Abstract
Neurally mediated syncope is a disorder of the autonomic regulation of postural tone, which results in hypotension, bradycardia, and loss of consciousness. A wide variety of stimuli can trigger this reflex, the most common stimulus being orthostatic stress. Typically, a patient with neurally mediated syncope experiences nausea, lightheadedness, a feeling of warmth, and pallor before abruptly losing consciousness. If the cause of syncope is unclear, a stepwise approach is necessary to arrive at the diagnosis. The diagnosis of neurally mediated syncope can be confirmed by a head-up tilt-table test. Treatment options include behavioral modification and several pharmacologic therapies. For severe recurrent syncope unresponsive to conventional treatment, a pacemaker can be implanted.
Key words: Syncope/etiology/physiopathology/therapy; syncope, vasovagal/etiology; tilt-table test
Syncope is a sudden loss of postural tone followed by rapid, spontaneous recovery. It affects all ages, from the pediatric to the elderly. Because syncope is a symptom, not a disease, identification of the underlying pathologic process is essential for successful management. Syncope is often caused by an abnormal autonomic response. 1 Several terms are used to describe this type of syncope, including vasovagal, neurocardiogenic, and neurally mediated syncope (NMS). We prefer the latter term because it indicates the underlying pathologic process. The triggering factor for NMS varies widely and includes orthostatic stress, emotional stress, urination, coughing, swallowing, physical exercise, and stimulation of the carotid sinus in susceptible persons.
Pathophysiology
The human body has a remarkable ability to maintain a stable blood pressure in the presence of ever-changing forces that constantly shift and redistribute the circulating blood volume. To achieve this steady control, reflex mechanisms continuously adjust the cardiac output and vascular tone. Even a simple change in posture, such as standing up, can result in a relatively “empty” ventricle owing to shifting of blood from the thorax to the abdomen and lower extremities. This shift in blood volume can markedly decrease the cardiac output. The decreased output is normally sensed by arterial baroreceptors located in the carotid sinus and aortic arch. 2 These receptors transmit signals to the nervous system and result in reflex-increased sympathetic output. In addition, the vascular system responds locally by restricting blood flow to nonvital organs such as the skin, muscles, and adipose tissue, thereby augmenting peripheral resistance. Clinically, this response manifests as an increase in the heart rate (by 10 to 15 beats/min), which is thought to be mediated by increased sympathetic output, and a gradual diastolic-pressure increase of about 10 mmHg, which is probably mediated by local vasoconstriction. 3
Neurally mediated syncope is caused by “hypersensitivity” of the autonomic nervous system, which overresponds to different stimuli. Orthostatic stress is one of the most commonly encountered triggering factors seen in clinical practice. When related to orthostatic stress, syncope is believed to involve the following steps (Fig. 1):
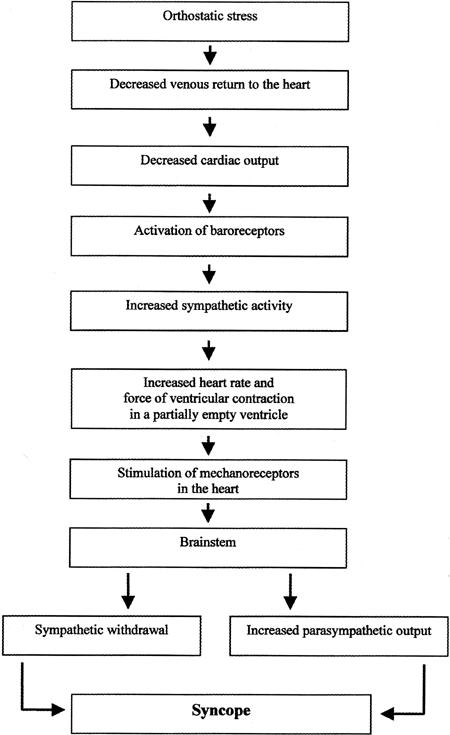
Fig. 1 Steps involved in the development of syncope related to orthostatic stress.
The heart is partially emptied as a result of a fluid shift.
Activation of the above-described normal sympathetic reflex response results in hypercontractility of the ventricle in an attempt to increase the cardiac output.
Cardiac mechanoreceptors, which are usually activated by distension of the heart (for example, in conditions involving severe hypertension), undergo abnormal stimulation. This paradoxical stimulation is believed to result from the combined hypercontractility and emptiness of the ventricle.
Abnormal mechanoreceptor stimulation transmits neural signals, as the afferent limb of the abnormal reflex, to the tractus nucleus solitarius in the brainstem.
The tractus nucleus solitarius synapses with other centers in the brainstem, which are not clearly understood but may be located in the rostral ventrolateral medulla.
Through the efferent limb of this reflex, the para-sympathetic output is increased, and sympathetic output is inhibited, resulting in bradycardia, hypotension, and syncope. 4
Experience with NMS in patients with transplanted hearts, which are denervated, suggests that this process is not merely an abnormal reflex arc. 5 Cardiac afferents may not be needed to trigger the vasodepressor response; rather, that response may be triggered by receptors in the aorta, in the carotid sinus, and at the pulmonary vascular junctions, or even by direct central nervous system (CNS) output. 3 In addition, several neuroendocrine changes are observed during orthostatic stress. At the time of syncope, a higher level of adrenaline is found in patients with NMS than in normal volunteers. 6 Endocrine changes that have been observed during syncope include decreased renin activity and increased opiate, serotonin, vasopressin, and endothelin levels. 4 These changes can result in abnormal peripheral vasodilation and predispose the patient to hypotension and syncope. 7
Postural hypotension and syncope can result from degenerative changes in 1 or more components of the autonomic nervous system. In these cases, the hypotension is part of a general autonomic disorder that affects many other organs and functions. 8,9 One form of autonomic dysfunction is postural orthostatic tachycardia syndrome (POTS), 10 which results from a failure of peripheral vasoconstriction. Patients present with hypotension, fatigue, and dizziness. An important feature of POTS is persistent tachycardia while the patient is upright, which may be misdiagnosed as inappropriate sinus tachycardia.
Diagnosis
Typically, a patient with NMS initially experiences warmth, nausea, and lightheadedness and may exhibit pallor before abruptly losing consciousness. 11 However, occasional patients may not exhibit any symptoms at all. Before the diagnosis of NMS can be established, other causes must be ruled out by means of a thorough history, a careful physical examination, and appropriate clinical tests. In about half of the cases, the cause of syncope can be identified during the initial clinical evaluation, and no further testing is needed to make the diagnosis. 12 When the cause is not clear, a stepwise approach is necessary for determining the diagnosis. 13 The differential diagnosis includes syncope associated with cardiac diseases and arrhythmias such as life-threatening ventricular dysrhythmia. Screening methods for heart disease include electrocardiography, stress testing, nuclear imaging, and echocardiography. If an arrhythmia is suspected, 24-hour Holter or long-term event monitoring, with either an external device or an implantable device with memory, may help establish the diagnosis. 14 Invasive electrophysiologic studies can be performed to clarify whether the syncope is caused by an arrhythmia such as ventricular tachycardia, supraventricular tachycardia, sinus node dysfunction, or intracardiac conduction delay. 15
If NMS is suspected in the absence of structural heart disease, a head-up tilt-table (HUTT) test is performed to confirm the diagnosis. 16 This is a provocative test in which the patient undergoes an orthostatic challenge designed to determine his or her susceptibility to syncope. During the test, resuscitative equipment should be available. With the patient in a fasting state, the head of the table is tilted upward to an angle of 60 to 80 degrees while hemodynamic and electrocardiographic monitoring is performed. The blood pressure is measured beat by beat with noninvasive techniques. The test is continued for 30 to 45 minutes unless hemodynamic collapse occurs earlier. If the patient's condition remains stable during this period, carotid-sinus massage is performed with the patient upright. If the findings are negative for NMS, a provocative agent such as nitroglycerin or isopro-terenol is administered, and tilting is continued for another 10 to 15 minutes. The result is considered positive if the original symptoms are reproduced with objective evidence of an abrupt blood pressure decrease or bradycardia (or both).
Another pattern of response to the HUTT test is observed in patients with autonomic failure who may show progressive hypotension as the blood pools in the lower extremities. Milder forms of autonomic failure (POTS) compensate for venous pooling by suddenly increasing the heart rate (by >30 beats/min, up to 120 beats/min), usually within the first 5 minutes. The blood pressure tends to decrease only slightly. 10
The specificity and sensitivity of the HUTT test are hard to determine because of methodologic differences in the test's performance. The absence of a “gold standard” also makes it difficult to determine normal versus abnormal results. Nevertheless, with “normal” volunteers and with patients who have a history typical of NMS, the reported specificity is about 90%, and the reported sensitivity ranges from 32% to 85%. 17 When a provocative agent is used, the sensitivity increases while the specificity decreases. Reproducibility of the results ranges from 35% to 85%. 17 In a recent study that assessed the reproducibility of the HUTT test using sublingual nitroglycerine as a provocative agent, the reproducibility of an initially negative test result was 83%, and that of an initially positive test result was 79%; the overall clinical reproducibility was 77%. 18
Treatment
Several strategies are available for treating NMS. The patient should be assured of the condition's benign nature. 19 However, NMS can affect the patient's quality of life and can pose a hazard during driving or in certain high-risk occupations. The condition may be controlled by an increased fluid and salt intake and avoidance of triggering factors such as dehydration, extreme heat, alcohol consumption, and prolonged standing. 20 For patients with frequent episodes, orthostatic tilt training may be useful. 21
Because no single large, randomized study has been undertaken to evaluate the best treatment options for NMS, medical therapy is based on a number of small, mostly nonrandomized clinical studies. Drug studies are hampered by the unpredictable nature of the syncopal episodes. The choice of therapeutic agent should be tailored to each patient, taking into account the age of the patient, any concurrent medical conditions, and the safety and side effects of each agent. Beta blockers are the preferred initial agents. Alternatively, midodrine, selective serotonin uptake inhibitors, or fludrocortisone may be administered. Controversy exists regarding the benefit of other medicines such as theophylline or anticholinergic agents (e.g., disopyramide). The optimal duration of treatment is hard to determine. In patients at low risk for occupational injury, treatment for a defined period such as 12 months is reasonable. 22 In many of these cases, syncope will not recur. In patients with a high risk of syncope-related injury, the duration of treatment must be tailored individually.
Beta Blockers. Because beta blockers such as metoprolol or atenolol 23–25 are effective and safe, with relatively few side effects, they are preferred for initial therapy. In preventing hypotension and bradycardia, these drugs' paradoxical effect is believed to arise from their blockage of catecholamines, which sensitize the receptors of the afferent limb of the reflex. If severe bradycardia develops during therapy with conventional beta blockers, the use of an agent with intrinsic sympathomimetic action (such as pindolol) may alleviate symptoms while having fewer adverse effects on the heart rate. 26 Potential side effects of beta blocker therapy include fatigue, depression, and sexual dysfunction.
Alpha Agonists. These drugs exert their effect peripherally by increasing vascular resistance and decreasing vascular capacitance. Potential side effects of alpha agonists include hypertension, paresthesia, urinary retention, “goose bumps,” hyperactivity, dizziness, tremor, and nervousness. A prototype of this drug group is midodrine, which has proved to be effective in randomized clinical trials. 27,28 However, patient compliance with therapy may be difficult because the medication must be taken 3 times a day. Midodrine does not cross the blood-brain barrier, so it should have no CNS effects.
Selective Serotonin Reuptake Inhibitors. Serotonin is a CNS neurotransmitter that regulates many functions, including those of the cardiovascular system. In animals, intravenous or intracerebral injection of 5-hydroxy-tryptophan, the precursor of serotonin, abruptly lowers the blood pressure and heart rate. 29 Pretreatment with selective serotonin reuptake inhibitors leads to an initial increase in the concentration of extracellular serotonin, which, in turn, causes downregulation of postsynaptic receptor density. This change is postulated to prevent overstimulation of the postsynaptic area by a surge of serotonin. 30 Paroxetine, 31 fluoxetine, 32 and sertraline 33 are selective serotonin reuptake inhibitors useful in treating NMS. Although generally well tolerated, these drugs may cause anxiety, headache, or insomnia in some patients.
Fludrocortisone. Fludrocortisone is a mineralocorticoid that increases blood volume by augmenting renal sodium absorption. By sensitizing alpha-adrenergic receptors, it may also increase the vasoconstrictive peripheral vascular response. 34 This agent has been studied and found beneficial, mainly in pediatric groups. 35 Adverse effects include hypertension, peripheral edema, depression, and acne formation.
Anticholinergic Agents. Disopyramide, scopolamine, 36 and propantheline 37 counteract the high level of vagal activity associated with NMS. Side effects include dry mouth, blurred vision, confusion, disorientation, and urinary difficulty.
Pacemaker Therapy. Pacing is useful in patients with hypersensitive carotid sinus syndrome and neurally mediated symptoms associated with severe, recurrent syncopal episodes. 38,39 According to guidelines published by the American College of Cardiology/American Heart Association, the following condition is considered a class I indication (Table I) for pacemaker therapy: recurrent syncope caused by carotid sinus stimulation 40 when minimal carotid sinus pressure induces ventricular asystole lasting for more than 3 seconds in the absence of medication that depresses sinus-node or AV conduction. In contrast, recurrent syncope without a clear provocative event and with a hypersensitive cardioinhibitory response is considered a class IIa indication for pacemaker implantation. Neurally mediated syncope, with severe bradycardia reproduced during HUTT testing with or without a provocative agent, is a class IIb indication. In contrast, vague symptoms (dizziness or lightheadedness) in the absence of syncope are a class III indication. In the North American neurally mediated pacemaker study, 39 which involved 54 patients with frequent syncopal spells and positive HUTT results, recurrence of syncope was significantly reduced in patients with pacemakers (22%) versus those without pacemakers (70%). Nevertheless, in patients with frequent syncope, pacing tends to be reserved until other therapies have been exhausted. Pacemakers are generally more useful in patients who have a cardioinhibitory, rather than a vasodepressor, response. However, bradycardia induced by HUTT testing does not necessarily predict a beneficial response to pacemaker therapy, and the length of asystole should not be a criterion for pacemaker implantation.
Table I. American College of Cardiology/American Heart Association Guidelines for Pacemaker Implantation in Patients with Syncope

Conclusion
Neurally mediated syncope includes a heterogeneous group of disorders characterized by an abnormal autonomic response that results in hypotension, bradycardia, and temporary loss of consciousness. Because syncope can herald various disease processes, patients with this symptom should undergo a thorough evaluation designed to identify the underlying pathologic process. Although HUTT testing is effective for diagnosing NMS, it is limited by its moderate sensitivity.
Several options are available for treating NMS. The physician's awareness and the patient's education are essential elements of medical care. Beta blockers are the first line of pharmacologic therapy. Alternative agents include alpha agonists, serotonin uptake inhibitors, fludrocortisone, and anticholinergic agents. Therapy is guided by symptom recurrence and the pharmacologic side-effect profile. If symptoms are severe and medical treatment options have been exhausted, pacemaker implantation may be considered.
More research is needed to further define the patho-logic mechanism of NMS and to expand its diagnostic and treatment options.
Acknowledgment
The authors thank Virginia D. Fairchild, medical editor, for helping to prepare this manuscript.
Footnotes
Address for reprints: Ali Massumi, MD, 6624 Fannin Street, Suite 2480, Houston, TX 77030-2336
Presented at the Texas Heart® Institute's symposium Cardiac Arrhythmias: New Pharmacologic and Interventional Strategies, held on 5 February 2000, at the Houstonian Hotel, Houston, Texas
References
- 1.Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol 1983;1:90–102. [DOI] [PubMed]
- 2.Jacobsen TN, Morgan BJ, Scherrer U, Vissing SF, Lange RA, Johnson N, et al. Relative contributions of cardiopulmonary and sinoaortic baroreflexes in causing sympathetic activation in the human skeletal muscle circulation during orthostatic stress. Circ Res 1993;73:367–78. [DOI] [PubMed]
- 3.Grubb BP, Karas B. Clinical disorders of the autonomic nervous system associated with orthostatic intolerance: an overview of classification, clinical evaluation, and management. Pacing Clin Electrophysiol 1999;22:798–810. [DOI] [PubMed]
- 4.Morillo CA, Ellenbogen KA, Fernando Pava L. Pathophysiologic basis for vasodepressor syncope. Cardiol Clinic 1997; 15:233–49. [DOI] [PubMed]
- 5.Fitzpatrick AP, Banner N, Cheng A, Yacoub M, Sutton R. Vasovagal reactions may occur after orthotopic heart transplantation. J Am Coll Cardiol 1993;21:1132–7. [DOI] [PubMed]
- 6.Leonelli FM, Wang K, Evans JM, Patwardhan AR, Ziegler MG, Natale A, et al. False positive head-up tilt: hemodynamic and neurohumoral profile. J Am Coll Cardiol 2000; 35:188–93. [DOI] [PubMed]
- 7.Thomson HL, Atherton JJ, Khafagi FA, Frenneaux MP. Failure of reflex venoconstriction during exercise in patients with vasovagal syncope. Circulation 1996;93:953–9. [DOI] [PubMed]
- 8.Shy GM, Drageer GA. A neurological syndrome associated with orthostatic hypotension. Arch Neurol 1960;3:511–27. [DOI] [PubMed]
- 9.Furlan R, Piazza S, Bevilacqua M, Turiel M, Norbiato G, Lombardi F, et al. Pure Autonomic Failure: complex abnormalities in the neural mechanisms regulating the cardiovascular system. J Auton Nerv Syst 1995;51:223–35. [DOI] [PubMed]
- 10.Grubb BP, Kosinski DJ, Boehm K, Kip K. The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin Electrophysiol 1997;20:2205–12. [DOI] [PubMed]
- 11.Grubb BP. Pathophysiology and differential diagnosis of neu-rocardiogenic syncope. Am J Cardiol 1999;84(8A):3Q–9Q. [DOI] [PubMed]
- 12.Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997;126:989–96. [DOI] [PubMed]
- 13.Kapoor WN. Evaluation and management of the patient with syncope. JAMA 1992;268:2553–60. [PubMed]
- 14.Krahn AD, Klein GJ, Yee R, Takle-Newhouse T, Norris C. Use of an extended monitoring strategy in patients with problematic syncope. Reveal Investigators. Circulation 1999; 99:406–10. [DOI] [PubMed]
- 15.Sra JS, Anderson AJ, Sheikh SH, Avitall B, Tchou PJ, Troup PJ, et al. Unexplained syncope evaluated by electrophysiologic studies and head-up tilt testing. Ann Intern Med 1991; 114:1013–9. [DOI] [PubMed]
- 16.Benditt DG, Ferguson DW, Grubb BP, Kapoor WN, Kugler J, Lerman BB, et al. Tilt table testing for assessing syncope. American College of Cardiology. J Am Coll Cardiol 1996; 28:263–75. [DOI] [PubMed]
- 17.Parry SW, Kenny RA. Tilt table testing in the diagnosis of unexplained syncope. QJM 1999;92:623–9. [DOI] [PubMed]
- 18.Foglia-Manzillo G, Giada F, Beretta S, Corrado G, Santarone M, Raviele A. Reproducibility of head-up tilt testing potentiated with sublingual nitroglycerin in patients with unexplained syncope. Am J Cardiol 1999;84:284–8. [DOI] [PubMed]
- 19.Dhala A, Natale A, Sra J, Deshpande S, Blanck Z, Jazayeri M, Akhtar M. Relevance of asystole during head-up tilt testing. Am J Cardiol 1995;75:251–4. [DOI] [PubMed]
- 20.Atiga WL, Rowe P, Calkins H. Management of vasovagal syncope. J Cardiovasc Electrophysiol 1999;10:874–86. [DOI] [PubMed]
- 21.Di Girolamo E, Di Iorio C, Leonzio L, Sabatini P, Barsotti A. Usefulness of a tilt training program for the prevention of refractory neurocardiogenic syncope in adolescents: A controlled study. Circulation 1999;100:1798–801. [DOI] [PubMed]
- 22.Bloomfield D, Sheldon R, Grubb BP, Calkins H, Sutton R. Putting it together: a new treatment algorithm for vasovagal syncope and related disorders. Am J Cardiol 1999;84(8A): 33Q–39Q. [DOI] [PubMed]
- 23.Muller G, Deal BJ, Strasburger JF, Benson DW Jr. Usefulness of metoprolol for unexplained syncope and positive response to tilt testing in young persons. Am J Cardiol 1993; 71:592–5. [DOI] [PubMed]
- 24.Cox MM, Perlman BA, Mayor MR, Silberstein TA, Levin E, Pringle L, et al. Acute and long-term beta-adrenergic blockade for patients with neurocardiogenic syncope. J Am Coll Cardiol 1995;26:1293–8 [DOI] [PubMed]
- 25.Mahanonda N, Bhuripanyo K, Kangkagate C, Wansanit K, Kulchot B, Nademanee K, et al. Randomized double-blind, placebo-controlled trial of oral atenolol in patients with unexplained syncope and positive upright tilt table test results. Am Heart J 1995;130:1250–3. [DOI] [PubMed]
- 26.Iskos D, Dutton J, Scheinman MM, Lurie KG. Usefulness of pindolol in neurocardiogenic syncope. Am J Cardiol 1998; 82:1121–4,A9. [DOI] [PubMed]
- 27.Sra J, Maglio C, Biehl M, Dhala A, Blanck Z, Deshpande S, et al. Efficacy of midodrine hydrochloride in neurocardiogenic syncope refractory to standard therapy. J Cardiovasc Electrophysiol 1997;8:42–6. [DOI] [PubMed]
- 28.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group [published erratum appears in JAMA 1997;278:388]. JAMA 1997;277:1046–51. [PubMed]
- 29.Baum T, Shropshire AT. Inhibition of efferent sympathetic nerve activity by 5-hydroxytryptophan in centrally administered 5-hydroxytryptamine. Neuropharmacology 1975; 14:227–33. [DOI] [PubMed]
- 30.Grubb BP, Karas BJ. The potential role of serotonin in the pathogenesis of neurocardiogenic syncope and related autonomic disturbances. J Interv Card Electrophysiol 1998;2: 325–32. [DOI] [PubMed]
- 31.Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin uptake inhibitor, on refractory vasovagal syncope: a randomized, double blind, placebo-controlled study. J Am Coll Cardiol 1999;33:1227–30. [DOI] [PubMed]
- 32.Grubb BP, Wolfe DA, Samoil D, Temsy-Armos P, Hahn H, Elliot L. Usefulness of fluoxetine hydrochloride for prevention of resistant upright tilt induced syncope. Pacing Clin Electrophysiol 1993;16(3 Pt I):458–64. [DOI] [PubMed]
- 33.Grubb BP, Samoil D, Kosinski D, Kip K, Brewster P. Use of sertraline hydrochloride in the treatment of refractory neurocardiogenic syncope in children and adolescents. J Am Coll Cardiol 1994;24:490–4. [DOI] [PubMed]
- 34.Mion D Jr, Rea RF, Anderson EA, Kahn D, Sinkey CA, Mark AL. Effects of fludrocortisone on sympathetic nerve activity in humans. Hypertension 1994;23:123–30. [DOI] [PubMed]
- 35.Scott WA, Pongiglione G, Bromberg BI, Schaffer MS, Deal BJ, Fish FA, et al. Randomized comparison of atenolol and fludrocortisone acetate in the treatment of pediatric neurally mediated syncope. Am J Cardiol 1995;76:400–2. [DOI] [PubMed]
- 36.Lee TM, Su SF, Chen MF, Liau CS, Lee YT. Usefulness of transdermal scopolamine for vasovagal syncope. Am J Cardiol 1996;78:480–2. [DOI] [PubMed]
- 37.Yu JC, Sung RJ. Clinical efficacy of propantheline bromide in neurocardiogenic syncope: pharmacodynamic implications. Cardiovasc Drugs Ther 1997;10:687–92. [DOI] [PubMed]
- 38.Brignole M, Menozzi C, Lolli G, Bottoni N, Gaggioli G. Long-term outcome of paced and nonpaced patients with severe carotid sinus syndrome. Am J Cardiol 1992;69:1039–43. [DOI] [PubMed]
- 39.Connolly SJ, Sheldon R, Roberts RS, Gent M. The North American Vasovagal Pacemaker Study (VPS). A randomized trial of permanent cardiac pacing for the prevention of vasovagal syncope. J Am Coll Cardiol 1999;33:16–20. [DOI] [PubMed]
- 40.Gregoratos G, Cheitlin M, Conill A, Epstein AE, Fellows C, Ferguson TB Jr, et al. ACC/AHA Guidelines for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices: Executive Summary—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Pacemaker Implantation). Circulation 1998;97:1325–35. [DOI] [PubMed]


