Abstract
The use of a composite graft is an established treatment for patients with aortic valve disease and ascending aortic aneurysms. Since bleeding from suture lines is a potential complication of this procedure, we modified the technique and evaluated the effect on hemostasis.
From January 1994 through December 1998, 35 patients underwent composite aortic graft replacement for chronic aortic disease. In the first 16 patients (Group 1), we used the standard open technique, with excision of the aortic aneurysm and anastomosis of aortic buttons containing the coronary ostia to the vascular graft. In the next 19 patients (Group 2), we modified the technique by placing an additional suture at the proximal graft anastomosis and harvesting large coronary buttons that were then attached to the graft by an “endo-button” buttress method.
There were no operative deaths; the actuarial survival rate at 36 months was 92% ± 5%. Between groups 1 and 2, a significant difference was found in postoperative bleeding (1,052 ± 433 mL vs 806 ± 257 mL, respectively; p = 0.02) and in number of blood transfusions required (2.1 ± 2.0 units vs 0.4 ± 0.7 units, respectively; p = 0.002). Multivariate analysis showed that the surgical technique used in Group 1 was the only independent risk factor for postoperative bleeding of 1,000 mL or more (p = 0.01) and for transfusion requirements of 3 or more units of blood (p = 0.004).
Composite aortic valve and root replacement can be accomplished with excellent results. Technical modifications may reduce bleeding complications and related morbidity significantly.
Key words: Anastomosis, surgical/methods; aortic aneurysm/surgery; aortic diseases/surgery; aortic valve/surgery; aortic valve/transplantation; blood loss, surgical/prevention & control; blood vessel prosthesis/methods; heart valve prosthesis; postoperative complications; suture techniques
Composite graft replacement of the ascending aorta and aortic valve was introduced by Bentall and De Bono in 1968. 1 Since then, this operation and its modifications have become established methods, especially for treating patients who have annuloaortic ectasia or aortic valve disease with chronic aneurysm of the ascending aorta. 2–6 Recently, we added a few technical modifications to our standard procedure with the aim of improving hemostasis. We compared our standard and modified techniques for possible reduction in bleeding complications and related morbidity.
Patients and Methods
Patients
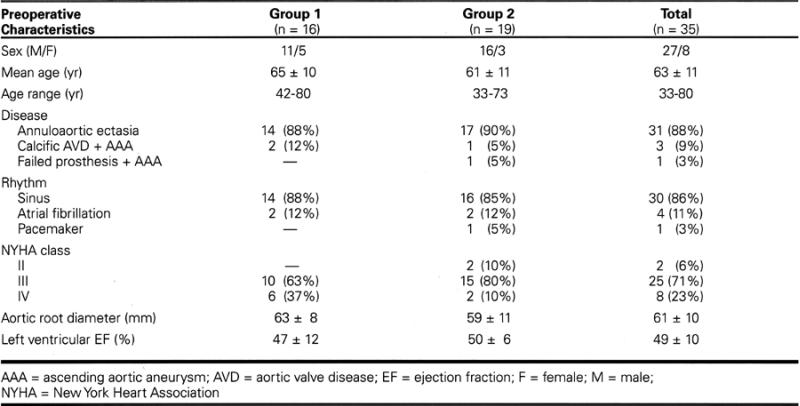
From January 1994 through December 1998, 35 patients who had chronic aortic disease underwent composite graft replacement of the aortic valve and ascending aorta with reimplantation of the coronary arteries; all procedures were performed by a single surgeon (UB). Patients were categorized into 2 groups: Group 1 comprised 16 patients undergoing our standard technique from January 1994 through September 1996; Group 2 comprised 19 patients undergoing our modified technique from October 1996 through December 1998. Preoperative clinical data are summarized in Table I.
Table I. Summary of Preoperative Patient Characteristics
Surgical Technique
The technique suggested by Kouchoukos and colleagues 7 was used by our surgeon as follows. In all patients, moderate hypothermia was applied without routine intraoperative administration of antifibrinolytic agents. After aortic cross-clamping, the ascending aorta was opened longitudinally and the heart was arrested with a single dose of cold blood cardioplegia (500 mL) delivered into the coronary ostia; topical cooling with iced saline was maintained throughout the procedure. The aortic valve was excised and the annulus size was measured. The ascending aorta was transected 2 cm below the aortic clamp, and buttons of aortic wall including the coronary ostia were isolated and mobilized. The composite graft was inserted using multiple sutures of 2-0 Ethibond Excel (Ethicon Ltd.; Edinburgh, U.K.) reinforced by subannular Teflon felt. Anastomosis of the aortic buttons to the vascular graft was performed with a continuous suture of 4-0 Prolene (Ethicon Ltd.). The graft was then trimmed and sutured to the distal aorta with a continuous suture of 3-0 Prolene. This technique was applied in the first 16 patients (Group 1) through September 1996.
Beginning in October 1996, the Kouchoukos procedure 7 was performed with the following modifications in the next 19 patients (Group 2). First, after the prosthesis was seated and the stitches tied, an additional suture of 3-0 Prolene was used to join the cut edge of the aortic wall and the prosthetic sewing ring (Fig. 1). 8,9 Second, the coronary ostia were harvested, surrounded by a large portion of aortic wall, allowing the coronary buttons to be sutured in a double layer, with an “endo-button” buttress technique (Fig. 2). 10
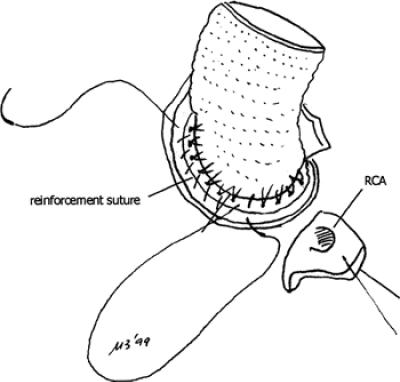
Fig. 1 Drawing showing the reinforcement suture joining the incised edge of the aortic wall and the prosthetic sewing ring.
RCA = right coronary artery
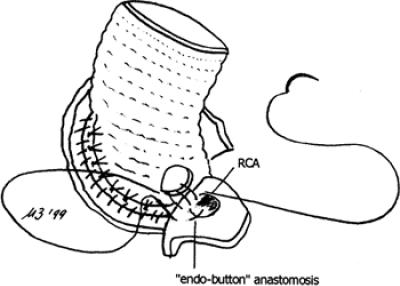
Fig. 2 Detail of the “endo-button” buttress technique used for anastomosis of the coronary ostia to the graft.
RCA = right coronary artery
All patients underwent anticoagulation by means of subcutaneous calcium heparin starting on the 1st postoperative day. Oral anticoagulants were administered upon discharge to a hospital room and were maintained indefinitely, with a target international normalized ratio (INR) between 3 and 4.5.
Postoperative Evaluation and Follow-Up
Patient records were reviewed, including data collected before the operation, at the end of cardiopulmonary bypass, and at discharge from the hospital. Data comprised hematologic values, such as hemoglobin, hematocrit, platelet count, activated partial throm-boplastin time (aPTT), prothrombin time (PT), fibrinogen, the volume of blood and blood products transfused, and the need for reoperation because of bleeding. Data from the 2 groups were then compared.
Patient interviews, direct clinical examination, and transthoracic 2-dimensional echocardiographic studies were part of the postoperative follow-up protocol for the evaluation of functional status and the incidence and type of postoperative complications.
Statistical Analysis
Data are presented as the mean ± standard deviation. Results from the 2 groups of patients were compared using a χ2 test or Fisher's exact test for discrete variables. The Student's t-test or the Mann-Whitney test was used for continuous variables. P values <0.5 were considered significant. The actuarial survival curve was determined using the Kaplan-Meier method and included hospital mortality rates. Multivariate analysis was performed in order to identify independent risk factors for postoperative bleeding of 1,000 mL or more and for transfusion requirements of 3 or more units of blood, introducing in a stepwise logistic regression all variables showing p <0.10 on univariate analysis.
Results
In 25 patients (15 of 16 from Group 1 and 10 of 19 from Group 2), a St. Jude Medical® aortic valved graft (St. Jude Medical, Inc.; St. Paul, Minn) was placed. The other 10 patients (1 from Group 1 and 9 from Group 2) received a Carbo-Seal™ ascending aortic prosthesis (CarboMedics, Inc.; Austin, Tex). Associated surgical procedures were performed in 5 patients (14%): coronary artery bypass grafting in 4 and mitral valve replacement in 1. There were no significant differences in duration of cardiopulmonary bypass between groups (Group 1, 111 ± 41 minutes vs Group 2, 103 ± 17 minutes), aortic cross-clamp time (87 ± 19 minutes vs 89 ± 18 minutes, respectively), or activated clotting time during cardiopulmonary bypass (918 ± 195 seconds vs 867 ± 148 seconds, respectively).
There were no operative deaths. During the follow-up period completed in February 1999 (mean follow-up, 28 ± 15 months; range, 2 to 54 months), there were 2 late deaths. One patient in Group 1 died of chronic renal failure after 16 months, and 1 patient in Group 2 died suddenly after 15 months. The actuarial survival rate at 36 months was 92% ± 5%.
The total postoperative blood loss in Group 1 was 1,052 ± 433 mL (range, 500 to 2,100 mL), and in Group 2 it was 806 ± 257 mL (range, 390 to 1,350; p = 0.02). Nine patients in Group 1 and 3 patients in Group 2 experienced blood losses of 1,000 mL or more. Chest reexploration for bleeding was required in 3 patients from Group 1 and in 2 patients from Group 2. In Group 1, bleeding was observed at the proximal conduit anastomosis in 1 patient and at the site of the right coronary ostium anastomosis in another. No patient in Group 2 experienced postoperative bleeding related to the surgical procedure. A significant difference was found in the number of blood transfusions required (2.1 ± 2.0 units in Group 1 vs 0.4 ± 0.7 units in Group 2; p = 0.002); however, the volume of blood products transfused was similar between groups. Multivariate analysis showed the surgical technique used in Group 1 to be an independent risk factor for higher postoperative bleeding (p = 0.01) and for the need of 3 or more units of blood (p = 0.004).
Finally, comparison of the hematologic profiles before operation, at the end of operation, and at hospital discharge revealed that the only significant difference between Group 1 and Group 2 was in the preoperative hemoglobin values (12.5 ± 1.4 mg/dL vs 13.5 ± 1.3 mg/dL, respectively; p = 0.03).
Discussion
Simultaneous replacement of the ascending aorta and the aortic valve with a composite graft is an effective method for treating patients who require both procedures. 2–7 In the classical approach described by Bentall and de Bono, 1 the composite graft is sutured to the aortic annulus, after which the aortic tissue surrounding the coronary ostia is sewn directly to openings created in the graft. The remaining aneurysmal wall is wrapped tightly around the graft at the end of the procedure. This technique has been associated with pseudoaneurysm formation at the coronary anastomoses and at the proximal and distal suture lines. 7,11
In patients requiring simultaneous replacement of the aortic valve and the ascending aorta, we prefer the open modification 7 of the classical Bentall operation with total resection of the aneurysm wall, use of a composite conduit, and reattachment of the coronary ostia—adequately mobilized—to the vascular graft. This technique has yielded acceptable operative mortality rates and satisfactory long-term results. 4,5,7 However, bleeding from the suture lines may be a major cause of morbidity after such an operation. 8,9 Therefore, some technical modifications have been suggested to improve hemostasis in patients undergoing this operation. These modifications consist of: 1) the use of an additional suture at the proximal graft anastomosis (as 1st described by Copeland's group 8 and later by Bayfield and Kron 9); and 2) harvesting of the coronary ostia with a large button of aortic wall, which enables the coronary anastomoses to be reinforced by an “endo-button” buttress technique (described by Northrup and Kshettry 10). The use of tandem suture lines at the proximal anastomosis was effective in reducing postoperative bleeding and the need for transfusion in 4 of 5 patients in Bayfield and Kron's study. 9 In 1998, the “endo-button” buttress anastomosis was proposed for implantation of an aortic homograft root; 10 this technique is thought to facilitate hemostasis by providing a large coaptation surface against the graft.
In order to evaluate the effectiveness of such combined methods, we reviewed the experience of a single surgeon using composite aortic grafts for chronic disease. We compared the results from 2 groups of patients who underwent surgery during a 5-year period. In the later patients (Group 2), we observed fewer bleeding complications than those found in Group 1 patients. Moreover, the technique used in Group 1 was found to be the only independent risk factor for higher postoperative bleeding and for transfusions of at least 3 units of blood. Furthermore, no significant difference was found between the hematologic profiles of the 2 groups at the end of surgery and at discharge, thus excluding the possibility that the reduced need for blood transfusion in Group 2 could be ascribed to a different approach to reintegration of blood losses. The results of this study demonstrate that modification of the surgical technique was responsible for better results in the Group 2 patients.
In 1997, Girardi and associates 12 reported low operative mortality rates and complications after using the St. Jude Medical® conduit for aortic root replacement. In our study, we used both the St. Jude Medical® and the Carbo-Seal™ grafts but did not find that the type of device influenced the surgical results. Therefore, it is our impression that the performance of the Carbo-Seal™ conduit may also be satisfactory.
This study is a retrospective evaluation, which is a major limitation; a prospective randomized study on a larger population of patients would certainly yield more conclusive data. However, this limitation may be offset by these factors: the 2 groups of patients were matched for most preoperative characteristics, and all the procedures were performed by a single surgeon.
In conclusion, simultaneous replacement of the aortic valve and ascending aorta with a composite graft can be performed with excellent operative results. Our results show that certain modifications in the standard open technique—in particular, the reinforcement of the proximal graft anastomosis and an improved method for reattaching the coronary ostia to the graft—may improve hemostasis, thus reducing bleeding complications and related morbidity rates of this technically demanding procedure.
Footnotes
Address for reprints: Uberto Bortolotti, MD, U.O. Cardiochirurgia, Ospedale Cisanello, Via Paradisa 2, 56124 Pisa, Italy
References
- 1.Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338–9. [DOI] [PMC free article] [PubMed]
- 2.Gott VL, Pyeritz RE, Cameron DE, Greene PS, McKusick VA. Composite graft repair of Marfan aneurysm of the ascending aorta: results in 100 patients. Ann Thorac Surg 1991;52:38–45. [DOI] [PubMed]
- 3.Lewis CT, Cooley DA, Murphy MC, Talledo O, Vega D. Surgical repair of aortic root aneurysms in 280 patients. Ann Thorac Surg 1992;53:38–46. [DOI] [PubMed]
- 4.Gott VL, Gillinov AM, Pyeritz RE, Cameron DE, Reitz BA, Greene PS, et al. Aortic root replacement. Risk factor analysis of a seventeen-year experience with 270 patients. J Thorac Cardiovasc Surg 1995;109:536–45. [DOI] [PubMed]
- 5.Hilgenberg AD, Akins CW, Logan DL, Vlahakes GJ, Buckley MJ, Madsen JC, et al. Composite aortic root replacement with direct coronary artery implantation. Ann Thorac Surg 1996;62:1090–5. [DOI] [PubMed]
- 6.Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. N Engl J Med 1997;336:1876–88. [DOI] [PubMed]
- 7.Kouchoukos NT, Marshall WG Jr, Wedige-Stecher TA. Eleven-year experience with composite graft replacement of the ascending aorta and aortic valve. J Thorac Cardiovasc Surg 1986;92:691–705. [PubMed]
- 8.Copeland JG 3d, Rosado LJ, Snyder SL. New technique for improving hemostasis in aortic root replacement with composite graft. Ann Thorac Surg 1993;55:1027–9. [DOI] [PubMed]
- 9.Bayfield MS, Kron IL. Reducing bleeding after replacement of the aortic root. Ann Thorac Surg 1995;60:1130–1. [DOI] [PubMed]
- 10.Northrup WF 3d, Kshettry VR. Implantation technique of aortic homograft root: emphasis on matching the host root to the graft. Ann Thorac Surg 1998;66:280–4. [DOI] [PubMed]
- 11.Marvasti MA, Parker FB Jr, Randall PA, Witwer GA. Composite graft replacement of the ascending aorta and aortic valve. Late follow-up with intra-arterial digital subtraction angiography. J Thorac Cardiovasc Surg 1988;95:924–8. [PubMed]
- 12.Girardi LN, Talwalkar NG, Coselli JS. Aortic root replacement: results using the St. Jude Medical/Hemashield composite graft. Ann Thorac Surg 1997;64:1032–5. [DOI] [PubMed]


