Abstract
Background
The aim of this randomized clinical trial was to evaluate the effect of diode laser photobiomodulation (PBM) on post‐surgical healing, inflammation and implant stability.
Methods
Forty dental implants were inserted into 13 patients. The implants were randomly divided into two groups. The test group (PBM+) underwent two sessions of PBM (combined diode laser of 630 and 808 nm), the first of which after surgery, and the second, 7 days after the surgical procedure. The control group (PBM−) received simulated laser treatment. The implant stability quotient (ISQ) was determined immediately after the surgical procedure, and 7 days, 4 and 8 weeks later. Post‐surgical inflammation was assessed following the criteria described by Bloemen and Cols. Healing was calculated using the healing index (HI).
Results
No differences were found in terms of the mean values of implant stability between the test and control groups over time. Only two of the implants (18.2%) from the PBM− group were classified with the maximum healing index (HI = 5), whereas in the PBM+ group, nine implants (45%) were classified with the aforementioned index (P < 0.0001). Using the logistic regression, it was determined that the non‐application of the laser in the PBM− group caused an OR of 4.333 times of presenting inflammation (IC95% 1.150–16.323; P = 0.030).
Conclusions
The application of 808 nm infra‐red laser for bone tissue, and 630 nm for mucosal tissue in two sessions is considered to be an effective way of reducing inflammation and improving early healing. More studies are needed to confirm these results.
Keywords: mucosal healing, mucosal inflammation, osseointegrated dental implants, photobiomodulation
One‐Sentence Summary.
This article is a double blind randomized clinical study that compares the effect of photobiomodulation on bone and tissue healing after implant placement surgery.
1. INTRODUCTION
Osseointegrated dental implants are used for replacing lost teeth and they offer a high success rate (Howe et al., 2019). Bone quantity and quality are predictive factors in achieving proper primary stability and in defining whether rehabilitation should be carried out with early or immediate loading (Elias et al., 2012; Monje et al., 2019; Shokri & Daraeighadikolaei, 2013). The clinical success of a dental implant over time is determined by bone quantity, bone quality, and, in particular, adequate osseointegration (Fornaini et al., 2015; Mills et al., 2018).
The primary stability of dental implants is defined by the biomechanical stability that is obtained when inserting the implant. Secondary stability occurs later on, and it depends on osseointegration, that is to say the bone formation process in the bone‐implant interface (Monje et al., 2019; Shokri & Daraeighadikolaei, 2013). Low‐level laser therapy (LLLT) or photobiomodulation (PBM) has been proposed as a method that could be used to enhance the bone cicatrisation process by reducing the healing time for osseointegration in preclinical studies (Karakaya & Demirbaş, 2020). However, other randomized clinical trials have not confirmed these results (Torkzaban et al., 2018).
PBM consists of low energy density laser and/or LED light therapy, so it does not generate heat. To induce photochemical effects and stimulate cell replication (Bayat & Jalalifirouzkouhi, 2017; Karoussis et al., 2017; Santinoni et al., 2021), there is a broad spectrum in terms of wavelength, ranging from red to infrared (600‐1100 nm) (Qu et al., 2022), nevertheless there is no specific guideline to achieve the best results in terms of osseointegration healing (Kazem Shakouri et al., 2010; Riboldi et al., 2009). The most recent published studies addressing PBM for osseointegration applied a protocol quite similar to the one used in our study (Kinalski et al., 2021; Lobato et al., 2020), however the present study presents as a novelty the use of both frequencies (dual laser, red and infrared wavelength) with the aim of improving the effects at the bone and soft tissue level.
Several clinical methods have mentioned the use of invasive techniques to measure the stability of dental implants, such as removal torque or histomorphometric analysis (Matys et al., 2017). However, the Periotest and resonance frequency analysis (RFA) are used more often (Marquezan et al., 2012). RFA analysis is carried out with specific devices with a transducer or “smart peg” being inserted into the implant or abutment to measure and quantify stability. The implant stability quotient (ISQ) is presented using a numerical scale from 0 to 100, and it is translated from an intensity frequency of 3500–8500 Hz (Torkzaban et al., 2018). Higher ISQ values result in a higher mean stability (Marquezan et al., 2012).
Studies evaluating the effect of PBM on the stability of dental implants have reported that the development of secondary stability does not take as long because fibrocartilage callus development increases during the initial stage of bone healing (Kazem Shakouri et al., 2010). Therefore, the bone‐implant contact (BIC) factor increased after the laser irradiation on the peri‐implant site (Lopes et al., 2005; Matys et al., 2018). Using laser after a surgical procedure that involves hard and soft tissues enhances healing and has a biostimulating effect over osteoblastic and fibroblastic proliferation, and osteogenesis (Bozkaya et al., 2021; Chen et al., 2017; Dompe et al., 2020; Tang & Arany, 2013). Moreover, laser reduces inflammation and pain, accelerates wound healing, (Agha‐Hosseini et al., 2012) and stimulates nerve regeneration (Farivar et al., 2014). Although, other clinical studies have not been able to demonstrate differences in the levels of stability of dental implants (Bozkaya et al., 2021; Mandić et al., 2015) or orthodontic mini‐implants (Marañón‐Vásquez et al., 2019) between the laser group and the control group, it is important to highlight the great differences between the few existing controlled clinical studies. For example, in the study by Lobato et al. (2020), the implants were placed in fresh sockets, which creates a situation of healing and primary stability very different from that of implants placed in healed sites, as in the study by Kinalski et al. (2021).
In relation to the healing of hard tissues, some clinical studies have shown that PBM can reduce the healing time after grafting the extraction socket. Histological evidence suggests that new bone formation in the sockets appeared within 60 days after PBM treatment compared to a minimum of 120 days in the control group (Monea et al., 2015). Moreover, in a computed tomography and histomorphometric analysis of human alveolar bone repair developed by Romão et al. (2015), the relative bone volume was significantly higher in the laser group (P < 0.0001) suggesting that PBM is able to accelerate alveolar bone repair after molar extraction, leading to a more thin and close trabeculae. In molecular terms, the study of Palled et al. (2021) showed a significant rise in osteoprotegerin levels of the test group at 3 months contrary to significant decline in the control group, suggesting that the healing of peri‐implant hard and soft tissues may be enhanced with the use of PBM during the postoperative period. However, although some studies have been developed in relation to the effect of PBM at the microbiological level (Bozkaya et al., 2021), limited studies have been developed regarding the clinical healing and inflammation around implants after PBM.
The aim of this clinical trial was to evaluate the effect of 808 nm diode laser PBM on implant stability using RFA measurements, and the effect of 660 nm diode laser PBM on inflammation and post‐surgical healing.
2. MATERIAL AND METHOD
2.1. Trial design, participants, and setting
The study was a randomised, double‐blinded clinical trial comprised of a control group and a test group. Participants were exclusively recruited from the University of Santiago de Compostela's Faculty of Dentistry, within the Medicine, Surgery and Oral Implantology Department. The trial was conducted between February 2020 and July 2021, according to the Declaration of Helsinki, as reviewed in 2002. The study was approved by the Local Ethics and Research Committee (Ref. 2019/169) and registered in ClinicalTrials.gov, with the identifier: NCT03796494.
All the patients who met the inclusion criteria were fully informed of the characteristics of the study and were invited to participate. Each patient underwent a complete clinical and oral assessment, as well as a radiological study based on cone‐beam computed tomography (CBCT) (i‐CAT‐FLX). The tests wed the recommended criteria established in the Spanish CONSORT Statement (Cuschieri, 2019), as shown in Figure 1 and followed the SPIRIT statement.
FIGURE 1.
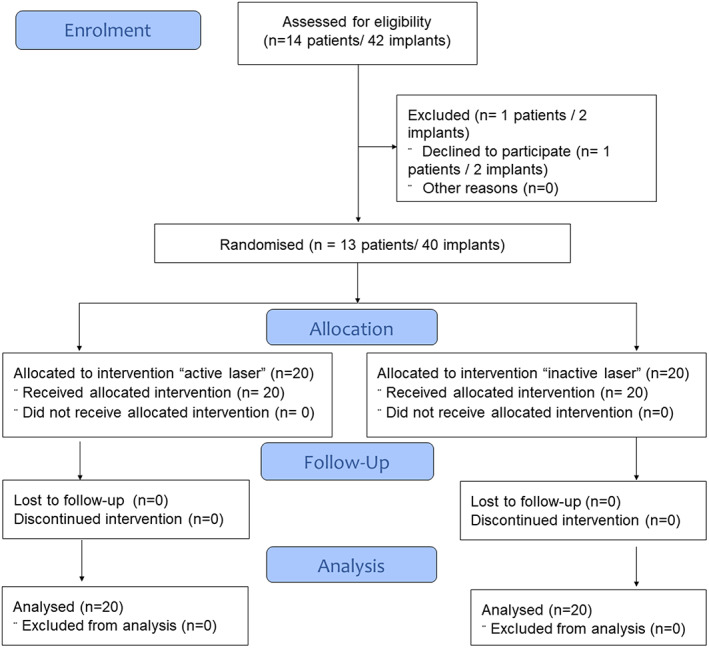
The consort flow diagram of subject Progress through the phases of a randomized trial
The patients included in this study all met the following inclusion criteria: (1) there was no presence of any systemic diseases which might be considered a contraindication, or if these were present they were controlled; (2) the informed consent form required for participation in the study has been signed; (3) they smoke <5 cigarettes per day; (4) they have sufficient bone quantity to insert 4 × 10 mm implants and ensure that no bone or soft tissue grafts are needed in molar and premolar areas; and (5) they have a minimum primary stability value of 50 ISQ. The exclusion criteria were: (1) they had undergone dental rehabilitation in aesthetic areas 13–23 and 33–43; (2) they have implants with mechanical torques inferior to 20 N; (3) they smoke more than 5 cigarettes per day; (4) they are pregnant and/or lactating; (5) they have cancer or a potentially malignant injury in the area where PBM is to be applied.
2.2. Intervention
The patients were distributed into two groups: a test group that received an active application of PBM (PBM+); and a control group that received an inactive/sham application of PBM, or laser in off mode (PBM−).
2.3. Study products
Forty implants with internal hex connection and a diameter of 4 × 10 mm were used. Implants were placed in healed mature bone (more than 6 months after the extractions), according to the manufacturer's guidelines (Model IPX, Nueva Galimplant). The model used was made of Ti IV and it had a macroscopic design that enhances primary stability in any circumstances. In all cases, aesthetical straight abutments were placed with their respective gingival protectors. A diode laser (Laser Duo, MMO‐São Carlos) was used with the following parameters: (1) wavelength of 630–808 nm, (2) output power of 100 mW, (3) continuous mode and (4) dose of 0.1 J/s and (5) handpiece with an output of 3 mm2.
2.4. Surgical procedures
The insertion of implants strictly followed the manufacturer's instructions regarding different bone types. Implants were placed mechanically and always 1 mm below the bone crest. Following the surgical intervention, all of the patients received detailed instructions about oral hygiene; antibiotics (a 750 mg/8 h dose of amoxicillin for 7 days); and painkillers (a 600 mg/8 h dose of ibuprofen for 4 days). No provisional prosthesis was placed during the 8‐week observation period.
2.5. Laser irradiation
After surgery, half of the implants were randomly assigned to receive a treatment with a low intensity active laser. The test group, PBM+, received intraoral irradiation for 100 s through a handpiece that entered into contact with the mucosa and which was positioned to allow light to enter perpendicularly to the longitudinal axe of the dental implant at 5 mm from the bone crest. Three points were irradiated: the buccal side (4J) and the palatal or lingual side (4J) using the infrared light mode (808 nm), to stimulate bone tissue regeneration; and the occlusal side (2J) using the red‐light mode (630 nm), to stimulate mucosal tissue regeneration and anti‐inflammatory effect. The laser treatment was performed immediately after surgery and it was repeated 7 days later. On each session, safety measures were taken with both the patients and odontologists wearing dark protective glasses. The parameters used for the irradiation were based on previous studies (Chen et al., 2019; Kinalski et al., 2021; Lobato et al., 2020). The total irradiation per session was 10 J during 100 s, the total energy density per session was 33.3 J/cm2, and the total energy density per implant was 66.6 J/cm2.
The control group, PBM−, received a sham laser treatment, that is, only the irradiation of the patient was simulated.
2.6. Measurement of primary and secondary objectives
2.6.1. Assessment of implants stability
Resonance frequency analysis (RFA) was performed using the Osstell™ Mentor device (Integration Diagnostics), and it was conducted by a trained and calibrated operator (GCVC) who was not aware of which side would be irradiated. Measurements were registered immediately following the implant surgery and after 7 days, 4 and 8 weeks.
A standardised fixed length device was inserted (Smartpeg™ Integration Diagnostics) and it was screwed into each implant by hand. The transducer probing (Osstell™ Mentor Probe) was carried out by directing the tip of the handpiece to the small magnet located on the upper part of the Smartpeg™ at a distance of 2–3 mm, until the instrument produced a beep and showed the implant stability quotient (ISQ). These measurements were performed in buccal, lingual, mesial and distal implant surfaces in order to obtaining a global average for the four surfaces.
2.6.2. Assessment of the healing process of the mucosa
The degree of post‐surgery inflammation and early healing was assessed by two independent operators (GCVC and MPS). Post‐surgical inflammation was assessed subjectively and dichotomously (yes/no), through visual examination, following the criteria described above by Bloemen and Cols (Bloemen et al., 2012). Healing was calculated using the healing index (HI) by Hamzani & Chaushu (2018) and Landry (1988). Table 1 shows the final score HI, that ranges from 0 to 5:0 for poor healing and 5 for excellent healing. The degree of concordance was verified by both researchers, who performed independent evaluations on the total sample, using Cohen's Kappa index (Mandrekar, 2011). The degree of agreement for the inflammation level was 0.91 and >0.90 at all HI levels.
TABLE 1.
Landry et al.'s healing index, the final score ranges from 0 to 5, where 0 indicates poor healing and 5 indicates excellent healing
| Clinical outcome parameters | Score 0 | Score 1 |
|---|---|---|
| Bleeding, spontaneously or on palpation | Yes | No |
| Granulation tissue | Yes | No |
| Tissue colour | Redder than opposite side tissue | Like the opposite side tissue |
| Incision margins | Incomplete flap closure/fibrin clot/partial necrosis | Complete flap closure/fine fibrin line |
| Suppuration | Yes | No |
2.7. Sample size calculation
The following statistical criteria were established in order to calculate the sample size: (1) a size of the expected increase or reduction effect on the HI of at least 1.5 at 1 week after surgery, (2) an alpha error of 0.05, and (3) a statistical power of 90%. These criteria were taken into account and the variance contrast for independent samples was applied. It was determined that a sample of 18 implants would be required for each group, that is to say a total of 36 implants. The final sample size was 42, considering an estimated loss of 15%. The sample size was calculated using the G Power 3.1.5 programme.
2.8. Randomisation
Simple randomization was performed for both study groups (PBM+/PBM−). Briefly, a random number generator (N = 40) was used for the two study groups, using a SPSS 28.0 macro.
2.9. Blinding
All of the assessments of the results of this study were double blinded because neither the patients (due to the use of a placebo) nor the evaluators (who were not involved in the LLLT process) knew which treatment had been assigned to each patient.
2.10. Statistical analysis
The collected data was analysed using the SPSS software programme, version 24.0 (SPSS Inc.). The IQS data for primary and secondary stability was presented using central tendency measurements (mean) and variation (SD and CI of 95%). The variance was analysed in order to examine changes to the ISQ during the observation period. The statistical significance of the differences amongst the observed parameters for IQS between the groups at each observation point was analysed using the T test for unpaired groups. Contingency tables were created and the Chi Square and Kruskal Wallis tests were applied to study the differences between the groups in terms of post‐surgery healing and inflammation. A multiple sequential logistic regression was employed to determine the risk (OR) of inflammation associated with the use of PBM. The statistical significance of all tests was defined as P < 0.05.
3. RESULTS
3.1. Sample description
The sample was comprised of five male (38.4%) and eight female subjects (61.5%). Regarding the number of implants, a total of 40 implants were inserted, in 13 patients: 20 using PBM and 20 without using it. With regards to the area, 28 of the implants were placed in the jaw (70%), 37 (92.5%) with a coarse periodontium, and 36 (90%) with bone type II‐III‐IV. Table 2 shows the complete sample data.
TABLE 2.
Full description of the sample variants
| Variable | PBM− n (%) | PBM+ n (%) | P‐value |
|---|---|---|---|
| Sex | |||
| Men | 5 (25) | 5 (25) | 1.000 |
| Woman | 15 (75) | 15 (75) | |
| Localisation | |||
| Maxillary | 6 (30) | 6 (30) | 1.000 |
| Mandible | 14 (70) | 14 (70) | |
| Abutment type | |||
| Straight | 12 (60) | 7 (35) | 0.113 |
| Slim | 8 (40) | 13 (65) | |
| Periodontal phenotype | |||
| Fine | 2 (10) | 1 (5) | 0.548 |
| Thick | 18 (90) | 19 (95) | |
| Bone type | |||
| I | 3 (15) | 1(5) | 0.292 |
| II‐III‐IV | 17 (85) | 19 (95) | |
| Visual examination | |||
| Without inflammation | 6 (60) | 13 (65) | 0.027 |
| With inflammation | 14 (40) | 7 (35) | |
| HI | |||
| 1 | 6 (30) | 0 (0) | <0.001 |
| 2 | 10 (50) | 1 (5) | |
| 3 | 2 (10) | 5 (25) | |
| 4 | 0 (0) | 5 (25) | |
| 5 | 2 (0) | 9 (45) | |
Bold means that the have been statistically significant difference.
3.2. Primary and secondary stability
The primary basal implant stability which was measured using the ISQ scale, showed results of 71.5 ± 8.8. The average basal stability for the abutment was 71.9 ± 5.9, without significant differences. The average stability 7 days after the laser application was 68.3 ± 8.6 for the PBM− group, and 71.2 ± 5.9 for the PBM+ group (P = 0.228). After 4 weeks, the values became closer in both groups: 69.5 ± 7.4 for PBM− and 69.7 ± 3.5 for PBM+ (P = 0.908). After 8 weeks, the highest level of stability for the ISQ scale was achieved without differences between both groups: 72.3 ± 7.4 for PBM− and 72.5 ± 4.3 for PBM+ (P = 0.938).
3.3. Postoperative inflammation
A postoperative inflammation assessment was carried out 7 days after the clinical trial, the Figure 2 present the clinical aspect of the Landry et al. Healing Index(HI). The dichotomous method showed that the PBM+ group presented inflammation in seven implants (35%), while the PBM− group presented inflammation in 14 implants (70%) (P = 0.028). The healing index (HI) revealed variable healing levels in both groups. Nevertheless, the PBM+ group implants showed better early healing (Table 3). Only two of the implants (18.2%) from the PBM− group were classified with the maximum healing index (HI = 5), whereas in the PBM+ group, nine implants (45%) were classified with the aforementioned index (P < 0.0001). Considering HI as a quantitative variable, the PBM− group showed an average healing of 2.1 ± 1.2, while PBM+ showed an average HI of 4.10 ± 0.9 (P < 0.0001). Using the logistic regression, it was determined that the non‐application of the laser in the PBM− group caused an OR of 4.333 times of presenting inflammation (IC95% 1.150–16.323; P = 0.030).
FIGURE 2.
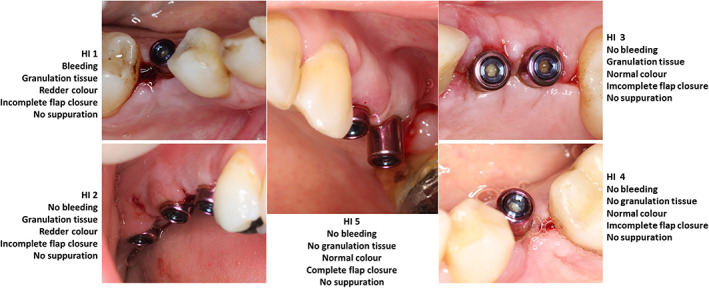
Clinical aspect of the Landry et al. healing index (HI), where 0 indicates poor healing and 5 indicates excellent healing
TABLE 3.
Descriptive classification of each implant according to healing index (HI)
| Tissue colour | Presence of bleeding | Presence of granulation tissue | Incision margin | Presence of suppuration | Total score (HI) | Inflammation visual evaluation | |
|---|---|---|---|---|---|---|---|
| Case 1 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 2 | 0 | 1 | 0 | 1 | 1 | 3 | No |
| Case 3 | 0 | 0 | 0 | 1 | 1 | 2 | No |
| Case 4 | 0 | 1 | 1 | 0 | 1 | 3 | No |
| Case 5 | 0 | 0 | 0 | 0 | 1 | 1 | No |
| Case 6 | 0 | 0 | 1 | 0 | 1 | 2 | Yes |
| Case 7 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 8 | 0 | 0 | 1 | 0 | 1 | 2 | Yes |
| Case 9 | 0 | 0 | 0 | 0 | 1 | 1 | Yes |
| Case 10 | 0 | 0 | 0 | 0 | 1 | 1 | Yes |
| Case 11 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 12 | 0 | 0 | 0 | 0 | 1 | 1 | Yes |
| Case 13 | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
| Case 14 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 15 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 16 | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
| Case 17 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 18 | 0 | 0 | 0 | 0 | 1 | 1 | Yes |
| Case 19 | 0 | 0 | 0 | 0 | 1 | 1 | Yes |
| Case 20 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 21 | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
| Case 22 | 0 | 1 | 0 | 0 | 1 | 2 | Yes |
| Case 23 | 0 | 1 | 0 | 1 | 1 | 3 | Yes |
| Case 24 | 0 | 1 | 1 | 0 | 1 | 3 | Yes |
| Case 25 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 26 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 27 | 0 | 1 | 1 | 1 | 1 | 4 | No |
| Case 28 | 0 | 1 | 1 | 1 | 1 | 4 | No |
| Case 29 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 30 | 0 | 1 | 1 | 1 | 1 | 4 | No |
| Case 31 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 32 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 33 | 0 | 1 | 1 | 0 | 1 | 3 | Yes |
| Case 34 | 0 | 1 | 1 | 1 | 1 | 4 | Yes |
| Case 35 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 36 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 37 | 0 | 0 | 1 | 1 | 1 | 3 | Yes |
| Case 38 | 1 | 1 | 1 | 1 | 1 | 5 | No |
| Case 39 | 0 | 1 | 1 | 1 | 1 | 4 | No |
| Case 40 | 0 | 0 | 1 | 1 | 1 | 3 | Yes |
3.4. Adverse events
No adverse events related to the use of PBM were observed, except for the inflammatory processes that are associated with the surgical procedure itself.
4. DISCUSSION
PBM is a non‐invasive therapy that uses photochemical and biological interactions to generate energy to help speed up cellular reparation and regeneration processes (Hamblin, 2017). Reports of the use of LLLT after implant surgery have been experiencing an increase, this is due to the demand for better and more predictable results in terms of healing and postoperative inflammation (Bozkaya et al., 2021; Gholami et al., 2019a). To the best of these authors' knowledge, and as reported in a recent systematic review (Qu et al., 2022), this is the first study analyzing the effects of diode laser irradiation at wavelengths of 808 and 630 nm combined, in an attempt to achieve a significant therapeutic effect with respect to improvement in implant stability, inflammation and tissue healing, respectively.
With regards to the biological processes that take place following dental implant surgery, that is to say, osseointegration; although primary stability is achieved, nonetheless, in the first days following the implant placement the bone suffers restructuration, therefore a temporary reduction in implant stability is expected (Insua et al., 2017). Gum healing can take place either by first intention—when the implant stays submerged and edge‐to‐edge suturing is performed—or by second intention—when a healing abutment is placed on the implant, to prevent the edges from moving closer. First intention healing usually results in faster healing and it has a lower risk of infection compared to second intention healing (Pippi, 2017).
After having placed one‐abutment‐one‐time implants with a successful primary stability, it was observed that PBM+ group was less affected by inherent adverse biological processes inherent to the surgical procedure. The average stability remained well‐balanced in all of the ISQ measurements, recording results similar to those obtained by Torkzaban et al. (2018). In their revision, Gholami et al. (2019b) reported that some studies observed increased bone activity at metabolic and cellular levels of the irradiated bone, as well as faster bone regeneration. Regarding the measurements made in this study, the mean stability values progressed equally in both groups throughout the study, except on the seventh day compared to the immediate postoperative period in the same group. The same results were observed in the studies by García‐Morales et al. (2012), Matys et al. (2019) and Torkzaban et al. (2018) that is to say, in the PBM+ group, the tendency to reduce implant stability was slower in the measurements made in the first 10–15 days. This may be justified by the increased bone activity associated with PBM, which accelerates both bone remodelling and the implant osseointegration process (García‐Morales et al., 2012).
After the reduction of the initial stability, both groups experienced a progressive increase in ISQ and at week 8 they reached values very similar to the initial ones. The reduction process followed by increased implant stability has also been reported in other studies (Matys et al., 2019; Torkzaban et al., 2018). Animal model studies have reported a significant increase in osseointegration in PBM+ groups, using an infrared wavelength (Khadra et al., 2004; Lopes et al., 2005; Maluf et al., 2010; Pereira et al., 2009). In our study, the application of PBM did not significantly improve the final stability of the dental implant in the long term, given that after 8 weeks, there were no significant differences between the two groups in terms of the average stability values, and this was also observed in other studies with the similar monitoring time (Bozkaya et al., 2021; García‐Morales et al., 2012; Lopes et al., 2005; Mandić et al., 2015; Torkzaban et al., 2018).
The inflammation and healing responses obtained for the PBM+ group in the objective assessment and HI were considerably better than those obtained for the PBM− group. 65% of the PBM+ group's implants did not present with any subjective inflammation, and 45% of the same group attained the maximum score in the HI scale. It was not possible to carry out a comparative analysis given that no prior studies in which the effect of PBM on gum postoperative implant surgery healing and inflammation were assessed were found. The healing scale applied (HI) was evaluated in studies that tested the effect of PBM on gum healing and inflammation following dental extraction (Hamzani & Chaushu, 2018). This better inflammatory and healing response with the application of PBM seems to be related to possible antimicrobial effects, thus in the study by Bozkaya et al., the PBM+ group presents a significant reduction in the level of periodontal pathogenic bacteria when compared to the PBM− group (Bozkaya et al., 2021). Furthermore, several studies have reported an increase in growth factors and anti‐inflammatory molecules in PBM+ groups (Gokmenoglu et al., 2014; Hamblin, 2017; Memarian et al., 2018; Tsai & Hamblin, 2017). This therefore justifies the results obtained in this study, given that the acceleration of osteogenic signalling and bone and mucosa vascularisation favours healing processes (Insua et al., 2017). In addition, accelerating the healing of the surgery wound and reducing the inflammatory process could may help to prevent marginal bone loss (Fernandes et al., 2021), which would increase the chance of dental implant survival (Aguirre‐Zorzano et al., 2013; Insua et al., 2017).
In this study, we used the combined application of short and long wavelengths as recommended by Qu et al. (2022), to verify whether this protocol may produce more significant therapeutic effects. PBM therapy was applied on two separate occasions. The dose applied in each session was 13.32 J/cm2, with longer wavelength (808 nm), for bone tissue and 6.66 J/cm2, with shorter wavelength (660 nm), for mucosal tissue. These values are within the therapeutic window range and they are compatible with those found in the scientific literature (Carroll et al., 2014; Gholami et al., 2019b). The control of employed energy level is essential, given that a weak stimulation may not activate a cellular response, and, likewise, a high stimulation may inhibit or even deactivate the cellular response (Dompe et al., 2020; Gholami et al., 2019a; Lima et al., 2020; Na et al., 2018), meaning that there is an optimum stimulation within an energetic density range (dose). This dose, which is known as the therapeutic window, biphasic dose response, or Arndt‐Schulz curve (Dompe et al., 2020; Huang et al., 2011; Lima et al., 2020), is the responsible for cellular activation. If stimulation is not achieved or if it was overshot, the PBM protocol would be inadequate and would not work.
The absence of a specific PBM protocol has been already highlighted by several authors as a problem (Bozkaya et al., 2021; Khadra et al., 2005; Qu et al., 2022; Woodruff et al., 2004). This is due, among other factors, to the heterogeneity of the characteristics and parameters of the laser devices available on the market (Khadra et al., 2005; Lima et al., 2020). It is important to note that PBM parameters that work on one cell group will not necessarily work on another. In other words, the ideal therapeutic window for hard tissues is not the same as for soft tissues, just as surely the window to produce favorable results for osseointegration in animals does not have the same effectiveness in humans (Chen et al., 2019; Memarian et al., 2018).
The main limitation of this study was the impossibility of measuring the ISQ in the period between the second and eighth weeks, considering the time required without mechanical stimulation for implants with deferred load. This measurement could yield more revealing data in the PBM+ group. Other studies with designs that allow ISQ measurements without the need for reverse‐torque to the implants are necessary. Another limitation inherent to the technology itself lies in the impossibility of evaluating the bone/implant surface (especially in the buccal area) that absorbs/reflects the laser rays. The use of postoperative antiseptics and antibiotics (both medically and ethically necessary) could produce biases based on individual variability. The role of the implant diameter in relation to the crestal bone should be evaluated in more specific studies.
5. CONCLUSIONS
Long‐term applications of PBM do not interfere with implant stability, and they may provide a good alternative for professionals that need to increase these values in early phases for prosthodontic reasons. With regards to healing and inflammation, PBM showed excellent results in this study. Therefore, the application of 808 nm infra‐red laser for bone tissue, and 630 nm for mucosal tissue in two sessions (in the immediate postoperative period and 7 days after) is considered to be an effective way of reducing inflammation and improving early healing of mucosal tissue, but ineffective to increase or accelerate the secondary stability of implants. Long‐term monitoring of these implants is required in order to assess the role of PBM in terms of marginal bone loss.
AUTHOR CONTRIBUTIONS
Gisela Cristina Vianna Camolesi: Conceptualization (equal); data curation (lead); formal analysis (supporting); investigation (equal); methodology (supporting); project administration (equal); resources (equal); validation (equal); writing – original draft (lead). Manuel Somoza‐Martin: Conceptualization (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal). M Dolores Reboiras‐López: Data curation (equal); supervision (supporting). Fabio Camacho‐Alonso: Formal analysis (supporting); validation (supporting); writing – review and editing (supporting). Andrés Blanco Carrión: Formal analysis (equal); methodology (equal); supervision (equal). Mario Pérez‐Sayáns: Conceptualization (equal); data curation (supporting); formal analysis (lead); investigation (lead); methodology (lead); project administration (equal); resources (equal); supervision (lead); validation (equal); writing – original draft (supporting); writing – review and editing (lead).
FUNDING INFORMATION
The study was supported by Nueva Galimplant and the University of Santiago de Compostela (Ref. USC‐2019‐CE178). The funding source played no role in the design of this study, the data collection and analyses, the decision to publish, and the preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Appendix S1.
Camolesi, G. C. V. , Somoza‐Martín, J. M. , Reboiras‐López, M. D. , Camacho‐Alonso, F. , Blanco‐Carrión, A. , & Pérez‐Sayáns, M. (2023). Photobiomodulation in dental implant stability and post‐surgical healing and inflammation. A randomised double‐blind study. Clinical Oral Implants Research, 34, 137–147. 10.1111/clr.14026
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Agha‐Hosseini, F. , Moslemi, E. , & Mirzaii‐Dizgah, I. (2012). Comparative evaluation of low‐level laser and CO₂ laser in treatment of patients with oral lichen planus. International Journal of Oral and Maxillofacial Surgery, 41, 1265–1269. 10.1016/j.ijom.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Aguirre‐Zorzano, L.‐A. , Vallejo‐Aisa, F.‐J. , & Estefanía‐Fresco, R. (2013). Supportive periodontal therapy and periodontal biotype as prognostic factors in implants placed in patients with a history of periodontitis. Medicina Oral, Patología Oral y Cirugía Bucal, 18, e786–e792. 10.4317/medoral.19136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat, M. , & Jalalifirouzkouhi, A. (2017). Presenting a method to improve bone quality through stimulation of osteoporotic mesenchymal stem cells by low‐level laser therapy. Photomedicine and Laser Surgery, 35, 622–628. 10.1089/pho.2016.4245 [DOI] [PubMed] [Google Scholar]
- Bloemen, M. C. T. , Boekema, B. K. H. L. , Vlig, M. , van Zuijlen, P. P. M. , & Middelkoop, E. (2012). Digital image analysis versus clinical assessment of wound epithelialization: A validation study. Burns, 38, 501–505. 10.1016/j.burns.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Bozkaya, S. , Uraz, A. , Guler, B. , Kahraman, S. A. , & Turhan Bal, B. (2021). The stability of implants and microbiological effects following photobiomodulation therapy with one‐stage placement: A randomized, controlled, single‐blinded, and split‐mouth clinical study. Clinical Implant Dentistry and Related Research, 23, 329–340. 10.1111/cid.12999 [DOI] [PubMed] [Google Scholar]
- Carroll, J. D. , Milward, M. R. , Cooper, P. R. , Hadis, M. , & Palin, W. M. (2014). Developments in low level light therapy (LLLT) for dentistry. Dental Materials, 30, 465–475. 10.1016/j.dental.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Liu, C. , Chen, X. , & Mo, A. (2019). Clinical evidence of photobiomodulation therapy (PBMT) on implant stability and success: A systematic review and meta‐analysis. BMC Oral Health, 19, 77. 10.1186/s12903-019-0779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Zhang, Y. , Li, J. , Wang, H.‐L. , & Yu, H. (2017). Influence of laser‐microtextured surface collar on marginal bone loss and peri‐implant soft tissue response: A systematic review and meta‐analysis. Journal of Periodontology, 88, 651–662. 10.1902/jop.2017.160805 [DOI] [PubMed] [Google Scholar]
- Cuschieri, S. (2019). The CONSORT statement. Saudi Journal of Anaesthesia, 13, S27–S30. 10.4103/sja.SJA_559_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dompe, C. , Moncrieff, L. , Matys, J. , Grzech‐Leśniak, K. , Kocherova, I. , Bryja, A. , Bruska, M. , Dominiak, M. , Mozdziak, P. , Skiba, T. H. I. , Shibli, J. A. , Volponi, A. A. , Kempisty, B. , & Dyszkiewicz‐Konwińska, M. (2020). Photobiomodulation‐underlying mechanism and clinical applications. Journal of Clinical Medicine, 9, 1724. 10.3390/jcm9061724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias, C. N. , Rocha, F. A. , Nascimento, A. L. , & Coelho, P. G. (2012). Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. Journal of the Mechanical Behavior of Biomedical Materials, 16, 169–180. 10.1016/j.jmbbm.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Farivar, S. , Malekshahabi, T. , & Shiari, R. (2014). Biological effects of low level laser therapy. Journal of Lasers in Medical Sciences, 5, 58–62. [PMC free article] [PubMed] [Google Scholar]
- Fernandes, P. F. , Grenho, L. , Fernandes, M. H. , Sampaio‐Fernandes, J. C. , & Gomes, P. S. (2021). Microgap and bacterial microleakage during the osseointegration period: An in vitro assessment of the cover screw and healing abutment in a platform‐switched implant system. The Journal of Prosthetic Dentistry. 10.1016/j.prosdent.2021.07.030 [DOI] [PubMed] [Google Scholar]
- Fornaini, C. , Merigo, E. , Vescovi, P. , Bonanini, M. , Antonietti, W. , Leoci, L. , Lagori, G. , & Meleti, M. (2015). Different laser wavelengths comparison in the second‐stage implant surgery: An ex vivo study. Lasers in Medical Science, 30, 1631–1639. 10.1007/s10103-014-1623-3 [DOI] [PubMed] [Google Scholar]
- García‐Morales, J. M. , Tortamano‐Neto, P. , Todescan, F. F. , de Andrade, J. C. S. J. , Marotti, J. , & Zezell, D. M. (2012). Stability of dental implants after irradiation with an 830‐nm low‐level laser: A double‐blind randomized clinical study. Lasers in Medical Science, 27, 703–711. 10.1007/s10103-011-0948-4 [DOI] [PubMed] [Google Scholar]
- Gholami, L. , Asefi, S. , Hooshyarfard, A. , Sculean, A. , Romanos, G. E. , Aoki, A. , & Fekrazad, R. (2019a). Photobiomodulation in periodontology and implant dentistry: Part 1. Photobiomodulation, Photomedicine, and Laser Surgery, 37, 739–765. 10.1089/photob.2019.4710 [DOI] [PubMed] [Google Scholar]
- Gholami, L. , Asefi, S. , Hooshyarfard, A. , Sculean, A. , Romanos, G. E. , Aoki, A. , & Fekrazad, R. (2019b). Photobiomodulation in periodontology and implant dentistry: Part 2. Photobiomodulation, Photomedicine, and Laser Surgery, 37, 766–783. 10.1089/photob.2019.4731 [DOI] [PubMed] [Google Scholar]
- Gokmenoglu, C. , Ozmeric, N. , Erguder, I. , & Elgun, S. (2014). The effect of light‐emitting diode photobiomodulation on implant stability and biochemical markers in peri‐implant crevicular fluid. Photomedicine and Laser Surgery, 32, 138–145. 10.1089/pho.2012.3473 [DOI] [PubMed] [Google Scholar]
- Hamblin, M. R. (2017). Mechanisms and applications of the anti‐inflammatory effects of photobiomodulation. AIMS Biophysics, 4, 337–361. 10.3934/biophy.2017.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzani, Y. , & Chaushu, G. (2018). Evaluation of early wound healing scales/indexes in oral surgery: A literature review. Clinical Implant Dentistry and Related Research, 20, 1030–1035. 10.1111/cid.12680 [DOI] [PubMed] [Google Scholar]
- Howe, M.‐S. , Keys, W. , & Richards, D. (2019). Long‐term (10‐year) dental implant survival: A systematic review and sensitivity meta‐analysis. Journal of Dentistry, 84, 9–21. [DOI] [PubMed] [Google Scholar]
- Huang, Y.‐Y. , Sharma, S. K. , Carroll, J. , & Hamblin, M. R. (2011). Biphasic dose response in low level light therapy ‐ An update. Dose‐response: A Publication of International Hormesis Society, 9, 602–618. 10.2203/dose-response.11-009.Hamblin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insua, A. , Monje, A. , Wang, H.‐L. , & Miron, R. J. (2017). Basis of bone metabolism around dental implants during osseointegration and peri‐implant bone loss. Journal of Biomedical Materials Research. Part A, 105, 2075–2089. 10.1002/jbm.a.36060 [DOI] [PubMed] [Google Scholar]
- Karakaya, M. , & Demirbaş, A. E. (2020). Effect of low‐level laser therapy on osseointegration of titanium dental implants in ovariectomized rabbits: Biomechanics and micro‐CT analysis. International Journal of Implant Dentistry, 6, 61. 10.1186/s40729-020-00257-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoussis, I. K. , Kyriakidou, K. , Psarros, C. , Lang, N. P. , & Vrotsos, I. A. (2017). Nd:YAG laser radiation (1.064 nm) accelerates differentiation of osteoblasts to osteocytes on smooth and rough titanium surfaces in vitro. Clinical Oral Implants Research, 28, 785–790. 10.1111/clr.12882 [DOI] [PubMed] [Google Scholar]
- Kazem Shakouri, S. , Soleimanpour, J. , Salekzamani, Y. , & Oskuie, M. R. (2010). Effect of low‐level laser therapy on the fracture healing process. Lasers in Medical Science, 25, 73–77. 10.1007/s10103-009-0670-7 [DOI] [PubMed] [Google Scholar]
- Khadra, M. , Lyngstadaas, S. P. , Haanaes, H. R. , & Mustafa, K. (2005). Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast‐like cells cultured on titanium implant material. Biomaterials, 26, 3503–3509. 10.1016/j.biomaterials.2004.09.033 [DOI] [PubMed] [Google Scholar]
- Khadra, M. , Rønold, H. J. , Lyngstadaas, S. P. , Ellingsen, J. E. , & Haanaes, H. R. (2004). Low‐level laser therapy stimulates bone‐implant interaction: An experimental study in rabbits. Clinical Oral Implants Research, 15, 325–332. 10.1111/j.1600-0501.2004.00994.x [DOI] [PubMed] [Google Scholar]
- Kinalski, M. D. A. , Agostini, B. A. , Bergoli, C. D. , & Dos Santos, M. B. F. (2021). Influence of low‐level laser therapy on implant stability in implants placed in healed sites: A randomized controlled trial. International Journal of Implant Dentistry, 7, 49. 10.1186/s40729-021-00331-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry, R. G. (1988). Effectiveness of benzydamine HC1 in the treatment of periodontal post‐surgical patients (Vol. 10, pp. 105–118). Faculty of Dentistry, University of Toronto. [Google Scholar]
- Lima, A. M. C. T. , da Silva Sergio, L. P. , & De Souza da Fonseca, A. (2020). Photobiomodulation via multiple‐wavelength radiations. Lasers in Medical Science, 35, 307–316. 10.1007/s10103-019-02879-1 [DOI] [PubMed] [Google Scholar]
- Lobato, R. P. B. , Kinalski, M. D. A. , Martins, T. M. , Agostini, B. A. , Bergoli, C. D. , & Dos Santos, M. B. F. (2020). Influence of low‐level laser therapy on implant stability in implants placed in fresh extraction sockets: A randomized clinical trial. Clinical Implant Dentistry and Related Research, 22, 261–269. 10.1111/cid.12904 [DOI] [PubMed] [Google Scholar]
- Lopes, C. B. , Pinheiro, A. L. B. , Sathaiah, S. , Duarte, J. , & Cristinamartins, M. (2005). Infrared laser light reduces loading time of dental implants: A Raman spectroscopic study. Photomedicine and Laser Surgery, 23, 27–31. 10.1089/pho.2005.23.27 [DOI] [PubMed] [Google Scholar]
- Maluf, A. P. , Maluf, R. P. , da Rocha Brito, C. , França, F. M. G. , & de Brito, R. B. (2010). Mechanical evaluation of the influence of low‐level laser therapy in secondary stability of implants in mice shinbones. Lasers in Medical Science, 25, 693–698. 10.1007/s10103-010-0778-9 [DOI] [PubMed] [Google Scholar]
- Mandić, B. , Lazić, Z. , Marković, A. , Mandić, B. , Mandić, M. , Djinić, A. , & Miličić, B. (2015). Influence of postoperative low‐level laser therapy on the osseointegration of self‐tapping implants in the posterior maxilla: A 6‐week split‐mouth clinical study. Vojnosanitetski Pregled, 72, 233–240. 10.2298/vsp131202075m [DOI] [PubMed] [Google Scholar]
- Mandrekar, J. N. (2011). Measures of interrater agreement. Journal of Thoracic Oncology, 6, 6–7. 10.1097/JTO.0b013e318200f983 [DOI] [PubMed] [Google Scholar]
- Marañón‐Vásquez, G. A. , Lagravère, M. O. , Borsatto, M. C. , de Souza, S. S. , Watanabe, P. C. A. , Matsumoto, M. A. , Saraiva, M. D. C. P. , & Romano, F. L. (2019). Effect of photobiomodulation on the stability and displacement of orthodontic mini‐implants submitted to immediate and delayed loading: A clinical study. Lasers in Medical Science, 34, 1705–1715. 10.1007/s10103-019-02818-0 [DOI] [PubMed] [Google Scholar]
- Marquezan, M. , Osório, A. , Sant'Anna, E. , Souza, M. M. , & Maia, L. (2012). Does bone mineral density influence the primary stability of dental implants? A systematic review. Clinical Oral Implants Research, 23, 767–774. 10.1111/j.1600-0501.2011.02228.x [DOI] [PubMed] [Google Scholar]
- Matys, J. , Flieger, R. , & Dominiak, M. (2017). Effect of diode lasers with wavelength of 445 and 980 nm on a temperature rise when uncovering implants for second stage surgery: An ex‐vivo study in pigs. Advances in Clinical and Experimental Medicine, 26, 687–693. 10.17219/acem/68943 [DOI] [PubMed] [Google Scholar]
- Matys, J. , Flieger, R. , Tenore, G. , Grzech‐Leśniak, K. , Romeo, U. , & Dominiak, M. (2018). Er:YAG laser, piezosurgery, and surgical drill for bone decortication during orthodontic mini‐implant insertion: Primary stability analysis—An animal study. Lasers in Medical Science, 33, 489–495. 10.1007/s10103-017-2381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys, J. , Świder, K. , Grzech‐Leśniak, K. , Dominiak, M. , & Romeo, U. (2019). Photobiomodulation by a 635nm diode laser on peri‐implant bone: Primary and secondary stability and bone density analysis‐A randomized clinical trial. BioMed Research International, 2019, 2785302. 10.1155/2019/2785302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarian, J. , Ketabi, M. , & Amini, S. (2018). The effect of low‐level laser 810 nm and light‐emitting diode photobiomodulation (626 nm) on the stability of the implant and inflammatory markers interleukin‐1 beta and prostaglandin E2, around implants. Dental Research Journal, 15, 283–288. [PMC free article] [PubMed] [Google Scholar]
- Mills, M. P. , Rosen, P. S. , Chambrone, L. , Greenwell, H. , Kao, R. T. , Klokkevold, P. R. , McAllister, B. S. , Reynolds, M. A. , Romanos, G. E. , & Wang, H.‐L. (2018). American Academy of periodontology best evidence consensus statement on the efficacy of laser therapy used alone or as an adjunct to non‐surgical and surgical treatment of periodontitis and peri‐implant diseases. Journal of Periodontology, 89, 737–742. 10.1002/JPER.17-0356 [DOI] [PubMed] [Google Scholar]
- Monea, A. , Beresescu, G. , Boeriu, S. , Tibor, M. , Popsor, S. , & Antonescu, D. M. (2015). Bone healing after low‐level laser application in extraction sockets grafted with allograft material and covered with a resorbable collagen dressing: A pilot histological evaluation. BMC Oral Health, 15, 134. 10.1186/s12903-015-0122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje, A. , Ravidà, A. , Wang, H.‐L. , Helms, J. A. , & Brunski, J. B. (2019). Relationship between primary/mechanical and secondary/biological implant stability. The International Journal of Oral & Maxillofacial Implants, 34, s7–s23. 10.11607/jomi.19suppl.g1 [DOI] [PubMed] [Google Scholar]
- Na, S. , TruongVo, T. , Jiang, F. , Joll, J. E. , Guo, Y. , Utreja, A. , & Chen, J. (2018). Dose analysis of photobiomodulation therapy on osteoblast, osteoclast, and osteocyte. Journal of Biomedical Optics, 23, 075008. 10.1117/1.JBO.23.7.075008 [DOI] [PubMed] [Google Scholar]
- Palled, V. , Rao, J. , Singh, R. D. , Tripathi, S. , Singh, K. , Radav, R. , Verma, U. , & Chand, P. (2021). Assessment of the healing of dental implant surgical site following low‐level laser therapy using bioclinical parameters: An exploratory study. The Journal of Oral Implantology, 47, 230–235. 10.1563/aaid-joi-D-18-00316 [DOI] [PubMed] [Google Scholar]
- Pereira, C. L. , Sallum, E. A. , Nociti, F. H. J. , & Moreira, R. W. F. (2009). The effect of low‐intensity laser therapy on bone healing around titanium implants: A histometric study in rabbits. The International Journal of Oral & Maxillofacial Implants, 24, 47–51. [PubMed] [Google Scholar]
- Pippi, R. (2017). Post‐surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. International Journal of Medical Sciences, 14, 721–728. 10.7150/ijms.19727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, C. , Luo, F. , Hong, G. , & Wan, Q. (2022). Effects of photobiomodulation therapy on implant stability and postoperative recovery: A systematic review and meta‐analysis. The British Journal of Oral & Maxillofacial Surgery, 60, e712–e721. 10.1016/j.bjoms.2022.01.014 [DOI] [PubMed] [Google Scholar]
- Riboldi, A. , Bombeccari, G. P. , & Spadari, F. (2009). Laser therapies in the treatment of oral facial painLaser therapies in the treatment of oral facial pain. Dental Cadmos, 77, 71–83. [Google Scholar]
- Romão, M. M. A. , Marques, M. M. , Cortes, A. R. G. , Horliana, A. C. R. T. , Moreira, M. S. , & Lascala, C. A. (2015). Micro‐computed tomography and histomorphometric analysis of human alveolar bone repair induced by laser phototherapy: A pilot study. International Journal of Oral and Maxillofacial Surgery, 44, 1521–1528. 10.1016/j.ijom.2015.08.989 [DOI] [PubMed] [Google Scholar]
- Santinoni, C. S. , Neves, A. P. C. , Almeida, B. F. M. , Kajimoto, N. C. , Pola, N. M. , Caliente, E. A. , Belem, E. L. G. , Lelis, J. B. , Fucini, S. E. , Messora, M. R. , Garcia, V. G. , Bomfim, S. R. M. , Ervolino, E. , & Nagata, M. J. H. (2021). Bone marrow coagulated and low‐level laser therapy accelerate bone healing by enhancing angiogenesis, cell proliferation, osteoblast differentiation, and mineralization. Journal of Biomedical Materials Research. Part A, 109, 849–858. 10.1002/jbm.a.37076 [DOI] [PubMed] [Google Scholar]
- Shokri, M. , & Daraeighadikolaei, A. (2013). Measurement of primary and secondary stability of dental implants by resonance frequency analysis method in mandible. International Journal of Dentistry, 2013, 506968. 10.1155/2013/506968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, E. , & Arany, P. (2013). Photobiomodulation and implants: Implications for dentistry. Journal of Periodontal & Implant Science, 43, 262–268. 10.5051/jpis.2013.43.6.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkzaban, P. , Kasraei, S. , Torabi, S. , & Farhadian, M. (2018). Low‐level laser therapy with 940 nm diode laser on stability of dental implants: A randomized controlled clinical trial. Lasers in Medical Science, 33, 287–293. 10.1007/s10103-017-2365-9 [DOI] [PubMed] [Google Scholar]
- Tsai, S.‐R. , & Hamblin, M. R. (2017). Biological effects and medical applications of infrared radiation. Journal of Photochemistry and Photobiology B: Biology, 170, 197–207. 10.1016/j.jphotobiol.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff, L. D. , Bounkeo, J. M. , Brannon, W. M. , Dawes, K. S. , Barham, C. D. , Waddell, D. L. , & Enwemeka, C. S. (2004). The efficacy of laser therapy in wound repair: A meta‐analysis of the literature. Photomedicine and Laser Surgery, 22, 241–247. 10.1089/1549541041438623 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Data available on request from the authors.


