Clinical Standards Committee
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) is a scientific organization that encourages sound clinical practice and high‐quality teaching and research related to diagnostic imaging in women's healthcare. The ISUOG Clinical Standards Committee (CSC) has a remit to develop Practice Guidelines and Consensus Statements as educational recommendations that provide healthcare practitioners with a consensus‐based approach, from experts, for diagnostic imaging. They are intended to reflect what is considered by ISUOG to be the best practice at the time at which they are issued. Although ISUOG has made every effort to ensure that Guidelines are accurate when issued, neither the Society nor any of its employees or members accepts liability for the consequences of any inaccurate or misleading data, opinions or statements issued by the CSC. The ISUOG CSC documents are not intended to establish a legal standard of care, because interpretation of the evidence that underpins the Guidelines may be influenced by individual circumstances, local protocol and available resources. Approved Guidelines can be distributed freely with the permission of ISUOG (info@isuog.org).
Introduction
Fetal magnetic resonance imaging (MRI) is an important diagnostic imaging adjunct to ultrasonography 1 , especially for evaluation of the fetal brain, lungs and bowel 2 and the placenta 3 . The aim herein is to provide information and guidelines on fetal MRI procedures for those performing the examination, as well as for clinicians interpreting the results of the examination.
What is the purpose of fetal MRI?
The purpose of fetal MRI is to complement an expert ultrasound examination, either by confirmation of the ultrasound findings or through the acquisition of additional information. Currently, MRI is not used as a primary screening tool in prenatal care, although a standardized and almost complete assessment of the fetal anatomy is feasible 4 . However, in selected high‐risk cases (e.g. those at risk for brain abnormalities because of affected first‐degree relatives), fetal MRI may be considered as a standard method, when previous targeted ultrasound examinations were considered normal 4 .
Is fetal MRI safe?
MRI is not associated with known adverse fetal effects at any point in pregnancy when performed without administration of contrast media. 1.5 Tesla (1.5 T) or 3 T may be used; when using 3 T, machine‐specific parameters are available that regulate the level of energy deposition in order to maintain safe levels 5 . Applied radiofrequency fields may lead to heating of the fetus, which may be harmful. Thus, whole‐body radiofrequency‐field transmission is limited by the International Electrotechnical Commission to an operating mode not exceeding a specific absorption rate of 2 W/kg 6 . Postnatal studies have not demonstrated any impact on hearing or growth following prenatal MRI at 3 T 7 .
Under which circumstances should fetal MRI be performed?
There is a general consensus that fetal MRI is indicated following an expert ultrasound examination in which the diagnostic information about an abnormality is incomplete or if there is a suspicion of an abnormality that cannot be confirmed by ultrasound alone. Under these circumstances, MRI may provide important information that may confirm or complement the ultrasound findings and modify patient management.
Factors influencing the decision to perform fetal MRI include, but are not limited to: experience/equipment of the ultrasound and MRI facilities, accessibility to MRI, maternal conditions such as obesity, abdominal scarring and oligohydramnios, gestational age, safety concerns, legal considerations regarding termination of pregnancy (TOP) and parental wishes after appropriate counseling 8 .
In general, following ISUOG's minimum recommendations for second‐trimester ultrasound with basic brain examination 9 provides insufficient information to justify requesting MRI. Additional views, such as orthogonal views, higher‐frequency probes and/or transvaginal imaging are required to detail specific findings 10 . In some cases, dedicated ultrasound imaging carried out by a specialist, after routine ultrasound examination, may make the performance of MRI unnecessary 11 , 12 , 13 .
The practice of TOP and associated medicolegal implications may influence the use of fetal MRI at local institutions. In countries in which the decision about TOP has to be made before 24 weeks, the performance of MRI prior to this time may help the parents to decide on the future of their pregnancy 12 ; however, in general, MRI is better reserved for later in the second trimester or in the third trimester 14 , 15 .
Although available data are still inconclusive and are heterogeneous due to differences in local expertise and experience with ultrasound and MRI, performing MRI for parental reassurance regarding the absence of associated pathologies in fetuses with apparently isolated conditions may be recommended in fetuses with sonographically isolated findings such as ventriculomegaly (Table 1) 16 , 17 , agenesis of the corpus callosum 18 , 19 , absent septum pellucidum 20 and cerebellar or vermian anomalies 21 .
Table 1.
Ultrasound and magnetic resonance imaging (MRI) in ventriculomegaly (VM)
| Ultrasound diagnosis | Main additional abnormalities to exclude on MRI | Reported findingsref |
|---|---|---|
| Mild VM |
MCD, CC abnormalities |
|
| Moderate VM | Hemorrhagic lesions, parenchymal damage, MCD, partial CC abnormalities, infratentorial abnormalities, vascular pathologies | |
| Severe VM | Same as moderate VM |
MRI has been shown to provide reassurance in most cases with mild or moderate VM. However, in severe VM, normal outcome can be expected in only about 50% of cases with apparently isolated VM on MRI. CC, corpus callosum; MCD, malformation of cortical development; ref, reference.
Fetal MRI has been found to be complementary and clinically informative in monochorionic twin pregnancy after iatrogenic or natural demise of a cotwin, to assess the surviving twin for pathological changes and other risks for brain injury 22 , such as severe fetal growth restriction, maternal hypoxia, thrombocytopenia and infection. In this situation, MRI may be indicated both in the absence of ultrasound abnormalities 23 and on visualization of ultrasound abnormalities, such as ventriculomegaly (Table 1), that may be associated with conditions which have an impact on further prognosis.
In addition to brain and spinal abnormalities, common reasons for referral for fetal MRI include face and neck abnormalities, as well as thoracic and abdominal abnormalities. In one series, fetal MRI was employed in 15% of cases of major fetal structural abnormalities, which represented < 0.3% of pregnancies 24 . MRI may also help with further characterization of placental adhesion disorders, which usually cannot be detected or excluded without prior suspicion on ultrasound examination 3 .
Maternal conditions such as obesity, abdominal scarring and oligohydramnios are well known to impair the quality of ultrasound assessment 25 . Obesity may also impair image quality in fetal MRI. However, diagnostic images can still be acquired by MRI in most cases (Figure 1).
Fetal MRI should be considered in all cases in which its performance might provide more information for a specific clinical question than has been achieved by previous ultrasound examinations (GOOD PRACTICE POINT).
Figure 1.
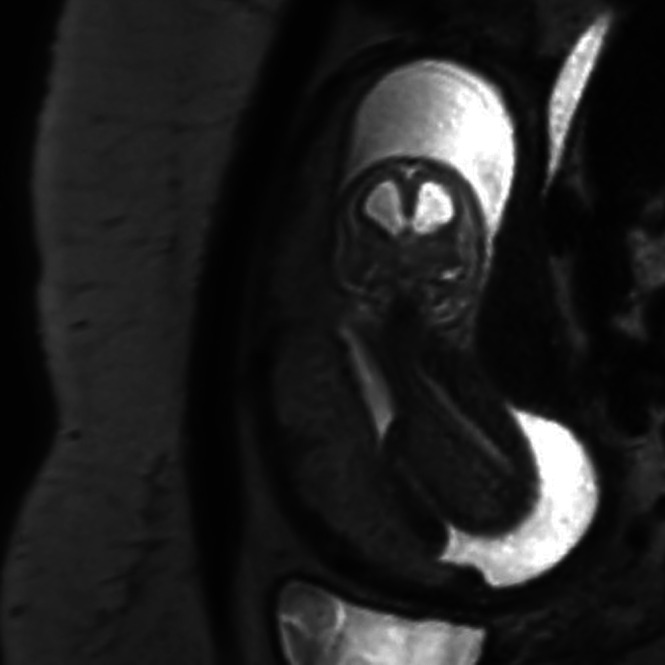
Coronal T2‐weighted magnetic resonance image in a fetus with hydrocephalus, in breech position, at 16 + 4 gestational weeks. Maternal body mass index was 33 kg/m2.
At what gestational age should fetal MRI be performed?
Fetal MRI performed before 18 weeks does not usually provide information additional to that obtained on (transvaginal) ultrasound examination. In some cases, additional information can be obtained before 22 weeks 25 , but MRI becomes helpful increasingly thereafter. Specific examples of pathologies that can be evaluated in the third trimester include, but are not limited to, those of cortical development and neck masses that may cause airway compromise 26 , 27 . Generally, organs can be visualized in detail between 26 and 32 weeks of pregnancy, when pathologies related to abnormal development are more fully evolved, but each pregnancy and each fetus will differ. It may become more difficult for the woman to remain comfortable in the scanner with advancing gestation and consideration of left‐lateral offset is recommended 28 , 29 .
Who should perform fetal MRI?
In the setting of single‐ or multicenter investigational studies including normal pregnancies without clinical indication, following ethical standards as defined elsewhere (declaration of Helsinki), fetal MRI should always be performed and assessed clinically by at least one individual with appropriate (see below) medical expertise in performing and interpreting the examination. When indicated, performed properly and interpreted correctly, MRI not only contributes to diagnosis but may be an important component of treatment choice, delivery planning and counseling. Technical setup of the scanner, onsite patient communication, including prescan safety checks and provision of information, as well as choice of appropriate protocols and techniques require extensive training, which lies beyond the scope of standard educational residency programs in radiology/pediatric radiology/neuroradiology and obstetrics/maternal–fetal medicine and can be offered only by centers with extensive practical clinical experience in fetal MRI. Thus, the performance of fetal MRI should be limited to individuals with specific training and expertise.
The same applies to interpretation of the examination. In many centers, this will require a multispecialty collaborative approach, including experts in the fields of prenatal diagnosis, perinatology, neonatology, pediatric neurology and neuroradiology, genetics and other related specialties (Table 2). This multispecialty approach allows for integration of clinical and family histories with the ultrasound and MRI findings, to optimize patient care 30 . The patient should be counseled by a subspecialist who is experienced with the particular pathology of her fetus, in order to provide her with the best counseling and management options.
Individuals who perform fetal MRI should have undergone specialized training at a teaching center, enabling them to perform state‐of‐the‐art fetal MRI (GOOD PRACTICE POINT).
Table 2.
The multidisciplinary team: proposed participants and their role in performing fetal magnetic resonance imaging (MRI)
| Participant | Role |
|---|---|
|
Obstetrician, radiologist |
Performs sonographic/neurosonographic examination; provides information to parent(s) regarding findings and possible diagnosis; provides counseling; indicates need for fetal MRI |
|
Radiologist, obstetrician |
Available during MRI examination for acquisition of appropriate planes and advises on changes of protocol as needed; interpretation and reporting of findings; provides counseling |
| Multidisciplinary team when available/necessary: obstetrician, pediatric radiologist or neuroradiologist, pediatric neurologist, geneticist, other pediatric subspecialist, social worker, psychologist |
Provides counseling and recommendations based on neurosonography, MRI, genetic findings, laboratory findings and/or family history |
Where should a practitioner train in fetal MRI?
Although we are unaware of the existence of a recognized fetal MRI qualification, individuals who perform fetal MRI should have undergone specialized training in collaboration with a teaching center, enabling them to perform a state‐of‐the‐art fetal MRI examination after exposure to a sufficient number of cases. A teaching center is defined as an institution that is able to teach students, physicians and radiographers/technologists how to perform skillfully fetal MRI. Desirable attributes of a teaching center include:
multidisciplinary case discussion meetings, including, but not limited to, fetomaternal specialists, radiologists and obstetricians;
institutional experience, with a total of at least 500 fetal MRI examinations and at least two performed per week;
publication of scientific research or reference material in this field 31 .
Performance of fetal MRI
Field strength
At present, 1.5 T (Figure 2a–c) is the most commonly used field strength, providing acceptable resolution even as early as 18 gestational weeks and not being associated with maternal discomfort related to overheating or to lengthy examination times resulting from long duration of sequences, field inhomogeneities or artifacts, as might be associated with lower or higher field strengths 32 . Yet, 3 T has the potential to achieve higher‐resolution images with a better signal‐to‐noise ratio than does 1.5 T at a comparable rate of energy deposition on tissue 33 . In addition, in some centers, only 3‐T machines are available. However, with conditions such as polyhydramnios, the use of 1.5 T is preferable to 3 T, as the former is less sensitive to fluid‐wave‐related artifacts 34 . Currently, in Europe, about 30% of MRI examinations are performed at 3T (Figure 2d–f) 35 .
Figure 2.
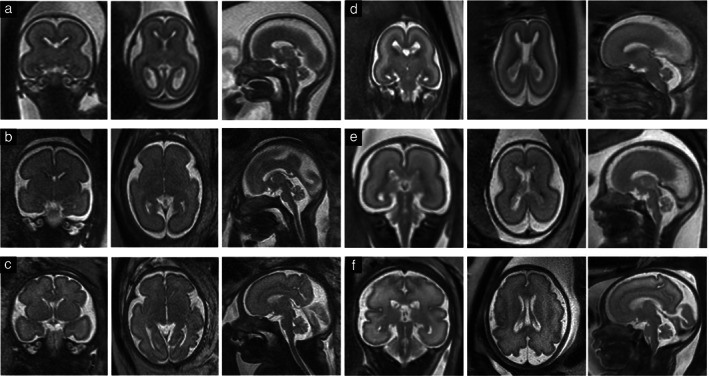
Coronal (left column), axial (middle column) and sagittal (right column) T2‐weighted fast (turbo) spin‐echo sequences of fetal brains at 1.5 Tesla (a–c) and at 3 Tesla (d–f): (a) 21 + 0 weeks; (b) 28 + 1 weeks; (c) 31 + 1 weeks; (d) 19 + 6 weeks; (e) 26 + 0 weeks; (f) 31 + 5 weeks.
Course of fetal MRI examination (see also Table 3)
Table 3.
Steps in performance of fetal magnetic resonance imaging (MRI)
| Step | Details |
|---|---|
| Indication | Dependent on individual level of previous ultrasound examination, clinical question and gestational age |
| Preparation of pregnant woman | Explanation of indication, performance, expected outcome and consequences of the procedure, information about the possibility of an accompanying person, exploration with respect to contraindications and claustrophobia and sedative drug prescription if necessary |
| Prerequisites for the MRI unit | Written referral with clear indication of the clinical question(s), ultrasound report and images (if possible), gestational age determined by first‐trimester ultrasound |
| At the MRI unit | Reiteration and clarification of possible contraindications, positioning of the woman in a comfortable position (either supine or lateral decubitus position), adequate coil positioning, performance of the appropriate protocol in the presence of the physician |
| After the examination | Informing of the patient about when the report will be ready. In case of immediate consequences resulting from the MRI examination*, informing of the referring physician |
| Storage of images, reporting | Electronic storage of images, reading of images, preferably also by a second physician, if available, then structured reporting (Table 4) |
As MRI is usually not a first‐line examination, but a complementary examination following an ultrasound examination performed in the second trimester, the emphasis of the examination and report should be on structures that are more difficult to assess with ultrasound. A detailed MRI‐based anatomical assessment may be performed on request. *Immediate consequences such as an emergency Cesarean delivery in the case of a fetal condition that could be treated more efficiently postnatally (e.g. cerebral hemorrhage, brain edema).
Exclude absolute contraindications for MRI 36 . Obtain informed consent from the pregnant woman and confirmation of a good understanding of the benefits and limitations of the MRI examination.
Note the gestational age, ideally as assessed by first‐trimester ultrasound, and pertinent prior clinical assessment and ultrasound findings.
Consider using sedation to reduce fetal movements and/or artifacts, and in exceptional cases, such as when the patient is anxious or claustrophobic.
Place the patient on the table of the scanner in a comfortable position.
In some cases, and according to the safety regulations at the particular institution, consider accommodation of an accompanying person in the examination room 8 .
Acquire localizer sequences.
Ensure correct placement of the coil, with the first organ of interest in the center of the coil, and plan for the next sequences.
Assess the primary organ of interest.
When indicated, proceed to perform a complete examination of the whole fetus and the extrafetal structures (including umbilical cord, placenta and maternal cervix).
Inform the referring physician expeditiously if a condition becomes apparent that needs rapid intervention, after gestational week 32, such as suspected placental abruption or hypoxic‐ischemic fetal brain injury.
Choice of sequences
T2‐weighted contrast is the mainstay of fetal MRI. It is usually achieved using T2‐weighted fast (turbo) spin‐echo (SE) or steady‐state free‐precession (SSFP) sequences. Fast (turbo) SE sequences with long echo time (TE) should be used in imaging of the fetal brain (Figure 2), while a shorter TE gives more contrast in the fetal body (Figure 3). SSFP sequences provide T2 information in moving fetuses, allowing, for instance, differentiation of vessels from solid tissue 37 .
T1‐weighted contrast is acquired by means of two‐dimensional (2D) gradient echo (GRE) sequences at 1.5 T. An average duration of 15 s allows its performance during maternal breath‐holding, which facilitates the acquisition of images that are free from movement artifacts 38 . At 3T, it is more difficult to achieve T1‐weighted contrast; GRE, fast spoiled GRE, SE, radial volumetric interpolated breath‐hold examination (VIBE) and Dixon sequences have been used to achieve this 35 , 39 . Recently, use of a 2D magnetization‐prepared rapid acquisition gradient echo (MP‐RAGE) sequence that does not require maternal breath‐holding was shown to be successful for T1‐weighted fetal brain imaging 40 . T1‐weighted contrast (Figure 4) identifies methemoglobin in subacute hemorrhages, calcifications, glands and meconium 38 .
Single‐shot high‐resolution (SSH) GRE echoplanar imaging (EPI) is used to visualize bony structures, calcifications and breakdown products of blood, such as deoxyhemoglobin, which suggests a recent bleed, or hemosiderin, as a residual of an older hemorrhage 41 (Figure 5).
Optional sequences include: diffusion‐weighted imaging, diffusion tensor imaging, dynamic SSFP sequences and SSH magnetic resonance cholangiopancreatography sequences, which provide three‐dimensional‐like images 42 (Figure 6).
Figure 3.
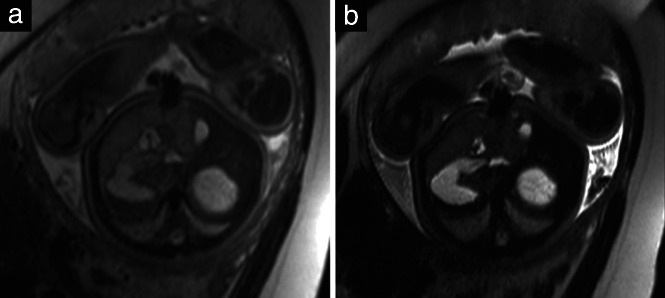
Axial T2‐weighted magnetic resonance images at level of stomach, liver and gallbladder in a 39 + 4‐week fetus, with echo time of 80 ms (a) and 140 ms (b). Haustration of meconium‐filled bowels can be seen only in (a).
Figure 4.
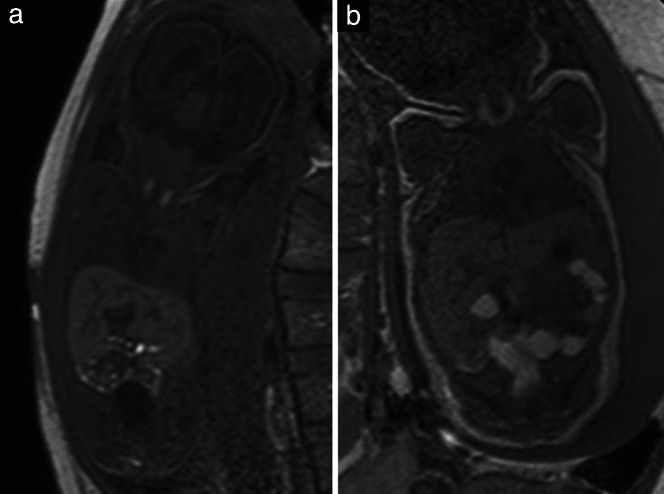
Coronal T1‐weighted magnetic resonance images in a 27 + 1‐week fetus (a) and a 38 + 3‐week fetus (b), showing the hyperintensity of the thyroid gland and meconium‐filled bowel loops.
Figure 5.
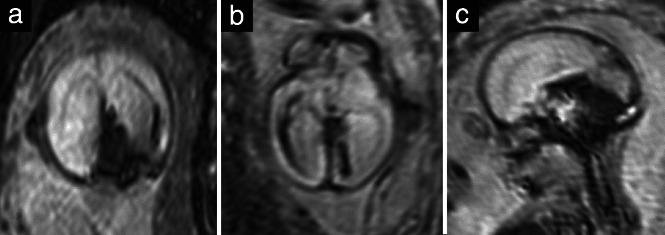
Single‐shot high‐resolution gradient echo echoplanar sequences in coronal (a), axial (b) and sagittal (c) planes in a 22 + 6‐week fetus with an intracranial hemorrhage, showing hypointense blood‐breakdown products.
Figure 6.
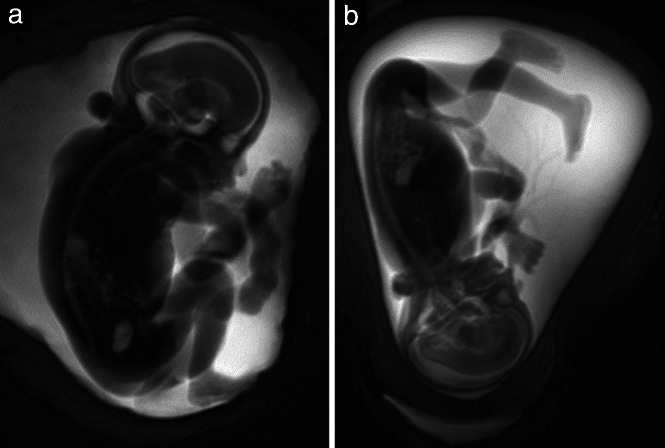
Magnetic resonance cholangiopancreatography sequence (MRCP) (40 mm thick), in a 24 + 4‐week fetus (a) and a 20 + 1‐week fetus (b), allowing detection of proportions and positions of hands and feet.
Figure 7.
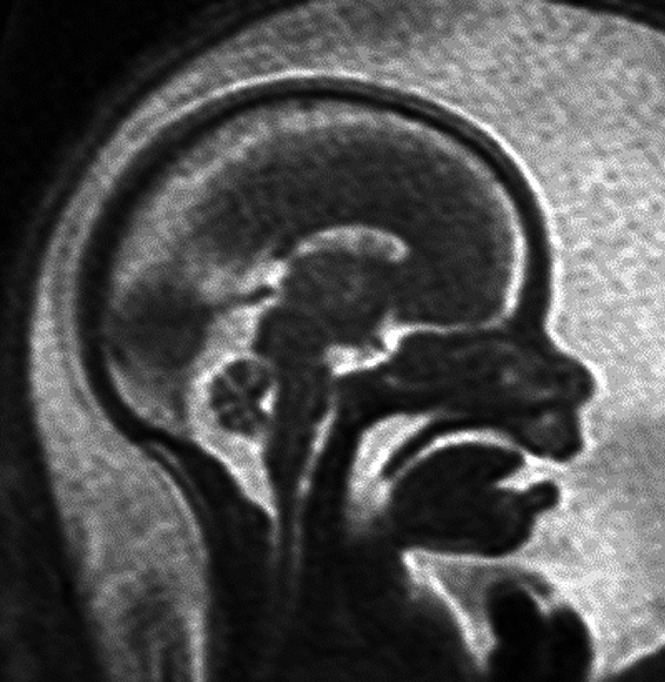
Sagittal T2‐weighted magnetic resonance image of a 21 + 5‐week fetus, showing profile with intact palate.
In all cases, the field‐of‐view should be adjusted to the region of interest and, generally, a slice thickness of 2–5 mm with a 10–15% intersection gap will be appropriate. The examination should include at least T2 information in three orthogonal planes of the fetal brain and body, and T1 and GRE‐EPI sequences in one or two orthogonal planes, preferably frontal and sagittal. This minimum protocol should be executable in under 30 min, even allowing for fetal movement and sequence repetition.
‘State‐of‐the‐art’ fetal MRI examinations should follow at least the suggested minimum protocol.
Standardized planes for fetal brain examination
Sagittal sections through the head, including a midsagittal plane depicting the corpus callosum, aqueduct of Sylvius and pituitary gland.
Coronal sections parallel to the brainstem, with symmetrical visualization of the inner ear structures.
Axial sections, perpendicular to the sagittal sections, parallel to the course of the corpus callosum (or skull base, in the case of absence of the corpus callosum), with lateral symmetry adjusted according to the coronal sections.
Standardized planes for fetal body examination
Standardized planes for examination of the fetal body are more difficult to achieve than those for fetal brain examination, as the fetus is usually in a position that will not allow strictly orthogonal placement of slices.
Sagittal sections can be achieved by placing the middle slice through the thoracic spine and the umbilical cord insertion.
Coronal sections have to be adjusted to the course of the spine (parallel to the thoracic spine and the frontal body wall at the level of the abdomen).
Axial slices should be perpendicular to the long axis of the spine at the level of the region of interest. To perform lung volumetry, for example, the axial sections should be perpendicular to the thoracic spine.
Placental assessment
A minimum fetal MRI protocol should include a SSFP sequence covering the whole uterus, to enable placental volumetry if necessary 43 . The position of the placenta needs to be documented in order to exclude placenta previa. A detailed examination of the placenta requires a specific protocol 3 that exceeds the imaging time of a fetus‐based examination.
Performance of fetal MRI according to the criteria in Table 3 will improve the management of pregnancies complicated by fetal malformation or acquired condition (GOOD PRACTICE POINT).
Figure 8.
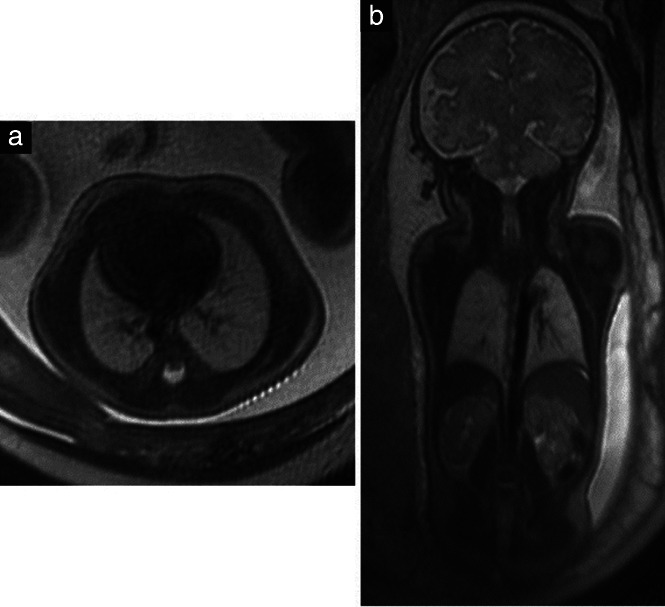
(a) Axial T2‐weighted magnetic resonance image in a 22 + 5‐week fetus showing normally shaped thorax and lungs with age‐matched regular signals. (b) Coronal T2‐weighted image showing additionally parts of the liver, kidneys and adrenal glands.
Measurements
Although, usually, measurements will already have been made with ultrasound, measuring certain structures at the MRI examination may be of benefit in particular cases 12 . Normal values for several cerebral structures have been defined by MRI 44 . Super‐resolution images 45 and machine‐learning‐based automatic measurement methods have been developed 46 . When measuring fluid‐containing structures, it is important to remember that MRI measurements are usually around 10% greater than the corresponding ultrasound measurements 47 . In lung volumetry, normal gestational‐age‐related MRI measurements correlate with fetal body volume and are considered predictive of outcome in the case of lung pathology 48 .
Storage of magnetic resonance images
The whole examination should be stored locally. In addition, digital storage enables second‐opinion assessment and future review of images in the event that new questions arise at a later time (GOOD PRACTICE POINT).
Reporting
Two types of examination should be distinguished and the type performed should be identified in the report:
A targeted examination, which assesses only for a certain category of fetal anomaly. The aim is to target a specific organ or address a particular question and not to evaluate the entire fetus.
A detailed examination, which includes a standardized evaluation of the entire fetal anatomy in a way similar to that described by the ISUOG guidelines for second‐trimester ultrasound 9 (or other locally used guidelines) (Table 3). This examination may include structures less amenable to MRI than to ultrasound examination, for example cardiac structures. Extrafetal structures, such as the umbilical cord, placenta and cervix, and the amniotic fluid (amount and signal intensity) should be described when indicated clinically. Structures not sought routinely in these examinations need to be indicated clearly in the report.
A standardized fetal MRI report, containing certain key components, should be produced (see Table 4) (GOOD PRACTICE POINT).
Table 4.
Suggested contents of structured report for detailed fetal magnetic resonance imaging examination
| Report header | Details |
|---|---|
| Method | Imaging conditions (degradation due to fetal movement, maternal obesity, premature termination of examination) and technical specifications (field strength, coil, sequences, planes) |
| Head | Normality of profile with intact hard and soft palate (Figure 7) |
| Brain | Age‐related normality of sulcation and gyration, regularity of lamination of brain parenchyma (after 30 gestational weeks: regularity of myelination and premyelination), normality of midline structures and normality of width of cerebrospinal fluid‐filled spaces (Figure 2) |
| Chest | Regularity of configuration of the thorax with age‐matched normal signals of the lungs (Figure 8). Regularity of heart on gross examination; detailed examination not performed |
| Abdomen | Stomach and gallbladder fluid‐filled, regularity of fluid (Figure 9a) and meconium signals of the bowels (Figure 4), presence of kidneys, urinary bladder fluid‐filled (Figure 9b); on request: normality of female/male external genitalia (in males: descended testes, yes/no) (Figure 10) |
| Extrafetal structures | Three‐vessel umbilical cord, normality of amniotic fluid volume, position and regularity of structure of placenta, according to age 54 , cervical length (Figure 11) if shortened |
| Skeleton (optional, if examined on request) | Course and completeness of spine, and shape, length and position of bones, including fingers and toes (if digits assessable sufficiently; this will not always be possible, particularly when amniotic fluid is minimal, after 32–35 gestational weeks) |
Figure 9.
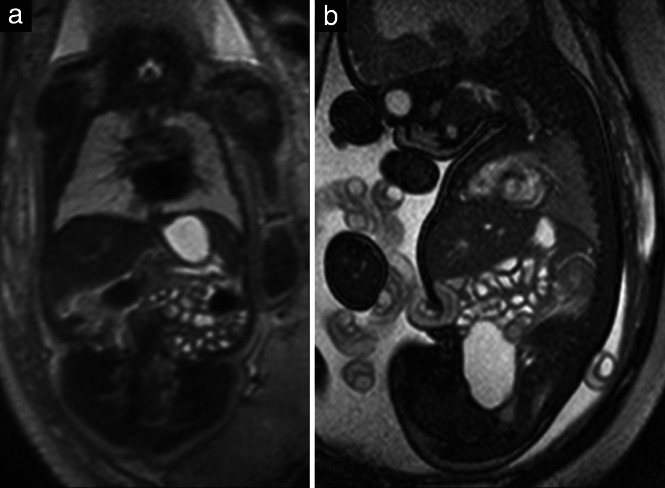
(a) Coronal T2‐weighted magnetic resonance image in a 32 + 2‐week fetus, displaying fluid‐filled stomach and bowel loops. (b) Sagittal steady‐state free‐precession (SSFP) image in a 35 + 6‐week fetus, showing in addition the fluid‐filled urinary bladder. Note the hyperintensity of the heart in this image in contrast to the T2‐weighted image (a).
Figure 10.
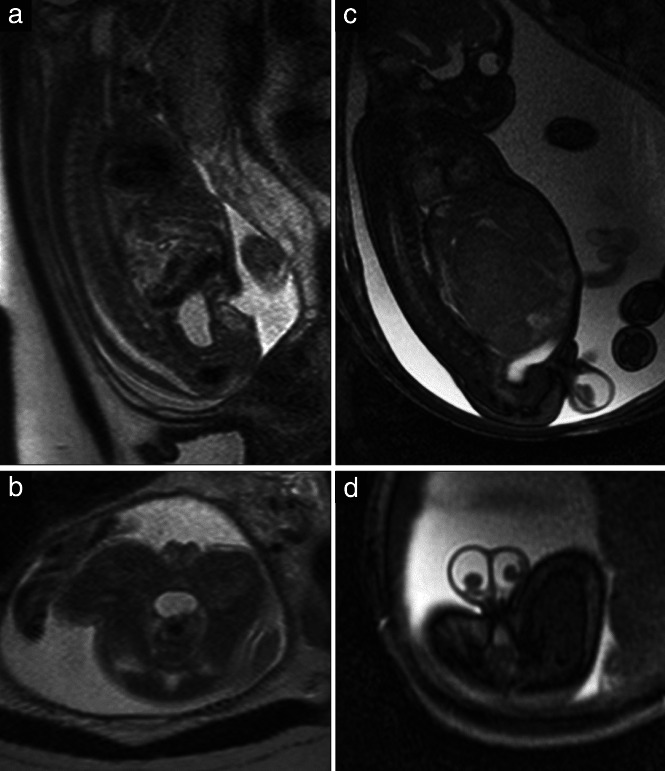
Sagittal (a) and axial (b) T2‐weighted magnetic resonance images in a 23 + 1‐week female fetus. Sagittal (c) and axial (d) steady‐state free‐precession (SSFP)‐weighted images in a 35 + 1‐week male fetus with descended testes and hydrocele as a consequence of a liver tumor.
Figure 11.
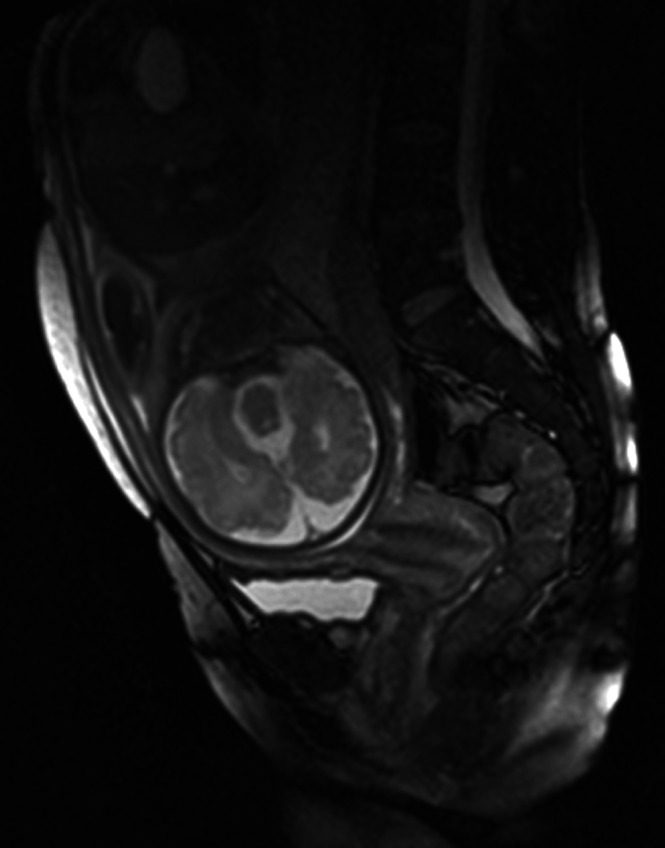
Sagittal T2‐weighted magnetic resonance image through the maternal abdomen showing a normal cervix at 33 gestational weeks.
As MRI is usually not a first‐line examination, but a complementary one following an ultrasound examination performed in the second trimester, the emphasis of the examination and report should be on structures that are more difficult to assess with ultrasound. A detailed anatomical assessment may be performed on request.
CITATION
These Guidelines should be cited as: ‘Prayer D, Malinger G, De Catte L, De Keersmaecker B, Goncalves LF, Kasprian G, Laifer‐Narin S, Lee W, Millischer A‐E, Platt L, Prayer F, Pugash D, Salomon LJ, Sanz Cortes M, Stuhr F, Timor‐Tritsch IE, Tutschek B, Twickler D, Raine‐Fenning N, on behalf of the ISUOG Clinical Standards Committee. ISUOG Practice Guidelines (updated): performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol 2023; 61: 278–287.
APPENDIX 1. Grades of recommendation and levels of evidence used in ISUOG Guidelines
| Classification of evidence levels | |
| 1 ++ | High‐quality meta‐analyses, systematic reviews of randomized controlled trials or randomized controlled trials with very low risk of bias |
| 1 + | Well‐conducted meta‐analyses, systematic reviews of randomized controlled trials or randomized controlled trials with low risk of bias |
| 1– | Meta‐analyses, systematic reviews of randomized controlled trials or randomized controlled trials with high risk of bias |
| 2 ++ | High‐quality systematic reviews of case–control or cohort studies or high‐quality case–control or cohort studies with very low risk of confounding, bias or chance and high probability that the relationship is causal |
| 2 + | Well‐conducted case–control or cohort studies with low risk of confounding, bias or chance and moderate probability that the relationship is causal |
| 2– | Case–control or cohort studies with high risk of confounding, bias or chance and significant risk that the relationship is not causal |
| 3 | Non‐analytical studies, e.g. case reports, case series |
| 4 | Expert opinion |
| Grades of recommendation | |
| A |
At least one meta‐analysis, systematic review or randomized controlled trial rated as 1 ++ and applicable directly to the target population; or a systematic review of randomized controlled trials or a body of evidence consisting principally of studies rated as 1 + applicable directly to the target population and demonstrating overall consistency of results |
| B |
Body of evidence including studies rated as 2 ++ applicable directly to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 1 ++ or 1 + |
| C | Body of evidence including studies rated as 2 + applicable directly to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2 ++ |
| D | Evidence level 3 or 4; or evidence extrapolated from studies rated as 2 + |
| Good practice point | Recommended best practice based on the clinical experience of the guideline development group |
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Hedrick HL, Flake AW, Crombleholme TM, Howell LJ, Johnson MP, Wilson RD, Adzick NS. History of Fetal Diagnosis and Therapy: Children's Hospital of Philadelphia Experience. Fetal Diagn Ther 2003; 18: 65–82. [DOI] [PubMed] [Google Scholar]
- 2. Meder JF, Ducou le Pointe H, Hédon B, Benachi A. Guidelines for coordinated radiologist/gynecologist‐obstetrician management of patients requiring fetal MRI or CT. Diagn Interv Imaging 2017; 98: 515–516. [DOI] [PubMed] [Google Scholar]
- 3. Arthuis C, Millischer AE, Bussières L, Mahallati H, Henry C, Ville Y, Salomon LJ, Grévent D. MRI based morphological examination of the placenta. Placenta 2021; 115: 20–26. [DOI] [PubMed] [Google Scholar]
- 4. Millischer AE, Sonigo P, Ville Y, Brunelle F, Boddaert N, Salomon LJ. Standardized fetal anatomical examination using magnetic resonance imaging: a feasibility study: Standardized fetal MRI anatomical exam. Ultrasound Obstet Gynecol 2013; 42: 553–559. [DOI] [PubMed] [Google Scholar]
- 5. The American College of Radiology . ACR–SPR Practice parameter for the safe and optimal performance of fetal magnetic resonance imaging (MRI) [Internet]. 2020. https://www.acr.org/‐/media/ACR/Files/Practice‐Parameters/mr‐fetal.pdf.
- 6. International Electrotechnical Commission . Medical electrical equipment‐Part 2–33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. IEC 60601‐2‐33. 3.0. 2010. [Google Scholar]
- 7. Chartier AL, Bouvier MJ, McPherson DR, Stepenosky JE, Taysom DA, Marks RM. The Safety of Maternal and Fetal MRI at 3 T. Am J Roentgenol 2019; 213: 1170–1173. [DOI] [PubMed] [Google Scholar]
- 8. Leithner K, Prayer D, Porstner E, Kapusta ND, Stammler‐Safar M, Krampl‐Bettelheim E, Hilger E. Psychological reactions related to fetal magnetic resonance imaging: a follow‐up study. J Perinat Med 2013; 41: 273–276. [DOI] [PubMed] [Google Scholar]
- 9. Salomon LJ, Alfirevic Z, Berghella V, Bilardo CM, Chalouhi GE, Da Silva Costa F, Hernandez‐Andrade E, Malinger G, Munoz H, Paladini D, Prefumo F, Sotiriadis A, Toi A, Lee W. ISUOG Practice Guidelines (updated): performance of the routine mid‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022; 59: 840–856. [DOI] [PubMed] [Google Scholar]
- 10. Jabaz D, Abed M. Sonography 2nd Trimester Assessment, Protocols, And Interpretation. StatPearls [Internet]. StatPearls Publishing: Treasure Island, FL, 2022. http://www.ncbi.nlm.nih.gov/books/NBK570574/. [PubMed]
- 11. Malinger G, Paladini D, Haratz KK, Monteagudo A, Pilu GL, Timor‐Tritsch IE. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet Gynecol 2020; 56: 476–484. [DOI] [PubMed] [Google Scholar]
- 12. Paladini D, Malinger G, Birnbaum R, Monteagudo A, Pilu G, Salomon LJ, Timor‐Tritsch IE. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol 2021; 57: 661–671. [DOI] [PubMed] [Google Scholar]
- 13. Timor‐Tritsch IE, Monteagudo A, Pilu G, Herausgeber. Ultrasonography of the prenatal brain. 3rd edn. McGraw‐Hill Professional: New York, 2012; 490 S. [Google Scholar]
- 14. Caro‐Domínguez P, García‐Díaz L, Rebollo Polo M. Encuesta sobre la situación actual de la resonancia magnética fetal en España. [Survey about the current use of fetal MRI in Spain] Radiología 2021. 10.1016/j.rx.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 15. Expert Panel on GYN and OB Imaging ; Sussman BL, Chopra P, Poder L, Bulas DI, Burger I, Feldstein VA, Laifer‐Narin SL, Oliver ER, Strachowski LM, Wang EY, Winter T, Zelop CM, Glanc P. ACR Appropriateness Criteria® Second and Third Trimester Screening for Fetal Anomaly. J Am Coll Radiol 2021; 18: S189–S198. [DOI] [PubMed] [Google Scholar]
- 16. Society for Maternal‐Fetal Medicine (SMFM) ; Fox NS, Monteagudo A, Kuller JA, Craigo S, Norton ME. Mild fetal ventriculomegaly: diagnosis, evaluation, and management. Am J Obstet Gynecol 2018; 219: B2–B9. [DOI] [PubMed] [Google Scholar]
- 17. Barzilay E, Bar‐Yosef O, Dorembus S, Achiron R, Katorza E. Fetal Brain Anomalies Associated with Ventriculomegaly or Asymmetry: An MRI‐Based Study. Am J Neuroradiol 2017; 38: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sileo FG, Di Mascio D, Rizzo G, Caulo M, Manganaro L, Bertucci E, Masmejan S, Liberati M, D'Amico A, Nappi L, Buca D, Van Mieghem T, Khalil A, D'Antonio F. Role of prenatal magnetic resonance imaging in fetuses with isolated agenesis of corpus callosum in the era of fetal neurosonography: A systematic review and meta‐analysis. Acta Obstet Gynecol Scand 2021; 100: 7–16. [DOI] [PubMed] [Google Scholar]
- 19. ENSO Working Group . Role of prenatal magnetic resonance imaging in fetuses with isolated anomalies of corpus callosum: multinational study. Ultrasound Obstet Gynecol 2021; 58: 26–33. [DOI] [PubMed] [Google Scholar]
- 20. Borkowski‐Tillman T, Garcia‐Rodriguez R, Viñals F, Branco M, Kradjen‐Haratz K, Ben‐Sira L, Lerman‐Sagie T, Malinger G. Agenesis of the septum pellucidum: Prenatal diagnosis and outcome. Prenat Diagn 2020; 40: 674–680. [DOI] [PubMed] [Google Scholar]
- 21. Schlatterer SD, Sanapo L, du Plessis AJ, Whitehead MT, Mulkey SB. The Role of Fetal MRI for Suspected Anomalies of the Posterior Fossa. Pediatr Neurol 2021; 117: 10–18. [DOI] [PubMed] [Google Scholar]
- 22. Cruciat G, Nemeti GI, Popa‐Stanila R, Florian A, Goidescu IG. Imaging diagnosis and legal implications of brain injury in survivors following single intrauterine fetal demise from monochorionic twins – a review of the literature. J Perinat Med 2021; 49: 837–846. [DOI] [PubMed] [Google Scholar]
- 23. Moltoni G, Talenti G, Righini A. Brain fetal neuroradiology: a beginner's guide. Transl Pediatr 2021; 10: 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herrera CL, Byrne JJ, Clark HR, Twickler DM, Dashe JS. Use of Fetal Magnetic Resonance Imaging After Sonographic Identification of Major Structural Anomalies. J Ultrasound Med 2020; 39: 2053–2058. [DOI] [PubMed] [Google Scholar]
- 25. Reddy UM, Abuhamad AZ, Levine D, Saade GR. Fetal imaging: Executive Summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal‐Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. Am J Obstet Gynecol 2014; 210: 387–397. [DOI] [PubMed] [Google Scholar]
- 26. Ng TW, Xi Y, Schindel D, Beavers A, Santiago‐Munoz P, Bailey AA, Twickler DM. Fetal Head and Neck Masses: MRI Prediction of Significant Morbidity. Am J Roentgenol 2019; 212: 215–221. [DOI] [PubMed] [Google Scholar]
- 27. Twickler DM, Magee KP, Caire J, Zaretsky M, Fleckenstein JL, Ramus RM. Second‐opinion magnetic resonance imaging for suspected fetal central nervous system abnormalities. Am J Obstet Gynecol 2003; 188: 492–496. [DOI] [PubMed] [Google Scholar]
- 28. Hughes EJ, Price AN, McCabe L, Hiscocks S, Waite L, Green E, Hutter J, Pegoretti K, Cordero‐Grande L, Edwards AD, Hajnal JV, Rutherford MA. The effect of maternal position on venous return for pregnant women during MRI. NMR Biomed 2021; 34: e4475. [DOI] [PubMed] [Google Scholar]
- 29. Chapman T, Alazraki AL, Eklund MJ. A survey of pediatric diagnostic radiologists in North America: current practices in fetal magnetic resonance imaging. Pediatr Radiol 2018; 48: 1924–1935. [DOI] [PubMed] [Google Scholar]
- 30. Cassart M, Garel C. European overview of current practice of fetal imaging by pediatric radiologists: a new task force is launched. Pediatr Radiol 2020; 50: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 31. Prayer D, Malinger G, Brugger PC, Cassady C, De Catte L, De Keersmaecker B, Fernandes GL, Glanc P, Gonçalves LF, Gruber GM, Laifer‐Narin S, Lee W, Millischer AE, Molho M, Neelavalli J, Platt L, Pugash D, Ramaekers P, Salomon LJ, Sanz M, Timor‐Tritsch IE, Tutschek B, Twickler D, Weber M, Ximenes R, Raine‐Fenning N. ISUOG Practice Guidelines: performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol 2017; 49: 671–680. [DOI] [PubMed] [Google Scholar]
- 32. Victoria T, Jaramillo D, Roberts TPL, Zarnow D, Johnson AM, Delgado J, Rubesova E, Vossough A. Fetal magnetic resonance imaging: jumping from 1.5 to 3 tesla (preliminary experience). Pediatr Radiol 2014; 44: 376–386. [DOI] [PubMed] [Google Scholar]
- 33. Priego G, Barrowman NJ, Hurteau‐Miller J, Miller E. Does 3T Fetal MRI Improve Image Resolution of Normal Brain Structures between 20 and 24 Weeks' Gestational Age? Am J Neuroradiol 2017; 38: 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagaraj UD, Calvo‐Garcia MA, Merrow AC, Zhang B, Tkach JA, Kline‐Fath BM. Utilization of 3‐T fetal magnetic resonance imaging in clinical practice: a single‐institution experience. Pediatr Radiol 2021; 51: 1798–1808. [DOI] [PubMed] [Google Scholar]
- 35. Colleran GC, Kyncl M, Garel C, Cassart M. Fetal magnetic resonance imaging at 3 Tesla – the European experience. Pediatr Radiol 2022; 52: 959–970. [DOI] [PubMed] [Google Scholar]
- 36. Mittendorff L, Young A, Sim J. A narrative review of current and emerging MRI safety issues: What every MRI technologist (radiographer) needs to know. J Med Radiat Sci 2022; 69: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brugger PC, Stuhr F, Lindner C, Prayer D. Methods of fetal MR: beyond T2‐weighted imaging. Eur J Radiol 2006; 57: 172–181. [DOI] [PubMed] [Google Scholar]
- 38. Asenbaum U, Brugger PC, Woitek R, Furtner J, Prayer D. Indikationen und Technik der fetalen Magnetresonanztomographie. Radiologe 2013; 53: 109–115. [DOI] [PubMed] [Google Scholar]
- 39. Liao Y, Li X, Jia F, Ye Z, Ning G, Liu S, Li P, Fu C, Li Q, Wang S, Zhang H, Qu H. Optimization of the image contrast for the developing fetal brain using 3D radial VIBE sequence in 3 T magnetic resonance imaging. BMC Med Imaging 2022; 22: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrazzi G, Price AN, Teixeira RPAG, Cordero‐Grande L, Hutter J, Gomes A, Padormo F, Hughes E, Schneider T, Rutherford M, Kuklisova Murgasova M, Hajnal JV. An efficient sequence for fetal brain imaging at 3T with enhanced T1 contrast and motion robustness. Magn Reson Med 2018; 80: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prayer D, Brugger PC, Kasprian G, Witzani L, Helmer H, Dietrich W, Eppel W, Langer M. MRI of fetal acquired brain lesions. Eur J Radiol 2006; 57: 233–249. [DOI] [PubMed] [Google Scholar]
- 42. Lo J, Lim A, Wagner MW, Ertl‐Wagner B, Sussman D. Fetal Organ Anomaly Classification Network for Identifying Organ Anomalies in Fetal MRI. Front Artif Intell 2022; 5: 832485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peterson HF, Eskild A, Sommerfelt S, Gjesdal K, Borthne AS, Mørkrid L, Hillestad V. Percentiles of intrauterine placental volume and placental volume relative to fetal volume: A prospective magnetic resonance imaging study. Placenta 2022; 121: 40–45. [DOI] [PubMed] [Google Scholar]
- 44. Di Mascio D, Khalil A, Rizzo G, Kasprian G, Caulo M, Manganaro L, Odibo AO, Flacco ME, Giancotti A, Buca D, Liberati M, Timor‐Tritsch IE, D'Antonio F. Reference ranges for fetal brain structures using magnetic resonance imaging: systematic review. Ultrasound Obstet Gynecol 2022; 59: 296–303. [DOI] [PubMed] [Google Scholar]
- 45. Khawam M, de Dumast P, Deman P, Kebiri H, Yu T, Tourbier S, Lajous H, Hagmann P, Maeder P, Thiran JP, Meuli R, Dunet V, Bach Cuadra M, Koob M. Fetal Brain Biometric Measurements on 3D Super‐Resolution Reconstructed T2‐Weighted MRI: An Intra‐ and Inter‐observer Agreement Study. Front Pediatr August 2021; 9: 639746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Avisdris N, Yehuda B, Ben‐Zvi O, Link‐Sourani D, Ben‐Sira L, Miller E, Zharkov E, Ben Bashat D, Joskowicz L. Automatic linear measurements of the fetal brain on MRI with deep neural networks. Int J Comput Assist Radiol Surg 2021; 16: 1481–1492. [DOI] [PubMed] [Google Scholar]
- 47. Behrendt N, Zaretsky MV, West NA, Galan HL, Crombleholme TM, Meyers ML. Ultrasound versus MRI: is there a difference in measurements of the fetal lateral ventricles? J Matern Fetal Neonatal Med 2017; 30: 298–301. [DOI] [PubMed] [Google Scholar]
- 48. Zamora IJ, Sheikh F, Cassady CI, Olutoye OO, Mehollin‐Ray AR, Ruano R, Lee TC, Welty SE, Belfort MA, Ethun CG, Kim ME, Cass DL. Fetal MRI lung volumes are predictive of perinatal outcomes in fetuses with congenital lung masses. J Pediatr Surg 2014; 49: 853–858. [DOI] [PubMed] [Google Scholar]
- 49. Pagani G, Thilaganathan B, Prefumo F. Neurodevelopmental outcome in isolated mild fetal ventriculomegaly: systematic review and meta‐analysis: Neurodevelopmental outcome of fetal ventriculomegaly. Ultrasound Obstet Gynecol 2014; 44: 254–260. [DOI] [PubMed] [Google Scholar]
- 50. Scelsa B, Rustico M, Righini A, Parazzini C, Balestriero MA, Introvini P, Spaccini L, Mastrangelo M, Lista G, Zuccotti GV, Veggiotti P. Mild ventriculomegaly from fetal consultation to neurodevelopmental assessment: A single center experience and review of the literature. Eur J Paediatr Neurol 2018; 22: 919–928. [DOI] [PubMed] [Google Scholar]
- 51. Li Z, Pan L, Chen Y, Meng D, Liu Y, Li L, Liu M, Luo Z. The value of prenatal magnetic resonance imaging and postnatal follow‐up using Gesell Developmental Schedules score for mild‐to‐moderate simple bilateral fetal ventriculomegaly. J Matern Fetal Neonatal Med 2022; 35: 6229–6235. [DOI] [PubMed] [Google Scholar]
- 52. Griffiths PD, Jarvis D, Connolly DJ, Mooney C, Embleton N, Hart AR. Predicting neurodevelopmental outcomes in fetuses with isolated mild ventriculomegaly. Arch Dis Child Fetal Neonatal Ed 2022; 107: 431–436. [DOI] [PubMed] [Google Scholar]
- 53. Mirsky DM, Stence NV, Powers AM, Dingman AL, Neuberger I. Imaging of fetal ventriculomegaly. Pediatr Radiol 2020; 50: 1948–1958. [DOI] [PubMed] [Google Scholar]
- 54. Ho A, Chappell LC, Story L, Al‐Adnani M, Egloff A, Routledge E, Rutherford M, Hutter J. Visual assessment of the placenta in antenatal magnetic resonance imaging across gestation in normal and compromised pregnancies: Observations from a large cohort study. Placenta 2022; 117: 29–38. [Corrigendum: Placenta 2022; 119: 31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


