Abstract
To help meet the objectives of the Great Lakes Restoration Initiative with regard to increasing knowledge about toxic substances, 223 pesticides and pesticide transformation products were monitored in 15 Great Lakes tributaries using polar organic chemical integrative samplers. A screening‐level assessment of their potential for biological effects was conducted by computing toxicity quotients (TQs) for chemicals with available US Environmental Protection Agency (USEPA) Aquatic Life Benchmark values. In addition, exposure activity ratios (EAR) were calculated using information from the USEPA ToxCast database. Between 16 and 81 chemicals were detected per site, with 97 unique compounds detected overall, for which 64 could be assessed using TQs or EARs. Ten chemicals exceeded TQ or EAR levels of concern at two or more sites. Chemicals exceeding thresholds included seven herbicides (2,4‐dichlorophenoxyacetic acid, diuron, metolachlor, acetochlor, atrazine, simazine, and sulfentrazone), a transformation product (deisopropylatrazine), and two insecticides (fipronil and imidacloprid). Watersheds draining agricultural and urban areas had more detections and higher concentrations of pesticides compared with other land uses. Chemical mixtures analysis for ToxCast assays associated with common modes of action defined by gene targets and adverse outcome pathways (AOP) indicated potential activity on biological pathways related to a range of cellular processes, including xenobiotic metabolism, extracellular signaling, endocrine function, and protection against oxidative stress. Use of gene ontology databases and the AOP knowledgebase within the R‐package ToxMixtures highlighted the utility of ToxCast data for identifying and evaluating potential biological effects and adverse outcomes of chemicals and mixtures. Results have provided a list of high‐priority chemicals for future monitoring and potential biological effects warranting further evaluation in laboratory and field environments. Environ Toxicol Chem 2023;42:340–366. Published 2022. This article is a U.S. Government work and is in the public domain in the USA. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Contaminants, Great Lakes, passive samplers, pesticides, rivers
INTRODUCTION
Pesticides have long been known as environmental contaminants of concern (Carson, 1962) given their widespread use (Braga et al., 2020; Tang et al., 2021; Wang et al., 2018; Wieben, 2020) and frequent occurrence in streams, lakes, and other waterbodies (Bradley et al., 2017; Hladik et al., 2018; Nowell et al., 2018; Stackpoole et al., 2021; Stone et al., 2014; Van Metre et al., 2017). By design, pesticides are intended to interfere with biological processes, which in the environment can result in adverse effects on nontarget organisms and population declines of sensitive species (Baker et al., 2013; Gibbons et al., 2015; Henry et al., 2012; Islam et al., 2018; Klementová et al., 2019; Köhler & Triebskorn, 2013; Morrissey et al., 2015; Singh et al., 2018; US Environmental Protection Agency [USEPA], 2009a). Even after parent chemical transformation, the toxicity of some pesticide transformation products (i.e., metabolites and degradates) can rival or exceed that of the parent chemical (Giacomazzi & Cochet, 2004; Gunasekara et al., 2007; Schlenk et al., 2001; Tixier et al., 2001; Weston & Lydy, 2014) but are less frequently included in environmental risk assessments (Mahler et al., 2021). In general, comprehensive knowledge of the unintended effects of chemical contamination is unknown (Rappaport & Smith, 2010), and thus tools are needed to prioritize chemicals, timing of transformation, and locations of greatest concern.
Under Focus Area 1 of the Great Lakes Restoration Initiative (Great Lakes Interagency Task Force, 2014), one of the aims has been to identify both sources and potential effects of contaminants and to evaluate their potential impacts to aquatic and terrestrial animal populations. Given that pesticide use in the United States tends to be greatest in the Midwest (Stackpoole et al., 2021; Wieben, 2020), including many Great Lakes states, the present study was focused on prioritizing pesticides (including transformation products) and pesticide mixtures in tributaries of the Great Lakes based on evaluations of their potential biological effects. A suite of pesticide and pesticide transformation products was monitored using polar organic chemical integrative samplers (POCIS) in 15 tributaries of the Great Lakes. Polar organic chemical integrative samplers accumulate soluble to moderately water‐soluble organic chemicals from the aqueous environment over the length of their deployment (typically weeks to months), resulting in time‐composite samples that represent larger volumes of water, and, as a result, they can be used to detect lower concentrations than in typical water samples (Alvarez et al., 2008; Huckins et al., 2006; Van Metre et al., 2017). Polar organic chemical integrative samplers can detect trace contamination of chemicals which would have gone undetected with traditional discrete sampling methods (Kolpin et al., 2013; Munaron et al., 2012).
The ecological toxicity of pesticide active ingredients is extensively evaluated through intact animal testing during the process of pesticide registration. Thus, for pesticides there are often substantially more acute and chronic toxicity data on which to base an effects evaluation than for other classes of contaminants. However, these laboratory‐based assessments cannot evaluate toxicity across all species and may not fully investigate long‐term, multigenerational, and/or other subtle effects, such as changes in reproductive potential, gene expression, and behavior (Nilsen et al., 2019). Furthermore, the interactions of pesticide mixtures are not examined extensively for registration purposes and are rarely tested otherwise (but see Blackwell et al., 2019; Schoenfuss et al., 2016), leading to incomplete understanding of the compounding biological effects from chemical mixtures (Lydy et al., 2004; Nilsen et al., 2019). Furthermore, the toxicity of pesticide transformation products often is unknown.
Recently, expanded chemical evaluation testing has been conducted to include chemical‐specific high‐throughput in vitro biological activity through the USEPA ToxCast program (Dix et al., 2007; Kavlock et al., 2012; USEPA, 2020) and the interagency Tox21 collaboration (Tice et al., 2013; Thomas et al., 2018), collectively referred to as “ToxCast.” The ToxCast database (USEPA, 2020) is regularly updated and includes in vitro bioactivity measurements (i.e., endpoints) for thousands of chemicals, including many pesticides, that cover a range of cellular responses and molecular signaling pathways (Blackwell et al., 2019). Many of the ToxCast assay endpoints are designed to measure activity related to cellular pathways and proteins coded and/or regulated by specific genes. For example, several ToxCast assays measure in vitro induction of transcriptional reporters involved in estrogen signaling. Thus, chemicals active in these assays have the potential to influence biological processes and molecular functions associated with estrogen receptors (coded by the ESR1 and ESR2 genes) provided the assay reflects in vivo absorption, distribution, metabolism, and excretion. The ToxCast assays and the genes associated with their biochemical targets can be further associated with adverse outcome pathways (AOPs; Ankley et al., 2010), providing a method to link chemicals and chemical mixtures with potential outcomes at the molecular, organismal, or population level. For example, AOP #29 describes linkages between agonism (activation) of the estrogen receptor on reproductive dysfunction in amphibians, birds, and fish (Hutchinson & Villeneuve, 2021). Thus, chemicals active in ToxCast assays designed to measure estrogen receptor activation have the potential to adversely affect reproduction in wildlife populations provided that exposure. Assays in ToxCast were originally intended for evaluation of mammalian biochemistry to provide a means to forecast organ system or whole organism effects, but the biological basis of many of these assays is conserved across species (LaLone et al., 2018), so incorporating the ToxCast library and AOP knowledgebase into evaluation of environmental monitoring results is a promising method for evaluating potential biological effects and adverse outcomes of chemicals and mixtures. Consequently, the present study used a combination of both traditional Aquatic Life Benchmarks, based on intact organism testing, and ToxCast high‐throughput screening data to identify chemicals, mixtures, and sites of concern. Using the ToxCast library and several open source databases on gene ontology, molecular biology, and AOPs, a list of plausible cellular interactions and biological processes were identified that may be linked to the exposure of aquatic life to pesticides in Great Lakes tributaries.
METHODS
Site description
Sixteen Laurentian Great Lakes tributaries were selected for analysis of pesticides; the tributaries drain a gradient of agricultural and urban land cover (Figure 1, based on 2016 National Land Cover Database; Homer et al., 2020). At least one tributary was represented in each of the five Great Lakes watersheds. Drainage areas for the tributary watersheds varied from 100 to 16,514 km2 based on watersheds derived from the Watershed Boundary Dataset (US Department of Agriculture‐Natural Resources Conservation Service et al., 2009), and mean flow at the sampling sites for water year 2016 varied between 3.9 and 182.6 m3 s–1 (Table 1; US Geological Survey [USGS], 2020).
Figure 1.
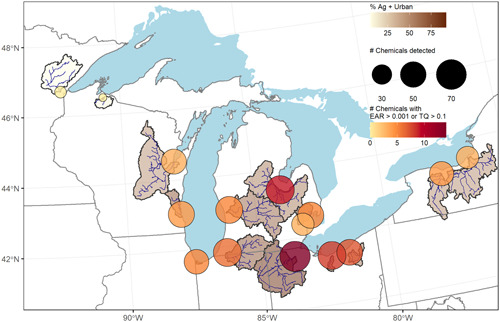
Chemical detections and exposure activity ratios (EARs) and/or toxicity quotients (TQs) for pesticides and pesticide transformation products measured in passive samplers collected from 15 Great Lakes tributaries, June–July 2016. Symbol size represents the number of chemicals detected per site and color represents the number of chemicals exceeding an EAR threshold of 10–3 or a TQ threshold of 0.1. Watershed fill color represents the percentage area classified as agriculture and urban.
Table 1.
Stream attributes and polar organic chemical integrated sampler (POCIS) deployment period for Great Lakes tributaries, June–July 2016
| Major land use | POCIS deployment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tributary name | Lake | USGS site ID | Mean daily flow (m3 s–1) | Drainage area (km2) | Agriculture (%) | Urban (%) | Other (%) | Date | Duration (days) |
| St. Louis | Superior | 04024000 | 99.7 | 8890 | 2.9 | 2.9 | 95.4 | 6/8/2016 | 27 |
| Bad | Superior | 04027000 | 31.7 | 1545 | 5.8 | 3.4 | 91.6 | 6/7/2016 | 29 |
| Fox | Michigan | 040851385 | 182.6 | 16,514 | 41.4 | 8.4 | 53.1 | 6/8/2016 | 28 |
| Manitowoc | Michigan | 04085427 | 13.6 | 1343 | 69.6 | 6.3 | 24.1 | 6/7/2016 | Destroyed |
| Milwaukee | Michigan | 04087170 | 25.2 | 2240 | 43.2 | 29.9 | 29.2 | 6/7/2016 | 28 |
| Indiana Harbor | Michigan | 04092750 | 15.6 | 100 | 1.8 | 85.5 | 16.4 | 6/7/2016 | 36 |
| St. Joseph | Michigan | 04101500 | 89.1 | 9628 | 60.4 | 13.8 | 27.3 | 6/14/2016 | 37 |
| Grand | Michigan | 04119400 | 140.3 | 13,719 | 54 | 14.8 | 34 | 6/14/2016 | 37 |
| Saginaw | Huron | 04157005 | 137.6 | 15,520 | 44.5 | 12.2 | 44.6 | 6/15/2016 | 21 |
| Clinton | Erie | 04165500 | 16.3 | 1904 | 19.9 | 51.9 | 29.6 | 6/15/2016 | 36 |
| Rouge | Erie | 04166500 | 3.9 | 476 | 0.1 | 92 | 7.8 | 6/15/2016 | 34 |
| Maumee | Erie | 04193500 | 125.6 | 16,295 | 78.7 | 10.7 | 11.3 | 6/17/2016 | 21 |
| Vermilion | Erie | 04199500 | 5.9 | 679 | 65.6 | 7.5 | 27.4 | 6/14/2016 | 35 |
| Cuyahoga | Erie | 04208000 | 23.7 | 1836 | 17.5 | 40 | 43 | 6/1/2016 | 35 |
| Genesee | Ontario | 04231600 | 58.0 | 6403 | 45.3 | 6.6 | 52 | 6/2/2016 | 29 |
| Oswego | Ontario | 04249000 | 163.0 | 13,215 | 39.5 | 8.3 | 51.9 | 6/7/2016 | 29 |
Watershed areas (km2) were determined in a geographic information system using linework derived from the Watershed Boundary Dataset (US Department of Agriculture‐Natural Resources Conservation Service et al., 2009) and percentage major land cover classifications were based on the 2016 National Land Cover Database (Homer et al., 2020). Mean daily discharge (m3 s–1) summarized for water year 2016 (USGS, 2020).
ID = identification; USGS = US Geological Survey.
Passive sampler deployment
Polar organic chemical integrative samplers were deployed following methods outlined in Van Metre et al. (2017) and Alvarez (2010). Each deployment canister contained two POCIS that were in the standard configuration with Oasis Hydrophilic–Lipophilic–Balanced (HLB) sorbent (Waters) between two polyethersulfone membranes and a sampling surface area of 41 cm2 (Alvarez, 2010). Oasis HLB is considered to be a universal sorbent used to extract a wide variety of chemical classes (Alvarez, 2010). Deployment durations ranged from 21 to 37 days, and deployments began June 1–17, 2016 (Table 1) to align with common application periods for many pesticides in the region (Covert et al., 2020). Longer deployments are more sensitive and can result in more detections under static conditions, but differences between the shortest and longest deployments would likely only yield additional detections of compounds at concentrations well below our prioritization thresholds (Supporting Information, Figure SI‐I). On retrieval, the POCIS were removed from the water, sealed in air‐tight metal cans, and shipped on ice to the laboratory for processing. One POCIS canister was compromised during deployment (USGS site 04085427), so the resulting analysis included only 15 sites.
Chemical analysis
Sorbents from each POCIS were transferred to empty 50‐ml solid‐phase extraction cartridges fitted with polypropylene frits using deionized water. The sorbents were dried under vacuum and chemical residues were recovered using 25 ml of methanol (Van Metre et al., 2017). Polar organic chemical integrative sampler extracts were reduced in volume by rotary evaporation and high‐purity nitrogen blowdown and sealed in amber ampoules at 1.0 ml of methanol for shipment to the USGS National Water Quality Laboratory (NWQL) for analysis. The extracts were analyzed for 225 pesticide and transformation products (Supporting Information, Table SI‐1) using direct aqueous injection liquid chromatography tandem mass spectrometry (DAI LC‐MS/MS; Sandstrom et al., 2015). Two of the 225 chemicals (cis‐ and trans‐permethrin) were excluded after assessment of potential contamination bias, recovery bias, and high variability of pesticide samples (Medalie & Bexfield, 2020; Stackpoole et al., 2021). Of the remaining 223 chemicals, 129 have known POCIS uptake rates, for which time‐weighted mean river concentrations over the duration of POCIS deployment can be calculated (Alvarez et al., 2008). Minimum detection limits (MDL) for concentration estimates ranged between 0.01 and 200 ng L–1 (median 0.3 ng L–1). Chemicals without POCIS uptake rates were noted as “detected” if concentrations in the POCIS extracts (ng POCIS–1) exceeded the laboratory method MDL. Although POCIS concentrations can be used to infer relative differences in concentration among sites (Alvarez et al., 2008), those without established POCIS uptake rates are noted simply as present or absent in the present study. Polar organic chemical integrative sampler data are available in Loken et al. (2022).
A blank sample was collected at two sites during POCIS deployments by exposing prepared POCIS to the air during the time it took to deploy and retrieve the sampler. Blank samples were extracted along with field samples at the end of the deployment period. Three chemicals (atrazine, metolachlor, and deisopropylatrazine) were detected in a blank sample, all of which were <0.6% of the median concentration and <7% of the minimum concentration detected in POCIS. Metolachlor, atrazine, and their transformation products have been detected frequently in precipitation and air samples in the region, which might explain their presence in the blanks (Metcalfe et al., 2016; Thurman & Cromwell, 2000).
Duplicate POCIS were deployed at the Saginaw River site. Sixty‐six chemicals were detected in extracts from both samplers with a median relative percent difference of 16.8%, while 154 chemicals were not detected in either sampler. Three chemicals were detected in one sampler but not in the other. For subsequent analyses, we used the mean concentration for chemicals detected in both samplers. For the three chemicals only detected in one sampler, we used the midpoint between the MDL and the detected concentration.
Discrete water samples
Because time‐weighted concentration estimates were only available for 128 of the compounds analyzed, pesticides quantified in concurrent discrete water samples were also evaluated. Water samples were collected at the 16 sites in the present study approximately monthly as part of a companion project focused on annual patterns of pesticide occurrence in Great Lakes tributaries (Oliver et al., 2022). Samples were collected using an equal‐width‐increment method (Edwards & Glysson, 1999) and analyzed for the same pesticide and transformation products using DAI LC‐MS/MS (Sandstrom et al., 2015). In the present study, we used the discrete data collected during the POCIS deployments (typically two samples per site) and calculated the maximum concentration for each chemical for use in subsequent analyses. Comparing the discrete sample results with the passive sampler results allowed us to identify any additional chemicals and (or) potential biological effects that were not prioritized using POCIS.
Prioritizing chemicals of ecological concern
Pesticides detected in POCIS (and discrete samples) were screened for potential hazard using two different methods, facilitated by use of functions within the R package toxEval (DeCicco et al., 2018). First, concentrations were compared with established USEPA Office of the Pesticide Programs' Aquatic Life Benchmarks (Supporting Information, Table SI‐2 and Supporting Information, Figure SI‐2; USEPA, 2019a). Aquatic Life Benchmarks are based on acute and chronic toxicity values for freshwater fish and invertebrates, and toxicity values for vascular and nonvascular plants found in scientific studies in support of publicly available ecological risk assessments. Because POCIS‐derived concentrations represent approximately monthly mean concentrations, comparing to both chronic and acute Aquatic Life Benchmarks is appropriate. Across freshwater fish, invertebrates, and plants, the most sensitive value was selected for each chemical (benchmark concentration) to evaluate the potential for adverse organismal effects by computation of toxicity quotients (TQs; Equation 1).
| (1) |
Toxicity quotient < 1 indicates an increased likelihood of adverse biological effects on aquatic species (fish, invertebrates, or plants). However, a TQ value of 0.1 was used in the present study as a conservative exceedance threshold for prioritizing chemicals.
Second, concentrations were compared with the relative potencies of chemicals in the ToxCast database (Ver 3.2; USEPA, 2020). The ToxCast program includes a wide array of laboratory assays designed to evaluate molecular, biochemical, cellular, or other pathway‐based effects of chemicals (Richard et al., 2016). Annotations describing assay details including the intended biological targets are available in the ToxCast database (USEPA, 2022b) and the USEPA CompTox dashboard (2022c). A list of assay endpoints (i.e., parameter name “assay_component_endpoint_name” in the ToxCast database) used in the present study is provided Supporting Information, Table SI‐3. Each assay endpoint is a unique laboratory measurement of change in the biological activity of living cells or biological macromolecules to varying chemical exposure.
The methods for using ToxCast data in the present analysis follow those published previously (Corsi et al., 2019) and are briefly summarized. The activity concentration at cutoff (ACC) was used as the benchmark for comparison with water concentrations of a given chemical (Equation 2; Blackwell et al., 2017; Fay et al., 2018). The ACC (parameter name “modl acc” in the ToxCast database) for each assay endpoint–chemical combination provides an indication of the concentration at which the chemical exceeds a biologically relevant level of bioactivity (Filer et al., 2016; Judson et al., 2009) and functions similarly to the Aquatic Life Benchmarks used to compute TQs. Assay endpoints from all platforms in the ToxCast database were used except Bioseek, which had ACC values that were anomalously low compared with those from other sources. Attagene “loss” endpoints and Novascreen “gain” endpoints were removed because these assays were not optimized or designed to report for the given assay direction (Blackwell et al., 2017). Data quality remarks were included with the reported metrics for each assay endpoint‐chemical test. Results with the following data quality flags were removed from consideration: “Borderline active,” “Only highest conc above baseline, active,” “Gain AC50 < lowest conc & loss AC50 <mean conc,” “Biochemical assay with <50% efficacy,” and “AC50 less than lowest concentration tested.” An additional 46 assay endpoints were excluded that lacked specific mechanistic information (e.g., TOX21_DT40; Supporting Information, Table SI‐4). Dose–response curves for assay endpoint‐chemical combinations remaining after this selection process were examined manually, and 78 of the curves were found to be of questionable quality based on anomalous values or lack of response.
Exposure activity ratios (EARs) were computed using the R package toxEval (DeCicco et al., 2018) as the ratio of observed chemical concentrations and ACC values for all relevant assay endpoints (Equation 2).
| (2) |
Following methods outlined in Corsi et al. (2019), EARs were summed across all available assay endpoints for each individual chemical (EARChem). Because ToxCast assays measure biological activities, which may or may not have the potential to be adverse, and do so in simplified biological systems (in vitro, in chemico) rather than whole organisms, EARs do not lend themselves to a clear threshold with respect to risk in the same manner as a TQ. For example, several ToxCast assays endpoints measure estrogen or androgen receptor activity; however, elevated in vitro activity in these assay endpoints does not necessarily translate to adverse outcomes in whole organisms, but rather to identify chemicals with the potential to interact with estrogenic and androgenic pathways (Rotroff et al., 2013). Exposure activity ratios provide a relative indicator of potential for biological effects that accounts for both chemical concentration and potency with respect to a given biological target or pathway and additivity of multiple chemicals at that target. Unlike TQs, EAR > 1 does not necessarily indicate a probable adverse effect. Linkage of a biological activity with an AOP description (Ankley et al., 2010; Organisation for Economic Co‐operation and Development [OECD], 2021) can provide increased confidence in potential for an adverse effect but not necessarily at the concentration that exceeds the ACC. In previous work, an EAR threshold value of 10–3 yielded a similar priority list of chemicals measured in water as did a TQ threshold of 0.1 (Corsi et al., 2019), and has been used as a screening‐level benchmark in other environmental assessments (Bradley et al., 2021). Thus, an EARChem threshold value of 10–3 was used in the present study as a level of concern, but this should be viewed in a relative sense compared with other compounds in the present study, rather than an absolute sense.
Using TQs and EARs, we prioritized chemicals of greatest concern. Of the 223 unique compounds in the laboratory schedule after exclusions (Sandstrom et al., 2015; Stackpoole et al., 2021), 112 had a USEPA Aquatic Life Benchmark, 126 were available in ToxCast 3.2 (USEPA, 2020), and 137 were in at least one of two screening databases. However, only 129 of the 223 compounds had POCIS uptake rates allowing concentration estimation, thus fewer chemicals could have TQs or EARs calculated compared with discrete water samples. Of the 129 compounds with POCIS uptake rates, 75 had a USEPA Aquatic Life Benchmark value, allowing TQ calculations, and 81 chemicals were available in ToxCast Ver 3.2 (USEPA, 2020), allowing EAR calculation. Eighty‐seven of the 129 chemicals with POCIS uptake rates were included in at least one of these screening databases. Priority chemicals were defined as those that exceeded TQ or EARChem thresholds in at least 10% of samples (i.e., two sites or more).
Chemical mixtures and biological relevance
Another application of EAR analysis includes inference of potential biological effects based on ToxCast annotations from assays with relevant bioactivity for observed individual chemicals and mixtures. This approach differs from other mixture indices based on combining the whole‐organism toxicity of multiple chemicals (Nowell et al., 2014). Our approach focuses on linking chemicals that share activity in common ToxCast in vitro laboratory assays and those that measure activity related to common genes and AOPs (Supporting Information, Tables SI‐5 and SI‐6). These associations were determined using routines within the R package ToxMixtures (Supporting Information, Figure SI‐3; Loken et al., 2021) to predict the mixture influence on a number of biological effects ranging from the cellular to population level and to identify the chemical mixture associated with the potential effects.
Chemical mixture effects were estimated assuming additivity (Nirmalakhandan et al., 1994; Thrupp et al., 2018) of EAR values for chemicals with bioactivity in a given ToxCast assay and relevant genes or AOPs. The EARMixture was calculated for each POCIS (and discrete) sample by summing EAR values among chemicals for each ToxCast assay endpoint. Similarly, EARgene and EARAOP were calculated for each sample by summing EAR values among chemicals for assay endpoints associated with a given gene (including activation, inhibition, or binding) and AOP, respectively. Only the maximum EAR for each individual chemical associated with an assay endpoint linked to the gene or AOP was included in the summation. Similar to the prioritization of individual chemicals, to prioritize chemical mixtures, the list of assay endpoints, genes, and AOPs were subset to those with EARMixture, EARgene, or EARAOP >10–3 in at least 10% of samples. For each EARMixture, EARgene, or EARAOP calculation, we defined the mixture as the chemicals with individual EARs > 10–4 (i.e., 10% of the EAR threshold) for the specific ToxCast assay endpoint, gene, or AOP. Functional annotations, gene ontologies, and pathways from online databases were summarized for instances exceeding the EAR threshold of 10–3 at two or more sites for both passive and discrete water samples.
First, we considered functional annotations and gene ontologies associated with the gene targets or ToxCast assay endpoints. Because most ToxCast assays target Homo sapiens (human) genes, we considered gene orthologs of Danio rerio (zebrafish) and Xenopus tropicalis (Western clawed frog), two model aquatic organisms. Orthologs for D. rerio and X. tropicalis were identified using the R package homologene (Mancarci, 2019). Homo sapiens, D. rerio, and X. tropicalis genes were queried in the Database for Annotation, Visualization and Integrated Discovery (DAVID: Laboratory of Human Retrovirology and Informatics, 2022) online database using the R package rDAVIDWebService (Fresno & Fernandez, 2013). The DAVID includes a collection of biological processes, molecular functions, and diseases associated with specific genes and genomes. For each EARgene threshold exceedance, the available annotation information was summarized to gain insight into the specific biological functions and cellular responses in which that gene is involved.
Second, ToxCast assay endpoints were mapped to AOPs and biological pathways provided through the AOP Knowledgebase (OECD, n.d.) and Panther Classification System (PANTHER; 2022). Data from these online databases are incorporated into the R package ToxMixtures (Loken et al., 2021), allowing linkage with ToxCast assay endpoints. These databases relate genes and proteins with specific cellular functions and key biological events that are nested within a broader network of pathways ultimately linking the interactions of specific biochemical molecules. In essence, these databases map individual genes and coded proteins into nested hierarchical biological functions and predicted conceptual frameworks at the cellular, organismal, or population level based on the synthesis and integration of existing knowledge.
RESULTS
Chemical detections
Mixtures of pesticides and transformation products were detected in passive (and discrete) samples from all 15 tributaries in the present study (Figure 1). Of the 223 compounds analyzed from POCIS extracts, 97 unique pesticide and transformation products were detected in samples from at least one site (Figure 2). Ten chemicals were detected in samples from all 15 sites, and 32 chemicals were detected in samples from 13 or more sites (Figure 2). Of the 3375 individual chemical analyses, 75.7% were below the analytical MDL, and 128 chemicals were never detected (Supporting Information, Table SI‐1). Comparatively, in concurrent filtered water samples analyzed using the same laboratory schedule, 78 chemicals were detected at least once and four chemicals were detected in all sites (Oliver et al., 2022). Thirty chemicals were only detected in POCIS, 11 chemicals were only detected in discrete samples, and 67 chemicals were detected using both methods.
Figure 2.
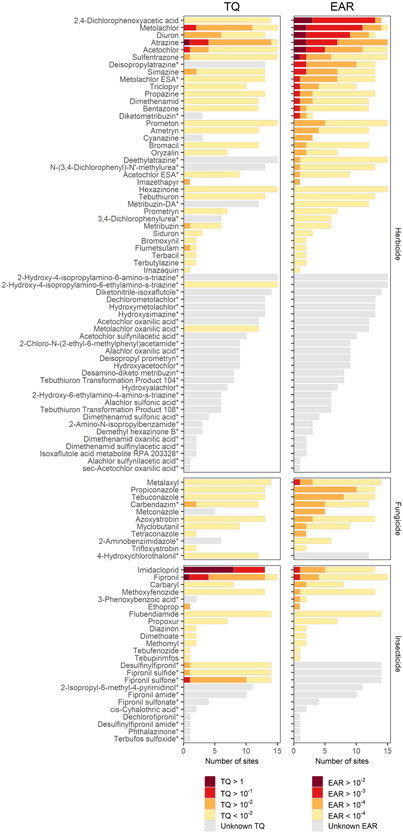
Pesticide and pesticide transformation product (designated with*) detections (yellow/gray) and potential activity (red/orange) based on toxicity quotients (TQs; left) and exposure activity ratios (EARs; right) from passive sampler monitoring results in 15 Great Lakes tributaries, June–July 2016. The combined length of each bar represents the number of sites where a chemical was detected above the minimum detection limit. Red, orange, and yellow show the number of sites where a chemical exceeded TQ or EAR values. Chemicals with gray bars lacked information for evaluation of TQ or EAR. Chemicals are grouped by intended use and ordered by frequency of elevated EAR values.
Polar organic chemical integrative sampler uptake rates were available for 95 of the 97 detected chemicals, allowing for estimation of time‐weighted mean concentrations (Supporting Information, Table SI‐1). Chemical concentrations above the MDLs varied between 0.007 and 3400 ng L–1, with a mean and median of 50 and 3 ng L–1, respectively. The two detected chemicals lacking POCIS uptake rates were sec‐acetochlor oxanilic acid and dimethenamid oxanilic acid. These are transformation products of herbicides acetochlor and dimethenamid, and were detected in POCIS extracts in one and two sites, respectively. These two transformation products were also detected in discrete water samples in one and three sites, respectively. In general, POCIS‐derived concentrations correlated with discrete samples when detected using both methods, with discrete samples tending to have slightly higher concentrations (Supporting Information, Figure SI‐4).
Chemical prioritization
Of the 95 detected chemicals with POCIS‐derived concentration estimates, 54 have Aquatic Life Benchmarks and 58 are in the ToxCast database, allowing for computation of TQ and (or) EAR values for 64 chemicals (27 herbicides, eight fungicides, 12 insecticides, and 17 transformation products). Three herbicides, two insecticides, and one insecticide transformation product exceeded the TQ threshold of 0.1 in at least one sample (Figure 2a). Eleven herbicides, one fungicide, two insecticides, and three herbicide transformation products exceeded the EARChem threshold of 10–3 (Figure 2b). Thirty‐one chemicals with concentration calculations were missing in both screening databases (Supporting Information, Table SI‐1). The concentrations of these chemicals (all transformation products; Figure 2) above the MDL varied between 0.02 and 2800 ng L–1. Transformation products lacking benchmarks were not evaluated further in the present study, but their importance was included in the overall assessment of parent pesticide compounds in the companion article using discrete water samples collected over the entire year (Oliver et al., 2022).
Chemical prioritization using POCIS‐derived TQ and EARChem values identified a complementary list of chemicals with potential for concern. Insecticides were more often prioritized using TQs, whereas herbicides tended to be prioritized using EARChem values (Figure 2). The TQ threshold (TQ > 0.1) was exceeded in samples from two or more sites for metolachlor, atrazine, imidacloprid, and fipronil (Table 2). Furthermore, atrazine and fipronil exceeded the acute nonvascular plant and chronic invertebrate Aquatic Life Benchmarks (TQ > 1), respectively, at one site, and imidacloprid exceeded the chronic invertebrate Aquatic Life Benchmark at eight sites. Imidacloprid exceeded our more conservative TQ threshold of 0.1 in samples from all 13 sites where it was detected (Figure 2a) yet only exceeded the EARChem threshold (EARChem > 10–3) in one sample. The EARChem threshold was exceeded at two or more sites for the herbicides 2,4‐dichlorophenoxyacetic acid (2,4‐D), metolachlor, diuron, atrazine, acetochlor, sulfentrazone, simazine, and deisopropylatrazine (Figure 2 and Table 2). Collectively, TQ‐ and EAR‐based prioritization identified seven herbicides, two insecticides, and one transformation product at concentrations exceeding their level of concern thresholds in at least two sites evaluated in the present study (Table 2).
Table 2.
Priority pesticides and pesticide transformation products that exceeded exposure activity ratios (EARChem) or toxicity quotient (TQ) thresholds in polar organic chemical integrated samplers (POCIS) samples collected from 15 Great Lakes tributaries, June–July 2016
| Number of sites | |||||
|---|---|---|---|---|---|
| Chemical class | CAS | Chemical name | Detections | EARChem > 10–3 | TQ > 0.1 |
| Herbicide | 51218‐45‐2 | Metolachlor | 15 | 11 | 2 |
| 1912‐24‐9 | Atrazine | 15 | 7 | 4 | |
| 34256‐82‐1 | Acetochlor | 15 | 5 | 1 | |
| 122836‐35‐5 | Sulfentrazone | 15 | 2 | 0 | |
| 94‐75‐7 | 2,4‐D | 14 | 13 | 0 | |
| 330‐54‐1 | Diuron | 13 | 9 | 0 | |
| 122‐34‐9 | Simazine | 13 | 2 | 0 | |
| 139‐40‐2 | Propazine | 13 | 1 | 0 | |
| 25057‐89‐0 | Bentazone | 12 | 1 | 0 | |
| 87674‐68‐8 | Dimethenamid | 12 | 1 | 0 | |
| 55335‐06‐3 | Triclopyr | 10 | 1 | 0 | |
| Herbicide TP | 1007‐28‐9 | Deisopropylatrazine | 13 | 2 | * |
| 171118‐09‐5 | Metolachlor ESA | 13 | 1 | * | |
| 56507‐37‐0 | Diketometribuzin | 3 | 1 | * | |
| Fungicide | 57837‐19‐1 | Metalaxyl | 14 | 1 | 0 |
| Insecticide | 120068‐37‐3 | Fipronil | 15 | 1 | 4 |
| 138261‐41‐3 | Imidacloprid | 13 | 1 | 13 | |
| Insecticide TP | 120068‐36‐2 | Fipronil sulfone | 14 | * | 1 |
2,4‐D = 2,4‐dichlorophenoxyacetic acid; CAS = Chemical Abstracts Service number; ESA = ethane sulfonic acid; TP = transformation product; *no benchmark available.
Similar lists of priority chemicals were identified with POCIS and discrete samples. Of the 10 POCIS‐based priority chemicals, nine were also prioritized based on discrete samples (Supporting Information, Figure SI‐5). The herbicide sulfentrazone was uniquely prioritized using POCIS because EARchem values only exceeded the 10–3 threshold in discrete samples from one site. Meanwhile, two herbicides (triclopyr, bentazone) and four fungicides (propiconazole, carbendazim, azoxystrobin, tebuconazole) were uniquely prioritized using discrete samples. Each of these six chemicals were near the prioritization screening thresholds for POCIS because EARchem or TQ values were within an order of magnitude of the benchmarks in at least two sites (Figure 2).
Site prioritization
The number of chemical detections in POCIS varied by site from 16 to 81, and the number of TQ or EARChem threshold exceedances varied from 0 to 14 (Figures 1 and 3, Supporting Information, Figure SI‐6). The two Lake Superior sites (St. Louis and Bad Rivers) had 18 or fewer chemical detections, none of which exceeded TQ or EARChem thresholds. These two watersheds comprised less than 10% agricultural and urban land cover (Table 1). In contrast, the remaining sites monitored had a greater proportion of agricultural and urban land cover in their watersheds (Table 1), where we detected between 43 and 81 chemicals, up to four TQ exceedances, and up to 14 EARChem exceedances per sample (Figures 1 and 3, Supporting Information, Figure SI‐6). The Maumee River sample had the highest number of chemical detections (n = 81), TQ exceedances (n = 4), and EARChem exceedances (n = 14), and it was the only site with a chemical exceeding the EARChem threshold by 100‐fold (acetochlor). The POCIS sample from the Maumee River also exceeded Aquatic Life Benchmarks (TQ > 1) for atrazine (acute nonvascular plants) and imidacloprid (chronic invertebrates; USEPA, 2019a). Samples from the Saginaw, Vermilion, Cuyahoga, and St. Joseph Rivers had at least six chemicals exceeding either TQ or EARChem thresholds. A more thorough characterization of EAR values for each site by chemical and chemical classes is provided in Supporting Information, Figure SI‐7.
Figure 3.
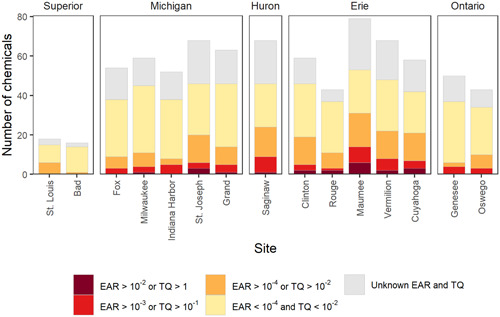
Number of pesticides and pesticide transformation products detected and toxicity quotient (TQ) or exposure activity ratio (EAR) threshold exceedances in passive sampler monitoring results from 15 Great Lakes tributaries, June–July 2016. The combined height of each bar shows the total number of chemicals detected above the minimum detection limit. Red, orange, and yellow show the frequency of chemicals above either TQ or EAR threshold level. Sites are arranged from west to east (counter‐clockwise around the Great Lakes) and grouped by Great Lake watershed.
The proportion of each watershed classified as urban or agricultural was positively correlated with the number of chemicals detected per site (n = 15, p < 0.001, R 2 = 0.56) and EARChem exceedances (n = 15, p = 0.024, R 2 = 0.28; Figure 4). Similar relationships were identified in discrete water samples (Supporting Information, Figure SI‐8; Oliver et al., 2022). In watersheds with agricultural land cover greater than 40%, atrazine, metolachlor, and acetochlor tended to have the highest EARChem values, and in watersheds with urban land cover >25%, diuron and 2,4‐D tended to have the highest EARChem values (Supporting Information, Figure SI‐7).
Figure 4.
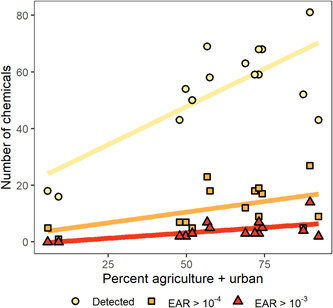
Pesticide and pesticide transformation product detections and exposure activity ratios (EARs) compared with land use for passive sampler results from 15 Great Lakes tributaries, June–July 2016. Watershed land cover metrics (% agriculture + urban) were a positive predictor of chemical detections (yellow) and EAR threshold exceedances (red).
Chemical mixtures and biological relevance
Potential for perturbation of biological pathways was evaluated for individual chemicals and chemical mixtures by linking them with ToxCast assay bioactivity, associated genes, and relevant AOPs. The chemical mixture analysis from POCIS identified 16 ToxCast assay endpoints that exceeded EARMixture thresholds in two or more sites (Table 3 and Supporting Information, Figure SI‐9). Between one and seven unique chemicals contributed to EARMixture for these assay endpoints. Ten unique genes were prioritized using EARgene (Table 3 and Supporting Information, Figure SI‐9). Each of these 10 genes was also associated with EAR threshold exceedances for a chemical individually, and nine genes were associated with EAR exceedances for multiple individual chemicals (Figure 5). In at least one sample, priority chemicals had EAR values greater than 10–3 for 35 assay endpoints that collectively targeted 22 genes (Figures 3 and 5).
Table 3.
Gene symbols, gene descriptions, and pathways for ToxCast assay endpoints with exposure activity ratios from mixtures of pesticides and pesticide transformation products (EARMixture) exceeding 10–3 in at least two sites
| Gene symbol | Orthologs | ToxCast assay endpoints | Chemicals | Method | Gene effects and functional annotations | Panther pathways | Adverse outcome pathways (AOP #) |
|---|---|---|---|---|---|---|---|
| ADORA1 |
H. sapiens D. rerio X. tropicalis |
NVS_GPCR_hAdoRA1 |
Atrazine Deisopropylatrazine |
Passive and discrete | Adenosine receptor belonging to the G‐protein coupled receptor family. Adenosine receptors regulate a diverse set of physiologic functions, including playing a role in fertilization | Two G‐protein signaling pathways (G i α/G s α and G q α/G o α mediated) | |
| CYP1A1 |
H. sapiens D. rerio X. tropicalis |
CLD_CYP1A1_24hr CLD_CYP1A1_6hr |
Diuron Acetochlor 2,4‐D |
Passive and discrete |
The superfamily of cytochrome P450 (CYP) genes encode for monooxygenases, which catalyze many reactions involved in drug, steroid, and xenobiotic metabolism and synthesis of cholesterol, steroids, and other lipids. CYP3A4 is involved in metabolism of approximately half the drugs in use |
||
| CYP1A2 | H. sapiens |
CLD_CYP1A2_24hr CLD_CYP1A2_6hr |
Diuron Atrazine Acetochlor Simazine 2,4‐D |
Passive and discrete | |||
| CYP2A1 | NVS_ADME_rCYP2A1 | Diuron | Passive and discrete | ||||
| CYP2B6 |
H. sapiens X. tropicalis |
CLD_CYP2B6_24hr CLD_CYP2B6_48hr CLD_CYP2B6_6hr NVS_ADME_hCYP2B6 |
Diuron Atrazine Metolachlor Acetochlor Simazine Metalaxyl Ametryn Sulfentrazone 2,4‐D |
Passive and discrete | Bupropion degradation | ||
| CYP3A4 |
H. sapiens X. tropicalis |
CLD_CYP3A4_48hr CLD_CYP3A4_6hr |
Metolachlor Metalaxyl Sulfentrazone 2,4‐D |
Passive and discrete | |||
| CYP2J2 |
H. sapiens D. rerio X. tropicalis |
NVS_ADME_hCYP2J2 | Simazined | Discrete only | |||
| HMGCS2 | H. sapiens | CLD_HMGCS2_24hr | 2,4‐D | Passive and discrete | Codes for a mitochondrial enzyme that catalyzes the first reaction of ketogenesis, which provides energy during carbohydrate deprivation (i.e., fasting) | Mitochondrial dysfunction and neurotoxicity (3); calcium‐mediated neuronal reactive oxygen species production and energy imbalance (26); liver X receptor activation leading to liver steatosis (34); ionotropic glutamatergic receptors and cognition (48); nAChR activation contributes to colony death (77‐80, 87, 178); phospholipase A inhibitors lead to hepatotoxicity (130); lysosomal uptake induced liver fibrosis (144); vitamin K epoxide reductase inhibition resulting in coagulopathy (187); estrogen receptor activation leading to breast cancer (200); basal cytotoxicity (205); NADPH oxidase activation leading to reproductive failure (207); reactive oxygen species production leading to population decline (238) | |
| NFE2L2 |
H. sapiens D. rerio X. tropicalis |
ATG_NRF2_ARE_CIS_up |
Metolachlor Acetochlor |
Passive and discrete |
Transcription factor that regulates genes containing antioxidant response elements. Many genes encode proteins involved in response to injury and inflammation and cellular response to oxidative stress |
NFE2L2/FXR activation leading to hepatic steatosis (61) | |
| NR1I2 |
H. sapiens D. rerio X. tropicalis |
ATG_PXRE_CIS_up |
Acetochlor Atrazine Metolachlor Metolachlor ESA Sulfentrazonep Bentazoned Azoxystrobind Simazined |
Passive and discrete | Transcription factor regulating CYP3A4, which is involved in xenobiotic metabolism (see above) | Angiogenesis | Nuclear receptor induced thyroid hormone catabolism and developmental hearing loss (8); pentachlorophenol acute response by percellome (11); pregnane X receptor activation to steatosis (60) |
| PDE4A |
H. sapiens D. rerio X. tropicalis |
NVS_ENZ_hPDE4A1 |
Atrazine Simazine |
Passive and discrete |
Member of the cyclic nucleotide phosphodiesterase family, which hydrolyze the second messenger cAMP. Mediates a number of cellular responses to extracellular signals and plays a role in many important physiological processes |
||
| AR |
H. sapiens D. rerio X. tropicalis |
ACEA_AR_agonist_80hr | Triclopyrd | Discrete only |
Steroid‐hormone activated transcription factor for androgen responsive genes. Androgens (e.g., testosterone) are involved in reproduction, sexual differentiation, and many important physiological processes |
Gonadotropin releasing hormone receptor pathway | Androgen receptor agonism leading to reproductive dysfunction in fish (23) and hepatocellular adenomas and carcinomas in mice (117) |
| GLI3 |
H. sapiens D. rerio X. tropicalis |
TOX21_SSH_3T3_GLI3_Antagonist | Atrazined | Discrete only |
DNA‐binding transcription factors that mediates Sonic hedgehog signaling. It is also thought to play a role during embryogenesis |
Hedgehog signaling pathway | |
| PTGS2 |
H. sapiens D. rerio X. tropicalis |
NVS_ENZ_oCOX2 | Atrazined | Discrete only |
Codes for a cyclooxygenase, which is a key enzyme in prostaglandin biosynthesis and acts both as a dioxygenase and as a peroxidase. Involved in inflammation and mitogenesis |
Inflammation mediated by chemokine and cytokine signaling pathway; toll receptor, endothelin, and CCKR signaling pathways | Cyclooxygenase inhibition leading to reproductive failure or dysfunction (28, 63, 100‐103); gastric ulcer formation (217), aryl hydrocarbon receptor mediated mortality, via COX‐2 (21) |
Chemicals were detected in polar organic chemical integrated samplers (POCIS) or discrete water samples in 15 Great Lakes tributaries in June–July 2016. Listed chemicals contributed at least 10% of the EARMixture value in at least two samples. Suffixes after chemical names represent chemicals that only contributed to an EARMixture threshold exceedance in discrete (“d”) or passive (“p”) samples. Gene effects, functional annotations, and pathways are summarized based on the Database for Annotation, Visualization, and Integrated Discovery (DAVID; https://david.ncifcrf.gov), Panther Classification System (http://www.pantherdb.org), and AOP‐wiki (https://aopwiki.org/).
2,4‐D = 2,4‐dichlorophenoxyacetic acid; H. sapiens = humans; D, rerio = zebrafish; X. tropicalis = Western clawed frog.
Figure 5.
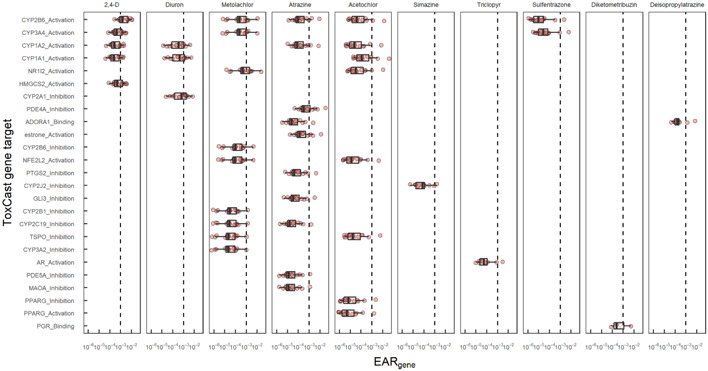
Exposure activity ratios (EARs) by site across chemicals and ToxCast assay endpoint gene targets for pesticides and pesticide transformation products detected in passive samplers from 15 Great Lakes tributaries, June–July 2016. Chemicals represent those that had an EAR exceeding the threshold of 10–3 for at least one endpoint in at least one site. Boxes were only plotted for combinations of chemicals and genes that exceeded the EAR threshold in at least one site. Each boxplot shows the distribution of EARs among sites and endpoints. The upper and lower edges are the 25th and 75th percentiles, and whiskers are drawn up to 1.5 times the interquartile range. Scanning the figure vertically highlights the specific genes linked to a chemical individually and scanning horizontally highlights the multiple chemicals associated with a given gene.
The specific functions of genes associated with EAR threshold exceedances include a wide range of cellular functions, including xenobiotic metabolism, extracellular signaling, and ketogenesis, as well as coding transcription factors (i.e., molecules that regulate other genes) and proteins to protect against oxidative stress (Table 3). Extended results for the genes that exceeded an EARgene of 10–3 in at least two samples are provided in Supporting Information, Table SI‐7. Of the 10 genes with EARgene exceedances at two or more sites in POCIS, five were related to cytochrome P450 isoenzymes (CYPs) that help to metabolize xenobiotics and endogenous cellular compounds (Mansuy, 1998). Other genes exceeding EARgene thresholds included an adenosine receptor (ADORA1), a signal transducer (PDE4A), a catalyst of ketogenesis (HMGCS2), and transcription factors involved in detoxification (NR1I2 or pregnane X receptor [PXR]) and antioxidant responses (NFE2L2). An additional four genes (CYP2J2, GLI3, PTSG2, and AR) were prioritized using EARgene based on discrete samples (Table 3). These genes had EARgene values greater than 10–3 in discrete samples from at least two sites and in one POCIS (Figure 6). Thus, the mixture analyses using the two sampling methods provided a similar projection of the potential biological effects from pesticide contamination.
Figure 6.
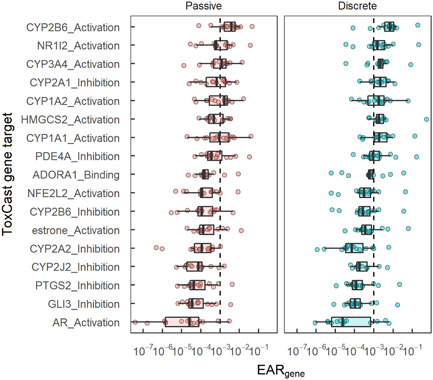
Exposure activity ratios (EARs) from mixtures of pesticides and pesticide transformation products by site and across ToxCast assay gene targets (EARgene) for passive (left) and discrete water (right) samples collected in 15 Great Lakes tributaries, June–July 2016. Assays included had EARgene > 10–3 in at least two sites for either method. Genes are ordered by median EARgene among all sites, endpoints, and methods. For one endpoint without a specific gene target, the gene name was replaced with the hormone induced by the ToxCast assay (i.e., estrone). The upper and lower edges are the 25th and 75th percentiles, and whiskers are drawn up to 1.5 times the interquartile range.
Overall, four PANTHER pathways and 23 AOPs were connected to EAR threshold exceedances from POCIS (Table 3 and Supporting Information, Table SI‐7 and Supporting Information, Figure SI‐9). Two of the PANTHER pathways linked to EARMixture threshold exceedances were angiogenesis (blood vessel growth) and bupropion degradation connected to the NR1I2 and CYP2B6 genes, respectively. The other two PANTHER pathways identified relate to G‐protein signaling pathways connected to the ADORA1 gene. Of the 23 AOPs prioritized using EARAOP, 21 were directly linked with a single priority assay endpoint and gene (Table 3). Four of the AOPs were linked to the transcription factors (NR1I2 and NFE2L2), two of which describe nuclear receptor activation (PXR and farnesoid X receptor) leading to hepatic steatosis, which is a relevant response impacting xenobiotic metabolism. The remaining 17 AOPs were prioritized based on a single assay endpoint, “CLD_HMGCS2_24hr,” which targets the HMGCS2 gene. These AOPs describe connections between mitochondrial dysfunction and energy imbalances associated with human diseases/impairments and adverse behaviors in social insects (Table 3). The two AOPs uniquely prioritized through the EARAOP relate activation of the androgen receptor (AR) to reproductive dysfunction in fish (AOP 23) and hepatocellular adenomas and carcinomas in mice (AOP 117). Both of these AOPs were prioritized in discrete samples through EARMixture threshold exceedances for an endpoint, “ACEA_AR_agonist_80hr,” targeting the AR gene (Table 3). Thus, summing across multiple endpoints (i.e., EARAOP) in the present study resulted in identification of at least one additional apical outcome that is ecologically relevant.
DISCUSSION
Considering detection of chemicals, estimation of concentrations, and comparison with Aquatic Life Benchmarks and ToxCast, 77% of pesticides and pesticide transformation products (172 of 223) analyzed in POCIS in the present study were either not detected or detected with EAR and TQ values below threshold levels. Five chemicals exceeded EAR or TQ thresholds only in the Maumee River, two chemicals exceeded thresholds only in the Saginaw River, and one chemical exceeded thresholds only in the Cuyahoga River (Supporting Information, Figure SI‐7). Ten chemicals were identified as having the greatest potential for biological effects based on TQ or EAR values exceeded thresholds in samples from two or more sites (Figure 2 and Table 2). The majority of these 10 chemicals have been identified at concentrations of concern in previous studies (Ankley et al., 2021; Baldwin et al., 2016; Battaglin et al., 2000; Bradley et al., 2017; Hladik et al., 2018; Metcalfe et al., 2016, 2019; Nowell et al., 2018; Stone et al., 2014) and have documented effects on aquatic organisms (Anderson et al., 2021; Gibbons et al., 2015; Islam et al., 2018; Klementová et al., 2019; Morrissey et al., 2015; Singh et al., 2018; USEPA, 2009a). Through ToxCast‐based EAR calculations, priority chemicals were associated with 20 genes (Figure 5) involved in functions such as xenobiotic metabolism, extracellular signaling, and protection against oxidative stress. Additional information on priority chemicals and their potential biological effects are provided in subsequent sections.
Thirty‐three of the 97 detected compounds—all transformation products—were not evaluated for potential biological effects or included in the mixtures analysis because they lacked screening benchmarks. Others have performed hazard assessments using other databases (e.g., ECOTOXicology Knowledgebase), quantitative structure–activity relationships, or by assuming transformation products have comparable effects as their parent compounds (Capuzzi et al., 2016; Mahler et al., 2021; Oliver et al., 2022; Pronschinske et al., 2022). However, we believe the two screening databases used in the present study were appropriate because they are from credible sources and include all of the detected parent compounds (USEPA, 2019a, 2020). Furthermore, the mixture analysis combined the effects of compounds on specific molecular assays, and it is uncertain if structurally similar chemicals with varying functional groups would elicit similar in vitro responses in these particular assays.
Chemical prioritization differed among benchmarks and chemical classes. Insecticides (i.e., imidacloprid and fipronil) were more frequently prioritized using TQs, whereas herbicides and fungicides were more frequently prioritized using EARs (Figure 2). Ultimately, differences between TQs and EARs relate to the specific Aquatic Life Benchmarks or ACC values used in their calculation (Equations 1(1), (2); Supporting Information, Figure SI‐10). Toxicity quotients for many insecticides were based on invertebrate Aquatic Life Benchmarks, which tended to be one to three orders of magnitude lower than ToxCast ACCs and benchmarks for other taxa (i.e., fish or plants; Supporting Information, Figure SI‐2). It makes sense that fipronil and imidacloprid (two insecticides) were prioritized using invertebrate‐based benchmarks because these two compounds are specifically used to control invertebrate pests. Similarly, plant‐based Aquatic Life Benchmarks tended to be lower than other benchmarks for herbicides, but the difference among benchmark types was less drastic (Supporting Information, Figure SI‐2). For many herbicides, minimum ToxCast ACC values were of a similar magnitude to plant Aquatic Life Benchmarks, thus EAR and TQ calculations were similar. The 100× difference in prioritization thresholds used in the present study (EAR < 10–3 and TQ < 0.1) translates into EARs being a more sensitive screening tool for herbicides and fungicides, whereas TQs were more sensitive for many insecticides (Supporting Information, Figure SI‐10). Although the EAR threshold used in the present study is based on overlap among screening techniques (Corsi et al., 2019), the specific EAR threshold is somewhat arbitrary because it does not imply any apical effects in whole organisms as do TQs. Rather, EARs are useful in a relative sense to prioritize contaminants, predict their apical effects, and offer guidance to future screening that could ultimately be used to generate future Aquatic Life Benchmarks. Using additional risk‐based screening tools (Nowell et al., 2014, 2018) also provides complimentary evaluations of pesticides because no single approach assesses the collective impact of chemicals on the environment.
Priority chemicals
Diuron
Diuron exceeded the EARChem threshold in passive samples from nine sites. Diuron is a phenylurea‐type herbicide used to control a variety of grasses and broadleaf weeds, and is included in antifouling paint as a biocide (Giacomazzi & Cochet, 2004; Lambert et al., 2006). Diuron is persistent in the environment (Harino et al., 2005) and is potentially toxic to fish and aquatic invertebrates (USEPA, 2009a). Diuron inhibits photosynthesis (Knauert et al., 2008) and impairs freshwater biofilms (Morin et al., 2018), potentially making it a concern to nontarget aquatic plants, algae, and other photosynthetic organisms. Diuron also has been reported to have negative effects on oyster development and reproduction through structural and functional modifications to DNA (Akcha et al., 2016; Bachère et al., 2017; Mai et al., 2013). Of the ToxCast assays for diuron, those linked with CYP targets involved in xenobiotic metabolism were the only assays that exceeded EAR thresholds (Figure 5).
A transformation product of diuron, 3,4‐dichloraniline (3,4‐DCA), is not included in the pesticide analytical schedule (Sandstrom et al., 2015) but has known ecotoxicological effects that exceed those of the parent compound (Crossland, 1990; Giacomazzi & Cochet, 2004; Tixier et al., 2001). Currently, the USEPA does not have Aquatic Life Benchmarks for 3,4‐DCA. However, it has been tested in ToxCast, and it was found to be active in several steroidal ToxCast assays (USEPA, 2022a). The ecotoxicological effects of diuron and other pesticides in the environment can be misleading if transformation products are not included (Mahler et al., 2021). The frequent occurrence of diuron in Great Lakes tributaries (the present study) and other aquatic ecosystems (Bradley et al., 2017), the expression of genes important for chemical detoxification, and the known ecotoxicological effects of one of its transformation products indicate that diuron is a contaminant with potential ecological health consequences.
Metolachlor and acetochlor
Metolachlor and acetochlor are two chloroacetanilide herbicides that are frequently detected in surface waters in the midwestern United States (Ankley et al., 2021; Baldwin et al., 2016; Battaglin et al., 2000; Nowell et al., 2018; Stone et al., 2014). In our study, metolachlor and acetochlor exceeded EARChem thresholds in samples from eleven and five sites, respectively. Negative effects of acetochlor include cytotoxicity in human liver cells (Huang et al., 2020) and disruption of thyroid systems in zebrafish (D. rerio) larvae (Yang et al., 2016). Metolachlor affects gene expression related to the thyroid systems in fish (Jin et al., 2011; Rozmánková et al., 2020) and reduces growth rates of crayfish at early life stages (Velisek et al., 2019). At environmentally relevant concentrations, metolachlor affects diatom mobility (Coquillé et al., 2015) and is toxic to marine dinoflagellates (Ebenezer & Ki, 2013).
The specific ToxCast assay endpoints for which metolachlor and acetochlor exceeded EAR thresholds related to 11 different genes (Figure 5 and Supporting Information, Figure SI‐8). Seven CYPs involved in a number of steroid, drug, and other xenobiotic metabolic pathways were linked through EAR threshold exceedances. Three genes, (NR1I2, NFE2L2, and PPARG) are transcription factors predicted to be involved in a number of cellular functions that include response to toxic substances and oxidative stress and peroxisome proliferation (Supporting Information, Table SI‐8). Orthologs for these three genes are present in fish and amphibian genomes, strengthening the molecular evidence that metolachlor and acetochlor could have potential ecological effects on aquatic biota, although the types of effects predicted could reflect both a compensatory as well as an adverse response.
Atrazine and simazine
Atrazine and simazine are members of the triazine family of herbicides used to control a wide range of grassy and broadleaf plants. Atrazine is one of the most widely used pesticides in the world (Singh et al., 2018), and in the United States is primarily used on corn production, but also on sugarcane and grass turf (Wieben, 2020). Although the majority of simazine use is also on major row crops (e.g., corn), it also is used extensively on orchards, vineyards, and berry fields (USEPA, 2019b). Atrazine and simazine were detected in 15 and 13 samples from the present study and in 92% and 41% of discrete water samples, respectively, from these sites over the year (Oliver et al., 2022). This is consistent with detection of atrazine and its transformation products in rainfall and surface waters in pristine areas, such as Isle Royale in Lake Superior (Thurman & Cromwell, 2000) and other US National Parks (Bradley et al., 2020; Mast et al., 2007). Other studies have found atrazine at concerning concentrations in surface waters (Ali & Kolok, 2015; Ankley et al., 2021; Ng & Clegg, 1997), and in agricultural watersheds, concentrations can commonly exceed 10 µg L–1 for over 10% of the calendar year (Lerch et al., 2011). Compared with the USEPA's Aquatic Life Benchmarks, the 21‐day Maumee River POCIS sample exceeded the atrazine benchmark for effects in nonvascular plants (<1 µg L–1) and was near the chronic toxicity benchmark for fish (5 µg L–1; USEPA, 2019a). Atrazine concentrations were within an order of magnitude of the nonvascular plants benchmark at an additional three sites, and seven sites exceeded the EAR threshold of 10–3. Atrazine negatively affects a variety of nontarget aquatic organisms. For example, phytoplankton, lichen microalgae, and aquatic macrophytes are highly sensitive to low concentrations (<0.1 µg L) of atrazine, which can lead to apical outcomes at the organism, population, or community level (Fleming et al., 1995; Pannard et al., 2009; Traba et al., 2017). Detrimental effects are not limited to primary producers (Singh et al., 2018) because atrazine has been linked with endocrine disruption in rats (Cooper, 2000), DNA damage in fish (Nwani et al., 2011), and decreased spatial aggregation of freshwater mussels (Flynn & Spellman, 2009). Furthermore, atrazine affects fish reproduction and egg production at concentrations as low as 0.5 µg L–1 (Moore & Waring, 1998; Richter et al., 2016; Tillitt et al., 2010).
Based on ToxCast data, atrazine appears to influence multiple biological pathways. In our samples, atrazine exceeded EAR thresholds for ToxCast assay endpoints related to nine genes (Figure 5), which was the most of any chemical individually. Genes linked with atrazine EAR exceedances included several CYPs involved in steroid, drug, and xenobiotic metabolism that were also affected by other priority herbicides (Figure 5). Simazine was linked to a different CYP involved in epoxidation. In addition, an endpoint (CEETOX_H295R_ESTRONE_up) relating to estrone production exceeded the EAR threshold for atrazine at three sites (Figure 5). Atrazine was the only chemical linked to estrogen‐related assay endpoints, and the potential modulation of steroid biosynthesis aligns with literature reports of aromatase induction in vitro (Fan et al., 2007; Sanderson et al., 2001) and abnormal sexual development, reproduction, and hormone concentrations in controlled experiments and in field studies with fish (Moore & Waring, 1998; Richter et al., 2016; Tillitt et al., 2010). Other genes (ADORA1, GLI3, MAOA, PDE4A, PDE5A, and PTSG2) connected to atrazine through EAR exceedances have predicted functions that include extracellular signaling, smooth muscle relaxation, and inflammation (Supporting Information, Table SI‐7). Through ToxCast EAR exceedances, multiple genes and predicted biological functions were linked to atrazine concentrations, suggesting that it may have a complex effect on biology and that a single mode of action is unlikely.
The ecotoxicological effects of atrazine and simazine may persist beyond their degradation because both parent and transformation products can share common mechanisms of toxicity (USEPA, 2019b). Several transformation products, such as deisopropylatrazine and deethylatrazine, are included in the ToxCast database and are toxic to aquatic life (Klementová et al., 2019). Our analysis identified deisopropylatrazine as a priority chemical because it exceeded EARChem thresholds in two samples co‐contaminated with atrazine. Deethylatrazine did not exceed EARChem thresholds, but it was detected in all samples. In agricultural settings, losses of deethylatrazine and atrazine can be of similar magnitudes (Shipitalo & Owens, 2003), and both chemicals have been detected in tree swallow (Tachycineta bicolor) diets, eggs, and carcasses (Custer et al., 2020). A third transformation product (deethylhydroxyatrazine) was also detected in all passive samples but could not be evaluated because it lacked data to calculate effects benchmarks (Figure 2). Assessing the ecotoxicology of related chemical mixtures and transformation products remains a challenge, but if multiple compounds have similar modes of action, the combined effects could be magnified.
2,4‐Dichlorophenoxyacetic acid
2,4‐Dichlorophenoxyacetic acid has been used since the 1940s to control a variety of broadleaf plants (Islam et al. 2018). Because it resembles natural auxins, 2,4‐D stimulates plant growth, ultimately causing uncontrolled cell division and death (USEPA, 2005). Due to its acidic carboxyl group, 2,4‐D and its other formulations (salts, esters, etc.) are quite mobile in the environment, leading to widespread occurrence in aquatic ecosystems (Islam et al., 2018; Van Metre et al., 2017; Zuanazzi et al., 2020). Over 1500 herbicide products contain 2,4‐D (Islam et al., 2018; Zuanazzi et al., 2020), including several used to control aquatic nuisance plants (Harrahy et al., 2014). The potential impact of 2,4‐D on aquatic, nontarget species is especially relevant given the USEPA allows direct application of 2,4‐D to aquatic ecosystems up to 4000 µg L–1 as a spot treatment for Eurasian watermilfoil and other aquatic plants (Harrahy et al., 2014; USEPA, 2005).
The potential adverse effects of 2,4‐D contamination include reproductive disorders, genetic alterations, and carcinogenic effects (see reviews by de Castro Marcato et al., 2017; Islam et al., 2018; Zuanazzi et al., 2020). Mound building termites and lady beetles exposed to recommended treatments of 2,4‐D had significant mortality and (or) impaired locomotion (Ejomah et al., 2020; Freydier & Lundgren, 2016). Exposure of amphibians to 2,4‐D can arrest egg development and induce oxidative stress (Aronzon et al., 2011; Lajmanovich et al., 2015; Stebbins‐Boaz et al., 2004). Early life stages of multiple fish species are particularly sensitive to 2,4‐D exposure at concentrations (500–2000 µg L–1) below the current 4000 µg L–1 spot treatment USEPA limit (USEPA, 2005), which has implications for fish populations in lakes treated for invasive plants (Dehnert et al., 2018, 2020). In the present study, 2,4‐D was detected in samples from 14 of the 15 sites, and it exceeded the EARChem threshold in 13 samples, which was the most of any chemical analyzed (Figure 2 and Table 2). Four of the ToxCast assays exceeding the EAR threshold measure CYP induction (Table 3), suggesting a plausible connection of 2,4‐D exposure to cellular oxidation and potential tissue damage (Goetz & Luch, 2008; Lackner, 1998). With the introduction and expansion of crops resistant to 2,4‐D, increased use and environmental detections of 2,4‐D are likely, as has been shown with other herbicide‐resistant crops (Benbrook, 2016). Furthermore, with the direct application to aquatic environments, 2,4‐D will likely remain a priority pesticide contaminant which has the potential to adversely affect a variety of nontarget organisms (Islam et al., 2018; Zuanazzi et al., 2020).
Sulfentrazone
Compared with the other priority herbicides in the present study, there are fewer reports of the environmental impact of the herbicide sulfentrazone. Sulfentrazone was first registered in the United States in 1997, and it is used as broad‐spectrum pre‐emergent herbicide. On exposure to light, sulfentrazone causes plant foliage dehydration and loss of membrane integrity by inhibiting protoporphyrinogen oxidase (Dayan & Watson, 2011; Thorngren et al., 2017). Protoporphyrinogen oxidase is involved in biosynthesis of chlorophyll (in plants) and hemoglobin (in animals), and in the presence of light can lead to porphyria (Birchfield & Casida, 1997). Thus, in clear or shallow waters, the effect of sulfentrazone on fish and other vertebrates may be enhanced. According to Thorngren et al. (2017), relatively few studies have tested the effects on nontarget aquatic species besides those conducted by the USEPA during the registration process (USEPA, 2009b). At that time, chronic and acute risk assessments for mammals, fish, and freshwater invertebrates were below the USEPA's level of concern (USEPA, 2009b). More recently, sulfentrazone has been shown to alter antioxidant enzymes in amphibians (Freitas et al., 2017) and enhance production of reactive oxygen species in earthworms (Li et al., 2020). Interestingly, the only endpoints exceeding EAR thresholds for sulfentrazone target CYPs (Figure 5), which can also be involved in the production of oxidative stress (Werck‐Reichhart & Feyereisen, 2000).
Imidacloprid
Imidacloprid concentrations were above the USEPA's chronic invertebrate Aquatic Life Benchmark of 0.01 µg L–1 in samples from eight tributaries. Imidacloprid, which was first registered in the United States in 1994, and other neonicotinoids are applied as seed coatings and used to control terrestrial insects on a broad range of agricultural crops and are one of the most widely used classes of insecticides in the world (Bass et al., 2015; Simon‐Delso et al., 2015). Neonicotinoids act on insect nicotinic acetylcholine receptors (nAChRs) via competitive modulation (USEPA, 2016), thus interfering with the central nervous systems of insects (Anderson et al., 2015; Goulson, 2013). Several studies have linked imidacloprid with adverse ecological outcomes (Gibbons et al., 2015; Pisa et al., 2015; Whitehorn et al., 2012), including changes in feeding and delayed migration of songbirds (Eng et al., 2019). Imidacloprid and other neonicotinoid pesticides are suspected of contributing to honey bee (Apis mellifera) losses (Henry et al., 2012; Mitchell et al., 2017; Whitehorn et al., 2012; Wu et al., 2017) and are toxic to a wide range of arthropods (Morrissey et al., 2015). The aquatic invertebrate orders of Ephemeroptera, Trichoptera, and Diptera are especially sensitive to chronic and acute exposure to imidacloprid and other neonicotinoids (Bartlett et al., 2018; Morrissey et al., 2015) at concentrations as low as 0.1 µg L–1 (Roessink et al., 2013). In 2017, the USEPA lowered the chronic toxicity benchmark of imidacloprid from 1.05 to 0.01 µg L–1 (USEPA, 2019a), and since 2013, the European Union has restricted its use in outdoor settings (Stokstad, 2018). Imidacloprid continues to be a chemical of concern in the Great Lakes and other areas where it is applied (Stackpoole et al., 2021).
Imidacloprid only exceeded EARChem thresholds at one site (summation of multiple endpoints), and no ToxCast endpoints exceeded EAR thresholds for imidacloprid alone. The two endpoints with the lowest ACC values for imidacloprid relate to mammalian genes (CHRNA2 and CHRNA7), which code for neuronal nicotinic acetylcholine receptors (nAChRs). These proteins play important roles in chemical signaling between neurons, are involved in fast synaptic transmission across ion channels, and are widely distributed in the nervous system (Le Novère et al., 2002). The active ToxCast endpoints for imidacloprid align with the function of imidacloprid because it, and other neonicotinoids, are structurally similar to nicotine, are designed to target nAChRs in insects, and disrupt their central nervous systems. Although the ToxCast endpoints based on mammalian genes for imidacloprid did not exceed EAR thresholds, the molecular evidence within ToxCast aligns with the known mode of action, although the magnitudes and effects likely vary among species.
Fipronil
Fipronil is one of the most widely used broad‐spectrum insecticides globally (Simon‐Delso et al., 2015; van der Sluijs et al., 2015). Fipronil, which was first registered for use in the United States in 1996, is used in topical pet care products and home roach traps, as well as in field settings on corn production, turf grass, and golf courses (Baker & Stone, 2014; Jackson et al., 2009). Similar to imidacloprid, fipronil interferes with the nervous system, acting on a ligand‐gated ion channel (specifically the GABA‐gated chloride channel) and has selective toxicity for insects relative to mammals (Hainzl et al., 1998). However, fipronil is highly toxic to a range of aquatic organisms, including aquatic insects, crayfish, and fish (Gunasekara et al., 2007; Miller et al., 2020). Furthermore, the toxicity of several of its transformation products (e.g., fipronil sulfone, fipronil sulfide, and desulfinyl fipronil) can exceed fipronil itself (Gunasekara et al., 2007; Jackson et al., 2009; Weston & Lydy, 2014; Schlenk et al., 2001). Fipronil and (or) its transformation products have been implicated in the collapse of a managed crayfish population (Bedient et al., 2005) as well as being a risk to honey bees (European Food Safety Authority, 2013) and aquatic macroinvertebrates (Miller et al., 2020). Fipronil and its transformation products have been detected frequently in US surface waters (Mahler et al., 2021; Stone et al., 2014; Van Metre et al., 2017), and in urban streams concentrations frequently exceed Aquatic Life Benchmarks (Miller et al., 2020; Nowell et al., 2018; Stone et al., 2014). In the present study, fipronil was detected in all POCIS samples; fipronil transformation products were detected in 14 of the 15 samples, and concentrations tended to be highest in samples collected in urban watersheds. The occurrence of fipronil in aquatic systems often overlaps with human development, wastewater treatment facilities, and golf courses (McMahen et al., 2016; Nowell et al., 2018; Wu et al., 2015), making the potential effects of fipronil more likely in urban waterways as opposed to streams and rivers in agricultural landscapes.
Other chemicals
Although our results highlight concerns with some specific chemicals, other compounds should not be assumed to have negligible effects. First, we specifically sampled in June and early July, and our prioritization may have missed compounds present at higher concentrations during other times of the year. Although pesticide detections in the region tend to highest in June (Covert et al., 2020), the composition of pesticide mixtures changes temporally. Pesticides were detected year‐round in discrete water samples (Oliver et al., 2022), and 12 chemicals were detected in discrete water samples outside of June and July that were not detected in POCIS. Second, only 225 compounds were analyzed in passive samples, yet over 400 pesticide compounds were applied in the conterminous United States between 2013 and 2017 (Wieben, 2020). The specific pesticides included in the pesticide analytical schedule were strategically identified in a prioritization analysis that assessed likelihood of occurrence, prevalence of use, and potential toxicity (Shoda et al., 2018). However, the method does not work for all types of pesticides, and several compounds were absent from the analysis. For example, glyphosate is one of the most widely used pesticides in the United States and Canada (Anderson et al., 2021; Benbrook, 2016; Wieben, 2020) but is not included in the pesticide analytical schedule because it is difficult to isolate (Meyer et al., 2009; Sandstrom et al., 2015). Third, toxicity evaluations continually change and benchmarks can be revised. If future toxicological evaluations include a larger variety of species or biochemical targets, some Aquatic Life Benchmarks or chemical assay endpoints could be revised, potentially changing the list of priority chemicals of concern. For example, in 2017 the chronic freshwater invertebrate Aquatic Life Benchmark for imidacloprid was reduced by two orders of magnitude (USEPA, 2019a), in part from studies reporting chronic effects in aquatic organisms well below previous benchmarks (Roessink et al., 2013). Using the original benchmark, only the Maumee River would have been flagged as a priority site for imidacloprid, but under the revised benchmark, samples from 13 sites had concerning concentrations. It is also noteworthy that with no representation of plants or plant‐specific pathways, it is quite likely that ToxCast‐based prioritization may underestimate the biological activity of herbicides designed specifically to target plants. Pesticides are currently re‐reviewed by the USEPA on 15‐year cycles, and it could be useful to reevaluate chemicals detected at levels below current thresholds as new information becomes available.
Many of the chemicals detected in the present study could not be evaluated for potential biological effects. To be evaluated, the chemical needed to (1) be detected, (2) have a known POCIS uptake rate, and (3) be included in a screening database to allow benchmark calculation. Of the 33 detected chemicals not evaluated for potential biological effects, two lacked POCIS uptake rates and all 33 lacked a screening benchmark. Thus, the primary limitation was the lack of effects benchmarks. The detected compounds that lacked benchmarks all were transformation products, and their occurrences tended to overlap with the parent compounds. Co‐occurrence of pesticides and transformation products has potential for synergistic, antagonistic, and additive interaction responses (Stratton, 1984), so an emphasis on developing toxicological benchmarks for transformation products with widespread occurrence could be warranted (Mahler et al., 2021).
Although the focus of the present study was to prioritize pesticides of ecological concern, most pesticides analyzed in the present study (172 of 223) were either never detected or concentrations were below thresholds (Supporting Information, Table SI‐1) and thus they were not found to be of concern. The use of many pesticides has declined substantially in part due to increased awareness of toxicological effects and resulting stronger regulations. For example, chlorpyrifos and diazinon are two organophosphate insecticides that were heavily restricted in the United States in the early 2000s to reduce potential risks to humans (USEPA, 2002, 2004). Chlorpyrifos was listed as one of the top seven priority pesticides in a recent risk assessment of aquatic ecosystems in Canada (Anderson et al., 2021). Many organophosphate insecticides are neurotoxins (Jokanović, 2018) that still pose an occupational hazard for farm workers and children (Curl et al., 2002; Curwin et al., 2007; Morgan et al., 2005). Although diazinon was detected in two passive samples, it is encouraging that these organophosphates were not found in concentrations of concern in the present study or in discrete water samples (Supporting Information, Figure SI‐5). With changing regulations and pesticide applications, additional monitoring is not only valuable to identify new contaminants of concern and develop new analytical methods (Norman et al., 2012), but also to deprioritize chemicals previously of concern.
Potential effects of chemical mixtures
The EAR‐based approach for evaluating chemical mixtures identified 16 priority ToxCast assay endpoints that exceeded EARMixture thresholds in at least two passive samples (Table 3 and Supporting Information, Figure SI‐9). These mixture‐assay pairs were linked with 10 unique genes and 23 AOPs (Table 3). Nine of the genes prioritized with the mixtures analysis have functional annotations described in online genome databases (e.g., DAVID) that relate to a number of cellular and physiological responses and biological pathways. The libraries of biological responses within ToxCast, the AOP knowledgebase, and DAVID are by no means exhaustive and only represent a small subset of potential systems influenced by chemicals in nature. These results also do not include mixture effects from other types of chemical contaminants (e.g., polycyclic aromatic hydrocarbons [PAHs], pharmaceuticals). The gene descriptions linked to detected chemicals discussed below are intended to provide additional context to some of the potential cellular and organismal interactions of pesticide mixtures, however, additional experiments and evidence are needed to fully document the specific chemical effects on aquatic organisms.
Many of the endpoints and genes linked to EAR exceedances can be related to the stimulation of xenobiotic metabolism or protective mechanisms (Table 3), which often is viewed as a compensatory or adaptive response in organisms exposed to foreign compounds. For example, the activation of transcription factors coded by the NR1I2 and NFE2L2 genes further induces genes involved in detoxification and oxidative stress responses, respectively (Table 3). NR1I2 and NFE2L2 were linked through ToxCast assay endpoints to metolachlor and acetochlor (individually and as a mixture), suggesting that the metabolism of these chemicals may invoke common cellular machinery. Comparatively, the gene PTSG2 also includes antioxidant annotations that code for different enzymes (i.e., dioxygenase and peroxidase). The gene PTSG2 was only linked to atrazine (Figure 5), suggesting alternative cellular oxidative stress protective responses compared with metolachlor and acetochlor. Cocontamination with atrazine, metolachlor, and acetochlor may lead to compounded oxidative stress within aquatic organisms that may lead to oxidative damage to tissues (Goetz & Luch, 2008; Lackner, 1998).
A number of CYP genes were frequently associated with EAR exceedances. This superfamily of genes can be found in the genomes of virtually all organisms (Werck‐Reichhart & Feyereisen, 2000). Cytochrome P450 isoenzymes catalyze oxidative metabolism of chemicals, leading to breakdown of various molecules within cells, which can facilitate the elimination of exogenous molecules (Mansuy, 1998; Zanger & Schwab, 2013). The specific CYPs associated with EAR exceedances are involved in a variety of biological pathways, including biosynthesis of steroids and metabolism of proteins, fatty acids, drugs, and other xenobiotics (Supporting Information, Table SI‐7). All seven priority herbicides contributed to EARMixture and EARgene threshold exceedances for CYP‐related assay endpoints (Figure 5), suggesting some degree of overlap in the cellular response to these xenobiotics and potential for compounding oxidative stress, which can be associated with the activity of CYPs. Collectively, the activation of transcription factors and enzymes related to xenobiotic metabolism aligns with the plausible effects expected with exposure to foreign compounds.
The priority endpoint CLD_HMGCS2_24hr exceeded the EARMixture threshold at five sites and was only linked to 2,4‐D. This endpoint measures mRNA induction as it relates to the HMGCS2 gene (USEPA, 2020), which is involved in cholesterol biosynthesis and mitochondrial ketogenesis (Vilà‐Brau et al., 2011; Wang et al., 2019). Ketogenesis provides lipid‐derived energy for various organs during times of carbohydrate deprivation, such as fasting (Geisler et al., 2019; Hegardt, 1999). The HMGCS2 gene was mapped to 17 AOPs (Table 3) through 10 key events mostly relating to mitochondrial dysfunction (AOP Wiki, 2016). Interestingly, four of these AOPs (#77–80) relate to abnormal behavior in social insects (bees), associated with colony loss or death (LaLone et al., 2017). Although these AOPs are still under development (LaLone et al., 2017), their descriptions align with other studies highlighting the potential risk of 2,4‐D on insects (Ejomah et al., 2020; Papaefthimiou et al., 2002).
Another group of genes linked with elevated EARs is related to cell signaling and endocrine function: the ADORA1 and PDE4A genes were connected to atrazine and one of its transformation products (Table 3). Both genes are involved in signaling through G protein‐coupled receptors (Amisten et al., 2008; Conti et al., 2003). G protein‐coupled receptors are typically embedded in cell membranes and involved in the transmission of extracellular signals inside the cell (Maurice et al., 2011). G protein‐coupled receptors (or their downstream signals) are the biochemical targets of many prescription drugs, including opiates (Zhao & Furness, 2019). These receptors are involved in a diverse set of physiologic functions, including responses to physiological stimuli, such as light, odors, taste, and other intercellular signals (Bockaert et al., 2010).
Another priority endpoint CEETOX_H295R_ESTRONE_up indicates increased production of estrone, an estrogenic steroid that is a precursor and metabolite of 17β‐estradiol (USEPA, 2020). Increased estrone production could suggest either increased aromatase activity or decreased 17β‐hydroxysteroid dehydrogenase activity. Atrazine was the only chemical linked to this endpoint, highlighting the potential effects it may have on sexual development and reproduction, neuroendocrine, vascular, skeletal, and immune systems (Hamilton et al., 2017). Atrazine increases aromatase in human granulosa cells (Holloway et al., 2008) and causes aromatase induction in H259R cells (Sanderson et al., 2001), which is the cell line used in the CEETOX assay. Atrazine‐induced aromatase expression may be an steroidogenic factor 1 (SF‐1) dependent process (Fan et al., 2007), and this mechanism was suggested to be phosphodiesterase inhibition (Roberge et al., 2004). However, atrazine‐induced aromatase activity or associated effects have generally not been observed in vivo.
A second endocrine‐related gene was prioritized after incorporating the discrete water samples. The endpoint ACEA_AR_agonist_80hr is designed to measure signaling activity related to an androgen receptor coded by the AR gene. Androgens, such as testosterone, are widely recognized for their importance in sexual development and differentiation but also play roles in metabolism, growth, development, and behavior and act as an intercellular signal (Bhasin et al., 2007; Monks & Holmes, 2018; Sumpter, 2005). Agonism of the androgen receptor is listed as a molecular initiating event in AOP #23, leading to reproductive dysfunction in fish (Villeneuve, 2021). The AR gene has been linked through ToxCast EAR exceedances with two pharmaceutical steroids from fish plasma samples in a Croatian river (Malev et al., 2020) and dl‐menthol (a common toothpaste and cosmetic additive) in another passive‐sampler based study in the Great Lakes (Alvarez et al., 2021). In our study, the herbicide triclopyr was the only chemical linked to AR through EAR exceedances (Table 3); however, triclopyr was listed as AR negative using the ToxCast AR computation model (Kleinstreuer et al., 2017). Furthermore, triclopyr did not yield a single active hit in the ToxCast EDSP 21 assays (USEPA, n.d.) that are specifically designed to detect endocrine disrupting activities (Rotroff et al., 2013), providing evidence that the ACEA_AR_agonist_80hr activity of triclopyr may not be a real AR response. There is general concern with chemicals that may interact with androgen and estrogen signaling (Rotroff et al., 2013), as reflected by chemical safety legislation, and ToxCast may be useful to guide future testing on chemicals that may disrupt endocrine functions.
The POCIS‐ and discrete‐sample‐based (Oliver et al., 2022) mixture analyses identified similar lists of priority chemicals, assay endpoints, and genes. The discrete sample analysis identified more priority genes (Table 3) due to slightly higher chemical concentrations, causing some sites to only have discrete‐based EAR exceedances (Figure 6 and Supporting Information, Figure SI‐11). Lower POCIS concentrations than filtered water samples may reflect methodological differences in accumulation because POCIS mimics cellular uptake of chemicals in the dissolved phase (Huckins et al., 2006), which may be more analogous to chemical exposure of aquatic organisms without consideration of metabolism and (or) excretion. Temporal variation may also contribute to differences between POCIS and discrete sample‐based results. Concentrations, detection frequencies, and assemblages of pesticides and transformation products can differ between baseflow and runoff conditions (Mahler et al., 2021). Polar organic chemical integrative samplers may be exposed to long periods of baseflow, which may not align with the hydrological conditions and chemistry when discrete water samples were collected. In addition, build‐up of particulates on the passive sampler surface over time could result in decreased sampling at the latter stages of the deployment and therefore lower estimated water concentrations. Overall, POCIS‐ and discrete sample‐based results offer similar qualitative conclusions about pesticide presence/contamination (Figure 6 and Table 3), but the POCIS‐derived results offer a less conservative view than that from water samples due to slightly lower estimated concentrations.
Site‐specific assessments
Chemical detection frequency and concentration varied among watersheds, aligning with patterns in land use (Figure 1). Comparison of the POCIS and discrete (Oliver et al., 2022) results to land cover provided patterns consistent with previous research, which reported greater concentrations and numbers of pesticides detected in watersheds with greater amounts of agricultural and urban land (Battaglin et al., 2016; Stone et al., 2014; Van Metre et al., 2017). This is also consistent with a greater density of pesticide applications in urban and agricultural settings (Wieben, 2020), posing a greater potential hazard to streams and rivers with these land uses (Nowell et al., 2018; Stackpoole et al., 2021; Stone et al., 2014).
The greatest overall concentrations and greatest number of pesticides were detected in the Maumee River sample. From a chemical mixture perspective, 16 chemicals contributed to 34 EARMixture threshold exceedances that were collectively associated with 21 genes and 37 AOPs (Supporting Information, Table SI‐8). In addition to the AOPs in Table 3, several AOPs linking the PTSG2 gene and cyclooxygenase activity with reproductive dysfunction and early life mortality in fish (AOPs #21, 28, 63, and 100–103) were only identified in the Maumee River sample. Prior studies in the Maumee River system have associated pesticides with embryonic deformations and reduced fecundity (Cipoletti et al., 2019) and induction of hepatic CYP genes in caged fish (Ankley et al., 2021). Contamination of the Maumee is not restricted to surface waters or pesticides because PAHs and other contaminants in sediments and porewaters of multiple Maumee tributaries also exceed EAR and TQ‐based thresholds (Baldwin et al., 2022), which may contribute to additional mixture effects. In addition, the Maumee is one of the most agriculturally productive watersheds in the Great Lakes basin, and it contributes substantial loads of nutrients, suspended sediment, and contaminants to western Lake Erie (Baker et al., 2014; Cipoletti et al., 2020; Matson et al., 2020). Although nutrient loading and other physical mechanisms are likely the dominant driver of increased prevalence of harmful cyanobacteria blooms in western Lake Erie (Bullerjahn et al., 2016; Sayers et al., 2019), pesticides—especially herbicides—may favor cyanobacteria and promote harmful blooms by suppressing the growth of more favorable eukaryotic phytoplankton species (Harris & Smith, 2016).
Even though strong linkages were identified between land use and pesticide contamination, chemicals were also present in samples collected from sites draining less disturbed Lake Superior watersheds. Six herbicides (metolachlor, atrazine, acetochlor, sulfentrazone, prometon, and hexazinone), one insecticide (fipronil), and three transformation products of atrazine were detected in all samples, including those from the St. Louis and Bad Rivers. These rivers have watersheds with less than 10% urban or agricultural land cover, which may be sufficient to result in detectable levels of pesticides to rivers, or pesticides may be transported across watershed boundaries through atmospheric dispersion. Polar organic chemical integrative sampler collections from the St. Louis and Bad Rivers had twice as many chemical detections as concurrent discrete water samples (Supporting Information, Figure SI‐8). Minimum detection levels for POCIS samples were approximately two orders of magnitude lower than discrete samples (Supporting Information, Figure SI‐1) because passive samplers continually absorb contaminants over their deployment, which are concentrated into a final extract that represents accumulated chemicals over a long duration. In addition, detection frequencies of pesticides in discrete water samples are likely biased low (Medalie & Bexfield, 2020), and samples are typically collected over a brief time window (seconds to minutes) that may not represent the extremes or short‐term pulses of concentration that POCIS are exposed to over a longer time. For example, nearly three times as many pesticides can be detected using daily water samples compared with weekly samples (Norman et al., 2020). Thus, POCIS provide surveillance for chemicals at low concentrations or high temporal variability and may provide relevant information for chemicals that have greater potency.
CONCLUSION
Evaluation of chemicals using ToxCast and Aquatic Life Benchmark comparison techniques provided valuable information for identifying chemicals of concern in the Great Lakes watershed. Many of the priority chemicals share activity in common ToxCast assays, highlighting the potential for compounding biological effects from mixtures of pesticides and transformation products, which supports using additive models for assessing potential toxicity of pesticide mixtures (e.g., Nowell et al., 2014). The potential activity on biological pathways related to a range of cellular processes including xenobiotic metabolism, extracellular signaling, endocrine function, and protection against oxidative stress, which may have consequences for nontarget aquatic species at the organism, population, or community level. Although these techniques do not provide direct evidence of modifications to molecular functions in the environment as a result of chemical exposure, the gene annotations and biological pathways described above provide a clue into how specific chemicals may interact with molecular biology, which could lead to adverse outcomes. The EAR‐based method may be used to guide additional hypothesis‐driven monitoring and experimentation, which are necessary to confidently link chemicals with adverse effects in the natural watershed setting. Complete understanding of the impact of pesticides and other chemical contaminants will likely remain an important knowledge gap, thus additional testing and development of evaluation techniques will likely be valuable to protect the health and function of the streams, rivers, and other aquatic ecosystems.
Author Contributions Statement
Luke C. Loken: Conceptualization; Formal analysis; Investigation; Software; Visualization; Writing—original draft; Writing—review & editing. Steven R. Corsi: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing—original draft; Writing—review & editing. David A. Alvarez: Data curation; Formal analysis; Investigation; Methodology; Writing—review & editing. Gerald T. Ankley: Conceptualization; Formal analysis; Investigation; Project administration; Validation; Writing—review & editing. Austin K. Baldwin: Conceptualization; Data curation; Formal analysis; Investigation; Writing—review & editing. Brett R. Blackwell: Conceptualization; Formal analysis; Investigation; Validation; Writing—review & editing. Laura A. De Cicco: Formal analysis; Investigation; Software; Visualization; Writing—review & editing. Michele A. Nott: Funding acquisition; Project administration; Writing—review & editing. Samantha K. Oliver: Formal analysis; Investigation; Software; Visualization; Writing—review & editing. Daniel L. Villeneuve: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Validation; Writing—review & editing.
This article has earned an Open Data badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://doi.org/10.5066/P9QOMM22. Learn more about the Open Practices badges from the Center For Open Science: https://osf.io/tvyxz/wiki.
Supporting information
This article contains online‐only Supporting Information.
Supporting File.
Supporting File.
Data Availability Statement
Data, associated metadata, and calculation tools are also available from the corresponding author (lloken@usgs.gov).
REFERENCES
- Adverse Outcome Pathways Wiki . (2016). Event: 177. Mitochondrial dysfunction, N/A. https://aopwiki.org/wiki/index.php/Event:177
- Akcha, F. , Barranger, A. , Bachère, E. , Berthelin, C. H. , Piquemal, D. , Alonso, P. , Sallan, R. R. , Dimastrogiovanni, G. , Porte, C. , Menard, D. , Szczybelski, A. , Benabdelmouna, A. , Auffret, M. , Rouxel, J. , & Burgeot, T. (2016). Effects of an environmentally relevant concentration of diuron on oyster genitors during gametogenesis: Responses of early molecular and cellular markers and physiological impacts. Environmental Science and Pollution Research, 23(8), 8008–8020. 10.1007/s11356-015-5969-2 [DOI] [PubMed] [Google Scholar]
- Ali, J. M. , & Kolok, A. S. (2015). On‐site, serial exposure of female fathead minnows to the Elkhorn River, Nebraska, USA, spring agrichemical pulse: Serial exposure of fathead minnows to 2013 agrichemicals. Environmental Toxicology and Chemistry, 34(6), 1354–1361. 10.1002/etc.2928 [DOI] [PubMed] [Google Scholar]
- Alvarez, D. A. (2010). Guidelines for the use of the Semipermeable Membrane Device (SPMD) and the Polar Organic Chemical Integrative Sampler (POCIS) in environmental monitoring studies (US Geological Survey Techniques and Methods Report No. Techniques and Methods 1–D4). 10.3133/tm1D4. [DOI]
- Alvarez, D. A. , Corsi, S. R. , De Cicco, L. A. , Villeneuve, D. L. , & Baldwin, A. K. (2021). Identifying chemicals and mixtures of potential biological concern detected in passive samplers from great lakes tributaries using high‐throughput data and biological pathways. Environmental Toxicology and Chemistry, 40, 2165–2182. 10.1002/etc.5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, D. A. , Cranor, W. L. , Perkins, S. D. , Clark, R. C. , & Smith, S. B. (2008). Chemical and toxicologic assessment of organic contaminants in surface water using passive samplers. Journal of Environmental Quality, 37(3), 1024–1033. 10.2134/jeq.2006.0463 [DOI] [PubMed] [Google Scholar]
- Amisten, S. , Braun, O. Ö. , Bengtsson, A. , & Erlinge, D. (2008). Gene expression profiling for the identification of G‐protein coupled receptors in human platelets. Thrombosis Research, 122(1), 47–57. 10.1016/j.thromres.2007.08.014 [DOI] [PubMed] [Google Scholar]
- Anderson, J. C. , Dubetz, C. , & Palace, V. P. (2015). Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Science of the Total Environment, 505, 409–422. 10.1016/j.scitotenv.2014.09.090 [DOI] [PubMed] [Google Scholar]
- Anderson, J. C. , Marteinson, S. C. , & Prosser, R. S. (2021). Prioritization of pesticides for assessment of risk to aquatic ecosystems in Canada and identification of knowledge gaps. In de Voogt P. (Ed.), Reviews of environmental contamination and toxicology (Volume 259, pp. 171–231). Springer International Publishing. (Reviews of Environmental Contamination and Toxicology). [DOI] [PubMed] [Google Scholar]
- Ankley, G. T. , Bennett, R. S. , Erickson, R. J. , Hoff, D. J. , Hornung, M. W. , Johnson, R. D. , Mount, D. R. , Nichols, J. W. , Russom, C. L. , Schmieder, P. K. , Serrrano, J. A. , Tietge, J. E. , & Villeneuve, D. L. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry, 29(3), 730–741. 10.1002/etc.34 [DOI] [PubMed] [Google Scholar]
- Ankley, G. T. , Berninger, J. P. , Blackwell, B. R. , Cavallin, J. E. , Collette, T. W. , Ekman, D. R. , Fay, K. A. , Feifarek, D. J. , Jensen, K. M. , Kahl, M. D. , Mosley, J. D. , Poole, S. T. , Randolph, E. C. , Rearick, D. , Schroeder, A. L. , Swintek, J. , & Villeneuve, D. L. (2021). Pathway‐based approaches for assessing biological hazards of complex mixtures of contaminants: A case study in the Maumee River. Environmental Toxicology and Chemistry, 40(4), 1098–1122. 10.1002/etc.4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronzon, C. M. , Sandoval, M. T. , Herkovits, J. , & Pérez‐Coll, C. S. (2011). Stage‐dependent toxicity of 2,4‐dichlorophenoxyacetic on the embryonic development of a South American toad, Rhinella arenarum . Environmental Toxicology, 26(4), 373–381. 10.1002/tox.20564 [DOI] [PubMed] [Google Scholar]
- Bachère, E. , Barranger, A. , Bruno, R. , Rouxel, J. , Menard, D. , Piquemal, D. , & Akcha, F. (2017). Parental diuron‐exposure alters offspring transcriptome and fitness in Pacific oyster Crassostrea gigas . Ecotoxicology and Environmental Safety, 142, 51–58. 10.1016/j.ecoenv.2017.03.030 [DOI] [PubMed] [Google Scholar]
- Baker, D. B. , Ewing, D. E. , Johnson, L. T. , Kramer, J. W. , Merryfield, B. J. , Confesor, R. B. , Peter Richards, R. , & Roerdink, A. A. (2014). Lagrangian analysis of the transport and processing of agricultural runoff in the lower Maumee River and Maumee Bay. Journal of Great Lakes Research, 40(3), 479–495. 10.1016/j.jglr.2014.06.001 [DOI] [Google Scholar]
- Baker, N. J. , Bancroft, B. A. , & Garcia, T. S. (2013). A meta‐analysis of the effects of pesticides and fertilizers on survival and growth of amphibians. Science of the Total Environment, 449, 150–156. 10.1016/j.scitotenv.2013.01.056 [DOI] [PubMed] [Google Scholar]
- Baker, N. T. , & Stone, W. W. (2014). Annual agricultural pesticide use for Midwest stream—quality assessment, 2012‐13 (Report No. Data Series 863). US Geological Survey, Reston, VA. 10.3133/ds863. [DOI]
- Baldwin, A. , Corsi, S. R. , Stefaniak, O. , Loken, L. C. , Villeneuve, D. L. , Ankley, G. T. , Blackwell, B. R. , Lenaker, P. , Nott, M. , & Mills, M. (2022). Risk‐based prioritization of organic chemicals and locations of ecological concern in sediment from Great Lakes tributaries. Environmental Toxicology and Chemistry, 41; 1016–1041. 10.1002/etc.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin, A. K. , Corsi, S. R. , De Cicco, L. A. , Lenaker, P. L. , Lutz, M. A. , Sullivan, D. J. , & Richards, K. D. (2016). Organic contaminants in Great Lakes tributaries: Prevalence and potential aquatic toxicity. Science of the Total Environment, 554–555, 42–52. 10.1016/j.scitotenv.2016.02.137 [DOI] [PubMed] [Google Scholar]
- Bartlett, A. J. , Hedges, A. M. , Intini, K. D. , Brown, L. R. , Maisonneuve, F. J. , Robinson, S. A. , Gillis, P. L. , & de Solla, S. R. (2018). Lethal and sublethal toxicity of neonicotinoid and butenolide insecticides to the mayfly, Hexagenia spp. Environmental Pollution, 238, 63–75. 10.1016/j.envpol.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Bass, C. , Denholm, I. , Williamson, M. S. , & Nauen, R. (2015). The global status of insect resistance to neonicotinoid insecticides. Pesticide Biochemistry and Physiology, 121, 78–87. 10.1016/j.pestbp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Battaglin, W. A. , Furlong, E. T. , Burkhardt, M. R. , & Peter, C. J. (2000). Occurrence of sulfonylurea, sulfonamide, imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Science of the Total Environment, 248(2–3), 123–133. 10.1016/S0048-9697(99)00536-7 [DOI] [PubMed] [Google Scholar]
- Battaglin, W. A. , Smalling, K. L. , Anderson, C. , Calhoun, D. , Chestnut, T. , & Muths, E. (2016). Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Science of the Total Environment, 566–567, 320–332. 10.1016/j.scitotenv.2016.05.062 [DOI] [PubMed] [Google Scholar]
- Bedient, P. B. , Horsak, R. D. , Schlenk, D. , Hovinga, R. M. , & Pierson, J. D. (2005). Environmental impact of fipronil to the Louisiana crawfish industry. Environmental Forensics, 6(3), 289–299. 10.1080/15275920500194530 [DOI] [Google Scholar]
- Benbrook, C. M. (2016). Trends in glyphosate herbicide use in the United States and globally. Environmental Sciences Europe, 28(1), 3. 10.1186/s12302-016-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin, S. , Enzlin, P. , Coviello, A. , & Basson, R. (2007). Sexual dysfunction in men and women with endocrine disorders. The Lancet, 369(9561), 597–611. 10.1016/S0140-6736(07)60280-3 [DOI] [PubMed] [Google Scholar]
- Birchfield, N. B. , & Casida, J. E. (1997). Protoporphyrinogen oxidase of mouse and maize: Target site selectivity and thiol effects on peroxidizing herbicide action. Pesticide Biochemistry and Physiology, 57(1), 36–43. 10.1006/pest.1997.2260 [DOI] [Google Scholar]
- Blackwell, B. R. , Ankley, G. T. , Bradley, P. M. , Houck, K. A. , Makarov, S. S. , Medvedev, A. V. , Swintek, J. , & Villeneuve, D. L. (2019). Potential toxicity of complex mixtures in surface waters from a nationwide survey of United States streams: Identifying in vitro bioactivities and causative chemicals. Environmental Science and Technology, 53(2), 973–983. 10.1021/acs.est.8b05304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, B. R. , Ankley, G. T. , Corsi, S. R. , DeCicco, L. A. , Houck, K. A. , Judson, R. S. , Li, S. , Martin, M. T. , Murphy, E. , Schroeder, A. L. , Smith, E. R. , Swintek, J. , & Villeneuve, D. L. (2017). An “EAR” on environmental surveillance and monitoring: A case study on the use of exposure–activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes waters. Environmental Science & Technology, 51(15), 8713–8724. 10.1021/acs.est.7b01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert, J. , Perroy, J. , Bécamel, C. , Marin, P. , & Fagni, L. (2010). GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annual Review of Pharmacology and Toxicology, 50(1), 89–109. 10.1146/annurev.pharmtox.010909.105705 [DOI] [PubMed] [Google Scholar]
- Bradley, P. M. , Journey, C. A. , Romanok, K. M. , Barber, L. B. , Buxton, H. T. , Foreman, W. T. , Furlong, E. T. , Glassmeyer, S. T. , Hladik, M. L. , Iwanowicz, L. R. , Jones, D. K. , Kolpin, D. W. , Kuivila, K. M. , Loftin, K. A. , Mills, M. A. , Meyer, M. T. , Orlando, J. L. , Reilly, T. J. , Smalling, K. L. , & Villeneuve, D. L. (2017). Expanded target‐chemical analysis reveals extensive mixed‐organic‐contaminant exposure in US streams. Environmental Science and Technology, 51(9), 4792–4802. 10.1021/acs.est.7b00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, P. M. , Journey, C. A. , Romanok, K. M. , Breitmeyer, S. E. , Button, D. T. , Carlisle, D. M. , Huffman, B. J. , Mahler, B. J. , Nowell, L. H. , Qi, S. L. , Smalling, K. L. , Waite, I. R. , & Van Metre, P. C. (2021). Multi‐region assessment of chemical mixture exposures and predicted cumulative effects in USA wadeable urban/agriculture‐gradient streams. Science of the Total Environment, 773, 145062. 10.1016/j.scitotenv.2021.145062 [DOI] [PubMed] [Google Scholar]
- Bradley, P. M. , Romanok, K. M. , Duncan, J. R. , Battaglin, W. A. , Clark, J. M. , Hladik, M. L. , Huffman, B. J. , Iwanowicz, L. R. , Journey, C. A. , & Smalling, K. L. (2020). Exposure and potential effects of pesticides and pharmaceuticals in protected streams of the US National Park Service southeast region. Science of the Total Environment, 704, 135431. 10.1016/j.scitotenv.2019.135431 [DOI] [PubMed] [Google Scholar]
- Braga, A. R. C. , de Rosso, V. V. , Harayashiki, C. A. Y. , Jimenez, P. C. , & Castro, Í. B. (2020). Global health risks from pesticide use in Brazil. Nature Food, 1(6), 312–314. 10.1038/s43016-020-0100-3 [DOI] [PubMed] [Google Scholar]
- Bullerjahn, G. S. , McKay, R. M. , Davis, T. W. , Baker, D. B. , Boyer, G. L. , D'anglada, L. V. , Doucette, G. J. , Ho, J. C. , Irwin, E. G. , Kling, C. L. , Kudela, R. M. , Kurmayer, R. , Michalak, A. M. , Ortiz, J. D. , Otten, T. G. , Paerl, H. W. , Qin, B. , Sohngen, B. L. , Stumpf, R. P. , … Wilhelm, S. W. (2016). Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful algae, 54, 223–238. 10.1016/j.hal.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuzzi, S. J. , Politi, R. , Isayev, O. , Farag, S. , & Tropsha, A. (2016). QSAR modeling of Tox21 challenge stress response and nuclear receptor signaling toxicity assays. Frontiers in Environmental Science4, (3). Retrieved August 23, 2022, from: https://www.frontiersin.org/articles/10.3389/fenvs.2016.00003
- Carson, R. (1962). Silent spring. Houghton Mifflin. [Google Scholar]
- de Castro Marcato, A. C. , de Souza, C. P. , & Fontanetti, C. S. (2017). Herbicide 2,4‐D: A review of toxicity on non‐target organisms. Water, Air, and Soil Pollution, 228(3), 120. 10.1007/s11270-017-3301-0 [DOI] [Google Scholar]
- Cipoletti, N. , Jorgenson, Z. G. , Banda, J. A. , Hummel, S. L. , Kohno, S. , & Schoenfuss, H. L. (2019). Land use contributions to adverse biological effects in a complex agricultural and urban watershed: A case study of the Maumee River. Environmental Toxicology and Chemistry, 38(5), 1035–1051. 10.1002/etc.4409 [DOI] [PubMed] [Google Scholar]
- Cipoletti, N. , Jorgenson, Z. G. , Banda, J. A. , Kohno, S. , Hummel, S. L. , & Schoenfuss, H. L. (2020). Biological consequences of agricultural and urban land‐use along the Maumee River, a major tributary to the Laurentian Great Lakes watershed. Journal of Great Lakes Research, 46(4), 1001–1014. 10.1016/j.jglr.2020.04.013 [DOI] [Google Scholar]
- Conti, M. , Richter, W. , Mehats, C. , Livera, G. , Park, J.‐Y. , & Jin, C. (2003). Cyclic AMP‐specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. Journal of Biological Chemistry, 278(8), 5493–5496. 10.1074/jbc.R200029200 [DOI] [PubMed] [Google Scholar]
- Cooper, R. L. (2000). Atrazine disrupts the hypothalamic control of pituitary‐ovarian function. Toxicological Sciences, 53(2), 297–307. 10.1093/toxsci/53.2.297 [DOI] [PubMed] [Google Scholar]
- Coquillé, N. , Jan, G. , Moreira, A. , & Morin, S. (2015). Use of diatom motility features as endpoints of metolachlor toxicity. Aquatic Toxicology, 158, 202–210. 10.1016/j.aquatox.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Corsi, S. R. , De Cicco, L. A. , Villeneuve, D. L. , Blackwell, B. R. , Fay, K. A. , Ankley, G. T. , & Baldwin, A. K. (2019). Prioritizing chemicals of ecological concern in Great Lakes tributaries using high‐throughput screening data and adverse outcome pathways. Science of the Total Environment, 686, 995–1009. 10.1016/j.scitotenv.2019.05.457 [DOI] [PubMed] [Google Scholar]
- Covert, S. A. , Shoda, M. E. , Stackpoole, S. M. , & Stone, W. W. (2020). Pesticide mixtures show potential toxicity to aquatic life in US streams, water years 2013–2017. Science of the Total Environment, 745, 141285. 10.1016/j.scitotenv.2020.141285 [DOI] [PubMed] [Google Scholar]
- Crossland, N. O. (1990). A review of the fate and toxicity of 3,4‐dichloroaniline in aquatic environments. Chemosphere, 21(12), 1489–1497. 10.1016/0045-6535(90)90054-W [DOI] [Google Scholar]
- Curl, C. L. , Fenske, R. A. , Kissel, J. C. , Shirai, J. H. , Moate, T. F. , Griffith, W. , Coronado, G. , & Thompson, B. (2002). Evaluation of take‐home organophosphorus pesticide exposure among agricultural workers and their children. Environmental Health Perspectives, 110(12), A787–A792. 10.1289/ehp.021100787; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin, B. D. , Hein, M. J. , Sanderson, W. T. , Striley, C. , Heederik, D. , Kromhout, H. , Reynolds, S. J. , & Alavanja, M. C. (2007). Pesticide dose estimates for children of Iowa farmers and non‐farmers. Environmental Research, 105(3), 307–315. 10.1016/j.envres.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Custer, C. M. , Custer, T. W. , Dummer, P. M. , Schultz, S. , Tseng, C. Y. , Karouna‐Renier, N. , & Matson, C. W. (2020). Legacy and contaminants of emerging concern in tree swallows along an agricultural to industrial gradient: Maumee River, Ohio. Environmental Toxicology and Chemistry, 39(10), 1936–1952. 10.1002/etc.4792 [DOI] [PubMed] [Google Scholar]
- Dayan, F. E. , & Watson, S. B. (2011). Plant cell membrane as a marker for light‐dependent and light‐independent herbicide mechanisms of action. Pesticide Biochemistry and Physiology, 101(3), 182–190. 10.1016/j.pestbp.2011.09.004 [DOI] [Google Scholar]
- DeCicco, L. A. , Corsi, S. R. , Villeneuve, D. L. , Blackwell, B. R. , & Ankley, G. T. (2018). toxEval: Evaluation of measured concentration data using the ToxCast high‐throughput screening database or a user‐defined set of concentration benchmarks. R Package version 1.0.0. https://code.usgs.gov/water/toxEval, 10.5066/P906UQ5I. [DOI]
- Dehnert, G. K. , Freitas, M. B. , DeQuattro, Z. A. , Barry, T. , & Karasov, W. H. (2018). Effects of low, subchronic exposure of 2,4‐dichlorophenoxyacetic acid (2,4‐D) and commercial 2,4‐D formulations on early life stages of fathead minnows (Pimephales promelas). Environmental Toxicology and Chemistry, 37(10), 2550–2559. 10.1002/etc.4209 [DOI] [PubMed] [Google Scholar]
- Dehnert, G. K. , Freitas, M. B. , Sharma, P. P. , Barry, T. P. , & Karasov, W. H. (2020). Impacts of subchronic exposure to a commercial 2,4‐D herbicide on developmental stages of multiple freshwater FISH species. Chemosphere, 127638. 10.1016/j.chemosphere.2020.127638 [DOI] [PubMed] [Google Scholar]
- Dix, D. J. , Houck, K. A. , Martin, M. T. , Richard, A. M. , Setzer, R. W. , & Kavlock, R. J. (2007). The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicological Sciences, 95(1), 5–12. 10.1093/toxsci/kfl103 [DOI] [PubMed] [Google Scholar]
- Ebenezer, V. , & Ki, J.‐S. (2013). Quantification of toxic effects of the herbicide metolachlor on marine microalgae Ditylum brightwellii (Bacillariophyceae), Prorocentrum minimum (Dinophyceae), and Tetraselmis suecica (Chlorophyceae). Journal of Microbiology, 51(1), 136–139. 10.1007/s12275-013-2114-0 [DOI] [PubMed] [Google Scholar]
- Edwards, T. K. , & Glysson, G. D. (1999). Field methods for measurement of fluvial sediment. Book 3, C2. US Geological Survey Techniques of Water‐Resources Investigations.
- European Food Safety Authority . (2013). Conclusion on the peer review of the pesticide risk assessment for bees for the active substance fipronil. European Food Safety Authority Journal, 11(5), 3158. 10.2903/j.efsa.2013.3158 [DOI] [Google Scholar]
- Ejomah, A. J. , Uyi, O. O. , & Ekaye, S.‐O. (2020). Exposure of the African mound building termite, Macrotermes bellicosus workers to commercially formulated 2,4‐D and atrazine caused high mortality and impaired locomotor response. PLoS One, 5(3), e0230664. 10.1371/journal.pone.0230664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng, M. L. , Stutchbury, B. J. M. , & Morrissey, C. A. (2019). A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science, 365(6458), 1177–1180. 10.1126/science.aaw9419 [DOI] [PubMed] [Google Scholar]
- Fan, W. , Yanase, T. , Morinaga, H. , Gondo, S. , Okabe, T. , Nomura, M. , Komatsu, T. , Morohashi, K. , Hayes, T. B. , Takayanagi, R. , & Nawata, H. (2007). Atrazine‐induced aromatase expression is SF‐1 dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans. Environmental Health Perspectives, 115(5), 720–727. 10.1289/ehp.9758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, K. A. , Villeneuve, D. L. , Swintek, J. , Edwards, S. W. , Nelms, M. D. , Blackwell, B. R. , & Ankley, G. T. (2018). Differentiating pathway‐specific from nonspecific effects in high‐throughput toxicity data: A foundation for prioritizing adverse outcome pathway development. Toxicological Sciences (Orlando, FL), 163(2), 500–515. 10.1093/toxsci/kfy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer, D. L. , Kothiya, P. , Setzer, R. W. , Judson, R. S. , & Martin, M. T. (2016). tcpl: The ToxCast pipeline for high‐throughput screening data. Bioinformatics, 33(4), 618–620. 10.1093/bioinformatics/btw680 [DOI] [PubMed] [Google Scholar]
- Fleming, W. J. , Ailstock, M. S. , & Momot, J. J. (1995). Net photosynthesis and respiration of sago pondweed (Potamogeton Pectinatus) exposed to herbicides. Environmental Toxicology and Risk Assessment: Third Volume. 10.1520/STP12697S [DOI] [Google Scholar]
- Flynn, K. , & Spellman, T. (2009). Environmental levels of atrazine decrease spatial aggregation in the freshwater mussel, Elliptio complanata . Ecotoxicology and Environmental Safety, 72(4), 1228–1233. 10.1016/j.ecoenv.2008.02.013 [DOI] [PubMed] [Google Scholar]
- Freitas, J. S. , Teresa, F. B. , & de Almeida, E. A. (2017). Influence of temperature on the antioxidant responses and lipid peroxidation of two species of tadpoles (Rhinella schneideri and Physalaemus nattereri) exposed to the herbicide sulfentrazone (Boral 500SC®). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 197, 32–44. 10.1016/j.cbpc.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Fresno, C. , & Fernandez, E. A. (2013). RDAVIDWebService: A versatile R interface to DAVID. Bioinformatics, 29(21), 2810–2811. 10.1093/bioinformatics/btt487 [DOI] [PubMed] [Google Scholar]
- Freydier, L. , & Lundgren, J. G. (2016). Unintended effects of the herbicides 2,4‐D and dicamba on lady beetles. Ecotoxicology, 25(6), 1270–1277. 10.1007/s10646-016-1680-4 [DOI] [PubMed] [Google Scholar]
- Geisler, C. E. , Ghimire, S. , Bogan, R. L. , & Renquist, B. J. (2019). Role of ketone signaling in the hepatic response to fasting. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 316(5), G623–G631. 10.1152/ajpgi.00415.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomazzi, S. , & Cochet, N. (2004). Environmental impact of diuron transformation: A review. Chemosphere, 56(11), 1021–1032. 10.1016/j.chemosphere.2004.04.061 [DOI] [PubMed] [Google Scholar]
- Gibbons, D. , Morrissey, C. , & Mineau, P. (2015). A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environmental Science and Pollution Research, 22(1), 103–118. 10.1007/s11356-014-3180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, M. E. , & Luch, A. (2008). Reactive species: A cell damaging rout assisting to chemical carcinogens. Cancer Letters, 266(1), 73–83. 10.1016/j.canlet.2008.02.035 [DOI] [PubMed] [Google Scholar]
- Goulson, D. (2013). REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology, 50(4), 977–987. 10.1111/1365-2664.12111 [DOI] [Google Scholar]
- Great Lakes Interagency Task Force . (2014). Great Lakes restoration initiative action Plan II. Retrieved July 7, 2021, from: http://greatlakes-restoration.us/actionplan/pdfs/glri-action-plan-2.pdf
- Gunasekara, A. S. , Truong, T. , Goh, K. S. , Spurlock, F. , & Tjeerdema, R. S. (2007). Environmental fate and toxicology of fipronil. Journal of Pesticide Science, 32(3), 189–199. 10.1584/jpestics.R07-02 [DOI] [Google Scholar]
- Hainzl, D. , Cole, L. M. , & Casida, J. E. (1998). Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chemical Research in Toxicology, 11(12), 1529–1535. 10.1021/tx980157t [DOI] [PubMed] [Google Scholar]
- Hamilton, K. J. , Hewitt, S. C. , Arao, Y. , & Korach, K. S. (2017). Estrogen hormone biology. In Forrest D., Tsai S., (Eds), Current topics in developmental biology (Vol. 125, pp. 109–146). Elsevier. https://www.sciencedirect.com/science/article/pii/S0070215316302046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harino, H. , Kitano, M. , Mori, Y. , & Mochida, K. (2005). Degradation of antifouling booster biocides in water. Journal of the Marine Biological Association of the United Kingdom, 85(1), 33–38. 10.1017/S0025315405010799h [DOI] [Google Scholar]
- Harrahy, E. A. , Edwards, D. S. , & Hedman, C. J. (2014). Persistence of 2,4‐D and its effects on benthic macroinvertebrates following spring treatment of Eurasian watermilfoil, Myriophyllum spicatum L. in two lakes in Southeastern Wisconsin, USA. Bulletin of Environmental Contamination and Toxicology, 92(4), 404–409. 10.1007/s00128-014-1206-9 [DOI] [PubMed] [Google Scholar]
- Harris, T. , & Smith, V. (2016). Do persistent organic pollutants stimulate cyanobacterial blooms. Inland Waters, 6(2), 124–130. 10.5268/IW-6.2.887 [DOI] [Google Scholar]
- Hegardt, F. G. (1999). Mitochondrial 3‐hydroxy‐3‐methylglutaryl‐CoA synthase: A control enzyme in ketogenesis. Biochemical Journal, 338(3), 569–582. 10.1042/bj3380569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. , Beguin, M. , Requier, F. , Rollin, O. , Odoux, J.‐F. , Aupinel, P. , Aptel, J. , Tchamitchian, S. , & Decourtye, A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science, 336(6079), 348–350. 10.1126/science.1215039 [DOI] [PubMed] [Google Scholar]
- Hladik, M. L. , Corsi, S. R. , Kolpin, D. W. , Baldwin, A. K. , Blackwell, B. R. , & Cavallin, J. E. (2018). Year‐round presence of neonicotinoid insecticides in tributaries to the Great Lakes, USA. Environmental Pollution, 235, 1022–1029. 10.1016/j.envpol.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, A. C. , Anger, D. A. , Crankshaw, D. J. , Wu, M. , & Foster, W. G. (2008). Atrazine‐induced changes in aromatase activity in estrogen sensitive target tissues. Journal of Applied Toxicology, 28(3), 260–270. 10.1002/jat.1275 [DOI] [PubMed] [Google Scholar]
- Homer, C. , Dewitz, J. , Jin, S. , Xian, G. , Costello, C. , Danielson, P. , Gass, L. , Funk, M. , Wickham, J. , Stehman, S. , Auch, R. , & Riitters, K. (2020). Conterminous United States land cover change patterns 2001–2016 from the 2016 National Land Cover Database. ISPRS Journal of Photogrammetry and Remote Sensing, 162, 184–199. 10.1016/j.isprsjprs.2020.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T. , Huang, Y. , Huang, Y. , Yang, Y. , Zhao, Y. , & Martyniuk, C. J. (2020). Toxicity assessment of the herbicide acetochlor in the human liver carcinoma (HepG2) cell line. Chemosphere, 243, 125345. 10.1016/j.chemosphere.2019.125345 [DOI] [PubMed] [Google Scholar]
- Huckins, J. N. , Petty, J. D. , & Booij, K. (2006). Monitors of organic chemicals in the environment: Semipermeable membrane devices. Springer Science & Business Media. [Google Scholar]
- Hutchinson, T. , & Villeneuve, D. (2021). AOP:29. Estrogen receptor agonism leading to reproductive dysfunction. AOP‐Wiki. https://aopwiki.org/aops/29
- Islam, F. , Wang, J. , Farooq, M. A. , Khan, M. S. S. , Xu, L. , Zhu, J. , Zhao, M. , Muños, S. , Li, Q. X. , & Zhou, W. (2018). Potential impact of the herbicide 2,4‐dichlorophenoxyacetic acid on human and ecosystems. Environment International, 111, 332–351. 10.1016/j.envint.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Jackson, D. , Cornell, C. B. , Luukinen, B. , Buhl, K. , & Stone, D. 2009. Fipronil [Technical Fact Sheet]. National Pesticide Information Center, Oregon State University Extension Services.
- Jin, Y. , Chen, R. , Wang, L. , Liu, J. , Yang, Y. , Zhou, C. , Liu, W. , & Fu, Z. (2011). Effects of metolachlor on transcription of thyroid system‐related genes in juvenile and adult Japanese medaka (Oryzias latipes). General and Comparative Endocrinology, 170(3), 487–493. 10.1016/j.ygcen.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Jokanović, M. (2018). Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology, 410, 125–131. 10.1016/j.tox.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Judson, R. S. , Houck, K. A. , Kavlock, R. J. , Knudsen, T. B. , Martin, M. T. , Mortensen, H. M. , Reif, D. M. , Rotroff, D. M. , Shah, I. , Richard, A. M. , & Dix, D. J. (2009). In vitro screening of environmental chemicals for targeted testing prioritization: The ToxCast project. Environmental Health Perspectives, 118(4), 485–492. 10.1289/ehp.0901392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock, R. , Chandler, K. , Houck, K. , Hunter, S. , Judson, R. , Kleinstreuer, N. , Knudsen, T. , Martin, M. , Padilla, S. , Reif, D. , Richard, A. , Rotroff, D. , Sipes, N. , & Dix, D. (2012). Update on EPAs ToxCast program: Providing high throughput decision support tools for chemical risk management. Chemical Research in Toxicology, 25(7), 1287–1302. 10.1021/tx3000939 [DOI] [PubMed] [Google Scholar]
- Kleinstreuer, N. C. , Ceger, P. , Watt, E. D. , Martin, M. , Houck, K. , Browne, P. , Thomas, R. S. , Casey, W. M. , Dix, D. J. , Allen, D. , Sakamuru, S. , Xia, M. , Huang, R. , & Judson, R. (2017). Development and validation of a computational model for androgen receptor activity. Chemical Research in Toxicology, 30(4), 946–964. 10.1021/acs.chemrestox.6b00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klementová, Š. , Hornychová, L. , Šorf, M. , Zemanová, J. , & Kahoun, D. (2019). Toxicity of atrazine and the products of its homogeneous photocatalytic degradation on the aquatic organisms Lemna minor and Daphnia magna . Environmental Science and Pollution Research, 26(26), 27259–27267. 10.1007/s11356-019-05710-0 [DOI] [PubMed] [Google Scholar]
- Knauert, S. , Escher, B. , Singer, H. , Hollender, J. , & Knauer, K. (2008). Mixture toxicity of three photosystem ii inhibitors (atrazine, isoproturon, and diuron) toward photosynthesis of freshwater phytoplankton studied in outdoor mesocosms. Environmental Science & Technology, 42(17), 6424–6430. 10.1021/es072037q [DOI] [PubMed] [Google Scholar]
- Köhler, H.‐R. , & Triebskorn, R. (2013). Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science, 341(6147), 759–765. 10.1126/science.1237591 [DOI] [PubMed] [Google Scholar]
- Kolpin, D. W. , Blazer, V. S. , Gray, J. L. , Focazio, M. J. , Young, J. A. , Alvarez, D. A. , Iwanowicz, L. R. , Foreman, W. T. , Furlong, E. T. , Speiran, G. K. , Zaugg, S. D. , Hubbard, L. E. , Meyer, M. T. , Sandstrom, M. W. , & Barber, L. B. (2013). Chemical contaminants in water and sediment near fish nesting sites in the Potomac River basin: Determining potential exposures to smallmouth bass (Micropterus dolomieu). Science of the Total Environment, 443, 700–716. 10.1016/j.scitotenv.2012.09.063 [DOI] [PubMed] [Google Scholar]
- Laboratory of Human Retrovirology and Informatics . (2022). DAVID bioinformatics resources. https://david.ncifcrf.gov/.
- Lackner, R. (1998). “Oxidative stress” in fish by environmental pollutants. In Braunbeck T., Hinton D. E., & Streit B. (Eds.), Fish ecotoxicology (pp. 203–224). Birkhäuser. [Google Scholar]
- Lajmanovich, R. C. , Attademo, A. M. , Simoniello, M. F. , Poletta, G. L. , Junges, C. M. , Peltzer, P. M. , Grenón, P. , & Cabagna‐Zenklusen, M. C. (2015). Harmful effects of the dermal intake of commercial formulations containing chlorpyrifos, 2,4‐D, and glyphosate on the common toad Rhinella arenarum (Anura: Bufonidae). Water, Air, and Soil Pollution, 226(12), 427. 10.1007/s11270-015-2695-9 [DOI] [Google Scholar]
- LaLone, C. A. , Villeneuve, D. L. , Doering, J. A. , Blackwell, B. R. , Transue, T. R. , Simmons, C. W. , Swintek, J. , Degitz, S. J. , Williams, A. J. , & Ankley, G. T. (2018). Evidence for cross species extrapolation of mammalian‐based high‐throughput screening assay results. Environmental Science & Technology, 52(23), 13960–13971. 10.1021/acs.est.8b04587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone, C. A. , Villeneuve, D. L. , Wu‐Smart, J. , Milsk, R. Y. , Sappington, K. , Garber, K. V. , Housenger, J. , & Ankley, G. T. (2017). Weight of evidence evaluation of a network of adverse outcome pathways linking activation of the nicotinic acetylcholine receptor in honey bees to colony death. Science of the Total Environment, 584–585, 751–775. 10.1016/j.scitotenv.2017.01.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, S. J. , Thomas, K. V. , & Davy, A. J. (2006). Assessment of the risk posed by the antifouling booster biocides Irgarol 1051 and diuron to freshwater macrophytes. Chemosphere, 63(5), 734–743. 10.1016/j.chemosphere.2005.08.023 [DOI] [PubMed] [Google Scholar]
- Le Novère, N. , Corringer, P.‐J. , & Changeux, J.‐P. (2002). The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences: Effect of subunit composition on nAChRs function. Journal of Neurobiology, 53(4), 447–456. 10.1002/neu.10153 [DOI] [PubMed] [Google Scholar]
- Lerch, R. N. , Sadler, E. J. , Sudduth, K. A. , Baffaut, C. , & Kitchen, N. R. (2011). Herbicide transport in Goodwater Creek experimental watershed: I. Long‐term research on Atrazine. JAWRA Journal of the American Water Resources Association, 47(2), 209–223. 10.1111/j.1752-1688.2010.00503.x [DOI] [Google Scholar]
- Li, M. , Ma, X. , Saleem, M. , Wang, X. , Sun, L. , Yang, Y. , & Zhang, Q. (2020). Biochemical response, histopathological change and DNA damage in earthworm (Eisenia fetida) exposed to sulfentrazone herbicide. Ecological Indicators, 115, 106465. 10.1016/j.ecolind.2020.106465 [DOI] [Google Scholar]
- Loken, L. C. , Alvarez, D. A. , Baldwin, A. K. , & Corsi, S. R. (2022). Pesticides and pesticide transformation product data from passive samplers deployed in 15 Great Lakes tributaries. 2016: US Geological Survey data release. 10.5066/P9QOMM22. [DOI]
- Loken, L. C. , DeCicco, L. A. , Corsi, S. R. , Oliver, S. K. , Blackwell, B. R. , Ankley, G. T. , & Villeneuve, D. L. (2021). ToxMixtures: A package to explore toxicity due to chemical mixtures. 10.5066/P9BX71PG. [DOI]
- Lydy, M. , Belden, J. , Wheelock, C. , Hammock, B. , & Denton, D. (2004). Challenges in regulating pesticide mixtures. Ecology and Society, 9(6), art1. 10.5751/ES-00694-090601 [DOI] [Google Scholar]
- Mahler, B. J. , Nowell, L. H. , Sandstrom, M. W. , Bradley, P. M. , Romanok, K. M. , Konrad, C. P. , & Van Metre, P. C. (2021). Inclusion of pesticide transformation products is key to estimating pesticide exposures and effects in small US streams. Environmental Science and Technology, 55(8), 4740–4752. 10.1021/acs.est.0c06625 [DOI] [PubMed] [Google Scholar]
- Mai, H. , Morin, B. , Pardon, P. , Gonzalez, P. , Budzinski, H. , & Cachot, J. (2013). Environmental concentrations of irgarol, diuron and S‐metolachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas . Marine Environmental Research, 89, 1–8. 10.1016/j.marenvres.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Malev, O. , Lovrić, M. , Stipaničev, D. , Repec, S. , Martinović‐Weigelt, D. , Zanella, D. , Ivanković, T. , Sindičić Đuretec, V. , Barišić, J. , Li, M. , & Klobučar, G. (2020). Toxicity prediction and effect characterization of 90 pharmaceuticals and illicit drugs measured in plasma of fish from a major European river (Sava, Croatia). Environmental Pollution, 266, 115162. 10.1016/j.envpol.2020.115162 [DOI] [PubMed] [Google Scholar]
- Mancarci, O. (2019). homologene: Quick Access to Homologene and Gene Annotation Updates. R package version 1.4.68.19.3.27. https://CRAN.R-project.org/package=homologene
- Mansuy, D. (1998). The great diversity of reactions catalyzed by cytochromes P450. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 121(1–3), 5–14. 10.1016/S0742-8413(98)10026-9 [DOI] [PubMed] [Google Scholar]
- Mast, M. A. , Foreman, W. T. , & Skaates, S. V. (2007). Current‐use pesticides and organochlorine compounds in precipitation and lake sediment from two high‐elevation national parks in the Western United States. Archives of Environmental Contamination and Toxicology, 52(3), 294–305. 10.1007/s00244-006-0096-1 [DOI] [PubMed] [Google Scholar]
- Matson, P. G. , Boyer, G. L. , Bridgeman, T. B. , Bullerjahn, G. S. , Kane, D. D. , McKay, R. M. L. , McKindles, K. M. , Raymond, H. A. , Snyder, B. K. , Stumpf, R. P. , & Davis, T. W. (2020). Physical drivers facilitating a toxigenic cyanobacterial bloom in a major Great Lakes tributary. Limnology and Oceanography, 65(12), 2866–2882. 10.1002/lno.11558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice, P. , Guillaume, J.‐L. , Benleulmi‐Chaachoua, A. , Daulat, A. M. , Kamal, M. , & Jockers, R. (2011). GPCR‐interacting proteins, major players of GPCR function. Advances in Pharmacology, 62, 349–380 [DOI] [PubMed] [Google Scholar]
- McMahen, R. L. , Strynar, M. J. , McMillan, L. , DeRose, E. , & Lindstrom, A. B. (2016). Comparison of fipronil sources in North Carolina surface water and identification of a novel fipronil transformation product in recycled wastewater. Science of the Total Environment, 569–570, 880–887. 10.1016/j.scitotenv.2016.05.085 [DOI] [PubMed] [Google Scholar]
- Medalie, L. , & Bexfield, L. M. (2020). Quality of data from the US Geological Survey National Water Quality Network for water years 2013–17: t 2020–5116 (Scientific Investigations Report Report No. 2020–5116). Reston, VA. 10.3133/sir20205116 [DOI]
- Metcalfe, C. D. , Helm, P. , Paterson, G. , Kaltenecker, G. , Murray, C. , Nowierski, M. , & Sultana, T. (2019). Pesticides related to land use in watersheds of the Great Lakes basin. Science of the Total Environment, 648, 681–692. 10.1016/j.scitotenv.2018.08.169 [DOI] [PubMed] [Google Scholar]
- Metcalfe, C. D. , Sultana, T. , Li, H. , & Helm, P. A. (2016). Current‐use pesticides in urban watersheds and receiving waters of western Lake Ontario measured using polar organic chemical integrative samplers (POCIS). Journal of Great Lakes Research, 42(6), 1432–1442. 10.1016/j.jglr.2016.08.004 [DOI] [Google Scholar]
- Meyer, M. T. , Loftin, K. A. , Lee, E. A. , Hinshaw, G. H. , Dietze, J. E. , & Scribner, E. A. 2009. Determination of glyphosate, its degradation product aminomethylphosphonic acid, and glufosinate, in water by isotope dilution and online solid‐phase extraction and liquid chromatography/tandem mass spectrometry (US Geological Survey Report No. 5‐A10).
- Miller, J. L. , Schmidt, T. S. , Van Metre, P. C. , Mahler, B. J. , Sandstrom, M. W. , Nowell, L. H. , Carlisle, D. M. , & Moran, P. W. (2020). Common insecticide disrupts aquatic communities: A mesocosm‐to‐field ecological risk assessment of fipronil and its degradates in US streams. Science Advances, 6, eabc1299. 10.1126/sciadv.abc1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, E. A. D. , Mulhauser, B. , Mulot, M. , Mutabazi, A. , Glauser, G. , & Aebi, A. (2017). A worldwide survey of neonicotinoids in honey. Science, 358(6359), 109–111. 10.1126/science.aan3684 [DOI] [PubMed] [Google Scholar]
- Monks, D. A. , & Holmes, M. M. (2018). Androgen receptors and muscle: A key mechanism underlying life history trade‐offs. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 204(1), 51–60. 10.1007/s00359-017-1222-4 [DOI] [PubMed] [Google Scholar]
- Moore, A. , & Waring, C. P. (1998). Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) Parr. Pesticide Biochemistry and Physiology, 62(1), 41–50. 10.1006/pest.1998.2366 [DOI] [Google Scholar]
- Morgan, M. K. , Sheldon, L. S. , Croghan, C. W. , Jones, P. A. , Robertson, G. L. , Chuang, J. C. , Wilson, N. K. , & Lyu, C. W. (2005). Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6‐trichloro‐2‐pyridinol in their everyday environments. Journal of exposure science & environmental epidemiology, 15(4), 297–309. 10.1038/sj.jea.7500406 [DOI] [PubMed] [Google Scholar]
- Morin, S. , Chaumet, B. , & Mazzella, N. (2018). A time‐dose response model to assess diuron‐induced photosynthesis inhibition in freshwater biofilms. Frontiers in Environmental Science, 6, 131. 10.3389/fenvs.2018.00131 [DOI] [Google Scholar]
- Morrissey, C. A. , Mineau, P. , Devries, J. H. , Sanchez‐Bayo, F. , Liess, M. , Cavallaro, M. C. , & Liber, K. (2015). Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environment International, 74, 291–303. 10.1016/j.envint.2014.10.024 [DOI] [PubMed] [Google Scholar]
- Munaron, D. , Tapie, N. , Budzinski, H. , Andral, B. , & Gonzalez, J.‐L. (2012). Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: Results from a pilot survey using passive samplers. Estuarine, Coastal and Shelf Science, 114, 82–92. 10.1016/j.ecss.2011.09.009 [DOI] [Google Scholar]
- Ng, H. Y. F. , & Clegg, S. B. (1997). Atrazine and metolachlor losses in runoff events from an agricultural watershed: The importance of runoff components. Science of the Total Environment, 193(3), 215–228. 10.1016/S0048-9697(96)05342-9 [DOI] [Google Scholar]
- Nilsen, E. , Smalling, K. L. , Ahrens, L. , Gros, M. , Miglioranza, K. S. B. , Picó, Y. , & Schoenfuss, H. L. (2019). Critical review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environmental Toxicology and Chemistry, 38(1), 46–60. 10.1002/etc.4290 [DOI] [PubMed] [Google Scholar]
- Nirmalakhandan, N. , Arulgnanendran, V. , Mohsin, M. , Sun, B. , & Cadena, F. (1994). Toxicity of mixtures of organic chemicals to microorganisms. Water Research, 28(3), 543–551. 10.1016/0043-1354(94)90005-1 [DOI] [Google Scholar]
- Norman, J. E. , Kuivila, K. M. , & Nowell, L. H. (2012). Prioritizing pesticide compounds for analytical methods development (US Geological Survey Scientific Investigations Report, 2012–5045).
- Norman, J. E. , Mahler, B. J. , Nowell, L. H. , Van Metre, P. C. , Sandstrom, M. W. , Corbin, M. A. , Qian, Y. , Pankow, J. F. , Luo, W. , Fitzgerald, N. B. , Asher, W. E. , & McWhirter, K. J. (2020). Daily stream samples reveal highly complex pesticide occurrence and potential toxicity to aquatic life. Science of the Total Environment, 715, 136795. 10.1016/j.scitotenv.2020.136795 [DOI] [PubMed] [Google Scholar]
- Nowell, L. H. , Moran, P. W. , Schmidt, T. S. , Norman, J. E. , Nakagaki, N. , Shoda, M. E. , Mahler, B. J. , Van Metre, P. C. , Stone, W. W. , Sandstrom, M. W. , & Hladik, M. L. (2018). Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern US streams. Science of the Total Environment, 613–614, 1469–1488. 10.1016/j.scitotenv.2017.06.156 [DOI] [PubMed] [Google Scholar]
- Nowell, L. H. , Norman, J. E. , Moran, P. W. , Martin, J. D. , & Stone, W. W. (2014). Pesticide toxicity index—A tool for assessing potential toxicity of pesticide mixtures to freshwater aquatic organisms. Science of the Total Environment, 476–477, 144–157. 10.1016/j.scitotenv.2013.12.088 [DOI] [PubMed] [Google Scholar]
- Nwani, C. D. , Nagpure, N. S. , Kumar, R. , Kushwaha, B. , Kumar, P. , & Lakra, W. S. (2011). Mutagenic and genotoxic assessment of atrazine‐based herbicide to freshwater fish Channa punctatus (Bloch) using micronucleus test and single cell gel electrophoresis. Environmental Toxicology and Pharmacology, 31(2), 314–322. 10.1016/j.etap.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Organisation of Economic Co‐operation and Development . (2021). Adverse outcome pathways, molecular screening and toxicogenomics. https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm
- Organisation of Economic Co‐operation and Development . (n.d.). AOP Knowledge base. https://aopkb.oecd.org/
- Oliver, S. K. , Corsi, S. R. , Baldwin, A. , Nott, M. , Ankley, G. T. , Blackwell, B. R. , Villeneuve, D. L. , Hladik, M. L. , Kolpin, D. W. , Loken, L. C. , DeCicco, L. A. , Meyer, M. T. , & Loftin, K. A . (2022). Pesticide prioritization by potential biological effects in tributaries of the Laurentian Great Lakes. Environmental Toxicology and Chemistry. 10.1002/etc.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannard, A. , Le Rouzic, B. , & Binet, F. (2009). Response of phytoplankton community to low‐dose atrazine exposure combined with phosphorus fluctuations. Archives of Environmental Contamination and Toxicology, 57(1), 50–59. 10.1007/s00244-008-9245-z [DOI] [PubMed] [Google Scholar]
- PANTHER . (2022). PANTHER classification system. http://www.pantherdb.org/
- Papaefthimiou, C. , Pavlidou, V. , Gregorc, A. , & Theophilidis, G. (2002). The action of 2,4‐dichlorophenoxyacetic acid on the isolated heart of insect and amphibia. Environmental Toxicology and Pharmacology, 11(2), 127–140. 10.1016/S1382-6689(01)00113-2 [DOI] [PubMed] [Google Scholar]
- Pisa, L. W. , Amaral‐Rogers, V. , Belzunces, L. P. , Bonmatin, J. M. , Downs, C. A. , Goulson, D. , Kreutzweiser, D. P. , Krupke, C. , Liess, M. , McField, M. , Morrissey, C. A. , Noome, D. A. , Settele, J. , Simon‐Delso, N. , Stark, J. D. , Van der Sluijs, J. P. , Van Dyck, H. , & Wiemers, M. (2015). Effects of neonicotinoids and fipronil on non‐target invertebrates. Environmental Science and Pollution Research, 22(1), 68–102. 10.1007/s11356-014-3471-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronschinske, M. A. , Corsi, S. R. , & De Cicco, L. A. (2022). Prioritizing pharmaceutical contaminants in Great Lakes tributaries using risk‐based screening techniques. Environmental Toxicology and Chemistry, 41(9), 2221–2239. 10.1002/etc.5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport, S. M. , & Smith, M. T. (2010). Environment and disease risks. Science, 330(6003), 460–461. 10.1126/science.1192603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, A. M. , Judson, R. S. , Houck, K. A. , Grulke, C. M. , Volarath, P. , Thillainadarajah, I. , Yang, C. , Rathman, J. , Martin, M. T. , Wambaugh, J. F. , Knudsen, T. B. , Kancherla, J. , Mansouri, K. , Patlewicz, G. , Williams, A. J. , Little, S. B. , Crofton, K. M. , & Thomas, R. S. (2016). ToxCast chemical landscape: Paving the road to 21st century toxicology. Chemical Research in Toxicology, 29(8), 1225–1251. 10.1021/acs.chemrestox.6b00135 [DOI] [PubMed] [Google Scholar]
- Richter, C. A. , Papoulias, D. M. , Whyte, J. J. , & Tillitt, D. E. (2016). Evaluation of potential mechanisms of atrazine‐induced reproductive impairment in fathead minnow (Pimephales promelas) and Japanese medaka (Oryzias latipes). Environmental Toxicology and Chemistry, 35(9), 2230–2238. 10.1002/etc.3376 [DOI] [PubMed] [Google Scholar]
- Roberge, M. , Hakk, H. , & Larsen, G. (2004). Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicology Letters, 154(1–2), 61–68. 10.1016/j.toxlet.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Roessink, I. , Merga, L. B. , Zweers, H. J. , & Brink, P. J. V. den (2013). The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environmental Toxicology and Chemistry, 32(5), 1096–1100. 10.1002/etc.2201 [DOI] [PubMed] [Google Scholar]
- Rotroff, D. M. , Dix, D. J. , Houck, K. A. , Knudsen, T. B. , Martin, M. T. , McLaurin, K. W. , Reif, D. M. , Crofton, K. M. , Singh, A. V. , Xia, M. , Huang, R. , & Judson, R. S. (2013). Using in vitro high throughput screening assays to identify potential endocrine‐disrupting chemicals. Environmental Health Perspectives, 121(1), 7–14. 10.1289/ehp.1205065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozmánková, E. , Pípal, M. , Bláhová, L. , Njattuvetty Chandran, N. , Morin, B. , Gonzalez, P. , & Bláha, L. (2020). Environmentally relevant mixture of S‐metolachlor and its two metabolites affects thyroid metabolism in zebrafish embryos. Aquatic Toxicology, 221, 105444. 10.1016/j.aquatox.2020.105444 [DOI] [PubMed] [Google Scholar]
- Sanderson, J. , Letcher, R. , Heneweer, M. , Giesy, J. , & van den Berg, M. (2001). Effects of chloro‐s‐triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environmental Health Perspectives, 109(10), 1027–1031. 10.1289/ehp.011091027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom, M. W. , Kanagy, L. K. , Anderson, C. A. , & Kanagy, C. J. (2015). Determination of pesticides and pesticide degradates in filtered water by direct aqueous‐injection liquid chromatography‐tandem mass spectrometry (US Geological Survey Techniques and Methods Report No. 5–B11). Reston, VA. 10.3133/tm5B11; 00
- Sayers, M. J. , Grimm, A. G. , Shuchman, R. A. , Bosse, K. R. , Fahnenstiel, G. L. , Ruberg, S. A. , & Leshkevich, G. A. (2019). Satellite monitoring of harmful algal blooms in the Western Basin of Lake Erie: A 20‐year time‐series. Journal of Great Lakes Research, 45(3), 508–521. 10.1016/j.jglr.2019.01.005 [DOI] [Google Scholar]
- Schlenk, D. , Huggett, D. B. , Allgood, J. , Bennett, E. , Rimoldi, J. , Beeler, A. B. , Block, D. , Holder, A. W. , Hovinga, R. , & Bedient, P. (2001). Toxicity of fipronil and its degradation products to Procambarus sp.: Field and laboratory studies. Archives of Environmental Contamination and Toxicology, 41(3), 325–332. 10.1007/s002440010255 [DOI] [PubMed] [Google Scholar]
- Schoenfuss, H. L. , Furlong, E. T. , Phillips, P. J. , Scott, T.‐M. , Kolpin, D. W. , Cetkovic‐Cvrlje, M. , Lesteberg, K. E. , & Rearick, D. C. (2016). Complex mixtures, complex responses: Assessing pharmaceutical mixtures using field and laboratory approaches: Biological effects of complex pharmaceutical mixtures. Environmental Toxicology and Chemistry, 35(4), 953–965. 10.1002/etc.3147 [DOI] [PubMed] [Google Scholar]
- Shipitalo, M. J. , & Owens, L. B. (2003). Atrazine, deethylatrazine, and deisopropylatrazine in surface runoff from conservation tilled watersheds. Environmental Science and Technology, 37(5), 944–950. 10.1021/es020870b [DOI] [PubMed] [Google Scholar]
- Shoda, M. E. , Nowell, L. H. , Stone, W. W. , Sandstrom, M. W. , & Bexfield, L. M. (2018). Data analysis considerations for pesticides determined by National Water Quality Laboratory schedule 2437 (United States Geological Survey Report No. 2018–5007).
- Simon‐Delso, N. , Amaral‐Rogers, V. , Belzunces, L. P. , Bonmatin, J. M. , Chagnon, M. , Downs, C. , Furlan, L. , Gibbons, D. W. , Giorio, C. , Girolami, V. , Goulson, D. , Kreutzweiser, D. P. , Krupke, C. H. , Liess, M. , Long, E. , McField, M. , Mineau, P. , Mitchell, E. A. , Morrissey, C. A. , … Wiemers, M. (2015). Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environmental Science and Pollution Research, 22(1), 5–34. 10.1007/s11356-014-3470-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. , Kumar, V. , Chauhan, A. , Datta, S. , Wani, A. B. , Singh, N. , & Singh, J. (2018). Toxicity, degradation and analysis of the herbicide atrazine. Environmental Chemistry Letters, 16(1), 211–237. 10.1007/s10311-017-0665-8 [DOI] [Google Scholar]
- van der Sluijs, J. P. , Amaral‐Rogers, V. , Belzunces, L. P. , Bijleveld van Lexmond, M. F. I. J. , Bijleveld van Lexmond, M. F. , Bonmatin, J. M. , Chagnon, M. , Downs, C. A. , Furlan, L. , Gibbons, D. W. , Giorio, C. , Girolami, V. , Goulson, D. , Kreutzweiser, D. P. , Krupke, C. , Liess, M. , Long, E. , McField, M. , Mineau, P. , … Wiemers, M. (2015). Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environmental Science and Pollution Research, 22(1), 148–154. 10.1007/s11356-014-3229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpoole, S. M. , Shoda, M. E. , Medalie, L. , & Stone, W. W. (2021). Pesticides in US rivers: Regional differences in use, occurrence, and environmental toxicity, 2013 to 2017. Science of the Total Environment, 787, 147147. 10.1016/j.scitotenv.2021.147147 [DOI] [PubMed] [Google Scholar]
- Stebbins‐Boaz, B. , Fortner, K. , Frazier, J. , Piluso, S. , Pullen, S. , Rasar, M. , Reid, W. , Sinclair, K. , & Winger, E. (2004). Oocyte maturation in Xenopus laevis is blocked by the hormonal herbicide, 2,4‐dichlorophenoxy acetic acid. Molecular Reproduction and Development, 67(2), 233–242. 10.1002/mrd.10396 [DOI] [PubMed] [Google Scholar]
- Stokstad, E. (2018). European Union expands ban of three neonicotinoid pesticides. Retrieved May 6, 2020, from: https://www.sciencemag.org/news/2018/04/european-union-expands-ban-three-neonicotinoid-pesticides
- Stone, W. W. , Gilliom, R. J. , & Ryberg, K. R. (2014). Pesticides in US streams and rivers: Occurrence and trends during 1992–2011. Environmental Science & Technology, 48(19), 11025–11030. 10.1021/es5025367 [DOI] [PubMed] [Google Scholar]
- Stratton, G. W. (1984). Effects of the herbicide atrazine and its degradation products, alone and in combination, on phototrophic microorganisms. Archives of Environmental Contamination and Toxicology, 13(1), 35–42. 10.1007/BF01055644 [DOI] [Google Scholar]
- Sumpter, J. P. (2005). Endocrine disrupters in the aquatic environment: An overview. Acta Hydrochimica et Hydrobiologica, 33(1), 9–16. 10.1002/aheh.200400555 [DOI] [Google Scholar]
- Tang, F. H. M. , Lenzen, M. , McBratney, A. , & Maggi, F. (2021). Risk of pesticide pollution at the global scale. Nature Geoscience, 14, 206–210. 10.1038/s41561-021-00712-5 [DOI] [Google Scholar]
- Thomas, R. S. , Paules, R. S. , Simeonov, A. , Fitzpatrick, S. C. , Crofton, K. M. , Casey, W. M. , & Mendrick, D. L. (2018). The US Federal Tox21 program: A strategic and operational plan for continued leadership. ALTEX: Alternativen zu Tierexperimenten, 35(2), 163–168. 10.14573/altex.1803011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorngren, J. L. , Harwood, A. D. , Murphy, T. M. , Hartz, K. E. H. , Fung, C. Y. , & Lydy, M. J. (2017). Fate and risk of atrazine and sulfentrazone to nontarget species at an agriculture site. Environmental Toxicology and Chemistry, 36(5), 1301–1310. 10.1002/etc.3664 [DOI] [PubMed] [Google Scholar]
- Thrupp, T. J. , Runnalls, T. J. , Scholze, M. , Kugathas, S. , Kortenkamp, A. , & Sumpter, J. P. (2018). The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Science of the Total Environment, 619–620, 1482–1492. 10.1016/j.scitotenv.2017.11.081 [DOI] [PubMed] [Google Scholar]
- Thurman, E. M. , & Cromwell, A. E. (2000). Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environmental Science and Technology, 34(15), 3079–3085. 10.1021/es000995l [DOI] [Google Scholar]
- Tice, R. R. , Austin, C. P. , Kavlock, R. J. , & Bucher, J. R. (2013). Improving the human hazard characterization of chemicals: A Tox21 update. Environmental Health Perspectives, 121(7), 756–765. 10.1289/ehp.1205784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillitt, D. E. , Papoulias, D. M. , Whyte, J. J. , & Richter, C. A. (2010). Atrazine reduces reproduction in fathead minnow (Pimephales promelas ). Aquatic Toxicology, 99(2), 149–159. 10.1016/j.aquatox.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Tixier, C. , Sancelme, M. , Sancelme, M. , Bonnemoy, F. , Cuer, A. , & Veschambre, H. (2001). Degradation products of a phenylurea herbicide, diuron: Synthesis, ecotoxicity, and biotransformation. Environmental Toxicology and Chemistry, 20(7), 1381–1389. 10.1002/etc.5620200701 [DOI] [PubMed] [Google Scholar]
- Traba, H. M. , Domínguez‐Morueco, N. , Barreno, E. , & Catalá, M. (2017). Lichen microalgae are sensitive to environmental concentrations of atrazine. Journal of Environmental Science and Health, Part B, 52(4), 223–228. 10.1080/03601234.2016.1270679 [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture‐Natural Resources Conservation Service, US Geological Survey, US Environmental Protection Agency . (2009). The Watershed Boundary Dataset (WBD); Vector digital data.
- US Environmental Protection Agency . (2022a). 3,4‐Dichloroaniline. CompTox Chemicals Dashboard. https://comptox.epa.gov/dashboard/chemical/details/DTXSID7021815
- US Environmental Protection Agency . (2022b). Exploring ToxCast data: Downloadable data. https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data
- US Environmental Protection Agency . (2022c). CompTox chemicals dashboard. https://comptox.epa.gov/dashboard
- US Environmental Protection Agency . (2002). Reregistration eligibility decision for chlorpyrifos (US Environmental Protection Agency Office of Pesticide Programs Report No. 738‐F‐01–006).
- US Environmental Protection Agency . (2004). Interim reregistration eligibility decision eligibility decision diazinon (Office of Pesticide Programs Report No. 738‐R‐04–006).
- US Environmental Protection Agency . (2005). Reregistration eligibility decision 2,4‐D (US Environmental Protection Agency Report No. 738‐R‐05–002).
- US Environmental Protection Agency . (2009a). Risks of diuron use to federally threatened California red‐legged frog (Rana aurora draytonii) (Office of Pesticide Programs Report No. EPA‐HQ‐OPP‐2009‐0081‐0140). Environmental Fate and Effects Division, Washington, DC.
- US Environmental Protection Agency . (2009b). Sulfentrazone registration review (Report No. EPA‐HQ‐OPP‐2009‐0624). Washington, DC.
- US Environmental Protection Agency . (n.d.) Triclopyr. CompTox Chemicals Dashboard. https://comptox.epa.gov/dashboard/chemical/details/DTXSID0032497
- US Environmental Protection Agency . (2016). Preliminary aquatic risk assessment to support the registration review of imidacloprid (US Environmental Protection Agency Office of Pesticide Programs Report No. 2008‐0844–1086). https://www.regulations.gov/document/EPA-HQ-OPP-2008-0844-1086
- US Environmental Protection Agency . (2019a). Aquatic life benchmarks and ecological risk assessments for registered pesticides. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk
- US Environmental Protection Agency . (2019b). Simazine proposed interim registration review decision case Number 0070 (Office of Pesticide Programs Report No. EPA‐HQ‐OPP‐2013‐0251).
- US Environmental Protection Agency . (2020). ToxCast & Tox21 summary files from invitrodb_v3.2. Retrieved May 5, 2020, from: https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data
- US Geological Survey . (2020). National Water Information System data available on the World Wide Web (USGS water data for the nation). Retrieved November 25, 2022, from: http://waterdata.usgs.gov/nwis
- Van Metre, P. C. , Alvarez, D. A. , Mahler, B. J. , Nowell, L. , Sandstrom, M. , & Moran, P. (2017). Complex mixtures of pesticides in Midwest US streams indicated by POCIS time‐integrating samplers. Environmental Pollution, 220, 431–440. 10.1016/j.envpol.2016.09.085 [DOI] [PubMed] [Google Scholar]
- Velisek, J. , Stara, A. , Zuskova, E. , Kubec, J. , Buric, M. , & Kouba, A. (2019). Effects of S‐metolachlor on early life stages of marbled crayfish. Pesticide Biochemistry and Physiology, 153, 87–94. 10.1016/j.pestbp.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Vilà‐Brau, A. , De Sousa‐Coelho, A. L. , Mayordomo, C. , Haro, D. , & Marrero, P. F. (2011). Human HMGCS2 regulates mitochondrial fatty acid oxidation and FGF21 expression in HepG2 cell line*. Journal of Biological Chemistry, 286(23), 20423–20430. 10.1074/jbc.M111.235044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve, D. (2021). AOP: 23. Androgen receptor agonism leading to reproductive dysfunction (in repeat‐spawning fish). AOP‐Wiki. https://aopwiki.org/aops/23
- Wang, S. , Salamova, A. , Hites, R. A. , & Venier, M. (2018). Spatial and seasonal distributions of current use pesticides (CUPs) in the atmospheric particulate phase in the Great Lakes region. Environmental Science and Technology, 52(11), 6177–6186. 10.1021/acs.est.8b00123 [DOI] [PubMed] [Google Scholar]
- Wang, Y.‐H. , Liu, C.‐L. , Chiu, W.‐C. , Twu, Y.‐C. , & Liao, Y.‐J. (2019). HMGCS2 mediates ketone production and regulates the proliferation and metastasis of hepatocellular carcinoma. Cancers (Basel), 11(12), 1876. 10.3390/cancers11121876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werck‐Reichhart, D. , & Feyereisen, R. (2000). Cytochromes P450: A success story. Genome Biology, 1(6), REVIEWS3003. 10.1186/gb-2000-1-6-reviews3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston, D. P. , & Lydy, M. J. (2014). Toxicity of the insecticide fipronil and its degradates to benthic macroinvertebrates of urban streams. Environmental Science & Technology, 48(2), 1290–1297. 10.1021/es4045874 [DOI] [PubMed] [Google Scholar]
- Whitehorn, P. R. , O'Connor, S. , Wackers, F. L. , & Goulson, D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science, 336(6079), 351–352. 10.1126/science.1215025 [DOI] [PubMed] [Google Scholar]
- Wieben, C. M. (2020). Estimated annual agricultural pesticide use for counties of the conterminous United States, 2013‐17 (ver. 2.0, May 2020). 10.5066/P9F2SRYH. [DOI]
- Wu, J. , Lu, J. , Lu, H. , Lin, Y. , & Chris Wilson, P. (2015). Occurrence and ecological risks from fipronil in aquatic environments located within residential landscapes. Science of the Total Environment, 518–519, 139–147. 10.1016/j.scitotenv.2014.12.103 [DOI] [PubMed] [Google Scholar]
- Wu, M.‐C. , Chang, Y.‐W. , Lu, K.‐H. , & Yang, E.‐C. (2017). Gene expression changes in honey bees induced by sublethal imidacloprid exposure during the larval stage. Insect Biochemistry and Molecular Biology, 88, 12–20. 10.1016/j.ibmb.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Yang, M. , Hu, J. , Li, S. , Ma, Y. , Gui, W. , & Zhu, G. (2016). Thyroid endocrine disruption of acetochlor on zebrafish (Danio rerio) larvae. Journal of Applied Toxicology, 36(6), 844–852. 10.1002/jat.3230 [DOI] [PubMed] [Google Scholar]
- Zanger, U. M. , & Schwab, M. (2013). Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology & Therapeutics, 138(1), 103–141. 10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Zhao, P. , & Furness, S. G. B. (2019). The nature of efficacy at G protein‐coupled receptors. Biochemical Pharmacology, 170, 113647. 10.1016/j.bcp.2019.113647 [DOI] [PubMed] [Google Scholar]
- Zuanazzi, N. R. , Ghisi, N. , de, C. , & Oliveira, E. C. (2020). Analysis of global trends and gaps for studies about 2,4‐D herbicide toxicity: A scientometric review. Chemosphere, 241, 125016. 10.1016/j.chemosphere.2019.125016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains online‐only Supporting Information.
Supporting File.
Supporting File.
Data Availability Statement
Data, associated metadata, and calculation tools are also available from the corresponding author (lloken@usgs.gov).


