Abstract
Objective
The Lee‐Jones model posits that antecedent individual and interpersonal factors predicate the development of fear of cancer recurrence (FCR) through cognitive and emotional processing, which further to behavioral, emotional, and/or physiological responses. We analyzed data from FoRtitude, a FCR intervention grounded in the Lee‐Jones FCR model, to evaluate associations between FCR antecedents, resources (e.g., breast cancer self‐efficacy, BCSE) and psychological and behavioral consequences.
Methods
Women with breast cancer who completed treatment and reported clinically elevated levels of FCR were randomized into a 4‐week online psychosocial intervention or contact control group. We assessed BCSE, FCR, and physical activity, anxiety and depression, or symptoms at baseline, 4 and 8 weeks. Separate structural equation models were constructed with both baseline data and change scores (baseline‐8 weeks) to examine the pathways linking BCSE, FCR and: (1) physical activity; (2) anxiety and depression; and (3) symptoms (fatigue, sleep disturbance, cognitive concerns).
Results
At baseline, higher levels of BCSE were associated with lower levels of FCR. Higher FCR was associated with worse psychological effects and symptoms but not behavioral response. Change models revealed that an increase in BCSE was associated with a decrease in FCR at 8‐week assessment, which was associated with reductions in psychological effects. A change in BCSE was also directly associated with reductions in psychological effects.
Conclusions
Results support the Lee‐Jones model as a foundation for FCR interventions among breast cancer survivors. Replicability among varied populations is needed to examine effects on behavioral outcomes of FCR such as health care utilization.
Clinical Trials Registration: NCT03384992.
Keywords: breast cancer, fear of recurrence, oncology, patient‐reported outcomes, quality of life, self‐efficacy, survivorship
1. BACKGROUND
Fear of cancer recurrence (FCR) is a prevalent, distressing concern among survivors of multiple cancer types, including breast cancer survivors. 1 A Delphi study, conducted to increase consensus related to FCR among clinicians and researchers, defined FCR as ‘Fear, worry or concern relating to the possibility that cancer will come back or progress. 2 The defining characteristics of clinical FCR are identified as: a preoccupation with the cancer returning or progressing, unhelpful coping behaviors, impairment in daily function, clinically significant distress and limited capacity for making future plans. 2 Previous evidence suggests that 24%–56% of women, including women at low risk, will report moderate to severe FCR, and that intrusive thoughts and impacts of this fear may persist for many years following treatment. 3 , 4 , 5 , 6 , 7 Even at low levels, fear of recurrence can result in psychological (anxiety, depression, and quality of life impairments) and behavioral consequences (health‐risk behaviors, excessive health care‐seeking). 8 , 9 While a degree of fear about recurrence may be beneficial to the extent that it motivates survivors to engage in self‐management and recommended surveillance, there remains a critical need to identify women with excessive levels of FCR, and to develop and deliver efficacious interventions that can help breast cancer survivors avoid a range of secondary problems that can stem from untreated FCR and ultimately increase the health burden on survivors and the cost of ongoing care.
In a review of the current FCR literature, Simonelli and colleagues 10 reported that several theories 11 , 12 , 13 , 14 , 15 , 16 have informed potential treatment options and several have shown some validity in breast cancer survivors specifically. 12 , 13 , 17 Leventhal's self‐regulation model of illness 11 forms the foundation of the Lee‐Jones model (Figure 1), 17 based on cognitive‐behavioral theory, which posits that antecedent individual and interpersonal factors (i.e., interpretation of somatic sensations or reminders about one's cancer history) predicate the development of FCR through cognitive and emotional processing. According to the model, this pathway is mediated by psychosocial resources. In turn, FCR leads to the development of behavioral, emotional, and/or physiological responses. The Lee‐Jones model is the conceptual underpinning of the FoRtitude trial, on which these secondary analyses focus.
FIGURE 1.
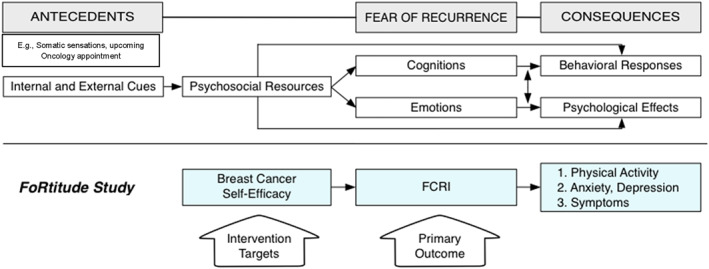
Lee‐Jones Model of Fear of Cancer Recurrence adapted. FCRI, Fear of Cancer Recurrence Inventory. 28
Growing empirical evidence supports that cognitive‐behavioral strategies can help patients to manage their FCR. Recently, two meta‐analyses and a systematic review demonstrated the efficacy of therapist‐delivered cognitive behavioral interventions to reduce FCR. 18 , 19 These findings provide indirect support for the Lee‐Jones theoretical model. 9 , 20 , 21 Cognitions and emotions encompassing FCR are also shown to lead to excessive healthcare utilization. 8 , 22 Findings linking FCR with health behaviors are mixed; one study linked FCR with positive changes in health behaviors (e.g., adopting a healthy diet or exercise) but not negative health behaviors (e.g. stopping smoking), 23 while a another study linked FCR with negative health behaviors (i.e., increased alcohol use and lower physical activity). 9 Psychological effects can include increased health‐related anxiety or depression, and or compromised quality of life (QOL). 24 To build on these advances, a critical step is to evaluate the cognitive‐behavioral pathways underlying the Lee‐Jones Model using longitudinal data from a clinical trial. CBT‐based interventions demonstrated small effect sizes in reducing FCR, therefore a more precise understanding of the mechanisms driving FCR could lead to more robust intervention effects as well as personalized interventions, optimizing efficacy. The Lee‐Jones theoretical model has been validated in a cross‐sectional sample of breast cancer survivors from three hospitals in the Netherlands. 25 In mediational models, both direct and indirect effects of internal (bodily sensations, feeling sick) and external cues (contact with health professionals, media and social interactions) via FCR on behavioral response variables (body checking, limited future planning) were found. Lebel and colleagues have also validated a blended theoretical model of FCR (including the Lee‐Jones model, Mishel's uncertainty in illness theory) in breast and gynecological cancer survivors, examining effects on body checking, reassurance seeking and avoidance. 26
The FoRtitude trial was an intervention trial explicitly grounded in the Lee‐Jones theoretical model of FCR. 27 We hypothesized that the intervention that taught relaxation training, cognitive restructuring (“changing how you feel by changing how you think”), and scheduled worry practice (“get control over your worry about recurrence by scheduling when and where it happens”), 27 would enhance breast cancer self‐efficacy (BCSE), facilitating a clinically meaningful reduction in FCR. BCSE specifically reflects confidence in one's ability to manage breast cancer survivorship including physical symptoms, social and emotional effects, potential recurrence, and other concerns that are associated with multiple domains of quality of life. Further, we were interested in examining whether targeting BCSE leads to a change in FCR that ultimately results in favorable changes in physical activity or to reduced anxiety, depression or symptoms. While limited evidence suggests that higher levels of FCR are correlated with positive changes in health behaviors, the degree to which this occurs in breast cancer survivors is not clear. 23
The purpose of this secondary data analysis was to evaluate the hypothesized relationships proposed by core aspects of the Lee‐Jones Model of FCR 17 (we did not measure internal or external cues (antecedents) and adapted the model to include physical activity as a behavioral response of FCR) in a sample of 196 breast cancer survivors who participated in FoRtitude, a clinical trial testing a cognitive‐behavioral intervention based on this theory. More specifically, we aimed to replicate the findings of the Lebel., et al. study, 26 using prospective, longitudinal data collected in the context of an intervention trial among a sample of breast cancer survivors recruited from community oncology settings. We extend the partial validation of the Lee‐Jones model by examining a lifestyle behavior (physical activity) as a response in addition to anxiety, depression and symptoms. A precise understanding of the mechanisms underlying effective interventions will help inform strategies to improve the accessibility of interventions, given barriers to therapist‐delivered psychosocial care. Analyses were conducted in two steps. First, we explored the hypothesized relationships between model constructs with cross‐sectional baseline data. Second, we reexamined these same relationships using change scores from baseline to 8‐week follow up (change models). We therefore tested the Lee‐Jones model in cross‐sectional models.
2. METHODS
This study was approved by the Institutional Review Board (IRB) at Northwestern University (STU00067549) and participating sites' local IRBs. All participants completed online informed consent prior to completing eligibility screening, randomization and participating. Eligible women were independently randomized to each of 3 CBT‐based coping strategies or health management content, designed to serve as attention control conditions. Detailed methods and procedures have been published. 29
2.1. Participants
Data on the recruitment characteristics has previously been reported. 29 Briefly, we recruited breast cancer survivors from three NCORP sites for this study. Breast cancer survivors who were stage I–III at diagnosis, 1–10 years post‐primary medical treatment, currently disease‐free, ≥18 years old, exceeded an established clinical cut‐off (≥13) on the severity subscale of Fear of Cancer Recurrence Inventory (FCRI), English speaking, and able to sign consent were eligible for the study. Between December 2014 and September 2015 participants were recruited from an academic medical center (Robert H. Lurie Comprehensive Cancer Center of Northwestern University, RHLCCC) and three NCI Community Oncology Research Program (NCORP) Community Sites (Aurora NCORP; Colorado Cancer Research Program NCORP; Metro Minnesota NCORP). Eligible women who provided informed consent received a hyperlink directing them to the baseline assessment.
2.2. Intervention
Intervention design, 27 iterative testing and refinement, 30 and the randomized trial to evaluate FoRtitude intervention components 29 have been previously described. The FoRtitude trial was based on the Multiphase Optimization Strategy (MOST) framework and was designed to evaluate four intervention components (three CBT‐based strategies vs. an attention control, and telecoaching vs. no telecoaching, for a total of 23∗2 = 16 unique groups) using a randomized, full factorial trial. Attention control components included health management content in the same eHealth format as CBT‐based treatment components. 29 CBT‐based content was designed to enhance coping strategies via an eHealth platform (website) with interactive text messaging. The website included didactic content to educate women on cognitive and emotional features of FCR and topics important for the development of coping strategies. Three CBT‐based coping strategies (relaxation, cognitive restructuring and worry practice) were presented in modules which included 3 sections: (1) introductory didactic content presenting the rationale for using the coping strategy or health‐related information to manage FCR; (2) an interactive tool to promote use of the CBT‐based coping strategy or application of attention control health‐related information; and (3) didactic content on how to utilize the coping strategy or health information on a regular basis to reduce FCR. Women were encouraged to use the website daily to access didactic content and to use the tools for promoting mastery of coping strategies. The primary analyses 29 indicated there were no significant intervention group by time effects, therefore the analyses presented in this report were conducted with the entire sample, grouping all participants regardless of randomization assignment across the four factors.
2.3. Measures
All assessments were completed at baseline (pre‐randomization), 4‐week post‐first FoRtitude site login (immediately following intervention) and 8‐week post‐login (4 weeks post‐intervention).
2.3.1. Fear of cancer recurrence
Breast cancer survivors were screened for clinically significant FCR with the Fear of Cancer Recurrence Inventory (FCRI) 9‐item severity sub‐scale. A score ≥13, reflecting clinically elevated FCR 31 was used to determine eligibility. FCR as a primary outcome was assessed by summing 5 domains (Distress, Triggers, Function, Insight, and Severity) of the FCRI (total 0–120). 28 Items in each domain (e.g. “I am afraid of cancer recurrence”) include scores ranging from 0 (Not at all) to 4 (A great deal). The FCRI has been previously validated in breast cancer survivors and has demonstrated reliability and validity. FCRI subscales Coping and Reassurance were not included in the FCRI total score due to being an intervention target (Coping) and to low internal consistency (Reassurance) (Cronbach's alphas 0.74, 0.54, respectively), similar to previous reports. 32
2.3.2. Breast cancer self‐efficacy
The modified Breast Cancer Self‐Efficacy (BCSE) scale (0–56) was used to assess self‐efficacy for dealing with breast cancer survivorship including recurrence. 33 The BCSE scale consists of 14 questions (e.g. “I am able to deal with the fact I had breast cancer” or “I am able to handle any fears I have about breast cancer returning”) with item scores ranging from 0 (Strongly disagree) to 4 (Strongly agree).
2.3.3. Behavioral responses—Physical activity
The Behavioral Risk Factors Surveillance System (BRFSS) was used to assess physical activity behavior (Model 1). 34 Participants were asked what frequency of exercise they engaged in per month or week and the average number of minutes that they exercised for on each occasion. A variable representing minutes of exercise per week was then calculated.
2.3.4. Psychological effects and symptoms
PROMIS 35 , 36 item banks were administered using computer adaptive tests (CATs) to assess psychological effects, including domains for Anxiety, Depression (Model 2) and domains for Fatigue, Sleep Disturbance and General Cognitive Concerns (Model 3). For each domain, question items from each item bank are tailored based on responses (i.e., severity of symptoms) with typically 4‐items used per domain. All items are computer scored automatically and calibrated with normative data from cancer patients and general population to generate T‐scores with a mean of 50 and a standard deviation of 10.
2.4. Statistical analyses
All analyses were conducted using Mplus v7.0. Structural equation models (SEM) were used to analyze the hypothesized relationships proposed by an adapted version of the Lee‐Jones Model of FCR. Both cross‐sectional associations and relationship between changes of the measures were evaluated in two separate analyses. For all model's tested, alpha was set at 0.05. We operationalized the schematic diagram in Figure 1 as a mediational SEM model such that FCR acted as a mediator between self‐efficacy and physical activity, anxiety and depression or symptoms. All results shown are of standardized coefficients (β). Symptoms were treated as a latent variable as indicated by fatigue, sleep disturbance, and general cognitive concerns. There were two advantages in using the SEM for our purpose: (1) it provides a flexible and scientifically justifiable framework for jointly testing model parameters as depicted by a path diagram that corresponds to the Lee‐Jones Model of FCR, and (2) it provides goodness‐of‐fit statistics for model diagnostics. The χ 2 statistic assessed absolute fit of the model to the data. The standardized root means residual (SRMR), root mean square error of approximation (RMSEA), and Comparative Fit Index (CFI) were also used to determine the fit of the model. 37 SRMR values approximating 0.08 or less, RMSEA value in the range 0.06–0.10 show close fit of the model, 37 , 38 whereas CFI values of 0.90 indicate a minimally acceptable fit value and values approximating 0.95 or greater are indicative of a good fit. 37
To evaluate the relationship between hypothesized theory‐based constructs at baseline and at follow‐up (8 weeks post‐intervention completion), we collapsed data across all randomized participants. Collapsing data was deemed appropriate as all randomized groups evidenced similar increases in BCSE and reductions in FCR. 29 Change score models were created by subtracting 8‐week values from baseline values. Therefore, a large positive change in BCSE was reflective of improvement, while for FCR, a large negative change was improvement. Behavioral responses, psychological effects and symptoms were modeled as 8‐week scores with baseline scores as covariates.
The following hypothesized relationships were tested in both baseline models, and change score models using change scores for all factors: 1a, 1b—a direct path from BCSE to FCR, a direct path from BCSE to physical activity. 2a, 2b—a direct path from BCSE to FCR, direct paths from BCSE to anxiety and depression, direct paths from FCR to anxiety and depression. 3a, 3b—a direct path from BCSE to FCR, a direct path from BCSE to symptoms, a direct path from FCR to symptoms. For all models FCR was measured as a latent construct using 5 subdomains (triggers, severity, distress, function, insight) of the FCRI as indicators. For model 3a, symptoms were measured as a latent construct with fatigue, sleep disturbance and general cognitive concerns as indicators. All models individually controlled for age, education, employment, marital status, and disease stage. Change models also included baseline scores for each factor as a covariate.
3. RESULTS
3.1. Participant characteristics
Data for sociodemographic, comorbidity status, and disease specific characteristics can be found in Table 1. Self‐reported demographic and data extracted from medical charts showed that most women were identified as white with some college education and were not currently employed. The average time since diagnosis was 4.5 years; the majority of patients were diagnosed with stage I or II disease.
TABLE 1.
Participant characteristics (N = 196)
| Characteristic | Mean (SD)/N (%) |
|---|---|
| Age | 55.22 (9.79) |
| Race | |
| Caucasian | 174 (90%) |
| African American | 9 (5%) |
| Asian | 6 (3%) |
| Hawaiian/Pacific Islander | 4 (2%) |
| American Indian/Alaska Native | 1 (0%) |
| Education | |
| Less than or some high school | 23 (12%) |
| Some college | 111 (57%) |
| College/post‐graduate | 56 (29%) |
| Marital status | |
| Not married or partnered | 153 (78%) |
| Married or partnered | 43 (22%) |
| Employment status | |
| Not employed | 164 (83%) |
| Employed part‐time | 21 (11%) |
| Employed full‐time | 6 (3%) |
| Time since diagnosis (years) | 4.5 (11.1) |
| Breast cancer stage | |
| 0/DCIS | 5 (3%) |
| I | 87 (44%) |
| II | 78 (40%) |
| III | 26 (13%) |
| Cancer treatment | |
| Surgery | 136 (69%) |
| Chemotherapy | 173 (88%) |
| Radiation | 136 (69%) |
| FCRI score (0–120) | 53.1 (17.4) |
| FCRI severity subscale (0–36) | 20.9 (5.0) |
Note: FCRI includes Distress, Triggers, Function, Insight, and Severity subdomains.
3.2. Model results
Table 2 contains the means and SDs for each of the factors we considered in our hypothesized conceptual models. Briefly, over the 8 weeks of the study, survivors experienced a decline in their FCR. There was also an increase in BCSE. Minutes of physical activity increased over 8‐week while all measures on PROMIS scales showed improvement. For change score models shown in Figure 2, dotted lines indicate non‐significant paths and solid lines indicate significant paths. The extent of missing data was 23% (only physical activity) at baseline and ranged from 22% (PROMIS measures) to 34% (8‐week physical activity) in longitudinal models and was largely the result of loss to follow up. Change score models therefore excluded baseline data from participants for whom there was no follow up data (n = 43). There were no significant differences on variables of interest for those who dropped out versus those who remained in the study (not shown).
TABLE 2.
Descriptives of fear of cancer recurrence (FCR) model at baseline and 8‐week follow up
| Variable | Baseline (n = 196) | 8‐week follow up (n = 153) | ||
|---|---|---|---|---|
| M | (SD) | M | (SD) | |
| Breast Cancer Self‐Efficacy (BCSE) | 31.1 | 7.9 | 34.6 | 6.5 |
| Fear of Cancer Recurrence Inventory (FCRI) | 53.1 | 17.4 | 41.9 | 16.2 |
| BRFSS Physical Activity (min per week) | 142.3 | 105.4 | 158.8 | 120.2 |
| PROMIS Anxiety | 53.0 | 9.5 | 48.3 | 9.7 |
| PROMIS Depression | 49.8 | 9.4 | 45.5 | 9.6 |
| PROMIS Fatigue | 52.7 | 10.6 | 49.4 | 10.7 |
| PROMIS Sleep Disturbance | 50.7 | 10.1 | 47.4 | 9.8 |
| PROMIS General Cognitive Concerns | 35.8 | 11.4 | 33.1 | 10.7 |
Note: FCRI includes Distress, Triggers, Function, Insight, and Severity subdomains.
FIGURE 2.
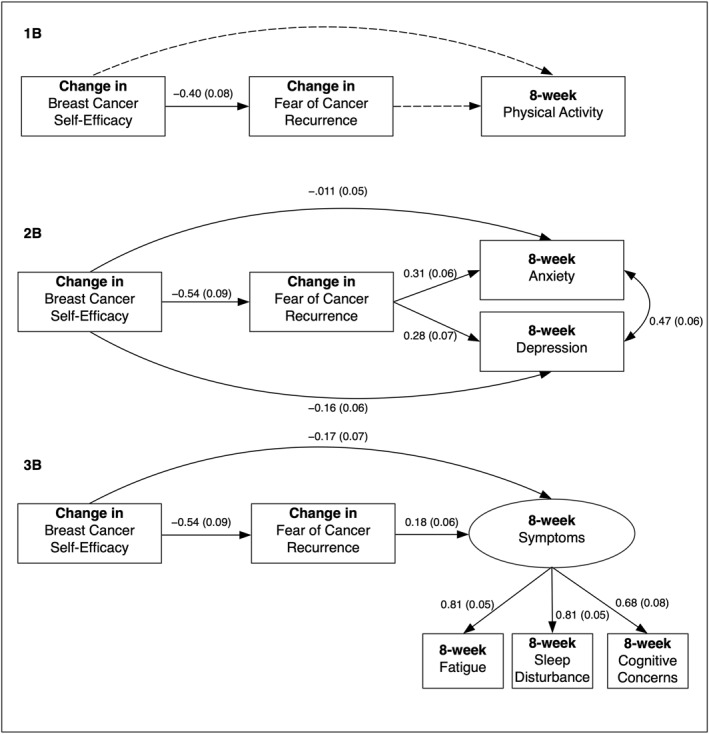
Change Models Depicting Hypothesized Relationships Between Lee‐Jones Model Constructs. All models included the following covariates (omitted from figures for clarity): age, education, employment, marital status, breast cancer stage, and baseline values for 8‐week physical activity, anxiety, depression, symptoms (latent factor)
3.2.1. Baseline models (1a, 2a and 3a—Supplementary file)
Fit statistics showed all models were a good fit to the data: (1a; χ 2 = 61.21, df = 46, p = 0.07; CFI = 0.97; SRMR 0.038; RMSEA 0.041), (2a; χ 2 = 68.06, df = 50, p = 0.05; CFI = 0.98; SRMR 0.036; RMSEA 0.043), and (3a; χ 2 = 85.62, df = 72, p = 0.13; CFI = 0.98; SRMR 0.037; RMSEA 0.031). At baseline there were several significant associations between BCSE, FCR and psychological effects but not behavioral response (physical activity). The physical activity model (1a), only indicated a significant path from BCSE to FCR (β = −0.73, SE = 0.05, p < 0.05), where higher levels of self‐efficacy were associated with lower FCR. For the anxiety and depression model (2a), all hypothesized paths, except those from BCSE to anxiety or depression, were significant. At baseline, breast cancer survivors who had higher levels of self‐efficacy had significantly lower levels of FCR (β = −0.73, SE = 0.05, p < 0.05). In turn, women with higher levels of baseline FCR had significantly higher levels of anxiety (β = 0.64, SE = 0.09 p < 0.05) and depression (β = 0.59, SE = 0.09 p < 0.05). There was also a significant correlation between anxiety and depression (r = 0.48, SE = 0.07 p < 0.05). In our third baseline model (3a) including a latent factor for symptoms indicated by fatigue, sleep disturbance and general cognitive concerns, all hypothesized paths, except a direct path from BCSE to symptoms, were significant. At baseline, in line with model 2a, women with higher levels of self‐efficacy had significantly lower FCR (β = −0.73, SE = 0.05, p < 0.05). In turn, women who had higher levels of FCR had significantly higher symptoms (β = 0.31, SE = 0.13, p < 0.05). The measurement model for symptoms was also supported in this model with higher levels of fatigue (β = 0.79, SE = 0.04, p < 0.05), more sleep disturbance (β = 0.73, SE = 0.05, p < 0.05) and more general cognitive concerns (β = 0.73, SE = 0.05, p < 0.05) all associated with significantly higher symptoms score.
3.2.2. Change score models (change from baseline to 8‐week, 1b, 2b, 3b)
Fit statistics again showed all models were a good fit to the data: (1b; χ 2 = 8.91, df = 8, p = 0.34; CFI = 0.99; SRMR 0.03; RMSEA 0.031), (2b; χ 2 = 45.76, df = 25, p = <0.01; CFI = 0.94; SRMR 0.078; RMSEA 0.074), and (3b; χ 2 = 79.04, df = 57, p = 0.03 CFI = 0.96; SRMR 0.092; RMSEA 0.05). Figure 2 depicts path diagrams for change score models. The model for physical activity (1b), showed a significant path from change in BCSE to change in FCR, where an increase in self‐efficacy was associated with reduced a FCR, but no association between change in BCSE or FCR and 8‐week physical activity. For the anxiety and depression model (2b), all hypothesized paths were significant, including direct paths from change in BCSE to 8‐week anxiety and depression. At 8‐week follow‐up, breast cancer survivors who had an increase in self‐efficacy had a significant reduction in FCR. They also had significantly lower anxiety and depression at 8‐week follow up. In turn, women with large decreases in FCR also had significantly lower anxiety and depression at 8‐week. In our third model including symptoms (3b), all hypothesized paths were significant, including a direct path from BCSE to symptoms. Women increasing their self‐efficacy had both significant reductions in FCR and significantly lower symptoms at 8‐week follow up. A reduction in FCR was also significantly associated with lower symptoms at 8‐week follow up.
4. DISCUSSION
Our findings partially validate an adapted Lee‐Jones theoretical model of FCR using an SEM approach for secondary analysis of data collected among breast cancer survivors enrolled in an FCR intervention trial. Our results largely support the Lee‐Jones conceptualization of FCR, with higher levels of FCR being associated with worse psychological effects at baseline, and greater reductions in FCR being associated with lower levels of psychological responses (ie. distress) following intervention, albeit small. In all models examined, BCSE, a target of the psychosocial intervention was associated with reductions in FCR and psychological responses. To our knowledge this is the first study to examine model fit among variables assessed pre‐post FCR intervention.
While the original Lee‐Jones model does not include physical activity as a behavioral response associated with FCR, there has been some evidence to suggest that physical activity may either reduce 9 or increase 23 in response to FCR. The FoRtitude intervention content did not present content on exercise or physical activity, making the examination of physical activity in the current analyses valuable, allowing us to examine whether general health concerns or fear of recurrence leads to women engaging in health supportive behaviors of their own accord. However, our findings did not support any significant association between FCR and change in physical activity. It is possible that FCR may lead to reduced physical activity in some, while prompting increased physical activity among other cancer survivors, and our non‐significant finding may be indicative of this heterogeneity. In a study examining group‐based trajectories of FCR in over 2300 cancer survivors, those with the highest FCR had low levels of physical activity. 39 Our eligibility criteria requiring elevated FCR may have resulted in a sample of breast cancer survivors with a lower likelihood of engaging in physical activity. Future trials should directly evaluate physical activity as a strategy for managing FCR as it is known to improve affect and reduce anxiety and depression, and symptoms such as fatigue, and include a range of participants with regard to FCR severity to understand if the efficacy of physical activity to reduce FCR is contingent upon FCR severity at trial entry. It is also possible that other health behaviors not assessed in this analysis, such as healthcare utilization and excessive body checking, may be more reliably associated with FCR 8 , 22 ; however, a longer follow up period may have revealed changes. A study conducted in a mixed group of cancer survivors (up to 10 years post‐diagnosis), found that higher levels of FCR were significant predictors of both outpatient and ER visits over the past 6 months. 22 Custers et al., 25 also examining the Lee‐Jones model among breast cancer survivors, found evidence for both direct and indirect effects of FCR on body checking.
We also examined effects on anxiety and depressive symptoms and found that women with lower self‐efficacy for managing breast cancer and a higher FCR experienced greater anxiety and depression. Our findings support a robust literature linking FCR with greater emotional disturbance, including prior examinations of the Lee‐Jones model using cross‐sectional survey data 9 , 20 , 21 and a qualitative study examining lived experiences of breast cancer survivors recently having completed treatment. 40 Notably, our findings extend this literature, providing strong empirical support for this pathway by using longitudinal data for modeling covariance in change.
With regard to fatigue, sleep disturbance and cognitive concerns, we again found evidence that women with lower self‐efficacy and higher FCR experienced worse symptoms. While physical symptoms such as pain may act as powerful antecedents of FCR, 11 , 17 our models incorporated fatigue, sleep disturbance and cognitive concerns as outcomes (symptoms) that commonly co‐occur 41 in cancer patients and may result from chronically elevated FCR. Leventhal's original model 11 suggested the presence of feedback loops and as suggested by Maheu et al., 40 refinements to the Lee‐Jones model to emphasize the salience of FCR and the need for support may be considered. Our findings support those of previous studies, where worse sleep quality, 42 and more global fatigue 43 was more likely in patients with higher levels of FCR.
Self‐efficacy has previously been shown to have a protective effect on FCR. 33 However, our findings are unique in that we examined the effects of change in an intervention target—self‐efficacy for managing breast cancer—on FCR, and on hypothesized psychological effects. Several other studies have utilized a cognitive behavioral approach to reducing FCR, however, self‐efficacy for managing cancer has seldom been targeted as a specific outcome of skills training. For example, one early CBT‐based uncertainty management intervention increased the use of cognitive reframing, active coping, resulting in reduced uncertainty. 44 Therefore, while many previous theory‐based interventions, designed to address FCR include overlapping constructs, 18 identifying additional important intervention targets that may protect against the development of clinically significant FCR may improve intervention design. Providing as needed access to evidence‐based content may be beneficial when feelings of fear arise, and is a strategy likely to enhance self‐efficacy for managing the breast cancer experience.
4.1. Study limitations
While our findings partially support the validity of the Lee‐Jones theoretical model of FCR, this was a secondary analysis of available data, thus did not include a comprehensive assessment of all Lee‐Jones theoretical constructs such as antecedents like somatic sensations. For example, we assessed health care seeking behaviors as a behavioral response of FCR, however our brief study duration (8 weeks) did not allow us to capture many instances of healthcare use. The 8‐week timeframe is also not reflective of a typical breast cancer survivors experience; however, the 8‐week changes may instead be a signal of an effective strategy, that if used when needed could lead to long‐term management of FCR. We also tested separate models for each set of psychological and behavioral responses therefore did not consider their shared variance simultaneously. While approximately half of our sample was recruited from geographically diverse community‐based oncology practices, our sample was predominantly white and reasonably well‐educated making the finding less easily translatable to other women of an ethnic minority or lower socio‐economic status. Our eligibility criteria required clinically elevated FCR, therefore our findings in support of the Lee‐Jones theoretical model of FCR may not be extrapolated to women with low or subclinical levels of FCR.
4.2. Clinical implications
The current study provided empirical support for the Lee‐Jones theoretical model of FCR and in doing so, suggests that CBT interventions grounded in components from the Lee‐Jones theoretical model have the potential to reduce FCR, and may be used to augment existing FCR interventions which have demonstrated modest effect sizes. Findings may also be used to translate key components of efficacious therapist‐delivered CBT to technology‐enabled platforms. Theoretically‐grounded, efficacious interventions to reduce FCR and associated consequences could significantly improve the quality of cancer survivorship among the many survivors who struggle with this prevalent and persistent concern.
AUTHOR CONTRIBUTIONS
Alexander R. Lucas: conceptualization, visualization, methodology, writing—original draft and review and editing. Jun‐hao Pan: formal analysis, validation, visualization, writing—original draft and review and editing. Edward H. Ip: formal analysis, validation, visualization, writing—original draft and review and editing. Daniel L. Hall: writing—review and editing. Janet A. Tooze: data curation, validation, writing—review and editing. Beverly Levine: data curation, validation, writing—review and editing. David C. Mohr: funding acquisition, methodology, resources, software, supervision, writing—review and editing. Frank J. Penedo: conceptualization, methodology, resources, supervision, writing—review and editing. David Cella: conceptualization, funding acquisition, methodology, resources, software, supervision, writing—review and editing. Lynne I. Wagner: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, supervision, visualization, writing—original draft and review and editing.
CONFLICT OF INTEREST
There are no conflicts of interest to report.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
This project was funded by the National Cancer Institute (1R21 CA173193), 1UG1CA189828, the ECOG‐ACRIN Medical Research Foundation and was partly supported by a National Cancer Institute training grant (R25 CA122061). We thank the breast cancer survivors who participated in this study. We thank study investigators, nurses, clinicians and study personnel at Aurora, Metro Minnesota, and Colorado Cancer Research Program NCORP Sites and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University for support with trial recruitment.
Lucas AR, Pan J‐h, Ip EH, et al. Validation of the Lee‐Jones theoretical model of fear of cancer recurrence among breast cancer survivors using a structural equation modeling approach. Psychooncology. 2023;32(2):256‐265. 10.1002/pon.6076
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Armes J, Crowe M, Colbourne L, et al. Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol. 2009;27(36):6172‐6179. 10.1200/jco.2009.22.5151 [DOI] [PubMed] [Google Scholar]
- 2. Lebel S, Ozakinci G, Humphris G, et al. From normal response to clinical problem: definition and clinical features of fear of cancer recurrence. Support Care Cancer. 2016;24(8):3265‐3268. 10.1007/s00520-016-3272-5 [DOI] [PubMed] [Google Scholar]
- 3. Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer‐related health worries and psychological distress among older adult, long‐term cancer survivors. Psychooncology. 2006;15(4):306‐320. 10.1002/pon.955 [DOI] [PubMed] [Google Scholar]
- 4. Simard S, Savard J, Ivers H. Fear of cancer recurrence: specific profiles and nature of intrusive thoughts. J Cancer Surviv. 2010;4(4):361‐371. 10.1007/s11764-010-0136-8 [DOI] [PubMed] [Google Scholar]
- 5. Stanton AL, Danoff‐Burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psychooncology. 2002;11(2):93‐102. 10.1002/pon.574 [DOI] [PubMed] [Google Scholar]
- 6. van den Beuken‐van Everdingen MH, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psychooncology. 2008;17(11):1137‐1145. 10.1002/pon.1340 [DOI] [PubMed] [Google Scholar]
- 7. Vickberg JSM. Fears about breast cancer recurrence. Cancer Pract. 2001;9(5):237‐243. 10.1046/j.1523-5394.2001.009005237.x [DOI] [PubMed] [Google Scholar]
- 8. Thewes B, Butow P, Bell ML, et al. Fear of cancer recurrence in young women with a history of early‐stage breast cancer: a cross‐sectional study of prevalence and association with health behaviours. Support Care Cancer. 2012;20(11):2651‐2659. 10.1007/s00520-011-1371-x [DOI] [PubMed] [Google Scholar]
- 9. Hall DL, Jimenez RB, Perez GK, et al. Fear of cancer recurrence: a model examination of physical symptoms, emotional distress, and health behavior change. J Oncol Pract. 2019;15(9):e787‐e797. 10.1200/jop.18.00787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonelli LE, Siegel SD, Duffy NM. Fear of cancer recurrence: a theoretical review and its relevance for clinical presentation and management. Psychooncology. 2017;26(10):1444‐1454. 10.1002/pon.4168 [DOI] [PubMed] [Google Scholar]
- 11. Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cognitive Ther Res. 1992;16(2):143‐163. 10.1007/bf01173486 [DOI] [Google Scholar]
- 12. Butow P, Kelly S, Thewes B, Hruby G, Sharpe L, Beith J. Attentional bias and metacognitions in cancer survivors with high fear of cancer recurrence. Psychooncology. 2015;24(4):416‐423. 10.1002/pon.3659 [DOI] [PubMed] [Google Scholar]
- 13. Mellon S, Kershaw TS, Northouse LL, Freeman‐Gibb L. A family‐based model to predict fear of recurrence for cancer survivors and their caregivers. Psychooncology. 2007;16(3):214‐223. 10.1002/pon.1074 [DOI] [PubMed] [Google Scholar]
- 14. Mishel MH. Reconceptualization of the uncertainty in illness theory. Image J Nurs Sch. 1990;22(4):256‐262. 10.1111/j.1547-5069.1990.tb00225.x [DOI] [PubMed] [Google Scholar]
- 15. Lepore SJ. A social–cognitive processing model of emotional adjustment to cancer. In: Psychosocial interventions for cancer. American Psychological Association; 2001:99‐116. [Google Scholar]
- 16. Greenberg J, Pyszczynski T, Solomon S. The causes and consequences of a need for self‐esteem: a terror management theory. In: Public Self and Private Self. 1986; 189‐212. 10.1007/978-1-4613-9564-5_10 [DOI]
- 17. Lee‐Jones C, Humphris G, Dixon R, Hatcher MB. Fear of cancer recurrence—a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology. 1997;6(2):95‐105. [DOI] [PubMed] [Google Scholar]
- 18. Hall DL, Luberto CM, Philpotts LL, Song R, Park ER, Yeh GY. Mind‐body interventions for fear of cancer recurrence: a systematic review and meta‐analysis. Psychooncology. 2018;27(11):2546‐2558. 10.1002/pon.4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tauber NM, O'Toole MS, Dinkel A, et al. Effect of psychological intervention on fear of cancer recurrence: a systematic review and meta‐analysis. J Clin Oncol. 2019;37(31):2899‐2915. 10.1200/jco.19.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall DL, Lennes IT, Pirl WF, Friedman ER, Park ER. Fear of recurrence or progression as a link between somatic symptoms and perceived stress among cancer survivors. Support Care Cancer. 2017;25(5):1401‐1407. 10.1007/s00520-016-3533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cho D, Chu Q, Lu Q. Associations among physical symptoms, fear of cancer recurrence, and emotional well‐being among Chinese American breast cancer survivors: a path model. Support Care Cancer. 2018;26(6):1755‐1761. 10.1007/s00520-017-4010-3 [DOI] [PubMed] [Google Scholar]
- 22. Lebel S, Tomei C, Feldstain A, Beattie S, McCallum M. Does fear of cancer recurrence predict cancer survivors' health care use? Support Care Cancer. 2013;21(3):901‐906. [DOI] [PubMed] [Google Scholar]
- 23. Hawkins NA, Smith T, Zhao L, Rodriguez J, Berkowitz Z, Stein KD. Health‐related behavior change after cancer: results of the American cancer society's studies of cancer survivors (SCS). J Cancer Surviv. 2010;4(1):20‐32. 10.1007/s11764-009-0104-3 [DOI] [PubMed] [Google Scholar]
- 24. Koch L, Bertram H, Eberle A, et al. Fear of recurrence in long‐term breast cancer survivors‐still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship—a multi‐regional population‐based study. Psychooncology. 2014;23(5):547‐554. 10.1002/pon.3452 [DOI] [PubMed] [Google Scholar]
- 25. Custers JA, Gielissen MF, de Wilt JH, et al. Towards an evidence‐based model of fear of cancer recurrence for breast cancer survivors. J Cancer Surviv. 2017;11(1):41‐47. 10.1007/s11764-016-0558-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lebel S, Maheu C, Tomei C, et al. Towards the validation of a new, blended theoretical model of fear of cancer recurrence. Psychooncology. 2018;27(11):2594‐2601. 10.1002/pon.4880 [DOI] [PubMed] [Google Scholar]
- 27. Wagner LI, Duffecy J, Penedo F, Mohr DC, Cella D. Coping strategies tailored to the management of fear of recurrence and adaptation for E‐health delivery: the FoRtitude intervention. Cancer. 2017;123(6):906‐910. 10.1002/cncr.30602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17(3):241‐251. 10.1007/s00520-008-0444-y [DOI] [PubMed] [Google Scholar]
- 29. Wagner LI, Tooze JA, Hall DL, et al. Targeted eHealth intervention to reduce breast cancer survivors' fear of recurrence: results from the FoRtitude randomized trial. J Natl Cancer Inst. 2021;113(11):1495‐1505. 10.1093/jnci/djab100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner LI, Duffecy J, Begale M, et al. Development and refinement of FoRtitude: an eHealth intervention to reduce fear of recurrence among breast cancer survivors. Psychooncology. 2020;29(1):227‐231. 10.1002/pon.5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv. 2015;9(3):481‐491. 10.1007/s11764-015-0424-4 [DOI] [PubMed] [Google Scholar]
- 32. Costa DS, Dieng M, Cust AE, Butow PN, Kasparian NA. Psychometric properties of the Fear of Cancer Recurrence Inventory: an item response theory approach. Psychooncology. 2016;25(7):832‐838. 10.1002/pon.4018 [DOI] [PubMed] [Google Scholar]
- 33. Ziner KW, Sledge GW, Bell CJ, Johns S, Miller KD, Champion VL. Predicting fear of breast cancer recurrence and self‐efficacy in survivors by age at diagnosis. Oncol Nurs Forum. 2012;39(3):287‐295. 10.1188/12.onf.287-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System Survey Questionnaire. US Department of Health and Human Services; 2011. [Google Scholar]
- 35. Cella D, Riley W, Stone A, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005‐2008. J Clin Epidemiol. 2010;63(11):1179‐1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cella D, Yount S, Rothrock N, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3‐S11. 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model Multidiscip J. 1999;6(1):1‐55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- 38. Browne MW, Cudeck R. Alternative ways of assessing model fit. Socio Methods Res. 2016;21(2):230‐258. 10.1177/0049124192021002005 [DOI] [Google Scholar]
- 39. Seguin Leclair C, Lebel S, Westmaas JL. The relationship between fear of cancer recurrence and health behaviors: a nationwide longitudinal study of cancer survivors. Health Psychol. 2019;38(7):596‐605. 10.1037/hea0000754 [DOI] [PubMed] [Google Scholar]
- 40. Maheu C, Hebert M, Louli J, et al. Revision of the fear of cancer recurrence cognitive and emotional model by Lee‐Jones et al with women with breast cancer. Cancer Rep (Hoboken). 2019;2(4):e1172. 10.1002/cnr2.1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liou KT, Ahles TA, Garland SN, et al. The relationship between insomnia and cognitive impairment in breast cancer survivors. JNCI Cancer Spectr. 2019;3(3):pkz041. 10.1093/jncics/pkz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berrett‐Abebe J, Cadet T, Pirl W, Lennes I. Exploring the relationship between fear of cancer recurrence and sleep quality in cancer survivors. J Psychosoc Oncol. 2015;33(3):297‐309. 10.1080/07347332.2015.1020586 [DOI] [PubMed] [Google Scholar]
- 43. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300‐322. 10.1007/s11764-013-0272-z [DOI] [PubMed] [Google Scholar]
- 44. Mishel MH, Germino BB, Gil KM, et al. Benefits from an uncertainty management intervention for African‐American and Caucasian older long‐term breast cancer survivors. Psychooncology. 2005;14(11):962‐978. 10.1002/pon.909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


