Abstract
We report a novel polyester material generated from readily available biobased 1,18‐octadecanedicarboxylic acid and ethylene glycol possesses a polyethylene‐like solid‐state structure and also tensile properties similar to high density polyethylene (HDPE). Despite its crystallinity, high melting point (T m=96 °C) and hydrophobic nature, polyester‐2,18 is subject to rapid and complete hydrolytic degradation in in vitro assays with isolated naturally occurring enzymes. Under industrial composting conditions (ISO standard 14855‐1) the material is biodegraded with mineralization above 95 % within two months. Reference studies with polyester‐18,18 (T m=99 °C) reveal a strong impact of the nature of the diol repeating unit on degradation rates, possibly related to the density of ester groups in the amorphous phase. Depolymerization by methanolysis indicates suitability for closed‐loop recycling.
Keywords: Biodegradation, Long-Chain Polyesters, Polyethylene-Like, Renewable Polymers, Sustainable Chemistry
While it resembles high density polyethylene with regard to its mechanical properties and solid‐state structure and has a high melting point (T m=96 °C), the novel polyester‐2,18 material at the same time fully hydrolyzes in in vitro enzymatic degradation studies and mineralizes under industrial composting conditions (ISO standard 14855‐1) within two months.
Plastics are ubiquitous in items used for everyday life and are essential components of practically any modern technology. As a downside, persistent plastic waste is found accumulated throughout the world in various receiving environments. [1] In conjunction with, but as a backstop to, more responsible waste management, [2] biodegradable alternatives to conventional plastics are desirable for specific applications. [3]
Crystalline order is most commonly the origin of polymers′ remarkable mechanical toughness and ductility. A most prominent and relevant example is high density polyethylene (HDPE) in which the linear hydrocarbon chains pack to crystalline domains by van der Waals interactions. However, crystallinity, even in the presence of cleavable bonds, in general hinders biodegradation of plastics due to inaccessibility for extracellular enzymes to the polymer chains. Plastics designed to be biodegradable are normally low crystalline materials, as exemplified by poly(butylene adipate‐co‐terephthalate) (PBAT) films.[ 4 , 5 ] For the case of polyethylene, the chemically inert nature of the hydrocarbon chains in addition to its hydrophobicity further precludes biodegradation. [6]
We have previously shown that renewable polyesters with a low density of in‐chain functional groups as breaking points in a polyethylene chain can be chemically recycled in a closed‐loop by solvolysis with quantitative recovery at mild conditions (120 to 180 °C). [7] Unlike the aforementioned and other low crystalline polyesters,[ 4 , 8 ] the in‐chain groups do not affect the crystalline structure and the beneficial material properties of polyethylene are retained at the same time. Concerning the amenability for hydrolytic degradation of the in‐chain ester groups of the solid crystalline polymer, the polyester‐18,18 (PE‐18,18, that is [−(CH2) x OOC(CH2) y−2COO−] n with x=18, y=18) studied here was previously found to be inert towards aqueous acid over an extended period of one year. [7] For a related polyester‐15, Koning and Heise had found no enzymatic degradation to occur over 100 days, which they ascribed to the materials crystallinity and hydrophobicity. [9] Notably, in their development of methods for a rapid screening of enzymatic polyester degradation Sander et al. recently employed polyester‐4,18 in a series of aliphatic polyesters. While this polyester was much less amenable to degradation than polyesters of shorter‐chain diacids, clear evidence for enzymatic hydrolysis was observed in the nanofilms studied.[ 10 , 11 ]
We now report the novel material polyester‐2,18 (PE‐2,18) exhibits high density polyethylene‐like crystalline and materials properties, and at the same time is readily hydrolyzable by natural enzymes and biodegraded completely under industrial composting conditions.
Polyesters of renewable 1,18‐dimethyl octadecanedioate[ 12 , 13 ] with different α,ω‐diols were generated by dibutyltin oxide catalyzed polycondensation, to yield PE‐2,18, PE‐3,18, and PE‐4,18 (Figure 1, for details of synthesis and characterization cf. the Supporting Information). PE‐2,18 stands out among the polyesters synthesized here with a high melting point of T m=96 °C (cf. Figure S1), which is similar to that of the long‐chain diol based PE‐18,18. [7] Further studies therefore focused on PE‐2,18 which was synthesized with high molecular weights (weight‐average molecular weight M w≈120 000 g mol−1, number‐average molecular weight M n≈50 000 g mol−1 as determined by gel permeation chromatography (GPC) vs. polystyrene standards, cf. Figure S2).
Figure 1.
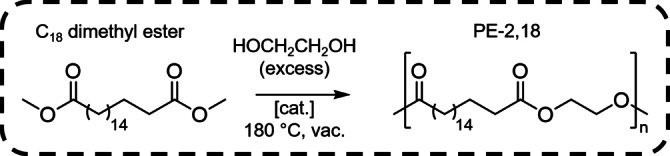
Polycondensation of C18 dimethyl ester and ethylene glycol to PE‐2,18.
Wide angle x‐ray scattering (WAXS) reveals the orthorhombic solid‐state structure found for HDPE is retained in PE‐2,18 (Figure 2a). [14] This underlines that the solid‐state structure is dominated by a crystalline alignment of the hydrocarbon segments. Deconvolution of the WAXS data yield a high degree of crystallinity of χ=66 %. Elucidation of the mechanical properties of PE‐2,18 by stress‐strain experiments on specimens generated by injection molding show a modulus of elasticity and ductile behavior (Et=730 MPa, σy=19 MPa, ϵtb=330 %) that compares to the tensile properties of commercial HDPE (Figure 2b and cf. Table S1). Further literature values are modulus of elasticity E=900 MPa for HDPE, vs. E=240 MPa for branched, less crystalline LDPE (low density polyethylene). [15] The presence of ester groups in PE‐2,18 reflects in a slightly decreased water contact angle and increased surface free energy compared to HDPE (93° vs. 97° and SFE=38 mN m−1 vs. 32 mN m−1, cf. Table S2).
Figure 2.
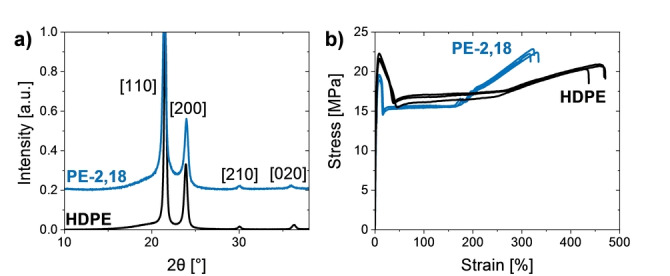
a) WAXS diffractograms of PE‐2,18 and commercial HDPE. Traces are shifted vertical for clarity. a.u., arbitrary units. b) Stress‐strain curves of injection‐molded PE‐2,18 and commercial HDPE.
The amenability of PE‐2,18 to enzymatic hydrolysis was probed by exposure to different naturally occurring esterases, namely Humicola insolens cutinase (HiC), [16] Thermomyces lanuginosus lipase (TlL), [17] and Aspergillus oryzae cutinase (AoC). [18] Monitoring of the fate of the polyester using HPLC with RI‐detector quantification of the ethylene glycol (EG) formed by hydrolysis showed that at 37 °C all three enzymes were capable of hydrolyzing PE‐2,18 (Figure 3a and cf. Supporting Information for details of procedures, reference experiments and results). Most notably with HiC a complete depolymerization to monomer was observed within days, and also with AoC a substantial portion (ca. 55 %) of the PE‐2,18 was broken down to the monomers over the course of one week. Monitoring hydrolysis at ambient temperature (25 °C) for HiC, the most active tested enzyme toward PE‐2,18, showed a substantial degradation to monomer occurs also under these conditions within one week (Figure 3b).
Figure 3.
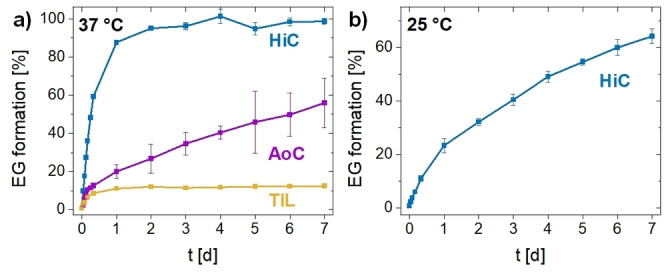
a) Hydrolysis of PE‐2,18 by Humicola insolens cutinase (HiC), Thermomyces lanuginosus lipase (TlL), and Aspergillus oryzae cutinase (AoC) at 37 °C and pH 7.2, monitored via HPLC‐RI quantification of formed ethylene glycol (EG). b) Hydrolysis of PE‐2,18 by HiC at ambient temperature (25 °C, pH 7.2).
Incubations of the three different enzymes were performed at activity‐normalized enzyme concentrations (as measured against model ester substrates, adjusted to reach a final enzyme activity of 0.21 kat L−1 in the medium). Therefore, differences in PE‐2,18 hydrolysis rates and extents indicate different affinities of each enzyme toward this polyester. Using an approach of modelling active site surface area, [11] the vast difference between activities of HiC and AoC vs. that of TlL toward PE‐2,18 could at least in part be explained by an increased accessibility of the active sites for the respective enzymes (cf. Figure S23). This result indicates that, similar to results for aromatic‐aliphatic polyesters, active site accessibility may be a controlling factor of the ability of an enzyme to hydrolyze crystalline polyesters such as PE‐2,18.
An alternative monitoring of the degradation to monomers by LC‐MS quantification of the formed C18 diacid allowed for a comparison to PE‐18,18. Note that the enzymatic degradation protocol and sample preparation were slightly adapted vs. the above procedure monitoring EG monomer in terms of amount of polymer and aqueous phase to account for the limited solubility of C18 diacid monomer (cf. Supporting Information). Under the conditions that resulted in complete degradation of PE‐2,18 within days (Figure 3a), PE‐18,18 was hydrolyzed much slower resulting in a conversion of less than 1 % to monomer after one week by HiC (cf. Figure S22).
The degradability of PE‐2,18 by microorganisms was assessed by a controlled laboratory composting test, monitoring the evolution of carbon dioxide formed by mineralization of the polyester according to ISO standard 14855‐1.[ 4 , 19 ] Remarkably, PE‐2,18 mineralization reached 96±2 % (n=3) within only two months at 58 °C, despite its crystalline nature (Figure 4). This mineralization profile compares qualitatively to the behavior of commercial, less crystalline, biodegradable polyesters and fulfills standard requirements for biodegradation under industrial composting conditions. [4] By comparison, PE‐18,18 exhibited only partial biodegradation in industrial composting, with two replicates plateauing around 30 % mineralization after two months in the same setup (cf. Figure S28).
Figure 4.
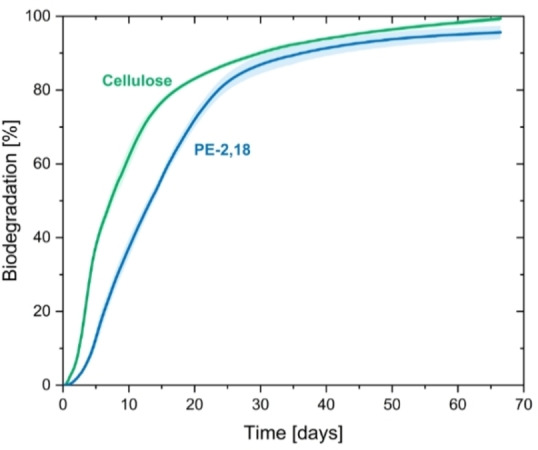
Mineralization curves based on CO2 evolution measured under industrial composting conditions following the standard ISO 14855‐1 for PE‐2,18 and for cellulose as a reference material. Shadows in light color correspond to the standard deviations.
Despite its semicrystalline nature, given by a polyethylene‐like solid state structure that also reflects in its tensile properties, polyester‐2,18 can undergo enzymatic and microbial degradation (Figure 5). Compared to PE‐18,18, which degraded much slower and to a limited extent in the experiments performed, PE‐2,18 will likely have a considerably higher ester bond density in the amorphous phase (while ester groups can be incorporated in the polyethylene crystalline lamella, they are known to preferentially locate in the amorphous phase). [20] This and other possible effects such as different chain conformations at the amorphous—crystalline interphase may facilitate the onset of degradation, and via the formation of less hydrophobic domains promote the further degradation process.
Figure 5.
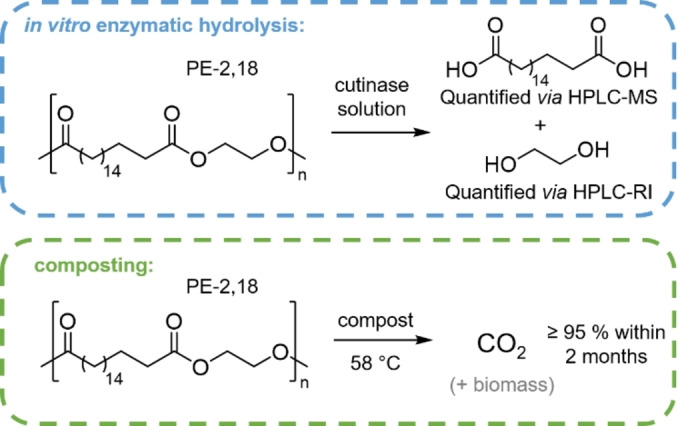
Biodegradation of PE‐2,18 probed here via enzymatic hydrolysis (a key step in overall biodegradation) (top) and mineralization under industrial composting conditions (bottom).
On a different note, ethylene glycol as a monomer for polyester synthesis offers the advantage of commercial availability, and like the commercially available C18 diacid it can be sourced from renewable feedstocks.[ 12 , 21 ] In addition, in preliminary chemical recycling experiments both the C18 monomer (>90 % recovery by weight vs. PE‐2,18 employed) and ethylene glycol could be recovered (cf. Figure S8 and S9).
The high enzyme concentrations employed in the in vitro depolymerization experiments, and the conditions of biodegradation in the controlled composting test certainly favor enzymatic and microbial degradation if compared to natural environments. Field studies, for example in the marine water column and sediments as well as soils, will be of interest to further elucidate the behavior and environmental fate of this material in natural environments. Notwithstanding, our findings underline the feasibility of plastics that can be alternatives to traditional polyolefins by covering their desirable properties and being biodegradable.
Author Contributions
S.M., M.E. and S.S. jointly devised the experimental programm. M.E. established polymerization protocolls and synthesized and characterized the materials. M.E. and S.I. performed recycling experiments. S.S. and T.N. established protocolls for enzyme degradation studies, and S.S. and K.W. performed enzyme degradation experiments. S.S. and D.S. designed, and S.S. and M.E. performed liquid chromatographic monomer quantification. C.L. and G.B. designed and performed composting studies. M.E., S.S., T.N., G.B. and S.M. wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
Funding by the ERC (Advanced Grant DEEPCAT, No. 832480) is gratefully acknowledged. Initial contributions to the establishment of the enzymatic degradation protocoll by Anne Staiger are gratefully acknowledged. Contributions by Felicitas Gülzow to the workup procedure for enzyme degradation experiments are acknowledged. We thank Lars Bolk for DSC analysis, Robin Kirsten for technical support, Maximilian Baur for tensiometry and Dario Rothauer for WAXS measurements. Fruitful discussions of long‐chain polyesters with Manuel Häußler and Timo Witt are acknowledged. We thank I. Göttker‐Schnetmann for support with NMR data interpretation. We further thank the University of Konstanz precision mechanics team for component manufacturing. Open Access funding enabled and organized by Projekt DEAL.
Eck M., Schwab S. T., Nelson T. F., Wurst K., Iberl S., Schleheck D., Link C., Battagliarin G., Mecking S., Angew. Chem. Int. Ed. 2023, 62, e202213438; Angew. Chem. 2023, 135, e202213438.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
- 1.
- 1a.“The New Plastics Economy. Rethinking the future of plastics”, can be found under https://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics, 2016;
- 1b. Geyer R., Jambeck J. R., Law K. L., Sci. Adv. 2017, 3, e1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vollmer I., Jenks M. J. F., Roelands M. C. P., White R. J., van Harmelen T., de Wild P., van der Laan G. P., Meirer F., Keurentjes J. T. F., Weckhuysen B. M., Angew. Chem. Int. Ed. 2020, 59, 15402; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 15524. [Google Scholar]
- 3.
- 3a. Cywar R. M., Rorrer N. A., Hoyt C. B., Beckham G. T., Chen E. Y.-X., Nat. Rev. Mater. 2022, 7, 83; [Google Scholar]
- 3b. Zhang X., Fevre M., Jones G. O., Waymouth R. M., Chem. Rev. 2018, 118, 839; [DOI] [PubMed] [Google Scholar]
- 3c. Zhu Y., Romain C., Williams C. K., Nature 2016, 540, 354. [DOI] [PubMed] [Google Scholar]
- 4.“Polymers, Biodegradable”: A. Künkel, J. Becker, L. Börger, J. Hamprecht, S. Koltzenburg, R. Loos, M. B. Schick, K. Schlegel, C. Sinkel, G. Skupin, et al., Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2016, pp. 1–29.
- 5. Zumstein M. T., Schintlmeister A., Nelson T. F., Baumgartner R., Woebken D., Wagner M., Kohler H.-P. E., McNeill K., Sander M., Sci. Adv. 2018, 4, eaas9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hees T., Zhong F., Stürzel M., Mülhaupt R., Macromol. Rapid Commun. 2019, 40, 1800608. [DOI] [PubMed] [Google Scholar]
- 7. Häußler M., Eck M., Rothauer D., Mecking S., Nature 2021, 590, 423. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. de Hoe G. X., Zumstein M. T., Tiegs B. J., Brutman J. P., McNeill K., Sander M., Coates G. W., Hillmyer M. A., J. Am. Chem. Soc. 2018, 140, 963; [DOI] [PubMed] [Google Scholar]
- 8b. Deacy A. C., Gregory G. L., Sulley G. S., Chen T. T. D., Williams C. K., J. Am. Chem. Soc. 2021, 143, 10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Meulen I., de Geus M., Antheunis H., Deumens R., Joosten E. A. J., Koning C. E., Heise A., Biomacromolecules 2008, 9, 3404. [DOI] [PubMed] [Google Scholar]
- 10. Zumstein M. T., Kohler H.-P. E., McNeill K., Sander M., Environ. Sci. Technol. 2017, 51, 4358. [DOI] [PubMed] [Google Scholar]
- 11. Zumstein M. T., Rechsteiner D., Roduner N., Perz V., Ribitsch D., Guebitz G. M., Kohler H.-P. E., McNeill K., Sander M., Environ. Sci. Technol. 2017, 51, 7476. [DOI] [PubMed] [Google Scholar]
- 12. Chikkali S., Mecking S., Angew. Chem. Int. Ed. 2012, 51, 5802; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 5902. [Google Scholar]
- 13.A. Beuhler, “C18 Diacid Market to Grow and Expand Into an Array of Novel Products with Superior Properties”, can be found under https://elevance.com/wp-content/uploads/2017/09/Elevance-ODDA-C18-white-paper_20130916_F.pdf.
- 14. Russell K. E., Hunter B. K., Heyding R. D., Polymer 1997, 38, 1409. [Google Scholar]
- 15.“Polyethylene”: Jeremic D., Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2016, pp. 1–42. [Google Scholar]
- 16. Carniel A., Valoni É., Nicomedes J., Da Gomes A. C., de Castro A. M., Process Biochem. 2017, 59, 84. [Google Scholar]
- 17. Kim H., Kim T., Choi S., Jeon H., Oh D. X., Park J., Eom Y., Hwang S. Y., Koo J. M., Green Chem. 2020, 22, 7778. [Google Scholar]
- 18. Liu Z., Gosser Y., Baker P. J., Ravee Y., Lu Z., Alemu G., Li H., Butterfoss G. L., Kong X.-P., Gross R., et al., J. Am. Chem. Soc. 2009, 131, 15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a.International Organization for Standardization, Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—Method by analysis of evolved carbon dioxide. Part 1: General method, 83.080.01, can be found under https://www.iso.org/standard/57902.html;
- 19b. Zumstein M. T., Narayan R., Kohler H.-P. E., McNeill K., Sander M., Environ. Sci. Technol. 2019, 53, 9967. [DOI] [PubMed] [Google Scholar]
- 20. Menges M. G., Penelle J., Le Fevere de Ten Hove C., Jonas A. M., Schmidt-Rohr K., Macromolecules 2007, 40, 8714. [Google Scholar]
- 21.
- 21a. Biermann U., Bornscheuer U. T., Feussner I., Meier M. A. R., Metzger J. O., Angew. Chem. Int. Ed. 2021, 60, 20144; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 20304; [Google Scholar]
- 21b. Pang J., Zheng M., Sun R., Wang A., Wang X., Zhang T., Green Chem. 2016, 18, 342. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.


