Abstract
Mitochondrial calcium (Ca2+) regulation is critically implicated in the regulation of bioenergetics and cell fate. Ca2+, a universal signaling ion, passively diffuses into the mitochondrial intermembrane space (IMS) through voltage‐dependent anion channels (VDAC), where uptake into the matrix is tightly regulated across the inner mitochondrial membrane (IMM) by the mitochondrial Ca2+ uniporter complex (mtCU). In recent years, immense progress has been made in identifying and characterizing distinct structural and physiological mechanisms of mtCU component function. One of the main regulatory components of the Ca2+ selective mtCU channel is the mitochondrial Ca2+ uniporter dominant‐negative beta subunit (MCUb). The structural mechanisms underlying the inhibitory effect(s) exerted by MCUb are poorly understood, despite high homology to the main mitochondrial Ca2+ uniporter (MCU) channel‐forming subunits. In this review, we provide an overview of the structural differences between MCUb and MCU, believed to contribute to the inhibition of mitochondrial Ca2+ uptake. We highlight the possible structural rationale for the absent interaction between MCUb and the mitochondrial Ca2+ uptake 1 (MICU1) gatekeeping subunit and a potential widening of the pore upon integration of MCUb into the channel. We discuss physiological and pathophysiological information known about MCUb, underscoring implications in cardiac function and arrhythmia as a basis for future therapeutic discovery. Finally, we discuss potential post‐translational modifications on MCUb as another layer of important regulation.
Keywords: MCU, MCU dominant‐negative beta subunit, MCUb, mitochondrial calcium uniporter, mitochondrial calcium uptake, post‐translational modifications, structure–function
Abbreviations
- AD
Alzheimer's disease
- ALL
acute lymphoblastic leukemia
- AMPK
AMP‐activated protein kinase
- ATP
adenosine 5′‐triphosphate
- C−
carboxyl
- Ca2+
calcium
- CAMKII
calmodulin‐dependent protein kinase II
- CCDC109B
coiled‐coil domain containing 109B protein
- cryoEM
cryoelectron microscopy
- DIME motif
Asp‐Ile‐Met‐Glu motif
- DMD
Duchenne muscular dystrophy
- EMRE
essential MCU regulator
- ER
endoplasmic reticulum
- GBM
glioblastoma multiforme
- HA
hemagglutinin
- HF
heart failure
- HIF1α
hypoxia‐inducible factor‐1‐alpha
- I/R
ischemia/reperfusion
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- IP3R
inositol 1,4,5‐trisphosphate receptor
- JML
juxtamembrane loop
- KD
equilibrium dissociation constant
- KO
knockout
- Li+
lithium
- MAM
mitochondria‐associated membrane
- MCU
mitochondrial calcium uniporter
- MCUb
mitochondrial calcium uniporter dominant‐negative beta subunit
- MCUB
MCUb gene
- MCUR1
MCU regulator‐1
- MICU1/2/3
mitochondrial calcium uptake 1, ‐2, and ‐3
- mPTP
mitochondrial permeability transition pore
- mROS
mitochondrial reactive oxygen species
- mtCU
mitochondrial calcium uniporter complex
- N−
amino
- Na+
sodium
- NCLX
sodium/calcium/lithium exchanger
- OMM
outer mitochondrial membrane
- PISA
protein, interfaces, structures, and assemblies server
- PXP motif
Pro‐X‐Pro motif
- Pyk2
proline‐rich tyrosine kinase 2
- Ru360/265
Ruthenium red derivative 360 and ‐265
- RyR2
ryanodine receptor 2
- shRNA
short hairpin ribonucleic acid
- siRNA
small interfering ribonucleic acid
- SR
sarcoplasmic reticulum
- STZ
streptozotocin
- TM1/2
transmembrane domain 1 and ‐2
- VDAC
voltage dependent anion channels
- WT
wild‐type
1. INTRODUCTION
Mitochondria play essential roles in ATP production, cell death, and shaping cytosolic calcium (Ca2+) transients, with mitochondrial Ca2+ uptake into the matrix fundamentally regulating all of these processes. 1 , 2 Mitochondria have a large Ca2+ buffering capacity, taking up Ca2+ upon elevated cytosolic levels and protecting cells from Ca2+ overload. 3 , 4 Under physiological conditions, mitochondrial matrix Ca2+ plays a role in regulating key enzymes of the citric acid cycle and oxidative phosphorylation, such as pyruvate dehydrogenase, isocitrate dehydrogenase, α‐ketoglutarate dehydrogenase, and perhaps even the ATP synthase complex, to regulate cellular bioenergetics. 2 , 5 , 6 , 7 However, sustained excess matrix Ca2+ can be pathological due to mitochondrial permeability transition pore (mPTP) opening, the release of pro‐apoptotic factors, and a progressive signaling cascade leading to cell death. 8 , 9 , 10
The endoplasmic reticulum (ER) and mitochondria are in close contact through mitochondria‐associated membranes (MAMs). 9 , 11 , 12 Physiologic stimuli cause the ER to release large amounts of Ca2+ through inositol‐1,4,5‐trisphosphate receptor (IP3R) Ca2+ release channels, generating microdomains of elevated Ca2+ (i.e., >~10 μM) at the outer mitochondrial membrane (OMM). 13 Subsequently, Ca2+ freely diffuses into the intermembrane space (IMS) through voltage‐dependent anion channels (VDAC). 10 , 14 Once in the IMS, Ca2+ uptake is tightly regulated across the inner mitochondrial membrane (IMM) by the mitochondrial Ca2+ uniporter complex (mtCU). 10 , 15 The driving force for Ca2+ uptake across the IMM through mtCU is the highly negative membrane potential (i.e., ~−180 mV). 1 , 13
After ~6 decades since the discovery of the mitochondrial Ca2+ uptake phenomenon by DeLuca, Engstrom, Vasington, and Murphy, 16 , 17 , 18 , 19 the identification of the molecular components making up mtCU began appearing in 2010. 20 Several necessary components and accessory regulator proteins have been identified since that time (reviewed in Ref. [4, 21, 22]). Central to the expansion of the field included the electrophysiological characterization of mtCU by the Clapham group, 23 and later the identification of the molecular components of mtCU, including the mitochondrial Ca2+ uptake‐1, 2 and 3 (MICU1/2/3) gatekeeping regulators by the Mootha group, 20 , 24 the mitochondrial Ca2+ uniporter (MCU) essential pore‐forming subunit by the Mootha and Rizzuto groups, 4 , 25 , 26 , 27 the essential MCU regulator (EMRE) by the Mootha group, 28 the MCU regulator‐1 (MCUR1) scaffold factor by the Foskett and Madesh groups 29 and the MCU dominant‐negative beta subunit (MCUb) by the Rizzuto group. 4 , 30 Remarkably, MCUb is a highly similar paralog of MCU, and research has shown the functional, physiologic, and pathophysiologic importance of MCU:MCUb expression ratios. 4 , 30 Nevertheless, there is currently a lack of understanding of the structural mechanisms underlying the MCUb inhibitory function despite high homology to the well‐studied MCU.
The most complete structural views of the human mtCU have been revealed by cryoelectron microscopy (cryoEM) and include MICU1, MICU2, MCU, and EMRE components. 31 , 32 , 33 In this article, we provide an overview of the major mtCU components, regulators, and structural arrangements as revealed by the cryoEM structures and compare primary and higher‐order structures of MCU and homology‐modeled MCUb, exposing differences in protein–protein interactions and pore sizes in the context of mtCU. Our homology models use the first published mtCU cryoEM structures, 31 reported by the Tsai and Feng groups. We review work characterizing MCUb in physiology and pathophysiology, led by the Rizzuto, Elrod, Belosludstev, Hajnóczky, and Mammucari groups, highlighting implications within cardiac function and arrhythmia, and survey potential post‐translational modifications within MCUb as an additional layer of regulation to this subunit.
2. MITOCHONDRIAL CALCIUM UNIPORTER COMPLEX (mtCU) COMPONENTS
MCU is the pore‐forming subunit that is essential for Ca2+ permeation into the matrix. 4 , 26 , 34 MCU subunits preferentially oligomerize into active tetrameric channels, and these tetramers can dimerize along the curvature of the IMM to form larger V‐shaped complexes (see Section 3; Ref. [4, 12, 22, 31]). The MCU subunit consists of matrix‐oriented amino (N)‐ and carboxyl (C)‐ terminal domains with two coiled‐coil domains and two transmembrane domains (TM1 and TM2) that are linked by a short loop exposed to the IMS. 15 , 35 The critical Asp‐Ile‐Met‐Glu, DIME motif necessary for the Ca2+ selectivity of the channel is located at the end of the loop, forming the start of TM2 (Figure 1; Ref. [4, 22, 37]). The conserved DIME sequence 38 of four MCU subunits is symmetrically arranged at the entrance of the oligomerized channel, creating an electronegative selectivity filter. 39 , 40 The Asp carboxylate ring of DIME formed at the entrance of the channel has a larger diameter compared to the Glu ring positioned just below, mediating Ca2+ coordination. 41 , 42 Conserved Trp and Pro residues located on each respective side of DIME may be essential for the orientation of the motif. 42 This mtCU selectivity filter has a high Ca2+ affinity (equilibrium dissociation constant, K D , ≤2 nM) and a preference for binding Ca2+ over other cations. 23 , 38 , 39 , 41 The ion permeation pathway has two constriction points at opposing ends of the mtCU channel. The first is the Glu ring of DIME and the second is a luminal gate formed by a juxtamembrane loop (JML) at the exit of the transmembrane domain. 43 To control the release of Ca2+ into the mitochondrial matrix, Glu288 and Val290 from the JML of each pore‐forming MCU subunit circle the pore exit to form the opening, with EMRE believed to promote the open conformation of this luminal gate. 33 , 41 , 43 , 44 EMRE interacts with three significant areas across two adjacent MCU subunits, including the side chain of Arg297 on MCU connecting the JML of MCU through a hydrogen bond to the backbone carbonyl of Val61 on EMRE. 43 The large, matrix‐oriented N‐terminal domains of MCU self‐associate within and between channels (Figure 2; Ref. [31, 32, 33]), contain sites of regulatory post‐translational modifications (see Section 4) and bind cations to regulate assembly and channel activity. 1 , 45 , 46
FIGURE 1.
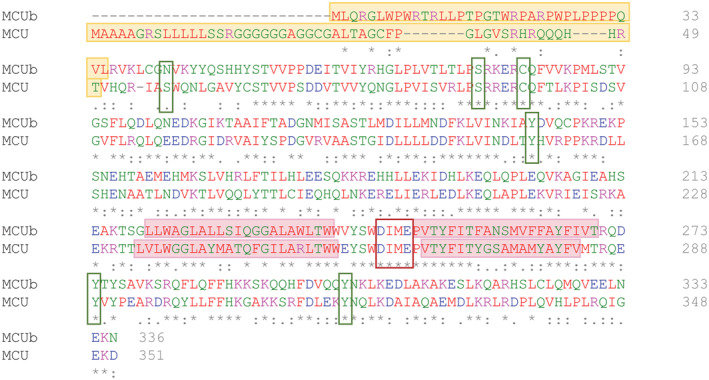
Human MCUb (NCBI accession # NP_060388.2) sequence alignment and conservation with human MCU (NCBI accession # NP_612366.1). Primary structure alignment of human MCUb and MCU conducted using Clustal Omega. 36 The mitochondrial transit peptides are shaded yellow; the TM1 and TM2 domains are shaded pink, and the DIME motifs are outlined with a red box. Residues known to undergo post‐translational modifications in MCU and the corresponding residue in MCUb are highlighted with a green box. The residue numbers are shown at the right of the sequences. Below the alignment, (*) indicates a fully conserved residue; (:) indicates highly similar residues; (.) indicates conservation between residues with low similarity.
FIGURE 2.
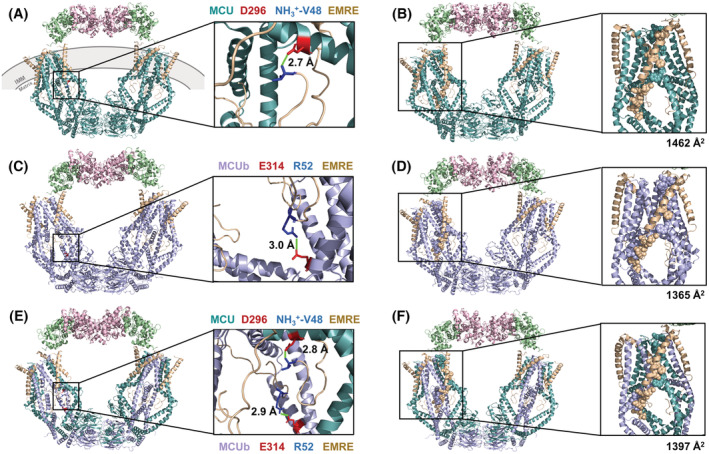
Three‐dimensional structures of human MCU (A) and MCUb (C) homotetrameric complexes and a 2 × MCU:2 × MCUb (E) heterotetrametric complex in high calcium (Ca2+). Each complex includes MCU (teal) or MCUb (lilac), EMRE (beige), MICU1 (green), and MICU2 (pink). At the right of (A), (C), and (E), a zoomed in view of the distinct salt bridges present in each respective complex model is shown with positive and negative charged residues as blue and red sticks, respectively. The closest distances between atoms involved in the salt bridge are indicated in each zoomed view (green line). Buried surface area exhibited by MCU (B) and MCUb (D) homotetrameric complexes and a 2 × MCU:2 × MCUb heterotetrameric complex (F), highlighting residues that are >50% buried in one interaction with EMRE (spacefill). At the right of (B), (D), and (F), a zoomed in view of a channel subunit interaction with EMRE is shown with the corresponding total buried surface area of the interaction indicated. The structure images were generated using the 6WDO.pdb and homology‐modeled coordinates in PyMOL. MCU, mitochondrial Ca2+ uniporter; MCUb, MCU dominant‐negative beta subunit; EMRE, essential MCU regulatory protein, MICU1/2, mitochondrial Ca2+ uptake 1 and −2 proteins; IMM, inner mitochondrial membrane.
The MCU tetramer interacts with MICU1 directly or indirectly. 47 MICU1 and MICU2 form a heterodimer with a gatekeeping function in mtCU channel activity. 37 , 47 , 48 MICU3 is a paralog of the MICU proteins present in vertebrates and expressed at high levels in the central nervous system. MICU2 or MICU3 both form a disulfide bond‐mediated dimer only with MICU1 to enhance Ca2+ uptake. 49 These gatekeeper proteins contain EF‐hand motifs that bind IMS Ca2+ and undergo conformational changes that may be involved in regulating the accessibility of Ca2+ to the mtCU channel pore or otherwise regulate channel open probability (see below; Ref. [31, 32, 33, 37, 50, 51, 52, 53]). Heritable loss of MICU1 and MICU2 function mutations leads to disorders characterized by muscle weakness, fatigue, lethargy, developmental delay, and learning disabilities. 21 , 54 , 55 , 56 , 57 MICU1 knockout (KO) mouse studies highlight a complex gatekeeping function as fibroblast KO mitochondria at low and high cytosolic Ca2+ show higher and lower rates of Ca2+ uptake, respectively, 58 striated muscle KO mitochondria show increased basal but decreased Ca2+ uptake rates after stimulus 59 and neuronal KO mitochondria show increased Ca2+ uptake at subthreshold cytosolic Ca2+ but reduced Ca2+ uptake at high cytosolic Ca2+. 60
In low Ca2+, cryoEM revealed mtCU arrangements with MICU1 tightly occluding the pore entrance through electrostatic interactions between Lys/Arg residues of MICU1 and three of the DIME Asp261 residues of the MCU tetramer. 31 , 32 MICU2 does not contribute to the occlusion, primarily contacting MICU1 in this assembly. However, low Ca2+ mtCU arrangements have also been identified with MICU1 completely displaced from the pore, remaining part of the complex only via EMRE interactions. In this context, non‐occluded dimers of tetrameric channels, bridged through N‐terminal domain interactions of MCU in the matrix and MICU2 interactions in the IMS, have been resolved. 32 , 33 Non‐occluded, MICU2‐ and N‐terminal domain‐bridged tetrameric channels are also the dominant mtCU arrangement elucidated in high Ca2+. 31 , 32 The low Ca2+ occluded/high Ca2+ non‐occluded states provide a convenient explanation for MICU gatekeeping. Yet, this simple model is not only incompatible with the observed non‐blocked states in low Ca2+, but also compelling electrophysiology data collected in the absence of Ca2+ showing sodium (Na+) currents are similar in wild‐type (WT) and MICU1‐KO mitoplasts. 52 At the same time, the Ca2+ currents in WT mitoplasts are ~2× that of MICU1‐KO mitoplasts due to MICU1‐dependent increases in the pore open probability. 52 Given that EMRE bridges MICU1 and MCU in the human mtCU in high Ca2+ structures, we speculate that increased open probability occurs due to Ca2+‐dependent structural changes in the MICUs, which allosterically couple to the JML luminal gate of MCU via EMRE. 33
Although not resolved in the mtCU cryoEM structures, the polyaspartate, IMS‐facing C‐terminus of EMRE interacts with a polybasic domain of MICU1, and the matrix‐oriented N‐terminus forms a beta‐hairpin motif, interacting with MCU. 37 , 40 , 43 , 47 , 61 EMRE interacts with MCU at as high as a 1:1 ratio, and this EMRE:MCU interaction is necessary to create an active Ca2+ permeable channel in metazoans. 22 , 44 However, a 4 × EMRE:4 × MCU ratio is not required for channel functionality, with channel complexes containing 1–4 EMRE subunits having partial to full gatekeeping function. 62 Concatemers enforcing 2 × EMRE:4 × MCU subunits recapitulated the activity, gatekeeping, and size of endogenous channels. 62 MCUR1 is another regulatory subunit of mtCU, binding to MCU and EMRE. 22 , 63 MCUR1 acts as a scaffolding protein for the oligomerization of mtCU 22 , 63 ; however, MCUR1 may have other important roles in the regulation of the mPTP and/or as a complex IV assembly factor. 29 , 64 , 65
Finally, MCUb, the dominant‐negative beta subunit, is a paralog of the MCU pore‐forming subunit. 66 MCUb was initially introduced to the field as the coiled‐coil domain‐containing 109B (CCDC109B) protein. 22 , 30 In humans, MCUb shares 48.8% sequence identity and 83.9% similarity with MCU (Figure 1). Despite this high similarity, hetero‐oligomerization with MCU remarkably exerts an inhibitory effect on Ca2+ permeation through the mtCU channel. 66 We explore potential structural, physiological, and pathophysiological mechanisms of MCUb‐mediated inhibition of the mtCU channel in subsequent sections.
3. MCU DOMINANT‐NEGATIVE BETA SUBUNIT (MCUb)
3.1. MCUb protein structure
MCUb consists of two coiled‐coil domains and two TM domains that are separated by a short IMS loop. 27 , 30 Despite the ~49% sequence identity between MCUb and MCU, one key amino acid differs between MCU and MCUb within the short loop. 27 , 30 , 37 In MCU, Glu257 is thought to be critical for Ca2+ permeation through the mtCU channel. 30 , 37 The Glu257 in MCU is replaced by Val242 in MCUb, resulting in the loss of a negative charge. 30 , 67 Thus, the surface of MCUb is less electronegative than MCU, which may interfere with electrostatic attraction and Ca2+ permeation through the mtCU channel. 30 , 67
Proteomic data show that, unlike MCU, MCUb does not interact with MICU1 through EMRE. 37 Immunoprecipitation experiments performed with MCU −/− KO HeLa cells revealed that EMRE fused with a C‐terminal FLAG tag interacts with both MCU and MCUb fused to C‐terminal HA tags. 66 Further, MCUb‐HA was confirmed to interact with MCU‐FLAG but not MICU1‐FLAG or MICU2‐FLAG by pull‐down. 66 Here, we used the high Ca2+/non‐occluded (6WDO.pdb; Ref. [31]) and low Ca2+/occluded (6WDN.pdb; Ref. [31]) human MCU‐EMRE‐MICU1‐MICU2 structures, to model the consequences of human MCUb incorporation into the mtCU assembly. The complex structures are composed of 4 × MCU:4 × EMRE:1 × MICU1:1 × MICU2 subunits per channel. The homology modeling was performed using Modeler 68 and the human MCUb sequence (NCBI accession # NP_060388.2), and the protein–protein interaction analysis was done using the protein, interfaces, structures, and assemblies (PISA) server. 69 We created 2 × MCU:2 × MCUb heterocomplexes with alternating MCU/MCUb proteins (i.e., MCU:MCUb:MCU:MCUb) and MCUb homotetramers for analysis. Note that symmetry restraints were not enforced, so analogous interfaces between the same subunit types were not always identical.
In comparison to the 6WDO.pdb non‐occluded template structure in high Ca2+ (Figure 2A,B), the high Ca2+ MCUb homotetramer complex strikingly revealed the loss of a salt bridge between the N‐terminus of EMRE and the channel forming subunit (Figure 2C,D). In the template mtCU channel, a salt bridge exists between Asp296 of MCU and the N‐terminus (i.e., NH3 +) at Val48 of EMRE. Note that residues 1–47 of EMRE comprise the mitochondrial transit sequence of the resolved model, while UniProtKB accession NX_Q9H4I9 reports the mitochondrial transit sequence of human EMRE as residues 1–52. In contrast, while a salt bridge was predicted between Glu314 on MCUb and Arg52 on EMRE (Figure 2C), the location of this charge interaction neither involves the EMRE N‐terminus nor the position on MCUb that aligns with MCU Asp296. Asp296 is not conserved in MCUb, where it exists as Ser281 (Figure 1). Arg252 of MCU is thought to be critical for Ca2+ permeation and exists as Trp237 in MCUb. 27 , 30 , 37 PISA identified hydrogen bonds between Arg252 of MCU and Thr82 as well as Ser85 of EMRE, with the Arg252:Thr82 hydrogen bond lost upon MCUb integration (Table 1). Notably, the buried surface area was smaller for the MCUb‐EMRE interaction compared to MCU‐EMRE (Table 1). Interestingly, our high Ca2+/non‐occluded MCU‐MCUb heterotetramer complex model (Figure 2E,F) showed a decrease in the number of intermolecular hydrogen bonds between MCU and MCUb and an increase in the number of intermolecular salt bridges between MCU/MCUb and EMRE compared to either the MCU or MCUb homotetramer structures (Table 1). Further, the buried surface area between the MCUb‐MCUb interfaces was greater than the MCU‐MCUb or MCU‐MCU interfaces (Table 1). In addition, the MCUb‐MCUb interactions showed the highest number of distinct hydrogen bonds and salt bridges among all channel‐forming subunit interactions (Table 1).
TABLE 1.
Summary of interface properties for the MCU homotetramer, MCUb homotetramer, and MCU‐MCUb heterotetramer complexes in high Ca2+/non‐occluded and low Ca2+/occluded states
| Interface composition interactions | Number of interface atoms | Number of interface residues | Number of surface atoms | Number of surface residues | Buried Surface Area (Å2) c | Number of distinct hydrogen bonds b | Number of distinct salt bridges b |
|---|---|---|---|---|---|---|---|
| High Ca 2+ /non‐occluded models d | |||||||
| MCU homotetramer | |||||||
| MCU‐MCU | 2072 | 532 | 17 877 | 3171 | 10 065 | 34 | 4 |
| MCU‐EMRE | 1206 | 323 | 14 502 | 2524 | 5926 | 13 | 1 |
| MCU‐MICU1 | 20 | 4 | 2856 | 528 | 69 | 0 | 0 |
| EMRE‐MICU1 | 74 | 23 | 3372 | 625 | 293 | 0 | 1 |
| MCUb homotetramer | |||||||
| MCUb‐MCUb | 2362 | 612 | 18 768 | 3258 | 10 841 | 39 | 7 |
| MCUb‐EMRE | 1169 | 318 | 15 082 | 2582 | 5596 | 13 | 1 |
| MCUb‐MICU1 | 15 | 4 | 3063 | 555 | 63 | 0 | 0 |
| EMRE‐MICU1 | 81 | 24 | 3663 | 671 | 382 | 1 | 1 |
| MCU‐MCUb heterotetramer a | |||||||
| MCU/MCUb | 2046 | 535 | 18 138 | 3213 | 9834 | 32 | 6 |
| MCU/MCUb‐EMRE | 1149 | 311 | 14 658 | 2552 | 5678 | 16 | 2 |
| MCU/MCUb‐MICU1 | 94 | 7 | 2882 | 532 | 94 | 0 | 0 |
| EMRE‐MICU1 | 75 | 23 | 3287 | 621 | 365 | 3 | 3 |
| Low Ca 2+ /occluded models e | |||||||
| MCU homotetramer | |||||||
| MCU‐EMRE | 1121 | 300 | 10 686 | 1748 | 5551 | 10 | 1 |
| MCU‐MICU1 | 269 | 71 | 10 718 | 2016 | 1109 | 8 | 3 |
| EMRE‐MICU1 | 58 | 17 | 3910 | 765 | 251 | 0 | 0 |
| MCUb homotetramer | |||||||
| MCUb‐EMRE | 1068 | 293 | 10 646 | 1744 | 5214 | 10 | 1 |
| MCUb‐MICU1 | 269 | 72 | 10 807 | 2034 | 1090 | 8 | 3 |
| EMRE‐MICU1 | 57 | 17 | 3944 | 775 | 252 | 1 | 0 |
| MCU‐MCUb heterotetramer a | |||||||
| MCU/MCUb‐EMRE | 1087 | 306 | 10 646 | 1746 | 5304 | 13 | 1 |
| MCU/MCUb‐MICU1 | 248 | 66 | 10 723 | 2047 | 1042 | 10 | 4 |
|
EMRE‐MICU1 |
59 | 16 | 3925 | 781 | 249 | 1 | 0 |
The MCU‐MCUb heterotetramer models were generated with a 2 × MCU:2 × MCUb ratio of MCU to MCUb subunits within each channel tetramer.
Only unique hydrogen bonds and salt bridges are indicated.
Figure 2 highlights a single chain interaction between EMRE and MCU or MCUb in the high Ca2+/non‐occluded models; here, totals of all interacting chains are given.
The 6WDO high Ca2+/non‐occluded model consists of two dimerized mtCU channels.
The 6WDN low Ca2+/occluded model consists of one mtCU channel; MCU‐MCU/MCUb interactions are not given because the N‐terminal domains involved in these interactions are not resolved.
We also generated homology models using the occluded/low Ca2+ MCU‐EMRE‐MICU1‐MICU2 structure (6WDN.pdb; Ref. [31]) but since the N‐terminal domains of MCU are not resolved, MCU‐MCU, MCUb‐MCUb or MCU‐MCUb interactions, which involve the N‐terminal domains, were not compared. Nevertheless, PISA reported that the low Ca2+/occluded model showed less MCU‐EMRE total buried surface area compared to the high Ca2+/non‐occluded. Integration of MCUb either as a homotetramer or 2 × MCU:2 × MCUb heterocomplex resulted in less MCUb/MCU‐EMRE total buried surface area, similar to observations with non‐occluded models (Table 1). As expected, the low Ca2+/occluded structure shows ~16× more buried surface area between MCU‐MICU1 compared to non‐occluded, and integration of MCUb marginally decreased this buried surface area with MICU1 (Table 1). Collectively, our homology models suggest a smaller interface between MCUb and EMRE, highlighted by decreased buried surface area and altered salt‐bridge formation. Further, while the MCU‐MCUb interface showed less hydrogen bonds, the MCUb‐MCUb interface showed a larger buried surface area, number of hydrogen bonds, and salt bridges compared to the MCU homotetramer (Table 1). The weakened/smaller interface between MCUb and EMRE, shown in both occluded and non‐occluded models, may play a role in the inhibitory mechanism of MCUb, while the enhanced MCUb‐MCUb solvent‐inaccessible interface may promote MCUb‐MCUb assembly. The N‐terminal domain of EMRE contains an extended linker region with a highly conserved PXP motif, thought to stabilize the luminal gate of MCU in an open conformation due to the rigidity of Pro. 41 , 44 Thus, a tighter MCU interaction (expanded interface) with EMRE could promote the opening of the mtCU luminal gate for Ca2+ uptake into the matrix, while a weaker MCUb‐EMRE interaction could suppress open probability.
Previous homology modeling of human MCU, human MCUb, and MCU‐MCUb heteromeric channels based on the Aspergillus fischeri (fungal) MCU structure, suggested that there are fewer hydrophobic interactions between TM1 and TM2 from the adjacent MCU subunit of the complex, resulting in a widening of the IMS side of the pore. 37 , 67 The decreased hydrophobic interactions are due to the replacement of hydrophobic side chains in MCU with polar/small neutral in MCUb (i.e., specifically Ala244 and Phe247 exist as Ser229 and Gly232 in MCUb; Figure 1). Indeed, the reported diameter of the pore measured in fungal structure‐derived human MCU/MCUb models changed from 7.0 Å in the human MCU homotetramer to 10.4 Å in the MCUb homotetramer to 8.9 Å in the human heterotetramer with 3 × MCU:1 × MCUb subunits (6D7W.pdb; Ref. [37]). Two constriction points at opposite ends of the Ca2+ permeation pathway through mtCU are the Asp and Glu rings of DIME at the IMS pore entrance and the luminal gate at the matrix pore exit. In our models based on resolved human mtCU structures, the Glu ring of DIME did not exhibit any notable diameter changes after MCUb integration in either the occluded or non‐occluded states, in contrast to the DIME Asp ring distances, which changed. Specifically, The high Ca2+/non‐occluded human mtCU template structure showed an average shortest pore diameter of 6.2 Å, measured between Asp261 of opposite MCU subunits. This distance increased to 6.6 Å upon integration of MCUb, modeled with 2 × MCU:2 × MCUb, and further increased to 6.8 Å in the MCUb homotetramer (Figure 3A). Further, the low Ca2+/occluded mtCU template structure showed an average shortest pore diameter of 7.1 Å between Asp261 residues, which increased to 7.8 Å for the MCUb homotetrameric complex and 7.7 Å in the 2 × MCU:2 × MCUb heterotetrameric complex (Figure 3B).
FIGURE 3.
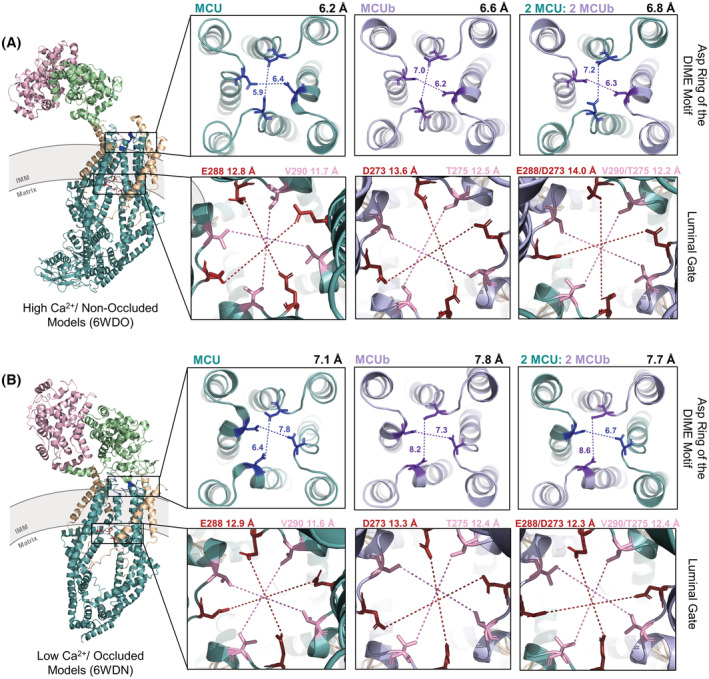
mtCU pore entry and exit diameters of the Ca2+ selectivity filter and the luminal gate of human MCU homotetrameric, MCUb homotetrameric, and 2 × MCU:2 × MCUb heterotetrameric complex structures elucidated in (A) high Ca2+/non‐occluded and (B) low Ca2+/occluded states. mtCU complexes include MCU (teal) or MCUb (lilac), EMRE (beige), MICU1 (green), and MICU2 (pink). The shortest distances are indicated between oxygens of MCU Asp261 (blue) and MCUb Asp246 (purple) from the DIME motifs of opposing subunits in each direction. The average of the shortest distances measured crosswise is indicated at the top right of each box in the first and third rows. The shortest distances were measured between opposing Glu288/Asp273 (brown) or Val290/Thr275 (pink) residues, the two residues that form the luminal gate in MCU/MCUb, respectively, with the average of these distances indicated at the top of each box in the second and fourth row. The structure images were generated using 6WDO.pdb and 6WDN.pdb for non‐occluded and occluded states, respectively, and homology‐modeled coordinates in PyMOL. MCU, mitochondrial Ca2+ uniporter; MCUb, MCU dominant‐negative beta subunit; IMM, inner mitochondrial membrane.
The second constriction point includes Glu288 and Val290 of MCU, making up the luminal gate; these residues are not conserved in MCUb, existing as Asp273 and Thr275 (Figure 1). The shortest pore distances between these JML‐forming residues of the luminal gate increased upon the incorporation of MCUb into mtCU. In the high Ca2+/non‐occluded human mtCU template structure, the Glu288 and Val290 average shortest pore diameter increased from 12.8 and 11.7 Å in the MCU homotetrameric complex, respectively, to 13.6 and 12.5 Å determined for the Asp273 and Thr275 residues in the MCUb homotetrameric complex, respectively (Figure 3A). The diameter was also increased in the 2 × MCU:2 × MCUb heterotetrameric complex (i.e., to 14.0 and 12.2 Å, respectively). In the low Ca2+/occluded mtCU template structure, the Glu288 and Val290 average shortest pore diameter increased from 12.9 and 11.6 Å in the MCU homotetrameric complex, respectively, to 13.3 and 12.4 Å determined for the Asp273 and Thr275 residues in the MCUb homotetrameric complex, respectively (Figure 3B). The diameter was also increased in the 2 × MCU:2 × MCUb heterotetrameric complex for the Val290/Thr275 pairs only (i.e., to 12.4 Å). Thus, consistent with previous models based on a fungal MCU structure, 37 integration of MCUb increases distances between DIME Asp residues, as well as between JML residues of the luminal gate, which could perturb Ca2+ attraction, coordination, gating, and permeation 70 and/or MICU1 and EMRE interactions.
MCUb alone does not form a Ca2+ permeable channel when inserted into lipid bilayers. 30 , 67 Nevertheless, MCUb does form a channel permeable to Na+ ions. 30 , 37 Therefore, many questions remain about the precise residue differences in the MCUb structure that result in the inhibitory effects of the subunit. 37 Similarly, the mechanisms underlying precisely how the MCUb subunits integrate into a channel remain unclear. Elucidation of the high‐resolution structure of MCUb in the context of an assembled homomeric and heteromeric channel will help pinpoint precise residues underlying inhibition and differences in complex assembly; further, this structural data will guide future research aimed at answering elusive questions surrounding the impact of post‐translational modifications and disease‐related mutations on the structure and function of MCUb.
3.2. MCUb protein function
MCUb, a paralog of MCU, acts as a dominant negative inhibitor of the mtCU channel. 30 MCUb hetero‐oligomerizes with MCU, integrating into mtCU, to negatively regulate Ca2+ uptake into the mitochondrial matrix. 9 , 30 Initial findings suggested that MCUb must integrate into the complex during assembly rather than a pre‐existing mtCU channel. 30 Co‐expression of MCU and MCUb resulted in a dramatic decrease in mitochondrial Ca2+ uptake compared to complexes made up of MCU alone, supporting the inhibitory role of MCUb when incorporated into mtCU. 30 More recent work has shown that MCUb can integrate into functional channels, with MCUb expression displacing MCU channel subunits from mtCU and decreasing the association with MICU1. 66 This decreased association between mtCU and MICU1 may be due to perturbed interactions between MCUb and EMRE (see above; Ref. [37]). When MICU1 binds Ca2+, mtCU open probability increases, boosting Ca2+ permeation when cellular energy demand rises. 52 Thus, higher MCUb:MCU stoichiometric ratios suppress Ca2+ uptake into the matrix (Figure 4; Ref. [66, 71]).
FIGURE 4.
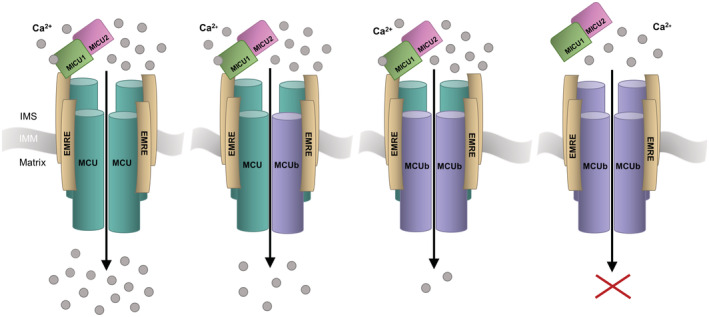
Schematic of mitochondrial Ca2+ uniporter (MCU) complexes with varying MCU:MCUb subunit ratios and the respective Ca2+ permeation. With an increasing expression of MCUb displacing MCU from the tetrameric channel, Ca2+ uptake into the matrix decreases. The MCUb homotetrameric complex does not form a Ca+ permeable channel, and the MICU gatekeeping proteins may not interact. MCUb, MCU dominant‐negative beta subunit; EMRE, essential MCU regulatory protein, MICU1/2, mitochondrial Ca2+ uptake 1 and −2 proteins; IMS, intermembrane space; IMM, inner mitochondrial membrane. Adapted from Ref. [4].
MCUB deletion affects mtCU assembly and functional capacity. 66 , 71 Deletion of MCUB using CRISPR/Cas9n resulted in a ~ 2.7‐fold and ~ 4.5‐fold increased expression of MCU and EMRE, respectively, compared to WT HeLa cells. 66 The loss of MCUB increased the mitochondrial Ca2+ uptake amplitudes by ~80%. 66 The increased Ca2+ uptake function decreased cytosolic Ca2+ peaks following the release of Ca2+ from the ER. 66 , 71 Thus, MCUB KO cells are readily susceptible to mitochondrial Ca2+ overload and mPTP opening. 66 , 71 Indeed, the MCUB KO cells withstood ~30% less bath Ca+ load in a permeabilized cell system before the mitochondrial membrane potential was lost. 66
Currently, there are no effective therapies to prevent or reduce mitochondrial Ca2+ overload in disease. 37 Ruthenium Red derivatives, such as Ru360, are the best current pharmacological approaches available to block mitochondrial Ca2+ uptake. 37 They directly bind to the solvent‐exposed Asp‐ring of the DIME selectivity filter of MCU, inhibiting channel function. 37 , 72 Ru360 lacks specificity and is unable to pass cell membranes easily. 30 More recent Ruthenium Red derivatives such as Ru265 show improved cell permeability and the potential to inhibit the channel by binding to regions outside the pore. 35 Nevertheless, Ruthenium‐based compounds are not used in clinical settings. It is interesting to speculate that MCUb could represent an alternative approach to inhibiting mtCU‐mediated Ca2+ uptake and mPTP opening. 30 For example, compounds that stabilize MCUb or mechanisms inducing increased MCUb expression could promote inhibition of the mtCU channel.
3.3. The role of MCUb in human physiology
The MCUB gene is located on chromosome 4 in Homo sapiens. 30 The translated ~340 amino acid (~39 kDa) protein 30 is highly conserved among vertebrates but not found in other organisms containing MCU, such as plants, kinetoplastids, nematodes, and arthropods. 30 MCUb is differentially expressed compared to MCU, with high variability between tissue types, perhaps due to the distinct mitochondrial Ca2+ uptake demands across tissues. 30 , 71 The ratio of MCU:MCUb expressed can vary from around 3:1 within the heart or lung to greater than 40:1 within skeletal muscle. 30 , 38 , 40 Analyses of the regulation of both MCU and MCUb under specific conditions, such as during development or pathological states, would likely reveal instances where the MCU:MCUb ratio is altered, in line with the metabolic Ca2+ demand. 30 Well‐regulated mitochondrial Ca2+ uptake contributes to the homeostasis of many organs involved in systemic metabolisms, such as the liver, skeletal muscle, adipose tissue, and heart. 9
In the normal physiologic heart, there is low MCUb expression and thus, MICU1 remains coupled to the channel with a low MICU1:MCU protein abundance ratio to enhance open probability. 71 , 73 Thus, this type of complex assembly results in a reduced threshold for Ca2+ uptake, allowing mitochondrial Ca2+ oscillations to occur on a beat‐by‐beat basis. 71 , 74 The potentially increased baseline mtCU channel activity within cardiac mitochondria could lead to increased metabolic flexibility, with the ability to take up more Ca2+ into the matrix, but inversely cause cardiac mitochondria to become more sensitive to Ca2+ overload with a higher probability of the mPTP opening. 30 , 75
3.4. MCUb‐associated pathophysiologies
There have been no heritable mutations identified in MCUb linked to human disease. 67 However, mitochondrial Ca2+ signaling dysregulation and alterations in MCUb expression have been observed as secondary characteristics of many human pathologies, ranging from musculoskeletal disease, neurodegenerative disease, cancer, and diabetes to heart disease.
In Duchenne muscular dystrophy (DMD), aberrant mitochondrial Ca2+ regulation results in impaired bioenergetics and progressive degradation of the skeletal muscle. 76 , 77 Dubinin et al. (2020b) determined that the quantity of MCUb protein in the skeletal muscle of DMD‐model mice was ~1.4‐fold greater than WT mice. 76 Additionally, the MCU:MCUb ratio decreased by ~1.7‐fold in the diseased mitochondria. 76 Thus, the reduced Ca2+ uptake observed in the DMD mitochondria may be associated with increased MCUb expression. 76
Alzheimer's disease (AD) has also been associated with neuronal mitochondrial Ca2+ signaling impairment. 78 Frontal cortex tissue from non‐familial, sporadic AD patients showed a considerable decrease in mitochondrial Na+/Ca2+/lithium (Li+) exchanger (NCLX) expression, the primary mitochondrial Ca2+ efflux mediator in excitable cells, along with a decrease in MICU1 and MCUb. 78 These expression profile changes may contribute to the matrix Ca2+ overload reported in neurons during disease progression. 78 Elevated mitochondrial Ca2+ levels preceded neuronal death and thus, may be a target for the development of neuroprotective therapies for AD. 79
MtCU function has been highly implicated in cancer. The disturbance of Ca2+ homeostasis within cancer cells has been correlated with continuous cell proliferation and inhibition of cell death. 80 Aberrant expression of MCUb has been associated with the malignant properties of gliomas. 81 Consistent with this finding, the Cosmic Cancer Catalogue has identified 94 somatic mutations across the MCUB gene. 82 In fact, Xu et al. (2017) determined that MCUb is a prognostic marker in glioma patients. 81 Specifically, MCUb was highly expressed in high‐grade gliomas compared to almost no expression in normal brain tissue; further, high MCUb expression correlated with an increased tumor grade. 81 Silencing of MCUB using shRNAs in U87MG and U251 human glioma cell lines inhibited proliferation, migration, and invasion and led to decreased tumor volume and prolonged overall survival in orthotopic tumor models of nude mice. 81 , 83 Glioblastoma multiforme (GBM) samples showed high expression of MCUb and hypoxia‐inducible factor 1‐α (HIF1α), a transcriptional regulator typically induced by hypoxia, both localized in areas bordering necrosis. 81 Culturing U87MG and U251 cells under hypoxia resulted in ~2‐fold increase in MCUB mRNA levels, and further treatment with siRNAs targeting HIF1α reduced mRNA levels of MCUB. 81 Thus, MCUb may be a potential tumor promotor in glioma progression, which is drastically upregulated under hypoxia and could be used as a prognostic marker or targeted as a novel treatment in human glioma. 81
MCUb overexpression has been reported in drug‐resistant acute lymphoblastic leukemia (ALL). 84 , 85 Additionally, Tosatto et al. (2016) demonstrated correlations between MCU and MCUb expression levels and clinically presented breast cancer stages. 86 Specifically, MCU expression was found to increase with tumor progression, while MCUb expression decreased. 86 A positive correlation between MCU expression and HIF1α was shown in triple‐negative breast cancer samples. 86 Thus, enhanced mitochondrial Ca2+ uptake due to increased MCU and decreased MCUb expression may promote HIF1α signaling, leading to increased tumor size and lymph node infiltration in breast cancer. 86 Collectively, MCUb may contribute to cancer progression through the promotion of cell proliferation and migration and may reveal a therapeutic target for clinical intervention in multiple aggressive cancers. 81 , 86
Diabetes mellitus is also associated with mitochondrial Ca2+ dysfunction. 87 Belosludtsev et al. (2019) found the rate of mitochondrial Ca2+ uptake increased ~1.4‐fold in hepatic cells from type 1 diabetic rats, 2 weeks after diabetes induction using streptozotocin (STZ). 87 The levels of MCU and MICU1 expression did not change; however, the MCUb protein decreased by ~2‐fold. 87 Thus, the MCU:MCUb ratio increased from ~3.0 in control rats to ~6.7 in diabetic rats. 87 Therefore, the increased mitochondrial Ca2+ uptake within liver mitochondria of diabetic rats may be related to the decrease in MCUb expression. 87
Finally, MCUb expression may be an endogenous cardioprotective mechanism, reducing mitochondrial Ca2+ overload‐induced injury. 88 Despite being undetected in the adult mouse heart under normal conditions, MCUb expression was found to increase following ischemic injury. 66 , 75 , 88 The increase in MCUb protein levels was observed only after 3 days post‐ischemia/reperfusion (I/R) in the hearts of mice, and further increased after 7 days. 75 , 88 The increased MCUb expression caused decreased mitochondrial Ca2+ uptake and suppressed mPTP opening, thus reducing cardiac tissue damage. 88 Due to the absence of MCUb until 3 days post‐I/R, MCUb may play a role in limiting infarct expansion and post‐ischemic pathological remodeling, which occurs days following initial injury. 75 , 88
3.4.1. Cardiac arrhythmia
Arrhythmia is a common cause of cardiovascular disease‐related deaths, and there are limited treatment options due to adverse anti‐arrhythmic drug side effects. 89 Cardiac arrhythmia is associated with cellular Ca2+ dysregulation in cardiomyocytes. 89 Mitochondria absorb ryanodine receptor 2 (RyR2)‐mediated Ca2+ released from the sarcoplasmic reticulum (SR). 89 RyR2 channel clusters are in close proximity to the mitochondria. 90 Thus, enhancing the ability of mitochondria‐mediated Ca2+ uptake can interfere with Ca2+ diffusion between RyR2 clusters, reducing the probability of arrhythmogenic spontaneous Ca2+ wave initiation and propagation. 90 Intracellular Ca2+ transporters, such as mtCU, may be candidate pathways for novel anti‐arrhythmic therapeutics with fewer adverse side effects. 89
Diabetes mellitus increases the risk of heart failure due to decreased cardiomyocyte function, linked to changes in cardiac mitochondrial energy metabolism. 91 Diabetes mellitus is a remarkable proponent of cardiac arrhythmias. 92 , 93 Type 1 diabetic mice hearts, induced using STZ, were found to have altered expression of MCU and MCUb, resulting in a decrease in mitochondrial Ca2+ uptake and cardiac function. 91 Reduced rates of contraction and relaxation were observed in isolated‐perfused diabetic hearts. 91 MCU and EMRE protein levels decreased by 50% and 36%, respectively, in the mouse hearts 8 weeks post‐STZ, and MCUb protein levels were increased by 31% compared to control hearts. 91 Additionally, in type 2 diabetic mouse hearts, MCUb was found to be upregulated. 94 Gene therapy displacement of endogenous MCUB with a dominant‐negative MCUB transgene (MCUB W245R/V251E) in vivo rescued cardiomyocytes from relying almost solely on mitochondrial fatty acids for energy production and increased cardiac contractile function. 94 Interestingly, the MCUbW245R/V251E mutant was found to interact with MICU1, but similar to the proteomic data (see Section 3.1) WT MCUb did not interact with MICU1 in cardiomyocytes from the type 2 diabetic mouse hearts. 94 Further, the MCUbW245R/V251E mutant increased mitochondrial Ca2+ uptake from the reduced rates observed with overexpression of FLAG‐tagged MCUb, to rates similar to cells expressing FLAG‐tagged MCU. 94 Thus, MCUb remains at a pivotal interface between metabolism and cardiac function, with the reduction of MCUb inhibitory function holding considerable promise for the chronically stressed heart. 94
Nonischemic heart failure (HF) triggered by Ca2+ dysregulation also contributes to arrhythmia risk. 95 Isolated myopathic myocytes in nonischemic HF mice showed increased mitochondrial Ca2+ transients and action potential duration, which both decreased upon knockdown of MCU. 95 This knockdown also reduced ventricular fibrillation in these nonischemic HF mice. 95 Interestingly, human cardiac samples isolated from HF patients showed an ~2‐fold increase in the MICU1/MCU protein ratio compared to non‐HF control samples. 96 Yet, there was no change in either MCU or MCUb protein levels in the human HF cardiac samples compared to the non‐HF control samples. 96 These data contrast the MCUb upregulation observed in I/R injury mouse models (see Section 3.4; Ref. [66, 88]). While differences in beat frequency/energetic demands of the mouse and human heart, could underlie some inconsistencies observed in mtCU subunit contribution to pathogenesis, it is noteworthy that MCUb expression changes were only observed ~2–3 days post‐I/R injury in mice. 66 , 88 Thus, a careful analysis of the temporal expression pattern of MCUb in human HF and mouse HF models is needed for comparison.
4. CONSERVATION OF MCU‐IDENTIFIED POST‐TRANSLATIONAL MODIFICATION SITES IN MCUb
Post‐translational modifications regulate mtCU channel oligomerization and function, 40 but the effects on the MCU paralog, MCUb, have yet to be characterized and understood. For instance, proline‐rich tyrosine kinase 2 (Pyk2)‐dependent phosphorylation of Tyr158 in the N‐terminal domain and Tyr289/Tyr317 in the C‐terminal domain of MCU was determined to increase mitochondrial Ca2+ uptake and mitochondrial reactive oxygen species (mROS) production, triggering mPTP opening and cell death (Table 2; Figure 5; Ref. [22, 40, 99]). Phosphorylation of Ser57 within the MCU N‐terminal domain promoted mitochondrial Ca2+ uptake, necessary for proper mitotic progression (Table 2; Ref. [22, 98]). Ser57 of MCU contains an AMP‐activated protein kinase (AMPK) consensus phosphorylation site, also indicated as a Ca2+/calmodulin‐dependent protein kinase II (CAMKII) phosphorylation site. 97 , 98 Phosphorylation of Ser92 within the MCU N‐terminal domain has been shown to disrupt the Ser92:Asp119 hydrogen bond between loop2 and loop4 of the β‐grasp‐fold (Table 2; Figure 5; Ref. [15]). The resultant conformational changes, due to the loss of the hydrogen bond and increased negative charge, were found to disrupt the dimerization between mtCU channel tetramers. 15 However, whether phosphorylation regulates MCUb inhibitory action remains elusive.
TABLE 2.
Summary of the post‐translational modifications known to regulate MCU and conservation in MCUb
| Post‐translational modification | Residue position in MCU | Domain position of residue | Conservation in MCUb | References |
|---|---|---|---|---|
| Phosphorylation | Ser57 | N‐terminal domain | No (Asn42) | Joiner et al., (2012) 97 ; Zhao et al., (2019) 98 ; Alevriadou et al., (2021) 22 |
| Phosphorylation | Ser92 | N‐terminal domain | Yes (Ser77) |
Joiner et al., (2012) 97 ; Nemani et al., (2018) 2 ; Lee et al., (2020) 15 |
| Phosphorylation | Tyr158 | N‐terminal domain | Yes (Tyr143) | Alevriadou et al., (2021) 22 ; Feno et al., (2021) 40 |
| Phosphorylation | Tyr289 | C‐terminal domain | Yes (Tyr274) | O‐Uchi et al., (2014) 99 ; Feno et al., (2021) 40 |
| Phosphorylation | Tyr317 | C‐terminal domain | Yes (Tyr302) | Feno et al., (2021) 40 |
| S‐glutathionylation | Cys97 | N‐terminal domain | Yes (Cys82) | Dong et al., (2017) 100 ; Nemani et al., (2018) 2 |
FIGURE 5.
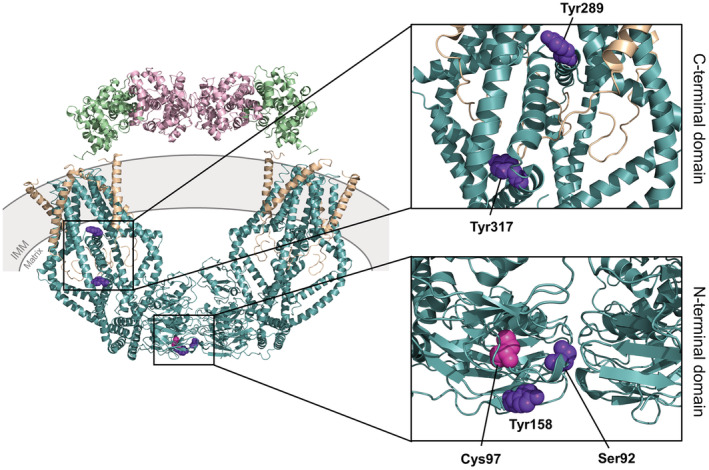
Three‐dimensional structures of the human mitochondrial calcium uniporter (MCU) holocomplex elucidated in a high Ca2+/non‐occluded state, highlighting the known post‐translational modification residue positions. The structure images were generated using the 6WDO.pdb. 31 Note that the Ser57 phosphorylation site is not visible in this structure. The MCU complex includes MCU (teal), EMRE (beige), MICU1 (green), and MICU2 (pink). The righthand panels show zoomed views of the post‐translational modification sites occurring in the C‐terminal domain (top) and the N‐terminal domain (bottom). The known phosphorylation residue positions are shown with purple spheres, and the S‐glutathionylation position is shown with magenta spheres. EMRE, essential MCU regulatory protein, MICU1/2, mitochondrial Ca2+ uptake 1 and −2 proteins; IMM, inner mitochondrial membrane.
MCU also contains three Cys in the N‐terminal domain (i.e., Cys67, Cys97, Cys191) that may be susceptible to oxidative modifications. 2 , 100 Indeed, S‐glutathionylation of Cys97 within the MCU N‐terminal domain has been shown to regulate mtCU channel activity (Table 2; Figure 5; Ref. [100]). Oxidation and mutation of Cys97 in MCU both induce a conformational change within the N‐terminal β‐grasp fold, resulting in elevated mitochondrial Ca2+ uptake through the mtCU channel, increased mROS, and an increased propensity for mitochondrial Ca2+ overload‐induced cell death. 100 While a biochemical gel shift assay revealed that MCU is the luminal mROS sensor, 2 , 100 the Cys97 is conserved in MCUb (i.e., existing as Cys82; Figure 1) and may also play a regulatory role under conditions that favor MCUb expression and oxidative modifications. Interestingly, mutating Cys97 of MCU abrogates the inhibitory action of Ru265 (see Section 3.2), suggesting that this post‐translational modification site may also play a role in binding this small molecule inhibitor. 35
The high sequence homology with MCU implies that post‐translational modifications of MCUb could also contribute to the regulation of MCUb and mtCUs in which the dominant‐negative beta subunit has been incorporated. Thus, understanding the mechanisms and structural consequences of post‐translational modifications within MCUb will provide a deeper understanding of the subunit regulation and function, both within physiological and pathophysiological states.
5. CONCLUSION
Since the discovery of mtCU components, MCUb has emerged as a critical regulator, despite the lack of understanding surrounding the precise molecular mechanisms that govern the complex assembly and negative regulation by this inhibitor. 30 Determining the precise structural mechanisms that underlie the inhibitory and differential function of MCUb compared to MCU requires considerable future efforts. Nevertheless, this type of research focus could be critical for the development of novel research tools and pave the way to new therapeutics. The research focused on the less well‐studied regulatory components of mtCU will also aid in the understanding of numerous pervasive pathologies effected by Ca2+ overload. 30 For example, with cardiac arrhythmias being a global health burden impacting close to 2% of the population and only moderately effective treatment options available, 101 research focusing on the association with mitochondrial Ca2+ regulation and the MCUb subunit will lead to a greater understanding of arrhythmogenesis and help in the development of safer therapeutics.
AUTHOR CONTRIBUTIONS
Danielle M. Colussi: writing original draft, investigation, editing/revising, conceptualization, and model analyses. Peter B. Stathopulos: editing, review, supervision, modeling methodology, and funding. Both authors read and approved the submitted version
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Supplementary Material S1
Supplementary Material S2
ACKNOWLEDGMENTS
This work was supported by an NSERC‐CGSM to D.M.C. and CIHR‐438225 operating grant to P.B.S.
Colussi DM, Stathopulos PB. From passage to inhibition: Uncovering the structural and physiological inhibitory mechanisms of MCUb in mitochondrial calcium regulation. The FASEB Journal. 2022;37:e22678. doi: 10.1096/fj.202201080R
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary materials of this article as Supplementary Dataset 1 (PISA and PyMOL analysis spreadsheet), Supplementary Dataset 2 (MCUb homotetramer homology model coordinates—non‐occluded), Supplementary Dataset 3 (MCU‐MCUb heterotetramer homology model coordinates—non‐occluded), Supplementary Dataset 4 (MCUb homotetramer homology model coordinates—occluded), Supplementary Dataset 5 (MCU‐MCUb heterotetramer homology model coordinates—occluded). The MCU homotetramer coordinates are openly available in the Protein Data Bank at https://www.rcsb.org/, reference 6WDO.pdb and 6WDN.pdb. The human MCU and MCUb protein sequences were taken from https://www.ncbi.nlm.nih.gov/protein/, references NP_612366.1 and NP_060388.2, respectively.
REFERENCES
- 1. Lee SK, Shanmughapriya S, Mok MCY, et al. Structural insights into mitochondrial calcium uniporter regulation by divalent cations. Cell Chem Biol. 2016;23:1157‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nemani N, Shanmughapriya S, Madesh M. Molecular regulation of MCU: implications in physiology and disease. Cell Calcium. 2018;74:86‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quintana A, Pasche M, Junker C, et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T‐cell activation: calcium microdomains at the immunological synapse. EMBO J. 2011;30:3895‐3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pallafacchina G, Zanin S, Rizzuto R. From the identification to the dissection of the physiological role of the mitochondrial calcium uniporter: an ongoing story. Biomolecules. 2021;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store‐operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Penna E, Espino J, De Stefani D, Rizzuto R. The MCU complex in cell death. Cell Calcium. 2018;69:73‐80. [DOI] [PubMed] [Google Scholar]
- 7. Tarasov AI, Griffiths EJ, Rutter GA. Regulation of ATP production by mitochondrial Ca2+ . Cell Calcium. 2012;52:28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Briston T, Roberts M, Lewis S, et al. Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci Rep. 2017;7:10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gherardi G, Monticelli H, Rizzuto R, Mammucari C. The mitochondrial Ca2+ uptake and the fine‐tuning of aerobic metabolism. Front Physiol. 2020;11:554904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanwar J, Singh JB, Motiani RK. Molecular machinery regulating mitochondrial calcium levels: the nuts and bolts of mitochondrial calcium dynamics. Mitochondrion. 2021;57:9‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3‐sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744‐747. [DOI] [PubMed] [Google Scholar]
- 12. Rizzuto R, Pinton P, Carrington W, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763‐1766. [DOI] [PubMed] [Google Scholar]
- 13. Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications: the mitochondrial calcium uniporter complex. J Physiol. 2014;592:829‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rapizzi E, Pinton P, Szabadkai G, et al. Recombinant expression of the voltage‐dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y, Park J, Lee G, et al. S92 phosphorylation induces structural changes in the N‐terminus domain of human mitochondrial calcium uniporter. Sci Rep. 2020;10:9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeLuca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci. 1961;47:1744‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jean‐Quartier C, Bondarenko AI, Alam MR, et al. Studying mitochondrial Ca2+ uptake—a revisit. Mol Cell Endocrinol. 2012;353:114‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carafoli E. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem Sci. 2003;28:175‐181. [DOI] [PubMed] [Google Scholar]
- 19. Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670‐2677. [PubMed] [Google Scholar]
- 20. Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noble M, Lin Q‐T, Sirko C, Houpt JA, Novello MJ, Stathopulos PB. Structural mechanisms of store‐operated and mitochondrial calcium regulation: initiation points for drug discovery. Int J Mol Sci. 2020;21:3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alevriadou BR, Patel A, Noble M, et al. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am J Physiol Cell Physiol. 2021;320:C465‐C482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360‐364. [DOI] [PubMed] [Google Scholar]
- 24. Plovanich M, Bogorad RL, Sancak Y, et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8:e55785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty‐kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Stefani D, Patron M, Rizzuto R. Structure and function of the mitochondrial calcium uniporter complex. Biochim Biophys Acta BBA Mol Cell Res. 2015;1853:2006‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sancak Y, Markhard AL, Kitami T, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mallilankaraman K, Cárdenas C, Doonan PJ, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raffaello A, De Stefani D, Sabbadin D, et al. The mitochondrial calcium uniporter is a multimer that can include a dominant‐negative pore‐forming subunit. EMBO J. 2013;32:2362‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan M, Zhang J, Tsai C‐W, et al. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature. 2020;582:129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Han Y, She J, et al. Structural insights into the Ca2+‐dependent gating of the human mitochondrial calcium uniporter. eLife. 2020;9:e60513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuo W, Zhou H, Guo R, et al. Structure of intact human MCU supercomplex with the auxiliary MICU subunits. Protein Cell. 2021;12:220‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murgia M, Rizzuto R. Molecular diversity and pleiotropic role of the mitochondrial calcium uniporter. Cell Calcium. 2015;58:11‐17. [DOI] [PubMed] [Google Scholar]
- 35. Woods JJ, Nemani N, Shanmughapriya S, et al. A selective and cell‐permeable mitochondrial calcium uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent Sci. 2019;5:153‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lambert JP, Murray EK, Elrod JW. MCUB and mitochondrial calcium uptake—modeling, function, and therapeutic potential. Expert Opin Ther Targets. 2020;24:163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cui C, Yang J, Fu L, Wang M, Wang X. Progress in understanding mitochondrial calcium uniporter complex‐mediated calcium signalling: a potential target for cancer treatment. Br J Pharmacol. 2019;176:1190‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baradaran R, Wang C, Siliciano AF, Long SB. Cryo‐EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018;559:580‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feno S, Rizzuto R, Raffaello A, Vecellio Reane D. The molecular complexity of the mitochondrial calcium uniporter. Cell Calcium. 2021;93:102322. [DOI] [PubMed] [Google Scholar]
- 41. Wu W, Zheng J, Jia Z. Structural characterization of the mitochondrial Ca2+ uniporter provides insights into Ca2+ uptake and regulation. iScience. 2021;24:102895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fan C, Fan M, Orlando BJ, et al. X‐ray and cryo‐EM structures of the mitochondrial calcium uniporter. Nature. 2018;559:575‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Nguyen NX, She J, et al. Structural mechanism of EMRE‐dependent gating of the human mitochondrial calcium uniporter. Cell. 2019;177:1252‐1261.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Keuren AM, Tsai C‐W, Balderas E, Rodriguez MX, Chaudhuri D, Tsai M‐F. Mechanisms of EMRE‐dependent MCU opening in the mitochondrial calcium uniporter complex. Cell Rep. 2020;33:108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan Y, Cao C, Wen M, et al. Structural characterization of the N‐terminal domain of the Dictyostelium discoideum mitochondrial calcium uniporter. ACS Omega. 2020;5:6452‐6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vais H, Payne R, Paudel U, Li C, Foskett JK. Coupled transmembrane mechanisms control MCU‐mediated mitochondrial Ca2+ uptake. Proc Natl Acad Sci. 2020;117:21731‐21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patron M, Checchetto V, Raffaello A, et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu W, Shen Q, Zhang R, et al. The structure of the micu 1‐micu 2 complex unveils the regulation of the mitochondrial calcium uniporter. EMBO J. 2020;39:e104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue‐specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26:179‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mallilankaraman K, Doonan P, Cárdenas C, et al. MICU1 is an essential gatekeeper for MCU‐mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Payne R, Hoff H, Roskowski A, Foskett JK. MICU2 restricts spatial crosstalk between InsP 3 R and MCU channels by regulating threshold and gain of MICU1‐mediated inhibition and activation of MCU. Cell Rep. 2017;21:3141‐3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garg V, Suzuki J, Paranjpe I, et al. The mechanism of MICU‐dependent gating of the mitochondrial Ca2+uniporter. eLife. 2021;10:e69312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Foskett JK. Uncorking MCU to let the calcium flow. Cell Calcium. 2020;91:102257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. UK10K Consortium , Logan CV, Szabadkai G, et al. Loss‐of‐function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46:188‐193. [DOI] [PubMed] [Google Scholar]
- 55. Lewis‐Smith D, Kamer KJ, Griffin H, et al. Homozygous deletion in MICU1 presenting with fatigue and lethargy in childhood. Neurol Genet. 2016;2:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Musa S, Eyaid W, Kamer K, et al. A middle eastern founder mutation expands the genotypic and phenotypic spectrum of mitochondrial MICU1 deficiency: a report of 13 patients. In: Morava E, Baumgartner M, Patterson M, Rahman S, Zschocke J, Peters V, eds. JIMD Reports, Volume 43. Vol 43. Springer Berlin Heidelberg; 2018:79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shamseldin HE, Alasmari A, Salih MA, et al. A null mutation in MICU2 causes abnormal mitochondrial calcium homeostasis and a severe neurodevelopmental disorder. Brain. 2017;140:2806‐2813. [DOI] [PubMed] [Google Scholar]
- 58. Antony AN, Paillard M, Moffat C, et al. MICU1 regulation of mitochondrial Ca2+ uptake dictates survival and tissue regeneration. Nat Commun. 2016;7:10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Debattisti V, Horn A, Singh R, et al. Dysregulation of mitochondrial Ca2+ uptake and sarcolemma repair underlie muscle weakness and wasting in patients and mice lacking MICU1. Cell Rep. 2019;29:1274‐1286.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singh R, Bartok A, Paillard M, Tyburski A, Elliott M, Hajnóczky G. Uncontrolled mitochondrial calcium uptake underlies the pathogenesis of neurodegeneration in MICU1‐deficient mice and patients. Sci Adv. 2022;8:eabj4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsai M‐F, Phillips CB, Ranaghan M, et al. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. eLife. 2016;5:e15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Payne R, Li C, Foskett JK. Variable assembly of EMRE and MCU creates functional channels with distinct gatekeeping profiles. iScience. 2020;23:101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tomar D, Dong Z, Shanmughapriya S, et al. MCUR1 is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Rep. 2016;15:1673‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chaudhuri D, Artiga DJ, Abiria SA, Clapham DE. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc Natl Acad Sci. 2016;113:E1872‐E1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015;21:109‐116. [DOI] [PubMed] [Google Scholar]
- 66. Lambert JP, Luongo TS, Tomar D, et al. MCUB regulates the molecular composition of the mitochondrial calcium uniporter channel to limit mitochondrial calcium overload during stress. Circulation. 2019;140:1720‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102:893‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinforma. 2016;54:5.6.1‐5.6.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774‐797. [DOI] [PubMed] [Google Scholar]
- 70. Phillips CB, Tsai C‐W, Tsai M‐F. The conserved aspartate ring of MCU mediates MICU1 binding and regulation in the mitochondrial calcium uniporter complex. eLife. 2019;8:e41112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaludercic N, Scorrano L. MCUB hearts mitochondria in sickness, less in health. Circulation. 2019;140:1734‐1736. [DOI] [PubMed] [Google Scholar]
- 72. Paillard M, Csordás G, Huang K‐T, Várnai P, Joseph SK, Hajnóczky G. MICU1 interacts with the D‐ring of the MCU pore to control its Ca2+ flux and sensitivity to Ru360. Mol Cell. 2018;72:778‐785.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Belosludtsev KN, Dubinin MV, Talanov EY, et al. Transport of Ca2+ and Ca2+‐dependent permeability transition in the liver and heart mitochondria of rats with different tolerance to acute hypoxia. Biomolecules. 2020;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Paillard M, Csordás G, Szanda G, et al. Tissue‐specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep. 2017;18:2291‐2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moyzis AG, Gustafsson ÅB. Protective function of MCUb in postischemic remodeling getting at the heart of the calcium control conundrum. Circ Res. 2020;127:391‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dubinin MV, Talanov EY, Tenkov KS, et al. Duchenne muscular dystrophy is associated with the inhibition of calcium uniport in mitochondria and an increased sensitivity of the organelles to the calcium‐induced permeability transition. Biochim Biophys Acta BBA Mol Basis Dis. 2020;1866:165674. [DOI] [PubMed] [Google Scholar]
- 77. Dubinin MV, Talanov EY, Tenkov KS, Starinets VS, Mikheeva IB, Belosludtsev KN. Transport of Ca2+ and Ca2+−dependent permeability transition in heart mitochondria in the early stages of Duchenne muscular dystrophy. Biochim Biophys Acta BBA Bioenerg. 2020;1861:148250. [DOI] [PubMed] [Google Scholar]
- 78. Jadiya P, Kolmetzky DW, Tomar D, et al. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer's disease. Nat Commun. 2019;10:3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Calvo‐Rodriguez M, Bacskai BJ. High mitochondrial calcium levels precede neuronal death in vivo in Alzheimer's disease. Cell Stress. 2020;4:187‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Danese A, Patergnani S, Bonora M, et al. Calcium regulates cell death in cancer: roles of the mitochondria and mitochondria‐associated membranes (MAMs). Biochim Biophys Acta BBA Bioenerg. 2017;1858:615‐627. [DOI] [PubMed] [Google Scholar]
- 81. Xu R, Han M, Xu Y, et al. Coiled‐coil domain containing 109B is a HIF1α‐regulated gene critical for progression of human gliomas. J Transl Med. 2017;15:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tate JG, Bamford S, Jubb HC, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941‐D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marchi S, Vitto VAM, Danese A, Wieckowski MR, Giorgi C, Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18:1068‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flotho C, Coustan‐Smith E, Pei D, et al. A set of genes that regulate cell proliferation predicts treatment outcome in childhood acute lymphoblastic leukemia. Blood. 2007;110:1271‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Campana D. Molecular determinants of treatment response in acute lymphoblastic leukemia. Hematology. 2008;2008:366‐373. [DOI] [PubMed] [Google Scholar]
- 86. Tosatto A, Sommaggio R, Kummerow C, et al. The mitochondrial calcium uniporter regulates breast cancer progression via hif‐1α. EMBO Mol Med. 2016;8:569‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Belosludtsev KN, Talanov EY, Starinets VS, Agafonov AV, Dubinin MV, Belosludtseva NV. Transport of Ca2+ and Ca2+‐dependent permeability transition in rat liver mitochondria under the streptozotocin‐induced type I diabetes. Cell. 2019;8:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huo J, Lu S, Kwong JQ, et al. MCUb induction protects the heart from postischemic remodeling. Circ Res. 2020;127:379‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schweitzer MK, Wilting F, Sedej S, et al. Suppression of arrhythmia by enhancing mitochondrial Ca2+ uptake in catecholaminergic ventricular tachycardia models. JACC Basic Transl Sci. 2017;2:737‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hamilton S, Terentyeva R, Clements RT, Belevych AE, Terentyev D. Sarcoplasmic reticulum‐mitochondria communication; implications for cardiac arrhythmia. J Mol Cell Cardiol. 2021;156:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Suarez J, Cividini F, Scott BT, et al. Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J Biol Chem. 2018;293:8182‐8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates 11Reprints are not available. Am J Cardiol. 1998;82:2 N‐9 N. [DOI] [PubMed] [Google Scholar]
- 93. Grisanti LA. Diabetes and arrhythmias: pathophysiology, mechanisms and therapeutic outcomes. Front Physiol. 2018;9:1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cividini F, Scott BT, Suarez J, et al. Ncor2/PPARα‐dependent upregulation of MCUb in the type 2 diabetic heart impacts cardiac metabolic flexibility and function. Diabetes. 2021;70:665‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xie A, Song Z, Liu H, et al. Mitochondrial Ca2+ influx contributes to arrhythmic risk in nonischemic cardiomyopathy. J Am Heart Assoc. 2018;7:e007805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Paillard M, Huang K‐T, Weaver D, Lambert JP, Elrod JW, Hajnóczky G. Altered composition of the mitochondrial Ca2+ uniporter in the failing human heart. Cell Calcium. 2022;105:102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Joiner MA, Koval OM, Li J, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao H, Li T, Wang K, et al. AMPK‐mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat Cell Biol. 2019;21:476‐486. [DOI] [PubMed] [Google Scholar]
- 99. O‐Uchi J, Jhun BS, Xu S, et al. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2‐dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid Redox Signal. 2014;21:863‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dong Z, Shanmughapriya S, Tomar D, et al. Mitochondrial Ca2+ uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol Cell. 2017;65:1014‐1028.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Graeff C, Bert C. Noninvasive cardiac arrhythmia ablation with particle beams. Med Phys. 2018;45:e1024‐e1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Supplementary Material S2
Data Availability Statement
The data that support the findings of this study are available in the supplementary materials of this article as Supplementary Dataset 1 (PISA and PyMOL analysis spreadsheet), Supplementary Dataset 2 (MCUb homotetramer homology model coordinates—non‐occluded), Supplementary Dataset 3 (MCU‐MCUb heterotetramer homology model coordinates—non‐occluded), Supplementary Dataset 4 (MCUb homotetramer homology model coordinates—occluded), Supplementary Dataset 5 (MCU‐MCUb heterotetramer homology model coordinates—occluded). The MCU homotetramer coordinates are openly available in the Protein Data Bank at https://www.rcsb.org/, reference 6WDO.pdb and 6WDN.pdb. The human MCU and MCUb protein sequences were taken from https://www.ncbi.nlm.nih.gov/protein/, references NP_612366.1 and NP_060388.2, respectively.


