Abstract
The nervous system governs both ontogeny and oncology. Regulating organogenesis during development and homeostasis and plasticity throughout life, the nervous system plays parallel roles in the regulation of cancers. Foundational discoveries have elucidated direct paracrine and electrochemical neuronal-cancer communication, as well as indirect interactions through neural effects on the immune system and stromal cells in the tumor microenvironment in a wide range of malignancies. Nervous system-cancer interactions can regulate oncogenesis, growth, invasion and metastatic spread, treatment resistance, stimulation of tumor-promoting inflammation and impairment of anti-cancer immunity. Progress in Cancer Neuroscience may create an important new pillar of cancer therapy.
In Brief
The nervous system plays a regulatory role in cancers via direct neuronal-cancer communication and indirect interactions through the immune system and cells in the tumor microenvironment. This review provides a comprehensive view of the reciprocal interactions of central and peripheral nervous system with cancers, highlighting opportunities for cancer therapy.
Introduction
As the nervous system governs such wide-ranging functions of the human body in health and disease, it is somewhat surprising that it took so long to fully appreciate its central involvement in cancer. Both the central nervous system (CNS) and the peripheral nervous system (PNS) regulate physiological functions and pathophysiological processes. Based on converging evidence, it is increasingly understood today that CNS and PNS activity regulates development, organogenesis, homeostasis, plasticity, regeneration, as well as immune function in diverse tissues (for review, see 1,2. As cancer formation, growth and progression subvert and repurpose mechanisms of development and regeneration, the nervous system may be implicated in all aspects of cancer pathophysiology. Reciprocally, cancer and cancer therapies can influence and remodel the nervous system, contributing to pathological feedback loops that not only yield neurological dysfunction but can also drive malignancy. These new insights have culminated in the emergence of Cancer Neuroscience as a new discipline 3 which focuses on defining and therapeutically targeting nervous system-cancer interactions, both in the local tumor microenvironment and systemically.
In this review, we will provide an update on the current state and future directions of Cancer Neuroscience. We identify important unanswered questions and current roadblocks, specifying ways to overcome these obstacles through the implementation of cross-disciplinary development of technologies, knowledge, and scholarly infrastructure. Reciprocal interactions of cancers with the nervous system are discussed, with new multidisciplinary research subfields like “neuro-immuno-oncology” outlined. Importantly, a roadmap for clinical translation is laid out for the implementation of neuroscience-instructed cancer therapies. We make the case that Cancer Neuroscience (Figure 1) can stimulate both fields: cancer research and clinical oncology, as well as neuroscience and neuro-medicine, with synergy at the intersection of these disciplines.
Figure 1: Mechanisms of nervous system-cancer interactions.
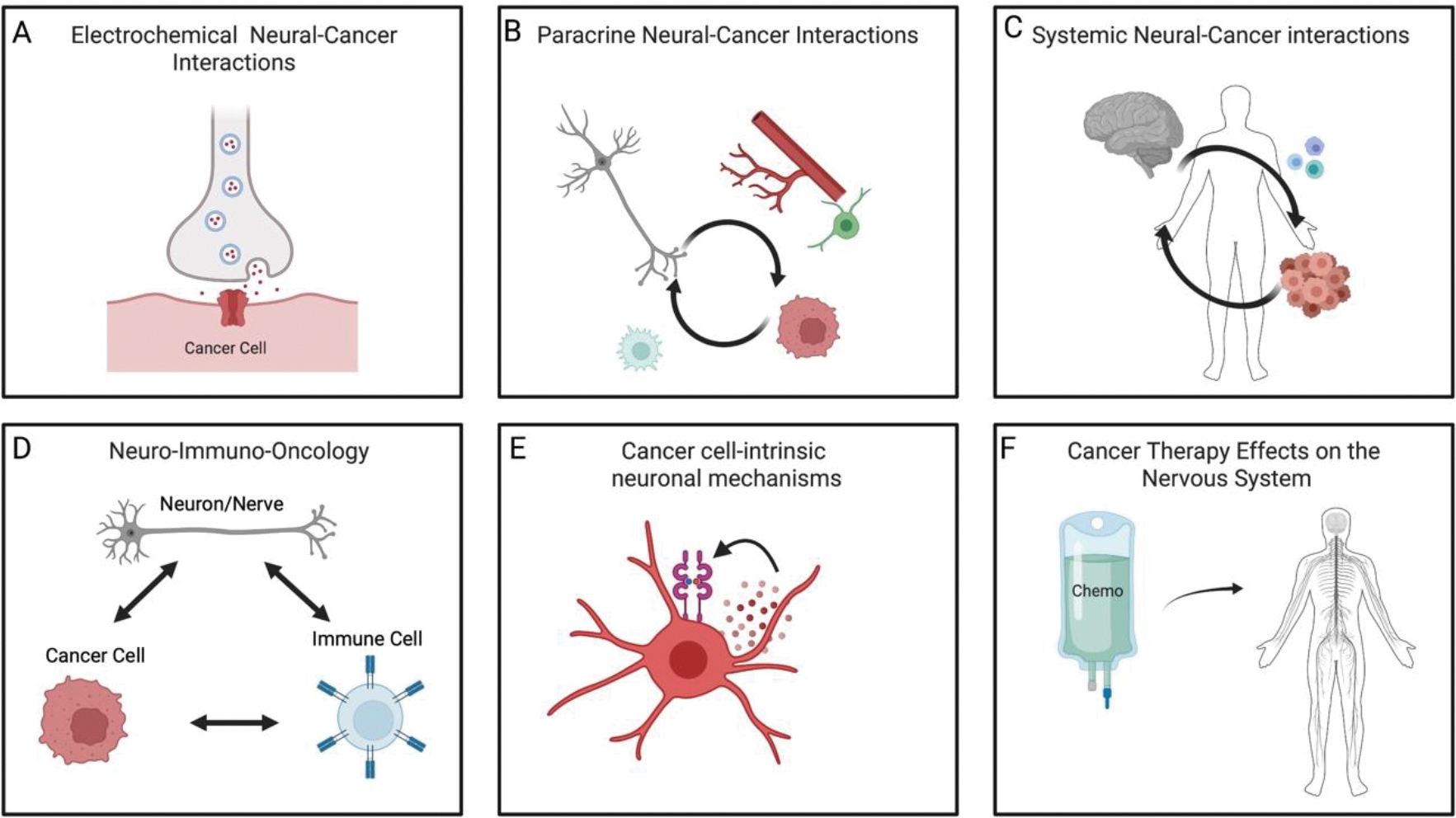
The nervous system (grey) and cancer (red) interact in at least six ways. A) electrochemical interactions, including bona fide neuron-to-cancer synapses. B) Paracrine interactions from neurons/nerves to cancer cells, directly or through signaling with cells in the tumor microenvironment (green stromal cell and red blood vessel shown). In turn, cancer cells often secrete signaling molecules such as synaptogenic factors or axonogenic factors that locally remodel the nervous system to augment nervous system-cancer interactions. C) Systemic nervous system-cancer interactions, such as circulating neurotransmitters or neuropeptides that can influence cancer pathogenesis directly or indirectly such as through altered immune system (blue) function. Reciprocally, cancers can influence the nervous system at a distance through circulating factors or altered afferent neural signals. D) Three-way interactions between neurons or nerves, cancer cells, and immune cells can modulate anti-cancer immunity and pro-cancer inflammation. E) Cancer cells may leverage cell-intrinsic signaling and other processes classically associated with neural cells. For example, autocrine neurotrophin signaling is illustrated. F) Cancer therapies (chemotherapy, green) can profoundly alter nervous system function, including impaired function of various types of peripheral nerves and impaired cognitive function.
1. Impact of the nervous system on tissue development, homeostasis and plasticity
Neuronal activity influences organ development, homeostasis, plasticity and regeneration – both in the CNS and throughout the entire body. The cellular and molecular basis for neuronal activity-dependent regulation of physiology in health has the potential to provide insights into how the nervous system might similarly influence tumor biology. Given how instructive understanding development of the brain itself has been for the study of cancer neuroscience, we begin with an in-depth discussion of nervous system development in order to explore foundational concepts mirrored in cancer pathogenesis discussed later.
Central Nervous System
Development of the CNS involves coordinated neuronogenesis and gliogenesis from neural stem and precursor cells, diversification of these neurons, astrocytes and oligodendrocytes, migration of new cells to the appropriate location, and neural circuit assembly (for review, see 4). Functional neural circuit development requires axonal outgrowth and pathfinding, establishment of synapses and refinement of these connections between neurons. Astrocytes promote synaptogenesis, develop a gap junction-coupled network throughout the brain and engage with synapses to support synaptic function, while oligodendrocytes myelinate axons to provide metabolic support5 and enable fast saltatory conduction of action potentials6.
Electrical activity influences all aspects of nervous system development (for review, see 7). During early stages of neurodevelopment, synchronous waves of electrical activity and consequent voltage-dependent calcium transients occur in developing neural tissues and regulate both cellular and synaptic patterning. In the nascent brain, gap junctions couple neural stem cells in the germinal zone, allowing membrane depolarization-induced calcium transients to propagate synchronously through the germinal zone, regulating stem cell proliferation8. Early in neurodevelopment, neurotransmitters are secreted from a variety of cell types in a non-synaptic manner to promote the generation of neurons9. Electrical activity also regulates the migration of these newly generated neurons10 and influences axon pathfinding and axonal targeting11–13.
In the developing nervous system, gap-junctional coupling occurs between migrating neuroblasts14, neurons in the prenatal and early postnatal neocortex15,16 and between neurons in numerous additional neuroanatomical locations. Such coupling, together with mechanisms of cell depolarization such as non-synaptic glutamate secretion and “pacemaker” neurons17 enables synchronized calcium transients to spread through developing central nervous system structures such as the nascent neocortex 18. Recent work has suggested that a small, distinct subpopulation of single neurons arborizes throughout the entire brain to provide a specific periodic signal coordinating brain development19. Such experience-independent, coordinated waves of activity promote the assembly of functional neural circuits that are later refined in an experience-dependent manner20,21.
Neurotransmitter signaling regulates brain organogenesis and later serves as the backbone of synaptic communication between neurons. The formation of new neurons from stem and progenitor cells, as well as their integration into neuronal circuits, is driven by the neurotransmitters during development (for review see 22), and in neurogenic regions of the adult brain9. This signaling is fine-tuned and can be spatially and temporally heterogeneous: for example, during development, the neurotransmitter GABA (which is an inhibitory neurotransmitter in later life) is chiefly excitatory (depolarizing) due to developmental expression patterns of chloride transporters and is implicated in many processes of neural development, including neuronal proliferation, migration, differentiation, and preliminary circuit-building in the CNS, for review see 22, while inhibiting the generation of neuronal progenies from embryonic stem cells and peripheral neural crest cells during early embryogenesis23.
Cellular plasticity in the CNS does not end at the time of birth or during childhood. As is the case during development, neuronal activity also governs ongoing cellular plasticity throughout life. Neuronal activity and neurotransmitter signaling robustly regulates the proliferation of neural precursor cells, including oligodendrocyte precursor cells (Figure 2)24, and neural stem cells in the subventricular zone25,26 and hippocampus27,28. Neuronal activity drives one of the most important features of plasticity and adaptation in the adult brain: ongoing generation and remodeling of myelin24,29,30(Figure 2), which contributes to motor function24, motor learning31, attention and short-term memory32, memory consolidation33,34, and social function35,36. In health, adaptive myelination appears to be highly and specifically regulated, with precise circuit-specific and neuron subtype-specific24,37 activity-regulated changes in myelin that tune circuit dynamics to promote coordinated circuit function33,38,39.
Figure 2. Parallel mechanisms of glial plasticity and glial malignancy.
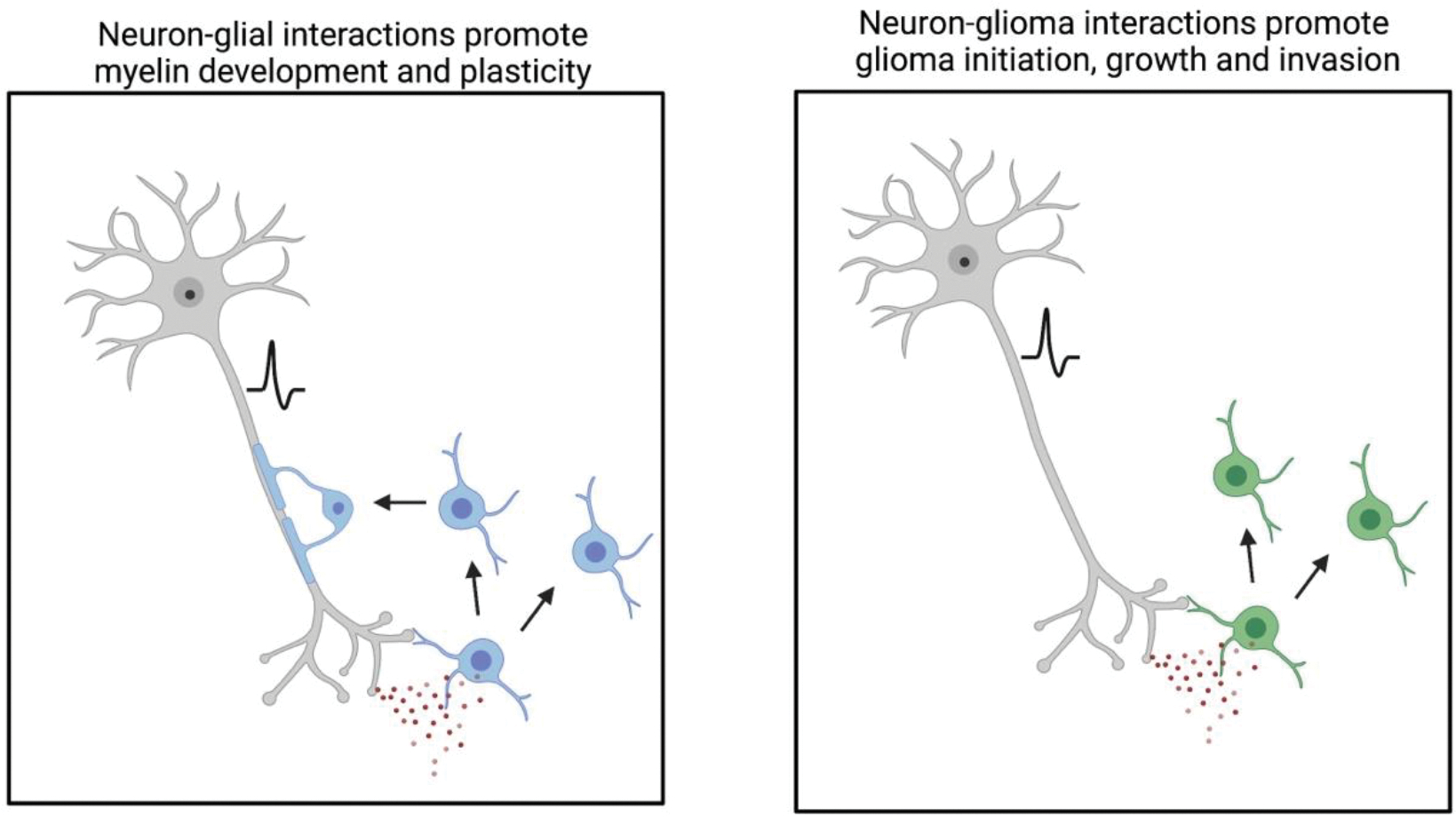
Left) Neuron (grey) to oligodendroglial (blue) interactions involve neuron-to-oligodendrocyte precursor cell synapses and paracrine (red circles) signaling, e.g. BDNF-TrkB signaling, during development and throughout life. Neuronal activity can promote the proliferation of oligodendrocyte precursor cells, generation of new oligodendrocytes and adaptive changes to myelination that tune neural circuit function. Such plasticity of myelin contributes to healthy cognitive function throughout life. Right) Neuron to glioma (green) interactions involve neuron-to-glioma synapses and paracrine signaling, e.g. BDNF-TrkB signaling. Glioma hijacking of mechanisms that normally support myelin development, homeostasis and plasticity instead contribute to glial cancer initiation, growth and invasion.
Neurons communicate with neural stem cell and progenitor cells by activity-dependent paracrine factors such as brain-derived neurotrophic factor (BDNF)32, and by synaptic communication (Figure 2). Synaptic signaling is well-established for oligodendrocyte progenitor cells (OPCs), which receive synaptic input via glutamatergic (calcium-permeable AMPA receptor-mediated) and GABAergic (GABAA receptor-mediated) neuron-to-glial synapses40,41 Such neuron-to-OPC synapses are unidirectional, with the OPC always in the postsynaptic position, and can be of a transient nature42, which is compatible with rapid migration of OPCs. Synaptic input to OPCs is extensive, involving both short range and long-range inputs43, although the role that such neuron-to-OPC synapses may play in activity-regulated myelination remains incompletely understood.
Beyond development and plasticity, glutamatergic neuronal activity also promotes myelin regeneration after a demyelinating injury44,45. GABAergic signaling to OPCs is involved in resistance to and adaptive repair of hypoxia-induced dysmyelination 46. Remarkably, new evidence suggests that following injury, not only neurons from the PNS but also from the CNS can revert to an embryonic-like growth state which allows axonal regeneration47. Together this speaks for a remarkable ability of the CNS to self-repair damage, at least to a certain extent, by neuronal activity-regulated mechanisms.
Neural activity is also an important regulator for vascular homeostatic physiological processes in the CNS. One example is the neural autoregulation of cerebral blood flow in the brain, called neurovascular coupling, which allows regional blood flow to increase to quickly supply oxygen and nutrients according to demand48; this process involves neurons, astrocytes and vascular cells, and includes direct neurotransmitter signaling49. Neuronal activity also directly regulates the blood-brain barrier by modulating endothelial gene expression and the functions of efflux transporters50.
Peripheral Nervous System
Innervation similarly regulates tissue development, organogenesis and regeneration outside the CNS, throughout the entire body (for reviews, see1,2). The CNS controls a myriad of non-neural cells and bodily functions, either by hormone secretion into the systemic circulation, or in a more region-specific manner via the PNS, which connects the CNS to all organs via sympathethic (adrenergic), parasympathetic (cholinergic) and/or sensory nerve fibers.
The role of nerves in development is increasingly appreciated, with organogenesis depending on proper innervation. A strong dependence on functional nerves and undisturbed nerve growth is long known to be indispensable for limb regeneration in amphibia and reptiles51. In mammals, a similar dependency of organogenesis on innervation has been reported. For the example of the salivary gland, parasympathetic innervation is crucial for glandular organogenesis52. Likewise, heart regeneration in neonatal mice is impaired by denervation53, and heart organogenesis depends on sympathetic nervous system signaling54. It is an exciting question to address whether synapses, or synapse-like structures, exist between neurons and certain non-neuronal cells throughout the body. The answer to this question also has great implications for cancer neuroscience and would help elucidate neuron-tumor interactions in the light of neurodevelopmental processes.
The innervation of tissue stem cell niches also regulates the functions of various cell types, both during development and in mature tissue, as demonstrated for the skin55–57, gastrointestinal tract 58, and bone marrow59. Additionally, Schwann cells, the chief glial cell type of the PNS, are involved in maintenance of hematopoietic stem cells in the bone marrow niche60.
The nervous system contributes to tissue regeneration throughout the entire body. Injured adult organs do not regenerate after denervation, while restoring the function of cholinergic signaling in salivary gland tissue improves epithelial regeneration61. Likewise, epidermis regeneration during wound healing depends on nerve-derived sonic hedgehog signaling, allowing hair follicle stem cells to become epidermal stem cells 56. As discussed in detail below, the nervous system is also involved in the regulation of multiple functions of the immune system (for review, see62). Moreover, nerves control blood vessels in the periphery: during development and tissue repair, blood vessels and nerves use similar signals and principles to differentiate, grow and navigate towards their target. The release of sympathetic neurotransmitters has been implied in the formation of new blood vessels during these processes63. Moreover, sympathetic innervations of the vessels can affect the extravasation of immune cells from the blood vessels to the local tissue by modulating their expression of adhesion molecules64, thereby, affecting the local immune response.
In summary, the CNS and PNS are not only involved in cognitive functions, movement and sensation, but govern the generation, adaptation, plasticity, and repair of tissues and organs. Local (paracrine) and systemic neural factors, classical synaptic contacts, as well as bona-fide synaptic contacts to cells that are not mature neurons are involved in this complex, multilayered system of governance. This explains why neural-cancer interactions are so intriguing to study, since all of the “non-canonical” biological functions of neuronal activity described above are highly relevant for cancer as well: organo(/tumoro)genesis; growth by activation of developmental programs; invasion and colonization; control of a permissive microenvironment, including blood vessels and the immune system; and resilience and self-repair capabilities.
2. CNS cancer neuroscience
Paracrine signaling in brain tumor growth and initiation
As described above, neuronal activity controls vast and varied physiological functions. In parallel, nervous system activity and neural mechanisms can control brain tumor initiation, growth, invasion, and metastatic colonization of the brain. The idea that neurons may play a key role in brain tumor biology, was first suggested by histological co-localization studies in 193865 and application of the tools of modern neuroscience to study glioma biology has now demonstrated clearly that neuronal activity can drive brain cancer growth 66(Figure 2). Mechanisms of activity-regulated paracrine signaling were first appreciated with the discovery that neuronal activity-dependent paracrine signaling of neuroligin-3 (NLGN3), BDNF, and GRP7866–68 promote glioma proliferation and growth. Recent data show how even CNS tumor initiation can be driven by neuronal activity67. In addition to promoting glioma growth, NLGN3 regulates the initiation of optic gliomas in a cancer predisposition syndrome67. Activity-dependent shedding of NLGN3 is mediated by the metalloprotease ADAM10, and the growth of high-grade and low-grade gliomas were significantly decreased with ADAM 10 inhibitors in mouse models67,69. Recently, IGF-1 was identified as another neuronal activity-regulated paracrine signaling molecule, which mediates olfactory sensory experience-dependent initiation of olfactory bulb high-grade glioma 70. Together, these discoveries suggest that circuit-specific neuronal activity-dependent paracrine signaling differentially influences the neurobiology of distinct brain tumor types.
Synaptic connections between neurons and brain tumor cells
Brain tumor cells can structurally and electrically integrate into neural circuits. Accordingly, tumor cells from various adult and pediatric glioma types form bona fide glutamatergic synapses with neurons (Figure 1A), driving tumor growth71,72 and brain invasion73. These synaptic connections consistently form unidirectionally from neurons on the presynaptic side to glioma cells on the postsynaptic side, inducing excitatory postsynaptic currents predominately mediated by calcium-permeable AMPA receptors (AMPAR) in glioma cells71,72. These EPSCs are depolarizing, and direct optogenetic depolarization of glioma cells increases glioma cell proliferation71. Furthermore, inhibiting AMPAR function genetically or pharmacologically with perampanel, an FDA-approved antiepileptic drug, reduces glioma cell proliferation and invasion71–73. As discussed above, oligodendrocyte precursor cells (OPCs), a likely cell of origin for many types of glioma, and immature neurons also receive synaptic input 10,40, demonstrating that physiological correlates of malignant synaptic contacts exist.
Distinct from direct, bona fide synaptic interactions, indirect, perisynaptic contacts, reminiscent of the position an astrocyte normally assumes in a tripartite synapse, were found in breast cancer brain metastases74 as well as adult glioblastoma72. In breast cancer brain metastatic disease, glutamatergic signaling via these perisynaptic structures promotes tumor growth through NMDA receptors on the breast cancer cells 74.
Linking paracrine and synaptic mechanisms, NLGN3 induces a synaptogenic gene expression profile in glioma cells, which suggested it may act as an upstream regulator of malignant synaptogenesis 69. Indeed, fewer neuron-to-glioma synapses form in the absence of NLGN3 in the tumor microenvironment71. Paracrine BDNF signaling also promotes synaptic connectivity between neurons and glioma cells, as well as regulates the strength of malignant synapses 68. Similar to the plasticity at physiological synapses that supports learning and memory in the healthy brain, glioma cell surface AMPA receptor trafficking is increased by BDNF, highlighting a postsynaptic mechanism of malignant synaptic plasticity 68. In turn, this mechanism amplifies glioma currents caused by glutamate and increases glutamate-driven calcium transients. In patient-derived glioma cells, genetic and pharmacological inhibition of NTRK2 (BDNF receptor TrkB) consistently reduces glioma cell responsiveness to glutamate, decreases neuron-to-glioma synaptic connections, and reduces neuronal activity-induced glioma proliferation68. Accordingly, pharmacological targeting of TrkB signaling in glioma inhibits glioma growth in mouse models without TrkB fusions68, highlighting a potentially broader indication for Trk inhibitors than only for gliomas expressing Trk-fusions.
Brain tumor-induced modifications of the neuronal environment
Several mechanisms have been identified by which gliomas influence their neuronal microenvironment. Seizures caused by neuronal hyperexcitability are frequent in gliomas and brain metastases. Several paracrine factors and aberrantly increased neuronal synaptogenesis contribute to glioma-induced neuronal hyperexcitability. Paracrine glutamate secretion via the xc-cystine-glutamate transporter system increases neuronal hyperexcitability as well as glioma growth in models of adult glioblastoma75. In the tumor microenvironment of IDH-WT adult glioblastoma, loss of GABAergic interneurons also contributes to circuit hyperexcitability76, as does glioma-induced alterations in neuronal chloride transporter expression, changing the effects of GABA from inhibitory to excitatory76. Another interesting mechanism promoting neuronal hyperexcitability is the ability of glioma cells to promote synaptogenesis, mirroring a physiological role of astrocytes77. In gliomas with specific point mutations of the enzyme PIK3CA, glioma cells secrete glypican-3 which drives aberrant synaptogenesis and associated neuronal hyperexcitability in mouse models78, indicating that distinct genomic characteristics of glioma can differentially affect the neuronal tumor microenvironment. Furthermore, glioma-secreted thrombospondin-1, another synaptogenic factor, promotes increased functional neuronal connectivity between the tumor and the brain; such functional connectivity of the tumor was strongly associated with decreased survival in humans with glioblastoma79.
Taken together, these data highlight a positive feedback loop between neuronal hyperexcitability, neuron–glioma interactions, and brain tumor progression. This concept is strengthened by recent clinical data linking preferentially active brain regions to glioma occurrence80.
Tumor-autonomous neurodevelopmental and neural mechanisms in brain cancer biology
In addition to neuron-tumor networks, brain tumor cells themselves show multiple neural and neurodevelopmental features, including network structures (Figure 1E). Ultralong, neurite-like membrane protrusions called tumor microtubes (TMs) are used by glioma cells to scan the brain microenvironment81, invade into the brain81–84 and colonize it by invasion and cell division81. Over time, TMs interconnect single glioma cells to a functional, communicating multicellular network81,84. TMs and the multicellular networks they generate are consistently found in high-grade human gliomas investigated so far, including astrocytomas grade 2–4 (which includes grade 4 glioblastomas), and K27M-mutated midline gliomas71,72,81–83,85,86. As mentioned, many similarities exist between TMs and neural protrusions. A subpopulation of invasive TMs exhibits tips resembling the growth cones of neurites, neuronal processes during neurodevelopment that are essential for neuronal migratory pathfinding and network building 81,82. In addition, invasion-related features of TMs such as branching, protrusion and retraction mimic mechanisms of neurite pathfinding 84. Several molecular drivers of tumor microtube growth are also involved in neurite outgrowth and neurodevelopment, such as GAP-43 and Ttyh181,83.
Using gap junctions (mainly connexin 43) and adherens junctions between TMs, tumor cells interconnect with each other, building the anatomical basis of the tumor-tumor network. The network of tumor cells connected by gap junctions communicate via intercellular calcium waves and exchange small molecules with each other, similar to physiological astrocyte networks in the brain71,81,87. Importantly, this functional tumor-tumor network is a crucial factor for mediating therapeutic resistance. TM network-integrated, gap junction-coupled tumor cells were predominately resistant to radiotherapy and standard chemotherapy with temozolomide. In contrast, unconnected glioma cells were much more responsive to cytotoxic therapeutic treatment which was associated with decreased tumor cellular homeostasis81,86,88,89. This resembles mechanisms of normal brain astrocyte networks that can dilute toxic metabolites throughout their gap junction-coupled network 90. Furthermore, tumor cell-coupling via gap junctions and TMs does not only occur with each other, but also with the astrocytic network of the brain, which has also been demonstrated for cancer cell survival in the brain during metastasis84,91.
In contrast, glioblastoma cells not (yet) integrated into tumor-tumor or tumor-astrocyte networks are the drivers of glioblastoma invasion84. On a molecular level, this subpopulation was enriched for OPC-like and neural progenitor (NPC)-like, and neuronal-like cell states. Interestingly, the invasive glioblastoma cell subpopulation showed migration patterns resembling immature neurons during neurodevelopment. Furthermore, analogous to immature neurons and OPC receiving synaptic input, glioma cell invasion as well as TM dynamics and TM genesis were increased after neuronal stimulation84.
In summary, while glioma cells connected with each other mediate therapeutic resistance, those that are not connected with each other or with astrocytes drive brain invasion. In other words, distinct neural features govern the various central traits of malignancy of incurable brain tumors.
It has recently been discovered that TM-connected glioblastoma cell networks are characterized by autonomous rhythmic activity that is generated by pacemaker-like tumor cells. Residing in the hubs of the functional tumor networks, autonomously rhythmic tumor cells effectively influence the other network members via generation of intercellular Ca2+ waves that travel throughout the network92. In addition to neuron-to-glioma synaptic signaling that also generates Ca2+ activity including Ca2+ waves in the glioma networks71,72, this periodic activity is an alternative, tumor-autonomous mechanism of glioma network activation. Importantly, glioblastoma growth and cellular survival depended on this autonomous rhythmic activity, possibly via frequency-specific upregulation of distinct tumor-promoting intracellular pathways92. Relevant for the field of cancer neuroscience, these findings show striking similarities to the spontaneous periodic network activity driven by pacemaker-like neuronal cells during neurodevelopment: regarding frequencies, molecular mechanisms for pacemaking (Ca2+-modulated potassium conductance), importance for network development, coordination of population activity, and plastic, even “self-repairing” features of pacemaker-like behavior17. It will be interesting to learn whether other tumor types show a similar pathobiological mechanism by recapitulating this physiological neurodevelopmental principle.
The complexity of interactions between various components of tumors and the central nervous system (Figure 3) illustrates an important challenge for the future. In addition to the various neural mechanisms governing brain cancer biology, research in ion channels expressed in tumor cells, neural-tumor co-regulation of the blood-brain barrier and tumor blood vessel biology, and other lines of research will certainly extent our knowledge in brain tumor cancer neuroscience.
Figure 3: Therapeutic opportunities at the intersection of neuroscience and cancer biology.
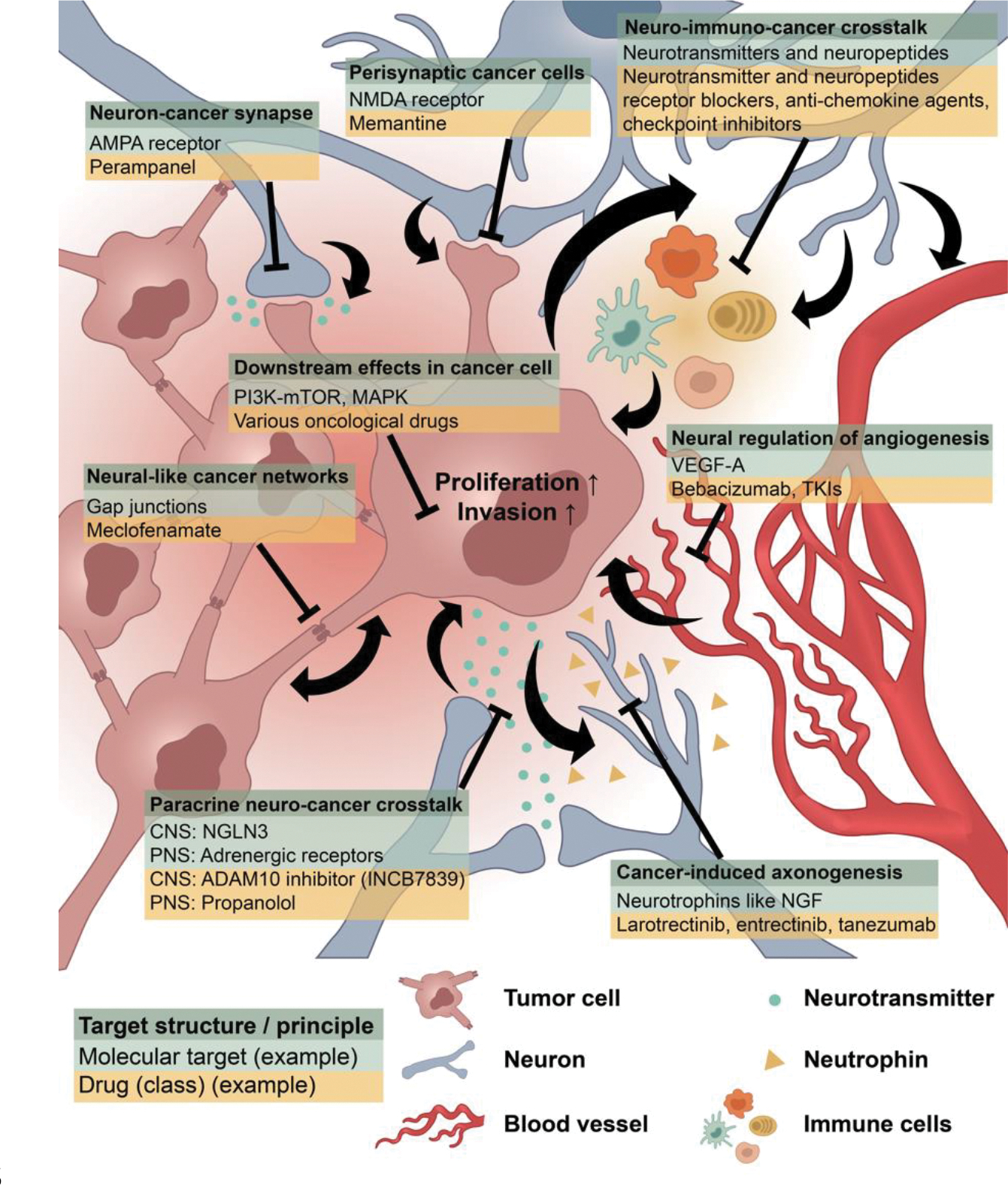
Increased understanding of nervous system-cancer crosstalk is beginning to elucidate therapeutic targets for a variety of cancer. While these targets vary in a tumor- specific manner, examples are shown here of the target structure or principle (dark green), a relevant molecular target (light green) and example of a drug or drug class that may prove useful for therapy (yellow). Please note that only examples are shown, and each target is not necessarily relevant for every tumor type; for instance, targeting AMPAR-mediated synapses using the anti-seizure medication parampanel has, to date, only been demonstrated as a potential strategy for gliomas. Each therapeutic strategy requires testing in prospective clinical trials, which has been initiated for several of those (see text).
3. PNS cancer neuroscience
Beyond the central nervous system, a wealth of studies across various cancer types have now demonstrated a fundamental role for the nervous system in driving tumor pathogenesis in cancers outside of the brain. As with gliomas, pathologists have appreciated the structural relationship between neurons and malignant cells in the periphery for more than a hundred years93, largely due to the histopathological observances of perineural invasion (PNI) 94 that suggests the perineural niche may be functionally beneficial to the tumor. PNI involves malignant cells surrounding or invading into nerve tracts, and has been associated with aggressiveness and poor prognosis in a number of different cancers, including pancreatic, breast, and prostate cancers 95–97. As with gliomas, cancer cells of various non-CNS tumors have been found to display distinct neurodevelopmental features, at least on the gene expression level98.
Preclinical studies have demonstrated an important role for the autonomic nervous system in the neural regulation of a wide range of cancers. For instance, in prostate cancer, β-adrenergic signaling (sympathetic) was found to be integral to tumor initiation, while cholinergic signaling (parasympathetic) contributed to invasiveness and dissemination99. In breast and ovarian cancer, β-adrenergic signaling was found to accelerate cancer progression100,101. Importantly, much like their differing roles in various tissues during normal development, different neuronal subpopulations may play distinct roles dependent on tissue type. As an example, cholinergic signaling has been shown to be either growth-promoting in gastric cancer102,103, or growth-inhibiting in pancreatic cancer 104. Even within specific tissues, careful attention must be given to identifying the specific contributions of various neurotransmitters stemming from either parasympathetic or sympathetic nerve activity. For instance, in breast cancer, genetic manipulation of autonomic nerves revealed sympathetic nerves accelerated tumor progression and growth, while parasympathetic nerves had the opposite effect105. Similarly, in pancreatic cancer, cholinergic signaling suppressed growth104, while adrenergic signaling promoted growth106.
Sensory nerves have also been shown to play a role in cancer pathogenesis. Basal cell carcinomas require hedgehog signals from cutaneous mechanosensory sensory nerves for tumor formation 57, and pancreatic cancers exhibit slowed growth with the ablation of sensory neurons107. In the context of metastasis, surgical denervation studies ruled out a role for circulating catecholamines in stress-induced metastasis in a mouse model of breast cancer108, though sensory nerve innervation enhanced triple-negative breast cancer invasion and metastatic spread109. Thus, the specific impact of various neurotransmitters coming from the activity of different branches of the nervous systems on malignant tissues of all types must be carefully parsed (potentially even on a single cell/cellular subpopulation-specific level) to better understand how manipulation of these neural circuits may be harnessed for treatment.
In the NF1 cancer predisposition syndrome, children and adults are prone to the development of benign peripheral nerve sheath tumors (neurofibromas) that derive from preneoplastic NF1-deficient Schwann cell precursors. These tumors are intimately associated with nerves, raising the intriguing possibility that neurons influence neurofibroma formation or growth. To this end, Nf1-mutant dorsal root ganglion neurons, which extend sensory axons to neurofibromas, exhibit greater action potential firing rates relative to wild-type controls. These Nf1-mutant sensory neurons also exhibit increased expression of collagen 1a2 that serves as a mitogen for NF1-deficient human and mouse Schwann cells, such that inhibition of their excitability with TTX or the anti-seizure drug lamotrigine reduces collagen 1a2 production as well as the growth of neurofibromas in Nf1 mutant mice in vivo 110.
Additional mechanisms promoting nerve-cancer interactions the tumor microenvironment include secreted neurotrophins that may be released in both activity and non-activity dependent manners from nerves or secreted from tumor cells. These neurotrophins, known to play a vital role in axonogenesis and nerve recruitment, have now been shown to critically modulate tumor growth outside of the brain (Figure 1). In pancreatic cancers, glial cell derived neurotrophic factor (GDNF) 111 and artemin (ARTN)112 secretion promotes perineural invasion, while nerve growth factor (NGF) has been shown to recruit sensory nerves into the tumor microenvironment 113,114. Similar to gliomas, BDNF/NTRK signaling has been implicated in promoting tumor survival in multiple myeloma and ovarian cancers115,116. Neurotrophins are often upregulated by neural signaling through a feed-forward mechanism, with cholinergic signaling promoting NGF expression in gastric cancer102, and adrenergic signaling promoting NGF expression in pancreatic cancer106; the NGF-induced increased nerve ingrowth into the tumor microenvironment further promotes tumor progression. Another avenue of neuronal contributions to the microenvironment of extracranial tumors includes metabolic support. Work by Zahalka and colleagues illustrated that β-adrenergic receptor signaling is critical for an angio-metabolic switch that fuels prostate cancer growth 117. In another example, pancreatic cancer cells increase NGF production to promote axon recruitment as a means of serine production to fuel metabolism118.
As illustrated in the above examples, in addition to benefiting from these secreted metabolites and neurotrophins, cancers reciprocally affect the nervous system (Figure 1). Just as brain tumors induce hyperexcitability, cancers outside of the CNS can increase innervation of the local tumor microenvironment by recruiting new nerve fibers via axonogenesis119, often driven by neurotrophin secretion. Another interesting example of tumor-induced modulation of the nervous system that in turn fosters cancer progression has been suggested for prostate and other cancers where peripheral tumors attract doublecortin-expressing neural progenitor cells that leave the brain and home to the tumor via the blood stream, generating new neurons in the tumor which has growth-stimulatory effects120. Remodeling of the neural microenvironment is further evidenced in a recent study demonstrating that tumor-associated neurons are reprogrammed towards an adrenergic phenotype that can stimulate tumor progression in oral cancer95. Together, these studies suggest that whether through activity-dependent mechanisms, paracrine signaling, or metabolic support, crosstalk between nerves and malignant cells (Figure 1B–C) in several tissues represent a novel angle to target malignant disease progression.
The ability of metastatic cells to leave the primary tumor and establish metastases is a major cause of death and a serious impediment to successful therapy. In brain metastases, these non-brain-cell-derived cancers hijack mechanisms of neurodevelopment (Figure 1E) for growth as described above. Even outside the context of specific brain metastases, ion channels have been implicated in the overall metastatic process. Changes in potassium channel expression were found to alter metastatic breast cancer progression121. Recent studies have also more broadly suggested that a single ion channel, NALCN, may regulate malignant cell dissemination and metastasis in a number of cancers122. Investigating the broader role of neural activity in driving the metastatic cascade will also be critical as innervation of peripheral tumors has been linked to invasion and dissemination from primary tumors99. For example, sympathetic neural signaling through β-adrenergic receptors on breast cancer cells induced cytoskeletal changes and protease production that increased invasion of those cells123. Sympathetic/β-adrenergic signaling to blood and lymphatic vessels in tumors contributes to metastatic dissemination100,109. Together, these studies suggest that ion channel and neurotransmitter signaling in malignant cells may facilitate metastatic progression. Furthermore, a dietary-induced pro-regenerative state of peripheral glial cells (Schwann cells) was related to increased tumor innervation and metastatic potential124. In the future, studies elucidating the interactions between various types of neurons/nerves and various types of metastatic cancers might lead to new ideas how to prevent and treat metastatic spread. It will also be fascinating to learn whether metastatic cells become functionally integrated into neural networks, such as in glioma.
In summary, the distinct mechanisms of interactions between malignant cancer cells and neurons in their microenvironment are now being studied across different tissue types and organs, though much is yet to be understood about how peripheral cancers integrate into neural networks and respond to electrochemical neurotransmission. There is clear evidence that in oral squamous cell, head and neck, gastric, colon, rectal, prostate, breast, and pancreatic cancers, neurons of different types contribute to malignant tumor growth. Moving forward, evaluating the effects of direct activity-mediated neurotransmission to and membrane depolarization of these malignant cells will be an exciting area of study. New technologies that allow for interrogation, visualization, and quantification of neuronal activity within peripheral tumors will be needed (Figure 4) to unravel the neural inputs and signaling patterns that contribute to tumor pathogenesis. As this field evolves, it is thus imperative that all axes of neuronal communication with both neoplastic and non-neoplastic cells of the tumor microenvironment are thoroughly investigated.
Figure 4: Techniques for studying nervous system-cancer interactions.
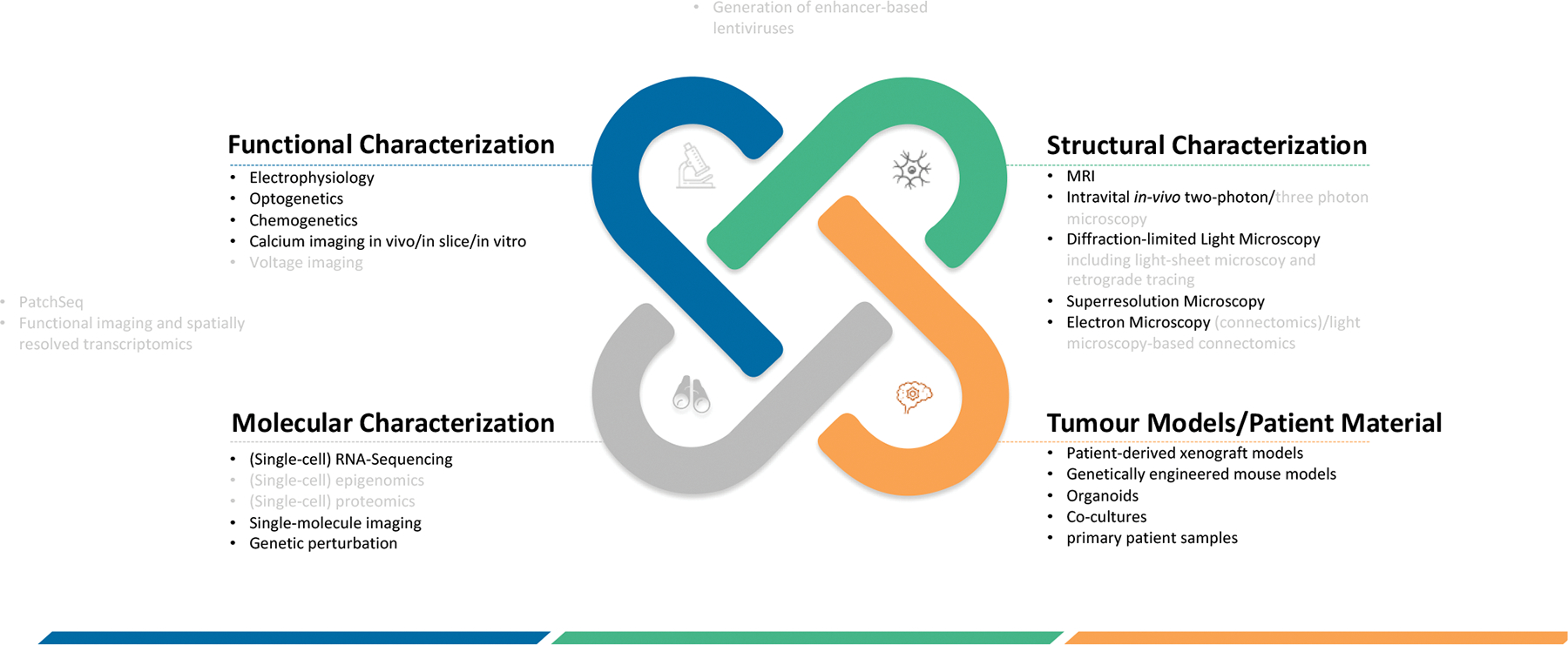
Methodologies to study nervous system-cancer interactions can be broadly categorized into four dimensions that encompass the functional, structural and molecular characterization as well as the material or model system that is studied. Furthermore, techniques crossing these modalities are mentioned here at the intersections. Methods shown in light grey are methodologies that have not yet been applied to Cancer Neuroscience studies but are of potential future use.
4. Neuro-Immuno-Oncology
Neural cells respond to immune system signaling molecules and immune cells respond to neurotransmitters and neuromodulators, so it is not surprising that neural-immune crosstalk can profoundly modulate both nervous system function and immune system function. In the context of cancer, a triangular relationship between neurons, immune cells and cancer cells (Figure 1D) is emerging that is relevant to nervous system influences on the tumor immune microenvironment, pro- and anti-tumor immunity, and immunotherapy.
The autonomic nervous system plays key roles mediating communication between the brain and immune system. Afferent fibers of the vagus nerve convey information about peripheral immune challenges to the brain, and efferent vagus pathways modulate the immune response through cholinergic signaling, for example powerfully mitigating proinflammatory cytokine release in the context of experimental lipopolysaccharide-induced sepsis125. Such an “inflammatory reflex”125 helps to exert precise control of powerful immune responses. This anti-inflammatory influence of parasympathetic nerves and acetylcholine on peripheral immune responses is one such mechanism of control, while neural orchestration of immune cell trafficking and function by the sympathetic nervous system is another important mechanism of regulation. Adrenergic signaling via sympathetic innervation regulates physiological, diurnal trafficking of lymphocytes through lymph nodes126 and egress of hematopoetic stem cells from the bone marrow into the circulation59,127, as well as movement of immune cells within tissues which is essential for their function128. In response to stressors, periventricular hypothalamic corticotropin hormone (CRH) neurons stimulate the hypothalamic-pituitary-adrenal axis and regulate trafficking of lymphocytes and monocytes between peripheral tissues and bone marrow129. CRH neurons of the periventricular nucleus and the central nucleus of the amygdala ultimately project to the splenic nerve and can influence adaptive immune responses through both adrenergic and cholinergic mechanisms 55. Norepinephrine, released locally from sympathetic nerves or systemically largely from the adrenal gland in a physiological manner or in response to range of stressors, binds chiefly to the beta2-adreneric receptor (B2AR) on immune cells. Norepinephrine-B2AR signaling can exert immune-suppressive effects such as upregulating PD-1130, regulating myeloid-derived suppressor cells and macrophage function and recruitment to tumors100,131,132, limiting anti-tumor immunity128,133,134 and promoting T-lymphocyte metabolic stress and exhaustion 135. Likewise, innervation of solid tumors by sensory neurons can induce T cell exhaustion, preventing effective antitumor immunity which could be overcome by inhibiting CGRP, a nociceptor-produced neuropeptide 136. Some of the aversive effects of stress on tumor growth in a breast cancer model were shown to be attenuated by optogenetic stimulation of the dopaminergic projections from the ventral tegmental area (VTA) to the medial prefrontal cortex 137. Interestingly, VTA activation reduced tumor growth in models of melanoma and lung cancer by modulating the sympathetic innervation to the bone marrow, altering the functional profile of myeloid-derived suppressor cells (MDSCs)138. Together these results provide a valuable first guidance on how antitumor immunotherapies can be augmented by neuromodulation strategies134,139.
Neuronal signaling molecules are sometimes used by the immune system directly. The neurotransmitter GABA can be synthesized by B-lymphocytes, and in the context of a mouse model of colon cancer can bind to GABAA receptors on CD8+ T-lymphocytes to reduce antitumor immunity and enable tumor growth 140. Serotonin, secreted by platelets, upregulates PD-L1 expression in models of pancreatic and gastric cancer through histone serotinylation and consequent epigenetic regulation of immune checkpoint expression 141. In addition, tryptophan, the precursor of serotonin, is metabolized to kynurenines by indoleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO), which are both neuroactive and immunomodulatory and implicated in neurodegenerative disorders and cancer 142. This also raises the question whether neuronal activity, with secretion of neuronal signaling molecules, can directly influence T-lymphocytes and other immune cells. The immunological environment in the CNS is unique, with a very specialized lymphatic system143, communication with unique immune populations in the skull bone marrow and various neural-immune interactions at the brain borders (for review, see 144 that may influence the effectiveness of immunotherapies. Accordingly, neuro-immuno-oncological interactions are probably quite different within the CNS and outside of it.
The nervous system not only regulates immune responses but also encodes them in an immunological engram in the brain that modifies subsequent immune function. Immune responses to challenges outside of the brain, such as in the gut, can be encoded by neurons in the insular cortex, and reactivation of the neurons activated by a particular immune challenge can recapitulate the immune response operant during the initial immune challenge145. This sort of immunological “memory” illustrates that the nervous system exerts profound regulatory control on the immune system, in ways that we are only just beginning to understand.
Another key demonstration of integration between neuronal activity and immune regulation of cancer growth derives from experiments performed using Nf1 optic glioma mice. In addition to light-induced, visual experience-dependent neuronal control of optic glioma initiation and progression, Nf1 mutation in neurons additionally increases basal action potential firing110. This increased excitability results in the production of midkine, a paracrine factor of the pleiotrophin family, which acts on T cells to secrete CCL4 and results in microglial secretion of CCL5, a key mitogen for glioma cell growth146,147. Consistent with diverse mechanisms underlying neuronal activity-dependent control of tumor biology, this basal hyperexcitability is mediated by Nf1 protein control of the Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 1 (HCN1), such that targeting of this channel with the anti-epileptic drug, lamotrigine, was sufficient to normalize midkine expression and suppress Nf1 optic glioma proliferation in vivo 110. Moreover, experience-dependent optic nerve activity-regulated shedding of NGLN367 appears to operate through mechanisms distinct from basal Nf1-regulated HCN1 channel-mediated activity-dependent expression of midkine. This illustrates how fine-tuned neuronal activity-dependent mechanisms may exert distinct effects on cancer biology in various contexts 146.
Further principles of neuron-immune cell-tumor cell crosstalk are also likely to come to light. How might drugs of neuroscience be leveraged to reduce the immune-suppressive microenvironment of solid tumors and improve immuno-oncology strategies (Figure 3)? Might targeting neurotransmitter or neuromodulator signaling influence the tumor immune microenvironment to promote anti-tumor immunity? Could targeting growth-promoting interactions between the nervous system and cancer slow down tumor growth to enable immune-based therapies to outpace cancer growth, facilitating tumor regression? Elucidating mechanisms of nervous system-immune system-cancer interactions may open an important new dimension in immuno-oncology strategies.
5. Effects of cancer and cancer therapies on the nervous system
Cancer therapies have the potential to limit the very mechanisms of neural homeostasis and plasticity that cancers depend on to grow. Unfortunately, the off-target effects of these therapies on normal neural processes (Figure 1F) can result in a syndrome of debilitating cognitive symptoms characterized by impaired attention, memory, speed of information processing, multitasking and executive function148, as well as neuropathies affecting sensory, motor and autonomic peripheral nerves (for review, see 149). Cancer therapies can result in tissue damage within the CNS – particularly insult to white matter and reduced volume of the hippocampus 150,151. Furthermore, cancer therapies can disrupt neural communication and network connectivity – which ultimately manifests as cognitive impairment152–154. The neurobiological underpinnings of cognitive impairment after cancer therapies (reviewed in 155 include radiation and chemotherapy-induced dysfunction of neural stem and precursor cell populations156–160, dysregulation of hippocampal neurogenesis 157,158,161,162 disruption of myelin homeostasis and plasticity32,160, and disruption of synaptic connectivity 163–165.
This fundamental understanding has led to therapeutic strategies targeting regeneration of neural stem and precursor cell populations that are showing promise in early clinical studies for cancer therapy-related cognitive impairment166–168. Given how cancers hijack the very same neural mechanisms and structures impaired after traditional cancer therapies, one wonders how the neurotoxicities of cancer therapy contribute to therapeutic efficacy. Understanding this may lead to more specific and less toxic cancer therapeutics.
In addition to neurotoxicity of therapies for cancer, cancer itself can change the nervous system: studies have demonstrated that, on a systemic level, mammary gland tumors can disrupt sleep and alter metabolism via altering a specific neuronal population of the CNS169. These effects can be observed in cancer patients, who exhibit clinical evidence for behavioral effects of cancer on sleep and appetite170,171. Furthermore, these interactions are bidirectional. On a more local level, tumor cell-and neuron-generated paracrine signaling can bidirectionally modulate peripheral sensory nerves, resulting in hypersensitivity, nerve sprouting and perineural invasion that contributes to cancer pain172,173,174. At the level of the whole patient, chronic stress can accelerate metastatic progression of breast and other peripheral cancers by elevating sympathetic signaling100,175. It will be important to fully understand these bidirectional interactions that seem to constitute a vicious cycle of nervous system-cancer interactions.
6. A framework for future clinical and preclinical development
Cancer neuroscience is a rapidly evolving field with emerging, exciting discoveries and the potential to influence and even fundamentally change oncological therapies176–178. Furthermore, these discoveries can feed back to inform basic neuroscience and developmental biology. A key challenge is to identify an optimal road to translation for neuroscience-instructed cancer therapies, a path which may be quite different from that of tumor cell-centric (cytotoxic or molecularly targeted) or anti-tumor immunological strategies. We will discuss trial-enabling aspects and develop a framework for implementing concepts from cancer neuroscience into clinical practice. An integrative framework that spans diverse preclinical and clinical-translational disciplines will be needed to make progress. In addition to neuroscientific and oncological expertise, development and adaptation of novel technologies will be needed (Figure 4). For both clinical trials and animal studies alike, it will be important to study pharmacological and non-pharmacological interventions over the disease course with a comprehensive clinical characterization using cancer-neuroscience-driven methodological frameworks (Figures 5).
Figure 5: Cancer neuroscience from bench-to-bedside & bedside-to-bench.
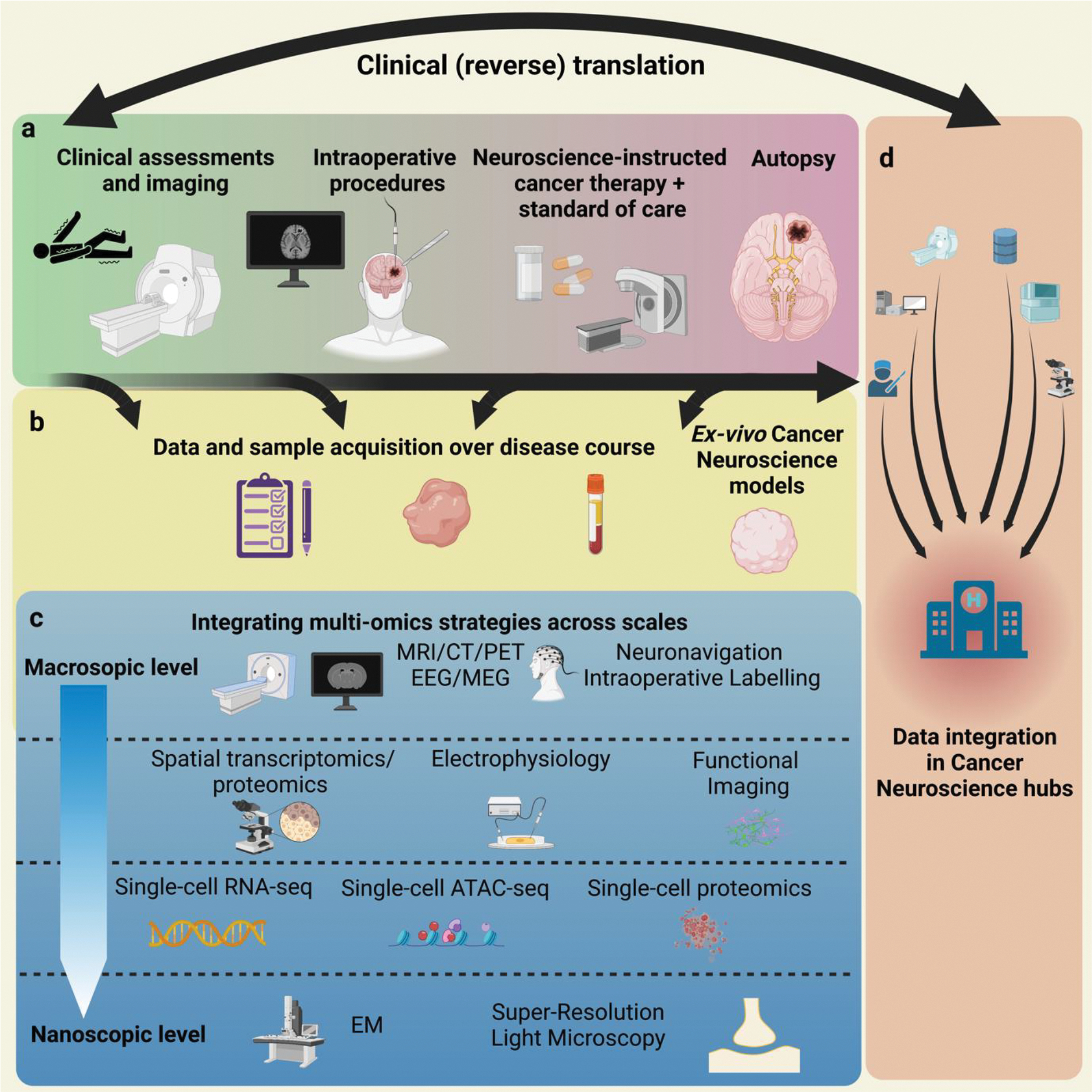
A framework for integrative cancer neuroscience at the intersection of preclinical and translational research. The figure provides an example for brain tumor studies, but can also serve as a blueprint for extracranial tumors.
Therapeutic opportunities
A fundamental conviction is that targeting the bidirectional neural-cancer crosstalk can slow tumor growth, or even reverse it, and at the same time preserve or reconstitute quality of life and neurological functioning. Considering the more than one hundred approved drugs in neurology, psychiatry and internal medicine that interfere with neurotransmitter and other neural signaling, it appears plausible that re-purposing of one or several of those for a given cancer (sub)type and stage can constitute a rapid road for clinical translation (Figure 3). Furthermore, drug development targeting neural-cancer signaling, and the functionality of the homotypic and heterotypic nervous system-cancer networks has started, albeit not on a large scale so far179. Prospective clinical trials have begun for multiple CNS and systemic cancer types176–178; for an overview of clinical trial numbers see180, some early phase trials have been published181 182, and further results are eagerly awaited. However, interference with the normal function of the CNS and PNS might limit dosing...
Targets and drugs
Figure 3 gives an overview of the principles of cancer neuroscience-related therapies that are tested in distinct indications for various tumor types. Conceptually, the field should prioritize strategies that allow a therapeutic window: since targeted mechanisms of neural-cancer interactions are frequently also relevant for the normal nervous system, a drug concentration needs to be selected that primarily affects cancer biology, or the particular strategy should be localized to affect the tumor microenvironment only (such as denervation strategies of non-brain tumors), always with careful monitoring of CNS and PNS side effects in patients. For children with malignant glioma, an inhibitor of ADAM10/17 (INCB7839) is being tested, because this inhibition blocks the secretion of NLGN369 (NCT04295759). Tumor-network-disconnecting strategies include: 1, the inhibition of gap junctions with meclofenamate in recurrent adult glioblastomas in combination with temozolomide chemotherapy (MecMeth/NOA-24; EudraCT 2021-000708-39); and 2, two trial initiatives in the US and Germany are underway to target glutamatergic neuron-to-glioma synapses with the approved anti-seizure drug perampanel, a non-competitive AMPAR inhibitor.
Outside the CNS, early phase clinical trials have shown that beta-blocker modulation of sympathetic neural signaling is safe in breast cancer patients and well-tolerated in combination with neoadjuvant chemotherapy181. Findings show that beta-blockers reduce biomarkers of breast cancer cell invasion while improving biomarkers of anti-cancer immunity 182,183.
Biomarkers and technologies
It will be crucial to understand whether a given neuroscience-instructed cancer therapy is hitting its target, leading to the desired effects on nervous system-cancer crosstalk, or not. If we do not validate this by accompanying biomarker research, we will not be able to link a positive study result to a desired target engagement. Likewise, we will not be able to interpret a negative result correctly: was the target pharmacologically missed? Or was it hit, but without a meaningful clinical effect?
Therefore, window-of-opportunity study concepts with investigation of molecular and structural tissue biomarkers of nervous system-cancer interactions in resected or (repetitively) biopsied tumor samples appear particularly meaningful for the first steps182,183, in addition to the development of imaging and electrophysiological biomarkers (Figure 5) Serial investigation over the disease course will enable the study of plasticity and evolution of multicellular neural-cancer networks.
Neural-cancer interactions have been chiefly characterized on a cellular and subcellular level using high-resolution light and electron microscopy as well as electrophysiological patch-clamp recordings (Figures 4 and 5). Although these approaches yield a precise readout it will be difficult to implement these methods on a larger scale for clinical trials. Therefore, a multi-omics approach from the macroscopic to the nanoscopic scale (Figure 5) will help define surrogate parameters that can be routinely employed for clinical trials. Additionally, such multi-omics approaches will extend our knowledge about the cancer neuroscience-related spatiotemporal cellular as well as molecular heterogeneity and plasticity of cancers. This will require the intensive collaboration of method developers, biologists, clinicians, and biostatisticians. In return, this approach yields the opportunity to methodologically advance not only the cancer neuroscience field but also the neuroscience and oncology fields.
Disease stage
Preclinical work should ideally address the question of whether an anti-cancer therapy that targets neural regulatory mechanisms is likely to be more efficient in the primary setting, in recurrence, or during further metastatic and invasive dissemination, which will define the ideal patient population included into a trial. Mounting data that particularly resistant, and recurrent tumors accumulate neuronal molecular signatures184 could speak for the latter. On the other hand, secondary evolution of heterogeneity, immune disturbances and general aspects of patient disposition as well as options for co-treatments and target evaluation including biological response assessment favor the newly diagnosed setting.
Outcome parameters
Another important question is the selection of the best efficacy measure, or outcome parameter(s). Targeting conserved neurodevelopmental pathways and structures that have a role in tumor:tumor cell /neuron:tumor cell contacts alike require careful neurological and neurocognitive as well as behavioural assessments exceeding the standard batteries in clinical studies. In addition to morphological, physiological and functional MR imaging and metabolic assessments, network analyses via EEG/MEG should be considered. Of note, for systemic (non-CNS) cancers advanced imaging should consider peripheral nerve MRI, which offers a sensitive novel tool for a potential effects of the cancer or neuroscience-instructed cancer therapy on sensory nerves 185. For any treatment, the first hurdle will be demonstration of a biological impact. This may require tumor or surrogate tissue (CSF/blood)-based diagnostics; i.e., demonstration of change in a preclinically defined biomarker of connectivity or network activity.
Trial design
The primary goal of the early trials should be a definition of the right patient population, which includes stage of the disease (see above), co-treatment as well as target identification and quantification. Biomarkers from the serum, CSF, and/or tumor tissue, potentially also imaging biomarkers may help to identify the patient subpopulation that is most likely to profit from a given neuroscience-instructed cancer therapy, similar to targeted therapy developments in other areas of oncology. As a starting point, phase 0 (window of opportunity) trials for neuroscience-instructed cancer therapy appear particularly meaningful, because they include the measurement of drug exposure and biological target engagement in resected tumor tissue. Standardization of clinical protocols will help accelerate the development of effective treatments.
Combination therapy
Therapies targeting neural-cancer interactions might work as monotherapies, but, more likely, they may be used as sensitizers to radiotherapy or chemotherapy (as shown for disconnection strategies of tumor cell networks81,89,178, or to work synergistically to benefit the efficacy and timeline of antitumor immunotherapies, as recently discussed 186. Targeting neural-cancer interactions may be a required component of effective combination therapy strategies. Therefore, it will be important to devise optimal combination partners, including concomitant cytotoxic, epigenetic or immunological therapies that together may achieve meaningful clinical effects.
Adapting and developing methodologies for preclinical and basic cancer neuroscience
Further advancing cancer neuroscience including clinical translation will require joint efforts in technology development and application in preclinical studies (Figure 4, 5) that define targetable mechanisms and test novel therapeutic strategies. Using functional imaging techniques such as calcium imaging or voltage imaging would help to decipher functional nervous system-cancer connectivity. Electrophysiological (e.g., microelectrode) arrays can be used to assess electric connectivity. Ideally, the correlation of functional imaging techniques and spatial transcriptomics would allow to identify the transcripts that are functionally relevant. The mapping of neuronal input can be achieved today with the help of evolving, elegant technologies, such as retrograde tracing with advanced viral vectors combined with tissue clearing and lightsheet microsocopy for large-volume imaging.
The analysis of tumor cells in the context of spatial patterns is helpful for the analysis of the tumor microenvironment, and certainly extends to cancer neuroscience-related questions. For example, it will be important to learn how proximity to neurons and neuronal processes influences cancer cell and tumor immunological features, and whether (how) cancer cell heterogeneity is related to specific neuronal features of cancer cells, and/or specific neural interactions. Furthermore, multiomic strategies that combine the methylome, transcriptome, translatome and proteome will further increase our knowledge of the molecular machinery associated with the neurobiology of cancer and might reveal novel therapeutic targets.
Finally, methods such as large-scale volume EM and super resolution light microscopy would allow analysis of neuron-tumor connections on a nanoscopic scale. Figure 5 shows a concept of how multi-omics strategies might be integrated across scales for future progress in the field of cancer neuroscience, including clinical and reverse translation.
Mapping the neural-tumor connectome by community-wide efforts
Cancer cell types and their neural partners will need to be classified based on their tumor biological function, connectivity, physiology, molecular signature, and morphology, in analogy to neuronal cell classification. This will require technological innovation, to integrate and understand tumor biological functions. Such initiatives could borrow from neuroscience (e.g. Allen Brain Atlas, neuromorpho.org, EM connectome data) and oncology (TCGA) consortia to adopt analogous frameworks for cancer neuroscience.
The complexity of such a clinical-translational framework requires a highly interdisciplinary infrastructural framework. Apart from various clinical disciplines that will need to work closely together (e.g. neurology, oncology, neurosurgery, neuropsychology, radiology, pathology), the close connection to the fields of neuroscience and basic cancer research will be an important element of the collaborative efforts in this direction. Therefore, we believe that establishing and interconnecting specialized cancer neuroscience hubs will be crucial to orchestrate such efforts (Figure 5).
7. Summary and Outlook
Research of the last few years has increasingly consolidated the new field of cancer neuroscience. The demonstrations of direct and indirect influences of the nervous system on cancer initiation, growth, dissemination, and treatment resistance significantly contribute to our understanding of cancer biology today. For every cancer entity investigated so far, cancer-promoting or (less frequently) cancer-inhibitory interactions with the CNS or PNS has been well documented. With more and more mechanisms from more and more cancers reported, the question arises whether nervous system-cancer interactions may someday be regarded as another general principle of cancer pathogenesis. In the next few years, we can expect exciting further discoveries in mechanisms known to be relevant for cancer neuroscience today (Fig. 1). In addition, a better understanding of the role of central and peripheral nervous system glial cells, and influence of innervation on other components of the tumor microenvironment will complement our body of knowledge and strengthen the therapeutic armamentarium.
The challenges are clear: we need to better map the nervous system-cancer interactome and connectome on multiple scales and levels. This is a key requirement to gain deeper insight into the complex world of interactions between the nervous system and specific cancer entities and stages. The future selection of the most promising neuroscience-instructed cancer therapies for individual patients will depend on this knowledge, particularly on our ability to conduct meaningful clinical trials, and potentially also on feasible biomarkers for nervous system-cancer interactions. Another key requirement for the future will be a joint development of collaborative networks, and of cross-disciplinary thinking, methodologies, and research strategies. Cancer neuroscience holds the promise to elucidate fundamentally new and therapeutically important insights into the pathobiology of many - if not all - cancers.
Acknowledgements:
This review article derives from and is written by the participants of a Cancer Neuroscience Think Tank meeting held jointly between the Cancer Neuroscience Programs of Heidelberg University, Stanford University and Harvard Medical School on July 18–19, 2022. The authors acknowledge Yvonne Yang for help with Figure 3 design. Initial drafts of figures were made with BioRender.com. The authors are grateful for support from the US National Institutes of Health (DP1NS111132, R01NS092597, P50CA165962, R01CA258384, U19CA264504), Deutsche Forschungsgemeinschaft (SFB 1389, UNITE Glioblastoma, project ID 404521405), Virginia and D.K. Ludwig Fund for Cancer Research, ChadTough Defeat DIPG Foundation, Sontag Foundation,
Footnotes
Competing Interests: M.M. holds equity in MapLight Therapeutics. M.M. and H.S.V. report the patent (US Patent #10,377,818) “Method for treating glioma”. F.W. and W.W. report the patent (WO2017020982A1) “Agents for use in the treatment of glioma.” F.W. is co-founder of DC Europa Ltd (a company trading under the name Divide & Conquer) that is developing new medicines for the treatment of glioma. Divide & Conquer also provides research funding to F.W.’s lab under a research collaboration agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boilly B, Faulkner S, Jobling P, and Hondermarck H (2017). Nerve Dependence: From Regeneration to Cancer. Cancer Cell 31, 342–354. 10.1016/j.ccell.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, and Brockes JP (2012). Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci 35, 691–699. 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, Frenette PS, Garzia L, Gutmann DH, Hanahan D, et al. (2020). Roadmap for the Emerging Field of Cancer Neuroscience. Cell 181, 219–222. 10.1016/j.cell.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silbereis JC, Pochareddy S, Zhu Y, Li M, and Sestan N (2016). The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 89, 248–268. 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funfschilling U, Supplie L, Mahad D, Boretius S, Saab A, Edgar J, Brinkmann B, Kassmann C, Tzvetanova I, Mobius W, et al. (2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521. 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley AF, and Stämpeli R (1949). Evidence for saltatory conduction in peripheral myelinated nerve fibres. The Journal of Physiology 108, 315–339. 10.1113/jphysiol.1949.sp004335. [DOI] [PubMed] [Google Scholar]
- 7.Spitzer NC (2006). Electrical activity in early neuronal development. Nature 444, 707–712. 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 8.Bittman K, Owens DF, Kriegstein AR, and LoTurco JJ (1997). Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci 17, 7037–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg DA, Belnoue L, Song H, and Simon A (2013). Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140, 2548–2561. 10.1242/dev.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtaka-Maruyama C, Okamoto M, Endo K, Oshima M, Kaneko N, Yura K, Okado H, Miyata T, and Maeda N (2018). Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science 360, 313–317. 10.1126/science.aar2866. [DOI] [PubMed] [Google Scholar]
- 11.Ming G, Henley J, Tessier-Lavigne M, Song H, and Poo M (2001). Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29, 441–452. 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 12.Catalano SM, and Shatz CJ (1998). Activity-dependent cortical target selection by thalamic axons. Science 281, 559–562. 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- 13.Dantzker JL, and Callaway EM (1998). The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity. J Neurosci 18, 4145–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marins M, Xavier AL, Viana NB, Fortes FS, Froes MM, and Menezes JR (2009). Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev Neurobiol 69, 715–730. 10.1002/dneu.20737. [DOI] [PubMed] [Google Scholar]
- 15.Peinado A, Yuste R, and Katz LC (1993). Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10, 103–114. 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- 16.Picken Bahrey HL, and Moody WJ (2003). Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol 89, 1761–1773. 10.1152/jn.00972.2002. [DOI] [PubMed] [Google Scholar]
- 17.Blankenship AG, and Feller MB (2010). Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nature reviews. Neuroscience 11, 18–29. 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corlew R, Bosma MM, and Moody WJ (2004). Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol 560, 377–390. 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajar BT, Phi NT, Isaacman-Beck J, Reichl J, Randhawa H, and Akin O (2022). A discrete neuronal population coordinates brain-wide developmental activity. Nature 602, 639–646. 10.1038/s41586-022-04406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz LC, and Shatz CJ (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 21.Kirkby LA, Sack GS, Firl A, and Feller MB (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144. 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojeda J, and Avila A (2019). Early Actions of Neurotransmitters During Cortex Development and Maturation of Reprogrammed Neurons. Front Synaptic Neurosci 11, 33. 10.3389/fnsyn.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, et al. (2008). Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature 451, 460–464. 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 24.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304. 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paez-Gonzalez P, Asrican B, Rodriguez E, and Kuo CT (2014). Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci 17, 934–942. 10.1038/nn.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul A, Chaker Z, and Doetsch F (2017). Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356, 1383–1386. 10.1126/science.aal3839. [DOI] [PubMed] [Google Scholar]
- 27.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, and Malenka RC (2004). Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552. S0896627304002661 [pii]. [DOI] [PubMed] [Google Scholar]
- 28.Tozuka Y, Fukuda S, Namba T, Seki T, and Hisatsune T (2005). GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47, 803–815. 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, et al. (2018). Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nature communications 9, 306. 10.1038/s41467-017-02719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes EG, Orthmann-Murphy JL, Langseth AJ, and Bergles DE (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci 21, 696–706. 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, and Richardson WD (2014). Motor skill learning requires active central myelination. Science 346, 318–322. 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Pasca SP, et al. (2019). Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 103, 250–265 e258. 10.1016/j.neuron.2019.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, and Frankland PW (2020). Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 105, 150–164 e156. 10.1016/j.neuron.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan S, Mayoral SR, Choi HS, Chan JR, and Kheirbek MA (2020). Preservation of a remote fear memory requires new myelin formation. Nat Neurosci 23, 487–499. 10.1038/s41593-019-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makinodan M, Rosen K, Ito S, and Corfas G (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360. 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Dietz K, DeLoyht J, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo M, Dietz D, Nestler E, et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15, 1621–1623. 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang SM, Michel K, Jokhi V, Nedivi E, and Arlotta P (2020). Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370. 10.1126/science.abd2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pajevic S, Basser P, and Fields R (2014). Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147. 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noori R, Park D, Griffiths JD, Bells S, Frankland PW, Mabbott D, and Lefebvre J (2020). Activity-dependent myelination: A glial mechanism of oscillatory self-organization in large-scale brain networks. Proc Natl Acad Sci U S A 117, 13227–13237. 10.1073/pnas.1916646117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergles DE, Roberts JD, Somogyi P, and Jahr CE (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 41.Lin SC, and Bergles DE (2004). Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci 7, 24–32. 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- 42.Etxeberria A, Mangin J, Aguirre A, and Gallo V (2010). Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci 13, 287–289. 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mount CW, Yalcin B, Cunliffe-Koehler K, Sundaresh S, and Monje M (2019). Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. Elife 8. 10.7554/eLife.49291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautier H, Lundgaard I, James F, Lao-Peregrin C, Franklin RJM, and Karadottir RT (2015). Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Glia 63, E18–E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortiz FC, Habermacher C, Graciarena M, Houry PY, Nishiyama A, Nait Oumesmar B, and Angulo MC (2019). Neuronal activity in vivo enhances functional myelin repair. JCI Insight 5. 10.1172/jci.insight.123434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zonouzi M, Scafidi J, Li P, McEllin B, Edwards J, Dupree JL, Harvey L, Sun D, Hubner CA, Cull-Candy SG, et al. (2015). GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat Neurosci 18, 674–682. 10.1038/nn.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poplawski GHD, Kawaguchi R, Van Niekerk E, Lu P, Mehta N, Canete P, Lie R, Dragatsis I, Meves JM, Zheng B, et al. (2020). Injured adult neurons regress to an embryonic transcriptional growth state. Nature 581, 77–82. 10.1038/s41586-020-2200-5. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan L, Chow BW, and Gu C (2020). Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nature reviews. Neuroscience 21, 416–432. 10.1038/s41583-020-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krimer LS, Muly EC 3rd, Williams GV, and Goldman-Rakic PS (1998). Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci 1, 286–289. 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- 50.Pulido RS, Munji RN, Chan TC, Quirk CR, Weiner GA, Weger BD, Rossi MJ, Elmsaouri S, Malfavon M, Deng A, et al. (2020). Neuronal Activity Regulates Blood-Brain Barrier Efflux Transport through Endothelial Circadian Genes. Neuron 108, 937–952 e937. 10.1016/j.neuron.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, and Brockes JP (2007). Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318, 772–777. 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, and Hoffman MP (2010). Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329, 1645–1647. 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, et al. (2015). Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Developmental cell 34, 387–399. 10.1016/j.devcel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kreipke RE, and Birren SJ (2015). Innervating sympathetic neurons regulate heart size and the timing of cardiomyocyte cell cycle withdrawal. J Physiol 593, 5057–5073. 10.1113/JP270917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S, Goncalves WA, Shwartz Y, Fast EM, Su Y, et al. (2020). Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 10.1038/s41586-020-1935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brownell I, Guevara E, Bai CB, Loomis CA, and Joyner AL (2011). Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565. 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, and Wong SY (2015). Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400–412. 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puzan M, Hosic S, Ghio C, and Koppes A (2018). Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci Rep 8, 6313. 10.1038/s41598-018-24768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, and Frenette PS (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421. 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, and Nakauchi H (2011). Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158. 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 61.Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, and Hoffman MP (2013). Parasympathetic stimulation improves epithelial organ regeneration. Nature communications 4, 1494. 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiller M, Ben-Shaanan TL, and Rolls A (2021). Neuronal regulation of immunity: why, how and where? Nat Rev Immunol 21, 20–36. 10.1038/s41577-020-0387-1. [DOI] [PubMed] [Google Scholar]
- 63.Carmeliet P (2003). Angiogenesis in health and disease. Nat Med 9, 653–660. 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 64.Schiller M, Azulay-Debby H, Boshnak N, Elyahu Y, Korin B, Ben-Shaanan TL, Koren T, Krot M, Hakim F, and Rolls A (2021). Optogenetic activation of local colonic sympathetic innervations attenuates colitis by limiting immune cell extravasation. Immunity 54, 1022–1036 e1028. 10.1016/j.immuni.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scherer HJ (1938). Structural development in gliomas. Am. J. Cancer 34, 333–351. [Google Scholar]
- 66.Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, et al. (2015). Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 161, 803–816. 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan Y, Hysinger JD, Barron T, Schindler NF, Cobb O, Guo X, Yalcin B, Anastasaki C, Mulinyawe SB, Ponnuswami A, et al. (2021). NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 594, 277–282. 10.1038/s41586-021-03580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor KR, Barron T, Zhang H, Hui A, Hartmann G, Ni L, Venkatesh HS, Du P, Mancusi R, Yalçin B, et al. (2021). Glioma synapses recruit mechanisms of adaptive plasticity. bioRxiv, 2021.2011.2004.467325. 10.1101/2021.11.04.467325. [DOI] [Google Scholar]
- 69.Venkatesh HS, Tam LT, Woo PJ, Lennon J, Nagaraja S, Gillespie SM, Ni J, Duveau DY, Morris PJ, Zhao JJ, et al. (2017). Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537. 10.1038/nature24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P, Wang W, Liu R, Lyu J, Zhang L, Li B, Qiu B, Tian A, Jiang W, Ying H, et al. (2022). Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature. 10.1038/s41586-022-04719-9. [DOI] [PubMed] [Google Scholar]
- 71.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, et al. (2019). Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545. 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Korber C, Kardorff M, Ratliff M, Xie R, et al. (2019). Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538. 10.1038/s41586-019-1564-x. [DOI] [PubMed] [Google Scholar]
- 73.Venkataramani VTK, Frank Winkler(2022). Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 185, 2899–2917 e2831. 10.1016/j.cell.2022.06.054. [DOI] [PubMed] [Google Scholar]
- 74.Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galvan JA, Robinson HPC, Zlobec I, et al. (2019). Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531. 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, and Sontheimer H (2011). Glutamate release by primary brain tumors induces epileptic activity. Nat Med 17, 1269–1274. 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell SL, Robel S, Cuddapah VA, Robert S, Buckingham SC, Kahle KT, and Sontheimer H (2015). GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia 63, 23–36. 10.1002/glia.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, et al. (2017). Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20, 396–405. 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu K, Lin CJ, Hatcher A, Lozzi B, Kong K, Huang-Hobbs E, Cheng YT, Beechar VB, Zhu W, Zhang Y, et al. (2020). PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature. 10.1038/s41586-020-1952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krishna S, Choudhury A, Seo K, Ni L, Kakaizada S, Lee A, Aabedi A, Cao C, Sudharshan R, Egladyous A, et al. (2021). Glioblastoma remodeling of neural circuits in the human brain decreases survival. bioRxiv. [Google Scholar]
- 80.Numan T, Breedt LC, Maciel B, Kulik SD, Derks J, Schoonheim MM, Klein M, de Witt Hamer PC, Miller JJ, Gerstner ER, et al. (2022). Regional healthy brain activity, glioma occurrence and symptomatology. Brain 145, 3654–3665. 10.1093/brain/awac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, et al. (2015). Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98. 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 82.Jung E, Osswald M, Blaes J, Wiestler B, Sahm F, Schmenger T, Solecki G, Deumelandt K, Kurz FT, Xie R, et al. (2017). Tweety-Homolog 1 Drives Brain Colonization of Gliomas. J Neurosci 37, 6837–6850. 10.1523/JNEUROSCI.3532-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gritsenko PG, Atlasy N, Dieteren CEJ, Navis AC, Venhuizen JH, Veelken C, Schubert D, Acker-Palmer A, Westerman BA, Wurdinger T, et al. (2020). p120-catenin-dependent collective brain infiltration by glioma cell networks. Nature cell biology 22, 97–107. 10.1038/s41556-019-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venkataramani V, Yang Y, Schubert MC, Reyhan E, Tetzlaff SK, Wissmann N, Botz M, Soyka SJ, Beretta CA, Pramatarov RL, et al. (2022). Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 185, 2899–2917 e2831. 10.1016/j.cell.2022.06.054. [DOI] [PubMed] [Google Scholar]
- 85.Nagaraja S, Quezada MA, Gillespie SM, Arzt M, Lennon JJ, Woo PJ, Hovestadt V, Kambhampati M, Filbin MG, Suva ML, et al. (2019). Histone Variant and Cell Context Determine H3K27M Reprogramming of the Enhancer Landscape and Oncogenic State. Molecular cell 76, 965–980 e912. 10.1016/j.molcel.2019.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jung E, Osswald M, Ratliff M, Dogan H, Xie R, Weil S, Hoffmann DC, Kurz FT, Kessler T, Heiland S, et al. (2021). Tumor cell plasticity, heterogeneity, and resistance in crucial microenvironmental niches in glioma. Nature communications 12, 1014. 10.1038/s41467-021-21117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, Reich B, Cohen-Gould L, Storaska A, Nakayama Y, et al. (2019). Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep 26, 3203–3211 e3205. 10.1016/j.celrep.2019.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weil S, Osswald M, Solecki G, Grosch J, Jung E, Lemke D, Ratliff M, Hanggi D, Wick W, and Winkler F (2017). Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol 19, 1316–1326. 10.1093/neuonc/nox070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneider M, Potthoff AL, Evert BO, Dicks M, Ehrentraut D, Dolf A, Schmidt ENC, Schafer N, Borger V, Pietsch T, et al. (2021). Inhibition of Intercellular Cytosolic Traffic via Gap Junctions Reinforces Lomustine-Induced Toxicity in Glioblastoma Independent of MGMT Promoter Methylation Status. Pharmaceuticals (Basel) 14. 10.3390/ph14030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, Saez JC, and Naus CC (2014). Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem 289, 1345–1354. 10.1074/jbc.M113.508390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K, et al. (2016). Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498. 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hausmann D, Hoffmann DC, Venkataramani V, Jung E, Horschitz S, Tetzlaff SK, Jabali A, Hai L, Kessler T, Azorin DD, et al. (2023). Autonomous rhythmic activity in glioma networks drives brain tumour growth. Nature 613, 179–186. 10.1038/s41586-022-05520-4. [DOI] [PubMed] [Google Scholar]
- 93.Young HH (1897). On the Presence of Nerves in Tumors and of Other Structures in Them as Revealed by a Modification of Ehrlich’s Method of “Vital Staining” with Methylene Blue. J Exp Med 2, 1–12. 10.1084/jem.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Batsakis JG (1985). Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 94, 426–427. [PubMed] [Google Scholar]
- 95.Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, et al. (2020). Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454. 10.1038/s41586-020-1996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, and Katoh H (1997). Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol 65, 164–170. . [DOI] [PubMed] [Google Scholar]
- 97.Villers A, McNeal JE, Redwine EA, Freiha FS, and Stamey TA (1989). The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol 142, 763–768. 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Z, Lei A, Xu L, Chen L, Chen Y, Zhang X, Gao Y, Yang X, Zhang M, and Cao Y (2017). Similarity in gene-regulatory networks suggests that cancer cells share characteristics of embryonic neural cells. J Biol Chem 292, 12842–12859. 10.1074/jbc.M117.785865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, and Frenette PS (2013). Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361. 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 100.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, et al. (2010). The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70, 7042–7052. 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12, 939–944. 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 102.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, et al. (2017). Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 31, 21–34. 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, et al. (2014). Denervation suppresses gastric tumorigenesis. Science translational medicine 6, 250ra115. 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, et al. (2018). Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov 8, 1458–1473. 10.1158/2159-8290.CD-18-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K, Kaneko R, Yanagawa Y, Kobayashi K, and Ochiya T (2019). Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci 22, 1289–1305. 10.1038/s41593-019-0430-3. [DOI] [PubMed] [Google Scholar]
- 106.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, et al. (2018). beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 33, 75–90 e77. 10.1016/j.ccell.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, and Davis BM (2016). Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A 113, 3078–3083. 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walker AK, Martelli D, Ziegler AI, Lambert GW, Phillips SE, Hill SJ, McAllen RM, and Sloan EK (2019). Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology 99, 191–195. 10.1016/j.psyneuen.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 109.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, et al. (2016). Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nature communications 7, 10634. 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anastasaki C, Mo J, Chen JK, Chatterjee J, Pan Y, Scheaffer SM, Cobb O, Monje M, Le LQ, and Gutmann DH (2022). Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nature communications 13, 2785. 10.1038/s41467-022-30466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He S, Chen CH, Chernichenko N, He S, Bakst RL, Barajas F, Deborde S, Allen PJ, Vakiani E, Yu Z, and Wong RJ (2014). GFRalpha1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci U S A 111, E2008–2017. 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, Buchler MW, and Friess H (2006). The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg 244, 274–281. 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, and Anand P (2006). The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett 399, 51–56. 10.1016/j.neulet.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 114.Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, and Buchler MW (1999). Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol 17, 2419–2428. 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 115.Au CW, Siu MK, Liao X, Wong ES, Ngan HY, Tam KF, Chan DC, Chan QK, and Cheung AN (2009). Tyrosine kinase B receptor and BDNF expression in ovarian cancers - Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett 281, 151–161. 10.1016/j.canlet.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 116.Pearse RN, Swendeman SL, Li Y, Rafii D, and Hempstead BL (2005). A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood 105, 4429–4436. 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- 117.Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, and Frenette PS (2017). Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326. 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Banh RS, Biancur DE, Yamamoto K, Sohn ASW, Walters B, Kuljanin M, Gikandi A, Wang H, Mancias JD, Schneider RJ, et al. (2020). Neurons Release Serine to Support mRNA Translation in Pancreatic Cancer. Cell 183, 1202–1218 e1225. 10.1016/j.cell.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, et al. (2008). Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14, 7593–7603. 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 120.Mauffrey P, Tchitchek N, Barroca V, Bemelmans AP, Firlej V, Allory Y, Romeo PH, and Magnon C (2019). Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678. 10.1038/s41586-019-1219-y. [DOI] [PubMed] [Google Scholar]
- 121.Payne SL, Ram P, Srinivasan DH, Le TT, Levin M, and Oudin MJ (2022). Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer. EBioMedicine 75, 103767. 10.1016/j.ebiom.2021.103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rahrmann EP, Shorthouse D, Jassim A, Hu LP, Ortiz M, Mahler-Araujo B, Vogel P, Paez-Ribes M, Fatemi A, Hannon GJ, et al. (2022). The NALCN channel regulates metastasis and nonmalignant cell dissemination. Nat Genet 54, 1827–1838. 10.1038/s41588-022-01182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Creed SJ, Le CP, Hassan M, Pon CK, Albold S, Chan KT, Berginski ME, Huang Z, Bear JE, Lane JR, et al. (2015). beta2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast cancer research : BCR 17, 145. 10.1186/s13058-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pascual G, Dominguez D, Elosua-Bayes M, Beckedorff F, Laudanna C, Bigas C, Douillet D, Greco C, Symeonidi A, Hernandez I, et al. (2021). Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 599, 485–490. 10.1038/s41586-021-04075-0. [DOI] [PubMed] [Google Scholar]
- 125.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, and Tracey KJ (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki K, Hayano Y, Nakai A, Furuta F, and Noda M (2016). Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med 213, 2567–2574. 10.1084/jem.20160723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mendez-Ferrer S, Lucas D, Battista M, and Frenette PS (2008). Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447. 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 128.Devi S, Alexandre YO, Loi JK, Gillis R, Ghazanfari N, Creed SJ, Holz LE, Shackleford D, Mackay LK, Heath WR, et al. (2021). Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230 e1217. 10.1016/j.immuni.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 129.Poller WC, Downey J, Mooslechner AA, Khan N, Li L, Chan CT, McAlpine CS, Xu C, Kahles F, He S, et al. (2022). Brain motor and fear circuits regulate leukocytes during acute stress. Nature. 10.1038/s41586-022-04890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M, et al. (2019). Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med 25, 1428–1441. 10.1038/s41591-019-0566-4. [DOI] [PubMed] [Google Scholar]
- 131.Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, and Repasky EA (2019). beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129, 5537–5552. 10.1172/JCI129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang SH, Zhu LL, Zhang M, Li RK, Yang Q, Yan JY, Zhang C, Yang JY, Dong FY, Dai M, et al. (2019). GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca(2+) signalling in a GABA-independent manner. Gut 68, 1994–2006. 10.1136/gutjnl-2018-317479. [DOI] [PubMed] [Google Scholar]
- 133.Chen M, Qiao G, Hylander BL, Mohammadpour H, Wang XY, Subjeck JR, Singh AK, and Repasky EA (2020). Adrenergic stress constrains the development of anti-tumor immunity and abscopal responses following local radiation. Nature communications 11, 1821. 10.1038/s41467-020-15676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nissen MD, Sloan EK, and Mattarollo SR (2018). beta-Adrenergic Signaling Impairs Antitumor CD8(+) T-cell Responses to B-cell Lymphoma Immunotherapy. Cancer Immunol Res 6, 98–109. 10.1158/2326-6066.CIR-17-0401. [DOI] [PubMed] [Google Scholar]
- 135.Qiao G, Chen M, Mohammadpour H, MacDonald CR, Bucsek MJ, Hylander BL, Barbi JJ, and Repasky EA (2021). Chronic Adrenergic Stress Contributes to Metabolic Dysfunction and an Exhausted Phenotype in T Cells in the Tumor Microenvironment. Cancer Immunol Res 9, 651–664. 10.1158/2326-6066.CIR-20-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Balood M, Ahmadi M, Eichwald T, Ahmadi A, Majdoubi A, Roversi K, Roversi K, Lucido CT, Restaino AC, Huang S, et al. (2022). Nociceptor neurons affect cancer immunosurveillance. Nature 611, 405–412. 10.1038/s41586-022-05374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu XR, Xiao Q, Hong YC, Liu YH, Liu Y, and Tu J (2021). Activation of dopaminergic VTA inputs to the mPFC ameliorates chronic stress-induced breast tumor progression. CNS Neurosci Ther 27, 206–219. 10.1111/cns.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ben-Shaanan TL, Schiller M, Azulay-Debby H, Korin B, Boshnak N, Koren T, Krot M, Shakya J, Rahat MA, Hakim F, and Rolls A (2018). Modulation of anti-tumor immunity by the brain’s reward system. Nature communications 9, 2723. 10.1038/s41467-018-05283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, and Schell TD (2018). Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205. 10.1080/2162402X.2017.1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, Nakano R, Hatae R, Menzies RJ, Sonomura K, et al. (2021). B cell-derived GABA elicits IL-10(+) macrophages to limit anti-tumour immunity. Nature 599, 471–476. 10.1038/s41586-021-04082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schneider MA, Heeb L, Beffinger MM, Pantelyushin S, Linecker M, Roth L, Lehmann K, Ungethum U, Kobold S, Graf R, et al. (2021). Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Science translational medicine 13, eabc8188. 10.1126/scitranslmed.abc8188. [DOI] [PubMed] [Google Scholar]
- 142.Platten M, Nollen EAA, Rohrig UF, Fallarino F, and Opitz CA (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 18, 379–401. 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 143.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rustenhoven J, and Kipnis J (2022). Brain borders at the central stage of neuroimmunology. Nature 612, 417–429. 10.1038/s41586-022-05474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, Azulay-Debby H, Zalayat I, Avishai E, Hajjo H, et al. (2021). Insular cortex neurons encode and retrieve specific immune responses. Cell 184, 5902–5915 e5917. 10.1016/j.cell.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 146.Guo X, Pan Y, Xiong M, Sanapala S, Anastasaki C, Cobb O, Dahiya S, and Gutmann DH (2020). Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nature communications 11, 2177. 10.1038/s41467-020-15770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Solga AC, Pong WW, Kim KY, Cimino PJ, Toonen JA, Walker J, Wylie T, Magrini V, Griffith M, Griffith OL, et al. (2015). RNA Sequencing of Tumor-Associated Microglia Reveals Ccl5 as a Stromal Chemokine Critical for Neurofibromatosis-1 Glioma Growth. Neoplasia 17, 776–788. 10.1016/j.neo.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wefel JS, Kayl AE, and Meyers CA (2004). Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer 90, 1691–1696. 10.1038/sj.bjc.6601772 6601772 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Staff NP, Grisold A, Grisold W, and Windebank AJ (2017). Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol 81, 772–781. 10.1002/ana.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kesler SR, Sleurs C, McDonald BC, Deprez S, van der Plas E, and Nieman BJ (2021). Brain Imaging in Pediatric Cancer Survivors: Correlates of Cognitive Impairment. J Clin Oncol 39, 1775–1785. 10.1200/JCO.20.02315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McDonald BC (2021). Structural Neuroimaging Findings Related to Adult Non-CNS Cancer and Treatment: Review, Integration, and Implications for Treatment of Cognitive Dysfunction. Neurotherapeutics 18, 792–810. 10.1007/s13311-021-01096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bells S, Lefebvre J, Prescott SA, Dockstader C, Bouffet E, Skocic J, Laughlin S, and Mabbott DJ (2017). Changes in White Matter Microstructure Impact Cognition by Disrupting the Ability of Neural Assemblies to Synchronize. J Neurosci 37, 8227–8238. 10.1523/JNEUROSCI.0560-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oyefiade A, Moxon-Emre I, Beera K, Bouffet E, Taylor M, Ramaswamy V, Laughlin S, Skocic J, and Mabbott D (2022). Structural connectivity and intelligence in brain-injured children. Neuropsychologia 173, 108285. 10.1016/j.neuropsychologia.2022.108285. [DOI] [PubMed] [Google Scholar]
- 154.Gauvreau S, Lefebvre J, Bells S, Laughlin S, Bouffet E, and Mabbott DJ (2019). Disrupted network connectivity in pediatric brain tumor survivors is a signature of injury. J Comp Neurol 527, 2896–2909. 10.1002/cne.24717. [DOI] [PubMed] [Google Scholar]
- 155.Gibson EM, and Monje M (2021). Microglia in Cancer Therapy-Related Cognitive Impairment. Trends Neurosci 44, 441–451. 10.1016/j.tins.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parent JM, Tada E, Fike JR, and Lowenstein DH (1999). Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci 19, 4508–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Monje ML, Mizumatsu S, Fike JR, and Palmer TD (2002). Irradiation induces neural precursor-cell dysfunction. Nat Med 8, 955–962. 10.1038/nm749 nm749 [pii]. [DOI] [PubMed] [Google Scholar]
- 158.Monje ML, Toda H, and Palmer TD (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765. 10.1126/science.1088417 1088417 [pii]. [DOI] [PubMed] [Google Scholar]
- 159.Dietrich J, Han R, Yang Y, Mayer-Proschel M, and Noble M (2006). CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol 5, 22. jbiol50 [pii] 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, et al. (2019). Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 176, 43–55 e13. 10.1016/j.cell.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, and Palmer TD (2007). Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol 62, 515–520. 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 162.Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FS, Koolhaas JM, and Buwalda B (2009). Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res 201, 279–284. 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 163.Andres AL, Gong X, Di K, and Bota DA (2014). Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp Neurol 255, 137–144. 10.1016/j.expneurol.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parihar VK, and Limoli CL (2013). Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A 110, 12822–12827. 10.1073/pnas.1307301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kempf SJ, Buratovic S, von Toerne C, Moertl S, Stenerlow B, Hauck SM, Atkinson MJ, Eriksson P, and Tapio S (2014). Ionising radiation immediately impairs synaptic plasticity-associated cytoskeletal signalling pathways in HT22 cells and in mouse brain: an in vitro/in vivo comparison study. PLoS One 9, e110464. 10.1371/journal.pone.0110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ayoub R, Ruddy RM, Cox E, Oyefiade A, Derkach D, Laughlin S, Ades-Aron B, Shirzadi Z, Fieremans E, MacIntosh BJ, et al. (2020). Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin. Nat Med 26, 1285–1294. 10.1038/s41591-020-0985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mabbott DJ, Monsalves E, Spiegler BJ, Bartels U, Janzen L, Guger S, Laperriere N, Andrews N, and Bouffet E (2011). Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 10.1002/cncr.26127. [DOI] [PubMed] [Google Scholar]
- 168.Riggs L, Piscione J, Laughlin S, Cunningham T, Timmons BW, Courneya KS, Bartels U, Skocic J, de Medeiros C, Liu F, et al. (2017). Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol 19, 440–450. 10.1093/neuonc/now177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Borniger JC, Walker Ii WH,Surbhi, Emmer KM, Zhang N, Zalenski AA, Muscarella SL, Fitzgerald JA, Smith AN, Braam CJ, et al. (2018). A Role for Hypocretin/Orexin in Metabolic and Sleep Abnormalities in a Mouse Model of Non-metastatic Breast Cancer. Cell Metab 28, 118–129 e115. 10.1016/j.cmet.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, and Spiegel D (2014). Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep 37, 837–842. 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ezeoke CC, and Morley JE (2015). Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle 6, 287–302. 10.1002/jcsm.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schweizerhof M, Stosser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW, Brugger S, et al. (2009). Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med 15, 802–807. 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 173.Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, et al. (2015). A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer Cell 27, 780–796. 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hirth M, Gandla J, Hoper C, Gaida MM, Agarwal N, Simonetti M, Demir A, Xie Y, Weiss C, Michalski CW, et al. (2020). CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 159, 665–681 e613. 10.1053/j.gastro.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 175.Moreno-Smith M, Lutgendorf SK, and Sood AK (2010). Impact of stress on cancer metastasis. Future Oncol 6, 1863–1881. 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shi DD, Guo JA, Hoffman HI, Su J, Mino-Kenudson M, Barth JL, Schenkel JM, Loeffler JS, Shih HA, Hong TS, et al. (2022). Therapeutic avenues for cancer neuroscience: translational frontiers and clinical opportunities. Lancet Oncol 23, e62–e74. 10.1016/S1470-2045(21)00596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Demir IE, Mota Reyes C, Alrawashdeh W, Ceyhan GO, Deborde S, Friess H, Gorgulu K, Istvanffy R, Jungwirth D, Kuner R, et al. (2021). Future directions in preclinical and translational cancer neuroscience research. Nat Cancer 1, 1027–1031. 10.1038/s43018-020-00146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Venkataramani V, Schneider M, Giordano FA, Kuner T, Wick W, Herrlinger U, and Winkler F (2022). Disconnecting multicellular networks in brain tumours. Nat Rev Cancer 22, 481–491. 10.1038/s41568-022-00475-0. [DOI] [PubMed] [Google Scholar]
- 179.Dolgin E (2020). Cancer-neuronal crosstalk and the startups working to silence it. Nature biotechnology 38, 115–117. 10.1038/s41587-020-0411-9. [DOI] [PubMed] [Google Scholar]
- 180.Pan C, and Winkler F (2022). Insights and opportunities at the crossroads of cancer and neuroscience. Nature cell biology 24, 1454–1460. 10.1038/s41556-022-00978-w. [DOI] [PubMed] [Google Scholar]
- 181.Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JB, Henderson MA, Nightingale SS, Ho KM, Myles PS, et al. (2020). Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin Cancer Res 26, 1803–1811. 10.1158/1078-0432.CCR-19-2641. [DOI] [PubMed] [Google Scholar]
- 182.Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, et al. (2017). Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin Cancer Res 23, 4651–4661. 10.1158/1078-0432.CCR-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JB, Henderson MA, Nightingale SS, Ho KM, Myles PS, et al. (2019). Pre-operative beta-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a Phase II randomized trial. Clin Cancer Res. 10.1158/1078-0432.CCR-19-2641. [DOI] [PubMed] [Google Scholar]
- 184.Varn FS, Johnson KC, Martinek J, Huse JT, Nasrallah MP, Wesseling P, Cooper LAD, Malta TM, Wade TE, Sabedot TS, et al. (2022). Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell. 10.1016/j.cell.2022.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jende JME, Groener JB, Kender Z, Rother C, Hahn A, Hilgenfeld T, Juerchott A, Preisner F, Heiland S, Kopf S, et al. (2020). Structural Nerve Remodeling at 3-T MR Neurography Differs between Painful and Painless Diabetic Polyneuropathy in Type 1 or 2 Diabetes. Radiology 294, 405–414. 10.1148/radiol.2019191347. [DOI] [PubMed] [Google Scholar]
- 186.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, Richards RM, Jiang L, Barsan V, Mancusi R, et al. (2022). GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


