Abstract
Issues
Assessing drug and alcohol inpatient withdrawal treatment programs is important, as these represent a first step of treatment among people with alcohol and drug problems. However, there are many ways of measuring outcomes making it difficult for service providers to decide which domains and methods to use. This narrative review aims to clarify frequencies of the domains and methods used to assess withdrawal treatment outcomes.
Approach
We reviewed published studies that examined outcomes of inpatient drug and alcohol withdrawal treatment. The types of outcome measures used and the frequency of use were summarised.
Key Findings
The review showed that assessment of withdrawal treatment outcomes goes beyond traditional abstinence measures. Outcomes mainly focus on biological and psychological outcomes, with social outcomes rarely measured. Even within outcome domains (e.g., cravings), there were many assessment methods.
Implications
The review provides service providers with an outline of common outcome domains and measures. Given the importance of social functioning to recovery from alcohol and drug problems, greater emphasis on such measures is desirable. Future research could develop greater consensus on outcome measures for use in withdrawal management services to facilitate clarity around factors associated with treatment success.
Conclusion
Outcome assessment in withdrawal treatment goes beyond abstinence to include holistic measurement of biological, psychological and some social outcomes; but more work needs to be done to cohere the different assessment methods and broaden the scope to include social functioning.
Keywords: drug and alcohol use, inpatient withdrawal treatment, outcome assessment, withdrawal treatment outcomes
1. INTRODUCTION
Mental health and substance use disorders were the fifth largest contributors to disability‐adjusted life years (DALY) globally in 2010 and were responsible for 7.4% (or 183.9 million) of DALYs [1]. Together, alcohol use disorders and drug use disorders account for 20.5% of this DALY burden, second only to depressive disorders [1]. In Australia, drug and alcohol use disorders accounted for 24% of the DALY burden in 2015 [2]. From 2020 to 2021, 139,300 clients aged at least 10 years received treatment for substance abuse in Australia, totalling 239,000 treatment episodes [3].
Given that withdrawal treatment often serves as a patient's first point of contact with formal treatment, it is imperative that the effectiveness of such services is understood. However, a challenge has been the wide range of outcome measures used in assessing withdrawal treatment outcome, and a lack of clarity regarding the relative strengths and weaknesses of these measures. Existing studies have used the following outcome assessments: (i) ‘safe’ withdrawal described as the prevention of severe withdrawal sequelae and minimisation of distress associated with withdrawal [4, 5, 6, 7]; (ii) successful completion of withdrawal treatment [4, 8, 9]; (iii) abstinence during withdrawal treatment [9]; (iv) abstinence rates at follow‐up (i.e., after discharge; [8, 10, 11]); (v) engagement in continuing care after discharge [8, 11, 12, 13]; (vi) withdrawal treatment satisfaction [5, 14]; and (vii) miscellaneous aspects of client functioning (e.g., rates of homelessness and employment; [8, 15, 16]).
Considering the numerous and varying measures of withdrawal treatment success across the literature, the current narrative review sought to clarify the frequencies of the different types of outcome assessments used in inpatient withdrawal treatment services.
2. METHOD
2.1. Narrative review
A narrative review was utilised due to the diversity of the topic and the wide range of methods used in the relevant studies. Additionally, the aim of this paper, investigating the frequency of outcome assessment types, is quite broad. As the research in this area does not always have an agreed‐upon gold standard methodology like double‐blind‐randomised‐controlled‐trials, narrative literature reviews are often viewed as appropriate and capable of advancing theoretical and conceptual understanding [17, 18]. Thus, a narrative versus other forms of systematic review was chosen.
2.2. Search strategy
To identify studies for review, we performed a Boolean search in the Medline, Scopus, PsycInfo, PsycArticles, PsycBooks and Psychiatry Online databases, using the search terms in abstract: (‘inpatient’ or ‘residential’) AND (‘detoxification’ or ‘withdrawal management’) OR in title: ‘inpatient’ AND ‘detoxification’. The use of a limited number of databases was due to the large number of results produced and a significant overlap of papers between databases. This study was registered on PROSPERO (CRD42021088576).
The search, conducted on 2 November 2021, was limited to studies reported in English language journal articles. We excluded preprints, commentaries, case studies, errata, study protocols and reviews/meta‐analyses. Trade publications and grey literature were not included since we focused on peer‐reviewed studies because these are more likely to include designs of sufficient rigour and that the results are most likely to be used to inform subsequent recommendations for assessment and treatment processes in services [19]. Additionally, funders and advocacy groups strongly endorse use of ‘evidence‐based approaches’ [20] that are more likely to be derived from peer‐reviewed publications. To focus on recent developments in the field, the search was limited to articles published from 2012. We wanted to look at a more recent and updated conceptualisation of drug and alcohol recovery, hence we used articles that came out after the Substance Abuse and Mental Health Services Administration (SAMHSA) recovery model was published. This would in theory provide a more holistic view of recovery including biological, psychological and social functioning [21]. Previously, recovery was defined mostly by abstinence, but recovery conceptualisations have now been expanded to include multiple facets such as, stable housing, health and sense of purpose [21].
2.3. Inclusion and exclusion criteria
The literature review process is shown in Figure 1. The search produced 1353 articles; after duplicates were removed, 745 remained. Article abstracts were screened for eligibility by one reviewer using the following criteria: (i) mentioned residential or inpatient withdrawal treatment; (ii) mentioned at least one outcome measure related to residential or inpatient withdrawal treatment; (iii) implemented at least one intervention (we chose to focus on intervention studies because they specify the primary outcomes more frequently. This is considered important because it gives some indications of which outcomes are more important by researchers); and (iv) outcomes were measured in relation to inpatient or residential withdrawal treatment only and not a mixture of inpatient or residential withdrawal treatment and other kinds of treatment (e.g., outpatient treatment, rehabilitation). This screening resulted in 573 articles being removed.
FIGURE 1.
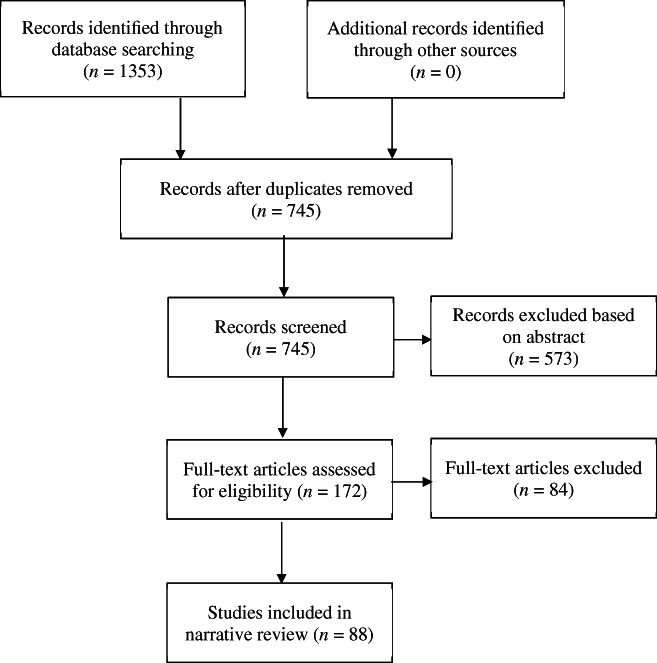
Flowchart of the literature review process
The remaining 172 articles were shortlisted for full text screening. Articles were then assessed for inclusion into the narrative synthesis if they met the specified criteria and included a sufficient description of the withdrawal treatment intervention for data extraction. Full texts were independently assessed for eligibility by the first author and a second reviewer. There was a high degree of agreement between the two reviewers (k = 0.85, p < 0.001) and any discrepancies were resolved through discussion. After the full‐text review, 88 studies were shortlisted for inclusion in the narrative review. Of the 84 studies excluded after the full text review, 66 were due to not describing the withdrawal treatment procedure or not providing a sufficient description for narrative synthesis data and 18 were because they mixed inpatient or residential withdrawal treatment with other kinds of treatment, such as outpatient treatment or rehabilitation.
2.4. Data extraction
Data extracted included brief sample characteristics, withdrawal treatment program components, outcome measures used, main findings and percentages of participants who completed withdrawal treatment and attended follow‐up treatment, if applicable. A summary of each of the 88 eligible studies is presented in three different tables, available as Supporting Information: Table S1 (alcohol users), Table S2 (opioid users) and Table S3 (other drug users), where the main findings column aims to detail how outcomes are reported/used.
3. RESULTS
3.1. Description of eligible studies
Twenty‐two studies were conducted in the USA, 13 in Germany, 6 in the Netherlands, 5 in Australia, 4 each in Brazil, Belgium, India and Iran, 3 each in Canada, Italy and Spain, 2 each in Austria, Sweden and the United Kingdom, and 1 each in 11 other countries.
Included studies mainly examined people with alcohol use disorders (30 studies), opioid use disorder (22 studies) and mixed substance use disorders (20 studies). To a lesser extent, cocaine dependence, cannabis dependence and heroin dependence were examined by eight, five and three studies, respectively.
Males comprised the majority of the sample in 78 (88.6%) of the 88 studies. Mean ages ranged from 11.14 [22] to 54.1 years [23] in the 82 studies that reported mean age. Sample sizes ranged from 11 to 72,205.
3.2. Description of program components
Twenty‐six studies (29%) used a combination of pharmacological and psychological interventions, and 61 studies (69%) used only pharmacological interventions. One study (1%) used only psychological interventions.
3.3. Description of outcome measures
3.3.1. Withdrawal treatment completion rate
Overall, 28 studies (31.8%) reported the withdrawal treatment completion rate. In order to estimate a mean completion rate across studies, when a range of completion rates was reported, we used the midpoint for calculation (e.g., range 90% to 100% then 95% used). The mean completion rate across studies was 74.9%.
Completion rates were defined by either: clinician judgement (13 studies) or completing a specified length of treatment (14 studies). One study [24] defined completion as either completing a specified treatment or withdrawal management according to clinician judgement.
In order to determine completion rates according to drug problem, we only considered drug problem types with at least three studies. For example, we did not analyse completion rates for gamma hydroxybutyrate dependence as only one study was identified [25].
Of the 30 studies concerning alcohol dependence, 5 reported completion rates, ranging from 71.3% to 98.3% (M = 86.2%). Of the 21 studies concerning opioid dependence, 11 reported completion rates, ranging from 56.0% to 100% (M = 68.8%). Of the 16 studies concerning mixed substance dependence, 5 reported completion rates, ranging from 44.0% to 99% (M = 73.2%). Of the nine studies concerning cocaine dependence, only one reported a completion rate which was 79.5%. Of the five studies concerning cannabis dependence, four reported completion rates, ranging from 45.5% to 88.6% (M = 77.4%). Of the three studies concerning heroin dependence, none reported completion rates.
3.3.2. Treatment continuity
Follow‐up treatment attendance rates were reported in seven studies, ranging from 9.7% to 84.4%. Follow‐up treatment was defined as attending treatment after discharge from withdrawal treatment, based on reviewing official records at the follow‐up treatment facility (three studies [11, 26, 27]) or via self‐report (two studies [12, 28]). Two studies [29, 30] did not specify how they obtained follow‐up treatment attendance rate data.
3.3.3. Other outcome measures
Withdrawal symptoms
Thirty‐two studies reported outcomes related to withdrawal symptoms. The most frequent measure, utilised in nine studies, was the self‐reported Subjective Opioid Withdrawal Scale [23, 31, 32, 33, 34, 35, 36, 37, 38]. Other outcome measures included the amount of ancillary or rescue medication used (five studies [35, 39, 40, 41, 42]), self‐report via the Clinical Institute Withdrawal Assessment for Alcohol, Revised Version (CIWA‐Ar) (four studies [39, 43, 44, 45]) or Clinical Institute Withdrawal Assessment for Alcohol (CIWA) (one study [46]). Clinician report via the Objective Opiate Withdrawal Scale was used in four studies [33, 35, 42, 47] and self‐report via the Cocaine Selective Severity Assessment in four studies [48, 49, 50, 51]. The clinician‐rated Clinical Opiate Withdrawal Scale was used in three studies [31, 32, 52] as was the clinician‐rated Clinical Global Impression Scale [42, 53, 54]. The self‐report Cannabis Withdrawal Scale [55, 56], the Adjective Rating Scale for Withdrawal [12, 57], Visual Analog Scale [12, 39] and Marijuana Withdrawal Scale [53, 54] were all used in two different studies. Single studies reported using a self‐report of subjective withdrawal symptoms (unspecified scale) [58], psychiatrist reports based on physical examinations [22], self‐report via the Alcohol Withdrawal Symptoms Score [39] and self‐report via Severity of Withdrawal Scale [59].
Relapse/return to drug use at follow‐up
Thirty studies reported outcomes related to relapse/drug use at follow‐up using a variety of 20 self‐report and 12 objective measures. Self‐reported measures included interviews such as the Interview for Research on Addictive Behaviour [60] and self‐administered questionnaires such as the Alcohol Use Disorders Identification Test [26, 61], Drug Use Disorders Identification Test and the Drug Use Disorders Identification Test‐Extended E [62], the Cannabis Problems Questionnaire and the Short Form‐12 [56] and the Severity of Dependence Scale [56, 63]. Assessments took place at different intervals, ranging from 2 weeks [14, 24, 56] to 3 years after discharge [64]. Objective measures of relapse included positive urinalysis results and readmission to hospitals or inpatient withdrawal treatment. The time‐frame for readmissions varied greatly from within 30 days of discharge [25] to within 2.5 years of discharge [65]. Other outcomes related to relapse include the use of a Maudsley Addiction Profile and author‐created questionnaire to determine factors related to relapse in one study [66] and measuring the effect of social factors on relapse in another [67].
Psychological symptoms
Twenty‐four studies reported outcomes relating to psychological symptoms. Twenty‐three different self‐report measures were used. The most common was the Beck Depression Inventory first or second edition (10 studies, [26, 27, 34, 47, 48, 49, 51, 61, 68, 69]). Depression was one of the most assessed psychological constructs and was also measured in other studies using aspects of other measures, such as the Depression, Anxiety and Stress Scale—21 Items [56, 59, 70], Hamilton Depression Rating Scale [39, 71] and Hospital Anxiety and Depression Scale [44]. The next most frequently used measures were the Addiction Severity Index or EuropASI (European version of Addiction Severity Index) [28, 72, 73] and State–Trait Anxiety Inventory‐Form Y or State–Trait Anxiety Inventory [26, 49, 61].
Retention and dropout
Thirty studies reported outcomes related to treatment retention and dropout. However, they differ widely in their definitions of both. Dropping out of treatment was defined simply as discharge against medical advice in five studies [4, 6, 25, 44, 75]. One study defined treatment dropout as staying less than 4 days in a 2‐week inpatient program [29], another study as a premature self‐discharge, withdrawal of consent for study participation, or development of a relevant comorbidity needing intervention and stabilisation [54] and another study as not completing the entire study trial [52]. Yet another study defined treatment dropout as discharge against medical advice or exclusion due to complications or administrative failure [39]. Dropout was additionally defined simply as non‐completion in two studies [16, 27] and discontinuation of intervention due to assessment refusal or dropout in another study [59]. Similarly, treatment retention was regarded as non‐premature discharge in 13 studies [9, 14, 24, 34, 35, 41, 53, 56, 71, 76, 77, 78] or for 1 study, non‐premature discharge in addition to number of days patients remained in the study [33]. Additionally, two studies defined retention as completing the entire withdrawal treatment process (5 days [12]; 6 days [36]) while another defined retention as remaining enrolled at the end of the 7‐day taper [32]. Other outcomes related to treatment retention include factors (e.g., increased age, personal obligations, etc.) associated with leaving against medical advice in one study [79], predictors of treatment dropout in one study [9] and factors relating to successful withdrawal treatment in another [76].
Length of stay
Similar to retention measures, 15 studies reported outcomes related to length of stay. The main difference between retention and length of stay was that there were no predetermined expectations about the required length of treatment (e.g., at least 7 days). Seven studies included outcomes relating to total length of stay in the facility [4, 6, 22, 24, 29, 40, 80]. Eight studies included outcomes relating to length of withdrawal treatment only [40, 41, 53, 54, 71, 72, 76].
Biological measures
Eighteen studies reported outcomes related to biological measures. In four studies, serum Brain Derived Neurotrophic Factor levels were measured [43, 81, 82, 84]. In two studies each, researchers measured serum tetrahydrocannabinol levels [53, 56], serum 1‐nor‐9‐carboxy‐delta‐9‐tetrahydrocannabinol levels [54, 56] and DNA methylation levels [84, 85]. In one study each, researchers assessed pupil diameter via a pupilometer [31], heart rate variability measured via sleep electroencephalogram [86], platelet counts (predictive of delirium tremens in alcohol withdrawal) [87], plasma total homocysteine concentrations (predictive of alcohol withdrawal seizures, short‐term cognitive deficits during withdrawal and long‐term cerebral atrophy) and blood concentrations of thiamine, riboflavin and pyridoxine [88], plasma oxytocin levels [56], Th1 and Th17‐related cytokine levels in blood [48], PEth serum levels (indicating chronic heavy drinking), aspartate transaminase, alanine transaminase, gamma‐glutamyl transferase levels (liver function indicators) and complete blood count [45], plasma neurotrophic factor levels [65], blood protein content, protein thiol content, protein carbonylation, reduced glutathione and total reactive antioxidant potential [51], and blood and urine biomarkers' associated with withdrawal/relapse/clinical scales (i.e., CDT and EtG, cytokines and growth factors, antioxidant enzymes, oxidative stress markers and neurochemical markers) [10].
Craving
Seventeen studies reported outcomes related to craving. There was a range of different methods used to assess craving including both self‐reported and objective measures. Measures used to assess craving most frequently used a visual analogue scale, utilised in eight studies [10, 12, 33, 34, 39, 46, 59, 77]. The next most frequent measure was the Penn Alcohol Craving Scale (four studies [45, 46, 89, 90]). Another nine craving orientated measures were used in one study each.
Withdrawal complications
Ten studies reported outcomes related to safety in terms of withdrawal complications or adverse events during withdrawal treatment, though the operationalisation of this differed widely between studies. Definitions of complications or adverse events include unspecified adverse events in two studies [29, 33]. In one study each, definitions include seizure, death or delirium [4], needing clinical intervention, referral to a medical or emergency team or death, during or within 72 h of stopping withdrawal treatment [6], events according to the lithium adverse event checklist [56] seizure, falls, delirium or requiring sedation that resulted in withheld doses of drug taper [25], side effects reported during physical examination [47], adverse events assessed via the Systematic Assessment for Treatment Emergent Events [77], delirium tremens, seizures or hallucinations [40], and epileptic seizures, hallucinations, and delirium tremens or pre‐delirium [41].
Follow‐up treatment
Eleven studies reported outcomes related to follow‐up treatment. Five studies operationalised follow‐up treatment as attendance of at least one treatment session after discharge from withdrawal treatment, but the time periods in which treatment had to be attended varied from within 14 days of discharge [30] to within 6 months after intake [91]. Yet another study operationalised it as attending one or five follow‐up treatment sessions within a 1‐year follow‐up period [29]. In one study each, follow‐up treatment has been operationalised as remaining in follow‐up treatment at 6‐month follow up [11], time to entry into follow‐up treatment and number of days in follow‐up treatment at 1, 3 and 6 months [91], prescribed buprenorphine and methadone use in the 30 days prior to 1‐, 3‐ and 6‐month follow‐up [92], contacting an outpatient alcohol treatment clinic within 30 days of discharge and remaining in outpatient treatment at 3 months after discharge [93], use of the clinic's patient transport/taxi to the follow‐up residential or day‐clinic for alcohol use disorders and/or co‐morbid disorder treatment (indicative of utilisation of follow‐up care) [27], induction onto extended‐release naltrexone after withdrawal treatment [94], 12‐step group affiliation (i.e., meeting attendance and 12‐step group involvement) assessed via the Alcoholics Anonymous Affiliation Scale [95] and self‐reported attendance at 12‐step meetings or outpatient treatment [28].
Sleep
Six studies reported outcomes related to sleep during withdrawal treatment. Three studies used Actigraphy, an objective measure [37, 44, 55], two studies used self‐report measures, such as the Pittsburgh Sleep Quality Index [89, 96], and two used the Sleep‐Related Behaviours Questionnaire [44, 89]. Another eight separate studies used eight different measures, including beliefs and attitudes about sleep, hours of sleep and other sleep details (e.g., onset latency, wake time, etc.).
Cognitive measures
Five studies reported outcomes related to cognitive measures. The most frequently used measure was the digit span test in two studies [34, 69] and the Stroop test in two studies [69, 97]. Another two measures were used in only single studies, and these included behavioural and electrophysiological responses to cue reactivity (visual oddball paradigm) and inhibition tasks (go/no‐go task) [98].
General health
Four studies reported outcomes concerning overall health (e.g., aspects of physical and psychological health, quality of life). The measures were self‐report and assessed aspects of both physical and psychological health. The World Health Organization Quality of Life scale was used in two studies [56, 61], the Short Form‐12 was used in one study [56] and the RAND Medical Outcomes Study Short Form Health Survey (Short Form‐36) was used in one study [72].
Physical health
Two studies reported outcomes concerning aspects of physical health. These measures were self‐report, including the Charlston‐Comorbidity Index in one study [39] and HIV risk behaviours assessed via the Revised Risk Behaviour Assessment in one study [99].
Satisfaction
Four studies reported outcomes related to client satisfaction, all using different self‐report measures. Measures were the Treatment Process Questionnaire (which assessed clients' understanding, satisfaction and perceived benefit of the treatment) [72], acceptability of intervention based on whether it: (i) improved their attention; (ii) reduced their craving for methamphetamine; and (iii) was interesting [14], Client Satisfaction Questionnaire [63] and a general client satisfaction measure [42].
Abstinence self‐efficacy
Two studies reported outcomes related to self‐efficacy. Self‐report measures used were the 12‐item versions of the Alcohol Abstinence Self‐Efficacy Scale and Drug Abstinence Self‐Efficacy Scale in one study [62] and the Brief Situational Confidence Questionnaire in another [28].
Others
Three studies reported outcomes that did not fit into the above categories. These measures were employment and death observed 11 years after withdrawal treatment in one study [16], motivation to continue treatment assessed via the German short form of the University of Rhode Island Change Assessment and the ‘Veränderungsstadien–Skala’, also known as the Stages of Change Scale, in one study [27], and self‐reported religious coping via the Brief Measure of Religious Coping in one study [100].
4. DISCUSSION
Overall, the assessment of withdrawal treatment outcomes seems to mainly concern psychological (e.g., craving, mental health) and biological markers (e.g., withdrawal symptom severity, biomarkers), with social outcomes (e.g., homelessness, quality of social or family relationships) very rarely measured. The results indicate that the field of withdrawal treatment research is concerned with the assessment of withdrawal treatment outcomes beyond abstinence alone. Thus, our results align with research showing that withdrawal treatment services consider outcomes beyond abstinence as important [101, 102]. One conceptual framework of recovery proposes seven aspects that should be measured: physical, biological (i.e., biomarkers), psychological, psychiatric, family, social and spiritual aspects as well as chemical dependence [103]. The SAMHSA recovery model [21] also promotes a holistic view of recovery that includes biological, psychological and social functioning. Another framework for measuring substance use outcomes holistically is the Australian Treatment Outcomes Profile which measures risk, health and wellbeing, and current substance use for those receiving treatment [104]. If such frameworks extend to withdrawal treatment services, then results from the current review clearly indicate that additional measures of social outcomes are warranted. Further support for the measurement of social outcomes comes from a survey of alcohol and drug users and their loved ones, which revealed that, in addition to abstinence and health, improved social circumstances (e.g., stable housing), supportive relationships, confidence, and wellbeing of family and friends were also seen as desirable outcomes for withdrawal treatment [102].
Despite few measures of social outcomes, the review revealed an extremely wide range of outcomes that researchers use to determine the outcomes of withdrawal treatment. Even within particular domains, diverse measures were used. For example, craving was measured in 17 different studies using 21 different instruments, spanning both self‐reported and objective measures, as well as different questionnaires. There is a stark lack of consensus regarding measurements, even within a single domain, which makes it difficult for clinicians and professionals to compare findings or evaluate relative effectiveness of withdrawal treatment services. This is particularly important when trying to understand the relative merits of withdrawal treatment services of varying lengths, content and for different drug types.
The review reveals that in some areas (e.g., psychological symptoms distress) there are multiple measures that have satisfactory psychometric characteristics to choose from. This may require some consensus at least within specific domains such as the assessment of depressive symptoms. Similarly, greater clarity regarding criteria for determining dropout and/or reporting retention is needed. It is unlikely that consensus could be found for measures in all domains, but it may be possible to obtain some agreement for particular domains. Examples of such efforts include the Outcome Reporting in Brief Intervention Trials: Alcohol (ORBITAL) Core Outcome Set [105, 106]. The ORBITAL Core Outcome Set proposes a set of outcome domains to measure in interventions as well as which tools should be used for assessment. For example, it proposes that recent alcohol consumption should be measured as the total number of standard drinks consumed within the last week, in grams, via timeline follow back and using a standard drink guide. The ORBITAL recommendations are composed entirely of self‐report measures and although it has been acknowledged that self‐reports carry risk of under‐reporting [107], the authors of the ORBITAL recommendations argue that objective measures such as biomarkers are not accessible in every country. Thus, the inclusion of objective measures would not be viable in creating a list of outcomes that is accessible to all studies [106].
Not all of the ORBITAL recommendations are suitable for measuring outcomes immediately after withdrawal treatment since most programs require strict abstinence. Further, ORBITAL was designed for alcohol use [106]. Thus, development of recommended outcomes and associated measures for withdrawal treatment would necessitate additional measures and methods that focus specifically on outcomes from withdrawal treatment services. The current review provides a starting resource for such a process.
Another finding from the literature review was a paucity of studies assessing client satisfaction as a withdrawal treatment outcome. Indeed, only 4 of the 88 studies in this review attempted to measure client satisfaction. Similar to other outcome domains, each of these four studies measured client satisfaction differently. The lack of assessment of client satisfaction and experience across studies looking at withdrawal treatment outcomes is concerning given the positive correlation between client satisfaction and treatment completion [108] as well as longer‐term treatment outcomes [109].
Similarly, other key social outcomes (e.g., housing) highlighted in more recent recovery frameworks [21, 103] were rarely assessed. Access to stable accommodation has been highlighted as one important component of recovery [102]. Homelessness has been linked to non‐attendance at follow‐up treatment after discharge from withdrawal treatment [8] and increased re‐admission rates to withdrawal treatment [110]. Thus, assessing whether accommodation needs have been satisfactorily addressed during withdrawal treatment is an area of outcome assessment worthy of increased attention. Finally, future research should investigate levels of treatment success in inpatient withdrawal treatment settings.
4.1. Limitations
The most significant limitation of this review was its scope. The analysis was restricted to articles published in English in 2012 or later and six databases. Additionally, our decision to restrict the scope of this article to only inpatient withdrawal treatment programs could limit the generalisability of our results to other types of withdrawal treatment programs, such as outpatient withdrawal treatment programs. However, the limited scope to studies published in 2012 and later allowed the review to focus on outcomes subsequent to more recent developments in the field, particularly recovery frameworks (e.g., [21]).
This review includes studies from multiple countries around the world with differing healthcare systems (e.g., universal health‐care systems, insurance systems, etc.), which could impact both the types of withdrawal treatment outcomes assessed as well as the results of these outcomes.
5. CONCLUSION
This literature review has highlighted three important aspects of withdrawal treatment outcome measurement that should be addressed. First, there needs to be greater consensus about the outcomes domains that should be assessed. Second, within domains, a narrower range of measures could be recommended. This might involve a review and consensus process similar to ORBITAL [103, 106]. Third, there are several social outcome domains that are rarely assessed but given their importance in recent consumer and recovery definitions, are clearly worthy of inclusion in future research (e.g., client satisfaction, social/family relationships, housing).
AUTHOR CONTRIBUTIONS
Each author certifies that their contribution to this work meets the standards of the International Committee of Medical Journal Editors.
CONFLICT OF INTEREST
No external funding was received by any authors for this study.
Supporting information
Table 1. Summary of withdrawal treatment outcomes in inpatient settings for alcohol users
Table 2. Summary of withdrawal treatment outcomes in inpatient settings for opioid users
Table 3. Summary of withdrawal treatment outcomes in inpatient settings for other drug users
ACKNOWLEDGEMENT
Open access publishing facilitated by University of Wollongong, as part of the Wiley ‐ University of Wollongong agreement via the Council of Australian University Librarians.
Wang J, Deane FP, Kelly PJ, Robinson L. A narrative review of outcome measures used in drug and alcohol inpatient withdrawal treatment research. Drug Alcohol Rev. 2023;42(2):415–426. 10.1111/dar.13591
REFERENCES
- 1. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382:1575–86. [DOI] [PubMed] [Google Scholar]
- 2. Ciobanu LG, Ferrari AJ, Erskine HE, Santomauro DF, Charlson FJ, Leung J, et al. The prevalence and burden of mental and substance use disorders in Australia: findings from the global burden of disease study 2015. Aust N Z J Psychiatry. 2018;52:483–90. [DOI] [PubMed] [Google Scholar]
- 3. Australian Institute of Health and Welfare . Alcohol and other drug treatment services in Australia annual report; 2022. Available from https://www.aihw.gov.au/reports/alcohol-other-drug-treatment-services/alcohol-other-drug-treatment-services-australia
- 4. Beyraghi N, Meybodi SS, Yazdi SSN, Janani M, Banihashem SS, Bahri R, et al. Results from the first inpatient alcohol withdrawal management program in Iran: an observational study. Alcohol. 2020;88:43–7. [DOI] [PubMed] [Google Scholar]
- 5. DiPaula BA, Schwartz R, Montoya ID, Barrett D, Tang C. Heroin detoxification with buprenorphine on an inpatient psychiatric unit. J Subst Abus Treat. 2002;23:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garhy MA, Williams JJ, Mohan S, Sharib HA. Novel opioid detoxification using loperamide and P‐glycoprotein inhibitor. Prog Neurol Psychiatry. 2019;23:19–24. [Google Scholar]
- 7. Ponizovsky AM, Grinshpoon A, Margolis A, Cohen R, Rosca P. Well‐being, psychosocial factors, and side‐effects among heroin‐dependent inpatients after detoxification using buprenorphine versus clonidine. Addict Behav. 2006;31:2002–13. [DOI] [PubMed] [Google Scholar]
- 8. Ford LK, Zarate P. Closing the gaps: the impact of inpatient detoxification and continuity of care on client outcomes. J Psychoactive Drugs. 2010;42(Suppl 6):303–14. [DOI] [PubMed] [Google Scholar]
- 9. Sofin Y, Danker‐Hopfe H, Gooren T, Neu P. Predicting inpatient detoxification outcome of alcohol and drug dependent patients: the influence of sociodemographic environment, motivation, impulsivity, and medical comorbidities. J Addict. 2017;2017:6415831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coccini T, Ottonello M, Spigno P, Malovini A, Fiabane E, Roda E, et al. Biomarkers for alcohol abuse/withdrawal and their association with clinical scales and temptation to drink. A prospective pilot study during 4‐week residential rehabilitation. Alcohol. 2021;94:43–56. [DOI] [PubMed] [Google Scholar]
- 11. Cushman PA, Liebschutz JM, Anderson BJ, Moreau MR, Stein MD. Buprenorphine initiation and linkage to outpatient buprenorphine do not reduce frequency of injection opiate use following hospitalization. J Subst Abus Treat. 2016;68:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baxley C, Weinstock J, Lustman PJ, Garner AA. The influence of anxiety sensitivity on opioid use disorder treatment outcomes. Exp Clin Psychopharmacol. 2019;27:64–77. [DOI] [PubMed] [Google Scholar]
- 13. McGovern MP, Caputo GC. Outcome prediction of inpatient alcohol detoxification. Addict Behav. 1983;8:167–71. [DOI] [PubMed] [Google Scholar]
- 14. Manning V, Garfield JB, Mroz K, Campbell SC, Piercy H, Staiger PK, et al. Feasibility and acceptability of approach bias modification during methamphetamine withdrawal and related methamphetamine use outcomes. J Subst Abus Treat. 2019;106:12–8. [DOI] [PubMed] [Google Scholar]
- 15. Foster JH, Peters TJ, Marshall EJ. Quality of life measures and outcome in alcohol‐dependent men and women. Alcohol. 2000;22:45–52. [DOI] [PubMed] [Google Scholar]
- 16. Naderi‐Heiden A, Gleiss A, Bäcker C, Bieber D, Nassan‐Agha H, Kasper S, et al. Mortality and employment after in‐patient opiate detoxification. Eur Psychiatry. 2012;27:294–300. [DOI] [PubMed] [Google Scholar]
- 17. Nieuwenhuys A, Oudejans RR. Anxiety and perceptual‐motor performance: toward an integrated model of concepts, mechanisms, and processes. Psychol Res. 2012;76:747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furley P, Goldschmied N. Systematic vs. narrative reviews in sport and exercise psychology: is either approach superior to the other? Front Psychol. 2021;12:685082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horton LG, Simmons KB, Curtis KM. Combined hormonal contraceptive use among obese women and risk for cardiovascular events: a systematic review. Contraception. 2016;94:590–604. [DOI] [PubMed] [Google Scholar]
- 20. Alcohol and Drug Foundation. (9 May 2022). Our purpose, mission, and focus. Available from: https://adf.org.au/about/our-purpose-mission-focus/
- 21. Substance Abuse and Mental Health Services Administration (SAMHSA) . SAMHSA's Working Definition of Recovery: 10 Guiding Principles of Recovery; 2012. Available from: https://store.samhsa.gov/sites/default/files/d7/priv/pep12-recdef.pdf
- 22. Firouzkouhi M, Hashemian SS, Pishjoo M, Ghasemi S, Hajebi A, Noroozi A. Inpatient opioid withdrawal management of street children and adolescents admitted to the pediatric and adolescent psychiatric ward: a preliminary case series. Iran J Pediatr. 2016;26:e5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Behnam B, Semnani V, Saghafi N, Ghorbani R, Shori MD, Choobmasjedi SG. Gabapentin effect on pain associated with heroin withdrawal in Iranian crack: a randomized double‐blind clinical trial. Iran J Pharm Res. 2012;11:979–83. [PMC free article] [PubMed] [Google Scholar]
- 24. Kiepek N, Groom B, Toppozini D, Kakekagumick K, Muileboom J, Len Kelly MD. Evaluation of an inpatient medical withdrawal program in rural Ontario: a 1‐year prospective study. Can J Rural Med. 2015;20:92–7. [PubMed] [Google Scholar]
- 25. Kawasaki SS, Jacapraro JS, Rastegar DA. Safety and effectiveness of a fixed‐dose phenobarbital protocol for inpatient benzodiazepine detoxification. J Subst Abus Treat. 2012;43:331–4. [DOI] [PubMed] [Google Scholar]
- 26. Olivia F, Nibbio G, Vizzuso P, Sodano AJ, Ostacoli L, Carletto S, et al. Gender differences in anxiety and depression before and after alcohol detoxification: anxiety and depression as gender‐related predictors of relapse. Eur Addict Res. 2018;24:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ostergaard M, Jatzkowski L, Seitz R, Speidel S, Weber T, Lübke N, et al. Integrated treatment at the first stage: increasing motivation for alcohol patients with comorbid disorders during inpatient detoxification. Alcohol Alcohol. 2018;53:719–27. [DOI] [PubMed] [Google Scholar]
- 28. Timko C, Below M, Vittorio L, Taylor E, Chang G, Lash S, et al. Randomized controlled trial of enhanced telephone monitoring with detoxification patients: 3‐and 6‐month outcomes. J Subst Abus Treat. 2019;99:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azuar J, Questel F, Hispard E, Scott J, Vorspan F, Bellivier F. Hospital stay and engagement in outpatient follow‐up after alcohol emergency detox: a 1‐year comparison study. Alcohol Clin Exp Res. 2016;40:418–21. [DOI] [PubMed] [Google Scholar]
- 30. Lee MT, Horgan CM, Garnick DW, Acevedo A, Panas L, Ritter G, et al. A performance measure for continuity of care after detoxification: relationship with outcomes. J Subst Abus Treat. 2014;47:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunn KE, Tompkins DA, Bigelow GE, Strain EC. Efficacy of tramadol extended‐release for opioid withdrawal: a randomized clinical trial. JAMA Psychiat. 2017;74:885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunn KE, Weerts EM, Huhn AS, Schroeder JR, Tompkins DA, Bigelow GE, et al. Preliminary evidence of different and clinically meaningful opioid withdrawal phenotypes. Addict Biol. 2020;25:e12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo S, Manning V, Yang Y, Koh PK, Chan E, de Souza NN, et al. Lofexidine versus diazepam for the treatment of opioid withdrawal syndrome: a double‐blind randomized clinical trial in Singapore. J Subst Abus Treat. 2018;91:1–11. [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Li L, Shen W, Shen X, Yang G, Zhou W. Scopolamine detoxification technique for heroin dependence: a randomized trial. CNS Drugs. 2013;27:1093–102. [DOI] [PubMed] [Google Scholar]
- 35. Mannelli P, Peindl K, Wu LT, Patkar AA, Gorelick DA. The combination very low‐dose naltrexone–clonidine in the management of opioid withdrawal. Am J Drug Alcohol Abuse. 2012;38:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mannelli P, Wu LT, Peindl KS, Gorelick DA. Smoking and opioid detoxification: behavioral changes and response to treatment. Nicotine Tob Res. 2013;15:1705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pjrek E, Frey R, Naderi‐Heiden A, Strnad A, Kowarik A, Kasper S, et al. Actigraphic measurements in opioid detoxification with methadone or buprenorphine. J Clin Psychopharmacol. 2012;2012(32):75–82. [DOI] [PubMed] [Google Scholar]
- 38. Kamal RM, Dijkstra BA, Loonen AJ, De Jong CA. The effect of co‐occurring substance use on gamma‐hydroxybutyric acid withdrawal syndrome. J Addict Med. 2016;10:229–35. [DOI] [PubMed] [Google Scholar]
- 39. Förg A, Hein J, Volkmar K, Winter M, Richter C, Heinz A, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo‐controlled trial. Alcohol Alcohol. 2012;47:149–55. [DOI] [PubMed] [Google Scholar]
- 40. Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal‐related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin. 2019;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soravia LM, Wopfner A, Pfiffner L, Bétrisey S, Moggi F. Symptom‐triggered detoxification using the alcohol‐withdrawal‐scale reduces risks and healthcare costs. Alcohol Alcohol. 2018;53:71–7. [DOI] [PubMed] [Google Scholar]
- 42. Lin SK, Pan CH, Chen CH. A double‐blind, placebo‐controlled trial of dextromethorphan combined with clonidine in the treatment of heroin withdrawal. J Clin Psychopharmacol. 2014;34:508–12. [DOI] [PubMed] [Google Scholar]
- 43. Kethawath SM, Jain R, Dhawan A, Sarkar S. Trajectory of liver function tests during the initial alcohol detoxification period: a preliminary analysis. Indian J Psychiatry. 2020;62:225–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith N, Hill R, Marshall J, Keaney F, Wanigaratne S. Sleep related beliefs and their association with alcohol relapse following residential alcohol detoxification treatment. Behav Cogn Psychother. 2014;42:593–604. [DOI] [PubMed] [Google Scholar]
- 45. Sonmez MB, Cinar RK, Gorgulu Y, Kilic EK, Unal A. Evaluation of phosphatidylethanol by ELISA for detection of excessive alcohol use compared with traditional biomarkers: a case‐control study. Psychiatr Clin Psychopharmacol. 2017;27:41–6. [Google Scholar]
- 46. Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov‐Polevoi A, et al. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;2013(37):484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mash DC, Duque L, Page B, Allen‐Ferdinand K. Ibogaine detoxification transitions opioid and cocaine abusers between dependence and abstinence: clinical observations and treatment outcomes. Front Pharmacol. 2018;9:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levandowski ML, Viola TW, Prado CH, Wieck A, Bauer ME, Brietzke E, et al. Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend. 2016;167:140–8. [DOI] [PubMed] [Google Scholar]
- 49. Pérez de los Cobos J, Alcaraz S, Verdejo‐García A, Muñoz L, Siñol N, Fernández‐Serrano MJ, et al. Factors associated with the absence of cocaine craving in treatment‐seeking individuals during inpatient cocaine detoxification. Am J Drug Alcohol Abuse. 2021;47:127–38. [DOI] [PubMed] [Google Scholar]
- 50. Viola TW, Tractenberg SG, Wearick‐Silva LE, de Oliveira Rosa CS, Pezzi JC, Grassi‐Oliveira R. Long‐term cannabis abuse and early‐onset cannabis use increase the severity of cocaine withdrawal during detoxification and rehospitalization rates due to cocaine dependence. Drug Alcohol Depend. 2014;144:153–9. [DOI] [PubMed] [Google Scholar]
- 51. Zaparte A, Viola TW, Grassi‐Oliveira R, da Silva MM, Moreira JC, Bauer ME. Early abstinence of crack‐cocaine is effective to attenuate oxidative stress and to improve antioxidant defences. Psychopharmacology (Berl). 2015;232:1405–13. [DOI] [PubMed] [Google Scholar]
- 52. Amiri S, Malek A, Tofighnia F, Asl BH, Seidy A. Amantadine as augmentation in managing opioid withdrawal with clonidine: a randomized controlled trial. Iran J Psychiatry. 2014;9:142–6. [PMC free article] [PubMed] [Google Scholar]
- 53. Bonnet U, Specka M, Stratmann U, Ochwadt R, Scherbaum N. Abstinence phenomena of chronic cannabis‐addicts prospectively monitored during controlled inpatient detoxification: cannabis withdrawal syndrome and its correlation with delta‐9‐tetrahydrocannabinol and‐metabolites in serum. Drug Alcohol Depend. 2014;143:189–97. [DOI] [PubMed] [Google Scholar]
- 54. Claus BB, Specka M, McAnally H, Scherbaum N, Schifano F, Bonnet U. Is the urine cannabinoid level measured via a commercial point‐of‐care semiquantitative immunoassay a cannabis withdrawal syndrome severity predictor? Front Psych. 2020;11:598150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Allsop DJ, Bartlett DJ, Johnston J, Helliwell D, Winstock A, McGregor IS, et al. The effects of lithium carbonate supplemented with nitrazepam on sleep disturbance during cannabis abstinence. J Clin Sleep Med. 2015;11:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, et al. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo‐controlled trial in an inpatient setting. Psychopharmacology. 2014;231:4623–36. [DOI] [PubMed] [Google Scholar]
- 57. Silverman MJ. Effects of a single lyric analysis intervention on withdrawal and craving with inpatients on a detoxification unit: a cluster‐randomized effectiveness study. Subst Use Misuse. 2016;51:241–9. [DOI] [PubMed] [Google Scholar]
- 58. de Haan HA, van der Palen J, Wijdeveld TG, Buitelaar JK, De Jong CA. Alexithymia in patients with substance use disorders: state or trait? Psychiatry Res. 2014;216:137–45. [DOI] [PubMed] [Google Scholar]
- 59. Ooms M, Roozen HG, Willering JH, Zijlstra WP, de Waart R, Goudriaan AE. Effects of multiple detoxifications on withdrawal symptoms, psychiatric distress and alcohol‐craving in patients with an alcohol use disorder. Behav Med. 2021;47:296–310. [DOI] [PubMed] [Google Scholar]
- 60. Stevens L, Goudriaan AE, Verdejo‐Garcia A, Dom G, Roeyers H, Vanderplasschen W. Impulsive choice predicts short‐term relapse in substance‐dependent individuals attending an in‐patient detoxification programme. Psychol Med. 2015;45:2083–93. [DOI] [PubMed] [Google Scholar]
- 61. Picci RL, Oliva F, Zuffranieri M, Vizzuso P, Ostacoli L, Sodano AJ, et al. Quality of life, alcohol detoxification and relapse: is quality of life a predictor of relapse or only a secondary outcome measure? Qual Life Res. 2014;23:2757–67. [DOI] [PubMed] [Google Scholar]
- 62. Berman AH, Lindqvist H, Källmén H, Durbeej N, Hermansson U, Forsberg L. Counselor and drug detox inpatient verbal behaviors in a single session of motivational interviewing and subsequent substance use‐related patient outcomes. Int J Ment Heal Addict. 2019;17:73–88. [Google Scholar]
- 63. Mills KL, Ewer P, Dore G, Teesson M, Baker A, Kay‐Lambkin F, et al. The feasibility and acceptability of a brief intervention for clients of substance use services experiencing symptoms of post traumatic stress disorder. Addict Behav. 2014;39:1094–9. [DOI] [PubMed] [Google Scholar]
- 64. McKetin R, Najman JM, Baker AL, Lubman DI, Dawe S, Ali R, et al. Evaluating the impact of community‐based treatment options on methamphetamine use: findings from the methamphetamine treatment evaluation study (MATES). Addiction. 2012;107:1998–2008. [DOI] [PubMed] [Google Scholar]
- 65. Viola TW, Tractenberg SG, Levandowski ML, Pezzi JC, Bauer ME, Teixeira AL, et al. Neurotrophic factors in women with crack cocaine dependence during early abstinence: the role of early life stress. J Psychiatry Neurosci. 2014;39:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ducray K, Darker C, Smyth BP. Situational and psycho‐social factors associated with relapse following residential detoxification in a population of Irish opioid dependent patients. Ir J Psychol Med. 2012;29:72–9. [DOI] [PubMed] [Google Scholar]
- 67. Van den Berg JF, Van den Brink W, Kist N, Hermes JS, Kok RM. Social factors and readmission after inpatient detoxification in older alcohol‐dependent patients. Am J Addict. 2015;2015(24):661–6. [DOI] [PubMed] [Google Scholar]
- 68. Ostergaard M, Seitz R, Jatzkowski L, Speidel S, Höcker W, Odenwald M. Changes of self‐reported PTSD and depression symptoms during alcohol detoxification treatment. J Dual Diagn. 2019;2019(15):123–9. [DOI] [PubMed] [Google Scholar]
- 69. Petit G, Luminet O, de Sousa C, Uva M, Zorbas A, Maurage P, et al. Differential spontaneous recovery across cognitive abilities during detoxification period in alcohol‐dependence. PLoS One. 2017;12:e0176638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beaufort IN, De Weert‐Van Oene GH, Buwalda VA, de Leeuw JRJ, Goudriaan AE. The depression, anxiety and stress scale (DASS‐21) as a screener for depression in substance use disorder inpatients: a pilot study. Eur Addict Res. 2017;23:260–8. [DOI] [PubMed] [Google Scholar]
- 71. Bonnet U, Borda T, Scherbaum N, Specka M. Abstinence phenomena of chronic cannabis‐addicts prospectively monitored during controlled inpatient detoxification (part II): psychiatric complaints and their relation to delta‐9‐tetrahydrocannabinol and its metabolites in serum. Drug Alcohol Depend. 2015;155:302–6. [DOI] [PubMed] [Google Scholar]
- 72. Lennox RD, Cecchini‐Sternquist M. Safety and tolerability of sauna detoxification for the protracted withdrawal symptoms of substance abuse. J Int Med Res. 2018;46:4480–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vederhus JK, Zemore SE, Rise J, Clausen T, Høie M. Predicting patient post‐detoxification engagement in 12‐step groups with an extended version of the theory of planned behavior. Addict Sci Clin Pract. 2015;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hakansson A, Hallén E. Predictors of dropout from inpatient opioid detoxification with buprenorphine: a chart review. J Addict. 2014;2014:965267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li X, Sun H, Marsh DC, Anis AH. Factors associated with pretreatment and treatment dropouts: comparisons between aboriginal and non‐aboriginal clients admitted to medical withdrawal management. Harm Reduct J. 2013;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stewart RD, Nelson DB, Adhikari EH, McIntire DD, Roberts SW, Dashe JS, et al. The obstetrical and neonatal impact of maternal opioid detoxification in pregnancy. Am J Obstet Gynecol. 2013;209:267.e1–5. [DOI] [PubMed] [Google Scholar]
- 77. Mongeau‐Pérusse V, Brissette S, Bruneau J, Conrod P, Dubreucq S, Gazil G, et al. Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: a randomized placebo‐controlled trial. Addiction. 2021;116:2431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stevens L, Verdejo‐García A, Roeyers H, Goudriaan AE, Vanderplasschen W. Delay discounting, treatment motivation and treatment retention among substance‐dependent individuals attending an in inpatient detoxification program. J Subst Abus Treat. 2015;49:58–64. [DOI] [PubMed] [Google Scholar]
- 79. Person US, Lin M, Fogel J, Parrill A, Bishev D, Takhi M, et al. Factors associated with leaving against medical advice from inpatient substance use detoxification treatment. Addict Disord Their Treat. 2021;20:507–16. [Google Scholar]
- 80. Quelch D, Pucci M, Marsh A, Coleman J, Bradberry S. Elective alcohol detoxification–a resource and efficacy evaluation. Future Healthc J. 2019;6:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sarkar S, Jain R, Kethawath SM, Gupta R, Kumar M. Serum BDNF levels in patients with opioid dependence during the early withdrawal period: a case control study. Neurosci Lett. 2018;681:100–4. [DOI] [PubMed] [Google Scholar]
- 82. Corominas‐Roso M, Roncero C, Daigre C, Grau‐Lopez L, Ros‐Cucurull E, Rodríguez‐Cintas L, et al. Changes in brain‐derived neurotrophic factor (BDNF) during abstinence could be associated with relapse in cocaine‐dependent patients. Psychiatry Res. 2015;225:309–14. [DOI] [PubMed] [Google Scholar]
- 83. Corominas‐Roso M, Roncero C, Eiroa‐Orosa FJ, Gonzalvo B, Grau‐Lopez L, Ribases M, et al. Brain‐derived neurotrophic factor serum levels in cocaine‐dependent patients during early abstinence. Eur Neuropsychopharmacol. 2013;23:1078–84. [DOI] [PubMed] [Google Scholar]
- 84. Koller G, Zill P, Soyka M, Adorjan K, Weiss C, Kern A, et al. Short‐term changes in global methylation and hydroxymethylation during alcohol detoxification. Eur Neuropsychopharmacol. 2019;2019(29):897–903. [DOI] [PubMed] [Google Scholar]
- 85. Witt SH, Frank J, Frischknecht U, Treutlein J, Streit F, Foo JC, et al. Acute alcohol withdrawal and recovery in men lead to profound changes in DNA methylation profiles: a longitudinal clinical study. Addiction. 2020;115:2034–44. [DOI] [PubMed] [Google Scholar]
- 86. Ganesha S, Thirthalli J, Muralidharan K, Benegal V, Gangadhar BN. Heart rate variability during sleep in detoxified alcohol‐dependent males: a comparison with healthy controls. Indian J Psychiatry. 2013;55:173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Harshe DG, Thadasare H, Karia SB, De Sousa A, Cholera RM, Kale SS, et al. A study of patterns of platelet counts in alcohol withdrawal. Indian J Psychol Med. 2017;39:441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Heese P, Linnebank M, Semmler A, Muschler MA, Heberlein A, Frieling H, et al. Alterations of homocysteine serum levels during alcohol withdrawal are influenced by folate and riboflavin: results from the German Investigation on Neurobiology in Alcoholism (GINA). Alcohol Alcohol. 2012;47:497–500. [DOI] [PubMed] [Google Scholar]
- 89. Brooks AT, Krumlauf M, Fryer CS, Beck KH, Yang L, Ramchandani VA, et al. Critical transitions: a mixed methods examination of sleep from inpatient alcohol rehabilitation treatment to the community. PLoS One. 2016;11:e0161725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Janakiraman R, Gowin JL, Sloan ME, Schwandt ML, Diazgranados N, Ramchandani VA, et al. History of suicidality and alcohol craving trajectories during inpatient treatment for alcohol use disorder. Drug Alcohol Depend. 2020;209:107918. [DOI] [PubMed] [Google Scholar]
- 91. Liebschutz JM, Crooks D, Herman D, Anderson B, Tsui J, Meshesha LZ, et al. Buprenorphine treatment for hospitalized, opioid‐dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stein M, Herman D, Conti M, Anderson B. Bailey GInitiating buprenorphine treatment for opioid use disorder during short‐term in‐patient ‘detoxification’: a randomized clinical trial. Addiction. 2020;115:82–94. [DOI] [PubMed] [Google Scholar]
- 93. Nielsen AS, Nielsen B. Outreach visits improve referral of alcohol dependent patients from psychiatric hospital to continued care. A randomized trial. Nord J Psychiatry. 2018;72:303–10. [DOI] [PubMed] [Google Scholar]
- 94. Shulman M, Choo TH, Scodes J, Pavlicova M, Wai J, Haenlein P, et al. Association between methadone or buprenorphine use during medically supervised opioid withdrawal and extended‐release injectable naltrexone induction failure. J Subst Abus Treat. 2021;124:108292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vederhus JK, Timko C, Kristensen Ø, Hjemdahl B, Clausen T. Motivational intervention to enhance post‐detoxification 12‐step group affiliation: a randomized controlled trial. Addiction. 2014;109:766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Neu P, Sofin Y, Danker‐Hopfe H. The effect of detoxification on sleep: how does sleep quality change during qualified detoxification treatment? J Addict. 2018;2018:9492453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Marhe R, Waters AJ, van de Wetering BJM, Franken IHA. Implicit and explicit drug‐related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J Consult Clin Psychol. 2013;81:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Campanella S, Schroder E, Kajosch H, Hanak C, Veeser J, Amiot M, et al. Neurophysiological markers of cue reactivity and inhibition subtend a three‐month period of complete alcohol abstinence. Clin Neurophysiol. 2020;131:555–65. [DOI] [PubMed] [Google Scholar]
- 99. Wechsberg WM, Krupitsky E, Romanova T, Zvartau E, Kline TL, Browne FA, et al. Double jeopardy‐‐drug and sex risks among Russian women who inject drugs: initial feasibility and efficacy results of a small randomized controlled trial. Subst Abuse Treat Prev Policy. 2012;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Puffer ES, Skalski LM, Meade CS. Changes in religious coping and relapse to drug use among opioid‐dependent patients following inpatient detoxification. J Relig Health. 2012;51:1226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Thurgood S, Crosby H, Raistrick D, Tober G. Service user, family and friends' views on the meaning of a ‘good outcome’ of treatment for an addiction problem. Drugs Educ Prev Policy. 2014;21:324–32. [Google Scholar]
- 102. Dodge K, Krantz B, Kenny PJ. How can we begin to measure recovery? Subst Abuse Treat Prev Policy. 2010;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Deacon RM, Mammen K, Bruno R, Mills L, Dunlop A, Holmes J, et al. Assessing the concurrent validity, inter‐rater reliability and test–re‐test reliability of the Australian treatment outcomes profile (ATOP) in alcohol and opioid treatment populations. Addiction. 2021;116:1245–55. [DOI] [PubMed] [Google Scholar]
- 104. Shorter GW, Bray JW, Heather N, Berman AH, Giles EL, Clarke M, et al. The “outcome reporting in brief intervention trials: alcohol” (ORBITAL) core outcome set: international consensus on outcomes to measure in efficacy and effectiveness trials of alcohol brief interventions. J Stud Alcohol Drugs. 2021;2021(82):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shorter GW, Heather N, Berman AH, Giles EL, Barbosa C, Monteiro MG, et al. The ORBITAL core outcome set: response to on biomarkers and methodological innovation in core outcome sets. J Stud Alcohol Drugs. 2022;83:298–300. [PubMed] [Google Scholar]
- 106. de Bejczy A, Wallhed Finn S, Söderpalm B, Andreasson S. Outcome measures in alcohol studies: a comment on the ORBITAL Core outcome set (Shorter et al., 2021). J Stud Alcohol Drugs. 2022;83:296–7. [PubMed] [Google Scholar]
- 107. Mutter R, Ali MM. Factors associated with completion of alcohol detoxification in residential settings. J Subst Abus Treat. 2019;98:53–8. [DOI] [PubMed] [Google Scholar]
- 108. Acevedo A, Harvey N, Kamanu M, Tendulkar S, Fleary S. Barriers, facilitators, and disparities in retention for adolescents in treatment for substance use disorders: a qualitative study with treatment providers. Subst Abuse Treat Prev Policy. 2020;15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Carrier E, McNeely J, Lobach I, Tay S, Gourevitch MN, Raven MC. Factors associated with frequent utilization of crisis substance use detoxification services. J Addict Dis. 2011;30:116–22. [DOI] [PubMed] [Google Scholar]
- 110. Maffina L, Deane FP, Lyons GCB, Crowe TP, Kelly PJ. Relative importance of abstinence in clients' and clinicians' perspectives of recovery from drug and alcohol abuse. Subst Use Misuse. 2013;2013(48):683–90. [DOI] [PubMed] [Google Scholar]
- 111. Hufnagel A, Frick U, Ridinger M, Wodarz N. Recovery from alcohol dependence: do smoking indicators predict abstinence? Am J Addict. 2017;26:366–73. [DOI] [PubMed] [Google Scholar]
- 112. Lucht M, Quellmalz A, Mende M, Broda A, Schmiedeknecht A, Brosteanu O, et al. Effect of a 1‐year short message service in detoxified alcohol‐dependent patients: a multi‐center, open‐label randomized controlled trial. Addiction. 2021;116:1431–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Summary of withdrawal treatment outcomes in inpatient settings for alcohol users
Table 2. Summary of withdrawal treatment outcomes in inpatient settings for opioid users
Table 3. Summary of withdrawal treatment outcomes in inpatient settings for other drug users


