either 1.51% l-arginine·HCl or 2.55% l-alanine (isonitrogenous control) for 12 weeks. At the end of the feeding experiment, RP adipose tissue was dissected and adipocytes were isolated using an enzymatic method. Data are expressed as means ± SEM (n = 8/treatment group). P values are shown for diet effect, AA effect, and their interaction.
AA: amino acid.
Figure 1.
Morphology of adipocytes isolated from rat retroperitoneal (RP) adipose tissue. Between 4 and 19 weeks of age, rats were fed low- and high-fat diets, respectively. Thereafter, rats in each dietary group received drinking water containing either 1.51% l-arginine·HCl or 2.55% l-alanine (isonitrogenous control) for 12 weeks. At the end of the feeding experiment, RP adipose tissue was dissected and adipocytes were isolated. Data are expressed as means ± SEM (n = 8/treatment group). Magnification scale was set at ×10. The scale bar in all panels of this figure is 100 µm.
Figure 2.
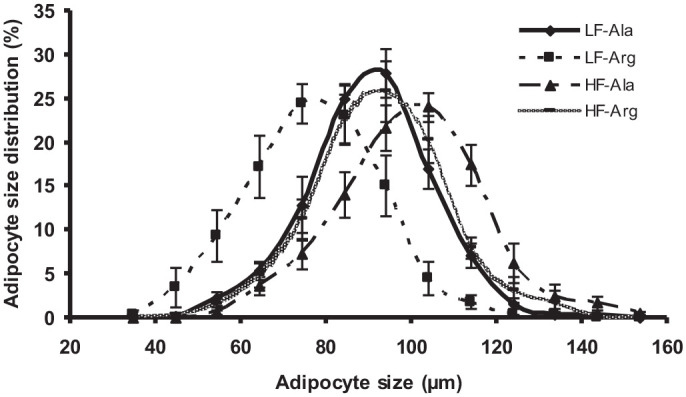
Size distribution of adipocyte from retroperitoneal (RP) adipose tissue of rats. Between 4 and 19 weeks of age, rats were fed low- and high-fat diets, respectively. Thereafter, rats in each dietary group received drinking water containing either 1.51% l-arginine·HCl or 2.55% l-alanine (isonitrogenous control) for 12 weeks. At the end of the feeding experiment, RP adipose tissue was dissected, adipocytes were isolated using an enzymatic method, and their sizes were determined under a microscope. Data are expressed as means ± SEM (n = 8/treatment group). P values for diet effect, AA effect, and their interaction are summarized below:
| Diameter of adipocytes (µm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P value | 45 | 55 | 65 | 75 | 85 | 95 | 105 | 115 | 125 | 135 | 145 |
| Diet | 0.13 | 0.005 | 0.002 | 0.001 | 0.052 | 0.51 | 0.001 | 0.0005 | 0.015 | 0.086 | 0.13 |
| AA | 0.13 | 0.020 | 0.003 | 0.004 | 0.23 | 0.16 | 0.001 | 0.0005 | 0.14 | 0.62 | 0.021 |
| Diet × AA | 0.19 | 0.056 | 0.016 | 0.14 | 0.052 | 0.011 | 0.050 | 0.17 | 0.60 | 0.80 | 0.21 |
Lactate release and lipolysis in RP adipose tissue
Table 2 summarizes the effects of high-fat feeding and arginine treatment on lactate release (an indicator of glycolysis) and lipolysis in RP adipose tissue. Expressed per gram tissue, lactate release from the RP adipose tissue was reduced (P < 0.001) in response to high-fat feeding, but dietary arginine supplementation increased lactate production by this tissue (P < 0.005). However, expressed per 106 adipocytes, the rate of lactate release from the RP adipose tissue was unaltered by either high-fat feeding or dietary arginine supplementation. Both basal and NE-stimulated rates of lipolysis per gram tissue or per 100 mg lipid were lower (P < 0.001) in rats fed a high-fat diet than in rats fed a low-fat diet. Dietary arginine supplementation increased (P < 0.05) basal lipolysis and had no effect on NE-stimulated lipolysis. However, expressed per 106 adipocytes, basal lipolysis did not differ either between rats fed the low- and high-fat diets or between alanine- and arginine-supplemented rats, whereas NE-stimulated lipolysis was lower in response to the arginine treatment due to the increased density of adipocytes (P < 0.05).
Table 2.
Lactate release and lipolysis in the retroperitoneal (RP) adipose tissue of rats fed a low- or high-fat diet supplemented with or without l-arginine.
| Variables | Low fat | High fat | P value | ||||
|---|---|---|---|---|---|---|---|
| Alanine | Arginine | Alanine | Arginine | Diet | AA | Diet × AA | |
| Lactate release | |||||||
| µmol lactate/g tissue · h | 1.10 ± 0.06 | 1.35 ± 0.14 | 0.84 ± 0.07 | 1.02 ± 0.04 | 0.001 | 0.004 | 0.67 |
| µmol lactate /106 adipocytes · h | 0.52 ± 0.05 | 0.42 ± 0.04 | 0.54 ± 0.06 | 0.52 ± 0.05 | 0.23 | 0.23 | 0.39 |
| Lipolysis | |||||||
| µmol glycerol/g tissue · h | |||||||
| Basal | 1.36 ± 0.07 | 1.54 ± 0.10 | 0.90 ± 0.33 | 1.20 ± 0.15 | 0.001 | 0.035 | 0.60 |
| NE-stimulated | 7.95 ± 0.66 | 7.27 ± 0.74 | 5.11 ± 0.33 | 5.93 ± 0.70 | 0.001 | 0.78 | 0.47 |
| µmol glycerol/100 mg lipid · h | |||||||
| Basal | 0.17 ± 0.01 | 0.19 ± 0.01 | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.002 | 0.03 | 0.46 |
| NE-stimulated | 0.98 ± 0.08 | 0.89 ± 0.09 | 0.62 ± 0.04 | 0.65 ± 0.09 | 0.001 | 0.75 | 0.46 |
| µmol glycerol/106 adipocytes · h | |||||||
| Basal | 0.68 ± 0.06 | 0.51 ± 0.03 | 0.57 ± 0.07 | 0.59 ± 0.07 | 0.92 | 0.23 | 0.13 |
| NE-stimulated | 3.97 ± 0.46 | 2.35 ± 0.14 | 3.20 ± 0.19 | 2.83 ± 0.55 | 0.71 | 0.016 | 0.11 |
Between 4 and 19 weeks of age, rats were fed low- and high-fat diets, respectively. Thereafter, rats in each dietary group received drinking water containing either 1.51% l-arginine·HCl or 2.55% l-alanine (isonitrogenous control) for 12 weeks. At the end of the feeding experiment, RP adipose tissue were collected, and lactate and glycerol were measured in incubation media. Data are expressed as means ± SEM (n = 8/treatment group). P values are shown for diet effect, AA effect, and their interaction.
AA: amino acid.
NO production by gastrocnemius skeletal muscles and RP adipose tissue
High-fat feeding did not affect NO synthesis in either gastrocnemius muscle or RP adipose tissue (Table 3). Dietary arginine supplementation increased (P < 0.05) NO production in gastrocnemius muscle by 25%, but had no effect (P> 0.05) on RP adipose tissue, compared with alanine-supplemented rats.
Table 3.
Nitrite production in the gastrocnemius muscle and retroperitoneal (RP) adipose tissue of rats fed a low- or high-fat diet supplemented with or without l-arginine.
| Nitrite production | Low fat | High fat | P value | ||||
|---|---|---|---|---|---|---|---|
| Alanine | Arginine | Alanine | Arginine | Diet | AA | Diet × AA | |
| Gastrocnemius muscle | |||||||
| nmol/g tissue · h | 0.23 ± 0.02 | 0.29 ± 0.02 | 0.21 ± 0.03 | 0.26 ± 0.02 | 0.23 | 0.032 | 0.83 |
| RP adipose tissue | |||||||
| nmol/g tissue · h | 0.17 ± 0.02 | 0.19 ± 0.02 | 0.11 ± 0.02 | 0.16 ± 0.02 | 0.61 | 0.081 | 0.59 |
| pmol/106 adipocytes · h | 78 ± 6 | 63 ± 9 | 72 ± 9 | 81 ± 9 | 0.53 | 0.79 | 0.23 |
Between 4 and 19 weeks of age, rats were fed low- and high-fat diets, respectively. Thereafter, rats in each dietary group received drinking water containing either 1.51% l-arginine·HCl or 2.55% l-alanine (isonitrogenous control) for 12 weeks. At the end of the feeding experiment, tissues were collected and nitrite was measured in incubation media. The production of nitrite was used as an indicator of NO synthesis by tissues. Data are expressed as means ± SEM (n = 8/treatment group). P values are shown for diet effect, AA effect, and their interaction.
AA: amino acid; NO: nitric oxide; RP: retroperitoneal.
Oxidation of glucose and fatty acids in muscle and RP adipose tissue
The effects of high-fat diet and arginine treatment on glucose and oleic acid oxidation in EDL muscle, soleus muscle, and RP adipose tissue are summarized in Table 4. Glucose oxidation per gram tissue in all three tissues was 18%, 28%, and 76% lower (P < 0.05), respectively, in rats fed the high-fat diet than in rats fed the low-fat diet. Glucose oxidation in RP adipose tissue per 106 adipocytes was approximately 64% lower (P < 0.01) in high-fat-fed rats, compared with low-fat-fed rats. Oleic acid oxidation in skeletal muscles did not differ between rats fed the low- and high-fat diets (P > 0.05). In RP adipose tissue, when data were expressed per gram tissue or 106 adipocytes, oleic acid oxidation increased (P < 0.001) by 33% or 79%, respectively, in high-fat-fed rats, in comparison with low-fat-fed rats. Dietary arginine supplementation increased (P < 0.05) glucose and oleic acid oxidation per gram tissue in skeletal muscles and RP adipose tissue. However, when data were expressed per 106 adipocytes, neither glucose nor oleic acid oxidation in RP adipose tissue differed between alanine- and arginine-supplemented rats.
Table 4.
Glucose and oleic acid oxidation in the extensor digitorum longus (EDL) muscle, soleus muscle, and retroperitoneal (RP) adipose tissue of rats fed a low- or high-fat diet supplemented with or without l-arginine.
| Tissues | Low fat | High fat | P value | ||||
|---|---|---|---|---|---|---|---|
| Alanine | Arginine | Alanine | Arginine | Diet | AA | Diet × AA | |
| Per g tissue | |||||||
| Glucose oxidation (nmol glucose/g tissue · h) | |||||||
| EDL muscle | 34.4 ± 4.0 | 44.1 ± 2.0 | 28.5 ± 2.7 | 35.9 ± 2.5 | 0.039 | 0.015 | 0.73 |
| Soleus muscle | 38.1 ± 3.8 | 49.0 ± 5.6 | 31.0 ± 2.4 | 31.4 ± 5.1 | 0.009 | 0.04 | 0.40 |
| RP adipose tissue | 346 ± 59 | 595 ± 51 | 104 ± 10 | 120 ± 14 | 0.001 | 0.014 | 0.098 |
| Oleic acid oxidation (nmol oleic acid/g tissue · h) | |||||||
| EDL muscle | 1.51 ± 0.28 | 2.77 ± 0.49 | 1.70 ± 0.22 | 1.94 ± 0.15 | 0.34 | 0.03 | 0.13 |
| Soleus muscle | 3.46 ± 0.45 | 4.64 ± 0.41 | 3.45 ± 0.36 | 3.97 ± 0.22 | 0.35 | 0.027 | 0.38 |
| RP adipose tissue | 0.80 ± 0.06 | 0.93 ± 0.07 | 1.05 ± 0.06 | 1.25 ± 0.08 | 0.001 | 0.036 | 0.63 |
| Per 106 adipocytes | |||||||
| Glucose oxidation (nmol glucose/106 adipocytes · h) | |||||||
| RP adipose tissue | 171 ± 32 | 192 ± 15 | 67 ± 9 | 62 ± 7 | 0.001 | 0.72 | 0.55 |
| Oleic acid oxidation (nmol oleic acid/106 adipocytes · h) | |||||||
| RP adipose tissue | 0.41 ± 0.05 | 0.31 ± 0.03 | 0.67 ± 0.06 | 0.62 ± 0.05 | 0.001 | 0.18 | 0.63 |
Between 4 and 19 weeks of age, rats were fed low- and high-fat diets, respectively. Thereafter, rats in each dietary group received drinking water containing either 1.51% l-arginine·HCl or 2.55% l-alanine (isonitrogenous control) for 12 weeks. At the end of the feeding experiment, tissues were used to measure glucose and oleic acid oxidation. Data are expressed as means ± SEM (n = 8/treatment group). P values are shown for diet effect, AA effect, and their interaction.
AA: amino acid; EDL: extensor digitorum longus; RP: retroperitoneal.
Discussion
Oxidation of energy substrates in skeletal muscle, liver, and adipose tissue plays an important role in fuel homeostasis, and fat accretion in animals depends on the balance between energy intake and expenditure. 3 Therefore, to provide a metabolic basis for reduced adiposity in arginine-supplemented DIO rats, 7 we studied glucose and oleic acid oxidation in EDL muscle (primarily glycolytic fiber), soleus muscle (primarily oxidative fiber), and RP adipose tissue. In addition, the production of lactate and glycerol from adipose tissue was measured as indicators of glycolysis and lipolysis, respectively. 16
Berger and Barnard 25 reported that 2 months of high-fat feeding increased the size of adipocytes and reduced their number in rats per gram tissue. Consistent with this finding, results of our study demonstrated a 15% increase in the size of adipocytes and a 32% reduction in their density in the RP adipose tissue of rats fed the high-fat diet, compared with rats fed the low-fat diet (Table 1). Because high-fat feeding did not affect lipolysis per 106 adipocytes (Table 2), the increased synthesis of triglycerides may be the major factor for the hypertrophy of the RP adipose tissue in DIO rats (Table 1), which is consistent with the report that high-fat diet increased the activity of lipoprotein lipase (LPL) in white adipose tissue. 26 A novel finding from the present study is that dietary arginine supplementation to diet-induced DIO rats reduced the size of adipocytes by reducing their accumulation of fat, without affecting their total cell numbers (Table 1). This outcome likely resulted from an increase in the basal hydrolysis of triglycerides and the subsequent oxidation of fatty acids per gram tissue in the fat depots (Tables 2 and 4). In support of this notion, physiological levels of NO, a metabolite of arginine (Table 3), stimulated basal lipolysis in human adipose tissue 27 and rat adipocytes. 28
Dietary fat intake is known to decrease muscle glucose oxidation. 29 Accordingly, glucose oxidation was lower in both EDL and soleus muscles of rats fed the high-fat diet, when compared with rats fed the low-fat diet (Table 4). This result can be explained by reduced glucose uptake 30 and increased concentrations of intramuscular lipids for oxidation 6 in DIO rats. The acetyl-CoA derived from fatty acids can potently inhibit pyruvate kinase and pyruvate dehydrogenase activity and results in the formation of citrate (an inhibitor of fructose-6-phosphate kinase-I), thereby decreasing glucose oxidation via glycolysis and the tricarboxylic-acid cycle. 14 In support of this view, there is an inverse relationship between intramuscular concentrations of triglycerides and the whole-body glucose oxidation in humans.31,32
Another important finding from the present study is that the fat tissue of adult rats exhibited a much higher rate of glucose oxidation than skeletal muscle per gram tissue (Table 4). Considering its large mass in obese animals, 6 white adipose tissue may play a quantitatively important role in regulating glucose homeostasis in DIO rats. 7 Interestingly, glucose oxidation decreased, but oleic acid oxidation increased, in the RP adipose tissue of rats fed the high-fat diet, in comparison with rats fed the low-fat diet (Tables 2 and 4). These results are consistent with the previous reports that high-fat feeding reduced glucose oxidation in adipose tissue, shifting its utilization of energy substrates from glucose to fatty acids. 33
Another novel and important finding from the present study is that dietary arginine supplementation increased the oxidation of both glucose and fatty acids in the skeletal muscle of DIO rats. An increase in muscular NO production within the physiological range may play a role in mediating this beneficial action of the arginine treatment (Table 3). Rat skeletal muscle contains nNOS in the mitochondria, sarcoplasm, and sarcolemmal membrane; iNOS in the sarcoplasm and sarcolemmal membrane; and eNOS in the sarcoplasm, sarcoplasmic reticulum, and sarcolemmal membrane caveolae. 3 In rats, the subcellular distribution of NOS in white adipose tissue is similar to that in skeletal muscle, as the former contains nNOS in the cytoplasm; iNOS in the cytoplasm plasma membrane; and eNOS in the cytoplasm and plasma membrane caveolae. 3 Determination of the expression of the three NOS isoforms would provide useful information about the dominant sources of NO in the skeletal muscle and white adipose tissue of rats supplemented with or without arginine. Emerging evidence shows that NO regulates the metabolism of energy substrates in insulin-sensitive tissues. 3 For example, sodium nitroprusside (SNP), an NO donor, increased glucose transport and oxidation in isolated muscle.34,35 Consequently, an inhibition of NO synthesis reduced both basal and exercise-stimulated glucose transport in skeletal muscle in in vitro and in vivo studies.26,34,36,37 The underlying mechanisms may involve the cellular signaling cascade, which includes soluble guanylyl cyclase (the target of NO) and cGMP-dependent protein kinase. 38 In support of this view, Garcia-Villafranca et al. 39 found that NO and cGMP increased fatty acid oxidation and reduced fatty acid synthesis in rat hepatocytes. In addition, the NO/cGMP pathway has been reported to trigger mitochondrial biogenesis in different mammalian cell types and tissues through the activation of PGC-1α. 40 The outcome is to increase the mitochondrial oxidation of energy substrates, thereby reducing their availability for fat accretion in the body. 41 Indeed, our previous study with ZDF rats showed that dietary arginine supplementation increased mRNA levels for PGC-1α in RP adipose tissue. 8 At present, it is unknown whether arginine treatment also increases PGC-1α expression in other insulin-sensitive tissues of animals.
A role for NO in regulating white adipose tissue metabolism remains elusive, primarily due to the conflicting reports of in vitro studies involving extremely high doses of NO donors (up to 2 mM). Another interesting issue is that macrophages present in white adipose tissue produce larger amounts of NO from arginine than white adipocytes, 3 and play crucial roles in both inflammatory responses and insulin resistance. Under conditions of metabolic syndrome, the relationship among macrophages, arginine, NO, and adipocytes is likely complex. 42 NO may act to stimulate the oxidation of energy substrates at physiological levels but to suppress this event at excessive levels.43,44 In addition, the function of NO in white adipose tissue may also be dependent on the location and types of tissues.43,45 On the basis of per 106 adipocytes, we found that arginine supplementation did not affect NO production, basal lipolysis, or substrate oxidation in the RP adipose tissue, suggesting that arginine may have only a modest effect on glucose or fatty acid metabolism in the adipose tissue of DIO rats after a 12-week period of its dietary supplementation. Thus, we suggest that liver and skeletal muscle may play a quantitatively more important role than white adipose tissue in the antiobesity action of arginine. It is also possible oral arginine exerts its regulatory effects partly through stimulating: (1) the phosphorylation of AMP-activated protein kinase 46 and (2) the secretion of glucagon-like peptide-1 from the endocrine l-cells of the intestine to augment insulin secretion and improve glucose tolerance, as reported for male mice. 47 In vitro experiments are warranted to determine whether arginine regulates glucose and fatty acid metabolism in skeletal muscle cells, hepatocytes, and intestinal organoid culture, as well as the intestinal synthesis and secretion of hormones (e.g. glucagon-like peptide-1) via NO-dependent and independent mechanisms. In this regard, it is noteworthy that arginine increased glucose and fatty acid oxidation in cultured BNL CL2 hepatocytes, C2C12 skeletal muscle cells, and 3T3-L1 white adipocytes via both NO-dependent and NO-independent mechanisms. 48 As a functional AA, arginine holds great promise for reducing obesity and improving health in humans5,49 and other animals such as pigs, sheep, cats, and dogs. 50
Conclusions
Results of our study indicate that dietary supplementation with arginine to DIO rats did not affect the total number of adipocytes in RP adipose tissue. High-fat feeding reduced the rates of glucose oxidation in EDL and soleus muscles as well as RP adipose tissue, but enhanced the rates of oleic acid oxidation in RP adipose tissue. Arginine treatment stimulated the rates of glucose and oleic acid oxidation in EDL and soleus muscles, but did not influence the rates of glucose and oleic acid oxidation or basal lipolysis per 106 adipocytes. Collectively, these results indicate that dietary arginine has important roles in regulating the metabolism of energy substrates in DIO rats. Our findings provide a metabolic basis for the use of arginine to drive the loss of body white fat in these animals. Further research is necessary to elucidate the underlying molecular mechanisms.
Acknowledgments
We thank Dr Stephen B. Smith and Dr Cynthia J. Meininger for helpful discussion, as well as Scott Jobgen for assistance in the laboratory analyses of metabolites.
Footnotes
Authors’ Contributions: GW designed and supervised the study. WSJ and MJL performed the experiment, summarized the results, and wrote the first draft of the manuscript. All authors contributed to data interpretation and manuscript revisions, as well as read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by American Heart Association–TX (Grant #0755024Y).
ORCID iD: Guoyao Wu  https://orcid.org/0000-0001-8058-6969
https://orcid.org/0000-0001-8058-6969
References
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001;357:593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durante W. Amino acids in circulatory function and health. Adv Exp Med Biol 2020;1265:39–56 [DOI] [PubMed] [Google Scholar]
- 3.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 2006;17:571–88 [DOI] [PubMed] [Google Scholar]
- 4.McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids 2010;39:349–57 [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Meininger CJ, McNeal CJ, Bazer FW, Rhoads JM. Role of L-arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol 2021;1332:167–87 [DOI] [PubMed] [Google Scholar]
- 6.Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 2009;139:230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, Spencer TE, Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids 2009;37:187–98 [DOI] [PubMed] [Google Scholar]
- 8.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 2005;135:714–21 [DOI] [PubMed] [Google Scholar]
- 9.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab 2006;291:E906–12 [DOI] [PubMed] [Google Scholar]
- 10.Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, Toplak H. Effects of low-dose l-arginine on insulin-mediated vasodilation and insulin sensitivity. Eur J Clin Invest 1997;27:690–5 [DOI] [PubMed] [Google Scholar]
- 11.McNeal CJ, Meininger CJ, Wilborn CD, Tekwe CD, Wu G. Safety of dietary supplementation with arginine in adult humans. Amino Acids 2018;50:1215–29 [DOI] [PubMed] [Google Scholar]
- 12.Araujo TR, Freitas IN, Vettorazzi JF, Batista TM, Santos-Silva JC, Bonfleur ML, Balbo SL, Boschero AC, Carneiro EM, Ribeiro RA. Benefits of L-alanine or L-arginine supplementation against adiposity and glucose intolerance in monosodium glutamate-induced obesity. Eur J Nutr 2017;56:2069–80 [DOI] [PubMed] [Google Scholar]
- 13.Cunningham GA, McClenaghan NH, Flatt PR, Newsholme P. L-alanine induces changes in metabolic and signal transduction gene expression in a clonal rat pancreatic beta-cell line and protects from pro-inflammatory cytokine-induced apoptosis. Clin Sci 2005;109:447–55 [DOI] [PubMed] [Google Scholar]
- 14.Wu G. Principles of animal nutrition. Boca Raton, FL: CRC Press, 2018 [Google Scholar]
- 15.Li H, Meinger CJ, Wu G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B 2000;746:199–207 [DOI] [PubMed] [Google Scholar]
- 16.Fried SK, Leibel RL, Edens NK, Kral JG. Lipolysis in intraabdominal adipose tissues of obese women and men. Obes Res 1993;1:443–8 [DOI] [PubMed] [Google Scholar]
- 17.Laurell S, Tibbling G. An enzymatic fluorometric micromethod for the determination of glycerol. Clin Chim Acta 1966;13:317–22 [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Knabe DA, Yan W, Flynn NE. Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am J Physiol 1995;268:R334–42 [DOI] [PubMed] [Google Scholar]
- 19.Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964;239:375–80 [PubMed] [Google Scholar]
- 20.Goldrick RB. Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol 1967;212:777–82 [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tisues. J Biol Chem 1957;226: 497–509 [PubMed] [Google Scholar]
- 22.Mandenoff A, Lenoir T, Apfelbaum M. Tardy occurrence of adipocyte hyperplasia in cafeteria-fed rat. Am J Physiol 1982;242:R349–51 [DOI] [PubMed] [Google Scholar]
- 23.Dillon EL, Wu G. Cortisol enhances citrulline synthesis from proline in enterocytes of suckling piglets. Amino Acids 2021;53:1957–66 [DOI] [PubMed] [Google Scholar]
- 24.Assaad HI, Zhou L, Carroll RJ, Wu G. Rapid publication-ready MS word tables for one-way ANOVA. SpringerPlus 2014;3:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger JJ, Barnard RJ. Effect of diet on fat cell size and hormone-sensitive lipase activity. J Appl Physiol 1999;87:227–32 [DOI] [PubMed] [Google Scholar]
- 26.Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol 1997;273:E220–5 [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Lee MJ, Fried SK. The arginine-NO pathway modulates lipolysis in adipose tissues of obese human subjects. FASEB J 2007;21: A1052 [Google Scholar]
- 28.Canová NK, Lincová D, Kmonícková E, Kameníková L, Farghali H. Nitric oxide production from rat adipocytes is modulated by beta3-adrenergic receptor agonists and is involved in a cyclic AMP-dependent lipolysis in adipocytes. Nitric Oxide 2006;14:200–11 [DOI] [PubMed] [Google Scholar]
- 29.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 1997;46:1768–74 [DOI] [PubMed] [Google Scholar]
- 30.Todd MK, Yaspelkis BB, 3rd, Turcotte LP. Short-term leptin treatment increases fatty acids uptake and oxidation in muscle of high fat-fed rats. Metabolism 2005;54:1218–24 [DOI] [PubMed] [Google Scholar]
- 31.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Shulman GI, Roden M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR speLFoscopy study. Diabetologia 1999;42:113–6 [DOI] [PubMed] [Google Scholar]
- 32.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 1997;46:983–8 [DOI] [PubMed] [Google Scholar]
- 33.Lavau M, Fried SK, Susini C, Freychet P. Mechanism of insulin resistance in adipocytes of rats fed a high-fat diet. J Lipid Res 1979;20:8–16 [PubMed] [Google Scholar]
- 34.Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol 1997;82:359–63 [DOI] [PubMed] [Google Scholar]
- 35.Young ME, Radda GK, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J 1997; 322:223–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 1999;48:1815–21 [DOI] [PubMed] [Google Scholar]
- 37.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes 2002;51:2572–80 [DOI] [PubMed] [Google Scholar]
- 38.Young ME, Leighton B. Fuel oxidation in skeletal muscle is increased by nitric oxide/cGMP – evidence for involvement of cGMP-dependent protein kinase. FEBS Lett 1998;424:79–83 [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Villafranca J, Guillen A, Castro J. Involvement of nitric oxide/cyclic GMP signaling pathway in the regulation of fatty acid metabolism in rat hepatocytes. Biochem Pharmacol 2003;65:807–12 [DOI] [PubMed] [Google Scholar]
- 40.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 2003;299:896–9 [DOI] [PubMed] [Google Scholar]
- 41.Wu G. Amino acids: biochemistry and nutrition. Boca Raton, FL: CRC Press, 2022 [Google Scholar]
- 42.Appari M, Channon KM, McNeill E. Metabolic regulation of adipose tissue macrophage function in obesity and diabetes. Antioxid Redox Signal 2018;29:297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lincová D, Miseková D, Kmonícková E, Canová N, Farghali H. Effect of nitric oxide donors on isoprenaline-induced lipolysis in rat epididymal adipose tissue: studies in isolated adipose tissues and immobilized perfused adipocytes. Physiol Res 2002;51:387–94 [PubMed] [Google Scholar]
- 44.McGrowder D, Ragoobirsingh D, Brown P. Modulation of glucose uptake in adipose tissue by nitric oxide-generating compounds. J Biosci 2006;31:347–54 [DOI] [PubMed] [Google Scholar]
- 45.Canová N, Lincová D, Farghali H. Inconsistent role of nitric oxide on lipolysis in isolated rat adipocytes. Physiol Res 2005;54:387–93 [PubMed] [Google Scholar]
- 46.Jobgen WS, Wu G. Dietary L-arginine supplementation increases the hepatic expression of AMP-activated protein kinase in rats. Amino Acids. 2022;54:1569–84 [DOI] [PubMed] [Google Scholar]
- 47.Clemmensen C, Smajilovic S, Smith EP, Woods SC, Bräuner-Osborne H, Seeley RJ, D’Alessio DA, Ryan KK. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology 2013;154:3978–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jobgen WS, Wu G. L-arginine increases AMPK phosphorylation and the oxidation of energy substrates in hepatocytes, skeletal muscle cells, and adipocytes. Amino Acids. 2022;54:1553–68 [DOI] [PubMed] [Google Scholar]
- 49.Szlas A, Kurek JM, Krejpco Z. The potential of L-arginine in prevention and treatment of disturbed carbohydrate and lipid metabolism – a review. Nutrients 2022;14:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu G. Amino acids in nutrition, health, and disease. Front Biosci 2021;26:1386–92 [DOI] [PubMed] [Google Scholar]