Abstract
Background
Hippocampal avoidance (HA) has been shown to preserve cognitive function in adult patients with cancer treated with whole‐brain radiation therapy for brain metastases. However, the feasibility of HA in pediatric patients with brain tumors has not been explored because of concerns of increased risk of relapse in the peri‐hippocampal region. Our aim was to determine patterns of recurrence and incidence of peri‐hippocampal relapse in pediatric patients with medulloblastoma (MB).
Methods and materials
We identified pediatric patients with MB treated with protons between 2002 and 2016 and who had recurrent disease. To estimate the risk of peri‐hippocampal recurrence, three hippocampal zones (HZs) were delineated corresponding to ≤5 mm (HZ‐1), 6 to 10 mm (HZ‐2), and >10 mm (HZ‐3) distance of the recurrence from the contoured hippocampi. To determine the feasibility of HA, three standard‐risk patients with MB were planned using either volumetric‐modulated arc therapy (VMAT) or intensity‐modulated proton therapy (IMPT) plans.
Results
Thirty‐eight patients developed a recurrence at a median of 1.6 years. Of the 25 patients who had magnetic resonance imaging of the recurrence, no patients failed within the hippocampus and only two patients failed within HZ‐1. The crude incidence of peri‐hippocampal failure was 8%. Both HA‐VMAT and HA‐IMPT plans were associated with significantly reduced mean dose to the hippocampi (p < .05). HA‐VMAT and HA‐IMPT plans were associated with decreased percentage of the third and lateral ventricles receiving the prescription craniospinal dose of 23.4 Gy.
Conclusions
Peri‐hippocampal failures are uncommon in pediatric patients with MB. Hippocampal avoidance should be evaluated in a prospective cohort of pediatric patients with MB.
Plain Language Summary
In this study, the patterns of disease recurrence in patients with a pediatric brain tumor known as medulloblastoma treated with proton radiotherapy were examined. The majority of failures occur outside of an important structure related to memory formation called the hippocampus. Hippocampal sparing radiation plans using proton radiotherapy were generated and showed that dose to the hippocampus was able to be significantly reduced. The study provides the rationale to explore hippocampal sparing in pediatric medulloblastoma in a prospective clinical trial.
Keywords: hippocampal sparing, late effects, medulloblastoma, neurocognitive sparing, proton radiation
Short abstract
The incidence of peri‐hippocampal failures in patients treated with proton radiotherapy for pediatric medulloblastoma was 8%.
Hippocampal sparing plans were associated with significant reduction in the mean dose to the right and left hippocampi.
Introduction
Pediatric medulloblastoma is the most common embryonal tumor of childhood, and accounts for up to 20% of all childhood brain tumors. 1 , 2 Surgical resection in combination with craniospinal irradiation (CSI) and chemotherapy is effective, with several prospective trials demonstrating a 5‐year overall survival of approximately 70% to 80% depending on risk stratification. 3 , 4 , 5 Although survival outcomes have significantly improved over the decades, medulloblastoma survivors continue to experience long‐term neurocognitive side effects, including deterioration of processing speed, attention, and working memory, all of which affect survivors’ quality of life. 6 , 7 Preclinical studies have shown dose‐dependent radiation injury to proliferating progenitor cells in the subgranular zone (SGZ) of the hippocampus is responsible for hippocampal memory deficits. 8 Although hippocampal avoidance (HA) has been shown to reduce cognitive deficits in adult patients with brain metastases undergoing whole‐brain radiotherapy, 9 its feasibility in pediatric patients with brain tumors has not been established. Hippocampal sparing with proton radiotherapy has the potential to reduce dose to the hippocampus during the CSI and boost phases of radiotherapy treatment.
Although hippocampal avoidance is an attractive strategy to improve neurocognitive outcomes in the pediatric brain tumor population, there is concern for increased rates of disease relapse in the peri‐hippocampal region because of the need to minimize dose to the hippocampus. The objective of this study therefore was to determine the patterns of failure in a large cohort of pediatric patients with medulloblastoma undergoing proton therapy and to determine the incidence of peri‐hippocampal failures.
Methods and materials
Patient selection and treatment
Pediatric patients treated at the Massachusetts General Hospital are prospectively enrolled on two clinical trials or a prospective pediatric tumor registry, and a small number of patients were identified from a pediatric database. A total of 130 patients (72.6%) were treated on two prospective clinical trials (NCT00105560 and NCT01063114), an additional 35 patients (19.6%) were prospectively followed and consented on the Pediatric Proton/Photon Consortium Registry, and the remaining 14 patients (7.8%) were identified from our pediatric radiation database. Our cohort consisted of pediatric patients with medulloblastoma who experienced either a local or distant failure and had radiographic imaging available to determine the location of failure in relation to the hippocampus. Of the 179 patients with medulloblastoma assessed for eligibility, 38 (21.2%) experienced either a local or distant failure; of these, 25 (65.8%) had detailed imaging of the recurrence available and comprised the final cohort of this study (Figure 1).
FIGURE 1.
CONSORT flow diagram
The study was approved by the Mass General Brigham institutional review board. We included all patients with histologically confirmed standard‐, intermediate‐, or high‐risk medulloblastoma treated with proton radiotherapy at the Massachusetts General Hospital between 2002 and 2016. Standard risk was defined as patients who had ≤1.5 cm2 residual disease on postoperative magnetic resonance imaging and no evidence of metastatic disease. Intermediate‐risk patients had diffuse large cell or anaplastic histology with ≤1.5 cm2 residual disease and no metastatic disease. All other patients were classified as high risk.
All patients were treated with proton radiotherapy, typically within 35 days of surgery, with either passively scattered or intensity‐modulated proton therapy (IMPT). Patients with standard‐ and high‐risk disease were typically treated with a CSI dose of 23.4 Gy (relative biological effectiveness [RBE]) and 36 Gy (RBE), respectively, although a minority of standard‐risk patients enrolled on the ACNS 0331 low‐dose arm received 18 Gy (RBE). Intermediate‐risk patients received a dose between 23.4 Gy (RBE) and 36 Gy (RBE), which was based on age and family preference. The boost dose was typically between 54 and 55.8 Gy (RBE) and was delivered to either the whole posterior fossa or the tumor bed. All patients in our cohort were treated with chemotherapy; the most common chemotherapy regimens were based on the Children’s Oncology Group standard‐ and high‐risk protocols (ACNS 0331 and 0332).
Hippocampal failure analysis
To determine the distribution of local failure in relation to the hippocampus, the hippocampus was delineated per the RTOG 0933 contouring atlas. 10 In brief, the hypointense gray matter was contoured from the caudal extent of the temporal horn of the lateral ventricle to the quadrigeminal cistern. The hippocampus was contoured on T1‐weighted magnetic resonance imaging axial sequences. For all patients who failed within the brain, the volume of gross tumor recurrence was contoured on T1 gadolinium‐enhanced imaging, fused with the radiation computed tomography planning scan, and the distance from the hippocampal region was recorded and assessed. When available, the hippocampus was preferably contoured on the three‐dimensional (3D) spoiled gradient sequence. To estimate the risk of peri‐hippocampal or hippocampal metastases, three hippocampal zones (HZs) were delineated corresponding to 0 to 5 mm (HZ‐1), 6 to 10 mm (HZ‐2), and >10 mm (HZ‐3) distance from the contoured SGZ per Gondi et al. 11
Hippocampal sparing and planning comparison
To determine the feasibility of hippocampal sparing, three standard‐risk patients were planned using either a volumetric‐modulated arc therapy (VMAT) or IMPT hippocampal sparing plan. Therefore, a total of nine plans were generated for dosimetry analysis. For VMAT, planning was optimized with multicriteria optimization in Raystation version 8A. Four arcs were used for the whole‐brain plans, including two full coplanar arcs and two partial noncoplanar arcs that avoided exit into the transition area within the spine. Noncoplanar arcs were also used for the boost if the boost target extended superiorly between the hippocampi. For the proton plan, pencil beam scanning (PBS) plans were generated using three IMPT beams. A direct posterior field and a right and left posteriorly oblique field were used, 15° posterior to lateral. The dose constraint to the hippocampus was set per RTOG 0933, and we endeavored to maintain a dose to 100% of the hippocampal volume ≤9 Gy and a maximum dose ≤16 Gy, although we allowed higher dose to maintain tumor coverage. The third and lateral ventricles were also contoured to determine the dosimetric coverage of the cerebrospinal fluid (CSF) space. The percentage of the volume of the third and lateral ventricles receiving the prescription CSI dose of 23.4 Gy was compared between the three plans.
Statistical analysis
Patient characteristics were summarized using descriptive statistics and displayed as percentages or ranges and medians as appropriate. All p values are reported based on two‐sided tests. All analyses were performed using the R Statistical Package v3.5.
Results
Patient characteristics
Thirty‐eight patients developed a local and/or distant recurrence at a median of 1.6 years (0.2–10.3) and composed our initial study cohort. The patient characteristics are shown in Table 1. The median follow‐up for the cohort was 10 years. The majority of patients were male (n = 26; 68.4%), had a gross total resection (GTR) or near‐GTR (n = 33; 86.4%), and had no evidence of metastatic disease at diagnosis (n = 22; 57.9%). Thirty‐five of the patients (92.1%) received either 23.4 Gy (RBE) or 36 Gy(RBE) depending on risk status, although a very small minority of patients received nonstandard CSI dosing (5%).
TABLE 1.
Patient characteristics of patients with medulloblastoma who relapsed with a local and/or distant recurrence
| Characteristics | N = 38 |
|---|---|
| Median follow‐up, years | 10 |
| Age at RT, years | 7.5 (3.5–16.0) |
| ≤8 | 22 (57.9) |
| >8 | 16 (42.1) |
| Sex | |
| Male | 26 (68.4) |
| Female | 12 (31.6) |
| Histological subtype | |
| Classic and or desmoplastic | 29 (76.3) |
| Anaplastic or large cell variant | 9 (23.7) |
| Extent of resection | |
| GTR/near‐GTR | 33 (86.4) |
| STR | 5 (13.2) |
| Risk | |
| Standard risk | 15 (39.5) |
| Intermediate | 6 (15.8) |
| High risk | 17 (44.7) |
| Modality | |
| PBS | 4 (10.5) |
| Passive scatter | 34 (89.5) |
| Craniospinal radiation dose (Gy RBE) a | 23.4 (23.4–36.0) |
| 23.4 | 21 (56.8) |
| 23.5 | 1 (2.7) |
| 30.6 | 1 (2.7) |
| 36.0 | 14 (37.8) |
| Stage | |
| M0 | 22 (57.9) |
| M1 | 0 (0) |
| M2 | 6 (15.8) |
| M3 | 10 (26.3) |
| Total dose (Gy) | 54 (50.4–55.8) |
| ≤54 | 38 (100.0) |
| >54 | 0 (0.0) |
| Median radiation treatment time (range), days | 42.5 (37.0–48.0) |
| ≤42 | 19 (50.0) |
| >42 | 19 (50.0) |
Abbreviations: GTR, gross total resection; PBS, pencil beam scanning; RBE, relative biological effectiveness; RT, radiotherapy; STR, subtotal resection.
One patient did not receive cranio‐spinal radiation.
Patterns of failure
Of the 38 patients who failed, three experienced a local failure alone (7.7%), 26 experienced a distant failure alone (68.4%), and nine experienced both a distant failure and a local failure (20.5%). Leptomeningeal disease was present in 12 patients (30.1%), of which eight (66.7%) were either intermediate‐ or high‐risk patients, and six (50%) had metastatic disease at the time of diagnosis. The 5‐year overall survival and progression‐free survival was 22.9% and 7.9% respectively.
Twenty‐five patients (64.1%) had detailed magnetic resonance imaging of the recurrence available and were included in the hippocampal failure analysis. Of the 25 patients, 13 (52%) were standard risk and 12 (48%) were high risk. The distribution of the failures in relation to the hippocampus is shown in Table 2. No patients failed within the hippocampus and only two patients failed within HZ‐1, one patient who was standard risk and one patient who was high risk. The 7‐year‐old standard‐risk patient had a classic medulloblastoma with GTR, received 23.4 Gy (RBE) of CSI, and died within 6 months of recurrence. The 4‐year‐old high‐risk patient had a classic medulloblastoma with GTR but had M3 disease, received a CSI dose of 36 Gy (RBE), and died within 5 months of recurrence. Neither of these failures was isolated, and both patients had a diffuse leptomeningeal pattern of relapse. The overall crude incidence of peri‐hippocampal failure was 8%. By risk group, it was 7.7% for standard‐risk patients and 8.3% for high‐risk patients. The median of the mean dose delivered to the right and left hippocampus was 39 Gy (RBE).
TABLE 2.
Failures within the hippocampus and peri‐hippocampal regions in recurrent medulloblastoma patients
| Risk stratification | Within hippocampus, % | HZ1 (0–5 mm) | HZ2 (6–10 mm) | HZ3 (>10 mm) | Median of mean dose of right hippocampus (Gy RBE) | Median of mean dose of left hippocampus (Gy RBE) | Leptomeningeal failure (%) |
|---|---|---|---|---|---|---|---|
| All (n = 25) | 0/25 = 0% | 2/25 = 8% | 0/25 = 0% | 0/25 = 0% | 39 | 39 | 30 |
| Standard risk (n = 13) | 0/13 = 0% | 1/13 = 7.7% | 0/13 = 0% | 0/13 = 0% | 39 | 39 | 33 |
| High risk (n = 12) | 0/12 = 0% | 1/12 = 8.3% | 0/12 = 0% | 0/12 = 0% | 39 | 39 | 67 |
Abbreviations: HZ, hippocampal zone; RBE, relative biological effectiveness.
Hippocampal planning
A comparison of a non‐hippocampal‐sparing PBS plan and hippocampal‐sparing PBS and VMAT plans are shown in Figure 2. Both VMAT and PBS hippocampal‐sparing plans were associated with significantly reduced mean dose to the right and left hippocampus (p < .05, Table 3). Coverage of the involved field boost was similar across the three plans, with no statistically significant reduction in tumor bed coverage in the HA‐PBS or HA‐VMAT plans. Cochlear mean dose did not differ between the three plans. HA‐VMAT was associated with a statistically higher mean dose to the pituitary, whole brain, and temporal lobes. However, HA‐VMAT and HA‐PBS were associated with decreased percentage of the third and lateral ventricular volumes receiving the prescription craniospinal dose of 23.4 Gy compared with the standard non‐HA PBS plan (Table 3).
FIGURE 2.
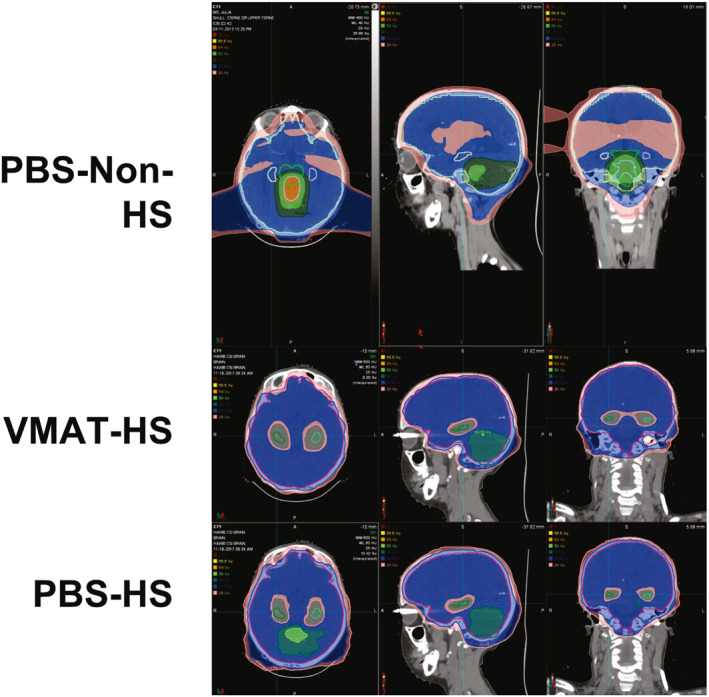
Comparison of a non‐hippocampal‐sparing pencil beam scanning (PBS) craniospinal plan and hippocampal sparing (HS) PBS and volumetric‐modulated arc therapy (VMAT) plan in a single standard‐risk medulloblastoma patient. Isodose lines: pink, 20 Gy; blue, 23.4 Gy; dark green, 40 Gy; light green, 50 Gy; orange, 54 Gy; yellow, 55.5 Gy; red, 56 Gy
TABLE 3.
Dosimetric comparison of organs at risk and the clinical target volume for non‐HS PBS plans vs HS plans in three patients
| Target/OAR | HA‐VMAT | HA‐PBS | Non‐HA proton | p |
|---|---|---|---|---|
| Hippocampus, R | 15.3 Gy | 13.4 Gy | 32.8 Gy | .006 |
| Hippocampus, L | 15.1 Gy | 9.7 Gy | 33.7 Gy | .007 |
| D95 a | 54.1 Gy | 53.1 Gy | 52.3 Gy | .19 |
| Cochlea, R | 26.4 Gy | 22.7 Gy | 26.1 Gy | .70 |
| Cochlea, L | 27.5 Gy | 25.3 Gy | 25.6 Gy | .78 |
| Pituitary | 36.2 Gy | 26.3 Gy | 23.9 Gy | .02 |
| Whole brain | 32.0 Gy | 29.4 Gy | 29.0 Gy | .05 |
| Temporal lobe, R | 30.1 Gy | 25.9 Gy | 27.5 Gy | .03 |
| Temporal lobe, L | 29.3 Gy | 25.1 Gy | 26.7 Gy | .04 |
| V23.4 Gy (ventricles) b | 82.3% | 84.6% | 100% | NA |
Note: All values are the mean of the mean dose among the three patients planned.
Abbreviations: HA, hippocampal avoidance; HS, hippocampal sparing; L, left; NA, not available; OAR, organs at risk; PBS, pencil beam scanning; R, right; VMAT, volumetric‐modulated arc therapy.
The minimum dose delivered to 95% of the PTV.
Percentage of volume of third and lateral ventricles receiving the prescription craniospinal dose of 23.4 Gy.
Discussion
To the best of our knowledge, this is the largest study to date to evaluate patterns of hippocampal failure in pediatric patients with medulloblastoma treated with proton radiotherapy. In addition, the relative lack of isolated peri‐hippocampal failures provides a rationale for consideration of dose reduction in this region to improve neurocognitive outcomes in pediatric medulloblastoma survivors.
Several studies have demonstrated the critical role of the hippocampus in pediatric neurocognition. Zureick et al. evaluated 70 patients treated with proton radiotherapy and showed that a higher percentage of left hippocampal volume receiving 20 Gy (V20GyE) was associated with a decline in delayed and immediate verbal memory. 12 Redmond et al. evaluated neuropsychological skills (motor speed, dexterity, verbal memory, visual perception, vocabulary, and visuospatial memory) in 19 pediatric patients who received cranial radiotherapy (RT) and 55 controls at baseline. The authors demonstrated that increasing dose of RT to the hippocampus and temporal lobes was responsible for a decline in neuropsychological skills after cranial irradiation. 13 More recently, Acharya et al. performed a 10‐year neurocognitive follow‐up of 80 patients between 6 and 21 years who underwent RT to 54 Gy for low‐grade glioma and demonstrated greater decline in delayed recall with increased hippocampal dose. 14 Collectively, these studies strongly suggest a critical role of the hippocampus in memory formation and highlight the urgent need to develop novel hippocampal sparing techniques.
Although multiple studies have evaluated the distribution of failures in pediatric medulloblastoma, few have evaluated failures in relation to the hippocampus. In the only other study to date, Padovani et al. evaluated 51 high‐risk patients with medulloblastoma treated on the French PNET HR+5 trial and demonstrated a relatively high rate of peri‐hippocampal failures, with 16% and 43% of patients developing metastases within 5 and 15 mm, respectively. 15 However, in patients without brain metastases, there were no patients who failed in the hippocampal region. The discordance between our results and that of the previously mentioned study is likely a reflection of the more unfavorable patient population in that trial and the lack of standard‐risk patients.
The reduction in mean dose to the bilateral hippocampi with an HA‐IMPT and HA‐VMAT plan is consistent with previous dosimetric studies. Brodin et al. evaluated mean hippocampal dose in 17 patients planned with either 3D‐conformal RT, HA–intensity‐modulated RT, or HA‐IMPT and demonstrated that HA‐IMPT plans were best at reducing dose to the hippocampus. 16 Similar to our study, the total mean dose was reduced to 10 to 18 Gy, depending on treatment margins used for the involved field boost. In addition, the authors estimate a significant benefit in task efficiency and memory with the use of IMPT compared with intensity‐modulated RT or 3D‐conformal RT. In another study, Blomstrand et al. demonstrated significant reductions in mean dose with an IMPT plan compared with a VMAT, intensity‐modulated RT, or two‐dimensional plan. 17
An unexpected finding in our study was that HA‐VMAT was as effective as HA‐IMPT in reducing the mean hippocampal dose. Although proton therapy was much more effective at reducing dose to the pituitary and temporal lobes, this technology is not available in the majority of radiation centers in the United States. Therefore, HA‐VMAT may be an alternate strategy for hippocampal sparing in centers where proton technology is not currently available.
One important concern regarding HA approaches is potential undercoverage of the ventricular system, in which CSI is meant to sterilize circulating tumor cells in the CSF. Metastatic spread of medulloblastoma is through the cerebrospinal fluid space and the risk of leptomeningeal metastasis is very high 18 , 19 ; it was nearly 30% among those who failed in our study. Underdosing in this area could lead to an elevated risk of relapse in the entire neuroaxis. In our study, approximately 80% of the CSF space was covered by the 23.4 Gy prescription isodose line in the HA plans. Undercoverage of the CSF space was required near the temporal horns of the ventricles to achieve dose gradients to allow for sparing of the hippocampi. It is unclear how this dose inhomogeneity near the hippocampi will influence patterns of failure in HA‐treated patients. In our study, we did not account for CSF coverage in our planning. In prospective studies, the CSF space should be contoured, and attempts should be made to maximize coverage of the CSF space while sparing dose to the hippocampi. A patterns of care analysis of infants with medulloblastoma treated without CSI or ACNS 0331 in the cohort that received low‐dose CSI may provide some preliminary data regarding the most likely areas of failure after undercoverage. In addition, evaluating the patterns of failure in the “Head Start” III protocol, which eliminated RT in young children with medulloblastoma, may provide additional insight about the predilection for failures in pediatric medulloblastoma. 20
Our study has several limitations. Although the lack of hippocampal failures is promising, our study cohort was small, and in 35% of patients, we did not have the availability of imaging to be reviewed for patterns of failure. Therefore, a collaborative multi‐institutional study with a much larger cohort would provide the sample size needed to evaluate the true incidence of hippocampal failures. Second, the majority of patients in this study were treated before molecular subgrouping 21 was available. Hippocampal sparing may be more suitable for specific molecular subgroups of patients with medulloblastoma with an excellent chance of survival, such as the WNT or SHH subtypes, or in patients who are not considered to be high risk, given the potential for increased risk of leptomeningeal metastases. Next, the 8% rate of peri‐hippocampal failures is with craniospinal irradiation without any sparing of the hippocampus. This percentage could be higher with a hippocampal‐sparing approach, especially with the reduction in dose near the temporal lobes, which could theoretically increase the risk of leptomeningeal failure. Finally, hippocampal‐sparing plans were generated for only three patients, which may not completely capture the variability in tumor position that can affect the dose delivered to the hippocampus.
In summary, this is the largest patterns of failure study to date to demonstrate a lack of isolated hippocampal failures in pediatric patients with medulloblastoma. This is further supported by a dosimetric feasibility study of a HA‐VMAT and HA‐PBS approach to reduce dose to the bilateral hippocampi. This finding should be validated in larger multi‐institutional cohort of patients with medulloblastoma before being prospectively evaluated in a cooperative group clinical trial.
AUTHOR CONTRIBUTIONS
Sujith Baliga: Data collection and data analysis and interpretation. Judith A. Adams: Data collection. Benjamin V. M. Bajaj: Data analysis and collection. Liam Van Benthuysen: Data collection. Juliane Daartz: Data collection. Sara L. Gallotto: Data collection. Jacqueline R. Lewy: Data collection. Nicholas Denunzio: Data collection. Elizabeth A. Weyman: Data analysis and interpretation. Miranda P. Lawell: Data collection. Joshua D. Palmer: Data analysis and interpretation. Beow Y. Yeap: Data analysis and interpretation. David H. Ebb: Data analysis and interpretation. Mary S. Huang: Data analysis and interpretation. Alisa F. Perry: Data collection. Shannon M. MacDonald: Data analysis and interpretation. Robin M. Jones: Data analysis and interpretation. Nancy J. Tarbell: Data analysis and interpretation. Torunn I. Yock: Data collection and data analysis and interpretation. All authors have seen and approved the manuscript.
CONFLICT OF INTEREST
The authors made no disclosures.
ACKNOWLEDGMENTS
The project was supported by the Federal Share of program income earned by the Massachusetts General Hospital on C06 CA059267 Proton Therapy Research and Treatment Center. The Pediatric Proton/Photon Consortium Registry (PPCR) also has received funding in the past (>3 years ago) from Ion Beam Applications; Protom; Elekta. The PPCR currently receives in kind support from MIM Software, Inc.
Baliga S, Adams JA, Bajaj BVM, et al. Patterns of failure in pediatric medulloblastoma and implications for hippocampal sparing. Cancer. 2023;129(5):764‐770. doi: 10.1002/cncr.34574
REFERENCES
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012‐2016. Neuro Oncol. 2019;21(Suppl 5):v1‐v100. doi: 10.1093/neuonc/noz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khanna V, Achey RL, Ostrom QT, et al. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neuro Oncol. 2017;135(3):433‐441. doi: 10.1007/s11060-017-2594-6 [DOI] [PubMed] [Google Scholar]
- 3. Michalski J, Vezina G, Burger P, et al. MB‐109 preliminary results of COG ACNS0331: a Phase III trial of involved field radiotherapy (IFRT) and low dose craniospinal irradiation (LD‐CSI) with chemotherapy in average risk medulloblastoma: a report from the Children’s Oncology Group. Neuro‐Oncol. 2016;18(Suppl 3):iii122. doi: 10.1093/neuonc/now076.104 [DOI] [Google Scholar]
- 4. Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study. J Clin Oncol. 2012;30(21):2648‐2653. doi: 10.1200/JCO.2011.40.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarbell NJ, Friedman H, Polkinghorn WR, et al. High‐risk medulloblastoma: a Pediatric Oncology Group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013;31(23):2936‐2941. doi: 10.1200/JCO.2012.43.9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong GT, Liu Q, Yasui Y, et al. Long‐term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946‐958. doi: 10.1093/jnci/djp148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer SL, Armstrong C, Onar‐Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494‐3500. doi: 10.1200/JCO.2012.47.4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rola R, Raber J, Rizk A, et al. Radiation‐induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316‐330. doi: 10.1016/j.expneurol.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 9. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole‐brain radiotherapy plus memantine for patients with brain metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol. 2020;38(10):1019‐1029. doi: 10.1200/JCO.19.02767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal‐sparing whole‐brain radiotherapy: a “how‐to” technique using helical tomotherapy and linear accelerator‐based intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244‐1252. doi: 10.1016/j.ijrobp.2010.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole‐brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol. 2010;95(3):327‐331. doi: 10.1016/j.radonc.2010.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zureick AH, Evans CL, Niemierko A, et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer. 2018;124(10):2238‐2245. doi: 10.1002/cncr.31143 [DOI] [PubMed] [Google Scholar]
- 13. Redmond KJ, Mahone EM, Terezakis S, et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol. 2013;15(3):360‐369. doi: 10.1093/neuonc/nos303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acharya S, Wu S, Ashford JM, et al. Association between hippocampal dose and memory in survivors of childhood or adolescent low‐grade glioma: a 10‐year neurocognitive longitudinal study. Neuro Oncol. 2019;21(9):1175‐1183. doi: 10.1093/neuonc/noz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Padovani L, Chapon F, Andre N, et al. Hippocampal sparing during craniospinal irradiation: what did we learn about the incidence of perihippocampus metastases? Int J Radiat Oncol Biol Phys. 2018;100(4):980‐986. doi: 10.1016/j.ijrobp.2017.12.265 [DOI] [PubMed] [Google Scholar]
- 16. Brodin NP, Munck af Rosenschold P, Blomstrand M, et al. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro Oncol. 2014;16(4):594‐602. doi: 10.1093/neuonc/not225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blomstrand M, Brodin NP, Munck Af Rosenschold P, et al. Estimated clinical benefit of protecting neurogenesis in the developing brain during radiation therapy for pediatric medulloblastoma. Neuro Oncol. 2012;14(7):882‐889. doi: 10.1093/neuonc/nos120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard‐risk medulloblastoma: results from the randomized multicenter HIT‐SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187‐3193. doi: 10.1200/JCO.2011.39.8719 [DOI] [PubMed] [Google Scholar]
- 19. Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average‐risk medulloblastoma. J Clin Oncol. 2006;24(25):4202‐4208. doi: 10.1200/JCO.2006.06.4980 [DOI] [PubMed] [Google Scholar]
- 20. Dhall G, O'Neil SH, Ji L, et al. Excellent outcome of young children with nodular desmoplastic medulloblastoma treated on "Head Start" III: a multi‐institutional, prospective clinical trial. Neuro Oncol. 2020;22(12):1862‐1872. doi: 10.1093/neuonc/noaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465‐472. doi: 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]


