Advancing populational age and presence of cases with increased perioperative risk result in greater interest in transcatheter aortic valve replacement (TAVI) procedures. Among them, a number of patients present as a reoperative cases with previously surgically implanted bioprostheses. They require heart-team assessment with thorough consideration of treatment options. At our department, we performed 557 TAVI procedures, including 25 (4.5%) valve-in-valve cases.
Many products were validated for transcatheter valve-in valve replacement. However, some prostheses are implanted in such a procedure incidentally. Although the use of the Boston Accurate Neo prosthesis for this purpose was reported [1, 2], so far there is no evidence on implanting the valve in the Medtronic Mosaic 19 bioprosthesis.
An 82-year-old female patient, with a history of bioprosthetic valve implantation at the age of 72 (Medtronic Mosaic Ultra Porcine Heart Valve 19) was referred urgently to the heart team due to advanced prosthetic degeneration. The patient had severe dyspnea and occasional chest pain; she was hemodynamically unstable and required urgent qualification and intervention. Transthoracic echocardiography was performed and revealed significant degeneration of the previously implanted valve (Vmax: 3.9 m/s; Pmax 60 mm Hg; Pmean 40 mm Hg, severe regurgitation). Other echocardiographic parameters included: left atrium – 46 mm; left ventricle end-diastolic diameter 48 mm; left ventricle end systolic diameter 29 mm; posterior wall 12 mm; intraventricular septum 12 mm; right ventricle 24 mm, aortic bulb 34 mm. The ejection fraction was estimated at 60% (Simpson method assessment). Moderate mitral valve regurgitation was present. The patient suffered from paroxysmal atrial fibrillation, arterial hypertension, and hyperlipidemia. The heart team chose TAVI valve-in-valve as an optimal treatment strategy.
Computed tomography scan was performed to evaluate the access site and diameters of the valve for possible implantation. The right coronary artery height was estimated at 7.2 mm and the left coronary artery height was estimated at 8.3 mm. The sino-tubular junction diameter was 29 mm and the maximal ascending aorta diameter was 37.3 mm.
Despite the fact that the valve-in-valve procedure is off-label in this model, it was the heart-team decision to proceed with the intervention as the prosthesis was readily available at the facility and the patient required urgent treatment.
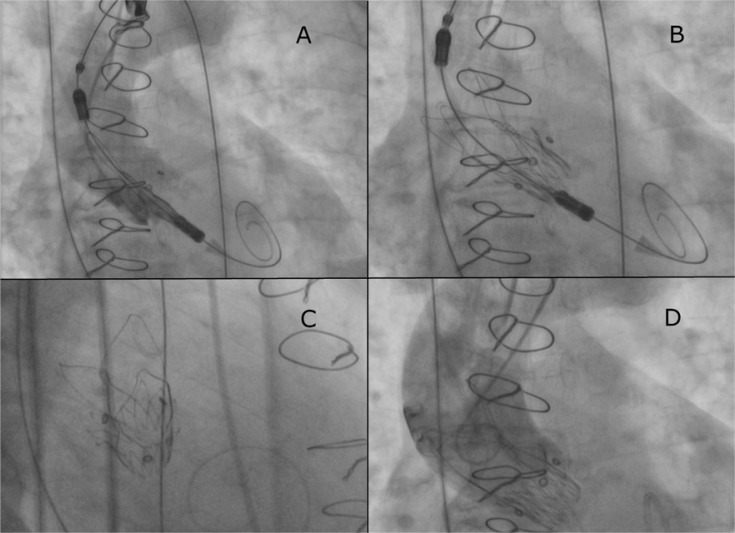
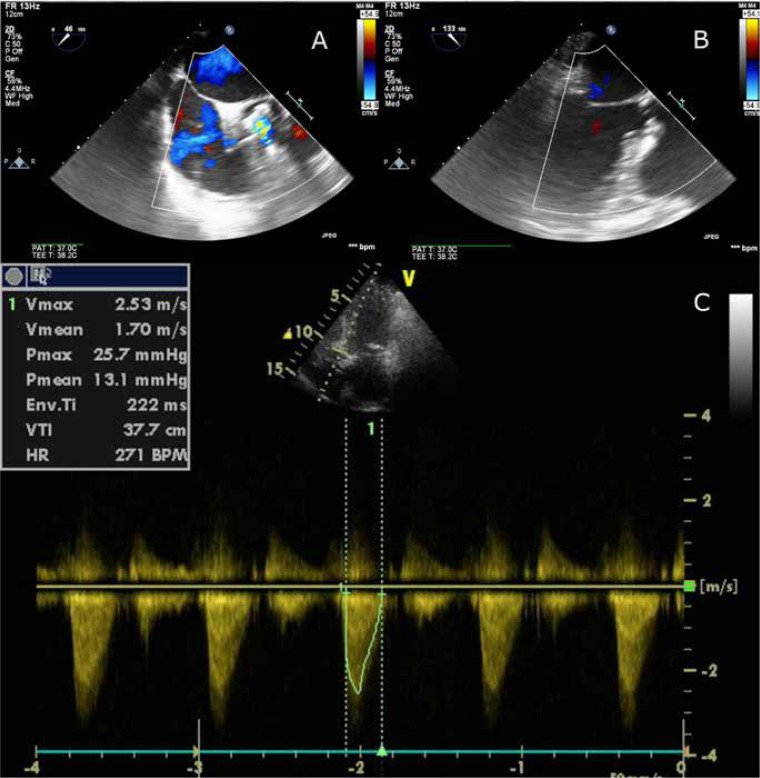
Although there are reports of performing Mosaic valve fracture during the TAVI procedure, the heart-team decision was to proceed with direct bioprosthesis implantation due to hemodynamic instability. The TAVI procedure was conducted through right femoral access. Two ProGlide access closure devices were used. The left radial artery was used for diagnostic pigtail catheter introduction. No predilatation was performed. The Boston Accurate Neo S-size Prosthesis was successfully implanted (Figures 1 A–D). The procedure was uncomplicated and verified using transesophageal echocardiography (Figures 2 A, B). Based on the final effect, no postdilatation was required.
Figure 1.
Valve implantation procedure (A, B), fluoroscopic verification of proper valve placement (C), angiography showing no perivalvular regurgitation (D)
Figure 2.
Intraprocedural transesophageal echocardiography for verification of proper valve implantation (A, B) and early postoperative transthoracic echocardiography for assessment of prosthesis function (C)
Postoperatively, two routine transthoracic echocardiographic examinations were conducted. Four days following the procedure, there was no perivalvular leak. The prosthetic valve provided adequate gradient and flow parameters (Figure 2 C). No pericardial effusion was present.
The second transthoracic echocardiography was performed 2 weeks following the procedure and revealed no perivalvular leak as well. The ejection fraction was 60%. The prosthetic valve parameters remained optimal (Vmax 2.4 m/s; Pmax 23 mm Hg; Pmean 14 mm Hg). The valve area was estimated at 2.8 cm2. No pericardial effusion was noted. The patient remains uncomplicated and presents with relief of all symptoms.
In summary, the Boston Accurate Neo transcatheter aortic valve may be used safely for vale-in-valve procedures in selected patients. Our case indicates that the valve, after detailed diagnostic imaging and careful qualification, is feasible for implantation even in small bioprostheses, such as the Medtronic Mosaic.
Disclosure
The authors report no conflict of interest.
Biography

References
- 1.Holzamer A, Kim WK, Rück A, Sathananthan J, Keller L, Cosma J, Bauer T, Nef H, Amat-Santos IJ, Brinkert M, Husser O, Pellegrini C, Schofer J, Nerla R, Montorfano M, Giannini F, Stella P, Kuwata S, Hilker M, Castriota F, Ussia GP, Webb JG, Nietlispach F, Toggweiler S. Valve-in-valve implantation using the ACURATE Neo in degenerated aortic bioprostheses: an international multicenter analysis. JACC Cardiovasc Interv 2019; 12: 2309-2316. [DOI] [PubMed] [Google Scholar]
- 2.Arunothayaraj S, Williams T, Cockburn J, Hildick-Smith D. Acurate Neo implantation to treat degenerative regurgitation of surgical bioprostheses. Cardiovasc Revasc Med 2021; 28 S: 105-108. [DOI] [PubMed] [Google Scholar]