Abstract
Obesity is a chronic, progressive, relapsing, and treatable multifactorial, neurobehavioral disease. According to the World Health Organization, obesity affects 15% of women and has long‐term effects on women's health. The focus of care in patients with obesity should be on optimizing health outcomes rather than on weight loss. Appropriate and common language, considering cultural sensitivity and trauma‐informed care, is needed to discuss obesity. Pregnancy is a time of significant physiological change. Pre‐, ante‐, and postpartum clinical encounters provide opportunities for health optimization for parents with obesity in terms of, but not limited to, fertility and breastfeeding. Pre‐existing conditions may also be identified and managed. Beyond pregnancy, women with obesity are at an increased risk for gastrointestinal and liver diseases, impaired kidney function, obstructive sleep apnea, and venous thromboembolism. Gynecological and reproductive health of women living with obesity cannot be dismissed, with accommodations needed for preventive health screenings and consideration of increased risk for gynecologic malignancies. Mental wellness, specifically depression, should be screened and managed appropriately. Obesity is a complex condition and is increasing in prevalence with failure of public health interventions to achieve significant decrease. Future research efforts should focus on interprofessional care and discovering effective interventions for health optimization.
Keywords: best practice, FIGO, health optimization, management, obesity, obesity‐related comorbidities, pregnancy, women's life course
Synopsis
Obesity is a complex and multifactorial medical condition that requires important considerations over a woman's life course and during pregnancy.
1. WHAT IS OBESITY AND WHY DOES IT MATTER TO WOMEN'S HEALTH?
Obesity is defined by the World Health Organization (WHO) as abnormal or excessive adipose accumulation that puts health at risk. 1 The most common method to calculate obesity is body mass index (BMI) (Table 1).
TABLE 1.
World Health Organization body mass index classification a
| Category | BMI |
|---|---|
| Underweight | <19 |
| Normal weight | 20–24.9 |
| Overweight | 25–29.9 |
| Obesity class I | 30–34.9 |
| Obesity class II | 35–39.9 |
| Obesity class III | >40 |
Calculated as weight in kilograms divided by height in meters squared.
According to the Obesity Medicine Association 2021 definition, ‘obesity is defined as a chronic, progressive, relapsing and treatable multi‐factorial, neurobehavioural disease’. 2 Thus, the presence of excess adipose or fat tissue causes endocrine and physical changes to the body's health and functioning, as well as impacting psychosocial well‐being. As a result, obesity has been shown to cause long‐term effects on many aspects of women's health (Figure 1). 3
FIGURE 1.
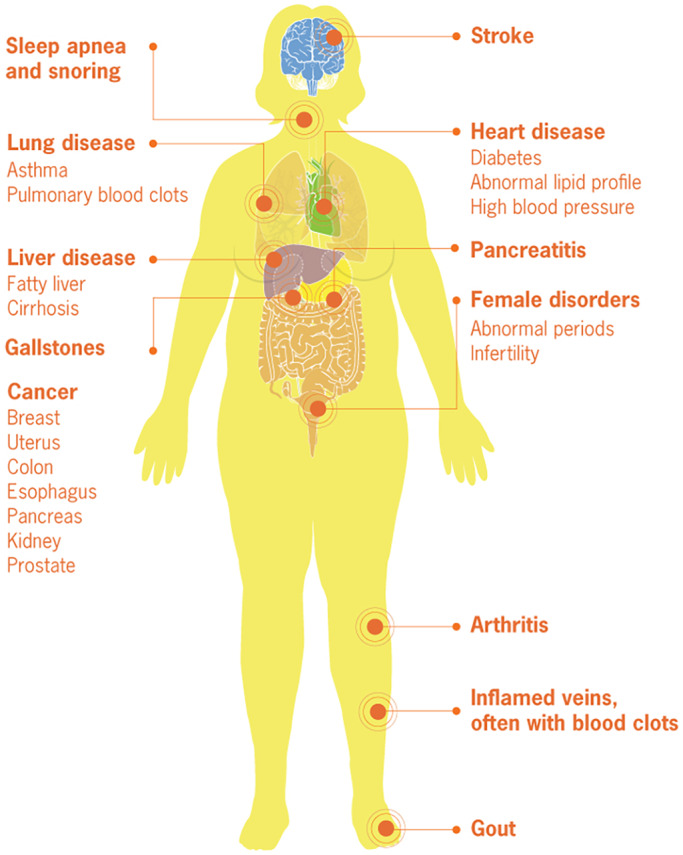
Medical complications of obesity. Reproduced from Centers for Disease Control and Prevention. 3
Understanding the global impact of obesity is important for all healthcare providers in countries of all income levels. According to WHO population statistics for 2016, 40% of women had overweight and 15% had obesity. 4 Figure 2, 5 provides an overview of the global percentages of women living with obesity. Low‐ and lower‐middle‐income countries, as defined by World Bank income group, also demonstrate significant rates of obesity in the most recent available data (Figure 3).
FIGURE 2.
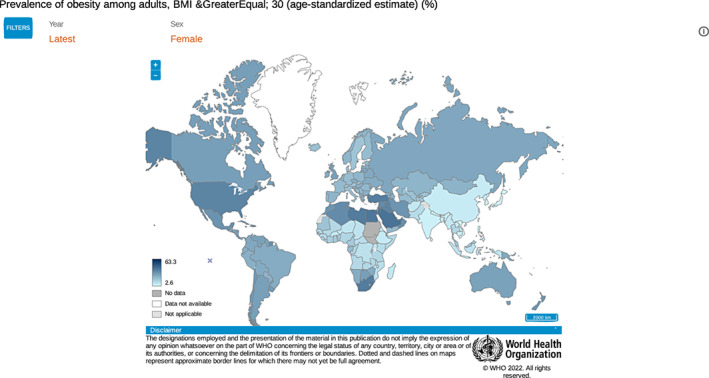
Prevalence of obesity among adult women with BMI greater than or equal to 30, all income levels. World Health Organization. 5
FIGURE 3.
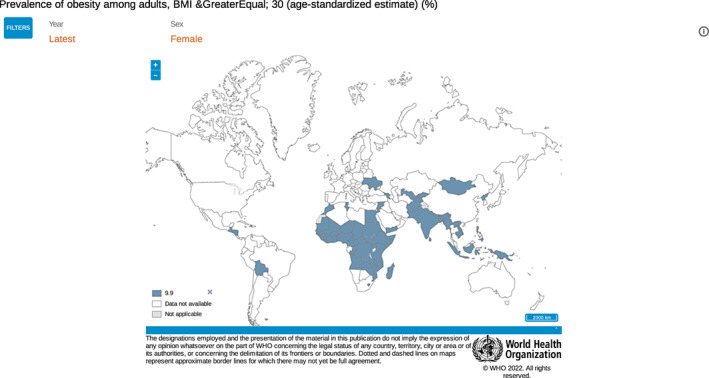
Prevalence of obesity amount adult women with BMI greater than or equal to 30 in low‐ and lower‐middle income countries. World Health Organization. 5
Low‐ and middle‐income countries face the double burden of malnutrition, where obesity rates are increasing in urban settings while undernutrition persists in rural settings. 4 More deaths occur globally from overweight and obesity than from underweight, with a few exceptions in Sub‐Saharan Africa and Asia. 4
Given that obesity affects many women before, during, and long after reproduction, providers of women's reproductive care need a common language to discuss obesity with patients, families, and the public. Four important considerations when discussing obesity and women's health are: (1) weight bias, weight stigma, and weight discrimination (Table 2); (2) using weight‐inclusive language and images; (3) cultural considerations and sensitivities when discussing obesity; and (4) obesity care through a trauma‐informed approach (Figure 4). Weight bias, stigma, and discrimination refer to the social beliefs that are ‘reinforced by misconceived ideas about body weight regulation’. 7
TABLE 2.
Definitions of weight bias, stigma, and discrimination
| Category | Definition |
|---|---|
| Weight bias | Negative attitudes toward people with obesity |
| Weight stigma | Stereotypes and labels used for people with obesity |
| Weight discrimination | Negative actions taken against people with obesity causing social disadvantage |
Note: Adapted from Obesity Canada. 6
FIGURE 4.
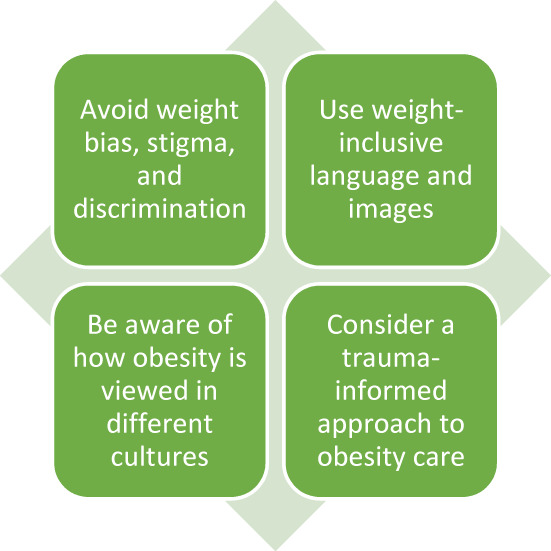
How to talk about obesity and women's health.
People living with obesity may be viewed as having made the choice to have obesity, resulting in an undermining of their human rights, social rights, and access to and quality of health care. 7 Of note, women face more weight bias, stigma, and discrimination than men. 8 Women who self‐identify as visible minorities, including Black and Asian, face even greater rates of weight bias in North America. 8
The Obesity Action Coalition recommends a ‘people first approach’ to avoid stigmatizing labelling 9 ; for example, we can describe a pregnant woman living with obesity, rather than a morbidly obese pregnant woman. 10 When using images and graphics to depict women living with obesity in clinical settings and in education, it is recommended that respectful portrayals and body positivity are recognized. 9
Globally, obesity can be viewed as both positive and negative depending on cultural and social norms. In some regions of the world, overweight and obesity body image is preferred, as has been observed in some African, Arabic, and in some refugee populations. 11
2. PREGNANCY COMPLICATED BY OBESITY AND THE IMPACT ON LONG‐TERM HEALTH
Pregnancy is a time of significant physiological change creating the potential for development of adverse pregnancy outcomes that can impact a person's health in the short‐term and are associated with worse health outcomes in the longer term. 12
The preconception, antepartum, and postnatal periods are unique times during which people have sustained engagement with the healthcare system. Thus, pregnancy‐associated clinical encounters have the potential to improve long‐term health if they can be harnessed for health optimization for parents and their families. 13 , 14 , 15
FIGO has highlighted the importance of weight management and reducing over‐ and undernutrition for people planning pregnancy to reduce the risk of noncommunicable diseases. 13 Identification of risk factors and subsequent management of pre‐existing diabetes mellitus, tobacco use, polycystic ovarian syndrome, high blood pressure, and micronutrient deficiencies can impact fertility, early pregnancy loss, pregnancy‐specific conditions such as gestational diabetes and hypertension, and preterm birth. 13
2.1. Clinical encounters as opportunities for health optimization for parents with obesity
FIGO has published guidance on using pre‐pregnancy, pregnancy, and postpartum clinical encounters for health optimization. 13
Primary care and other providers should support those desiring fertility (male, female, and nonbinary) with weight and health optimization prior to pregnancy. Medical and surgical interventions for weight management can be explored, in addition to lifestyle and nutritional counseling. 16 Patients desiring fertility should have a BMI assessment, receive advice on weight optimization, assessment for health complications associated with obesity where indicated, provision of adequate contraception to allow for health optimization prior to pregnancy, and appropriate folic acid supplementation once planning a pregnancy. 13
Breastfeeding of infants carries metabolic benefits for the lactating parent as well as the child. There is emerging evidence that breast milk can impact lifelong health. 17 Parents with obesity have been found to have lower breastfeeding rates in some communities while not in others. 18 Lactation support should be available for all parents. 13 , 19
There is an increased risk of peripartum depression in patients with obesity and depression is associated with increased adverse lifelong health outcomes. 20 , 21 , 22
2.2. Intrauterine environment and impact on long‐term health of the child
The metabolic intrauterine environment during gametogenesis and embryonic and fetal development is increasingly understood to influence the short‐ and long‐term health outcomes for the child, such as future childhood obesity, diabetes, asthma, heart disease and neurodevelopmental disorders. 23 Pregnancies from parents with obesity may impact the health outcomes for future generations through epigenetic modifications in both maternally and paternally derived gametes, as well as through metabolic programming of the embryo and fetus. 24 , 25 , 26
3. HOW CAN WE SUPPORT WOMEN LIVING WITH OBESITY BEYOND PREGNANCY?
People living with obesity can face bias and stigma that can increase morbidity and mortality independent of weight or BMI. The focus of care for those with obesity should be on improving health outcomes rather than focusing on weight loss. 27
3.1. Health optimization
Long‐term implications of obesity are mostly related to the presence or absence of associated complications. 28 BMI is an easy‐to‐use, low‐cost tool to approximate adiposity. 29 BMI can be used in pregnancy up to 18 weeks of gestation but is unreliable after given changes in maternal body composition. 30 Outside of pregnancy, measurement of waist circumference may improve adiposity assessment for patients with a BMI of 25–35. 31 , 32 The Women's Preventive Services Initiative recommends screening for obesity at routine visits using BMI. 33 More comprehensive tools such as the Edmonton obesity staging system that consider metabolic, physical, and psychological health may be considered when assessing weight‐related health and assessing the need and planning for treatment and intervention (Figure 5).
FIGURE 5.

Edmonton Obesity Staging System. Reproduced with permission granted by Springer Nature from Sharma and Kushner. 28
Health optimization for patients with obesity requires a stepwise approach to addressing obesity and related health complications, including recognition of the problem, assessment of physical status, exploration of the root causes of obesity, discussion about management options, agreement on a course of action, appropriate follow‐up, and advocacy for systems improvements (Figure 6).
FIGURE 6.

Clinical practice guideline for approach to adults with obesity. Reproduced with permission from Wharton et al. 27 under license CC BY‐NC‐ND.
Treatment for obesity requires individualized care plans that address the root causes of obesity, outlined above, and provide support for behavioral change. Treatment includes nutritional therapy, exercise and physical activity, psychological and behavioral interventions, pharmacotherapy, and surgery. Weight loss achieved with health behavioral changes is usually 3%–5% of body weight, which can result in meaningful improvement in obesity‐related comorbidities. 34 The Women's Preventive Services Initiative recommends that women aged 40–60 years with normal or overweight either maintain weight or limit weight to prevent obesity, respectively. 35 For those with BMI above 30, intensive behavioral interventions to promote weight loss and prevent chronic conditions are recommended. 33
3.2. Family‐centered care
Many of the root causes of obesity, such as sociocultural practices and beliefs, are strongly influenced by family and social structure.
Family‐centered care is recognized to be associated with better health outcomes in the pediatric population and there is some evidence that weight optimization interventions can be more effective when couples are engaged in a program together rather than individually. 36 , 37 , 38 , 39 There is currently insufficient evidence to assess family‐centered approaches to perinatal health optimization.
3.3. Healthcare provider education
Many health professionals are poorly prepared to address obesity. There is often little training available in behavior‐changing strategies and little experience available in working with the interprofessional teams involved in dedicated programs for patients with obesity. 40
Obesity assessment and health optimization strategies should be incorporated into training programs for all health professionals, including obesity awareness and health optimization components.
3.4. Emerging technologies
There is increasing evidence that emerging technologies can be used effectively for health optimization. 27 , 41 These include web‐based programs, applications for mobile devices, and wearable tracking devices. Further research on the potential applications of this technology for pregnancy and perinatal health optimization is needed.
3.5. Addressing the root causes of obesity and barriers to treatment
There are barriers to our ability to track and follow up patients with adverse pregnancy outcomes after delivery and to enable them to access health optimization care. 12 Pregnancy offers a window of opportunity for understanding and managing future health risks. 42 Compartmentalization of care and lack of collaboration among healthcare providers are important barriers in women's long‐term health management. 12
Solutions will need to be culturally and health‐system specific. In high‐income countries obesity is related to lower education and socioeconomic disadvantage. 43 , 44 National strategies to address obesity must address social inequity. Public health programs in low‐ and middle‐income countries are often focused on the prevention of undernutrition and face unique challenges in managing obesity and its disease burden. There is a lack of evidence on how to manage obesity in those with stunted growth and malnourishment. 40 Research specifically targeting malnourishment and obesity is needed.
| Best practice advice |
| Primary care providers, fertility clinics, contraception clinics, obstetric and gynecology services, and community healthcare providers should support those with fertility potential with health and weight optimization prior to pregnancy |
| Lactation support should be available for all parents |
| In addressing obesity‐related health with patients, care providers should ask permission to discuss weight and health, perform a physical assessment and an assessment of the root causes of obesity, discuss available care options, and plan for follow‐up |
| Obesity assessment and health optimization strategies should be incorporated into training programs for all health professionals |
| Pragmatic practice advice |
| Where resources are limited, body mass index can be assessed and consideration given to exercise and nutrition to optimize health and weight prior to pregnancy |
4. LIFELONG HEALTH IMPLICATIONS FOR WOMEN LIVING WITH OBESITY
As shown in Figure 1, obesity can affect many aspects of a woman's health beyond pregnancy. This section highlights key areas for providers of women's health care. Cardiovascular health, diabetes mellitus, COVID‐19 and pandemic considerations, and fertility are covered elsewhere. 45
4.1. Gastrointestinal and liver diseases
Nonalcoholic Fatty Liver Disease (NAFLD)
The prevalence of NAFLD increases with increasing BMI. 46 , 47 A higher incidence of gestational diabetes and early pregnancy miscarriages exists among women with NAFLD. 48 , 49 NAFLD can develop into nonalcoholic steatohepatitis over 5–7 years, leading to fibrosis and hepatocellular carcinoma. Nonalcoholic steatohepatitis is the second most common cause of liver transplantation and hepatocellular carcinoma. 50 , 51 Women with NAFLD could benefit from assessment 6–12 weeks after giving birth to avoid long‐term liver complications. 47
Cholelithiasis
Obesity, female sex, and multiparity are associated with increased risk of cholelithiasis. 52 , 53 Women choosing to manage obesity with rapid weight loss interventions and/or surgery have a paradoxical increased risk of cholelithiasis. 54 , 55 , 56 , 57 Therefore, screening women with class III obesity for cholelithiasis and complications of cholelithiasis, such as cholecystitis and pancreatitis secondary to choledocholithiasis, following weight loss interventions is recommended.
4.2. Micronutrient deficiency without weight loss surgery
Obesity, particularly class III obesity, is associated with several micronutrient deficiencies. This is likely due to aberrations in absorptive, metabolic, and hormonal functions in these individuals. 58 The most common deficiencies among this group include folate, vitamins B1, B12, A, and C, and iron. 59 Such deficiencies may be exacerbated by pregnancy. Thus, dietary and blood screening of micronutrient levels with appropriate supplementation may be necessary.
4.3. Micronutrient deficiency with weight loss surgery
Nutritional deficiencies associated with malabsorptive weight loss surgery can have significant consequences to an individual's long‐term health, including vision loss, osteoporotic fracture, cardiomyopathy, and neurological deficits related to Gayet‐Wernicke encephalopathy. 59 Key micronutrient deficiencies that occur after malabsorptive weight loss surgery include folate (15%–38%), vitamin B12 (37%–50%), iron (47%–66%), vitamin D (20%–80%), and vitamin E (5%–12%). 59
The risk of osteoporotic fractures varies with time; however, based on some studies, the risk is increased 3–5 years following surgery. 60 , 61 Women have the additional risk factor of osteoporosis in later life, potentially compounding long‐term risks to bone health with advancing age following weight loss surgery.
4.4. Obesity‐related cancers
There is an association between obesity and an increased incidence of several cancers including endometrial, renal, esophageal, ovarian, and breast cancers in postmenopausal women and colorectal cancer in premenopausal women 62 , 63 , 64 , 65 , 66 , 67 (Figure 7). 68
FIGURE 7.
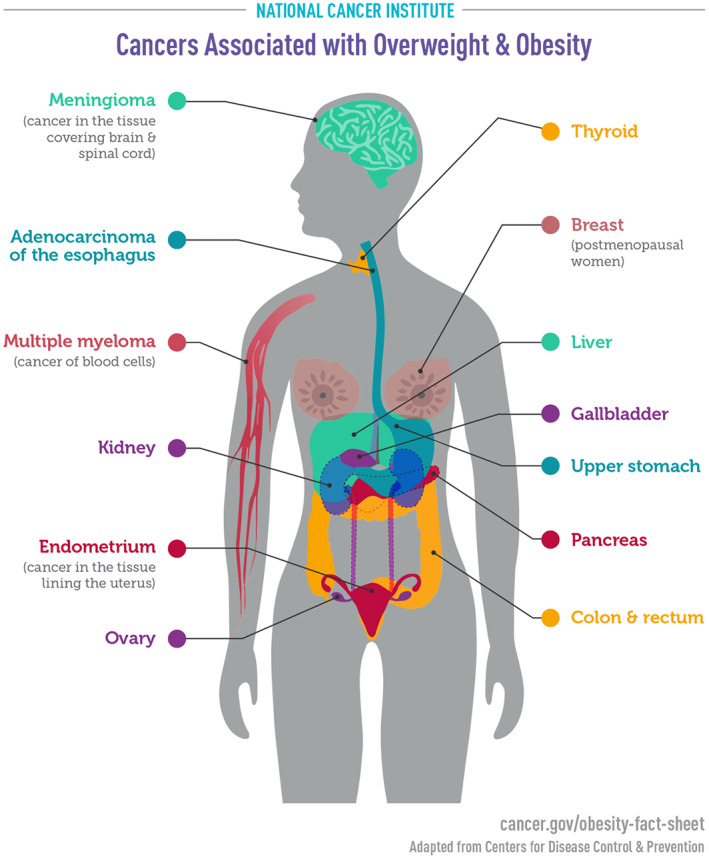
Cancers associated with overweight and obesity. Reproduced from National Cancer Institute. 68
Obesity may directly influence the development of cancer through the insulin–IGF‐1 axis, and sex hormone and adipokine mechanisms. 69
In women with obesity, conservative and/or surgical management of obesity can reduce cancer risk. 70 Bariatric surgery may decrease the incidence of breast and endometrial cancer. 70 Early mammogram screening can be considered for individuals with obesity and gigantomastia if there is concern or uncertainty on breast examination.
Women with obesity are at risk of abnormal uterine bleeding and polycystic ovarian syndrome due to insulin resistance and elevated unopposed estrogens, increasing the risk of endometrial cancer. 62 Early referral of women with obesity and abnormal uterine bleeding to a gynecologist for pelvic ultrasound and endometrial sampling is suggested. 71
4.5. Kidney health
Obesity is an independent risk factor for impaired renal function and chronic kidney disease. 72 , 73 , 74 , 75 Alterations in renal hemodynamics from compression of the kidneys by visceral adiposity, and inflammatory and metabolic changes are postulated causes. 72 Obesity is associated with a decline in estimated glomerular filtration rate, increased blood urea nitrogen, increased urinary albumin‐to‐creatinine ratio, and chronic kidney disease. 75 , 76
Obesity in pregnancy and associated metabolic syndrome are risk factors for pre‐eclampsia and persistent glomerulonephritis postpartum, diabetic nephropathy, and end‐stage kidney disease. 77 , 78 , 79
Although the underlying mechanisms that result in renal cell carcinoma in individuals with obesity are not well understood, risk factors were found to be obesity, elevated diastolic blood pressure, elevated triglycerides, and elevated fasting insulin. 80
Weight management may be beneficial in decreasing the incidence of chronic kidney disease among individuals with obesity. 75
4.6. Respiratory health and obstructive sleep apnea
Obesity‐related dyspnea is characterized by a reduction in lung volume and function. 81 , 82 , 83 , 84 There is an association with increasing BMI class and decrease in partial pressure of oxygen (PaO2) and increase in partial pressure of carbon dioxide (PaCO2), resulting in worsening of dyspnea. 83
Obesity is a predisposing factor for obstructive sleep apnea (OSA). One may consider a cycle of events where systemic inflammation, cardiometabolic risk factors, and endothelial dysfunction associated with individuals living with obesity and OSA may lead to worsening intermittent hypoxia and metabolic dysfunction. 85 , 86 Roca et al. 87 found that OSA was an independent risk factor for heart failure and mortality among women. Furthermore, pregnancy has been associated with exacerbation of OSA. 88
4.7. Venous thromboembolism
Cesarean birth and obesity are common independent risk factors for the development of postpartum venous thromboembolism (VTE). 89 , 90 , 91 In one study, Sultan et al. 92 found that women with a BMI greater than or equal to 30 in the postpartum period had a four‐fold increased risk of deep vein thrombosis (DVT). In another study, there appeared to be a dose‐dependent effect of BMI on the risk of postpartum VTE. 89 Prepregnancy and postpartum BMI were also found to be independent risk factors for VTE. 89 , 93
The following is recommended in the immediate postpartum period for women with obesity:
DVT prophylaxis should be started on day 1 after vaginal birth for up to 3 weeks postpartum. 94
DVT prophylaxis should be started on day 1 after cesarean birth for up to 6 weeks postpartum. 94
| Best practice advice |
| Postpartum liver assessment is recommended for women with nonalcoholic fatty liver disease (NAFLD) |
| Routine screening of women with class III obesity for cholelithiasis every 5 years, post weight loss interventions (i.e. conservative, medical, or surgical management) that result in greater than 10% total body weight loss |
| In the absence of weight loss surgery, women living with obesity are at risk of micronutrient deficiency and may require nutritional assessment and supplementation |
| Annual screening for micronutrient deficiencies in women who have had weight loss surgery is recommended, with subsequent supplementation as needed. Screening for osteopenia and osteoporosis via bone density scan is recommended 1–2 years after malabsorptive weight loss surgery |
| Weight loss and specialized cancer screening are recommended to reduce the risk of obesity‐related cancers |
| Early assessment of women with obesity and abnormal uterine bleeding is recommended |
| Women living with obesity should be screened for impaired renal function by laboratory testing of estimated glomerular filtrate rate (eGFR), blood urea nitrogen (BUN), creatinine, and microalbuminuria at 3 months postpartum and at intervals up to 3–5 years postpartum |
| Assessment and treatment for obstructive sleep apnea is recommended for women to reduce health risks |
| Postpartum DVT prophylaxis is recommended for both cesarean and vaginal birth in women with obesity |
5. GYNECOLOGIC AND REPRODUCTIVE HEALTH FOR WOMEN LIVING WITH OBESITY
5.1. Preventive gynecologic health
Gynecological assessment with cervical screening and breast examination are an important part of care for women with obesity in the primary care setting. Barriers in the care environment should be identified and addressed; for example, doorways, washrooms, and examination spaces should allow access. Care environments should avoid signage and language that is weight stigmatizing.
Pelvic examination can pose challenges due to increased vaginal adiposity, which increases the distance from the vulva to the cervix. Difficulty retracting vaginal adiposity can lead to poor visibility and inadequate examination of the cervix and vagina. 95 Several practical tips can facilitate pelvic examination in women with obesity:
Provision of adequately sized examination beds, stirrups, gowns, drapes, towels.
Use of an appropriately sized vaginal speculum with or without adjuncts that aid retraction of the protruding vaginal adiposity, allowing adequate visualization of the cervix.
The lateral decubitus position may be used for examination of the cervix, with the posterior blade of the speculum oriented toward the anus. 95
Consider a trauma‐informed care approach as the patient may have experienced difficult pelvic examinations in the past.
5.2. Endometrial health
As discussed previously, increasing BMI is associated with a higher incidence of endometrial cancer. 96 , 97 Due to the association of anovulatory bleeding and endometrial dysplasia in women with obesity, abnormal uterine bleeding requires careful assessment.
5.3. Breast health
There is an association between estrogen and progesterone receptor‐positive breast cancer in postmenopausal women with obesity. 66
The breast contains adipose tissue predominantly. The endocrine and paracrine role of excess adipose tissue in the development of breast cancer is linked to the secretion of growth factors, inflammatory mediators such as cytokines and adipokines, and increased aromatase activity involved in the increased conversion of androgens to estrogens. 98
Impaired lactogenesis is associated with obesity. Breastfeeding challenges may limit milk production and breastfeeding, which is an independent risk factor for the development of breast and endometrial cancer in the menopausal period. 98 , 99
Recommended practices for promoting breast health and reducing the risk of breast cancer in women with obesity include taking a detailed medical, family, and genetic history for breast, ovarian, and glandular cancers, encouraging milk and breastfeeding during the reproductive years, advising breast and chest self‐examination, and mammogram screening. The Women's Preventive Services Initiative recommends that mammography screening is initiated between the ages of 40 and 50, and occurs every 2 years thereafter until age 74, unless individualized risk factors are present. 33
5.4. Ovarian health
Postmenopausal women with obesity have an increased risk of ovarian cancer. 100 , 101 , 102
Preventive steps for ovarian health include healthcare providers taking a detailed medical and family history for breast, ovarian, and glandular cancers, being vigilant for constitutional signs of ovarian cancer, and appropriate referral to a gynecologic oncologist.
5.5. Contraception
The risk of VTE was two times higher in the obesity group among users of combined hormonal contraception compared with women of normal weight. 103 There were also associations between this group and the risk of arterial thrombosis and embolism. 103
Compared with other progestin‐only contraception, depot‐medroxyprogesterone acetate (DMPA) has been associated with loss of bone mineral density with long‐term use. 104 However, the degree to which bone mineral density is affected in mature women aged 65 years or older with obesity is unclear. One pilot study demonstrated that there were no significant differences in the degree of lumbar spine bone mineral density loss across BMI categories. 104
An individualized approach to contraceptive advice is recommended as there may be other obesity‐related medical conditions and medications that should be taken into consideration. Contraceptive options for women with obesity are shown in Table 3. 105
TABLE 3.
Contraceptive options for women with obesity, with or without a history of bariatric surgery a
| Cu‐IUD | LNG‐IUD | Subdermal implant | DMPA | POP | CHC | |
|---|---|---|---|---|---|---|
| Age > 45 years | Yes | Yes | Yes | Relative contraindication | Yes | Relative contraindication |
| BMI ≥ 30 | Yes | Yes | Yes | Yes | Yes | Relative contraindication |
| Restrictive bariatric surgery | Yes | Yes | Yes | Yes | Yes | Yes |
| Malabsorptive bariatric surgery | Yes | Yes | Yes | Yes | Yes |
Oral—no Patch/ring ‐ yes |
Abbreviations: CHC, combined hormonal contraceptive; Cu‐IUD, copper‐containing intrauterine device; DMPA, depot medroxyprogesterone acetate; LNG‐IUD, levonorgestrel‐releasing intrauterine device; POP, progestin‐only pill.
Centers for Disease Control and Prevention. 105
Emergency contraception
Insufficient data exist on the ideal method of emergency contraception for women of reproductive age with obesity.
The dose of levonorgestrel‐based emergency contraception can be doubled as this may improve its effectiveness in women with obesity. 106 , 107
5.6. Menopause
Postmenopausal women with obesity may experience symptoms of menopause, irrespective of additional circulating estrogens derived from adipose tissue. Obesity and smoking are risk factors for worsening vasomotor symptoms in women aged 65 years or older. 108
Treatment of symptoms associated with menopause can include lifestyle changes such as wearing cool, light clothing, oral hydration, and reducing caffeinated beverages and tobacco use. Alternatives to hormonal therapies such as clonidine, paroxetine, venlafaxine, gabapentin, and black cohosh can be used at low doses to treat vasomotor symptoms. 109
Hormone replacement therapy for women with obesity is controversial due to the risks of breast cancer, VTE, and cardiovascular disease in this population. 110 , 111
5.7. Bone health
Osteoporosis
There is no clear consensus regarding the effect of excess adiposity on the risk of development of osteoporosis. 112 General approaches to optimizing bone and skeletal health in women aged 65 years or older with obesity include management of chronic conditions predisposing to osteoporosis, discontinuation of alcohol and tobacco use which impairs new bone formation, promotion of weight‐bearing exercise, reducing risk of falls in the home environment, and dietary supplementation with calcium and vitamin D. 113 , 114 The Women's Preventive Services Initiative recommends screening for osteoporosis in women aged 65 and over, and possibly in younger women at risk of osteoporotic fractures. 33
Degenerative bone disease
Osteoarthritis is an obesity‐related medical condition and is more commonly seen in women with obesity in the sixth or seventh decade of life. 115 It is thought that the main mechanism leading to osteoarthritis in individuals with obesity is due to the effect of weight or increased load on weight‐bearing joints. However, since osteoarthritis of nonweight‐bearing joints has been observed in individuals with obesity, other postulates include the influence of local and systemic inflammatory mediators associated with excess adiposity playing a role in the disease process. 116 , 117 , 118 Weight management may improve symptoms associated with osteoarthritis in women with obesity.
5.8. Mental wellness and cognition
Depression
Depression is associated with obesity and the menopausal transition. Biopsychosocial factors such as body image, binge eating, weight stigma and discrimination, substance abuse, micro‐ and macronutrient deficiencies, neuroendocrine alterations, pre‐existing psychiatric conditions, and obesity‐related chronic conditions have been implicated. 119 High expectations of weight loss surgery in the early postoperative period may also contribute to substance abuse and worsening depression postoperatively. 59
Conservative management of depression related to obesity may include cognitive behavioral therapy, mental wellness support groups, and physical activity. Psychiatric evaluation before and after weight loss surgery to reduce risk of self‐harm is strongly recommended. 59 Co‐managing patients with a weight loss support team, including nutritionist, bariatric surgeon, and gastroenterologist, is suggested. As some psychiatric medications may cause weight gain and exacerbate obesity, medication management may require revision or substitution in some cases. 120
Cognition
There is a paucity of data indicating whether obesity has a direct effect on cognition in women aged 65 years or older. Some studies do report this association and it is thought that adipokines associated with obesity may be associated with changes in brain structure and cognitive decline. 121 , 122
Obesity and its associated chronic medical conditions, which predispose individuals to vascular disease and stroke, may be implicated in the development of vascular dementia. 123
Management and counseling regarding lifestyle, exercise, and treatment of chronic obesity‐related conditions may be helpful in minimizing the impact of obesity on cognitive decline.
| Best practice advice |
|
Preventive breast health care is recommended for postmenopausal women with obesity Vasomotor symptoms in women aged 65 years or older with obesity should be evaluated and treated using an individualized approach, recognizing the increased risks of breast and endometrial cancers associated with obesity in women |
| Osteoporosis in women aged 65 years or older with obesity should be evaluated and treated using an individualized approach |
6. CONCLUSION AND AREAS FOR FUTURE RESEARCH
The causes and drivers of obesity prevalence are complex and multifactorial. Beyond public health advice on diet and physical activity, other potentially modifiable drivers of increasing obesity rates include aspects closely related to pregnancy care, including but not limited to, influences of the microbiome and microorganisms on the development of obesity and its related illnesses, epigenetics, maternal age, endocrine influences, obesogenic medication, intrauterine and intergenerational as well as paternally inherited effects, sociocultural practices and beliefs, social determinants of health, built environment, individual life experiences such as adverse childhood experiences, and psychological factors such as mood, anxiety, binge eating disorder, attention deficit/hyperactivity disorder, self‐worth, and identity.
The root causes of obesity are diverse and different in different individuals. Public health interventions have thus far failed to achieve significant decreases in population obesity prevalence. 43
Future research should be focused on interprofessional care and on an exploration of the root causes of obesity and their interrelationship. Research should focus on finding effective interventions for health optimization and on longitudinal research to better understand the drivers of risk and morbidity over a life course. Differences in sociocultural practices, available resources, and health systems mean that effective obesity interventions are not globally generalizable. System‐ and country‐specific research should be encouraged and supported.
AUTHOR CONTRIBUTIONS
Cynthia V. Maxwell, Rachelle Shirley, Amy C. O'Higgins, Mary L. Rosser, and Patrick O'Brien contributed to content planning and manuscript writing. All other authors contributed to content planning and article editing.
CONFLICT OF INTEREST
Cynthia Maxwell reports grants from the Canadian Institutes for Health Research and the Crohns and Colitis Foundation of Canada. Sharleen O'Reilly reports research grants from the European Commission Horizon 2020, National Health and Medical Research Council of Australia, Health Research Board Ireland, Al Qasimi Foundation, and University of Sharjah. Ronald C. Ma reports research support from AstraZeneca, Bayer, Novo Nordisk, Pfizer, and Tricida Inc. Harold David McIntyre reports honoraria for lectures from Phillips Health Care, Mead Johnson (China), and Diabetes Ireland. Lina Bergman reports research funds from Thermo Fischer, Roche, and Perkin Elmer and payment from Homburg and Partner.
ACKNOWLEDGMENTS
The authors would like to thank Ms Julianah Oguntala for assisting with editing of this manuscript. Open access funding provided by IReL.
Maxwell CV, Shirley R, O’Higgins AC, et al. Management of obesity across women's life course: FIGO Best Practice Advice. Int J Gynecol Obstet. 2023;160(Suppl. 1):35‐49. doi: 10.1002/ijgo.14549
REFERENCES
- 1. World Health Organization [website] . Obesity. https://www.who.int/health‐topics/obesity#tab=tab_1. Accessed July 5, 2022.
- 2. Obesity Medicine Association . Obesity Algorithm. 2021. https://obesitymedicine.org/wp‐content/uploads/2021/08/2021‐Obesity‐Algorithm‐PowerPoint.pdf. Accessed July 5, 2022.
- 3. Centers for Disease Control and Prevention [website] . Vital signs. Medical complications of obesity. How does obesity affect the body. https://www.cdc.gov/vitalsigns/adultobesity/infographic.html. Accessed November 1, 2022.
- 4. World Health Organization [website] . Obesity and overweight June 2021. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight. Accessed July 5, 2022.
- 5. World Health Organization . The Global Health Observatory [website]. Prevalence of obesity among adults, BMI >= 30 (age‐standardized estimate) (%). https://www.who.int/data/gho/data/indicators/indicator‐details/GHO/prevalence‐of‐obesity‐among‐adults‐bmi‐=‐30‐(age‐standardized‐estimate)‐(−). Accessed July 5, 2022.
- 6. Obesity Canada . Overcoming weight bias. http://obesitycanada.ca/wp‐content/uploads/2018/10/Overcoming‐Weight‐Bias‐11×17‐May‐2018‐Eng‐Fr4.pdf. Accessed July 5, 2022.
- 7. Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:485‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puhl RM, Andreyeva T, Brownell KD. Perceptions of weight discrimination: prevalence and comparison to race and gender discrimination in America. Int J Obes (Lond). 2008;32:992‐1000. [DOI] [PubMed] [Google Scholar]
- 9. Obesity Action Coalition [website]. Weight bias. https://www.obesityaction.org/education‐support/learn‐about‐obesity/weight‐bias/. Accessed July 5, 2022.
- 10. Maxwell C, Sharma A. Thinking about people‐first language: weight bias, stigma, and discrimination, and women's reproductive health. J Obstet Gynaecol Can. 2019;41:1533‐1534. [DOI] [PubMed] [Google Scholar]
- 11. Naigaga DA, Jahanlu D, Claudius HM, Gjerlaug AK, Barikmo I, Henjum S. Body size perceptions and preferences favor overweight in adult Saharawi refugees. Nutr J. 2018;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheiner E, Kapur A, Retnakaran R, et al. FIGO (International Federation of Gynecology and Obstetrics) postpregnancy initiative: long‐term maternal implications of pregnancy complications‐follow‐up considerations. Int J Gynecol Obstet. 2019;147(Suppl 1):1‐31. [DOI] [PubMed] [Google Scholar]
- 13. McAuliffe FM, Killeen SL, Jacob CM, et al. Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO Pregnancy and Non‐Communicable Diseases Committee: a FIGO (International Federation of Gynecology and Obstetrics) guideline. Int J Gynecol Obstet. 2020;151(Suppl 1):16‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phelan S. Pregnancy: a "teachable moment" for weight control and obesity prevention. Am J Obstet Gynecol. 2010;202(135):e1‐e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olander EK, Darwin ZJ, Atkinson L, Smith DM, Gardner B. Beyond the 'teachable moment' ‐ A conceptual analysis of women's perinatal behaviour change. Women Birth. 2016;29:e67‐e71. [DOI] [PubMed] [Google Scholar]
- 16. Kennelly MA, Ainscough K, Lindsay KL, et al. Pregnancy exercise and nutrition with smartphone application support: a randomized controlled trial. Obstet Gynecol. 2018;131:818‐826. [DOI] [PubMed] [Google Scholar]
- 17. Stuebe AM. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin Perinatol. 2015;39:290‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen SS, Larson CO, Matthews CE, et al. Parity and breastfeeding in relation to obesity among black and white women in the southern community cohort study. J Womens Health (Larchmt). 2009;18:1323‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Reilly SL, Conway MC, O'Brien EC, et al. Exploring Successful Breastfeeding Behaviors Among Women Who Have High Body Mass Indices. J Hum Lact. 2022. Jun 16;8903344221102839. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao XH, Zhang ZH. Risk factors for postpartum depression: an evidence‐based systematic review of systematic reviews and meta‐analyses. Asian J Psychiatr. 2020. Oct;53:102353. doi: 10.1016/j.ajp.2020.102353 [DOI] [PubMed] [Google Scholar]
- 21. Ertop F, Cetisli NE. Postpartum depression and breastfeeding in overweight/obese and non‐obese mothers. J Pak Med Assoc. 2020;70:219‐224. [DOI] [PubMed] [Google Scholar]
- 22. Steinig J, Nagl M, Linde K, Zietlow G, Kersting A. Antenatal and postnatal depression in women with obesity: a systematic review. Arch Womens Ment Health. 2017;20:569‐585. [DOI] [PubMed] [Google Scholar]
- 23. Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long‐term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skogen JC, Overland S. The fetal origins of adult disease: a narrative review of the epidemiological literature. JRSM Short Rep. 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bošković A, Rando OJ. Transgenerational Epigenetic Inheritance. Annu Rev Genet. 2018;52:21‐41. [DOI] [PubMed] [Google Scholar]
- 26. Carone BR, Fauquier L, Habib N, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875‐E891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond). 2009;33:289‐295. [DOI] [PubMed] [Google Scholar]
- 29. Garvey WT, Mechanick JI. Proposal for a Scientifically Correct and Medically Actionable Disease Classification System (ICD) for Obesity. Obesity (Silver Spring). 2020;28:484‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Higgins AC, Doolan A, Mullaney L, Daly N, McCartney D, Turner MJ. The relationship between gestational weight gain and fetal growth: time to take stock? J Perinat Med. 2014;42:409‐415. [DOI] [PubMed] [Google Scholar]
- 31. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Needland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715‐725. [DOI] [PubMed] [Google Scholar]
- 33. Women's Preventive Services Initiative . Recommendations for well‐woman care clinical summary tables. Updated January 11, 2022. https://www.womenspreventivehealth.org/wp‐content/uploads/FINAL_WPSI_ClinicalSummaryTables_2022.pdf Accessed September 2, 2022.
- 34. Look AHEAD Research Group . Eight‐year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Women's Preventive Services Initiative [website]. Preventing obesity in midlife women. January 2022. https://www.womenspreventivehealth.org/recommendations/preventing‐obesity/. Accessed September 2, 2022.
- 36. Bertakis KD, Azari R. Patient‐centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24:229‐239. [DOI] [PubMed] [Google Scholar]
- 37. Black DR, Gleser LJ, Kooyers KJ. A meta‐analytic evaluation of couples weight‐loss programs. Health Psychol. 1990;9:330‐347. [DOI] [PubMed] [Google Scholar]
- 38. McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight‐loss interventions: a systematic review of randomised trials. Int J Obes Relat Metab Disord. 2003;27:987‐1005. [DOI] [PubMed] [Google Scholar]
- 39. Moore AK, Rasmussen R, Sandberg J, Straseki J, Peterson CM, Johnstone EB. Intensive lifestyle intervention including emotionally‐focused couples therapy leads to more pregnancies and weight loss in obese infertile couples. Fertil Steril. 2016;106:E101. [Google Scholar]
- 40. Dietz WH, Baur LA, Hall K, et al. Management of obesity: improvement of health‐care training and systems for prevention and care. Lancet. 2015;385:2521‐2533. [DOI] [PubMed] [Google Scholar]
- 41. Houser SH, Joseph R, Puro N, Darrell E. Use of technology in the management of obesity: a literature review. Perspect Health Inf Manag. 2019;16(Fall):1c. [PMC free article] [PubMed] [Google Scholar]
- 42. Jacob CM, Killeen SL, McAuliffe FM, et al. Prevention of noncommunicable diseases by interventions in the preconception period: a FIGO position paper for action by healthcare practitioners. Int J Gynecol Obstet. 2020;151(Suppl 1):6‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Obesity [website]. World Obesity Atlas 2022. https://www.worldobesity.org/resources/resource‐library/world‐obesity‐atlas‐2022. Accessed July 5, 2022.
- 44. Turner MJ, Layte R. Obesity levels in a national cohort of women 9 months after delivery. Am J Obstet Gynecol. 2013;209(124):e1‐e7. [DOI] [PubMed] [Google Scholar]
- 45. Gautam D, Purandure N, Maxwell C, et al. The challenges of obesity for fertility: a FIGO literature review. Int J Gynecol Obstet. 2023;160(Suppl 1): 50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 47. Goh GB, McCullough AJ. Natural History of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1226‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song S, Duo Y, Zhang Y, et al. The predictive ability of hepatic steatosis index for gestational diabetes mellitus and large for gestational age infant compared with other noninvasive indices among chinese pregnancies: a preliminary double‐center cohort study. Diabetes Metab Syndr Obes. 2021;14:4791‐4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koralegedara IS, Warnasekara JN, Dayaratne KG, De Silva FN, Premadasa JK, Agampodi SB. Non‐alcoholic fatty liver disease (NAFLD): a significant predictor of gestational diabetes mellitus (GDM) and early pregnancy miscarriages‐prospective study in Rajarata Pregnancy Cohort (RaPCo). BMJ Open Gastroenterol. 2022;9:e000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 51. Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non‐alcoholic fatty liver disease. Metabolism. 2016;65:1017‐1025. [DOI] [PubMed] [Google Scholar]
- 52. Lindseth G, Bird‐Baker MY. Risk factors for cholelithiasis in pregnancy. Res Nurs Health. 2004;27:382‐391. [DOI] [PubMed] [Google Scholar]
- 53. Littlefield A, Lenahan C. Cholelithiasis: presentation and management. J Midwifery Womens Health. 2019;64:289‐297. [DOI] [PubMed] [Google Scholar]
- 54. Aidonopoulos AP, Papavramidis ST, Zaraboukas TG, Habib HW, Pothoulakis IG. Gallbladder Findings after Cholecystectomy in Morbidly Obese Patients. Obes Surg. 1994;4:8‐12. [DOI] [PubMed] [Google Scholar]
- 55. Brandão I, de Oliveira C, Adami Chaim E, da Silva BB. Impact of rapid weight reduction on risk of cholelithiasis after bariatric surgery. Obes Surg. 2003;13:625‐628. [DOI] [PubMed] [Google Scholar]
- 56. Fuller W, Rasmussen JJ, Ghosh J, Ali MR. Is routine cholecystectomy indicated for asymptomatic cholelithiasis in patients undergoing gastric bypass? Obes Surg. 2007;17:747‐751. [Erratum in Obes Surg. 2007;17:996]. [DOI] [PubMed] [Google Scholar]
- 57. Heida A, Koot BG, vd Baan‐Slootweg OH, et al. Gallstone disease in severely obese children participating in a lifestyle intervention program: incidence and risk factors. Int J Obes (Lond). 2014;38:950‐953. [DOI] [PubMed] [Google Scholar]
- 58. McKay J, Ho S, Jane M, Pal S. Overweight & obese Australian adults and micronutrient deficiency. BMC Nutr. 2020;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Auge M, Menahem B, Savey V, Lee Bion A, Alves A. Long‐term complications after gastric bypass and sleeve gastrectomy: what information to give to patients and practitioners, and why? J Visc Surg. 2022;159:298‐308. [DOI] [PubMed] [Google Scholar]
- 60. Zhang Q, Chen Y, Li J, et al. A meta‐analysis of the effects of bariatric surgery on fracture risk. Obes Rev. 2018;19:728‐736. [DOI] [PubMed] [Google Scholar]
- 61. Paccou J, Martignène N, Lespessailles E, et al. Gastric bypass but not sleeve gastrectomy increases risk of major osteoporotic fracture: French population‐based cohort study. J Bone Miner Res. 2020;35:1415‐1423. [DOI] [PubMed] [Google Scholar]
- 62. Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status‐‐a meta‐analysis. Int J Cancer. 2009;124:698‐712. [DOI] [PubMed] [Google Scholar]
- 64. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8:e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xia X, Chen W, Li J, et al. Body mass index and risk of breast cancer: a nonlinear dose‐response meta‐analysis of prospective studies. Sci Rep. 2014;4:7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol. 2015;1:611‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alshamsan B, Suleman K, Agha N, et al. Association Between Obesity and Clinicopathological Profile of Patients with Newly Diagnosed Non‐Metastatic Breast Cancer in Saudi Arabia. Int J Womens Health. 2022;14:373‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. National Cancer Institute [website]. Obesity and cancer https://www.cancer.gov/about‐cancer/causes‐prevention/risk/obesity/obesity‐fact‐sheet. Accessed July 6, 2022.
- 69. Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer‐‐mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crafts TD, Tonneson JE, Wolfe BM, Stroud AM. Obesity and breast cancer: preventive and therapeutic possibilities for bariatric surgery. Obesity (Silver Spring). 2022;30:587‐598. [DOI] [PubMed] [Google Scholar]
- 71. Miles RC, Lehman CD, Mercaldo SF, Tamimi RM, Dontchos BN, Narayan AK. Obesity and breast cancer screening: cross‐sectional survey results from the behavioral risk factor surveillance system. Cancer. 2019;125:4158‐4163. [DOI] [PubMed] [Google Scholar]
- 72. Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta‐analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91:1224‐1235. [DOI] [PubMed] [Google Scholar]
- 73. Herrington WG, Smith M, Bankhead C, et al. Body‐mass index and risk of advanced chronic kidney disease: prospective analyses from a primary care cohort of 1.4 million adults in England. PLoS One. 2017;12:e0173515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chang AR, Grams ME, Ballew SH, et al. Adiposity and risk of decline in glomerular filtration rate: meta‐analysis of individual participant data in a global consortium. BMJ. 2019;364:k5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kjaergaard AD, Teumer A, Witte DR, et al. Obesity and kidney function: a two‐sample mendelian randomization study. Clin Chem. 2022;68:461‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu P, Lewington S, Haynes R, et al. Cross‐sectional associations between central and general adiposity with albuminuria: observations from 400,000 people in UK Biobank. Int J Obes (Lond). 2020;44:2256‐2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta‐analysis. Am J Kidney Dis. 2010;55:1026‐1039. [DOI] [PubMed] [Google Scholar]
- 78. Kittiskulnam P, Thokanit NS, Katavetin P, et al. The magnitude of obesity and metabolic syndrome among diabetic chronic kidney disease population: a nationwide study. PLoS One. 2018;13:e0196332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu H, Kuja‐Halkola R, Chen X, Magnusson PKE, Svensson P, Carrero JJ. Higher body mass index is associated with incident diabetes and chronic kidney disease independent of genetic confounding. Kidney Int. 2019;95:1225‐1233. [DOI] [PubMed] [Google Scholar]
- 80. Johansson M, Carreras‐Torres R, Scelo G, et al. The influence of obesity‐related factors in the etiology of renal cell carcinoma‐A mendelian randomization study. PLoS Med. 2019;16:e1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Collet F, Mallart A, Bervar JF, et al. Physiologic correlates of dyspnea in patients with morbid obesity. Int J Obes (Lond). 2007;31:700‐706. [DOI] [PubMed] [Google Scholar]
- 82. Boissière L, Perotin‐Collard JM, Bertin E, et al. Improvement of dyspnea after bariatric surgery is associated with increased expiratory reserve volume: a prospective follow‐up study of 45 patients. PLoS One. 2017;12:e0185058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Currow DC, Dal Grande E, Sidhu C, Ekström M, Johnson MJ. The independent association of overweight and obesity with breathlessness in adults: a cross‐sectional, population‐based study. Eur Respir J. 2017;50:1700558. [DOI] [PubMed] [Google Scholar]
- 84. Hagenburg J, Bertin E, Salmon JH, et al. Association between obesity‐related dyspnea in daily living, lung function and body composition analyzed by DXA: a prospective study of 130 patients. BMC Pulm Med. 2022;22:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Drager LF, Togeiro SM, Polotsky VY, Lorenzi‐Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yasir M, Pervaiz A, Sankari A. Cardiovascular outcomes in sleep‐disordered breathing: are we under‐estimating? Front Neurol. 2022;13:801167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roca GQ, Redline S, Claggett B, et al. Sex‐Specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community‐dwelling cohort: the atherosclerosis risk in communities‐sleep heart health study. Circulation. 2015;132:1329‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Facco FL, Ouyang DW, Zee PC, Grobman WA. Sleep disordered breathing in a high‐risk cohort prevalence and severity across pregnancy. Am J Perinatol. 2014;31:899‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Butwick AJ, Bentley J, Leonard SA, et al. Prepregnancy maternal body mass index and venous thromboembolism: a population‐based cohort study. BJOG. 2019;126:581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ellis‐Kahana J, Sparks AD, Gimovsky AC, James AH, Ahmadzia HK. Developing a model for predicting venous thromboembolism in obese pregnant women in a national study. Thromb Res. 2020;191:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhao Z, Zhou Q, Li X. Missed opportunities for venous thromboembolism prophylaxis during pregnancy and the postpartum period: evidence from mainland China in 2019. BMC Pregnancy Childbirth. 2021;21:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sultan AA, West J, Tata LJ, Fleming KM, Nelson‐Piercy C, Grainge MJ. Risk of first venous thromboembolism in and around pregnancy: a population‐based cohort study. Br J Haematol. 2012;156:366‐373. [DOI] [PubMed] [Google Scholar]
- 93. Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta‐analysis. Chest. 2016;150:572‐596. [DOI] [PubMed] [Google Scholar]
- 94. Abdul Sultan A, Grainge MJ, West J, Fleming KM, Nelson‐Piercy C, Tata LJ. Impact of risk factors on the timing of first postpartum venous thromboembolism: a population‐based cohort study from England. Blood. 2014;124:2872‐2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Breitkopf DM. Lateral decubitus position to facilitate pelvic examination of the patient with severe obesity. BMC Womens Health. 2021;21:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625‐1638. [DOI] [PubMed] [Google Scholar]
- 97. Arem H, Pfeiffer RM, Moore SC, et al. Post‐diagnosis body mass index and mortality among women diagnosed with endometrial cancer: results from the women's health initiative. PLoS One. 2017;12:e0171250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Colleluori G, Perugini J, Barbatelli G, Cinti S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev Endocr Metab Disord. 2021;22:241‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ma X, Zhao LG, Sun JW, et al. Association between breastfeeding and risk of endometrial cancer: a meta‐analysis of epidemiological studies. Eur J Cancer Prev. 2018;27:144‐151. [DOI] [PubMed] [Google Scholar]
- 100. Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta‐analysis. Eur J Cancer. 2007;43:690‐709. [DOI] [PubMed] [Google Scholar]
- 101. Peterson NB, Trentham‐Dietz A, Newcomb PA, et al. Relation of anthropometric measurements to ovarian cancer risk in a population‐based case‐control study (United States). Cancer Causes Control. 2006;17:459‐467. [DOI] [PubMed] [Google Scholar]
- 102. Bandera EV, Qin B, Moorman PG, et al. Obesity, weight gain, and ovarian cancer risk in African American women. Int J Cancer. 2016;139:593‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sugiura K, Kobayashi T, Ojima T. Risks of thromboembolism associated with hormonal contraceptives related to body mass index and aging in Japanese women. Thromb Res. 2016;137:11‐16. [DOI] [PubMed] [Google Scholar]
- 104. Segall‐Gutierrez P, Agarwal R, Ge M, Lopez C, Hernandez G, Stanczyk FZ. A pilot study examining short‐term changes in bone mineral density among class 3 obese users of depot‐medroxyprogesterone acetate. Eur J Contracept Reprod Health Care. 2013;18:199‐205. [DOI] [PubMed] [Google Scholar]
- 105. Centers for Disease Control and Prevention [website]. US Medical Eligibility Criteria (US MEC) for contraceptive use, 2016. https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html. Accessed July 20, 2022.
- 106. Edelman AB, Cherala G, Blue SW, Erikson DW, Jensen JT. Impact of obesity on the pharmacokinetics of levonorgestrel‐based emergency contraception: single and double dosing. Contraception. 2016;94:52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stowers P, Mestad R. Use of levonorgestrel as emergency contraception in overweight women. Obes Res Clin Pract. 2019;13:180‐183. [DOI] [PubMed] [Google Scholar]
- 108. Anderson DJ, Chung HF, Seib CA, et al. Obesity, smoking, and risk of vasomotor menopausal symptoms: a pooled analysis of eight cohort studies. Am J Obstet Gynecol. 2020;222:478.e1‐478.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cheema D, Coomarasamy A, El‐Toukhy T. Non‐hormonal therapy of post‐menopausal vasomotor symptoms: a structured evidence‐based review. Arch Gynecol Obstet. 2007;276:463‐469. [DOI] [PubMed] [Google Scholar]
- 110. Barrett‐Connor E, Stuenkel CA. Hormone replacement therapy (HRT)‐‐risks and benefits. Int J Epidemiol. 2001;30:423‐426. [DOI] [PubMed] [Google Scholar]
- 111. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321‐333. [DOI] [PubMed] [Google Scholar]
- 112. Zhao LJ, Jiang H, Papasian CJ, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wacker M, Holick MF. Vitamin D ‐ effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rondanelli M, Faliva MA, Barrile GC, et al. Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: a food pyramid. Nutrients. 2021;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Magnusson K, Turkiewicz A, Englund M. Nature vs nurture in knee osteoarthritis ‐ the importance of age, sex and body mass index. Osteoarthr Cartil. 2019;27:586‐592. [DOI] [PubMed] [Google Scholar]
- 116. Jiang L, Rong J, Wang Y, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta‐analysis. Joint Bone Spine. 2011;78:150‐155. [DOI] [PubMed] [Google Scholar]
- 117. Vuolteenaho K, Koskinen A, Moilanen E. Leptin ‐ a link between obesity and osteoarthritis. applications for prevention and treatment. Basic Clin Pharmacol Toxicol. 2014;114:103‐108. [DOI] [PubMed] [Google Scholar]
- 118. Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity‐induced osteoarthritis. Rheumatology (Oxford). 2015;54:588‐600. [DOI] [PubMed] [Google Scholar]
- 119. Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev. 2013;14:906‐918. [DOI] [PubMed] [Google Scholar]
- 120. de Silva VA, Suraweera C, Ratnatunga SS, Dayabandara M, Wanniarachchi N, Hanwella R. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta‐analysis. BMC Psychiatry. 2016;16:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late‐life dementia risk: a population‐based twin study. Neurology. 2011;76:1568‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 2014;24:1982‐1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]


