Abstract
Recent evidence has emerged concerning delayed cutaneous hypersensitivity reactions after infliximab or adalimumab applications in patients with coronavirus disease 2019 (COVID‐19). A few real‐world studies compared the events, clinical features, and prognosis of infliximab‐ or adalimumab‐related delayed cutaneous hypersensitivity reactions in COVID‐19 patients. Disproportionality analysis and Bayesian analysis were utilized to determine the suspected adverse events of delayed cutaneous hypersensitivity reactions after infliximab or adalimumab use based on the Food and Drug Administration's Adverse Event Reporting Systems (FAERS) from May 2020 to December 2021. Additionally, the times to onset and fatality rates of delayed cutaneous hypersensitivity reactions following infliximab or adalimumab were compared. In total, 475 reports of delayed cutaneous hypersensitivity reactions were associated with infliximab or adalimumab. Females were affected almost twice more than males. Among the two therapies, infliximab had the highest association with delayed cutaneous hypersensitivity reactions based on the highest reporting odds ratio (2.14, 95% two‐sided confidence interval [CI] = 1.2–3.81), proportional reporting ratio (1.95, χ 2 = 7.03), and empirical Bayesian geometric mean (1.94, 95% one‐sided CI = 1.2). Infliximab‐related delayed cutaneous hypersensitivity reactions had earlier onset (0 [interquartile range (IQR): 0–0] days vs. 166.5 (IQR: 18–889.5) days, p < 0.05), while adalimumab‐related delayed cutaneous hypersensitivity reactions have higher fatality rate (0.44% vs. 0.00%). Based on the FAERS database, we profiled delayed cutaneous hypersensitivity reactions related to infliximab or adalimumab application in patients with COVID‐19 with more points of occurrences, clinical characteristics, and prognosis.
Keywords: adalimumab, coronavirus disease 2019, delayed cutaneous hypersensitivity reactions, Food and Drug Administration's Adverse Event Reporting Systems, infliximab
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a viral replication disorder associated with a host inflammatory response. 1 A worldwide effort has been made to develop specific severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antiviral drugs to reduce mortality and constrain COVID‐19‐related damage although the pathogenesis of COVID‐19 remains unknown. 2 From a pathogenesis standpoint, COVID‐19 infection generally elicits a vigorous immune response that is important for viral clearance, involving both innate and adaptive immunities. 1 Thus, addressing the immune response with immunomodulatory agents may be an important avenue for investigation. Immune modulators potentially inhibit cytokines and treat cytokine storms. 3 Some specialists reported associations between severe COVID‐19 cases and increased tumor necrosis factor (TNF)‐α levels. 4 Anti‐TNF‐α therapy is an established therapy for several rheumatological clutters, which was proposed as a conceivable COVID‐19 treatment. 5 The use of currently available therapeutic agents (including monoclonal antibodies) was applied to novel indications (such as the cytokine storm treatment during COVID‐19), including a chimeric monoclonal antibody (infliximab), two human monoclonal antibodies (adalimumab and golimumab), a fragment of a human monoclonal antibody (certolizumab pegol), and a human fusion protein (etanercept). 6 However, among the potential therapeutic medicine to reduce COVID‐19 inflammation, such as monoclonal antibodies, peptides, vaccines, small‐molecule drugs, and interferon, 7 only a few drugs, such as anti‐TNF‐α therapy (infliximab and adalimumab), Janus kinase inhibitors (baricitinib) 8 are possibly compelling and widely available, with a complete safety profile. 9
TNF‐α is a cytokine that plays a key role in nearly all intense inflammatory responses, as a particular immune modulator, by inducing oxidative stress and inflammation. 10 Anti‐TNF‐α therapy has been administered for >20 years in severe immune‐mediated inflammatory disorders. 6 Immune reaction enhancement is accompanied by unique or even unknown side effects due to its unique mechanism of action. Infliximab and adalimumab are TNF‐α inhibitors that are currently food and drug administration (FDA)‐approved for the treatment of psoriasis, rheumatoid arthritis, inflammatory bowel disease, or ankylosing spondylitis. The two are slightly different in structure. Infliximab is a chimeric human/murine immunoglobulin (Ig)G1 monoclonal antibody that actuates the human anti‐infliximab antibody generation. 11 Adalimumab is a recombinant fully human IgG1 monoclonal antibody that inhibits the interaction of this inflammatory cytokine with TNF receptors on the p55 and p75 cell surface by binding to TNF‐α, 12 adalimumab was reported to suppress C‐reactive protein, interleukin (IL)‐6, and matrix metalloproteinases (MMP‐1 and MMP‐3) by tissue remodeling and matrix destruction. 13 Available data and evidence revealed that adalimumab has important restorative therapeutic potential in terms of overseeing COVID‐19 pneumonia. A previous study reported that reducing MMP activities can effectively control COVID‐19‐related tissue damage. 14 Their effectiveness, wide accessibility, various dosage forms, and security profile make anti‐TNF‐α antibodies a promising therapeutic choice to relieve COVID‐19 inflammation. 12 The clinical and cytokine information demonstrates that infliximab may rapidly abrogate pathological inflammatory signaling to facilitate clinical recuperation in severe and critical COVID‐19. A variety of accompanying symptoms may occur after the corresponding treatment, such as excessive immune response phenomenon, mild epidermal damage, eczema, and so forth, although the use of these drugs is generally considered to have few side effects with tolerability. 15 The present study revealed some cases of delayed cutaneous hypersensitivity reactions in patients with COVID‐19 treated with adalimumab and infliximab, including focal vacuolar alteration at the dermal–epidermal junction, a diffuse, scaly erythematous eruption with morbilliform and pityriasis‐rosea‐like features, pruritic pityriasis rosea‐like eruption, rash, urticarial lesions, and so forth.
The FDA label for infliximab and adalimumab described several risks and adverse reactions. 15 , 16 , 17 Notably, infliximab or adalimumab was associated with a heterogeneous range of cutaneous and extracutaneous adverse events. Cutaneous antagonistic occasions may develop in some patients treated with infliximab and adalimumab, with xerosis cutis, eczema, psoriasis, palmoplantar pustulosis, cutaneous infections, alopecia, and skin cancer as most habitually encountered, typically months to years after the treatment initiation. 18
DAEs are a current clinical concern, and knowledge of skin security profiles after infliximab or adalimumab use in patients with COVID‐19 remains limited in real‐world clinical practice. The safety evidence of these drugs primarily comes from the drug inserts, and their safety differences in the real world remain unknown. Therefore, this study aimed to assess and compare the relationship between infliximab or adalimumab and cutaneous allergic reactions in an expansive population by investigating the FDA Adverse Event Reporting System (FAERS) until recently, to supply a reference for clinicians to keep pace with the new data and identify and process them in early clinical stage. Further, this study examined the time to onset and fatality and hospitalization rates for delayed cutaneous hypersensitivity reactions of infliximab or adalimumab. Data mining techniques were used in this study to identify and analyze the signals of postmarketing adverse event reports in the FAERS and distinguish potential high‐risk safety.
2. MATERIALS AND METHODS
2.1. Data source
Publicly available data from the FAERS database from May 2020 to December 2021 were retrospectively analyzed. This study selected the most recent data available because adalimumab and infliximab were introduced for COVID‐19 treatment in February 2020. The duplicate records were removed according to FDA's recommendations, the latest FDA_DT was selected when the case identification (ID) was the same, and the higher primary ID was selected when the case ID and FDA_DT were the same, thereby reducing the number to 3548 (Figure 1). Finally, 14 cases of delayed cutaneous hypersensitivity reactions caused by infliximab as a suspected drug were identified, as well as 461 cases caused by adalimumab therapy. This study determined the number of reported adverse drug events (ADEs) described as delayed cutaneous hypersensitivity reactions each year, as well as medication (drugs) and administration information (demonstrations). The distribution of serious ADE reports was determined by demographic patient outcomes (OUTC) and demography (DEMO). This study identified and sequenced the source (consumer, medical professional, or others) of these serious ADE reports, OUTC, and the main suspect drug causing serious ADE (death, disability, and other serious consequences).
Figure 1.
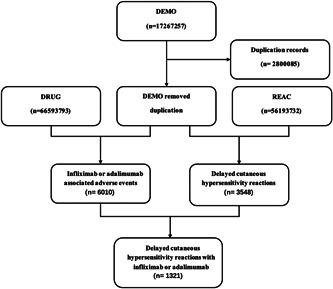
Flow diagram of the study population.
2.2. Adverse event and drug ID
Delayed cutaneous hypersensitivity reactions are based on the Preferred Term of the Medical Dictionary of Regulatory Activities (MedDRA, version 24.0), and this study focused on adverse effects following the use of monoclonal antibodies for COVID‐19 treatment. This study examined infliximab and adalimumab according to the COVID‐19 treatment guidelines and hypothesized that delayed cutaneous hypersensitivity reactions was associated with these therapies, using the terminology in the REAC document “DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS, DRESS SYNDROME, HYPERSENSITIVE SYNDROME, HYPERSENSITIVITY SYNDROME, DAPSONE SYNDROME, DRUG‐INDUCED HYPERSENSITIVITY SYNDROME, DRUG RASH WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS, WILSON‐BROCQ'S DISEASE, ERYTHRODERMA, GENERALIZED EXFOLIATIVE DERMATITIS, SUBACUTE GENERALIZED EXFOLIATIVE DERMATITIS, CHRONIC EXFOLIATIVE DERMATITIS, DERMATITIS EXFOLIATIVE GENERALIZED, GENERALIZED EXFOLIATIVE DERMATITIS, CHRONIC GENERALIZED EXFOLIATIVE DERMATITIS, SUBACUTE GENERALIZED EXFOLIATIVE DERMATITIS, LYELL SYNDROME, LYELL'S SYNDROME, NECROLYSIS EPIDERMAL, TEN, DRUG ERUPTION LYELL SYNDROME TYPE, LYELL'S DISEASE, EPIDERMAL NECROLYSIS, NECROLYSIS EPIDERMAL TOXIC (LYELL TYPE), SYNDROME STEVENS JOHNSON, STEVENS JOHNSON SYNDROME, TOXIC EPITHELIOLYSIS, STEVENS JOHNSON REACTION, PHOTOINDUCED STEVENS JOHNSON SYNDROME, STEVENS JOHNSON TYPE REACTION, TOXIC RESPIRATORY EPITHELIOLYSIS.” Micromedex® (Index Nominum) was used as a dictionary during the data mining to select generic and brand names of infliximab and adalimumab.
2.3. Data mining
Disproportionality and Bayesian analyses were used for statistical analysis to identify the association between infliximab or adalimumab and adverse events. This study applied the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network, and the multi‐item gamma Poisson shrinker algorithms to analyze the four adverse events that were extracted when at least one of the metrics met the criteria.
Moreover, the time to onset of delayed cutaneous hypersensitivity with infliximab or adalimumab in COVID‐19 treatment was estimated; the time interval between the date of onset of the adverse event and the date of drug initiation was defined; and erroneously entered (Reports with EVENT_DT earlier than START_DT) or inaccurate date entries were excluded. Additionally, this study analyzed the reports of fatal events in patients with COVID‐19 due to delayed cutaneous hypersensitivity, which were calculated by dividing the number of fatal events by the total number of infliximab‐ or adalimumab‐related delayed cutaneous hypersensitivity events.
2.4. Statistical analysis
Clinical characteristics of patients with delayed cutaneous hypersensitivity associated with the drugs (infliximab or adalimumab) for COVID‐19 were summarized using descriptive analysis; these data were collected from the FAERS database. The time to onset of infliximab‐ and adalimumab‐associated delayed cutaneous hypersensitivity were compared using a nonparametric test (Mann–Whitney U test). Pearson's χ 2 test or Fisher's exact test was used to compare hospitalization and fatality rates due to infliximab‐ or adalimumab‐associated delayed cutaneous hypersensitivity in patients with COVID‐19. Statistical significance was determined with 95% confidence intervals (CI) at p < 0.05. All data mining and statistical analyses were performed using GraphPad Prism 9 (GraphPad Software).
3. RESULTS
3.1. Descriptive analysis
This study retrospectively analyzed 3548 adverse events related to delayed cutaneous hypersensitivity in monoclonal antibody utilization for COVID‐19 treatment in the FAERS database from May 2020 to December 2021. Additionally, 14 reports with suspected infliximab‐related delayed cutaneous hypersensitivity were included, as well as 461 reports with adalimumab‐related delayed cutaneous hypersensitivity. Table 1 summarizes the clinical features of these cases which are concentrated in 2021. Of the adverse reactions related to delayed cutaneous hypersensitivity reported by these two monoclonal antibodies (infliximab and adalimumab) for COVID‐19 treatment, >90% were from patients in North America, including infliximab in 92.86% and adalimumab in 96.1%, followed by 3.69% of cases on adalimumab‐related delayed cutaneous hypersensitivity from South America and 0.21% from Europe. Moreover, 7.14% of infliximab‐related delayed cutaneous hypersensitivity cases were reported from Europe, excluding unknown data. Additionally, adalimumab has more reports of delayed cutaneous hypersensitivity than infliximab, mainly among 18–85 years old and most common 45–64 years old (23.86%), whereas infliximab is noted in 65–74 years old (7.14%). Further, the adverse reactions of the two drugs were almost twice higher in females than in males (infliximab‐female vs. adalimumab‐male, 14.29% vs. 7.14%, p < 0.05 adalimumab‐female vs. adalimumab‐male, 64.43% vs. 35.14%, p < 0.05). Adalimumab‐related cases were mainly uploaded by consumers (96.53%), and infliximab‐related cases were uploaded by consumers (21.42%) and physicians (14.29%). This may be because adalimumab is usually self‐administered while infliximab is a hospital‐based therapy.
Table 1.
Clinical characteristics of patients with infliximab or adalimumab‐associated delayed cutaneous hypersensitivity in patients with COVID‐19 collected from the FAERS database.
| Reports (N, %) | ||
|---|---|---|
| Characteristics | Infliximab | Adalimumab |
| Patient age (year) | ||
| <18 | 0 (0.00) | 0 (0.00) |
| 18–44 | 0 (0.00) | 37 (8.02) |
| 45–64 | 0 (0.00) | 110 (23.86) |
| 65–74 | 1 (7.14) | 98 (21.26) |
| 75–84 | 0 (0.00) | 21 (4.56) |
| ≥85 | 0 (0.00) | 3 (0.65) |
| Unknown | 13 (92.86) | 192 (41.65) |
| Patient gender | ||
| Female | 2 (14.29) | 297 (64.43) |
| Male | 1 (7.14) | 162 (35.14) |
| Unknown | 11 (78.57) | 2 (0.43) |
| Year | ||
| 2020 | 0 (0.00) | 0 (0.00) |
| 2021 | 14 (100.00) | 461 (100.00) |
| Area | ||
| Africa | 0 (0.00) | 0 (0.00) |
| Asian | 0 (0.00) | 0 (0.00) |
| Europe | 1 (7.14) | 1 (0.22) |
| North America | 13 (92.86) | 443 (96.10) |
| Oceania | 0 (0.00) | 0 (0.00) |
| South America | 0 (0.00) | 17 (3.69) |
| Unknown | 0 (0.00) | 0 (0.00) |
| Reporters | ||
| Consumer | 3 (21.43) | 445 (96.53) |
| Pharmacist | 0 (0.00) | 0 (0.00) |
| Physician | 2 (14.29) | 7 (1.52) |
| Unknown | 9 (64.29) | 9 (1.95) |
| Mean weight | ||
| Weight (kg) | 101.00 | 83.93 |
Abbreviations: COVID‐19, coronavirus disease 2019; FEARS, Food and Drug Administration's Adverse Event Reporting Systems.
3.2. Disproportionality analysis and Bayesian analysis
This study focused on infliximab or adalimumab following the COVID‐19 treatment. The two COVID‐19 treatment drugs were detected to relate to atopic dermatitis based on the four algorithm criteria. Table 2 shows the results. Notably, infliximab was more strongly associated with delayed cutaneous hypersensitivity than adalimumab due to its higher ROR, PRR, and empirical Bayesian geometric mean (EBGM). Additionally, the association of adalimumab with delayed cutaneous hypersensitivity was deemed statistically significant.
Table 2.
Disproportionality analysis and Bayesian analysis.
| N | ROR | PRR | IC | EBGM | |
|---|---|---|---|---|---|
| Drug | (95% two‐sided CI) | (χ 2) | (IC025) | (EBGM05) | |
| Infliximab | 14 | 2.14 (1.2, 3.81) | 1.95 (7.03) | 0.96 (0.54) | 1.94 (1.2) |
| Adalimumab | ABC | 1.66 (1.49, 1.84) | 1.57 (91.54) | 0.58 (0.52) | 1.5 (1.37) |
Abbreviations: CI, confidence interval; EBGM, empirical Bayesian geometric mean; PRR, proportional reporting ratio; ROR, reporting odds ratio.
3.3. Time to onset of infliximab‐ or adalimumab‐associated delayed cutaneous hypersensitivity
Figure 2 described the time to onset of delayed cutaneous hypersensitivity following infliximab or adalimumab therapy initiation for COVID‐19. Data revealed that infliximab‐related delayed cutaneous hypersensitivity accounted for 83.33% in 0–30 days and 16.67% in 180–210 days, and adalimumab‐related delayed cutaneous hypersensitivity accounted for 31.82% in 0–30 days, and the others were evenly reported in the remaining stages, with a median of 166.5 days (interquartile range [IQR]: 18–889.5). Adalimumab at 40 mg was given as a single subcutaneous injection 19 and infliximab at 5/mg/kg body weight was given as a single subcutaneous injection according to the usual regimen of drug treatment. 20 Notably, delayed cutaneous hypersensitivity may occur after the first infliximab dosage for COVID‐19 treatment, whereas adalimumab showed a small peak after the first dose and more extensive distribution, which may be related to the different drug structure. A statistically significant difference was found in the time to onset associated with delayed cutaneous hypersensitivity for COVID‐19 treatment in infliximab or adalimumab (Mann–Whitney U test, p < 0.05).
Figure 2.
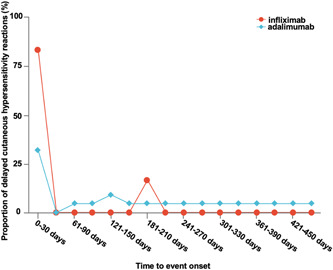
Time to onset of delayed cutaneous hypersensitivity following infliximab or adalimumab therapy.
3.4. Fatality due to infliximab‐ or adalimumab‐associated delayed cutaneous hypersensitivity
This study assessed the fatality rate due to toxic dermatitis after infliximab or adalimumab regimens to determine the prognosis of toxic dermatitis after using infliximab or adalimumab in COVID‐19 treatment. Figure 3 shows the results. The two‐drug regimens revealed few adverse outcomes, with a 0.04% of death for adalimumab‐related delayed cutaneous hypersensitivity. No significant difference was found between adalimumab‐ or infliximab‐related delayed cutaneous hypersensitivity mortality rates.
Figure 3.
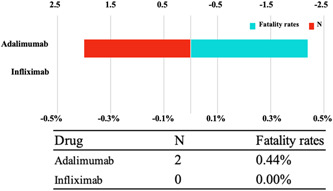
The number of reports and fatality rate for delayed cutaneous hypersensitivity following infliximab or adalimumab therapy.
4. DISCUSSION
This study compared the associations, timing, and prognosis of delayed cutaneous hypersensitivity in patients with COVID‐19 after using infliximab or adalimumab in real‐world practice based on the FAERS pharmacovigilance database. Study findings revealed the association between infliximab and adalimumab and the adverse event of delayed cutaneous hypersensitivity. Moreover, the diverse characteristics across the therapy were determined.
This study based on the FAERS database revealed the generally incremental reports of delayed cutaneous hypersensitivity in patients with COVID‐19 over time, especially in 2021. Additionally, data indicated that infliximab‐ or adalimumab‐associated delayed cutaneous hypersensitivity affected females twice more than males because the higher risk of autoimmune diseases that females carry should be noted. 21 Infliximab and adalimumab demonstrate efficacy in inducing remission and maintaining numerous chronic inflammatory conditions. Immune‐related adverse reactions of delayed cutaneous hypersensitivity 22 , 23 colitis, pneumonitis, hepatitis, and endocrinopathies will occur with the increasing number of patients receiving infliximab or adalimumab. Hence, the physician must consider these potential problems. Cutaneous eruptions complicating treatment with infliximab or adalimumab are common. Additionally, the interference with the physiological function of the target protein may lead to loss of peripheral tolerance and a subsequent unleashing of the immune system on immune cells, leading to unintended tissue damage, although with desirable antivirus effects of immunotherapies. 24 This tissue damage can affect nearly every organ system, mostly the dermatologic, gastrointestinal, endocrine, and pulmonary systems. The binding of SARS‐CoV‐2 to angiotensin‐converting enzyme‐2 induces TNF‐α‐converting enzyme‐dependent shedding of the angiotensin‐converting enzyme‐2 ectodomain, which then facilitates viral entry and causes tissue damage through TNF‐α production. 25
This study compared the association of delayed cutaneous hypersensitivity among infliximab or adalimumab application in patients with COVID‐19. The results revealed higher hazard ratio (ROR = 2.14, 95% two‐sided CI = 1.2–3.81), PRR (PRR = 1.95, χ 2 = 7.03), and EBGM (EBGM = 1.94, 95% one‐sided CI = 1.2) of delayed cutaneous hypersensitivity in infliximab than in adalimumab. Infliximab, as a monotherapy, is the most immunogenic of the two drugs, confirming that chimeric antibodies are generally more immunogenic than humanized or human antibodies. 26 Some scientists started using infliximab in large quantities to treat immune diseases in the early 2000s and noticed significant skin‐related side effects in a large number of patients. 27 Additionally, previous studies revealed that TNF‐α inhibition induces cutaneous IFN‐α overexpression, which results in desquamative skin reactions. 28 , 29 TNF‐α antagonists induce cytokine imbalance, and excessive inhibition of TNF‐α, particularly in predisposed subjects, may induce a locally increased IFN‐α and T‐cell migration via IL‐15 by the skin. 30 , 31 Additionally, the present study indicated that infliximab‐ or adalimumab‐related delayed cutaneous hypersensitivity was mainly detected in middle‐aged and elderly patients. The use of infliximab revealed a significance in age difference at onset (50.33% in ≥45 years vs. 8.02% in <45 years). Moreover, the use of adalimumab has an obvious difference (7.14% in ≥45 years vs. 0.00% in <45 years). Unfortunately, age information for most patients is unavailable. Hence, controlling confounding factors, such as age, is difficult in this study because of the FAERS database limitation. Therefore, the association of these characteristics of infliximab and adalimumab with delayed cutaneous hypersensitivity in patients with COVID‐19 needs further confirmation.
Moreover, the median time to onset of delayed cutaneous hypersensitivity effects is 0 (IQR: 0–0) and 166.5 (IQR: 18–889.5) days in infliximab and adalimumab, respectively. The varied predominance in the average onset times among the use of infliximab and adalimumab suggests that individualized monitoring strategies can be performed after the therapy administration. For example, skin reactions should be considered shortly after applying infliximab, while the necessity of long‐term adalimumab usage should be regularly evaluated to avoid possible harm. However, delayed cutaneous hypersensitivity associated with infliximab and adalimumab has similar severity. Delayed cutaneous hypersensitivity had no poor outcomes, and only approximately a few cases (0.00%–0.44%) resulted in death between the two therapies.
Infliximab and adalimumab have valuable therapeutic potential in managing COVID‐19 pneumonia according to the existing data and evidence, but patients with unfortunate outcomes remained. 19 , 32 Moreover, evaluating delayed cutaneous hypersensitivity in patients with COVID‐19 on infliximab or adalimumab is challenging, unusual, and mostly nonspecific, and thus easily overlooked. 33 , 34 , 35 Data on the use of adalimumab and infliximab in children with COVID‐19 are lacking in the present data; however, Giulia D'Arcangelo et al. collected 209 suspected and confirmed COVID‐19 cases and included 185 children, of which 149 received infliximab and 88 received adalimumab, and 4 of them were COVID‐19 cases with a very mild course. 36 , 37 Additionally, delayed cutaneous hypersensitivity related to adalimumab and infliximab treatment was reported in children. In addition, pediatricians should consider whether skin allergies in a child with COVID‐19 could have been caused by treatment with adalimumab or infliximab. Recent studies revealed that many patients who receive the COVID‐19 vaccine develop delayed skin hypersensitivity reactions, 38 such as those after adalimumab and infliximab treatment, which are difficult to distinguish. The present data on adverse effects of adalimumab and infliximab in patients with COVID‐19 alone are a good reminder to patients who receive both mabs and vaccines that their adverse effects are caused by the vaccine, the mabs, or both. Finally, this study aimed to provide a reference for physicians and patients by assessing the adverse effects of COVID‐19 medications based on the data from the FAERS database.
5. CONCLUSION
This present study identified signals for delayed cutaneous hypersensitivity after infliximab or adalimumab use with COVID‐19 in real‐world practice based on the FAERS database. This study revealed the association between infliximab and adalimumab and delayed cutaneous hypersensitivity in patients with COVID‐19. Moreover, a significant difference in the time of delayed cutaneous hypersensitivity onset after infliximab and adalimumab is notable; thus, awareness should be raised for some immediate occurrences after the initial administration of infliximab or adalimumab. Additionally, female patients with COVID‐19 may be more sensitive to infliximab‐ or adalimumab‐associated delayed cutaneous hypersensitivity. Therefore, caution must be considered, and the adverse reactions must be distinguished as induced using a monoclonal antibody for COVID‐19, which is conducive to rational drug use and treatment. The study results are useful for future pharmacovigilance research on children, and other studies are encouraged to explore the hypotheses derived from this study.
AUTHOR CONTRIBUTIONS
Xiaoping Jing, Jing Wang, Zhiling Li, and Bin Zhao contributed to study concept and design. Jing Wang, Bin Zhao, Xuedong Yin, Linlin Yu, Weiwei Cheng, and Ling Wang performed the data collection. Xiaoping Jing, Zhiling Li, and Bin Zhao conducted data analysis. Jing Wang, Xuedong Yin, and Linlin Yu helped to analyze the data. All authors contributed to the article and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
National Natural Science Foundation of China (No. 81674020). TCM guidance project of Shanghai Science and Technology Commission (No. 21Y11921700). Natural Science Foundation of Shanghai Children's Hospital (No. 2021YGZM01).
Wang J, Yin X, Yu L, et al. Delayed cutaneous hypersensitivity reactions following the use of infliximab or adalimumab in patients with coronavirus disease 2019. J Med Virol. 2023;95:e28518. 10.1002/jmv.28518
Jing Wang, Xuedong Yin, and Linlin Yu contributed equally to this work.
Contributor Information
Bin Zhao, Email: zhaobin@pumch.cn.
Zhiling Li, Email: lizhiling22@163.com.
Xiaoping Jing, Email: xiaopingjdoctor@126.com.
DATA AVAILABILITY STATEMENT
The data in present study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8):782‐793. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2. Dhama K, Khan S, Tiwari R, et al. Coronavirus disease 2019‐COVID‐19. Clin Microbiol Rev. 2020;33(4):e00028‐20. 10.1128/cmr.00028-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramasamy S, Subbian S. Critical determinants of cytokine storm and type I interferon response in COVID‐19 pathogenesis. Clin Microbiol Rev. 2021;34(3):e00299‐20. 10.1128/cmr.00299-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X‐Y, Yan B‐X, Man X‐Y. TNFα inhibitor may be effective for severe COVID‐19: learning from toxic epidermal necrolysis. Therapeutic Advances in Respiratory Disease. 2020;14:175346662092680. 10.1177/1753466620926800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neurath MF. COVID‐19: biologic and immunosuppressive therapy in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2021;18(10):705‐715. 10.1038/s41575-021-00480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13(4):217‐233. 10.1038/nrrheum.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahimkhoei V, Jabbari N, Nourani A, Sharifi S, Akbari A. Potential small‐molecule drugs as available weapons to fight novel coronavirus (2019‐nCoV): a review. Cell Biochem Funct. 2021;39(1):4‐9. 10.1002/cbf.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kramer A, Prinz C, Fichtner F, et al. Janus kinase inhibitors for the treatment of COVID‐19. Cochrane Database Syst Rev. 2022;2022:Cd015209. 10.1002/14651858.Cd015209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ablamunits V, Lepsy C. Blocking TNF signaling may save lives in COVID‐19 infection. Mol Biol Rep. 2022;49(3):2303‐2309. 10.1007/s11033-022-07166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26(4):249‐261. 10.1016/j.tcb.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 11. Murdaca G, Spanò F, Contatore M, et al. Immunogenicity of infliximab and adalimumab: what is its role in hypersensitivity and modulation of therapeutic efficacy and safety? Expert Opin Drug Saf. 2016;15(1):43‐52. 10.1517/14740338.2016.1112375 [DOI] [PubMed] [Google Scholar]
- 12. Feldmann M, Maini RN, Woody JN, et al. Trials of anti‐tumour necrosis factor therapy for COVID‐19 are urgently needed. Lancet. 2020;395(10234):1407‐1409. 10.1016/S0140-6736(20)30858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collange O, Tacquard C, Delabranche X, et al. Coronavirus disease 2019: associated multiple organ damage. Open Forum Infect Dis. 2020;7(7):ofaa249. 10.1093/ofid/ofaa249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solun B, Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID‐19. Med Drug Discov. 2020;7:100052. 10.1016/j.medidd.2020.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Haens GR, van Deventer S. 25 years of anti‐TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut. 2021;70(7):1396‐1405. 10.1136/gutjnl-2019-320022 [DOI] [PubMed] [Google Scholar]
- 16. Kuehn BM. Severe fungal infections linked to drugs. JAMA. 2008;300(14):1639. 10.1001/jama.300.14.1639 [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Tuberculosis associated with blocking agents against tumor necrosis factor—alpha—California, 2002‐2003. MMWR Morb Mortal Wkly Rep. 2004;53(30):683‐686. [PubMed] [Google Scholar]
- 18. Segaert S, Hermans C. Clinical signs, pathophysiology and management of cutaneous side effects of anti‐tumor necrosis factor agents. Am J Clin Dermatol. 2017;18(6):771‐787. 10.1007/s40257-017-0296-7 [DOI] [PubMed] [Google Scholar]
- 19. Fakharian A, Barati S, Mirenayat M, et al. Evaluation of adalimumab effects in managing severe cases of COVID‐19: a randomized controlled trial. Int Immunopharmacol. 2021;99:107961. 10.1016/j.intimp.2021.107961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coldewey SM, Neu C, Bloos F, et al. Infliximab in the treatment of patients with severe COVID‐19 (INFLIXCOVID): protocol for a randomised, controlled, multicentre, open‐label phase II clinical study. Trials. 2022;23(1):737. 10.1186/s13063-022-06566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallis CJD, Butaney M, Satkunasivam R, et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta‐analysis. JAMA Oncology. 2019;5(4):529‐536. 10.1001/jamaoncol.2018.5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blaszczak A, Trinidad JCL, Cartron AM. Adalimumab for treatment of hidradenitis suppurativa during the COVID‐19 pandemic: safety considerations. J Am Acad Dermatol. 2020;83(1):e31. 10.1016/j.jaad.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fisher BA, Veenith T, Slade D, et al. Namilumab or infliximab compared with standard of care in hospitalised patients with COVID‐19 (CATALYST): a randomised, multicentre, multi‐arm, multistage, open‐label, adaptive, phase 2, proof‐of‐concept trial. Lancet Respirator Med. 2022;10(3):255‐266. 10.1016/s2213-26002100460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(9):1425‐1433. 10.1093/annonc/mdi279 [DOI] [PubMed] [Google Scholar]
- 25. Haga S, Yamamoto N, Nakai‐Murakami C, et al. Modulation of TNF‐α‐converting enzyme by the spike protein of SARS‐CoV and ACE2 induces TNF‐α production and facilitates viral entry. Proc Natl Acad Sci. 2008;105(22):7809‐7814. 10.1073/pnas.0711241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36(1):3‐10. 10.1016/j.ymeth.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 27. Fidder H, Schnitzler F, Ferrante M, et al. Long‐term safety of infliximab for the treatment of inflammatory bowel disease: a single‐centre cohort study. Gut. 2009;58(4):501‐508. 10.1136/gut.2008.163642 [DOI] [PubMed] [Google Scholar]
- 28. Nestle FO, Conrad C, Tun‐Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon‐α production. J Exp Med. 2005;202(1):135‐143. 10.1084/jem.20050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59(7):996‐1001. 10.1002/art.23835 [DOI] [PubMed] [Google Scholar]
- 30. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244‐279. 10.1016/j.pharmthera.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 31. Fiorino G, Allez M, Malesci A, Danese S. Review article: anti TNF‐α induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29(9):921‐927. 10.1111/j.1365-2036.2009.03955.x [DOI] [PubMed] [Google Scholar]
- 32. Bezzio C, Manes G, Bini F, Pellegrini L, Saibeni S. Infliximab for severe ulcerative colitis and subsequent SARS‐CoV‐2 pneumonia: a stone for two birds. Gut. 2021;70(3):623‐624. 10.1136/gutjnl-2020-321760 [DOI] [PubMed] [Google Scholar]
- 33. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gisondi P, PIaserico S, Bordin C, Alaibac M, Girolomoni G, Naldi L. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34(11):2499‐2504. 10.1111/jdv.16774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson Regnault M. Comment on ‘Cutaneous manifestations in COVID‐19: a first perspective' by Recalcati S. J Eur Acad Dermatol Venereol. 2020;34(7):e299‐e300. 10.1111/jdv.16519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D'Arcangelo G, Distante M, Raso T, Rossetti D, Catassi G, Aloi M. Safety of biological therapy in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2021;72(5):736‐741. 10.1097/mpg.0000000000003044 [DOI] [PubMed] [Google Scholar]
- 37. Sun Q, Fathy R, McMahon DE, Freeman EE. COVID‐19 vaccines and the skin. Dermatol Clin. 2021;39(4):653‐673. 10.1016/j.det.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID‐19 vaccination. J Cutan Pathol. 2022;49:34‐41. 10.1111/cup.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data in present study are available from the corresponding author upon reasonable request.


