Abstract
Objective
To assess efficacy, safety, pharmacokinetics, and immunogenicity of pegloticase plus methotrexate (MTX) versus pegloticase plus placebo cotreatment for uncontrolled gout in a randomized, placebo‐controlled, double‐blind trial.
Methods
This study included adults with uncontrolled gout, defined as serum urate ≥7 mg/dl, oral urate‐lowering therapy failure or intolerance, and presence of ongoing gout symptoms including ≥1 tophus, ≥2 flares in the past 12 months, or gouty arthritis. Key exclusion criteria included MTX contraindication, current immunosuppressant use, G6PDH deficiency, and estimated glomerular filtration rate <40 ml/minute/1.73 m2. Patients were randomized 2:1 to 52 weeks of pegloticase (8 mg biweekly) with either oral MTX (15 mg/week) or placebo. The primary end point was the proportion of treatment responders during month 6 (defined as serum urate <6 mg/dl for ≥80% of visits during weeks 20–24). Efficacy was evaluated in all randomized patients (intent‐to‐treat population), and safety was evaluated in all patients receiving ≥1 blinded MTX or placebo dose.
Results
A total of 152 patients were randomized, 100 to receive pegloticase plus MTX, 52 to receive pegloticase plus placebo. Significantly higher treatment response occurred during month 6 in the MTX group versus the placebo group (71.0% [71 of 100 patients] versus 38.5% [20 of 52 patients], respectively; between‐group difference 32.3% [95% confidence interval 16.3%, 48.3%]) (P < 0.0001 for between‐group difference). During the first 6 months of pegloticase plus MTX or pegloticase plus placebo treatment, 78 (81.3%) of 96 MTX patients versus 47 (95.9%) of 49 placebo patients experienced ≥1 adverse event (AE), most commonly gout flare (64 [66.7%] of 96 MTX patients and 34 [69.4%] of 49 placebo patients). Reports of AEs and serious AEs were comparable between groups, but the infusion reaction rate was considerably lower with MTX cotherapy (4.2% [4 of 96 MTX patients, including 1 patient who had anaphylaxis]) than with placebo cotherapy (30.6% [15 of 49 placebo patients, 0 who had anaphylaxis]) (P < 0.001). Antidrug antibody positivity was also lower in the MTX group.
Conclusion
MTX cotherapy markedly increased pegloticase response rate over placebo (71.0% versus 38.5%) during month 6 with no new safety signals. These findings verify higher treatment response rate, lower infusion reaction incidence, and lower immunogenicity when pegloticase is coadministered with MTX.
INTRODUCTION
Pegloticase is a US Food and Drug Administration–approved PEGylated uricase enzyme indicated for the treatment of uncontrolled gout. Pegloticase rapidly lowers serum urate levels by converting circulating urate to allantoin, a water‐soluble molecule readily cleared by the kidneys. Antidrug antibody development can limit pegloticase efficacy, resulting in premature treatment discontinuation. Clinical studies showed that 42% of patients maintain serum urate <6.0 mg/dl during months 3 and 6 of pegloticase monotherapy, with 26% having infusion reactions in the absence of a serum urate monitoring protocol (1). Loss of efficacy and infusion reactions have been attributed to antidrug antibody development that accelerates pegloticase clearance (2, 3, 4). Because pegloticase is often the last remaining therapy option for patients with uncontrolled gout, rheumatologists began to cotreat patients with immunomodulating therapies in an effort to attenuate antidrug antibody formation and increase urate‐lowering response durability, similar to approaches with other biologics for rheumatic diseases.
The literature supports using immunomodulating therapies with pegloticase (5). Data from clinical settings and open‐label trials show higher rates of sustained urate‐lowering in patients treated with pegloticase plus immunomodulation. A small randomized, placebo‐controlled trial examining pegloticase plus mycophenolate mofetil (n = 22) versus pegloticase plus placebo (n = 10) found a treatment response rate of 86% versus 40%, respectively, at week 12 (6). Additionally, an open‐label trial (n = 14) examining pegloticase plus oral methotrexate (MTX) showed a 6‐month response rate of 79% (7). A recent systematic literature review showed an overall pegloticase response rate of 83% with immunomodulation. Treatment response rates were higher than the established pegloticase monotherapy response rate (42%) for all immunomodulators examined: oral MTX (90%), subcutaneous MTX (78%), mycophenolate mofetil (86%), leflunomide (67%), and azathioprine (64%) (5). Furthermore, immunomodulator use with pegloticase has been recommended by consensus from gout experts across multiple subspecialities (8).
Despite these consistent and positive findings, there are no large, randomized, double‐blind, placebo‐controlled trials examining the effect of immunomodulation cotherapy on pegloticase response rates. The primary efficacy objective of the Methotrexate to Increase Response Rates in Patients with Uncontrolled GOut Receiving Pegloticase randomized controlled trial (MIRROR RCT) was to compare the treatment response rate of pegloticase plus oral MTX (15 mg/week) versus pegloticase plus placebo in an uncontrolled gout population. Here, we report 6‐month findings of the 52‐week trial, including treatment response rates, safety profile, pharmacokinetics, and antidrug antibody findings.
PATIENTS AND METHODS
Patients
The trial enrolled adults (≥18 years of age) with uncontrolled gout, defined as serum urate ≥7 mg/dl, gout refractory to conventional therapy (failure to normalize serum urate levels and/or intolerance to oral urate‐lowering therapy), and presence of ongoing gout symptoms (presence of ≥1 tophus, recurrent acute gout flares [≥2 flares in the 12 months prior to screening], and/or chronic gouty arthritis). Patients discontinued oral urate‐lowering therapy for ≥7 days prior to beginning the MTX tolerance period (6 weeks before the initial pegloticase infusion [week –6]). Key exclusion criteria included pregnancy; serious acute bacterial infection (unless treated and resolved 2 weeks prior to starting the MTX tolerance period); severe chronic or recurrent bacterial infection; current or chronic (≥3 months) use of a systemic immunosuppressive agent or glucocorticoid (<10 mg/day prednisone or equivalent dose allowed); HIV or hepatitis B/C positivity; advanced kidney disease (estimated glomerular filtration rate [eGFR] <40 ml/minute/1.73 m2); G6PDH deficiency at screening; serious cardiovascular disease or uncontrolled blood pressure (>160/100 mm Hg) prior to randomization; contraindication to or known intolerance of MTX; elevated liver transaminase levels; low albumin levels; low blood cell counts (white blood cells <4,000/μl, hematocrit <32%, platelets <75,000/μl); regular alcohol use (>3 beverages/week); and known intolerance to all gout flare prophylaxis. For full inclusion/exclusion criteria, see Supplementary Table 1 available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/art.42335.
Study design
This was a phase IV, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, efficacy and safety study of pegloticase plus MTX versus pegloticase plus placebo in adult patients with uncontrolled gout and was conducted at 42 sites in the US (Supplementary Table 2, https://onlinelibrary.wiley.com/doi/art.42335). This trial was registered at ClinicalTrials.gov (identifier: NCT03994731). All eligible patients underwent a 2‐week tolerance test of oral MTX (15 mg/week during 6 weeks to 4 weeks prior to initial pegloticase infusion [week –6 to week –4], and 1 mg/day oral folic acid also administered). Patients who were unable to tolerate oral MTX were considered screen failures. Patients who tolerated oral MTX and who continued to meet eligibility criteria were randomized and included in the intent‐to‐treat population. Medidata Randomization and Trial Supply Management (Medidata Solutions) was used to randomly assign patients 2:1 (stratified by presence of tophi) to receive pegloticase plus MTX or pegloticase plus placebo (Figure 1). Patients and investigators remained blinded to treatment assignment until after study completion.
Figure 1.
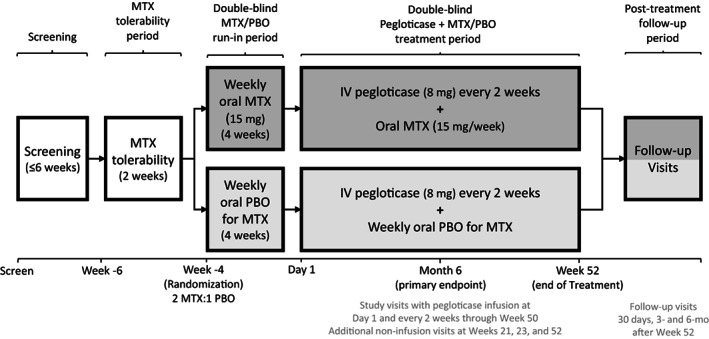
Overview of the study design for assessing pegloticase plus methotrexate (MTX) versus pegloticase plus placebo (PBO) cotreatment for uncontrolled gout in a randomized, placebo‐controlled, double‐blind trial. Following screening, patients underwent a 2‐week MTX tolerability period before randomization then entered a 4‐week run‐in period and 52‐week pegloticase plus MTX or PBO treatment period. Patients with pre‐infusion serum urate >6 mg/dl at 2 consecutive study visits beginning at week 2 discontinued study treatment and remained in the study on observation. Key efficacy and safety assessments were conducted during months 6 and 12.
Following randomization (4 weeks before first pegloticase infusion), patients entered a 4‐week run‐in period, during which they continued taking folic acid and either MTX or placebo. As specified in the clinical trial protocol, MTX and placebo dose adjustments or interruptions were allowed for tolerance purposes during the run‐in period and during the pegloticase plus MTX or placebo treatment period (after discussion with the sponsor medical monitor), maximizing the proportion of patients who remained on therapy. Temporary pauses in therapy were allowed based on liver function tests, cytopenias, eGFR (<30 ml/minute/1.73 m2), or clinically important symptoms as deemed by the investigators.
Patients completing the run‐in period received their first pegloticase infusion (8 mg) on study day 1, continuing oral folic acid (1 mg/day) and blinded oral MTX (15 mg/week) or placebo during the 52‐week treatment period. MTX dose adjustments were allowed during the treatment period. Standard gout flare prophylaxis (colchicine, nonsteroidal antiinflammatory drugs, and/or low‐dose prednisone [≤10 mg/day]) was required for ≥1 week before and throughout the pegloticase plus MTX or placebo treatment period per the 2012 American College of Rheumatology guidelines for management of gout (9). Infusion reaction prophylaxis was administered before each pegloticase infusion (180 mg oral fexofenadine the day before infusion, 180 mg oral fexofenadine and 1,000 mg acetaminophen the morning of infusion, and 125 mg intravenous methylprednisolone over 10–30 minutes immediately prior to infusion). During the treatment period, pegloticase infusions were administered biweekly from day 1 to week 50 (26 infusions) using the standard 2‐hour infusion rate. If a patient had serum urate levels >6 mg/dl at 2 consecutive visits beginning at week 2, treatment was discontinued, and the patient remained in study under observation. Following the week 52 visit (or end of pegloticase infusions visit), patients resumed normal gout care, including oral urate‐lowering therapy resumption, per the treating physician's discretion. Patients were followed up at 3 and 6 months after week 52 (or end of pegloticase treatment visit) for evaluation of clinical status, including serum urate.
The trial protocol was reviewed and approved by the US Food and Drug Administration, by the WCG Institutional Review Board (Puyallup, WA), and by local institutional review boards as required by investigators. All patients provided written informed consent to participate, and study conduct adhered to the tenets of the Declaration of Helsinki.
Study outcomes
The primary efficacy end point was the proportion of patients who were treatment responders in the intent‐to‐treat population during month 6 (study weeks 20–24), defined as the proportion of randomized patients with serum urate <6 mg/dl for ≥80% of the visits during month 6. Secondary end points included the proportion of treatment responders during month 12, the proportion of patients with tophi at baseline who had complete resolution of ≥1 tophus at week 52, and change from baseline to week 52 in Health Assessment Questionnaire (HAQ) scores (pain, health, and disability index) (10). Because the primary end point was during month 6, this report focuses on findings through week 24, and secondary end points are not reported here. Other exploratory end points of interest for this 6‐month analysis included mean change from baseline in serum urate at weeks 14 and 24, time to 2 consecutive serum urate measurements >6 mg/dl (pegloticase discontinuation due to urate‐lowering efficacy loss), and proportion of treatment responders (serum urate <6 mg/dl for >80% of the time) during month 3 (weeks 10, 12, and 14) and overall (month 3 and month 6 combined).
Serum samples for serum urate measurement were collected at screening and at 6 weeks, 4 weeks, and 2 weeks prior to the initial pegloticase infusion (weeks –6, –4, and –2). On the day of the first pegloticase infusion (day 1), pre‐ and post‐infusion blood samples were collected for serum urate measurement by a central laboratory. Beginning with week 2, two pre‐infusion blood samples were collected for serum urate measurement within 48 hours of pegloticase infusion. Digital photographs of tophi on the hands and feet were obtained at 6 weeks before the first pegloticase infusion (week –6), day 1, and weeks 14, 24, 36, and 52.
Blood samples for pharmacokinetic evaluations were collected post‐infusion on day 1, pre‐ and post‐infusion at weeks 2, 4, 6, 14, 22, 24, 36, 52 (or end of pegloticase infusions visit, only 1 sample collected [noninfusion visit]), and at the 3‐month posttreatment follow‐up. Pre‐infusion blood samples for antidrug antibody evaluations were collected at the same visits. The pegloticase quantitation limit was 0.6 μg/ml, and measurements below quantitation limit were imputed as 0.3 μg/ml. Trough (Cmin) and peak (Cmax) concentrations of pegloticase and the proportion of patients with Cmin below quantitation limit were summarized by visit and treatment group. Anti–polyethylene glycol (anti‐PEG) and anti‐uricase IgG antibody incidence and titer were summarized by visit, treatment group, and treatment response. Both of these antidrug antibodies were examined because the naked uricase enzyme (11, 12) and PEG (13) can individually elicit antibody production. Pre‐infusion blood samples were collected to measure MTX polyglutamate (MTXGlu1‐5) concentrations in red blood cells.
Safety, including laboratory testing and adverse event (AE) reporting, was evaluated during the run‐in period (≥1 dose blinded MTX or placebo received), the 52‐week pegloticase plus MTX or placebo treatment period (≥1 pegloticase dose received), and the 6‐month follow‐up period (first assessment >30 days after the last medication dose). The overall AE and serious AE profile for pegloticase and pegloticase plus MTX cotherapy were summarized. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) and graded for severity using the Rheumatology Common Toxicity Criteria version 2.0 (14). AEs of special interest included infusion reactions, anaphylaxis per National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network criteria (15), gout flare, and cardiovascular events. An independent safety committee adjudicated AEs of special interest other than gout flare.
The full schedule of assessments is provided in Supplementary Table 3, available at https://onlinelibrary.wiley.com/doi/art.42335. At screening, assessments included medical history, physical examination, gout assessment (patient‐reported and clinical), and laboratory testing (serum urate, hematology, clinical chemistry, pregnancy test, G6PDH). Before the 2‐week MTX tolerance test (6 weeks prior to first pegloticase infusion [week –6]), physical examination, gout assessments, and laboratory parameters were examined. Biweekly assessments included physical examination and gout assessment. Blood samples to measure serum urate were also collected during noninfusion visits at weeks 21 and 23.
Statistical analyses
The primary efficacy analysis was conducted in the intent‐to‐treat population. Additional efficacy analyses were conducted in the modified intent‐to‐treat population (≥1 pegloticase infusion received). The proportion of patients with treatment response during month 6 was analyzed with Cochran‐Mantel‐Haenszel weighting to estimate common risk difference within strata (presence of subcutaneous tophi, yes/no) and SEM of the common risk difference. Difference in the rate of treatment response between the pegloticase plus MTX group and the pegloticase plus placebo group and corresponding 95% confidence interval (95% CI) and P value were estimated. At least 2 serum urate observations from visits during month 6 must have been available for a patient to be eligible for responder consideration. Patients were considered nonresponders if they had no serum urate observations, had only 1 serum urate observation (regardless of the value of that single observation), or met serum urate monitoring criteria specifying pegloticase discontinuation (pre‐infusion serum urate >6 mg/dl at 2 consecutive scheduled visits between week 2 and week 24 for the month 6 end point). The mean change from baseline to week 24 in serum urate was analyzed using a mixed model for repeated measures analysis of covariance (ANCOVA) model, with terms for baseline score, tophi presence at baseline, treatment group, visit, visit by treatment group interaction, and visit by baseline interaction.
Up to 5 measurable tophi (≥5 mm in longest dimension with distinguishable borders) and 2 unmeasurable tophi (≥10 mm in longest dimension with indistinguishable borders) were selected for assessment using study photographs. Two trained, central readers categorized individual tophi as having complete response, defined as 100% tophus area reduction for measurable tophi or no longer visible for unmeasurable tophi. Complete resolution of a tophus response was defined as complete resolution of ≥1 tophus at week 24 without evidence of progressive disease among other tophi. Patients with a missing week 24 tophi assessment were considered nonresponders.
Safety analyses were conducted in the safety population (patients receiving ≥1 dose of blinded MTX or placebo during the run‐in period and patients receiving ≥1 pegloticase infusion during the treatment period). Based on the 43% response rate during month 6 observed in phase III trials of pegloticase monotherapy (8 mg every 2 weeks), a sample size of 135 patients (90 randomized to pegloticase plus MTX, 45 randomized to pegloticase plus placebo) was estimated to provide 88% power with a 2‐sided alpha level of 0.05 to detect a 28% difference in rates (71% response rate for pegloticase plus MTX versus 43% for pegloticase plus placebo).
Trial oversight
An independent, multidisciplinary data monitoring committee (Axio [a Cytel company]) reviewed study progress and unblinded, comparative efficacy and safety data to protect patient welfare while preserving study integrity. An external adjudication committee evaluated AEs of special interest, including infusion reactions, anaphylaxis, and cardiovascular events. The data monitoring committee could recommend study design modification or cessation for safety reasons, neither of which was recommended. Blinded safety data were summarized regularly throughout the study for sponsor safety monitoring.
RESULTS
Patients
A total of 278 patients were screened between June 13, 2019 and July 1, 2020. During screening, 119 patients were screen failures before the 2‐week open‐label MTX tolerance period, and another 7 patients discontinued after ≥1 dose of open‐label MTX. Among these 7 patients, 5 patients did not meet inclusion/exclusion criteria, and 2 patients were lost to follow‐up. Therefore, 152 patients were randomized to either pegloticase plus MTX (n = 100) or pegloticase plus placebo (n = 52) and included in the intent‐to‐treat population. Of these randomized patients, 3 patients (2 MTX, 1 placebo) withdrew before taking blinded MTX or placebo, and 4 patients (2 MTX, 2 placebo) withdrew before the first pegloticase infusion. Ultimately, 145 patients (96 receiving MTX, 49 receiving placebo) entered the pegloticase plus MTX or pegloticase plus placebo treatment period and received ≥1 pegloticase infusion, with 70 (72.9%) of 96 MTX patients and 19 (38.8%) of 49 placebo patients completing treatment through week 24 (Figure 2).
Figure 2.
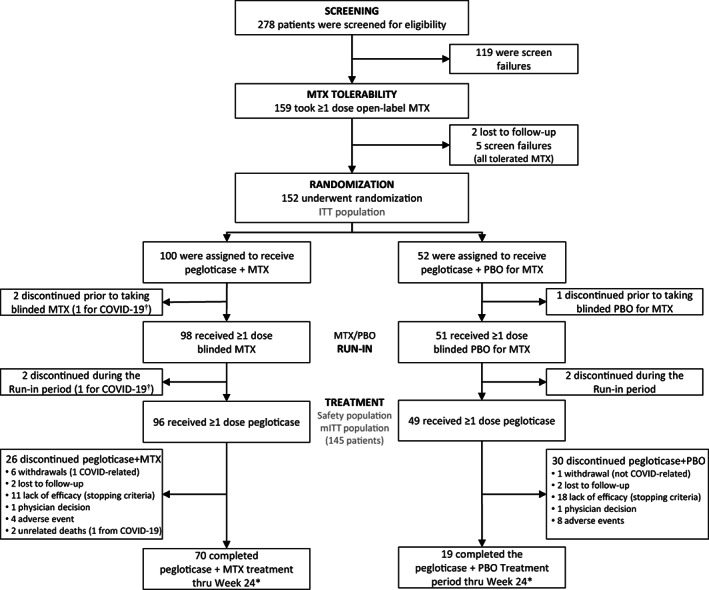
Flow chart depicting patient distribution and study disposition for a randomized, placebo‐controlled, double‐blind trial assessing pegloticase plus methotrexate (MTX) versus pegloticase plus placebo (PBO) cotreatment for uncontrolled gout. * = 1 patient in each treatment group discontinued MTX/PBO but continued to receive biweekly pegloticase infusions. † = withdrawal due to COVID‐19 concerns, not infection. ITT = intent‐to‐treat; mITT = modified intent‐to‐treat.
Of the 152 randomized patients, 135 (88.8%) were male, and 105 (69.1%) were White. Mean ± SD age was 54.7 ± 12.6 years. At screening, patients had a mean ± SD gout history (time since first diagnosis) of 13.9 ± 10.7 years. All patients had experienced ≥1 gout flare in the prior year (mean ± SD 10.8 ± 14.2 flares per patient), and 115 patients (75.7%) had tophi. Failure to improve despite prior oral urate‐lowering therapy (allopurinol, febuxostat, and/or probenecid) was noted in 86.1% of patients, with most patients having taken allopurinol. Mean daily doses of allopurinol, febuxostat, and probenecid were approximately 290 mg, 60 mg, and 750 mg, respectively. The remaining patients were unable to tolerate or had a contraindication to oral urate‐lowering therapies. Comorbidity prevalence was high, with hypertension (62.5%), gastrointestinal disorders (38.2%), stage 3 chronic kidney disease (defined as eGFR <60 ml/minute/1.73 m2; 32.2%), osteoarthritis (25.0%), hyperlipidemia (24.3%), cardiac disorders (23.7%), obesity (20.4%), type 2 diabetes mellitus (17.8%), depression (15.8%), and eye disorders (15.8%) most frequently noted. Baseline characteristics were balanced across treatment groups (Table 1).
Table 1.
Characteristics of patients with uncontrolled gout in the intent‐to‐treat population treated with pegloticase plus MTX versus pegloticase plus placebo*
| Pegloticase plus MTX | Pegloticase plus placebo | |
|---|---|---|
| (n = 100) | (n = 52) | |
| Male | 91 (91.0) | 44 (84.6) |
| Age, mean ± SD years | 55.6 ± 12.7 | 53.0 ± 12.1 |
| Race/ethnicity | ||
| White | 69 (69.0) | 36 (69.2) |
| Black or African American | 16 (16.0) | 6 (11.5) |
| Asian | 8 (8.0) | 6 (11.5) |
| Native Hawaiian/Pacific Islander | 4 (4.0) | 1 (1.9) |
| Other | 3 (3.0) | 3 (5.8)† |
| Tobacco use history | ||
| Never | 51 (51.0) | 28 (53.8) |
| Former | 26 (26.0) | 13 (25.0) |
| Current | 23 (23.0) | 11 (21.2) |
| Body mass index, mean ± SD kg/m2 | 32.7 ± 5.6 | 32.7 ± 7.8 |
| Baseline eGFR, mean ± SD ml/minute/1.73 m2 ‡, § | 69.3 ± 17.8 | 71.1 ± 17.2 |
| eGFR <60 ml/minute/1.73 m2 | 33 (33.3) | 16 (30.8) |
| Gout characteristics | ||
| Time since first gout diagnosis, mean ± SD years | 13.7 ± 10.6 | 14.3 ± 10.8 |
| Presence of tophi at screening¶ | 74 (74.0) | 41 (78.8) |
| Number of acute gout flares in prior year, mean ± SD | 10.6 ± 12.9 | 11.3 ± 16.7 |
| Number of acute gout flares in prior 6 months, mean ± SD | 5.5 ± 6.7 | 6.1 ± 8.7 |
| Baseline serum urate, mean ± SD mg/dl‡ | 8.74 ± 1.61 | 9.11 ± 1.65 |
| Prior oral urate‐lowering therapy use (within past year) | 83 (83.0) | 45 (86.5) |
| Allopurinol | 78 (78.0) | 39 (75.0) |
| Febuxostat | 15 (15.0) | 10 (19.2) |
| Probenecid | 3 (3.0) | 3 (5.8) |
Except where otherwise indicated, values are the number (%) of patients. eGFR = estimated glomerular filtration rate.
Includes 1 patient with missing race/ethnicity information.
Last observation made prior to first open‐label methotrexate (MTX) dose (6 weeks before first pegloticase infusion [week –6]).
In the safety population (≥1 pegloticase infusion received), the pegloticase plus MTX group included 95 patients, and the pegloticase plus placebo group included 49 patients.
Based on investigator or photographic assessment.
Of patients who received ≥1 pegloticase infusion (modified intent‐to‐treat population), a mean ± SD of 10.8 ± 4.07 infusions (median 13 infusions) were received by the pegloticase plus MTX group through week 24 (n = 96). In contrast, the pegloticase plus placebo group received a mean ± SD of 7.3 ± 4.90 infusions (median 6 infusions; n = 49). Both groups had a high degree of MTX or placebo compliance through week 24, with an average weekly dose range of 14.7–15.1 mg in the MTX group (n = 98) and 14.5–15.0 mg in the placebo group (n = 51) during the run‐in and treatment periods.
Efficacy
The primary efficacy end point was met, with 71.0% of patients (71 of 100) in the pegloticase plus MTX group meeting response criteria during month 6 compared to 38.5% of patients (20 of 52) in the pegloticase plus placebo group (between‐group difference 32.3% [95% CI 16.3%, 48.3%]; P < 0.0001) (Figure 3A). Response rates during month 3 and overall (months 3 and 6 combined) were similar between the intent‐to‐treat population and the modified intent‐to‐treat population (≥1 pegloticase infusion received).
Figure 3.
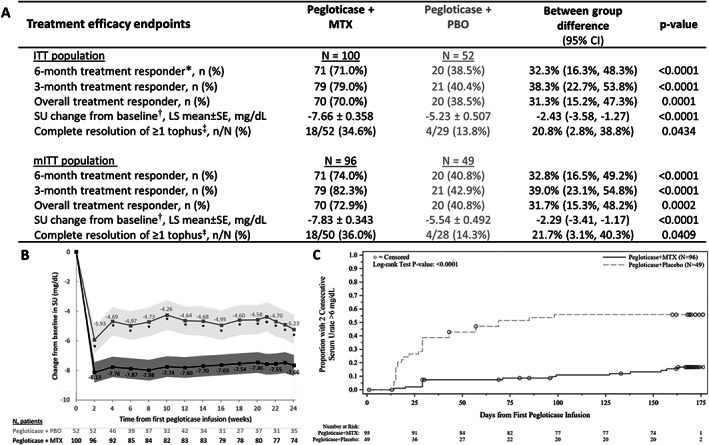
A, Key treatment efficacy end points through week 24 in the intent‐to‐treat (ITT) and modified intent‐to‐treat (mITT) populations of patients randomized to receive pegloticase plus methotrexate (MTX) or pegloticase plus placebo (PBO) for treatment of uncontrolled gout. Treatment response was defined as serum urate (SU) <6 mg/dl for ≥80% of visits during month 6 (weeks 20–24). B, Least squares (LS) mean change from baseline in SU at each study visit. The shaded region represents 95% confidence intervals (95% CIs). C, Kaplan‐Meier curve for time to 2 consecutive SU measurements >6 mg/dl through week 24. Median time to 2 consecutive SU >6 mg/dl was not estimable in the pegloticase plus MTX group and 69 days (9.9 weeks) in the pegloticase plus PBO group (mITT population). In A, * = primary efficacy end point. † = determined through week 24 using mixed model repeated measures analysis. ‡ = assessed during pegloticase treatment; MTX/PBO was discontinued when pegloticase was discontinued. In B, asterisks denote a statistically significant difference between treatment groups (P ≤ 0.0001). In C, circles represent patient censoring due to 2 consecutive SU measurements >6 mg/dl.
Mean ± SEM change from baseline in serum urate through week 24 was –7.66 ± 0.358 mg/dl in the pegloticase plus MTX group versus –5.23 ± 0.507 mg/dl in the pegloticase plus placebo group (intent‐to‐treat population; between‐group difference –2.43 mg/dl [95% CI –3.58, –1.27 mg/dl]; P < 0.0001) (Figure 3B). The median time to 2 consecutive serum urate measurements >6 mg/dl was not estimable in the pegloticase plus MTX group (too few patients met discontinuation criteria) and 69 days (lower quartile [Q1] 29.0 days, upper quartile [Q3] not estimable) in the pegloticase plus placebo group (P < 0.0001) (Figure 3C). In the pegloticase plus MTX group, 15 (15.6%) of 96 patients had 2 consecutive serum urate measurements >6 mg/dl through week 24 compared to 27 (55.1%) of 49 patients in the pegloticase plus placebo group; median time from first pegloticase infusion to serum urate measurement >6 mg/dl was 71 days and 27 days, respectively. In patients with tophi at baseline, complete tophus resolution at week 24 was observed in significantly more patients receiving pegloticase plus MTX than patients receiving pegloticase plus placebo (18 [34.6%] of 52 patients versus 4 [13.8%] of 29 patients; between‐group difference 20.8% [95% CI 2.8%, 38.8%]) (P = 0.0434 for between‐group difference).
Safety
Of patients who received ≥1 dose of blinded MTX or placebo during the run‐in period, 43 (43.9%) of 98 patients in the MTX group versus 20 (39.2%) of 51 patients in the placebo group experienced ≥1 AE, with gout flare the most common AE in both groups (28 [28.6%] of 98 patients versus 10 [19.6%] of 51 patients, respectively) (Figure 4A). One serious AE of coincident nephrolithiasis (grade 2), increased bilirubin (grade 2), leukocytosis (grade 1), and acute kidney injury (grade 1) occurred in 1 MTX patient (patient hospitalized) and was deemed unrelated to MTX treatment by the investigator. No AE led to withdrawal of blinded MTX or placebo during the run‐in period.
Figure 4.
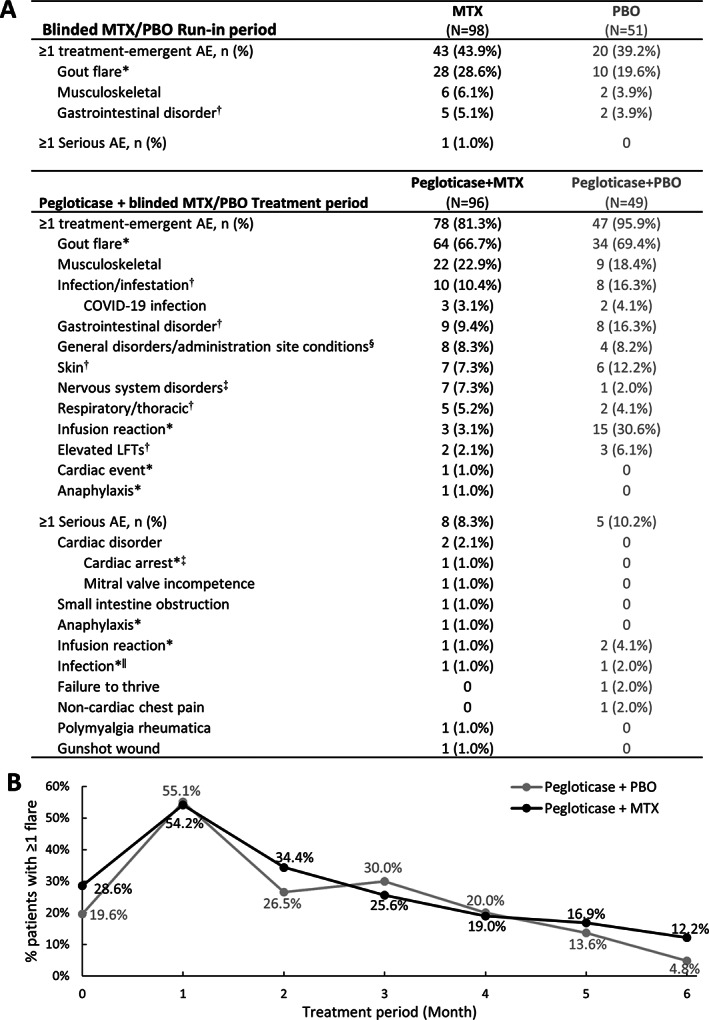
A, Summary of treatment‐emergent adverse events (AEs) occurring in ≥5% of either treatment group through week 24 of the pegloticase plus methotrexate (MTX) or placebo (PBO) treatment period (safety population). All AEs of special interest and serious AEs are also shown. Elevated liver function test (LFT) includes elevated aspartate aminotransferase, alanine aminotransferase, bilirubin, and hepatic enzymes. B, Proportion of patients in each treatment group who experienced ≥1 gout flare during each month of the pegloticase plus MTX or pegloticase plus PBO treatment period. Month 0 represents the proportion of patients with ≥1 flare during the MTX/PBO 4‐week run‐in period. In A, * = AE of special interest, adjudicated. † = known MTX AE (ref. 17). ‡ = nervous system disorders included dizziness (2 MTX), headache (1 MTX, 1 PBO), hypoesthesia (2 MTX), neuropathy peripheral (1 MTX), paresthesia (1 MTX). § = general disorders included fatigue (5 MTX, 1 PBO), chest discomfort (1 PBO), feeling hot (1 MTX), peripheral edema (1 PBO), peripheral swelling (1 MTX), pain (1 MTX), foreign body sensation (1 MTX), non‐cardiac chest pain (1 PBO). ‖ = infections included COVID‐19 (1 MTX) and soft tissue abscess (1 PBO).
In the first 24 weeks of the pegloticase plus methotrexate or placebo treatment period, 78 (81.3%) of 96 patients in the MTX group and 47 (95.9%) of 49 patients in the placebo group experienced ≥1 AE (Figure 4A), most of which were mild or moderate (grade 1 or 2) in both groups. Of 338 AEs in the MTX group, 321 (95.0%) were mild or moderate, and of 185 AEs in the placebo group, 178 (96.2%) were mild or moderate. AEs of special interest during treatment included gout flare, infusion reaction, anaphylaxis, and cardiovascular events. Gout flare was the most common AE with a similar frequency between the MTX and placebo groups (66.7% [64 of 96 patients] versus 69.4% [34 of 49 patients], respectively). As expected with urate‐lowering therapy initiation (16), the proportion of patients with ≥1 gout flare increased in the first month following pegloticase initiation, followed by a progressive decrease in both treatment groups (Figure 4B). The proportion of patients with ≥1 gout flare during each month was similar between groups.
Infusion reactions were less frequent in the MTX group (4.2% [includes anaphylaxis in 1 MTX patient]) than the placebo group (30.6%) (Figure 4A) and occurred during the first or second pegloticase infusion in 3 (75.0%) of the 4 MTX patients and 11 (73%) of the 15 placebo patients who had an infusion reaction. The single anaphylaxis AE occurred during the first pegloticase infusion following good MTX compliance. The patient recovered the same day following treatment with intramuscular and subcutaneous epinephrine, intravenous diphenhydramine hydrochloride, and intravenous methylprednisolone. A single cardiovascular event of cardiac arrest occurred in 1 MTX patient >2 weeks after the patient's last pegloticase infusion (third infusion; event deemed unrelated to either MTX or pegloticase treatment by investigator). AEs known to have an association with MTX (e.g., infection, liver toxicity, renal toxicity, gastrointestinal disorders, hematologic disorders) (17) did not occur more frequently in the MTX group than the placebo group (Figure 4A).
Reports of serious AEs were similar between groups through month 6 of the pegloticase plus MTX or placebo treatment period (8 [8.3%] MTX patients, 5 [10.2%] placebo patients) (Figure 4A). These included cardiac disorders (2 MTX patients [2.1%]), small intestine obstruction (1 MTX patient [1.0%]), noncardiac chest pain during pegloticase administration (1 placebo patient [2.0%]), infusion reaction (2 MTX patients [2.0%; includes 1 anaphylaxis]; 2 placebo patients [4.1%]), infection (1 MTX patient [1.0%; COVID‐19], 1 placebo patient [2.0%; soft tissue abscess]), gunshot wound (1 MTX patient [1.0%]), failure‐to‐thrive (1 placebo patient [2.0%]), and polymyalgia rheumatica (1 MTX patient [1.0%]).
AEs leading to permanent withdrawal of study medication occurred in both groups but less frequently with pegloticase plus MTX treatment. In the MTX group, 7 patients (7.3%) had pegloticase and MTX withdrawn because of tachycardia, anaphylaxis, infusion reaction, hypersensitivity, COVID‐19, pneumonia, influenza, and/or gout flare, and 2 patients had only MTX withdrawn because of fatigue and decreased hemoglobin. In the placebo group, 9 patients (18.4%) had pegloticase withdrawn because of infusion reaction, with 5 also discontinuing placebo. Three patients (6.1%) had only placebo withdrawn because of gastrointestinal disorder (pain, constipation, vomiting), infusion reaction, decreased platelet and white blood cell counts, back pain, alopecia, and skin drug eruption. Treatment‐emergent serious AEs led to 2 deaths in the pegloticase plus MTX group. One patient with a history of appendicitis/appendectomy and cervical disc disease experienced cardiac arrest more than 2 weeks after the patient's last pegloticase infusion (third infusion) that was deemed unrelated to pegloticase or MTX treatment by the site investigator. The second patient had COVID‐19 that was deemed unrelated to either study drug; the patient had multiple comorbidities, including hypertension, obesity, coronary artery disease, cardiomegaly, atrial fibrillation, and type 2 diabetes mellitus.
Pharmacokinetics and immunogenicity of pegloticase in the presence and absence of MTX
Higher pegloticase concentrations (Cmax and Cmin) were observed in the pegloticase plus MTX group than in the pegloticase plus placebo group (Figures 5A and B). At week 14, median Cmin was 1.32 μg/ml (Q1, Q3 0.73, 1.74 μg/ml) in the pegloticase plus MTX group versus 0.63 μg/ml (Q1, Q3 0.30, 1.28 μg/ml) in the pegloticase plus placebo group. Median Cmax was 3.01 μg/ml (Q1, Q3 1.94, 3.98 μg/ml) in the pegloticase plus MTX group versus 2.66 μg/ml (Q1, Q3 1.45, 3.20 μg/ml) in the pegloticase plus placebo group. Improved pegloticase response was associated with higher pegloticase concentrations; at week 14, Cmin was below quantitation limit in 8 (80.0%) of 10 nonresponders and 16 (18%) of 89 responders (median Cmin 1.26 μg/ml [Q1, Q3 0.72, 1.71] in responders).
Figure 5.
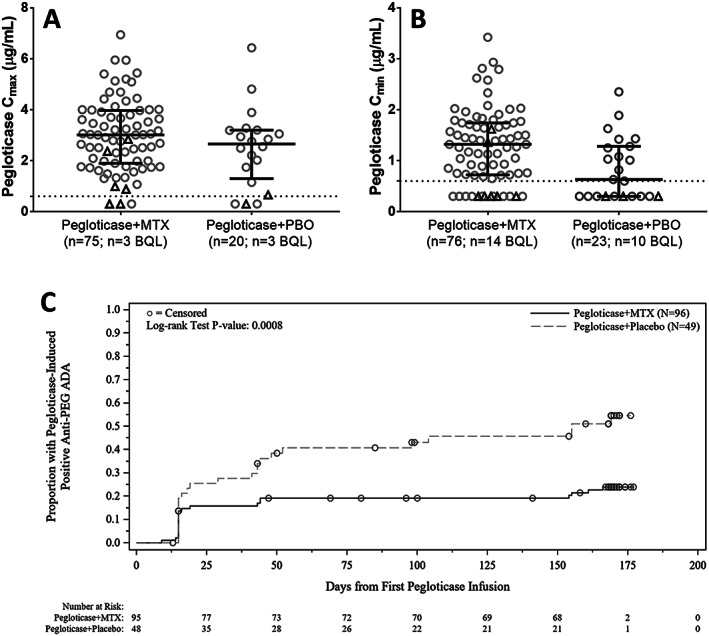
Comparison of peak (Cmax [A]) and trough (Cmin [B]) concentrations of pegloticase at week 14 in patients who received cotreatment with methotrexate (MTX) or placebo (PBO). C, Kaplan‐Meier curve for time to anti–polyethylene glycol (anti‐PEG) IgG antibody positivity. Median time to anti‐PEG antibody positivity was not estimable in patients treated with pegloticase plus MTX and 155 days (lower quartile [Q1] 44, upper quartile [Q3] not estimable) in patients treated with pegloticase plus PBO. The below quantitation limit (BQL) was 0.6 μg/ml (imputed as 0.3 μg/ml in analysis). In A and B, circles represent treatment responders; triangles represent treatment nonresponders. Lines represent the median; whiskers represent Q1 and Q3. Dashed horizontal lines show the pegloticase BQL. In C, circles represent patient censoring. Numbers below the plot show the number of patients remaining in the analysis at each time point. ADA = antidrug antibody.
Pegloticase plus MTX cotherapy reduced the incidence of new antidrug antibody formation. Anti‐uricase antibodies were not detected at baseline in either treatment group; however, 11 (11.6%) of 95 MTX patients versus 10 (20.4%) of 49 placebo patients developed anti‐uricase antibodies during the first 24 weeks of pegloticase plus MTX or placebo treatment. Mostly low anti‐uricase antibody titers (≤1:10) were observed, and anti‐uricase antibody positivity was not associated with lower pegloticase concentration (no effect on pegloticase pharmacokinetics) or treatment response.
Anti‐PEG antibodies were present in 24 (25.0%) of 96 MTX patients and 14 (29.2%) of 48 placebo patients at baseline. The proportion of patients who had postbaseline anti‐PEG antibody positivity (positive titer if antibody‐negative at baseline; increase in titer if antibody‐positive at baseline) was 23.2% in the pegloticase plus MTX group and 50.0% in the pegloticase plus placebo group. Median time to positive anti‐PEG antibody response was not estimable in the pegloticase plus MTX group (few anti‐PEG antibody–positive patients postbaseline) and 155 days (95% CI 44 days, not estimable) in the pegloticase plus placebo group (P = 0.0008 for Kaplan‐Meier estimate) (Figure 5C). In patients who were positive for anti‐PEG antibodies postbaseline, those in the pegloticase plus MTX group had overall lower titer levels than the pegloticase plus placebo group through week 24 (mean titer range 92.5–280.6 versus 325.7–728.4, respectively). Additionally, positive anti‐PEG antibody status was associated with a lower median pegloticase Cmin at week 6 (0.64 μg/ml [Q1 below quantitation limit, Q3 1.09 μg/ml] in anti‐PEG antibody–positive patients versus 1.06 μg/ml [Q1, Q3 0.66, 1.44 μg/ml] in anti‐PEG antibody–negative patients).
Treatment response rates were lower in patients who developed anti‐PEG antibodies postbaseline than in those who remained negative throughout the pegloticase plus MTX or placebo treatment period. In the modified intent‐to‐treat population, patients who remained anti‐PEG antibody–negative had a treatment response rate during month 6 of 80.8% (59 of 73 patients) in the MTX group and 58.3% (14 of 24 patients) in the placebo group. In contrast, those who developed anti‐PEG antibodies postbaseline had a response rate of 54.5% (12 of 22) and 25.0% (6 of 24) in the MTX and placebo groups, respectively. These treatment group differences were similar to the overall modified intent‐to‐treat response rate (74.0% in the MTX group versus 40.8% in the placebo group). In the 4 MTX patients who experienced infusion reactions or anaphylaxis during the treatment period, 3 (75%) had anti‐PEG antibodies at baseline (all had titers ≤1:20), and all 4 of these patients (100%) had anti‐PEG antibodies after baseline (≥1:320). Of the 15 placebo patients who experienced an infusion reaction during the treatment period, 6 (40%) had anti‐PEG antibodies at baseline (4 patients <1:40, 2 patients ≥1:320), and 11 (73.3%) had anti‐PEG antibodies postbaseline (2 patients ≤1:20, 9 patients ≥1:160).
Methotrexate polyglutamates exposure
MTXGlu concentrations in red blood cells were maintained during treatment in the pegloticase plus MTX group, suggesting compliance with MTX administration. Furthermore, MTXGlu concentrations were in the expected range based on a prior study of low‐dose oral MTX use in rheumatoid arthritis patients (18), suggesting minimal or no impact of pegloticase on MTX pharmacokinetics. MTXGlu concentrations (including MTXGlu3, the predominant MTXGlu [19]) did not significantly differ between responders and nonresponders.
DISCUSSION
In this trial conducted in patients with uncontrolled gout who were intolerant of or refractory to oral urate‐lowering therapy, we observed a significantly higher proportion of patients meeting the 6‐month primary end point and lower infusion reaction incidence among patients receiving pegloticase plus MTX compared to those receiving pegloticase plus placebo. Patients cotreated with MTX had lower incidence of antidrug antibody development, indicating MTX attenuation of pegloticase immunogenicity. Consequently, patients receiving pegloticase plus MTX had overall higher pegloticase Cmin and Cmax than those receiving pegloticase plus placebo, indicating higher and more consistent pegloticase exposure. This finding is consistent with the improved efficacy outcome found in the pegloticase plus MTX group.
Gout has a high health burden and is associated with chronic kidney disease (20, 21), metabolic syndrome (22, 23), cardiovascular disease (24, 25, 26), increased mortality (26, 27), and decreased quality of life (28, 29, 30). Uncontrolled gout results in an even higher burden due to higher comorbidity rates (31) and frequent, painful acute gout flares (29, 32). Given that pegloticase is the only medical therapy indicated for refractory or urate‐lowering therapy–intolerant gout, maximizing the number of patients who achieve sustained lowering of serum urate levels and receive the full course of therapy is extremely important. Preliminary clinical trials (6, 7, 33) and case series (34, 35, 36) in the literature support immunomodulator use in conjunction with pegloticase to increase the proportion of patients with therapeutic response. The MIRROR RCT study, a large, prospective, randomized, placebo‐controlled, double‐blind trial, verified that pegloticase plus oral MTX (15 mg/week) cotherapy resulted in a higher rate of sustained urate lowering than pegloticase plus placebo (pegloticase monotherapy) during the first 6 months of therapy. Importantly, the MTX group had lower infusion reaction incidence (4% [includes 1 patient who experienced anaphylaxis] in the MTX group versus 31% in the placebo group) and otherwise similar safety profile as the placebo group. Among noted AEs, ≥95% were mild or moderate. As in prior studies, gout flare was the most common AE in both treatment groups and decreased over time during treatment.
Anti‐pegloticase antibody development has been previously linked to loss of pegloticase urate‐lowering efficacy and infusion reaction occurrence (1, 3). The pharmacokinetic and antidrug antibody findings presented here support these associations. In both treatment groups, fewer patients with anti‐PEG antibody positivity were treatment responders during month 6 than patients without these antibodies (54.5% versus 80.8%, respectively, in the MTX group; 25.0% versus 58.3%, respectively, in the placebo group). Additionally, the patient who experienced anaphylaxis had low‐titer anti‐PEG antibodies (<1:10) prior to pegloticase initiation. This study also provides evidence that a low‐to‐moderate MTX dose attenuated antidrug antibody development against pegloticase.
This study had several limitations. It did not address serum urate management following pegloticase therapy. In patients who are tolerant of xanthine oxidase inhibitors but have inadequate response, some clinicians begin oral urate‐lowering therapy immediately after the last pegloticase infusion, but others wait until serum urate is >6 mg/dl. Further study of this important issue is needed. This study does not address pegloticase therapy duration in patients who are unable to tolerate xanthine oxidase inhibitors. Future studies may address potential therapeutic strategies in this population. In addition, few patients had eGFR <60 ml/minute/1.73 m2 (all had eGFR ≥40 ml/minute/1.73 m2). Although MTX should be avoided when eGFR is <30 ml/minute/1.73 m2, up to 49% of uncontrolled gout patients have chronic kidney disease (31). Therefore, further study of other immunomodulating therapies with pegloticase is needed in patients with advanced chronic kidney disease. Last, these 6‐month analyses may not have been long enough for maximum therapeutic benefit to be observed, particularly with respect to tophi resolution. Future analysis of the full 52‐week treatment period will provide further insight.
In conclusion, these results confirm superiority of pegloticase plus MTX cotherapy to pegloticase monotherapy in treatment efficacy and safety in uncontrolled gout management. In patients cotreated with MTX, response rates during month 6 of pegloticase therapy were significantly higher (71.0% versus 38.5% in patients receiving pegloticase monotherapy), and infusion reaction incidence was markedly lower (4.2% versus 30.6% in patients receiving pegloticase monotherapy), with a comparable safety profile to patients cotreated with placebo. Furthermore, this trial provides evidence that MTX enhances drug survival and decreases pegloticase immunogenicity through attenuation of antidrug antibody development. Based on these findings, this trial strongly supports MTX coadministration with pegloticase in uncontrolled gout patients.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Weinblatt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Botson, Peterson, Obermeyer, Xin, Chamberlain, LaMoreaux, Verma, Sainati, Teser, Weinblatt.
Acquisition of data
Botson, Saag, Peterson, Parikh, Ong, LoCicero, Grewal, Majjhoo, Tesser, Weinblatt.
Analysis and interpretation of data
Botson, Saag, La, Obermeyer, Xin, Chamberlain, LaMoreaux, Verma, Sainati, Weinblatt.
ROLE OF THE STUDY SPONSOR
This study was sponsored by Horizon Therapeutics plc. The study sponsor was involved in study design and conduct, data analysis and interpretation, and manuscript preparation and approval.
Supporting information
Disclosure Form
Supplemental Table 1 Inclusion and exclusion criteria of the MIRROR RCT trial.
Supplemental Table 2. Clinical trial investigators who randomized ≥1 patient (in alphabetical order).
Supplementary Table 3. Schedule of assessments through Week 24.
ACKNOWLEDGMENT
We thank Lissa Padnick‐Silver, PhD, an employee of and stockholder in Horizon Therapeutics plc, for medical writing and editing support.
ClinicalTrials.gov identifier: NCT03994731.
Supported by Horizon Therapeutics plc.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42335&file=art42335‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011;306:711–20. [DOI] [PubMed] [Google Scholar]
- 2. Baraf HSB, Yood RA, Ottery FD, et al. Infusion‐related reactions with pegloticase, a recombinant uricase for the treatment of chronic gout refractory to conventional therapy. J Clin Rheumatol 2014;20:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipsky PE, Calabrese LH, Kavanaugh A, et al. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther 2014;16:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hershfield MS, Ganson NJ, Kelly SJ, et al. Induced and pre‐existing anti‐polyethylene glycol antibody in a trial of every 3‐week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther 2014;16:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keenan RT, Botson JK, Masri KR, et al. The effect of immunomodulators on the efficacy and tolerability of pegloticase: a systematic review. Semin Arthritis Rheum 2021;51:347–52. [DOI] [PubMed] [Google Scholar]
- 6. Khanna PP, Khanna D, Cutter G, et al. Reducing immunogenicity of pegloticase with concomitant use of mycophenolate mofetil in patients with refractory gout: a phase II, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheumatol 2021;73:1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Botson JK, Tesser JRP, Bennett R, et al. Pegloticase in combination with methotrexate in patients with uncontrolled gout: a multicenter, open‐label study (MIRROR). J Rheumatol 2021;48:767–74. [DOI] [PubMed] [Google Scholar]
- 8. Botson JK, Baraf HSB, Keenan RT, et al. Expert opinion on pegloticase with concomitant immunomodulatory therapy in the treatment of uncontrolled gout to improve efficacy, safety, and durability of response [review]. Curr Rheumatol Rep 2022;24:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 2012;64:1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 11. Moia R, Boggio E, Gigliotti L, et al. Anti‐rasburicase antibodies induce clinical refractoriness by inhibiting the enzyme catalytic activity. Hematol Oncol 2020;38:204–6. [DOI] [PubMed] [Google Scholar]
- 12. Garay RP, El‐Gewely MR, Labaune JP,et al. Therapeutic perspectives on uricases for gout [review]. Joint Bone Spine 2012;79:237–42. [DOI] [PubMed] [Google Scholar]
- 13. Garay RP, El‐Gewely R, Armstrong JK, et al. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG‐conjugated agents [review]. Expert Opin Drug Deliv 2012;9:1319–23. [DOI] [PubMed] [Google Scholar]
- 14. Woodworth T, Furst DE, Alten R, et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the Rheumatology Common Toxicity Criteria v.2.0 [published correction appears in J Rheumatol 2014;41:2336]. J Rheumatol 2007;34:1401–14. [PubMed] [Google Scholar]
- 15. Sampson HA, Muñoz‐Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117:391–7. [DOI] [PubMed] [Google Scholar]
- 16. Latourte A, Bardin T, Richette P. Prophylaxis for acute gout flares after initiation of urate‐lowering therapy [review]. Rheumatology (Oxford) 2014;53:1920–6. [DOI] [PubMed] [Google Scholar]
- 17. Methotrexate [package insert] . Morristown, New Jersey, US: Alvogen Inc, 2021.
- 18. Dervieux T, Weinblatt ME, Kivitz A, et al. Methotrexate polyglutamation in relation to infliximab pharmacokinetics in rheumatoid arthritis. Ann Rheum Dis 2013;72:908–10. [DOI] [PubMed] [Google Scholar]
- 19. Choi R, Chun MR, Park J, et al. Methotrexate polyglutamate quantification for clinical application in patients with pediatric acute lymphoblastic leukemia in association with genetic polymorphisms. J Pharm Biomed Anal 2021;201:114124. [DOI] [PubMed] [Google Scholar]
- 20. Roughley MJ, Belcher J, Mallen CD, et al. Gout and risk of chronic kidney disease and nephrolithiasis: meta‐analysis of observational studies. Arthritis Res Ther 2015;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu KH, Kuo CF, Luo SF, et al. Risk of end‐stage renal disease associated with gout: a nationwide population study. Arthritis Res Ther 2012;14:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoo HG, Lee SI, Chae HJ, et al. Prevalence of insulin resistance and metabolic syndrome in patients with gouty arthritis. Rheumatol Int 2011;31:485–91. [DOI] [PubMed] [Google Scholar]
- 23. Choi HK, Ford ES, Li C, et al. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2007;57:109–15. [DOI] [PubMed] [Google Scholar]
- 24. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007‐2008. Am J Med 2012;125:679–87. [DOI] [PubMed] [Google Scholar]
- 25. Clarson LE, Hider SL, Belcher J, et al. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK clinical practice research datalink. Ann Rheum Dis 2015;74:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 27. Kuo CF, See LC, Luo SF, et al. Gout: an independent risk factor for all‐cause and cardiovascular mortality. Rheumatology (Oxford) 2010;49:141–6. [DOI] [PubMed] [Google Scholar]
- 28. Becker MA, Schumacher HR, Benjamin KL, et al. Quality of life and disability in patients with treatment‐failure gout. J Rheumatol 2009;36:1041–8. [DOI] [PubMed] [Google Scholar]
- 29. Khanna PP, Nuki G, Bardin T, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: results from a cross‐sectional survey. Health Qual Life Outcomes 2012;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh JA, Strand V. Gout is associated with more comorbidities, poorer health‐related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis 2008;67:1310–6. [DOI] [PubMed] [Google Scholar]
- 31. Francis‐Sedlak M, LaMoreaux B, Padnick‐Silver L, et al. Characteristics, comorbidities, and potential consequences of uncontrolled gout: an insurance‐claims database study. Rheumatol Ther 2021;8:183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirsch JD, Terkeltaub R, Khanna D, et al. Gout disease‐specific quality of life and the association with gout characteristics. Patient Relat Outcome Meas 2010;2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rainey H, Baraf HSB, Yeo A, et al. Companion immunosuppression with azathioprine increases the frequency of persistent responsiveness to pegloticase in patients with chronic refractory gout [abstract]. Ann Rheum Dis 2020;79:442–3. [Google Scholar]
- 34. Botson JK, Peterson J. Pretreatment and coadministration with methotrexate improved durability of pegloticase response: an observational, proof‐of‐concept case series. J Clin Rheumatol 2022;28:e129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Albert JA, Hosey T, LaMoreaux B. Increased efficacy and tolerability of pegloticase in patients with uncontrolled gout co‐treated with methotrexate: a retrospective study. Rheumatol Ther 2020;7:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masri KR, Padnick‐Silver L, Winterling K, et al. Effect of leflunomide on pegloticase response rate in patients with uncontrolled gout: a retrospective study. Rheumatol Ther 2022;9:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplemental Table 1 Inclusion and exclusion criteria of the MIRROR RCT trial.
Supplemental Table 2. Clinical trial investigators who randomized ≥1 patient (in alphabetical order).
Supplementary Table 3. Schedule of assessments through Week 24.


