Abstract
There is a paucity of literature regarding the optimal selection of combination antiseizure medications (ASMs) for drug‐resistant epilepsy (DRE). The aim of this scoping review is to evaluate current evidence related to “rational polytherapy” among adults with DRE. Using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses for Scoping Reviews (PRISMA‐SCr) guidelines, PubMed, ProQuest, CINAHL, and Cochrane databases were searched using DRE‐ and polytherapy‐related keywords. The exclusion criteria applied included: non‐English; non‐human studies; non‐research studies; participants less than 18 years; status epilepticus; ASM monotherapy; and certain ASMs. In Covidence, two researchers independently reviewed articles for inclusion at each phase, with a third resolving conflicts. Data were extracted, with quality appraisal using the Mixed Methods Appraisal Tool (MMAT). Of the 6477 studies imported for screening, 33 studies were included. Clinical, humanistic, and economic outcomes were reported by 26, 12, and one study, respectively. Common efficacy‐related clinical outcomes included ≥50% reduction in seizure frequency (n = 14), seizure freedom (n = 14), and percent reduction in seizure frequency (n = 8). Common humanistic outcomes included quality of life (n = 4), medication adherence (n = 2), sleep‐related outcomes (n = 2), and physician and patient global assessments (n = 2). The economic study reported quality‐adjusted life years. The median MMAT score was 80 (range: 60–100). Two studies referenced the standard definition of DRE, whereas five studies did not specifically define DRE. Gaps in the literature include limited generalizability, minimal reports in pregnancy, and lack of optimal ASM combinations, among others. Strengths of the evidence include addressing a variety of outcomes. Inconsistent definitions of DRE, small sample sizes, and heterogeneity among studies limit the ability to draw meaningful conclusions. Optimal combinations of ASMs for rational polytherapy for DRE is unclear.
Keywords: drug‐resistant epilepsy, polypharmacy, refractory epilepsy, scoping review
1. INTRODUCTION
Approximately 50 million patients are diagnosed with epilepsy worldwide. 1 Ideally, people with epilepsy (PWE) achieve seizure control with monotherapy; however, some patients may require treatment with multiple antiseizure medications (ASMs). Up to 30% of PWE have drug‐resistant epilepsy (DRE), which has serious morbidity and mortality implications, 2 including significant physical, psychological, and economic ramifications. 3 , 4 DRE is defined as the failure of adequate trials of two tolerated and appropriately chosen ASMs (whether as monotherapies or in combination) to achieve sustained seizure freedom. 2 Other terms for DRE include medically refractory, treatment‐resistant, or pharmacoresistant epilepsy. In 2010, the International League Against Epilepsy (ILAE) published a formal definition for DRE and encouraged standardization of terminology and definitions in research and practice. 2
Over the years, there have been various pharmacologic treatment approaches to DRE. 5 Early on, PWE were treated with multiple first‐generation ASMs (i.e., carbamazepine (CBZ), phenytoin (PTH), phenobarbital (PHB)), including agents with similar mechanisms of action, contributing to poor tolerability. Additionally, there was concern for drug interactions between ASMs and other medications. In the 1990s, there was a shift in practice from prioritizing polytherapy to trialing single ASMs in succession, known as sequential monotherapy, in order to minimize these complications while optimizing efficacy. In the interim, new ASMs became available with different mechanisms of action, improved tolerability profiles, and fewer drug interactions. In the early 2000s, a universal definition of DRE was put forth, so clinicians recognized that monotherapy is insufficient for patients with DRE. As such, clinicians sought to take a thoughtful approach when selecting polytherapy. Known as “rational polytherapy,” this intentional combination of two or more ASMs has a “supra‐additive therapeutic efficacy” with the goal of minimizing toxicity. 5
There is a paucity of literature regarding the optimal combinations of ASMs for rational polytherapy for DRE. In 2018, the American Academy of Neurology and the American Epilepsy Society published guidelines for management of treatment‐resistant epilepsy with second‐ and third‐ generation ASMs for both adults and children. 6 Although they were able to make recommendations regarding use of newer ASMs as adjunctive therapy for DRE, the guideline did not identify optimal combinations of ASMs. The objective of this scoping review is to summarize current evidence related to rational polytherapy among patients with DRE.
2. METHODS
To conduct this scoping review, the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses for Scoping Reviews (PRISMA‐SCr) framework was followed. 7
2.1. Research question and definitions
The research question was broken down into the PICOS (Patient‐Intervention‐Comparison‐Outcome‐Study Design) format to ensure that the research question was properly framed before beginning the search. 8 For this study, patients were defined as adults ages 18 years and older prescribed two or more ASMs. There was no comparator, but the intervention was two or more ASMs. The outcomes were: effectiveness and tolerability, health‐related economic and humanistic outcomes, and utilization of the appropriate definition if DRE is mentioned. Study designs included randomized controlled trials (RCTs) and non‐RCTs; however, case reports and case series were not included. See Table 1 for additional exclusion criteria based on publication type.
TABLE 1.
Research question and inclusion and exclusion criteria for the scoping review
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Year of Publication | January 1981–March 29, 2022 | Prior to 1981 |
| Type of study design | Published peer‐reviewed research articles |
|
| Age | Adults aged 18 years or older | Pediatric population (ages less than 18 years) |
| Type of epilepsy |
For articles prior to 2017
For articles after 2017
|
|
| Number of ASMs | ASM polytherapy (i.e., participants are taking at least two ASMs) | ASM monotherapy only |
| ASMs |
Brivaracetam Carbamazepine Cenobamate Clobazam Clonazepam Divalproex sodium Eslicarbazepine Felbamate Gabapentin Lacosamide Lamotrigine Levetiracetam Oxcarbazepine Perampanel Phenobarbital Phenytoin Pregabalin Primidone Tiagabine Topiramate Valproic acid Vigabatrin Zonisamide |
Ethosuximide Everolimus Fenfluramine Rufinamide Stiripentol Cannabidiol Other treatment modalities, including vagus nerve stimulators as an ASM |
Abbreviation: ASM, antiseizure medications.
From the PICOS, the specific review questions were developed:
What is the current state of evidence of effectiveness and tolerability of rational polytherapy with ASMs?
What is the current state of evidence regarding health‐related economic and humanistic outcomes of rational polytherapy with ASMs?
What term and definition is used to describe DRE?
What gaps in the literature exist regarding rational polytherapy for DRE?
In order to ensure consistency in the scoping review process, the two key definitions were utilized. DRE was defined as the failure of adequate trials of two tolerated and appropriately chosen and used ASM schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom. 2 Synonyms for DRE included pharmacoresistant or medically refractory/intractable epilepsy. Rational polytherapy was defined as the intentional combination of two or more ASMs to have “supra‐additive therapeutic efficacy,” while minimizing toxicity. 5 , 9
2.2. Search strategy
The inclusion and exclusion criteria for the literature search can be found in Table 1. A research librarian was utilized to finalize the search strategy and terms. The search was conducted in PubMed, ProQuest, CINAHL, and Cochrane using the following search terms: drug‐resistant epilepsy, refractory epilepsy, and/or antiepileptic polytherapy. Appropriate wildcards were used in all searches to account for plurals and variations in spelling. The initial year of publication was selected in order to include articles evaluating both older and newer generations of ASMs and various treatment approaches (i.e., sequential monotherapy and rational polytherapy). 5 Once the search strategy was generated, the search was piloted before finalized. References of all identified articles were searched to identify any additional studies for inclusion and review. Articles included in meta‐analyses were reviewed for inclusion rather than including the meta‐analysis in the review.
2.3. Screening, full‐text review, data extraction, and quality appraisal
Once articles were identified, they were imported into Covidence Systematic Review Software (Veritas Health Innovation), an online software program that facilitates the process of reviews. Covidence automatically detected duplicate citations by matching titles, year of publication, volume, and author. 10 A two‐stage screening process in Covidence utilizing two independent reviewers was implemented. In stage one, titles and abstracts of publications were reviewed for inclusion independently by two reviewers. Any articles that met inclusion criteria were moved to stage two. In stage two, two reviewers independently reviewed the full texts of articles for inclusion. A third reviewer resolved any disagreements in each stage.
For articles that met inclusion criteria after full‐text review, data extraction was performed. The following information was extracted: type of article, year of publication, full journal title, location of research, study details (i.e., purpose, study design, start and end date, total number of participants, type of outcomes, and results), and participants/patient population (type of epilepsy, underlying etiology, gender, age range, number of ASMs, specific ASM(s), other comorbidities). The DRE‐related term and definition also were extracted from each publication.
The quality of evidence for each included article was assessed using the Mixed Methods Appraisal Tool (MMAT). 11 Two reviewers rated each article and had to come to full agreement upon the MMAT score. If the two reviewers did not come to an agreement, then a third reviewer resolved disagreements. With the MMAT, scores can range from 0% (no criteria met) to 100% (all criteria met). The tool allows for quality appraisal of qualitative, quantitative, and mixed methods studies and is appropriate for this level of scoping review. If eligible studies did not provide enough information to use the MMAT, reviewers did not calculate a score. The MMAT score was not used for inclusion or exclusion purpose but rather to describe the quality of the literature.
Given the differences in study design and results, a descriptive approach was used to summarize answers to the research questions and quality scores.
3. RESULTS
After removal of duplicates, 6477 citations were identified. The PRISMA flowchart diagram can be found in Figure 1. 12 After review of titles and abstracts, 286 citations were identified as potentially relevant. During full‐text review, 253 citations were excluded. In total, 33 citations met inclusion criteria. The most common reasons for exclusion were wrong study design (n = 94), pediatric population (n = 48), and not focused on polytherapy (n = 27). Of the final 33 articles that met inclusion criteria, 26 studies addressed clinical outcomes, 12 addressed humanistic outcomes, and one study addressed economic outcomes. Twenty‐one studies reported only clinical outcomes, and six studies reported only humanistic outcomes. Additional study characteristics are found in Table 2.
FIGURE 1.
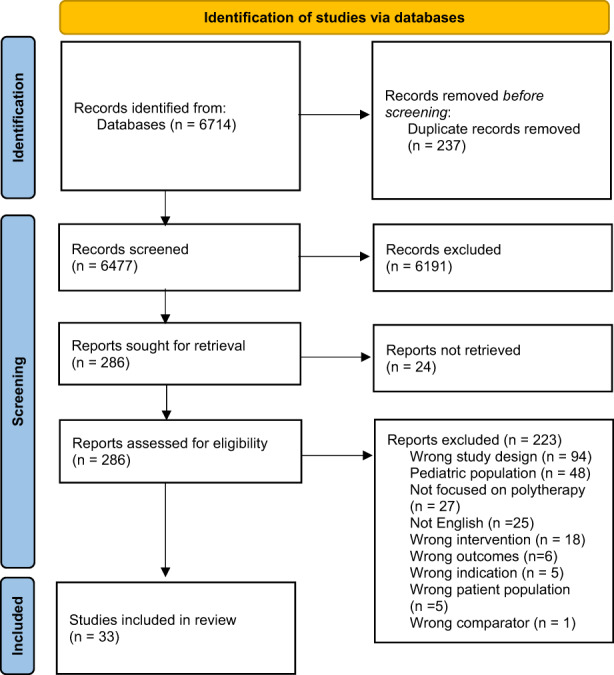
PRISMA flow diagram
TABLE 2.
Characteristics of included articles by their primary outcome
| Clinical (n = 26) | Humanistic (n = 6) | Economic (n = 1) | |
|---|---|---|---|
| Year of study publication (n, %) | |||
| 2010 or before | 12 (46) | 1 (16.6) | 1 (100) |
| After 2010 | 14 (54) | 5 (83.3) | 0 (0) |
| Country (n, %) | |||
| Brazil | 2 (7.6) | 1 (16.6) | |
| China | 1 (3.8) | ||
| Europe | 1 (3.8) | ||
| Italy | 2 (7.6) | ||
| Korea | 1 (3.8) | ||
| Multinational a | 7 (26.9) | ||
| Netherlands | 1 (16.6) | ||
| Poland | 1 (3.8) | ||
| Scotland | 2 (7.6) | ||
| Spain | 1 (3.8) | ||
| Sweden | 1 (16.6) | ||
| Taiwan | 1 (16.6) | ||
| Thailand | 1 (3.8) | ||
| Turkey | 1 (3.8) | ||
| United Kingdom | 1 (3.8) | 1 (100) | |
| United States | 5 (19.2) | 2 (33.3) | |
| Study design (n, %) | |||
| RCT | 10 (38.4) | ||
| Non‐RCT | 16 (61.5) | 6 (100) | 1 (100) |
| Cohort | 12 (46) | 1 (16.6) | 1 (100) |
| Case control | 1 (3.8) | 4 (66.6) | |
| Case series | 1 (3.8) | ||
| Prospective observational | 1 (3.8) | ||
| Response‐conditional crossover | 1 (3.8) | ||
| Cross sectional | 1 (16.6) | ||
| Number of participants (Median, range) | 144 (18–768) | 168 (108–3853) | 125 |
Abbreviation: RCT, randomized controlled trial.
Multinational defined as countries from multiple continents.
The median MMAT score was 80 and ranged from 60 to 100. Quality scores for studies addressing clinical outcomes ranged from 60 to 100 (n = 26), whereas scores ranged from 80 to 100 for humanistic studies (n = 12). Quality score for the economic study was 100.
The following ASMs were specifically evaluated as an intervention by one study reporting clinical outcomes (see Table 3): CBZ, 13 cenobamate, 14 clobazam (CLB), 15 eslicarbazepine (ESL), 16 lacosamide (LCS), 17 levetiracetam (LEV), 18 lamotrigine (LTG), 18 perampanel (PER), 19 pregabalin (PGB). 20 Clinical outcomes of specific combinations of ASMs, including CBZ/valproic acid (VPA) compared to CBZ monotherapy 21 and VPA/LTG compared to VPA and/or LTG monotherapy, 22 were evaluated in one study each. Brivaracetam (BRV), 23 , 24 , 25 oxcarbazepine (OXC), 18 , 26 , 27 and topiramate (TPM) 28 , 29 , 30 were evaluated in three studies each, while zonisamide (ZNS) was evaluated in two studies, 31 , 32 all of which reported clinical outcomes. Among studies reporting humanistic outcomes, OXC was evaluated in one study, 27 while ZNS was evaluated in two studies. 31 , 32 In the study reporting economic outcomes, CLB, gabapentin (GBP), LTG, TPM, and vigabatrin (VGB) were compared among patients taking at least one ASM at baseline. 33 See Table 4 for a summary of humanistic and economic outcomes.
TABLE 3.
Summary of studies reporting clinical outcomes
| Author (year) Country, Study design | Total number of participants | Age (mean ± SD and/or median, range), in years | Intervention and control groups (if applicable) | Number of ASMs at baseline | Clinical outcome(s) evaluated | Summary of results | Results based on polytherapy |
|---|---|---|---|---|---|---|---|
|
Azar et al. (2010) 18 US, Non‐RCT, cohort |
43 | 33.3 ± 11.8 (range 18–59) | LEV, LTG, OXC |
Monotherapy: 15 Polytherapy: 28 |
Association of seizure interval prolongation (SIP) with additional metrics |
SIP was not difference between LEV, LTG, and OXC (p‐value not reported) Mean SIP was higher in patients with a history of ASM tolerance vs. no reported history (23.9 ± 35.9 days vs. 13.5 ± 16.2 days, p = 0.34) |
SIP was greater in monotherapy vs. polytherapy group (25.6 ± 23.7 vs. 13.9 ± 28.0 days, p = 0.02) |
|
Biton et al. (2014) 23 Multinational RCT |
400 randomized 396 ITT |
Placebo: 37.5 ± 12.6 BRV 5: 38.9 ± 11.6 BRV 20: 37.3 ± 13.3 BRV 50: 38.9 ± 12.3 |
Placebo vs. BRV (5, 20, or 50 mg/day) |
Placebo: monotherapy 13 (13.3%) polytherapy 84 (85.7%) BRV 5: monotherapy 14 (14.4%) Polytherapy 83 (85.6%) BRV 20: monotherapy 16 (16%) polytherapy 84 (84%) BRV 50: monotherapy 13 (12.9%) polytherapy 88 (86.1%) |
Primary outcome:
Secondary outcomes:
|
Significant difference in the percent reduction of focal seizure frequency with BRV 50 mg/day compared to placebo (12.8, p = 0.025). Treatment‐emergent adverse events:
|
Subgroup analysis of LEV‐naive vs. prior LEV use vs. concomitant LEV (no statistical analysis) Responder rate based on LEV exposure (found in table S1) 11 : Patients who were LEV‐naive had the best results among BRV 50 mg compared to prior or concomitant LEV use:
Patients who were LEV‐prior compared to others had the best results among BRV 20 mg:
|
|
Borges Pereira et al. (1996) 13 Brazil Non‐RCT, Cohort |
48 | Range: 18–51 | CBZ only | 33 on monotherapy, 15 on polytherapy |
|
|
No statistical analyses were conducted based on monotherapy vs. polytherapy. Among the 9 patients who achieved seizure freedom, 3 were prescribed polytherapy. In addition to CBZ, these patients were prescribed:
Patients taking monotherapy had a higher number of average plasma levels outside of the therapeutic range compared to polytherapy regimens (see figure 3). 15 |
|
Brahmbhatt et al. (2021) 15 US Non‐RCT, Case series |
33 | 43.5 ± 15 (range: 21.8–76.8) | CLB only | 2 on monotherapy, 31 on polytherapy | Demographic information, clinical response, and safety |
28 (80%) started CLB due to refractory seizures, and seven (20%) started CLB due to experiencing side effects of other ASMs. Two patients did not tolerate CLB due to side effects (i.e., tolerability), but one of these patients reported CLB was effective |
No statistical analyses were conducted based on monotherapy vs. polytherapy. At the last study visit, patients were taking CLB and:
|
|
Elger et al. (2009) 16 Multinational, RCT |
468 (402 randomized) |
Placebo: 37.0 ± 11.93 ESL 400: 37.8 ± 11.43 ESL 800: 41.3 ± 12.04 ESL 1200: 38.4 ± 11.71 |
Placebo vs. ESL (400, 800, or 1200 mg/day) |
Placebo: 33.3% monotherapy, 66.7% polytherapy (defined as 2 or 3 ASMs) ESL 400: 39.0% monotherapy, 61.0% polytherapy ESL 800: 31.6% monotherapy, 68.4% polytherapy ESL 1200: 38.2% monotherapy, 61.8% polytherapy |
Primary:
Secondary:
|
Seizure frequency was significantly lower with ESL 800 mg (LS Mean 5.66, p = 0.0028 mg) and ESL 1200 mg (LS Mean 5.35, p = 0.0003) compared to placebo (LS Mean 7.64), but not ESL 400 mg (LS Mean 6.73, not significant) | All patients were taking multiple ASMs |
|
French et al. (2014) 27 Multinational, RCT |
366 |
Placebo: 39.1 ± 12.5 OXC 1200: 39.1 ± 11.5 OXC 2400: 38.5 ± 11.6 |
Placebo vs. OXC (1200 or 2400 mg/day) |
1 ASM Placebo: 43 (35.5%) OXC 1200: 36 (29.5%) OXC 2400: 40 (32.5%) 2 ASMs Placebo: 61 (50.4%) OXC 1200: 68 (55.7%) OXC 2400: 67 (54.5%) 3 ASMs Placebo: 17 (14.0%) OXC 1200: 18 (14.8%) OXC 2400: 16 (13.0%) (1 patient received 4 ASMs) |
Primary:
Secondary:
|
Percent change in seizure frequency was significantly lower with OXC 2400 mg (−42.9%) compared with placebo (−28.7%, p < 0.05), but OXC 1200 mg (−38.2%) compared to placebo | All patients were taking multiple ASMs
|
|
French et al. (2021) 14 Multinational Non‐RCT, Cohort |
149 | 37.6 ± 10.9 | Cenobamate only |
1 ASM: 13 (8.7%) 2 ASM: 70 (47.0%) 3 ASM: 62 (41.6%) >3 ASM: 4 (2.7%) |
|
|
Over 50% (53.7%) of patients were able to discontinue one or more concomitant ASMs, while 26.2% of patients required the addition of one or more concomitant ASMs during the study period |
|
Harden et al. (1993) 21 US Non‐RCT, Patient‐controlled |
18 | 43 (26–61) | CBZ‐VPA bitherapy vs. CBZ monotherapy | NA | Seizure frequency compared to CBZ monotherapy, categorized as:
|
|
All patients received the same treatment |
|
Kellet et al. (1999) 30 UK Non‐RCT, Cohort |
174 | Not reported | TPM | Not reported (119/174 patients were taking two or more ASMs at baseline) |
|
|
Taking other ASMs at baseline was not associated with time‐to‐stop TPM (χ2 0.62, p = 0.733) Monotherapy or polytherapy: Continued TPM (n = 84):
Discontinued TPM (n = 90):
|
|
Klein et al. (2015) 24 Multinational RCT |
768 |
Placebo: 39.8 ± 12.5 BRV 100: 39.1 ± 13.4 BRV 200: 39.8 ± 12.8 |
Placebo vs. BRV (100 or 200 mg/day) |
1 ASM: Placebo: 75 (29.0%) BRV 100: 70 (27.8%) BRV 200: 69 (27.7%) 2 ASMs: Placebo: 181 (35.5%) BRV 100: 182 (72.2%) BRV 200: 179 (71.9%) ≥3 ASMs: Placebo: 3 (1.2%) BRV 100: 0 BRV 200: 1 (0.4%) |
Co‐primary:
Secondary:
|
|
Patients taking ≤2 ASMs had better outcomes than >2 ASMs in both BRV 100 and 200 mg/day compared to placebo (p‐values not reported).
Based on LEV exposure
|
|
Kraiprab et al. (2005) 26 Thailand RCT |
39 |
Total: 31.6 ± 7.1 (range 18–44) OXC 600: 30.4 ± 7.3 OXC 1200: 31.7 ± 6.9 |
OXC 600 vs. 1200 mg/day |
1 ASM: 20 (51%) 2 ASM: 10 (26%) 3 ASM: 9 (23%) |
Primary:
Secondary:
MHD plasma concentration |
Median percent reduction was −47% for OXC 600 mg/day compared with −58% for OXC 1200 mg/day (p = 0.729) |
Based on CBZ exposure
|
|
Kusznir Vitturi et al. (2019) 34 Brazil Non‐RCT, Cohort |
82 | 24.5 ± 5.5 | N/A |
|
|
|
|
|
Lattanzi et al. (2021) 19 Italy Non‐RCT, Cohort |
92 | 69 (range 66–73) | PER | All patients were taking 1 or 2 concomitant ASMs |
Effectiveness:
Safety
|
|
|
|
Lee et al. (2013) 35 Korea Non‐RCT, Cohort |
95 | 39.1 ± 10.8 | N/A |
1 ASM – 9 (9.5%) 2 ASMs – 43 (45.3%) 3 ASMs – 21 (22.1%) 4 or more ASMs – 22 (23.2%) |
|
|
|
|
Pirio Richardson et al. (2004) 38 US Non‐RCT, Cohort |
35 | 39.8 (range 20–63) | N/A | All were on polytherapy at the start of the study were converted to monotherapy, and followed for 12 months |
|
|
|
|
Pisani et al. (1999) 22 Italy Non‐RCT, Response‐conditional crossover |
20 | Range: 19–51 | VPA vs. LTG vs. combo VPA/LTG |
15 patients were on monotherapy 5 were on polytherapy |
|
|
|
|
Ryvlin et al. (2014) 25 Multinational RCT |
398 |
Total population: 37.2 ± 13.1 Placebo: 36.4 ± 13.0 BRV 20: 35.7 ± 12.5 BRV 50: 38.9 ± 13.6 BRV 100: 38.0 ± 13.1 |
Placebo vs. BRV (20, 50, or 100 mg/day) |
1 ASM: 68 (17.1%) 2 ASMs: 314 (78.9%) ≥3 ASMs: 16 (4.0%) |
Primary:
Secondary:
|
The percent reduction of focal seizure frequency/week was 6.5% for BRV 50 mg compared to placebo and was not statistically significant (p = 0.261) | Patients taking concomitant LEV did not benefit as much as LEV‐naive patients or those with prior LEV use regarding primary efficacy outcome or response rate (no p‐values reported) |
|
Sackellarres et al. (2004) 31 US RCT |
152 |
Placebo: 36.4 ± 11.3 ZNS: 35.6 ± 12.1 |
Placebo vs. ZNS | Not reported |
Primary:
Secondary:
|
There was a statistically significant difference in median percent reduction in seizure frequency for all seizure groups among ZNS vs. placebo, respectively.
|
Not reported |
|
Schmidt et al. (1993) 32 Europe RCT |
139 | Range: 18–59 | Placebo vs. ZNS | Up to three concomitant ASMs (with therapeutic levels prior to study start) |
Primary:
Secondary:
|
There was a statistically significant reduction in the medial percent change in complex partial seizure frequency from baseline with ZNS compared to placebo (−27.7% vs. 3.9%, p < 0.05) | Not reported |
|
Senadim et al. (2018) 39 Turkey Non‐RCT, Cohort |
39 | 34.3 ± 9.3 (range: 19–56) | ZNS | ZNS was started as the second drug in 12.8% (n = 5) of patients, the third in 61.5% (n = 24), the fourth in 23.1% (n = 9) and the fifth drug in 2.6% (n = 1) | Demographic information, clinical response, and safety |
|
|
|
Shorvon et al. (2000) 36 Multinational Non‐RCT, Cohort |
324 | 37 ± 11 | Placebo vs. LEV |
60 (19%) were on monotherapy 264 (81%) were on polytherapy (2 or 3 ASMs) |
Primary:
Secondary:
|
|
Not reported |
|
Stephen et al. (2000) 28 Scotland Non‐RCT, Prospective observational |
170 | 18–75 | TPM only |
93 patients were on monotherapy 67 taking two ASMs 10 taking three ASMs |
Co‐primary (at 6 months):
Additional outcomes:
|
Over the 6‐month timeframe:
|
No statistical analyses were conducted based on monotherapy vs. polytherapy. Of the patients who were seizure‐free (n = 39): TPM and
Of the patients who were responders (n = 80): TPM and
Of the patients for who TPM was withdrawn (n = 12): TPM and
|
|
Stephen et al. (2011) 20 Scotland Non‐RCT, Cohort |
135 | 44 (18–76) | PGB only |
1 ASM – 93 patients 2 ASMs – 67 3 ASMs – 10 |
Co‐primary (at ≥6 months):
Additional outcomes:
|
Over the ≥6‐month timeframe:
|
|
|
Villanueva et al. (2012) 17 Spain Non‐RCT, Cohort |
158 | 42.1 ± 15.3 | LCS only | All but five patients were taking at least one ASM at baseline |
Co‐primary (at 12 months):
Secondary:
|
At 12 months:
|
|
|
Wezyk et al. (2020) 37 Poland Non‐RCT, Cohort |
530 | 36.1 ± 12.6 | NA |
292 (55.1%) on monotherapy 238 (44.9%) on polytherapy
|
|
|
Independent predictor of seizure freedom included:
|
|
Zhang et al. (2011) 29 China RCT |
86 | 73.4 ± 8.5 | Placebo vs. TPM |
1 ASM: 8 (9.3%) 2 ASMs: 37 (43.0%) 3 ASMs: 41 (47.7%) |
|
|
Not reported |
Abbreviations: AEs, adverse effects; ASM, antiseizure medication; EEG, electroencephalogram; Ei‐ASM, enzyme‐inducing antiseizure medication; EMU, epilepsy monitoring unit; ITT, intention to treat; LS, least square; MHD, monohydroxycarbazepine; PK, pharmacokinetics; POS, partial‐onset seizures; TEAEs, Treatment‐emergent adverse events; US, United States.
Abbreviations for ASMs: BRV, brivaracetam; CBZ, carbamazepine; CLB, clobazam; ESL, eslicarbazepine acetate; GBP, gabapentin; LCS, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PER, perampanel; PGB, pregabalin; PHB, phenobarbital; PTH, phenytoin; TPM, topiramate; VGB, vigabatrin; VPA, valproate; ZNS, zonisamide.
TABLE 4.
Summary of studies reporting humanistic and economic outcomes
| Author (year) Country, study design | Total number of participants | Age (mean ± SD and/or median, IQR) | Intervention and control groups (if applicable) | Number of ASMs at baseline | Humanistic outcome(s) evaluated | Summary of results | Results based on polytherapy |
|---|---|---|---|---|---|---|---|
|
Bardai et al. (2012) 44 Netherlands Non‐RCT, case control |
3853 (1019 cases vs. 2834 controls) PWE: 12 cases and 12 controls |
Cases: 63.5 ± 13.7 Controls: 58.3 ± 14.5 Cases with epilepsy: 60.0 ± 16.0 |
NA |
Reported for PWE only: Monotherapy: Cases: 4 Controls: 10 Polytherapy: Cases: 8 Controls: 2 |
Odds of SCA associated with epilepsy | Epilepsy was associated increased odds of SCA (adjusted OR 2.9 [95% CI 1.1–8.0], p = 0.034) |
No statistical analyses were conducted based on monotherapy vs. polytherapy. Numerically more PWE cases taking polytherapy (8/12, 75%) compared to controls (2/12, 17%) |
|
Bautista et al. (2012) 41 US Non‐RCT, cohort |
108 | 42 | NA | 68% on monotherapy and 31% on two ASMs | MMPR |
|
MMPR for monotherapy was significantly higher compared to polytherapy (0.95 ± 0.25 vs. 0.91 ± 0.2, p = 0.02) |
|
Freitas‐Lima et al. (2013) 43 Non‐RCT, cross sectional |
112 | 38.8 ± 11.8 | NA |
1 ASM: 8 (7.1%) 2 ASMs: 36 (32.1%) ≥3 ASMs: 68 (60.7%) 4 ASMs: 6 (5.4%) |
ASM prescribing patterns (e.g., mean ± SD (range) of ASMs per patient, commonly prescribed ASMs) Evaluation of ASM load: ratio of prescribed daily dose over defined daily dose (PDD/DDD) |
|
The more ASMs prescribed, the higher the ASM load (r = 0.63, p < 0.01) |
|
French et al. (2014) 27 International RCT |
366 |
Placebo: 39.1 ± 12.5 OXC 1200: 39.1 ± 11.5 OXC 2400: 38.5 ± 11.6 |
Placebo vs. OXC (1200 or 2400 mg) |
1 ASM: Placebo 43 (35.5%) OXC 1200: 36 (29.5%) OXC 2400: 40 (32.5%) 2 ASMs: Placebo: 61 (50.4%) OXC 1200: 68 (55.7%) OXC 2400: 67 (54.5%) 3 ASMs: Placebo: 17 (14%) OXC 1200: 18 (14.8%) OXC 2400: 16 (13%) |
(Secondary outcomes) changes in PGIC and QOLIE‐31 scores |
|
All patients were taking multiple ASMs |
|
Kusznir Vitturi et al. (2019) 34 Brazil Non‐RCT, Cohort |
82 | 24.5 ± 5.5 | NA |
Monotherapy: 41 (50%) Polytherapy: 40 (48.8%) |
|
|
|
|
Pirio Richardson et al. (2004) 38 US Non‐RCT, cohort |
35 | 39.8 (range, 20–63) | NA | All were on polytherapy at the start of the study and were converted to monotherapy | Quality of life, as measured by 10 questions from QOLIE‐31 |
|
All patients were transitioned from polytherapy to monotherapy |
|
Remak et al. (2004) 33 UK Non‐RCT, cohort |
125 |
CLB: 37.3 ± 8.2 GBP: 36 ± 11 LTG: 37 ± 9.8 TPM: 38 ± 12.6 VGB: 35.7 ± 12 |
CLB, GBP, LTG, TPM, VGB | All patients were on at least one ASM |
Humanistic
Economic
|
Humanistic
Economic
|
Not reported |
|
Sackellarres et al. (2004) 31 US RCT |
152 |
Placebo: 36.4 ± 11.3 ZNS: 35.6 ± 12.1 |
Placebo vs. ZNS | Not reported | (Secondary outcomes) Physician and patient global assessments |
|
Not reported |
|
Schmidt et al. (1993) 32 European RCT |
139 | Range: 18–59 | Placebo vs. ZNS | Up to three concomitant ASMs (with therapeutic levels prior to study start) | (Secondary outcomes) Physician and patient global assessments |
|
Not reported |
|
Sveinsson et al. (2020) 45 Sweden Non‐RCT, case control |
1275 (255 cases vs. 1148 controls) | Not reported | NA |
Cases: 0 ASMs: 46 1 ASM: 113 2 ASMs: 96 Controls: 0 ASMs: 265 1 ASM: 483 ≥2 ASMs: 400 |
|
OR of SUDEP and: No ASMs vs. specific ASM monotherapy:
Nonadherence mentioned in the medical record:
|
OR of SUDEP based on ASM therapy:
|
|
Xu et al. (2006) 40 US Non‐RCT, cross sectional |
201 | 44.2 ± 12.5 | NA |
2 ASMs: 139 (69.2%) 3 ASMs: 49 (24.4%) 4 ASMs: 12 (6.0%) ≥5 ASMs: 1 (0.5%) |
|
Quality of life
Sleep scores
(For reference, the average score for the general US population is 26.)
Comparison among subjects with and without diagnosed sleep disturbance
Among patients taking two ASMs
|
All patients were taking multiple ASMs No significant difference in the mean MOS Sleep Problems Index Score based on number of ASMs (p = 0.202); however, patients taking 4+ ASMs reported higher scores compared to patient taking two or three ASMs |
|
Yeh et al. (2021) 42 Taiwan Non‐RCT, case control |
134 |
Refractory: 36.7 ± 12.1 Medically controlled: 34.9 ± 11.6 |
NA |
Refractory: 1 ASM: 5.30% 2 ASM: 60.5% 3 ASMs: 23.70% Medically controlled: 1 ASM: 53.1% 2 ASMs: 27.10% |
|
PSQI score
ESS score
Sleep architecture for refractory vs. controlled, respectively
REM sleep %: 13.52 ± 6.097 vs. 16.24 ± 6.103, p < 0.05
|
Not reported |
Abbreviations: ASM, antiseizure medication; BID, twice daily; CI, confidence interval; EQ‐5D, EuroQol‐5 dimensions; ESS, Epworth Sleepiness Scale; ICER, incremental cost‐effectiveness ratio; MCM, major congenital malformations; MMPR, mean medication possession ratio; OR, odds ratio; PGIC, Patient Global Impression of Change; PSQI, Pittsburgh Sleep Quality Index; PWE, people with epilepsy; QALY, quality‐adjusted life years; QOLIE‐10, quality of life in epilepsy‐10; QOLIE‐31, quality of life in epilepsy‐31; SCA, sudden cardiac arrest; SF36, Short form survey; SUDEP, Sudden Unexpected Death in Epilepsy; TID, three times daily; MOS, Medical Outcomes Study.
Abbreviations for ASMs: BRV, brivaracetam; CBZ, carbamazepine; CLB, clobazam; ESL, eslicarbazepine acetate; GBP, gabapentin; LCS, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PER, perampanel; PGB, pregabalin; PHB, phenobarbital; PTH, phenytoin; TPM, topiramate; VGB, vigabatrin; VPA, valproate; ZNS, zonisamide.
3.1. Research question 1: clinical (effectiveness and tolerability) outcomes
Results based on clinical outcomes are summarized in Table 3. Common efficacy outcomes included ≥50% reduction in seizure frequency (n = 14), 17 , 19 , 20 , 21 , 23 , 24 , 25 , 26 , 28 , 29 , 31 , 32 , 34 , 35 , 36 followed by achieving seizure freedom (n = 14) 17 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 34 , 35 , 36 , 37 and percent reduction in seizure frequency (n = 8). 23 , 24 , 25 , 26 , 27 , 29 , 31 , 32 Additionally, eight studies reported if seizure frequency was increased or exacerbated. 16 , 19 , 21 , 28 , 29 , 34 Sixteen studies addressed safety outcomes, 14 , 15 , 16 , 17 , 19 , 20 , 24 , 25 , 26 , 27 , 28 , 29 , 31 , 36 , 38 , 39 while seven studies reported plasma concentrations of the study drug 13 , 24 , 26 , 27 and/or all ASMs taken by patients. 22 , 28 , 36
Outcomes evaluating specific ASM combinations were not part of the primary outcomes but were generally reported with or without statistical analysis. For example, patients receiving concomitant PER and enzyme‐inducing ASMs (e.g., CBZ, PTH, phenobarbital, primidone) were less likely to be classified as responders or achieve seizure control compared to non‐enzyme‐inducing ASMs. In addition, another study reported the most effective combination was OXC and TPM where three of four patients were classified as responders. 26 Finally, other studies reported that VPA and LTG were an effective combination. 22 , 37
There were some unique efficacy‐related outcomes, such as seizure interval prolongation after a brief ASM holiday among patients with previous ASM tolerance 18 and initial achievement of seizure control with LEV with regression at 3 months. 35 Additionally, studies that focused only on safety and tolerability also reported unique outcomes compared to studies that reported both safety and efficacy outcomes, such as time to discontinuation, 30 retention rate, 14 , 30 factors associated with discontinuation, 30 probability of treatment continuation, 14 and most common reasons for discontinuation. 14 Some studies also did not report specific percent reductions but rather categorized patients into various groups given their response to treatment such as seizure frequency, adjustment to ASM therapy, seizure freedom, responder status, or discontinuation of therapy. 28 , 34 Finally, one study reported the effect of withdrawing concomitant ASMs after achieving response with TPM. 28
Among the six studies that mentioned worsening seizure control in their methods, one study did not report any results associated with increased seizure frequency. 16 In a study involving pregnant women, seizure frequency increased in 51% of the population, compared to remaining unchanged in 33%, decreased in 12%, and seizure freedom in 4% (no statistical analysis reported). 34 In the other three studies, reports of increased seizure frequency among patients ranged from 1.1% to 39%. 19 , 21 , 29
3.2. Research question 2: humanistic and economic outcomes
Of the 12 studies reporting humanistic outcomes, six studies also reported clinical or economic outcomes and six reported solely humanistic outcomes. When clinical or economic outcomes were also reported, humanistic outcomes were considered secondary outcomes. The most common humanist outcomes across studies included quality of life (n = 4), 27 , 33 , 38 , 40 medication adherence (n = 2), 34 , 41 sleep‐related outcomes (n = 2), 40 , 42 and physician and patient global assessments (n = 2). 31 , 32 Some articles reported multiple humanistic outcomes. See Table 4 for a summary of included studies.
Among any study reporting quality of life‐related outcomes, various tools were utilized including: EuroQol‐5 dimensions (EQ‐5D), 33 , 40 Quality of Life in Epilepsy Inventory QOLIE‐10, 40 (QOLIE‐31), 27 , 38 Patient Global Impression of Change (PGIC), 27 and Quality of Life Assessment Schedule. 33 Impact on quality of life was variable. One study showed improved QOLIE‐31 scores among those successfully transitioned to monotherapy compared to patients remaining on polytherapy. 38 Another study demonstrated no difference in the change in QOLIE‐31 or PGIC scores between placebo and OXC. 27 Additionally, QOLIE‐10 (p = 0.006) and EQ‐5D (p < 0.001) scores were significantly better among patients without a diagnosed sleep disturbance compared to those with a diagnosed sleep disturbance. 40 Finally, patients prescribed TPM experienced improvement in the EQ‐5D score from baseline (p < 0.05), while there was a small but nonsignificant increase for VGB and nonsignificant declines for GBP, CLB, and LTG. 33 This study also collected data using the Quality of Life Assessment Schedule; however, the results were not clearly reported. 33
Of the two studies describing medication adherence, one study reported mean medication possession ratio, 41 while the other evaluated adherence using the Morisky Medication Adherence Scale. 34 Monotherapy was associated with better adherence compared to polytherapy regimens. 41 Additionally, poor adherence was associated with increased seizure frequency. 34 Furthermore, the addition of an ASM was associated with increased seizure frequency, whereas polytherapy in general was not associated with increased seizure frequency. Finally, patients treated with a higher number of ASMs, among other factors, were associated with increased odds of status epilepticus (p < 0.05, statistical analysis not reported), and polytherapy was associated with increased odds of obstetric complications (odds ratio (OR) 2.8 [95% confidence interval (CI) 1.1–7.6, p = 0.06]). 34
Among the studies reporting sleep‐related outcomes, authors used the Medical Outcomes Study (MOS) Sleep Scale 40 or Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and Short Form Survey (SF36) and polysomnography results. 42 There were no differences in MOS sleep scale results among patients taking two or three ASMs; however, patients taking four or more ASMs reported numerically higher scores compared to three ASMs or less, but this was not statistically significant (p = 0.202). 40 In general, the percentage of patients with elevated PSQI and ESS scores were not significantly different between patients with refractory and medically controlled epilepsy. 42
Finally, the two studies that reported global assessments were both related to zonisamide (ZNS), 31 , 32 where physicians and patients rated improvement of patient symptoms as marked, slight, none, or worse at the end of the study period. In both studies among patients with refractory epilepsy, there was a statistically significant difference in marked improvement in symptoms between adjunctive ZNS and placebo groups. 31 , 32
One study was included that evaluated economic outcomes, including health care costs, quality‐adjusted life years (QALY), cost‐utility analyses, and incremental cost‐effectiveness ratios. 33 TPM and VGB were associated with increased utility scores, but the incremental cost‐effectiveness ratio (ICER) of TPM was £7869/QALY compared to VGB (see Table 4). 33
3.3. Research question 3: terms and definitions for DRE
Included studies used a variety of terms and definitions for DRE (see Table 5). The ILAE published their definition of DRE in 2010. In our review, 14 studies were published through 2010, and 19 studies were published after 2010. The majority of studies used the term “refractory.” These studies also used other terms, such as adjunctive, uncontrolled, or intractable. Two studies solely used the term “adjunctive” to describe the intervention, 19 , 27 while three studies solely used the term “uncontrolled” to describe epilepsy. 20 , 23 , 24
TABLE 5.
Drug‐resistant epilepsy terminology and definition
| Author (year) | Specific term(s) | Definition of DRE |
|---|---|---|
| Azar et al. (2010) 18 | Refractory, “history of AED tolerance” | Failed at least one ASM in the past |
| Bardai et al. (2012) 44 | NA | Not reported; authors differentiated between mono‐ and polytherapy |
| Bautista et al. (2012) 41 | Intractable epilepsy | Not defined; authors differentiated between mono‐ and polytherapy |
| Biton et al. (2014) 23 | Uncontrolled | Patients were uncontrolled on one to two concomitant ASMs at optimal stable dosages for ≥1 month prior to screening and throughout the treatment period |
| Borges et al. (1996) 13 | Refractory | No significant reduction of seizure frequency despite of the adequate treatment with several ASMs prescribed on mono‐ or polytherapy |
| Brahmbhatt et al. (2021) 15 | Post‐operative tumor‐related refractory, add‐on ASM | Two or more ASMs at baseline |
| Elger et al. (2009) 16 | Refractory epilepsy; adjunctive therapy | Inclusion criteria: receiving one or two ASMs in a stable dose regimen for at least 2 weeks before screening and had at least 4 POS in the two 4‐week periods of the baseline phase with no seizure‐free interval > 21 days |
| Freitas‐Lima et al. (2013) 43 | Refractory | Persistence of seizures after adequately applied treatment(s) with one or more appropriate ASMs at maximally tolerated doses, excluding treatments whereby titration to usually effective dosages was prevented by the onset of idiosyncratic reactions |
| French et al. (2014) 27 | Adjunctive therapy | Patients had to have an average of at least three seizures monthly (28 days) during the 8‐week screening period despite ongoing treatment with stable doses of one to three ASMs with/without adjunctive VNS |
| French et al. (2021) 14 | Treatment‐resistant epilepsy; adjunctive treatment | Uncontrolled despite treatment with at least one ASM within the past 2 years. Patients must be taking one to three ASM at stable doses for 4 weeks before screening |
| Harden et al. (1993) 21 | Refractory, intractable epilepsy | Refractory to treatment with multiple medications; all with unsatisfactory control on highest tolerated CBZ dose for at least 3 months, with documented toxicity |
| Kellet et al. (1999) 30 | Refractory | Two or more ASMs at baseline |
| Klein et al. (2015) 24 | Uncontrolled | One or two conventional ASMs at stable dosage for at least 1 month before visit 1 (3 months for phenobarbital, phenytoin, and primidone) |
| Kraiprab et al. (2005) 26 | Refractory, uncontrolled | Inclusion criteria: Patients experienced at least four seizures during the 8‐week baseline period, taking at least one or more concomitant ASM at baseline. If patients were only on one concomitant ASM, they had failed treatment with other ASM, either as monotherapy or polytherapy |
| Kusznir Vitturi et al. (2019) 34 | Refractory | When treatment fails to achieve seizure freedom for 12 months or more, for whatever reason |
| Lattanzi et al. (2021) 19 | Adjunctive | Inclusion criteria: Stable treatment with one or more ASMs in the past 90 days |
| Lee et al. (2013) 35 | Refractory, uncontrolled | Inclusion criteria: One or more concomitant ASM at stable doses for at least 4 weeks prior to selection, if they had uncontrolled partial seizures with or without secondary generalization at least once a month during the 3‐month retrospective baseline period before LEV commencement |
| Pirio Richardson et al. (2004) 38 | Medically refractory epilepsy | Failed two or more first‐line ASM at adequate doses as monotherapy and in combination |
| Pisani et al. (1999) 22 | Refractory | Inclusion criteria: Occurrence of at least four complex partial seizures per month during the previous 3 months, despite adequate dosages of no more than two conventional ASM |
| Remak et al. (2004) 33 | Refractory, adjunctive treatment | Starting adjunctive treatment with one of five ASMs |
| Ryvlin et al. (2014) 25 | Refractory | Inclusion criteria: Previous trial of at least two ASMs |
| Sackellarres et al. (2004) 31 | Refractory, adjunctive treatment | Inclusion criteria: were refractory to current AED therapy; had a history of at least four complex partial seizures per month; and had no more than eight generalized tonic, clonic, or tonic–clonic seizures per month |
| Schmidt et al. (1993) 32 | Refractory | Inclusion criteria: Average of at least four seizures per month despite therapeutic plasma concentrations of standard ASMs |
| Senadim et al. (2018) 39 | Refractory | Polytherapy; referred to ILAE 2010 publication 1 |
| Shorvon et al. (2000) 36 | Refractory | Inclusion criteria: All patients had seizures that had persisted for at least the previous 2 years despite treatment with one or two ASMs and were on stable dose regimens of a maximum of two ASMs for at least 4 weeks before the selection visit, as well as throughout the study |
| Stephen et al. (2000) 28 | Refractory | Inclusion criteria: Continued to have seizures for more than 2 years despite treatment with a range of ASMs |
| Stephen et al. (2011) 20 | Uncontrolled | Unclear definition, summarized as failed at least one ASM |
| Sveinsson et al. (2020) 45 | Refractory | Not defined; authors differentiated between mono‐ and polytherapy |
| Villanueva et al. (2012) 17 | Refractory | Patients who had not achieved complete seizure control after at least two ASMs |
| Wezyk et al. (2020) 37 | Refractory | Not defined; authors differentiated between mono‐ and polytherapy |
| Xu et al. (2006) 40 | Refractory | Inclusion criteria: Stable polytherapy (at least 2 ASMs), on treatment for ≥60 days, on a stable dose for ≥30 days |
| Yeh et al. (2021) 42 | Refractory | Based on the ILAE definition and criteria from Taiwan Handicap Handbook (e.g., additionally ≥2 monthly seizure episodes over the past year) |
| Zhang et al. (2011) 29 | Refractory, adjunctive | At least 4 seizures during an 8‐week baseline period, despite treatment with one to three standard ASMs |
Abbreviations: AED, antiepileptic drug; ASM, antiseizure medication; CBZ, carbamazepine; ILAE, International League Against Epilepsy; LEV, levetiracetam; NA, not applicable; POS, partial‐onset seizure; VNS, vagus nerve stimulator.
Among RCTs (n = 10), “refractory” epilepsy was used in seven studies. 13 , 15 , 21 , 22 , 25 , 28 , 35 Additionally, five studies also used the term “adjunctive” or “adjunctive therapy” to describe the intervention. 14 , 16 , 27 , 29 , 31 Three studies also used the term “uncontrolled” or “not adequately controlled,” referring to seizures. 23 , 24 , 26 Among non‐RCTs (n = 23), “refractory” epilepsy was used in 15 studies. 13 , 15 , 17 , 18 , 21 , 22 , 28 , 30 , 33 , 34 , 35 , 39 , 40 , 42 , 43 Two studies specifically highlighted “medically refractory epilepsy” 38 and “treatment‐resistant epilepsy.” 14 Four studies did not specifically define DRE. 37 , 41 , 44 , 45 Humanistic and clinical outcomes were captured by three 41 , 44 , 45 and one 37 study, respectively, and compared ASM mono‐ and polytherapy.
Definitions of DRE varied between studies. Two studies specifically referenced the ILAE definition of DRE. 39 , 42 Four studies highlighted if previous therapy failed to control seizures. 15 , 21 , 26 , 38 Two of these studies specified at least two ASMs could fail as either monotherapy or in combination. 26 , 38 One study included patients if only one ASM failed. One study did not specify the number of previously failed ASMs. 21 In addition, two studies defined refractory epilepsy based on seizure freedom. 17 , 34 The first study used the definition of inability to achieve seizure freedom in the past 12 months, 34 and the second study used the definition of inability to completely control seizures after trying at least two ASMs. 17 Furthermore, some authors sought to clarify whether patients had adequate trials of ASMs to be considered DRE. One study included establishing therapeutic concentrations of baseline ASMs as part of the inclusion criteria for refractory epilepsy. 32 Eight studies specified patients had to be on stable doses of ASMs prior to entering the study. 14 , 16 , 23 , 24 , 27 , 35 , 36 , 40 The time frame varied by study.
Additionally, some definitions were specific to the study inclusion criteria and may not specifically reflect the definition of DRE, but this was not clarified. Ten studies mentioned a maximum number of ASMs patients could be on as part of their inclusion criteria. Six studies limited participants to one or two ASMs at baseline, 16 , 22 , 23 , 24 , 31 , 36 and three studies limited participants to three ASMs. 14 , 27 , 29
3.4. Research question 4: gaps in the literature
Gaps in the literature were evident related to every research question, especially regarding specific ASMs combinations for rational polytherapy. Some gaps in the literature are related to study design and generalizability. Included studies utilized a range of study designs; however, the RCTs evaluated an adjunctive ASM compared to placebo, whereas retrospective, non‐randomized studies typically evaluated effective combinations of ASMs. In addition, although studies were conducted in a variety of countries, African countries were minimally represented, which may impact generalizability. Furthermore, only one article included pregnant women. 34 Outcomes could not be thoroughly evaluated among this population or the impact on the children themselves. Additionally, many studies had small sample sizes. Finally, consistent terminology for and definition of DRE is lacking. Table 6 includes a summary of gaps in the literature related to clinical, humanistic, and economic outcomes and DRE terms and definitions.
TABLE 6.
Identified gaps in the literature related to drug‐resistant epilepsy
| Type of outcome | Gaps in the literature |
|---|---|
| General gaps | RCTs evaluated ASM compared to placebo; did not evaluate specific ASM combinations |
| Non‐RCTs evaluated ASM combinations; however, they were retrospective, did not have a control group, and/or had small sample sizes | |
| Limited research conducted among African countries/ethnic groups and women who are pregnant | |
| Inconsistent reporting of concomitant non‐pharmacologic interventions (i.e., medical marijuana use or ketogenic diet). It is unclear what is the optimal interplay of non‐pharmacologic and pharmacologic treatments | |
| Clinical outcomes | Evaluation of specific ASM combinations were not included as a primary end point for RCTs, or ASMs were grouped based on similar mechanisms |
| Unclear how individual and/or specific ASM combinations worsen seizure control since this was specifically reported in a few studies (five of 26 studies) | |
| Humanistic outcomes | Limited number of studies (two or less) evaluating each humanistic outcome |
| Limited number of studies (n = 1) evaluating SUDEP, and results appear to be inconsistent with other reports | |
| Economic outcomes | Limited number of studies (n = 1) reporting economic outcomes, including healthcare costs, QALY, cost‐utility analyses, and ICER |
| Evaluation of cost may have limited generalizability given health care and payment systems vary widely between countries | |
| DRE term and definition(s) | Inconsistent use of terms, such as refractory or DRE |
| Inconsistent use of DRE definition for inclusion criteria | |
| In general, it is difficult to align inclusion criteria with ILAE definition of DRE to ensure appropriate trials of ASMs prior to inclusion |
Abbreviations: ASM, antiseizure medication; DRE, drug‐resistant epilepsy; ICER, incremental cost‐effectiveness ratio; ILAE, International League Against Epilepsy; QALY, quality‐adjusted life years; RCT, randomized controlled trial; SUDEP, sudden unexpected death in epilepsy.
4. DISCUSSION
This scoping review summarizes the evidence related to clinical, humanistic, and economic outcomes for treatment among adult patients with DRE and the terms and definitions used to describe DRE. Our research questions primarily focused on describing the current literature regarding rational polytherapy in DRE. We found that the majority of studies did not specifically identify or evaluate effective combinations of ASMs but rather looked at outcomes of ASM polytherapy generally. When compared to monotherapy, polytherapy regimens were typically associated with worse outcomes. Among studies that did report effective combinations of ASMs, sample sizes were small, and authors could not draw strong conclusions.
Clinical outcomes regarding specific ASM combinations were minimally reported, which opens the door for future exploration. Specifically, the combination of VPA and LTG is promising. At least two included studies noted VPA and LTG were effective combinations, 22 , 37 which corroborate other reports of their synergistic action despite the known drug interaction necessitating a slow titration of LTG. 46 , 47 , 48 In addition, it is unclear how individual ASMs may result in no change or worsening of seizure control since this was infrequently reported as a clinical outcome (six of 26 studies) and this phenomenon is not well characterized and is difficult to ascertain. 49 Even so, there are some seizure types that are exacerbated by certain ASMs, such as Dravet syndrome and sodium channel blockers or generalized epilepsies and CBZ. 49 , 50 Clinicians are encouraged to ensure an accurate diagnosis in the setting of DRE for this very reason. 2 There is still an opportunity to define ideal ASM combinations based on various patient‐ and disease‐specific characteristics.
Based on our review of humanistic outcomes, conclusions may not be readily drawn given the limited number of studies evaluating each outcome. First, polytherapy may be associated with no change or worse QOL 27 , 33 , 38 , 40 and poor adherence, 34 , 41 but these outcomes were evaluated by a limited number of studies. It is unclear how improvements in QOL could be optimized if patients with DRE were switched from poly‐ to monotherapy 38 since this study was limited by its retrospective design and small sample size. Even so, these results align with common sense that patients requiring more therapy (i.e., polytherapy), would have more severe disease and thus be at risk for impaired effectiveness and poor tolerability. Additionally, there were limited studies included in this review evaluating sudden unexpected death in epilepsy (SUDEP) among patients with DRE. The findings of the single included article offer a new insight regarding adherence and SUDEP, whereas findings related to SUDEP and polytherapy may conflict with other reports. In the single included study evaluating SUDEP, poor adherence to ASMs was associated with increased odds of SUDEP, which the authors note has not been reported before. 45 On the other hand, no ASMs were associated with increased odds of SUDEP, 45 and practice guidelines identify there is low‐quality evidence regarding specific ASMs (i.e., LTG) use in patients with refractory epilepsy and increased risk of SUDEP. 51 Surprisingly, both polytherapy and three or more ASMs were statistically associated with decreased odds of SUDEP, whereas monotherapy and two ASMs did not decrease the odds of SUDEP. 45 This is an interesting finding since other reports note that polytherapy is associated with increased risk of SUDEP, 52 but the evidence is very low or conflicting. 51 The current scoping review is unable to further explore these results given the limited number of included articles also evaluating SUDEP. Overall, the current scoping review cannot draw robust conclusions regarding DRE and polytherapy on humanistic outcomes, regarding QOL, adherence, and SUDEP.
Finally, an additional gap in the literature relates to the evaluation of economic outcomes among adults with DRE. There was a limited number of articles evaluating economic outcomes compared to those reporting either clinical or humanistic outcomes. The single article reporting economic outcomes in patients with refractory epilepsy has some significant limitations related to study design, sample size, and risk of recall bias, and also did not use the ILAE definition of DRE. On the other hand, economic evaluations conducted in Germany and Italy among patients with DRE included both children and adults 4 , 53 or were not focused on polytherapy. 54 In one study, drug costs were highest among patients with DRE (defined as “patients with recurrent seizures who in the judgment of the caring physician would not respond to additional treatment changes”), compared to patients with complete seizure control, occasional seizures, or active non‐drug‐resistant seizures. 54 ASMs accounted for the majority of direct costs and newer generations of ASMs contributed to this increased cost in the two studies from Italy, but these results are difficult to generalize to other countries given different health care and payment systems. 53 , 54 Additionally, it is unclear from these studies if certain ASMs or ASM combinations are associated with decreased costs while also improving other clinical and/or humanistic outcomes.
There are opportunities for future research to explore gaps related to clinical, humanistic, and economic outcomes for patients with DRE. Although most studies included in this scoping review did not specifically evaluate ASM combinations for rational polytherapy, some studies utilized innovative study designs or statistical analysis that could be used in the future to allow for evaluation of polytherapy. For example, three studies evaluating various BRV doses conducted a priori subgroup analyses based on current and/or prior exposure to LEV. 23 , 24 , 25 Future researchers should consider a similar approach to a priori determinations when evaluating ASMs with similar mechanisms of action, such as BRV and LEV. Overall, researchers can use similar statistical analyses to provide insight into future seizure control based on past exposure to ASMs in general. Another innovative study design is related to evaluating sequential monotherapy and rational polytherapy. For example, VPA and LTG have been shown to be effective in a single‐arm prospective study, but it was unknown if these patients would have benefitted from monotherapy initially. 46 Another study evaluated sequential monotherapy of VPA then LTG before trialing VPA and LTG polytherapy, only in those patients who did not experience side effects and/or lacked efficacy on monotherapy. 22 By definition, these patients could meet criteria for DRE if appropriate trials of VPA and LTG monotherapy were completed. This also serves as an example for clinicians of optimizing monotherapy before classifying a patient with DRE and subjecting them to unnecessary polytherapy.
Addressing gaps in these outcomes is predicated on a clear definition for DRE. Inconsistent DRE definitions prevent researchers from completing high‐quality meta‐analyses to evaluate overall impact of smaller studies, such as those included in this scoping review. In addition, heterogeneity and limited data ultimately makes it challenging for the medical team to incorporate results into clinical practice. There is a need to consistently translate the ILAE definition of DRE into the research setting to address clinical, humanistic, and economic gaps to ultimately impact patient care.
Strengths of our study related to using a standard process and asking comprehensive research questions. First, we followed a systematic approach for scoping reviews and utilized a standard process to identify, review, and select articles for inclusion. Additionally, we used a validated tool for quality appraisal. Finally, clinical, humanistic, and economic outcomes were evaluated in light of the impact of DRE on all aspects of health. There are many reviews regarding non‐pharmacological management strategies for DRE, including the ketogenic diet and various surgeries. To our knowledge, this is the first scoping review of pharmacological management of DRE that followed a systematic approach.
There were some limitations with this review. First, we had to exclude certain articles reporting humanistic and economic outcomes, which limited the number of studies we could review. Since only one study reporting economic outcomes was included, we could not draw additional conclusions about the economic impact of polytherapy for DRE. Secondly, we excluded studies involving patients under the age of 18 to maintain consistent inclusion criteria. Additionally, we did not set an upper age limit as most studies did not include one. Finally, we could not conduct statistical analysis given heterogeneity among studies and thus cannot draw definitive conclusions regarding specific ASM combinations in general, or for specific seizure types (i.e., partial vs. generalized).
Data supports that monotherapy is ideal for PWE, but polytherapy may be necessary. As such, researchers and clinicians can prioritize evaluating optimal ASM regimens for rational polytherapy. Studies can be strategically designed to evaluate both sequential monotherapy and rational polytherapy and subsequent de‐escalation, while taking specific seizure types into consideration. Additionally, there is a need for well‐designed studies to evaluate humanistic and/or economic outcomes in DRE populations. Finally, authors should use the term refractory appropriately, and inclusion criteria should reflect the ILAE definition for DRE.
5. CONCLUSION
Ultimately, the optimal combinations of ASMs for rational polytherapy for DRE is unclear given the present data. Use of the ILAE definition of DRE was applied inconsistently. Operationalizing the definition as inclusion criteria may have been challenging, which creates a barrier for well‐designed studies in this population but also limits clinicians from practicing evidence‐based medicine.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
The authors would like to acknowledge the contribution of Cedarville University Research Assistant Jonathan Stell for his assistance with data extraction and quality assessment.
Grinalds MS, Yoder C, Krauss Z, Chen AM, Rhoney DH. Scoping review of rational polytherapy in patients with drug‐resistant epilepsy. Pharmacotherapy. 2023;43:53‐84. doi: 10.1002/phar.2748
REFERENCES
- 1. World Health Organization . Epilepsy: A Public Health Imperative . World Health Organization; 2019. https://www.who.int/publications/i/item/epilepsy‐a‐public‐health‐imperative [Google Scholar]
- 2. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069‐1077. doi: 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 3. Devinsky O, Spruill T, Thurman D, Friedman D. Recognizing and preventing epilepsy‐related mortality: a call for action. Neurology. 2016;86(8):779‐786. doi: 10.1212/wnl.0000000000002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strzelczyk A, Griebel C, Lux W, Rosenow F, Reese JP. The burden of severely drug‐refractory epilepsy: a comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using German health insurance data. Front Neurol. 2017;8:712. doi: 10.3389/fneur.2017.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50(Suppl 8):63‐68. doi: 10.1111/j.1528-1167.2009.02238.x [DOI] [PubMed] [Google Scholar]
- 6. Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment‐resistant epilepsy: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology and the American Epilepsy Society. Neurology. 2018;91(2):82‐90. doi: 10.1212/wnl.0000000000005756 [DOI] [PubMed] [Google Scholar]
- 7. Tricco A, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 8. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16. doi: 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brodie MJ, Sills GJ. Combining antiepileptic drugs–rational polytherapy? Seizure. 2011;20(5):369‐375. doi: 10.1016/j.seizure.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 10. FAQ: How does Covidence detect duplicates? Updated June 15, 2022. Accessed August 9, 2022. https://support.covidence.org/help/how‐does‐covidence‐detect‐duplicates
- 11. Hong QN, Pluye P, Fàbregues S, et al. Mixed Methods Appraisal Tool (MMAT), version 2018. Registration of Copyright (#1148552), Canadian Intellectual Property Office, Industry Canada.
- 12. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and shiny app for producing PRISMA 2020‐compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022;18:e1230. doi: 10.1002/cl2.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borges Pereira C, Otto Heise C, Cukiert A. High doses of carbamazepine for refractory partial epilepsy. Arq Neuropsiquiatr. 1996;54(1):42‐46. doi: 10.1590/s0004-282x1996000100007 [DOI] [PubMed] [Google Scholar]
- 14. French JA, Chung SS, Krauss GL, et al. Long‐term safety of adjunctive cenobamate in patients with uncontrolled focal seizures: open‐label extension of a randomized clinical study. Epilepsia. 2021;62(9):2142‐2150. doi: 10.1111/epi.17007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brahmbhatt N, Stupp R, Bushara O, Bachman E, Schuele SU, Templer JW. Efficacy of clobazam as add‐on therapy in brain tumor‐related epilepsy. J Neurooncol. 2021;151(2):287‐293. doi: 10.1007/s11060-020-03664-9 [DOI] [PubMed] [Google Scholar]
- 16. Elger C, Halász P, Maia J, Almeida L, Soares‐da‐Silva P; on behalf of the BIAISG . Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial‐onset seizures: a randomized, double‐blind, placebo‐controlled, parallel‐group phase III study. Epilepsia. 2009;50(3):454‐463. doi: 10.1111/j.1528-1167.2008.01946.x [DOI] [PubMed] [Google Scholar]
- 17. Villanueva V, López‐Gomáriz E, López‐Trigo J, et al. Rational polytherapy with lacosamide in clinical practice: results of a Spanish cohort analysis RELACOVA. Epilepsy Behav. 2012;23(3):298‐304. doi: 10.1016/j.yebeh.2011.11.026 [DOI] [PubMed] [Google Scholar]
- 18. Azar NJ, Lagrange AH, Wang L, Song Y, Abou‐Khalil BW. Transient improvement after brief antiepileptic drug withdrawal in the epilepsy monitoring unit‐‐possible relationship to AED tolerance. Epilepsia. 2010;51(5):811‐817. doi: 10.1111/j.1528-1167.2009.02494.x [DOI] [PubMed] [Google Scholar]
- 19. Lattanzi S, Cagnetti C, Foschi N, et al. Adjunctive perampanel in older patients with epilepsy: a multicenter study of clinical practice. Drugs Aging. 2021;38(7):603‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stephen LJ, Parker P, Kelly K, Wilson EA, Leach V, Brodie MJ. Adjunctive pregabalin for uncontrolled partial‐onset seizures: findings from a prospective audit. Acta Neurol Scand. 2011;124(2):142‐145. doi: 10.1111/j.1600-0404.2011.01507.x [DOI] [PubMed] [Google Scholar]
- 21. Harden CL, Zisfein J, Atos‐Radzion EC, Tuchman AJ. Combination valproate—carbamazepine therapy in partial epilepsies resistant to carbamazepine monotherapy. J Epilepsy. 1993;6(2):91‐94. doi: 10.1016/S0896-6974(05)80094-5 [DOI] [Google Scholar]
- 22. Pisani F, Oteri G, Russo MF, Di Perri R, Perucca E, Richens A. The efficacy of valproate‐lamotrigine comedication in refractory complex partial seizures: evidence for a pharmacodynamic interaction. Epilepsia. 1999;40(8):1141‐1146. doi: 10.1111/j.1528-1157.1999.tb00832.x [DOI] [PubMed] [Google Scholar]
- 23. Biton V, Berkovic SF, Abou‐Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double‐blind, placebo‐controlled trial. Epilepsia. 2014;55(1):57‐66. doi: 10.1111/epi.12433 [DOI] [PubMed] [Google Scholar]
- 24. Klein P, Schiemann J, Sperling MR, et al. A randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial‐onset seizures. Epilepsia. 2015;56(12):1890‐1898. doi: 10.1111/epi.13212 [DOI] [PubMed] [Google Scholar]
- 25. Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double‐blind, randomized, placebo‐controlled trial. Epilepsia. 2014;55(1):47‐56. doi: 10.1111/epi.12432 [DOI] [PubMed] [Google Scholar]
- 26. Kraiprab P, Chinvarun Y, Tantisira MH. Oxcarbazepine as add‐on therapy in Thai epileptic patients with refractory partial seizures. J Med Assoc Thai. 2005;88(S3):S193‐S201. [PubMed] [Google Scholar]
- 27. French JA, Baroldi P, Brittain ST, Johnson JK; the PISG . Efficacy and safety of extended‐release oxcarbazepine (Oxtellar XR™) as adjunctive therapy in patients with refractory partial‐onset seizures: a randomized controlled trial. Acta Neurol Scand. 2014;129(3):143‐153. doi: 10.1111/ane.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stephen LJ, Sills GJ, Brodie MJ. Topiramate in refractory epilepsy: a prospective observational study. Epilepsia. 2000;41(8):977‐980. doi: 10.1111/j.1528-1157.2000.tb00282.x [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Huang J, Zhuang JH, Huang LQ, Zhao ZX. Topiramate as an adjunctive treatment for refractory partial epilepsy in the elderly. J Int Med Res. 2011;39(2):408‐415. doi: 10.1177/147323001103900208 [DOI] [PubMed] [Google Scholar]
- 30. Kellett MW, Smith DF, Stockton PA, Chadwick DW. Topiramate in clinical practice: first year's postlicensing experience in a specialist epilepsy clinic. J Neurol Neurosurg Psychiatry. 1999;66(6):759‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sackellares JC, Ramsay RE, Wilder BJ, Browne TR, Shellenberger MK. Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia. 2004;45(6):610‐617. doi: 10.1111/j.0013-9580.2004.11403.x [DOI] [PubMed] [Google Scholar]
- 32. Schmidt D, Jacob R, Loiseau P, et al. Zonisamide for add‐on treatment of refractory partial epilepsy: a European double‐blind trial. Epilepsy Res. 1993;15(1):67‐73. doi: 10.1016/0920-1211(93)90011-U [DOI] [PubMed] [Google Scholar]
- 33. Remák E, Hutton J, Selai CE, Trimble MR, Price MJ. A cost‐utility analysis of adjunctive treatment with newer antiepileptic drugs in the UK. J Drug Assess. 2004;7(2):109‐120. [Google Scholar]
- 34. Kusznir Vitturi B, Barreto Cabral F, Mella CC. Outcomes of pregnant women with refractory epilepsy. Seizure. 2019;69:251‐257. doi: 10.1016/j.seizure.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 35. Lee G‐H, Kim B‐M, Kang JK, Lee S‐A. Loss of the initial efficacy of levetiracetam in patients with refractory epilepsy. Seizure. 2013;22(3):185‐188. doi: 10.1016/j.seizure.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 36. Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P; Group ftELS . Multicenter double‐blind, randomized, placebo‐controlled trial of levetiracetam as add‐on therapy in patients with refractory partial seizures. Epilepsia. 2000;41(9):1179‐1186. doi: 10.1111/j.1528-1157.2000.tb00323.x [DOI] [PubMed] [Google Scholar]
- 37. Wężyk K, Słowik A, Bosak M. Predictors of remission in patients with epilepsy. Neurol Neurochir Pol. 2020;54(5):434‐439. [DOI] [PubMed] [Google Scholar]
- 38. Pirio Richardson S, Farias ST, Lima AR, Alsaadi TM. Improvement in seizure control and quality of life in medically refractory epilepsy patients converted from polypharmacy to monotherapy. Epilepsy Behav. 2004;5(3):343‐347. doi: 10.1016/j.yebeh.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 39. Şenadim S, Alpaydin Baslo S, Tekİn GÜvelİ B, Sari H, Atakli HD. Safety and efficacy of zonisamide in refractory epilepsy patients: clinical experience from a tertiary center. Arch Epilepsy. 2018;24(1):27‐32. doi: 10.14744/epilepsi.2017.65477 [DOI] [Google Scholar]
- 40. Xu X, Brandenburg NA, McDermott AM, Bazil CW. Sleep disturbances reported by refractory partial‐onset epilepsy patients receiving polytherapy. Epilepsia. 2006;47(7):1176‐1183. doi: 10.1111/j.1528-1167.2006.00591.x [DOI] [PubMed] [Google Scholar]
- 41. Bautista RED, Rundle‐Gonzalez V. Effects of antiepileptic drug characteristics on medication adherence. Epilepsy Behav. 2012;23(4):437‐441. doi: 10.1016/j.yebeh.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 42. Yeh W‐C, Lai C‐L, Wu M‐N, et al. Rapid eye movement sleep disturbance in patients with refractory epilepsy: a polysomnographic study. Sleep Med. 2021;81:101‐108. doi: 10.1016/j.sleep.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 43. Freitas‐Lima P, Baldoni AO, Alexandre V, Pereira LRL, Sakamoto AC. Drug utilization profile in adult patients with refractory epilepsy at a tertiary referral center. Arq Neuropsiquiatr. 2013;71(11):856‐861. doi: 10.1590/0004-282X20130169 [DOI] [PubMed] [Google Scholar]
- 44. Bardai A, Lamberts RJ, Blom MT, et al. Epilepsy is a risk factor for sudden cardiac arrest in the general population. PLoS One. 2012;7(8):e42749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Pharmacologic treatment and SUDEP risk: a nationwide, population‐based, case‐control study. Neurology. 2020;95(18):e2509‐e2518. doi: 10.1212/WNL.0000000000010874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 study group. Epilepsy Res. 1997;26(3):423‐432. doi: 10.1016/s0920-1211(96)01007-8 [DOI] [PubMed] [Google Scholar]
- 47. Fattorusso A, Matricardi S, Mencaroni E, et al. The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front Neurol. 2021;12:674483. doi: 10.3389/fneur.2021.674483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poolos NP, Warner LN, Humphreys SZ, Williams S. Comparative efficacy of combination drug therapy in refractory epilepsy. Neurology. 2012;78(1):62‐68. doi: 10.1212/WNL.0b013e31823ed0dd [DOI] [PubMed] [Google Scholar]
- 49. Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. Epilepsia. 1998;39(1):5‐17. doi: 10.1111/j.1528-1157.1998.tb01268.x [DOI] [PubMed] [Google Scholar]
- 50. Cross JH, Caraballo RH, Nabbout R, Vigevano F, Guerrini R, Lagae L. Dravet syndrome: treatment options and management of prolonged seizures. Epilepsia. 2019;60(Suppl 3):S39‐S48. doi: 10.1111/epi.16334 [DOI] [PubMed] [Google Scholar]
- 51. Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674‐1680. doi: 10.1212/wnl.0000000000003685 [DOI] [PubMed] [Google Scholar]
- 52. Jones LA, Thomas RH. Sudden death in epilepsy: insights from the last 25 years. Seizure. 2017;44:232‐236. doi: 10.1016/j.seizure.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 53. Luoni C, Canevini MP, Capovilla G, et al. A prospective study of direct medical costs in a large cohort of consecutively enrolled patients with refractory epilepsy in Italy. Epilepsia. 2015;56(7):1162‐1173. doi: 10.1111/epi.13030 [DOI] [PubMed] [Google Scholar]
- 54. Beghi E, Garattini L, Ricci E, Cornago D, Parazzini F. Direct cost of medical management of epilepsy among adults in Italy: a prospective cost‐of‐illness study (EPICOS). Epilepsia. 2004;45(2):171‐178. doi: 10.1111/j.0013-9580.2004.14103.x [DOI] [PubMed] [Google Scholar]


