Abstract
Fluorescence‐guided surgery has emerged as a promising imaging technique for real‐time intraoperative tumour delineation and visualisation of submillimetre tumour masses in cytoreductive surgery for epithelial ovarian cancer (EOC). Researchers have developed several EOC‐targeted fluorescent probes, most of which are currently in the preclinical stage. Interestingly, imaging devices designed for open surgery are proof of concept. This review summarises the recent advances in EOC‐targeted fluorescent probes and open‐field fluorescence imaging strategies and discusses the challenges and potential solutions for clinical translation.
Keywords: bioinspired imager, epithelial ovarian cancer, fluorescence‐guided cytoreductive surgery, fluorescent probe, folic‐AIEgen, hybrid imager, open‐field imaging
1. INTRODUCTION
The microscopic disease clearance rate positively correlates with favourable survival outcomes in patients with epithelial ovarian cancer (EOC). 1 , 2 , 3 , 4 , 5 Therefore, according to clinical guidelines for EOC management, cytoreductive surgery should include hysterectomy and bilateral salpingo‐oophorectomy. 6 , 7 Interestingly, other surgical decisions are based on preoperative imaging examinations, intraoperative visible lesions and the tactile sensation of the surgeon. Furthermore, tumour‐free margins can only be verified in practice by the time‐consuming examination of frozen sections, and insufficiently frozen specimens lead to increased sampling errors, resulting in residual lesions. 8 , 9 Meanwhile, submillimetre‐sized tumour masses in EOC dispersed intraperitoneally are invisible to the naked eye and are imperceptible through tactile sensation. Consequently, gynaecologists are faced with a difficult decision, to either ‘blindly’ resect entire tissues, such as majus omentum, which has a high risk of severe complications, 10 , 11 or to ‘blindly’ maintain these normal appearing tissues, which may harbour occult metastasis with a high risk of recurrence. Therefore, intraoperative real‐time visualisation of the surgical margin and occult tumour masses is imperative in EOC cytoreduction surgery.
In surgical oncology, fluorescence‐guided surgery has become popular for staining the vascular structures of interest, mapping lymph nodes, and identifying vital structures. 12 , 13 , 14 Conversely, fluorescence‐guided cytoreductive surgery (FGCS) for EOC emphasises the active targeting of fluorescent probes to tumour cells. FGCS stimulates fluorescent probes, which actively aggregate in the tumour tissue, showing tumour delineation and submillimetre tumour masses. Notably, the following three parts are required to achieve FGCS: (1) fluorescent probes; (2) delivery systems carrying fluorescent probes to tumour tissues; and (3) imaging systems that launch a specific wavelength range of light (mostly near‐infrared light) and display fluorescence images to the naked eye. 15 , 16
The fluorescence signals from tumour tissues are influenced by several factors, including the target expression level in EOC tissues, the recognition rate and recognition group affinity to targets, and the tumour‐to‐background fluorescence intensity. Additionally, minute molecules, peptides, or proteins specifically expressed in tumour cells, specific enzymes, and low pH in the tumour microenvironment are targets of ovarian cancer (OC) tissues. Interestingly, near‐infrared II (NIR‐II; 700–900 mm) imaging is considered the most promising imaging modality owing to its better penetration depth (NIR‐I vs NIR‐II: 6 vs 20 mm), higher spatiotemporal resolution and lower photon scattering, compared with NIR‐I imaging. 17 , 18 However, the fluorescence intensity of traditional fluorophores is weakened or quenched when solidified or aggregated, which is a well‐recognised quenching effect. 19 Luo et al introduced aggregation‐induced emission (AIE) in 2001. 20 AIE luminogens (AIEgens) are non‐ or mildly emissive in solution but strongly emissive when solidified or aggregated. 21 NIR‐II AIEgens are the most promising next‐generation fluorophores because of their prominent characteristics, such as tuneable molecular structures, high fluorescence quantum yield, large Stokes shift, excellent photostability and good biocompatibility. 22 Most fluorescent probes targeting tumour microenvironment hallmarks are ‘turn‐on’ probes that activate only in the presence of specific biological reactions, thus reducing the false‐positive background fluorescence signal. 23 Therefore, EOC‐targeted fluorescent probes are classified into the following three categories based on the targeting groups and fluorophore optical properties: type I, an active recognition group (small molecules, peptides or proteins that actively recognise the specific biomarkers of EOC cells) conjugated with an ‘always‐on’ fluorophore; type II, a ‘turn‐on’ fluorophore that is activated by the hallmarks of the tumour microenvironment (low pH, tumour‐associated enzymes); and type III, an active recognition group conjugated with a next‐generation fluorophore (NIR‐II fluorophore, aggregation‐induced emission fluorophore) (Figure 1).
FIGURE 1.
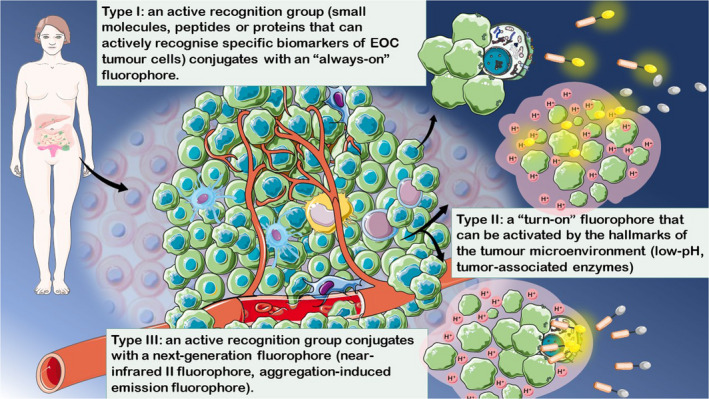
Classification of epithelial ovarian cancer (EOC)‐specific fluorescent probes. According to the optical and biochemical properties of the fluorophores and recognition groups, EOC‐targeted fluorescent probes can be classified into three categories: type I, an active recognition group (small molecules, peptides or proteins that can actively recognise specific biomarkers of EOC cells) that conjugates with an ‘always‐on’ fluorophore; type II, a ‘turn‐on’ fluorophore that can be activated by the hallmarks of the tumour microenvironment (low pH, tumour‐associated enzymes); and type III, an active recognition group conjugated with a next‐generation fluorophore (near‐infrared II and aggregation‐induced emission fluorophore)
The lack of consensus on a standardised evaluation protocol has hindered the clinical translation to extensive intraoperative fluorescence imaging studies at the proof‐of‐principle stage. For example, as important parameters for evaluating the optical properties, varying the observation distance and imaging devices influence the tumour recognition efficiency of fluorescent probes, the optimal dose and the tumour‐to‐background ratio. 15 , 24 , 25 Lauwerends et al. proposed that changes in surgical decisions based on fluorescence observation are the most objective parameters and are least affected by differences in fluorescence observations. They recommended that clinical trials should report additional resection, preservation based on fluorescence signals and any complications resulting from these decisions. 26 Therefore, we extended their recommendations to evaluate preclinical studies of EOC‐targeted fluorescent probes.
Some retrospective cohorts in early EOC have reported similar survival outcomes in the laparotomy and laparoscopy groups. 27 , 28 , 29 , 30 In the advanced EOC study, only selected patients had similar disease‐free and overall survival rates between the two groups; moreover, patients with widespread disease were mostly excluded from the laparoscopy group. The clinical effect of laparoscopic surgery strongly depends on the proficiency of the surgeon. 31 , 32 , 33 , 34 , 35 , 36 , 37 However, there is insufficient high‐quality evidence that supports laparoscopy as a substitute for laparotomy in managing EOC, regardless of the stage. Currently, laparotomy is the routine treatment for EOC. Therefore, open‐field imaging is unavoidable in FGCS for EOC. However, commercially available fluorescence imaging devices designed for neurosurgery and endoscopy perform poorly in open‐field abdominal surgery.
This review presents an overview of potential EOC‐targeted fluorescent probes employed in clinical trials and preclinical studies. Next, we summarise the recent developments in open‐field fluorescence imaging devices and highlight the current challenges and potential solutions for clinical translation.
2. EOC‐TARGETED FLUORESCENT PROBES
2.1. EOC‐targeted fluorescent probes in the preclinical stage
2.1.1. Type‐I fluorescent probes
Cluster of differentiation 24
Cluster of differentiation 24 (CD24) is a densely glycosylated cell surface protein anchored by glycosylphosphatidylinositol, overexpressed in 80%–100% of EOC tissues. 38 Kleinmanns et al. reported a CD24‐target fluorescent probe comprising a monoclonal mouse‐derived antibody against CD24 and Alexa Fluor 680. 39 Additionally, relatively intense autofluorescence was observed in the stomach and gastrointestinal tract. Therefore, Alexa Fluor 750, with a longer excitation wavelength, was selected to reduce the autofluorescence. Furthermore, the CD24‐AF750‐guided FGCS group detected significantly more metastatic lesions than the bright‐field surgery group (n = 36 vs 19) and orthotopic OV‐90luc+ xenografts (n = 8 per group), and additional resections were verified as tumour tissues. Moreover, the results in patient‐derived xenografts (PDXs) with heterogeneous CD24 expression revealed that the fluorescence intensity could still attain a high immunoreactivity score even in PDX with low CD24 expression. Therefore, many unresectable lesions were observed, although they were constrained by the limited mouse model. 40
Folate receptor alpha
Folate receptor alpha (FRα) is highly expressed (80%–90%) in EOC. 41 Liu et al. reported a multimodal positron emission tomography (PET) and optical FRα‐targeted agent (PPF). Intraoperative fluorescence imaging of PPF detected submillimetre tumour tissues in PDXs from 31 patients. 42 Hekman et al. synthesised an FRα‐targeted intact humanised antibody that was dual labelled by radioisotope and fluorescence, known as 111In‐farletuzumab‐IRDye800CW. 43 FGCS guided by 111In‐farletuzumab‐IRDye800CW also detected superficial submillimetre tumour deposits in IGROV‐1 xenograft models, suggesting the possibility of additional resection. Furthermore, the distribution of dual‐labelled farletuzumab in tumour tissues with homogeneous FRα expression was heterogeneous, suggesting that tumour vascularisation influences the probe distribution. de Jong et al. systematically evaluated the clinical and preclinical studies of FGCS that targeted either HER2 or FRα. 44 However, as HER‐2 is expressed only in 20% of OCs, the relevant studies are not summarised in this review.
Gonadotropin‐releasing hormone receptor
Gonadotropin‐releasing hormone receptor (GnRHR) is overexpressed in 74%–95% of OC cases. 45 , 46 Therefore, Liu et al. designed a GnRHR‐targeted fluorescent probe, known as GnRHa‐indocyanine green (ICG) (ICG‐conjugated GnRH peptide). 47 Fluorescence imaging of A2780 xenograft mice revealed submillimetre tumour deposits on the mesentery, and the lesions were confirmed via pathological examination.
Prolactin receptor
Sundaram et al. reported that >98% of OCs express the prolactin receptor (PRLR), irrespective of histological type, grade and stage. 48 More importantly, no significant difference was observed in PRLR expression between the primary site and peritoneal metastases. Subsequently, they conjugated human placental lactogen (hPL), a specific and strongly binding PRLR ligand, to Cy5. However, this study did not focus on intraoperative imaging with hPL‐Cy5, and in vivo fluorescence imaging showed high fluorescence in non‐target areas, indicating the limited value of hPL‐Cy5 as an intraoperative imaging probe.
OC‐associated antigen (OC183B2)
Chen et al. demonstrated OC183B2 expression (61.2%–89.9%) in OC. 49 , 50 They subsequently developed COC183B2‐Cy7 (Cy7‐conjugated COC183B2) and COC183B2‐800 (IRDye800CW‐conjugated COC183B2). 49 , 50 Interestingly, the two fluorescent probes showed relatively high fluorescence signals in the liver. Furthermore, submillimetre tumour masses were detected in OC tissues using COC183B2‐Cy7 ex vivo, without the detection data from in vivo fluorescence imaging.
2.1.2. Type‐II fluorescent probes
γ‐Glutamyl transpeptidase
γ‐Glutamyl transpeptidase (GGT) regulates the cellular homeostasis of glutathione and cysteine. GGT expression is significantly upregulated in OC. 51 , 52 Urano et al. developed γ‐glutamyl hydroxymethyl rhodamine green (gGlu‐HMRG), which is activated by GGT. 53 Additionally, submillimetre tumour masses were detected on the mesentery and peritoneal wall 10 min after peritoneal injection of gGlu‐HMRG in SHIN3 xenograft mice. Therefore, in mice, gGlu‐HMRG can be activated rapidly in the presence of GGT within seconds of intraperitoneal spaying, although the fluorescence signal weakens within 60 min.
pH
The pH of the solid tumour microenvironment is 6.4–7.1, whereas that of the normal extracellular matrix is 7.3–7.4. 54 Tung et al. developed a novel low‐pH response fluorophore (CypH‐1) by modifying a Cy7 analogue with an amino group. 55 Notably, the fluorescence signals were evenly distributed in the tumour and were also observed in the intestine; however, micrometastases were not detected. Furthermore, Yokomizo reported a rapidly activated pH‐responsive fluorophore known as PH08. 56 Here, submillimetre tumour masses were visualised in ID8 xenograft models within 10 min of the peritoneal injection of PH08, and intense fluorescence signals were maintained for 4 h. Therefore, the adverse events of PH08 were limited because of the similarity in the backbones of PH08 and ICG.
2.1.3. Type‐III fluorescent probes
In the past decade, conventional fluorophores, such as ICG, IRDye800CW, and rhodamine green, have dominated the field of EOC‐targeted FGCS. 43 , 47 , 49 , 50 , 53 However, only a few studies on EOC‐targeted fluorescent probes were modified with NIR‐II fluorophores and/or AIEgens. Zhou et al. developed a novel type‐III fluorescent probe (NIR‐II Pdots‐GnRH) that comprised cetrorelix, which is a GnRHR‐targeted peptide, and an ultrabright AIEgen, NIR‐II emissive polymer dots. 57 Notably, the NIR‐II Pdot‐GnRH showed ultrabright fluorescence. Additionally, tumour masses of <2 mm could be detected using NIR‐II Pdot‐GnRH. Zhong et al. modified an AIEgen with folic acid (an FR recognition group) and synthesised folic AIEgen. 58 Interestingly, folic‐AIEgens have facilitated delicate sentinel lymph node (SLN) detection from mice to nonhuman primate rhesus macaques. Furthermore, micro‐ to submillimetre peritoneal metastases, detected using folic‐AIEgens in SKOV3 xenograft models, were visualised and resected. Moreover, a significant difference in survival outcomes was observed (survival rate of FGCS group vs control group 40 days after surgery: 80% vs 0%) (Table 1).
TABLE 1.
Preclinical EOC‐targeted fluorescent probes
| Biomarker | Recognition group | Fluorophore | λex/λem (nm) | Animal models | Smallest lesion detected (mm) | Sensitivity | Specificity | Additional lesions detection/Pathological examination | Administration | Year | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | Cluster of differentiation 24(CD24) | The monoclonal mouse anti‐human CD24 antibody | Alexa Fluor 680 | 678/706 | OV‐90luc xenografts, PDXs | NA | NA | NA | NA/NA | Intravenous injection | 2020 |
| The monoclonal mouse anti‐human CD24 antibody | Alexa Fluor 750 | 753/778 | OV‐90luc xenografts, PDXs | NA | NA | NA | Yes/Yes | Intravenous injection | 2020 | ||
| Folate receptor alpha (FRa) | Folate | Pyropheophorbide‐α | 480/725 | PDXs | ≤1 | NA | NA | Yes/Yes | Intraperitoneal injection | 2013 | |
| Farletuzumab | IRDye800CW | 774/800 | IGROV‐1 xenograft | ≈1 | NA | NA | Yes/Yes | Intravenous injection | 2020 | ||
| Gonadotropin‐releasing hormone receptor (GnRHR) | GnRHa | ICG | 795/810 | A2780 xenografts | ≈1 | NA | NA | Yes/Yes | Intraperitoneal injection | 2020 | |
| Prolactin receptor (PRLR) | HPL | Cy5 | 646/664 | CaOV3/HeyA8/SKOV3 GFP‐Luc xenofrafts | NA | NA | NA | NA/NA | Intravenous injection | 2018 | |
| OC‐associated antigen (OC183B2) | COC183B2 | Cy7 | 750/773 | SKOV3 Luc xenografts | ≈1 | NA | NA | Yes/Yes | Intravenous injection | 2020 | |
| COC183B2 | IRDye800CW | 774/800 | SKOV3 Luc xenografts | NA | NA | NA | NA/NA | Intravenous injection | 2020 | ||
| Type II | γ‐Glutamyl transpeptidase (GGT) | γ‐glutamyl hydroxymethyl | Rhodamine green | 490/551 | SHIN3 xenograft mice | ≈1 | 100% | 100% | Yes/NA | Intraperitoneal injection | 2020 |
| PH | CypH‐1 | CypH‐1 | 760/777 | SKOV3 GFP‐Luc xenografts | ≈1 | NA | NA | NA/NA | Intraperitoneal injection | 2015 | |
| PH08 | PH08 | 710/794 | ID8 xenografts | ≤1 | NA | NA | Yes/Yes | Intraperitoneal injection | 2022 | ||
| Type III | Gonadotropin‐releasing hormone receptor (GnRHR) | Cetrorelix | NIR‐II Pdots | 760/1020 | A2780 xenografts | ≤2 | NA | NA | Yes/Yes | Intravenous injection | 2021 |
| Folate receptor (FR) | Folic‐acid | AIEgen | 365/540 | SKOV3 xenografts | ≤1 | NA | NA | Yes/Yes | Intraperitoneal injection | 2021 |
Abbreviations: AIEgen, aggregation‐induced emission luminogen; EOC, epithelial ovarian cancer; NA, not applicable; PDXs, patient‐derived xenografts; λem, emission wavelength; λex, excitation wavelength.
2.2. EOC‐targeted fluorescent probes in clinical trials
2.2.1. Type‐I fluorescent probes
van Dam et al. performed the first FGCS trial in humans using fluorescein isothiocyanate (FITC), an FRα‐targeted fluorescent probe. 59 FITC is the first EOC‐specific probe commercially available as EC‐17 to be tested in clinical trials. Although it proceeded to phase‐2 clinical trials (NCT01511055), the trial was terminated in 2020 as a result of technical constraints.
OTL38, an FRα‐targeted ligand bound to a cyanine dye, is the first EOC‐specific fluorescent probe to complete phase‐3 clinical trials (NCT03180307) for FGCS. Hoogstins et al. reported a phase‐1 trial of OTL38 in healthy volunteers and patients with OC and provided the first available data on its safety and efficacy. 60 Additionally, a phase‐2 trial of OTL38 (NCT02317705) revealed that OTL38 detected EOCs with 85.93% sensitivity. 61 Therefore, fluorescence imaging was performed under white light to detect residual lesions after routine cytoreductive surgery.
The latest phase‐3 trial (NCT03180307) showed that 47 of 109 patients with OC underwent additional resections, for tumours that were detected by the fluorescence signal of OTL38 but were invisible in white light and were undetectable by palpation. 62 Moreover, OTL38 showed a limited safety risk in all trials and steady sensitivity, ranging from 83% to 85%. Therefore, the OTL38‐guided FGCS, the first EOC‐targeted fluorescent probe that completed clinical translation with satisfactory primary outcomes, would be an important supplement in managing patients with EOC in the future.
2.2.2. Type‐II fluorescent probes
ONM‐100, a polymer micelle covalently conjugated to ICG, is an ultra‐pH‐sensitive, tumour acidosis‐activated targeted fluorophore. Voskuil et al. reported a phase‐1 study of ONM‐100 for intraoperative imaging in 30 patients with head and neck squamous cell carcinoma and breast, oesophageal and colorectal cancers (NTR number 7085). 63 They validated the safety and feasibility of ONM‐100. Surprisingly, the results showed 100% sensitivity, no false negatives and the presence of five additional occult lesions. Additionally, a phase‐2 study (NCT03735680) is currently in progress. Another phase‐2 study (NCT04950166) is recruiting participants to evaluate the potential of ONM‐100 in detecting peritoneal metastases (primary cancers including OC). Furthermore, ONM‐100 is a pH‐sensitive fluorescent probe that is not limited by biomarkers with heterogeneous expression and has the best performance in identifying occult tumour masses, with an excellent sensitivity of 100% in solid tumours. These data reveal the enormous potential of pH‐sensitive fluorescent probes in FGCS for all solid tumours (Table 2).
TABLE 2.
Clinical EOC‐targeted fluorescent probes
| Name | Biomarker | Fluorophore | Registration no. | Indication | Participants | Patients (ovarian cancer) | Phase | Update | Status | Administration | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | FITC | Folate receptor alpha (FRα) | Cyanine dye | NCT02000778 | Ovarian cancer | 10 | NA | Phase 1 | 2018 | Completed | NA | NA | NA | NA | NA |
| NCT01511055 | Ovarian cancer | NA | 27 | Phase 2 | 2020 | Terminated |
0.1 mg/kg IV 2–3 h prior to surgery |
NA | NA | NA | NA | ||||
| OTL38 | Folate receptor alpha (FRα) | Cyanine dye | 2013‐004774‐10/2014‐002352‐12 | Ovarian cancer | 30 | 12 | Phase 1/2 | 2016 | Completed |
0.0125 mg/kg 2–6 h prior surgery |
NA | NA | NA | NA | |
| NCT02317705 | Ovarian cancer | NA | 44 | Phase 2 | 2019 | Completed |
0.025 mg/kg 2–3 h prior to surgery |
85% | 87% | 88% | 9% | ||||
| NCT03180307 | Ovarian cancer | NA | 109 | Phase 3 | 2022 | Completed |
0.025 mg/kg 1 h prior to surgery |
86% | 31% | 64% | 43% | ||||
| Type II | ONM‐100 | pH | ICG | NTR number 7085 | Head and neck squamous cell carcinoma, breast cancer, oesophageal cancer and colorectal cancer | 135 | 0 | Phase 1 | 2020 | Completed |
0.1–1.2 mg/kg 24 ± 8 h prior to surgery |
100% | 75% | 64% | 100% |
| NCT03735680 | Head and neck squamous cell carcinoma, breast cancer, oesophageal cancer, colorectal cancer, ovarian cancer | 30 | NA | Phase 2 | 2022 | Completed | NA | NA | NA | NA | NA | ||||
| NCT04950166 | Peritoneal carcinomatosis from the metastasis of a primary cancer of the peritoneum (e.g. appendiceal, ovarian, uterine, colorectal and gastric cancers) | 40 | NA | Phase 2 | 2022 | Recruiting |
1 mg/kg 24–72 h prior to surgery |
NA | NA | NA | NA |
Abbreviations: EOC, epithelial ovarian cancer; NA, not applicable.
3. OPEN‐FIELD IMAGING STRATEGY
3.1. Challenge I: attenuation of the fluorescence signal
Moore et al. evaluated open‐field FGS in 15 patients with head and neck cancers. 64 Here, the fluorescent camera was fixed at a distance of 15–30 cm during the surgical procedure. 64 The results showed that the fluorescence intensity in situ was 87% less than that ex situ. A fixed camera cannot collect most fluorescence signals from complex anatomical structures, and the fluorescence signals fade with the lengthening of the observation distance. Dorval et al. developed a palm‐sized handheld high‐sensitivity fluorescence imager known as Fluostick™. 65 Reportedly, a 150‐g handheld device could detect tumours as small as 185 μm in mice. Additionally, surgeons have reported that Fluostick™ provides a better side view of the cavity and organs. Fluostick™ requires turning off the ambient light and scanning the surgical field, thus including additional surgical procedures. Notably, the maximum penetration depth of the fluorescence signal was 1.0–1.5 cm. Furthermore, radioactive image guidance provides in‐depth information about the tissue of interest. KleinJan et al. attached a fluorescence camera (VITOM; Karl Storz, Tuttlingen, Germany) to either a gamma‐ray detection probe (GP; VITOM‐GP) or a portable gamma‐camera (GC; VITOM GC) and tested this real‐time hybrid handheld imager on 11 patients with penis cancer to detect SLNs. 66 However, surgeons have reported that VITOM‐GP and VITOM‐GC are large and heavy, thereby adding inconvenience to the surgical process. Therefore, this study is the first to report that the detection rates of hybrid and single modalities are highly similar.
3.2. Challenge II: interruption of surgical flow
Many clinical practices using open‐field FGS turn off the ambient light for maximal fluorescence signals, which results in pauses during the surgical procedure. However, van den Berg et al. developed a light‐filtered fluorescence imager. 67 This imager collected fluorescent images containing ambient light and then only the image of ambient light. Therefore, subtracting the two images presented a fluorescent image without interference from the ambient light. Multispectral fluorescence imagers are light‐filtered fluorescence imagers equipped with multiple fluorescence channels. Specifically, they can present purely fluorescent images and collect fluorescence signals at different wavelengths. Behrooz et al. introduced a multispectral imager, Solaris™, which supports four fixed fluorescence channels and a multispectral channel with tunability. 68 In 2018 Garcia et al. designed a multispectral imager inspired by the compound eye of the butterfly, which is currently undergoing clinical trials (NCT02316795). 69 Additionally, in 2021 they designed another hexachromatic camera inspired by the eye of the mantis shrimp that supports three colour channels to improve spatial accuracy and three fluorescence channels to collect different fluorescence signals. 70 This imaging device simultaneously obtained fluorescence signals from two fluorescent probes in a mouse model. In a cohort of 18 patients with breast cancer, SLNs were detected with true‐ and false‐positive rates of 84% and 0%, respectively.
The surgeon's sight in open‐field FGS is disturbed by switching between the surgical field and the imaging display platform, in contrast to laparoscopy. Therefore, Liu et al. have developed a wearable display system, which is a goggle system that integrates detector, displayer, light source and control components to overlay the surgical field and display platform. 71 This wearable device enables direct visual access to the surgical field and integrates the surgical procedure. Notably, a detection sensitivity of 100% was obtained for seven women with breast cancer. 72 Furthermore, Sarder et al. have designed an imager that can directly project luminescence onto a surgical field. 73 Specifically, this prototype device comprised a fluorescence camera and projector kit fixed vertically to the surgical field. 73 Subsequently, they optimised this device and tested it in pigs in 2016. The projecting device could successfully track fluorescence signals while tissues were moved and resected, with a true mean calibration error of 1.01–1.75 mm. 74 Therefore, researchers suggest that intermittent projections are preferable. However, the accuracy of the projection was compromised in a non‐flat surgical field.
4. DISCUSSION
Approximately 40% of metastatic tumours and lymph nodes express biomarkers (small molecules, peptides or proteins) that differ from those in the primary tumour. 75 , 76 The application of mono‐targeted fluorescent probes is constrained by heterogeneous spatial, intertumoral and intratumour expression.
Immortalised cell line xenografts are not the ideal representative experimental models for FGCS because of their limited value in presenting inter‐ and intratumour heterogeneities. Folic‐AIEgen has been verified as the most promising EOC‐targeted fluorescent probe in the preclinical stage, as it promotes further resection and improves survival outcomes, according to additional experimental data on rhesus macaques. Furthermore, the fluorescence wavelength emitted by folic‐AIEgen is extremely near the visible wavelength, indicating a scenario in the future where surgeons can observe a submillimetre tumour mass with the naked eye without fluorescence collection and display devices. In preclinical studies, the following aspects can accelerate the clinical translations of fluorescent probes: (1) replacing immortalised cell line xenografts with PDXs; (2) reporting additional resection; and (3) using an animal cohort to compare the impact of FGCS on animal survival.
The hallmarks of low pH and enzymes are more homogeneous between patients and tumours. Notably, the ‘turn‐on’ probe can be activated within a few minutes when it encounters targets, with minimal non‐target fluorescence. Additionally, intraperitoneal spray administration was recommended for the abovementioned type‐II fluorescent probe. However, intraperitoneal administration can only reveal superficial tumour masses. Additionally, limited blood flow in small‐volume tumour masses may affect the fluorescence intensity of submillimetre tumour masses. 43 , 77 , 78 Therefore, the vascular distribution effect on probe deposition should be considered based on intravenous injection. Therefore, intraperitoneal spray can be used as a supplement for intravenous administration within the overall dose safety range.
The satisfactory clinical translation of OTL38 will undoubtedly accelerate other potential EOC‐targeted fluorescent probes, such as ONM‐100. Furthermore, the high efficiency of ONM‐100 processes across all solid tumours demonstrated the tremendous potential of the type‐II fluorescent probes. First, they target the hallmark of the tumour microenvironment, which is not limited by the heterogeneity of the tumour cell biomarkers, and are only activated when they encounter specific biological reactions. Second, type‐II fluorescent probes have a higher tumour‐to‐background ratio and higher efficiency, making them more sensitive to smaller tumour masses, than type‐I fluorescent probes. Therefore, type‐II fluorescent probes can be applied to various clinical scenarios. Type‐III fluorescent probes hold great potential owing to their excellent optical characteristics; however, their recognition groups limit their broader clinical application. Although EOC‐targeted fluorescent probes in clinical trials were considered safe, their efficiency evaluation was not standardised because of the heterogeneity of the studies. 79 Furthermore, similar limitations were observed when we evaluated the preclinical EOC‐targeted fluorescent probes. Therefore, the theory that changes in surgical decisions are the most objective parameter was adopted in our evaluation.
Currently, dual‐mode imaging, comprising fluorescence and traditional imaging techniques, such as magnetic resonance imaging (MRI), PET and single‐photon emission computed tomography/computed tomography (SPECT/CT), to achieve intraoperative co‐localisation, is not recommended. The potential radiation hazard to the surgeons compromises the value of dual‐mode imaging, and there is no evidence to indicate that dual‐mode imaging benefits the patients. Therefore, wearable and projection imaging devices aim to optimise the surgeons’ surgical experiences; however, the delayed imaging and distortion in the non‐plane surface may cause tumour site omission. Furthermore, the ideal open‐field fluorescence imaging device for EOC requires a portable handheld detector for the close observation of complex anatomical structures deep within the abdominal cavity, a fixed camera for a broad view of the surgical field, multispectral fluorescent channels for multiple targeting images and a light‐filter component to minimise the interference from ambient light. Therefore, a device that meets the abovementioned conditions will be massive and heavy in the operating room. Moreover, engineering problems have significantly limited the feasibility of an ideal device, and such a tool with high fluorescence and good integration into the surgical process is yet to be developed.
5. CONCLUSION
Implementing FGCS maximises tumour cytoreduction to a submillimetre grade and reduces the functional impairment caused by excessive resection. To date, type‐II and ‐III fluorescent probes have shown prominent potential in guiding the visualisation of submillimetre tumour masses and mediating additional resection in the preclinical stage. Notably, OTL38 completed a phase‐3 clinical trial with satisfactory primary outcomes. Therefore, OTL38‐guided FGCS is a crucial addition to current EOC cytoreductive surgery. Additionally, ONM‐100 has proceeded to phase‐2 clinical trials, and current data show that ONM‐100 has the highest efficiency. However, large, multicentre, randomised controlled trials are also required for the promising probes discussed in this review, such as folic‐AIEgens. Furthermore, the devices discussed provide incomplete solutions for open‐field FGCS. Moreover, an ideal device with high fluorescence and good integration into the surgical process is yet to be developed. Therefore, when EOC‐targeted probes and open‐field fluorescence imaging devices are clinically recommended, EOC surgical efficiency and clinical outcomes will significantly improve.
AUTHOR CONTRIBUTIONS
CS: literature search, formal analysis, visualisation and writing (original draft). YH: writing (review and editing). CJ: writing (review and editing). ZL: conceptualisation, supervision, project administration and funding acquisition.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interests form available to view online as supporting information.
ETHICS APPROVAL
No human or animal subject were involved in this manuscript.
Supporting information
ICMJE DISCLOSURE FORM
ACKNOWLEDGEMENTS
This study was supported by the Sichuan Province Science and Technology Support Program (grant no. 2019YJ0072) for Science, Engineering, and Humanities and Social Sciences.
Sun C, Huang Y, Jiang C, Li Z. Updates on fluorescent probes and open‐field imaging methods for fluorescence‐guided cytoreductive surgery for epithelial ovarian cancer: A review. BJOG. 2022;129(Suppl. 2):50–59. 10.1111/1471-0528.17332
REFERENCES
- 1. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta‐analysis. J Clin Oncol. 2002;20(5):1248–59. [DOI] [PubMed] [Google Scholar]
- 2. Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced‐stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85. [DOI] [PubMed] [Google Scholar]
- 3. Horowitz NS, Miller A, Rungruang B, Richard SD, Rodriguez N, Bookman MA, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced‐stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33(8):937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tseng JH, Cowan RA, Zhou Q, Iasonos A, Byrne M, Polcino T, et al. Continuous improvement in primary debulking surgery for advanced ovarian cancer: do increased complete gross resection rates independently lead to increased progression‐free and overall survival? Gynecol Oncol. 2018;151(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall M, Savvatis K, Nixon K, Kyrgiou M, Hariharan K, Padwick M, et al. Maximal‐effort cytoreductive surgery for ovarian cancer patients with a high tumor burden: variations in practice and impact on outcome. Ann Surg Oncol. 2019;26(9):2943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong DK, Alvarez RD, Bakkum‐Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(2):191–226. [DOI] [PubMed] [Google Scholar]
- 7. Fotopoulou C, Planchamp F, Aytulu T, Chiva L, Cina A, Ergönül Ö, et al. European Society of Gynaecological Oncology guidelines for the peri‐operative management of advanced ovarian cancer patients undergoing debulking surgery. Int J Gynecol Cancer. 2021;31(9):1199–206. [DOI] [PubMed] [Google Scholar]
- 8. Cendán JC, Coco D, Copeland EM 3rd. Accuracy of intraoperative frozen‐section analysis of breast cancer lumpectomy‐bed margins. J Am Coll Surg. 2005;201(2):194–8. [DOI] [PubMed] [Google Scholar]
- 9. Nakanishi K, Morita S, Taniguchi H, Otsuki S, Fukagawa T, Katai H. Diagnostic accuracy and usefulness of intraoperative margin assessment by frozen section in gastric cancer. Ann Surg Oncol. 2019;26(6):1787–94. [DOI] [PubMed] [Google Scholar]
- 10. Bilbao M, Aikins JK, Ostrovsky O. Is routine omentectomy of grossly normal omentum helpful in surgery for ovarian cancer? A look at the tumor microenvironment and its clinical implications. Gynecol Oncol. 2021;161(1):78–82. [DOI] [PubMed] [Google Scholar]
- 11. Di Donato V, Bardhi E, Tramontano L, Capomacchia FM, Palaia I, Perniola G, et al. Management of morbidity associated with pancreatic resection during cytoreductive surgery for epithelial ovarian cancer: a systematic review. Eur J Surg Oncol. 2020;46(4 Pt A):694–702. [DOI] [PubMed] [Google Scholar]
- 12. Frumovitz M, Plante M, Lee PS, Sandadi S, Lilja JF, Escobar PF, et al. Near‐infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non‐inferiority trial. Lancet Oncol. 2018;19(10):1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Valk KS, Handgraaf HJ, Deken MM, Sibinga Mulder BG, Valentijn AR, Terwisscha van Scheltinga AG, et al. A zwitterionic near‐infrared fluorophore for real‐time ureter identification during laparoscopic abdominopelvic surgery. Nat Commun. 2019;10(1):3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsumura M, Kawaguchi Y, Kobayashi Y, Kobayashi K, Ishizawa T, Akamatsu N, et al. Indocyanine green administration a day before surgery may increase bile duct detectability on fluorescence cholangiography during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2021;28(2):202–10. [DOI] [PubMed] [Google Scholar]
- 15. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image‐guided cancer surgery using near‐infrared fluorescence. Nat Rev Clin Oncol. 2013;10(9):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bu L, Shen B, Cheng Z. Fluorescent imaging of cancerous tissues for targeted surgery. Adv Drug Deliv Rev. 2014;76:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu Z, Chan WK, Zhang Y, Tan TTY. Near‐infrared‐II activated inorganic photothermal nanomedicines. Biomaterials. 2021;269:120459. [DOI] [PubMed] [Google Scholar]
- 18. Su Y, Yu B, Wang S, Cong H, Shen Y. NIR‐II bioimaging of small organic molecule. Biomaterials. 2021;271:120717. [DOI] [PubMed] [Google Scholar]
- 19. Wang D, Tang BZ. Aggregation‐induced emission luminogens for activity‐based sensing. Acc Chem Res. 2019;52(9):2559–70. [DOI] [PubMed] [Google Scholar]
- 20. Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, et al. Aggregation‐induced emission of 1‐methyl‐1,2,3,4,5‐pentaphenylsilole. Chem Commun (Camb). 2001;18:1740–1. [DOI] [PubMed] [Google Scholar]
- 21. Hong Y, Lam JW, Tang BZ. Aggregation‐induced emission. Chem Soc Rev. 2011;40(11):5361–88. [DOI] [PubMed] [Google Scholar]
- 22. Xu W, Wang D, Tang BZ. NIR‐II AIEgens: a win‐win integration towards bioapplications. Angew Chem Int Ed Engl. 2021;60(14):7476–87. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Li S, Ma H, Wang H, Zhang R, Zhang XD. Activatable NIR‐II organic fluorescent probes for bioimaging. Theranostics. 2022;12(7):3345–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keereweer S, Van Driel PB, Snoeks TJ, Kerrebijn JD, Baatenburg de Jong RJ, Vahrmeijer AL, et al. Optical image‐guided cancer surgery: challenges and limitations. Clin Cancer Res. 2013;19(14):3745–54. [DOI] [PubMed] [Google Scholar]
- 25. Low PS, Singhal S, Srinivasarao M. Fluorescence‐guided surgery of cancer: applications, tools and perspectives. Curr Opin Chem Biol. 2018;45:64–72. [DOI] [PubMed] [Google Scholar]
- 26. Lauwerends LJ, van Driel P, Baatenburg de Jong RJ, Hardillo JAU, Koljenovic S, Puppels G, et al. Real‐time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021;22(5):e186–95. [DOI] [PubMed] [Google Scholar]
- 27. Bogani G, Cromi A, Serati M, Di Naro E, Casarin J, Pinelli C, et al. Laparoscopic and open abdominal staging for early‐stage ovarian cancer: our experience, systematic review, and meta‐analysis of comparative studies. Int J Gynecol Cancer. 2014;24(7):1241–9. [DOI] [PubMed] [Google Scholar]
- 28. Koo YJ, Kim JE, Kim YH, Hahn HS, Lee IH, Kim TJ, et al. Comparison of laparoscopy and laparotomy for the management of early‐stage ovarian cancer: surgical and oncological outcomes. J Gynecol Oncol. 2014;25(2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gallotta V, Petrillo M, Conte C, Vizzielli G, Fagotti A, Ferrandina G, et al. Laparoscopic versus laparotomic surgical staging for early‐stage ovarian cancer: a case‐control study. J Minim Invasive Gynecol. 2016;23(5):769–74. [DOI] [PubMed] [Google Scholar]
- 30. Ran X, He X, Li Z. Comparison of laparoscopic and open surgery for women with early‐stage epithelial ovarian cancer. Front Oncol. 2022;12:879889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clough KB, Ladonne JM, Nos C, Renolleau C, Validire P, Durand JC. Second look for ovarian cancer: laparoscopy or laparotomy? A prospective comparative study. Gynecol Oncol. 1999;72(3):411–7. [DOI] [PubMed] [Google Scholar]
- 32. Fagotti A, Fanfani F, Ludovisi M, Lo Voi R, Bifulco G, Testa AC, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol. 2005;96(3):729–35. [DOI] [PubMed] [Google Scholar]
- 33. Magrina JF, Cetta RL, Chang YH, Guevara G, Magtibay PM. Analysis of secondary cytoreduction for recurrent ovarian cancer by robotics, laparoscopy and laparotomy. Gynecol Oncol. 2013;129(2):336–40. [DOI] [PubMed] [Google Scholar]
- 34. Gueli Alletti S, Capozzi VA, Rosati A, De Blasis I, Cianci S, Vizzielli G, et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: a systematic review of the literature. Minerva Med. 2019;110(4):341–57. [DOI] [PubMed] [Google Scholar]
- 35. Jochum F, Vermel M, Faller E, Boisrame T, Lecointre L, Akladios C. Three and five‐year mortality in ovarian cancer after minimally invasive compared to open surgery: a systematic review and meta‐analysis. J Clin Med. 2020;9(8):2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pomel C, Akladios C, Lambaudie E, Rouzier R, Ferron G, Lecuru F, et al. Laparoscopic management of advanced epithelial ovarian cancer after neoadjuvant chemotherapy: a phase II prospective multicenter non‐randomized trial (the CILOVE study). Int J Gynecol Cancer. 2021;31(12):1572–8. [DOI] [PubMed] [Google Scholar]
- 37. Lecointre L, Pellerin M, Venkatasamy A, Fabacher T, Eberst L, Gantzer J, et al. Complete laparoscopic interval debulking surgery for advanced ovarian cancer achieves similar survival outcomes to open approach: a propensity‐matched study. J Invest Surg. 2022;35(6):1394–401. [DOI] [PubMed] [Google Scholar]
- 38. Schreiber L, Raanan C, Amsterdam A. CD24 and Nanog identify stem cells signature of ovarian epithelium and cysts that may develop to ovarian cancer. Acta Histochem. 2014;116(2):399–406. [DOI] [PubMed] [Google Scholar]
- 39. Kleinmanns K, Bischof K, Anandan S, Popa M, Akslen LA, Fosse V, et al. CD24‐targeted fluorescence imaging in patient‐derived xenograft models of high‐grade serous ovarian carcinoma. EBioMedicine. 2020;56:102782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleinmanns K, Fosse V, Davidson B, de Jalón EG, Tenstad O, Bjørge L, et al. CD24‐targeted intraoperative fluorescence image‐guided surgery leads to improved cytoreduction of ovarian cancer in a preclinical orthotopic surgical model. EBioMedicine. 2020;56:102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284–93. [DOI] [PubMed] [Google Scholar]
- 42. Liu TW, Stewart JM, Macdonald TD, Chen J, Clarke B, Shi J, et al. Biologically‐targeted detection of primary and micro‐metastatic ovarian cancer. Theranostics. 2013;3(6):420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hekman MCH, Boerman OC, Bos DL, Massuger L, Weil S, Grasso L, et al. Improved intraoperative detection of ovarian cancer by folate receptor alpha targeted dual‐modality imaging. Mol Pharm. 2017;14(10):3457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Jong JM, Hoogendam JP, Braat A, Zweemer RP, Gerestein CG. The feasibility of folate receptor alpha‐ and HER2‐targeted intraoperative fluorescence‐guided cytoreductive surgery in women with epithelial ovarian cancer: a systematic review. Gynecol Oncol. 2021;162(2):517–25. [DOI] [PubMed] [Google Scholar]
- 45. Alonso L, Gallego E, González FJ, Sánchez‐Muñoz A, Torres E, Pajares BI, et al. Gonadotropin and steroid receptors as prognostic factors in advanced ovarian cancer: a retrospective study. Clin Transl Oncol. 2009;11(11):748–52. [DOI] [PubMed] [Google Scholar]
- 46. Limonta P, Montagnani Marelli M, Mai S, Motta M, Martini L, Moretti RM. GnRH receptors in cancer: from cell biology to novel targeted therapeutic strategies. Endocr Rev. 2012;33(5):784–811. [DOI] [PubMed] [Google Scholar]
- 47. Liu Q, Zhou X, Feng W, Pu T, Li X, Li F, et al. Gonadotropin‐releasing hormone receptor‐targeted near‐infrared fluorescence probe for specific recognition and localization of peritoneal metastases of ovarian cancer. Front Oncol. 2020;10:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sundaram KM, Zhang Y, Mitra AK, Kouadio JK, Gwin K, Kossiakoff AA, et al. Prolactin receptor‐mediated internalization of imaging agents detects epithelial ovarian cancer with enhanced sensitivity and specificity. Cancer Res. 2017;77(7):1684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen J, Guo Y, Li H, Zhang C, Chang X, Ma R, et al. Near‐infrared dye‐labeled antibody COC183B2 enables detection of tiny metastatic ovarian cancer and optimizes fluorescence‐guided surgery. J Surg Oncol. 2020;122(6):1207–17. [DOI] [PubMed] [Google Scholar]
- 50. Chen J, Zhang C, Guo Y, Chang X, Ma R, Ye X, et al. Evaluation of a novel ovarian cancer‐specific fluorescent antibody probe for targeted near‐infrared fluorescence imaging. World J Surg Oncol. 2020;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kunutsor SK, Apekey TA, Van Hemelrijck M, Calori G, Perseghin G. Gamma glutamyltransferase, alanine aminotransferase and risk of cancer: systematic review and meta‐analysis. Int J Cancer. 2015;136(5):1162–70. [DOI] [PubMed] [Google Scholar]
- 52. Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma‐glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7(4):360–6. [DOI] [PubMed] [Google Scholar]
- 53. Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, et al. Rapid cancer detection by topically spraying a γ‐glutamyltranspeptidase‐activated fluorescent probe. Sci Transl Med. 2011;3(110):110ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–26. [DOI] [PubMed] [Google Scholar]
- 55. Tung CH, Qi J, Hu L, Han MS, Kim Y. A quick responsive fluorogenic pH probe for ovarian tumor imaging. Theranostics. 2015;5(10):1166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yokomizo S, Henary M, Buabeng ER, Fukuda T, Monaco H, Baek Y, et al. Topical pH sensing NIR fluorophores for intraoperative imaging and surgery of disseminated ovarian cancer. Adv Sci (Weinh). 2022;9:e2201416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou X, Liu Q, Yuan W, Li Z, Xu Y, Feng W, et al. Ultrabright NIR‐II emissive polymer dots for metastatic ovarian cancer detection. Adv Sci (Weinh). 2021;8(4):2000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhong D, Chen W, Xia Z, Hu R, Qi Y, Zhou B, et al. Aggregation‐induced emission luminogens for image‐guided surgery in non‐human primates. Nat Commun. 2021;12(1):6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor‐specific fluorescence imaging in ovarian cancer by folate receptor‐α targeting: first in‐human results. Nat Med. 2011;17(10):1315–9. [DOI] [PubMed] [Google Scholar]
- 60. Hoogstins CE, Tummers QR, Gaarenstroom KN, de Kroon CD, Trimbos JB, Bosse T, et al. A novel tumor‐specific agent for intraoperative near‐infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22(12):2929–38. [DOI] [PubMed] [Google Scholar]
- 61. Randall LM, Wenham RM, Low PS, Dowdy SC, Tanyi JL. A phase II, multicenter, open‐label trial of OTL38 injection for the intra‐operative imaging of folate receptor‐alpha positive ovarian cancer. Gynecol Oncol. 2019;155(1):63–8. [DOI] [PubMed] [Google Scholar]
- 62. Tanyi JL, Randall LM, Chambers SK, Butler KA, Winer IS, Langstraat CL, et al. A phase III study of pafolacianine injection (OTL38) for intraoperative imaging of folate receptor‐positive ovarian cancer (study 006). J Clin Oncol. 2022;Jco2200291. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 63. Voskuil FJ, Steinkamp PJ, Zhao T, van der Vegt B, Koller M, Doff JJ, et al. Exploiting metabolic acidosis in solid cancers using a tumor‐agnostic pH‐activatable nanoprobe for fluorescence‐guided surgery. Nat Commun. 2020;11(1):3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moore LS, Rosenthal EL, Chung TK, de Boer E, Patel N, Prince AC, et al. Characterizing the utility and limitations of repurposing an open‐field optical imaging device for fluorescence‐guided surgery in head and neck cancer patients. J Nucl Med. 2017;58(2):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dorval P, Mangeret N, Guillermet S, Atallah I, Righini C, Barabino G, et al. A palm‐sized high‐sensitivity near‐infrared fluorescence imager for laparotomy surgery. Phys Med. 2016;32(1):218–25. [DOI] [PubMed] [Google Scholar]
- 66. KleinJan GH, Hellingman D, van den Berg NS, van Oosterom MN, Hendricksen K, Horenblas S, et al. Hybrid surgical guidance: does hardware integration of γ‐ and fluorescence imaging modalities make sense? J Nucl Med. 2017;58(4):646–50. [DOI] [PubMed] [Google Scholar]
- 67. van den Berg NS, Miwa M, KleinJan GH, Sato T, Maeda Y, van Akkooi AC, et al. (Near‐infrared) fluorescence‐guided surgery under ambient light conditions: a next step to embedment of the technology in clinical routine. Ann Surg Oncol. 2016;23(8):2586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Behrooz A, Waterman P, Vasquez KO, Meganck J, Peterson JD, Faqir I, et al. Multispectral open‐air intraoperative fluorescence imaging. Opt Lett. 2017;42(15):2964–7. [DOI] [PubMed] [Google Scholar]
- 69. Garcia M, Edmiston C, York T, Marinov R, Mondal S, Zhu N, et al. Bio‐inspired imager improves sensitivity in near‐infrared fluorescence image‐guided surgery. Optica. 2018;5(4):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blair S, Garcia M, Davis T, Zhu Z, Liang Z, Konopka C, et al. Hexachromatic bioinspired camera for image‐guided cancer surgery. Sci Transl Med. 2021;13(592):eaw7067. [DOI] [PubMed] [Google Scholar]
- 71. Liu Y, Njuguna R, Matthews T, Akers WJ, Sudlow GP, Mondal S, et al. Near‐infrared fluorescence goggle system with complementary metal‐oxide‐semiconductor imaging sensor and see‐through display. J Biomed Opt. 2013;18(10):101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mondal SB, Gao S, Zhu N, Habimana‐Griffin L, Akers WJ, Liang R, et al. Optical see‐through cancer vision goggles enable direct patient visualization and real‐time fluorescence‐guided oncologic surgery. Ann Surg Oncol. 2017;24(7):1897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sarder P, Gullicksrud K, Mondal S, Sudlow GP, Achilefu S, Akers WJ. Dynamic optical projection of acquired luminescence for aiding oncologic surgery. J Biomed Opt. 2013;18(12):120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ringhausen E, Wang T, Pitts J, Sarder P, Akers WJ. Evaluation of dynamic optical projection of acquired luminescence for sentinel lymph node biopsy in large animals. Technol Cancer Res Treat. 2016;15(6):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kashyap A, Rapsomaniki MA, Barros V, Fomitcheva‐Khartchenko A, Martinelli AL, Rodriguez AF, et al. Quantification of tumor heterogeneity: from data acquisition to metric generation. Trends Biotechnol. 2022;40(6):647–76. [DOI] [PubMed] [Google Scholar]
- 76. Parker TM, Henriques V, Beltran A, Nakshatri H, Gogna R. Cell competition and tumor heterogeneity. Semin Cancer Biol. 2020;63:1–10. [DOI] [PubMed] [Google Scholar]
- 77. Donnem T, Reynolds AR, Kuczynski EA, Gatter K, Vermeulen PB, Kerbel RS, et al. Non‐angiogenic tumours and their influence on cancer biology. Nat Rev Cancer. 2018;18(5):323–36. [DOI] [PubMed] [Google Scholar]
- 78. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74. [DOI] [PubMed] [Google Scholar]
- 79. Boussedra S, Benoit L, Koual M, Bentivegna E, Nguyen‐Xuan HT, Bats AS, et al. Fluorescence guided surgery to improve peritoneal cytoreduction in epithelial ovarian cancer: a systematic review of available data. Eur J Surg Oncol. 2022;48(6):1217–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICMJE DISCLOSURE FORM


