Abstract

Dexmedetomidine is commonly used in clinical practice as an anesthetic adjuvant and sedative. Unfortunately, major side effects include significant blood pressure fluctuation and bradycardia. Herein, we report the design and synthesis of four series of dexmedetomidine prodrugs aimed to alleviate hemodynamic fluctuations and simplify the administration procedure. From the in vivo experiments, all the prodrugs took effect within 5 min and did not cause significant recovery delay. The increase in blood pressure generated by one bolus of most of the prodrugs (14.57%–26.80%) was similar to that resulting from a 10 min infusion of dexmedetomidine (15.54%), which is significantly lower than the effect from a single dose of dexmedetomidine (43.55%). The decrease in heart rate induced by some prodrugs (−22.88% to −31.10%) was significantly alleviated compared with dexmedetomidine infusion (−41.07%). Overall, our work demonstrates that the prodrug strategy is useful to simplify administration procedures and mitigate hemodynamic fluctuations induced by dexmedetomidine.
Keywords: Dexmedetomidine prodrug, Transient hypertension, Bradycardia, Persistent hypotension, Hemodynamic fluctuation
Dexmedetomidine, a general anesthetic adjuvant drug and sedative agent, was first used clinically in the United States in 1999.1 Dexmedetomidine has obvious clinical advantages. Dexmedetomidine’s analgesic impact can boost patients’ tolerance to intubation and mechanical ventilation.2 Its sedative effect is similar to natural sleep,3 and patients can be awakened at any time.2 Moreover, dexmedetomidine exhibits minimal respiratory depression.4 With these advantages, the use of dexmedetomidine has been expanded. In addition to its perioperative use, dexmedetomidine is widely used as a sedative in an intensive care unit.5
Dexmedetomidine stimulates peripheral α2-adrenergic receptors, which can cause a rapid increase in blood pressure and feedback bradycardia.6 Furthermore, stimulation of the central α2-adrenergic receptors exerts sedative, analgesic, and antisympathetic effects, among which antisympathetic effects lead to persistent hypotension and bradycardia.7 In clinical use, the drug first enters the peripheral circulatory system and then diffuses into the center to exert sedative and analgesic effects. Consequently, it will inevitably lead to an excessive increase in blood pressure at the initial administration stage and subsequent bradycardia and hypotension. Intolerable blood pressure and heart rate fluctuations in patients contribute to safety issues of dexmedetomidine. To improve hemodynamic instability, researchers have tried to change the routes of administration, such as intramuscular administration, subcutaneous administration, and nasal administration.8−10 However, these delivery methods failed to solve the above problems and led to delayed efficacy, reduced plasma drug concentration (1 order of magnitude) and low bioavailability (65%–81%).8−11 In the clinic, to relieve dose-dependent transient hypertension, the loading dose of dexmedetomidine must be slowly infused for 10–15 min by intravenous administration,12 which increases the complexity of administration. Despite this undesirable route of administration, a portion of these patients continue to suffer from hemodynamic fluctuation. For example, clinical data showed that among 387 ICU patients receiving dexmedetomidine infusion, the incidence of hypertension was 16% (the fluctuation range was ≥30%).13 On the basis of previous studies, the efficacy and side effects of dexmedetomidine are closely related to its metabolic behavior. Since prodrugs can alter drug metabolic behavior, we aim to identify prodrugs of dexmedetomidine that can optimize the administration method while simulating the infusion of dexmedetomidine by one bolus and alleviating the hemodynamic fluctuations.
On the basis of previous research,14 commonly employed linkers include functionalities such as esters, carbonates, carbamates, and phosphates, as exemplified in the well-known prodrugs Aristada,14 Viread,15 Xeloda,15 and Lusedra.14 Inspired by these functional modifications, four series of dexmedetomidine prodrugs with a range of decomposition rates were designed and synthesized (Scheme 1). The ester group connected to the structure of the drug could potentially undergo enzymatic cleavage to release the parent drug.14 We, thus, introduced different ester side chains on the NH group of dexmedetomidine to design ester prodrugs 1a–j. Carbonates have different hydrolysis rates compared with esters.16 To obtain substances with different metabolic rates, we designed carbonate derivatives 2a–e. Furthermore, carbamates are generally more enzymatically stable compared with esters and carbonates.15 Thus, we designed carbamate prodrugs 3a–e with the goal of prolonging metabolic stability. Because phosphate, dibasic ester, and carbonate ester have a longer metabolic time,17 we introduced these functional groups into the structures of dexmedetomidine prodrugs to produce the phosphate prodrug 4a (a mixture of positional isomers), dibasic ester derivatives 4b–c, and carbonate ester prodrugs 4d–e. The general synthetic routes toward 1a–4e are presented in Scheme 1.
Scheme 1. Synthesis of Prodrugs 1a–4e.

Reactions and conditions: (a) chloromethyl alkylate or chloromethyl benzoate or chloromethyl alkyl carbonate, anhydrous K2CO3, anhydrous ACN, rt, overnight; (b) vinyl alkyl acetate, tetrabutylammonium hydrogen sulfate, NaHCO3, DCM:H2O = 1:1, 0 °C to rt, overnight; (c) 2 N HCl in ethyl acetate, ethyl acetate, 0 °C, 4 h; (d) alkyl chloroformate, DCM, 0 °C to rt, 8 h; (e) diethyl dicarbonate, NaHCO3, THF:H2O = 1:1, rt, overnight; (f) di-tert-butyl chloromethyl phosphate, 60% NaH, anhydrous DMF, rt, 24 h; (g) 2 N HCl in dioxane/dioxane = 1:1, rt, overnight; (h) 1 N NaOH + H2O solution, rt.
With the synthesized prodrugs in hand, we conducted a plasma decomposition assay in vitro to infer the connection between the decomposition rate and prodrug efficacy (Figure 1). Notably, the percentage of prodrug decomposition was calculated on the basis of the detection and formation of dexmedetomidine released from the respective prodrugs. Compound 1b–d exhibited full decomposition within 5 min. Additionally, the metabolic time of compounds 1h–j was more than 30 min because of the large steric hindrance. Most carbonate derivatives, except for 2d and 2e, were more stable than the ester derivatives, and exhibited a decomposition time in about 5 min. Compounds 2d and 2e were fully degraded into dexmedetomidine within 2 min. As a result of the fast decomposition rate, we speculated that 2d and 2e would not control blood pressure fluctuation. The hydrolysis rates of carbamate prodrugs 3a and 3d, for which the decomposition was over 15 min, were also slower than that of ester prodrugs. Furthermore, we found that the conversion rate of compound 3c was identical to the conversion rate of the corresponding ester derivative 1c. These findings indicated that the decomposition rate of the prodrugs was ascribed to enzymatic cleavage, as well as chemical hydrolysis (Table S1). As expected, 69.47 ± 1.36% of 4a decomposed to dexmedetomidine in 1 h. It also took more than 1 h for compounds 4b and 4c to be metabolized into dexmedetomidine. Unexpectedly, only about 40% of 4d and 4e were hydrolyzed into dexmedetomidine at 1 h. Overall, the hydrolysis time of the prodrugs varied from a few tens of seconds to several hours.
Figure 1.
Percentage of prodrug metabolism to dexmedetomidine in rat plasma in vitro (n = 3). (A) Percentage of ester derivative compound metabolism to dexmedetomidine in rat plasma in vitro. (B) Percentage of carbonate derivative compound metabolism to dexmedetomidine in rat plasma in vitro. (C) Percentage of carbamate derivative compound metabolism to dexmedetomidine in rat plasma in vitro. (D) Percentage of other ester derivative compound metabolism to dexmedetomidine in rat plasma in vitro.
We next conducted pharmacodynamic experiments to evaluate the efficacy of compounds 1a–4e. In a single dosage, the molecular efficiency of the majority of the prodrugs was comparable with dexmedetomidine (Table 1). The molecular efficiency of 2d, for example, was 107% (the reason that the value was greater than 100% may be due to measurement variability), which is consistent with its in vitro decomposition rate in plasma. In addition, some prodrug analogues with a large steric hindrance were difficult to be degraded by enzymes in vivo. The low molecular efficiencies of compounds 1e, 1h, and 3d were 45%, 44%, and 51%, respectively. However, the molecular efficiencies of a portion of prodrugs did not match up with their hydrolysis rate in plasma in vitro. For example, the molecular efficiencies of 4b and 4c, for which the decomposition time was over 1 h, were 89% and 103%. We deduce that, except for plasma decomposition, other metabolic pathways significantly affected the metabolic rate of the prodrugs in vivo.
Table 1. Pharmacodynamic Characteristics in Rats.
| one
bolus (2 × ED50; n = 10) |
||||||
|---|---|---|---|---|---|---|
| compound | ED50 [μg/kg, ED50 (95% CI)] | survival rate (2 × ED50) | onset time (s) (2 × ED50)a | duration time (s)b | Dexc from prodrug (μg/kg)d | molecular efficiency (%)e |
| 1a | 32.13 (29.69–34.78) | 100% | 252.40 ± 83.34h | 2483.60 ± 644.85g | 41.68 | 68 |
| 1b | 26.85 (24.47–29.47) | 100% | 184.40 ± 56.97g | 2325.80 ± 443.01h | 33.32 | 85 |
| 1c | 21.15 (19.75–22.65) | 100% | 265.00 ± 162.39h | 1941.90 ± 916.50h | 25.15 | 113 |
| 1d | 39.89 (28.24–56.35) | 100% | 63.90 ± 23.73 | 3798.60 ± 971.90 | 47.44 | 60 |
| 1e | 55.13 (35.79–84.92) | 100% | 157.90 ± 64.68g | 3091.80 ± 633.31 | 62.94 | 45 |
| 1f | 29.82 (27.06–32.86) | 100% | 195.20 ± 84.00g | 2410.00 ± 489.12g | 35.67 | 80 |
| 1g | 33.98 (31.15–37.08) | 100% | 214.40 ± 83.49h | 2841.20 ± 527.69 | 39.02 | 73 |
| 1h | 59.52 (49.96–70.92) | 100% | 204.10 ± 48.25h | 4457.70 ± 1050.18f | 64.29 | 44 |
| 1i | 26.28 (22.95–30.10) | 100% | 117.00 ± 53.41 | 2274.10 ± 717.36g | 32.61 | 87 |
| 1j | 34.97 (32.37–37.78) | 100% | 159.80 ± 59.2g | 2295.60 ± 548.60g | 41.58 | 68 |
| 2a | 23.25 (20.23–26.73) | 100% | 259.60 ± 115.62h | 2017.20 ± 571.54h | 28.68 | 99 |
| 2b | 29.16 (26.19–32.47) | 100% | 122.50 ± 67.88 | 2887.70 ± 593.23 | 34.47 | 82 |
| 2c | 31.12 (28.75–33.68) | 100% | 305.20 ± 98.74h | 2458.70 ± 725.68g | 35.33 | 80 |
| 2d | 23.23 (20.89–25.84) | 100% | 92.10 ± 19.68 | 2795.70 ± 594.80 | 26.53 | 107 |
| 2e | 26.85 (24.47–29.47) | 100% | 185.00 ± 67.72g | 3189.50 ± 350.24 | 29.48 | 96 |
| 3a | 18.96 (17.38–20.69) | 100% | 219.50 ± 89.15h | 2018.20 ± 523.23h | 25.77 | 110 |
| 3b | 21.29 (19.12–23.70) | 100% | 289.10 ± 146.93h | 1971.40 ± 224.74h | 27.61 | 103 |
| 3c | 25.55 (23.22–28.11) | 100% | 142.10 ± 37.45f | 2605.70 ± 586.83f | 31.70 | 90 |
| 3d | 44.64 (41.10–48.49) | 100% | 239.30 ± 80.84h | 3984.10 ± 1077.21 | 55.39 | 51 |
| 3e | 35.91 (33.60–38.38) | 100% | 151.90 ± 60.85f | 2802.90 ± 555.47 | 42.70 | 66 |
| 4a | 40.24 (36.84–43.95) | 100% | 206.50 ± 53.14h | 3966.60 ± 927.58 | 45.50 | 62 |
| 4b | 30.15 (25.83–35.20) | 100% | 78.90 ± 39.78 | 2733.90 ± 539.72f | 31.71 | 89 |
| 4c | 27.28 (24.85–29.94) | 100% | 116.00 ± 37.36 | 2484.10 ± 262.85h | 27.67 | 103 |
| 4d | 48.68 (44.97–52.69) | 100% | 94.90 ± 37.44 | 3795.20 ± 1130.57 | 50.93 | 56 |
| 4e | 46.78 (43.30–50.55) | 100% | 68.20 ± 32.52 | 3177.70 ± 663.03 | 47.22 | 60 |
| Dex | 16.77 (13.79–20.39) | 100% | 87.80 ± 38.08 | 3312.00 ± 556.74 | 28.38 | 100 |
Time from the end of injection to the disappearance of the righting reflex.
Time from the disappearance of the righting reflex to recovery.
Dex, dexmedetomidine.
The calculation formula was: dexmedetomidine from prodrug = (molecular weight of dexmedetomidine/molecular weight of prodrug) × (2 × ED50 of each drug).
Molecular efficiency = (ED50 of dexmedetomidine/molecular weight of dexmedetomidine hydrochloride)/(ED50 of prodrug/molecular weight of prodrug).
p < 0.05 versus the dexmedetomidine group in a single dose.
p < 0.01 versus the dexmedetomidine group in a single dose.
p < 0.001 versus the dexmedetomidine group in a single dose.
Although most prodrug derivatives had a longer onset time, all compounds 1a–4e were efficacious within 5 min (Table 1). Among them, the onset time of compound 2d was similar to that of dexmedetomidine, which was consistent with the result of plasma metabolism. However, compounds 4b–e, with a decomposition time greater than 1 h, had an onset time essentially equivalent to dexmedetomidine. The result of 4b–e further demonstrated that there were other metabolic routes in vivo. Compared with dexmedetomidine, most compounds, except for compound 1h, displayed less or similar duration times (Table 1). The result of the duration times of the prodrugs confirmed that a dexmedetomidine prodrug would not lead to slow recovery, despite the greater dosage and slow release rate of dexmedetomidine. This feature of prodrugs could have an advantage in clinical practice.
Because it takes time for the prodrug to release the parent drug in vivo, the prodrug may mimic the manner of infusion to some extent. Therefore, we investigated if the prodrugs could minimize the increase in blood pressure following a single dosage. Compared with dexmedetomidine administered by one bolus (43.55 ± 11.32%), most of these prodrugs elevated blood pressure to a smaller degree (Figure 2 and Table S7). An infusion of dexmedetomidine effectively reduced the rise in blood pressure (15.54 ± 4.03%). Some prodrugs could control the rise in blood pressure as effectively as dexmedetomidine administered by infusion. The increasing range of MAP (mean blood pressure) in groups 1e, 1i, 2a, 3a, and 4a was 14.57 ± 6.32%, 14.67 ± 4.49%, 16.35 ± 10.80%, 17.35 ± 11.45%, and 15.15 ± 11.03%, respectively. However, unlike dexmedetomidine infusion, not all prodrugs with a single bolus can control the elevation in blood pressure. Because of the fast hydrolysis rate, the MAPs in the groups treated with compounds 1b, 2d, 2e, 3c, and 3e were higher than the MAPs in the dexmedetomidine infusion group, for which the values were 34.92 ± 9.23%, 37.25 ± 8.45%, 34.79 ± 5.12%, 28.23 ± 6.64%, and 34.65 ± 13.62%, respectively. Likewise, despite being broken down in plasma in vitro for more than 45 min, compounds 1a (29.66 ± 14.60%), 1j (32.17 ± 13.74%), 4d (31.30 ± 8.17%), and 4e (28.49 ± 9.15%) failed to reduce the increase in blood pressure, thereby suggesting that there were other metabolic routes in vivo. In addition, none of these prodrug derivatives improved persistent hypotension, possibly because of the sustained antisympathetic effects of dexmedetomidine released from prodrugs.
Figure 2.
Rise in MAP was compared with the baseline of each rat (n = 6). (A) Ester derivative groups. (B) Carbonate derivative groups. (C) Carbamate derivative groups. (D) Other ester derivative groups. Dex-1 was administered to rats that received dexmedetomidine by intravenous infusion. Dex-2 was administered to rats that received dexmedetomidine in a single dose. #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the Dex-1 group. *p < 0.05, **p < 0.01, and ***p < 0.001 versus the Dex-2 group.
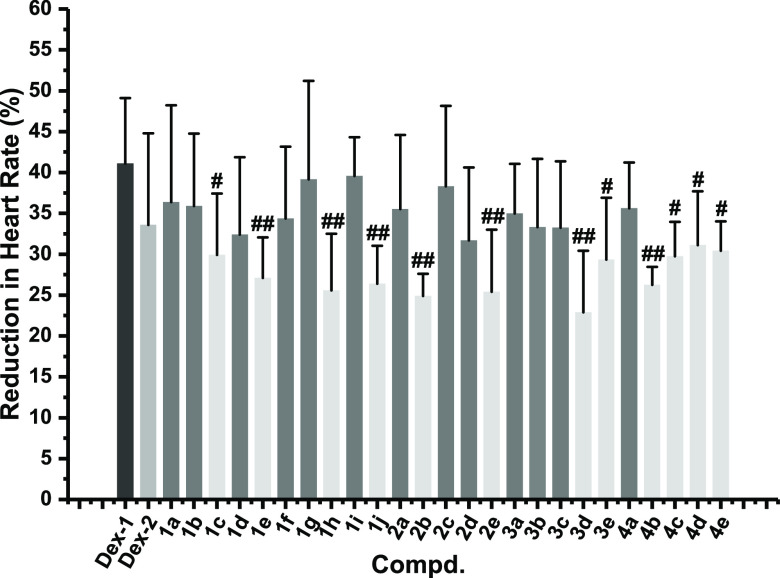
Dexmedetomidine enters the peripheral system to induce transient hypertension and feedback bradycardia. In addition, it enters the central system to inhibit sympathetic activity and reduce heart rate. In this work, some prodrugs in a single dose, such as 1c, 1e, 1h, 1j, 2b, 2e, 3d, 3e, 4b, 4c, 4d, and 4e, possessed better stability on heart rate compared with dexmedetomidine given by infusion (Figure 3 and Table S7). Among them, the bradycardia of the rats in the 2b and 3d groups (−24.88 ± 2.74% and −22.88 ± 7.55%) was substantially improved compared with the dexmedetomidine infusion group (−41.07 ± 8.04%). However, all prodrugs in a single dose did not show significant improvement in bradycardia compared with dexmedetomidine administered by one bolus. Because dexmedetomidine is administered by infusion clinically, the improvement of bradycardia generated by a single administration of prodrug could be beneficial to the clinical application.
Figure 3.
Reduction in heart rate was compared with the baseline of each rat (n = 6). The reduction in heart rate was compared with the baseline before administration and expressed as absolute values of percentage reduction in heart rate. Dex-1 was administered to rats that received dexmedetomidine by intravenous infusion. Dex-2 was administered to rats that received dexmedetomidine in a single administration. #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the Dex-1 group.
Combined with these results of pharmacodynamic experiments, we obtained three preferred prodrugs, compounds 1c, 1e, and 3d (Table 2). With a single injection, they could have a sedative effect within 5 min and would not cause a recovery delay. Compared with dexmedetomidine infusion, they had comparable hemodynamic stability and low heart rate fluctuation.
Table 2. Pharmacodynamic Characteristics of Prodrugs 1c, 1e, and 3d.
| compound | core | R1 | onset time (s) | duration time (s) | mean change in MAPmax (%) | mean change in heart rate (%) |
|---|---|---|---|---|---|---|
| 1c | I | CH2CH2CH3 | 265.00 ± 162.39h | 1941.90 ± 916.50h | 20.07 ± 21.86f | –29.92 ± 7.50c |
| 1e | I | C(CH3)3 | 157.90 ± 64.68g | 3091.80 ± 633.31 | 14.57 ± 6.32h | –27.08 ± 4.96d |
| 3d | II | CH(CH3)2 | 239.30 ± 80.84h | 3984.10 ± 1077.21 | 22.40 ± 9.98g | –22.88 ± 7.55d |
| Dex-1a | 15.54 ± 4.03h | –41.07 ± 8.04 | ||||
| Dex-2b | 87.80 ± 38.08 | 3312.00 ± 556.74 | 43.55 ± 11.32 | –33.58 ± 11.22 |
Rats received dexmedetomidine by intravenous infusion.
Rats received dexmedetomidine in a single administration.
p < 0.05 versus the Dex-1 group.
p < 0.01 versus the Dex-1 group.
p < 0.001 versus the Dex-1 group.
p < 0.05 versus the Dex-2 group.
p < 0.01 versus the Dex-2 group.
p < 0.001 versus the Dex-2 group.
In the present study, four series of dexmedetomidine prodrugs were synthesized, of which the sedative and cardiovascular effects were investigated. We obtained three preferred prodrugs, compounds 1c, 1e, and 3d, which produced a sedative effect with low hemodynamic fluctuation and a simple administration procedure. In particular, they could be administered in a single dose to treat patients with acute delirium who have difficulty accepting long-term infusions.18 Additionally, we found that some prodrugs with a rapid decomposition rate could not improve hemodynamic instability, such as 2d and 2e, which was in accordance with the result of the plasma decomposition assay. However, certain prodrugs that broke down quickly had excellent hemodynamic stability, like 1d. Prodrugs 4d and 4e exhibited slow plasma decomposition rates, but they could still quickly exert sedative effects and fail to mitigate the hemodynamic fluctuation in vivo, thereby indicating that these prodrugs may undergo hepatic metabolism. These above findings suggest that the prodrugs’ structure and release are closely related to their sedative effect and hemodynamic stability in vivo. Their interactions require more thorough pharmacokinetic studies, which will be our key research content in the future.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (No. 82171263).
Glossary
ABBREVIATIONS
- Dex
dexmedetomidine
- ACN
acetonitrile
- DCM
dichloromethane
- THF
tetrahydrofuran
- DMF
N,N-dimethylformamide
- MAP
mean blood pressure
- ED50
median effective dose
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00449.
Details of preparation, characterization, and evaluation experiments in vitro and in vivo of 1a–4e (PDF)
Author Contributions
§ X.L. and J.Z. contributed equally to this work. X.L. carried out the study and wrote the manuscript. J.Z. assisted in the animal experiment. C.Z. conducted the animal experiment. R.L. helped to synthesize compounds. D.G. and Y.K. helped to conduct the analysis experiments. Q.Q. designed the study and analyzed the data. J.Y. designed the study, analyzed the data, and wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Lakhlani P. P.; MacMillan L. B.; Guo T. Z.; McCool B. A.; Lovinger D. M.; Maze M.; Limbird L. E. Substitution of a Mutant Alpha2a-Adrenergic Receptor via ″Hit and Run″ Gene Targeting Reveals the Role of this Subtype in Sedative, Analgesic, and Anesthetic-Sparing Responses in Vivo. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 9950–9955. 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergese S. D.; Khabiri B.; Roberts W. D.; Howie M. B.; McSweeney T. D.; Gerhardt M. A. Dexmedetomidine for Conscious Sedation in Difficult Awake Fiberoptic Intubation Cases. J. Clin Anesth 2007, 19, 141–144. 10.1016/j.jclinane.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Seidel W. F.; Maze M.; Dement W. C.; Edgar D. M. Alpha-2 Adrenergic Modulation of Sleep: Time-of-Day-Dependent Pharmacodynamic Profiles of Dexmedetomidine and Clonidine in the Rat. J. Pharmacol. Exp. Ther. 1995, 275, 263–273. [PubMed] [Google Scholar]
- Venn R. M.; Hell J.; Grounds R. M. Respiratory Effects of Dexmedetomidine in the Surgical Patient Requiring Intensive Care. Crit Care 2000, 4, 302–308. 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn R. M.; Bradshaw C. J.; Spencer R.; Brealey D.; Caudwell E.; Naughton C.; Vedio A.; Singer M.; Feneck R.; Treacher D.; Willatts S. M.; Grounds R. M. Preliminary UK Experience of Dexmedetomidine, a Novel Agent for Postoperative Sedation in the Intensive Care Unit. Anaesthesia 1999, 54, 1136–1142. 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- MacMillan L. B.; Hein L.; Smith M. S.; Piascik M. T.; Limbird L. E. Central Hypotensive Effects of the α2a-Adrenergic Receptor Subtype. Science 1996, 273, 801–803. 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- Ebert T. J.; Hall J. E.; Barney J. A.; Uhrich T. D.; Colinco M. D. The Effects of Increasing Plasma Concentrations of Dexmedetomidine in Humans. Anesthesiology 2000, 93, 382–394. 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- Dyck J. B.; Maze M.; Haack C.; Vuorilehto L.; Shafer S. L. The Pharmacokinetics and Hemodynamic Effect of Intravenous and Intramuscular Dexmedetomidine Hydrochloride in Adult Human Volunteers. Anesthesiology 1993, 78, 813–820. 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- Uusalo P.; Al-Ramahi D.; Tilli I.; Aantaa R. A.; Scheinin M.; Saari T. I. Subcutaneously Administered Dexmedetomidine is Efficiently Absorbed and is Associated with Attenuated Cardiovascular Effects in Healthy Volunteers. Eur. J. Clin. Pharmacol. 2018, 74, 1047–1054. 10.1007/s00228-018-2461-1. [DOI] [PubMed] [Google Scholar]
- Yoo H.; Iirola T.; Vilo S.; Manner T.; Aantaa R.; Lahtinen M.; Scheinin M.; Olkkola K. T.; Jusko W. J. Mechanism-Based Population Pharmacokinetic and Pharmacodynamic Modeling of Intravenous and Intranasal Dexmedetomidine in Healthy Subjects. Eur. J. Clin. Pharmacol. 2015, 71, 1197–1207. 10.1007/s00228-015-1913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iirola T.; Vilo S.; Manner T.; Aantaa R.; Lahtinen M.; Scheinin M.; Olkkola K. T. Bioavailability of Dexmedetomidine after Intranasal Administration. Eur. J. Clin Pharmacol 2011, 67, 825–831. 10.1007/s00228-011-1002-y. [DOI] [PubMed] [Google Scholar]
- Abbott Laboratories . Precedex (Dexmedetomidine Hydrochloride); U.S. Food & Drug, 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-038_Precedex.cfm (accessed 2022-05-26).
- Hospira Inc . Approval Package for: Application Number: 21-038/S017; U.S. Food & Drug, 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/021038Orig1s017.pdf (accessed 2022-05-26).
- Rautio J.; Meanwell N. A.; Di L.; Hageman M. J. The Expanding Role of Prodrugs in Contemporary Drug Design and Development. Nat. Rev. Drug Discovery 2018, 17, 559–587. 10.1038/nrd.2018.46. [DOI] [PubMed] [Google Scholar]
- Rautio J.; Kumpulainen H.; Heimbach T.; Oliyai R.; Oh D.; Jarvinen T.; Savolainen J. Prodrugs: Design and Clinical Applications. Nat. Rev. Drug Discovery 2008, 7, 255–270. 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- Shaw J. P.; Sueoko C. M.; Oliyai R.; Lee W. A.; Arimilli M. N.; Kim C. U.; Cundy K. C. Metabolism and Pharmacokinetics of Novel Oral Prodrugs of 9-[(R)-2-(Phosphonomethoxy) Propyl] Adenine (PMPA) in Dogs. Pharm. Res. 1997, 14, 1824–1829. 10.1023/A:1012108719462. [DOI] [PubMed] [Google Scholar]
- Liu L. Q.; Hong P. X.; Song X. H.; Zhou C. C.; Ling R.; Kang Y.; Qi Q. R.; Yang J. Design, Synthesis, and Activity Study of Water-Soluble, Rapid-Release Propofol Prodrugs. J. Med. Chem. 2020, 63, 7857–7866. 10.1021/acs.jmedchem.0c00698. [DOI] [PubMed] [Google Scholar]
- Jooste E. H.; Muhly W. T.; Ibinson J. W.; Suresh T.; Damian D.; Phadke A.; Callahan P.; Miller S.; Feingold B.; Lichtenstein S. E.; Cain J. G.; Chrysostomou C.; Davis P. J. Acute Hemodynamic Changes after Rapid Intravenous Bolus Dosing of Dexmedetomidine in Pediatric Heart Transplant Patients Undergoing Routine Cardiac Catheterization. Anesth. Analg. 2010, 111, 1490–1496. 10.1213/ANE.0b013e3181f7e2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.