Abstract
Wheat is a globally important crop and one of the “big three” US field crops. But unlike the other two (maize and soybean), in the United States its development is commercially unattractive, and so its breeding takes place primarily in public universities. Troublingly, the incentive structures within these universities may be hindering genetic improvement just as climate change is complicating breeding efforts. “Business as usual” in the US public wheat‐breeding infrastructure may not sustain productivity increases. To address this concern, we held a multidisciplinary conference in which researchers from 12 US (public) universities and one European university shared the current state of knowledge in their disciplines, aired concerns, and proposed initiatives that could facilitate maintaining genetic improvement of wheat in the face of climate change. We discovered that climate‐change‐oriented breeding efforts are currently considered too risky and/or costly for most university wheat breeders to undertake, leading to a relative lack of breeding efforts that focus on abiotic stressors such as drought and heat. We hypothesize that this risk/cost burden can be reduced through the development of appropriate germplasm, relevant screening mechanisms, consistent germplasm characterization, and innovative models predicting the performance of germplasm under projected future climate conditions. However, doing so will require coordinated, longer‐term, inter‐regional efforts to generate phenotype data, and the modification of incentive structures to consistently reward such efforts.
Keywords: abiotic stressors, biotic stressors, climate uncertainty, genetic improvement, institutions, land‐grant universities, research infrastructure, United States, wheat breeding
“Business as usual” in the US public wheat‐breeding infrastructure may not sustain productivity increases in the face of climate change. To address this concern, we convened an interdisciplinary group of researchers (including breeders) from across the United States to identify the tools, germplasm, and networks that breeders need to develop climate‐ready cultivars. Interdisciplinary collaboration and inter‐regional cooperation will be critical.
1. INTRODUCTION
Wheat is the most widely grown and globally traded crop on Earth and it is a (human) food crop, accounting for 18% of global calorie consumption (Erenstein et al., 2022). It is eaten by rich and poor people alike, but in many countries, it is the single most important source of calories, particularly among their poorest citizens. Because of this, disruption to its production and distribution can easily trigger increased hunger in many parts of the world.
Wheat is important for the United States, too. It is a relatively low‐input crop (it requires less water and nitrogen than maize) and can be grown in regions with vastly differing climates and without irrigation. As a result, it is grown in nearly every US state. Wheat production can also provide societal benefits beyond the value of its harvested grain: depending on the farm, it prevents soil erosion, improves soil quality, suppresses weeds, provides wildlife habitat, and—more recently—sequesters carbon. These general characteristics, combined with the ability to develop regionally suitable varieties, have made wheat an important part of US agriculture. Indeed, the United States has numbered among the “top‐five” wheat‐producing countries since the 1960s.
For over two centuries, US breeding programs have played critical roles in wheat production. They contributed varieties that could survive the new growing conditions encountered during the Westward expansion in the 1800s and the early 1900s and put national wheat yields on a sustained upward trend starting in the mid‐1930s. They generated dramatically higher‐yielding varieties (HYV) that would not lodge (e.g., Orville Vogel's Norin‐10 × Brevor cross in 1952, as documented in Borojevic & Borojevic 2005). As part of the Green Revolution technologies, these HYVs raised the global supply of calories per capita even as the world's population rapidly increased in the 1960s. And least heralded—but possibly the most important—they have permitted farmers to withstand threats posed by new and/or ever‐evolving pests and pathogens (Graybosch & Peterson, 2012; Olmstead & Rhode, 2002).
More recently, public wheat‐breeding programs have partnered with extension agronomists and others, whose research has encouraged the widespread adoption of no‐till agriculture, which has been shown to conserve soil and stabilize farm incomes. While no‐till agriculture is essentially a planting method that uses specialized machinery, without the work of breeders developing compatible varieties, it would never have become the “breakthrough” technology that it has become in the United States and in South America. For example, the combination of no‐till methods and adapted varieties allowed hard‐red‐winter wheat growers in the US Great Plains to move away from a wheat‐fallow rotation toward continuous wheat or wheat with another crop. And in the soft‐red‐winter wheat region of the United States, it has permitted a double‐crop rotation of corn–wheat–soybean (three crops in 2 years).
Climate change is likely to challenge the ability to increase or simply maintain yields. Tack et al. (2015) show a tradeoff between heat resilience and yield, suggesting that increased extreme heat events will cause greater yield penalties. Ominously, the same authors find that new varieties are more susceptible to warming. Other authors find a 6% decrease in global wheat yields per °C rise in temperature (Asseng et al., 2015; Zhao et al., 2017). Other climate‐change manifestations that will negatively impact wheat yields include increases in extreme rainfall and drought events (Zampieri et al., 2017), higher night‐time temperatures (García et al., 2015; Hatfield et al., 2011; Hein et al., 2020), greater water vapor deficits (Yuan et al., 2019), and the movement of pests and diseases with the evolution of local micro‐climates (Russell & Van Sanford, 2018; Russell et al., 2014; Tessmann & Van Sanford, 2018). On the other hand, breeders have an opportunity to exploit increased CO2 fertilization due to climate change (Dingkuhn et al., 2020; Taylor & Schlenker, 2021) and, in some places, to breed cultivars that mature before heat stress or water stress reduces yields.
The success of a breeding infrastructure can be measured in terms of genetic improvement and variety adoption/turnover. These are difficult to observe in US wheat for many reasons. Various data are currently collected in only 10 out of 30 states with major wheat production. In the absence of these data, genetic improvement cannot be fully measured. Moreover, US wheat is differentiated in terms of end‐use (bread, biscuit, or pasta) and growth habit (spring or winter). Genetic improvement is a meaningful metric only when calculated within these subcategories; spotty varietal data mean that this can be impossible for all but the more dominant subcategories (e.g., hard red winter wheat in the Great Plains). Lacking a comprehensive view of genetic improvement, we rely on yield data.
US wheat's yield gains over the past decades—while steady—have been modest compared to maize and soybean in the United States, and even compared to wheat in Germany or France (Figure 1). Yield increases are imperfect indicators of genetic gain, as yields reflect input intensity, management practices, geographic locations, and environmental conditions, in addition to genetic potential. For example, Europe's climate permits a very long grain‐filling period, leading to higher yields. However, these factors explain the difference in yield levels between the countries. They cannot explain the differences in yield growth rates.
FIGURE 1.
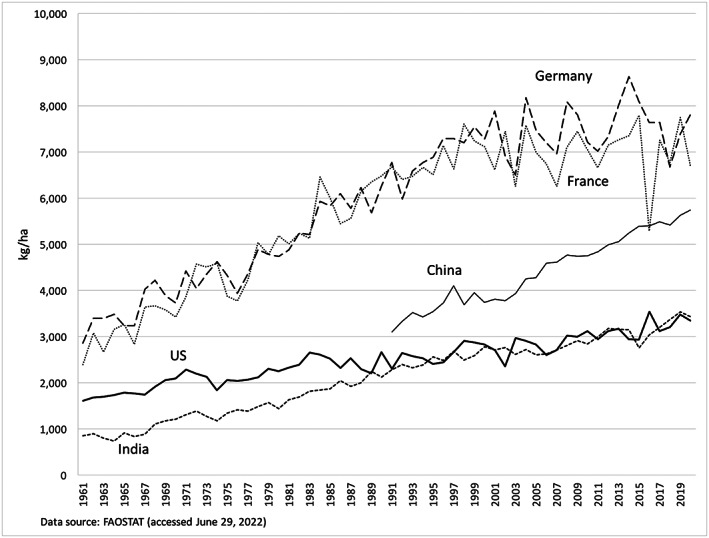
Wheat yields for various countries
Certain aspects of the US wheat‐breeding infrastructure itself may bear some of the blame for the slower yield gains. The wheat‐breeding system of the US is organized very differently from those in Europe. For example, in Germany and France, “breeders” (those who develop cultivars ready for producers) work in companies and “geneticists” (those who develop germplasm and use gene‐editing techniques) work in universities. In the United States, public breeding programs are led by breeders, among whom few have received training in the techniques of geneticists. In addition, Germany has a long tradition of businesses and public researchers working together to adapt varieties to suit localized needs (Gerullis, 2016; Wieland, 2004). In contrast, private and public breeders in the United States tend to have a more “arms‐length” relationship and the sheer geographic scale and heterogeneity of growing conditions in the United States mean that varieties are typically developed for broader sets of conditions.
In the United States, profit margins are thin for developers of wheat seed. As a result, wheat is not as commercially attractive for private seed companies to develop. The majority of wheat‐breeding research is conducted in 30 land grant universities across the country, with the remainder taking place in a handful of private companies. Indeed, an estimated 57% of wheat varieties planted in the United States are those released from land‐grant universities (WheatCAP, 2018).
Why are profit margins thin? Wheat self‐pollinates and generally stores well on‐farm, which allows growers to save seed. Unlike other major commodity crops, intellectual property is protected not by biological means (e.g., hybrid technology, “terminator genes”) but primarily by legal means such as Plant Variety Protection Certificates (PVPC) and—more recently—utility patents. However, enforcement of these systems is costly on the part of seed developers (both private and public) because saving seed is effective (the quality of the crop from saved seed does not fall as it would from replanting hybrid seeds), relatively easy and—in the case of cultivars protected by the PVPC—legal if the saved seed is for the farmer's own use. Thus, even PVPC holders are often prevented from fully recouping the value of their genetic contributions via royalties (Smith et al., 2016). Moreover, the “US wheat market” is comprised of at least six “classes,” or sub‐markets. And because each class is distinguished by the wheat plant's growth habit (spring or winter) and end use, breeding priorities and marketing approaches will necessarily be different for each. The size of the market for a specific wheat class (e.g., soft white) may be inadequate for a firm to recoup these class‐specific investments.
On the “buyer” side, for many farms, wheat is barely profitable despite its ability to be grown on marginal land and primarily under rainfed conditions (Wulff & Dhugga, 2018). When per‐acre yields are low, farmers are less willing to pay for a given percentage increase in yield, at a given wheat price. It is telling that during a period of high commodity prices in the late 2000s, a handful of European companies entered the US wheat breeding/seed market, only to depart when prices returned to “normal.”
While GM technology could potentially make wheat‐breeding research so productive that new cultivars could be profitable for private companies (as it has been for corn, soybean, cotton, and canola), the fact that wheat is a food crop used in very distinctive and culturally important food products means that its acceptance by potential buyers is not a given. The world's first GM wheat, the drought‐tolerant HB4, developed by the Argentina‐based company Bioceres and approved by Argentina in 2020 for production, is an important test case; we will not know the result for a few years yet. GM research is also trait specific. In the United States, where different wheat market classes encounter different problems, it may be hard to identify “breakthrough” traits for which there are large enough customer bases. Thin profit margins are also likely forestalling the development of other major “breakthrough” technologies. For example, Pioneer—as early as the 1960s—had discussed developing hybrid wheat. However, it has not released a commercial hybrid variety, to date. Syngenta in 2018 paused its US hybrid wheat program. It is likely that public programs will continue to be the biggest source of wheat‐breeding innovation in the United States.
Because public breeding programs are primarily housed in land‐grant universities, these programs are affected by funding mechanisms and institutional constraints that affect all research at land‐grant universities. Since the passage of the Bayh‐Dole Act in 1980, federal funds allocated to public agricultural research and development have fallen, and the proportion of these monies being awarded through competitive grants (as opposed to formula funds) has increased. Greater reliance on federal competitive grant funding has been shown to drag down agricultural productivity in general (Huffman & Evenson, 2006). Presently, sources of plant breeding program funding include university funds, regular appropriations (or “formula” funds), federal competitive grants, royalties, and grants from various wheat organizations (e.g., small grain growers' associations/wheat commissions). These state wheat organizations typically represent a significant source of funding.
Within this research environment, breeding programs have thus far been able to maintain yields. However, two hallmarks of climate change are uncertainty and more extreme weather patterns. Preparing for these conditions requires agility in research, wider access to promising germplasm, and the ability to accumulate longer records of fit‐for‐purpose data. This type of research will require time frames longer than the 3–5 years typical of competitive grants. The mismatch of funding time frames and research time frames is particularly acute for wheat, for which breeding cycles can typically take 8–12 or more years. And for political reasons, state wheat organizations might be wary of funding projects that explicitly deal with climate change. It is not clear that public US wheat‐breeding programs are sufficiently equipped to meet the new challenges posed by climate change.
These concerns led three of the authors to convene a conference of wheat breeders and allied scientists to better characterize the nature of the constraints faced by wheat breeders in conducting breeding research for climate resiliency. The present section outlined our “prior beliefs” going into this conference. The next section describes the conference and our discoveries—in the form of common concerns and big unknowns—raised and discussed by the conference participants; we can think of these as our updated beliefs. Section 3 outlines the needs articulated by the group and a roadmap for better preparing the US public wheat‐breeding infrastructure for climate change. Section 4 summarizes our findings.
2. IDENTIFYING BOTTLENECKS TO US PUBLIC WHEAT BREEDING
We convened a group of 16 researchers in February 2022 to discuss how the US wheat‐breeding infrastructure could better prepare for climate change. The group comprised five wheat breeders, one agronomic modeler, one plant physiologist, one plant pathologist, one geographer, and seven agricultural economists, representing 12 different US land‐grant universities and one European university. It is important to note here that USDA‐Agricultural Research Service's (ARS) scientists play an important role in US wheat improvement—developing germplasm, coordinating uniform regional nurseries, facilitating genotyping of cultivars and breeding lines—but those whom we invited were unable to attend.
Over the 3 days of the conference, participants each gave short presentations outlining the state of research on climate change and wheat in their specific area of expertise. Following these presentations, we engaged in facilitated discussions centered on the following questions:
What breeding objectives, if prioritized, would enable the wheat production systems to respond to changing climate conditions in diverse locales?
How can we more effectively coordinate research across the many institutions involved in US wheat breeding?
With whom should wheat breeders collaborate (i.e., other types of scientists) to identify potential scenarios and options for effective wheat breeding the face of climate change?
What current constraints and incentives shape the breeding and research portfolios of wheat breeders and other researchers?
From these presentations and discussions, we collectively prioritized specific topic areas which require more research and collaboration. The remainder of this section catalogues the main concerns and unanswered questions that our group identified as critical for accelerating targeted genetic improvement in wheat.
2.1. Common concerns
2.1.1. Long breeding cycles and rapidly changing environmental conditions
The long time‐to‐market has always been a concern for wheat breeders and, accordingly, many are experimenting with innovations such as double haploids and “speed breeding.” However, wheat's uniquely complex genome means that the typical wheat‐breeding cycle can still take 12 or more years, consisting of 1–2 years for the initial crossing, 5–6 years for inbreeding and selection of the best variants, and another 4–5 years for extensive trial evaluations. These long commercialization times, combined with changing environmental conditions, might mean that the environmental conditions at market release no longer match those at testing. Variation in weather can also complicate breeding: For example, if the 4–5 years of extensive testing prior to a cultivar's release happen to be extremely wet, the breeder will have little information on its resistance to drought.
2.1.2. Challenges predicting trait performance and tradeoffs between traits
Wheat's genomic complexity also limits the potential of direct genetic interventions via recombinant technologies or gene editing techniques. Identifying the “best” varieties for different locations is already challenging because of the tradeoffs that inevitably occur when selecting for specific traits. These tradeoffs are difficult to predict. As local environmental conditions change due to climate change, the identification of novel traits will be simultaneously more important and complicated. Breeders will require more data on the relationship between phenotype, genotype, and environmental context of production to predict yield outcomes for specific varieties under different scenarios.
2.1.3. Differing regional priorities
US wheat breeders routinely cooperate with one another with phenotyping efforts, typically on bilateral bases or through regional uniform nurseries. Breeders also have multiple pathways for exchanging germplasm and related accession data. Much research on biotic stressors also takes place through networks of land‐grant breeders. However, these collaborations almost exclusively take place within current climate regions, which roughly coincide with wheat market classes and/or the incidence of specific pests or pathogens. A recurring comment throughout the conference was that climate change will likely cause local environmental conditions to “migrate.” Consequently, germplasm and data generated in one region might be more useful to a neighboring region in a matter of decades, if not years.
2.1.4. Communication among stakeholders
Breeders expressed concern that farmers often sow the “wrong” varieties, despite the existence of locally adapted varieties produced by breeding programs. Breeders posited a few reasons for these choices: (1) farmers lack information when choosing wheat varieties to plant, (2) farmers' goals diverge from those of breeders, (3) crop insurance and farm programs create disincentives for planting new varieties, and (4) varieties underperform on farms compared to field trials. Understanding farmers' choices is hindered by the fact that the USDA's National Agricultural Statistics Service no longer surveys farmers on the varieties used. Currently, only 10 of 30 major wheat‐growing states regularly collect such data; in the remainder, surveying is done through informal means.
Beyond understanding what varieties farmers prefer and why, breeders desired better communication with producers on other topics. Among these topics are (1) how/whether farmers are adapting to climate change, (2) the degree to which on‐farm yields reflect field trial performance, (3) how improved varieties perform in different environmental conditions, and (4) what language would better resonate with farmers who are thoroughly familiar with weather variability but might not engage in conversations about “climate change.”
Finally, breeders suggested that breeders and germplasm repositories require better coordination to stimulate climate‐resilient breeding efforts. Breeders gave examples of successful exchange networks within regions for plant genetic resources and auxiliary data; however, some regions have more successful networks than others. Germplasm, data, and findings are also exchanged within networks established around specific resistance initiatives (e.g., The US Wheat and Barley Scab Initiative https://www.scabusa.org). There were fewer references to germplasm/data exchange between breeders and germplasm repositories. A few breeders noted that germplasm repositories often have phenotype data, but fewer have genotype data to match these accessions. Not all users of germplasm from repositories report back field trial data to those repositories.
2.2. Big unknowns
2.2.1. How will climate change impact local wheat production?
Wheat is particularly sensitive to weather variability partially because it stays in the field for longer than other crops, increasing the probability of weather‐ and pest‐related crop losses. Consequently, a pressing question for breeders is how local manifestations of climate change will impact the yield of different varieties in different conditions. More environment and climate data—specifically variables tied to trait/yield productivity—are necessary to replicate current and historical conditions as a precursor to climate predictions. In addition, better models are needed to predict localized changes in climate.
Climate change increases uncertainty surrounding the incidence and severity of threats from pathogens and pests. The historical boom‐and‐bust cycles of wheat rust disease epidemics illustrate the importance of continuing breeding efforts to keep up with the ever‐changing pest profile. Stem rust frequently caused severe yield losses globally (Saari & Prescott, 1985) until the adoption of resistant wheat varieties in the 1950s, which effectively controlled stem rust for the next three decades (Roelfs et al., 1992; Singh et al., 2006; Stokstad, 2007). However, in 1998, a new virulent lineage of stem rust named Ug99 was first detected in Uganda. In addition, stripe rust has also now expanded into non‐traditional, warmer and drier areas, causing more frequent yield losses (Beddow et al., 2015; Chen, 2007; Goyal & Manoharachary, 2014; Milus et al., 2009; Wellings, 2011).
Climate change will force breeding programs to change their resistance‐breeding priorities. Once future threats are characterized, it will be crucial to identify new sources of resistance genes to deploy high‐yielding, resistant varieties (Bhavani et al., 2019). In practice, this means sourcing resistant germplasm resources from areas where a particular pathogen or pest is already prominent to develop lines that might be resilient to an emerging pathogen, a strategy that breeders have long deployed. The challenge at hand is the pace at which pathogens and pests are changing.
2.2.2. What traits will be important locally?
In the face of these many potential threats, as well as potential opportunities, breeders need to make informed decisions on what breeding paths should be taken given limited resources. These choices are often made with very few tools to quantify the future value of certain traits. This decision‐making could be facilitated by having (1) forecasts of climate and local environmental conditions, including pests and pathogens; (2) crop‐, yield‐, or phenotype models which would, in turn be informed by data from controlled‐environment research to mimic climate change scenarios; and (3) predictions of human behavior, markets, and policy. Using these, researchers could estimate the value of using improved genotypes in specific contexts, potentially helping to justify breeding goals requiring longer time horizons.
2.2.3. Which challenges are best met through breeding?
Even with more tools and better models, climate variability and shifts in market preferences could accelerate the displacement of wheat by other crops in many cropping systems. Breeding alone will not prevent this displacement; therefore, it will be critical to understand which climate‐related challenges would be best met through breeding and which could be better met with other tools. Producers are also likely to alter planting times to adapt to distributions of climate stressors over the crop year; they may even experiment with different market classes of wheat, with implications for regional markets. Breeders must be cognizant of the many other potential tools for risk mitigation, so as to focus on traits that will complement anticipated strategies.
3. THE NEED FOR TOOLS AND A DIFFERENT KIND OF COORDINATION
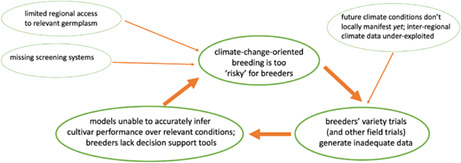
The wheat breeders were unanimous in their concern about the problems presented by climate change and the challenges it will pose for growers and breeders in the coming decades. They also explained that they are indeed constrained from preparing for these challenges, not so much because of funding issues or institutional constraints, but for a lack of tools. For a breeder to focus on a trait, he/she needs predictive models for guidance as to which trait(s) to develop, germplasm containing sufficient genetic variation, screening mechanisms for select traits, and the ability to characterize germplasm.
3.1. Tools for assessing threats and prioritizing traits
To understand where breeding might have the most impact, breeders and researchers must develop a comprehensive typology of potential threats to (and opportunities for) wheat in different regions at multiple intervals in the future. These threats can include both biotic and abiotic stressors which vary greatly across space and over time, such as night‐time temperature increase, increased drought risk, and pest/disease incursion. Local abiotic manifestations of climate change on wheat productivity can be explored using process‐based crop models coupled with climate scenario projections, helping to identify high‐risk “hotspots” for specific stressors. Similarly, pest‐ and disease models, coupled with crop growth models, can be used to evaluate the risk profiles for different biotic stressors under various climate change scenarios. Depending on the purpose of this risk mapping, a probabilistic approach using long‐run historical pest and disease loss distributions (e.g., Beddow et al., 2015; Chai et al., 2020; Pardey et al., 2013) can also be used to help assess pest risks and prioritize investment decisions. Another approach is to exploit spatial variation of climate to anticipate time‐series change at given locations. Olmstead and Rhode (2002) offer an informative account of the challenges faced by wheat breeders as wheat production moved westward, to new and challenging locations in the United States.
To guide these breeding efforts, it is necessary to identify the traits valued most by different stakeholders (e.g., farmers, bakers) which will likely vary across space and time. For instance, drought tolerance traits may need to be developed and maintained as breeding material to “future‐proof” wheat production, but commercial varieties may not need to be released to farmers for immediate use until those climate conditions are realized. Care should be taken to recognize the implications of current crop insurance for the choices made by farmers, in addition to their perceptions of the biggest threats to growing wheat.
In short, regional assessments of threats and opportunities would provide information on which scenarios can be most effectively addressed by breeding efforts versus other strategies such as modern financial risk management, insurance, and/or adjustments to on‐farm management practices (e.g., no‐till farming, diversification). Once regionally specific typologies of threats and opportunities are identified, breeders and researchers could develop a set of breeding priorities within and among regions.
3.2. Improved predictive models
As part of and parallel to risk assessments, breeders need better modelling tools to assist the efficacy of field trials and other in situ wheat evaluation and production efforts. Experimental field trials, called “yield trials” by wheat breeders, inform breeders and farmers of the performance—under current climate conditions—of locally adapted varieties that are on the market or close to commercialization. Their results inform farmers as they choose among varieties, as well as breeders, whose breeding programs are shaped by the data generated. If climate change‐induced shifts in local growing conditions are slow and incremental, then field trials, as described above, will continue to generate adequate data for both groups. If climate‐induced shifts are rapid or unpredictable, farmers and breeders will need to make inferences on the performance of (locally) “new” varieties, varieties under various warming scenarios, and/or varieties in the face of not‐yet‐endemic pests and pathogens. Field trials, as they are currently conducted, may no longer generate the necessary data.
Participants discussed complementing field trials with in silico, or “virtual,” field trials (VFT) in which genomic prediction models are coupled with crop growth models, the analytical underpinnings of which have been developed and evaluated by several groups (e.g., Heslot et al., 2014; Messina et al., 2018; Rincent et al., 2017). A VFT approach could assist both breeders and farmers in predicting performance metrics for a given genotype under various climate scenarios and management regimes. VFTs would create data that complement conventional field trials by (1) modeling growing conditions that include predicted‐but‐not‐yet‐seen scenarios such as higher temperatures and elevated CO2 concentrations, (2) accommodating heretofore untested cultivar‐environment‐management combinations, and (3) predicting a variety's performance using metrics specific to climate change. In addition, breeders could use VFTs to “subject” their experimental lines to conditions that did not occur during the development of a given cultivar.
Generating reliable VFT results will require an ensemble of models and input data tested against the anticipated range of future climate conditions. Model ensembles have provided effective insights on climate impacts in the face of uncertainty (Asseng et al., 2015; Martre et al., 2015; Ruane et al., 2016). Model development and validation will also require fit‐for‐purpose data. Although current field trial data provide a potential long‐term source of such data, experimental lines and released varieties will need to be observed under conditions more extreme than those experienced currently. One way to achieve this is through geographically dispersed trials located in strategically selected future climate analog sites (e.g., Taylor & Schlenker 2021 conduct a spatial analysis of CO2 fertilization). Another way is through controlled‐environment studies using interventions such as heaters, heat tents, or CO2 fertilization (e.g., Bergkamp et al., 2018; Hein et al., 2019). Moreover, more specialized data (beyond yield, basic agronomic characteristics, and resistance values) will need to be collected. Given these needs, it is unlikely that all breeding programs could set up such facilities. Rather, this work could take place in regional hubs and emulate the USDA‐ARS's current approach of having experts specializing in screening for targeted traits.
3.3. Networks to generate tools for breeders
Creating tools that facilitate breeding locally appropriate, climate‐resilient wheat varieties requires new kinds of networks. First, these networks should be inter‐regional. Breeding can potentially facilitate adaptation of varieties to new locations where wheat might persist and even have an emerging comparative advantage relative to other crops. Identifying production possibilities across regions under future scenarios might facilitate breeding goals that are not limited by immediate regional priorities.
Second, coordinating networks should be interdisciplinary. Discussions made clear that much climate‐change‐oriented research was taking place, but on “parallel tracks.” Many scientists (including some wheat breeders) conduct climate‐change‐related research on wheat; however, there is little systematic communication of findings between disciplines, and even less ex‐ante coordination of research activities. Different disciplines generate specialized models—for example, process‐based crop growth models, genomic selection models, economic models—but these models are rarely integrated. And despite their abundance and diversity, most are of limited use as decision‐support tools for field‐based wheat breeders. Similarly, many disciplines generate phenotypic data, but these data are typically not useful for researchers in other disciplines. For example, wheat breeders' field trials generate data on a massive scale, but these data may or may not be usable as training data for, say, geneticists. Plant physiologists conduct experiments that cover wide ranges of environmental conditions, but these experiments are typically limited in the number and diversity of genotypes represented.
Finally, US wheat breeders must also strengthen linkages to relevant international networks that target climate‐change‐related threats such as the Heat and Drought Wheat Improvement Consortium (HeDWIC http://www.hedwic.org). New technologies generated within HeDWIC are disseminated globally to wheat breeders via the International Wheat Improvement Network (IWIN https://www.cimmyt.org/funder_partner/international‐wheat‐improvement‐network‐iwin/). Organized by CIMMYT, IWIN currently distributes about 1000 wheat varieties for testing at approximately 700 field sites in over 90 countries. US‐based breeders interested in climate resilience would benefit by working with such international networks.
4. CONCLUDING THOUGHTS
Yield‐related genetic gain in wheat has held steady over the past decades, due in no small part to the work of wheat breeders worldwide. This steady pace of yield growth, however, will likely be threatened by the gradually worsening effects of climate change. Maintaining present rates of genetic gain will require that wheat‐breeding infrastructures worldwide be nimble and efficient, but also far‐sighted. How can the US wheat‐breeding infrastructure—the majority of which is housed in state land‐grant universities across the nation—achieve this?
Meeting this challenge requires (1) the prioritization of specific breeding objectives, (2) better coordination and data sharing between breeding institutions and germplasm banks, (3) more interdisciplinary collaboration to develop models and identify priority breeding objectives, and (4) an iterative evaluation of institutional constraints and opportunities for breeding efforts that hold the most promise for “future‐proofing” US wheat production.
As noted above, many US wheat breeders lack predictive models to help prioritize breeding objectives. However, the expertise to build such models already exists in other disciplines. Researchers in these disciplines should work with breeders to develop fit‐for‐purpose decision‐support tools. Many breeders also lack access to promising germplasm outside of their regional networks. However, USDA‐ARS already plays a coordinating role with germplasm for resistance to biotic stressors; it could play a similar role for abiotic stressors. In addition, we have observed, for decades, international networks sharing germplasm and data across countries. A US network based on these international models could facilitate inter‐regional—and even international—exchange of germplasm and data. Developing more robust relationships between these research/breeding institutions and germplasm banks can accelerate the critical work already being done by breeders. To actualize this potential, however, we must not only scrutinize and modify the existing incentive structures to facilitate long‐term, sustained cooperation and data‐sharing, but also change the research culture to naturally consider climate change effects when developing research programs. This must begin with the training curricula for those entering the profession.
Updating curricula, building networks, and collaborating across disciplines, institutions, regions, and countries requires significant “activation energy.” However, we believe our institutional situation provides reasons for optimism, too: Tenured researchers in public institutions are in the greatest position to take academic risks and generate public goods. As public scientists, we are free to cooperate across institutions and countries. And while interdisciplinary work within and across universities is difficult, it is relatively easy between academics. Making US wheat climate‐ready will require that we immediately and fully make use of these advantages. We are at a point where inaction is too costly.
FUNDING INFORMATION
Kusunose, Rossi, and Van Sanford received funding through the Agriculture and Food Research Initiative (AFRI) from the USDA National Institute of Food and Agriculture (award no. 2020‐67013‐31681) for the research and publication of this article. Gerullis was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy (EXC 2070—390732324—PhenoRob).
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENTS
We thank Paula Castellari, Joyce Robinson, and Elzbieta Szuleta for their excellent work facilitating logistics, leading discussions, and wrangling technology during the conference described herein. We also thank Rachel Laudan for her constructive comments and keen eye for language.
Kusunose, Y. , Rossi, J. J. , Van Sanford, D. A. , Alderman, P. D. , Anderson, J. A. , Chai, Y. , Gerullis, M. K. , Jagadish, S. V. K. , Paul, P. A. , Tack, J. B. , & Wright, B. D. (2023). Sustaining productivity gains in the face of climate change: A research agenda for US wheat. Global Change Biology, 29, 926–934. 10.1111/gcb.16538
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Asseng, S. , Ewert, F. , Martre, P. , Rötter, R. P. , Lobell, D. B. , Cammarano, D. , Kimball, B. A. , Ottman, M. J. , Wall, G. W. , White, J. W. , Reynolds, M. P. , Alderman, P. D. , Prasad, P. V. V. , Aggarwal, P. K. , Anothai, J. , Basso, B. , Biernath, C. , Challinor, A. J. , De Sanctis, G. , … Reynolds, M. P. (2015). Rising temperatures reduce global wheat production. Nature Climate Change, 5(2), 143–147. 10.1038/nclimate2470 [DOI] [Google Scholar]
- Beddow, J. M. , Pardey, P. G. , Chai, Y. , Hurley, T. M. , Kriticos, D. J. , Braun, H. J. , Park, R. F. , Cuddy, W. S. , & Yonow, T. (2015). Research investment implications of shifts in the global geography of wheat stripe rust. Nature Plants, 1(10), 1–5. 10.1038/nplants.2015.132 [DOI] [PubMed] [Google Scholar]
- Bergkamp, B. , Impa, S. M. , Asebedo, A. R. , Fritz, A. K. , & Jagadish, S. K. (2018). Prominent winter wheat varieties response to post‐flowering heat stress under controlled chambers and field based heat tents. Field Crops Research, 222, 143–152. 10.1016/j.fcr.2018.03.009 [DOI] [Google Scholar]
- Bhavani, S. , Hodson, D. P. , Huerta‐Espino, J. , Randhawa, M. S. , & Singh, R. P. (2019). Progress in breeding for resistance to Ug99 and other races of the stem rust fungus in CIMMYT wheat germplasm. Frontiers of Agricultural Science and Engineering, 6, 210–224. 10.15302/J-FASE-2019268 [DOI] [Google Scholar]
- Borojevic, K. , & Borojevic, K. (2005). The transfer and history of “reduced height genes” (Rht) in wheat from Japan to Europe. Journal of Heredity, 96(4), 455–459. 10.1093/jhered/esi060 [DOI] [PubMed] [Google Scholar]
- Chai, Y. , Pardey, P. G. , Hurley, T. M. , Senay, S. D. , & Beddow, J. M. (2020). A probabilistic bio‐economic assessment of the global consequences of wheat leaf rust. Phytopathology, 110, 1896. 10.1094/PHYTO-02-20-0032-R [DOI] [PubMed] [Google Scholar]
- Chen, X. M. (2007). Challenges and solutions for stripe rust control in the United States. Australian Journal of Agricultural Research, 58(6), 648–655. 10.1071/AR07045 [DOI] [Google Scholar]
- Dingkuhn, M. , Luquet, D. , Fabre, D. , Muller, B. , Yin, X. , & Paul, M. J. (2020). The case for improving crop carbon sink strength or plasticity for a CO2‐rich future. Current Opinion in Plant Biology, 56, 259–272. 10.1016/j.pbi.2020.05.012 [DOI] [PubMed] [Google Scholar]
- Erenstein, O. , Jaleta, M. , Mottaleb, K. A. , Sonder, K. , Donovan, J. , & Braun, H. J. (2022). Global trends in wheat production, consumption and trade (pp. 47–66). Springer International Publishing. [Google Scholar]
- García, G. A. , Dreccer, M. F. , Miralles, D. J. , & Serrago, R. A. (2015). High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Global Change Biology, 21(11), 4153–4164. 10.1111/gcb.13009 [DOI] [PubMed] [Google Scholar]
- Gerullis, M. K. (2016). Entstehung privater Eigentumsrechte an Weiterentwicklungen von Weizensaat in den USA und Deutschland. In Biopatente (pp. 235–260). Nomos Verlagsgesellschaft mbH and Co. KG. [Google Scholar]
- Goyal, A. , & Manoharachary, C. (Eds.). (2014). Future challenges in crop protection against fungal pathogens. Springer. [Google Scholar]
- Graybosch, R. A. , & Peterson, C. J. (2012). Specific adaptation and genetic progress for grain yield in Great Plains hard winter wheats from 1987 to 2010. Crop Science, 52(2), 631–643. 10.2135/cropsci2011.08.0412 [DOI] [Google Scholar]
- Hatfield, J. L. , Boote, K. J. , Kimball, B. A. , Ziska, L. H. , Izaurralde, R. C. , Ort, D. , & Wolfe, D. (2011). Climate impacts on agriculture: Implications for crop production. Agronomy Journal, 103(2), 351–370. 10.2134/agronj2010.0303 [DOI] [Google Scholar]
- Hein, N. T. , Bheemanahalli, R. , Wagner, D. , Vennapusa, A. R. , Bustamante, C. , Ostmeyer, T. , Pokharel, M. , Chiluwal, A. , Fu, J. , Srikanthan, D. S. , Neilsen, M. L. , & Jagadish, S. V. K. (2020). Improved cyber‐physical system captured post‐flowering high night temperature impact on yield and quality of field grown wheat. Scientific Reports, 10(1), 1–15. 10.1038/s41598-020-79179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein, N. T. , Wagner, D. , Bheemanahalli, R. , Šebela, D. , Bustamante, C. , Chiluwal, A. , Neilsen, M. L. , & Jagadish, S. V. K. (2019). Integrating field‐based heat tents and cyber‐physical system technology to phenotype high night‐time temperature impact on winter wheat. Plant Methods, 15(1), 41–15. 10.1186/s13007-019-0424-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslot, N. , Akdemir, D. , Sorrells, M. E. , & Jannink, J. L. (2014). Integrating environmental covariates and crop modeling into the genomic selection framework to predict genotype by environment interactions. Theoretical and Applied Genetics, 127(2), 463–480. 10.1007/s00122-013-2231-5 [DOI] [PubMed] [Google Scholar]
- Huffman, W. E. , & Evenson, R. E. (2006). Do formula or competitive grant funds have greater impacts on state agricultural productivity? American Journal of Agricultural Economics, 88(4), 783–798. 10.1111/j.1467-8276.2006.00898.x [DOI] [Google Scholar]
- Martre, P. , Wallach, D. , Asseng, S. , Ewert, F. , Jones, J. W. , Rötter, R. P. , Boote, K. J. , Ruane, A. C. , Thorburn, P. J. , Cammarano, D. , Hatfield, J. L. , Rosenzweig, C. , Aggarwal, P. K. , Angulo, C. , Basso, B. , Bertuzzi, P. , Biernath, C. , Brisson, N. , Challinor, A. J. , … Wolf, J. (2015). Multimodel ensembles of wheat growth: Many models are better than one. Global Change Biology, 21(2), 911–925. 10.1111/gcb.12768 [DOI] [PubMed] [Google Scholar]
- Messina, C. D. , Technow, F. , Tang, T. , Totir, R. , Gho, C. , & Cooper, M. (2018). Leveraging biological insight and environmental variation to improve phenotypic prediction: Integrating crop growth models (CGM) with whole genome prediction (WGP). European Journal of Agronomy, 100, 151–162. 10.1016/j.eja.2018.01.007 [DOI] [Google Scholar]
- Milus, E. A. , Kristensen, K. , & Hovmøller, M. (2009). Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology, 99(1), 89–94. 10.1094/PHYTO-99-1-0089 [DOI] [PubMed] [Google Scholar]
- Olmstead, A. L. , & Rhode, P. W. (2002). The red queen and the hard reds: Productivity growth in American wheat, 1800‐1940. The Journal of Economic History, 62(4), 929–966. 10.1017/S0022050702001602 [DOI] [Google Scholar]
- Pardey, P. G. , Beddow, J. M. , Kriticos, D. J. , Hurley, T. M. , Park, R. F. , Duveiller, E. , Sutherst, R. W. , Burdon, J. J. , & Hodson, D. (2013). Right‐sizing stem‐rust research. Science, 340(6129), 147–148. 10.1126/science.122970 [DOI] [PubMed] [Google Scholar]
- Rincent, R. , Kuhn, E. , Monod, H. , Oury, F. X. , Rousset, M. , Allard, V. , & Le Gouis, J. (2017). Optimization of multi‐environment trials for genomic selection based on crop models. Theoretical and Applied Genetics, 130(8), 1735–1752. 10.1007/s00122-017-2922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfs, A. P. , Singh, R. P. , & Saari, E. E. (1992). Rust diseases of wheat: Concepts and methods of disease management. CIMMYT. [Google Scholar]
- Ruane, A. C. , Hudson, N. I. , Asseng, S. , Cammarano, D. , Ewert, F. , Martre, P. , & Wolf, J. (2016). Multi‐wheat‐model ensemble responses to interannual climate variability. Environmental Modelling & Software, 81, 86–101. 10.1016/j.envsoft.2016.03.008 [DOI] [Google Scholar]
- Russell, K. , Lee, C. , McCulley, R. L. , & Van Sanford, D. (2014). Impact of climate change on wheat production in Kentucky. Plant and Soil Sciences Research Report, 3(3), 1–20. [Google Scholar]
- Russell, K. , & Van Sanford, D. (2018). Breeding for resilience to increasing temperatures: A field trial assessing genetic variation in soft red winter wheat. Ecology and Evolution, 8(23), 12090–12100. 10.1002/ece3.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari, E. E. , & Prescott, J. M. (1985). World distribution in relation to economic losses. In Roelfs A. P. & Bushnell W. R. (Eds.), The cereal rusts (Vol. II). Academic Press. [Google Scholar]
- Singh, R. P. , Hodson, D. P. , Jin, Y. , Huerta‐Espino, J. , Kinyua, M. , Wanyera, R. , Njau, P. , & Ward, R. (2006). Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviews: Perspective in Agriculture, Veterinary Science and Natural Resources, 54, 1–13. 10.1079/PAVSNNR20061054 [DOI] [Google Scholar]
- Smith, S. , Lence, S. , Hayes, D. , Alston, J. , & Corona, E. (2016). Elements of intellectual property protection in plant breeding and biotechnology: Interactions and outcomes. Crop Science, 56(4), 1401–1411. 10.2135/cropsci2015.10.0608 [DOI] [Google Scholar]
- Stokstad, E. (2007). Deadly wheat fungus threatens world's breadbaskets. Science, 315(5820), 1786–1787. 10.1126/science.315.5820.1786 [DOI] [PubMed] [Google Scholar]
- Tack, J. , Barkley, A. , & Nalley, L. L. (2015). Effect of warming temperatures on US wheat yields. Proceedings of the National Academy of Sciences of the USA, 112(22), 6931–6936. 10.1073/pnas.1415181112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. and Schlenker, W. (2021). Environmental drivers of agricultural productivity growth: CO 2 fertilization of US field crops (October 2021) . NBER Working Paper No. w29320. 10.3386/w29320 [DOI]
- Tessmann, E. W. , & Van Sanford, D. A. (2018). GWAS for fusarium head blight related traits in winter wheat (Triticum aestivum L.) in an artificially warmed treatment. Agronomy, 8(5), 68. 10.3390/agronomy8050068 [DOI] [Google Scholar]
- Wellings, C. R. (2011). Global status of stripe rust: A review of historical and current threats. Euphytica, 179(1), 129–141. 10.1007/s10681-011-0360-y [DOI] [Google Scholar]
- WheatCAP . (2018). Economic impact report. https://www.triticeaecap.org/publications‐and‐germplasm/
- Wieland, T. (2004). Wir beherrchen den pflanzlichen organismus besser … Wissenschaftliche Pflanzenzüchtung in Deutschland, 1889–1945. Abhandlungen und Berichte, NF, Bd.20. Munich.
- Wulff, B. B. , & Dhugga, K. S. (2018). Wheat—The cereal abandoned by GM. Science, 361(6401), 451–452. 10.1126/science.aat5119 [DOI] [PubMed] [Google Scholar]
- Yuan, W. , Zheng, Y. , Piao, S. , Ciais, P. , Lombardozzi, D. , Wang, Y. , Ryu, Y. , Chen, G. , Dong, W. , Hu, Z. , Jain, A. K. , Jiang, C. , Kato, E. , Li, S. , Lienert, S. , Liu, S. , Nabel, J. E. M. S. , Qin, Z. , Quine, T. , … Yang, S. (2019). Increased atmospheric vapor pressure deficit reduces global vegetation growth. Science Advances, 5(8), eeax1396. 10.1126/sciadv.aax1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri, M. , Ceglar, A. , Dentener, F. , & Toreti, A. (2017). Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environmental Research Letters, 12(6), 064008. 10.1088/1748-9326/aa723b [DOI] [Google Scholar]
- Zhao, C. , Liu, B. , Piao, S. , Wang, X. , Lobell, D. B. , Huang, Y. , Huang, M. , Yao, Y. , Bassu, S. , Ciais, P. , Durand, J. L. , Elliott, J. , Ewert, F. , Janssens, I. A. , Li, T. , Lin, E. , Liu, Q. , Martre, P. , Müller, C. , … Asseng, S. (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proceedings of the National Academy of Sciences of the USA, 114(35), 9326–9331. 10.1073/pnas.1701762114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


