Abstract
Issues
Poor oral health is a significant problem among people who access alcohol and other drugs (AOD) health services, yet little is known about their oral health‐care needs and whether any interventions and guidelines are implemented within AOD treatment services.
Approach
A scoping review was conducted to identify scientific literature in three focus areas: oral health knowledge, attitudes and practices of clients and clinicians at AOD‐related services; oral health guidelines for AOD clinicians; and interventions that describe clinicians promoting oral health among clients.
Key Findings
Thirty‐two reports were identified. Twenty‐three studies focused on the oral health knowledge, attitudes and practices of AOD clients, but none of the studies explored perspectives of clinicians. Seven clinical practice guidelines were identified but recommendations varied. Only two interventions in which an AOD clinician promoted oral health were identified.
Implications
Most reports provided insight into the perspectives of clients accessing AOD treatment services. Little evidence demonstrated that clinical practice guidelines were being implemented into practice, or that oral health promotion was part of the tasks of AOD clinicians.
Conclusion
This review identified oral health knowledge gaps and perceived poor attitudes that appeared to be reflected in certain oral health practices among clients. This finding indicates a need to educate clients about oral health while receiving AOD treatment. There was an absence of research about the needs for AOD clinicians to advocate for good oral health, and little evidence exists about effective interventions that could enhance integrating oral health care into AOD treatment services.
Keywords: health knowledge, attitudes, practice, health promotion, oral health, substance abuse treatment centres, substance‐related disorders
Key Points.
Most literature to date has focused on the oral health needs and perspectives of people who receive services for alcohol and/or other drugs dependence.
While some clinical practice guidelines recommend that clinicians play a greater role in promotion of good oral health in people with alcohol and/or other drug dependence, our review suggests that the translation of the guidelines into practice is limited.
The findings from the interventions identified by this review indicate that clinicians could improve facilitation of access to oral health services for the clients receiving alcohol and other drug treatment services.
Future research should focus on the perspectives and needs of alcohol and other drug clinicians around improving their capacity to promote oral health among clients.
1. INTRODUCTION
Patterns of oral health pathology in people who use alcohol and/or other drugs (AOD) are notably more distinct and severe compared to people who do not [1, 2, 3, 4]. Commonly used psychotropic drugs such as methamphetamine, marijuana, opioids, cocaine, alcohol and tobacco can affect oral health both directly and indirectly [1]. The use of psychotropic drugs can reduce salivary production leading to xerostomia (dry mouth) [5], which in turn causes increased risk of mucosal infections such as oral candidiasis [6], contributes to periodontal diseases [7, 8] and impedes mastication and swallowing [9]. Individuals with AOD dependence may also experience higher rates of tooth decay and tooth loss [4, 7]. A study in the United States found that people who used methamphetamine were four times more likely to have untreated dental decay [10]. Periodontal diseases are also common among people with an AOD dependence. In some populations, more than 80% of people who used AOD had signs of periodontal diseases [8]. Furthermore, the use of certain drugs, like alcohol, also increases the risk of oral cancers [11]. There is also an increased risk in oral health problems among people who have an AOD dependence, which can also affect quality of life [12, 13, 14, 15]. Amiri and Shekarchizadeh [16] also found that among people receiving inpatient treatment for AOD dependence, oral health problems had an impact on eating, speaking, sleeping, going out, relaxing, smoking, emotional stability and affected their contact with other people.
Maintaining good oral health care is often challenging among people with AOD dependence. Indirectly, the use of certain drugs can alter cognitive function, alter an individual's routine behaviours and increase the likelihood of neglecting self‐care [7]. The use of some drugs is also associated with increased dietary sugar consumption [17, 18], further contributing to dental decay. Lifestyle factors, including the need to curb withdrawal symptoms through consistent AOD use, can make it difficult to prioritise oral health‐care practices like toothbrushing [19]. Furthermore, many individuals who frequently use AOD may also have mental health illnesses, such as clinical depression, panic disorder or generalised anxiety disorder, that can make it even more difficult to engage in oral health care [20, 21].
Another potential barrier to dental care is the expectation or experience of stigmatisation by health‐care professionals. A systematic review by van Boekel et al. [22] found that a range of health professionals are reluctant to provide care to people with AOD dependence, especially when they do not regularly work among this population. Some individuals with AOD dependence and mental health illnesses may have fear of dentists, be unable to prioritise dental appointments due to lifestyle as well as socio‐economic factors, and may opt to self‐medicate for dental pain in lieu of accessing professional dental services [23]. These factors can contribute to delayed care seeking and greater rates of untreated dental decay, and potentially lead to an increase in emergency department presentations for dental reasons among people with an AOD dependence [10].
People who receive treatment to manage AOD dependence may be at a greater risk of poor oral health. Methadone maintenance therapy, which treats opioid dependence and manages opioid withdrawals, can induce xerostomia, immunosuppression and increased craving for sugar [24]. Methadone is also prescribed for daily use, and was previously administered in a high sucrose, concentrated syrup solution. While there is limited robust evidence to support the association between sugar‐containing oral methadone and dental caries, it is important to acknowledge that sugar‐content is only one risk factor for dental decay in this population [25]. Nevertheless, guidelines and studies still recommend the use of sugar‐free opioid agonist preparations to reduce frequency of sugar consumption [25]. Thus, use of methadone in combination with other risk factors that are common among people with an AOD dependence could exacerbate oral health problems [7, 24].
Robinson et al. [19] found that people with AOD dependence may not address their oral health problems until they access AOD‐related services. Considering their risk of developing oral problems, it is important that people who access AOD treatment services receive appropriate oral health advice and treatment. Considering existing barriers to care by dental professionals, there may be a potential for clinicians within AOD treatment services to promote oral health. Systematic reviews conducted to date have largely focused on the links between AOD use and poor oral health [8, 26, 27, 28, 29], and have not identified potential strategies that could promote delivery of integrated oral health promotion and guidance in AOD services. Furthermore, no review has yet comprehensively outlined the issues that currently face AOD clinicians in providing or promoting oral health care as well as the potential role and scope of practice by AOD clinicians in oral health promotion [30]. The aim of this scoping review, therefore, was to explore the current evidence on:
oral health knowledge, attitudes and practices of people who access AOD inpatient and outpatient services;
oral health knowledge, attitudes, and practices of clinicians who provide these services (AOD clinicians);
current guidelines outlining the potential role of AOD clinicians in oral health promotion; and
existing interventions that support AOD clinicians in promoting good oral health among their clients.
2. METHODS
2.1. Study design
The scoping review framework outlined by Arksey and O'Malley [31] was followed for this review. This approach was considered appropriate for exploring a diverse body of studies, including qualitative and quantitative research publications as well as other reports and guidelines on this emerging research topic. Furthermore, such review process is iterative rather than linear, which enables the researchers to go back‐and‐forth and redefine search terms and study aims based on the gradually emerging findings [31].
2.2. Data sources
A systematic search for potentially eligible records was undertaken in the following electronic bibliographic databases: Medline (Ovid platform) and PubMed Central (PMC), Scopus, ProQuest Central, CINAHL (Cumulative Index to Nursing and Allied Health Literature), APA PsycInfo (American Psychological Association) and Web of Science. Grey literature was searched in ProQuest Dissertation and Theses. Individual search strategies were developed for each database. Use of diverse terminology and the spelling of keywords were considered to aid in the identification of relevant literature. Some of the keywords used in the study included: drug use, drug addiction, drug dependence, substance abuse, substance addiction, dental, oral health, oral hygiene, knowledge, awareness, attitudes, perspectives, experiences, practice, behaviour, program, intervention, training, education, model, guideline, consensus and recommendations.
2.3. Search strategy
A search strategy was developed by Prakash Poudel and Ariana Kong for each database using a combination of keywords, Boolean operators, truncations and subject headings. The search strategy for the databases is included in Appendix 1. The bibliographies in all relevant publications were also reviewed manually for additional references for potential inclusion. After exporting the records from the electronic database searches into the electronic reference management program EndNote, duplicates, literature published in languages other than English or before year 2000 were removed. Ariana Kong conducted the initial screening of the titles, followed by review of abstracts by Prakash Poudel. Potentially relevant citations were independently identified by Prakash Poudel and Ariana Kong, and any discrepancies in inclusion or exclusion of records were resolved through discussion.
2.4. Eligibility criteria
The records identified by the searches included empirical qualitative or quantitative literature available in the English language published from January 2000 to 3 February 2022. Peer‐reviewed published journal articles and grey literature were considered for inclusion. Studies that were included needed to report on either oral health knowledge, attitudes or practices of people who were recruited from AOD treatment services (as either outpatient or inpatient). AOD treatment services were defined as health‐care services that delivered specialised care to people with AOD dependence. Services that did not focus on delivering AOD health services were excluded as the scoping review aimed to explore the potential needs of clinicians who specialise in this area.
Potentially relevant guidelines were screened through the database search and identified through the hand search. Guidelines aimed at clinicians providing AOD‐related services were then reviewed to assess whether any recommendations about oral health were included.
Studies that explored the role of AOD clinicians in promoting oral health were also considered, whereas those that focused on only the roles of dental professionals, such as dentists, oral health therapists, dental hygienists and dental assistants, were excluded. No restrictions were placed on the quality or geographical location of the study.
2.5. Data extraction
The information extracted from the selected studies included author, publication year, country, study design, sample, setting, demographic and clinical characteristics, and oral health knowledge, attitudes or practices. Data were collated, summarised and reported using text and tables. Focus area number 1 encompassed objectives number 1 and 2 and aimed to explore the current knowledge, attitudes and practices of individuals who accessed AOD treatment services as well as AOD clinicians. Oral health knowledge was defined as data that explored participants' awareness of the link between the use of AOD and oral health, and the factors that impact on oral health status. Studies were also included in this area if AOD clients reported receiving oral health information from the service, or if AOD clinicians received oral health promotion education. Oral health attitudes encompassed respondents' perspectives towards the importance of oral health and preferred ways to manage oral health. The review also aimed to report AOD clinicians' attitudes towards promoting oral health among clients, including the acceptability and feasibility of this role. Oral health practices included oral hygiene self‐care practices of AOD clients, particularly toothbrushing and flossing frequency, and ways in which they managed oral health problems. This review also aimed to scope whether oral health promotion was part of the current practice of AOD clinicians. The second focus area, which corresponded to the third objective, aimed to report on existing guidelines relating to oral health, the role of AOD clinicians and people who use AOD treatment services. The third focus area, corresponding to the fourth objective, aimed to identify interventions involving non‐dental AOD clinicians promoting oral health.
3. RESULTS
A total of 1560 records (citations) were initially identified as potentially relevant (Figure 1). Initial screening excluded 429 records due to duplicates, languages other than English and publication date prior to the year 2000. The screening of titles and subsequently of the remaining abstracts further excluded 1042 records. The full text of 89 articles was retrieved and reviewed for eligibility. A total of 57 records were excluded due to study setting (n = 20), outcomes reported (n = 35), or population (n = 2) (Appendix 2). Thirty‐two articles were included in this review across the three focus areas and are summarised in Table 1. In the first focus area, there were 20 quantitative studies and 3 qualitative studies. The second focus area consisted of seven guidelines. The third focus area included two intervention studies.
FIGURE 1.
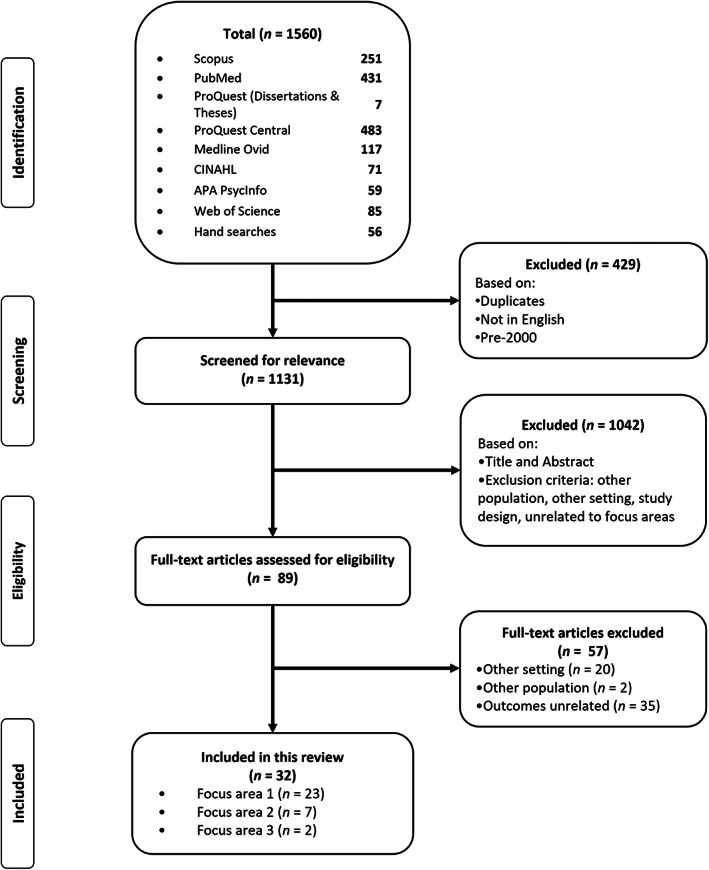
Study selection.
TABLE 1.
Summary of included articles
| Author (year), country [reference #] | Study design | PDC | Findings/recommendations |
|---|---|---|---|
| Setting | Other clinical characteristics | ||
| Sample size (M/F) | |||
| Age (range, median, mean) | |||
| FOCUS AREA 1: Oral health knowledge, attitudes and practices of clients and clinicians at AOD treatment services | |||
| Antoniazzi et al., Brazil [32] |
Cross‐sectional Hospitals for people with a chemical dependence 106 (79 M/27 F) Median age 25.0 years |
• PDC: Crack cocaine, cocaine, tobacco, marijuana, solvent, other street drugs. • Dental examination: 42% had ‘high severity’ dental caries; 52.8% periodontal disease; 55.7% missing teeth. |
Practices • 60.4% used dental services ≤24 months • 84% brushed ≥3×/daily • 26.4% used floss |
| Araujo et al., United States [33] |
Cross‐sectional Rehabilitation centre 34 (24 M/10 F) Mean age 37.1 years |
• PDC: Cigarettes, marijuana, cocaine. • Dental examination: 82.3% moderate/severe gingival inflammation; 26.5% dental plaque, 13.5% dental caries (mean: 3.2 lesions). |
Practices • 94.1% brushed teeth ≥1×/day • 14.7% flossed daily • 55.9% seen dentist >1 year ago • 20.6% not seen dentist ≥5 years |
| Arora et al., India [34] |
Case–control De‐addiction centre 100 (100 M/0 F) Mean age 30.8 years |
• PDC: heroin, opium, cigarettes. • Dental examination: None had healthy gingiva; 56% had gingival bleeding; mean DMFT index 5.71. |
Practices • Toothbrushing frequency: 80% >1×/day; 20% ≥2×/day • 28% used the same toothbrush >1 year • 16% seen dentist >2 years • 28% never visited dentist |
| Åstrøm et al., Norway [35] |
Cross‐sectional Clinics for people with substance use disorders 167 (126 M/38 F) a Mean age 45 years |
PDC: cannabis, amphetamines, heroin. |
Practices • Toothbrushing frequency: 52% >2×/day • 42% seen dentist >1×/year Attitudes • Reasons for no dental visit: dental anxiety (42%), no perceived need (23%), negative experience with dental clinician's attitude (20%), could not afford attending (18%), negative experience with treatment (14%), forgot appointment (9%), forgot scheduling appointment (9%), and did not have time (5%) • 78% dissatisfied with their oral health • 78% dissatisfied with their dental care |
| Aukštakalnis & Jurgelevičius, Lithuania [36] |
Cross‐sectional Centre for addictive diseases 49 (42 M/7 F) Mean age 40.3 (SD 8.3) years |
Dental examination: decayed (3.1), filled (12.1), missing (0.3), residual roots (4.4) |
Practices • Dental visiting: 45% never visited dentist; 18% visit only for urgent situations; 19% visit <1×/year; 12% visit 1×/year • DMF score lower among people who visited ≥1×/year compared to those who attended less frequently |
| Barbadoro et al., Italy [37] |
Pre‐post quasi‐experimental. Specialised residential rehabilitation clinics 76 (58 M/18 F) Age range: 30–39 years: 35.5%; 40–49 years: 28.9%; 50–59 years: 18.4% |
Dental examination: 56.6% DMFT index ≥10; 76.3% CPITN index 2 or 3 |
Practices • Frequency of tooth brushing: 23.7% never used a toothbrush or brush once a day; 19.7% brushed twice a day; 25.0% brushed after every meal • Flossing: 68.4% never used interdental thread (floss) • Dental examination: 44.7% attended <1 dental examination a year or never (missing = 23.7%) |
| Cheah et al., Australia [38] |
Descriptive qualitative Public specialist alcohol and drug service 14 (6 M/8 F) Mean age 39 years (range 23–60) |
PDC: alcohol, tobacco/nicotine, opiates, heroin, methamphetamine, marijuana. Oral concerns: 12 reported oral health needs included missing or damaged teeth. |
Knowledge • No participant received oral health advice from drug treatment services Practices • Toothbrushing frequency: n = 4 reported maybe ‘once a day’ • Dental visiting: n = 4 attend regularly; n = 6 infrequently or ‘never’ • Participants wanted information on the effects of substances and treatments (e.g., methadone) on oral health |
| De Souza et al., Brazil [39] |
Cross‐sectional Institute for research and treatment of alcoholism 202 (202 M/0 F) Mean age 34 (SD 9.1) years |
PDC: alcohol, crack, marijuana, cocaine Dental characteristics: Mean DMFT 11 (6.7 SD); prevalence of OHIP‐14 > 15 was 49.0% |
Practices • 94.6% reported brushing their teeth; 61.4% brushed ≥3×/day • 23.3% reported flossing • 96.5% used toothpaste to brush teeth • 90.6% reported visiting the dentist |
| Espósito Santos et al., Brazil [40] |
Cross‐sectional Recovery centre for drug addiction 39 (39 M/0 F) Age < 30 years: 67% |
PDC: alcohol, marijuana, tobacco, cocaine, crack cocaine. Oral concerns: 69.2% reported have fair to very poor dental condition; 64.1% reported have fair to very poor oral health. |
Knowledge • 35.9% received counselling on risks of drug use for dental treatment Attitudes • 97.4% perceived teeth are important • 38.5% perceived need for treatment • 38.5% satisfied with their oral health • 20.5% rated their oral health as poor Practices • Toothbrushing frequency: 84.6% daily • Flossing frequency: 20.5% daily • Dental visiting: 56.3% saw dentist in last year; 10.3% went for routine/maintenance of oral health |
| Gupta et al., India [41] |
Cross‐sectional Drug dependence treatment clinic 126 (125 M/1 F) Mean age 36 (SD 11) years |
PDC: opioids (street heroin), cannabis. Dental characteristics: 69% DMFT > 0; 56% CPI 2 or 3. |
Practices • 9% did not used anything to clean teeth • To clean teeth, 62% used a toothbrush, 24% used a finger, 6% used a neem stick • 60% used toothpaste to clean teeth; 18% used tooth powder • 48% cleaned their teeth 1×/day (44%) or ≥2×/day (4%) |
| Khocht et al., United States [42] |
Cohort University hospital ambulatory alcohol treatment centre 40 (22 M/18 F) Mean age 41 years |
PDC: alcohol only (25%); AOD (75%). Dental characteristics: Plaque index 2.7, 13.96% teeth had decay. |
Practices • All participants owned a toothbrush • None of the participants shared a toothbrush • A third (33.3%) brushed >1×/day; 35.9% brushed 1×/day; 23.1% brushed >1×/week • 23.5% used a hard toothbrush; 55.9% used a medium toothbrush; 20.6% used a soft brush type • Mean changes of toothbrush a year 2.72 (1.14 SD) • 37.84% received professional dental care |
| Laslett et al., Australia [43] |
Cross‐sectional Needle and syringe programs across six sites 287 (178 M/109 F) Age range: 21–24 years: 23%; 25–34 years: 51% |
PDC: heroin (76.8%), methamphetamine (18.2%). Oral concerns: 68.1% (n = 194) reported a dental problem in the last 12 months. |
Practices • Of the participants who had a dental problem, 58.2% saw a dentist for their problems (39.6% of total sample) |
| Lo Giudice et al., Italy [44] |
Cohort National drug rehabilitation centres 50 (46 M/4 F) Age range 21–42 years |
PDC: heroin only (28%), heroin and other drugs (72%). Dental characteristics: mean DMFT 23.52 (SD 11.64). |
Practices Before admission • 56% brushed ≥1×/day (38% 1×/day; 8% ≥2×/day); 36% brushed ‘sometimes’; 8% did not brush. During admission • 0–3 months: 70% did not brush; 18% ‘sometimes’ brushed; 12% brushed their teeth ≥1×/day (4% brushed 1/day; 8% brushed ≥2×/day). • 3–6 months: 12% did not brush; 38% brushed ‘sometimes’; 50% brushed their teeth ≥1×/day (42% brushed 1×/day; 8% brushed ≥2×/day). |
| Minic and Pejcic, Serbia [45] |
Cohort Special hospital for the treatment of substance abuse 26 (M/F not reported) Mean age 27.9 years |
PDC: heroin (84.6%), marijuana (11.5%), cocaine (7.7%). Dental characteristics: gingivitis (88.5%), periodontitis (61.5%), coated tongue (50%). |
Practices Before admission • 92.31% used a toothbrush and toothpaste • 19.23% used interdental means for oral hygiene • 7.69% never brushed teeth; 46.15% brushed 1×/day; 46.15% brushed ≥2×/day • 34.62% changed their toothbrush every 3 months; 23.08% changed every 6 months; 23.08% changed once a year; 11.54% changed >1 year During admission • 100% used a toothbrush and toothpaste • 61.54% used interdental means for oral hygiene • 23.08% brushed 1×/day; 73.08% brushed ≥2×/day • 34.62% changed their toothbrush every 3 months; 42.31% changed every 6 months; 19.23% changed once a year; 3.85% changed >1 year |
| Mufida et al., Indonesia [46] |
Cross‐sectional Rehabilitation centre 60 (M/F not reported) Age not reported |
PDC: heroin (38.3%), methamphetamine (36.7%), cannabis (25%). Dental characteristics: average dental and oral hygiene index: 1.8; mean DMFT: 4.33. |
Knowledge • 60% received dental health education • 13.33% said cavities are caused by sweet food • 50% said the cause of cavities was poor or bad oral hygiene • 16.67% said cavities were caused by maggots • 20% were unsure what caused cavities Attitudes • 40% reported that they would self‐treat a toothache, 26.66% would go to a health centre/dentist; 16.67% would leave the toothache alone until it healed itself Practices • 26.67% used a toothbrush to clean • 73.33% used toothbrush and toothpaste to clean teeth • 20% brushed once/day; 80% brushed ≥2×/day |
| O'Sullivan, Ireland [47] |
Cross‐sectional Residential abuse treatment centres 210 (148 M/62 F) Mean age 37.9 (SD 13.9) years |
PDC: alcohol only (43.8%), AOD (52.9%). Dental characteristics: mean DMFT 14.4 (SD 7.3), range 2–32. |
Practices • Brushing: 87% brushed their teeth daily during rehabilitation; 52% brushed more frequently; all engaged in oral hygiene practices infrequently when under the influence of alcohol/drugs • Dental visit: 33.5% regularly attended the dentist; 31.0% were regular mouthwash users • 51% attended the dentist only when in pain |
| Ravenel et al., United States [48] |
Cross‐sectional Community‐based outpatient methamphetamine abuse treatment program 28 (14 M/14 F) Mean age 29.57 (SD 6.92) years |
PDC: marijuana (67.9%), alcohol (57.1%), cocaine (50%). Dental characteristics: mean DMFS 29 (SD 25.78), range 0–114. |
Practices • 71.43% brushed their teeth ‘often’, 25% brushed their teeth ‘some’ • 10.71% flossed ‘often’, 46.43% ‘some’, 42.85% never flossed their teeth • 67.86% did not see the dentist in the last 12 months; 28.57% saw the dentist once in the last year |
| Robinson et al., United Kingdom [19] |
Qualitative focus groups Drug detoxification, recovery and rehabilitation units 30 (26 M/14 F) Age range 21–52 years |
PDC: tobacco, heroin, alcohol. |
Knowledge • Some participants attributed methadone linctus to dental decay Attitudes • Oral hygiene would be ignored if participants went on a ‘binge’ or went into a ‘crack house’ • Low self‐esteem meant that some participants neglected oral and personal hygiene • Many avoided dentists until pain was impossible to self‐manage • Some described have a fear of dentists • Lifestyle prevented dental attendance (preoccupied with avoiding withdrawal symptoms, waiting lists for drug treatment, low self‐esteem) • Some felt stigmatised by their dentist due to drug use Practices • Toothbrushing only possible if participants had sheltered accommodation with running water • Some consumed large amounts of sugar in their diet |
| Santella et al., United States [49] |
Prospective longitudinal study Hospital 657 (157 M/222 F/182 non‐men) Median age 44.5 (SD 10) years |
PDC: tobacco, stimulants, alcohol, heroin, opioids |
Practices • 41% received dental care in the last 12 months • Higher dental care utilisation was associated with being non‐Black (p = 0.003), having insurance coverage (p = 0.001), and a higher food insecure score (p = 0.014) • Participants were less likely to report dental care utilisation if they had less than a high school education (aOR = 0.6, p = 0.0382), and if they resided in a Southern state (aOR = 0.55, p = 0.0013) |
| Shekarchizadeh et al., Iran [50] |
Cross‐sectional Addiction treatment centre 682 (656 M/29 F) Mean age 38.2 (SD 10.1) years |
PDC: opium (65%), heroin (27%), other (8%). |
Practices • Brushing: 48% <1×/day; 39% 1×/day; 13% ≥2×/day • 93% almost or always use fluoride toothpaste • 81% never use dental floss; 5% use dental floss several times a week; 14% use dental floss >1×/day. • Dental visit: 25% saw the dentists more than 2 years ago/never; 18% saw the dentist 1–2 years ago; 57% saw the dentist in the last year |
| Smit and Naidoo, South Africa [51] |
Cross‐sectional Specialised substance addiction treatment centre 308 (249 M/59 F) Mean age 28 (SD 6.7) years |
PDC: methamphetamine (100%). Dental characteristics: mean DMFT 10 (98.05% had dental caries). |
Practices Before therapy • Toothbrushing: Never (5.5%); less often (35.7%); 1×/day (20.8%); ≥2×/day (38.0%) During therapy • Toothbrushing: Never (0.6%); less often (10.4%); 1×/day (40.9%); ≥2×/day (48.1%) • Significant association between tooth brushing frequency and whether this patient was ‘on meth’ compared to when ‘off meth’ (p < 0.001; OR = 3.25) |
| Teixeira et al., Portugal [52] |
Cross‐sectional Withdrawal unit ‘Instituto da Droga e Toxicodependência—Norte’ 295 (149 M/35 F) b Mean age 44 (SD 8.6) years |
PDC: alcohol (100%), heroin (28.8%), cocaine (28.3%), hashish (27.7%). |
Practices • 22.8% were toothbrushing ‘fairly often’ or ‘very often’ • 80.4% used mouthwash ‘fairly often’ or ‘very often’ • 22.8% reported toothbrushing after alcohol ingestion ‘fairly often’ or ‘very often’ |
| Van Hout and Hearne, Ireland [53] |
Qualitative focus groups Two detoxification, treatment and rehabilitation services for homeless men and women 15 (M/F not reported) Age range 20–56 years |
Engaged in detoxification (40%), rehabilitation (60%) and methadone maintenance treatment (20%). |
Knowledge • Half the participants were aware that alcohol could increase tooth decay and contribute to tooth enamel demineralisation • All participants were aware that tobacco could cause a range of oral health problems (e.g., delayed wound healing, soft tissue damage, gum disease) • Some participants were aware of oral cancers related to tobacco • All participants were aware of the relationship between drug use and xerostomia but were not aware of its impact on tooth decay Attitudes • Some people disliked receiving needles, were afraid of the dentist • Some participants would only visit the dentist when in pain or needed a dental extraction • Some participants believed that teeth become more of a priority during rehabilitation • Some participants believed it would be good to have an onsite dental practice • Many participants recognised that oral health was important and regretted prior neglect and its impact on their confidence • Some participants intended to receive fillings and teeth whitening to improve appearance • Participants with children were strongly motivated to teach good dental care to children (including daily oral hygiene and routine dental visits) Practices • Some participants did not brush or floss their teeth during active addiction • In rehabilitation, frequency of brushing and flossing improved • No participants flossed their teeth in the past or currently • Last visit to the dentist ranged between a week to 5 years ago • Participants attended 0 to 2 times in the last 12 months • For oral health issues/dental emergencies, some participants applied toothpaste to the dental cavity, self‐medicated pain‐relieving medication, consumed excessive levels of alcohol or prescribed/illicit drugs; people without a medical card did nothing • Some attempted self‐extraction of teeth and self‐treating dental plaque and stains |
| FOCUS AREA #2: Guidelines for AOD clinicians to promote oral health | |||
| Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group (2017), United Kingdom [54] | Clinical guidelines | N/A |
• Non‐dental health professionals: a. Can ask ‘simple questions about dental and oral health problems, and current and past use of dental services, [and] can help identify early positive health goals for the patient's care plan’. b. Can provide ‘encouragement, help and advice to attend a suitable local dental service is also an opportunity to engage the patient in positive care for their health and wellbeing as part of a wider process of recovery. Liaison with the GP may also be needed in some cases’. • The guidelines reinforce that dental services need to be ‘competent in providing dental treatment and oral health promotion advice for people in drug treatment. They can also undertake identification and brief advice for hazardous or harmful alcohol use and for tobacco use, and can refer patients to smoking cessation and alcohol services’. |
| Grigg et al., Australia [55] | Practice guidelines | N/A | Health professionals can provide basic oral health advice to clients: ‘Brush and floss regularly, especially after food and sweet drinks, to prevent dental disease’ |
| Health Service Executive, Ireland [56] | Clinical guidelines | N/A | Health professionals may provide a dental referral: ‘Oral health and referral to appropriate dental service’ |
| Jenner and Lee, Australia [60] | Treatment approach guidelines | N/A | Frontline workers can encourage clients to ‘Brush and floss teeth regularly, and chew sugar‐free gum to increase saliva and to take some pressure off the enamel if teeth grinding is a problem. Dental health can suffer due to a lack of bacteria‐fighting saliva in the mouth’. |
| Kleber et al., United States [57] | Practice guidelines | N/A | Using a nicotine lozenge may be preferred over nicotine gum: ‘Because it does not have to be chewed, the lozenge may be preferable for smokers with dental problems or for those who do not like to chew gum’. |
| Registered Nurses' Association of Ontario, Canada [58] | Best practice guidelines | N/A |
Practice recommendations • Nurses should be aware of their personal oral hygiene beliefs and practices, as these may influence the care they provide to their clients. • As part of their client admission assessment, nurses obtain an oral health history that includes oral hygiene beliefs, practices and current state of oral health. • Nurses use a standardised, valid and reliable oral assessment tool to perform their initial and ongoing oral assessment. • Oral health status information is regularly reviewed with all members of the health‐care team to monitor client progress and facilitate the development of an individualised plan of care.
• Nurses provide or supervise the provision of oral care for clients at risk for aspiration. • Nurses provide ongoing education to the client and/or family members regarding oral care. • Nurses are knowledgeable of oral hygiene products and their applications as they pertain to their specific client populations. • Nurses are aware of treatments and medications that impact on the oral health of clients. • Nurses use appropriate techniques when providing oral care to clients. • Nurses advocate for referral for those clients who require consultation with an oral health professional (e.g., dental hygienist, denturist, dentist). • Nurses ensure that all oral health‐related history, assessment and care be documented. Educational recommendations • Nurses require appropriate oral health knowledge and skills acquired through entry‐level nursing education programs, workplace orientation programs and ongoing professional development opportunities. • Nurses who provide oral hygiene care to their clients, either directly or indirectly, must participate in, and complete, appropriate oral hygiene education and training. |
| Registered Nurses' Association of Ontario, Canada [59] | Clinical best practice guidelines | N/A | Discussing dental issues with clients is part of a comprehensive health assessment. |
| FOCUS AREA 3: Existing interventions involving AOD clinicians and oral health care | |||
| Hede et al., Denmark [61] |
Pre‐post‐test design. Municipality centres (participants recruited from local drug treatment centres, centres for homeless people and/or centres for socially underserved citizens) 235 (171 M/64 F) Mean age 43.5 years |
N/A |
• Program: A dental program aimed to restore dentition to a functional and socially acceptable level through low‐cost treatment services. Social workers provided some oral health advice, referred clients to dental services and escorted clients to their first dental appointment • The average OHIP‐14 scores among participants significantly reduced from 27.8 to 9.9 (p < 0.001), indicated better overall oral health quality of life at the end of the program |
| Ivanovic et al., Australia [62] |
Descriptive MSIR 14 (M/F not reported) |
N/A |
• Program: Introduced a dental hygienist and dental assistant in the MSIR to conduct oral assessments, X‐rays, oral health promotion and the application of topical fluoride. MSIR staff received education about oral health (including use of silver diamine fluoride) and promoted the program to clients • In the three sessions that were available, 14 people received care and were receptive to the program |
Abbreviations: AOD, alcohol and other drugs; aOR, adjusted odds ratio; CPI, Community Periodontal Index; CPITN, community index of periodontal treatment needs; DMFS, decayed, missing and filled permanent teeth or surfaces; DMFT, decayed, missing and filled teeth; F, female; M, male; MSIR, medically supervised injecting room; N/A, not applicable; OHIP‐14, Oral Health Impact Profile 14 index; OR, odds ratio; PDC, principal drug(s) of concern.
Missing from M/F: n = 3.
Missing from M/F: n = 111.
3.1. Study characteristics
In the first focus area, 23 studies were identified that included 3611 participants with a mean age ranging from 27.9 to 44 years [19, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53]. The studies were conducted in Australia (n = 2), Brazil (n = 3), India (n = 2), Indonesia (n = 1), Iran (n = 1), Ireland (n = 2), Italy (n = 2), Lithuania (n = 1), Norway (n = 1), Portugal (n = 1), Serbia (n = 1), South Africa (n = 1), United Kingdom (n = 1) and the United States (n = 4). About half of the studies were of cross‐sectional design (n = 14) [32, 33, 35, 36, 39, 40, 41, 43, 46, 47, 48, 50, 51, 52]; few studies used a qualitative design (n = 3) [19, 38, 53]; and the designs of remaining six studies were cohort (n = 3) [42, 44, 45], case–control (n = 1) [34], prospective longitudinal (n = 1) [49] and pre‐post quasi‐experimental (n = 1) [37]. In the second focus area, the guidelines identified were developed for application in Australia (n = 2), Canada (n = 2), Ireland (n = 1), United Kingdom (n = 1) and the United States (n = 1). Six of the guidelines were specified as practice guidelines [54, 55, 56, 57, 58, 59] and one was termed a treatment approach guideline [60]. In the third, one study was conducted in Denmark [61] and the other in Australia [62]. The study in Denmark employed a pre‐post test design [61] whereas the Australian study used a descriptive design [62].
The authors used a wide range of terms to describe the settings where AOD treatment services were delivered, including addiction treatment, detoxification, rehabilitation, abuse treatment and withdrawal unit. One study also explored needle and syringe exchange programs. The most common AOD used by clients included tobacco, alcohol, marijuana, cocaine, methamphetamine and opiates.
3.2. Focus area 1: Oral health knowledge, attitudes and practices of people who receive AOD‐related services and AOD clinicians
Twenty‐three articles focused on people who attend AOD‐related services. None of the papers reported on oral health knowledge, attitudes or practices of AOD clinicians. Of the 23 studies [19, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53], 5 reported on their oral health knowledge [19, 38, 40, 46, 53]; 5 explored their oral health attitudes [19, 35, 40, 46, 53] and all reported on oral health practices [19, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53].
3.2.1. Oral health knowledge
Of the five studies exploring oral health knowledge among people who use AOD, two studies reported on their knowledge regarding the impact of AOD use on oral health [19, 53]. A qualitative study among people accessing drug treatment and recovery reported that some participants attributed their dental decay to their use of methadone [19]. Another study conducted in Ireland found that all participants (n = 15) were aware of the impact of drug use on oral health, namely causing a range of problems, including gum disease, soft tissue damage and oral cancer [53]. Half the participants were aware that alcohol had the potential to increase the risk of dental decay. Although these participants were aware of the link between sugar consumption and dental decay, the link between mouth dryness and dental decay was not well known among respondents [53]. An Indonesian study explored participants' awareness of the causes of cavities; their responses varied and included poor oral hygiene (50%), unsure (20%), maggots (17%) and sweet food (13%) [46].
Only three studies reported on clients receiving oral health advice from AOD treatment services. A study in Australia found that none of the participants received oral health advice from their drug treatment service [38], whereas about one‐third (36%) of participants in a Brazilian study received counselling regarding the risk of drug use causing dental treatment needs [40]. In Indonesia, 60% of clients at one rehabilitation centre were reported to receive dental health education, although it was unclear whether this education was given at the rehabilitation centre or elsewhere [46].
3.2.2. Oral health attitudes
Among the five studies that explored the attitudes about oral health, only two concluded that participants believed that oral health was important [40, 53]. Nonetheless, it appeared that many participants attempted to avoid professional dental care by self‐managing oral symptoms. In the Indonesian study, more than half the participants preferred to self‐treat a toothache (40%) or leave it alone (17%) instead of accessing a medical health‐care provider or dentist (26%) [46]. In the study by Robinson et al. [19] in the United Kingdom, participants highlighted that low self‐esteem and lifestyle adversely affected their prioritisation of oral health. Two studies also reported that some clients feared accessing the dentist [19, 53] and two found that some people experienced being stigmatised by their dentist [19, 35]. The study conducted in Norway found that most participants (78%) were dissatisfied with their oral health as well as their dental care, but dental anxiety or lack of perceived need were barriers to obtaining a dental visit [35]. However, one of the Irish studies reported that participants who were parents were motivated to pass on good oral hygiene practices to their children, including routine dental visits and daily personal oral health measures [53].
3.2.3. Oral health practices
Of the 23 studies that reported on respondents' oral health practices, the vast majority (n = 20) described the participants' self‐care oral hygiene practices, including frequency of toothbrushing and flossing and factors that influenced these practices. Thirteen studies [33, 34, 38, 39, 40, 41, 42, 44, 45, 46, 47, 50, 51] reported large variations in the prevalence of participants brushing their teeth at least once a day, ranging from 29% in one Australian study [38] to 100% in populations located in India [34] and Indonesia [46]. Seven studies [34, 35, 41, 44, 45, 46, 50] explored the frequency of participants brushing their teeth twice a day, which also varied from 4% [41] to 80% [46]. The three studies conducted in Brazil found that up to 84% of participants brushed their teeth three times daily [32, 39, 40]. Three studies explored the participants' oral health practices before accessing AOD treatment services [44, 45, 51]. Two of the three studies reported that upon accessing AOD treatment services, the frequency of toothbrushing at least once daily did improve between 30% and 50% [45, 51]. The third study observed that the percentage of people who brushed at least once daily dropped slightly, by 6%, during in‐patient admission for drug rehabilitation [44].
The frequency of dental flossing was reported in seven studies [32, 33, 37, 39, 40, 48, 50] and was generally low, although there was some variation. Only 14%–23% of participants across three studies reported flossing daily [33, 39, 50]; and 43%–74% of participants in three studies reported that they had never flossed [32, 37, 48].
Eight studies reported the frequency of dental visits in the last year, which ranged between 18% and 57% [33, 35, 36, 37, 40, 48, 49, 50]. However, one of these studies found that, of those having seen a dentist in the last year, only 10% attended for a routine dental check‐up [40]. Three studies reported that between 28% and 45% of clients had never accessed dental services [34, 36, 37]. Some studies reported on participants accessing dental services; however, the frequency of access in these studies was not clearly specified [38, 39, 42, 43, 47, 53]. In the United States, Santella et al. [49] reported that greater dental care utilisation was associated with being non‐Black, having insurance coverage and greater food security. The study by Van Hout and Hearne [53] identified that some participants applied toothpaste directly on the cavity or self‐treated dental plaque and stains to manage oral health issues, and employed other alternative management strategies for dental emergencies, such as self‐medicating to manage dental pain, consuming excess levels of AOD, and in some cases, attempting self‐extraction of the tooth.
3.3. Focus area 2: Guidelines for non‐dental AOD clinicians
There were seven guidelines that targeted AOD clinicians and described the oral health needs or recommendations for people with AOD dependence. Two guidelines provided more detailed recommendations for AOD clinicians to promote good oral health among clients. One of the guidelines published by the Registered Nurses' Association of Ontario supported the involvement of Canadian nurses in oral health promotion, and recommended that nurses advocate for referral of AOD clients for consultation with an oral health‐care professional [58]. Another set of guidelines aimed at health‐care professionals in the United Kingdom provided advice on how AOD clients could achieve and maintain good oral hygiene [54]. These clinical guidelines recommended that clinicians ask clients about their oral health status and dental service use, and subsequently include oral health care as part of the person's overall care plan. Furthermore, these guidelines recommended that clinicians encourage individuals to seek dental treatment and liaise with general dental practitioners when appropriate.
Methamphetamine treatment guidelines for AOD clinicians in Australia briefly listed regular tooth brushing and flossing as harm reduction tips for clients to prevent dental disease, but did not explicitly identify the role of AOD clinicians in oral health promotion [55]. Two other guidelines acknowledged the risk of AOD use on oral health, but similarly did not make any recommendations for non‐dental AOD clinicians. A practical guide for frontline workers in Australia reported that use of methamphetamine can increase the risk of poor oral health, but provided no recommendations [60]. A practical guideline published by the American Psychiatric Association noted that the ‘downward drift’ that may occur among people who use AOD could impact their access to dental services; however, no guidance was provided on how to improve such access [57].
Guidelines for Canadian nurses [59] and for people working in addiction therapy services in Ireland [56], respectively, recommended that oral health should be part of the general health assessment conducted by the clinician; however, no further advice was provided about how oral health could be assessed.
3.4. Focus area 3: Existing interventions that involved AOD clinicians promoting oral health
Two studies described interventions involving AOD clinicians promoting or advocating for good oral health among AOD dependence clients. The study in Denmark by Hede et al. [61] reported on a dental program in which all treatment and preventive care were provided by publicly employed dental service clinicians. While it appeared that the AOD clinicians, who were social workers, did not provide oral health advice directly, they played a role in referring AOD clients to dental services. Furthermore, they escorted their clients to their first dental appointment and supported participants to maintain their dental appointment and complete the dental treatment plan. The study found that among those who completed the treatment plan, their oral health‐related quality of life improved significantly.
The second report was in the form of a conference abstract by Ivanovic et al. [62]. Medically supervised injecting room staff were provided education about oral health, including information regarding low‐cost application of silver diamine fluoride to manage dental decay. This program offered storage of toothbrushes and toothpaste on site for clients to clean their teeth; encouraged them to have an on‐site dental hygienist apply silver diamine fluoride; and referred clients with urgent oral health needs to specialist care. The authors reported that clients were highly receptive to this program and planned to co‐design a similar model of care with consumers.
4. DISCUSSION
This review provides new insights into the oral health knowledge, attitudes and practices of people who use AOD treatment services. In addition, this review sought to explore existing guidelines and interventions designed to support AOD clinicians in promoting good oral health among their clients. Despite the detrimental impact of AOD dependence on oral health established in previous reviews [8, 26, 27, 28, 29], this study identified a paucity of interventions aimed at enhancing integrated oral and general health care among AOD clinicians. Furthermore, while some international guidelines have recognised the need for AOD clinicians to integrate oral health into their practice, further research would be necessary to scope current oral health promotion practices among AOD clinicians.
The first focus area confirmed that among people who use AOD treatment services, there were gaps in oral health knowledge as well as some negative attitudes towards oral health care that appeared to be reflected in certain oral health practices. The lack of awareness about the impact of AOD dependence on oral health was unsurprising, given that the oral health advice provided within the AOD services studied was limited across the studies [38, 40, 46]. The reported stigma from and fear of dentists contributed to self‐management of oral health problems, as reflected in the low proportion of AOD clients having seen a dentist the last year [33, 35, 36, 37, 40, 48, 49, 50], with only a small subset having visited the dentist for routine dental care [40]. The reported reluctance and fear regarding obtaining dental care among clients demonstrate that AOD clinicians are ideally placed to promote good oral health in ways that are not stigmatising and could mitigate some of the barriers to accessing dental care.
People with substance use disorders often have comorbidities, including mental health illnesses, which can independently increase the risk of oral health problems. High rates of tooth loss and untreated dental decay are often reported among people living with mental health illnesses [63]. The risk factors for oral health problems (such as poor diet and smoking) and perceived barriers to timely access to oral health care (fear of dentists, stigma and cost of dental treatment) [64, 65], are similar for people with substance use disorders as well as for people with mental health illnesses, suggesting a need of an integrated and interprofessional approach of oral health care for people with dual diagnoses.
The guidelines relating to oral health for AOD clinicians reinforced that oral health status is recognised by health organisations as an important, integral part of health assessment. While there was significant variation in recommendations across the guidelines, just a few articulated the exact role of AOD clinicians in promoting oral health care [54, 58]. Most guidelines recommended AOD clinicians provide basic advice like daily toothbrushing and flossing, as well as encouraging visits to the dentist. Only one Canadian guideline comprehensively outlined the practice, educational and larger policy and organisational recommendations to integrate oral health into routine practice [58]. Nevertheless, little is known about whether these guidelines are being translated into practice or whether AOD clinicians experience barriers or have additional needs to integrate oral health into their practice as none of the reviewed studies explored the oral health knowledge, attitudes and practices of non‐dental AOD clinicians.
AOD clinicians have an opportunity to promote oral health among clients who access AOD treatment services due to their established contact, communication and trust. Their existing skills in working with AOD clients could also mitigate the stigma, reluctance and fear of dental services. Moreover, a couple of studies from this review suggest that when clients access AOD treatment services, there is a natural improvement in toothbrushing frequency even without specific oral health promotion [45, 51], perhaps due to the support and rehabilitation provided by AOD treatment services to improve overall functioning and wellbeing. In other populations where non‐dental clinicians have promoted oral health among specific adult populations, studies have found that there needs to be a consensus among clinicians that oral health promotion is an aspect of their role [66, 67, 68, 69, 70]. There also needs to be dissemination of rudimentary oral health education, access to appropriate oral health promotion resources, and a priority referral pathway for clients at risk of poor oral health needs to be implemented [66, 67, 68, 69, 70].
Unfortunately, the third focus area, which explored interventions that involve AOD clinicians promoting oral health, found limited research around the role of AOD clinicians. Nevertheless, the interventions studied suggest that AOD clinicians could work effectively to complement the roles of dental practitioners by encouraging clients to access services and receive timely dental treatment. In addition to the potential role of clinicians within AOD treatment services, pharmacists could provide some auxiliary oral health promotion as many people who use AOD treatment services also regularly see pharmacists to obtain medications [71, 72].
Developing effective and sustainable integrated oral health interventions for people who access AOD treatment services may be challenging due to their complex needs. Recent studies involving people who use AOD treatment services have shifted towards action research principles that rely on a process of co‐design or co‐production to ensure that interventions and services are not only user‐friendly, but also user‐centred [73, 74]. Following a process in which the end‐users are central to the development of the intervention will ensure that it ultimately will address the oral health needs of clients who use AOD treatment services, and hence increasing the acceptability and effectiveness of the intervention [75]. The interventions included in this review provided limited detail on how they were developed. They also did not measure whether they effectively improved the oral health knowledge and confidence of AOD clinicians nor the subsequent impact on the oral health outcomes of clients. Thus, further development of interventions aimed at improved integrated oral health care within AOD treatment services needs to involve both clinicians and clients in the process.
Although this review provides a summary of the existing evidence regarding the important needs of AOD treatment service clients and AOD clinicians to improve oral health care, there were some limitations to the review. As only studies that were reported in English language were eligible for inclusion in this review, studies and guidelines published in other languages were excluded, limiting the available evidence that could be considered.
5. CONCLUSIONS
This study aimed to map the evidence related to the oral health perspectives and needs of AOD clients and their clinicians, as well as oral health guidelines and interventions for AOD treatment settings. The review identified gaps in patients' knowledge and concluded that poor attitudes towards oral health appeared to be reflected in certain oral health practices. Overall, this finding indicates a need for education, counselling, and motivation to increase access to dental care services among people with AOD dependence. There is an opportunity for AOD clinicians to play a proactive role in promoting oral health among their patients. However, the review identified gaps in the evidence base for effective oral health interventions and clinicians' capacity, needs and barriers to promote oral health. While a couple of guidelines support the need for AOD clinicians to play a greater role in oral health promotion, the actual scope of this role needs to be further explored.
AUTHOR CONTRIBUTIONS
Each author certifies that their contribution to this work meets the standards of the International Committee of Medical Journal Editors. Prakash Poudel, Stephanie Hocking, Gilbert Whitton, Ravi Srinivas and Ajesh George conceptualised the study and the research design. Prakash Poudel and Ariana Kong developed the search strategy. Prakash Poudel and Ariana Kong conducted the preliminary search and independently screened the articles for inclusion. All authors contributed to the interpretation of the data. Prakash Poudel, Ariana Kong and Ajesh George drafted the first version of the manuscript, which was reviewed by all authors for important intellectual content. The final version has been approved by all authors, and all authors have agreed to be accountable for all aspects of the work.
FUNDING INFORMATION
This study was funded by a grant provided by Drug Health Services, South Western Sydney Local Health District.
CONFLICT OF INTEREST
The authors declare no other conflict of interest.
Supporting information
Appendix 1. Search Strategy.
Appendix 2. Full text articles excluded.
Poudel P, Kong A, Hocking S, Whitton G, Srinivas R, Borgnakke WS, et al. Oral health‐care needs among clients receiving alcohol and other drugs treatment—A scoping review. Drug Alcohol Rev. 2023;42(2):346–366. 10.1111/dar.13583
Funding information Drug Health Services, SWSLHD
REFERENCES
- 1. D'Amore MM, Cheng DM, Kressin NR, Jones J, Samet JH, Winter M, et al. Oral health of substance‐dependent individuals: impact of specific substances. J Subst Abus Treat. 2011;41:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donaldson M, Goodchild JH. Oral health of the methamphetamine abuser. Am J Health Syst Pharm. 2006;63:2078–82. [DOI] [PubMed] [Google Scholar]
- 3. Ma H, Shi X‐c, Hu D‐y, Li X. The poor oral health status of former heroin users treated with methadone in a Chinese city. Med Sci Monit. 2012;18:PH51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priyanka K, Sudhir KM, Reddy VCS, Kumar RK, Srinivasulu G. Impact of alcohol dependency on oral health ‐ a cross‐sectional comparative study. J Clin Diagn Res. 2017;11:ZC43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Titsas A, Ferguson MM. Impact of opioid use on dentistry. Aust Dent J. 2002;47:94–8. [DOI] [PubMed] [Google Scholar]
- 6. Brazier WJ, Dhariwal DK, Patton DW, Bishop K. Ecstasy related periodontitis and mucosal ulceration – a case report. Br Dent J. 2003;194:197–9. [DOI] [PubMed] [Google Scholar]
- 7. Shekarchizadeh H, Khami MR, Mohebbi SZ, Ekhtiari H, Virtanen JI. Oral health of drug abusers: a review of health effects and care. Iran J Public Health. 2013;42:929–40. [PMC free article] [PubMed] [Google Scholar]
- 8. Yazdanian M, Armoon B, Noroozi A, Mohammadi R, Bayat A‐H, Ahounbar E, et al. Dental caries and periodontal disease among people who use drugs: a systematic review and meta‐analysis. BMC Oral Health. 2020;20:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossow I. Illicit drug use and oral health. Addiction. 2021;116:3235–42. [DOI] [PubMed] [Google Scholar]
- 10. Shetty V, Harrell L, Clague J, Murphy DA, Dye BA, Belin TR. Methamphetamine users have increased dental disease: a propensity score analysis. J Dent Res. 2016;95:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD 2016 Alcohol Collaborators . Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukherjee A, Dye BA, Clague J, Belin TR, Shetty V. Methamphetamine use and oral health‐related quality of life. Qual Life Res. 2018;27:3179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marques TC, Sarracini KL, Cortellazzi KL, Mialhe FL, de Castro MM, Pereira AC, et al. The impact of oral health conditions, socioeconomic status and use of specific substances on quality of life of addicted persons. BMC Oral Health. 2015;15:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdelsalam S, Van Den Boom W, Higgs P, Dietze P, Erbas B. The association between depression and oral health related quality of life in people who inject drugs. Drug Alcohol Depend. 2021;229:109121. [DOI] [PubMed] [Google Scholar]
- 15. Truong A, Higgs P, Cogger S, Jamieson L, Burns L, Dietze P. Oral health‐related quality of life among an Australian sample of people who inject drugs. J Public Health Dent. 2015;75:218–24. [DOI] [PubMed] [Google Scholar]
- 16. Amiri S, Shekarchizadeh H. Oral health‐related quality of life among a group of patients with substance use disorders in rehabilitation treatment: a cross‐sectional study. BMC Oral Health. 2021;21:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sæland M, Haugen M, Eriksen FL, Wandel M, Smehaugen A, Böhmer T, et al. High sugar consumption and poor nutrient intake among drug addicts in Oslo, Norway. Br J Nutr. 2011;105:618–24. [DOI] [PubMed] [Google Scholar]
- 18. Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long‐term methadone maintenance. Subst Use Misuse. 2007;42:1555–66. [DOI] [PubMed] [Google Scholar]
- 19. Robinson PG, Acquah S, Gibson B. Drug users: oral health‐related attitudes and behaviours. Br Dent J. 2005;198:219–24. [DOI] [PubMed] [Google Scholar]
- 20. Anttila S, Knuuttila M, Ylöstalo P, Joukamaa M. Symptoms of depression and anxiety in relation to dental health behavior and self‐perceived dental treatment need. Eur J Oral Sci. 2006;114:109–14. [DOI] [PubMed] [Google Scholar]
- 21. Cranford JA, Eisenberg D, Serras AM. Substance use behaviors, mental health problems, and use of mental health services in a probability sample of college students. Addict Behav. 2009;34:134–45. [DOI] [PubMed] [Google Scholar]
- 22. van Boekel LC, Brouwers EPM, van Weeghel J, Garretsen HFL. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131:23–35. [DOI] [PubMed] [Google Scholar]
- 23. Griffiths J, Jones V, Leeman I, et al. Oral health care for people with mental health problems guidelines and recommendations. London: British Society of Special Care Dentistry; 2000. [Google Scholar]
- 24. Brondani M, Park PE. Methadone and oral health – a brief review. J Dent Hyg. 2011;85:92–8. [PubMed] [Google Scholar]
- 25. Tripathee S, Akbar T, Richards D, Themessl‐Huber M, Freeman R. The relationship between sugar‐containing methadone and dental caries: a systematic review. Health Educ J. 2012;72:469–85. [Google Scholar]
- 26. Baghaie H, Kisely S, Forbes M, Sawyer E, Siskind DJ. A systematic review and meta‐analysis of the association between poor oral health and substance abuse. Addiction. 2017;112:765–79. [DOI] [PubMed] [Google Scholar]
- 27. Kisely S, Baghaie H, Forbes M, Sawyer E, Siskind D. A systematic review and meta‐analysis of the association between poor oral health and substance use. Aust N Z J Psychiatry. 2017;51:136. [DOI] [PubMed] [Google Scholar]
- 28. van Kempen EEJ, Brand HS. Effects of krokodil (desomorphine) use on oral health ‐ a systematic review. Br Dent J. 2019;227:806–12. [DOI] [PubMed] [Google Scholar]
- 29. El‐Zaemey S, Schüz J, Leon ME. Qat chewing and risk of potentially malignant and malignant oral disorders: a systematic review. Int J Occup Med Environ Health. 2015;6:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization (Regional Office for Europe) . Integrated care model: an overview. Geneva: WHO; 2016. [Google Scholar]
- 31. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 32. Antoniazzi RP, Palmeira RV, Schöffer C, Dos Santos BZ, Zanatta FB, Feldens CA. Use of crack cocaine increases tooth loss. Am J Dent. 2021;34:317–21. [PubMed] [Google Scholar]
- 33. Araujo MWB, Dermen K, Connors G, Ciancio S. Oral and dental health among inpatients in treatment for alcohol use disorders: a pilot study. J Int Acad Periodontol. 2004;6:125–30. [PubMed] [Google Scholar]
- 34. Arora PC, Ragi KGS, Arora A, Gupta A. Oral health behavior and treatment needs among drug addicts and controls in Amritsar district: a case‐controlled study. J Neurosci Rural Pract. 2019;10:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Åstrøm AN, Virtanen J, Özkaya F, Fadnes LT. Oral health related quality of life and reasons for non‐dental attendance among patients with substance use disorders in withdrawal rehabilitation. Clin Exp Dent Res. 2022;8:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aukštakalnis R, Jurgelevičius T. The oral health status and behaviour of methadone users in Lithuania. Stomatologija. 2018;20:27–31. [PubMed] [Google Scholar]
- 37. Barbadoro P, Lucrezi D, Prospero E, Annino I. Improvement of knowledge, attitude, and behavior about oral health in a population of alcohol addicted persons. Alcohol Alcohol. 2008;43:347–50. [DOI] [PubMed] [Google Scholar]
- 38. Cheah ALS, Pandey R, Daglish M, Ford PJ, Patterson S. A qualitative study of patients' knowledge and views of about oral health and acceptability of related intervention in an Australian inpatient alcohol and drug treatment facility. Health Soc Care Community. 2017;25:1209–17. [DOI] [PubMed] [Google Scholar]
- 39. De Souza SJR, Dos Santos AD, Albini MB, Gabardo MCL, De Lima AAS, Machado MAN. Oral health impact profile and associated variables in southern Brazilian drug users. Iran J Public Health. 2018;47:1466–75. [PMC free article] [PubMed] [Google Scholar]
- 40. Espósito Santos BF, Da‐Ré EL, Silva GP, Bello GF, Fernandes LA, Coelho De Lima D. Drug addiction: self‐perception and clinical data on oral health. Rev Bras Promoc Saude. 2015;28:479–86. [Google Scholar]
- 41. Gupta T, Shah N, Mathur VP, Dhawan A. Oral health status of a group of illicit drug users in Delhi, India. Community Dent Health. 2012;29:49–54. [PubMed] [Google Scholar]
- 42. Khocht A, Schleifer SJ, Janal MN, Keller S. Dental care and oral disease in alcohol‐dependent persons. J Subst Abus Treat. 2009;37:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laslett A‐M, Dietze P, Dwyer R. The oral health of street‐recruited injecting drug users: prevalence and correlates of problems. Addiction. 2008;103:1821–5. [DOI] [PubMed] [Google Scholar]
- 44. Lo Giudice G, Cicciu M, Polimeni A, Lizio A, Lo Giudice R, Lauritano F, et al. Oral and dental health of Italian drug addicted in methadone treatment. Oral Sci Int. 2019;16:8–14. [Google Scholar]
- 45. Minic I, Pejcic A. The condition of oral health in regular users of psychoactive substances. West Indian Med J. 2016;65:193–7. [DOI] [PubMed] [Google Scholar]
- 46. Mufida L, Setijanto R, Palupi R, Bramantoro T, Ramadhan C, Aulia R. Caries and dental and oral hygiene profile of drug (narcotics and dangerous drugs) users at drug rehabilitation centers. J Int Oral Health. 2019;11:6–9. [Google Scholar]
- 47. O'Sullivan EM. Dental health of Irish alcohol/drug abuse treatment centre residents. Community Dent Health. 2012;29:263–7. [PubMed] [Google Scholar]
- 48. Ravenel MC, Salinas CF, Marlow NM, Slate EH, Evans ZP, Miller PM. Methamphetamine abuse and oral health: a pilot study of “meth mouth”. Quintessence Int. 2012;43:229–37. [PubMed] [Google Scholar]
- 49. Santella AJ, Parish C, Dan R, Feaster DJ, Rodriguez AE, Del Rio C, et al. Dental care utilization of hospitalized persons living with HIV and substance use. J Community Health. 2021;46:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shekarchizadeh H, Khami MR, Mohebbi SZ, Virtanen JI. Oral health behavior of drug addicts in withdrawal treatment. BMC Oral Health. 2013;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smit DA, Naidoo S. Oral health effects, brushing habits and management of methamphetamine users for the general dental practitioner. Br Dent J. 2015;218:531–6. [DOI] [PubMed] [Google Scholar]
- 52. Teixeira L, Manso M‐C, Manarte‐Monteiro P. Oral health‐related quality of life among a Portuguese sample of institutionalised alcoholic patients under rehabilitation therapy. Oral Health Prev Dent. 2018;16:241–8. [DOI] [PubMed] [Google Scholar]
- 53. Van Hout MC, Hearne E. Oral health behaviours amongst homeless people attending rehabilitation services in Ireland. J Ir Dent Assoc. 2014;60:144–9. [PubMed] [Google Scholar]
- 54. Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group . Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health & Social Care; 2017. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/673978/clinical_guidelines_2017.pdf. Accessed 25 October 2022. [Google Scholar]
- 55. Grigg J, Manning V, Arunogiri S, Volpe I, Frei M, Phan V, et al. Methamphetamine treatment guidelines: practice guidelines for health professionals Richmond, Victoria; 2018.
- 56. Health Service Executive . Clinical guidelines for opioid substitution treatment (OST) Ireland. Available from: https://www.hse.ie/eng/services/publications/primary/clinical-guidelines-for-opioid-substitution-treatment.pdf. Accessed 25 October 2022.
- 57. Kleber HD, Weiss RD, Anton RF, George TP, Greenfield SF, Kosten TR, et al. Treatment of patients with substance use disorders. Am J Psychiatry. 2006;163(8 Suppl):1–81. [PubMed] [Google Scholar]
- 58. Registered Nurses' Association of Ontario . Oral health: nursing assessment and interventions. Toronto, ON: Registered Nurses'Association of Ontario; 2008. [Google Scholar]
- 59. Registered Nurses' Association of Ontario . Engaging clients who use substances. Toronto, ON: Registered Nurses'Association of Ontario; 2015. [Google Scholar]
- 60. Jenner L, Lee N. Treatment approaches for users of methamphetamine: a practical guide for frontline workers. Canberra: Australian Government Department of Health and Ageing; 2008. [Google Scholar]
- 61. Hede B, Thiesen H, Christensen LB. A program review of a community‐based oral health care program for socially vulnerable and underserved citizens in Denmark. Acta Odontol Scand. 2019;77:364–70. [DOI] [PubMed] [Google Scholar]
- 62. Ivanovic A, Martin R, Clark N, Pemberton D, Fox S, Kangutkar T, et al. Early development of an integrated oral health (OH) care program with people who inject drugs (PWID) in a primary health care setting. Int J Integr Care. 2019;19:254. [Google Scholar]
- 63. Kisely S, Sawyer E, Siskind D, Lalloo R. The oral health of people with anxiety and depressive disorders – a systematic review and meta‐analysis. J Affect Disord. 2016;200:119–32. [DOI] [PubMed] [Google Scholar]
- 64. Ho HD, Satur J, Meldrum R. Perceptions of oral health by those living with mental illnesses in the Victorian community ‐ the consumer's perspective. Int J Dent Hyg. 2018;16:e10–6. [DOI] [PubMed] [Google Scholar]
- 65. McKibbin CL, Kitchen‐Andren KA, Lee AA, Wykes TL, Bourassa KA. Oral health in adults with serious mental illness: needs for and perspectives on care. Community Ment Health J. 2015;51:222–8. [DOI] [PubMed] [Google Scholar]
- 66. Poudel P, Griffiths R, Wong VW, Arora A, Flack JR, Khoo CL, et al. Perceptions and practices of general practitioners on providing oral health care to people with diabetes ‐ a qualitative study. BMC Fam Pract. 2020;21:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Poudel P, Griffiths R, Wong VW, Arora A, Flack JR, Khoo CL, et al. Perceptions and practices of diabetes educators in providing oral health care: a qualitative study. Diabetes Educ. 2018;44:454–64. [DOI] [PubMed] [Google Scholar]
- 68. Sanchez P, Everett B, Salamonson Y, Ajwani S, Bhole S, Bishop J, et al. Perceptions of cardiac care providers towards oral health promotion in Australia. Collegian. 2018;25:471–8. [Google Scholar]
- 69. Kong AC, Sousa MS, Ramjan L, Dickson M, Goulding J, Gwynne K, et al. “Got to build that trust”: the perspectives and experiences of aboriginal health staff on maternal oral health. Int J Equity Health. 2020;19:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. George A, Johnson M, Duff M, Blinkhorn A, Ajwani S, Bhole S, et al. Maintaining oral health during pregnancy: perceptions of midwives in Southwest Sydney. Collegian. 2011;18:71–9. [DOI] [PubMed] [Google Scholar]
- 71. Mann RS, Marcenes W, Gillam DG. Is there a role for community pharmacists in promoting oral health? Br Dent J. 2015;218:E10. [DOI] [PubMed] [Google Scholar]
- 72. Sheridan J, Aggleton M, Carson T. Dental health and access to dental treatment: a comparison of drug users and non‐drug users attending community pharmacies. Br Dent J. 2001;191:453–7. [DOI] [PubMed] [Google Scholar]
- 73. Hussey D, Trinder‐Widdess Z, Dee C, Bagnall D, Bojangles T, Kesten JM. Co‐design of harm reduction materials for people who inject drugs to implement research findings. Harm Reduct J. 2019;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jones S, Lee N, Jenner L. Results of a co‐design process to identify alcohol and other drugs service opportunities in the eastern Melbourne PHN catchment. Melbourne: Eastern Melbourne Primary Health Network; 2019. [Google Scholar]
- 75. Goodyear‐Smith F, Jackson C, Greenhalgh T. Co‐design and implementation research: challenges and solutions for ethics committees. BMC Med Ethics. 2015;16:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Search Strategy.
Appendix 2. Full text articles excluded.


