Abstract
Objective
To examine the effect of interventions used to enhance cognitive function in patients experiencing cancer‐related cognitive impairment.
Methods
Studies including adults with a non‐metastatic cancer who have received chemotherapy as part of their treatment and who have undergone interventions targeting cancer‐related cognitive impairment were included. Studies involving patients with metastatic cancer and pre‐existing cognitive deficits were excluded. Academic Search Complete, CINAHL Plus with full text, MEDLINE, Education Full Text, PsycARTICLES, PsycINFO, and ERIC were searched for studies published between January 2011 and September 2022. Data extraction and quality appraisal were conducted by two authors and cross‐checked by the review team. Quality appraisal was conducted using 12 items from the Mixed Methods Appraisal Tool. Findings were presented narratively without meta‐analysis.
Results
Thirty‐one studies were included. Interventions were categorised as integrative/complementary, cognitive behavioural therapy and compensatory strategies, exercise, psychoeducational/psychosocial, brain‐training, and pharmacological. Over 100 instruments were identified, including the Functional Assessment of Cancer Therapy‐Cognitive, Trail Making Tests‐A and B, and instruments measuring secondary outcomes, including depression. Instruments often measured attention and concentration, language, memory, executive function, and/or patient‐reported outcomes. Improvements were reported, with most studies measuring some or various aspects of cognitive functioning and very few studies measuring all domains of cognitive functioning, making it difficult to draw definitive conclusions about effectiveness.
Conclusions
Various interventions are available to treat cancer‐related cognitive impairment. Outcome measurement was inconsistent and future research should prioritise using standardised measures. Current evidence, whilst not being definitive, suggests that certain interventions show greater promise than others, including cognitive behavioural therapy and brain training.
Keywords: antineoplastic agents, cancer, chemotherapy‐related cognitive impairment, cognitive dysfunction, memory, oncology, survivorship, systematic review
1. INTRODUCTION
An estimated 19.2 m new cases of cancer were diagnosed in 2020 globally. 1 Screening, early detection, treatment advancement, and ongoing surveillance contribute to improved survivorship rates. 2 , 3 , 4 Prediction models report an estimated 28 m people will be diagnosed with cancer in the year 2040. 5 In Western Europe, five‐year survival rates for breast cancer are between 80% and 90%, and between 70% and 90% for prostate cancer. 6 Increased survival demonstrates a need for aftercare prioritising health‐related quality of life. Many cancer survivors report after effects following treatment including fatigue (50%–90%), neuropathy (50%), gastric symptoms (50%) as well as cancer‐related cognitive impairment (CRCI). 7 , 8 , 9 This demonstrates the continued unmet needs of cancer survivors and a necessity to address these needs by providing long‐term support and intervention. CRCI commonly termed ‘chemo‐brain’ or ‘chemo‐fog’ affects general cognitive processes such as memory, attention and concentration, language, executive functioning often articulated as poor working memory, problems remembering, poor concentration, attention difficulties and reduced processing speed. 10 Between 12% and 75% of cancer survivors experience CRCI, with some experiencing CRCI up to 20 years following completion of treatment. 11 , 12 A range of reported incidence of CRCI may vary due to patient demographics, cancer type, chemotherapeutics type, and methods for measuring CRCI.
Chemotherapeutic agents including taxanes (e.g., paclitaxel), anthracyclines (e.g., daunorubicin), antimetabolites (e.g., methotrexate), and nitrosureas (e.g., fotemustine) are known neurotoxins associated with CRCI. 13 , 14 Although previously thought to be protected by the blood‐brain barrier, some chemotherapeutic agents have been shown to pass into cerebral tissue or contribute to neurotoxicity through the action of their metabolites. 15 The effect of cytotoxic agents in the development of CRCI is compounded by factors including adjunct therapies (e.g., radiotherapy, immunotherapy, endocrine therapy, and surgery); patient demographics (e.g., age, gender, body mass index, type of cancer, and underlying comorbidity); and patient response to cancer diagnosis and treatment (e.g., pain, fatigue, depression, and anxiety). 16 , 17 Previous research highlights this complication and the need for further studies isolating the cognitive impact of chemotherapy. 18 Despite prevalence and ongoing investigation, inconsistencies also remain between subjective experience of CRCI and objective neurophysiological measurements. 19 Neuroimaging evidence shows that cancer survivors treated with chemotherapy and who suffer from CRCI display structural changes in grey matter volume, 20 , 21 altered cerebral blood flow, 22 and disrupted dopamine activity. 23 Subjective experiences of CRCI vary amongst survivors 10 and are often associated with stress, loneliness, 24 and poor sleep quality. 25 These combined factors make quantifying the aetiology of CRCI, its standard progression, and developing appropriate interventions a complex undertaking. 10 , 26 , 27 , 28
Most up to date systematic reviews are often limited by cancer type such as breast cancer 29 or by intervention type such as non‐pharmacological, 29 or exercise‐based interventions. 30 Moreover, a current review in this area was limited to synthesising evidence from randomised controlled trials (RCTs) exclusively. 31 The present systematic review has broader inclusion criteria, encompassing a range of interventions used in various cancer types. Therefore, the aim of this systematic review was to examine the effect of interventions used to enhance cognitive function in patients with cancer who experience CRCI. This review aims to answer the following questions:
What patient‐focused interventions are available to reduce the effect of CRCI in patients with cancer?
What instruments are used to measure the impact of these interventions on cognitive function?
What is the effect of these interventions on cognitive function in patients with cancer?
2. METHODS
The formulation of the systematic review questions, inclusion and exclusion criteria, database searching, study selection process, and data extraction was guided by the Cochrane Handbook for Systematic Reviews of Interventions, 32 and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist. 33 The protocol for this systematic review was not registered a priori.
2.1. Eligibility criteria
Eligibility was determined according to the population, intervention, comparison, and outcome (PICO) framework (Table 1). 34 Studies were eligible for inclusion if they included: Population: adult patients (≥18 years) with a non‐metastatic cancer diagnosis who have received chemotherapy as part of their treatment; Intervention: any intervention targeting CRCI; Comparison: control group or baseline comparison(s); Outcome: objective and/or subjective measures of cognitive functioning.
TABLE 1.
Review eligibility criteria
| PICO framework | Eligibility criteria |
|---|---|
| Population |
|
| Intervention | Any intervention targeted at improving chemotherapy‐related cognitive impairment. |
| Comparison |
|
| Outcome |
|
Studies were excluded if patients had metastatic cancer, pre‐existing cognitive deficits, or were paediatric patients (<18 years). Studies without baseline measures of cognitive function, control group/comparison, or outcome measure of cognitive function were excluded. Secondary research publications (e.g., narrative reviews, systematic reviews, or meta‐analyses), non‐empirical studies, opinion pieces, animal studies, case series/studies, editorials, abstracts, dissertations, and thesis were also excluded. Feasibility and pilot studies were excluded since they are not sufficiently powered to detect changes in outcomes. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48
2.2. Search strategy
Studies were identified through an electronic database search of Academic Search Complete, CINAHL Plus with full text, MEDLINE, Education Full Text, PsycARTICLES, PsycINFO, and ERIC. The search strategy was guided by the PICO framework. 34 A single search strategy was agreed by all authors and was used across the seven electronic databases. This search strategy was based on a review of subject headings (e.g., MeSH terms and CINAHL headings) in the seven databases as well as a review of existing protocols investigating cognitive function and chemotherapy available through the Cochrane Database of Systematic Reviews. 49 , 50
Truncation was used and terms were combined using Boolean operators ‘OR’ and ‘AND’ and the proximity indicator for EBSCO ‘N’ as follows: (oncolog* OR cancer* OR tumo* OR neoplas* OR carcino* OR malignan*) AND (chemotherap* OR antineoplas*) AND (chemobrain OR chemofog OR ‘cognit* function’ OR ‘cognit* impair*’ OR ‘cognit* dysfunction’ OR ‘cognit* decline’ OR ‘cognit* deterioration’ OR ‘cognit* deficit’ OR ‘problem solving’ OR processing N3 speed OR ‘reaction time’ OR ‘executive functioning’ OR reasoning OR attention N3 span OR memory OR language). Searches were conducted of titles or abstracts of studies published in English from January 2011 to September 2022 to access current relevant data. Although there is no gold standard relating to selecting publications by date, the recency of scientific articles that are more than 10 years old is often questionable. 51 The search was last updated on the 16 September 2022.
2.3. Study selection
Database search results were uploaded to Covidence online software, a tool used to mainstream the production of systematic reviews. Duplicates were removed automatically, and titles and abstracts were screened. Full texts of potentially eligible studies were sourced and screened further. Two independent reviewers screened title, abstract, and full texts at random to determine eligibility. A third reviewer resolved screening conflicts. Studies not meeting the eligibility criteria were excluded. The reference lists of systematic and narrative reviews identified from the search were screened for relevant studies.
2.4. Data extraction
Independent reviewers (LO, NMC) extracted data using a pre‐designed extraction table. 51 Extracted data included: author(s), year, country, setting, study design, aim, participants (number, types of cancer and stage of treatment), intervention type, duration and format, method of data collection, outcomes measured, measurement instruments, and findings (see Table S1). Data extracted were cross‐checked by a third reviewer (MMS, PO’R, JH) to ensure accuracy.
2.5. Quality appraisal
The methodological quality of the included studies was appraised using the Mixed Methods Appraisal Tool (MMAT) 52 which includes questions tailored to different study designs. In this review, studies were categorised by design (i.e., RCTs and non‐RCTs) and their quality was appraised using the MMAT. Items were voted on a ‘yes’, ‘no’, or ‘can't tell’ basis. RCTs and non‐RCTs were assessed for sample representativeness, appropriate randomisation (for RCTs), group similarities at baseline, outcome data completeness, blinding of outcome assessors, accounting for confounders, and implementation of interventions as intended. According to MMAT guidance, ‘it is discouraged to calculate an overall score from the ratings of each criterion’ (p. 1). It is also discouraged to exclude studies based on methodological quality. 52
Quality appraisal was completed by one reviewer (LO) and accuracy was checked by a second reviewer (MMS, PO’R, JH). Studies were included in this review regardless of their quality to reduce study selection and reporting bias. 32
2.6. Data synthesis
Patients in the included studies had various cancer diagnoses and treatments received, with many studies having small sample sizes. Moreover, study designs, methodologies, statistical analyses, and outcome measures were heterogeneous. Therefore, a meta‐analysis was not completed. Instead, studies were grouped, and findings were synthesised narratively according to intervention type. This involved presenting statistical data from the included studies in tabular format as well as adopting a textual approach in the reporting of findings, 53 which were grouped and presented by intervention type as follows: (i) pharmacological, (ii) cognitive behavioural therapy (CBT) and compensatory strategies, (iii) integrative/complementary, (iv) exercise, (v) brain‐training and (vi) psychoeducational/psychosocial. Intervention categories were agreed by two reviewers (LO, PO’R). Outcome measures were assessed, and instruments were grouped according to the primary cognitive domain measured. Within these intervention categories and cognitive domain outcomes, p‐values were extracted and presented in a purposely designed table identifying categories of statistically significant results and the instrument used. Non‐statistically significant and non‐reported items were detailed, including studies reporting no p‐values.
3. RESULTS
The final search yielded 4809 records. Duplicates were removed and 2844 records were title and abstract screened. A total of 1964 irrelevant citations were excluded, and 438 studies were full text screened. Of those, 30 studies were included in the current review. An additional study was identified from hand searching. Therefore, a total of 31 studies were included in this systematic review (Figure 1).
FIGURE 1.
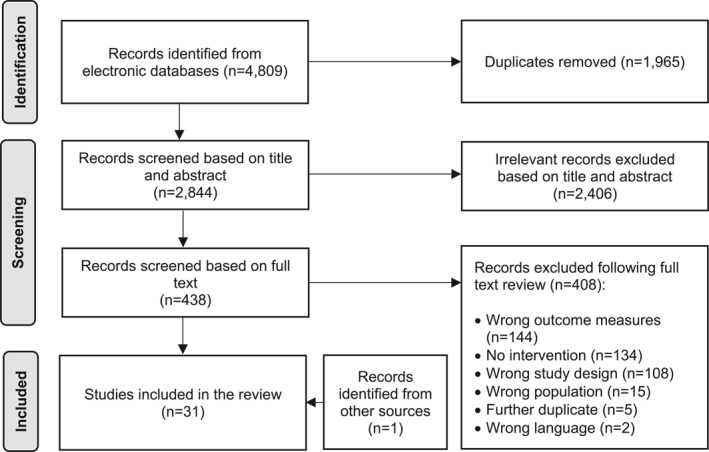
PRISMA flow diagram illustrating record identification, screening, and selection process. 33
3.1. Quality appraisal
All 31 studies had clear research questions. Analysis of reviewed RCTs (n = 27) revealed appropriate randomisation (n = 23) and comparability of groups at baseline (n = 24) in the majority of RCTs. Where groups were non‐comparable at baseline, 54 this was statistically accounted for retrospectively. One study did not report complete outcome data 55 and most RCTs (n = 18) noted appropriately blinding assessors to intervention allocation status of participants. Participants adhered to intervention as intended in most RCTs (n = 21) (See Table S2a).
Quality appraisal of non‐RCTs (n = 4) revealed that three reported a sample representative of the target population and all non‐RCTs implemented measures appropriate to outcomes and interventions assessed. Complete outcome data were reported in three non‐RCTs, however no non‐RCTs reported accounting for confounders (see Table S2b).
3.2. Study characteristics
Most of the included studies were RCTs (n = 27), conducted in China (n = 11) and the United States of America (n = 7), and focussing on breast cancer (n = 25). FACT‐Cog was the most used instrument (n = 12). Sample size varied from 26 to 242 56 , 57 , 58 participants. The full study characteristics are presented in Table 2. Included studies were grouped into five intervention types as follows: psychosocial and psychoeducational (n = 7), CBT and compensatory strategies (n = 6), integrative and complementary (n = 6), brain‐training (n = 5), exercise (n = 4), and pharmacological (n = 3) (Table 3).
TABLE 2.
Characteristics of the included studies (n = 31)
| Country |
|
| Design |
|
| Frequently used neuropsychological tools |
|
| Frequently used self‐report tools |
|
| Sample size | 2799 participants over 31 studies (range = 26–242 participants) |
| Cancer types a |
|
| Intervention type |
|
Some of the reviewed studies included more than one cancer type.
TABLE 3.
Impact of interventions on cognitive function (n = 31)
| Sample size | Cancer type | Study design | Control group | Outcomes measured | Results (p values) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Attention & concentration | Executive function | Language | Memory | Subjective measures | ||||||
| Psychoeducational/psychosocial | ||||||||||
| Akechi et al. 59 | n = 59 | Breast | RCT | Yes | SM | NR | NR | NR | NR | NS |
| Ding et al. 60 | n = 74 | Breast | RCT | Yes | EF, L, M, SM | NR | p < 0.05 (MMSE) | p < 0.05 (MMSE) | p < 0.05 (MMSE) | p < 0.05 (FACT‐Cog) p < 0.05 (PRMQ) |
| Feng & Yang 61 | n = 136 | Lung | RCT | Yes | SM | NR | NR | NR | NR | p < 0.001 (EORTC‐QLQ‐C30) |
| Gjerset et al. 58 | n = 242 | Breast | Pre & post test | No | SM | NR | NR | NR | NR | p = 0.003 (EORTC‐QLQ‐C30) |
| Jacobs et al. 62 | n = 90 | GI | 3 arm RCT | Yes | M, SM | NR | NR | NR | p = 0.006 a , p < 0.001 b , c (Arm B) & p = 0.009 b & p = 0.001 c (Arm C) (GWT) | p = 0.003 (Arm B) & p = 0.002 (Arm C) (CPR) |
| Shari et al. 55 | n = 60 | Breast | RCT | Yes | SM | NR | NR | NR | NR | p < 0.05 (FACT‐Cog[M]) |
| Xie et al. 63 | n = 86 | GI | RCT | Yes | SM | NR | NR | NR | NR | p = 0.038 (EORTC‐QLQC30) |
| Cognitive behavioural therapy/compensatory strategies | ||||||||||
| Duval et al. 64 | n = 60 | Breast | RCT | Yes | All* | NS | NS | NS | NS | NS |
| Ferguson et al. 65 | n = 31 | Breast | RCT | Yes | All | NS | NS | NS | p < 0.01 (CVLT2) | NS |
| Ferguson et al. 66 | n = 35 | Breast | RCT | Yes | A&C, L, M, SM | p = 0.003 (SDT) | NR | NS | NS | p = 0.02 (FACT‐Cog: PCI) |
| Huang et al. 67 | n = 55 | Lung | RCT | Yes | SM | NR | NR | NR | NR | NS |
| Lin et al. 68 | n = 55 | Colorectal | Experimental | Yes | SM | NR | NR | NR | NR | p = 0.002 (FACT‐Cog) |
| Tack et al. 69 | n = 121 | Mixed | RCT | Yes | SM | NR | NR | NR | NR | p < 0.005 (CFQ) |
| Integrative/complementary | ||||||||||
| Barton et al. 70 | n = 166 | Breast | RCT | Yes | All | NS | NS | NS | NS | NS |
| Chan et al. 71 | n = 59 | Ovarian | RCT | Yes | SM | NR | NR | NR | NR | p = 0.025 (EORTC‐QLQC30) d |
| Cui et al. 72 | n = 93 | Breast | RCT | Yes | A&C, L, M | p = 0.026 (MMSE) p < 0.05 (MoCA) | NR | p = 0.026 (MMSE) p < 0.05 (MoCA) | p = 0.026 (MMSE) p < 0.05 (MoCA) | NR |
| Henneghan et al. 56 | n = 26 | Breast | RCT | Yes | A&C, EF, M & SM | p = 0.002 (TMT‐A) e | p < 0.001 (COWA) e | NR | p < 0.001 (HVLT) e | p < 0.001 (FACT‐Cog) e |
| Tong et al. 73 | n = 75 | Breast | RCT | Yes | All | NS | NS | NS | p = 0.002 f (AVLT3) p = 0.002 (CDT) | p = 0.001 (FACT‐Cog) |
| Zhang et al. 74 | n = 83 | Breast | RCT | Yes | A&C, EF, M | p = 0.009 g (DS‐T) p = 0.045 h (DS‐T) | NR | NR | p = 0.009 g (DS‐T) p = 0.045 h (DS‐T) | NR |
| Brain‐training | ||||||||||
| Bray et al. 57 | n = 242 | Mixed | Longitudinal RCT | Yes | All | NS | NS | NS | NS | p < 0.001 (FACT‐Cog) |
| Dos Santos et al. 75 | n = 143 | Mixed | 3 arm RCT | Yes | All | NS | NS | NS | p = 0.03 (Arm A) (WAIS IV) | p = 0.02, p < 0.01 (Arm A) (FACT‐Cog) |
| Kesler et al. 76 | n = 41 | Breast | RCT | Yes | A&C, EF, L, M | p = 0.009 (WAIS) | p = 0.008 (WCST) | p = 0.003 (DKEFS) | p = 0.009 (WAIS) & p = 0.07 (HVLT‐R) | NR |
| Li et al. 77 | n = 80 | Breast | Experimental | No | EF & M | NR | p < 0.001 (BADS) | NR | p < 0.05 (RBMT) | NR |
| Von Ah et al. 78 | n = 82 | Breast | 3‐arm RCT | Yes | A&C, M, SM | p = 0.016 (Arm B) (UFOV) | NR | NR | p = 0.036 a & p = 0.013 e (Arm A) p = 0.004 a & p = 0.01 b (Arm B) (RAVLT) | p = 0.021 (Arm A) & p = 0.042 (Arm B) (FACT‐Cog) p = 0.003 (Arm A) & p = 0.065 (Arm B) (SSMQ) |
| Exercise | ||||||||||
| Gokal et al. 79 | n = 50 | Breast | RCT | Yes | A&C, EF, M, SM | NS | NS | NR | p = 0.03 (WAIS) | p = 0.05 (CFQ) |
| Koevoets et al. 80 | n = 181 | Breast | RCT | Yes | A&C, EF, M & SM | NR | NR | NR | NR | NR |
| Leach et al. 81 | n = 63 | Breast | Pre/post‐test | No | SM | NR | NR | NR | NR | NS |
| Wei et al. 82 | n = 70 | Breast | RCT | Yes | SM | NR | NR | NR | NR | p < 0.001 (FACT‐Cog) |
| Pharmacological | ||||||||||
| Juan et al. 83 | n = 159 | Breast | RCT | Yes | A&C, EF, M | NR | p < 0.001 (VFT) | NR | p = 0.003 a & p = 0.001 b (HVLT‐R) p = 0.001 a & p = 0.003 b (BVMT‐R) | NR |
| Lawrence et al. 84 | n = 47 | Breast | RCT | Yes | All | NS | p = 0.007 (TMT‐B) | NS | p = 0.033 i p = 0.036 j (HVLT‐R) | NS |
| Palmer et al. 54 | n = 35 | Breast | RCT | Yes | All | p = 0.02 (TMT‐A) | p < 0.001 (TMT‐B) | p < 0.001 (RAVLT) p = 0.001 k (COWA) | p = 0.002 i p < 0.001 f (RAVLT) p = 0.001 k (COWA) | NS |
Abbreviations: A&C, attention and concentration; AVLT3, Auditory Verbal Learning Test‐III; BADS, Behavioural Assessment of the Dysexecutive Syndrome; BVMT‐R, Brief Visuospatial Memory Test‐Revised; CDT, Clock Drawing Test; CFQ, Cognitive Failures Questionnaire; CFS, Cancer Fatigue Scale; CPR, Cognitive Problem Reporting; CRT, Choice Reaction Time task; CVLT2, California Verbal Learning Test‐II; DAFS, Direct Assessment of Functional Status; DEX, Dysexecutive Questionnaire; DKEFS, Delis–Kaplan Executive Function System; DS‐T, Digit Span Test; EF, executive function; EORTC‐QLQC30, European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐C30; FACT‐Cog, Functional Assessment of Cancer Therapy‐Cognitive; FACT‐Cog(M), Functional Assessment of Cancer Therapy‐Cognitive (Malay Version); GWT, Grogingen 15 Words Test; GI, gastrointestinal; Gyn, gynaecological; HVLT‐R, Hopkins Verbal Learning Test‐Revised; K‐COWA, Controlled Oral Word Association test (Korean version); L, language; M, memory; MIA, Metamemory In Adulthood questionnaire; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; MSEQ, Memory Self‐Efficacy Questionnaire; NR, not reported; NS, not significant; PCA, Perceived Cognitive Abilities; PCI, Perceived Cognitive Impairment; PRMQ, Prospective and Retrospective Memory Questionnaire; PROMIS, Patient Reported Outcomes Measurement Information System; QOL, Quality Of Life; RAVLT, Rey Auditory Verbal Learning Test; RCT, randomised controlled trial; SDT, Symbol Digit Test; SM, subjective measure; SSMQ, Squire Subjective Memory Questionnaire; SVLT, Seoul Verbal Learning Test; TMT‐A, Trail Making Test‐A; TMT‐B, Trail Making Test‐B; UFOV, Usual Field of Vision; VFT, Verbal Fluency Test; WAIS, Weschler Adult Intelligence Scale; WCST, Wisconsin Card Scoring Test.
Immediate recall.
Delayed recall.
Recognition.
Significant regression of cognitive function seen in intervention group.
Improvement seen in both groups.
Recognition.
Over time.
Between groups.
Recall.
Discrimination.
Orthographic.
3.3. Synthesis of results
3.3.1. Psychoeducational and psychosocial
Seven interventions were broadly categorised as psychoeducational and psychosocial, with interventions including collaborative care 59 and self‐affirmation. 62 Unique psychoeducational programs encouraged practicing acceptance, educated on stress management skills and strategies to improve function, muscle relaxation and self‐care. 55 , 63 This intervention category has been mainly investigated since 2019 with interventions being highly heterogeneous making comparison difficult. Six studies found statistically significant improvement in patient‐reported experience of CRCI as measured by FACT‐Cog, European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐C30 (EORTC‐QLQ‐C30), Cognitive Problem Reporting, Prospective and Retrospective Memory Questionnaire (PRMQ) and Patient Reported Outcomes Measurement Information System (PROMIS). Akechi et al. 59 found no statistically significant impact of a collaborative care intervention. However, Jacobs et al. 62 found self‐affirmation provided statistically significant protection of memory function (p = 0.006 [recall] & p = 0.001 [recognition]) when information was provided to participants relating to CRCI, as measured by the Groningen 15 words test. Ding et al. 60 trialled the Managing Cancer And Living Meaningfully (CALM) program over 3–6 months and demonstrated improved language and executive function (p < 0.05 [MMSE]), memory (p < 0.05 [PRMQ]), and patient‐reported outcomes (p < 0.05 [FACT‐Cog]). More recently, Gjerset et al. 58 found that an outpatient rehabilitation program focussing on patient education and group discussion resulted in significant improvement of subjective cognitive function (p = 0.003 [EORTC‐QLQ‐C30]). Similarly, a nursing care intervention implemented during administration of chemotherapy using advanced pain care, nutritional advice and health education led to improved subjective cognitive function (p < 0.001 [EORTC‐QLQ‐C30]). 61 Overall, six of the seven psychoeducational and psychosocial interventions were found to have a statistically significant impact on patient‐reported cognitive function.
3.3.2. Cognitive behavioural therapies and compensatory strategies
The use of CBT or a compensatory strategy was reported in six studies. All interventions were delivered face to face and ranged in duration from 8 weeks 64 , 65 , 66 to 6 months. 68 Only one study reported no statistically significant findings. 67 Ferguson et al. 65 developed a Memory and Attention Adaptation Training (MAAT) intervention tool, identifying the intervention as grounded in CBT. MAAT educates participants about normal cognitive and attention complaints after chemotherapy, self‐awareness and self‐regulation training, stress management and compensation strategies. Participants showed improvement in verbal memory (p = 0.05 [California Verbal Learning Test]). Ferguson et al. 66 further developed a video‐conference version of MAAT, conducted via eight 30–45‐min weekly sessions. The intervention was compared with physician provided reflective listening and summarisation aimed at developing awareness of and empathy for cognitive problems. The video‐conference intervention had a statistically significant impact on perceived cognitive impairment (p = 0.02 [FACT‐Cog]). Improvement was seen in language skills measured by Delis‐Kaplan Executive Function System (DKEFS) (p < 0.05), executive function measured by the Dysexecutive Questionnaire (DEX) (p < 0.05) and cognitive failures measured by the CFQ (p < 0.05). One study 64 included an element of mindfulness‐based stress reduction in addition to CBT and found no statistically significant improvement in cognitive function. Lin et al. 68 implemented a CBT‐based program combined with gentle Baduanjin exercise and found significantly improved subjective cognitive function amongst colorectal cancer patients currently undergoing chemotherapy (p < 0.001 [FACT‐Cog]). Finally, Tack et al. 69 trained breast cancer survivors in emotional freedom techniques to address worries and distress over a course of 16 weeks and found that the intervention significantly improved self‐reported cognitive function (p < 0.005 [CFQ]).
3.3.3. Integrative and complementary
Music, acupuncture, ginkgo biloba, and traditional Chinese medicine were all deemed integrative and complementary interventions. Acupuncture interventions were all implemented in China by experienced practitioners. Tong et al. 73 found acupuncture improved memory function (p = 0.002 [Auditory Verbal Learning Test (AVLT)]) and (p = 0.002 [Clock Drawing Test (CDT)]). Cui et al. 72 found improvements in attention, language and memory (p < 0.05 [Montreal Cognitive Assessment (MoCA)]) and (p = 0.026 [Mini Mental State Examination (MMSE)]). Later studies by Zhang et al. 74 found improvements in attention and concentration measured using the Digit Span Test, both over time (p = 0.009) and between groups (p = 0.045). A recent RCT 56 comparing classical music listening (control group) to mantra meditation (intervention group) found significant improvements in attention and concentration in both groups over time (p = 0.002 [TMT‐A]), executive functioning (p < 0.001 [COWA]), memory (p < 0.001 [HVLT]), and subjective functioning (p < 0.001 [FACT‐Cog]). However, no significant group by time effects were found. Barton et al. 70 investigated the Chinese herb Ginkgo Biloba and reported no statistically significant outcomes and Chan et al. 71 investigated traditional Chinese medicine and reported statistically significant decline in patient‐reported function following treatment (p = 0.025 [EORTC‐QLQ‐C30]).
3.3.4. Brain‐training
Five studies explored brain‐training interventions ranging in duration from six 78 to 16 weeks. 77 Five programs had a computerised or online element, one was home‐based, and one provided real‐time neurofeedback during training exercises. Bray et al. 57 implemented the ‘Insight’ program, a CD‐based program for use at home. Participants reported improved perceived cognitive impairment and abilities (p < 0.001 [FACT‐Cog]) following the intervention, however no statistically significant improvement was found in objective neuropsychological measures (Cogstate). Von Ah et al. 78 also implemented the computerised ‘Insight’ program to compare effect of memory training, speed of processing training, and wait‐list control group. Statistically significant improvement over time was noted in immediate (p = 0.036) and delayed memory (p = 0.013) measured using the RAVLT for the memory training group compared to the control group. Improvement was also reported in attention and concentration (p = 0.04 [post intervention] and p = 0.016 [2‐month follow up]) measured using the Useful Field Of View test (UFOV) for the speed of processing training group compared to the control group. Moreover, in comparison to the control group, significant improvement was noted in self‐reported cognitive function in the memory training group (p = 0.021 [FACT‐Cog] and p = 0.003 [Squire Subjective Memory Questionnaire]) as well as the speed of processing group (p = 0.042 [FACT‐Cog] and p = 0.065 [Squire Subjective Memory Questionnaire]). Dos Santos et al. 75 tested a computer‐based home program and reported improved perceived cognitive impairment (p = 0.02 [FACT‐Cog]). Similarly, Kesler et al. 76 tested a computer‐based program with improvement in executive function (p = 0.008 [Wisconsin Card Sorting Test]), language (p = 0.003 [Delis Kaplan Executive Function System]), and memory (p = 0.009 [WAIS] and p = 0.07 [Hopkins Verbal Learning Test‐Revised (HVLT‐R)]). Improvements in executive function (p < 0.001 [Behavioural Assessment of the Dysexecutive Syndrome]) and memory (p < 0.01 [Rivermead Behavioural Memory Test]) were reported by Li et al. 77 following olfactory and tactile stimulation in combination with a brain training program.
3.3.5. Exercise
Four studies investigated exercise interventions including a group‐based community exercise program, 81 walking, 79 breathing with light exercise 82 and aerobic exercise with Nordic power walking. 80 A community‐based exercise program found no statistically significant improvements in subjectively measured cognitive function. 81 However, Gokal et al. 79 found that a home‐based self‐implemented walking intervention over 12 weeks improved memory capacity (p = 0.03) as measured by the Welschler Adult Intelligence Scale (WAIS). Koevoets et al. 80 conducted an RCT investigating the impact of aerobic exercise with Nordic power walking. Only borderline improvement in the cognitive functioning scale of the EORTC QLQ C‐30 was noted (p‐value not reported). Wei et al. 82 reported improvements in subjective cognitive function (p < 0.001 [FACT‐Cog]) following an exercise intervention incorporating breathing and stretching exercises and mindfulness practice.
3.3.6. Pharmacological interventions
Three studies investigated pharmacological interventions for CRCI. All studies were placebo controlled RCTs investigating donepezil, 84 melatonin, 54 and probiotic supplementation. 83 Lawrence et al. 84 administered 5 mg of Donepezil daily for 6 weeks, then 10 mg daily for 18 weeks. Significant improvement was reported in recall (p = 0.033) and discrimination (p = 0.036), measured using the HVLT‐R and improvement of executive function (p = 0.007), measured with TMT‐B. Sub‐group analysis revealed greater improvements amongst breast cancer survivors with greater cognitive impairment at baseline. 84 Palmer et al. 54 hypothesised melatonin would prove neuroprotective for patients undergoing an initial cycle of adjuvant chemotherapy for breast cancer. A dose of 20 mg of melatonin was administered 1 h before bedtime for 10 days, starting 3 days prior to the first chemotherapy cycle. Participants demonstrated improvement in attention (p = 0.02 [TMT‐A]), executive function (p < 0.001 [TMT‐B]), auditory learning (p < 0.001 [RAVLT]), as well as orthographic verbal fluency (p = 0.001 [Controlled Oral Word Association test (COWA)]). Juan et al. 83 tested the effect of probiotic supplementation administered twice daily during chemotherapy. Executive function was found to be significantly improved (p < 0.001 [Verbal Fluency Test]), as were immediate recall (p = 0.003 [HVLT‐R] and p = 0.001 [Brief Visuospatial Memory Test‐Revised (BVMT‐R)]) and delayed recall (p = 0.001 [HVLT‐R] and p = 0.003 [BVMT‐R]).
4. DISCUSSION
This systematic review examined the effect of interventions used to enhance cognitive function in patients with cancer. Previous reviews of interventions are becoming increasingly dated 85 or tend to focus on pharmacologic or non‐pharmacologic interventions, but seldom both. 86 , 87 To the best of the authors' knowledge, the current systematic review is the first recent review to synthesize evidence regardless of intervention, cancer type, and experimental study design. Findings from previous systematic reviews 29 , 30 were broadly in agreement with findings from the present review. Heterogeneity in outcome measurement instruments was encountered, presenting difficulty in carrying out a meta‐analysis. However, further investigation of these instruments was carried out and comprehensive documentation and assessment of the range and variety of tools used was conducted (See Table S3). Of note, multiple pilot and feasibility studies were excluded from this review. Following up on these studies is worthwhile once at trial/full‐scale stage. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48
Studies varied in design, participant demographics, interventions, and measurement instruments utilised. Therefore, studies were grouped according to intervention type and categories subsequently emerged. Many of the psychoeducational and psychosocial, and CBT and compensatory strategy interventions were complex, specifically designed as multifaceted to target the experience of CRCI from multiple angles. For example, Lin et al. 68 combined a CBT‐based intervention with specific Baduanjin exercises supported by video material. The unique nature of many interventions underscores the importance of investigating their components in primary research to isolate effective content and modes of delivery.
Analysis of findings identified over 100 measurement tools and instruments; these are reported in detail in Table S3. Almost half the tools (n = 50) targeted secondary outcomes including anxiety (n = 6), depression (n = 5), and fatigue (n = 5). Some were utilised across many studies (e.g., FACT‐Cog [n = 12]) although instrument reliability and validity were not reported uniformly across studies. Some tools focused on measurement of one area of cognitive function (e.g., TMT‐B to measure executive function) while others had broad criteria (e.g., CogState battery). Studies also differed in identifying which cognitive domains were addressed by each instrument. It is worth noting that some studies have used instruments in a non‐conventional capacity. For example, the MMSE which is traditionally used in dementia screening was identified as measuring memory, orientation, recall and executive function, 60 , 72 as well as measuring generalised cognitive decline, without specificity. 73 Therefore, instrument test results are reported according to their identification within the primary studies.
Studies reporting on CBT and compensatory strategies used multiple instruments to measure various cognitive domains. Only Duval et al. 64 carried out cognitive measurement across objective domains, using multiple separate tools. Some studies 57 used a full range of appropriate tests, such as those encompassed in the Cogstate battery assessing attention, memory, language, emotional, and social cognition. Of note, Cogstate batteries utilise up to 14 sub‐tests including the ‘one‐back test’ and ‘identification test’. 88 These sub‐tests may be used or omitted depending on researcher preference. 88 Although the Cogstate battery in its entirety is comprehensive, comparison of Cogstate results should be informed by a thorough understanding of the sub‐scales implemented. Studies herein mainly give extensive detail of sub‐scales used 57 ; however, these vary, and close attention should be paid to ensure accurate comparison of results. Other testing methods offer flexibility in delivery such as the Stroop test 79 which can be tested both in writing and in computerised format. Many studies incorporated computerised testing which can address the risk of the Hawthorne effect, a potential confounder during in person testing. Understanding an intervention's impact requires knowledge of the outcome measures used and broadly the cognitive domains that are being assessed. For this review, measurement instruments were grouped into the broader domains of attention and concentration, executive function, language, memory, and subjective measures. The categorisation process was complex as the name of an instrument may not fully reflect all domains measured or the sub‐scales used. Table S3 details all instruments and domains assigned, to support accurate interpretation of Table 3. Some tools measured cognitive function across multiple domains (i.e., WAIS) and statistically significant results were reported in multiple domains accordingly. 76
Included exercise‐based studies (n = 4) involved only patients with breast cancer, with some recruiting participants already reporting cognitive dysfunction post‐treatment 80 and others including women prior to beginning chemotherapy. 79 , 82 Various types of exercise were investigated in these studies including a group‐based community exercise program, walking, breathing with light exercise, and aerobic exercise with Nordic power walking. Results varied whereby a Baduanjin strength training intervention 82 led to statistically significant improvements in subjectively measured cognitive function, whilst aerobic exercise and Nordic walking led to borderline improvement in subjectively measured cognitive function and a community‐based exercise program was not associated with a statistically significant improvement in subjectively measured cognitive function. Findings here contrast with a previous review 30 which included human and animal studies and concluded that exercise was a promising intervention in treating CRCI. These findings also contrast with previous reviews of exercise interventions which highlighted the benefits of exercise in ameliorating other post‐chemotherapy symptoms like fatigue. 89 , 90 Variation of exercise type may warrant further investigation to determine the most beneficial type(s) of exercise as well as significance in other variables not reported in the present review such as the effect of group‐based exercise or indoor versus outdoor exercise.
Traditional 71 and herbal 70 interventions reported no statistically significant impact, and reduced function was observed following use of traditional herbal medicine. 71 Recent investigation of acupuncture therapies found statistically significant improvements in objective and subjective cognitive function. Tong et al., 73 revealed improvement in memory, attention, concentration, and subjective cognitive function. These findings significantly positively correlated with Brain Derived Neurotrophic Factor (BDNF) levels which is an important molecule in memory, learning, and neuroplasticity. Due to the unclear nature of underpinning physiological mechanisms of CRCI, further investigation into acupuncture and the role of BDNF across a range of cancer survivors is warranted.
Most psychoeducational and psychosocial studies (n = 7) measured cognitive function utilising either FACT‐Cog, 55 , 60 or EORTC‐QLQ‐C30, 58 , 61 , 63 demonstrating some development in cohesiveness of measurement. These recent studies also reported improvement in memory function; however, only one study employed objective neuropsychological testing not focused on memory. 58 , 60 , 61 These most recent effective interventions may be limited in their application due to a failure to measure objective cognitive outcomes, focussing on subjective measures like FACT‐Cog. Therefore, there is an ongoing need for further research to determine the effect of consistently using commonly approved instruments with broad measurement criteria. Only two psychoeducational/psychosocial interventions measured memory as an objective outcome and reported statistically significant improvement in this domain, demonstrating a need for cohesive standardisation of objective measurement instruments. 60 , 62 Standardisation of measurement is becoming apparent in subjective domains; however, variations in tools and failure to measure objective outcomes is worth addressing in future research. Despite failure of most psychoeducational and psychosocial studies (n = 6) to measure outcomes beyond subjective cognitive function and memory, psychoeducational/psychosocial interventions have shown to be effective in improving subjective cognitive functioning and thereby improving the quality of life of cancer survivors.
Brain‐training interventions comprised extensive outcome measurement, with three studies 57 , 75 , 76 measuring all domains of cognitive function. Studies investigating brain‐training were heterogenous, with some including patients with cognitive impairment 57 , 77 and others not. 76 In addition, brain‐training interventions were administered either during chemotherapy, 77 once chemotherapy is completed, 78 or up to 5 years following treatment. 57 This variability makes it difficult to draw definitive conclusions regarding the effectiveness of such interventions. This requires further consideration in future research.
Recent pharmacological interventions, as well as CBT and compensatory strategy interventions provided a range of measurement across all cognitive domains, with FACT‐Cog tool and TMT‐A&B used most often. 54 , 84 Pharmacological studies showed a trend towards consistent measurement of objective cognitive function. Notably, Palmer et al. 54 investigated synthetic melatonin and was the only intervention reporting improvement in all four cognitive domains. Recent clinical trials also demonstrated improvements using melatonin to treat cancer‐related fatigue. 91 Furthermore, a recent systematic review and meta‐analysis found that melatonin supplementation significantly improved sleep quality. 92 Of note, Palmer et al.'s 54 trial included just 35 participants, melatonin was administered for only 10 days, and consecutive assessments were 10 days apart. Therefore, the use of melatonin in the treatment of CRCI necessitates further investigation to clarify any negative effects, especially considering the present results, small sample size, and short follow‐up. 41 , 54 Juan et al.'s 83 recent investigation of probiotic supplementation also demonstrated improved objectively measured cognitive function in a relatively large number of participants (n = 159). This finding is promising and warrants investigation outside the breast cancer cohort.
4.1. Limitations
This review sought to be rigorous; however, some limitations were identified. All records reviewed were in English as resources were not available to translate papers into English. Studies included in this review were heterogeneous and the review questions and eligibility criteria were broad. Therefore, a meta‐analysis was precluded. Consequently, findings from this review might not be generalisable. This review focused exclusively on studies identified through electronic database searching, which could have led to omitting potentially relevant studies published in the grey literature. Participants in the included studies had different types of primary cancers, received different chemotherapeutic agents with varying doses and durations, and were recruited at varying stages of treatment, both with and without pre‐existing CRCI. Our findings are therefore limited by the heterogeneous nature of studies included. Accounting for potential confounding variables is important within experimental studies. This was not always addressed in the included studies. For example, Oh et al. 93 found that patients receiving chemotherapy with moderate‐to‐high perceived social support experience less severe symptoms, including memory loss, compared with patients with low perceived social support.
4.2. Clinical implications
While guidelines to assessing and treating CRCI exist, 94 , 95 , 96 such guidelines are not yet based on robust evidence, nor are they specific to assessing and managing CRCI, thus limiting the ability of healthcare professionals to address this issue in routine clinical practice. These findings highlight the need for a comprehensive assessment tool for diagnosing CRCI within the clinical environment. Findings also demonstrate the variety of treatment options for patients with cancer experiencing CRCI allowing healthcare professionals to recommend the most suitable intervention for each patient. As further research is carried out, informed by these findings, the development of standardised clinical care pathways and interventions is warranted.
5. CONCLUSION
To the authors' knowledge this is the first in‐depth review exploring interventions used to manage CRCI at differing stages of cancer treatment. The variety of interventions reviewed highlights a continued lack of clarity around the complex mechanisms of CRCI and problems in assessment and treatment. In this review, although several of the interventions were found to be effective, there appears to be no clear pattern in the consistent testing and evidence of effectiveness of interventions for CRCI. Thus, there is a need for more RCTs testing interventions for CRCI.
Carers can, however, help cancer survivors navigate their experience by stressing the individuality of CRCI and recommending an individualised approach to assessment and treatment. Studies reviewed, whilst not providing conclusive evidence, suggest that certain types of interventions show greater promise than others, for example, CBT and brain training. Psychoeducational approaches like ‘CALM’ 60 and brain‐training using models like ‘Insight’ 57 have demonstrated improvements in subjective functioning, executive function, and memory. Pharmacological interventions also offer potential in addressing CRCI. There is a need for patients to be assessed by multidisciplinary teams specialising in survivorship care. Such teams can remain appraised of available treatments and develop capacity in delivering community‐based interventions, holistically tailored to the needs of patients experiencing CRCI.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGEMENTS
Open access funding provided by IReL.
Oldacres L, Hegarty J, O'Regan P, Murphy‐Coakley NM, Saab MM. Interventions promoting cognitive function in patients experiencing cancer related cognitive impairment: a systematic review. Psychooncology. 2023;32(2):214‐228. 10.1002/pon.6073
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ferlay J, Ervik M, Lam F, et al. Cancer today. In: Global Cancer Observatory. International Agency for Research on Cancer; 2020. Accessed July 21, 2021. https://gco.iarc.fr/today [Google Scholar]
- 2. Sung H, Ferlay J, Seigel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Markham MJ, Wachter K, Agarwal N, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2021;38(10):1081. 10.1200/JCO.19.03141 [DOI] [PubMed] [Google Scholar]
- 4. O'Dell W, Takita C, Casey‐Sawicki K, Daily K, Heldermon DC, Okunieff P. Projected clinical benefit of surveillance imaging for early detection and treatment of breast cancer metastases. Breast J. 2019;25(1):75‐79. 10.1111/tbj.13153 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organisation (WHO) . Estimated Number of New Cases from 2020 to 2040, Both Sexes, Age 0–85. World Health Organisation; 2021. Cancer Tomorrow. Accessed November 22, 2021. iarc.fr [Google Scholar]
- 6. Carioli G, Malvezzi M, Bertuccio P, et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol. 2021;32(4):478‐487. 10.1016/j.annonc.2021.01.006 [DOI] [PubMed] [Google Scholar]
- 7. Gegechkori N, Haines L, Lin JJ. Long‐term and latent side effects of specific cancer types. Med Clin North Am. 2017;101(6):1053‐1073. 10.1016/j.mcna.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lagergren P, Schandl A, Aaronson NK, et al. Cancer survivorship: and integral part of Europe's research agenda. Mol Oncol. 2019;13(3):624‐635. 10.1002/1878-0261.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Department of Health . National Cancer Strategy 2017–2026. Department of Health; 2017. [Google Scholar]
- 10. Bray VJ, Dhillon HM, Vardy JL. Systematic review of self‐reported cognitive function in cancer patients following chemotherapy treatment. J Cancer Surviv. 2018;12(4):537‐559. 10.1007/s11764-018-0692-x [DOI] [PubMed] [Google Scholar]
- 11. van der Willik KD, Koppelmans V, Hauptmann M, Compter A, Ikram MA, Schagen SB. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 2018;20(1):135‐144. 10.1186/s13058-018-1062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Small BJ, Lim HS. Understanding the time course of cancer‐associated cognitive decline: does impairment precede diagnosis? J Natl Cancer Inst. 2018;112(5):341‐432. 10.1093/jnci/djz179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alhowail AH, Bloemer J, Majrashi M, et al. Doxorubicin‐induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol Mech Methods. 2019;29(6):457‐466. 10.1080/15376516.2019.1600086 [DOI] [PubMed] [Google Scholar]
- 14. da Costa R, Passos GF, Quintao NLM, et al. Taxane‐induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol. 2020;177(14):3127‐3146. 10.1111/bph.15086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song BC, Bai J. Microbiome‐gut‐brain axis in cancer treatment‐related psychoneurological toxicities and symptoms: a systematic review. Support Care Cancer. 2020;29(2):605‐617. 10.1007/s00520-020-05739-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jebahi F, Sharma S, Bloss JE, Wright HH. Effects of tamoxifen on cognition and language in women with breast cancer: a systematic search and scoping review. Psycho‐Oncol. 2021;30(8):1262‐1277. 10.1002/pon.5696 [DOI] [PubMed] [Google Scholar]
- 17. Saeed O, Bernstein LJ, Fazelzad R, et al. Cognitive functioning in thyroid cancer survivors: a systematic review and meta‐analysis. J Cancer Surviv. 2019;13(2):231‐243. 10.1007/s11764-019-00745-1 [DOI] [PubMed] [Google Scholar]
- 18. Matsos A, Johnston IN. Chemotherapy induced cognitive impairments: a systematic review of the animal literature. Neurosci Biobehav Rev. 2019;102:382‐399. 10.1016/j.neubiorev.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 19. Ono M, Ogilvie JM, Wilson JS, et al. A meta‐analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol. 2015;5:1‐19. 10.3389/fonc.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niu R, Du M, Ren J, et al. Chemotherapy‐induced grey matter abnormalities in cancer survivors: a voxel‐wise neuroimaging meta‐analysis. Brain Imaging Behav. 2020:1‐13. Accessed July 6, 2021. https://link.springer.com/content/pdf/10.1007%2Fs11682‐020‐00402‐7.pdf [DOI] [PubMed] [Google Scholar]
- 21. Torrente NC, Pastor JBN, de la Osa Chaparro N. Systematic review of cognitive sequelae of non‐central nervous system cancer and cancer therapy. J Cancer Surviv. 2020;14(4):464‐482. 10.1007/s11764-020-00870-2 [DOI] [PubMed] [Google Scholar]
- 22. Chen X, He X, Tao L, et al. The attention network changes in breast cancer patients receiving neoadjuvant chemotherapy: evidence from an arterial spin labelling perfusion study. Sci Rep. 2017;7(1):42684. 10.1038/srep42684. Accessed July 6, 2021. https://www.nature.com/articles/srep42684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vitor T, Kozasa EH, Bressan RA, et al. Impaired brain dopamine transported in chemobrain patients submitted to brain SPECT imaging using the technetium‐99 labeled tracer TRODAT‐1. Ann Nucl Med. 2019;33(4):269‐279. 10.1007/s12149-019-01331-2 [DOI] [PubMed] [Google Scholar]
- 24. Henneghan A, Stuifbergen A, Becker H, Kesler S, King E. Modifiable correlates of perceived cognitive function in breast cancer survivors up to 10 years after chemotherapy completion. J Cancer Surviv. 2017;12(2):224‐233. 10.1007/s11764-017-0661-9 [DOI] [PubMed] [Google Scholar]
- 25. Henneghan AM, Carter P, Stuifbergan A, Parmelee B, Kesler S. Relationships between self‐reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psycho‐Oncol. 2018;27(8):1937‐1943. 10.1002/pon.4745 [DOI] [PubMed] [Google Scholar]
- 26. Sousa H, Almeida S, Bessa J, Pereira MG. The developmental trajectory of cancer‐related cognitive impairment in breast cancer patients: a systematic review of longitudinal neuroimaging studies. Nueropsych Rev. 2020;30(3):287‐309. 10.1007/s11065-020-09441-9 [DOI] [PubMed] [Google Scholar]
- 27. Collins B, Paquet L, Dominelli R, White A, MacKenzie J. Metamemory function in chemotherapy‐treated patients with breast cancer: an explanation for the dissociation between subjective and objective memory measures? Psycho‐Oncol. 2017;26(1):109‐117. 10.1002/pon.4012 [DOI] [PubMed] [Google Scholar]
- 28. Kim HJ, Jung SO, Kim H, Abraham I. Systematic review of longitudinal studies on chemotherapy=associated subjective cognitive impairment in cancer patients. Psycho‐Oncol. 2020;29(4):617‐631. 10.1002/pon.5339 [DOI] [PubMed] [Google Scholar]
- 29. Floyd R, Dyer AH, Kennelly SP. Non‐pharmacological interventions for cognitive impairment in women with breast cancer post‐chemotherapy: a systematic review. J Geriat Oncol. 2020;12(2):173‐181. 10.1016/j.jgo.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 30. Zimmer P, Baumann FT, Oberste M, et al. Effects of exercise interventions and physical activity behaviour on cancer related cognitive impairments: a systematic review. BioMed Res Int. 2016;2016:1‐15. 10.1155/2016/1820954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng ASK, Wang X, Niu N, Liang M, Zeng Y. Neuropsychological interventions for cancer‐related cognitive impairment: a network meta‐analysis of randomised controlled trials. Neuropsychol Rev. 2022;32(4):893‐905. 10.1007/s11065-021-09532-1 [DOI] [PubMed] [Google Scholar]
- 32. Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021. Accessed June 21, 2021. www.training.cochrane.org/handbook [Google Scholar]
- 33. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71). 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inf Decis Mak. 2007;7(1):1‐6. 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarvghadi P, Ghaffari A, Rostami HR. The effects of neurofeedback training of short‐term memory and quality of life in women with breast cancer. Int J Ther Rehabil. 2019;26(11):1‐8. 10.12968/ijtr.2018.0088 [DOI] [Google Scholar]
- 36. Myers JS, Cook‐Wiens G, Baynes R, et al. Emerging from the haze: a multicentre, controlled pilot study of a multi‐dimensional, psycho‐education‐based cognitive rehabilitation intervention for breast cancer survivors delivered with telehealth conferencing. Am J Phys Med Rehabil. 2020;101:948‐959. 10.1016/j.apmr.2020.01.021 [DOI] [PubMed] [Google Scholar]
- 37. Myers JS, Mitchell M, Krigel S, et al. Qigong intervention for breast cancer survivors with complaints of decreased cognitive function. Support Care Cancer. 2019;27(4):1395‐1403. 10.1007/s00520-018-4430-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Becker H, Henneghan AM, Volker DL, Mikan SQ. A pilot study of a cognitive‐behavioural intervention for breast cancer survivors. Onc Nurs Forum. 2017;44(2):255‐264. 10.1188/17.ONF.255-264 [DOI] [PubMed] [Google Scholar]
- 39. Campbell KL, Kam JWY, Neil‐Sztramko SE, et al. Effect of aerobic exercise on cancer‐associated cognitive impairment: a proof‐of‐concept RCT. Psycho‐Oncol. 2018;27:53‐60. 10.1002/pon4370 [DOI] [PubMed] [Google Scholar]
- 40. Komatsu H, Yagasaki K, Yamauchi H, Yamauchi T, Takebayashi T. A self‐directed home yoga programme for women with breast cancer during chemotherapy: a feasibility study. Int J Nurs. 2016;22(3):258‐266. 10.1111/ijn.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milbury K, Chaoul A, Biegler K, et al. Tibetan sound meditation for cognitive dysfunction: results from a randomised controlled pilot trial. Psycho‐Oncol. 2013;22(10):2354‐2363. 10.1002/pon.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van der Gucht K, Ahmadoun S, Melis M, et al. Effects of a mindfulness‐based intervention on cancer‐related cognitive impairment: results of a randomised controlled functional magnetic resonance imaging pilot study. J Cancer. 2020;126(18):4246‐4255. 10.1002/cncr.33074 [DOI] [PubMed] [Google Scholar]
- 43. Liang MI, Erich B, Bailey C, Jo MY, Walsh CS, Asher A. Emerging from the haze: a pilot study evaluating feasibility of a psychoeducational intervention to improve cancer‐related cognitive impairment in gynaecologic cancer survivors. J Palliat Care. 2019;34(1):32‐37. 10.1177/0825859718796794 [DOI] [PubMed] [Google Scholar]
- 44. Wolf TJ, Doherty M, Kallogjeri D, et al. The feasibility of using metacognitive strategy training to improve cognitive performance and neural connectivity in women with chemotherapy‐induced cognitive impairment. J Oncol. 2016;91(1):143‐152. 10.1159/000447744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McDougall GJ, Becker H, Acee TW, Vaughan PW, Delville CL. Symptom management of affective and cognitive disturbance with a group of cancer survivors. Arch Psychiatr Nurs. 2011;25(1):24‐35. 10.1016/j.apnu.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park JH, Jung YS, Kim KS, Bae SH. Effects of compensatory cognitive training intervention for breast cancer patients undergoing chemotherapy: a pilot study. Support Care Cancer. 2017;25(6):1887‐1896. 10.1007/s00520-017-3589-8 [DOI] [PubMed] [Google Scholar]
- 47. Leach HJ, Danyluk JM, Nishimura KC, Culos‐Reed N. Evaluation of a community based exercise program for breast cancer patients undergoing treatment. J Breast Cancer. 2015;38(6):417‐425. 10.1097/NCC.0000000000000217 [DOI] [PubMed] [Google Scholar]
- 48. Zeng Y, Cheng ASK, Song T, et al. Effects of acupuncture on cancer‐related cognitive impairment in Chinese gynaecological cancer patients: a pilot cohort study. Integr Cancer Ther. 2018;17(3):737‐746. 10.1177/1534735418777109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Forbes SC, Forbes D, Forbes S, et al. Exercise interventions for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database Syst Rev. 2015(5):CD011705. 10.1002/14651858.CD011705 [DOI] [Google Scholar]
- 50. Weigl A, Köhler N, Monsef I, et al. Intravenous iron versus oral iron versus no iron with or without erythropoiesis‐stimulating agents (ESA) for cancer patients with anaemia: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2017(4):CD012633. 10.1002/14651858.CD012633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saab MM, Fitzgerald S, Noonan B, et al. Promoting lung cancer awareness, help‐seeking and early detection: a systematic review of interventions Health. Promot Int. 2021. 10.1093/heapro/daab016/6153940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hong QN, Pluye P, Fabregues S, et al. Mixed Methods Appraisal Tool (MMAT) User Guide. Department of Family Medicine, McGill University; 2018. [Google Scholar]
- 53. Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme [internet]. Lancaster University; 2006. www.lancaster.ac.uk/media/lancaster‐university/content‐assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1‐April2006.pdf [Google Scholar]
- 54. Palmer ACS, Zortea M, Souza A, et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomised, double‐blind, placebo‐controlled trial. PLoS One. 2020;15(4):1‐24. 10.1371/journal.pone.0231379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shari NI, Zainal NZ, Ng CG. Effects of brief acceptance and commitment therapy (ACT) on subjective cognitive impairment in breast cancer patients undergoing chemotherapy. J Psychosoc Oncol. 2020;39(6):1‐20. 10.1080/07347332.2020.1856283 [DOI] [PubMed] [Google Scholar]
- 56. Henneghan AM, Becker H, Phillips C, Kesler S. Sustained effects of mantra meditation compared to music listening on neurocognitive outcomes of breast cancer survivors: a brief report of a randomised controlled trial. J Psychosom Res. 2021;150:110628. 10.1016//j.jpsychores.2021.110628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bray VJ, Dhillon HM, Bell ML, et al. Evaluation of a web‐based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. Am J Clin Oncol. 2017;35(2):217‐231. 10.1200/jco.2016.67.8201 [DOI] [PubMed] [Google Scholar]
- 58. Gjerset GM, Skaali T, Seland M, Thorsen L. Health‐related quality of life, fatigue, level of physical activity and physical capacity before and after an outpatient rehabilitation program for women within working age treated for breast cancer. J Cancer Educ. 2022. 10.1007/s13187-022-02211-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Akechi T, Momino K, Katsuki F, et al. Brief collaborative care intervention to reduce perceived unmet needs in highly distressed breast cancer patients: randomised controlled trial. Jpn J Clin Oncol. 2020;51(2):244‐251. 10.1093/jjco/hyaa166 [DOI] [PubMed] [Google Scholar]
- 60. Ding K, Zhang X, Zhao J, Zuo H, Bi Z, Cheng H. Managing cancer and living meaningfully (CALM) intervention on chemotherapy‐related cognitive impairment in breast cancer survivors. Integr Cancer Ther. 2020;19:1‐10. 10.1177/1534735420938450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng L, Yang D. Observation on the effect of high‐quality nursing intervention plus health education in chemotherapy for non‐small cell lung cancer and its influence on the physical and mental health of patients. Evid Based Complement Altern Med. 2022;2022:1‐8. 10.1155/2022/2459013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62. Jacobs W, Schagen SB, Thijssen M, Das E. Preventing adverse information effects on health outcomes: a self‐affirmation intervention reduced information‐induced cognitive decline in gastrointestinal cancer patients. Soc Sci Med. 2019;226:47‐55. 10.1016/j.socscimed.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 63. Xie J, Zhu T, Lu Q, Xu X, Cai Y, Xu Z. The effects of add‐on self‐care education on quality of life and fatigue in gastrointestinal cancer patients undergoing chemotherapy. J Altern Complement Med. 2020;20(15):1‐8. 10.1186/s12906-019-2800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Duval A, Davis CG, Khoo EL, et al. Mindfulness‐based stress reduction and cognitive function among breast cancer survivors: a randomised control trial. Cancer. 2022;128(13):2520‐2528. 10.1002/cncr.34209 [DOI] [PubMed] [Google Scholar]
- 65. Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy‐related cognitive change: results of a waitlist control trial. Psycho‐Oncol. 2012;21(2):176‐186. 10.1002/pon.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomised trial of videoconference‐delivered cognitive behavioural therapy for survivors of breast cancer with self‐reported cognitive dysfunction. Cancer. 2016;122(11):1782‐1791. 10.1002/cncr.29891 [DOI] [PubMed] [Google Scholar]
- 67. Huang CC, Kuo HP, Lin YE, Chen SE. Effects of a web‐based health education program on quality of life and symptom distress of initially diagnosed advanced non‐small cell lung cancer patients: a randomised controlled trial. J Cancer Educ. 2019;34(1):41‐49. 10.1007/s13187-017-1263-y [DOI] [PubMed] [Google Scholar]
- 68. Lin ZG, Li RD, Ai FL, Li S, Zhang XA. Effects of cognitive behavioural therapy combined with Baduanjin in patients with colorectal cancer. World J Gastrointest Oncol. 2022;13(5):2647‐2653. 10.4251/wjgo.v14.il.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tack L, Lefebvre T, Lycke M, et al. A randomised waitlist controlled trial to evaluate Emotional Freedom Techniques for self‐report cancer‐related cognitive impairment in cancer survivors (EMOTICON). E Clin Med. 2021;39:101081. 10.1016/j.eclinm.2021.101081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barton DL, Burger K, Novotny PJ, et al. The use of Ginkgo biloba for the prevention of chemotherapy‐related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support. Care Cancer. 2013;21(4):1185‐1192. 10.1007/s00520-012-1647-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chan KKL, Yao TJ, Jones B, et al. The use of Chinese herbal medicine to improve quality of life in women undergoing chemotherapy for ovarian cancer: a double‐blind placebo‐controlled randomised trial with immunological monitoring. Ann Oncol. 2011;22(10):2241‐2249. 10.1093/annonc/mdq749 [DOI] [PubMed] [Google Scholar]
- 72. Cui Z, Dong H, Yu Z, Na L, Qing Z. Clinical study on qi‐boosting and spirit regulating acupuncture on chemotherapy‐induced mild cognitive impairment in breast cancer patients. Int J Clin Acupunct. 2018;27(4):222‐227. [Google Scholar]
- 73. Tong T, Pei C, Chen J, Lv Q, Zhang F, Cheng Z. Efficacy of acupuncture therapy for chemotherapy‐related cognitive impairment in breast cancer patients. Med Sci Monit. 2018;24:2919‐2927. 10.12659/msm.909712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang ZJ, Man SC, Yam LL, et al. Electroacupuncture trigeminal nerve stimulation plus body acupuncture for chemotherapy‐induced cognitive impairment in breast cancer patients: an assessor‐participant blinded, randomised controlled trial. Brain Behav Immun. 2020;88:88‐96. 10.1016/j.bbi.2020.04.035 [DOI] [PubMed] [Google Scholar]
- 75. Dos Santos M, Hardy‐Leger I, Rigal O, et al. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: a 3‐arm randomised trial. Cancer. 2020;126(24):5328‐5336. 10.1002/cncr.33186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kesler S, Hosseini SMH, Heckler C, et al. Cognitive training for improving executive function in chemotherapy‐treated breast cancer survivors. Clin Breast Cancer. 2013;13(4):299‐306. 10.1016/j.clbc.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li Z, Hao X, Lei P, et al. Patients with breast cancer receiving chemotherapy: effects of multisensory stimulation training on cognitive impairment. Clin J Oncol Nurs. 2022;26(1):71‐78. 10.1188/22.CJON.71-77 [DOI] [PubMed] [Google Scholar]
- 78. Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomised controlled trial. Breast Cancer Res Treat. 2012;135(3):799‐809. 10.1007/s10549-012-2210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gokal K, Munir F, Ahmed S, Kancherla K, Wallis D. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS one. 2018;13(11):1‐23. 10.17605/osf.10/79pt6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Koevoets EW, Schagen SB, de Ruiter MB, et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomised control trial (PAM study). Breast Cancer Res. 2022;24(36):36. 10.1186/s13058-022-01530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leach HJ, Danyluk JM, Nishimura KC, Culos‐Reed N. Benefits of 24 versus 12 weeks of exercise and wellness programming for women undergoing treatment for breast cancer. Support Care Cancer. 2016;24(11):4597‐4606. 10.1007/s00520-016-3302-3 [DOI] [PubMed] [Google Scholar]
- 82. Wei X, Yuan R, Yang J, et al. Effects of Baduanjin exercise on cognitive function and cancer‐related symptoms in women with breast cancer receiving chemotherapy: a randomised controlled trial. Support Care Cancer. 2022;30(7):6079‐6091. 10.21203/rs.3.rs-1044265/v1 [DOI] [PubMed] [Google Scholar]
- 83. Juan Z, Chen J, Ding B, et al. Probiotic supplement attenuates chemotherapy‐related cognitive impairment in patients with breast cancer: a randomised, double‐blind, placebo controlled trial. Eur J Cancer. 2022;161:10‐22. 10.1016/j.ejca.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 84. Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self‐reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10:176‐184. 10.1007/s11764-015-0463-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther. 2012;12(2):255‐269. 10.1586/era.11.202 [DOI] [PubMed] [Google Scholar]
- 86. Karschnia P, Parsons MW, Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol. 2019;20(2):92‐102. 10.1016/S1470-2045(18)30938-0 [DOI] [PubMed] [Google Scholar]
- 87. Zeng Y, Dong J, Huang M, et al. Non‐pharmacological interventions for cancer‐related cognitive impairment in adult cancer patients: a network meta‐analysis. Int J Nurs Stud. 2020;104:1‐8. 10.1016/j.ijnurstu.2019.103514 [DOI] [PubMed] [Google Scholar]
- 88. Patel SK, Meier AM, Fernandez M, Lo TT, Moore C, Delgado N. Convergent and criterion validity of the Cogstate computerised brief battery cognitive assessment in women with and without breast cancer. Clin Neuropsychol. 2017;31(8):1375‐1386. 10.1080/13854046.2016.1275819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tomlinson D, Diorio C, Beyene J, Sung L. Effects of exercise on cancer‐related fatigue: a meta‐analysis. Am J Phys Med Rehabil. 2014;93(8):675‐686. 10.1097/phm.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 90. Kim HJ, Jung SO, Kim H, Abraham I. Systematic review of longitudinal studies on chemotherapy‐associated subjective cognitive impairment in cancer patients. Psycho‐Oncol. 2020;29(4):617‐631. 10.1002/pon.5339 [DOI] [PubMed] [Google Scholar]
- 91. Pashaki AS, Mohammadian K, Afshar S, et al. A randomised controlled parallel‐group trial on the effects of melatonin on fatigue associated with breast cancer and its adjuvant treatments. Integr Cancer Ther. 2021;20:1‐6. 10.1177/1534735420988343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fatemeh G, Sajjad M, Niloufar R, Neda S, Leila S, Khadijeh M. Effect of melatonin supplementation on sleep quality: a systematic review and meta‐analysis of randomized controlled trials. J Neurol. 2021;269:1‐12. 10.1007/s00415-020-10381-w [DOI] [PubMed] [Google Scholar]
- 93. Oh GH, Yeom CW, Shim EJ, et al. The effect of perceived social support on chemotherapy‐related symptoms in patients with breast cancer: a prospective observational study. J Psychosom Res. 2020. 10.1016/j.psychores.2019.109911 [DOI] [PubMed] [Google Scholar]
- 94. National Comprehensive Cancer Network (NCCN) . Survivorship care for cancer‐related late and long‐term effects. Chapter 5: Cognitive dysfunction. [internet] USA National Comprehensive Cancer Network; 2020 [updated 2020]. NCCN%20Guidelines%20for%20Patients%20Survivorship%20for%20Cancer‐Related%20Late%20and%20Long‐Term%20Effects
- 95. ASCO guidelines ; Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2015;34(6):611‐635. 10.1200/JCO.2015.64.3809 [DOI] [PubMed] [Google Scholar]
- 96. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326‐2347. 10.1200/jco.2018.78.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


