Abstract
Atrial fibrillation is the most common sustained arrhythmia, increases with age, and presents with a wide spectrum of symptoms and severity. Paroxysmal, persistent, and permanent forms require very individualized approaches to management. New information about electrical and anatomic remodeling emphasizes the importance of time-related thrombogenicity and progressive interference with mechanical function of the atria and ventricles. The most important aspect of diagnosis is risk stratification with respect to risk of thromboembolism. The general goals in treatment are, in order of importance: prevention of thromboemboli, control of ventricular response, restoration of sinus rhythm, and maintenance of sinus rhythm by preventing recurrences. This review focuses on the above issues. The therapeutic choices are discussed under each category. Antiarrhythmic drugs, radiofrequency ablation techniques, and device therapy are reviewed with respect to prevention of recurrent atrial fibrillation.
Key words: Anticoagulants/therapeutic use; anti-arrhythmia agents/therapeutic use; atrial fibrillation/physiopathology; atrial fibrillation/therapy; atrial flutter/therapy; cardiac pacing, artificial; catheter ablation; thromboembolism/prevention & control
Much has been learned in the last decade regarding the mechanisms that underlie the initiation and perpetuation of atrial fibrillation. For nearly a century, it has been known that atrial fibrillation exists in the presence of various forms of heart disease in which stretching and scarring of the atrial myocardium appear to promote, in some way, electrical disorganization of atrial depolarization. It has also been recognized that atrial fibrillation occurs in individuals without overt heart disease, but the mechanisms are only now becoming understood. In such individuals, with “lone” atrial fibrillation, certain precursor arrhythmias are sometimes found—especially macro-reentrant atrial flutter, focal atrial tachycardias, and reentrant supraventricular tachycardias, such as atrioventricular (A-V) nodal reentrant tachycardia and orthodromic reciprocating tachycardia.
Whether atrial fibrillation occurs in the presence or absence of structural heart disease, it is now clear that atrial electrical and mechanical remodeling, as a consequence of the arrhythmia, tend to perpetuate atrial fibrillation. This remodeling process results primarily from the increase in atrial rate, but atrial ischemia, neurohumoral and autonomic factors, and atrial stretch can also contribute. 1 The remodeling process can be followed in time by stages that reflect a series of membrane, intracellular, and intercellular adaptations to increased rate, as recently described by Allessie. 2 Table I summarizes the sequence and probable mechanisms of these progressive changes, which, if not interrupted, can lead to permanent structural and functional changes in the atria or the heart as a whole. It is probable that tachycardia-induced cardiomyopathy occurs in this manner.
Table I. Adaptation to Heart Rate 2
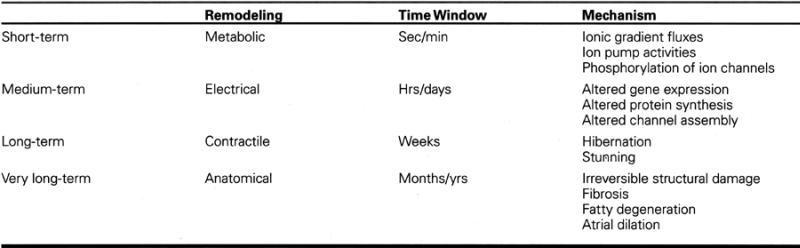
At the electrophysiologic level, the consequences of prolonged rapid atrial rate include a spatially heterogeneous shortening of atrial refractoriness, conduction delay, loss of adaptation of refractoriness to rate, and a dispersion of atrial refractoriness. These changes promote vulnerability to atrial fibrillation and favor maintenance of multiple wavelets to perpetuate the arrhythmia. The underlying cellular events that permit these changes include a progressive reduction in the density both of L-type calcium (ICa) channels and of the transient outward potassium channels (Ito). 3 Sodium current (INa) is also reduced during atrial tachycardia, which leads to slowing of intercellular conduction and to maladaptive functional conduction delay. 4 The conduction delay may be further impaired by a down-regulation of the gap-junction proteins Connexin-40 and -43. 5 The molecular changes that are thought to lead to the above ionic dysfunction include transcriptional down-regulation of messenger-RNA encoding for ICa, INa, and Ito channel proteins. 6,7 Intracellular calcium overload might be the signal that leads to atrial electrical remodeling. In experimental animals, the T-type calcium blocking drug mibefradil seems capable—by diminishing intracellular calcium load—of preventing electrical and mechanical remodeling. 8
We have a new understanding of the mechanisms by which acutely and chronically increased atrial rates adapt, and of the pharmacologic and electrical methods that seem capable of arresting or reversing the remodeling process. This gives us cause for hope that clinically effective therapies will soon be developed, to prevent or control what is truly the most common and troublesome arrhythmia of our time.
Classification of Atrial Fibrillation
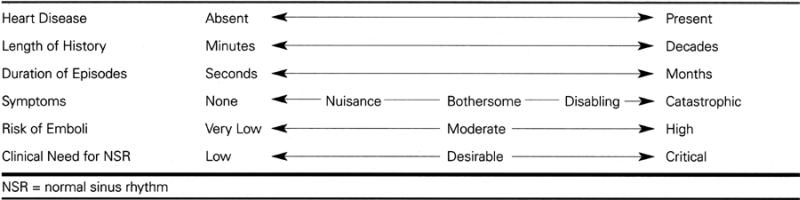
The clinical manifestations of atrial fibrillation run the gamut from a completely asymptomatic arrhythmia discovered in the course of medical evaluation for other reasons, through a sporadic arrhythmia with minimal, troubling, disabling, or catastrophic symptoms, to a chronic arrhythmia that also may have minimal or disabling symptoms. Table II displays the clinical spectrum of atrial fibrillation.
Table II. Clinical Spectrum of Atrial Fibrillation
A useful classification of atrial fibrillation into 3 varieties—paroxysmal, persistent, and permanent, as shown in Table III—helps to organize thinking about the management of this highly variable arrhythmia.
Table III. Classification of Atrial Fibrillation

Implied in the above classification system is the conclusion that therapy must be tailored to the individual patient, in accordance with these factors: whether the duration of the arrhythmia is short, medium, long, or unknown; whether the patient is symptomatic; how severe the symptoms are; and whether restoration to sinus rhythm is known to be possible. In all cases, of course, a decision must be made regarding protection from thromboembolism, in light of current data about the risk of stroke.
Diagnostic Goals
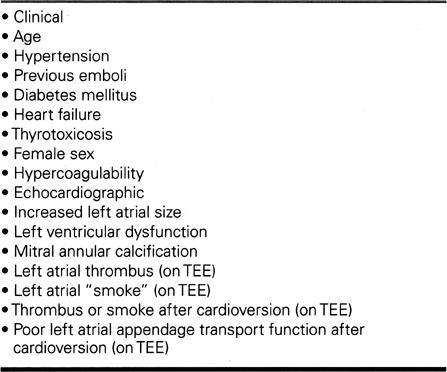
In a patient newly presenting with atrial fibrillation, an early goal is to determine—by historical, physical, radiographic, and echocardiographic criteria—if the patient has structural heart disease. The importance of the echocardiogram should not be underestimated, since many of the risk factors for thromboembolism (Table IV) are revealed best by echocardiography. The special role of transesophageal echocardiography is discussed below under Reversion to Sinus Rhythm.
Table IV. Identifiable Risk Factors for Systemic Thromboembolism
It is also important to ask the patient about any concurrent medical (such as pulmonary or endocrinologic) problems and about prescription or nonprescription drugs currently taken, since some of these may be proarrhythmic or may interact with medicines that need to be introduced in management of the fibrillation.
Therapeutic Goals
Stroke Prevention
The several controlled trials published in the latter half of the 1990s attest to the importance of long-term anticoagulation with adjusted-dose warfarin in the majority of patients with atrial fibrillation, whether paroxysmal or permanent. The exceptions are younger patients (<75 years) who have none of the recognized risk factors for thromboembolism that are listed in Table IV. Present recommendations are summarized in Table V.
Table V. Antithrombotic Strategies

In a critical review of current data on embolic risk and management, Blackshear and Safford 9 have made several important observations substantiated by citations in the literature:
A large proportion (30% to 44%) of atrial fibrillation detected in the elderly is asymptomatic;
Elderly patients with pacemakers have a very high (48%) incidence of atrial fibrillation;
Warfarin appears to be grossly under used, especially in the elderly who are not hospitalized or who are in nursing homes;
Formal anticoagulation clinics, in comparison with “usual medical care,” reduce the risk of stroke, as well as hemorrhage, hospitalizations, and mortality;
The guidelines for thromboembolic protection should be the same for atrial flutter as for atrial fibrillation;
A prethrombotic state can be identified within 12 hours of the onset of atrial fibrillation, and progressively in the first 24 hours, even in younger patients with “lone” atrial fibrillation;
Current practice is rapidly moving toward urgent heparinization and transesophageal echocardiography (TEE) in patients with recent onset (<3 days) of atrial fibrillation; and
Asymptomatic episodes may be up to 12 times more common than symptomatic episodes, which makes symptoms an unreliable marker of arrhythmia suppression.
Control of Ventricular Response
The conventional use of digitalis for acute control of ventricular rate in a patient presenting with atrial fibrillation and a rapid ventricular response has become obsolete with the introduction of intravenous beta-blockers, verapamil, and diltiazem.
For control of rate in long-standing atrial fibrillation, beta-blocking drugs are generally preferred over digitalis and calcium-blocking drugs, especially in active or young patients, because exercise usually overrides the dromotropic effects of the latter 2 drug types. Digitalis is most useful in the patient with a dilated heart, and calcium-blocking drugs are preferred over beta-blocking drugs in patients with bronchoconstriction or serious cerebrovascular or peripheral vascular disease. Double or triple therapy is sometimes needed.
When antiarrhythmic drugs are used (with the occasional exception of amiodarone), AV nodal blocking drugs are generally used concomitantly to prevent a rapid ventricular response, should the patient have recurrent atrial fibrillation or atrial flutter.
In patients who present with atrial fibrillation and very rapid pre-excited ventricular response via an accessory pathway (Wolff-Parkinson-White syndrome WPW), the following drugs are contraindicated for acute management: adenosine, verapamil, diltiazem, and digitalis. Digitalis is also contraindicated in long-term treatment of a WPW patient who is known to have a short accessory pathway refractory period—or whose refractory period is unknown. The means by which these drugs accelerate ventricular response via the accessory pathway (with a small but finite risk of ventricular fibrillation) differs from drug to drug. In the case of intravenous adenosine, verapamil, and diltiazem, the vasodilatory effects produce a reflex sympathetic shortening of accessory pathway refractoriness. It is unlikely that the AV nodal blocking effect of these drugs “enhances” accessory pathway conduction as is popularly taught, since beta-blocking drugs (being vasoconstrictors) are without this deleterious effect. In the case of digitalis, a direct shortening of ventricular muscle refractoriness (possibly also of the accessory pathway itself) seems to be responsible for accelerating the ventricular rate.
Reversion to Sinus Rhythm
Whether and when to restore sinus rhythm is a decision that should be based on the highly individual circumstances of the particular patient, as expressed in Table II. On the whole, early (even urgent) restoration of sinus rhythm is wise in patients who have severe or catastrophic symptoms, significant hemodynamic compromise, or a history of “persistent” episodes (Table III). In most instances, early return to sinus rhythm is desirable because thrombogenicity and remodeling increase over time. When the urgency is less, a delayed (conventional) approach to conversion to sinus rhythm can be justified, provided that adjusted-dose anticoagulation therapy is in place. It has long been recognized that certain patients with “permanent” atrial fibrillation may be managed best by permanent anticoagulation and rate-controlling drugs alone, provided that the arrhythmia is well tolerated subjectively.
Anticoagulation is the 1st requirement, regardless of whether acute, late, or no conversion to sinus rhythm is contemplated. In the acute situation, the administration of intravenous heparin—or of subcutaneous enoxaparin or dalteparin in combination with Coumadin or aspirin (or both)—are recommended strategies, while a decision is being made about the timing of conversion to sinus rhythm. Restoration of sinus rhythm by any method, whether electrical or pharmacologic, should be delayed until the patient has been given the best possible protection from systemic embolism.
The convention of administrating full anticoagulation for 4 weeks preceding pharmacologic or electrical cardioversion should not be applied to every patient. Transesophageal echocardiography, when used in a patient not chronically anticoagulated, provides valuable information to the physician. Some of the risk-stratification variables (Table IV) are best defined by TEE, especially left atrial thrombus, “smoke,” appendage shortening and velocity, and mobile atherosclerotic plaque in the ascending aorta. If such risk factors are absent or minimal, electrical cardioversion is now commonly performed immediately after TEE, under general anesthesia. The TEE is repeated after cardioversion, in order to re-evaluate left atrial function and pre-thrombotic risk factors. While it is not yet proved that such an approach is optimal in all or even in selected patients, several prospective trials (SPAF-III, ACUTE, AFFIRM) 10–14 are investigating the safety and efficacy of this more aggressive approach.* Transesophageal echocardiography will probably be economically feasible only when it is not preceded by transthoracic echocardiography. From a theoretical standpoint, of course, the earlier the restoration of sinus rhythm, the less the progress of thrombogenicity and remodeling.
Electrical cardioversion should always be performed under brief general anesthesia, administered by competent personnel in a well oxygenated patient who is hemodynamically and medically stable, to the degree possible.
Medical cardioversion with intravenous or oral antiarrhythmic drugs has a lower acute success rate than electrical cardioversion. Whether medical cardioversion will be shown to be safer than electrical cardioversion is an interesting question. Theoretically, the proarrhythmic effects of antiarrhythmic drugs might be offset by less of a mechanical stunning effect on the atria. It is now well-established that the class III drug ibutilide can effectively restore sinus rhythm, especially in atrial fibrillation or flutter of short duration (hours), with an acceptably low risk of torsades de pointes in properly selected patients. 15 Other oral regimens, such as high-dose propafenone, quinidine, and flecainide, have been reported. 16,17
From the foregoing, it is apparent that recurrence of atrial fibrillation is the rule, regardless of causation. The frequency of asymptomatic recurrences (e.g., during sleep) implies that anticoagulation, once started, will in general be permanent. It is the opinion of this author that the common teaching to continue anticoagulation for only 3 to 4 weeks after cardioversion should be abandoned. In light of current data about the ongoing risk of thromboembolism, it seems much wiser to continue dose-adjusted Coumadin therapy forever. Until data emerge on the safety and efficacy of clopidogrel and other antiplatelet agents, it seems that we are stuck with Coumadin.
Maintenance of Sinus Rhythm
In patients who are highly or even mildly symptomatic from recurrent atrial fibrillation, maintenance therapy is important in order to relieve symptoms. In theory, maintenance of sinus rhythm will prevent both thromboembolism and electrical and structural remodeling; this goal is laudable, but its efficacy has not yet been proved. When the primary objective of symptomatic relief is absent (in asymptomatic patients with atrial fibrillation), it is not yet clear whether maintenance of sinus rhythm will prevent heart failure, emboli, or death. In patients with persistent atrial fibrillation, an alternative strategy, still under investigation, is the control of ventricular rate. The ongoing AFFIRM 14 and PIAF 18 trials will provide answers to these very intriguing questions.*
After an initial episode of atrial fibrillation has resolved, either spontaneously (“paroxysmal” variety) or by intervention (“persistent” variety), antiarrhythmic drug therapy will generally be withheld until it is known whether the patient has a recurrence. In the case of recurrent episodes of atrial fibrillation, the question of whether the benefits of antiarrhythmic therapy outweigh the disadvantages becomes a matter of clinical judgment. Patients and physicians need to understand at the outset that atrial fibrillation is rarely eliminated. A good response to therapy might include occasional recurrences, especially if these are self-terminating. It is sometimes quite acceptable to have the patient undergo electrical cardioversion once or twice a year, if the maintenance drug therapy is well tolerated. Any new therapy, including antiarrhythmic drugs, should be introduced with careful attention to potential interaction with other drugs—in this case, the prescribing physician should look at the interactions of Coumadin, digitalis, and beta-blocking and calcium-blocking drugs, together with concomitant therapy that affects depolarization and repolarization of the heart, absorption of drugs, activation or depression of the hepatic cytochrome P-450 system, and the excretion of drugs and metabolites. Thus, in a patient with recurrent atrial fibrillation, the frequency and severity of recurrences in that individual will determine whether a trade-off for cost of therapy, proarrhythmic effects, extracardiac side-effects, and drug interactions is a worthwhile exchange.
Most available antiarrhythmic drugs appear to have approximately similar efficacy against atrial fibrillation, with flecainide and amiodarone possibly having a slight edge in superiority. The choice of drug then comes down to avoidance of unwanted side-effects, considerations of ease in dosing, and, in most instances, cost. Table VI shows a popular algorithm for choice of 1st-line drug therapy for recurrent atrial fibrillation, as outlined in a superb recent review by Ganz and Antman. 19
Table VI. Choice of Antiarrhythmic Drug
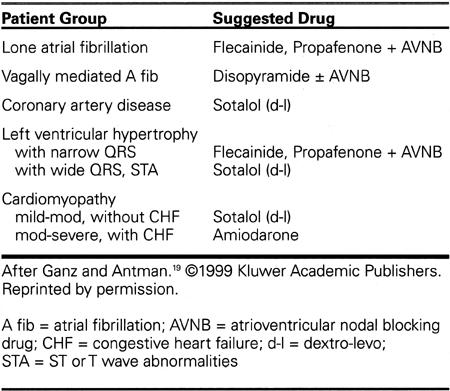
The above choices depend on the nature of the underlying cardiac disease, if any, on the clinical presentation of the atrial fibrillation according to its type (parasympathetic vs sympathetic), on the presence of left ventricular hypertrophy, QRS widening, repolarization changes, and QT interval, and on the degree of left ventricular (LV) dysfunction, if that is present. Except for disopyramide, which is very good for patients with nocturnal or post-prandial atrial fibrillation, Class IA drugs are not generally used as 1st-time drugs, given their propensity to proarrhythmic and non-cardiac effects. Class IC drugs, especially flecainide, are useful in lone (idiopathic) atrial fibrillation and in uncomplicated left ventricular hypertrophy, but are contraindicated in the presence of known or suspected coronary artery disease. In the latter case or with mild cardiomyopathy, d-l sotalol might be preferred. Since all of the antiarrhythmic drugs considered here are negatively inotropic except for amiodarone, amiodarone becomes the only viable choice for patients with significant left ventricular dysfunction or a history of heart failure.* On the other hand, negative inotropic drugs (disopyramide, flecainide, beta-blocking drugs) would be preferred in hypertrophic cardiomyopathy, especially with outflow obstruction.
It is the opinion of this author that antiarrhythmic therapy should generally be initiated during telemetry in the hospital, in order to detect early bradycardiac or proarrhythmic effects and to monitor for excessive ventricular response as the drugs are introduced. However, amiodarone frequently can be initiated among outpatients, with appropriate biochemical, electrocardiographic, and clinical monitoring. In selected outpatients without structural heart disease or sinus node disease, flecainide and propafenone can sometimes be initiated safely, provided that there is early follow-up (3 to 10 days) and electrocardiographic and exercise testing to monitor for exercise-related or use-dependent proarrhythmic effects.
Ablative Therapy Directed at Atrial Fibrillation
Since 1990, radiofrequency ablation of a variety of supra-ventricular tachycardias has become well-established, even 1st-line therapy. During the surgical era for WPW syndrome, it became apparent that transsection of the accessory pathway reduced or eliminated atrial fibrillation. It has been a clinical impression that radiofrequency elimination of various supra-ventricular tachycardias, including classical atrial flutter, has also been followed by a reduction in episodes of atrial fibrillation. 20 On the other hand, it is not yet known whether ablation of specific pathways might have a proarrhythmic effect in some patients, perhaps promoting the development of atrial flutter or atrial fibrillation at a later time.
In patients with paroxysmal or persistent atrial fibrillation who also have one of the amenable supra-ventricular tachycardias or atrial flutter, it is most reasonable to perform catheter ablation of the underlying mechanism that predisposes to the arrhythmia.
Ablation of Classical Atrial Flutter
Atrial flutter is generally a macro-reentrant atrial tachycardia with a more-or-less continuous waveform on the electrocardiogram and is distinguished from atrial fibrillation in that all 4 of the following ECG features remain constant with respect to the atrial activity: morphology, amplitude, polarity, and rate. Atrial fibrillation should be diagnosed if 1 or more of those features varies from moment to moment. Classical atrial flutter comprises 2 forms, both of which are dependent upon the isthmus of tissue between the tricuspid annulus and the lip of the inferior vena cava or Eustacian ridge (i.e., the inferior cavotricuspid isthmus or sub-Eustacian isthmus). Isthmus-dependent classical flutter exists in a common counterclockwise (CCW) variety and a less common clockwise (CW) variety. 21 The terminology derives from the direction of rotation of the flutter wavefront around the tricuspid valve as viewed in the left-anterioroblique projection of the heart. In the CCW form, a rapid wavefront proceeds posterosuperiorly from the coronary sinus orifice over the atrial septum and the posterior right atrial wall toward the superior vena cava. The impulse then moves anteriorly over the superior end of the crista terminalis and then anteroinferiorly over the trabeculated portion of the right atrial free wall, as a slower wavefront. Upon reaching the anterolateral low right atrium, the impulse enters the cavotricuspid isthmus anterior to the Eustachian valve that guards the inferior vena cava, to reach the region of the coronary sinus orifice anterior to the Eustachian ridge and thereby completes the circuit. Classical CW flutter follows the same circuit in the opposite direction. The crista terminalis and the Eustachian valve and ridge form an important anatomic boundary that divides the anterior and posterior limbs of the circuit on the basis of anisotropic conduction and functional block. 21
Ablation of the cavotricuspid isthmus 22 is preceded by definition of the essential role of the isthmus in the reentrant circuit, which is accomplished by discrete mapping of the area during atrial flutter and by pacing in the isthmus to demonstrate transient entrainment of the flutter without atrial fusion. Pacing during sinus rhythm at the low lateral right atrium establishes the counterclockwise activation of the isthmus and tricuspid annulus, whereas pacing at the coronary sinus orifice establishes the clockwise activation of the same tissues. Pacing at the orifice is then continued while the isthmus is ablated from the tricuspid annulus to the inferior vena cava or to the Eustachian ridge. Special attention is sometimes needed along the inferior lip of the coronary sinus and on other occasions at the lateral margin of the isthmus. Partial block or progressive conduction delay across the isthmus sometimes mimics isthmus block. Elegant mapping systems are capable of demonstrating conduction gaps in the line of block, enabling the placement of discrete radiofrequency lesions to complete the line of block. 23 Various end points to the ablation are shown in Table VII, in roughly increasing order of reliability in predicting successful outcome from the procedure.
Table VII. End Points in Ablation for Atrial Flutter

In patients who have both atrial flutter and paroxysmal atrial fibrillation, it has been shown that elimination of atrial flutter can decrease the occurrence of atrial fibrillation, especially if antifibrillatory drug therapy is continued. 24–26
Ablation of Atypical (Non-Classical) Atrial Flutter
Flutters that do not conform to the foregoing electrocardiographic patterns are designated atypical flutters. Although these can occur in the absence of structural heart disease, they seem to be much more prevalent in patients with congenital or acquired heart diseases or in patients in whom previous ablation procedures have been performed in the atria. Circuits have been described crossing the crista terminalis, circulating around atrial septal defects, around a septal defect patch, around atriotomy scars, and around the pulmonary veins.
Focal atrial tachycardias arising from a variety of right and left atrial sites (below) also may occur at rates that mimic atrial flutters (200 to 350 bpm).
Ablation of atypical atrial flutters obviously requires a detailed understanding of the flutter mechanism and a procedure that is designed to interrupt the particular circuit or focus in the individual patient. The general principle in ablation of flutter circuits is to connect 2 anatomic obstacles straddling the essential link by a line of block. This is at present an area of development in both mapping techniques and tools for ablation. 27
Surgical Ablation of Atrial Fibrillation
The pioneering work of Cox and colleagues in mapping human atrial flutter and in developing a series of “maze” operations to eliminate atrial fibrillation has led to very impressive long-term cure of the arrhythmia with substantial restoration of right and (usually) left atrial transport function and the possibility of discontinuation of anticoagulation in selected patients with idiopathic, medically refractory atrial fibrillation. 28 Recently, Melo 29 has reported a high success rate in conversion of “permanent” atrial fibrillation and maintenance of sinus rhythm in a population of patients with rheumatic and other forms of mitral valve disease, including patients with very large atria. The surgical technique involves the use of open radiofrequency oval lesions to surround and isolate the pulmonary veins. These and other pioneering surgical efforts 30 have promoted the development of catheter techniques to achieve similar goals.
Linear Atrial Lesions for Atrial Fibrillation
In experimental animals and in limited human studies to date, linear radiofrequency applications have been used to suppress atrial fibrillation. 31–36 Right atrial linear lesions were reported to eliminate atrial fibrillation in patients with idiopathic atrial fibrillation, 32–35 but follow-up in 1 study 34 showed recurrence of atrial fibrillation in 33% of patients after 21 ± 11 months and in another study 35 showed recurrence in 63% of patients after 26 ± 5 months, despite retreatment with antiarrhythmic drugs. In the process of these studies, patients demonstrated recurrences that sometimes were based on left atrial circuits or point-sources, which in turn became targets for additional left atrial radiofrequency applications. Then, biatrial ablation was reported to enhance the likelihood of cure by both linear and focal left atrial lesions. By adding lesions in the left atrium, Haïssaguerre and colleagues 36 improved the results from 53% after right atrial ablation alone to an additional 70% in patients who failed right atrial linear ablations.
Ablations in the left atrium carry with them a significant risk of systemic thromboembolism and other complications of the transseptal technique, such as cardiac tamponade. Moreover, lesions delivered at high power in the branching portions of the pulmonary veins can be followed by stenosis of the pulmonary vein, sometimes with clinical consequences.
Focal Ablation for Atrial Fibrillation
In 1996, the Bordeaux group reported that in the patients in whom right atrial linear lesions (including isthmus lesions for atrial flutter) had failed to prevent atrial fibrillation, focal electrical sources often were found to initiate atrial fibrillation spontaneously. 36 These sites were most commonly found in the pulmonary veins, especially the left upper and right upper pulmonary veins. The depolarization of myocardial fiber sleeves extending 2 to 4 cm into the proximal pulmonary veins 37 is associated with slow conduction of impulses toward the left atrial myo-cardium, where atrial fibrillation is initiated. It has been suggested that such foci might be the principal cause of lone atrial fibrillation and that they play a role in atrial fibrillation in some patients with heart disease. 38,39 Such patients may be identified by clinical, electrocardiographic, and electrophysiologic features, as shown in Tables VIII and IX, and detected by ECG, ambulatory or trans-telephonic ECG, or exercise testing. With multipolar catheters in 2 to 4 of the pulmonary veins, the electrophysiologic features can be defined.
Table VIII. Clinical and Electrocardiographic Features of Focally Initiated Atrial Fibrillation

Table IX. Electrophysiologic Features of Focally Initiated Atrial Fibrillation from the Pulmonary Veins
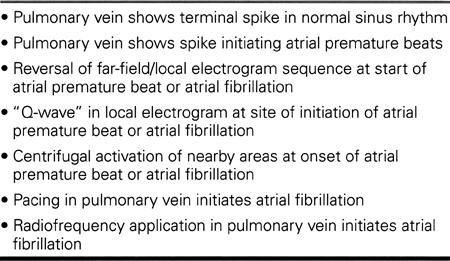
The results of radiofrequency applications within the proximal portions of 1 or more pulmonary veins—alone or in combination with circumferential applications around part or all of the orifice of the relevant pulmonary vein—have been remarkably effective in the hands of the Bordeaux group 38 and a Taipei group. 39 At North Texas Heart Center, we have performed pulmonary vein ablations in 20 patients, with prevention of atrial fibrillation in 15 patients, suppression of arrhythmic symptoms with return to antiarrhythmic drugs in an additional 3 patients, and no benefit in 2 patients. The follow-up ranges from 1 to 28 months, as of January 2000.
The risks of ablation of pulmonary veins or the adjacent left atrial wall include transient vagal effects, systemic emboli, cardiac tamponade, and pulmonary vein stenosis. The last complication can be minimized by restricting ablation to the non-branching portion of the pulmonary vein and by limiting power to 30 watts.* Preablation and post-ablation imaging of the pulmonary veins by contrast venography or transesophageal echocardiography seems prudent. End points for ablation of focal sources of atrial fibrillation can include the features shown in Table X, whether in the pulmonary veins or at other sites in the right or left atria.
Table X. End Points for Ablation of Atrial Foci

It is clear that ablation techniques for atrial fibrillation are still in a state of evolution. It is appreciated that many things need to be developed in the design of ablation catheters and in steering of catheters, as well as alternative forms of energy such as cryotherapy, ultrasound, and microwave energy.
Ablation or Modification of Atrioventricular Conduction
For nearly 20 years, interruption of A-V conduction by high-energy shock and more recently by radiofrequency energy has been a procedure of last resort in selected patients with persistent or permanent atrial fibrillation in whom ventricular rate has not been controllable by double or triple therapy with A-V nodal blocking agents or amiodarone. 40 Preservation of an intact junctional escape rhythm can sometimes be obtained by ablative lesions directed at the A-V nodal approaches (“fast” and “slow” A-V nodal pathways), in contrast with targeting the bundle of His itself. Subsequently, there have been reports that slowing A-V nodal conduction (increasing A-V nodal refractoriness) by directing lesions at the inferior approaches to the A-V node have helped to control ventricular rate. 41,42
Many patients so treated, however, have needed subsequent completion of ablation of A-V conduction to bring about successful rate control.
Because of a small incidence of sudden cardiac death after A-V nodal ablation, 41 it has been recommended that the pacing rate be increased above standard VVI rates for several weeks to prevent QT-related ventricular fibrillation. If dual-chambered pacemakers are used after A-V nodal ablation (i.e., in those patients who have periods of sinus rhythm), care should be taken to maintain regular rate by attention to programming of mode-switching and rate-smoothing to provide symptomatic relief at the times when atrial fibrillation alternates with sinus rhythm.
Device Therapy for Prevention of Atrial Fibrillation
Pacemaker Therapy
Of the many exciting developments in the management of atrial fibrillation, one of the most promising is the present investigation of various pacing techniques for the prevention of atrial fibrillation.
It has been recognized since 199342 that atrial pacing (as opposed to ventricular pacing) may decrease the frequency of recurrent atrial fibrillation in patients with sinus node disease. Theoretically, especially in patients with vagotonic atrial fibrillation, atrial pacing can 1) suppress atrial premature beats, 2) alter atrial refractoriness, 3) diminish dispersion of refractoriness, 4) alter atrial activation sequence and 5) change the electrical and mechanical behavior of atrial muscle that enables the development of atrial fibrillation. The foregoing discussion of the pathophysiology of atrial fibrillation emphasizes that the longer atrial fibrillation is allowed to persist, the shorter will be the intervals between recurrences and the longer the durations of episodes. 1–8,43 As we shall see, pacing and defibrillation interventions seem to be capable of partially reversing the remodeling pro-cess.
In patients with atrial or dual-chambered pacemakers for sinus node disease or A-V block, a con-sistent trend toward a lower prevalence of atrial fibrillation has been observed. 42,44,45 In a prospective crossover study of VVI versus DDD pacing, electrical and mechanical remodeling was observed during VVI pacing, consisting of a reversible, non-uniform increase in atrial refractoriness, an increase in P wave duration, and an increase in atrial size. These changes were not observed when the same patients were paced in DDD node. 45
There is much current interest in alternative-site and dual-site atrial pacing for the prevention of atrial fibrillation. Theoretically, if atrial depolarization were hastened by pacing on the septum or at more than 1 site, functional conduction delay and dispersion of refractoriness might be lessened. In a trial of atrial septal pacing above the coronary sinus orifice in 25 patients, it was shown that a reduction of atrial fibrillation by maintaining sinus rhythm for an average of 10 months was achieved in 90% of patients with or without antiarrhythmic drugs. 46 An early report of a multicenter trial of pacing at Bachmann's bundle showed a trend toward prevention of atrial fibrillation compared to high right atrial pacing. 47
Dual-site atrial pacing has been reported to have the following short-term effects: shortening of P wave duration and interatrial conduction time; diminished dispersion of atrial refractoriness; less functional delay during atrial extrastimulation; and lower inducibility of atrial fibrillation. 48 Initial reports of long-term dual-site atrial pacing support the hypothesis that such pacing can reduce the recurrence of atrial fibrillation in 64% to 80% of patients who have refractory atrial fibrillation and flutter, with and without underlying bradycardia. 49–52 Prospective clinical trials are continuing. 52–54 It appears to be important to achieve substantially continuous atrial pacing by maintaining a relatively high atrial rate and by using beta-blocking drugs to suppress spontaneous sinus rhythm and atrial premature beats. 49
Two trials of implantable atrial defibrillators are continuing. 55,56 In 1 trial, the atrial defibrillator is a stand-alone device, and in the other both atrial and ventricular defibrillation are available. Preliminary results suggest that prompt atrial defibrillation can both shorten the intervals between subsequent episodes and diminish left atrial size. 57 This is exciting support for the hypothesis that the deleterious atrial remodeling process is reversible.
Conclusion
The year 2000 is an exciting one for those interested in the management of arrhythmia patients. The techniques of catheter ablation for many of the common sustained tachycardias have come of age. Out of experimental studies and the maturing technologies used in mapping and ablation, we have learned much about the mysteries of atrial fibrillation, which are the most pervasive and challenging arrhythmias that remain. The special circumstances of the particular patient require thoughtful decision-making in therapy. Available and future drugs, the use of ablation in selected patients, and the appropriate use of sophisticated pacing methods and perhaps atrial defibrillators will change the face of this group of arrhythmias over the next 5 to 20 years, as appropriate combinations of these therapies emerge for application in different clinical circumstances. Prevention of stroke remains the most important clinical objective in patients with atrial fibrillation; the results of many present trials of alternative antithrombotic agents are eagerly awaited.
Acknowledgment
The author is grateful for the secretarial assistance of Angela Faison and Jeremy Wyndham.
Footnotes
* SPAF = Stroke Prevention in Atrial Fibrillation;
ACUTE = Assessment of Cardioversion Using Transesophageal Echocardiography; AFFIRM = Atrial Fibrillation Follow-up Investigation of Rhythm Management
* PIAF = Pharmacological Intervention in Atrial Fibrillation
* In the U.S., use of amidarone for atrial fibrillation is an off-label use. Dofetilide, recently approved in the U.S., may be less depressive of left-ventricular dysfunction than standard drugs, as may other drugs available outside the U.S.
* P. Jaïs. Personal communication (conversation), November 1998.
Address for reprints: Christopher R.C. Wyndham, MD, North Texas Heart Center, 8440 Walnut Hill Lane, Suite 700, Dallas, TX 75231-4469
Presented at the Texas Heart® Institute's symposium Cardiac Arrhythmias: New Pharmacologic and Interventional Strategies, held on 5 February 2000, at the Houstonian Hotel, Houston, Texas
References
- 1.Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation 1997;96:3710–20. [DOI] [PubMed]
- 2.Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol 1998;9:1378–93. [DOI] [PubMed]
- 3.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 1997;81:512–25. [DOI] [PubMed]
- 4.Gaspo R, Bosch RF, Bou-Abboud E, Nattel S. Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ Res 1997;81:1045–52. [DOI] [PubMed]
- 5.van der Velden HM, van Kempen MJ, Wijffels MC, van Zijverden M, Groenewegen WA, Allessie MA, et al. Altered pattern of connexin40 distribution in persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol 1998;9: 596–607. [DOI] [PubMed]
- 6.Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res 1999;84:776–84. [DOI] [PubMed]
- 7.Brundel BJ, van Gelder IC, Henning RH, Tuinenburg AE, Deelman LE, Tieleman RG, et al. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc Res 1999;42:443–54. [DOI] [PubMed]
- 8.Fareh S, Thibault B, Nattel S. Treatment with a T-type calcium channel blocker prevents atrial fibrillation caused by tachycardia-induced atrial remodeling [abstract]. Circulation 1998;98(Suppl I):I–210.
- 9.Blackshear JL, Safford RE. Immediate and long-term prevention of thromboembolism in atrial fibrillation. Cardiac Electrophysiol Review 1999;3:88–92.
- 10.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol 1998;31:1622–6. [DOI] [PubMed]
- 11.Klein AL, Grimm RA, Black IW, Leung DY, Chung MK, Vaughn SE, et al. Cardioversion guided by transesophageal echocardiography: the ACUTE Pilot Study. A randomized, controlled trial. Assessment of Cardioversion Using Transesophageal Echocardiography. Ann Intern Med 1997;126: 200–9. [DOI] [PubMed]
- 12.Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol 1998;81:877–83. [PubMed]
- 13.Weigner MJ, Patel U, Thomas LR, Schwartz JG, Burger AJ, Douglas PS, et al. Transesophageal echocardiography facilitated cardioversion from atrial fibrillation: short term safety and implications regarding maintenance of sinus rhythm [abstract]. Circulation 1998;98(Suppl I):I–500. [DOI] [PubMed]
- 14.Atrial fibrillation follow-up investigation of rhythm management—the AFFIRM study design. The Planning and Steering Committees of the AFFIRM study for the NHLBI AFFIRM investigators. Am J Cardiol 1997;79:1198–202. [PubMed]
- 15.Stambler BS, Wood MA, Ellenbogen KA, Perry KT, Wakefield LK, VanderLugt JT. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation 1996;94:1613–21. [DOI] [PubMed]
- 16.Boriani G, Biffi M, Capucci A, Botto G, Broffoni T, Ongari M, et al. Conversion of recent-onset atrial fibrillation to sinus rhythm: effects of different drug protocols. Pacing Clin Electrophysiol 1998;21(11 Pt 2):2470–4. [DOI] [PubMed]
- 17.Ganz LI, Antman EM. Antiarrhythmic drug therapy in the management of atrial fibrillation. J Cardiovasc Electrophysiol 1997;8:1175–89. [DOI] [PubMed]
- 18.Hohnloser SH, Kuck KH. Atrial fibrillation: maintaining stability of sinus rhythm or ventricular rate control? The need for prospective data: the PIAF trial. Pacing Clin Electrophysiol 1997;20(8 Pt 1):1989–92. [DOI] [PubMed]
- 19.Ganz LI, Antman EM. The long-term pharmacologic management of atrial fibrillation for control of rate and rhythm. Cardiac Electrophysiol Review 1999;3:96–9.
- 20.Weiss R, Knight BP, Bahu M, Zivin A, Souza J, Goyal R, et al. Long-term follow-up after radiofrequency ablation of paroxysmal supraventricular tachycardia in patients with tachycardia-induced atrial fibrillation. Am J Cardiol 1997; 80:1609–10. [DOI] [PubMed]
- 21.Waldo AL. Pathogenesis of atrial flutter. J Cardiovasc Electrophysiol 1998;9(8 Suppl):S18–S25. [PubMed]
- 22.Tai CT, Chen SA, Chiang CE, Lee SH, Wen ZC, Huang JL, et al. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J Cardiovasc Electrophysiol 1998;9:115–21. [DOI] [PubMed]
- 23.Shah D, Haissaguerre M, Jais P, Takahashi A, Hocini M, Clementy J. High-density mapping of activation through an incomplete isthmus ablation line. Circulation 1999;99: 211–5. [DOI] [PubMed]
- 24.Anselme F, Saoudi N, Poty H, Douillet R, Cribier A. Radiofrequency catheter ablation of common atrial flutter: significance of palpitations and quality-of-life evaluation in patients with proven isthmus block. Circulation 1999; 99:534–40. [DOI] [PubMed]
- 25.Paydak H, Kall JG, Burke MC, Rubenstein D, Kopp DE, Verdino RJ, et al. Atrial fibrillation after radiofrequency ablation of type I atrial flutter: time to onset, determinants, and clinical course. Circulation 1998;98:315–22. [DOI] [PubMed]
- 26.Huang DT, Zimetbaum P, Monahan KM, Papageorgiou P, Epstein LM, Josephson ME. Hybrid pharmacologic and ablative therapy—a novel and effective approach for the management of atrial fibrillation [abstract]. Pacing Clin Electrophysiol 1998;21(II):963. [DOI] [PubMed]
- 27.Gomes JA, Santoni-Rugiu F, Mehta D, Langan NM, Marx SO, Nayak H. Uncommon atrial flutter: characteristics, mechanisms, and results of ablative therapy. Pacing Clin Electrophysiol 1998;21(11 Pt 1):2029–42. [DOI] [PubMed]
- 28.Cox JL, Schuessler RB, Lappas DG, Boineau JP. An 8 ½ year clinical experience with surgery for atrial fibrillation. Ann Surg 1996;224:267–75. [DOI] [PMC free article] [PubMed]
- 29.Melo J, Adragao P, Aguiar C, Neves J, Ferrieira M, Santiago T. Mid term results of surgery for atrial fibrillation in mitral patients: comparison of three surgical procedures [abstract]. Pacing Clin Electrophysiol 1999;22(II):674.
- 30.Sankar NM, Farnsworth AE. Left atrial reduction for chronic atrial fibrillation associated with mitral valve disease. Ann Thorac Surg 1998;66:254–6. [DOI] [PubMed]
- 31.Swartz JF, Pellersels G, Silvers J, Patten L, Cervantez D. A catheter-based curative approach to atrial fibrillation in humans [abstract]. Circulation 1994;90:I–335.
- 32.Haissaguerre M, Gencel L, Fischer B, Le Metayer P, Poquet F, Marcus FI, et al. Successful catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1994;5:1045–52. [DOI] [PubMed]
- 33.Gaita F, Riccardi R, Calo L, Scaglione M, Garberoglio L, Antolini R, et al. Atrial mapping and radiofrequency catheter ablation in patients with idiopathic atrial fibrillation. Electrophysiological findings and ablation results. Circulation 1998;97:2136–45. [DOI] [PubMed]
- 34.Jais P, Shah DC, Takahashi A, Hocini M, Haissaguerre M, Clementy J. Long-term follow-up after right atrial radiofrequency catheter treatment of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 1998;21(11 Pt 2):2533–8. [DOI] [PubMed]
- 35.Garg A, Finneran W, Mollerus M, Birgersdotter-Green U, Fujimura O, Tone L, et al. Right atrial compartmentalization using radiofrequency catheter ablation for management of patients with refractory atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:763–71. [DOI] [PubMed]
- 36.Haissaguerre M, Jais P, Shah DC, Gencel L, Pradeau V, Garrigues S, et al. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 1996;7:1132–44. [DOI] [PubMed]
- 37.Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:1525–33. [DOI] [PubMed]
- 38.Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Ho-cini M, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95:572–6. [DOI] [PubMed]
- 39.Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879–86. [DOI] [PubMed]
- 40.Langberg JJ, Chin MC, Rosenqvist M, Cockrell J, Dullet N, Van Hare G, et al. Catheter ablation of the atrioventricular junction with radiofrequency energy. Circulation 1989;80:1527–35. [DOI] [PubMed]
- 41.Williamson BD, Man KC, Daoud E, Niebauer M, Strickberger SA, Morady F. Radiofrequency catheter modification of atrioventricular conduction to control the ventricular rate during atrial fibrillation. N Engl J Med 1994;331:910–7. [DOI] [PubMed]
- 42.Sgarbossa EB, Pinski SL, Maloney JD, Simmons TW, Wilkoff BL, Castle LW, et al. Chronic atrial fibrillation and stroke in paced patients with sick sinus syndrome. Relevance of clinical characteristics and pacing modalities. Circulation 1993;88:1045–53. [DOI] [PubMed]
- 43.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92: 1954–68. [DOI] [PubMed]
- 44.Andersen HR, Nielsen JC, Thomsen PE, Thuesen L, Mortensen PT, Vesterlund T, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet 1997;350:1210–6. [DOI] [PubMed]
- 45.Sparks PB, Mond HG, Vohra JK, Jayaprakash S, Kalman JM. Electrical remodeling of the atria following loss of atrioventricular synchrony: a long-term study in humans. Circulation 1999;100:1894–900. [DOI] [PubMed]
- 46.Padeletti L, Porciani MC, Michelucci A, Colella A, Ticci P, Vena S, et al. Interatrial septum pacing: a new approach to prevent recurrent atrial fibrillation. J Interv Card Electrophysiol 1999;3:35–43. [DOI] [PubMed]
- 47.Bailin SJ, Adler SW, Guidici MC, Hill MRS, Solinger B. Bachmann's Bundle Pacing Trial Centers. Bachmann's bundle pacing for the prevention of atrial fibrillation. Initial trends in a multicenter randomized prospective study [abstract]. Pacing Clin Electrophysiol 1999;22(II):727.10353131
- 48.Yu WC, Chen SA, Tai CT, Feng AN, Chang MS. Effects of different atrial pacing modes on atrial electrophysiology: implicating the mechanism of biatrial pacing in prevention of atrial fibrillation. Circulation 1997;96:2992–6. [DOI] [PubMed]
- 49.Prakash A, Vardas P, Delfaut P, Simantirakis EN, Krol R, Munsif A, et al. Multicenter experience with single and dual site right atrial pacing in refractory atrial fibrillation [abstract]. Pacing Clin Electrophysiol 1997;20(II):1074.
- 50.Delfaut P, Saksena S, Prakash A, Krol RB. Long-term outcome of patients with drug-refractory atrial flutter and fibrillation after single- and dual-site right atrial pacing for arrhythmia prevention. J Am Coll Cardiol 1998;32:1900–8. [DOI] [PubMed]
- 51.Saksena S, Prakash A, Krol R, Delfaut P. Time dependence and pattern of atrial fibrillation recurrences after single and dual site right atrial pacing [abstract]. J Am Coll Cardiol 1999;33:140A.
- 52.Lau CP, Tse HF, Yu CM, Teo WS, Ng KH, Huang SK, et al. Dual site right atrial pacing in paroxysmal atrial fibrillation without bradycardia (NIPP-AF Study) [abstract]. Pacing Clin Electrophysiol 1999;22(II):804.
- 53.Mabo P, Daubert C, Bouhour A, on behalf of the SYNBIAPACE Study Group. Biatrial synchronous pacing for atrial arrhythmia prevention: the SYNBIAPACE Study [abstract]. Pacing Clin Electrophysiol 1999;22(II):755.
- 54.Fitts SM, Hill MR, Mehra R, Friedman P, Hammill S, Kay GN, et al. Design and implementation of the Dual Site Atrial Pacing to Prevent Atrial Fibrillation (DAPPAF) clinical trial. DAPPAF Phase I Investigators. J Interv Card Electrophysiol 1998;2:139–44. [DOI] [PubMed]
- 55.Daoud E, Ayers G, Fellows C, Hoyt R, Lemery R, for the US Metrix Investigators. Effect of underlying cardiac disease on the ambulatory use of the Metrix automatic implantable atrial defibrillator [abstract]. Pacing Clin Electrophysiol 1999;22(II):890.
- 56.Bailin SJ, Sulke N, Swerdlow CD, for the worldwide 7250 Jewel AF-only investigators. Clinical experience with a dual chamber implantable cardioverter defibrillator in patients with atrial fibrillation and flutter [abstract]. Pacing Clin Electrophysiol 1999;22(II):871.10392384
- 57.Tse HF, Lau CP, Yu CM, Lee KL, Michaud GF, Knight BP, et al. Effect of the implantable atrial defibrillator on the natural history of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:1200–9. [DOI] [PubMed]


