Summary
The economic and ecologically important genus Eucalyptus is rich in structurally diverse specialized metabolites. While some specialized metabolite classes are highly prevalent across the genus, the cyanogenic glucoside prunasin is only produced by c. 3% of species.
To investigate the evolutionary mechanisms behind prunasin biosynthesis in Eucalyptus, we compared de novo assembled transcriptomes, together with online resources between cyanogenic and acyanogenic species. Identified genes were characterized in vivo and in vitro.
Pathway characterization of cyanogenic Eucalyptus camphora and Eucalyptus yarraensis showed for the first time that the final glucosylation step from mandelonitrile to prunasin is catalyzed by a novel UDP‐glucosyltransferase UGT87. This step is typically catalyzed by a member of the UGT85 family, including in Eucalyptus cladocalyx. The upstream conversion of phenylalanine to mandelonitrile is catalyzed by three cytochrome P450 (CYP) enzymes from the CYP79, CYP706, and CYP71 families, as previously shown. Analysis of acyanogenic Eucalyptus species revealed the loss of different ortholog prunasin biosynthetic genes.
The recruitment of UGTs from different families for prunasin biosynthesis in Eucalyptus demonstrates important pathway heterogeneities and unprecedented dynamic pathway evolution of chemical defense within a single genus. Overall, this study provides relevant insights into the tremendous adaptability of these long‐lived trees.
Keywords: chemical defense, cyanogenic glucoside, cytochrome P450, Eucalyptus, evolution, plant‐specialized metabolism, UDP‐glycosyltransferase, UGT87
Introduction
Plants have acquired exceptional abilities to adapt and adjust to their biotic and abiotic environments. This adaptability is particularly impressive in long‐lived trees that must withstand changing environmental conditions and stresses over hundreds of years. Abiotic and biotic interactions are significantly mediated by the biosynthesis of highly diverse specialized metabolites, such as terpenoids, alkaloids, phenylpropanoids, and cyanogenic glucosides. These metabolites play many essential roles in plants, including chemical defense against herbivores and pathogens, resistance to extreme temperatures, and the attraction of pollinators. Plant evolution is repeatedly marked by the dynamic acquisition and loss of associated biosynthetic genes, resulting in a large and diverse chemical landscape across the plant kingdom, and in plants' abilities to occupy and withstand highly variable environmental conditions and niches.
Eucalyptus L'Hér is a hyper‐diverse and large genus of over 700 species (Nicolle, 2019), with high economic and ecological relevance. Eucalyptus trees are the most widely planted commercial hardwoods in the world and display remarkable adaptability across native Australia and nearby islands occupying many diverse and sometimes extreme ecosystems (Bennett, 2016). Tandem gene duplications are suggested to play an important role in the diversification of eucalypts (Butler et al., 2017), with tandem duplications constituting 34% of the predicted protein‐coding gene sequences in the genome of Eucalyptus grandis (Myburg et al., 2014). Notably, the E. grandis genome is particularly enriched in genes encoding terpene synthases (TPSs), cytochromes P450 (CYP), and UDP‐glucosyltransferases (UGT), which are enzyme families that are significantly involved in the biosynthesis of specialized metabolites (Chae et al., 2014; Myburg et al., 2014; Kulheim et al., 2015; Bustos‐Segura et al., 2017; Wilson & Tian, 2019; Hansen et al., 2021). Indeed, Eucalyptus species produce a corresponding panoply of specialized metabolites, with high intra and interspecies variation (Goodger & Woodrow, 2011; Neilson et al., 2011; Bustos‐Segura et al., 2017; Marsh et al., 2017; dos Santos et al., 2019; Sørensen et al., 2020). This variation is exemplified by the low proportion of species that display cyanogenic glucosides, with only some 3% of Eucalyptus species identified as cyanogenic (Gleadow et al., 2008). All authenticated cyanogenic Eucalyptus species belong to the subgenus Symphyomyrtus and are grouped into two distinct phylogenetic clusters: section Maidenaria; and sections Adnataria, Sejunctae, and Glandulosae (Gleadow et al., 2008; Steane et al., 2011). Most recent phylogenetic analysis of 732 species and subspecies indicates further stratification between the Adnataria and Sejunctae sections, and section Glandulosae, but resolution is challenged by a high degree of polyphyly (Fig. 1a; Thornhill et al., 2019).
Fig. 1.
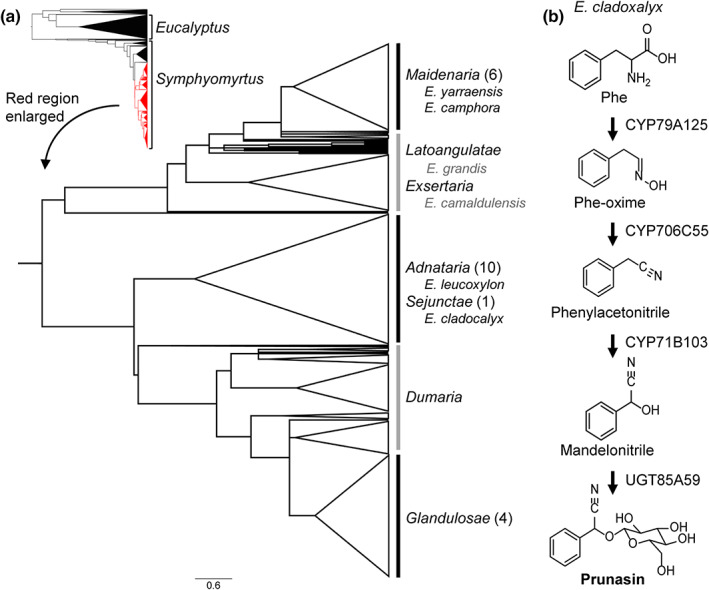
Distribution and biosynthesis of cyanogenic glucosides in Eucalyptus. (a) Species phylogeny of the genus Eucalyptus adapted from Thornhill et al. (2019). The small phylogenetic tree shows the phylogeny of the entire Eucalyptus genus, where the two major subgenera (Eucalyptus and Symphyomyrtus) are labeled. Subgenus Eucalyptus and the section Bisectae are colored black. The subtree marked in red is enlarged and consists of all sections belonging to Symphyomyrtus except the large acyanogenic section Bisectae. Eucalyptus species are highly paraphyletic (Thornhill et al., 2019), and the sections are labeled based on the most number of representative species for a given section. Sections encompassing cyanogenic species (black lines) and mayor sections without cyanogenic species (gray lines) are marked. The number in brackets denotes the number of identified cyanogenic species in a given section (Gleadow et al., 2008). Cyanogenic and acyanogenic species studied in this article are written in black and gray, respectively. (b) Prunasin biosynthetic pathway in Eucalyptus cladocalyx (section Sejunctae) (Hansen et al., 2018). phe, phenylalanine; phe‐oxime, phenylacetaldoxime.
Cyanogenic glucosides are amino acid‐derived α‐hydroxynitrile glucosides (Gleadow & Møller, 2014). Upon tissue disruption and enzymatic hydrolysis, toxic hydrogen cyanide is liberated, thereby providing an effective chemical defense against generalist herbivores (Gleadow & Woodrow, 2002). In addition to defense, cyanogenic glucosides have increasingly been shown to provide reduced nitrogen for general metabolism via different recycling pathways (Jenrich et al., 2007; Picmanova et al., 2015; Bjarnholt et al., 2018; Schmidt et al., 2018). Cyanogenic Eucalyptus species produce l‐Phe‐derived cyanogenic glucosides, with prunasin being the major accumulating cyanogen. To date, characterization of prunasin biosynthesis in Eucalyptus has only been resolved for Eucalyptus cladocalyx (section Sejunctae), whereby prunasin is produced by the successive action of three CYP enzymes (CYP79A125, CYP706C55, and CYP71B103) and a UGT85A59 (Fig. 1b; Hansen et al., 2018). Notably, this biosynthetic configuration contrasts with other characterized cyanogenic angiosperms, which only require two CYPs and a UGT (e.g. almond (Prunus dulcis)) (Thodberg et al., 2018).
Given the distinct distribution of cyanogenic species within the subgenus Symphyomyrtus, the atypical configuration of prunasin biosynthesis previously described for E. cladocalyx, and the known genetic complexity of the Eucalyptus genus in general, the functional characterization of cyanogenic glucoside biosynthesis within this genus constitutes an excellent model to investigate the dynamic evolution of specialized metabolism. We tested the following three hypotheses: (1) Due to the close relatedness within the subgenus Symphyomyrtus, cyanogenic eucalypts would possess orthologous CYP79A, CYP706C, CYP71B, and UGT85A encoding genes to E. cladocalyx with conserved functionalities, (2) acyanogenic eucalypts would lack either one or more members of the genes encoding the biosynthetic enzymes in the prunasin pathway, and/or (3) acyanogenic eucalypts possess orthologous genes, but their functionality is compromised. Here, we show that cyanogenic Eucalyptus species of the Maidenaria section have recruited a novel UGT87Y1 that catalyzes the glucosylation of mandelonitrile to prunasin. This represents the first UGT87 family member to be functionality‐characterized in plants. We also show that the atypical three‐CYP system is retained within cyanogenic Eucalyptus species, and through analysis of the acyanogenic species E. camaldulensis and E. grandis, and that there have been losses of orthologous prunasin biosynthetic genes in these species. Taken together, a highly dynamic evolution of chemical defense within the Eucalyptus genus is demonstrated, providing insights into the tremendous adaptability of these long‐lived trees.
Materials and Methods
Plant material
Eucalyptus L'Hér seeds were purchased from CSIRO Australian Tree Seed Centre (Clayton, Vic., Australia). Seedlings were germinated in a glasshouse at the University of Copenhagen on 24 August 2013 and grown at minimum 22°C during the day (10‐h light) and minimum 19°C at night. Leaf samples were harvested 1, 4, 7, and 10 months after sowing (33, 110, 215, and 285 d after sowing, respectively) from eight biological replicates. For the 1‐month‐aged seedlings, all plants were pooled to obtain sufficient leaf material. Nicotiana benthamiana Domin plants were grown in a glasshouse at 21°C : 19°C, day : night and infiltrated at c. 4 wk of age.
RNA extractions and transcriptomic analysis
Total RNA was extracted from a single representative expanding leaf from Eucalyptus camphora and Eucalyptus yarraensis plants at 4 and 7 months of age and a pooled sample at 1 month of age, using Spectrum™ Plant Total RNA Kit (Sigma) according to the manufacturer's instructions with a few modifications according to Hansen et al. (2018). The quantity and integrity of RNA was measured on an Agilent 2100 Bioanalyzer instrument using an RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer's instructions.
Transcriptomic sequencing (Hiseq2500, 100‐bp paired‐end) was performed by Macrogen Inc. (Seoul, Korea). Two independent transcriptome assemblies were combined for candidate gene identification. One assembly was performed by Sequentia Biotech (Bellaterra, Spain) as reported previously (Hansen et al., 2018), using a minimum length setting of 35 bp and a minimum quality score of 35. The transcripts were clustered based on the coding sequence to remove redundancy (Cerveau & Jackson, 2016). In the second assembly, the transcriptomes were also de novo assembled using a genome‐guided Trinity pipeline (Haas et al., 2013) after trimming the reads using AdapterRemoval (Lindgreen, 2012), with a minimum length setting of 40 bp and a minimum quality score of 28. Expression levels were quantified using Kallisto (Bray et al., 2016). The first transcriptome assembly was used to guide UGT selection (Table S2), while the second transcriptome was used to better quantify UGT expression due to a detailed assessment of intron retainment and bicistronic assembly in the datasets.
Transient expression in N. benthamiana
The open reading frames (ORFs) of prunasin CYPs from E. camphora and E. yarraensis were cloned from cDNA. Primers used for the amplification of the nucleotide sequences are listed in Table S1. ORFs for genes from E. grandis and the UGT genes were ordered as synthetic constructs from Genscript (Piscataway, NJ, USA). The ORF of UGT87Y1 was in addition amplified from cDNA prepared from RNA from E. camphora and E. yarraensis, respectively.
Agrobacterium‐mediated transient expression in N. benthamiana was carried out as described previously (Hansen et al., 2018). Equal volumes of agrobacteria suspensions were introduced into N. benthamiana leaves using a blunt syringe, with an expression construct containing the p19 gene (Hearne et al., 1990) always co‐infiltrated to prevent post‐transcriptional gene silencing (Voinnet et al., 1999; Lakatos et al., 2004). Leaf disks (1.4 cm in diameter) were harvested 4–5 d after infiltration, snap‐frozen in liquid nitrogen, and stored at −70°C until extraction. Due to the effect of leaf age and sampling position in the leaf on the variance in agroinfiltration experiments (Bashandy et al., 2015), leaf disks were consistently harvested from the first expanded leaf, between the midrib and leaf edge. Leaf disks from minimum two replicate plants were harvested for each combination and verified in at least two independent experiments.
Metabolite extractions
Metabolites from frozen, homogenized E. camphora and E. yarraensis leaf tissue (15–50 mg) were extracted with cold aqueous 80% MeOH, while 85% MeOH was used for infiltrated N. benthamiana plants. Plant tissue was incubated on ice (5 min) and subsequently centrifuged (3 min, 10 000 g , 4°C). Extracts were diluted five times in MilliQ water and filtered through a Durapore® membrane with 22 μm pore size (Merck Millipore, Burlington, MA, USA).
Liquid chromatography tandem mass spectrometry
Extracts were analyzed either by an Agilent 1100 Series LC system coupled to a Bruker HCT‐Ultra ion trap mass spectrometer (LC‐ion trap) or using a Dionex Ultimate 3000RS UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) system coupled to a Compact™ (UPLC‐QqToF) mass spectrometer (Bruker Daltonics, Bremen, Germany). Both instruments were equipped with an electrospray ionization source, DAD detector, and a temperature‐controlled auto‐sampler (10°C). Extracts of Eucalyptus and initial N. benthamiana transient expression experiments were analyzed according to the LC‐ion trap methods reported in Hansen et al. (2018). Later, leaf extracts of N. benthamiana analyzed by UHPLC‐QqToF were injected (2 μl) onto a Kinetex XB‐C18 UHPLC column (150 × 2.1 mm, 1.7 μm; Phenomenex, Torrance, CA, USA) at 40°C with a flow rate of 0.3 ml min−1. The mobile phase A consisted of 0.05% formic acid in water, and solvent B consisted of 0.05% formic acid in acetonitrile. The initial composition was 95% A and 5% B, which was held for 1 min, followed by a linear gradient to 70% solvent B over 6 min, and a subsequent increase to 100% B over 1 min and held at 100% solvent B for 2 min. The QqToF mass spectrometer was operated in negative ion mode within a range of m/z 50–1200, and data‐dependent MS/MS acquisition was triggered for the three most intense ions in the MS spectra. Liquid chromatography tandem mass spectrometry (LC‐MS/MS) data were analyzed using the Bruker Data Analysis 5.0 software. Prunasin was identified by comparison with an authentic standard (Møller et al., 2016), and other compounds were putatively annotated based on their m/z value, MS2 fragmentation pattern, similar to the CFM‐ID 4.0 library (for benzoic acid glucoside; Wang et al., 2021) and comparison with the previous literature (Bak et al., 2000; Kristensen et al., 2005; Hansen et al., 2018; Thodberg et al., 2018).
Microbial protein expression and purification
Codon‐optimized UGT85A59 and UGT87Y1 in pET‐28a(+)‐TEV were ordered from Genscript® and expressed in NiCo21 cells (New England Biolabs, Ipswich, MA, USA) in TB media with 50 μg ml−1 kanamycin at 16°C for 20 h, 200 rpm, after induction with 0.5 mM IPTG. Cell pellets were sedimented by centrifugation (15 000 g , 15 min, 4°C) and stored at −20°C. Thawed cell pellets were suspended in 5 ml lysis buffer (100 mM Tris (pH 7.5), 300 mM NaCl, 1 mM MgCl2, 0.5 mM tris(2‐carboxyethyl)phosphine (TCEP), 0.04 lysozyme mg ml−1, benzonase (25 U ml−1; Sigma‐Aldrich), 1 mM PMSF) per 1 g cells and lysed using a high‐pressure homogenizer at 20 kPSI (EmulsiFlex D20, Avestin, Ottawa, Canada). The lysate was cleared by centrifugation (9600 g , 40 min), filtered (0.45 μM pore size), and applied to an equilibrated 5 ml His‐Trap FF column (GE Healthcare, Chicago, IL, USA) connected to an ÄKTAexpress (GE Healthcare). The column was washed with 10 column volumes (CV) of wash buffer (50 mM Na3PO4 (pH 7.5), 300 mM NaCl, 30 mM imidazole, and 0.5 mM TCEP). The protein was eluted using a combination of gradient and step elution: The column was washed with a linear gradient of 0–7.5% elution buffer (50 mM Na3PO4 (pH 7.5), 300 mM NaCl, 500 mM Imidazole, and 0.5 mM TCEP) followed by at least 2 CV at 7.5%. A second linear gradient of 7.5–25% for UGT87Y1 and 7.5–35% for UGT85A59 was followed by another plateau of at least 5 CV before a third linear increase to 100% elution buffer. The eluate was collected in 1.5 ml fractions, and fractions containing the target protein were pooled and concentrated to a final total volume of 6 ml by centrifugation using a pre‐equilibrated membrane filter (30 kDa cutoff; Thermo Fisher Scientific). The concentrate was applied to an equilibrated HiLoad 16/60 Superdex 200 column (120 ml; GE Healthcare) connected to an ÄKTA Pure (GE Healthcare). Protein was eluted in 50 mM Na3PO4 (pH 7.5) with 100 mM NaCl, 0.5 mM TCEP, and 10% w/v glycerol. Fractions containing the target protein were combined and concentrated by centrifugation (30 kDa cutoff; Thermo Fisher Scientific). During the purification process, selected fractions from all purification steps were analyzed by SDS‐PAGE using 4–12% NuPAGE protein gels (Thermo Fisher Scientific). The final protein fraction was additionally analyzed by mass spectrometry on a micrOTOF‐Q II instrument (Bruker Daltonics), which confirmed 87% purity for UGT87Y1 and 92% purity for UGT85A59 protein preparations. Isolated protein was frozen in liquid nitrogen and stored at −80°C.
Nicotiana benthamiana protein extract
Agrobacterium‐infiltrated N. benthamiana leaves were harvested 4–5 d after infiltration and frozen in liquid nitrogen. Approximately 200 mg of homogenized leaf tissue was mixed with 0.1 g polyvinylpolypyrrolidone per gram fresh weight and 800 μl buffer composed of 100 mM Tricine (pH 7.9), 250 mM sucrose, 50 mM NaCl, 2 mM DTT, 1 tablet cOmplete™ protease inhibitor cocktail (Roche) per 50 ml buffer. Samples were centrifuged for 10 min at 15 000 g , 4°C. The supernatant was transferred to a clean tube and centrifuged again (10 min at 15 000 g , 4°C) with the resultant supernatant used for in vitro assays and Western blotting.
In vitro assays
The assay reactions consisted of 10 μl total protein extract or 1.2 μg purified protein, 0.2–10 mM of substrate, 8 μM 14C‐UDP‐glucose (0.8 μCi, specific activity 250 mCi mmol−1; Perkin‐Elmer, Waltham, MA, USA), and 50 mM Tris–HCl buffer (pH 7.5) with 100 mM NaCl in a total volume of 25 μl. Due to the instability of p‐hydroxymandelonitrile and acetone cyanohydrin, these substrates were tested at 1 and 10 mM, respectively. Other substrates were tested at both 200 and 400 μM concentrations. Samples were incubated for 30 min at 30°C, 300 rpm, and then terminated by the addition of 2 μl 10% acetic acid. Samples were stored at −20°C until analysis by thin‐layer chromatography (TLC). Eight microliter sample was applied to a TLC plate (Silica gel 60F254 0.2 mm thickness; Merck, Rahway, NJ, USA), and substrate and products were separated using a mobile phase consisting of EtOAc : Me2CO : CH2Cl2 : MeOH : MilliQ H2O in a ratio of 20 : 15 : 6 : 5 : 4 (v/v). Migration of prunasin was determined by the application of an unlabeled authentic standard and visualized by the UV absorbance. Analysis of 14C‐labeled products was carried out by exposure of the TLC plate to a Storage Phosphor Screen (GE Healthcare) for 4 d followed by visualization using an Amersham Typhoon IP imager (GE Healthcare). The in vitro assays were conducted minimum two times.
Phylogenetic analysis
The resources listed in Table 1 were mined for CYP79, CYP706, CYP71, UGT85, and UGT87 sequences. Sequences were manually curated, and non‐full‐length sequences (i.e. containing deletions, insertions, and/or missing reads) were removed. CYPs involved in prunasin production and CYPs from E. grandis were kindly named by David Nelson according to the standardized CYP nomenclature system (Nelson, 2009). EgUGT87 SNPs were assessed using the Phytozome database (Goodstein et al., 2012; Myburg et al., 2014). Amino acid sequences were aligned in Mega7 (Kumar et al., 2016) using Muscle with default settings, and maximum likelihood trees were generated with n = 1000 bootstrap replicates.
Table 1.
Phylogenetic classification, authority, and source of data for Eucalyptus species investigated in this study. The classification follows Nicolle (2019).
| Species and authority | Common name | Section | Series | Data source |
|---|---|---|---|---|
| E. cladocalyx F. von Müller | Sugar gum | Sejunctae | – | Hansen et al. (2018) |
| E. leucoxylon Müller | Yellow gum | Adnataria | Melliodorae | Onekp (sample AYMT) |
| E. camaldulensis Dehnh. | River red gum | Exsertaria | Exertae subser. Rostratae | E. camaldulensis Genome Database |
| E. grandis W. Hill ex Maiden | Flooded gum, rose gum | Latoangulatae | Transversae | Phytozome |
| E. camphora R.T. Baker | Mountain swamp gum | Maidenaria | Foveolatae | This study |
| E. yarraensis Maiden & Cambage | Yarra Gum | Maidenaria | Foveolatae | This study |
Protein modeling
Homology modeling was performed with automated modeling integrated in Yet Another Scientific Artificial Reality Application (Yasara, v.16.4.16) (Krieger et al., 2009; Krieger & Vriend, 2014, 2015). Nineteen homology models were created based on five templates (2PQ6 (Li et al., 2007), 5U6S (George Thompson et al., 2017), 2ACV (Shao et al., 2005), and 2VG8 and 2VCH (Brazier‐Hicks et al., 2007)). A hybrid model, based mainly on the X‐ray structure of 2VG8, with some better folded fragments from 2ACV and 2PQ6, was used for further modeling studies. The ligand, uridine‐5′‐diphosphate (UDP), was overtaken in the hybrid model based on their position in the X‐ray structure of 2VG8. An alignment of the EgrUGT87Y with the sequence of UGT87Y1 showed 97.6% sequence identity and an almost identical model structure. The donor sugar was manually added to UDP while keeping a fixed protein structure and energy optimized with AMBER‐EHT force field (Kumar et al., 2016). One hundred docking runs were performed for mandelonitrile using MOE with the London dG fitness function (Molecular Operating Environment 2021; 2019.01; Chemical Computing Group ULC, Montreal, Canada). The fifth best docked arrangement fulfilled the requirements for an optimal catalytic reaction by forming a hydrogen bond of the hydroxyl group of mandelonitrile to the catalytic active histidine (His‐21), which is part of the catalytic dyad with Asp‐113, and by having a short distance of 3.8 Å between the carbon atoms of the ligand and the C1 of the sugar donor (dotted red line in Fig. 6b).
Fig. 6.
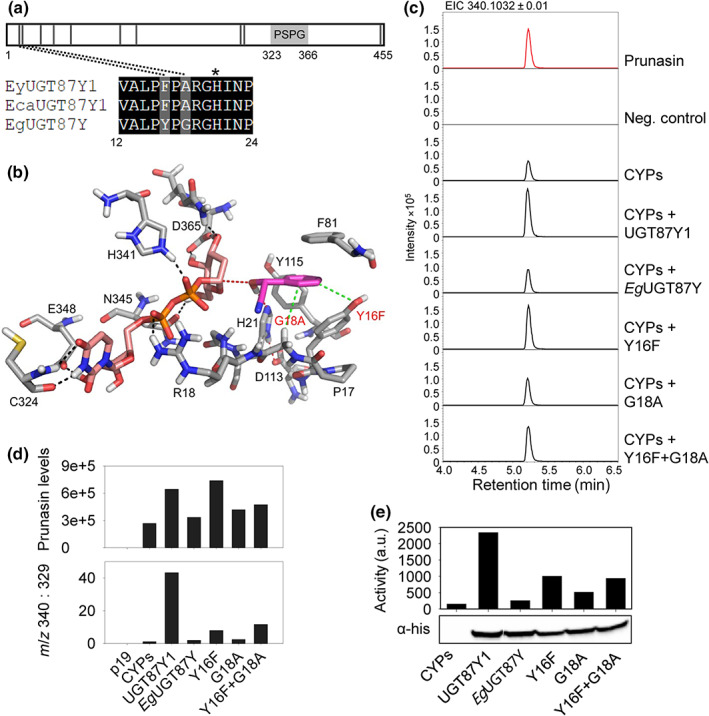
Site‐directed mutagenesis of EgUGT87. (a) Schematic of the UGT87 amino acid sequence indicating the positions of amino acids (black vertical lines) that differ between UGT87Y1 and EgUGT87Y. The PSPG motif is marked with a light gray box. The catalytic His is marked with an asterisk. (b) Active site of EgUGT87 (Eucgr.B03993) (grey carbon atoms) with docked mandelonitrile (magenta carbon atoms) and UDP‐glucose (pink carbon atoms). Nitrogen, oxygen, sulfur and phosphorus atoms are shown in blue, red, yellow and orange, respectively. The benzene moiety of the substrate is mainly recognized by hydrophobic interactions with Y16, F81, and Y115. Mutations of Y16F and G18A (red text) enhance the hydrophobic interactions (green dotted lines) with the benzene ring of mandelonitrile. The catalytic dyad H21 ‐ D113 is shown (red dotted lines) and hydrogen bonds indicated (black dotted lines). (c) Representative extracted ion chromatograms (EIC) corresponding to prunasin (m/z 340.1032 [M + FA]−) of leaf extracts from Nicotiana benthamiana plants transiently expressing Eucalyptus UGTs together with the prunasin CYPs from Eucalyptus yarraensis. (d) Prunasin accumulation in N. benthamiana leaf extracts measured by peak area integration of prunasin (m/z 340; upper panel), and prunasin levels normalized to the pathway derivative putatively annotated as benzoic acid glucoside (m/z 329), shown here as a ratio (m/z 340 : 329; lower panel). (e) In vitro assays using protein extracts prepared from N. benthamiana leaves transiently expressing prunasin CYPs and the UGT variants (top) and Western blot of the His‐tagged UGTs in the protein extract that was used for activity assay (bottom). The UGT activity is plotted as the intensity of the prunasin signal on a TLC plate measured by phospho‐imaging.
Results
The three‐CYP system is retained in cyanogenic Eucalyptus
To compare the biosynthetic genes between different phylogenetic groups, prunasin biosynthesis was investigated in E. camphora and E. yarraensis from section Maidenaria, species known to accumulate this compound (Goodger et al., 2002, 2007; Neilson et al., 2006). Prunasin accumulation in expanding leaves from plants at 1, 4, 7, and 10 months of age was measured by LC‐MS/MS (Fig. 2a). Prunasin was detected at all four ages in E. yarraensis with up to 30 μg mg−1 DW at 7 months of age. In E. camphora, the onset of prunasin formation occurred at a later age and with significantly lower quantities than in E. yarraensis (c. 100‐fold less). Similar to E. yarraensis, the highest prunasin accumulation occurred in E. camphora plants at 7 months of age (0.36 μg mg−1 DW). Compared with the high prunasin accumulation in E. cladocalyx at an equivalent age (Gleadow & Woodrow, 2000; Hansen et al., 2018), E. yarraensis and E. camphora can be regarded as intermediate and low producers of prunasin, respectively.
Fig. 2.
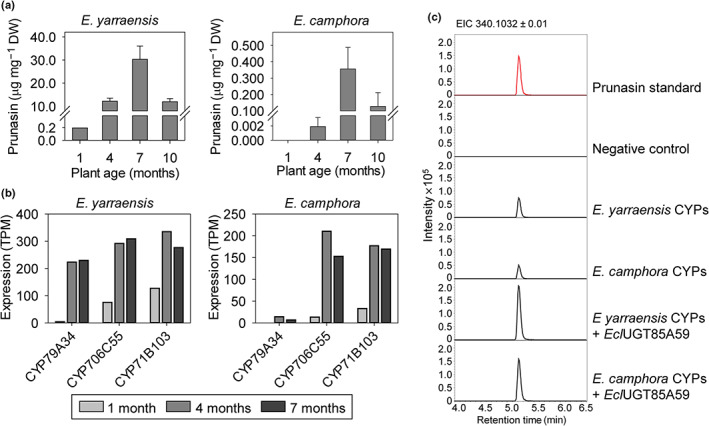
Prunasin accumulation in Eucalyptus yarraensis and Eucalyptus camphora, and CYPs involved in prunasin production. (a) Mean prunasin content (± 1 SE) in E. yarraensis and E. camphora seedlings at 1, 4, 7, and 10 months of age (n = 8). (b) Transcript profiles of prunasin biosynthetic CYP genes in representative E. yarraensis and E. camphora individual tissue at different ontogenetic stages. Prunasin and transcript values at 1 month represent a pooled sample due to small plant size. (c) Extracted ion chromatograms (EIC) corresponding to prunasin (m/z 340.1032 [M + FA]−) of leaf extracts from Nicotiana benthamiana plants transiently expressing the three candidate CYP genes from E. yarraensis and E. camphora with the UGT85A59 from Eucalyptus cladocalyx. DW, dry weight; TPM, transcripts per million.
To identify the biosynthetic genes responsible for prunasin production, leaves of three different ages (1, 4, and 7 months) were selected for transcriptomic analysis. CYP79A, CYP706C, and CYP71B transcripts, with expression patterns matching prunasin accumulation, were identified from E. camphora and E. yarraensis (Fig. 2b). The three candidate CYP genes from E. yarraensis showed higher expression than the candidates from E. camphora, consistent with the prunasin levels. The orthologous CYPs from E. camphora and E. yarraensis showed > 99% amino acid sequence identity to each other and > 94% sequence identity to the respective CYP sequences from E. cladocalyx. The CYPs from E. yarraensis and E. camphora were assigned the names CYP79A34, CYP706C55, and CYP71B103 according to the standardized CYP nomenclature system (Nelson, 2009). A previous study identified four CYP79 genes from E. yarraensis (Neilson, 2012), but only a single CYP79 sequence was present in our transcriptomic dataset.
The full‐length CYP genes from E. camphora and E. yarraensis were functionally characterized by transient expression in N. benthamiana. Co‐expression of EyCYP79A34, EyCYP706C55, and EyCYP71B103 or EcaCYP79A34, EcaCYP706C55, and EcaCYP71B103, together with UGT85A59 from E. cladocalyx, resulted in accumulation of prunasin in N. benthamiana leaves (Fig. 2c). Akin to similar studies (Hansen et al., 2018; Thodberg et al., 2018), when the prunasin CYPs were transiently expressed, some prunasin accumulated in the N. benthamiana leaves due to endogenous UGT activity (Fig. 2c). Putatively annotated identified malonated prunasin and other glucosylated and malonated pathway intermediates were also observed due to the presence of endogenous N. benthamiana enzyme activities (Fig. S1). In addition, a diagnostic peak with m/z 329.0873 [M + FA]− was identified, putatively annotated as benzoic acid glucoside and likely derived from the dissociation of mandelonitrile (Figs S2, S3). A drop‐out experiment in which each CYP was omitted from the assay system confirmed that all three CYPs are necessary for prunasin production (Fig. S2). This indicates that the three‐CYP system is conserved in subgenus Symphyomyrtus in contrast to the two‐CYP system found in other flowering plant species.
Recruitment of a distinct UDP‐glucosyltransferase for prunasin production in E. camphora and E. yarraensis
No UGT85A59 ortholog was expressed in E. camphora and E. yarraensis at any of the three analyzed ages, and expression of other UGT85s showed either overall low expression or an expression pattern contrasting with the expression of the CYPs (Fig. 3a). A set of criteria were therefore defined to guide the selection of candidate UGTs for functional analysis: (1) The expression pattern should parallel prunasin accumulation or show overall high expression at all time points given that UGTs in cyanogenic glucoside biosynthesis do not necessarily co‐express with the CYPs (Hansen et al., 2018); (2) the UGT should be present in both E. camphora and E. yarraensis due to the close relationship of these species to each other; and (3) no ortholog match in the transcriptome from E. cladocalyx was expected since this species has UGT85A59 that glucosylates mandelonitrile. These selection criteria resulted in three candidates from the families UGT88, UGT87, and UGT74, in addition to two UGT85s to include members of this family with the best matching expression pattern (Fig. 3a). These candidate UGT genes were transiently co‐expressed with the prunasin CYPs in N. benthamiana (Fig. 3b). When the UGT85A59 from E. cladocalyx was co‐expressed together with the CYPs as a positive control, prunasin levels were consistently 2.1–2.5 times higher, and we also observed the formation of prunasin malonate ester and the cyanogenic diglucoside amygdalin (Fig. S1). By contrast, markedly lower levels (18 times less) of the diagnostic m/z 329.0872 ion were detected when these four prunasin pathway members were co‐expressed. As the pattern of m/z 329.0873 ion accumulation was consistently observed across multiple experiments with and without co‐expression of a UGT candidate, the m/z 329.0873 ion was used to assess fluctuations in endogenous N. benthamiana UGT activity toward mandelonitrile. Accordingly, candidate UGT activity was assessed by total prunasin accumulation and by peak area ratio between prunasin and the m/z 329.0873 ion. The ratio calculation method showed consistent results across three independent experiments (Fig. S4). The inclusion of additional pathway derivatives (m/z 711 and m/z 761) in the calculation did not alter activity patterns across independent experiments (Fig. S4).
Fig. 3.
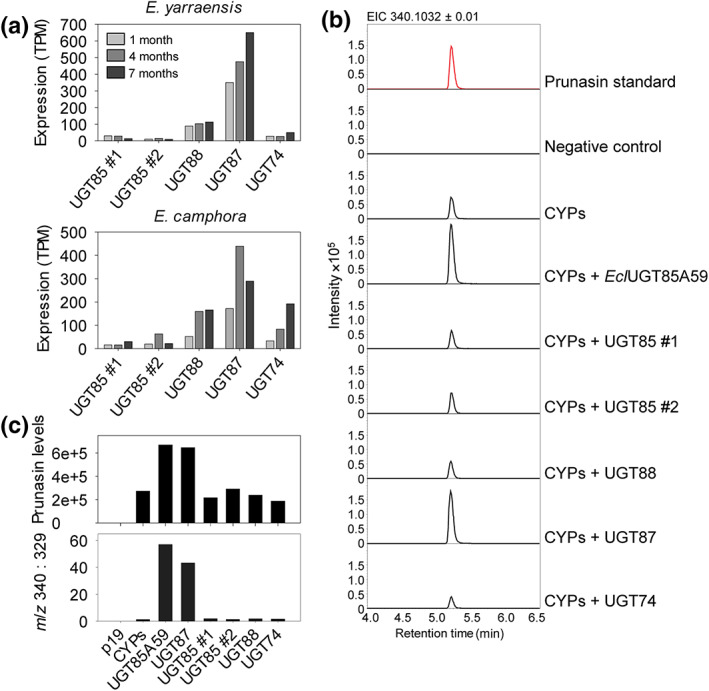
UGT87 is involved in prunasin production in Eucalyptus yarraensis and Eucalyptus camphora. (a) Transcript profiles of UGT candidate genes from E. yarraensis and E. camphora (n = 1 per species, at 1, 4, and 7 months of age). UGT candidates were selected based on expression criteria within and between the two species. (b) Representative extracted ion chromatograms (EIC) corresponding to prunasin (m/z 340.1032 [M + FA]−) of leaf extracts from Nicotiana benthamiana plants transiently expressing the E. yarraensis and E. camphora UGT candidate genes together with the prunasin EyCYP79A34, EyCYP706C55, and EyCYP71B103. (c) Corresponding levels of prunasin in N. benthamiana leaf extracts measured by peak area integration of prunasin (m/z 340; upper panel), and prunasin levels normalized to the pathway derivative putatively annotated as benzoic acid glucoside (m/z 329), shown here as a ratio (m/z 340 : 329; lower panel). All tested UGT candidates and the positive control UGT85A59 are co‐expressed with the prunasin CYPs. TPM, transcripts per million.
Upon co‐expression with the five candidate UGTs, only co‐expression of an EyUGT87 resulted in high accumulation of prunasin in N. benthamiana leaves (Fig. 3b,c). Since the sequences of EyUGT87Y and EcaUGT87Y are identical at the amino acid level, only one UGT87Y gene was included in the experiments. Calculation of the prunasin to m/z 329.0872 ratio supported that only EyUGT87 displayed mandelonitrile glucosylation activity above endogenous N. benthamiana UGT activity (Fig. 3c). Accordingly, while E. cladocalyx has a UGT85 that can glucosylate mandelonitrile, E. camphora and E. yarraensis have recruited a UGT87, which can catalyze the same reaction. This demonstrates that UGTs from distinct families and thus sharing < 45% amino acid sequence identity can glucosylate mandelonitrile (Fig. S5). The functionally active UGT87 was named UGT87Y1 by the UGT Nomenclature Committee (Burchell et al., 1991; Mackenzie et al., 1997, 2005).
To further characterize the activity of UGT87Y1 and UGT85A59, codon‐optimized genes were heterologously expressed in Escherichia coli and the enzymes were purified for in vitro analysis using 14C‐UDP‐glucose as the donor substrate. Both enzymes catalyzed the glucosylation of mandelonitrile, confirming the activity observed upon transient expression in N. benthamiana (Fig. S6). Substrate specificity of the two UGTs was investigated by testing other small metabolites as acceptors. UGT85A59 and UGT87Y1 also glucosylated benzoic acid but to a lower extent compared with mandelonitrile (Fig. S6). UGT85A59 showed some activity toward the slightly larger cyanohydrin p‐hydroxymandelonitrile, while UGT87Y1 did not glucosylate this compound. By contrast, UGT87Y1 glucosylated the smaller acetone cyanohydrin, whereas UGT85A59 did not. No activity was observed with gallic acid, coumaric acid, caffeic acid, ferulic acid, quercetin, genistein, naringenin, or kaempfeol.
Loss and gain of prunasin biosynthetic genes
To gain a broader understanding of prunasin evolution in the genus, we mined transcriptome and genome data from online sources available for three cyanogenic and acyanogenic Eucalyptus species (Table 1). A transcriptome from the cyanogenic species Eucalyptus leucoxylon (section Adnataria) is available from the Onekp database (Carpenter et al., 2019). A good‐quality genome is available for the acyanogenic E. grandis (section Latoangulatae) (Myburg et al., 2014), and a shallow genome library is available for the acyanogenic E. camaldulensis (section Exsertaria) (Hirakawa et al., 2011).
In line with our hypotheses, orthologs to EclCYP79A125, EclCYP706C55, EclCYP71B103, and EclUGT85A59 were found in the transcriptomic dataset from cyanogenic E. leucoxylon (Figs S5, S7–S9), consistent with E. leucoxylon and E. cladocalyx belonging to the same cyanogenic cluster in the phylogeny (Fig. 1a). The sequences share > 96% identity, with the CYP79 orthologs being most identical (99%) and the CYP71 orthologs being least identical (96%).
Assessment of the two acyanogenic species, E. camaldulensis and E. grandis, revealed different outcomes of prunasin biosynthesis gene conservation or loss. The E. camaldulensis genome harbors nine CYP79s, including a full‐length ortholog (Ec018270) to EyCYP79A34, sharing 97.7% amino acid sequence identity (Fig. S7). In addition, a full‐length ortholog (EcC047195) to EclUGT85A59, sharing 92% amino acid sequence identity, was also identified (Fig. S5). Only gene fragments with high identity to EclCYP706C55 and EclCYP71B103 were present in the E. camaldulensis genome, and no clear orthologs to UGT87Y1 were identified. These findings suggest that the lack of prunasin accumulation in E. camaldulensis is due to the lack of full‐length CYP genes involved in oxime to cyanohydrin conversion, consistent with hypothesis 2.
In contrast to E. camaldulensis, several full‐length prunasin pathway orthologs were identified in the E. grandis genome. A potential ortholog to EyCYP79A34 was a pseudogenized CYP79 gene with a 9.76‐kb insertion and a premature stop codon, but a total of 11 other full‐length CYP79s were identified in E. grandis. Two of the full‐length E. grandis CYP79s (EgCYP79A37 and EgCYP79A36) show high identity (> 85%) to EclCYP79A125 at the amino acid level and are apparent orthologous genes to EyCYP79A36 and EyCYP79A37, respectively (Fig. S7). Full‐length orthologs to EclCYP706C55 (EgCYP706C55) and EclCYP71B103 (EgCYP71B115) are also present in the E. grandis genome (Figs S8, S9). Interestingly, an ortholog to EclUGT85A59 (EgUGT85A, > 92% identity) and an ortholog to UGT87Y1 (EgUGT87Y, > 97% identity) were identified (Fig. S5). Low but possible co‐expression of EgCYP79A37, EgCYP706C55, EgCYP71B115, and EgUGT87Y was detected in shoot tips and reproductive tissue, with no reported co‐expression of EgUGT85A (Vining et al., 2015).
To determine whether these homolog genes in E. grandis had the ability to produce prunasin, their functional activity was determined using transient expression in N. benthamiana. Functionality assessment of the two closest homologs to EyCYP79A34 (EgCYP79A37 and EgCYP79A36) by co‐expression of downstream biosynthetic genes from E. yarraensis only resulted in traces of prunasin (Fig. 4), while targeted mining of the data identified a small peak with a m/z 308.1345 corresponding to a leucine/isoleucine oxime glucoside (Fig. S10). Substitutions of pathway members from E. yarraensis with EgCYP706C55, EgCYP71B115, or EgUGT85A resulted in prunasin accumulation in N. benthamiana leaves (Fig. 4a), suggesting that a CYP79 with phe‐oxime biosynthetic activity is the limiting factor for prunasin production in E. grandis. In a separate experiment where EgUGT85A was co‐expressed with the CYPs from E. cladocalyx and extracts analyzed on a LC‐ion trap instrument, EgUGT85A co‐expression resulted in more putatively glucosylated intermediates than EclUGT85A59 (Fig. S11), suggesting that EgUGT85A is less specific toward mandelonitrile than EclUGT85A59. Transient co‐expression of EgUGT87Y with the prunasin CYPs only resulted in prunasin levels slightly above background levels (1.4 times), with compromised EgUGT87Y activity also supported when normalized to m/z 329 (Figs 4a, 6c,d).
Fig. 4.
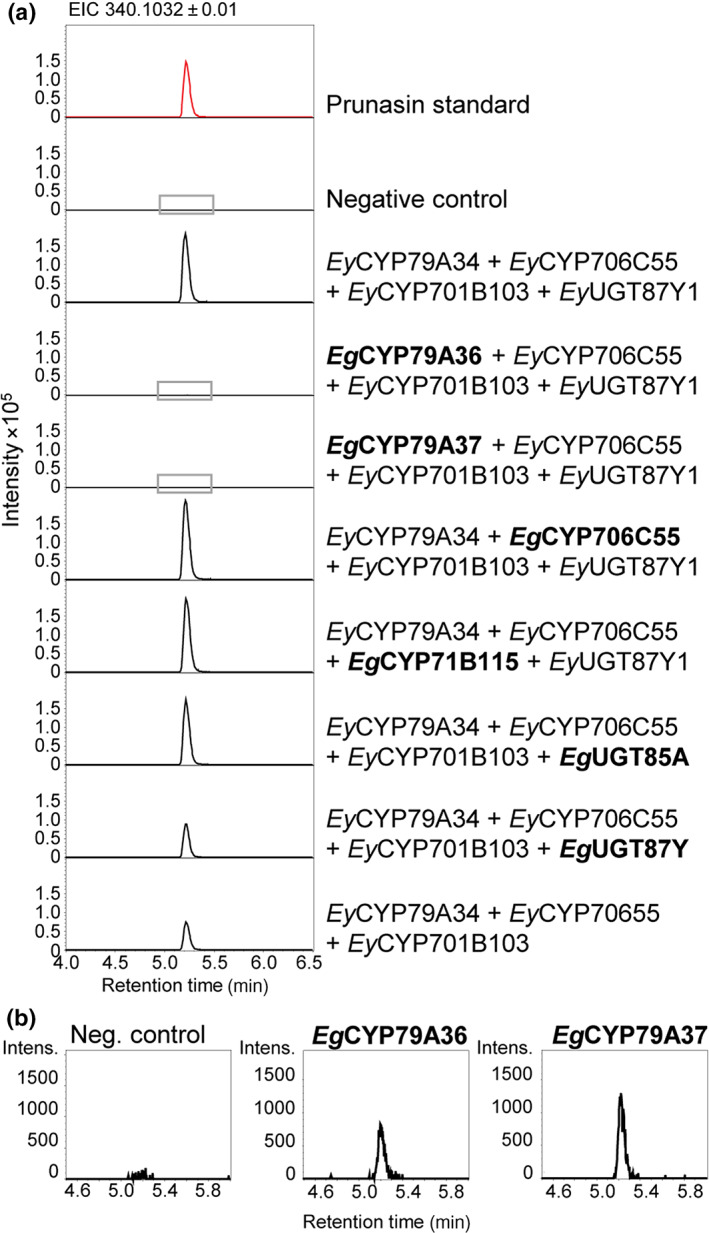
Transient expression of homologous prunasin biosynthetic genes from acyanogenic Eucalyptus grandis. (a) Extracted ion chromatograms (EIC) of Nicotiana benthamiana leaf extracts prepared from plants transiently co‐expressing homologs of prunasin biosynthetic genes from E. grandis (marked in bold) with the prunasin biosynthetic genes from Eucalyptus yarraensis. The chromatograms surrounded by a gray box for the combinations co‐expressing EgCYP79A36 and EgCYP79A37, respectively, are enlarged in panel B. (b) Zoom on two chromatograms from panel A where the CYP79s EgCYP79A36 and EgCYP79A37 are each co‐expressed with downstream prunasin biosynthetic genes.
These data suggest that E. grandis has potential, but limited capacity to biosynthesize prunasin. Functional prunasin production, however, is constrained by several factors in line with our hypotheses: lack of a functional CYP79A34 ortholog (due to gene pseudogenization), limited activity of paralogous CYP79s, compromised activity of an orthologous EgUGT87Y, and the lack of EgUGT85A expression (Vining et al., 2015). A summary of the prunasin pathway orthologs present in the analyzed species is shown in Fig. 5.
Fig. 5.
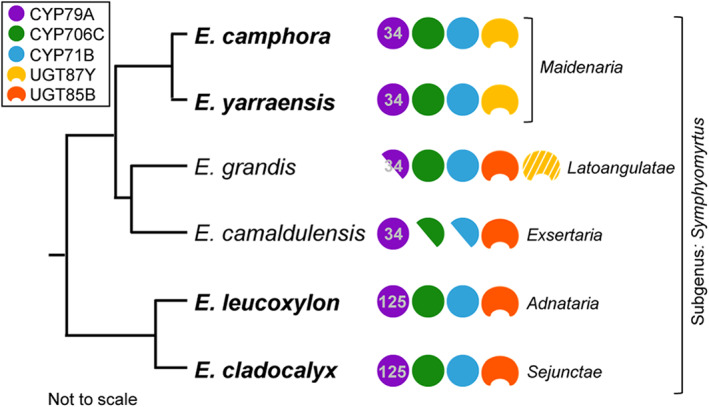
Evolution of prunasin biosynthesis in Eucalyptus showing conservation of the three‐CYP system but functional recruitment of two distinct UGT families. CYP79 subfamily names are denoted, showing divergence across sections. Eucalyptus camphora, Eucalyptus yarraensis, Eucalyptus leucoxylon, and Eucalyptus cladocalyx are cyanogenic (highlighted in bold). Ortholog genes identified in the acyanogenic Eucalyptus grandis and Eucalyptus camaldulensis are shown. The half circles illustrate pseudogenes. The ortholog CYP706C, CYP71B, and UGT85A from E. grandis demonstrated functional activity for prunasin production, while the ortholog UGT87Y showed severely compromised activity (illustrated with white stripes).
Site‐directed mutagenesis of UGT87
Due to high amino acid sequence identity (97.6%) between the functional and functionally compromised UGT87Ys from E. yarraensis/E. camphora and E. grandis, respectively, key residues for mandelonitrile glucosylation were identified and tested. Homology modeling of UGT87Y1 showed that the phenyl ring of mandelonitrile is primarily positioned in the active site by hydrophobic interactions with Phe‐16, Phe‐81, and Tyr‐115. The rings in Phe‐16 and Phe‐81 may also form π stacks with the phenyl ring in mandelonitrile. Comparison between UGT87Y1 and EgUGT87 identified two residues localized close to the catalytic His‐21 that differed: a substitution of Phe‐16 to a Tyr residue, and Gly‐18 to an Ala (Fig. 6a). No SNPs are reported in these two amino acid positions for 36 sequenced E. grandis individuals (4). Modeled mutations of Y16F and G18A in EgUGT87 resulted in enhanced hydrophobic interactions with the phenyl ring of mandelonitrile (Fig. 6b).
To investigate the importance of these residues on catalytic activity, mutants Y16F, G18A, and Y16F + G18A were generated for EgUGT87. The mutant genes were transiently co‐expressed with the prunasin CYPs in N. benthamiana. LC–MS/MS analysis of infiltrated leaves showed increased prunasin levels for mutants Y16F and Y16F + G18A compared with EgUGT87Y but with varying levels across three independent experiments (Figs 6c,d, S4). These data suggest partial to full recovery of activity. However, when prunasin levels were normalized to the levels of the m/z 329 ion, only partial recovery of activity was observed for the mutant Y16F and Y16F + G18A (Figs 6d, S4), with the normalized data showing a consistent pattern across the three experiments. To independently test the activity of the E. grandis enzyme mutants, we performed in vitro assays using total protein extracts from N. benthamiana leaves and 14C‐UDP‐glucose and mandelonitrile as substrates (Fig. 6e). In this experiment, UGT87 activity levels showed a pattern highly similar to the normalized prunasin levels. These data support the reliability of the m/z 340 : 329 normalization method. The increased prunasin accumulation observed for the EgUGT87Y mutants, but not the m/z 340 : 329 ratio, suggests possible stimulation of prunasin CYP expression by the UGT enzymes in vivo and a higher flux toward mandelonitrile production.
The partial recovery of the mutants Y16F and Y16F + G18A suggests that other amino acid differences between UGT87Y1 and EgUGT87 are important for catalytic activity. The other residues that differ between EgUGT87 and UGT87Y1 are not localized within the catalytic pocket or in the conserved PSPG motif and may be involved in the stabilization of the enzyme structure. For example, the homology model shows that the differences between R77Q and S139P cause a slightly altered structure, potentially causing a different conformation of K79, which is positioned close to the substrate entry channel.
Discussion
Dynamic and conserved features of prunasin CYP evolution
Cyanogenic Eucalyptus species within the subgenus Symphyomyrtus group into distinct phylogenetic clades. The evolutionary genetic patterns behind prunasin biosynthesis were achieved using a metabolite‐guided transcriptomic approach in the species E. yarraensis and E. camphora. Both species contain the functional CYP79A, CYP706C, and CYP71B necessary for prunasin production (Figs 2, S2), similar to E. cladocalyx (23). Eucalyptus species that do not accumulate cyanogenic glucosides (i.e. E. grandis and E. camaldulensis) were characterized by an apparent lack of either one or two full‐length CYP orthologs associated with prunasin biosynthesis (Fig. 5).
CYP79s are the entry point to cyanogenic glucoside production in seed plants, and regulation of gene expression and enzyme activity thus control the flux into the pathway (Busk & Møller, 2002; Thodberg et al., 2018). In agreement with this, CYP79A34 from E. camphora and E. yarraensis was the lowest expressed pathway member, which was also observed for EclCYP79A125 (Hansen et al., 2018). Moreover, the expression of EcaCYP79A34 was markedly lower than EyCYP79A34, consistent with significantly lower prunasin levels in E. camphora (Fig. 2).
Several CYP79s were identified in the acyanogenic species E. grandis and E. camaldulensis, with E. grandis harboring among the highest CYP79 gene copy number within diploid species sequenced to date (Fig. S7; Nelson, 2009). The physiological functions of these CYP79s are unknown. Published expression data from various E. grandis tissues show that the CYP79s are either expressed at low levels or not at all (Vining et al., 2015), although it is possible that CYP79s are expressed at different developmental stages or induced upon exposure to environmental factors. Two of the CYP79s from E. grandis (EgCYP79A36 and EgCYP79A37) showed trace activity with l‐Phe and potentially l‐Leu/l‐Ile upon transient expression in N. benthamiana (Fig. 4). While we did not detect other amino acid‐derived oxime glucosides in this study, l‐Phe, l‐Leu, l‐Ile, l‐Trp, and l‐Tyr have been identified as substrates for CYP79s present in the acyanogenic tree Populus trichocarpa (Irmisch et al., 2013). In P. trichocarpa, the formation of volatile oximes is induced upon herbivore damage. It is also possible that CYP79s may perform N‐hydroxylation reactions on other substrates beyond amino acids, and the high copy number and diversity of eucalypt CYP79s provide an interesting system to study the role of this CYP gene family in more detail.
In other seed plants, the conversion of an amino acid‐derived oxime into the corresponding cyanohydrin in cyanogenic glucoside biosynthesis is performed by a single, multifunctional CYP usually from the 71 family (Bak et al., 1998; Jørgensen et al., 2011; Yamaguchi et al., 2014; Thodberg et al., 2018; Lai et al., 2020). By contrast, this step is catalyzed by two distinct CYPs, a CYP706C and a CYP71B, in Eucalyptus. The functional characterization of a CYP79A, CYP706C, and CYP71B in different Eucalyptus species shows that the three‐CYP system is conserved within the subgenus Symphyomyrtus, thereby requiring a total of four biosynthetic enzymes for prunasin production. Recent work suggests that a four‐step pathway for prunasin biosynthesis is also operating in the cyanogenic fern Phlebodium aureum (Thodberg et al., 2021), demonstrating that this biosynthetic configuration is not unique to Eucalyptus.
Repeated recruitment of UGTs for two‐component chemical defense systems in plants
In contrast to the conservation of CYPs in prunasin biosynthesis, we found evidence of novel UGT87Y1 recruitment for mandelonitrile glucosylation within the Maidenaria section. Measurement of UGT87Y1 activity above endogenous N. benthamiana enzyme levels was facilitated by calculating a ratio between prunasin and a pathway derivative formed upon the expression of the prunasin CYPs (Fig. S3). This is the first time that a UGT87 has been shown to be involved in cyanogenic glucoside biosynthesis and the first member of the UGT87 family to be functionally characterized. UGT87Y1 expression showed a pattern similar to CYP expression and was the highest expressed pathway gene in both species at all time points (Figs 2, 3), supporting a role in cyanohydrin stabilization.
Very little is known about the UGT87 family in plants. UGT87A2 from Arabidopsis thaliana was reported to regulate flowering time (Wang et al., 2012) and play a role in adaptation to abiotic stress including salt tolerance (Li et al., 2017). A UGT87A from Carex rigescens has also been linked to increased salt tolerance (Zhang et al., 2021). Several metabolite acceptors for UGT87A2 have been experimentally tested in vitro including abscisic acid, auxins, cytokinins, various phenylpropanoids, and benzoates, but the in planta substrate has not yet been identified (Lim et al., 2002; Li et al., 2017).
Similar to the glucosylation of mandelonitrile by EclUGT85A59, the glucosylation of a cyanohydrin to form the cyanogenic glucoside is typically catalyzed by UGT85 members in various angiosperms (Franks et al., 2008; Kannangara et al., 2011; Takos et al., 2011). A recent exception is a UGT94AF3 from almond that was able to glucosylate mandelonitrile (Thodberg et al., 2018) in addition to the previously characterized UGT85A19 (Franks et al., 2008). However, substrate specificity of UGT94AF3 was not examined and its physiological function awaits further investigation. Most of the cyanogenic UGT85s belong to distinct phylogenetic subfamilies demonstrating that homologous, but not orthologous, UGT genes have been repeatedly recruited for cyanogenic glucoside biosynthesis (Fig. S5). This is similar to the CYP79 and CYP71 members involved in cyanogenic glucoside biosynthesis in different plant species (Takos et al., 2011).
Beyond the biosynthesis of cyanogenic glucosides, UGTs from different families able to catalyze the same reaction are also known from plants with other classes of specialized metabolites, including benzoxazinoids. Benzoxazinoids are a small class of defense compounds found in multiple monocot Poaceae species including maize (Zea mays) and wheat (Triticum aestivum), and a few dicot species (e.g. Consolida orientalis) (Sicker et al., 2000). Glucosylation of the benzoxazinoid DIBOA is catalyzed by UGT710Es in Poaceae species and by UGT85N1 in C. orientalis (von Rad et al., 2001; Dick et al., 2012). Together, these examples point to the remarkable plasticity of UGTs that have been recruited for lineage‐specific functions.
Eucalyptus contains a rich repertoire of UGTs
UDP‐glucosyltransferases constitute a large gene family in plants, often with > 100 members present in angiosperm species (Wilson & Tian, 2019). A phylogenomic analysis of UGTs from 65 plant genomes representing taxonomic diversity from green algae to angiosperms showed that E. grandis contains the highest number UGT genes (379) among the species that were investigated. For comparison, 123 UGT genes were identified in Arabidopsis (A. thaliana) and 136 in pomegranate (Punica granatum), the latter species belonging to the same order (Myrtales) as Eucalyptus. Notably, the genomes of oak (Quercus suber) and loblolly pine (Pinus taeda), two long‐lived trees, also contain a high number of UGT genes (312 and 243, respectively). Long‐lived trees experience diverse environmental conditions over their lifespan, and it can be speculated that a rich UGT diversity is important for tree longevity.
Little is known about UGT functionalities in Eucalyptus, thereby leaving a large catalog of uncharted UGTs to be investigated. In addition to the UGTs reported in this and our previous study (Hansen et al., 2018), two UGT84As from E. camaldulensis were shown to glucosylate gallic acid in the biosynthesis of hydrolysable tannins (Tahara et al., 2018). The genome of E. grandis contains numerous CYP and TPS genes (Myburg et al., 2014; Kulheim et al., 2015; Hansen et al., 2021), which are involved in the production of various specialized metabolites in addition to some general metabolites. Therefore, the rich UGT resource of 379 genes may complement the large collection of TPSs and CYPs by serving to stabilize, detoxify, and facilitate the transport and storage of the metabolites produced by these and other biosynthetic enzymes.
Many of the UGT genes in the genome of E. grandis occur in clusters, consistent with a high proportion of tandemly duplicated genes in this species (Myburg et al., 2014). The multiplicity of duplicated genes provides a repertoire of genes ready to undergo neofunctionalization by positive selection of advantageous mutations. Sometimes very few mutations result in gain of a new function. This is exemplified by the site‐directed mutagenesis study of the UGT87Y1 ortholog, EgUGT87, where a single‐point mutation in the active site resulted in partial gain of the ability to glucosylate mandelonitrile compared with UGT87Y1 (Fig. 6). Interestingly, increased prunasin accumulation was observed for the EgUGT87Y mutants, while no‐to‐partial recovery was observed for the m/z 340 : 329 ratio. This could be explained by variation in the delivery of different T‐DNA constructs to the same cell upon co‐expression of multiple genes (Montague et al., 2011) and/or by stimulation of CYP activity (Laursen et al., 2016). For example, there may be an increased flux of phenylalanine to mandelonitrile by the presence of certain UGT proteins as observed for the dhurrin metabolon.
Studies into the functionality of tandemly duplicated UGT genes are relatively rare. A key study in the grass Brachypodium distachyon showed that two UGTs in a cluster comprising six similar UGT genes conferred resistance against trichothecene mycotoxins from the fungal pathogen Fusarium graminearum (Schweiger et al., 2013). The authors found no evidence of diversifying selection and therefore speculated that such a cluster constituted a ‘trench in the warfare’ between grasses and fungal pathogens. This strategy equips the plant with a collection of genes readily available to be activated for defense upon fungal infection or herbivore attack. Other studies demonstrate similar differential functionality of tandemly duplicated UGT genes in terms of sugar donor and acceptor specificity (Chen et al., 2021; Sayama et al., 2012), as well as regio‐selectivity for glycosidic linkage (Ono et al., 2019). Beyond UGTs, other defense gene families including CYPs and TPSs are heavily expanded in Eucalyptus and are positioned in tandem repeats (Myburg et al., 2014). Further studies into the regulation and evolution of these tandemly duplicated genes in Eucalyptus would shed light on genetic strategies to secure the metabolic and physiological plasticity as required by these long‐lived trees.
Evolution of chemical defense in Eucalyptus
The biosynthesis of cyanogenic glucosides is known from several distantly related plant species. Nonetheless, little is known about the evolution of cyanogenic glucoside biosynthesis in closely related plant species (Lai et al., 2020). Here, we show on the molecular level how the distribution and biosynthesis of cyanogenic glucosides occurs within a subgenus. The prunasin biosynthetic gene patterns in the six species analyzed here suggest that the capacity to produce prunasin, and the three‐CYP system, arose early in the diversification of the subgenus Symphyomyrtus, whereby the trait was retained by some species (i.e. within the Sejunctae and Adnataria series), and lost by others. Gene duplication of an ancestral CYP79A125 likely gave rise to the CYP79A34 and CYP79A35 where prunasin functionality was then reobtained in E. camphora and E. yarraensis (section Maidenaria) but then lost in E. grandis (section Latoangulatae) via gene pseudogenization. Pseudogenization is an apparent driver for biosynthetic capacity loss in E. camaldulensis, but here it is due to mutations in the CYP71 and CYP706 pathway genes (Fig. 5). Cyanogenic glucoside loss in these Eucalyptus species differs from the well‐characterized system in sweet almond (P. dulcis), where the lack of prunasin biosynthesis is due to a mutation in the BHLH transcription factor activating CYP79 and CYP71 transcriptions, resulting in a lack of CYP expression (Sánchez‐Pérez et al., 2019). These CYP evolution patterns, coupled to the recruitment of different UGT families in distinct phylogenetic clades, demonstrate the dynamic evolution of cyanogenic glucoside biosynthesis in Eucalyptus. To understand the full extent of pathway gain and loss, access to much greater genomic resources of high quality across this important genus is required.
Based on the high diversity of CYPs and UGTs in the sequenced Eucalyptus species to date, it is reasonable to suggest that there may be many members of the prunasin gene families that possess some capacity to catalyze a reaction relevant for cyanogenic glucoside formation, especially as many other characterized CYPs and UGTs show substrate promiscuity (Hansen et al., 2003, 2021; Zhang et al., 2022). The Eucalyptus genus is large and genetically complex, and it is possible that some species previously classified as acyanogenic may possess a limited degree of capacity to produce cyanogenic glucosides. It is interesting to speculate that the remarkable adaptivity of Eucalyptus species may be an evolutionary outcome where certain defense pathways are more readily activated or somatically mutated to provide a fitness advantage under particular environmental conditions or pressures, as shown in the so‐called ‘mosaic trees’ (Padovan et al., 2015; Orr et al., 2020).
Eucalyptus are predominantly native to the Australian continent, where many soils are depleted in nitrogen and phosphorous, and the apparent ‘cost’ related to the formation and storage of these nitrogen‐containing specialized metabolites might be an important factor driving selection for or against the presence of cyanogenic glucosides (Neilson et al., 2013). Studies into the geochemical distribution of cyanogenic glucosides in Eucalyptus species are required to link soil conditions to the accumulation of cyanogenic glucosides. Notably, trees belonging to the section Adnataria generally grow on younger and fertile soil (Pryor, 1976). Coincidently, this section and the sections Bisectae, Exsertaria, Glandulosae, and Maidenaria were shown to have accelerated net species diversification (Thornhill et al., 2019), and the authors speculated that a driver of diversification could be the ability to quickly adapt to newer geochemical environments.
The evolution of specific chemotypes within the genus may also be driven by different abiotic and biotic factors. For example, an analysis of E. camaldulensis seedlings sourced from a wide geographical range linked the variation in mono‐ and sesquiterpene abundance to temperature and humidity (Bustos‐Segura et al., 2017), while formylated phloroglucinol compound concentration was implicated in the preferred tree selection of koalas (Phascolarctos cinereus) (Moore & Foley, 2005). The genetic complexity of Eucalyptus species, characterized by numerous tandem duplications and great diversity of specialized metabolite gene families (Myburg et al., 2014), provides a rich genetic space from which to garner information on metabolic and physiological plasticity within plants.
Author contributions
CCH, MS, JQDG, IEW, BLM and EHJN conceptualized the study. CCH, MS, MB, CEO and EHJN designed the study methodology. CCH, MS, MB, WB and EHJN involved in investigation, and curated and analyzed the data. CCH and EHJN involved in writing – original draft. CCH, MS, MB, WB, CEO, JQDG, IEW, BLM and EHJN involved in writing – review and editing. BLM, IEW and EHJN acquired fund for the study.
Supporting information
Fig. S1 Metabolites produced upon transient expression of prunasin biosynthetic genes in Nicotiana benthamiana.
Fig. S2 Drop‐out experiment of the prunasin biosynthetic genes from Eucalyptus yarraensis and Eucalyptus camphora.
Fig. S3 Observation of a diagnostic fragment m/z 329 putatively annotated as benzoic acid glucoside.
Fig. S4 Assessment of UGT activity using diagnostic prunasin pathway derivatives provides a consistent measure of prunasin accumulation above purported Nicotiana benthamiana endogenous UGT activity.
Fig. S5 Phylogenetic tree of UGT85 and UGT87 sequences identified from five different Eucalyptus species.
Fig. S6 In vitro assays of UGT87Y1 and UGT85A59 showing activity toward different metabolite acceptors.
Fig. S7 CYP79 phylogeny with sequences identified from five different Eucalyptus species.
Fig. S8 CYP706 phylogeny with sequences identified from five different Eucalyptus species.
Fig. S9 CYP71 phylogeny with sequences identified from five different Eucalyptus species.
Fig. S10 Transient expression of EgCYP79A36 and EgCYP79A37 from the acyanogenic Eucalyptus grandis.
Fig. S11 Comparison of product profiles upon transient co‐expression of EclUGT85A59 or EgUGT85A with the prunasin CYPs from Eucalyptus cladocalyx.
Table S1 Primers used to isolate target open reading frames from cDNA.
Table S2 UGT gene expression at 1, 4, and 7 months in Eucalyptus camphora and Eucalyptus yarraensis.
Please note: Wiley is not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank David R. Nelson for naming the CYPome from Eucalyptus grandis and David Vernon for his generous support for wildlife and early career researchers. This work was supported by the VILLUM Center for Plant Plasticity (VKR023054) (BLM); the European Research Council Advanced Grant (ERC‐2012‐ADG_20120314) (BLM); VILLUM Young Investigator Grant (VKR013167) (EHJN), a Novo Nordisk Emerging Investigator Grant (0054890) (EHJN) and funding from the Danish Independent Research Council (6111‐00379B, 1051‐00083B, and 1131‐00002B) (EHJN).
Data availability
The transcriptomic data that support the findings of this study are openly available in BioProject at www.ncbi.nlm.nih.gov/bioproject, ID: PRJNA765518.
References
- Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA. 1998. Cloning of three A‐type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Molecular Biology 36: 393–405. [DOI] [PubMed] [Google Scholar]
- Bak S, Olsen CE, Halkier BA, Møller BL. 2000. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiology 123: 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashandy H, Jalkanen S, Teeri TH. 2015. Within leaf variation is the largest source of variation in agroinfiltration of Nicotiana benthamiana . Plant Methods 11: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AF. 2016. Eucalypts, wildlife and nature conservation: from individual trees to landscape patterns. Proceedings of the Royal Society of Victoria 128: 71–86. [Google Scholar]
- Bjarnholt N, Neilson EHJ, Crocoll C, Jørgensen K, Motawia MS, Olsen CE, Dixon DP, Edwards R, Møller BL. 2018. Glutathione transferases catalyze recycling of auto‐toxic cyanogenic glucosides in sorghum. The Plant Journal 94: 1109–1125. [DOI] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near‐optimal probabilistic RNA‐seq quantification. Nature Biotechnology 34: 525–527. [DOI] [PubMed] [Google Scholar]
- Brazier‐Hicks M, Offen WA, Gershater MC, Revett TJ, Lim E‐K, Bowles DJ, Davies GJ, Edwards R. 2007. Characterization and engineering of the bifunctional N‐ and O‐glucosyltransferase involved in xenobiotic metabolism in plants. Proceedings of the National Academy of Sciences, USA 104: 20238–20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell B, Nebert DW, Nelson DR, Bock KW, Iyanagi T, Jansen PL, Lancet D, Mulder GJ, Chowdhury JR, Siest G et al. 1991. The UDP glucuronosyltransferase gene superfamily: suggested nomenclature based on evolutionary divergence. DNA and Cell Biology 10: 487–494. [DOI] [PubMed] [Google Scholar]
- Busk PK, Møller BL. 2002. Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiology 129: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos‐Segura C, Dillon S, Keszei A, Foley WJ, Külheim C. 2017. Intraspecific diversity of terpenes of Eucalyptus camaldulensis (Myrtaceae) at a continental scale. Australian Journal of Botany 65: 257–269. [Google Scholar]
- Butler JB, Vaillancourt RE, Potts BM, Lee DJ, King GJ, Baten A, Shepherd M, Freeman JS. 2017. Comparative genomics of Eucalyptus and Corymbia reveals low rates of genome structural rearrangement. BMC Genomics 18: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter EJ, Matasci N, Ayyampalayam S, Wu S, Sun J, Yu J, Jimenez Vieira FR, Bowler C, Dorrell RG, Gitzendanner MA et al. 2019. Access to RNA‐sequencing data from 1,173 plant species: the 1000 Plant transcriptomes initiative (1KP). Gigascience 8: giz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveau N, Jackson DJ. 2016. Combining independent de novo assemblies optimizes the coding transcriptome for nonconventional model eukaryotic organisms. BMC Bioinformatics 17: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae L, Kim T, Nilo‐Poyanco R, Rhee SY. 2014. Genomic signatures of specialized metabolism in plants. Science 344: 510–513. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen J, Feng J, Wang Y, Li S, Xiao Y, Diao Y, Zhang L, Chen W. 2021. Tandem UGT71B5s catalyze lignan glycosylation in Isatis indigotica with substrates promiscuity. Frontiers in Plant Science 12. doi: 10.3389/fpls.2021.637695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick R, Rattei T, Haslbeck M, Schwab W, Gierl A, Frey M. 2012. Comparative analysis of benzoxazinoid biosynthesis in monocots and dicots: independent recruitment of stabilization and activation functions. Plant Cell 24: 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TK, Yadollahi A, Wirthensohn MG, Guerin JR, Kaiser BN, Sedgley M, Ford CM. 2008. A seed coat cyanohydrin glucosyltransferase is associated with bitterness in almond (Prunus dulcis) kernels. Functional Plant Biology 35: 236–246. [DOI] [PubMed] [Google Scholar]
- George Thompson AM, Iancu CV, Neet KE, Dean JV, Choe J‐Y. 2017. Differences in salicylic acid glucose conjugations by UGT74F1 and UGT74F2 from Arabidopsis thaliana . Scientific Reports 7: 46629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleadow RM, Haburjak J, Dunn JE, Conn ME, Conn EE. 2008. Frequency and distribution of cyanogenic glycosides in Eucalyptus L'Herit. Phytochemistry 69: 1870–1874. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Møller BL. 2014. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annual Review of Plant Biology 65: 155–185. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE. 2000. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx . Tree Physiology 20: 591–598. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE. 2002. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. Journal of Chemical Ecology 28: 1301–1313. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Capon RJ, Woodrow IE. 2002. Cyanogenic polymorphism in Eucalyptus polyanthemos Schauer subsp. vestita L. Johnson and K. Hill (Myrtaceae). Biochemical Systematics and Ecology 30: 617–630. [Google Scholar]
- Goodger JQD, Choo TY, Woodrow IE. 2007. Ontogenetic and temporal trajectories of chemical defence in a cyanogenic eucalypt. Oecologia 153: 799–808. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Woodrow IE. 2011. α,β‐Unsaturated monoterpene acid glucose esters: structural diversity, bioactivities and functional roles. Phytochemistry 72: 2259–2266. [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N et al. 2012. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research 40: D1178–D1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M et al. 2013. De novo transcript sequence reconstruction from RNA‐seq using the Trinity platform for reference generation and analysis. Nature Protocols 8: 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CC, Nelson DR, Møller BL, Werck‐Reichhart D. 2021. Plant cytochrome P450 plasticity and evolution. Molecular Plant 14: 1244–1265. [DOI] [PubMed] [Google Scholar]
- Hansen CC, Sørensen M, Veiga TAM, Zibrandtsen JFS, Heskes AM, Olsen CE, Boughton BA, Møller BL, Neilson EHJ. 2018. Reconfigured cyanogenic glucoside biosynthesis in Eucalyptus cladocalyx involves a cytochrome P450 CYP706C55. Plant Physiology 178: 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KS, Kristensen C, Tattersall DB, Jones PR, Olsen CE, Bak S, Møller BL. 2003. The in vitro substrate regiospecificity of recombinant UGT85B1, the cyanohydrin glucosyltransferase from Sorghum bicolor . Phytochemistry 64: 143–151. [DOI] [PubMed] [Google Scholar]
- Hearne PQ, Knorr DA, Hillman BI, Morris TJ. 1990. The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus. Virology 177: 141–151. [DOI] [PubMed] [Google Scholar]
- Hirakawa H, Nakamura Y, Kaneko T, Isobe S, Sakai H, Kato T, Hibino T, Sasamoto S, Watanabe A, Yamada M et al. 2011. Survey of the genetic information carried in the genome of Eucalyptus camaldulensis . Plant Biotechnology 28: 471–480. [Google Scholar]
- Irmisch S, McCormick AC, Boeckler GA, Schmidt A, Reichelt M, Schneider B, Block K, Schnitzler JP, Gershenzon J, Unsicker SB et al. 2013. Two herbivore‐induced cytochrome P450 enzymes CYP79D6 and CYP79D7 catalyze the formation of volatile aldoximes involved in poplar defense. Plant Cell 25: 4737–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenrich R, Trompetter I, Bak S, Olsen CE, Møller BL, Piotrowski M. 2007. Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proceedings of the National Academy of Sciences, USA 104: 18848–18853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K, Morant AV, Morant M, Jensen NB, Olsen CE, Kannangara R, Motawia MS, Møller BL, Bak S. 2011. Biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in cassava: isolation, biochemical characterization, and expression pattern of CYP71E7, the oxime‐metabolizing cytochrome P450 enzyme. Plant Physiology 155: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara R, Motawia MS, Hansen NK, Paquette SM, Olsen CE, Møller BL, Jørgensen K. 2011. Characterization and expression profile of two UDP‐glucosyltransferases, UGT85K4 and UGT85K5, catalyzing the last step in cyanogenic glucoside biosynthesis in cassava. The Plant Journal 68: 287–301. [DOI] [PubMed] [Google Scholar]
- Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J, Tyka M, Baker D, Karplus K. 2009. Improving physical realism, stereochemistry, and side‐chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins 77(Suppl 9): 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger E, Vriend G. 2014. Yasara view – molecular graphics for all devices – from smartphones to workstations. Bioinformatics 30: 2981–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger E, Vriend G. 2015. New ways to boost molecular dynamics simulations. Journal of Computational Chemistry 36: 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen C, Morant M, Olsen CE, Ekstrøm CT, Galbraith DW, Møller BL, Bak S. 2005. Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proceedings of the National Academy of Sciences, USA 102: 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulheim C, Padovan A, Hefer C, Krause ST, Kollner TG, Myburg AA, Degenhardt J, Foley WJ. 2015. The Eucalyptus terpene synthase gene family. BMC Genomics 16: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. Mega7: molecular evolutionary genetics analysis v.7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D, Maimann AB, Macea E, Ocampo CH, Cardona G, Pičmanová M, Darbani B, Olsen CE, Debouck D, Raatz B et al. 2020. Biosynthesis of cyanogenic glucosides in Phaseolus lunatus and the evolution of oxime‐based defenses. Plant Direct 4: e00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos L, Szittya G, Silhavy D, Burgyán J. 2004. Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO Journal 23: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen T, Borch J, Knudsen C, Bavishi K, Torta F, Martens HJ, Silvestro D, Hatzakis NS, Wenk MR, Dafforn TR et al. 2016. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354: 890–893. [DOI] [PubMed] [Google Scholar]
- Li L, Modolo LV, Escamilla‐Trevino LL, Achnine L, Dixon RA, Wang X. 2007. Crystal structure of Medicago truncatula UGT85H2 – insights into the structural basis of a multifunctional (iso)flavonoid glycosyltransferase. Journal of Molecular Biology 370: 951–963. [DOI] [PubMed] [Google Scholar]
- Li P, Li YJ, Wang B, Yu HM, Li Q, Hou BK. 2017. The Arabidopsis UGT87A2, a stress‐inducible family 1 glycosyltransferase, is involved in the plant adaptation to abiotic stresses. Physiologia Plantarum 159: 416–432. [DOI] [PubMed] [Google Scholar]
- Lim E‐K, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ. 2002. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4‐hydroxybenzoic acid, and other benzoates. The Journal of Biological Chemistry 277: 586–592. [DOI] [PubMed] [Google Scholar]
- Lindgreen S. 2012. AdapterRemoval: easy cleaning of next‐generation sequencing reads. BMC Research Notes 5: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Bélanger A, Fournel‐Gigleux S, Green M, Hum DW, Iyanagi T et al. 1997. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7: 255–269. [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Walter Bock K, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. 2005. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenetics and Genomics 15: 677–685. [DOI] [PubMed] [Google Scholar]
- Marsh KJ, Kulheim C, Blomberg SP, Thornhill AH, Miller JT, Wallis IR, Nicolle D, Salminen JP, Foley WJ. 2017. Genus‐wide variation in foliar polyphenolics in eucalypts. Phytochemistry 144: 197–207. [DOI] [PubMed] [Google Scholar]
- Møller BL, Olsen CE, Motawia MS. 2016. General and stereocontrolled approach to the chemical synthesis of naturally occurring cyanogenic glucosides. Journal of Natural Products 79: 1198–1202. [DOI] [PubMed] [Google Scholar]
- Montague NP, Thuenemann EC, Saxena P, Saunders K, Lenzi P, Lomonossoff GP. 2011. Recent advances of Cowpea mosaic virus‐based particle technology. Human Vaccines 7: 383–390. [DOI] [PubMed] [Google Scholar]
- Moore BD, Foley WJ. 2005. Tree use by koalas in a chemically complex landscape. Nature 435: 488–490. [DOI] [PubMed] [Google Scholar]
- Myburg AA, Grattapaglia D, Tuskan GA, Hellsten U, Hayes RD, Grimwood J, Jenkins J, Lindquist E, Tice H, Bauer D et al. 2014. The genome of Eucalyptus grandis . Nature 510: 356–362. [DOI] [PubMed] [Google Scholar]
- Neilson EH. 2012. Characterisation of cyanogenic glucoside synthesis in Eucalyptus. PhD thesis, University of Melbourne, Melbourne, Vic., Australia.
- Neilson EH, Goodger JQD, Motawia MS, Bjarnholt N, Frisch T, Olsen CE, Møller BL, Woodrow IE. 2011. Phenylalanine derived cyanogenic diglucosides from Eucalyptus camphora and their abundances in relation to ontogeny and tissue type. Phytochemistry 72: 2325–2334. [DOI] [PubMed] [Google Scholar]
- Neilson EH, Goodger JQD, Woodrow IE. 2006. Novel aspects of cyanogenesis in Eucalyptus camphora subsp. humeana . Functional Plant Biology 33: 487–496. [DOI] [PubMed] [Google Scholar]
- Neilson EH, Goodger JQD, Woodrow IE, Møller BL. 2013. Plant chemical defense: at what cost? Trends in Plant Science 18: 250–258. [DOI] [PubMed] [Google Scholar]
- Nelson DR. 2009. The cytochrome P450 homepage. Human Genomics 4: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle D. 2019. Classification of the eucalypts (Angophora, Corymbia and Eucalyptus) v.4. [WWW document] URL http://www.dn.com.au/Classification-Of-The-Eucalypts.pdf
- Ono E, Waki T, Oikawa D, Murata J, Shiraishi A, Toyonaga H, Kato M, Ogata N, Takahashi S, Yamaguchi M et al. 2019. Glycoside-specific glycosyltransferases catalyze regio-selective sequential glucosylations for a sesame lignan, sesaminol triglucoside. The Plant Journal 101: 1221–1233. [DOI] [PubMed] [Google Scholar]
- Orr AJ, Padovan A, Kainer D, Külheim C, Lindell Bromham L, Bustos‐Segura C, Foley W, Haff T, Hsieh J‐F, Morales‐Suarez A et al. 2020. A phylogenomic approach reveals a low somatic mutation rate in a long‐lived plant. Proceedings of the Royal Society B: Biological Sciences 287: 20192364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan A, Patel HR, Chuah A, Huttley GA, Krause ST, Degenhardt J, Foley WJ, Külheim C. 2015. Transcriptome sequencing of two phenotypic mosaic Eucalyptus trees reveals large scale transcriptome re‐modelling. PLoS ONE 10: e0123226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picmanova M, Neilson EH, Motawia MS, Olsen CE, Agerbirk N, Gray CJ, Flitsch S, Meier S, Silvestro D, Jørgensen K et al. 2015. A recycling pathway for cyanogenic glycosides evidenced by the comparative metabolic profiling in three cyanogenic plant species. The Biochemical Journal 469: 375–389. [DOI] [PubMed] [Google Scholar]
- Pryor LD. 1976. The biology of eucalypts. London, UK: Edward Arnold. [Google Scholar]
- von Rad U, Hüttl R, Lottspeich F, Gierl A, Frey M. 2001. Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize. The Plant Journal 28: 633–642. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Pérez R, Pavan S, Mazzeo R, Moldovan C, Aiese Cigliano R, Del Cueto J, Ricciardi F, Lotti C, Ricciardi L, Dicenta F et al. 2019. Mutation of a bHLH transcription factor allowed almond domestication. Science 364: 1095–1098. [DOI] [PubMed] [Google Scholar]
- dos Santos BM, Zibrandtsen JFS, Gunbilig D, Sørensen M, Cozzi F, Boughton BA, Heskes AM, Neilson EHJ. 2019. Quantification and localization of formylated phloroglucinol compounds (FPCs) in Eucalyptus species. Frontiers in Plant Science 10: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama T, Ono E, Takagi K, Takada Y, Horikawa M, Nakamoto Y, Hirose A, Sasama H, Ohashi M, Hasegawa H et al. 2012. The Sg‐1 glycosyltransferase locus regulates structural diversity of triterpenoid saponins of soybean. The Plant Cell 24: 2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FB, Cho SK, Olsen CE, Yang SW, Møller BL, Jørgensen K. 2018. Diurnal regulation of cyanogenic glucoside biosynthesis and endogenous turnover in cassava. Plant Direct 2: e00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger W, Pasquet J‐C, Nussbaumer T, Paris MPK, Wiesenberger G, Macadré C, Ametz C, Berthiller F, Lemmens M, Saindrenan P et al. 2013. Functional characterization of two clusters of Brachypodium distachyon UDP‐glycosyltransferases encoding putative deoxynivalenol detoxification genes. Molecular Plant–Microbe Interactions 26: 781–792. [DOI] [PubMed] [Google Scholar]
- Shao H, He X, Achnine L, Blount JW, Dixon RA, Wang X. 2005. Crystal structures of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula . Plant Cell 17: 3141–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicker D, Frey M, Schulz M, Gierl A. 2000. Role of natural benzoxazinones in the survival strategy of plants. International Review of Cytology 198: 319–346. [DOI] [PubMed] [Google Scholar]
- Sørensen M, Rinnan R, Woodrow I, Møller BL, Neilson EHJ. 2020. The entangled dynamics of eucalypt leaf and flower volatile emissions. Environmental and Experimental Botany 176: 104032. [Google Scholar]
- Steane DA, Nicolle D, Sansaloni CP, Petroli CD, Carling J, Kilian A, Myburg AA, Grattapaglia D, Vaillancourt RE. 2011. Population genetic analysis and phylogeny reconstruction in Eucalyptus (Myrtaceae) using high‐throughput, genome‐wide genotyping. Molecular Phylogenetics and Evolution 59: 206–224. [DOI] [PubMed] [Google Scholar]
- Tahara K, Nishiguchi M, Frolov A, Mittasch J, Milkowski C. 2018. Identification of UDP glucosyltransferases from the aluminum‐resistant tree Eucalyptus camaldulensis forming beta‐glucogallin, the precursor of hydrolyzable tannins. Phytochemistry 152: 154–161. [DOI] [PubMed] [Google Scholar]
- Takos AM, Knudsen C, Lai D, Kannangara R, Mikkelsen L, Motawia MS, Olsen CE, Sato S, Tabata S, Jørgensen K et al. 2011. Genomic clustering of cyanogenic glucoside biosynthetic genes aids their identification in Lotus japonicus and suggests the repeated evolution of this chemical defence pathway. The Plant Journal 68: 273–286. [DOI] [PubMed] [Google Scholar]
- Thodberg S, Del Cueto J, Mazzeo R, Pavan S, Lotti C, Dicenta F, Jakobsen Neilson EH, Møller BL, Sanchez‐Perez R. 2018. Elucidation of the amygdalin pathway reveals the metabolic basis of bitter and sweet almonds (Prunus dulcis). Plant Physiology 178: 1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodberg S, Hansen CC, Takos AM, Pičmanová M, Møller BL, Nelson DR, Neilson EHJ. 2021. The fern CYPome: fern‐specific cytochrome P450 family involved in convergent evolution of chemical defense. bioRxiv. doi: 10.1101/2021.03.23.436569. [DOI] [Google Scholar]
- Thornhill AH, Crisp MD, Külheim C, Lam KE, Nelson LA, Yeates DK, Miller JT. 2019. A dated molecular perspective of eucalypt taxonomy, evolution and diversification. Australian Systematic Botany 32: 29–48. [Google Scholar]
- Vining KJ, Romanel E, Jones RC, Klocko A, Alves‐Ferreira M, Hefer CA, Amarasinghe V, Dharmawardhana P, Naithani S, Ranik M et al. 2015. The floral transcriptome of Eucalyptus grandis . New Phytologist 206: 1406–1422. [DOI] [PubMed] [Google Scholar]
- Voinnet O, Pinto YM, Baulcombe DC. 1999. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proceedings of the National Academy of Sciences, USA 96: 14147–14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Jin SH, Hu HQ, Sun YG, Wang YW, Han P, Hou BK. 2012. UGT87A2, an Arabidopsis glycosyltransferase, regulates flowering time via FLOWERING LOCUS C. New Phytologist 194: 666–675. [DOI] [PubMed] [Google Scholar]
- Wang F, Liigand J, Tian S, Arndt D, Greiner R, Wishart DS. 2021. CFM‐ID 4.0: more accurate ESI‐MS/MS spectral prediction and compound identification. Analytical Chemistry 93: 11692–11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AE, Tian L. 2019. Phylogenomic analysis of UDP‐dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. The Plant Journal 100: 1273–1288. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Yamamoto K, Asano Y. 2014. Identification and characterization of CYP79D16 and CYP71AN24 catalyzing the first and second steps in L‐phenylalanine‐derived cyanogenic glycoside biosynthesis in the Japanese apricot, Prunus mume Sieb. et Zucc. Plant Molecular Biology 86: 215–223. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sun Y, Li M, Long R. 2021. CrUGT87A1, a UDP‐sugar glycosyltransferases (UGTs) gene from Carex rigescens, increases salt tolerance by accumulating flavonoids for antioxidation in Arabidopsis thaliana . Plant Physiology and Biochemistry 159: 28–36. [DOI] [PubMed] [Google Scholar]
- Zhang L‐J, Wang D‐G, Zhang P, Wu C, Li Y‐Z. 2022. Promiscuity characteristics of versatile plant glycosyltransferases for natural product glycodiversification. ACS Synthetic Biology 11: 812–819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Metabolites produced upon transient expression of prunasin biosynthetic genes in Nicotiana benthamiana.
Fig. S2 Drop‐out experiment of the prunasin biosynthetic genes from Eucalyptus yarraensis and Eucalyptus camphora.
Fig. S3 Observation of a diagnostic fragment m/z 329 putatively annotated as benzoic acid glucoside.
Fig. S4 Assessment of UGT activity using diagnostic prunasin pathway derivatives provides a consistent measure of prunasin accumulation above purported Nicotiana benthamiana endogenous UGT activity.
Fig. S5 Phylogenetic tree of UGT85 and UGT87 sequences identified from five different Eucalyptus species.
Fig. S6 In vitro assays of UGT87Y1 and UGT85A59 showing activity toward different metabolite acceptors.
Fig. S7 CYP79 phylogeny with sequences identified from five different Eucalyptus species.
Fig. S8 CYP706 phylogeny with sequences identified from five different Eucalyptus species.
Fig. S9 CYP71 phylogeny with sequences identified from five different Eucalyptus species.
Fig. S10 Transient expression of EgCYP79A36 and EgCYP79A37 from the acyanogenic Eucalyptus grandis.
Fig. S11 Comparison of product profiles upon transient co‐expression of EclUGT85A59 or EgUGT85A with the prunasin CYPs from Eucalyptus cladocalyx.
Table S1 Primers used to isolate target open reading frames from cDNA.
Table S2 UGT gene expression at 1, 4, and 7 months in Eucalyptus camphora and Eucalyptus yarraensis.
Please note: Wiley is not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The transcriptomic data that support the findings of this study are openly available in BioProject at www.ncbi.nlm.nih.gov/bioproject, ID: PRJNA765518.


