Abstract
The gut microbiota communicates with the brain through several pathways including the vagus nerve, immune system, microbial metabolites and through the endocrine system. Pathways along the humoral/immune gut microbiota–brain axis are composed of a series of vascular and epithelial barriers including the intestinal epithelial barrier, gut–vascular barrier, blood–brain barrier and blood–cerebrospinal fluid barrier. Of these barriers, the relationship between the gut microbiota and blood–cerebrospinal fluid barrier is yet to be fully defined. Here, using a germ‐free mouse model, we aimed to assess the relationship between the gut microbiota and the integrity of the blood–cerebrospinal fluid barrier, which is localized to the choroid plexus epithelium. Using confocal microscopy, we visualized the tight junction protein zonula occludens‐1, an integral aspect of choroid plexus integrity, as well as the choroid plexus fenestrated capillaries. Quantification of tight junction proteins via network analysis led to the observation that there was a decrease in the zonula occludens‐1 network organization in germ‐free mice; however, we did not observe any differences in capillary structure. Taken together, these data indicate that the blood–cerebrospinal fluid barrier is another barrier along the gut microbiota–brain axis. Future studies are required to elucidate its relative contribution in signalling from microbiota to the brain.
Keywords: choroid plexus, germ‐free, tight junction proteins, vasculature
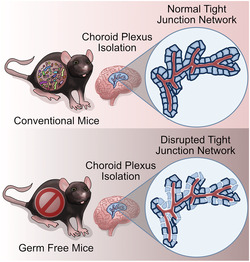
Choroid plexus tissue was isolated from conventional and germ‐free adult male mice. Confocal microscopy of the tight junction protein, zonula occludens‐1 (ZO‐1), was quantified and revealed a disrupted tight junction protein network in germ‐free mice. Choroid plexus vasculature is unaltered. These data implicate the importance of the presence/absence of the gut microbiota in the regulation of blood–cerebrospinal fluid barrier physiology.
Abbreviations
- BBB
blood–brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- ROI
region of interest
- ZO‐1, ‐2, ‐3
zonula occludens‐1, ‐2, ‐3
1. INTRODUCTION
The composition and function of the gut microbiota play a major role in influencing brain and behaviour (Cryan et al., 2019; Morais et al., 2021). This is possible via the gut–brain axis, a bidirectional communication system between the gut microbiota and the brain. There are several pathways of communication along the gut–brain axis including the vagus nerve, immune system, microbial metabolites, and through endocrine pathways (Cryan & Dinan, 2012; Sherwin et al., 2016). Several of these communication pathways involve the systemic circulation, a route that includes a series of barriers between the resident gut microbes and the brain. The barriers closest to the resident microbes are the intestinal epithelial barrier and gut–vascular barrier, which are greatly responsible for regulating the flux of molecules between the gut and blood (Spadoni et al., 2017). When molecules enter circulation, they can travel as far as the brain. The brain, however, is equipped with several blood–brain/cerebrospinal fluid (CSF) barriers including the blood–brain barrier (BBB), meninges and choroid plexus (Spadoni et al., 2017). These barriers are all composed of specialized endothelial or epithelial cells, which express the tight junction proteins, which greatly restrict paracellular permeability, preventing unwanted molecules and pathogens from moving across the barriers (Knox, Aburto, Clarke, et al., 2022; Solár et al., 2020; Stamatovic et al., 2016).
To date, there are many studies that investigate the impact of the presence of the gut microbiota on the anatomy and physiology of the gut barriers (Hayes et al., 2018; Kelly et al., 2015; Spichak et al., 2018). For instance, specific bacteria strains (Han et al., 2019; Zhou et al., 2022), altered microbiota composition, altered fibre intake, and gut microbiota‐derived metabolites have been shown to alter intestinal epithelial cells and gut barrier integrity (Zhou et al., 2022). Additionally, the BBB has also been identified as a target of the gut microbiota whereby gut microbes modulate brain barrier integrity and specific microbial metabolites have been found to directly disrupt, improve or protect BBB integrity (Braniste et al., 2014; Hoyles et al., 2018, 2021; Knox, Aburto, Clarke, et al., 2022; Lin et al., 2020; O'Riordan et al., 2022; Quinn et al., 2014). The tight junction protein expression of brain endothelial cells and BBB permeability are altered in germ‐free mice (mice raised devoid of microorganisms) when compared with conventional mice (Braniste et al., 2014). Although both the gut barrier and BBB are altered in germ‐free mice (Braniste et al., 2014; Ghosh et al., 2021; Parker et al., 2020; Spichak et al., 2018), the effects of germ‐free status on blood–CSF barrier remains to be characterized.
The blood–CSF barrier is localized to the choroid plexus at the four brain ventricles and is composed of fenestrated capillaries surrounded by connective tissue and immune cells enclosed by cuboidal epithelial cells, which are tightly joined by tight junction proteins (Solár et al., 2020). This brain barrier is more similar to the intestinal epithelial barrier (Spadoni et al., 2017) rather than the BBB, as they are both composed of epithelial cells, not endothelial cells, making it a likely target of the gut microbiota. The blood–CSF barrier, gut–vascular barrier and intestinal epithelial barrier are all immunomodulatory selective gateways (Spadoni et al., 2017). The blood–CSF barrier is also more accessible than the BBB to foreign toxins, but the blood–CSF barrier is far less studied in the context of the gut microbiota–brain axis (Gorlé et al., 2018). Compared with the BBB, the blood–CSF barrier is also much tighter to soluble tracers and has higher electrical resistance (Spadoni et al., 2017). The blood–CSF barrier is crucial for maintaining brain homeostasis and reducing the spread of inflammatory reactions from reaching the central nervous system (CNS) (Solár et al., 2020). The tight junction proteins, zonula occludens (ZO‐1, ‐2, ‐3), claudins and occludin are an integral aspect of maintaining blood–CSF barrier integrity as they greatly restrict paracellular permeability and maintain electrical resistance of the epithelial layer of cells in the choroid plexus. Occludin and claudins are transmembrane proteins mediating contact between epithelial cells, whereas zonula occludens are sub‐membrane proteins anchoring occludin and claudins to actin filaments. Bacterial infections such as Streptococcus suis have been previously shown to induce disruption of blood–CSF barrier but not BBB, through rearrangement of the tight junction proteins (Solár et al., 2020; Tenenbaum et al., 2008).
Considering the role of choroid plexus integrity in health and disease as well as the understanding that the gut microbiota influences several other barriers along the gut microbiota–brain axis, it is urgent to understand the impact of the gut microbiota on choroid plexus physiology. Here, we used isolated choroid plexus tissue from germ‐free mice to investigate the relationship between the gut microbiota and choroid plexus physiology. Through confocal microscopy of tight junction protein networks and capillaries, we quantified ZO‐1 network and in‐depth analysis of capillary structure.
2. MATERIALS AND METHODS
2.1. Animals
Male F1‐generation offspring from germ‐free (n = 5–7) and conventionally raised (n = 8) C57/BL6 mice breeding pairs previously obtained from Taconic (Germantown, New York, USA) were used in all experiments. Mice were sacrificed at 3–4 months with similar body weights (conventional; mean ± SEM = 27.66 ± 0.66 g, germ‐free; mean ± SEM = 29.25 ± 0.59 g). Germ‐free mice were housed in gnotobiotic isolators, as two to four mice per cage. All mice were housed in identical controlled conditions (20–21°C, 55%–60% humidity) under a strict 12‐hour light/dark cycle, with access ad libitum to autoclaved chow (Special Diet Services, product code 801010) and water. All experiments were conducted in accordance with the European Directive 2010/63/EC, the requirements of the SI No 543 of 2012, and approved by the Animal Experimentation Ethics Committee of University College Cork and the Health Products Regulatory Authority (HPRA AE19130 P047).
2.2. Tissue collection and fixation
Choroid plexus tissues from both lateral ventricles were placed in Dulbecco's Modified Eagle Medium: Nutrient Mixture F‐12 immediately following isolation. Following isolation, tissue was fixed with 10% trichloroacetic acid for 15 min and stored in PBS with azide (0.05%) at 4°C.
2.3. Immunofluorescence assays
Choroid plexus tissue was washed with 1× phosphate buffered saline (PBS), permeabilized with 0.1% Triton X and blocked with 10% normal donkey serum. Isolectin GS‐IB4 from Griffonia simplicifolia, Alexa Flour 647 conjugate (1:200, Thermo Fisher Scientific, #I32450), was used to detect blood vessels, and primary antibodies were used against ZO‐1 (1:150, Invitrogen #61‐7300, RRID: AB_138452). Images were captured using a confocal laser scanning microscope (Olympus FV1000) fitted with 488, 633‐nm lasers and 20× dry objective lens (isolectin) or 100× oil immersion objective lens (ZO‐1). All tissues were imaged at the same resolution (1024 × 1024) under the same speed (10.0 μs/pixel), laser intensity, gain and off set. Images include five confocal planes with a step size of 0.47 μm for 20× lens and 1.14 μm for 100× lens.
2.4. Image analysis
2.4.1. Tight junction protein network analysis
The 100× confocal images were used to analyse the tight junction protein networks. To remove background speckles and focus on the tight junction protein networks, the images were processed in Fiji/ImageJ to reduce the background noise under process > noise > reduce outliers. A radius of 1 pixel and a threshold of 100 was chosen to uniformly remove some of the background. The images were then converted to a binary image and then skeletonized. The skeleton image was then analysed using the Analyse Skeleton plugin for FIJI/ImageJ (https://imagej.net/plugins/analyze-skeleton/). The animals processed were then normalized to the respective conventional control animals and expressed as a percentage.
2.4.2. Vessel analysis
The 20× confocal images were used to analyse the choired plexus vessels. A region of interest (ROI) was created 7× smaller than the original image. This ROI was used to crop four smaller regions within the 20× images. ROIs were collected from diverse sections of the larger images, and these ROIs were then analysed using AngioTool. The ROIs of each image were averaged, then the choroid plexus for each animal were averaged then normalized to the respective conventional control animals and expressed as a percentage.
2.5. Statistical analysis
Experimental data were expressed as mean ± SEM, n = animal replicates. A maximum of one outlier (if any) were removed using the Grubbs outlier test. Following Levene's test for equality of variances and Shapiro–Wilk test for normality, data were analysed by a two‐sided t test with equal variances assumed using SPSS Statistics software (IBM, Armonk, New York, USA). A p‐value of less than 0.05 was considered significant.
3. RESULTS
3.1. Choroid plexus tight junction protein network is disrupted in germ‐free mice
Choroid plexus tissue of germ‐free mice was collected, and the tight junction protein ZO‐1 was immunodetected to assess its expression and localization in germ‐free mice compared with conventional controls. The tight junction proteins are localized to the cell–cell junctions, forming network‐like structures. In conventional mice, ZO‐1 formed a continuous network among cells, whereas in germ‐free mice, it appeared more fragmented (Figure 1a). The junctions themselves, when present, appeared similar in both germ‐free and conventional mice, forming hexagonal networks. However, there appeared to be less tight junction protein‐linked polygon‐like structures. To quantify this tight junction network, we converted these confocal images to binary and then skeletonized projections. Using the ‘analyse skeleton’ plugin for Fiji/ImageJ (https://imagej.net/plugins/analyze-skeleton/), we quantified the total length of the ZO‐1 skeletonized branches, the mean and maximum branch length and the number of endpoints (Figure 1b–e). There was no difference in the total length of the ZO‐1 skeletonized branches between conventional and germ‐free mice (Figure 1b). However, there was a decrease in both the mean (p < 0.1, mean ± SEM = 63.5 ± 8.4) and max (p < 0.01, mean ± SEM = 73.3 ± 4.7) branch length in germ‐free mice compared with the conventional controls (mean; mean ± SEM = 100.0 ± 4.6, max; mean ± SEM = 100.0 ± 3.9) (Figure 1c,d). The number of endpoints (p < 0.5, mean ± SEM = 137.7 ± 9.6) in the images was increased in the germ‐free mice compared with the conventional control animals (mean ± SEM = 100.0 ± 8.1) (Figure 1e). Taken together, the decrease in ZO‐1 branching and increase in endpoints indicates a disrupted ZO‐1 network in germ‐free mice.
FIGURE 1.
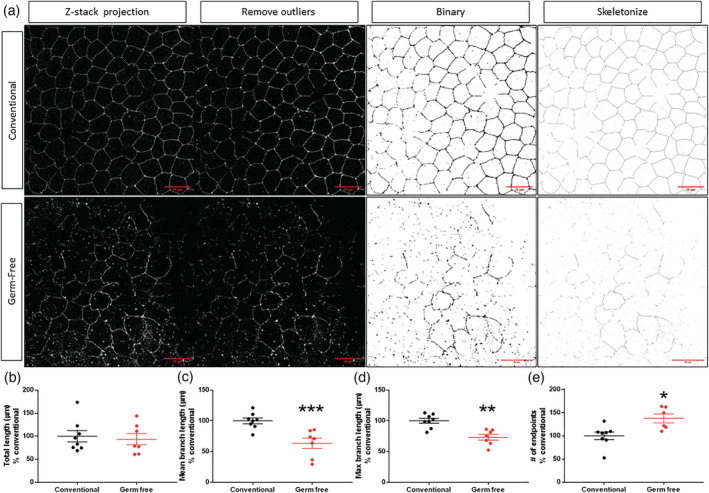
Tight junction protein network is reduced in germ‐free mice. (a) Representative 100× confocal Z‐stack projection of ZO‐1 pre‐processing, post‐processing (remove noise), binary and skeleton in conventional and germ‐free mice. Skeletal image analysis of (b) total length, (c) mean branch length, (d) max branch length and (e) number of endpoints of ZO‐1 networks. Mean ± SEM two‐sided t test comparing means. *p < 0.05, **p < 0.01, *** p < 0.001 compared with untreated control group. Scale bar, 20 μm
3.2. Choroid plexus vasculature is unaltered in germ‐free mice
The fenestrated capillaries in the blood–CSF barrier are a major aspect of the choroid plexus tissue, structure and function (Lun et al., 2015). Since we observed differences in choroid plexus tight junction protein networks, we explored possible differences in the structure of the choroid plexus vascular network upon the absence of the microbiota. To assess this, we stained the vessels in the choroid plexus with isolectin‐B4 from Griffonia simplicifolia and imaged a wide section of the tissue with a 20× objective (Figure 2a). To better assess the structure of the capillaries, but not the larger veins and arteries, we analysed four smaller ROIs from diverse sections of each image (Figure 2a) and quantified the vessel density, junction density, total number of endpoints, total vessel length, average vessel length and mean E lacunarity (a measure of the nonuniformity) using AngioTool (Figure 2b–g). Overall, there were no differences in any of the metrics measured between the germ‐free and conventional mice.
FIGURE 2.
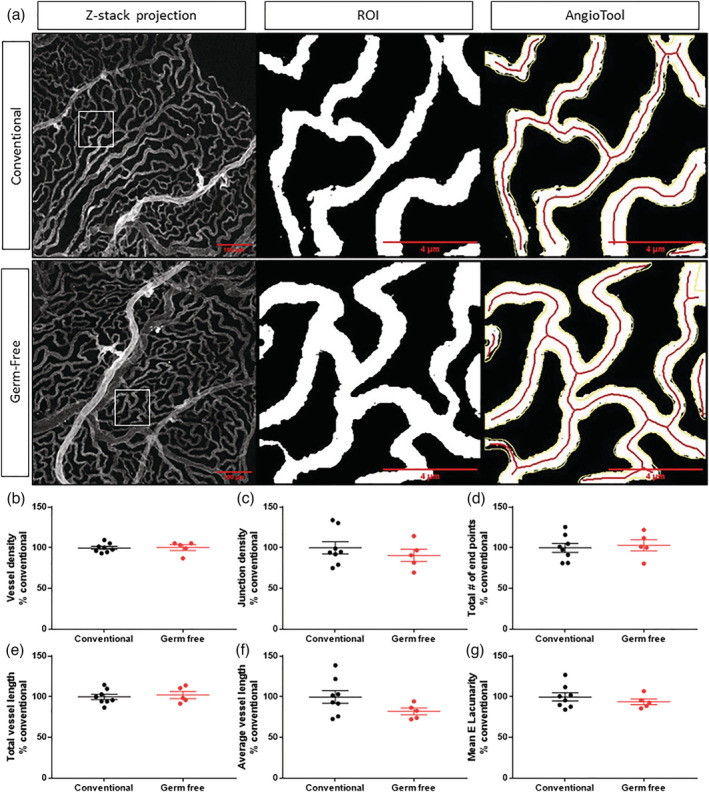
Vessels are unaltered in germ‐free mice. (a) Representative 20× confocal Z‐stack projection of vessels (isolectin) pre‐processing, representative region of interest (ROI) and AngioTool analysis in conventional and germ‐free mice. AngioTool image analysis of (b) vessel density, (c) junction density, (d) total number of endpoints, (e) total vessel length, (f) average vessel length, and (g) mean E lacunarity. Mean ± SEM. Scale bar, 100 μm, 4 μm
4. DISCUSSION
Investigating the integrity and physiology of the blood–CSF barrier in the absence of commensal microbes is imperative for understanding the regulatory role of the gut microbiota in brain signalling. Since the choroid plexus is vital to maintaining brain homeostasis in health and disease and is a major immunomodulatory selective gateway to the brain (Spadoni et al., 2017), it is important to understand what factors influence this barrier. In the current study, we show that the tight junction protein network, but not the vascular network, is disrupted in the choroid plexus of germ‐free mice.
Functional tight junction proteins are localized at the cell–cell junctions in epithelial cells. The connection between endothelial cells forms a tight junction protein network, where each cuboidal cell is closely connected to the adjacent ones like a puzzle. Our results indicate that there is a disruption of the ZO‐1 tight junction protein network in male germ‐free mice. The overall length of the total quantified ZO‐1 expression between groups remained unaltered, indicating that the same level of ZO‐1 was quantified between both groups. Further studies are merited to determine if this reduction in the tight junction protein network is also an indication of a decrease in functional ZO‐1, overall protein expression of ZO‐1 or mRNA decreased expression in ZO‐1. Additionally, the expression and localization of the other tight junction proteins including claudins and occludin should be explored. Our results in the blood–CSF barrier complement previous studies that found germ‐free mice have lower tight junction protein expression in the BBB (Braniste et al., 2014), further implicating the gut microbiota in the regulation and maintenance of brain barrier integrity. Although we did not measure the gut microbiota composition in this study, the conventional mice were all raised under the same variables known to influence the gut microbiota composition (i.e., diet and light cycles) (Guo et al., 2022). Additionally, even if we were to have seen variation in microbiota composition among the conventional mice, we did not see different clusters in ZO‐1 network quantification.
Although we saw changes in the ZO‐1 network, we did not see any differences in the choroid plexus capillary structure between germ‐free and conventional mice. Similarly, in the brain, Braniste et al. did not see differences in vessel size or density between germ‐free and pathogen‐free mice (Braniste et al., 2014). Although our data only focus on the structure of the choroid plexus vessels, future research should further investigate if the gut microbiota impacts the function of the vascular barrier, fenestrations and other vascular cellular components. Additionally, our work was performed in adult male mice, and we cannot exclude the potential for sex‐specific or age‐dependent effects. For instance, in healthy developing rats, there are low numbers of fenestrae observed at E16 in the choroid plexus, which increase in number up to postnatal day 30 (Lun et al., 2015), and since germ‐free mice have other developmental differences from conventional mice (Spichak et al., 2018), there may be undetected differences in choroid plexus vessels that are compensated by adulthood. Although only tight junction protein network and vascular structure were investigated in this study, it is also important to note another major role of the choroid plexus, which is to produce CSF. Given that there are alterations in the tight junction protein organization of the epithelial cells, there may also be alterations in the CSF production or secretion in germ‐free mice, since the epithelial cells are responsible for the secretion of the CSF through active transport (MacAulay et al., 2022).
Taken together, these data reveal that the gut microbiota plays a role in the integrity of the blood–CSF barrier. This finding is the first step in understanding the extent of influence the gut microbiota has on blood–CSF barrier physiology. Although we did not investigate potential mechanisms underlying these changes in the tight junction network, we speculate that the absence of microbial metabolites could lead to the disrupted maintenance of the blood–CSF barrier integrity. For instance, microbial metabolites that affect BBB (Knox, Aburto, Clarke, et al., 2022) and gut barrier integrity (Ghosh et al., 2021) may also affect the blood–CSF barrier. Germ‐free mice present with a disrupted BBB, which is associated with decreased tight junction protein levels and increased permeability. This disruption is ameliorated when colonized with short‐chain fatty acid (SCFA) producing strains, Clostridium tyrobutyricum, or the SCFA butyrate (Braniste et al., 2014; Knox, Aburto, Tessier, et al., 2022). In in vitro BBB models, the SCFAs, butyrate and propionate, also have protective effects from barrier disruption and affect tight junction protein expression (Hoyles et al., 2018), and may show similar protection of the blood–CSF barrier. Additionally, SCFAs and other microbial metabolites such as indoles and secondary bile acids affect intestinal epithelial cells through mammalian target of rapamycin (mTOR), aryl hydrocarbon receptor (AhR) and farnesoid X receptor (FXR) receptors (Zhou et al., 2022) and may therefore have similar effects on the choroid plexus epithelial cells. Previous studies have indicated that other models of gut microbiota perturbations affect the other barriers of the gut microbiota–brain axis such as the BBB, gut vascular and intestinal epithelial barrier (Tang et al., 2020; Zhou et al., 2022). Since the tight junctions of the blood–CSF barrier are disrupted in diseases such as Huntington's and Alzheimer's disease (Brkic et al., 2015; Solár et al., 2020; Stopa et al., 2018), the role of the gut microbiota and microbiota‐derived signalling molecules (e.g., SCFAs) should be explored in order to exploit potential therapeutics for maintaining barrier integrity. Several microbially derived metabolites have already been shown to have positive or protective effects over the BBB, highlighting the potential to use microbial metabolites to therapeutically target the BBB in disease (Knox, Aburto, Clarke, et al., 2022; O'Riordan et al., 2022) where the barrier is compromised such as in neuropsychiatric (Kealy et al., 2020) and neurodegenerative (Sweeney et al., 2018) disorders. Metabolites such as the short‐chain fatty acids, specifically, have been shown to protect tight junction protein expression, further emphasizing the importance of tight junction protein networks (Braniste et al., 2014; Hoyles et al., 2018). Moreover, our data identify the choroid plexus as another brain barrier to be influenced by the gut microbiota. Future studies should focus on understanding specific bacterial strains and their metabolites in the maintenance and regulation of blood–CSF barrier function.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Emily G. Knox: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing‐original draft; writing‐review and editing. Caoimhe M. K. Lynch: Data curation; methodology; visualization; writing‐review and editing. Ye Seul Lee: Data curation; formal analysis. Caitriona M. O'Driscoll: Conceptualization; supervision; writing‐original draft; writing‐review and editing. Gerard Clarke: Resources; supervision; visualization; writing‐original draft; writing‐review and editing. John F. Cryan: Conceptualization; project administration; supervision; visualization; writing‐original draft; writing‐review and editing. Maria R. Aburto: Conceptualization; data curation; investigation; methodology; project administration; supervision; visualization; writing‐original draft; writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15878.
ACKNOWLEDGEMENTS
The research was conducted in the APC Microbiome Ireland, which is funded by Science Foundation Ireland (SFI/12/RC/2273_P2) to JFC and GC. MRA is supported by ERC_StG_RADIOGUT (project 101040951) and by SFI‐IRC Pathway Program SFI21/PATH‐S/9424. We would like to thank Carmen Tessier and Elisa Cintado Reyes for their help in the optoization of choroid plexus analysis. Open access funding provided by IReL.
Knox, E. G. , Lynch, C. M. K. , Lee, Y. S. , O'Driscoll, C. M. , Clarke, G. , Cryan, J. F. , & Aburto, M. R. (2023). The gut microbiota is important for the maintenance of blood–cerebrospinal fluid barrier integrity. European Journal of Neuroscience, 57(2), 233–241. 10.1111/ejn.15878
Edited by: Eilis Dowd
Funding information Science Foundation Ireland, Grant/Award Numbers: SFI/12/RC/2273_P2, SFI21/PATH‐S/9424; ERC_StG_RADIOGUT, Grant/Award Number: 101040951
Contributor Information
John F. Cryan, Email: j.cryan@ucc.ie.
Maria R. Aburto, Email: maria.rodriguezaburto@ucc.ie.
DATA AVAILABILITY STATEMENT
All data have been deposited in Zenoda and are publicly available as of the date of publication. DOIs are listed in the key resources table.
REFERENCES
- Braniste, V. , Al‐Asmakh, M. , Kowal, C. , Anuar, F. , Abbaspour, A. , Tóth, M. , Korecka, A. , Bakocevic, N. , Ng, L. G. , Kundu, P. , Gulyás, B. , Halldin, C. , Hultenby, K. , Nilsson, H. , Hebert, H. , Volpe, B. T. , Diamond, B. , & Pettersson, S. (2014). The gut microbiota influences blood‐brain barrier permeability in mice. Science Translational Medicine, 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkic, M. , Balusu, S. , Van Wonterghem, E. , Gorlé, N. , Benilova, I. , Kremer, A. , Van Hove, I. , Moons, L. , De Strooper, B. , Kanazir, S. , Libert, C. , & Vandenbroucke, R. E. (2015). Amyloid β oligomers disrupt blood‐CSF barrier integrity by activating matrix metalloproteinases. The Journal of Neuroscience, 35, 12766–12778. 10.1523/JNEUROSCI.0006-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan, J. F. , & Dinan, T. G. (2012). Mind‐altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13, 701–712. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Cryan, J. F. , O'Riordan, K. J. , Cowan, C. S. M. , Sandhu, K. V. , Bastiaanssen, T. F. S. , Boehme, M. , Codagnone, M. G. , Cussotto, S. , Fulling, C. , Golubeva, A. V. , Guzzetta, K. E. , Jaggar, M. , Long‐Smith, C. M. , Lyte, J. M. , Martin, J. A. , Molinero‐Perez, A. , Moloney, G. , Morelli, E. , Morillas, E. , … Dinan, T. G. (2019). The microbiota‐gut‐brain axis. Physiological Reviews, 99, 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- Ghosh, S. , Whitley, C. S. , Haribabu, B. , & Jala, V. R. (2021). Regulation of intestinal barrier function by microbial metabolites. Cellular and Molecular Gastroenterology and Hepatology, 11, 1463–1482. 10.1016/j.jcmgh.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlé, N. , Blaecher, C. , Bauwens, E. , Vandendriessche, C. , Balusu, S. , Vandewalle, J. , Van Cauwenberghe, C. , Van Wonterghem, E. , Van Imschoot, G. , Liu, C. , Ducatelle, R. , Libert, C. , Haesebrouck, F. , Smet, A. , & Vandenbroucke, R. E. (2018). The choroid plexus epithelium as a novel player in the stomach‐brain axis during helicobacter infection. Brain, Behavior, and Immunity, 69, 35–47. 10.1016/j.bbi.2017.12.010 [DOI] [PubMed] [Google Scholar]
- Guo, J. , Song, C. , Liu, Y. , Wu, X. , Dong, W. , Zhu, H. , Xiang, Z. , & Qin, C. (2022). Characteristics of gut microbiota in representative mice strains: Implications for biological research. Animal Models and Experimental Medicine, 5, 337–349. 10.1002/ame2.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X. , Lee, A. , Huang, S. , Gao, J. , Spence, J. R. , & Owyang, C. (2019). Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon‐gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes, 10, 59–76. 10.1080/19490976.2018.1479625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, C. L. , Dong, J. , Galipeau, H. J. , Jury, J. , McCarville, J. , Huang, X. , Wang, X. Y. , Naidoo, A. , Anbazhagan, A. N. , Libertucci, J. , Sheridan, C. , Dudeja, P. K. , Bowdish, D. M. E. , Surette, M. G. , & Verdu, E. F. (2018). Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Scientific Reports, 8, 14184. 10.1038/s41598-018-32366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyles, L. , Pontifex, M. G. , Rodriguez‐Ramiro, I. , Anis‐Alavi, M. A. , Jelane, K. S. , Snelling, T. , Solito, E. , Fonseca, S. , Carvalho, A. L. , Carding, S. R. , Müller, M. , Glen, R. C. , Vauzour, D. , & McArthur, S. (2021). 'Regulation of blood–brain barrier integrity and cognition by the microbiome‐associated methylamines trimethylamine‐N‐oxide and trimethylamine', Preprint. [DOI] [PMC free article] [PubMed]
- Hoyles, L. , Snelling, T. , Umlai, U.‐K. , Nicholson, J. K. , Carding, S. R. , Glen, R. C. , & McArthur, S. (2018). Microbiome‐host systems interactions: Protective effects of propionate upon the blood‐brain barrier. Microbiome, 6, 55. 10.1186/s40168-018-0439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy, J. , Greene, C. , & Campbell, M. (2020). Blood‐brain barrier regulation in psychiatric disorders. Neuroscience Letters, 726, 133664. 10.1016/j.neulet.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Kelly, J. R. , Kennedy, P. J. , Cryan, J. F. , Dinan, T. G. , Clarke, G. , & Hyland, N. P. (2015). Breaking down the barriers: The gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Frontiers in Cellular Neuroscience, 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox, E. G. , Aburto, M. R. , Tessier, C. , Nagpal, J. , Clarke, G. , O'Driscoll, C. M. , and Cryan, J. F. (2022). 'Microbial‐derived metabolites induce actin cytoskeletal rearrangement and protect blood‐brain barrier function', iScience. [DOI] [PMC free article] [PubMed]
- Knox, E. G. , Aburto, M. R. , Clarke, G. , Cryan, J. F. , & O'Driscoll, C. M. (2022). The blood‐brain barrier in aging and neurodegeneration. Molecular Psychiatry, 27, 2659–2673. 10.1038/s41380-022-01511-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X.‐H. , Ye, X.‐J. , Li, Q.‐F. , Gong, Z. , Cao, X. , Li, J.‐H. , Zhao, S.‐T. , Sun, X.‐D. , He, X.‐S. , & Xuan, A.‐G. (2020). Urolithin a prevents focal cerebral ischemic injury via attenuating apoptosis and neuroinflammation in mice. Neuroscience, 448, 94–106. 10.1016/j.neuroscience.2020.09.027 [DOI] [PubMed] [Google Scholar]
- Lun, M. P. , Monuki, E. S. , & Lehtinen, M. K. (2015). Development and functions of the choroid plexus–cerebrospinal fluid system. Nature Reviews Neuroscience, 16, 445–457. 10.1038/nrn3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay, N. , Keep, R. F. , & Zeuthen, T. (2022). Cerebrospinal fluid production by the choroid plexus: A century of barrier research revisited. Fluids and Barriers of the CNS, 19, 26. 10.1186/s12987-022-00323-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais, L. H. , Schreiber, H. L. , & Mazmanian, S. K. (2021). The gut microbiota–brain axis in behaviour and brain disorders. Nature Reviews Microbiology, 19, 241–255. 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- O'Riordan, K. J. , Collins, M. K. , Moloney, G. M. , Knox, E. G. , Aburto, M. R. , Fülling, C. , Morley, S. J. , Clarke, G. , Schellekens, H. , & Cryan, J. F. (2022). Short chain fatty acids: Microbial metabolites for gut‐brain axis signalling. Molecular and Cellular Endocrinology, 546, 111572. 10.1016/j.mce.2022.111572 [DOI] [PubMed] [Google Scholar]
- Parker, A. , Fonseca, S. , & Carding, S. R. (2020). Gut microbes and metabolites as modulators of blood‐brain barrier integrity and brain health. Gut Microbes, 11, 135–157. 10.1080/19490976.2019.1638722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, M. , McMillin, M. , Galindo, C. , Frampton, G. , Pae, H. Y. , & DeMorrow, S. (2014). Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1‐dependent mechanisms. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 46, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin, E. , Sandhu, K. V. , Dinan, T. G. , & Cryan, J. F. (2016). May the force be with you: The light and dark sides of the microbiota‐gut‐brain axis in neuropsychiatry. CNS Drugs, 30, 1019–1041. 10.1007/s40263-016-0370-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solár, P. , Zamani, A. , Kubíčková, L. , Dubový, P. , & Joukal, M. (2020). Choroid plexus and the blood–cerebrospinal fluid barrier in disease. Fluids and Barriers of the CNS, 17, 35. 10.1186/s12987-020-00196-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni, I. , Fornasa, G. , & Rescigno, M. (2017). Organ‐specific protection mediated by cooperation between vascular and epithelial barriers. Nature Reviews Immunology, 17, 761–773. 10.1038/nri.2017.100 [DOI] [PubMed] [Google Scholar]
- Spichak, S. , Guzzetta, K. E. , O'Leary, O. F. , Clarke, G. , Dinan, T. G. , & Cryan, J. F. (2018). Without a bug's life: Germ‐free rodents to interrogate microbiota‐gut‐neuroimmune interactions. Drug Discovery Today: Disease Models, 28, 79–93. [Google Scholar]
- Stamatovic, S. M. , Johnson, A. M. , Keep, R. F. , & Andjelkovic, A. V. (2016). Junctional proteins of the blood‐brain barrier: New insights into function and dysfunction. Tissue Barriers, 4, e1154641. 10.1080/21688370.2016.1154641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopa, E. G. , Tanis, K. Q. , Miller, M. C. , Nikonova, E. V. , Podtelezhnikov, A. A. , Finney, E. M. , Stone, D. J. , Camargo, L. M. , Parker, L. , Verma, A. , Baird, A. , Donahue, J. E. , Torabi, T. , Eliceiri, B. P. , Silverberg, G. D. , & Johanson, C. E. (2018). Comparative transcriptomics of choroid plexus in Alzheimer's disease, frontotemporal dementia and Huntington's disease: Implications for CSF homeostasis. Fluids and Barriers of the CNS, 15, 18. 10.1186/s12987-018-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney, M. D. , Sagare, A. P. , & Zlokovic, B. V. (2018). Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature Reviews Neurology, 14, 133–150. 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. , Zhu, H. , Feng, Y. , Guo, R. , & Wan, D. (2020). The impact of gut microbiota disorders on the blood‐brain barrier. Infection and Drug Resistance, 13, 3351–3363. 10.2147/IDR.S254403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum, T. , Matalon, D. , Adam, R. , Seibt, A. , Wewer, C. , Schwerk, C. , Galla, H.‐J. , & Schroten, H. (2008). Dexamethasone prevents alteration of tight junction‐associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro. Brain Research, 1229, 1–17. 10.1016/j.brainres.2008.06.118 [DOI] [PubMed] [Google Scholar]
- Zhou, A. , Yuan, Y. , Yang, M. , Huang, Y. , Li, X. , Li, S. , Yang, S. , & Tang, B. (2022). Crosstalk between the gut microbiota and epithelial cells under physiological and infectious conditions. Frontiers in Cellular and Infection Microbiology, 12, 832672. 10.3389/fcimb.2022.832672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data have been deposited in Zenoda and are publicly available as of the date of publication. DOIs are listed in the key resources table.


