Abstract
Background
Currently, the proportion of standard chemotherapy for elderly patients is much lower than that for young patients, with little evidence from clinical trials supporting the use of chemotherapy for elderly patients. The effectiveness of chemotherapy for the elderly suffering from breast cancer remains to be further verified.
Methods
A total of 75,525 female breast cancer patients aged 70 years or older were hereby identified, all from the Surveillance, Epidemiology, and End Results (SEER) database from January 1, 2010 to December 31, 2016. Kaplan–Meier analysis and multivariable Cox proportional model were performed to evaluate the effectiveness of chemotherapy on overall survival (OS) and breast cancer‐specific survival (BCSS). Propensity score matching (PSM) (PSM ratio: 1:1, caliper: 0.2 standard deviation of propensity score) was applied to construct balanced cohorts with or without chemotherapy based on demographic and pathophysiological characteristics.
Results
A total of 33,177 eligible patients were included, with 5273 (15.89%) receiving chemotherapy. Through PSM, 8360 patients were successfully matched, and balances between groups were almost reached. In the matched data set, multivariable Cox analysis reveals that chemotherapy was associated with a 36% and 21% risk reduction on OS (HR = 0.64, 95% CI 0.58 to 0.71) and BCSS (HR = 0.79, 95% CI 0.69 to 0.91), respectively. Furthermore, subgroups with more adjacent lymph nodes involved by tumor, or nonluminal A, were inclined to benefit more from chemotherapy. Moreover, chemotherapy did not increase the chances of dying from heart disease.
Conclusions
The present study provided evidence that chemotherapy may improve the prognosis of elderly breast cancer, especially for those subpopulations that benefit more from chemotherapy treatment.
Keywords: aged, breast cancer, chemotherapy, survival analysis
1. INTRODUCTION
Breast cancer has become the largest cancer type worldwide. 1 Its incidence rates increase with age, with over 30% of cases diagnosed in women aged over 70 years. 2 In 2019, the population of new‐onset breast cancer patients older than 70 years was estimated to be over 80,000. 3 The proportion and number of elderly patients with breast cancer is expected to increase with the extension of human life expectancy continuously. However, the treatment for this group of elderly patients has not reached a consensus, and there is limited evidence supporting chemotherapy recommendations.
Currently, the proportion of elderly patients receiving standard chemotherapy is lower than that of younger patients, 4 , 5 , 6 , 7 , 8 though adjuvant chemotherapy has been proven effective in reducing the annual breast cancer death rate by about 38% in patients under 70 years old. 9 Considering ethical restrictions, there is still a lack of prospective clinical trials on chemotherapy for elderly breast cancer and sound evidence for making definitive chemotherapy recommendations. Meanwhile, given that this population has many indolent tumor characteristics, 10 short life expectancy, and many comorbidities that could lead to increased toxicities and reduced tolerance, 11 , 12 , 13 , 14 the benefits of chemotherapy as a treatment approach for elderly breast cancer have not been fully acknowledged. 15 , 16 Therefore, current guidelines, such as the National Comprehensive Cancer Network Guidelines, European Society for Medical Oncology, and American Society of Clinical Oncology Guidelines, mark limited evidence regarding chemotherapy for elderly patients with breast cancer. 17 , 18 , 19 With the increasing demand for high‐quality life expectancy supported by the development of medical conditions and precision medicine, study on whether elderly patients with breast cancer could benefit from chemotherapy has become necessarily important.
However, almost all current studies are retrospective and present conflicting results on the effectiveness of chemotherapy in treating elderly breast cancer. For example, some retrospective studies found that chemotherapy may benefit specific subgroups among elderly patients with triple‐negative breast cancer or involved lymph nodes, 20 , 21 while others pointed out that the benefits of chemotherapy for elderly patients are limited compared with those for young patients. 22 Elderly patients are inclined to die from other causes except for breast cancer, thus resulting in the competing risk of death during statistical analysis. In this case, the overall survival (OS) and breast cancer‐specific survival (BCSS) should be necessarily reported for elderly patients having received chemotherapy for breast cancer treatment. However, only a few studies have reached similar conclusions from both respects or contradictory conclusions. 23
To handle these problems in clinical practice, the Epidemiology, and End Results (SEER) database was hereby established to collect routine data about the clinical diagnosis and treatment of various cancers in real‐world environments. Taking this database as a basis, one secondary analysis focusing on the prognosis of patients over 65 years from 1991 to 1999 concludes that chemotherapy may benefit ER– or lymph node‐positive elderly patients. 24 Another analysis of the cohort of patients older than 66 years with ER–/PR– breast cancer diagnosed in 1992−1999 reveals that chemotherapy is associated with approximately 15% mortality reduction after the adjustment for confounding effects. 25 However, all these studies were conducted 20 years ago. With the optimization of the chemotherapy regimen and the rich data accumulated in SEER database, further research must be conducted to verify chemotherapy's effectiveness in elderly breast cancer.
To this end, this study aims to further verify the overall and specific effect of chemotherapy on the prognosis of breast cancer in patients over 70 years by conducting a retrospective cohort study based on the SEER database and the presented OS and BCSS. Subpopulations, including those with HER2+ breast cancer who could benefit more from chemotherapy, were also explored to provide additional evidence for the decision‐making in clinical practice and offer more reference data for the design of clinical trials in the future.
2. METHODS
2.1. Data sources and patient selection
This study was based on the SEER database released in November 2020. The target patients were extracted from SEER*Stat Version 8.3.9.2 (SEER ID: 26588‐Nov2019), which also contained population‐based data from 18 cancer registries covering approximately 28% of US cancer populations from 1975 to 2018 and provided complete data regarding patient demographics, tumor characteristics, diagnosis, first course of treatment, and follow‐up for vital status. Given that the data released by the SEER database were publicly available, the present study did not require informed patient consent and was exempt from the review of the Ethics Committee of West China Hospital, Sichuan University.
Breast cancer patient data, including chemotherapy records from January 1, 2010 to December 31, 2016 were extracted, since SEER recorded the HER‐2 status since 2010. A total of 252,472 diagnosed breast cancer cases were identified in the database during this period (Supplementary Figure S1). The inclusion criteria were as follows: (1) female; (2) diagnosed with breast cancer as the first primary tumor; and (3) older than 70 years old. The exclusion criteria were: (1) diagnosed with metastatic breast cancer; (2) diagnosed with bilateral breast cancer; (3) breast cancer as the secondary tumor; (4) no histologic confirmation; (5) missing key information, including stage, grade, and molecular type; or (6) subject to death or loss to follow‐up in 6 months after diagnosis. Finally, 33,177 eligible patients were gathered for analysis.
2.2. Data acquisition
Detailed patient data were collected, including age at diagnosis, race (white, black, other, or unknown), marital status (married, divorced, separated, single, widowed, unmarried, domestic partner, or unknown), insurance status (insured, insured/no specifics, any medical, uninsured, or insurance unknown), grade (G1, G2, or G3), stage (I, II, III, or IV), T/N/M stage (T0‐T4, N0‐N3, or Mo‐M1), estrogen receptor (ER) status (negative, positive, or borderline), progesterone receptor (PR) status (negative, positive, or borderline), HER‐2 status (negative, positive, or borderline), breast cancer molecular subtype (luminal A, luminal B, HER‐2 enriched, or triple‐negative), breast surgery type (partial mastectomy with or without axillary dissection, simple and subcutaneous mastectomy, modified radical mastectomy, radical and extended radical mastectomy with or without breast reconstruction, and other mastectomy or unknown), and chemotherapy and radiotherapy records. Stage and T/N stage were defined according to the 7th ed. American Joint Committee on Cancer (2010−2015). Detailed information about the variables can be found on the SEER official website (https://seer.cancer.gov/data‐software/documentation /seerstat/nov2020/), and the overall analysis strictly follows the definitions.
2.3. Outcomes
OS and BCSS were adopted as outcomes. OS was measured from the time of diagnosis to the time of death for any reason or the time to the last follow‐up for patients who did not die. BCSS was defined as the time from diagnosis to death from breast cancer or the time to the last follow‐up for patients who did not die or died from other causes. The description from “SEER cause‐specific death classification” defined the patients’ cause of death.
2.4. Statistical analysis
First, preprocessing was conducted for the data, and some categories of a few variables were combined, including age groups (70−74.9, 75−79.9, and > 80 years), insurance status (insured, uninsured, and unknown), marital status (married, others, and unknown), grade categories (G1, G2, and G3), and surgical mode, which was regrouped into partial mastectomy (partial mastectomy with or without axillary dissection), mastectomy (simple and subcutaneous mastectomy, modified radical mastectomy, and radical and extended radical mastectomy with or without breast reconstruction), and other variables (other mastectomy or unknown).
Student's t‐test was used for normally distributed continuous variables, and a nonparametric test, for nonnormal continuous variables and categorical variables in the case of comparing patients' baseline demographics and clinicopathological characteristics. Mean and standard deviation (SD) were employed to describe patients' age and follow‐up time. Besides, the Kaplan–Meier curves were applied to present the survival rates at various time points during the follow‐up and log‐rank test for calculating the 5‐year OS/BCSS rate and comparing the survival differences between groups with or without chemotherapy treatment.
To establish balanced groups, handle the baseline imbalance between comparison groups, and control for potential confounding factors, propensity score matching (PSM) 26 was performed by using the logistic regression model that incorporates variables of age groups, race, marital status, insurance status, grade, T/N stage, ER/PR/HER2 status, breast surgery type, and radiotherapy for the calculation of the propensity score (PS) for each patient. A 1:1 match between the patients who had received chemotherapy and the controls without chemotherapy treatment was conducted using the MatchIt package, setting calliper width as the 0.2 standard deviations of PS. Then, standardized mean difference (SMD) was used to assess the equilibrium degree of baseline characteristics between groups after PSM, and SMD < 0.1 was regarded as reaching acceptable requirements. After that, a multivariable Cox regression model involving variables of age groups, grade, T/N stage, subtype, and radiotherapy was established. The hazard ratio (HR) and its corresponding 95% confidence intervals (95% CI) were calculated to estimate the effectiveness of chemotherapy in treating elderly breast cancer.
Sensitivity analysis was also conducted by using PS weighting to verify the robustness of the results. In the PSM data set, several subgroup analyses among the potential variables of clinical concern, such as HER‐2 receptor status, breast cancer molecular subtypes, and the number of involved adjacent lymph nodes, were also carried out, which verified the existing interaction effects by testing the statistical significance of interaction terms. Then, the 5‐year OS and BCSS rates were calculated for different subgroups, and statistical analyses were conducted using R software (R version 4.0.4). In this study, a two‐sided p‐value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Baseline demographic and clinicopathological characteristics
The baseline demographic and clinicopathological characteristics of these patients are summarized in Table 1. Among the 33,177 eligible elderly patients with breast cancer, the mean age for diagnosis was 77.6 [SD: 6.1] years, and the mean follow‐up time was 43.7 [21.4] months. Only 15.9% (5373/33,177) of them received chemotherapy. Compared with the controls without chemotherapy, those patients having received chemotherapy were younger (<75 years: 59.5% vs. 34.6%); suffered from advanced cancer (G3: 55.0% vs. 18.3%; stage III: 26.9% vs. 5.8%); were ER– (35.3% vs. 8.1%), PR– (50.7% vs. 18.8%), and HER2+ (29.9% vs. 6.2%); and were more likely to receive mastectomy surgery (25.5% vs. 9.8%) and radiotherapy (58.1% vs. 45.9%) (all p values < 0.001).
TABLE 1.
Baseline and treatment characteristics of patients
| Variables | Overall (n = 33,177) | Nonchemotherapy (n = 27,904) | Chemotherapy (n = 5273) | p Value | |
|---|---|---|---|---|---|
| Age (%) | |||||
| 70–74.9 years | 12,800 (38.6) | 9661 (34.6) | 3139 (59.5) | <0.001* | |
| 75–79.9 years | 9185 (27.7) | 7720 (27.7) | 1465 (27.8) | ||
| 80+ years | 11,192 (33.7) | 10,523 (37.7) | 669 (12.7) | ||
| Race (%) | |||||
| White | 27,346 (82.4) | 23,137 (82.9) | 4209 (79.8) | <0.001 | |
| Black | 2536 (7.6) | 1997 (7.2) | 539 (10.2) | ||
| Other/unknown | 3295 (9.9) | 2770 (9.9) | 525 (10.0) | ||
| Insurance (%) | |||||
| No | 91 (0.3) | 72 (0.3) | 19 (0.4) | <0.001 | |
| Yes | 32,602 (98.3) | 27,389 (98.2) | 5213 (98.9) | ||
| Unknown | 484 (1.5) | 443 (1.6) | 41 (0.8) | ||
| Marital (%) | |||||
| Others | 17,370 (52.4) | 14,947 (53.6) | 2423 (46.0) | <0.001 | |
| Yes | 14,196 (42.8) | 11,557 (41.4) | 2639 (50.0) | ||
| Unknown | 1611 (4.9) | 1400 (5.0) | 211 (4.0) | ||
| Grade (%) | |||||
| G1 | 9616 (29.0) | 9251 (33.2) | 365 (6.9) | <0.001* | |
| G2 | 15,559 (46.9) | 13,551 (48.6) | 2008 (38.1) | ||
| G3 | 8002 (24.1) | 5102 (18.3) | 2900 (55.0) | ||
| Stage (%) | |||||
| I | 19,130 (57.7) | 17,775 (63.7) | 1355 (25.7) | <0.001* | |
| II | 11,001 (33.2) | 8501 (30.5) | 2500 (47.4) | ||
| III | 3046 (9.2) | 1628 (5.8) | 1418 (26.9) | ||
| T (%) | |||||
| T0 | 14 (0.0) | 5 (0.0) | 9 (0.2) | <0.001* | |
| T1 | 21,137 (63.7) | 19,085 (68.4) | 2052 (38.9) | ||
| T2 | 9634 (29.0) | 7269 (26.1) | 2365 (44.9) | ||
| T3 | 1539 (4.6) | 1027 (3.7) | 512 (9.7) | ||
| T4 | 853 (2.6) | 518 (1.9) | 335 (6.4) | ||
| N (%) | |||||
| N0 | 25,539 (77) | 23,123 (82.9) | 2416 (45.8) | <0.001* | |
| N1 | 5657 (17.1) | 3815 (13.7) | 1842 (34.9) | ||
| N2 | 1251 (3.8) | 626 (2.2) | 625 (11.9) | ||
| N3 | 730 (2.2) | 340 (1.2) | 390 (7.4) | ||
| Subtype (%) | |||||
| Luminal A | 26,915 (81.1) | 24,477 (87.7) | 2438 (46.2) | <0.001 | |
| Luminal B | 2349 (7.1) | 1297 (4.6) | 1052 (20.0) | ||
| HER2‐ enriched | 954 (2.9) | 432 (1.5) | 522 (9.9) | ||
| Triple‐negative | 2959 (8.9) | 1698 (6.1) | 1261 (23.9) | ||
| ER (%) | |||||
| Negative | 4133 (12.5) | 2274 (8.1) | 1859 (35.3) | <0.001 | |
| Positive | 29,025 (87.5) | 25,618 (91.8) | 3407 (64.6) | ||
| Borderline | 19 (0.1) | 12 (0.0) | 7 (0.1) | ||
| PR (%) | |||||
| Negative | 7927 (23.9) | 5255 (18.8) | 2672 (50.7) | <0.001 | |
| Positive | 25,206 (76.0) | 22,615 (81.0) | 2591 (49.1) | ||
| Borderline | 44 (0.1) | 34 (0.1) | 10 (0.2) | ||
| HER2 (%) | |||||
| Negative | 29,874 (90) | 26,175 (93.8) | 3699 (70.1) | <0.001 | |
| Positive | 3303 0 | 1729 (6.2) | 1574 (29.9) | ||
| Surgery (%) | |||||
| No | 1560 (4.7) | 1337 (4.8) | 223 (4.2) | <0.001 | |
| Partial mastectomy | 27,473 (82.8) | 23,776 (85.2) | 3697 (70.1) | ||
| Mastectomy | 4092 (12.3) | 2747 (9.8) | 1345 (25.5) | ||
| Other/unknown | 52 (0.2) | 44 (0.2) | 8 (0.2) | ||
| Radiotherapy (%) | |||||
| No/unknown | 17,292 (52.1) | 15,083 (54.1) | 2209 (41.9) | <0.001 | |
| Yes | 15,885 (47.9) | 12,821 (45.9) | 3064 (58.1) | ||
Nonparametric test.
T: tumor stage; N: nearby lymph node stage; ER: estrogen receptor; PR: progesterone receptor; HER2: growth factor receptor 2.
3.2. Univariable survival and mortality analyses between groups
After summarizing the baseline characteristics, the OS and BCSS in these two groups were further evaluated using the Kaplan–Meier survival curves. Without adjustment, patients with chemotherapy treatment were found to have a better OS (5‐year OS rate, HR = 0.84, 95% CI 0.83 to 0.84) but not BCSS (5‐year BCSS rate, HR = 0.94, 95% CI 0.94 to 0.95) compared with the control group.
The death record of these elderly patients was further processed to analyze their mortality, and it was found that a total of 861 patients (16.33%) in the chemotherapy group and 5,131 patients (18.39%) in the nonchemotherapy group were deceased. In the death crowd, breast cancer was the most common cause of death in both groups. Other common causes of death included heart diseases, cerebrovascular diseases, chronic obstructive pulmonary diseases, Alzheimer's diseases, and diseases of lung and bronchus (Table 2).
TABLE 2.
Mortality analysis*
| Variables | Chemotherapy (n = 861) | Nonchemotherapy (n = 5131) | p Value |
|---|---|---|---|
| Breast cancer (%) | 523(60.7) | 1353(26.4) | <0.001 |
| Heart diseases (%) | 73(8.5) | 1034(20.2) | <0.001 |
| Cerebrovascular diseases (%) | 19(2.2) | 281(5.5) | <0.001 |
| Chronic obstructive pulmonary diseases (%) | 16(1.9) | 261(5.1) | <0.001 |
| Alzheimer's disease (%) | 14(1.6) | 238(4.6) | <0.001 |
| Lung and bronchus diseases (%) | 27(3.1) | 161(3.1) | 0.998 |
Student's t‐test.
3.3. Survival analysis in propensity score matched data set
3.3.1. Effect of chemotherapy on the OS and BCSS of the matched groups
After PSM, 8360 patients were successfully matched, and a good balance of baseline characteristics was reached between groups (Table 3). In PSM data set, the univariable Kaplan–Meier curve showed that the patients having received chemotherapy exhibited better prognosis on OS and BCSS (Figure 1, both log‐rank test: p < 0.001) compared with those having not received chemotherapy. Meanwhile, the multivariable COX model showed that chemotherapy was associated with a 36% and 21% risk reduction on OS (HR = 0.64, 95% CI 0.58 to 0.71) and BCSS (HR = 0.79, 95% CI 0.69 to 0.91), respectively (Figure 2).
TABLE 3.
Baseline and treatment characteristics of patients after PSM
| PSM data set | ||||
|---|---|---|---|---|
| Variables | None (n = 4180) | Chemotherapy (n = 4180) | SMD* | |
| Age (%) | ||||
| 70–74.9 years | 2140 (51.2) | 2352 (56.3) | 0.116 | |
| 75–79.9 years | 1255 (30.0) | 1197 (28.6) | ||
| 80+ years | 785 (18.8) | 631 (15.1) | ||
| Race (%) | ||||
| White | 3329 (79.6) | 3317 (79.4) | 0.027 | |
| Black | 443 (10.6) | 424 (10.1) | ||
| Other/unknown | 408 (9.8) | 439 (10.5) | ||
| Insurance (%) | ||||
| No | 16 (0.4) | 16 (0.4) | 0.01 | |
| Yes | 4123 (98.6) | 4127 (98.7) | ||
| Unknown | 41 (1.0) | 37 (0.9) | ||
| Marital (%) | ||||
| No | 2021 (48.3) | 1929 (46.1) | 0.048 | |
| Yes | 1979 (47.3) | 2079 (49.7) | ||
| Unknown | 180 (4.3) | 172 (4.1) | ||
| Grade (%) | ||||
| G1 | 323 (7.7) | 356 (8.5) | 0.046 | |
| G2 | 1739 (41.6) | 1796 (43.0) | ||
| G3 | 2118 (50.7) | 2028 (48.5) | ||
| Stage (%) | ||||
| I | 1364 (32.6) | 1250 (29.9) | 0.074 | |
| II | 1953 (46.7) | 1957 (46.8) | ||
| III | 863 (20.6) | 973 (23.3) | ||
| T (%) | ||||
| T0 | 3 (0.1) | 7 (0.2) | 0.038 | |
| T1 | 1789 (42.8) | 1795 (42.9) | ||
| T2 | 1789 (42.8) | 1761 (42.1) | ||
| T3 | 389 (9.3) | 384 (9.2) | ||
| T4 | 210 (5.0) | 233 (5.6) | ||
| N (%) | ||||
| N0 | 2104 (50.3) | 2166 (51.8) | 0.084 | |
| N1 | 1480 (35.4) | 1340 (32.1) | ||
| N2 | 383 (9.2) | 406 (9.7) | ||
| N3 | 213 (5.1) | 268 (6.4) | ||
| Subtype (%) | ||||
| Luminal A | 2285 (54.7) | 2183 (52.2) | 0.107 | |
| Luminal B | 667 (16.0) | 762 (18.2) | ||
| HER2‐enriched | 360 (8.6) | 277 (6.6) | ||
| Triple‐negative | 868 (20.8) | 958 (22.9) | ||
| ER (%) | ||||
| Negative | 1297 (31.0) | 1295 (31.0) | 0.007 | |
| Positive | 2877 (68.8) | 2880 (68.9) | ||
| Borderline | 6 (0.1) | 5 (0.1) | ||
| PR (%) | ||||
| Negative | 1949 (46.6) | 1927 (46.1) | 0.011 | |
| Positive | 2224 (53.2) | 2246 (53.7) | ||
| Borderline | 7 (0.2) | 7 (0.2) | ||
| HER2 (%) | ||||
| Negative | 3153 (75.4) | 3141 (75.1) | 0.007 | |
| Positive | 1027 (24.6) | 1039 (24.9) | ||
| Surgery (%) | ||||
| No | 195 (4.7) | 185 (4.4) | 0.019 | |
| Partial mastectomy | 3061 (73.2) | 3043 (72.8) | ||
| Mastectomy | 918 (22.0) | 946 (22.6) | ||
| Other/unknown | 6 (0.1) | 6 (0.1) | ||
| Radiotherapy (%) | ||||
| No/unknown | 2080 (49.8) | 1892 (45.3) | 0.09 | |
| Yes | 2100 (50.2) | 2288 (54.7) | ||
PSM: propensity score matching; SMD: standardized mean difference.
FIGURE 1.
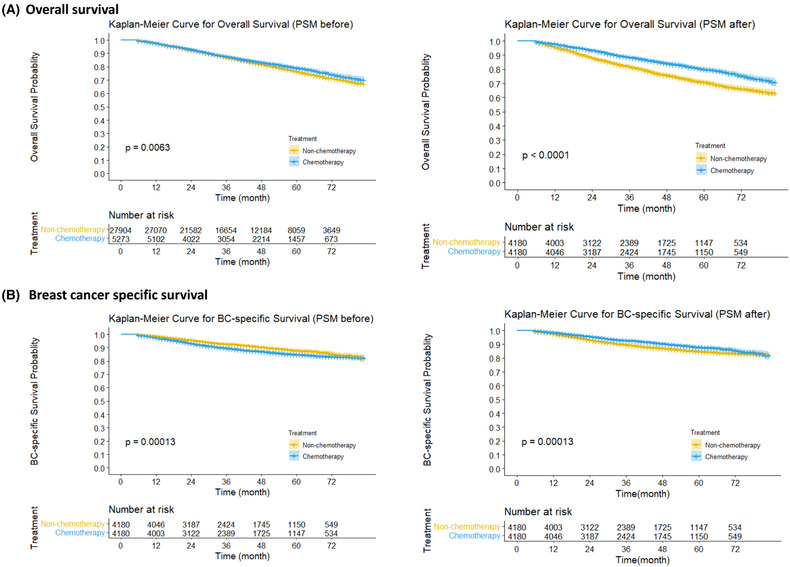
Kaplan–Meier curve of overall survival (A) and breast cancer (BC) specific survival (B) in patients before and after propensity score matching (PSM). A 95% confidence interval (estimated from a log hazard), the number of patients at risk at different time points, and the p value for the log‐rank test are displayed on the graph.
FIGURE 2.
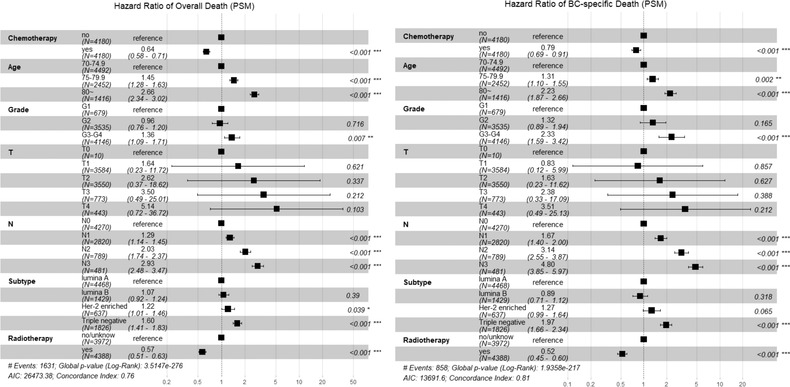
Multivariable COX proportional analysis of chemotherapy on overall death and breast cancer (BC)‐specific death in PSM data set. PSM: propensity score matching. The model adjusted variables with SMD ≥ 0.1 after PSM and those of clinical concern.
3.3.2. Sensitivity analysis by the PS‐weighting method
The PS‐weighting method was also used to verify the robustness of the results and analyze the minimal differences in SMD regarding various baseline characteristics between groups with and without chemotherapy (Supplementary Figure S2). PS weighting analysis showed that chemotherapy was associated with 35% and 18% risk reduction on OS (HR = 0.65, 95% CI 0.59 to 0.71) and BCSS (HR = 0.82, 95% CI 0.72 to 0.94), respectively, which is in line with the results of PSM analysis.
3.3.3. Subgroup analysis
Interaction tests showed that the radiotherapy treatment, number of involved lymph nodes, and breast cancer molecular types might interactively affect the treatment outcomes of chemotherapy on elderly breast cancer (all p interactions < 0.05). However, no statistical significance was observed for the interaction effects of different age groups, grades, T/N stages, or different surgical methods.
First, among the elderly patients had received radiotherapy, chemotherapy improved the 5‐year OS (HR = 0.89, 95% CI 0.88 to 0.91 vs. HR = 0.85, 95% CI 0.83 to 0.86) instead of the 5‐year BCSS (HR = 0.94, 95% CI 0.93 to 0.94 vs. HR = 0.94, 95% CI 0.93 to 0.94). Among the elderly patients without radiotherapy, chemotherapy provided a favorable 5‐year OS (HR = 0.82, 95% CI 0.81 to 0.84 vs. HR = 0.71, 95% CI 0.69 to 0.73) or BCSS (HR = 0.90, 95% CI 0.88 to 0.91 vs. HR = 0.84, 95% CI 0.83 to 0.86).
Second, the effectiveness of chemotherapy in treating elderly breast cancer increased with the number of involved lymph nodes. The 5‐year OS rate of the chemotherapy group was HR = 0.90, 95% CI 0.88 to 0.91 vs. HR = 0.84, 95% CI 0.82 to 0.85, HR = 0.86, 95% CI 0.84 to 0.88 vs. HR = 0.80, 95% CI 0.78 to 0.82, HR = 0.79, 95% CI 0.75 to 0.83 vs. HR = 0.58, 95% CI 0.53 to 0.63, and HR = 0.67, 95% CI 0.61 to 0.72 vs. HR = 0.44, 95% CI 0.37 to 0.51, respectively, compared with that of the control group in N0, N1, N2, and N3 subpopulations. The 5‐year BCSS rate of the chemotherapy group was HR = 0.95, 95% CI 0.94 to 0.96 vs. HR = 0.94, 95% CI 0.93 to 0.95, HR = 0.92, 95% CI 0.90 to 0.93 vs. HR = 0.90, 95% CI 0.88 to 0.91, HR = 0.85, 95% CI 0.81 to 0.88 vs. HR = 0.74, 95% CI 0.70 to 0.79, and HR = 0.76, 95% CI 0.71 to 0.81 vs. HR = 0.61, 95% CI 0.54 to 0.67, respectively, compared with that of the control group in N0, N1, N2, and N3 subpopulations.
Third, the breast cancer molecular subtypes might interact with chemotherapy on the OS and BCSS rate. The 5‐year OS rate of the chemotherapy group was HR = 0.87, 95% CI 0.86 to 0.89 vs. HR = 0.83, 95% CI 0.81 to 0.92 vs. HR = 0.78, 95% CI 0.74 to 0.81, HR = 0.83, 95% CI 0.79 to 0.87 vs. HR = 0.83, 95% CI 0.83 to 0.84, and HR = 0.81, 95% CI 0.79 to 0.83 vs. HR = 0.68, 95% CI 0.65 to 0.71, respectively, compared with that of the control group in luminal A, luminal B, HER2‐enriched, and triple‐negative subpopulations. The 5‐year BCSS rate of the chemotherapy group was HR = 0.93, 95% CI 0.92 to 0.94 vs. HR = 0.92, 95% CI 0.91 to 0.93, HR = 0.96, 95% CI 0.94 to 0.97 vs. HR = 0.90, 95% CI 0.87 to 0.92, HR = 0.91, 95% CI 0.87 to 0.94 vs. HR = 0.85, 95% CI 0.81 to 0.88, and HR = 0.86, 95% CI 0.84 to 0.88 vs. HR = 0.82, 95% CI 0.79 to 0.84, respectively, compared with that of the control group in luminal A, luminal B, HER2‐enriched, and triple‐negative subpopulations.
4. DISCUSSIONS
This study was based on a large SEER database and characterized by the retrospective cohort design and appropriate analysis for bias control on the prognosis of elderly breast cancer treated by chemotherapy. The results showed that elderly patients could benefit from chemotherapy for breast cancer treatment in terms of overall and disease‐specific survival after adjustment. Before PSM, patients with chemotherapy had worse survival in BCSS; it was possibly attributed to the unmatched baseline and numerous confounding factors. The populations with lymph nodes involved in specific molecular subtypes and nonradiotherapy were inclined to considerably benefit from chemotherapy for breast cancer treatment.
Indeed, several studies have attempted to verify the effectiveness of chemotherapy for elderly breast cancer. One study based on UK cancer registry data included 11,735 patients aged 70−79 years from 2002 to 2012 with stage I–III breast cancer with confounder adjustment found that chemotherapy might improve BCSS in patients exposed to a high recurrence risk (HR= 0.74, 95% CI 0.67 to 0.81). 27 However, this study lacked ER data and no OS reported.
Compared with that by Sharon H et al., 24 the chemotherapy acceptance rate did not increase during the last 30 years (1991: 7.4%; 1999: 16.3%; 2010−2016: 16%), though the chemotherapy regimens for elderly patients have changed in recent years, especially the abandonment of using anthracyclines lowered drug risks. The CALGB 49907 trial 20 proved that chemotherapy with adriamycin and cyclamide (AC) or cyclamide + methotrexate + fluorouracil (CMF) presents a better effect than capecitabine in patients with breast cancer over 65 years old, though AC/CMF might not be superior to other chemotherapy regimens. With the development of clinical drug research, the prominent cardiotoxicity of anthracyclines in elderly patients has attracted increasing academic attention. 28 , 29 Guidelines have gradually promoted relatively mild chemotherapy options based on taxane‐based regimens such as paclitaxel and docetaxel. Additionally, the selection of various targeted drugs for HER2 hormone receptors has been developed, and the chemotherapy strategy for elderly patients with breast cancer has been extensively changed.
Although breast cancer is the most common death cause in elderly patients with breast cancer, only a 26.37% mortality rate was observed among the nonchemotherapy group. Therefore, other causes of death still must be considered for patients with a low recurrence rate. In this study, other recorded causes of death were most common heart diseases, cerebrovascular diseases, chronic obstructive pulmonary diseases and allied diseases, Alzheimer's disease, and diseases of lung and bronchus, similar to the common causes of death in the general population. 30 In this study, we found no difference in the death of heart disease between groups. However, a retrospective cohort study 31 reported 14.8% (19/128) cardiotoxicity among chemotherapy in patients over 65, while the dosage of chemotherapy was reduced in 23 patients (18.0%), and 14 (10.9%) had premature interruptions, though nearly half of patients use anthracyclines. In this case, patients' comorbidities and life expectancy must be fully considered while making treatment decisions. In addition, some studies suggested the OS is not related to BCSS among elderly patients with breast cancer because of the death competition of comorbidities, 32 but they also supported the benefit of receiving the standard treatment in those patients. In elderly patients with breast cancer, specific indicators of geriatric assessment 33 (including frailty, cognitive status, and quality of life) may be important for prognosis prediction. High‐quality clinical studies are still needed to fill in the details.
In the subgroup analysis, no interaction was observed in age groups, which is in line with the findings of Sharon H et al. 24 However, one study21 found that compared with young patients, those older than 70 years enjoyed limited benefits. A retrospective study for patients older than 70 years 34 found an interaction between the effect of chemotherapy and age after PS, with the OS benefit observed only in the subgroup aged 70−75 years instead of the older subgroups or any subgroups in BSCC. However, only 420 patients were included in this study. After PS, only 23 patients with chemotherapy were older than 80 years, and they had a low recurrence risk, poor comorbidities, and activities of daily living before PS, all of which might have offset the results. In this case, age may not be a hindrance to benefiting from adjuvant chemotherapy, provided that appropriate subgroups are selected. Further RCT is needed for verification.
Meanwhile, chemotherapy adds no extra benefits to radiotherapy. This has not been mentioned in other studies, which is possibly attributed to the fact that the local therapeutic effect attributed to radiotherapy is difficult to be assessed by OS and BCSS. Thus, further studies are needed to evaluate this hypothesis by using appropriate outcomes such as local recurrence rate. Besides, it is still a hint that elderly breast cancer patients having received radiotherapy may be waived from chemotherapy.
Some studies defined the types of elderly patients and reached similar conclusions to the hereby conducted subgroup analysis. Focusing on triple‐negative breast cancer, a retrospective study involving 16,062 triple‐negative patients who were 70 years or older with stage I–III was conducted, with patients suffering from T1aN0M0 disease excluded. 35 After PSM and multivariable Cox regression analysis, the chemotherapy group was found to possess a better OS (HR = 0.69, 95% CI 0.60 to 0.80; p < 0.001) than those who were recommended but not given chemotherapy. Another retrospective research involving 1130 Switzerland patients aged 70 years and older with triple‐negative breast cancer found both benefits in 5‐year OS (HR = 0.75, 95% CI 0.69 to 0.82 vs. HR = 0.63, 95% CI 0.57 to 0.71, p = 0.029) and 5‐year BCSS (HR = 0.83, 95% CI 0.78 to 0.89 vs. HR = 0.73, 95% CI 0.67 to 0.80, p = 0.014) after PSM. 36 For those with triple‐negative breast cancer, adjuvant chemotherapy played a particularly important role in their survival due to the lack of benefits from endocrine therapy and targeted therapy.
As for patients with positive hormone receptors, the benefit of chemotherapy has been widely discussed. Retrospective research including 1592 patients aged 70 years older, scoring comorbidity 2 or 3, ER+ and HER2–, and undergoing surgery found through PSM and multivariable Cox regression analysis that chemotherapy was associated with improved OS (HR = 0.67, 95% CI 0.48 to 0.93; p = 0.02). However, the result of BCSS was not reported in this study.20 Besides, a cohort study recruiting 3416 UK women aged 70 years or above found that chemotherapy might offer little benefit and negatively impact the quality of life for most older women with ER+ breast cancer. 37 However, the present study found both benefits in the 5‐years OS and BCSS rate, and such changes were statistically different, although the difference was relatively small. In this case, it can be concluded that chemotherapy may benefit some elderly patients with breast cancer. However, clinicians need to strictly evaluate patients’ conditions and relevant indications to determine those inclined to benefit from chemotherapy.
Not surprisingly, patients with enriched HER‐2 can benefit a lot from chemotherapy, as well as targeted therapies. However, data on targeted therapies are available in the SEER database, making it hard to distinguish whether this benefit comes from chemotherapy or targeted therapy. Thus, further clinical studies are still required for further confirmation. In any case, it is recommended that patients with positive HER2 receptors better receive comprehensive treatment. 38
The present study has several strengths. First, this work is currently the largest investigation that further verifies the effectiveness of chemotherapy in treating elderly breast cancer by evaluating overall and disease‐specific survival based on retrospectively collecting routine clinical data in real‐world environments. Therefore, the conclusion is generalizable to other heterogeneous populations. Second, the effectiveness of chemotherapy was confirmed using the common and effective method of PS‐matched analysis by strictly standardized procedures controlling for various biases, including selection bias and confounding effects. The effectiveness of chemotherapy was also proved robust in the sensitivity analysis of PS weighting analysis. Third, several subgroup analyses, taking into account the demographic and clinicopathological characteristics of clinical concern, were conducted through interaction tests to identify the elderly breast cancer subpopulations that could considerably benefit from chemotherapy, thus providing evidence for individualized treatment in clinical practice.
However, the present study is also subject to some limitations. The SEER database could not provide sufficient data on important variables, such as menstrual and reproductive history, family history of breast cancer, comorbidities, endocrine therapy, individual chemotherapeutic regimen, combined prescriptions, etc. These factors could not be adjusted in analysis and may affect the effectiveness of chemotherapy in treating elderly breast cancer. Additionally, the results of subgroup analysis are exploratory and must be confirmed by further prospective studies.
5. CONCLUSIONS
Chemotherapy reduces the overall risk of death by 36% and breast cancer‐specific mortality by 21% in patients with breast cancer aged 70 years or above. In addition to breast cancer, heart diseases are these patients' most common cause of death. Subgroup analysis shows that elderly patients with lymph node involvement and non‐luminal A subtypes are likely to benefit from chemotherapy.
FUNDING
This work was supported by The Key Program of National Natural Science Foundation of China (No: 32071284), Clinical Research Program for West China Hospital, Sichuan University (No: 2022HXFH021), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No: Z20192011), National Natural Science Foundation of China (No: 31971141), Natural Science Foundation of Sichuan (No: 2022NSFSC1347) and The Science and Technology Department of Sichuan Province (No: 2021YJ0012).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest to disclose and has no financial relationships with any biomedical companies.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Since data released by the SEER database was publicly available, ethics approval and informed patient consent was not required for this study.
CONSENT TO PUBLISH
The abstract of this article has been selected at the 2022 ASCO Annual Meeting and released by ASCO on May 26, 2022. Apart from this, the work described has not been submitted elsewhere for publication.
Supporting information
Supplementary Figure S1 Patient screening
Supplementary Figure S2 Balance between comparison groups with and without chemotherapy by using different methods
Wu Y, Qi Y, Yang J, et al. Effect of adjuvant chemotherapy on the survival outcomes of elderly breast cancer: A retrospective cohort study based on SEER database. J Evid Based Med. 2022;15:354–364. 10.1111/jebm.12506
Yunhao Wu and Yana Qi contributed equally.
Contributor Information
Xin Sun, Email: sunx79@hotmail.com.
Jie Chen, Email: chenjiewestchina@163.com.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J EM, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. [Google Scholar]
- 3.American Cancer Society. Breast Cancer Facts & Figures 2019—2020. Atlanta: American Cancer Society, Inc. 2019.
- 4. Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early‐stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamelinck VC, Bastiaannet E, Pieterse AH, et al. A prospective comparison of younger and older patients' preferences for adjuvant chemotherapy and hormonal therapy in early breast cancer. Clin Breast Cancer. 2016;16(5):379–388. [DOI] [PubMed] [Google Scholar]
- 6. Derks MGM, van de Velde CJH, Giardiello D, et al. Impact of comorbidities and age on cause‐specific mortality in postmenopausal patients with breast cancer. Oncologist. 2019;24(7):e467–e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hancke K, Denkinger MD, König J, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: a German clinical cohort study. Ann Oncol. 2010;21(4):748–753. [DOI] [PubMed] [Google Scholar]
- 8. Bastiaannet E, Liefers GJ, de Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124(3):801–807. [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687‐1717. [DOI] [PubMed] [Google Scholar]
- 10. Abdel‐Razeq H, Iweir S, Abdel‐Razeq R, et al. Differences in clinicopathological characteristics, treatment, and survival outcomes between older and younger breast cancer patients. Sci Rep. 2021;11(1):14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. [DOI] [PubMed] [Google Scholar]
- 12. Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: the perspective of oncologists and primary care providers. J Clin Oncol. 2008;26(33):5386–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barthélémy P, Heitz D, Mathelin C, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol/Hematol. 2011;79(2):196–204. [DOI] [PubMed] [Google Scholar]
- 15. Abe O, Abe R, Enomoto K, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 16. Bernardi D, Errante D, Gallligioni E, et al. treatment of breast cancer in older women. Acta oncologica (Stockholm, Sweden). 2008;47(2):187–198. [DOI] [PubMed] [Google Scholar]
- 17. Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Nat Comprehen Cancer Netw. 16(11):1362–1389. [DOI] [PubMed] [Google Scholar]
- 18. Tung NM, Boughey JC, Pierce LJ, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 38(18):2080–2106. [DOI] [PubMed] [Google Scholar]
- 19. Kirova YM, Carroll S, Fourquet A, Offersen B, Aristei C, Chen JY. The St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017: the point of view of an International Panel of Experts in Radiation Oncology. Ann Oncol. 29(1):280–281. [DOI] [PubMed] [Google Scholar]
- 20. Muss HB, Polley MC, Berry DA, et al. Randomised trial of standard adjuvant chemotherapy regimens versus capecitabine in older women with early breast cancer: 10‐year update of the CALGB 49907 trial. J Clin Oncol. 2019;37(26):2338–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamirisa N, Lin H, Shen Y, et al. Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor‐positive, node‐positive breast cancer. JAMA Oncol. 2020;6(10):1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taubenhansl C, Ortmann O, Gerken M, Inwald EC, Klinkhammer‐Schalke M. Guideline‐concordant chemotherapy in patients with hormone receptor‐positive and node‐positive, early breast cancer leads to better overall and metastases‐free survival with limited benefit in elderly patients. Arch Gynecol Obstet. 2020;301(2):573–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Zheng D, Wu Y, et al. Treatment patterns and outcomes in older women with early breast cancer: a population‐based cohort study in China. BMC Cancer. 2021;21(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750–2756. [DOI] [PubMed] [Google Scholar]
- 25. Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS. Adjuvant chemotherapy and survival in older women with hormone receptor‐negative breast cancer: assessing outcome in a population‐based, observational cohort. J Clin Oncol. 2006;24(18):2757–2764. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ward SE, Holmes GR, Ring A, et al. Adjuvant chemotherapy for breast cancer in older women: an analysis of retrospective English Cancer Registration Data. Clin Oncol (R Coll Radiol). 2019;31(7):444–452. [DOI] [PubMed] [Google Scholar]
- 28. Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population‐based study. J Clin Oncol. 2005;23(34):8597–8605. [DOI] [PubMed] [Google Scholar]
- 29. Du XL, Xia R, Liu CC, et al. Cardiac toxicity associated with anthracycline‐containing chemotherapy in older women with breast cancer. Cancer. 2009;115(22):5296–5308. [DOI] [PubMed] [Google Scholar]
- 30. WHO . The top 10 causes of death. 2019.
- 31. Zanuso V, Fregoni V, Gervaso L. Side effects of adjuvant chemotherapy and their impact on outcome in elderly breast cancer patients: a cohort study. Fut Sci OA. 2020;6(9):Fso617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takuwa H, Tsuji W, Yotsumoto F. Overall survival of elderly patients with breast cancer is not related to breast‐cancer specific survival: a single institution experience in Japan. Breast Dis. 2018;37(4):177–183. [DOI] [PubMed] [Google Scholar]
- 33. Biganzoli L, Battisti NML, Wildiers H, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021;22(7):e327‐e40. [DOI] [PubMed] [Google Scholar]
- 34. Peng Y, Hu T, Cheng L, et al. Evaluating and balancing the risk of breast cancer‐specific death and other cause‐specific death in elderly breast cancer patients. Front Oncol. 2021;11:578880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crozier JA, Pezzi TA, Hodge C, et al. addition of chemotherapy to local therapy in women aged 70 years or older with triple‐negative breast cancer: a propensity‐matched analysis. Lancet Oncol. 2020;21(12):1611–1619. [DOI] [PubMed] [Google Scholar]
- 36. Janeva S, Zhang C, Kovács A, et al. Adjuvant chemotherapy and survival in women aged 70 years and older with triple‐negative breast cancer: a Swedish population‐based propensity score‐matched analysis. Lancet Healthy Longev. 2020;1(3):e117–e124. [DOI] [PubMed] [Google Scholar]
- 37. Wyld L, Reed MWR, Collins K, et al. Programme Grants for Applied Research. Improving Outcomes for Women Aged 70 Years or Above with Early Breast Cancer: Research Programme Including a Cluster RCT. Southampton (UK): National Institute for Health and Care Research; 2022. [PubMed] [Google Scholar]
- 38. Carli P, Turchet E, Quitadamo D, et al. Target therapy in elderly breast cancer patients. Crit Rev Oncol/Hematol. 2012;83(3):422–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Patient screening
Supplementary Figure S2 Balance between comparison groups with and without chemotherapy by using different methods


