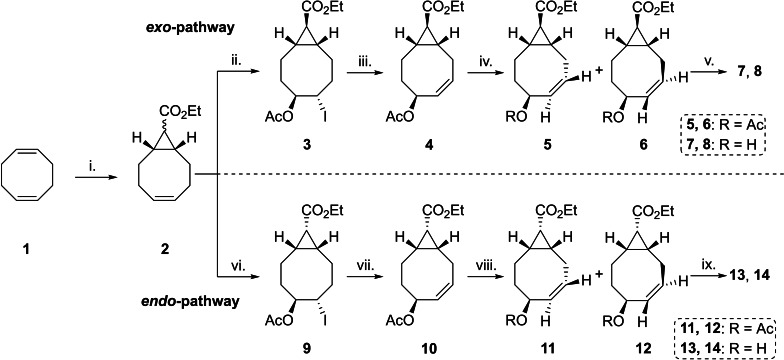
Scheme 1.
Synthesis of the difunctionalized TCO derivatives starting from commercially available cis,cis‐1,5‐cyclooctadiene (1). i. copper(ii) acetylacetonate (0.01 equiv), ethyl diazoacetate (0.17 equiv), 90 °C, 18 h, 61 % (exo/endo 2 : 1). ii. sodium acetate (3.0 equiv), acetic acid, NIS (1.2 equiv), 21 °C, 3 h, 83 %. iii. DBU (3.0 equiv), toluene, 100 °C, 18 h, 89 %. iv. methyl benzoate (2.03 equiv), heptane, MTBE, hv, silver nitrate (2.97 equiv.), 21 °C, 16 h, 5/6 7 : 13, 46 %. v. potassium carbonate (2.0 equiv), ethanol, 21 °C, 18 h, 7: 28 %, 8: 59 %. vi. sodium acetate (3.0 equiv), acetic acid, NIS (1.2 equiv), 21 °C, 72 h, 82 %. vii. DBU (3.0 equiv), toluene, 100 °C, 48 h, 48 %. viii. methyl benzoate (0.4 equiv), heptane, hv, silver nitrate (2.0 equiv), 21 °C, 43 h, 11/12 1 : 1, 31 %. ix. potassium carbonate (2.0 equiv), ethanol, 21 °C, 18 h, 13/14 1 : 1, 43 %.