Abstract
Objective
Postprandial hyperinsulinemic hypoglycemia with neuroglycopenia is an increasingly recognized complication of Roux‐en‐Y gastric bypass and gastric sleeve surgery that may detrimentally affect patient quality of life. One likely causal factor is glucagon‐like peptide‐1 (GLP‐1), which has an exaggerated rise following ingestion of carbohydrates after bariatric surgery. This paper sought to assess the role of GLP‐1 receptor agonists (GLP‐1RAs) in managing postprandial hypoglycemia following bariatric surgery.
Methods
MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, and Scopus were systematically and critically appraised for all peer‐reviewed publications that suitably fulfilled the inclusion criteria established a priori. This systematic review was developed according to the Preferred Reporting Items for Systematic Review and Meta‐Analyses Protocols (PRISMA‐P). It followed methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions and is registered with PROSPERO (International Prospective Register of Systematic Reviews; identifier CRD420212716429).
Results and Conclusions
Postprandial hyperinsulinemic hypoglycemia remains a notoriously difficult to manage metabolic complication of bariatric surgery. This first, to the authors' knowledge, systematic review presents evidence suggesting that use of GLP‐1RAs does not lead to an increase of hypoglycemic episodes, and, although this approach may appear counterintuitive, the findings suggest that GLP‐1RAs could reduce the number of postprandial hypoglycemic episodes and improve glycemic variability.
Study Importance.
What is already known?
There is limited evidence on the use of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) for the management of postprandial hypoglycemia even though this is a therapeutic approach adopted often in clinical practice.
What does this review add?
This is the first systematic review, to our knowledge, assessing the role of GLP‐1RAs for the management of postprandial hyperinsulinemic hypoglycemia with neuroglycopenia. Although there is some initial evidence that this class of medication could work, there is insufficient data to come to a definitive conclusion. There is some evidence that insulin and glucose peaks are synchronous when taking GLP‐1RAs. Based only on very limited data, there appears to be less variability of GLP‐1 release, which prevents excessive peaks of endogenous insulin and, ultimately, postprandial hypoglycemia. Whether this happens via continuous stimulation of the GLP‐1 receptor is not known. It could further work via reduced gastric emptying, reduction in appetite, earlier satiety, stimulation of glucagon, and reduction of small bowel motility.
How might these results change the direction of research or the focus of clinical practice?
Postprandial hypoglycemia following bariatric surgery is a notoriously difficult to manage metabolic complication. Future studies reviewing glycemic control with GLP‐1RAs and insulin peaks need to factor in participants' diet, whether they are symptomatic, and the length of follow‐up on treatment to clearly determine benefit. Reviewing combined GLP‐1 with glucose‐dependent insulinotropic polypeptide agonist efficacy and safety for the management of postprandial hypoglycemia could be another important target of future research.
INTRODUCTION
Bariatric surgery is the most effective treatment for complex obesity, inducing sustained weight loss and reducing obesity‐induced complications [1]. The most common type performed is sleeve gastrectomy (53.6%), followed by Roux‐en‐Y gastric bypass (RYGB; 30.1%) and one‐anastomosis gastric bypass (4.8%) [2].
A complication of bariatric surgery is dumping syndrome. Dumping syndrome can be divided into early and late stages. Early‐stage dumping occurs within the first hour of eating and it has various vasomotor and gastrointestinal symptoms. Late‐stage dumping syndrome is seen 1 to 3 hours after food with the occurrence of hypoglycemia (blood sugar < 4 mmol/L), which causes autonomic symptoms and neuroglycopenic symptoms. Whereas these episodes of hypoglycemia, in extreme cases, can be a risk of death, they also have a more significant effect on someone's quality of life because of the inability to perform activities of daily living [3, 4, 5, 6]. Blood glucose concentrations vary significantly throughout the day following bariatric surgery, and these variations may also contribute to patients' symptoms [7].
Following bariatric surgery, it can take 3 to 12 months for symptoms of hypoglycemia to appear; symptoms can appear even years later [3]. The prevalence of post‐bariatric surgery hypoglycemia appears higher than initially suggested by the standard mixed‐meal test. Continuous blood glucose monitoring (CGM) suggests it can occur in up to 55% of patients after laparoscopic vertical sleeve gastrectomy and up to 75% after RYGB, with many of these episodes of hypoglycemia occurring without symptoms [5, 8].
A large body of research has shown that a faster transit of contents into the small bowel leads to a steeper rise in blood sugar, followed by hyperinsulinemia, which then causes a rapid drop in blood sugar 1 to 3 hours after food [3, 8, 9, 10]. Therefore, patients who develop hypoglycemic episodes are said to have hyperinsulinemic hypoglycemia syndrome.
Following bariatric surgery, the stomach can no longer perform one of its functions: to hold nutrition like a reservoir and delay transit. Normally, postprandial glucose concentrations are determined by the rate of carbohydrates entering the small intestine, and slowing the flow helps reduce postprandial blood glucose [11, 12]. Following surgery, this is no longer possible, and alteration of the location and timing of carbohydrate absorption leads to an earlier and higher glycemic peak [8, 13, 14, 15]. Why this faster passage of nutrients leads to hypoglycemia episodes is not fully understood. One of the first theories behind hyperinsulinemic hypoglycemia syndrome suggests that patients had an increased β‐cell mass; however, reversing the procedure [13] or using gastric feeding tubes [13, 16] led to rapid reduction in both the postprandial insulin concentrations and the number of hypoglycemic episodes. Cessation of episodes would not have been expected straight after reversal or by altering the feeding route if they were due to increased β‐cell mass.
Since this earlier theory, there has been a significant amount of research into the cause of hyperinsulinemic hypoglycemia syndrome, with multiple reasons as to why it occurs, but, in general, this fast transit of nutrients through the gastrointestinal tract has led to alterations of insulin and gastrointestinal hormones. There appears to be both an increased β‐cell sensitivity with the increase to plasma glucose concentrations and decreased β‐cell sensitivity to hypoglycemia following surgery [9, 17, 18]. There is reduced insulin clearance [19] and alterations of gastrointestinal hormones. There also appears to be impaired α‐cell response to hypoglycemia [9, 20].
Of these potential causes, glucagon‐like peptide‐1 (GLP‐1) appears to have a causal association with post‐bariatric surgery hypoglycemia according to more recent evidence. GLP‐1 is a gut‐derived incretin hormone secreted from L cells primarily found in the ileum. Within minutes of ingesting food, there is secretion of GLP‐1, suggesting that release is not due to direct stimulation but by neuronal or endocrine signaling [21]. GLP‐1 becomes one of the molecules responsible for glucose‐dependent insulin secretion from β cells and for delaying gastric emptying.
Surgical alterations in anatomy lead to nutrients entering the distal jejunum almost immediately. This leads to up to a 10 to 20‐fold increase in GLP‐1 release [1, 10]. The rapid rise precedes the excessive insulin secretion and is thought to be the key mediator to the insulin release from β cells. The insulinotropic effects of GLP‐1 are dependent on both the concentrations of blood glucose and GLP‐1. This early and rapid peak of GLP‐1 and faster transition of food into the small bowel leads to the peak in insulin becoming decoupled and dyssynchronous from the blood glucose level; therefore, hypoglycemia occurs a few hours after a meal [3, 5, 7, 9, 13, 22, 23, 24, 25]. GLP‐1 release is significantly higher in those who have versus in those who have not had surgery, and there is higher GLP‐1 and insulin secretion in those who develop hypoglycemia compared with those who do not [3, 9, 24, 26]. Hyperinsulinemia appears to only occur postprandially [17, 24, 27]. This would explain why hypoglycemia is rare when fasting [27] and why there was no difference in insulin concentrations among nonsurgical and postsurgical groups when intravenous dextrose was infused [24].
Although GLP‐1 is not the only cause of enhanced insulin secretion and hypoglycemia following bariatric surgery [24, 28], it still has a significant contribution. This is reinforced by studies that have shown that antagonizing the action of GLP‐1 leads to significant reduction in hypogylcemic episodes, with an antagonistic drug almost completely preventing cases of neuroglycemic symptoms in some cases [25, 29, 30, 31, 32]. Displaying that blocking GLP‐1 can resolve episodes further reinforces its involvement in late‐stage dumping syndrome.
Treatment for postprandial hypoglycemic episodes is split into dietary, pharmacological, and surgical, with dietary being the gold standard and medical or surgical being complementary but failing too often. Limiting carbohydrate intake, with less absorbable carbohydrates in those meals, and high‐protein and high‐fiber content reduces glucose variability and leads to a reduction in hypoglycemic events [3, 33]. Smaller meals and meals of solid texture also lead to fewer hypoglycemic episodes [3, 34]. Fluid intake is advised to be reduced until 30 minutes after a meal [3]. Pharmacological treatments that have been used include acarbose, diazoxide, calcium channel blockers, and octreotide. The efficacy and tolerability of these medications have mixed results. Acarbose is an α‐Glucosidase that slows the release of monoglycerides from carbohydrates and has been shown to reduce frequency or intensity of hypoglycemic episodes [3, 7, 35, 36]. However, it can lead to bloating, flatulence, and diarrhea [35]. Diazoxide acts through ATP‐sensitive potassium channels, inhibiting insulin release from β cells [35]. However, upon review of its evidence, it was not deemed to lead to a significant improvement to hyperinsulinemic hypoglycemia syndrome [3]. Calcium channel blockers work by direct action on β cells to inhibit glucose‐induced insulin release [3, 4], but the evidence of their efficacy is not strong [3]. Somatostatin analogues reduce the secretion of gastrointestinal hormones and inhibit insulin secretion and postprandial vasodilation [3, 7, 35, 37]. Although short‐acting injections were better for symptom control, long‐acting somatostatin analogues were preferred because of the convenience of lower frequency of injections [3]. Although these medications have been shown to have been of benefit, patients do not always have a sufficient response to them [3], which is why further treatment methods need to be explored.
One potential method is the use of GLP‐1 receptor agonists (GLP‐1RAs) [7, 9, 38, 39, 40]. They work by mimicking native GLP‐1 and are resistant to dipeptidyl peptidase‐4 (DDP‐4), which usually breaks it down within minutes [21, 41]. They decelerate gastric emptying, which contributes to increased feelings of fullness and satiety, lower appetite, reduction of postprandial hyperglycemia, promotion of insulin secretion, reduction of β‐cell workload, reduction of intestinal glucose uptake, and slowing of nutrient transit through the small intestine, which helps attenuate glycemic control [12, 42, 43, 44, 45]. GLP‐1RAs have been well established in the treatment of type 2 diabetes mellitus (T2DM) by promoting weight loss and improving insulin sensitivity without causing hypoglycemia [21, 41, 45, 46, 47]. Even after bariatric surgery, GLP‐1RAs continue to aid weight loss in secondary poor responders to surgery or those that regain weight. They also protect from cardiovascular and metabolic risk factors [46, 48, 49, 50, 51, 52]. Although it would be thought that increasing GLP‐1 would be counterintuitive and worsen postprandial hypoglycemia, it appears that this class of drugs could reduce the frequency of episodes [9, 38, 39]. One theory is that the medication occupies the GLP‐1 receptor, keeping a more consistent level and thereby blocking the excessive peak of postprandial endogenous GLP‐1 from accessing the receptor. By the aforementioned actions, the medication could thereby prevent the excessive peak of endogenous insulin, reduce glycemic variability, and, ultimately, reduce postprandial hypoglycemia [39, 53]. As GLP‐1 and GLP‐1RAs both have preliminary results suggesting that they could reduce hypoglycemic episodes, these contrasting methods further highlight that we do not yet fully understand the etiology of hyperinsulinemic hypoglycemia syndrome.
To test the efficacy of GLP‐1RAs and how they work on patients with hyperinsulinemic hypoglycemia syndrome following bariatric surgery, a systematic review was performed.
METHODS
The reporting of this review has been according to the Preferred Reporting Items for Systematic review and Meta‐Analysis Protocols (PRISMA‐P) (54) and followed methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions. This systematic review has been registered with PROSPERO (International Prospective Register of Systematic Reviews; identifier CRD42021271642).
Search strategy
Systematic searches of the MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, and Scopus databases were conducted. Our key Medical Subject Heading terms were “GLP‐1” OR “GLP‐1 receptor agonist” AND “bariatric surgery” AND “hypoglycemia.”
Reference lists of selected articles and other literature sources were browsed to ensure a comprehensive literature search was completed. None of the database searches filtered results based on year of publication date, and the last search was carried out in July 2021.
Study selection
Expert opinion manuscripts, letters to the editor, commentaries, conference papers, animal studies, meta‐analyses, and articles not published in English were excluded. Data were only included on adults (18 years or older), men, and women who were not pregnant. Studies assessing GLP‐1RA efficacy for management of hypoglycemia in participants who had not undergone bariatric surgery were not included.
No restrictions were made regarding the intervention or exposure type, where a study took place, the number of participants, or the duration of follow‐up. Rayyan systemic review software (http://www.rayyan.ai) was used for manuscript screening and extraction. Publications were initially screened for any duplicates before being assessed independently and in parallel by two reviewers (David C. Llewellyn and Hugh Logan Ellis). Any conflicts regarding the inclusion of a study were met with discussion and consensus. If an agreement had not been reached, arbitration by a third reviewer was used (Georgios K. Dimitriadis).
Data extraction
Data were extracted independently by two reviewers following the “Cochrane Public Health Group Data Extraction and Assessment Template” to construct our own data extraction template that was pilot‐tested and systematically used for each article. Data extracted included study description (e.g., title, primary author, publication year, type of study, number of participants), patient‐reported outcomes and quality of life, adverse effects, and number of hypoglycemic events measures (measured by multiple methods, including mixed‐meal tests, continuous glucose monitoring, flash glucose test, and oral glucose tolerance test [OGTT]). The primary outcome was the number of hypoglycemic events reduced and the impact of GLP‐1RAs on improvement in symptoms of hypoglycemia following bariatric surgery. The review did not remove studies from analysis based on how they determined a patient had a hypoglycemic event. The number of events were therefore allowed to be reported by any method, e.g., via a glucose monitor or dynamic testing such as a mixed‐meal test. We included any effect measurement (i.e., relative risks, odds ratio, risk difference).
Quality assessment
Quality assessment of experimental studies was conducted in line with the Cochrane Collaboration's risk of bias 2 (RoB‐2) tool [55]. Observational studies were evaluated using the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS)‐I tool for nonrandomized studies (Table 2) [56]. Within the assessment of each study was review of treatment allocation, blinding, and how the outcome (reduction in hypoglycemic episodes) was analyzed. A narrative synthesis was made of this assessment, which was used to suggest areas that future research would evaluate. Two reviewers were involved in assessment of bias. In case of disagreement, arbitration by a third reviewer was sought.
TABLE 2.
Assessment of RoB [Color table can be viewed at wileyonlinelibrary.com]
| Abrahamsson et al. [38] | Chiappetta and Stier [39] | Tharakan et al. [9] | Øhrstrøm et al. [7] | Almby et al. [40] | Ding et al. [56] | |
|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) |

|

|

|

|

|

|
| Allocation concealment (selection bias) |

|

|

|

|

|

|
| Blinding of participants and personnel (performance bias) |

|

|

|

|

|

|
| Blinding of outcome assessment (detection bias) |

|

|

|

|

|

|
| Incomplete outcome data (attrition bias) |

|

|

|

|

|

|
| Selective reporting (reporting bias) |

|

|

|

|

|

|
| Risk of overall bias |

|

|

|

|

|

|
Note: Experimental studies were conducted in line with the Cochrane Collaboration's RoB‐2 tool [55]. Observational studies were evaluated using the ROBINS‐I (Risk of Bias in Nonrandomized Studies of Interventions) tool [56]. “+” indicates low risk of bias, “?
indicates low risk of bias, “? ” indicates unclear bias, and “−”
” indicates unclear bias, and “−” indicates high risk of bias.
indicates high risk of bias.
Abbreviation: RoB, risk of bias.
Publication bias planned to be assessed using Egger's and Begg's tests along with a visual evaluation of the funnel plots when at least 10 studies were available.
Data synthesis and statistical analysis
Heterogeneity was visually inspected and, owing to the high variability and insufficient data, results were not pooled into a meta‐analysis and a narrative synthesis was conducted instead.
RESULTS
The results of the screening process are seen in the PRISMA‐P flow diagram, shown in Figure 1. Our search found five published papers and one clinical trial that has been completed but with results that have not yet been published. Of the published articles, one was a case report, one a case series of five patients, and the other three were randomized controlled trials (RCTs). One of the RCTs was not focused on GLP‐1RAs; therefore, only one of their patients was on the medication. Two of the RCTs were randomized crossover studies. Only a total of 30 patients could be included in the analysis (Table 1).
FIGURE 1.
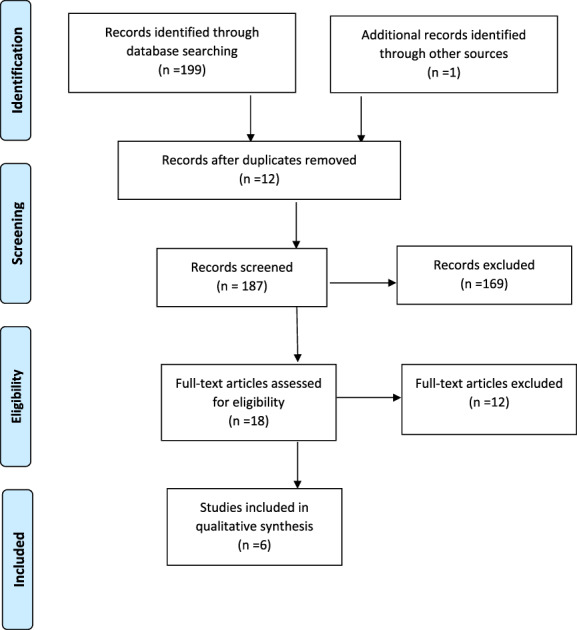
PRISMA‐P (Preferred Reporting Items for Systematic Review and Meta‐Analyses Protocols) flow diagram of the systemic review, modified from Moher et al. [54] [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Characteristics of studies using GLP‐1 agonists for postprandial hypoglycemia
| Study | Country | Study design | Follow‐up | Sample size on GLP‐1RA (n) | Length of time on intervention | Symptomatic of hypoglycemia | Assessment of outcome | Quality assessment, ROBINS‐I/RoB‐2 |
|---|---|---|---|---|---|---|---|---|
| Abrahammson et al., 2013 [ 38 ] | Sweden | Case series | Varied, minimum 6 months | 5 | Varied with each case, minimum 1 year | 5 | All participants had reduced episodes of postprandial hypoglycemia or none at all. 3 attempted to stop drug but, owing to symptoms, recurring liraglutide was restarted. Symptoms then resolved. | Moderate risk |
| Tharakan et al., 2017 [ 9 ] | UK | RCT | Not done | 1 | Unclear | 18 | Reduction in postprandial hypoglycemic episodes. | Low risk |
| Øhrstrøm et al., 2019 [ 7 ] | Denmark | Randomized crossover study | Not done | 11 | 3 weeks | 11 | No difference to number of hypoglycemic episodes. No change to insulin concentrations. Hypoglycemic episodes were less severe. There was significantly reduced glycemic variability and less time spent with hyperglycemia. Reduced negative glucose AUC. | Low risk |
| Almby et al., 2019 [ 40 ] | Sweden | Randomized crossover study | Not done | 12 | Infusion lasted for 165 minutes | 0 | Exenatide had no plasma glucose‐raising effect and did not alter glucagon. It did not increase number of hypoglycemic episodes. Exenatide did not alter symptoms. Drop of GLP‐1 less during hypoglycemia with exenatide. | Low risk |
| Ding et al., 2021 [ 53 ] | China | Case report | Not done | 1 | 1 month | 0 | Symptoms in remission. 100% of blood glucose readings within range. Insulin concentrations decreased and glucose peak delayed. Insulin and glucose peaks occurred at same time. | Moderate risk |
| Shalamar D. Sibley, MD (unpublished data, 2021) | United States | Randomized crossover study | Unknown | 11 | Unknown | 11 | Results not yet published. | Unknown |
Abbreviations: AUC, area under the curve; GLP‐1, glucagon‐like peptide‐1; GLP‐1RA, GLP‐1 receptor agonist; RCT, randomized controlled trial; RoB, risk of bias; ROBINS, Risk of Bias in Nonrandomized Studies of Interventions.
There were studies that showed that GLP‐1RAs did not increase hypoglycemic episodes following bariatric surgery, but they were not included in the review because they did not specifically investigate hyperinsulinemic hypoglycemia syndrome. Gorgojo‐Martinez et al. [46] evaluated the effectiveness and tolerability of liraglutide following bariatric surgery with comparison to a nonsurgical group of patients with diabetes. The study's primary outcome was to monitor glycated hemoglobin (HbA1c) and weight while on liraglutide. Miras et al.’s [51] primary outcome was change to HbA1c.
Another study not included within the review was that of Chiappetta and Stier [39]. They presented a case report of a female individual who had suffered with hypoglycemia following a Toupet fundoplication, which was performed because of severe acid reflux. Touper fundoplication is not a type of bariatric surgery and could not be included in the qualitative analysis. The patient in this study was trialed on 0.6 mg of liraglutide (Victoza) initially but increased to 1.2 mg owing to insufficient response. The increased dose led to synchronization of the insulin and glucose peaks during the OGTT and, therefore, cessation of hypoglycemia. The insulin peak was noted sooner, with reduced fasting insulin with treatment compared with without treatment. Peak insulin concentration was much lower, at 413 μU/mL from the initial 717 μU/mL without treatment, and glucose concentration remained above 66 mg/dL, with 84% of the CGM readings remaining in the normoglycemia range.
Since the first publication on the use of GLP‐1RAs for hyperinsulinemic hypoglycemia syndrome, by Abrahamsson et al. [38], there has been minimal published research. Clinicians may have trialed the medication but not seen an effect and, therefore, might not have published their findings. This could have led to publication bias.
We failed in our attempts to perform a meta‐analysis despite considering a variety of methods. A two‐staged individual patient‐level meta‐analysis and regular (non‐meta‐scientific) statistical analysis had been attempted, but, owing to differences in studies and missing data, it was not possible. We failed in our attempts to get all of the required data. Owing to not having standard deviations for single‐patient studies, we could not combine studies into forest plots and produce results with a random‐effects model.
The first paper to explore the effect of GLP‐1RAs on hyperinsulinemic hypoglycemia syndrome was Abrahamsson et al. [38]. In a case series of five patients in which four of the five did not have prior T2DM, they found that GLP‐1RAs had a protective effect on symptomatic postprandial hypoglycemia in all of these patients. Three of the five patients stopped the medication but then relapsed and thereby had to be restarted on it, which led to improvement of symptoms. Three of the five patients needed to continue the medication. One patient stopped it and found symptoms of hypoglycemia had resolved completely, and another had to stop altogether because of side effects from the medication.
Tharakan et al. [9] recruited 18 participants who had RYGB surgery with symptomatic postprandial hypoglycemia and gave them a standardized liquid mixed‐meal test. Only one of these participants was on a GLP‐1RA medication, liraglutide (0.6 mg/d, brand unknown), but it was found that this patient had improvement on frequency of postprandial hypoglycemia. Although the authors did not directly test for how GLP1‐RAs worked, they hypothesized that the improvement of patient's hypoglycemic symptoms while on the medication was likely owing to delaying of small bowel transit, which reduces the speed at which nutrients enter the jejunum. By producing a GLP‐1 concentration that was less variable via continuous stimulation of their receptors, the blood glucose became less variable, with less hypoglycemic episodes.
Øhrstrøm et al. [7] compared five different treatments in the same group of participants, including 1.2 mg of liraglutide. Although there were increased GLP‐1 concentrations after the mixed‐meal test with liraglutide, overall, there was no difference to insulin or C‐peptide concentrations, and the raised GLP‐1 did not lead to worsening hypoglycemic episodes. However, there was no improvement in the number of hypoglycemic episodes and no change to postprandial insulin concentrations. Treatment with liraglutide led to several important improvements. It increased the lowest interstitial blood glucose readings on the CGM; therefore, the hypoglycemic episodes were less severe. There was significantly reduced glycemic variability and less time spent with hyperglycemia. A 45% to 52% reduction of time spent with interstitial glucose > 7.8 mmol/L was seen and a 54% to 68% reduction of time spent >10.0 mmol/L was observed. There was significantly reduced negative incremental area under the curve for glucose, reduced fasting blood glucose, and raised fasting C‐peptide.
Almby et al. [40] used a hyperinsulinemic hypoglycemic clamp technique on two separate occasions: one with an infusion of the GLP‐1RA medication exenatide and the other with saline. The order of this was randomized and blinded. Along with this infusion, a glucose infusion was commenced to achieve a blood glucose concentration of 5 mmol/L before the infusion rate dropped to gradually reduce the blood glucose to 2.7 mmol/L. The exenatide or saline infusion would then stop, and participants' blood glucose would be allowed to rise via an increase in the infusion of glucose. Although it had been queried by Abrahamsson et al. [37] that hypoglycemia led to upregulation of glucagon via stimulation of α cells, Almby et al. found that hypoglycemia did not alter glucagon levels significantly when infused with exenatide. There was no difference in glucose infusion rate either to induce the hypoglycemia or resolve it, suggesting that exenatide had no plasma glucose‐inducing effect during hypoglycemic episodes. There was also no difference to other hypoglycemia counter‐regulatory hormones such as cortisol, catecholamines, and growth hormone, as well as no difference in noradrenaline or adrenaline. Exenatide did not lead to a reduction in symptoms when hypoglycemia occurred. Almby et al. showed that, whereas the GLP‐1 concentrations were reduced in both groups with clamping, the reduction was more significant in the saline infusion. They also found that exenatide led to a reduction in another incretin hormone linked to hyperinsulinemic hypoglycemia: glucose‐dependent insulinotropic polypeptide (GIP). These two findings suggested possible negative feedback with GLP‐1/GLP‐1RAs and GIP. It was concluded that, during episodes of hypoglycemia following bariatric surgery, GLP‐1RAs do not impact counter‐regulatory hormonal and metabolic responses.
For one patient for 1 month, Ding et al. used twice‐daily 0.1 mg of beinaglutide, a GLP‐1RA medication that shares 100% homology with human GLP‐1, and showed that there was significant improvement on hypoglycemic episodes [53]. An OGTT and CGM were performed at the end of the month. Insulin concentrations were decreased, the glucose peak was delayed and was synchronous with the insulin peak, and glucose concentrations remained within normal range (3.9‐10 mmol/L) 100% of the time. The patient had no side effects from medication, and hypoglycemia remained in remission.
A recent pilot study by Shalamar D. Sibley, MD (unpublished data, 2021) reviewed the effect of exenatide on postprandial blood glucose and insulin concentrations on patients who had RYGB surgery with known post‐bariatric surgery hypoglycemia. Their results are not yet published.
DISCUSSION
Interpretation of these studies is difficult for multiple reasons. There is minimal amount of data. Only 30 patients could be used for analysis. Of the papers available, one is a case series, one was a case report, and one of the RCTs was not focused on GLP‐1RAs; therefore, only one of its patients happened to be on the medication. This led to only two RCTs, one of which was Almby et al. [40], in which patients were fasting, which is not when the hypoglycemic events occur in this group of patients.
Diet control is one of the important treatments of hyperinsulinemic hypoglycemia syndrome. In this group of patients, alterations in diet not only reduce glucose variability but also the number of hypoglycemic events [3, 33, 34]. The six studies reviewed all differ in the type of diet the patients were on. Abrahamsson et al. [38] asked patients to have meals six to seven times a day with low‐carbohydrate and high‐protein content and no drinking during meals. Tharakan et al. [9] had patients who were meant to be on a low glycemic index (GI) diet, but it was unknown how many were doing this during the measurements. Øhrstrøm et al. [7] had patients on their usual diet. As they had only 11 patients, it could be that the majority of these had a diet that was more likely to induce hypoglycemia and was thereby masking the effect of the medication. Almby et al. [40] reviewed their patients while fasting.
Length of treatment and dosing
A further consideration is whether GLP‐1RAs were given for long enough to lead to a reduction in hypoglycemic events. With Øhrstrøm et al. [7], 1.2 mg of liraglutide was given for 3 weeks. It is unclear how long patients were on liraglutide with Tharakan et al. [9], as it is unclear how quickly the symptoms of hypoglycemia resolved with Abrahamsson et al. [38]. Ding et al. [53] gave beinaglutide for 1 month and saw symptoms completely resolve.
Øhrstrøm et al. [7] reported side effects, including loss of appetite and nausea, in 45% of patients. As GLP‐1RAs could reduce postprandial hypoglycemia episodes by inducing alterations in food preferences, a reduction in appetite, delay on nutritional transit, and causing of earlier satiety [12, 42, 43, 44, 45], it is unclear whether the medications were given long enough to induce these effects.
Tolerance and safety
GLP‐1RAs are generally well tolerated when used long‐term. Gorgojo‐Martinez et al. [46] compared two groups: one was on liraglutide for 2 years, and the other had bariatric surgery but was also on liraglutide for 2 years. GLP‐1RAs appear to be well tolerated in long‐term use after bariatric surgery and have significant benefits on weight loss and HbA1c [46].
With long‐term use of GLP‐1RAs following bariatric surgery, liraglutide appears relatively well tolerated, with the most common side effects being gastrointestinal (nausea, constipation, and diarrhea being the most common) [51, 52]. Of the 117 patients on 3 mg of liraglutide, Wharton et al. [52] found that 29.1% developed nausea, 13% constipation, and 8% diarrhea. Miras et al. [51] recruited 53 patients and treated them with 1.8 mg of liraglutide. Of them, 17% developed nausea, 2% constipation, and 8% diarrhea. It does not appear that concomitant use of GLP‐1RAs increases the risk for more severe complications if a person has had bariatric surgery, such as pancreatitis, cholecystitis, or an allergic reaction. Although Wharton et al. [52] reported a potential marginal increase in severe adverse events with 3 mg of liraglutide, as two patients had an allergic reaction and one developed pancreatitis, they admit this may just be owing to the smaller sample size. Miras et al. [51] did not have similar findings when patients were on 1.8 mg of liraglutide. The use of GLP‐1RAs does not appear to increase the risk of developing hypoglycemia following bariatric surgery. Although Abrahamson et al. [38] and Chiappetta and Stier [39] were directly reviewing the reduction of hypoglycemic events due to GLP‐1RAs, other studies have reviewed this class of medication post bariatric surgery for a separate reason but noted no hypoglycemic events. In the GLP‐1 Receptor Agonist Intervention for Poor Responders After Bariatric Surgery (GRAVITAS) RCT, Miras et al. [51] gave liraglutide to 53 participants who had bariatric surgery with persistent or refractory T2DM. Of those on liraglutide, only two had reported hypoglycemic episodes. One of these patients was on insulin, and one was on a sulfonylurea; therefore, this event may have been owing to these medications. Gorgojo‐Martinez et al. [46] did not report a significant increase in hypoglycemic episodes in the surgical versus medical groups that were both taking the same dose of liraglutide. However, both Miras et al. [51] and Gorgojo‐Martinez et al. [46] may have under‐recorded events because hypoglycemia assessment was not among their outcomes, and participants may have had asymptomatic hypoglycemia.
Areas of future work
Further research is needed to determine how GLP‐1RAs reduce the frequency of postprandial hypoglycemic episodes. There were two studies presenting case reports that showed that insulin peak decreases and synchrony occurs between insulin and glucose peaks, with a reduction/complete resolution of hypoglycemia. However, only one of these studies was on a patient who had bariatric surgery. Therefore, further studies reviewing hyperinsulinemic hypoglycemia syndrome following bariatric surgery should evaluate the peaks of GLP‐1 and insulin in a similar way as done by Ding et al. [53] and Chiappetta and Stier [39] to identify how they differ pre and post GLP‐1RA use.
Importantly, there has been no research, to our knowledge, into the combined effects of low GI diet with GLP‐1RAs. Reviewing whether treating patients with GLP‐1RAs facilitates adherence to a low GI diet by reducing carbohydrate cravings, quantity of food intake with each meal, and number of meals would be important.
Further studies should evaluate the usefulness of different methods to determine hyperinsulinemic hypoglycemia and response to treatment using CGM and mixed‐meal tests that could look at any alteration of glucose, GLP‐1, insulin, and glucagon concentrations. A further area that future research needs to consider is possible differences among subgroups: e.g., those with reduced insulin clearance, those with reduced counter‐regulatory hormone responses, or those who develop different severities of hypoglycemia.
It is not clear what length of GLP‐1RA treatment is required to improve postprandial hypoglycemia. A future RCT investigating the use of GLP‐1RAs to manage postprandial hypoglycemia following bariatric surgery would require a longer follow‐up period to clearly demonstrate benefit.
There has been a lot of recent research into the combined use of GLP‐1RAs with GIP agonists in T2DM that has shown that the addition of a GIP agonist could potentiate the positive effects of GLP‐1RAs on T2DM [57, 58, 59]. Although it is suggested that a GIP agonist could also antagonize the emetic signaling pathways of GLP‐1RAs, a Cochrane review suggested that it did not [58]. It would be interesting to see whether dual agonists would work in patients with hyperinsulinemic hypoglycemia syndrome following bariatric surgery.
Shoda et al. [60] demonstrated that patients who had Roux‐en‐Y reconstruction following distal gastrectomy for gastric carcinoma were more likely to develop hypoglycemic episodes. Future research should investigate whether GLP‐1RAs could be used for the management of such patients.
Strengths and weaknesses
The main weakness of this work is the low number of studies available to investigate and pool into a qualitative synthesis. There were only 31 patients who could be included for analysis. Nevertheless, a systematic review can be conducted with two or more manuscripts. A further issue relating to the previous point is the heterogenous evidence, which did not allow us to pool it to a quantitative synthesis (meta‐analysis). There is growing data on GLP‐1RA use post bariatric surgery, but this mostly reviews effects on weight and metabolic and cardiovascular risk factors rather than treatment's effect on hyperinsulinemic hypoglycemia syndrome. Of the data that are available, there are some important differences among the studies. This makes it hard to draw conclusions. The strengths of this review are that it has highlighted some important areas that future research needs to address to ensure firmer conclusions can be drawn from the effectiveness of GLP‐1RAs on reducing hypoglycemic events using very stringent methodology. A further strength is that this work represents the first systematic review, to our knowledge, on this topic. Our search and interpretation of existing evidence suggests that GLP‐1RAs do not cause worsening of postprandial hypoglycemia. In contrast, existing evidence suggests that GLP‐1RAs may improve hypoglycemia following bariatric surgery.
CONCLUSION
Treatment for hyperinsulinemic hypoglycemia syndrome following bariatric surgery includes various dietary, medical, and surgical interventions, but these are not always successful in reducing hypoglycemic episodes. Therefore, alternative treatments must be considered. Although the use of GLP‐1RAs appears counterintuitive, early research has suggested that it could reduce the number of postprandial hypoglycemic episodes and improve glycemic variability. We have performed the first systematic review, to our knowledge, of the literature, and, although there is some evidence to support the use of GLP‐1RAs in late‐stage dumping syndrome, further work is required to determine an ideal position in the therapeutic algorithm for this class of drugs.
Future studies reviewing glycemic control with GLP‐1RAs and insulin peaks need to factor in the participants' diet, whether they are symptomatic, and the length of follow‐up on treatment to clearly determine benefit. We have suggested possible methods to assess whether this class of drugs works in reducing postprandial hypoglycemia and how they work. Future studies could also include a review of GLP‐1RAs combined with GIP agonists such as tirzepatide.
The evidence into a GLP‐1 mechanism of action in the context of postprandial hypoglycemia is currently inconclusive. GLP‐1RAs did not appear to reverse an episode of hypoglycemia by counter‐regulatory hormonal and metabolic responses during a fasting state, but it is more likely that they work by preventing an episode occurring when patients are eating. There is some evidence that the insulin and glucose peaks are synchronous when taking GLP‐1RAs. Based only on very limited data, there appears to be less variability of GLP‐1 release, which prevents excessive peaks of endogenous insulin and, ultimately, postprandial hypoglycemia. Whether this happens via continuous stimulation of the GLP‐1 receptor is not known. It could further work via reduced gastric emptying, reduction in appetite, earlier satiety, stimulation of glucagon, and reduction of small bowel motility.
We recommend that, should other treatment modalities fail, GLP‐1RAs are an option in the treatment of hyperinsulinemic hypoglycemia syndrome following bariatric surgery.
AUTHOR CONTRIBUTIONS
Georgios K. Dimitriadis conceived the idea for this work. David C. Llewellyn, Hugh Logan Ellis, and Georgios K. Dimitriadis designed the protocol of the systematic review. Literature search was carried out by David C. Llewellyn, Hugh Logan Ellis, and William Sheridan. Study screening and selection were performed by all authors. David C. Llewellyn composed the first draft, which was interpreted and edited with the help of Georgios K. Dimitriadis. The final version of the manuscript was reviewed and approved by all authors.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
Llewellyn DC, Logan Ellis H, Aylwin SJB, et al. The efficacy of GLP‐1RAs for the management of postprandial hypoglycemia following bariatric surgery: a systematic review. Obesity (Silver Spring). 2023;31(1):20‐30. doi: 10.1002/oby.23600
REFERENCES
- 1. Svane MS, Madsbad S. Bariatric surgery ‐ effects on obesity and related co‐morbidities. Curr Diabetes Rev. 2014;10:208‐214. [DOI] [PubMed] [Google Scholar]
- 2. Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg. 2018;28:3783‐3794. [DOI] [PubMed] [Google Scholar]
- 3. Scarpellini E, Arts J, Karamanolis G, et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16:448‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malik S, Mitchell JE, Steffen K, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract. 2016;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evers SS, Kim KS, Bozadjieva N, et al. Continuous glucose monitoring reveals glycemic variability and hypoglycemia after vertical sleeve gastrectomy in rats. Mol Metab. 2020;32:148‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Øhrstrøm CC, Worm D, Hansen DL. Postprandial hyperinsulinemic hypoglycemia after Roux‐en‐Y gastric bypass: an update. Surg Obes Relat Dis. 2017;13:345‐351. [DOI] [PubMed] [Google Scholar]
- 7. Øhrstrøm CC, Worm D, Højager A, et al. Postprandial hypoglycaemia after Roux‐en‐Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide. Diabetes Obes Metab. 2019;21:2142‐2151. [DOI] [PubMed] [Google Scholar]
- 8. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103:2815‐2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tharakan G, Behary P, Wewer Albrechtsen NJ, et al. Roles of increased glycaemic variability, GLP‐1 and glucagon in hypoglycaemia after Roux‐en‐Y gastric bypass. Eur J Endocrinol. 2017;177:455‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long‐term effects of Roux‐en‐Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122‐E131. [DOI] [PubMed] [Google Scholar]
- 11. Chaikomin R, Wu KL, Doran S, et al. Concurrent duodenal manometric and impedance recording to evaluate the effects of hyoscine on motility and flow events, glucose absorption, and incretin release. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1099‐G1104. [DOI] [PubMed] [Google Scholar]
- 12. Ma J, Pilichiewicz AN, Feinle‐Bisset C, et al. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med. 2012;29:604‐608. [DOI] [PubMed] [Google Scholar]
- 13. Davis DB, Khoraki J, Ziemelis M, Sirinvaravong S, Han JY, Campos GM. Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: confirming a non‐pancreatic etiology. Mol Metab. 2018;9:15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Svane MS, Toft‐Nielsen MB, Kristiansen VB, et al. Nutrient re‐routing and altered gut‐islet cell crosstalk may explain early relief of severe postprandial hypoglycaemia after reversal of Roux‐en‐Y gastric bypass. Diabet Med. 2017;34:1783‐1787. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22:2003‐2009. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95:1851‐1855. [DOI] [PubMed] [Google Scholar]
- 17. Honka H, Salehi M. Postprandial hypoglycemia after gastric bypass surgery: from pathogenesis to diagnosis and treatment. Curr Opin Clin Nutr Metab Care. 2019;22:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poitou C, Bouaziz‐Amar E, Genser L, Oppert JM, Lacorte JM, Le Beyec J. Fasting levels of glicentin are higher in Roux‐en‐Y gastric bypass patients exhibiting postprandial hypoglycemia during a meal test. Surg Obes Relat Dis. 2018;14:929‐935. [DOI] [PubMed] [Google Scholar]
- 19. Salehi M, Gastaldelli A, D'Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99:2008‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabiee A, Magruder JT, Salas‐Carrillo R, et al. Hyperinsulinemic hypoglycemia after Roux‐en‐Y gastric bypass: unraveling the role of gut hormonal and pancreatic endocrine dysfunction. J Surg Res. 2011;167:199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piya MK, Tahrani AA, Barnett AH. Emerging treatment options for type 2 diabetes. Br J Clin Pharmacol. 2010;70:631‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond). 2009;33(suppl 1):S33‐S40. [DOI] [PubMed] [Google Scholar]
- 23. Roberts GP, Kay RG, Howard J, Hardwick RH, Reimann F, Gribble FM. Gastrectomy with Roux‐en‐Y reconstruction as a lean model of bariatric surgery. Surg Obes Relat Dis. 2018;14:562‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon‐like peptide 1‐stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon‐like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146:669‐680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678‐4685. [DOI] [PubMed] [Google Scholar]
- 27. Patti ME, Goldfine AB. Hypoglycemia after gastric bypass: the dark side of GLP‐1. Gastroenterology. 2014;146:605‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holst JJ, Madsbad S. Mechanisms of surgical control of type 2 diabetes: GLP‐1 is key factor. Surg Obes Relat Dis. 2016;12:1236‐1242. [DOI] [PubMed] [Google Scholar]
- 29. Jurowich CF, Otto C, Rikkala PR, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365. doi: 10.1155/2015/490365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan M, Lamendola C, Luong R, McLaughlin T, Craig C. Safety, efficacy and pharmacokinetics of repeat subcutaneous dosing of avexitide (exendin 9‐39) for treatment of post‐bariatric hypoglycaemia. Diabetes Obes Metab. 2020;22:1406‐1416. [DOI] [PubMed] [Google Scholar]
- 31. Craig CM, Liu LF, Nguyen T, Price C, Bingham J, McLaughlin TL. Efficacy and pharmacokinetics of subcutaneous exendin (9‐39) in patients with post‐bariatric hypoglycaemia. Diabetes Obes Metab. 2018;20:352‐361. [DOI] [PubMed] [Google Scholar]
- 32. Craig CM, Lawler HM, Lee CJE, et al. PREVENT: a randomized, placebo‐controlled crossover trial of Avexitide for treatment of postbariatric hypoglycemia. J Clin Endocrinol Metab. 2021;106:e3235‐e3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kandel D, Bojsen‐Møller KN, Svane MS, et al. Mechanisms of action of a carbohydrate‐reduced, high‐protein diet in reducing the risk of postprandial hypoglycemia after Roux‐en‐Y gastric bypass surgery. Am J Clin Nutr. 2019;110:296‐304. [DOI] [PubMed] [Google Scholar]
- 34. Stano S, Alam F, Wu L, et al. Effect of meal size and texture on gastric pouch emptying and glucagon‐like peptide 1 after gastric bypass surgery. Surg Obes Relat Dis. 2017;13:‐1975‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shantavasinkul PC, Torquati A, Corsino L. Post‐gastric bypass hypoglycaemia: a review. Clin Endocrinol (Oxf). 2016;85:3‐9. [DOI] [PubMed] [Google Scholar]
- 36. Valderas JP, Ahuad J, Rubio L, Escalona M, Pollak F, Maiz A. Acarbose improves hypoglycaemia following gastric bypass surgery without increasing glucagon‐like peptide 1 levels. Obes Surg. 2012;22:582‐586. [DOI] [PubMed] [Google Scholar]
- 37. Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol. 2012;166:951‐955. [DOI] [PubMed] [Google Scholar]
- 38. Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. 2013;169:885‐889. [DOI] [PubMed] [Google Scholar]
- 39. Chiappetta S, Stier C. A case report: Liraglutide as a novel treatment option in late dumping syndrome. Medicine (Baltimore). 2017;96:e6348. doi: 10.1097/MD.0000000000006348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Almby KE, Abrahamsson N, Lundqvist MH, et al. Effects of GLP‐1 on counter‐regulatory responses during hypoglycemia after GBP surgery. Eur J Endocrinol. 2019;181:161‐171. [DOI] [PubMed] [Google Scholar]
- 41. Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes (Lond). 2011;35(suppl 3):S22‐S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819‐837. [DOI] [PubMed] [Google Scholar]
- 43. Thazhath SS, Marathe CS, Wu T, et al. The glucagon‐like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: a randomized controlled trial. Diabetes. 2016;65:269‐275. [DOI] [PubMed] [Google Scholar]
- 44. Davidson JA, Stager W, Paranjape S, Berria R, Leiter LA. Achieving postprandial glucose control with lixisenatide improves glycemic control in patients with type 2 diabetes on basal insulin: a post‐hoc analysis of pooled data. Clin Diabetes Endocrinol. 2020;6:2. 10.1186/s40842-019-0088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Raalte DH, Verchere CB. Improving glycaemic control in type 2 diabetes: stimulate insulin secretion or provide beta‐cell rest? Diabetes Obes Metab. 2017;19:1205‐1213. [DOI] [PubMed] [Google Scholar]
- 46. Gorgojo‐Martínez JJ, Feo‐Ortega G, Serrano‐Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis. 2016;12:1856‐1863. [DOI] [PubMed] [Google Scholar]
- 47. Koot R, van Borren M, de Boer H. Continuation of liraglutide during fasting is not associated with hypoglycaemia. Eur J Case Rep Intern Med. 2017;4:000712. doi: 10.12890/2017_000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pajecki D, Halpern A, Cercato C, Mancini M, de Cleva R, Santo MA. Short‐term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir. 2013;40:191‐195. [DOI] [PubMed] [Google Scholar]
- 49. Apovian CM, Okemah J, O'Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miras AD, Pérez‐Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7:549‐559. [DOI] [PubMed] [Google Scholar]
- 52. Wharton S, Kuk JL, Luszczynski M, Kamran E, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post‐bariatric surgery. Clin Obes. 2019;9:e12323. doi: 10.1111/cob.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ding B, Hu Y, Yuan L, Yan RN, Ma JH. Effectiveness of beinaglutide in a patient with late dumping syndrome after gastrectomy: a case report. Medicine (Baltimore). 2021;100:e26086. doi: 10.1097/MD.0000000000026086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28(366):l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 56. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;12(355):i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Borner T, Tinsley IC, Doyle RP, Hayes MR, De Jonghe BC. Glucagon‐like peptide‐1 in diabetes care: can glycaemic control be achieved without nausea and vomiting? Br J Pharmacol. 2022;179:542‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dutta D, Surana V, Singla R, Aggarwal S, Sharma M. Efficacy and safety of novel twincretin tirzepatide a dual GIP and GLP‐1 receptor agonist in the management of type‐2 diabetes: a Cochrane meta‐analysis. Indian J Endocrinol Metab. 2021;25:475‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos V. Tirzepatide, a new era of dual‐targeted treatment for diabetes and obesity: a mini‐review. Molecules. 2022;27:4315. doi: 10.3390/molecules27134315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shoda K, Kubota T, Ushigome E, et al. Dynamics of glucose levels after Billroth I versus Roux‐en‐Y reconstruction in patients who undergo distal gastrectomy. Surg Today. 2022;52:889‐895. [DOI] [PubMed] [Google Scholar]


