Abstract
VIA is recommended for triage of HPV‐positive women attending cervical screening. In the multicentric ESTAMPA study, VIA performance for detection of cervical intraepithelial neoplasia grade 3 or worse (CIN3+) among HPV‐positive women was evaluated. Women aged 30‐64 years were screened with HPV testing and cytology and referred to colposcopy if either test was positive. At colposcopy visit, study‐trained midwives/nurses/GPs performed VIA ahead of colposcopy. VIA was considered positive if acetowhite lesions were observed in or close to the transformation zone. Ablative treatment eligibility was assessed for VIA positives. Performance indicators were estimated. Three thousand one hundred and forty‐two HPV‐positive women were included. Sensitivity for CIN3+ was 85.9% (95% CI 81.2‐89.5) among women <50 years and, although not significant, slightly lower in women 50+ (78.0%, 95% CI 65.9‐86.6). Overall specificity was 58.6% (95% CI 56.7‐60.5) and was significantly higher among women 50+ (70.3%, 95% CI 66.8‐73.5) compared to women <50 (54.3%, 95% CI 52.1‐56.5). VIA positivity was lower among women 50+ (35.2%, 95% CI 31.9‐38.6) compared to women <50 (53.2, 95% CI 51.1‐55.2). Overall eligibility for ablative treatment was 74.5% and did not differ by age. VIA sensitivity, specificity, and positivity, and ablative treatment eligibility varied highly by provider (ranges: 25%‐95.4%, 44.9%‐94.4%, 8.2%‐65.3%, 0%‐98.7%, respectively). VIA sensitivity for cervical precancer detection among HPV‐positive women performed by trained providers was high with an important reduction in referral rates. However, scaling‐up HPV screening triaged by VIA will be challenging due to the high variability of VIA performance and providers' need for training and supervision.
Keywords: ablative treatment eligibility, cervical cancer screening and triage, HPV, visual inspection with acetic acid, ESTAMPA
What's new?
WHO guidelines for cervical cancer screening include partial HPV genotyping, cytology, visual inspection with acetic acid (VIA), and colposcopy to triage HPV‐positive women. Here, the authors report on the performance of VIA to triage HPV‐positive women. Data from 5 sites across Latin America showed that VIA detected about 85% of high‐grade cervical lesions in HPV‐positive women, particularly those under age 50. The method also correctly identified almost 60% of women without lesions, reducing referral or treatment to nearly 50% of HPV‐positive women. However, the performance varied depending on the skill of the examiner.
Abbreviations
- AI
artificial intelligence
- ASC‐US
atypical squamous cells of undetermined significance
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- cNPV
complement of the negative predictive value
- ESTAMPA
EStudio multicéntrico de TAMizaje y triaje de cáncer de cuello uterino con pruebas del virus del PApiloma humano (Spanish acronym)
- GP
general practitioner
- HPV
human papillomavirus
- HSIL
high‐grade squamous intraepithelial lesion
- IARC
International Agency for Research on Cancer
- INEN
National Cancer Institute of Peru
- LAST
lower anogenital squamous terminology
- LLETZ
large loop excision of the transformation zone
- LMIC
low‐ and middle‐income country
- LSIL
low‐grade squamous intraepithelial lesion
- PPV
positive predictive value
- SCJ
squamocolumnar junction; SD, standard deviation
- SOP
standard operating procedure
- TZ
transformation zone
- VIA
visual inspection with acetic acid
- WHO
World Health Organization
1. INTRODUCTION
Cervical cancer remains a leading cause of cancer death in women, ranking first or second in many low‐ and middle‐income countries (LMICs). 1 In 2020, the WHO launched a global initiative to eliminate cervical cancer, consisting on achieving by 2030 90% of young girls vaccinated against the human papillomavirus (HPV), 70% of adult women screened with HPV testing, and 90% of detected lesions adequately treated, with a target incidence below 4 cases per 100 000 women. 2
In line with these goals, WHO has updated its guidelines for HPV‐based cervical cancer screening and treatment. Current guidelines suggest using partial HPV genotyping, cytology, VIA, or colposcopy to triage HPV‐positive women, with some strategies more suitable than others, depending on regional variations. 3 Triage with cytology is the preferred approach where it has been widely available. However, in settings where cytology is not used, and/or access to care is difficult, it may be feasible to implement VIA which offers immediate results, facilitating single point‐of‐care screen‐and‐treat approaches and reducing losses to follow‐up. 3 Moreover, eligibility for ablative treatment in such scenarios still relies on VIA examination, even when other triage techniques are used.
Studies have shown highly variable VIA performance in both primary screening and triage of HPV‐positive women. 4 , 5 , 6 Nevertheless, some studies have reported sensitivity for detection of high‐grade cervical lesions above 80% in triage settings, although recent analyses included in the WHO guidelines reported pooled sensitivity of 68% (95% CI 57‐78). 3 More evidence on performance of VIA for triage of women with a positive HPV screening test in different settings worldwide is required.
We present results of VIA as triage of HPV‐positive women aged 30‐64 years for detection of precancerous cervical lesions performed by health providers with different levels of expertise and training within the ESTAMPA study. Additionally, we evaluated the VIA examiners' ability to assess eligibility for ablative treatment.
2. MATERIALS AND METHODS
2.1. Study design
ESTAMPA is a multicentric study of cervical cancer screening with HPV testing in 12 study centres across Latin America, as previously described. 7 Briefly, women aged 30‐64 years were screened with HPV testing and cytology and referred to colposcopy (with biopsies from observed acetowhite lesions) if either test was positive. In 5 study centres, VIA was performed ahead of colposcopy. Women with cervical intraepithelial neoplasia grade 2 or worse (CIN2+) were usually treated with large loop excision of the transformation zone (LLETZ). Those without cervical disease (<CIN2) were recalled 18 months after enrolment for repeat HPV testing; HPV‐negative women were considered free‐of‐disease, and HPV positives were referred to colposcopy with biopsy and treatment as needed. Here, we present results of VIA performed at initial colposcopy by study‐trained examiners in 5 study centres among HPV screen‐positive women between December 2012 and December 2021.
2.2. Screening tests at enrolment
Cervical cells were collected at enrolment using a Cervex‐Brush (Rovers Medical Devices, The Netherlands) for conventional cytology and HPV testing. After smearing the material onto the glass slide, the brush was washed in a vial with ThinPrep PreservCyt medium (Hologic Inc., Marlborough, MA, USA) for HPV testing and additional tests, except for the first 872 participants from Colombia (recruited between December 2012 and May 2013) whose samples were stored on Digene Specimen Transport Medium (STM). HPV was detected using Digene HC2© High‐Risk HPV DNA Test (QIAGEN, Germantown USA) and/or COBAS 4800 HPV Test (Roche Diagnostics, Mannheim, Germany) following manufacturers' instructions. Cytology was considered abnormal at the threshold of ASC‐US or worse according to the 2014 Bethesda classification. 8
2.3. Visual inspection with acetic acid and colposcopy
At the initial colposcopy, ahead of the procedure, and without the presence of the colposcopist, a study‐trained examiner, following study SOPs, applied 5% acetic acid using a cotton swab and after ~1 minute inspected the cervix to identify any acetowhite lesions. Following IARC's definition, VIA was considered negative if nonsignificant or no acetowhite lesions were observed in the transformation zone (TZ); positive if any distinct, well‐defined, dense (opaque, dull‐ or oyster‐white) acetowhite lesions were observed close to or abutting the squamocolumnar junction (SCJ) in the TZ or close to the external os if the SCJ was not visible; or suspicion of cancer if any lesion suggestive of cancer was observed. 9 , 10 If the TZ was not observed or if other conditions prevented examination of the cervix (eg, bleeding or inflammation), VIA was considered not evaluable. Following WHO guidelines, eligibility for ablative treatment was assessed by VIA examiners for women with positive VIA (without suspicion of cancer) based on whether the TZ was fully visible and the whole lesion was visible, did not extend into the endocervix or did not cover more than 75% of the ectocervix, or whether the lesion was TZ type 2 and the upper limit of the TZ was reachable. 11 , 12 VIA results and eligibility for ablative treatment were recorded on a standardised form. Following VIA examination, colposcopy was performed blindly to VIA results according to ESTAMPA protocols. The colposcopic impression (defined as negative, positive minor or major, or suspicion of cancer) and other characteristics such as the TZ type were recorded on standardised forms. Where acetowhite lesions were present on colposcopy, collection of at least two biopsies was mandatory. Further details of the colposcopy procedures are described elsewhere (Valls J et al, 2022). Whenever cancer was suspected on VIA, the examiner discussed results with the colposcopist without modifying recorded VIA results. Women with nonevaluable VIA were considered invalid for analyses.
2.4. VIA examiners and training
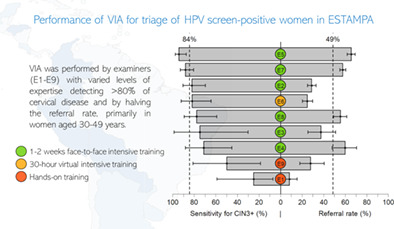
Eight providers across the 5 study centres performed 98% of the exams: two general practitioners (GP), five nurses (two specialised in research), and one professional midwife. Six received intensive face‐to‐face theoretical‐practical training for 1‐2 weeks at the National Cancer Institute of Peru (INEN) and one attended an intensive virtual course before starting the study. The remaining examiner was only hands‐on trained by a local colposcopist. Whenever possible, initial training was followed by evaluation of at least 100 VIA images over 6 months. The research nurses became VIA trainers in their country. At the start of recruitment, additional hands‐on refresher training was offered to all VIA providers by the research nurses or ESTAMPA gynaecologists with VIA expertise. The face‐to‐face training was a certified course offered by the INEN to ESTAMPA examiners; the practical component included doing VIA in women with cervical cancer and in women with and without suspicion of high‐grade cervical lesions attending the INEN. The virtual training was a 30‐hour certified free‐of‐charge online course offered by the National Cancer Institute of Colombia (https://campusvirtual.cancer.gov.co/).
2.5. Gold‐standard outcomes
The main outcome was histologically confirmed cervical intraepithelial neoplasia grade 3 or worse (CIN3+), diagnosed by local pathologists from biopsy or LLETZ specimens taken after the first colposcopy (triggered by enrolment screening results) or after the second colposcopy (triggered by HPV results at the 18‐month visit). CIN2+ was a secondary outcome. Women without cervical disease (<CIN2) included CIN1, negative histology, or negative colposcopy when cervical tissues were not collected. When necessary, cellular blocks from endocervical samples were processed as histology and considered part of the disease definition. Participants who did not complete or had not yet completed the 18‐month visit were considered free‐of‐disease.
2.6. Statistical analysis
Clinical characteristics (eg, TZ type from colposcopy, colposcopic impression, histological diagnosis, and ablative treatment eligibility based on VIA) were cross‐tabulated by VIA results and participants' age. Additionally, the TZ type was described for VIA‐positive women according to treatment eligibility and age. VIA performance indicators (sensitivity, specificity, positive predictive value [PPV], and the complement of the negative predictive value [cNPV]) for detection of CIN3+ (or CIN2+), and VIA positivity were estimated with 95% confidence intervals (95% CI) overall, by age (<50 or 50+ years), and by VIA examiner. VIA positivity included VIA positive results and suspicion of cancer, and CIN2 cases were excluded from estimates of predictive values for CIN3+ and specificity. The correlation between VIA and colposcopic impression was summarised using Kendall's correlation coefficients overall and by pairs of VIA/Colposcopy examiners. VIA performance indicators were estimated and stratified by VIA examiners with Kendall's coefficients above or below the overall correlation. Finally, the variability of sensitivity, specificity, VIA positivity, and eligibility for ablative treatment was graphically assessed by VIA examiner using scatter and funnel plots (meta library of R). Differences in proportions and trends were assessed, as appropriate, using chi‐squared tests (either Pearson's or likelihood chi‐squared from logistic regressions). All statistical analyses were performed using R version 4.1.2.
3. RESULTS
3.1. Study population
Included participants are presented in Figure 1. Between December 2012 and December 2021, 25 628 participants were recruited in the five study centres where VIA was performed. Fourteen percent (n = 3612) tested positive for HPV, of whom 3204 (89%) received VIA and colposcopy. Sixty‐two (1.9%) had nonevaluable VIA and were excluded from analyses; based on colposcopy, 56 of them had TZ type 3 with 7 (12.5%) being younger than 50 years. Data from 3142 participants were analysed. Nearly half were from the study centre in Colombia (n = 1427). In total, 229 CIN2 (7.3%) and 328 CIN3+ cases (10.4%) were detected either at enrolment (n = 468 CIN2+) or at 18‐month visit (n = 89 CIN2+). Nineteen percent of women without cervical disease at enrolment (n = 512) did not attend (151/512) or have not yet attended/completed (361/512) the 18‐month visit and were considered free‐of‐disease for analyses. Participants' characteristics between those who attended and have not attended (or not yet attended) the 18‐month visit only differed by study centre; other characteristics that may influence the risk of cervical disease were similar (Supplementary Table 1).
FIGURE 1.
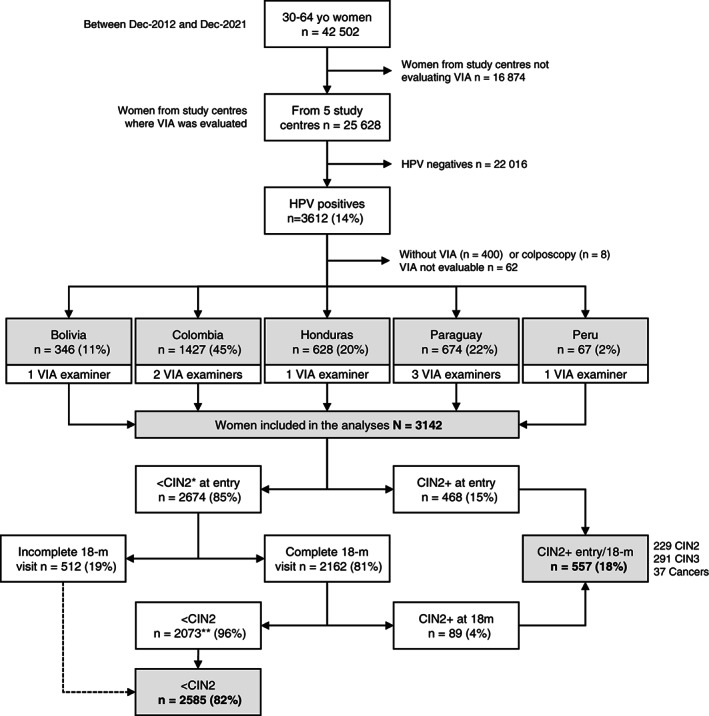
Study population. ESTAMPA included participants recruited between December 2012 and December 2021. Ninety‐eight percent of VIAs were performed by 8 examiners (1 GP in Bolivia and Honduras each, 1 midwife in Peru, and 5 nurses elsewhere); examiners who performed less than 50 VIAs were grouped into a single category for analyses. In total 3142 women were included in the analyses of whom 557 (18%) had CIN2+ and 2585 (82%) were considered without cervical disease (<CIN2). Dashed line: participants who did not complete or had not yet completed the 18‐month visit were considered <CIN2. *Includes 19 women with pending diagnoses at entry. **Includes 23 women treated at entry and therefore not eligible for the 18‐month visit
Among analysed participants, the mean (±SD) age was 43 (±9) years (25% being women over 50 years), nearly 15% had ≥ASC‐US (11% ASC‐US/LSIL, 4.5% HSIL+), about two‐fifths (39%) had only elementary education, 39% had 4 or more children, and 87% had been screened with cytology at least once within the last five years.
3.2. Clinical characteristics according to VIA results
Table 1 presents age‐stratified VIA results by type of transformation zone (TZ), colposcopic impression, histological diagnosis, and VIA‐based eligibility for ablative treatment. VIA positivity was higher in women younger than 50 years (52.6%) compared to older (33.8%), and steadily decreased from 58.3% to 29.7% in women aged 30‐34 and 60‐64 years, respectively (p‐trend<0.001, Figure 2). As expected, the proportion of women with TZ type 1 (fully visible) was higher for women below 50 years (68%) than for older women (19.7%). Among VIA positives, 80.5% of young women had a TZ type 1 compared to 29.7% of women over 50 years. The proportion of women with positive colposcopic impression (including suspicion of cancer) was higher in those under 50 years (61.9%) than in older women (46.3%). Regardless of age, most women with positive VIA also had a positive colposcopic impression (92.9% and 91.1% among women younger and older than 50 years, respectively), and similarly, although, to a lesser extent, most women with negative VIA were also negative on colposcopy (73.2% and 78.3%, respectively). Almost 20% of younger and 13% of older women had CIN2+. VIA missed three cancers, two of them with TZ type 3 or 2 (Table 1, footnote). Among women with positive VIA, about three‐quarters were considered eligible for ablative treatment by VIA examiners regardless of age.
TABLE 1.
Clinical characteristics according to VIA results overall and by age of HPV positive women within the ESTAMPA study
| Characteristics | VIA results for women <50 years | VIA results for women 50+ years | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | Negative | Positive | Ca susp | n | (%) | Negative | Positive | Ca susp | |||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||||||||
| Total [row %] | 2346 | [100] | 1099 | [46.8] | 1233 | [52.6] | 14 | [0.6] | 796 | [100] | 516 | [64.8] | 269 | [33.8] | 11 | [1.4] | ||||
| TZ type based on colposcopy | ||||||||||||||||||||
| Type 1 (TZ fully visible) | 1596 | (68.0) | 593 | (54.0) | 993 | (80.5) | 10 | (71.4) | 157 | (19.7) | 73 | (14.1) | 80 | (29.7) | 4 | (36.4) | ||||
| Type 2 (TZ partially visible) | 437 | (18.6) | 256 | (23.3) | 180 | (14.6) | 1 | (7.1) | 199 | (25.0) | 107 | (20.7) | 89 | (33.1) | 3 | (27.3) | ||||
| Type 3 (TZ not visible) | 313 | (13.3) | 250 | (22.7) | 60 | (4.9) | 3 | (21.4) | 440 | (55.3) | 336 | (65.1) | 100 | (37.2) | 4 | (36.4) | ||||
| Colposcopic impression | ||||||||||||||||||||
| Negative | 893 | (38.1) | 805 | (73.2) | 84 | (6.8) | 4 | (28.6) | 428 | (53.8) | 404 | (78.3) | 24 | (8.9) | 0 | (0.0) | ||||
| Positive minor | 1169 | (49.8) | 277 | (25.2) | 888 | (72.0) | 4 | (28.6) | 293 | (36.8) | 104 | (20.2) | 186 | (69.1) | 3 | (27.3) | ||||
| Positive major | 276 | (11.8) | 16 | (1.5) | 257 | (20.8) | 3 | (21.4) | 69 | (8.7) | 8 | (1.6) | 59 | (21.9) | 2 | (18.2) | ||||
| Cancer suspicion | 8 | (0.3) | 1 | (0.1) | 4 | (0.3) | 3 | (21.4) | 6 | (0.8) | 0 | (0.0) | 0 | (0.0) | 6 | (54.5) | ||||
| Histological diagnosis | ||||||||||||||||||||
| CIN1 or less | 1889 | (80.5) | 1026 | (93.4) | 853 | (69.2) | 10 | (71.4) | 696 | (87.4) | 489 | (94.8) | 205 | (76.2) | 2 | (18.2) | ||||
| CIN2 | 188 | (8.0) | 35 | (3.2) | 153 | (12.4) | 0 | (0.0) | 41 | (5.2) | 14 | (2.7) | 26 | (9.7) | 1 | (9.1) | ||||
| CIN3 | 244 | (10.4) | 36 | (3.3) | 207 | (16.8) | 1 | (7.1) | 47 | (5.9) | 12 | (2.3) | 33 | (12.3) | 2 | (18.2) | ||||
| Cancer a | 25 | (1.1) | 2 | (0.2) | 20 | (1.6) | 3 | (21.4) | 12 | (1.5) | 1 | (0.2) | 5 | (1.9) | 6 | (54.5) | ||||
| Ablative treatment | ||||||||||||||||||||
| Ineligible | – | – | 309 | (25.1) | – | – | – | 74 | (27.5) | – | ||||||||||
| Eligible | – | – | 924 | (74.9) | – | – | – | 195 | (72.5) | – | ||||||||||
Three cases diagnosed with cancer were missed by VIA. Case 1: detected at enrolment, 49 years, inadequate cytology, suspicion of cancer on colposcopy, TZ type 1, confirmed as invasive squamous carcinoma stage IIB. Case 2: detected at enrolment, 52 years, high‐grade on cytology and colposcopy, TZ type 3, confirmed as invasive adenocarcinoma stage IB1. Case 3: detected at 18‐month visit, 48 years, TZ type 2 and positive major at 18‐month colposcopy, confirmed as invasive squamous carcinoma stage IIB. Ca susp: cancer suspicion on VIA. CIN: cervical intraepithelial neoplasia. TZ: transformation zone of the cervix. –: Not applicable. Column percentages are shown unless otherwise noted.
FIGURE 2.
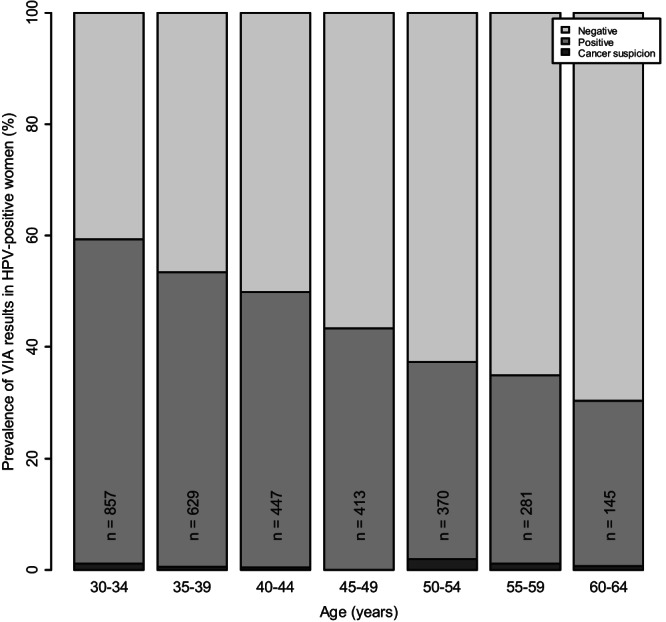
VIA results according to age in HPV‐positive women within the ESTAMPA study
Figure 3 presents the TZ type based on colposcopy among VIA positives by age and eligibility for ablative treatment status based on VIA. As expected, most women <50 years who were considered eligible for ablative treatment had TZ type 1 (85%), compared to only 33% of older women. However, among women <50 years considered ineligible, most (67%) had TZ type 1.
FIGURE 3.
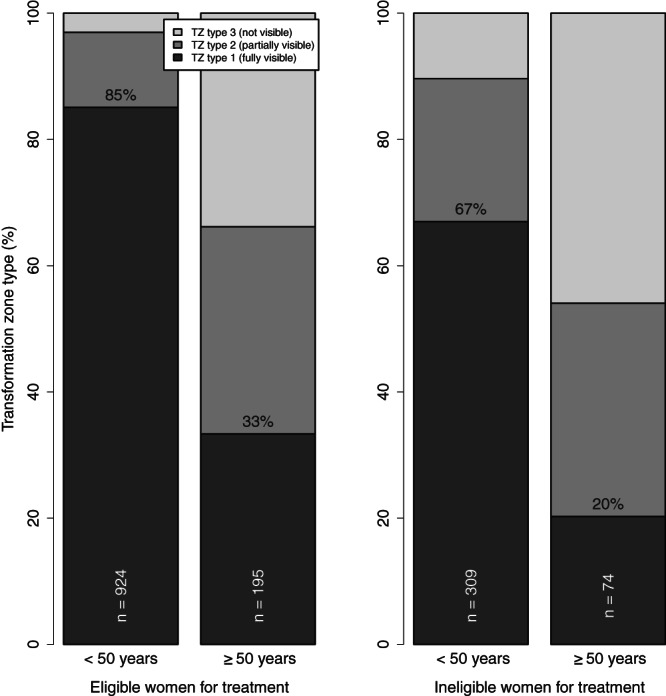
Transformation zone (TZ) type by age and eligibility for ablative treatment among women with positive VIA
3.3. Performance of VIA for triage of HPV screen‐positive women
The performance of VIA is presented in Table 2. VIA detected more than 80% of precancerous lesions (277 of 328), particularly under 50 years, although differences by age were only significant for CIN2+ (sensitivity for CIN2+ 84% in young women vs 73% in older women, P = 0.014). Specificity was higher for women over 50 years (70.3%) compared to younger women (54.3%, P‐value<0.001). The risk of CIN3+ among women with positive VIA (PPV) was about 6‐fold the risk of those with negative VIA results (cNPV) and differences by age were not significant (P‐values 0.340 and 0.385, respectively). The risk of CIN3+ among VIA positives doubled the overall risk of CIN3+ in HPV positives (20.6% vs 10.4%). VIA almost halved the referral rate in women younger than 50 years (53.2%, 95% CI 51.1‐55.2) and reduced the referral by almost two‐thirds in older women (35.2%, 95% CI 31.9‐38.6). VIA performed by examiners whose VIA results were highly correlated with the colposcopy impression (Supplementary Figure 1), yielded better specificity and referral results while maintaining high sensitivity similar to the overall estimate (Supplementary Table 2).
TABLE 2.
Overall and age‐stratified performance of VIA for detection of precancerous cervical lesions in HPV positive women within the ESTAMPA study
| Performance indicators | All women | Women <50 years | Women 50+ years | P a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | (95% CI) | n/N | % | (95% CI) | n/N | % | (95% CI) | ||
| For CIN3+ | ||||||||||
| Sensitivity | 277/328 | 84.5 | (80.1‐88.0) | 231/269 | 85.9 | (81.2‐89.5) | 46/59 | 78.0 | (65.9‐86.6) | .187 |
| PPV | 277/1347 | 20.6 | (18.5‐22.8) | 231/1094 | 21.1 | (18.8‐23.6) | 46/253 | 18.2 | (13.9‐23.4) | .340 |
| cNPV | 51/1566 | 3.3 | (2.49‐4.26) | 38/1064 | 3.6 | (2.61‐4.86) | 13/502 | 2.6 | (1.52‐4.38) | .385 |
| For CIN2+ | ||||||||||
| Sensitivity | 457/557 | 82.0 | (78.6‐85.0) | 384/457 | 84.0 | (80.4‐87.1) | 73/100 | 73.0 | (63.6‐80.7) | .014 |
| PPV | 457/1527 | 29.9 | (27.7‐32.3) | 384/1247 | 30.8 | (28.3‐33.4) | 73/280 | 26.1 | (21.3‐31.5) | .137 |
| cNPV | 100/1615 | 6.2 | (5.12‐7.47) | 73/1099 | 6.6 | (5.32‐8.27) | 27/516 | 5.2 | (3.62‐7.51) | .324 |
| Other indicators | ||||||||||
| Specificity for <CIN2 | 1515/2585 | 58.6 | (56.7‐60.5) | 1026/1889 | 54.3 | (52.1‐56.5) | 489/696 | 70.3 | (66.8‐73.5) | <.001 |
| Positivity of VIA | 1527/3142 | 48.6 | (46.9‐50.3) | 1247/2346 | 53.2 | (51.1‐55.2) | 280/796 | 35.2 | (31.9‐38.6) | <.001 |
Chi‐squared test to compare performance measures between women younger than 50 vs older than 50 years. cNPV: complement of the negative predictive value (%). PPV: positive predictive value (%). CIN2 cases are excluded from CIN3+ estimates. Numerators (n) correspond to true positives (TP) for sensitivity and PPV, true negatives (TN) for specificity, false negatives (FN) for cNPV, and TP + false positives (FP) for VIA positivity. Denominators (N) correspond to TP + FN for sensitivity, TN + FP for specificity, TP + FP for PPV, TN + FN for cNPV, and TP + TN + FP + FN for VIA positivity.
3.4. Performance of VIA by examiner
High variability of both sensitivity and specificity was observed (Figure 4A). The sensitivity ranged between 25.0% and 95.4%, and the specificity between 44.9% and 94.4%. Although sensitivity was higher for examiners with a larger number of VIAs, most examiners did not differ from the overall sensitivity, while many of them significantly differed from the overall specificity (Supplementary Figure 2A1,A1). High variability of the proportion of eligibility for ablative treatment and VIA positivity was also observed (Figure 4B) but this was not influenced by the number of VIAs performed by the examiner. Eligibility for ablative treatment ranged from 0% to 98.7%, and VIA positivity from 8.2% to 65.3%. Most examiners significantly differed from the overall estimates (Supplementary Figure 2B1,B2).
FIGURE 4.

Sensitivity and specificity of VIA for detection of precancerous cervical lesions (A), and eligibility for ablative treatment of VIA positives and VIA positivity (B) among HPV positive women by VIA examiner. E9 groups examiners who performed less than 50 VIAs. Dashed lines: overall sensitivity (84.5%) and specificity (58.6%), and, overall eligibility rate (74.5%) and VIA positivity (48.6%), respectively. Sensitivity range: 25.0%‐95.4%. Specificity range: 44.9%‐94.4%. Eligibility rate range: 0%‐98.7%. VIA positivity range: 8.2%‐65.3%. Circles' sizes are proportional to the number of VIAs performed by examiner.
4. DISCUSSION
We evaluated VIA performance as triage of HPV screen‐positive women in several settings across Latin America. VIA detected about 85% of high‐grade cervical lesions, particularly among women under 50 years, and correctly identified almost 60% of women without lesions, reducing referral or treatment to nearly 50% of HPV‐positive women. Although sensitivity was higher under the age 50 years, it remained relatively high for older women (78%). Specificity for <CIN2 was higher and the referral rate was lower in older women. Despite the encouraging results, VIA missed three (including one with HSIL+ cytology) of 37 cancers and the variability of VIA was high, with sensitivities for CIN3+ ranging from 25% to 95%, and specificities from 45% to 94% between VIA examiners. Additionally, VIA positivity and eligibility assessment for ablative treatment (important for the triage of HPV‐positive women, particularly in screen‐and‐treat scenarios), also varied highly between examiners.
Several studies have evidenced high variability of VIA to detect high‐grade cervical lesions with sensitivities ranging between 17%‐96% in primary screening, 4 , 6 , 13 , 14 , 15 , 16 , 17 and between 25%–92% in HPV‐positive women. 13 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Only five studies in HPV‐positive women, characterised by including intensive training, report sensitivities ≥80%. 20 , 23 , 24 , 27 , 30 VIA positivity, which is a useful indicator in triage scenarios, has also shown to be highly variable. 5 It is worth noting that in the context of triage, knowledge of HPV status may increase the positivity rate of observer‐depended techniques, as reported for cytology, increasing the sensitivity. 31 , 32 However, the variability of VIA is so high that the range of sensitivity in primary screening overlaps with the range in triage. Although women's characteristics may explain differences in VIA results, 33 , 34 the experience level of VIA examiners, training and supervision, and quality assurance may largely explain the variability of this visual technique. 6 , 27 , 35 , 36 In ESTAMPA, the majority of VIAs (98%) were performed by eight examiners, and despite the lower number, performance of VIA varied widely between them. A research nurse who was also a VIA trainer, accounted for 37% (n = 1177) of all VIAs, becoming the most experienced examiner. Three other examiners, whose results were highly correlated with the colposcopic impression (Supplementary Figure 1), also performed a large number of VIAs (n = 1447 in total). These four examiners, who received initial intensive training, represented 83.5% (2624/3142) of all VIAs evaluated and therefore, heavily influenced the overall high sensitivity (Figure 4A). Conversely, the examiner with the lowest sensitivity (Figure 4A) performed 3% of VIAs (98/3142) and was the one who only received hands‐on training (ie, the least experienced examiner). A plausible explanation of the high performance of VIA in our study could be that expertise gained through intensive training, performing many exams, and continuous interaction with colposcopists may improve VIA examiner's skills and, as a result, VIA performance. As observed by others, 27 our results suggest that adopting proficiency schemes based on training and supervision is desirable to improve VIA. Consequently, alternatives such as computer tools based on remote support from external experts and/or artificial intelligence algorithms (AI) that can offer permanent supervision and training 30 , 37 , 38 , 39 , 40 may be useful. The use of AI algorithms incorporated to portable devices such as smartphones will represent a more objective assessment of the presence or not of cervical precancerous lesions contributing to reduction of variability attributed to VIA examiners. 41 , 42
VIA continues to be used to assess eligibility for ablative treatment, which is particularly relevant in screen‐and‐treat approaches. In ESTAMPA, there was discordant evaluation on the TZ between VIA and colposcopy. According to VIA examiners, about 75% of women with positive VIA were eligible for ablative treatment, even among women older than 50 years (Table 1). However, based on colposcopy, while the TZ was fully visible (ie, TZ type 1) for about 80% of young women with positive VIA, only 30% of older women had TZ type 1. Moreover, for younger women, the proportion of TZ type 1 was high even among those who were considered not eligible for ablative treatment (Figure 3). Conversely, the proportion of TZ type 1 among older women was low, particularly for those who were considered eligible for ablative treatment (Figure 3). About a third of ineligible women with TZ type 1 had acetowhite lesions extending into the endocervix or covered more than 75% of the ectocervix (81 of 222, data not shown); thus, two‐thirds could have benefited from ablative treatment as they appeared to meet eligibility criteria. These results not only show that the lack of training to observe the TZ by VIA providers leave a proportion of young women who, despite having a fully visible TZ, would not benefit from ablative treatment since they were considered ineligible on VIA (67%), but also confirm that ablative treatment would have minimal benefit to women over 50 years even if they are considered eligible on VIA. In our study, the inaccuracy of eligibility assessment could be attributed to the fact that the examiners' training might have been mainly focused on the detection of acetowhite lesions and that decision of treatment was not based on VIA examiners. These results still reflect the need to improve examiners' capability to adequately assess eligibility for ablative treatment. AI algorithms once validated may aid VIA providers to decide on who to treat, and eventually can change current screening practices.
ESTAMPA is the largest HPV‐based cervical screening study conducted in the Americas. 7 All HPV‐positive women with complete VIA and colposcopy at entry (>80%) from five study centres were included leading to adequate sample size. The large sample size allowed us to report results on CIN3+, the most reproducible cancer precursor, estimating overall performance indicators with precision levels lower than ±5%. In our analyses, we enhanced the outcomes by including high‐grade cervical lesions missed at enrolment but detected at a subsequent visit (18‐month visit), reducing the possibility of verification bias and preventing overestimation of the sensitivity. About half of those high‐grade lesions were negative on VIA at the first colposcopy visit (data not shown). Therefore, it seems that including a follow‐up visit after negative VIA results, as recommended by WHO guidelines, 3 is warranted. Another strength is to have included women over 50 years allowing us to determine and better understand the performance of VIA in a broader population. Additionally, ESTAMPA assured data quality by using standardised forms and extensive training of VIA and colposcopy providers which reinforces the validity of the findings.
The main limitation was the lack of independence of VIA examiners and colposcopists. However, the sensitivity for CIN3+ excluding the most experienced observer and those mostly correlated with colposcopy remained relatively high (66.1%, 95% CI 53.4‐76.9). As discussed above, this highlights the potential for VIA to be improved by adopting proficiency schemes based on training and increased expert interaction and supervision. Also, adding more objective tests such as partial HPV16/18 genotyping and/or HPV viral load and/or computer‐based aids may further improve the methodology. 13 , 30 , 43 Our results are based on local diagnoses. Among analysed participants, 2248 had results based on histology, of which 68% have been reviewed by external pathologists using the LAST nomenclature; 44 , 45 355 high‐grade cervical lesions (CIN2 p16‐positive, CIN3, or cancer) have been confirmed. Preliminary sensitivity and specificity estimates using reviewed outcomes provide similar results to those obtained using CIN3+ diagnosed by local pathologists (data not shown). Finally, 19% of women did not attend/complete the 18‐month visit and were assumed free‐of‐disease; although the risk of cervical disease at the 18‐month visit is relatively low (4.1%; 89/2162), this approach could have slightly underestimated disease prevalence. If we had alternatively excluded nonattendees/incomplete from our analyses, this would have likely overestimated the prevalence of the disease and therefore the predictive values, as well as biasing the referral rate. Rerunning analyses with these women excluded yielded similar specificity (57.1%) although significantly higher predictive values (PPV for CIN3+ 23.7%) and VIA positivity (51.2%) (data not shown).
In conclusion, within ESTAMPA, VIA detected most precancerous cervical lesions present in HPV‐positive women with a substantial reduction in referral or treatment rate, particularly in women younger than 50 years. However, as reported by others, results varied highly by VIA examiner. Our results provide evidence that VIA performance improves with the number of examinations and constant training and supervision of VIA examiners, which in our study was present through intensive initial training for most examiners and permanent interaction with colposcopists. However, VIA as a triage method should be recommended with caution. Its use should be prioritised for women under 50 years, who would achieve the greatest benefit from ablative treatment, and in settings where more reproducible triage options are not affordable and where adoption of VIA proficiency schemes is feasible.
AUTHOR CONTRIBUTIONS
The work reported in the paper has been performed by the authors, unless clearly specified in the text. Maribel Almonte and Rolando Herrero conceived the ESTAMPA study and are the principal investigators responsible for its overall conduction. Gino Venegas, Carolina Terán, Annabelle Ferrera, Laura Mendoza, Elena Kasamatsu, Raúl Murillo and Carolina Wiesner are the local principal investigators responsible for recruitment, clinical management, and data collection. Maribel Almonte, Rolando Herrero, Silvana Luciani and Nathalie Broutet contributed to funding acquisition and local capacity building. Manuel Álvarez‐Larraondo, Gino Venegas and Sandra Martínez coordinated VIA training activities. Yuli Salgado, Sandra Martínez, Griselda Raquel Villalba, Maria Luisa Amarilla, Brenda Salgado, Bettsy Flores and Yenny Bellido‐Fuentes performed VIA. Oscar Lora, Gonzalo Virreira‐Prout, Jacqueline Figueroa, Elmer Turcios, Ana María Soilán, Marina Ortega, Marcela Celis, Mauricio González and Gino Venegas performed colposcopies. Armando Baena and Joan Valls were responsible for data curation. Armando Baena performed the statistical analysis that was verified by David Mesher. Armando Baena wrote the manuscript with support from David Mesher, Rolando Herrero and Maribel Almonte. Rolando Herrero, Nathalie Broutet and Silvana Luciani contributed to finalisation of the manuscript. All authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
ETHICS STATEMENT
The ESTAMPA protocol was approved by the Ethics Committee of the International Agency for Research on Cancer of the World Health Organization (IARC/WHO) (IEC Project 12‐27‐A7), the Pan American Health Organization (PAHO) Ethical Committee, and Ethical Committees at study centres. 7 The study is considered of minimal risk as procedures are standard clinical practice. All subjects signed informed consent. Our study is registered with clinicaltrials.gov (NCT01881659).
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
This project acknowledges the financial contribution from IARC/WHO; the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP/WHO), a cosponsored programme executed by the World Health Organization (WHO); the Pan American Health Organization (PAHO); the National Cancer Institute (NCI) UH2/3 CA202730; the NCI Center for Global Health; the National Council for Science and Technology (CONACYT) from Paraguay; the National Cancer Institute of Colombia; and all local collaborative institutions. The funders had no role in the design of the study, data collection, analysis, interpretation, writing, or the decision to publish the manuscript.
Baena A, Mesher D, Salgado Y, et al. Performance of visual inspection of the cervix with acetic acid (VIA) for triage of HPV screen‐positive women: results from the ESTAMPA study. Int J Cancer. 2023;152(8):1581‐1592. doi: 10.1002/ijc.34384
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Funding information International Agency for Research on Cancer; Instituto Nacional de Cancerología de Colombia; National Cancer Institute, Grant/Award Number: UH2/3 CA202730; National Cancer Institute (NCI) Center for Global Health; National Council for Science and Technology (CONACYT) from Paraguay; Pan American Health Organization (PAHO); UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP/WHO)
DATA AVAILABILITY STATEMENT
Anonymised individual participant data or aggregate data would be available from the corresponding author upon reasonable request, requiring signing a specific contract with the approval of the ESTAMPA principal investigators and local investigators of study centres involved.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneve: WHO; 2020. [Google Scholar]
- 3. WHO Guideline for Screening and Treatment of Cervical Pre‐Cancer Lesions for Cervical Cancer Prevention , 2nd ed.; 2021. [PubMed]
- 4. Catarino R, Schafer S, Vassilakos P, Petignat P, Arbyn M. Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: a meta‐analysis. BJOG. 2018;125:545‐553. [DOI] [PubMed] [Google Scholar]
- 5. Almonte M, Ferreccio C, Luciani S, et al. Visual inspection after acetic acid (VIA) is highly heterogeneous in primary cervical screening in Amazonian Peru. PLoS One. 2015;10:e0115355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fokom‐Domgue J, Combescure C, Fokom‐Defo V, et al. Performance of alternative strategies for primary cervical cancer screening in sub‐Saharan Africa: systematic review and meta‐analysis of diagnostic test accuracy studies. BMJ. 2015;351:h3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almonte M, Murillo R, Sanchez GI, et al. Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol. BMJ Open. 2020;10:e035796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123:271‐281. [DOI] [PubMed] [Google Scholar]
- 9. Sankaranarayanan R, Wesley R. A Practical Manual on Visual Screening for Cervical Neoplasia (IARC Technical Publication No. 41). Lyon, France: International Agency for Research on Cancer; 2003. [Google Scholar]
- 10. Mittal S, Basu P, Lucas E. Atlas of Visual Inspection of the Cervix with Acetic Acid for Screening, Triage, and Assessment for Treatment: IARC Cancer Base No. 16 [Internet]. Lyon, France: International Agency for Research on Cancer; 2020. [Google Scholar]
- 11. Santesso N, Schunemann H, Blumenthal P, et al. World Health Organization guidelines: use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2012;118:97‐102. [DOI] [PubMed] [Google Scholar]
- 12. WHO Guidelines for the Use of Thermal Ablation for Cervical Pre‐Cancer Lesions ; 2019. [PubMed]
- 13. Wang MZ, Feng RM, Wang S, et al. Clinical performance of human papillomavirus testing and visual inspection with acetic acid in primary, combination, and sequential cervical cancer screening in China. Sex Transm Dis. 2019;46:540‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeffries A, Beck‐Sague CM, Marroquin‐Garcia AB, et al. Cervical visual inspection with acetic acid (VIA) and oncogenic human papillomavirus screening in rural indigenous Guatemalan women: time to rethink VIA. Int J Environ Res Public Health. 2021;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murillo R, Luna J, Gamboa O, et al. Cervical cancer screening with naked‐eye visual inspection in Colombia. Int J Gynaecol Obstet. 2010;109:230‐234. [DOI] [PubMed] [Google Scholar]
- 16. Sankaranarayanan R, Nessa A, Esmy PO, Dangou JM. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26:221‐232. [DOI] [PubMed] [Google Scholar]
- 17. Adsul P, Manjunath N, Srinivas V, Arun A, Madhivanan P. Implementing community‐based cervical cancer screening programs using visual inspection with acetic acid in India: a systematic review. Cancer Epidemiol. 2017;49:161‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toliman PJ, Kaldor JM, Badman SG, et al. Performance of clinical screening algorithms comprising point‐of‐care HPV‐DNA testing using self‐collected vaginal specimens, and visual inspection of the cervix with acetic acid, for the detection of underlying high‐grade squamous intraepithelial lesions in Papua New Guinea. Papillomavirus Res. 2018;6:70‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basu P, Mittal S, Banerjee D, et al. Diagnostic accuracy of VIA and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. Int J Cancer. 2015;137:859‐867. [DOI] [PubMed] [Google Scholar]
- 20. Kunckler M, Schumacher F, Kenfack B, et al. Cervical cancer screening in a low‐resource setting: a pilot study on an HPV‐based screen‐and‐treat approach. Cancer Med. 2017;6:1752‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tebeu PM, Fokom‐Domgue J, Crofts V, et al. Effectiveness of a two‐stage strategy with HPV testing followed by visual inspection with acetic acid for cervical cancer screening in a low‐income setting. Int J Cancer. 2015;136:E743‐E750. [DOI] [PubMed] [Google Scholar]
- 22. Awolude OA, Oyerinde SO, Ayeni AO, Adewole IF. Human papillomavirus‐based cervical precancer screening with visual inspection with acetic acid triage to achieve same‐day treatments among women living with human immunodeficiency virus infection: test‐of‐concept study in Ibadan, Nigeria. Pan Afr Med J. 2021;40:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muwonge R, Wesley RS, Nene BM, et al. Evaluation of cytology and visual triage of human papillomavirus‐positive women in cervical cancer prevention in India. Int J Cancer. 2014;134:2902‐2909. [DOI] [PubMed] [Google Scholar]
- 24. Parra SG, Lopez‐Orellana LM, Molina Duque AR, et al. Cervical cancer prevention in El Salvador: a prospective evaluation of screening and triage strategies incorporating high‐resolution microendoscopy to detect cervical precancer. Int J Cancer. 2020;148:2571‐2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiao YL, Jeronimo J, Zhao FH, et al. Lower cost strategies for triage of human papillomavirus DNA‐positive women. Int J Cancer. 2014;134:2891‐2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petignat P, Kenfack B, Wisniak A, et al. ABCD criteria to improve visual inspection with acetic acid (VIA) triage in HPV‐positive women: a prospective study of diagnostic accuracy. BMJ Open. 2022;12:e052504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frund C, Kenfack B, Sormani J, et al. Training, supervision, and competence assessment of Cameroonian health care providers using HPV self‐sampling, triage by visual inspection, and treatment by thermal ablation in a single visit. Front Public Health. 2022;10:875177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang M, Hu S, Zhao S, et al. Accuracy of triage strategies for human papillomavirus DNA‐positive women in low‐resource settings: a cross‐sectional study in China. Chin J Cancer Res. 2017;29:496‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bigoni J, Gundar M, Tebeu PM, et al. Cervical cancer screening in sub‐Saharan Africa: a randomized trial of VIA versus cytology for triage of HPV‐positive women. Int J Cancer. 2015;137:127‐134. [DOI] [PubMed] [Google Scholar]
- 30. Dufeil E, Kenfack B, Tincho E, et al. Addition of digital VIA/VILI to conventional naked‐eye examination for triage of HPV‐positive women: a study conducted in a low‐resource setting. PLoS One. 2022;17:e0268015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benoy IH, Vanden Broeck D, Ruymbeke MJ, et al. Prior knowledge of HPV status improves detection of CIN2+ by cytology screening. Am J Obstet Gynecol. 2011;205(569):e1‐e7. [DOI] [PubMed] [Google Scholar]
- 32. Aitken CA, Holtzer‐Goor KM, Uyterlinde A, et al. The impact of knowledge of HPV positivity on cytology triage in primary high‐risk HPV screening. J Med Screen. 2019;26:221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raifu AO, El‐Zein M, Sangwa‐Lugoma G, et al. Determinants of cervical cancer screening accuracy for visual inspection with acetic acid (VIA) and Lugol's iodine (VILI) performed by nurse and physician. PLoS One. 2017;12:e0170631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castle PE, Qiao YL, Zhao FH, et al. Clinical determinants of a positive visual inspection after treatment with acetic acid for cervical cancer screening. BJOG. 2014;121:739‐746. [DOI] [PubMed] [Google Scholar]
- 35. WHO Technical Guidance and Specifications of Medical Devices for Screening and Treatment of Precancerous Lesions in the Prevention of Cervical Cancer ; 2020.
- 36. Parashari A, Singh V. Reasons for variation in sensitivity and specificity of visual inspection with acetic acid (VIA) for the detection of pre‐cancer and cancer lesions of uterine cervix. Asian Pac J Cancer Prev. 2013;14:7761‐7762. [DOI] [PubMed] [Google Scholar]
- 37. Yeates K, Erwin E, Mtema Z, et al. Smartphone‐enhanced training, QA, monitoring, and evaluation of a platform for secondary prevention of cervical cancer: opportunities and challenges to implementation in Tanzania. JCO Glob Oncol. 2020;6:1114‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desai KT, Befano B, Xue Z, et al. The development of “automated visual evaluation” for cervical cancer screening: the promise and challenges in adapting deep‐learning for clinical testing: interdisciplinary principles of automated visual evaluation in cervical screening. Int J Cancer. 2022;150:741‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahatgaonkar V, Uchale P, Oka G. Comparative study of smart scope(R) visual Screening test with naked eye visual screening and pap test. Asian Pac J Cancer Prev. 2020;21:3509‐3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Champin D, Ramirez‐Soto MC, Vargas‐Herrera J. Use of smartphones for the detection of uterine cervical cancer: a systematic review. Cancers (Basel). 2021;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;111:923‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xue Z, Novetsky AP, Einstein MH, et al. A demonstration of automated visual evaluation of cervical images taken with a smartphone camera. Int J Cancer. 2020;147:2416‐2423. [DOI] [PubMed] [Google Scholar]
- 43. Murillo R, Gamboa O, Hernandez G, et al. Accuracy of combined molecular and morphology‐based triage for HPV‐positive women in routine cervical cancer screening services from Colombia. Prev Med. 2021;153:106801. [DOI] [PubMed] [Google Scholar]
- 44. Darragh TM, Colgan TJ, Thomas Cox J, et al. The lower anogenital squamous terminology standardization project for HPV‐associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76‐115. [DOI] [PubMed] [Google Scholar]
- 45. Baena A, Rol ML, Barbier S, et al. Adjudication of final histological diagnosis of cervical biopsies using a three‐step standardised protocol: an inter‐observer reproducibility analysis within the ESTAMPA study (NCT01881659). Paper presented at: International Papillomavirus Conference Barcelona, Spain; 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Anonymised individual participant data or aggregate data would be available from the corresponding author upon reasonable request, requiring signing a specific contract with the approval of the ESTAMPA principal investigators and local investigators of study centres involved.


