Abstract
Liver diseases, including viral hepatitis, fatty liver, metabolic-associated fatty liver disease, liver cirrhosis, alcoholic liver disease, and liver neoplasms, are major global health challenges. Despite the continued development of new drugs and technologies, the prognosis of end-stage liver diseases, including advanced liver cirrhosis and liver neoplasms, remains poor. Follistatin-like protein 1 (FSTL1), an extracellular glycoprotein, is secreted by various cell types. It is a glycoprotein that belongs to the family of secreted proteins acidic and rich in cysteine (SPARC). It is also known as transforming growth factor-beta inducible TSC-36 and follistatin-related protein (FRP). FSTL1 plays a key role in cell survival, proliferation, differentiation, and migration, as well as the regulation of inflammation and immunity. Studies have demonstrated that FSTL1 significantly affects the occurrence and development of liver diseases. This article reviews the role and mechanism of FSLT1 in liver diseases.
Keywords: FSTL1, liver disease, liver cirrhosis, hepatocellular carcinoma, mechanism
Impact statement
Liver diseases are major global health challenges, and the prognosis of end-stage liver diseases remains poor. Persistent inflammatory reactions and substantial damage eventually lead to liver fibrosis and cirrhosis when liver diseases progress to the chronic phase. There are no ideal therapies at present. In this review, we summarized the role and mechanism of Follistatin-like protein 1 (FSTL1) in liver diseases. FSTL1 plays a key role in cell survival, proliferation, differentiation, and migration, as well as the regulation of inflammation and immunity. However, studies on its mechanism in liver diseases such as liver fibrosis and cancer are scarce. Therefore, we present this review while trying to show the role of FSTL1 in liver diseases more intensively. With further research, FSTL1 will be a promising target and potential marker for treating liver diseases in the future.
Introduction
The liver is one of the most important glands in the human body, and its primary function is material metabolism. It participates in a series of physiological processes of the human body, such as the metabolism of lipid, 1 fatty acid, and glucose, 2 the secretion of immune response, 3 and the detoxification of growth factors 4 and cytokines. 5 According to the GLOBOCAN 2020 database statistics, approximately 905 million people worldwide suffer from chronic liver diseases, and approximately 830 million people die from liver diseases. The most common liver diseases include viral hepatitis, alcoholic liver disease (ALD), metabolic-associated fatty liver disease (MAFLD), liver cirrhosis and hepatocellular carcinoma (HCC). 6 In February 2022, the National Cancer Center released the latest issue of national cancer statistics, approximately 388 million people suffer from liver diseases in China. 7 Follistatin-like protein 1 (FSTL1) is also known as transforming growth factor-beta inducible protein (TSC-36) or follistatin-related protein (FRP). 8 Studies have revealed that FSTL1 plays an important role in cardiovascular diseases, obesity, endocrine diseases, autoimmune diseases, and fibrous system diseases.9–12 It is closely related to the pathological type of tumor, the degree of malignancy, and the degree of inflammation in inflammatory diseases. However, studies on its mechanism in liver diseases such as liver cirrhosis and cancer are scarce. This article reviews the relationship between FSTL1 and liver diseases.
FSTL1 overview
FSTL1 structure
FSTL1 is a secretory extracellular glycoprotein which is originally isolated in mouse osteoblastic MC3T3E1 cells, and is upregulated upon transforming growth factor-beta 1 (TGF-β1) stimulation.13,14 It is a member of the secretory protein acidic, rich in the cysteine (SPARC) family, and is expressed in various human tissues. 8 Similar to other members of the family, FSTL1 has a follicular statin domain and an extracellular calcium (EC) domain. 15 The human FSTL1 gene is located on the long arm of chromosome 3 (3q13 and 33) and contains 11 exons, which can encode FSTL1 protein and microRNA-198 after transcription. 12 FSTL1 protein consists of 308 amino acids, and its amino-terminal comprises 12 amino acid residues. FSTL1 has two O-glycosylation sites and four N-glycosylation sites, and its molecular weight is 35 kDa. More than two-thirds of the common amino acid sequences between FSTL1 and follistatin family members are identical. Like other family members, FSTL1 has an extracellular calcium-binding region and follistatin-like domain (FS). Its first EF-hand in the EC domain contains one fewer amino acid than those of other family members, which is a nonfunctional region. This may be why FSTL1 has different functions from the family in evolution. 16 Furthermore, FSTL1 has a von Willebrand C-type domain, which is involved in protein-protein interactions.
FSTL1 biological function
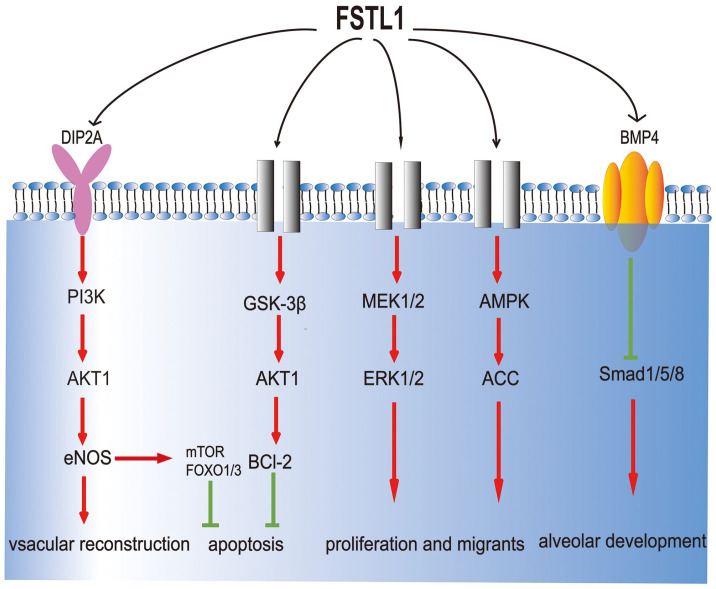
Although the biological function and mechanism of FSTL1 are unclear, studies have found that it regulates many biological processes such as the regulation of embryonic organ tissue formation, 17 cell proliferation, differentiation, apoptosis, 18 the improvement of ischemia/reperfusion injury 19 (Figure 1). It plays a major role in many systemic diseases such as cardiovascular disease, obesity, rheumatoid arthritis, and tissue fibrosis. 20 In cardiovascular diseases such as heart failure and acute coronary syndrome (ACS), the FSTL1 concentration in circulating blood increases. 21 Increased serum FSTL1 is related to the mortality of ACS patients and the severity of chronic heart failure. 22 Overexpressed FSTL1 can prevent extensive cardiac injury and abnormal vascular remodeling and offer protection and regeneration, whereas the lack of FSTL1 aggravates cardiac injury. 8 FSTL1 plays dual roles in systemic autoimmune disease (SADS). It mainly plays an anti-inflammatory role in acute inflammation but has a pro-inflammatory effect in chronic diseases, which may result from the activation of various signal pathways by FSTL1. 23 In respiratory diseases, FSTL1 is one of the proteins with the highest expression level in the sputum of asthmatic patients through proteomic analysis. The concentration of FSTL1 is negatively correlated with pulmonary function parameters and is positively correlated with airway remodeling indexes. 24 The results of various studies on the expression of FSTL1 in various tumors vary. Compared with healthy tissues, the expression of FSTL1 in renal cell carcinoma, 25 nasopharyngeal carcinoma, 26 ovarian cancer, and endometrial carcinoma decreases. 27 In contrast, it is upregulated in esophageal, 28 colorectal, 29 and brain tumor cells. 30 FSTL1 may have different regulatory mechanisms and effects in various tumor types.
Figure 1.
The biological function and mechanism of FSTL1. Known signaling pathways that interact with FSTL1 are displayed in the schematic. Colored sections indicate known receptors. Gray components indicate unknown receptors.
Association of FSTL1 with body mass index and fibrosis
A recent study suggested that FSTL1 levels were significantly higher in overweight and obese subjects and correlated with their body mass index (BMI) values. 31 During the differentiation of preadipocytes (3T3L1) into adipocytes, the expression of FSTL1 was temporarily upregulated and then downregulated to its baseline level. The differentiation of 3T3L1 cells can be prevented by blocking the initial peak of FSTL1 expression or maintaining a high level of FSTL1 in the medium. 32 In tissues and organs, fibrosis is characterized by an increase in fibrous connective tissue and a relative decrease in parenchymal cells. Compared with healthy tissues, the expression of FSTL1 was higher in patients with idiopathic pulmonary fibrosis. The effect of FSTL1 on fibrosis may be because FSTL1 can inhibit the Smad1/5/8-mediated BMP-4 signal transduction pathway, and stimulate the Smad2/3-mediated TGF-β1 signal transduction pathway, thereby destroying the balance of TGF-β/BMP. 33 During nephrectomy, high-level expressions of circulating FSTL1 can inhibit the formation of renal fibrosis by reducing the expression of collagen-I, collagen-III, TGF-β1, and connective tissue growth factor. However, similar to its role in inflammation, the effect of FSTL1 on fibrosis progresses to exhibit the opposite effect. 34
FSTL1 and liver diseases
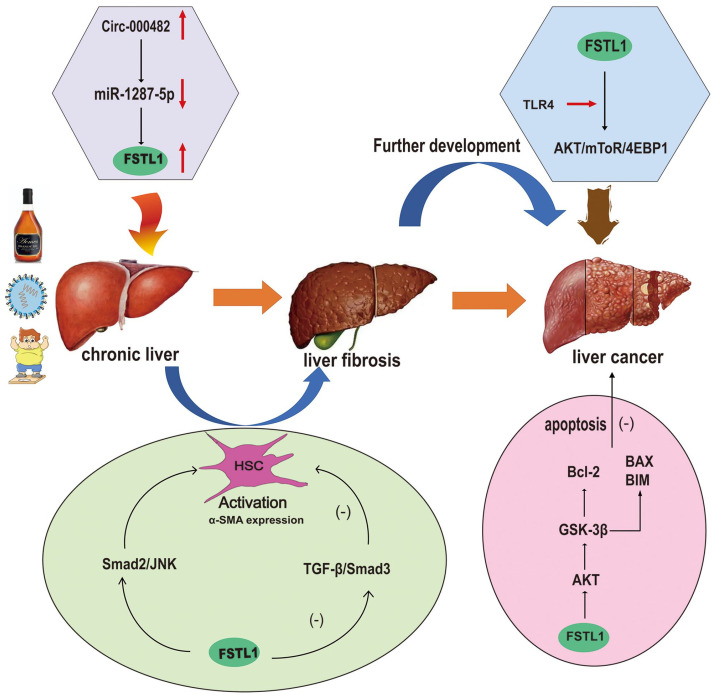
FSTL1 is involved in many physiological and pathological processes and affects the occurrence and development of various diseases. Furthermore, FSTL1 plays a major role in liver diseases, especially hepatic fibrosis and liver neoplasms, and serves as a biomarker and therapeutic target for these diseases (Figure 2). Therefore, FSTL1 may be helpful for the diagnosis and treatment of liver diseases in the future.
Figure 2.
Upon various types of chronic injury, including that caused by alcohol, viral, and non-alcoholic steatohepatitis (NASH), hepatic stellate cells (HSCs) are activated, the latter of which secrete abundant extracellular proteins that contribute to liver fibrosis. Untreated or relapsed fibrosis progresses to liver cirrhosis, which may eventually develop into liver cancer.
Chronic liver diseases
Non-neoplastic chronic liver diseases include ALD, MAFLD, viral hepatitis, liver cirrhosis, and autoimmune liver disease. ALD, MAFLD, and hepatitis C virus (HCV) infections are more common in North America and Europe, whereas hepatitis B virus (HBV) infection, HCV, MAFLD, and ALD are more common in Asia and Africa. 35
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases worldwide. International experts reached a consensus that the disease abbreviation should be changed from NAFLD to MAFLD.36–40 Despite no a clear worldwide consensus, global multi-stakeholder and some associations have endorsed the new terminology, including the Asian Pacific Association for the Study of the Liver (APASL), the Latin American Association for the Study of the Liver (ALEH), the Chinese Society of Hepatology, the Arabic Association for the Study of Diabetes and Metabolism, and societies and clinicians in the Middle East and North Africa (MENA). 41 Its pathological changes include non-alcoholic steatohepatitis (NASH) and steatosis, which are characterized by fat accumulation, hepatocyte swelling, and the development of inflammation and/or fibrosis. 42 Its main characteristics are insulin resistance and abnormal lipid metabolism. 43 Severe steatosis can cause liver failure and even hepatocyte necrosis. Studies have demonstrated that in patients with HCV and steatosis, FSTL1 is significantly increased in the late and early stages of fibrosis, but there is an insignificant difference in patients with simple HCV infection. The increased expression of FSTL1 may be an important marker of poor prognosis. 44
For viral hepatitis, HBV and HCV are the most common viral infections. HBV or HCV infection is an important pathogen of liver cirrhosis, and HCC. Zhang and Wang 45 found that HBV infection may upregulate the expression of FSTL, but in HBV infected cells with annular RNAcirc-0004812 knockout, the expression of FSTL1 decreases, whereas overexpressed circ-0004812 can promote the expression of FSTL1 by inhibiting miR-1287-5p, indicating that circ-0004812/miR-1287-5p/FSTL1 axis can regulate HBV-induced immunosuppression and can be used as a potential therapeutic target for the treatment of chronic hepatitis B (CHB). Fatty liver is the most common histological feature in chronic hepatitis C. Compared with HCV patients without steatosis, a steatosis-enriched gene set associated with advanced fibrosis, typically FSTL1, is identified in the biopsies of HCV patients with steatosis. Patients with advanced fibrosis had higher Serum FSTL1 levels than those with steatosis (versus those without). Liver FSTL1 mRNA levels were also elevated in murine chronic liver disease models. 46
Liver cirrhosis
Liver cirrhosis, which seriously affects human health, is a healing reaction caused by chronic liver injury resulting from various causes. 47 It may develop into liver cirrhosis and, if not prevented, may lead to liver cancer and liver failure. 48 The common causes of liver cirrhosis include viral infection, ethanol, parasite infection, drug or chemical poison, and autoimmune liver disease. These causes lead to chronic inflammation of the liver, followed by abnormal healing response. 49
The activation of hepatic stellate cells (HSC) and excessive deposition of extracellular matrix (ECM) are two major processes in developing hepatic fibrosis. 50 HSCs are liver-specific mesenchymal cells in the peri-sinusoidal space of the liver. In normal liver, hepatic stellate cells contain many lipid droplets. HSC stores vitamin A in the form of retinol and expresses glial fibrillary acidic protein (GFAP) in a resting state. 51 Under the stimulation of long-term liver pathogenic factors, hepatic stellate cells are activated and transdifferentiated into myofibroblasts. 49 It is characterized by the up-regulation of a-smooth muscle actin (a-SMA), desmin, and type I collagen, as well as the production of a large number of extracellular matrix and fibrotic cytokines. 52 Therefore, hepatic stellate cells play a crucial role in the regression of hepatic fibrosis.
The activation of the TGF-β signal transduction pathway plays a central role in the regulation of hepatic fibrosis. 34 TGF-β1 is the most widely and most intensely investigated isoform in liver fibrogenesis. The canonical signaling pathway is initiated via phosphorylation of R-SMADs, that is, SMAD2 and SMAD3. The non-canonical SMAD-independent pathways are activated through MAPK, mTOR, PI3K/AKT, and Rho/GTPase pathways. The study found that TGF-β can promote the synthesis of various collagen components and tissue metalloprotein inhibitors by HSC, and inhibit the synthesis of matrix metalloproteinases, causing the accumulation of extracellular matrix and leading to the occurrence of cirrhosis. 53 TGF-β is a critical cytokine triggering canonical and non-canonical intracellular pathways leading to activated HSC, macrophages with variant polarization, and liver sinusoidal endothelial cell capillarization. 54 TGF-β was reported to induce glycolysis, and thus can be considered a driver of metabolic reprogramming in the activation process of HSC. Furthermore, reactive oxygen species (ROS) plays crucial roles in liver fibrosis and HSC activation, and TGF-β augments mitochondrial ROS production by activating the mTOR pathway and reducing the activity of complex III and IV. 55 In addition, SMAD7 is an effective inhibitor of TGF-β signaling and is a key regulator of TGF-β-induced fibrogenesis. 56
FSTL1 can regulate hepatic fibrosis by affecting the TGF-β signal transduction pathway. FSTL1 is significantly upregulated in HSC activated by TGF-β1 stimulation, whereas the knockout of FSTL1 can inhibit the phosphorylation of Smad3 in the TGF-β 1/Smad3 signal pathway, thereby attenuating the activation of HSCs and alleviating liver fibrosis. The plasmid verification experiment of MiR29a target gene 3’UTR demonstrated that FSTL1 is the direct target of miR29a. There may be a TGF-β 1-miR29a-FSTL1 regulatory loop to regulate hepatic fibrosis through the TGF-β 1/Smad2/JNK signal pathway. FSTL1 can regulate the fibrosis degree of hepatic stellate cells and hepatic fibrosis induced by CCL4 by inhibiting the expression of miR29a, whereas blocking FSTL1 signal transduction with neutralizing antibody can upregulate the expression of miR29a in CCL4-treated mice. 57
Xu et al. 57 and other studies 58 have demonstrated that FSTL1 is significantly upregulated in human and mouse activated HSCs and liver fibrosis. 4-Methyl umbrella ketone (4MU) can downregulate the expression of FSTL1 in CCL4-induced liver fibrosis model mice, thereby inhibiting hyaluronic acid (HA) deposition and reducing fiber formation and collagen deposition. In the study of the mechanism by which FSTL1 regulates hepatic fibrosis, it was found that FSTL1 regulates hepatic fibrosis by affecting the Smad2/c-Jun N-terminal kinase (JNK) signal transduction pathway. In the mice with half-fold deletion of FSTL1 or the use of FSTL1 neutralizing antibody to block the function of FSTL1 in CCL4-induced liver fibrosis mice, Smad2/JNK signal pathway can be inhibited and, simultaneously, the activation of HSCs can also be inhibited, ECM deposition can be decreased, and the degree of liver fibrosis can be alleviated. Furthermore, FSTL1 may affect the senescence and apoptosis of HSCs through the PI3K-AKT and MAPK signaling pathways, thereby affecting liver fibrosis. 13 Rao’s group demonstrated the role of macrophage FSTL1 in liver fibrosis. Macrophage FSTL1 promotes the progression of liver fibrosis by inducing M1 polarization and inflammation based on the intracellular PKM2 reprogramming function of macrophages. 59
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is one of the highest incidences of malignant tumors worldwide and is also a cancer type with extremely high mortality. 60 In 2020, liver cancer accounted for 4.7% of new cancer cases and 8.3% of deaths. 61 Nearly half of the world’s new cases and deaths occur in China, seriously threatening people’s health and quality of life. The most common type of liver neoplasms is HCC, accounting for 80–90% of cases. HCC usually develops from chronic liver diseases. 60 Liver transplantation is the most effective treatment for liver cancer, but the overall survival rate remains poor. 62
Recently, it was found that the occurrence and development of HCC are closely related to the expression of FSTL1. Yang et al. 63 discovered an abnormal increase of FSTL1 protein in 172 cases of HCC (81.9%). FSTL1 expression increases with an increase in tumor size, Tumor Node Metastasis stage, portal vein invasion, and intrahepatic metastasis. Furthermore, the overall survival rate of patients was negatively correlated with the expression level of FSTL1, and a high expression of FSTL1 is identified as an indicator of a poor prognosis for HCC. FSTL1 inhibits tumor cell apoptosis by the over-activation of protein kinase B (AKT)/glycogen synthase kinase (GSK-3β) signal pathway, which increases the expression of Bcl-2 and downregulates the expression of Bcl-2-related X protein (BAX and Bim). Loh et al. 64 found that treating HCC cells and 3D organs derived from patients with recombinant FSTL1 or conditioned medium collected from HSC or cells overexpressing FSTL1 can promote growth and metastasis HCC. FSTL1 binds to the TLR4 receptor, which leads to the activation of AKT/mTOR/4EBP1 signal pathway. In a preclinical mouse model, inhibiting FSTL1 can reduce the malignancy and metastasis of HCC, renders HCC tumor sensitive to sorafenib, prolongs survival time, and eradicates Tumor-initiating cells subsets. FSTL1 is a valuable new diagnostic, prognostic biomarker and therapeutic target for HCC.
Cancer-associated fibroblasts
Normal fibroblasts (NFs) are resting mesenchymal cells buried in interstitial fibers. They can be activated environment-dependent during wound healing, tissue inflammation, and organ fibrosis, and apoptosis occurs when the stimulation is removed. The HSC in the liver is activated into myofibroblasts under the stimulation of chronic injury, which promotes the occurrence and development of hepatic fibrosis and even liver tumors. 65 Cancer-associated fibroblasts (CAF) can promote the occurrence and development of tumor cells, which is an important part of the tumor microenvironment and plays a role by secreting various growth factors and cytokines. 66 It can cause malignant tumors and participate in their development. For HCC, liver cirrhosis is carcinogenic, and CAF’s involvement in carcinogenesis is supported. 67 FSTL1 expression in healthy liver tissues is very low but significantly increased in activated HSCs cells and liver fibrosis tissues. In cirrhotic liver tissues, the expression of FSTL1 is co-located with the expression of α-SMA and is higher than that in normal liver tissues, indicating that FSTL1 plays a crucial role in activating HSC. 13 Studies have revealed that fibroblasts, especially CAF in the tumor matrix, are the main sources of FSTL1. There is a significant correlation between FSTL1 and CAF features of HCC. Recently, CAF has received increasing attention as a new target for anticancer therapy.
Concluding remarks
Persistent inflammatory reactions and substantial damage eventually lead to liver fibrosis and cirrhosis when liver diseases progress to the chronic phase. Chronic hepatocyte loss occurs in chronic liver disease of any etiology and is related to HSC activation and abnormal liver microenvironment. 68 The inhibition of HSC activation is considered an effective method to alleviate the progression of liver cirrhosis. Considering the clinical correlation between cholangiopathies and liver diseases, we conducted literature search in NCBI PubMed and other databases with keywords related to biliary diseases, such as cholestatic liver diseases, primary sclerosing cholangitis, primary biliary cholangitis, biliary atresia, or cholangiocarcinoma. None of these diseases were reported to be related to FSTL1 as of the submission of this review. We suppose this might be a novel direction for researchers.
FSTL1 is a secreted glycoprotein whose function varies in response to pathological states. Studies have demonstrated that the up-regulation of FSTL1 promotes the development and progression of chronic liver diseases, especially liver cirrhosis and HCC. However, the role and mechanism of HSC FSTL1 in the process of liver cirrhosis evolving into HCC require further investigation. With further research, FSTL1 will be a promising target and potential marker for treating liver diseases in the future.
Footnotes
Authors’ Contributions: CSG, HX, XLY, and YN drafted the manuscript and figures. CSG edited the manuscript. XLQ revised and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Key Scientific Research Project Plans of Colleges and Universities in Henan Province (Grant 20B320017), and National Natural Science Foundation of China (Grant 31900569).
ORCID iD: Chuansha Gu  https://orcid.org/0000-0002-0658-7418
https://orcid.org/0000-0002-0658-7418
References
- 1.Lee S, Mardinoglu A, Zhang C, Lee D, Nielsen J.Dysregulated signaling hubs of liver lipid metabolism reveal hepatocellular carcinoma pathogenesis. Nucleic Acids Res 2016;44:5529–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han HS, Kang G, Kim JS, Choi BH, Koo SH.Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med 2016;48:e218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, Li R, Qie J, Zhen B, Wang Y, He F, Qin J, Ding C.A proteomics landscape of circadian clock in mouse liver. Nat Commun 2018;9:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JH, Toita R, Murata M.Liver cell-targeted delivery of therapeutic molecules. Crit Rev Biotechnol 2016;36:132–43 [DOI] [PubMed] [Google Scholar]
- 5.Gao B.Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol 2012;27:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seto W-K, Lo Y-R, Pawlotsky J-M, Yuen M-F.Chronic hepatitis B virus infection. Lancet 2018;392:2313–24 [DOI] [PubMed] [Google Scholar]
- 7.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J.Cancer incidence and mortality in China, 2016. J Nat Cancer Center 2022;2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattiotti A, Prakash S, Barnett P, van den Hoff MJB. Follistatin-like 1 in development and human diseases. Cell Mol Life Sci 2018;75:2339–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Xiao X, Huang T, Du C, Wang S, Mo Y, Ma N, Murata M, Li B, Wen W, Huang G, Zeng X, Zhang Z.Epigenetic inactivation of follistatin-like 1 mediates tumor immune evasion in nasopharyngeal carcinoma. Oncotarget 2016;7:16433–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka K, Valero-Muñoz M, Wilson RM, Essick EE, Fowler CT, Nakamura K, van den Hoff M, Ouchi N, Sam F.Follistatin like 1 regulates hypertrophy in heart failure with preserved ejection fraction. JACC Basic Transl Sci 2016;1:207–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Xia J, Hu P, Zhou F, Chen Y, Wu J, Lei W, Shen Z.Follistatin-like 1 protects cardiomyoblasts from injury induced by sodium nitroprusside through modulating Akt and Smad1/5/9 signaling. Biochem Biophys Res Commun 2016;469:418–23 [DOI] [PubMed] [Google Scholar]
- 12.Sundaram GM, Common JE, Gopal FE, Srikanta S, Lakshman K, Lunny DP, Lim TC, Tanavde V, Lane EB, Sampath P.“See-saw” expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature 2013;495:103–6 [DOI] [PubMed] [Google Scholar]
- 13.Shang H, Liu X, Guo H.Knockdown of Fstl1 attenuates hepatic stellate cell activation through the TGF-β1/Smad3 signaling pathway. Mol Med Rep 2017;16:7119–23 [DOI] [PubMed] [Google Scholar]
- 14.Shibanuma MMJ, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem 1993;217:13–9 [DOI] [PubMed] [Google Scholar]
- 15.Li W, Alahdal M, Deng Z, Liu J, Zhao Z, Cheng X, Chen X, Li J, Yin J, Li Y, Wang G, Wang D, Tang K, Zhang J.Molecular functions of FSTL1 in the osteoarthritis. Int Immunopharmacol 2020;83:106465. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Li L, Chang Y, Ning W, Liu X.Structural and functional study of FK domain of Fstl1. Protein Sci 2019;28:1819–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W.Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci USA 2011;108:7058–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K.Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 2008;283:32802–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochette L, Vergely C.“Pro-youthful” factors in the “labyrinth” of cardiac rejuvenation. Exp Gerontol 2016;83:1–5 [DOI] [PubMed] [Google Scholar]
- 20.Hambrock HO, Kaufmann B, Muller S, Hanisch FG, Nose K, Paulsson M, Maurer P, Hartmann U.Structural characterization of TSC-36/Flik: analysis of two charge isoforms. J Biol Chem 2004;279:11727–35 [DOI] [PubMed] [Google Scholar]
- 21.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ.Expression of follistatin-related genes is altered in heart failure. Endocrinology 2008;149:5822–7 [DOI] [PubMed] [Google Scholar]
- 22.Widera C, Horn-Wichmann R, Kempf T, Bethmann K, Fiedler B, Sharma S, Lichtinghagen R, Leitolf H, Ivandic B, Katus HA, Giannitsis E, Wollert KC.Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin Chem 2009;55:1794–800 [DOI] [PubMed] [Google Scholar]
- 23.Shi DL, Shi GR, Xie J, Du XZ, Yang H.MicroRNA-27a inhibits cell migration and invasion of fibroblast-like synoviocytes by targeting follistatin-like protein 1 in rheumatoid arthritis. Mol Cells 2016;39:611–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller M, Esnault S, Kurten RC, Kelly EA, Beppu A, Das S, Rosenthal P, Ramsdell J, Croft M, Zuraw B, Jarjour N, Hamid Q, Broide DH.Segmental allergen challenge increases levels of airway follistatin-like 1 in patients with asthma. J Allergy Clin Immunol 2016;138:596–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Han X, Yu Y, Ding Y, Ni C, Liu W, Hou X, Li Z, Hou J, Shen D, Yin J, Zhang H, Thompson TC, Tan X, Cao G.A genetic polymorphism affects the risk and prognosis of renal cell carcinoma: association with follistatin-like protein 1 expression. Sci Rep 2016;6:26689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Huang S, Wu S, Yin S, Tang A, Wen W.Follistatin-like protein-1 upregulates dendritic cell-based immunity in patients with nasopharyngeal carcinoma. J Interferon Cytokine Res 2017;37:494–502 [DOI] [PubMed] [Google Scholar]
- 27.Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN.Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis 2009;30:114–21 [DOI] [PubMed] [Google Scholar]
- 28.Lau MC, Ng KY, Wong TL, Tong M, Lee TK, Ming XY, Law S, Lee NP, Cheung AL, Qin YR, Chan KW, Ning W, Guan XY, Ma S. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB-BMP signaling cross-talk. Cancer Res 2017;77:5886–99 [DOI] [PubMed] [Google Scholar]
- 29.Gu C, Wang X, Long T, Wang X, Zhong Y, Ma Y, Hu Z, Li Z.FSTL1 interacts with VIM and promotes colorectal cancer metastasis via activating the focal adhesion signalling pathway. Cell Death Dis 2018;9:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, Kumari A, Shilpa BM, Vrinda M, Umesh S, Samuel C, Shetty M, Tandon A, Pandey P, Hegde S, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Kondaiah P, Somasundaram K, Rao MR.Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res 2008;14: 2978–87 [DOI] [PubMed] [Google Scholar]
- 31.Fan N, Sun H, Wang Y, Wang Y, Zhang L, Xia Z, Peng L, Hou Y, Shen W, Liu R, Yin J, Peng Y.Follistatin-like 1: a potential mediator of inflammation in obesity. Mediators Inflamm 2013;2013:752519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prieto-Echagüe V, Lodh S, Colman L, Bobba N, Santos L, Katsanis N, Escande C, Zaghloul NA, Badano JL.BBS4 regulates the expression and secretion of FSTL1, a protein that participates in ciliogenesis and the differentiation of 3T3-L1. Sci Rep 2017;7:9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollmann EH, Cao L, Amatucci A, Reynolds T, Hamann S, Dalkilic-Liddle I, Cameron TO, Hossbach M, Kauffman KJ, Mir FF, Anderson DG, Novobrantseva T, Koteliansky V, Kisseleva T, Brenner D, Duffield J, Burkly LC.Identification of novel fibrosis modifiers by in vivo siRNA silencing. Mol Ther Nucleic Acids 2017;7:314–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, Li X, Dong S, Liu X, Li X, Yang X, Zheng X, Xie T, Liang J, Dai H, Liu X, Yin Z, Noble PW, Jiang D, Ning W.Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med 2015;212:235–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya C, Bajaj JS.Chronic liver diseases and the microbiome-translating our knowledge of gut microbiota to management of chronic liver disease. Gastroenterology 2021;160:556–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J.The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020;14:889–919 [DOI] [PubMed] [Google Scholar]
- 37.Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, Chávez-Tapia NC, Dirchwolf M, Torre A, Ridruejo E, Pinchemel-Cotrim H, Castellanos Fernández MI, Uribe M, Girala M, Diaz-Ferrer J, Restrepo JC, Padilla-Machaca M, Dagher L, Gatica M, Olaechea B, Pessôa MG, Silva M.The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol 2021;6:65–72 [DOI] [PubMed] [Google Scholar]
- 38.Shiha G, Alswat K, Al Khatry M, Sharara AI, Örmeci N, Waked I, Benazzouz M, Al-Ali F, Hamed AE, Hamoudi W, Attia D, Derbala M, Sharaf-Eldin M, Al-Busafi SA, Zaky S, Bamakhrama K, Ibrahim N, Ajlouni Y, Sabbah M, Salama M, Anushiravani A, Afredj N, Barakat S, Hashim A, Fouad Y, Soliman R.Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol 2021;6:57–64 [DOI] [PubMed] [Google Scholar]
- 39.Spearman CW, Desalegn H, Ocama P, Awuku YA, Ojo O, Elsahhar M, Abdo AA, Ndububa DA, Fouad Y, Borodo MM, Ng’wanasayi M, Ally R, Elwakil R.The sub-Saharan Africa position statement on the redefinition of fatty liver disease: from NAFLD to MAFLD. J Hepatol 2021;74:1256–8 [DOI] [PubMed] [Google Scholar]
- 40.Nan Y, An J, Bao J, Chen H, Chen Y, Ding H, Dou X, Duan Z, Fan J, Gao Y, Han T, Han Y, Hu P, Huang Y, Huang Y, Jia J, Jiang J, Jiang Y, Li J, Li J, Li R, Li S, Li W, Li Y, Lin S, Liu J, Liu S, Lu L, Lu Q, Luo X, Ma X, Rao H, Ren H, Ren W, Shang J, Shi L, Su M, Wang B, Wang R, Wei L, Wen Z, Wu B, Wu J, Xin S, Xing H, Xu J, Yan M, Yang J, Yang J, Yang L, Yang Y, Yu Y, Zhang L, Zhang L, Zhang X, Zhang Y, Zhang Y, Zhao J, Zhao S, Zheng H, Zhou Y, Zhou Y, Zhuang H, Zuo W, Xu X, Qiao L.The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol 2021;75:454–61 [DOI] [PubMed] [Google Scholar]
- 41.Fouad YM, Gomaa A, El Etreby RM, AbdAllah M, Attia D.Editorial: the Metabolic (Dysfunction)-Associated Fatty Liver Disease (MAFLD) and Non-Alcoholic Fatty Liver Disease (NAFLD) debate: why the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) consensus process is not representative. Medical Science Monitor 2022;28:e938066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Jarvinen H, Fan JG, Gronbaek H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J.A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9 [DOI] [PubMed] [Google Scholar]
- 43.Zeng X, Yuan X, Cai Q, Tang C, Gao J.Circular RNA as an epigenetic regulator in chronic liver diseases. Cells 2021;10:1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afonso MB, Rodrigues PM, Simão AL, Castro RE.Circulating microRNAs as potential biomarkers in non-alcoholic fatty liver disease and hepatocellular carcinoma. J Clin Med 2016;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Wang Z.Circular RNA hsa_circ_0004812 impairs IFN-induced immune response by sponging miR-1287-5p to regulate FSTL1 in chronic hepatitis B. Virol J 2020;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramnath D, Irvine KM, Lukowski SW, Horsfall LU, Loh Z, Clouston AD, Patel PJ, Fagan KJ, Iyer A, Lampe G, Stow JL, Schroder K, Fairlie DP, Powell JE, Powell EE, Sweet MJ.Hepatic expression profiling identifies steatosis-independent and steatosis-driven advanced fibrosis genes. JCI Insight 2018;3:e120274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman SL, Pinzani M.Hepatic fibrosis 2022: unmet needs and a blueprint for the future. Hepatology 2022;75:473–88 [DOI] [PubMed] [Google Scholar]
- 48.Caballería L, Torán P, Caballería J.Markers of hepatic fibrosis. Med Clin (Barc) 2018;150:310–6 [DOI] [PubMed] [Google Scholar]
- 49.Shang L, Hosseini M, Liu X, Kisseleva T, Brenner DA.Human hepatic stellate cell isolation and characterization. J Gastroenterol 2018;53:6–17 [DOI] [PubMed] [Google Scholar]
- 50.Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N.Management strategies for liver fibrosis. Ann Hepatol 2017;16:48–56 [DOI] [PubMed] [Google Scholar]
- 51.Mounajjed T, Graham RP, Sanderson SO, Smyrk TC.Clinical associations of hepatic stellate cell (HSC) hyperplasia. Virchows Arch 2014;465: 57–65 [DOI] [PubMed] [Google Scholar]
- 52.Seo HY, Lee SH, Lee JH, Kang YN, Choi YK, Hwang JS, Park KG, Jang BK, Kim MK.Clusterin attenuates hepatic fibrosis by inhibiting hepatic stellate cell activation and downregulating the Smad3 signaling pathway. Cells 2019;8:1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Mabwi HA, Palange NJ, Jia R, Ma J, Bah FB, Sah RK, Li D, Wang D, Bah FB, Togo J, Jin H, Ban L, Feng X, Zheng Y.Expression patterns and potential biological roles of Dip2a. PLoS ONE 2015;10: e0143284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN.TGF-beta in hepatic stellate cell activation and liver fibrogenesis—updated 2019. Cells 2019;8:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu RM, Desai LP.Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 2015;6:565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu F, Liu C, Zhou D, Zhang L.TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem 2016;64:157–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu XY, Du Y, Liu X, Ren Y, Dong Y, Xu HY, Shi JS, Jiang D, Xu X, Li L, Xu ZH, Geng Y.Targeting follistatin like 1 ameliorates liver fibrosis induced by carbon tetrachloride through TGF-β1-miR29a in mice. Cell Commun Signal 2020;18:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreichenko IN, Tsitrina AA, Fokin AV, Gabdulkhakova AI, Maltsev DI, Perelman GS, Bulgakova EV, Kulikov AM, Mikaelyan AS, Kotelevtsev YV.4-methylumbelliferone prevents liver fibrosis by affecting hyaluronan deposition, FSTL1 expression and cell localization. Int J Mol Sci 2019;20:6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao J, Wang H, Ni M, Wang Z, Wang Z, Wei S, Liu M, Wang P, Qiu J, Zhang L, Wu C, Shen H, Wang X, Cheng F, Lu L.FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 2022;71:2539–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS.Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 61.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY.The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y.Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 2020;10:2993–3036 [PMC free article] [PubMed] [Google Scholar]
- 63.Yang W, Wu Y, Wang C, Liu Z, Xu M, Zheng X.FSTL1 contributes to tumor progression via attenuating apoptosis in a AKT/GSK-3β–dependent manner in hepatocellular carcinoma. Cancer Biomark 2017; 20:75–85 [DOI] [PubMed] [Google Scholar]
- 64.Loh JJ, Li TW, Zhou L, Wong TL, Liu X, Ma VWS, Lo CM, Man K, Lee TK, Ning W, Tong M, Ma S.FSTL1 secreted by activated fibroblasts promotes hepatocellular carcinoma metastasis and stemness. Cancer Res 2021;81:5692–705 [DOI] [PubMed] [Google Scholar]
- 65.Higashi T, Friedman SL, Hoshida Y.Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017;121:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Song E.Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov 2019;18:99–115 [DOI] [PubMed] [Google Scholar]
- 67.Baglieri J, Brenner DA, Kisseleva T.The role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma. Int J Mol Sci 2019;20:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michalopoulos GK, Bhushan B.Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol 2021;18:40–55 [DOI] [PubMed] [Google Scholar]