ABSTRACT
Osteoporosis has traditionally been characterized by underlying endocrine mechanisms, though evidence indicates a role of inflammation in its pathophysiology. Lipopolysaccharide (LPS), a component of gram‐negative bacteria that reside in the intestines, can be released into circulation and stimulate the immune system, upregulating bone resorption. Exogenous LPS is used in rodent models to study the effect of systemic inflammation on bone, and to date a variety of different doses, routes, and durations of LPS administration have been used. The study objective was to determine whether systemic administration of LPS induced inflammatory bone loss in rodent models. A systematic search of Medline and four other databases resulted in a total of 110 studies that met the inclusion criteria. Pooled standardized mean differences (SMDs) and corresponding 95% confidence intervals (CI) with a random‐effects meta‐analyses were used for bone volume fraction (BV/TV) and volumetric bone mineral density (vBMD). Heterogeneity was quantified using the I 2 statistic. Shorter‐term (<2 weeks) and longer‐term (>2 weeks) LPS interventions were analyzed separately because of intractable study design differences. BV/TV was significantly reduced in both shorter‐term (SMD = −3.79%, 95% CI [−4.20, −3.38], I 2 62%; p < 0.01) and longer‐term (SMD = −1.50%, 95% CI [−2.00, −1.00], I 2 78%; p < 0.01) studies. vBMD was also reduced in both shorter‐term (SMD = −3.11%, 95% CI [−3.78, −2.44]; I 2 72%; p < 0.01) and longer‐term (SMD = −3.49%, 95% CI [−4.94, −2.04], I 2 82%; p < 0.01) studies. In both groups, regardless of duration, LPS negatively impacted trabecular bone structure but not cortical bone structure, and an upregulation in bone resorption demonstrated by bone cell staining and serum biomarkers was reported. This suggests systemically delivered exogenous LPS in rodents is a viable model for studying inflammatory bone loss, particularly in trabecular bone. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: MICRO‐COMPUTED TOMOGRAPHY, DUAL‐ENERGY X‐RAY ABSORPTIOMETRY (DXA), BONE HISTOMORPHOMETRY, BIOCHEMICAL MARKERS OF BONE TURNOVER, PRECLINICAL STUDIES
Introduction
Osteoporosis is a disease characterized by low bone mineral density (BMD) and weakened bone structure and is estimated to affect more than 500 million people worldwide.( 1 ) Approximately one in three women and one in five men will suffer an osteoporotic fracture in their lifetime.( 2 ) Fragility fractures, resulting from low trauma (e.g., a fall from standing height or less), can result in decreased quality of life, increased risk of future fractures, morbidity, mortality and impose a significant financial burden on the healthcare system.( 3 , 4 ) An estimated 158 million individuals worldwide were at a high risk of a fragility fracture in 2010 and this is expected to double by 2040( 5 ); therefore, research investigating intervention strategies can have a major public health impact.
Human studies investigating approaches to preventing fragility fractures are limited by the duration required to observe a response, as well as the available analysis techniques that can be performed due to limitations associated with patient radiation exposure. Preclinical rodent models are useful for studying loss of bone mineral and structure because they mimic skeletal changes observed in humans over their lifespan and provide an accelerated model for studying bone outcomes.( 6 ) These models provide useful information about disease pathophysiology and for the development of novel treatment interventions.
Traditionally, the pathophysiology of osteoporosis has emphasized endocrine mechanisms; however, more recent evidence suggests that inflammation is also a contributor to this disease.( 7 ) Bone is continually remodeled throughout the lifespan by a tightly regulated process mediated by bone‐forming osteoblasts and bone‐resorbing osteoclasts. However, this becomes dysregulated in inflammatory environments, disproportionately favoring bone resorption.( 8 ) In older women, inflammatory markers interleukin (IL)‐6 and tumor necrosis factor (TNF) have been associated with a higher risk of hip fracture.( 9 ) In men, the risk of hip or vertebral fracture was also positively associated with IL‐6 and TNF‐α.( 10 ) Furthermore, diseases associated with an underlying inflammatory pathophysiology such as rheumatoid arthritis,( 11 ) inflammatory bowel disease,( 12 ) pancreatitis,( 13 ) nonalcoholic fatty liver disease,( 14 ) and cardiovascular disease,( 15 ) are often accompanied by low BMD. Aside from the associated underlying inflammatory pathophysiology, other contributing factors include mobility impairment, nutrient malabsorption, and hormonal alterations that may contribute to a high risk of developing secondary osteoporosis with the aforementioned diseases. There is strong evidence for the role of inflammatory cytokines in osteoporotic bone loss,( 16 ) but there is no consensus on a preclinical rodent model of systemic inflammation that induces a deterioration of bone tissue that mirrors osteoporosis.
One approach to inducing systemic inflammation is with lipopolysaccharide (LPS), a component of the outer membrane of gram‐negative bacteria found in the intestines or in the diet that can enter circulation. In humans, the translocation of LPS into systemic circulation has been linked with multiple disease states; at high concentrations LPS induces sepsis, whereas lower concentrations trigger low‐grade inflammation.( 17 ) Additionally, there is an increase in gut permeability with aging, leading to elevated circulating LPS thought to contribute to age‐related inflammation.( 18 , 19 ) LPS is also a potent inducer of bone resorption by upregulating the differentiation and activity of osteoclasts( 20 ) and inhibiting bone‐forming osteoblasts.( 21 ) Systemic LPS stimulates the immune system to release pro‐inflammatory cytokines (e.g., IL‐1, IL‐6, TNF‐α) that can further exacerbate the altered bone metabolism that favors bone resorption.( 22 ) LPS also stimulates granulocyte colony‐stimulating factor,( 23 , 24 ) which induces bone resorption while inhibiting bone formation.( 25 ) This dysregulated metabolism can alter bone structure and BMD. Although ovariectomy in rodent models mimics the estrogen deficiency seen in postmenopausal women and is associated with an upregulation of inflammatory cytokines,( 26 ) the role of inflammation cannot be distinguished from the extensive hormonal changes. Other rodent models of inflammatory bone loss include osteoarthritis( 27 ) and colitis,( 28 , 29 ) but the results from these models are confounded by alterations in mobility/weight‐bearing activity and nutrient malabsorption, respectively. Since chronically administered LPS induces an immune response and upregulates pro‐inflammatory cytokines with no changes in observable health status,( 30 , 31 , 32 , 33 , 34 , 35 , 36 ) exogenous LPS administered to rodents is a viable model for inducing systemic inflammatory bone loss to better understand the inflammatory component of osteoporosis. The objective of this systematic review and meta‐analysis was to determine whether systemic administration of LPS induced inflammatory bone loss in rodent models.
Methods
This systematic review was conducted in accordance with PRISMA guidelines,( 37 ) and the protocol was registered in advance on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/), ID CRD42020203892.
Search strategy
The search strategy was developed with support from Brock University's library services (EF). MEDLINE, Embase, CINAHL Complete, ProQuest Dissertations and Theses Global, and Web of Science databases were searched from inception through January 13, 2021, for studies that administered exogenous LPS in vivo in rodent models and examined bone outcomes (Table 1). The full systematic search strategy across databases is presented in Table S1. Language was restricted to English. All citations returned by this search were imported into Covidence systematic review software (www.covidence.org), and duplicates were removed.
Table 1.
Structure of Systematic Literature Search
| Lipopolysaccharide |
| lipopolysaccharide OR endotoxin OR LPS OR lipoglycans OR lipid A OR O Antigen |
| Bone |
| bone and bones OR femur OR leg bones OR fibula OR tibia OR arm bones OR diaphysis OR epiphysis OR spine OR lumbar vertebra bone tissue OR bone density OR bone loss OR bone microarchitecture or bone histomorphometry OR cancellous bone OR spongy bone OR trabecular bone OR cortical bone OR cortical thickness OR compact bone* OR bone volume OR trabecular number OR trabecular thickness OR trabecular separation OR bone strength OR mineral apposition rate OR bone mineral OR bone structure |
| Rodent Models |
| rodentia OR rodent OR mouse OR mice OR mus OR rat OR rats OR guinea pig OR cricetinae OR hamster |
Note: Search terms within each concept were linked with “OR” and concepts with “AND.” Database‐specific syntax, truncation options, and proximity operators were used.
Eligibility criteria and study selection
All study titles and abstracts were independently screened for inclusion criteria by two independent reviewers. The principal researcher (KB) screened all studies, whereas two other researchers (SP, WW) each screened half. Any disagreements between the two reviewers on a study were resolved by the third reviewer. Studies were excluded if any of the following applied: (i) not an original study, (ii) not a full‐text publication in English, (iii) animal models other than rodents, human, or cell culture studies, (iv) no systemic in vivo delivery of exogenous LPS (local injections or diet‐induced), (v) a lethal dose of LPS causing septic shock or death, (vi) none of the specified bone outcomes reported, or (vii) no comparator group. If it was unclear from a study title and abstract whether the study should be excluded, the study was included in the full text screening. Published research articles and dissertations were considered for inclusion, whereas research in progress and conference proceedings were excluded because these forms of research had not yet undergone peer review.
Studies that passed title and abstract screening were assessed in full text by the principal reviewer (KB), and those studies meeting all inclusion criteria were then included in the synthesis. Study inclusion criteria were as follows: (i) experimental study that was performed in a rodent model (mouse, rat, guinea pig, hamster), (ii) the intervention administered exogenous LPS in vivo systemically, (iii) a control group was used for comparison, and (iv) bone outcomes were examined. Bone outcomes from the following analysis methods were included: calipers/measuring tape, dual energy X‐ray absorptiometry (DXA), micro–computed tomography (μCT), mechanical strength testing, microindentation, bone ashing, histology, dynamic bone histomorphometry, specific bone cell staining, blood biomarkers related to bone formation or resorption. In the case where a decision for study inclusion was not straightforward, input from the other reviewers (SP, WW) was sought, and decisions were made by consensus. All such decisions were documented.
Data extraction
Data extraction was performed independently and in duplicate by two researchers (KB, RF). The following characteristics were extracted from the included studies: bibliographic information, information about the animal model (species, strain, age, sex, number of animals per group), LPS intervention (serotype, dosage, route of administration, duration, delivery schedule), and bone outcome measures (trabecular and cortical bone structure, bone quantity, bone strength, bone cell staining, serum bone biomarkers). None of the reviewed studies performed microindentation or bone ashing. Data were extracted from tables/text first, and in cases where data were only presented in a graphical format, corresponding authors were contacted via email with a request for additional information. In cases where no response was received within 2 weeks, graphical data were extracted using Web Plot Digitizer (https://apps.automeris.io/wpd/).
Risk of bias assessment
The risk of bias of each included study was assessed using the Systematic Review Center for Laboratory animal Experimentation (SYRCLE) risk of bias tool.( 38 ) This tool consists of 10 questions to identify different types of potential bias, including selection (sequence generation, baseline characteristics, allocation concealment), performance (random housing, blinding), detection (random outcome assessment, blinding), attrition (incomplete outcome data), and reporting (selective outcome reporting), and other (other sources) bias in studies. A yes indicated a low risk of bias, no a high risk of bias, and unclear that there was insufficient information to evaluate the risk of bias. A detailed description of the types of risk of bias are summarized in Table S2.
Statistical analysis
Effect estimates
For each individual study, the effect was calculated as the standardized mean difference (SMD) Hedge's g between the LPS and control group using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, 2020). The SMD was calculated as follows:
Meta‐analysis
Bone outcomes were presented as mean ± standard deviation (SD). We pooled study‐specific SMD and corresponding 95% confidence intervals (CIs) with a DerSimonian and Laird random‐effects meta‐analysis for each outcome. Subgroup analysis by skeletal site was performed if there were at least three studies per skeletal site. For studies that reported multiple skeletal sites, time points, or LPS dosages, each set of data was included in the appropriate meta‐analysis, but the study was only counted once. Heterogeneity was quantified using the I 2 statistic. We pooled shorter‐term (<2 weeks) and longer‐term (>2 weeks) LPS interventions separately because of the intractable differences in design features, such as mode of administration and dose. This was supported by a meta‐analysis testing subgroup analysis by duration, which demonstrated a significant difference between studies less than 2 weeks in duration and studies greater than 2 weeks in duration for BV/TV (Fig. S1) but not vBMD (Fig. S2). Differences between these study designs are summarized in Table S3.
Missing data
In cases where the sample size was reported as a range, the lowest number was used. To maximize the use of data, for studies that did not report a sample size, the average sample size for studies lasting <2 weeks was assumed when missing in studies lasting <2 weeks; and the average sample size for studies lasting ≥ 2 weeks was assumed when missing in studies ≥ 2 weeks. If a study reported the sample size but no measure of variance, a pooled SD from studies that did report the SD was used:
Studies that reported standard error of the mean (SEM) were converted to SD by multiplying the SEM by the square root of the corresponding control or LPS group sample size.
Publication bias
Publication bias was assessed by visual inspection of funnel plots in conjunction with Egger's regression. Where publication bias was suspected, and at least 10 studies were available, we conducted a “trim‐and‐fill” analysis to account for publication bias. The method iteratively estimates the number of studies potentially missing because of publication bias at the iteration stage. Then, at the final pooling stage, the effect sizes and effect‐size standard errors are imputed (“filled in”) for these studies and an overall effect size is estimated. We used the approach of Duval and Tweedie (2000).( 39 )
Software
Data were analyzed using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, 2020) and Stata version 17 (StataCorp LLC, 2019).
Results
Study selection and characteristics
The search strategy returned a total of 1554 studies for assessment after the removal of duplicates. Following title and abstract screening, 344 studies were selected for full text review, of which a total of 113 studies met the inclusion criteria. During data extraction, it was noted that there were three sets of studies that included the same control and LPS data in two papers such that we used one data set from these studies. This resulted in the exclusion of three additional studies. These studies had distinct objectives and the duplication of the control data between studies would lead to a reduction in the number of animals required. Taken together, this brought the total number to 110 studies included in the systematic review. The process of identification, screening, eligibility, and inclusion is summarized in Fig. 1.
Fig. 1.
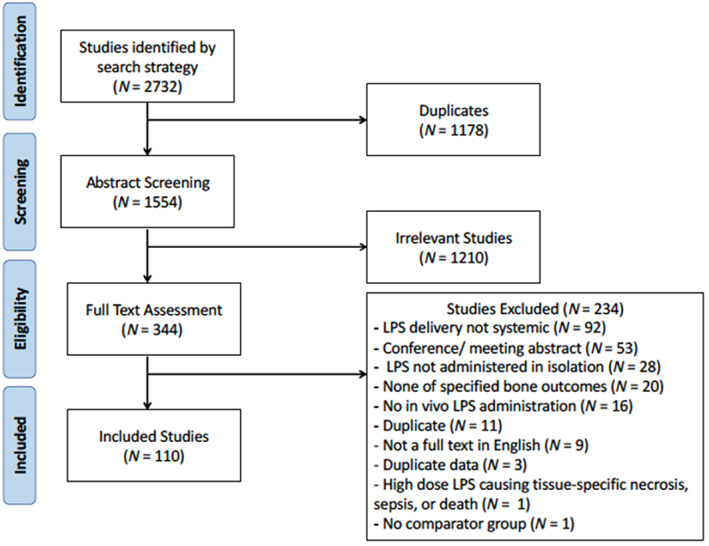
Flow chart of study screening and selection process.
Of the included studies, 83% (n = 91) had an experimental duration of less than 2 weeks and 17% of studies (n = 19) had an experimental duration of greater than 2 weeks. Two studies were included in the short‐term experiment that used a 2‐week LPS intervention but examined the effects on bone outcomes either 6 weeks( 40 ) or 8 weeks( 41 ) after the initial LPS exposure. The LPS exposure duration is summarized in Fig. 2. In terms of LPS administration, 92% of the studies (n = 101) used injections, whereas 8% of studies (n = 9) used LPS incorporated into slow‐release pellets. Only 5% of the studies (n = 5) were in rats, all of which were Sprague–Dawley strain, whereas 95% of the studies (n = 105) used mice, including ICR, C57BL/6J, BALB/c, ddY, DBA/1 J, and DBA/2 mouse strains; two studies did not specify the mouse strain. For an experimental duration of less than 2 weeks, 64% (n = 58) of the studies included only males, 5% (n = 5) of the studies included only females, and 31% (n = 28) of the studies did not specify the sex studied. For experimental durations of greater than 2 weeks, 19% (n = 6) of the studies included only males and 58% (n = 11) included only females, and sex was not specified in 11% (n = 2) of the studies.
Fig. 2.
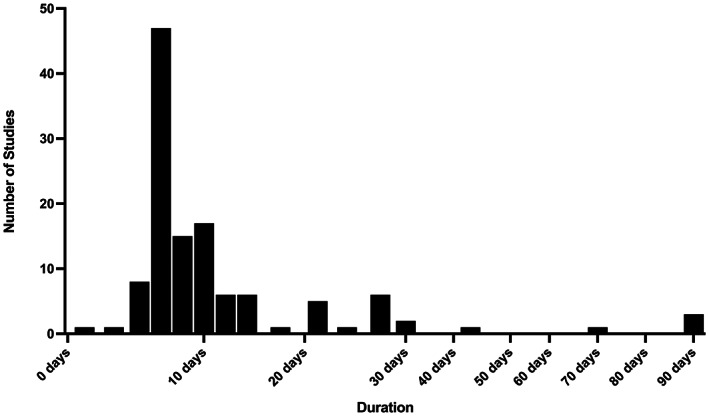
Duration of lipopolysaccharide (LPS) administration in included studies (n = 110). Given the clustering of studies with LPS administration less than 2 weeks with more homogeneous study design compared to studies with LPS administration greater than 2 weeks, this 2‐week cutoff was used for analysis.
Risk of bias
All studies were considered to have an unclear risk of bias according to the SYRCLE risk of bias tool. For selection bias, 37% (n = 41) of the studies reported randomization to groups, but none provided information on the methods used to generate the allocation sequence. Although 17% (n = 19) of the studies reported body weight either at baseline or at the study endpoint, only 2% (n = 2) of the studies stratified randomization of animals by body weight to ensure group body weights were similar at baseline in all groups. There was no reported allocation concealment, random housing, or random outcome assessment in any of the studies. In one study, weight loss and disease severity were monitored in a “blinded manner” to avoid performance bias, and in one other study (1%), μCT analysis was blinded to avoid detection bias. Determining the attrition bias for incomplete data reporting was difficult given that only 21% (n = 23) of the studies reported the number of animals used in the experiment and in the data analysis. However, details were lacking, so the studies that did report the number of animals in the experimental design and analysis were classified as unclear. The reporting bias and reporting of measures against other types of bias were not mentioned in any of the studies. The results of the risk of bias evaluation are presented in Fig. 3.
Fig. 3.
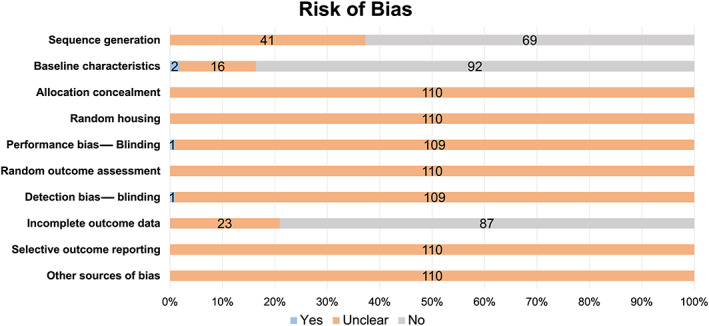
Results of the risk of bias for 110 studies according to the SYRCLE risk of bias tool. “Yes” indicates low risk of bias, “no” high risk of bias, and “unclear” an unclear risk of bias. The stacked bars represent the percentage of studies, and the numbers within each bar represent the number of studies.
Bone structure and BMD
Skeletal sites analyzed using μCT and DXA included the tibia, femur, and lumbar vertebra. Experimental durations less than 2 weeks generally consisted of two to three injections of LPS ranging from 5 to 10 mg/kg body weight per injection with bones collected for ex vivo analysis within 2 weeks of the first LPS exposure. This type of experimental design consistently demonstrated negative effects of LPS on trabecular bone structure, including BV/TV, Tb.N, and Tb.Sp, in the femur( 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 ) and tibia.( 68 , 119 , 120 , 121 , 122 , 123 , 124 ) In studies that reported negative effects of LPS exposure on trabecular bone structure, approximately half (22/47) also reported a decreased Tb.Th in the femur( 48 , 55 , 59 , 70 , 77 , 81 , 88 , 89 , 94 , 97 , 98 , 99 , 100 , 101 , 107 , 108 , 114 , 117 , 123 ) and tibia.( 119 , 120 , 122 ) These alterations in trabecular bone structure were in conjunction with reduced vBMD of the femur( 48 , 58 , 60 , 61 , 65 , 66 , 68 , 77 , 79 , 80 , 82 , 83 , 95 , 99 , 102 , 103 , 106 , 107 , 108 , 109 , 114 , 115 , 116 , 121 ) and tibia( 68 ) measured using μCT and areal BMD (aBMD) of the femur( 49 , 50 , 58 , 111 , 125 , 126 ) and tibia( 124 ) measured using DXA. Cortical bone structure, including Ct.Ar/Tt.Ar and Ct.Th, remained unchanged in three out of four studies,( 98 , 106 , 117 ) but both studies that analyzed TMD reported negative effects of LPS exposure.( 64 , 65 ) Notably, the two studies that utilized a LPS exposure for less than 2 weeks but examined the lingering effects either 4 or 8 weeks after the first LPS injection also reported negative effects of LPS on trabecular bone including, BV/TV, Tb.N, and Tb.Sp with no change in Tb.Th, but did not examine cortical bone.( 40 , 41 ) It is unlikely that cortical bone would be altered within this short exposure to LPS.
Experimental durations greater than 2 weeks demonstrated negative effects of LPS on trabecular bone structure, including BV/TV, Tb.N, and Tb.Sp, in the femur,( 30 , 32 , 35 , 127 , 128 , 129 , 130 , 131 ) tibia,( 33 , 132 , 133 ) and lumbar vertebra.( 30 , 31 , 132 , 134 ) Similar to shorter‐duration LPS exposure, seven out of 11 studies that reported negative effects on trabecular bone structure also reported a decrease in Tb.Th in the femur,( 30 , 35 , 129 , 130 , 131 ) tibia,( 135 ) and lumbar vertebra.( 136 ) These alterations in bone structure were also reflected in a reduced vBMD of the femur( 127 , 129 , 131 , 137 , 138 , 139 ) and lumbar vertebra( 134 ) measured using μCT analysis and aBMD of the femur,( 34 , 35 , 36 ) tibia,( 132 ) and lumbar vertebra( 31 , 36 , 128 , 132 ) measured using DXA. In all eight of the studies that reported cortical bone outcomes (Ct.Ar, Tt.Ar, Ct.Ar/Tt.Ar, Ct.Th), these measures remained unaffected by LPS exposure.( 30 , 31 , 32 , 35 , 36 , 132 , 133 )
In particular, one study examining multiple skeletal sites reported that trabecular bone outcomes in lumbar vertebra were negatively impacted, whereas the tibia bone outcomes remained unchanged in response to the LPS exposure.( 31 ) Additionally, the effects of dose and duration in mice were demonstrated comparing LPS dosages of 0.01 mg/kg/day and 0.1 mg/kg/day for either 30 or 90 days in duration. This experimental design demonstrated a general decline in tibia trabecular bone outcomes in the 30‐day but not 90‐day LPS exposure regardless of dosage, while cortical bone outcomes were unaffected regardless of dosage or duration.( 133 ) Alternatively, in rats, 90‐day LPS exposure only demonstrated limited negative affects using a greater LPS exposure of 0.0333 mg/day but not 0.0033 mg/day.( 36 ) However, a different study in mice comparing durations of LPS exposure for 4, 6, or 10 weeks at a dosage of 0.1 mg/kg/day did not affect either trabecular or cortical bone structure or aBMD in the tibia.( 140 ) In general, LPS exposure greater than 2 weeks negatively impacted trabecular bone structure and BMD, though there are some discrepant findings among studies, and these may be attributed to the broader range in the duration, dose, and delivery of LPS in the experimental design.
Meta‐analysis of the effects of LPS on BV/TV and vBMD
LPS negatively impacted BV/TV regardless of experimental duration. Of the 91 studies utilizing a LPS exposure of less than 2 weeks, 79 studies reported BV/TV and 27 studies reported vBMD. The meta‐analysis indicated reduced BV/TV in the femur (SMD = −3.81%, 95% CI [−4.25, −3.37]; p < 0.01) with substantial heterogeneity (I 2 = 63%) and tibia (SMD = −3.68%, 95% CI [−4.92, −2.44]; p < 0.01) with substantial heterogeneity (I 2 = 63%). Overall LPS had a significant effect on BV/TV regardless of skeletal site (subgroup difference p > 0.01) in short‐term studies (SMD = −3.79%, 95% CI [−4.20, −3.38]; p < 0.01) with substantial heterogeneity (I 2 = 62%) (Fig. 4). Similarly, vBMD was reduced in the femur (SMD = −3.33%, 95% CI [−4.02, −2.63]; p < 0.01) with substantial heterogeneity (I 2 = 66%) and tibia (SMD = −1.67%, 95% CI [−3.57, 0.23]; p < 0.01) with substantial heterogeneity (I 2 = 87%). This resulted in a total effect of LPS regardless of skeletal site (subgroup difference p > 0.01) in studies less than 2 weeks in duration (SMD = −3.11%, 95% CI [−3.78, −2.44]; p < 0.01) with substantial heterogeneity (I 2 = 72%) (Fig. 5).
Fig. 4.
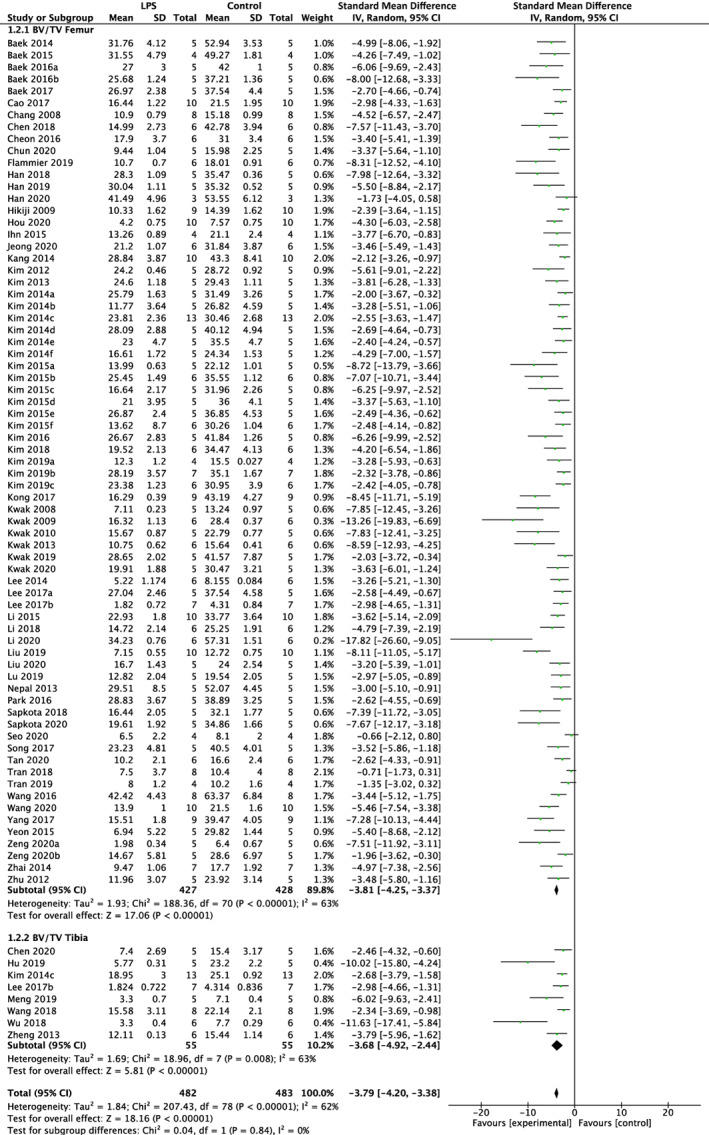
Effect of lipopolysaccharide (LPS) on BV/TV of femur and tibia in studies lasting less than 2 weeks. LPS, lipopolysaccharide; BV/TV, bone volume fraction; CI, confidence interval; SD, standard deviation; IV, weighted mean difference.
Fig. 5.
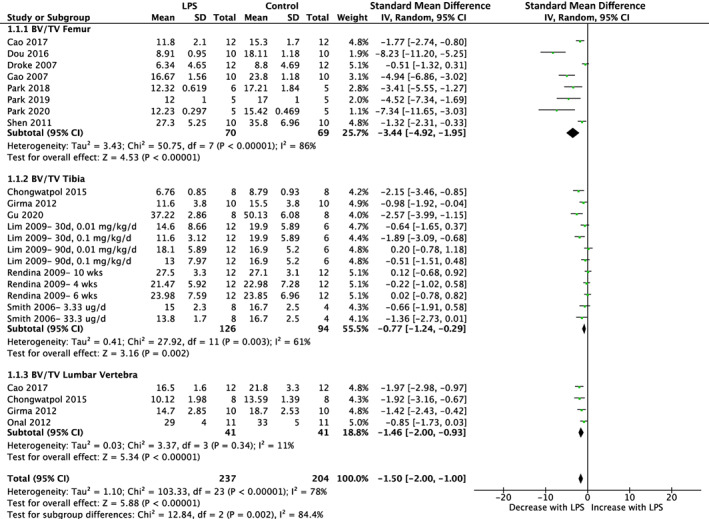
Effect of lipopolysaccharide (LPS) on vBMD of femur and tibia in studies lasting less than 2 weeks. LPS, lipopolysaccharide; vBMD, volumetric bone mineral density; CI, confidence interval; SD, standard deviation; IV, weighted mean difference.
Of the 19 studies utilizing a LPS exposure of greater than 2 weeks, 15 reported BV/TV and eight reported vBMD. The femur demonstrated reduced BV/TV in response to LPS (SMD = −3.44%, 95% CI [−4.92, −1.95]; p < 0.01) with substantial heterogeneity (I 2 = 86%), as well as the tibia (SMD = −0.77%, 95% CI [−1.24, −0.29]; p < 0.01) with substantial heterogeneity (I 2 = 61%) and lumbar vertebra (SMD = −1.46%, 95% CI [−2.00, −0.93]; p < 0.01) with low heterogeneity (I 2 = 11%). Subgroup analysis for different skeletal sites was significant (p < 0.01) and indicated that the femur was associated with the greatest decline in BV/TV, followed by the lumbar vertebra and tibia. Overall, the effect of LPS on BV/TV in studies of longer duration resulted in reduced BV/TV (SMD = −1.50%, 95% CI [−2.00, −1.00]; p < 0.01) with substantial heterogeneity (I 2 = 78%) (Fig. 6). Of the studies that measured vBMD except for one study that analyzed the lumbar vertebra, all other studies analyzed the femur skeletal site. LPS reduced vBMD across skeletal sites (SMD = −3.49%, 95% CI [−4.94, −2.04]; p < 0.01) with substantial heterogeneity (I 2 = 82%) (Fig. 7).
Fig. 6.
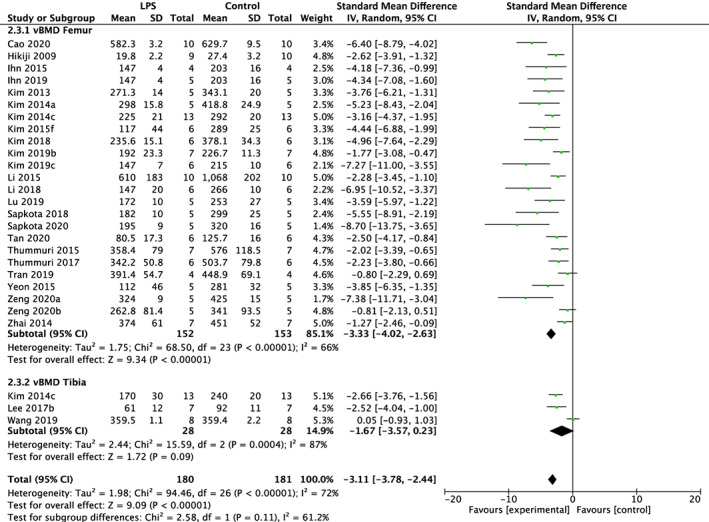
Effect of lipopolysaccharide (LPS) on BV/TV of femur, tibia, and lumbar vertebra in studies lasting more than 2 weeks. LPS, lipopolysaccharide; BV/TV, bone volume fraction; CI, confidence interval; SD, standard deviation; IV, weighted mean difference.
Fig. 7.
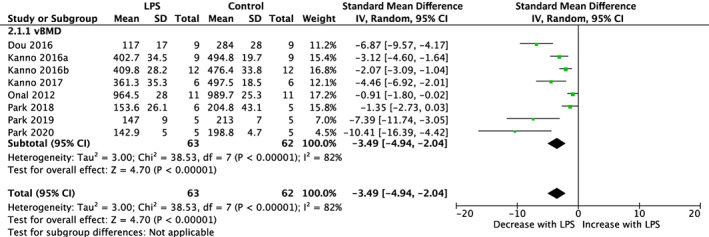
Effect of lipopolysaccharide (LPS) on vBMD of femur and lumbar vertebra in studies lasting more than 2 weeks. LPS, lipopolysaccharide; vBMD, volumetric bone mineral density; CI, confidence interval; SD, standard deviation; IV, weighted mean difference.
According to the Egger regression results, publication bias was determined to be present in both short‐ and long‐term LPS studies for BV/TV and vBMD. Within the short‐term study duration, trim‐and‐fill analysis imputed a total of 23 studies for BV/TV while no studies were imputed for vBMD, whereas within the long‐term study duration no studies were imputed either for BV/TV or vBMD following the trim‐and‐fill analysis. Funnel plots are presented for short‐duration LPS studies (Fig. S3) and long‐duration LPS studies (Fig. S4).
Bone histomorphometry
Studies that utilized LPS exposures for less than 2 weeks reported negative alterations in histological trabecular bone structure, including BV/TV, Tb.Th, Tb.N, and Tb.Sp, in the femur( 106 , 126 , 141 ) and tibia.( 119 , 122 , 123 , 142 ) Notably, four of these seven studies( 106 , 119 , 122 , 123 ) also reported μCT findings and demonstrated an agreement in the structure data obtained through both histomorphometry and μCT analyses. In line with these data, there was also a reduction in mineral apposition rate (MAR)( 106 ) and an increase in eroded surface/bone surface (ES/BS),( 65 , 67 , 70 , 81 , 86 , 88 , 94 , 96 , 100 , 102 , 103 , 118 ) along with an increase in both the number of osteoclasts( 41 , 42 , 43 , 44 , 45 , 46 , 52 , 56 , 57 , 60 , 61 , 62 , 63 , 69 , 70 , 75 , 76 , 77 , 78 , 86 , 88 , 89 , 90 , 93 , 101 , 102 , 103 , 104 , 105 , 106 , 111 , 113 , 117 , 121 , 122 , 123 , 126 , 141 , 142 ) and osteoclast surface.( 50 , 54 , 65 , 67 , 81 , 87 , 106 , 107 , 117 , 118 , 122 , 123 , 142 ) Only one study reported no change in osteoblast surface.( 118 )
One study utilizing a LPS exposure of greater than 2 weeks measured histological trabecular bone structure in the tibia. There was a negative effect of LPS (0.0333 mg/day) over a duration of 12 weeks on trabecular BV/TV, Tb.Th, and Tb.Sp, with no alterations in cortical bone parameters. Within this same study, these histological data support the femur μCT analysis that reported negative alterations in femur trabecular structure and a lack of change in cortical bone.( 35 ) Dynamic bone parameters were also analyzed in the tibia, with no change in the periosteal cortical dynamic bone histomorphometry measures, including MAR, mineralized surface/bone surface MS/BS, and bone formation rate/bone surface and an increase in endocortical MAR, MS/BS, and ES/BS in response to LPS exposure,( 35 ) whereas other studies reported an increased ES/BS,( 35 ) as well as the number of osteoclasts( 129 , 131 , 139 , 143 ) and osteoclast surfaces.( 127 , 129 , 143 ) None of the longer‐term LPS exposure studies reported osteoblast bone cell staining.
Bone strength
Few studies measured bone strength, with only one study testing the mechanical strength and other studies using finite‐element analysis to simulate the mechanical strength of a skeletal site. A study by Wang et al.( 40 ) found that total force prior to fracture, but not stiffness or elastic modulus, was reduced following LPS exposure. The duration of LPS exposure was less than 2 weeks and femur bone strength were measured 6 weeks after the initial dose of LPS.
Studies that had a LPS exposure of greater than 2 weeks and assessed bone strength used finite‐element analysis as a surrogate measure of mechanical strength. Studies that reported negative alterations in trabecular bone structure also consistently reported reduced total force prior to fracture, physiological force, and/or size‐independent stiffness in the femur,( 32 , 35 ) tibia,( 31 ) and lumbar vertebra.( 31 , 132 ) Stiffness was reduced in three out of four studies in the femur,( 32 ) tibia,( 31 , 36 ) and lumbar vertebra,( 31 ) whereas von Mises stress was increased in three out of four studies that reported this measure in the femur,( 32 ) tibia,( 36 ) and lumbar vertebra.( 31 ) Interestingly, in two studies that reported nonsignificant changes in tibia trabecular bone structure, there was reduced total force, physiological force, stiffness, and size‐independent stiffness and increased von Mises stresses in response to LPS.( 31 , 36 ) In contrast, Rendina et al.( 144 ) reported no changes in any measures of bone strength (total force and size independent stiffness), and these findings aligned with the findings that trabecular bone structure was also unchanged with LPS exposure.
Serum biomarkers
Negative alterations in bone structure and strength associated with short‐term LPS exposure for less than 2 weeks were supported by an increase in bone resorption markers and an overall decrease in bone formation markers. Serum biomarkers for bone resorption, including receptor activator of nuclear factor kappa B ligand (RANKL)( 41 , 43 , 73 , 76 , 84 , 98 , 105 ) and cross‐linked c‐telopeptide of type I collagen (CTX‐1),( 57 , 76 , 77 , 88 , 105 ) were elevated, whereas osteoprotegerin (OPG),( 43 , 76 , 84 , 98 , 105 ) a serum biomarker for the inhibition of bone resorption, was reduced in five out of six studies in response to LPS exposure. Overall, there was an increase in the RANKL to OPG ratio favoring bone resorption over bone formation.( 43 , 73 , 76 , 84 , 98 , 105 )
The effects of LPS exposure for more than 2 weeks resulted in an increase in serum markers of bone resorption and no change in serum markers of bone formation. Specifically, serum CTX‐1 was elevated in all studies,( 128 , 129 , 130 , 131 ) whereas one out of two studies reported an elevation in serum tartrate‐resistant acid phosphatase (TRAP).( 34 ) In terms of serum bone formation markers, bone specific alkaline phosphatase( 30 ) and alkaline phosphatase( 129 , 130 , 131 ) were unchanged in all studies, whereas OC remained unchanged in response to LPS in four out of five studies.( 128 , 129 , 130 , 131 )
Discussion
Rodent models are integral to current research investigating the underlying inflammatory component of osteoporosis. Our systematic review found that regardless of study duration, LPS negatively impacted trabecular bone structure and BMD and upregulated bone resorption. The negative alterations in bone outcomes reported in the rodent models mimic the characteristics of osteoporosis in humans, including reduced BMD with weakened trabecular bone structure, specifically reduced Tb.N and Tb.Th. In humans, there is greater bone loss with aging due to a shift in the ratio of bone resorption to bone formation.( 145 )
The negative impact of LPS on bone outcomes was more consistent and pronounced among studies that lasted less than 2 weeks. This may be explained by the relative homogeneity of the designs and LPS interventions among these studies, which generally consisted of two to three injections utilizing a LPS dosage ranging from 5 to 10 mg/kg body, resulting in a higher LPS exposure. These short‐term studies often used in vivo LPS exposure as a proof of concept to support cell culture work. However, osteoporosis is a chronic disease, and the studies that lasted more than 2 weeks may better represent the pathophysiology of this disease.
Studies greater than 2 weeks in duration had a more heterogeneous LPS intervention design, including delivery (injections or slow‐release pellets), LPS dosage, and study duration (ranging from 16 days to 13 weeks). LPS exposure could not be compared across studies since some dosages were reported as an absolute LPS exposure (e.g., mg/day) versus a relative LPS exposure (e.g., mg/kg body weight/day). Although the relative exposure accounts for body size, slow‐release pellets implanted at the beginning of the study would not be adjustable for changes in body weight over the course of the study. A benefit of the slow‐release pellets is that they offer long‐term LPS exposure compared to injections. However, research testing the delivery rate of 17β‐estradiol via slow‐release pellets reported an initial bolus release followed by a substantial decline.( 146 , 147 )
Differences in study duration and design were supported by the meta‐analysis of BV/TV, which indicated that the short‐term studies that were less than 2 weeks in duration had a greater effect size based on the SMD and 95% CI compared to the longer‐term studies that lasted more than 2 weeks. Of the trabecular bone outcomes only BV/TV was included in the meta‐analysis since the other structural outcomes (Tb.N, Tb.Th, Tb.Sp) influence BV/TV. In addition, although osteoporosis in humans is assessed using aBMD quantified by DXA, this measure is not typically reported in rodent studies because it is not sensitive enough to capture changes in bone. Additionally, there were only enough studies to conduct a meta‐analysis on vBMD, not aBMD. We found no difference in skeletal site subgroup analysis for the short‐term studies, but the long‐term studies indicated a greater effect of LPS in the femur compared to the lumbar vertebra and tibia. However, it is important to consider both the substantial heterogeneity among studies when interpreting these results and the small sample size for some of the subgroup analyses that may influence the I 2 statistic. This encompasses multiple study design aspects, including differences in LPS delivery method (injection versus slow‐release pellet), LPS dose, LPS delivery schedule (multiple injections versus continuous exposure), and animal characteristics such as species (mouse versus rat), strain (inbred versus outbred), age, and sex (summarized in Table S3). In particular, animal strain can influence the inflammatory effect of LPS, given that some inbred strains, such as the C57BL/6J mouse, have blunted inflammatory responses,( 148 ) although none of the studies analyzed in this systematic review utilized the C3H/HeJ or C57BL/10ScCr mouse strains because these models are known to carry TLR4 mutations, making them resistant to LPS.( 149 )
The effects of study duration are difficult to assess across studies because of several additional important design features that may not be a consequence of duration (e.g., LPS dosing regimen and/or administration method). Conversely, Lim et al.( 133 ) compared both duration (30 days and 90 days) and LPS dosage (0.01 and 0.1 mg/kg/day slow‐release pellets) and reported a general decline in tibia trabecular bone outcomes. In contrast, Rendina et al.( 140 ) used the same dosage (0.1 mg/kg/day) as the aforementioned study, examining LPS durations of 4, 6, or 10 weeks; however, there was no effect on bone outcomes. Although both studies used C57BL/6J mice that were similar in age, Lim et al.( 133 ) used male mice while Rendina et al.( 140 ) used hormonally intact female mice. The protective anti‐inflammatory role of estrogen signaling may in part explain the discrepancy between the two studies,( 82 ) highlighting the need to consider sex when designing experiments.
Although we chose to focus on systemic LPS exposure and did not include studies using localized LPS injections (e.g., direct injections in the gingiva to induce periodontitis), skeletal site may also be differentially affected by LPS. This may be partially due to differences in weight bearing and loading patterns of different skeletal sites.( 150 ) Chongwatpol et al.( 31 ) reported that the lumbar vertebra but not the tibia was negatively impacted by LPS exposure. Since the lumbar vertebra is a less‐weight‐bearing site compared to the tibia, the lumbar vertebra would have less mechanical loading, which promotes bone formation and may in part explain the detrimental effect of the LPS exposure measured at this site and not the tibia.
Bone strength is a function of both bone quantity (mineral) and bone quality (three‐dimensional structure).( 151 ) Most of the included studies in this systematic review analyzed bone quantity and quality, but few assessed the mechanical properties of bone that provide helpful insight into the actual strength at a skeletal site. Compression testing would provide useful data about trabecular strength, while a three‐ or four‐point bending test would measure cortical strength. Of the reported studies in this systematic review, only one reported mechanical strength testing using a three‐point bending test,( 40 ) while three studies simulated trabecular compression testing using finite‐element analysis.( 31 , 32 , 132 ) However, previous work examining human osteoporotic specimens with femoral neck fractures demonstrated that cancellous bone microarchitecture, including BV/TV, Tb.N, and Tb.Sp, were correlated with fracture toughness (i.e., the ability to resist fracture following a crack).( 152 ) This relationship between bone structure and mechanical properties suggests that the bone structure analysis is translatable to bone strength. Reductions in bone strength, compromised bone structure, and lower BMD with both short‐ and longer‐term LPS exposure was supported by an increase in bone resorption as demonstrated by a consistent increase in serum bone resorption markers, histological osteoclast staining, and eroded bone surface in all studies that assessed these outcomes.
The increase in bone resorption was demonstrated by the upregulation in osteoclast activity through serum biomarkers and bone‐specific staining, while osteoblast activity remained relatively unchanged through serum biomarkers. The increase in RANKL to OPG ratio favored bone resorption, and osteoclast staining in bone was also increased. Previous cell culture studies demonstrated the potent effect of LPS upregulating osteoclasts,( 20 ) which is in line with these results across studies. However, exogenous LPS exposure did not inhibit osteoblasts as previously described using cell culture.( 21 )
In humans, aging is associated with an elevated circulating LPS concentration, which is suggested to contribute to the pro‐inflammatory state associated with aging.( 18 ) Additionally, an increase in gut permeability has also been proposed to contribute to age‐ related inflammation.( 19 ) LPS is a component of gram‐negative bacteria located in the gut,( 17 ) and with aging there is an increase in LPS translocation from the intestines into circulation.( 18 ) Since the prevalence of osteoporosis increases with age and emerging evidence supports the role of inflammation in the pathophysiology of this disease, developing a rodent model for LPS‐induced bone loss is pertinent to understanding osteoporosis. Furthermore, it will be important to consider the role of both inflammation and estrogen deficiency in future models to elucidate the relationship between these two aspects related to osteoporosis. The selection of a rodent model is an important consideration given that estrogen deficiency differentially affects some species and strains; in particular, female C57BL/6J mice lose bone at a young age and develop spontaneous osteopenia.( 153 )
Even though there was no time restriction, included studies were all published after 2003, with the majority being published in 2020. This may in part be explained by a shift toward understanding the underlying inflammatory pathophysiology of osteoporosis and increased accessibility to μCT and DXA analysis. Of note, none of the studies analyzed bone outcomes in vivo to measure changes longitudinally. Although at the time of this systematic search a study from our lab longitudinally measuring in vivo bone outcomes in response to exogenous LPS delivered via osmotic pumps had not yet been published, we found that trabecular and cortical bone outcomes were unaffected in male and female CD‐1 mice.( 154 ) As previously stated, slow‐release pellets are suggested to have a less consistent release rate than other delivery methods, and we hypothesized that, given the more consistent release rate of osmotic pumps, a higher dosage of LPS would be required to induce alterations in bone outcomes. This should be a future consideration in study design to better understand the development of bone loss in response to LPS and inflammation.
Strengths and limitations
The comprehensive set of bone outcomes analyzed was a major strength of this systematic review. The encompassing set of analysis techniques allowed for both the physical (structure and BMD) and physiological (bone‐specific staining, serum biomarkers) aspects of bone to be assessed.
There are several limitations of this systematic review and meta‐analysis. In general, the sample size of the included studies was small, and in some studies the sample size was not reported. In terms of study design, it was most often the LPS bacterial strain and not the serotype that was reported, and in some cases the LPS dosage and duration were unclear, whereas for animal characteristics the sex of the animals was sometimes not specified, and body weight was not usually reported. In terms of bone outcome analysis, some studies did not report the μCT scanning parameters, and in some cases no quantitative data were reported and only μCT images of the bone were published. Additionally, only the BV/TV outcome met the criteria for meta‐analysis for both short‐term study durations of less than 2 weeks and longer study durations of more than 2 weeks, and substantial heterogeneity was reported. Overall, there was a substantial unclear risk of bias identified using the SYRCLE risk of bias tool.( 38 ) The missing information is pivotal for interpreting the results, comparing different studies, and reproducibility. This highlights the need for more rigorous reporting and study design considerations in preclinical models to reduce bias and strengthen findings from future systematic reviews. Future studies should follow the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines( 155 ) to ensure study reproducibility and improved research reporting. Finally, language may have been a limitation as the systematic review was restricted to English, so studies meeting the search criteria that were not in English were excluded.
Conclusions
This systematic review found exogenous LPS administration in rodents to be a viable model for studying inflammatory bone loss to better understand the inflammatory pathophysiology of osteoporosis. We found that LPS exposure regardless of duration negatively impacted bone outcomes, including trabecular bone structure at multiple skeletal sites and BMD, and upregulated osteoclast activity, as seen in specific bone cell staining and serum biomarkers.
Author Contributions
Evelyn Feldman: Methodology; resources; writing – review and editing. Russel J. De Souza: Formal analysis; methodology; writing – review and editing. Elena M. Comelli: Supervision; writing – review and editing. Panagiota Klentrou: Supervision; writing – review and editing. Sandra J Peters: Conceptualization; data curation; funding acquisition; supervision; writing – review and editing. Wendy E Ward: Conceptualization; funding acquisition; supervision; writing – review and editing.
Conflicts of Interest
E.M.C. received research support from Lallemand Health Solutions and Ocean Spray and consultant fees or speaker and travel support from Danone and Lallemand Health Solutions. (All are outside of this study.)
R.J.d.S. has served as an external resource person to the World Health Organization's (WHO) Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodations to attend meetings from 2012–2017 to present and discuss this work. He presented updates of this work to the WHO in 2022. He has also done contract research for the Canadian Institutes of Health Research's Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the WHO, for which he received remuneration. He has received speaker's fees from the University of Toronto and McMaster Children's Hospital. He has held grants from the Canadian Institutes of Health Research, Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator and is a co‐investigator on several funded team grants from the Canadian Institutes of Health Research. He has served as an independent director of the Helderleigh Foundation (Canada). He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada) and a co‐opted member of the Scientific Advisory Committee on Nutrition (SACN) Subgroup on the Framework for the Evaluation of Evidence (Public Health England). (All are outside of this study.)
Author Roles
K.N.B., conceptualization, project administration, data curation, formal analysis, investigation, methodology, visualization, writing—original draft.
E.F., methodology, resources, writing—review and editing.
R.J.d.S., methodology, formal analysis, writing—review and editing.
E.M.C., supervision, writing—review and editing.
N.P., supervision, writing—review and editing.
S.J.P., conceptualization, financial acquisition, data curation, supervision, writing—review and editing.
W.E.W., conceptualization, financial acquisition, data curation, supervision, writing—review and editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4740.
Supporting information
Figure S1. Subgroup analysis by duration of lipopolysaccharide (LPS) for studies less than 2 weeks and greater than 2 weeks in duration on BV/TV. LPS, lipopolysaccharide; BV/TV, bone volume fraction; CI, confidence interval; SD, standard deviation; IV, weighted mean difference.
Figure S2. Subgroup analysis by duration of lipopolysaccharide (LPS) for studies less than 2 weeks and greater than 2 weeks in duration on vBMD. LPS, lipopolysaccharide; vBMD, volumetric bone mineral density; CI confidence interval; SD, standard deviation; IV, weighted mean difference.
Figure S3. Funnel plots for studies shorter than 2 weeks in duration. Contour‐enhanced funnel plot for studies for (A) BV/TV and (B) vBMD. (C) Trim and fill for BV/TV (23 studies imputed; observed SMD = −4.106, 95% CI [−4.607, −3.605]; imputed SMD = −2.979, 95% CI [−3.662, −2.295]) and (D) vBMD (no missing studies imputed). LPS, lipopolysaccharide; BV/TV, bone volume fraction; SMD, standardized mean difference, calculated as Hedge's g; CI confidence interval.
Figure S4. Funnel plots for studies greater than 2 weeks in duration. Contour‐enhanced funnel plot for (A) BV/TV and (B) vBMD. (C) Trim and fill for BV/TV and (D) vBMD. LPS, lipopolysaccharide; vBMD, volumetric bone mineral density (no missing studies were imputed for BV/TV or vBMD); SE, standard error; SMD, standardized mean difference, calculated as Hedge's g.
Table S1. Full systematic search strategy by database.
Table S2. Summary of risk of bias assessment guidelines.
Table S3. Systematic review summary of study characteristics. Sample size represents number of studies.
Acknowledgments
We would like to acknowledge Rebekah Feld (R.F.), who independently performed data extraction to verify the accuracy of the initial data extraction by K.N.B., and the Brock University Library services for their support in developing the systematic search strategy and access to databases. A Natural Science and Engineering Research Council Alexander Graham Bell Canada Graduate Scholarship supported K.N.B. This project was supported by research funding provided by Brock University, the Canada Foundation for Innovation (Grant 222084) and the Canada Research Chairs Program to W.E.W. (Grant 230786) and a Discovery Grant from the Natural Science and Engineering Research Council to S.J.P. (Grant 229767).
Data Availability Statement
The data that support the findings of this study are openly available in Brock University Dataverse at https://doi.org/10.5683/SP3/REEPYJ.
References
- 1. International Osteoporosis Foundation . Epidemiology of osteoporosis and fragility fractures. Facts and Statistics 2022. Available from: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures#ref_bottom_2.
- 2. Epidemiology of Osteoporosis and Fragility Fractures . Facts & Statistics [cited 2021 July 17]. Available from: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures.
- 3. Papaioannou A, Kennedy CC, Ioannidis G, et al. The impact of incident fractures on health‐related quality of life: 5 years of data from the Canadian multicentre osteoporosis study. Osteoporos Int. 2009;20(5):703‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Organization WH . WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Brussels, Belgium: World Health Organization; 2004. [Google Scholar]
- 5. Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010‐2040. Osteoporos Int. 2015;26(9):2243‐2248. [DOI] [PubMed] [Google Scholar]
- 6. Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17(6):1044‐1050. [DOI] [PubMed] [Google Scholar]
- 7. Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Foger‐Samwald U, Ellinger I. Immunology of osteoporosis: a mini‐review. Gerontology. 2016;62(2):128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discovery. 2012;11(3):234‐250. [DOI] [PubMed] [Google Scholar]
- 9. Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. 2014;29(9):2057‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cauley JA, Barbour KE, Harrison SL, et al. Inflammatory markers and the risk of hip and vertebral fractures in men: the osteoporotic fractures in men (MrOS). J Bone Miner Res. 2016;31(12):2129‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh N, Gravallese E. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301‐312. [DOI] [PubMed] [Google Scholar]
- 12. Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122(7):599‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duggan SN, Smyth ND, Murphy A, Macnaughton D, O'Keefe SJ, Conlon KC. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2014;12(2):219‐228. [DOI] [PubMed] [Google Scholar]
- 14. Targher G, Lonardo A, Rossini M. Nonalcoholic fatty liver disease and decreased bone mineral density: is there a link? J Endocrinol Invest. 2015;38(8):817‐825. [DOI] [PubMed] [Google Scholar]
- 15. McFarlane S, Muniyappa R, Shin J, Bahtiyar G, Sowers J. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 16. Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65(12):147‐151. [DOI] [PubMed] [Google Scholar]
- 17. Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. 2020;4(2):bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghosh S, Lertwattanarak R, Garduno Jde J, et al. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol, Ser A. 2015;70(2):232‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qi Y, Goel R, Kim S, et al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J Am Med Dir Assoc. 2017;18(9):810 e1‐810 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou GQ, Guo C, Song GH, et al. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX‐2 expression in RAW264.7 cells. Int J Mol Med. 2013;32(2):503‐510. [DOI] [PubMed] [Google Scholar]
- 21. Guo C, Yuan L, Wang JG, et al. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3‐E1 cells. Inflammation. 2014;37(2):621‐631. [DOI] [PubMed] [Google Scholar]
- 22. Bar‐Shavit Z. Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity. 2008;41(3):195‐203. [DOI] [PubMed] [Google Scholar]
- 23. Dekkers PEP, Juffermans NP, ten Hove T, de Jonge E, van Deventer SJH, van der Poll T. Granulocyte colony‐stimulating factor receptors on granulocytes are down‐regulated after endotoxin administration to healthy humans. J Infect Dis. 2000;181:2067‐2070. [DOI] [PubMed] [Google Scholar]
- 24. Boettcher S, Gerosa RC, Radpour R, et al. Endothelial cells translate pathogen signals into G‐CSF‐driven emergency granulopoiesis. Blood. 2014;124(9):1393‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Lévesque J‐P. Osteoclast‐mediated bone resorption is stimulated during short‐term administration of granulocyte colony‐stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92(9):3465‐3473. [PubMed] [Google Scholar]
- 26. Komori T. Animal models for osteoporosis. Eur J Pharmacol. 2015;759:287‐294. [DOI] [PubMed] [Google Scholar]
- 27. Guingamp C, Gegout‐Pottie P, Philippe L, Terlain B, Netter P. Mono‐iodoacetate‐induced experimental osteoarthritis: a dose‐response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40:1670‐1679. [DOI] [PubMed] [Google Scholar]
- 28. Lin C, Moniz C, Chambers TJ, Chow JWM. Colitis causes bone loss in rats through suppression of bone formation. Gastroenterology. 1996;111:1263‐1271. [DOI] [PubMed] [Google Scholar]
- 29. Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis‐induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis. 2013;19(8):1586‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao JJ, Gregoire BR, Shen C‐L. A high‐fat diet decreases bone mass in growing mice with systemic chronic inflammation induced by low‐dose, slow‐release lipopolysaccharide pellets. J Nutr. 2017;147(10):1909‐1916. [DOI] [PubMed] [Google Scholar]
- 31. Chongwatpol P, Rendina‐Ruedy E, Stoecker BJ, Clarke SL, Lucas EA, Smith BJ. Implications of compromised zinc status on bone loss associated with chronic inflammation in C57BL/6 mice. J Inflamm Res. 2015;8:117‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Droke EA, Hager KA, Lerner MR, et al. Soy isoflavones avert chronic inflammation‐induced bone loss and vascular disease. J Inflamm. 2007;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu Y, Wu Z, Zeng F, et al. Systemic exposure to lipopolysaccharide from Porphyromonas gingivalis induces bone loss‐correlated Alzheimer's disease‐like pathologies in middle‐aged mice. J Alzheimers Dis. 2020;78(1):61‐74. [DOI] [PubMed] [Google Scholar]
- 34. Shen C‐L, Yeh JK, Cao JJ, Tatum OL, Dagda RY, Wang J‐S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation‐induced bone loss model. J Nutr Biochem. 2010;21(10):968‐974. [DOI] [PubMed] [Google Scholar]
- 35. Shen CL, Yeh JK, Samathanam C, et al. Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporos Int. 2011;22(1):327‐337. [DOI] [PubMed] [Google Scholar]
- 36. Smith BJ, Lerner MR, Bu SY, et al. Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone. 2006;38(3):378‐386. [DOI] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. [DOI] [PubMed] [Google Scholar]
- 40. Wang X‐F, Wang Y‐J, Li T‐Y, et al. Colony‐stimulating factor 1 receptor inhibition prevents against lipopolysaccharide‐induced osteoporosis by inhibiting osteoclast formation. Biomed Pharmacother. 2019;115:108916. [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Wu X, Duan Y. Magnesium lithospermate B protects against lipopolysaccharide‐induced bone loss by inhibiting RANKL/RANK pathway. Front Pharmacol. 2018;9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baek JM, Kim J‐Y, Ahn S‐J, et al. Dendrobium moniliforme exerts inhibitory effects on both receptor activator of nuclear factor kappa‐B ligand‐mediated osteoclast differentiation in vitro and lipopolysaccharide‐induced bone erosion in vivo. Molecules. 2016;21(3):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baek JM, Kim J‐Y, Cheon Y‐H, et al. Aconitum pseudo‐laeve var. erectum inhibits receptor activator of nuclear factor kappa‐B ligand‐induced osteoclastogenesis via the c‐Fos/nuclear factor of activated T‐cells, cytoplasmic 1 signaling pathway and prevents lipopolysaccharide‐induced bone loss in mice. Molecules. 2014;19(8):11628‐11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baek JM, Kim J‐Y, Lee CH, Yoon K‐H, Lee MS. Methyl gallate inhibits osteoclast formation and function by suppressing Akt and Btk‐PLCgamma2‐Ca2+ signaling and prevents lipopolysaccharide‐induced bone loss. Int J Mol Sci. 2017;18(3):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baek JM, Kim J‐Y, Yoon K‐H, Oh J, Lee MS. Ebselen is a potential anti‐osteoporosis agent by suppressing receptor activator of nuclear factor kappa‐B ligand‐induced osteoclast differentiation in vitro and lipopolysaccharide‐induced inflammatory bone destruction in vivo. Int J Biol Sci. 2016;12(5):478‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baek JM, Kim JY, Jung Y, et al. Mollugin from Rubea cordifolia suppresses receptor activator of nuclear factor‐B ligand‐induced osteoclastogenesis and bone resorbing activity in vitro and prevents lipopolysaccharide‐induced bone loss in vivo. Phytomedicine. 2015;22(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 47. Cao J, Lu Q, Liu N, et al. Sciadopitysin suppresses RANKL‐mediated osteoclastogenesis and prevents bone loss in LPS‐treated mice. Int Immunopharmacol. 2017;49:109‐117. [DOI] [PubMed] [Google Scholar]
- 48. Cao J, Wang S, Wei C, et al. Agrimophol suppresses RANKL‐mediated osteoclastogenesis through Blimp1‐Bcl6 axis and prevents inflammatory bone loss in mice. Int Immunopharmacol. 2020;90:107137. [DOI] [PubMed] [Google Scholar]
- 49. Chang E‐J, Ha J, Oerlemans F, et al. Brain‐type creatine kinase has a crucial role in osteoclast‐mediated bone resorption. Nat Med. 2008;14(9):966‐972. [DOI] [PubMed] [Google Scholar]
- 50. Chen H, Guo T, Wang D, Qin R. Vaccaria hypaphorine impairs RANKL‐induced osteoclastogenesis by inhibition of ERK, p38, JNK and NF‐kappaB pathway and prevents inflammatory bone loss in mice. Biomed Pharmacother. 2018;97:1155‐1163. [DOI] [PubMed] [Google Scholar]
- 51. Chen K, Geng H, Liang W, et al. Modulated podosome patterning in osteoclasts by fullerenol nanoparticles disturbs the bone resorption for osteoporosis treatment. Nanoscale. 2020;12(17):9359‐9365. [DOI] [PubMed] [Google Scholar]
- 52. Cheon Y‐H, Kim J‐Y, Baek JM, Ahn S‐J, So H‐S, Oh J. Niclosamide suppresses RANKL‐induced osteoclastogenesis and prevents LPS‐induced bone loss. Biochem Biophys Res Commun. 2016;470(2):343‐349. [DOI] [PubMed] [Google Scholar]
- 53. Chun K‐H, Jin HC, Kang KS, Chang T‐S, Hwang GS. Poncirin inhibits osteoclast differentiation and bone loss through down‐regulation of NFATc1 in vitro and in vivo. Biomol Ther. 2020;28(4):337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flammier S, Peyruchaud O, Bourguillault F, et al. Osteoclast‐derived autotaxin, a distinguishing factor for inflammatory bone loss. Arthritis Rheumatol. 2019;71(11):1801‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han B, Geng H, Liu L, Wu Z, Wang Y. GSH attenuates RANKL‐induced osteoclast formation in vitro and LPS‐induced bone loss in vivo. Biomed Pharmacother. 2020;128:110305. [DOI] [PubMed] [Google Scholar]
- 56. Han S‐Y, Kim Y‐K. Berberine suppresses RANKL‐induced osteoclast differentiation by inhibiting c‐Fos and NFATc1 expression. Am J Chin Med. 2019;47(2):439‐455. [DOI] [PubMed] [Google Scholar]
- 57. Han S‐Y, Lee K‐H, Kim Y‐K. Poligoni Multiflori radix enhances osteoblast formation and reduces osteoclast differentiation. Int J Mol Med. 2018;42(1):331‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hikiji H, Ishii S, Yokomizo T, Takato T, Shimizu T. A distinctive role of the leukotriene B4 receptor BLT1 in osteoclastic activity during bone loss. Proc Natl Acad Sci U S A. 2009;106(50):21294‐21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hou H, Peng Q, Wang S, et al. Anemonin attenuates RANKL‐induced osteoclastogenesis and ameliorates LPS‐induced inflammatory bone loss in mice via modulation of NFATc1. Front Pharmacol. 2020;10:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ihn HJ, Lee D, Lee T, et al. Inhibitory effects of KP‐A159, a thiazolopyridine derivative, on osteoclast differentiation, function, and inflammatory bone loss via suppression of RANKL‐induced MAP kinase signaling pathway. PLoS One. 2015;10(11):e0142201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ihn HJ, Lee T, Lee D, et al. Inhibitory effect of KP‐A038 on osteoclastogenesis and inflammatory bone loss is associated with downregulation of BLIMP1. Front Pharmacol. 2019;10(APR):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jeong DH, Kwak SC, Lee MS, Yoon K‐H, Kim J‐Y, Lee CH. Betulinic acid inhibits RANKL‐induced osteoclastogenesis via attenuating Akt, NF‐kappaB, and PLCgamma2‐Ca2+ signaling and prevents inflammatory bone loss. J Nat Prod. 2020;83(4):1174‐1182. [DOI] [PubMed] [Google Scholar]
- 63. Kang DM, Yoon K‐H, Kim J‐Y, et al. CT imaging biomarker for evaluation of emodin as a potential drug on LPS‐mediated osteoporosis mice. Acad Radiol. 2014;21(4):457‐462. [DOI] [PubMed] [Google Scholar]
- 64. Kim H‐J, Yoon K‐A, Lee M‐K, Kim SH, Lee I‐K, Kim S‐Y. A novel small molecule, NecroX‐7, inhibits osteoclast differentiation by suppressing NF‐kappaB activity and c‐Fos expression. Life Sci. 2012;91(19–20):928‐934. [DOI] [PubMed] [Google Scholar]
- 65. Kim H‐J, Yoon K‐A, Yoon H‐J, et al. Liver X receptor activation inhibits osteoclastogenesis by suppressing NF‐kappaB activity and c‐Fos induction and prevents inflammatory bone loss in mice. J Leukoc Biol. 2013;94(1):99‐107. [DOI] [PubMed] [Google Scholar]
- 66. Kim H‐J, Hong JM, Yoon H‐J, et al. Inhibitory effects of obovatol on osteoclast differentiation and bone resorption. Eur J Pharmacol. 2014;723:473‐480. [DOI] [PubMed] [Google Scholar]
- 67. Kim H‐J, Yoon H‐J, Choi J‐Y, Lee I‐K, Kim S‐Y. The tyrosine kinase inhibitor GNF‐2 suppresses osteoclast formation and activity. J Leukoc Biol. 2014;95(2):337‐345. [DOI] [PubMed] [Google Scholar]
- 68. Kim IS, Lee B, Yoo SJ, Hwang SJ. Whole body vibration reduces inflammatory bone loss in a lipopolysaccharide murine model. J Dent Res. 2014;93(7):704‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim J‐Y, Cheon Y‐H, Kwak SC, et al. Emodin regulates bone remodeling by inhibiting osteoclastogenesis and stimulating osteoblast formation. J Bone Miner Res. 2014;29(7):1541‐1553. [DOI] [PubMed] [Google Scholar]
- 70. Kim J‐Y, Cheon Y‐H, Oh HM, et al. Oleanolic acid acetate inhibits osteoclast differentiation by downregulating PLCgamma2‐Ca(2+)‐NFATc1 signaling, and suppresses bone loss in mice. Bone. 2014;60:104‐111. [DOI] [PubMed] [Google Scholar]
- 71. Kim J‐Y, Park S‐H, Oh HM, et al. Ampelopsis brevipedunculata extract prevents bone loss by inhibiting osteoclastogenesis in vitro and in vivo. Molecules. 2014;19(11):18465‐18478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim J, Lee H, Kang KS, Chun K‐H, Hwang GS. Cordyceps militaris mushroom and cordycepin inhibit RANKL‐induced osteoclast differentiation. J Med Food. 2015;18(4):446‐452. [DOI] [PubMed] [Google Scholar]
- 73. Kim J‐Y, Ahn S‐J, Baek JM, Yoon K‐H, Lee MS, Oh J. Ostericum koreanum reduces LPS‐induced bone loss through inhibition of osteoclastogenesis. Am J Chin Med. 2015;43(3):495‐512. [DOI] [PubMed] [Google Scholar]
- 74. Kim J‐Y, Lee MS, Baek JM, Park J, Youn B‐S, Oh J. Massive elimination of multinucleated osteoclasts by eupatilin is due to dual inhibition of transcription and cytoskeletal rearrangement. Bone Rep. 2015;3:83‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim J‐Y, Oh HM, Kwak SC, et al. Purslane suppresses osteoclast differentiation and bone resorbing activity via inhibition of Akt/GSK3beta‐c‐Fos‐NFATc1 signaling in vitro and prevents lipopolysaccharide‐induced bone loss in vivo. Biol Pharm Bull. 2015;38(1):66‐74. [DOI] [PubMed] [Google Scholar]
- 76. Kim J‐Y, Park S‐H, Baek JM, et al. Harpagoside inhibits RANKL‐induced osteoclastogenesis via Syk‐Btk‐PLCgamma2‐Ca(2+) signaling pathway and prevents inflammation‐mediated bone loss. J Nat Prod. 2015;78(9):2167‐2174. [DOI] [PubMed] [Google Scholar]
- 77. Kim K‐J, Yeon J‐T, Choi S‐W, et al. Decursin inhibits osteoclastogenesis by downregulating NFATc1 and blocking fusion of pre‐osteoclasts. Bone. 2015;81:208‐216. [DOI] [PubMed] [Google Scholar]
- 78. Kim J‐Y, Baek JM, Ahn S‐J, et al. Ethanolic extract of Schizonepeta tenuifolia attenuates osteoclast formation and activation in vitro and protects against lipopolysaccharide‐induced bone loss in vivo. BMC Complement Altern Med. 2016;16(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim TH, Jeong CG, Son HU, et al. Ethanolic extract of rubus coreanus fruits inhibits bone marrow‐derived osteoclast differentiation and lipopolysaccharide‐induced bone loss. Nat Prod Commun. 2017;12(12):1925‐1928. [Google Scholar]
- 80. Kim KJ, Jeong MH, Lee Y, et al. Effect of usnic acid on osteoclastogenic activity. J Clin Med. 2018;7(10):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim E‐N, Kim YG, Lee J‐H, Min BS, Jeong G‐S. 6,7,4′‐Trihydroxyflavone inhibits osteoclast formation and bone resorption in vitro and in vivo. Phytother Res. 2019;33(11):2948‐2959. [DOI] [PubMed] [Google Scholar]
- 82. Kim H‐J, Kim BK, Ohk B, et al. Estrogen‐related receptor gamma negatively regulates osteoclastogenesis and protects against inflammatory bone loss. J Cell Physiol. 2019;234(2):1659‐1670. [DOI] [PubMed] [Google Scholar]
- 83. Kim J‐H, Kim M, Jung H‐S, Sohn Y. Leonurus sibiricus L. ethanol extract promotes osteoblast differentiation and inhibits osteoclast formation. Int J Mol Med. 2019;44(3):913‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kong L, Ma R, Yang X, et al. Psoralidin suppresses osteoclastogenesis in BMMs and attenuates LPS‐mediated osteolysis by inhibiting inflammatory cytokines. Int Immunopharmacol. 2017;51:31‐39. [DOI] [PubMed] [Google Scholar]
- 85. Kwak HB, Kim JY, Kim KJ, et al. Risedronate directly inhibits osteoclast differentiation and inflammatory bone loss. Biol Pharm Bull. 2009;32(7):1193‐1198. [DOI] [PubMed] [Google Scholar]
- 86. Kwak HB, Lee BK, Oh J, et al. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down‐regulation of RANKL‐induced c‐Fos and NFATc1 expression. Bone. 2010;46(3):724‐731. [DOI] [PubMed] [Google Scholar]
- 87. Kwak HB, Sun H‐M, Ha H, et al. Tanshinone IIA suppresses inflammatory bone loss by inhibiting the synthesis of prostaglandin E2 in osteoblasts. Eur J Pharmacol. 2008;601(1–3):30‐37. [DOI] [PubMed] [Google Scholar]
- 88. Kwak SC, Baek JM, Lee CH, Yoon K‐H, Lee MS, Kim J‐Y. Umbelliferone prevents lipopolysaccharide‐induced bone loss and suppresses RANKL‐induced osteoclastogenesis by attenuating Akt‐c‐Fos‐NFATc1 signaling. Int J Biol Sci. 2019;15(11):2427‐2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kwak SC, Cheon YH, Lee CH, et al. Grape seed proanthocyanidin extract prevents bone loss via regulation of osteoclast differentiation, apoptosis, and proliferation. Nutrients. 2020;12(10):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kwak SC, Jeong DH, Cheon YH, et al. Securinine suppresses osteoclastogenesis and ameliorates inflammatory bone loss. Phytother Res. 2020;34(11):3029‐3040. [DOI] [PubMed] [Google Scholar]
- 91. Kwak SC, Lee C, Kim JY, et al. Chlorogenic acid inhibits osteodast differentiation and bone resorption by down‐regulation of receptor activator of nuclear factor kappa‐B ligand‐induced nuclear factor of activated T cells c1 expression. Biol Pharm Bull. 2013;36(11):1779‐1786. [DOI] [PubMed] [Google Scholar]
- 92. Lee E‐J, Song D‐H, Kim Y‐J, et al. PTX3 stimulates osteoclastogenesis by increasing osteoblast RANKL production. J Cell Physiol. 2014;229(11):1744‐1752. [DOI] [PubMed] [Google Scholar]
- 93. Lee MS, Baek JM, Kim JY, Yoo WH, Hong SJ, Lee CH. Methyl gallate inhibits osteoclast formation and function through suppressing the AKT and BTK‐PLCgamma2‐CA2+ signaling, and prevents LPS‐induced bone loss. Ann Rheum Dis. 2017;76(Suppl 2):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li L, Sapkota M, Kim SW, Soh Y. Herbacetin inhibits RANKL‐mediated osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Eur. J. Pharmacol. 2016;777:17‐25. [DOI] [PubMed] [Google Scholar]
- 95. Li Y, Gao X, Wang J. Human adipose‐derived mesenchymal stem cell‐conditioned media suppresses inflammatory bone loss in a lipopolysaccharide‐induced murine model. Exp Ther Med. 2018;15(2):1839‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li L, Park Y‐R, Shrestha SK, Cho H‐K, Soh Y. Suppression of inflammation, osteoclastogenesis and bone loss by PZRAS extract. J Microbiol Biotechnol. 2020;30(10):1543‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu F‐L, Chen C‐L, Lai C‐C, Lee C‐C, Chang D‐M. Arecoline suppresses RANKL‐induced osteoclast differentiation in vitro and attenuates LPS‐induced bone loss in vivo. Phytomedicine. 2020;69:153195. [DOI] [PubMed] [Google Scholar]
- 98. Liu H, Dong Y, Gao Y, et al. Hesperetin suppresses RANKL‐induced osteoclastogenesis and ameliorates lipopolysaccharide‐induced bone loss. J Cell Physiol. 2019;234(7):11009‐11022. [DOI] [PubMed] [Google Scholar]
- 99. Lu S‐H, Hsia Y‐J, Shih K‐C, Chou T‐C. Fucoidan prevents RANKL‐stimulated osteoclastogenesis and LPS‐induced inflammatory bone loss via regulation of Akt/GSK3beta/PTEN/NFATc1 signaling pathway and calcineurin activity. Mar Drugs. 2019;17(6):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nepal M, Choi HJ, Choi B‐Y, et al. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL‐induced NF‐kappaB and NFATc1 pathways. Eur J Pharmacol. 2013;715(1–3):96‐104. [DOI] [PubMed] [Google Scholar]
- 101. Park S‐H, Kim J‐Y, Cheon Y‐H, et al. Protocatechuic acid attenuates osteoclastogenesis by downregulating JNK/c‐Fos/NFATc1 signaling and prevents inflammatory bone loss in mice. Phytother Res. 2016;30(4):604‐612. [DOI] [PubMed] [Google Scholar]
- 102. Sapkota M, Gao M, Li L, et al. Macrolactin a protects against LPS‐induced bone loss by regulation of bone remodeling. Eur J Pharmacol. 2020;883:173305. [DOI] [PubMed] [Google Scholar]
- 103. Sapkota M, Li L, Kim S‐W, Soh Y. Thymol inhibits RANKL‐induced osteoclastogenesis in RAW264.7 and BMM cells and LPS‐induced bone loss in mice. Food Chem Toxicol. 2018;120:418‐429. [DOI] [PubMed] [Google Scholar]
- 104. Seo W, Lee S, Tran PT, et al. 3‐Hydroxyolean‐12‐en‐27‐oic acids inhibit RANKL‐induced osteoclastogenesis in vitro and inflammation‐induced bone loss in vivo. Int J Mol Sci. 2020;21(15):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Song J, Jing Z, Hu W, Yu J, Cui X. alpha‐Linolenic acid inhibits receptor activator of NF‐kB ligand induced (RANKL‐induced) osteoclastogenesis and prevents inflammatory bone loss via downregulation of nuclear factor‐KappaB‐inducible nitric oxide synthases (NF‐kB‐iNOS) signaling pathways. Med Sci Monit. 2017;23:5056‐5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tan Y, Deng W, Zhang Y, et al. A marine fungus‐derived nitrobenzoyl sesquiterpenoid suppresses receptor activator of NF‐kappaB ligand‐induced osteoclastogenesis and inflammatory bone destruction. Br J Pharmacol. 2020;177(18):4242‐4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Thummuri D, Jeengar MK, Shrivastava S, et al. Thymoquinone prevents RANKL‐induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF‐KB and MAPK Signalling. Pharmacol Res. 2015;99:63‐73. [DOI] [PubMed] [Google Scholar]
- 108. Thummuri D, Naidu VGM, Chaudhari P. Carnosic acid attenuates RANKL‐induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF‐kappaB and MAPK signalling. J Mol Med. 2017;95(10):1065‐1076. [DOI] [PubMed] [Google Scholar]
- 109. Tran PT, Dang NH, Kim O, et al. Ethanol extract of Polyscias fruticosa leaves suppresses RANKL‐mediated osteoclastogenesis in vitro and LPS‐induced bone loss in vivo. Phytomedicine. 2019;59:152908. [DOI] [PubMed] [Google Scholar]
- 110. Tran PT, Park D‐H, Kim O, Kwon S‐H, Min B‐S, Lee J‐H. Desoxyrhapontigenin inhibits RANKL‐induced osteoclast formation and prevents inflammation‐mediated bone loss. Int J Mol Med. 2018;42(1):569‐578. [DOI] [PubMed] [Google Scholar]
- 111. Wang X, Zheng T, Kang J‐H, et al. Decursin from Angelica gigas suppresses RANKL‐induced osteoclast formation and bone loss. Eur J Pharmacol. 2016;774:34‐42. [DOI] [PubMed] [Google Scholar]
- 112. Wang J, Zhang Y, Xu X, et al. ASP2‐1, a polysaccharide from Acorus tatarinowii Schott, inhibits osteoclastogenesis via modulation of NFATc1 and attenuates LPS‐induced bone loss in mice. Int J Biol Macromol. 2020;165:2219‐2230. [DOI] [PubMed] [Google Scholar]
- 113. Yang XB, Gao WJ, Wang B, et al. Picroside II inhibits RANKL‐mediated osteoclastogenesis by attenuating the NF‐B and MAPKs signaling pathway in vitro and prevents bone loss in lipopolysaccharide treatment mice. J Cell Biochem. 2017;118(12):4479‐4486. [DOI] [PubMed] [Google Scholar]
- 114. Yeon JT, Choi SW, Ryu BJ, et al. Praeruptorin a inhibits in vitro migration of preosteoclasts and in vivo bone erosion, possibly due to its potential to target calmodulin. J Nat Prod. 2015;78(4):776‐782. [DOI] [PubMed] [Google Scholar]
- 115. Zeng Q, Lu W, Deng Z, Wu J, Guo R, Xu X. Tablysin‐15 inhibits osteoclastogenesis and LPS‐induced bone loss via attenuating the integrin alphavbeta3 pathway. Chem Biol Interact. 2020;327:109179. [DOI] [PubMed] [Google Scholar]
- 116. Zeng X‐Z, Zhang Y‐Y, Yang Q, et al. Artesunate attenuates LPS‐induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCgamma1‐Ca2+‐NFATc1 signaling pathway. Acta Pharmacol Sin. 2020;41(2):229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhai ZJ, Li HW, Liu GW, et al. Andrographolide suppresses RANKL‐induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Br J Pharmacol. 2014;171(3):663‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhu L, Wei H, Wu Y, et al. Licorice isoliquiritigenin suppresses RANKL‐induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Int J Biochem Cell Biol. 2012;44(7):1139‐1152. [DOI] [PubMed] [Google Scholar]
- 119. Chen Y, Lu J, Li S, et al. Carnosol attenuates RANKL‐induced osteoclastogenesis in vitro and LPS‐induced bone loss. Int Immunopharmacol. 2020;89:106978. [DOI] [PubMed] [Google Scholar]
- 120. Hu B, Wu F, Shi Z, et al. Dehydrocostus lactone attenuates osteoclastogenesis and osteoclast‐induced bone loss by modulating NF‐kappaB signalling pathway. J Cell Mol Med. 2019;23(8):5762‐5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee K, Kim MY, Ahn H, Kim H‐S, Shin H‐I, Jeong D. Blocking of the ubiquitin‐proteasome system prevents inflammation‐induced bone loss by accelerating M‐CSF receptor c‐Fms degradation in osteoclast differentiation. Int J Mol Sci. 2017;18(10):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Meng J, Zhou C, Zhang W, et al. Stachydrine prevents LPS‐induced bone loss by inhibiting osteoclastogenesis via NF‐kappaB and Akt signalling. J Cell Mol Med. 2019;23(10):6730‐6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wu H, Hu B, Zhou X, et al. Artemether attenuates LPS‐induced inflammatory bone loss by inhibiting osteoclastogenesis and bone resorption via suppression of MAPK signaling pathway. Cell Death Dis. 2018;9(5):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zheng T, Noh ALSM, Park H, Yim M. Aminocoumarins inhibit osteoclast differentiation and bone resorption via downregulation of nuclear factor of activated T cells c1. Biochem Pharmacol. 2013;85(3):417‐425. [DOI] [PubMed] [Google Scholar]
- 125. Kobayashi M, Watanabe K, Yokoyama S, et al. Capsaicin, a TRPV1 ligand, suppresses bone resorption by inhibiting the prostaglandin E production of osteoblasts, and attenuates the inflammatory bone loss induced by lipopolysaccharide. ISRN Pharmacol. 2012;2012:439860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Miyaura C, Inada M, Matsumoto C, et al. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2‐mediated bone resorption associated with inflammation. J Exp Med. 2003;197(10):1303‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dou C, Ding N, Xing J, et al. Dihydroartemisinin attenuates lipopolysaccharide‐induced osteoclastogenesis and bone loss via the mitochondria‐dependent apoptosis pathway. Cell Death Dis. 2016;7:e2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gao Y, Grassi F, Ryan MR, et al. IFN‐gamma stimulates osteoclast formation and bone loss in vivo via antigen‐driven T cell activation. J Clin Invest. 2007;117(1):122‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Park H‐J, Son H‐J, Sul O‐J, Suh J‐H, Choi H‐S. 4‐Phenylbutyric acid protects against lipopolysaccharide‐induced bone loss by modulating autophagy in osteoclasts. Biochem Pharmacol. 2018;151:9‐17. [DOI] [PubMed] [Google Scholar]
- 130. Park H‐J, Gholam‐Zadeh M, Suh J‐H, Choi H‐S. Lycorine attenuates autophagy in osteoclasts via an axis of mROS/TRPML1/TFEB to reduce LPS‐induced bone loss. Oxid Med Cell Longev. 2019;2019:8982147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Park H‐J, Gholam Zadeh M, Suh J‐H, Choi H‐S. Dauricine protects from LPS‐induced bone loss via the ROS/PP2A/NF‐kappaB Axis in osteoclasts. Antioxidants. 2020;9(7):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Girma AM, Effects of selenium supplementation and chronic inflammation on bone microarchitecture and strength in mice. Oklahoma State University: Ann Arbor; 2012. p 107. [Google Scholar]
- 133. Lim YF. Alterations in Vitamin D Metabolism with Chronic Inflammation: Potential Implications in Inflammation‐Induced Bone Loss. Oklahoma State University: Ann Arbor; 2009. p 113. [Google Scholar]
- 134. Onal M. The Significance of RANKL Levels and Cellular Sources during Physiological and Pathophysiological Bone Resorption. Ann Arbor: University of Arkansas for Medical Sciences; 2012. p 138. [Google Scholar]
- 135. Lim YF and Smith BJ. (2009). Alterations in vitamin D metabolism with chronic inflammation: Potential implications in inflammation‐induced bone loss (1469170), pp. 113.
- 136. Onal M and O'Brien CA. (2012). The significance of RANKL levels and cellular sources during physiological and pathophysiological bone resorption (3551939), pp. 138.
- 137. Kanno Y, Ishisaki A, Kawashita E, Kuretake H, Ikeda K, Matsuo O. uPA attenuated LPS‐induced inflammatory Osteoclastogenesis through the plasmin/PAR‐1/Ca(2+)/CaMKK/AMPK Axis. Int J Biol Sci. 2016;12(1):63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kanno Y, Ishisaki A, Miyashita M, Matsuo O. The blocking of uPAR suppresses lipopolysaccharide‐induced inflammatory osteoclastogenesis and the resultant bone loss through attenuation of integrin beta3/Akt pathway. Immun Inflammation Dis. 2016;4(3):338‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kanno Y, Maruyama C, Matsuda A, Ishisaki A. uPA‐derived peptide, A6 is involved in the suppression of lipopolysaccaride‐promoted inflammatory osteoclastogenesis and the resultant bone loss. Immun Inflammation Dis. 2017;5(3):289‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rendina E. Reversal of Inflammation‐Induced Bone Loss and Vascular Pathology by Dried Plum's Polyphenols. Oklahoma State University: Ann Arbor; 2009. p 106. [Google Scholar]
- 141. Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. Membrane‐bound prostaglandin E synthase‐1‐mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide‐induced bone loss associated with inflammation. J Immunol. 2006;177(3):1879‐1885. [DOI] [PubMed] [Google Scholar]
- 142. Ochi H, Hara Y, Tagawa M, Shinomiya K, Asou Y. The roles of TNFR1 in lipopolysaccharide‐induced bone loss: dual effects of TNFR1 on bone metabolism via osteoclastogenesis and osteoblast survival. J Orthop Res. 2010;28(5):657‐663. [DOI] [PubMed] [Google Scholar]
- 143. Park HJ, Choi HS. Lycorine protects mice from inflammatory bone loss via decreasing autophagy. J Bone Miner Res. 2019;34(Suppl 1):334. [Google Scholar]
- 144. Rendina E and Smith BJ. (2009). Reversal of inflammation‐induced bone loss and vascular pathology by dried plum's polyphenols (1465226), pp. 106.
- 145. Seeman E. Physiology of aging invited review: pathogenesis of osteoporosis. J Appl Physiol. 2003;95:2142‐2151. [DOI] [PubMed] [Google Scholar]
- 146. Strom JO, Theodorsson E, Holm L, Theodorsson A. Different methods for administering 17beta‐estradiol to ovariectomized rats result in opposite effects on ischemic brain damage. BMC Neurosci. 2010;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long‐term 17beta‐estradiol administration to mice. Gen Comp Endocrinol. 2012;175(1):188‐193. [DOI] [PubMed] [Google Scholar]
- 148. Nikodemova M, Watters JJ. Outbred ICR/CD1 mice display more severe neuroinflammation mediated by microglial TLR4/CD14 activation than inbred C57Bl/6 mice. Neuroscience. 2011;190:67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085‐2088. [DOI] [PubMed] [Google Scholar]
- 150. Bott KN, Sacco SM, Turnbull PC, Longo AB, Ward WE, Peters SJ. Skeletal‐site specific effects of endurance running on bone structure and strength in growing rats. Med Sci Sports Exercise. 2015;47(5):S496. [Google Scholar]
- 151. Donnelly E. Methods for assessing bone quality: a review. Clin Orthop Relat Res. 2011;469(8):2128‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Greenwood C, Clement JG, Dicken AJ, et al. The micro‐architecture of human cancellous bone from fracture neck of femur patients in relation to the structural integrity and fracture toughness of the tissue. Bone Rep. 2015;3:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age‐related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22(8):1197‐1207. [DOI] [PubMed] [Google Scholar]
- 154. Bott KN, Yumol JL, Comelli EM, Klentrou P, Peters SJ, Ward WE. Trabecular and cortical bone are unaltered in response to chronic lipopolysaccharide exposure via osmotic pumps in male and female CD‐1 mice. PLoS One. 2021;16(2):e0243933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Subgroup analysis by duration of lipopolysaccharide (LPS) for studies less than 2 weeks and greater than 2 weeks in duration on BV/TV. LPS, lipopolysaccharide; BV/TV, bone volume fraction; CI, confidence interval; SD, standard deviation; IV, weighted mean difference.
Figure S2. Subgroup analysis by duration of lipopolysaccharide (LPS) for studies less than 2 weeks and greater than 2 weeks in duration on vBMD. LPS, lipopolysaccharide; vBMD, volumetric bone mineral density; CI confidence interval; SD, standard deviation; IV, weighted mean difference.
Figure S3. Funnel plots for studies shorter than 2 weeks in duration. Contour‐enhanced funnel plot for studies for (A) BV/TV and (B) vBMD. (C) Trim and fill for BV/TV (23 studies imputed; observed SMD = −4.106, 95% CI [−4.607, −3.605]; imputed SMD = −2.979, 95% CI [−3.662, −2.295]) and (D) vBMD (no missing studies imputed). LPS, lipopolysaccharide; BV/TV, bone volume fraction; SMD, standardized mean difference, calculated as Hedge's g; CI confidence interval.
Figure S4. Funnel plots for studies greater than 2 weeks in duration. Contour‐enhanced funnel plot for (A) BV/TV and (B) vBMD. (C) Trim and fill for BV/TV and (D) vBMD. LPS, lipopolysaccharide; vBMD, volumetric bone mineral density (no missing studies were imputed for BV/TV or vBMD); SE, standard error; SMD, standardized mean difference, calculated as Hedge's g.
Table S1. Full systematic search strategy by database.
Table S2. Summary of risk of bias assessment guidelines.
Table S3. Systematic review summary of study characteristics. Sample size represents number of studies.
Data Availability Statement
The data that support the findings of this study are openly available in Brock University Dataverse at https://doi.org/10.5683/SP3/REEPYJ.


