Abstract
Background
Cervical cancer affects 3197 women in the UK, and 604 000 women worldwide annually, with peak incidence seen in women between 30 and 34 years of age. For many, fertility‐sparing surgery is an appealing option where possible. However, absence of large‐scale data, along with a notable variation in reported outcomes in relevant studies, may undermine future efforts for consistent evidence synthesis.
Objectives
To systematically review the reported outcomes measured in studies that include women who underwent fertility‐sparing surgery for cervical cancer and identify whether variation exists.
Search strategy
We searched MEDLINE, EMBASE and CENTRAL from inception to February 2019.
Selection criteria
Randomised controlled trials, cohort and observational studies, and case studies of more than ten participants from January 1990 to date.
Data collection and analysis
Study characteristics and all reported treatment outcomes.
Main results
A total of 104 studies with a sum of 9535 participants were identified. Most studies reported on oncological outcomes (97/104), followed by fertility and pregnancy (86/104), postoperative complications (74/104), intra‐operative complications (72/104) and quality of life (5/104). There was huge variation and heterogeneity in reported outcomes, with only 12% being good quality and 87% being of poor quality.
Conclusions
There is significant heterogeneity in the reported outcomes. An agreed Core Outcome Set is necessary for future studies to effectively harmonise reported outcomes that are measurable and relevant to patients, clinicians and researchers. This systematic review sets the groundwork for the development of a Core Outcome Set for fertility‐sparing surgery in cervical cancer.
Keywords: cervical cancer, core outcomes, fertility‐sparing
Short abstract
 This article includes Author Insights, a video abstract available at: https://vimeo.com/771508530
This article includes Author Insights, a video abstract available at: https://vimeo.com/771508530
1. INTRODUCTION
Cervical cancer is the fourth most common cancer in women, with a global incidence of 13.1 per 100 000 women annually. 1 The incidence of cervical cancer peaks at 30–34 years, when many women may not have completed their families. 1 Cervical cancer staging involves clinical examination, colposcopy, histological assessment and radiological imaging (magnetic resonance imaging for local extent and computed tomography for distant disease), 2 , 3 , 4 and is based on the International Federation of Obstetrics & Gynaecology (FIGO) 2018 revised classification. 5 , 6 , 7
Generally, early stage (IA1) cervical cancer treatment can be in the form of large loop excision of transformation zone or cone biopsy. The presence of lymphovascular space invasion or stage IA2 disease may necessitate pelvic lymph node dissection to prevent under‐staging and assess the need for adjuvant treatment. Radical hysterectomy with pelvic lymphadenectomy has been the reference standard management for stage IA2 (lymphovascular space invasion) to IB1 disease. 8 , 9 As a principle, stage IA1 through IB1 disease is amenable to surgery subject to individual assessment, although some IB1 cases may be equally or preferably managed with radiation therapy. Stage IB2 and above is usually treated with cisplatin‐based chemoradiation. 10 , 11 , 12 , 13 , 14
The age distribution of cervical cancer implies that a proportion of women may not have completed their family. Regardless, loss of fertility can cause psychological distress and impacts women's quality of life. 15 , 16 , 17 Several fertility‐sparing surgical options have been introduced to address this. These include radical trachelectomy (vaginal, open abdominal, laparoscopic, robotic approaches) with pelvic lymph node assessment. It also includes local treatments in the form of large loop excision of transformation zone, conisation, or simple trachelectomy. Key cornerstone criteria to proceed with fertility‐sparing surgery are the desire for, or the likelihood of, fertility, and oncological safety. 15
1.1. Reported outcomes after a fertility‐sparing approach
FIGO recommends that women diagnosed with cervical cancer FIGO Stage IA1–IB1 can be offered a fertility‐sparing treatment if they wish to conceive. 18 Although these fertility‐sparing surgical alternatives have been in practice for over three decades, questions remain regarding oncological safety, their efficacy and outcomes, and the superiority of one procedure over another. 15 , 19 , 20 , 21 , 22 To address this issue, clinicians require robust data from high‐quality systematic reviews and/or large‐scale prospective studies. A move forward towards this direction would need global consensus on achieving homogeneously reported outcomes in such studies. For example, several original studies report a melange of outcomes tailored to measure cancer survival, surgical morbidity, sexual function after treatment, pregnancy success rates and other vital outcomes. 23 , 24 , 25 , 26 , 27 However, the variation in reporting quality and outcome measures across studies impairs evidence synthesis and poses a hindrance to robust evidence‐based developments in the field.
This challenge has been recognised in other fields of our specialty. To address this, several journal editors together set the foundation for ‘CoRe Outcomes in Women's and Newborn health’ (CROWN) initiative. 28 The CROWN initiative aims to produce, disseminate and implement core outcome sets (COS), which is a stepping stone to advance research quality and usefulness. 29 It also sets the ground for homogenisation of reported outcomes to facilitate evidence synthesis and accommodate the vision of delivering robust evidence. This can form the basis of guidelines and policies to improve decision‐making and evidence‐based practice. 29 By the term COS, we refer to a minimum collection of outcomes with standardised measurement and reporting, which are prioritised by stakeholders, researchers and clinicians. 29 , 30 , 31
To date, there is no reported COS for studies that discuss fertility‐sparing surgery for women diagnosed with cervical cancer. To this end, we performed a systematic review to identify and characterise the variation of reported outcomes in studies investigating fertility‐sparing surgery for cervical cancer. This systematic review aims to form the groundwork for the development of the relevant COS.
2. METHODS
We followed a prospectively designed protocol with distinct study selection criteria. The objectives of this systematic review fell outside the PROSPERO registry criteria. 30 , 32 It was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA, Appendix S2).
2.1. Study eligibility
We included all published randomised control trials (RCT), cohort studies, observational studies and case series with a minimum of ten participants. All participants involved had some form of fertility‐sparing surgery (for example, trachelectomy, conisation, excision) for a confirmed histological diagnosis of adenocarcinoma, squamous cell carcinoma, or adenosquamous carcinoma of the cervix. Studies that involved pregnant women were also included in the analysis.
Study types excluded were case reports, histological diagnoses not previously listed, such as clear cell carcinoma or neuroendocrine neoplasms, studies primarily aimed at assessing pharmacokinetics, mechanism of drugs, technical results of novel devices, radio‐imaging or histological or physiological data. We used a pragmatic date cutoff to capture all studies based on modern practice and excluded studies before 1990.
Systematic review publications were included during the literature review to cross‐reference and identify studies not captured during the initial literature search. Studies reported in conferences or when only an abstract was available were excluded from the final review.
2.2. Search strategy
A systematic literature review was undertaken by searching MEDLINE, EMBASE and CENTRAL until 27 February 2019. 33 , 34 Search terms included ‘cervical cancer’, ‘tumour’, ‘neoplasm’, ‘malignancy’, ‘large loop excision of transformation zone’, ‘lletz’, ‘leep’, ‘cone’, ‘conisation’, ‘cervicectomy’, ‘trachelectomy’, ‘surgery’, ‘biopsy’, ‘fertility’ and ‘fertility sparing’. There was no language restriction applied to the literature search. Appendix S1 describes our search strategy.
2.3. Data extraction
Two reviewers (NY and CB) independently assessed the titles and abstracts using the predefined study eligibility criteria described above. Full articles were then obtained, and data on all reported outcomes were extracted using an agreed prespecified extraction sheet. Discrepancies were resolved by discussion and input from a third party if necessary. Descriptive statistics were used to map the characteristics of reported COS. Data are presented in comprehensive tables.
2.4. Quality assessment
Jadad scoring was used for assessing the methodological quality of RCT. 35 Any study that scored 3 or more (maximum score 5) was considered medium to high quality. Quality of reporting of outcomes in RCT was assessed using the six‐point Management of Otitis Media with Effusion in Cleft Palate (MOMENT) criteria. 36 A trial that scores 4 or more (maximum score 6) is considered high quality.
The quality of non‐randomised studies was scrutinised using the Newcastle–Ottawa Scale. 37
2.5. Patient involvement
There was no direct patient involvement in this systematic review.
2.6. Core outcomes
There are no previously stated core outcomes within our field of study. Therefore, this systematic review will form part of the process in developing a set of core outcomes for women diagnosed with cervical cancer and undergoing fertility‐sparing surgery as part of the Core Outcome sets for Gynaecological conditions (COGS) project.
3. RESULTS
The literature search yielded a total of 937 studies, of which 355 duplicates were removed; 582 titles were screened against our inclusion criteria, and 452 abstracts were fully assessed. Of those abstracts, 130 full texts were scrutinised, and 51 failed to meet the inclusion criteria, leaving 79 studies for inclusion in our analysis. 25 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 Additionally, the literature search yielded several systematic reviews, which were manually assessed, and we identified a further 25 studies not captured by the initial literature search. 26 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139
In total, 104 studies were included for the final analysis, with a cumulative sum of 9535 participants. Figure 1 summarises the study selection process (PRISMA flowsheet).
FIGURE 1.
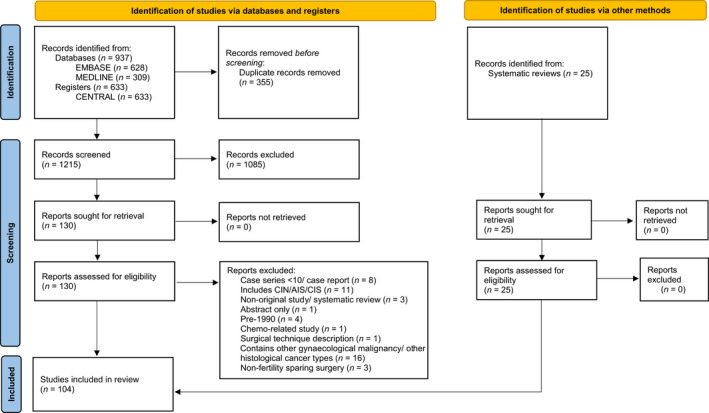
PRISMA flowchart
3.1. Study characteristics
We included 22 cohort studies, 32 prospective observational studies, 57 retrospective observational studies and 4 case series. No published RCT met our inclusion criteria. The populations of included studies were from North America, Europe and Asia, with only two representing South America and one from the Middle East. There was one international collaborative study that took place in the USA, Columbia and Brazil, and 11 multicentre studies.
Of the cohort studies, 11/22 (50%) compared fertility‐sparing interventions against hysterectomy. The remaining studies compared two different fertility‐sparing procedures. Twelve of 104 studies (12%) included patients who received neoadjuvant chemotherapy before surgery. 25 , 26 , 62 , 76 , 82 , 85 , 86 , 125 , 128 , 129 , 135 , 140 Nine studies (9%) described patients who underwent sentinel lymph node mapping as part of the surgical workup. 62 , 64 , 65 , 69 , 80 , 85 , 102 , 109 , 116 The full characteristics of the included studies are summarised in Table S1.
Ninety‐seven studies included participants with FIGO stage IA1–IB1 cervical cancer. There were seven studies with patients with stage IIA disease and two studies with stage IIB disease. Seven studies did not specify the stage of the disease. Sixty‐five studies did not specify primary outcomes. Of those that had set primary outcomes, only one included secondary outcomes in its reporting.
Vaginal trachelectomy was the most common form of fertility‐sparing surgery reported, with 63 out of 104 trials (61%), followed by open abdominal trachelectomy with 32 (31%) trials. A comprehensive breakdown is detailed in Table S2.
3.2. Outcomes
This review has drawn five broad categories of outcomes: (1) intra‐operative, (2) postoperative, (3) fertility and pregnancy, (4) oncological and (5) quality‐of‐life outcomes. Seventy‐two (69%) reported intra‐operative outcomes. Seventy‐four (71%) reported postoperative outcomes. Eighty‐six (83%) reported outcomes relating to fertility and pregnancy following surgery. Ninety‐seven (93%) reported oncological outcomes. Five (5%) studies included outcomes related to the quality of life following fertility‐sparing treatment. Outcomes that did not fit into the categories previously mentioned included those focused on neonatal outcomes and those related to neoadjuvant chemotherapy. Table 1 outlines a summary of intra‐operative, postoperative, quality of life and miscellaneous outcomes; while Table 2 highlights a summary of fertility and pregnancy outcomes, and oncological outcomes.
TABLE 1.
Reported intra‐operative, postoperative and quality of life outcomes
| Outcomes | Number of trials | Outcomes | Number of trials | Outcomes | Number of trials | |||
|---|---|---|---|---|---|---|---|---|
| Intra‐operative | Blood loss | 49 | Quality of life | Menopausal symptoms | 1 | Quality of life | Fertility‐specific anxiety | 3 |
| Blood transfusion | 38 | Health‐related quality of life | 3 | Financial cost | 1 | |||
| Visceral injury (complications) | 45 | Body image | 1 | Femininity | 1 | |||
| Operating time | 55 | Sexual function | 4 | Impact of diagnosis on others | 1 | |||
| Conversion to laparotomy | 9 | Cancer‐related anxiety | 1 | Emotional impact | 1 | |||
| Conversion to radical hysterectomy | 31 | Depression | 1 | Level of distress | 1 | |||
| Surgery aborted | 3 | Anxiety | 1 | |||||
| Postoperative | Menstrual disorder | 22 | Postoperative | Infection | 24 | Postoperative | Renal tract injury | 1 |
| Irregular bleeding | 8 | Lymphocyst formation | 30 | Bladder dysfunction | 22 | |||
| Pelvic pain | 7 | Lower limb oedema/lymphoedema | 16 | Gastrointestinal tract dysfunction | 9 | |||
| Cervical stenosis | 43 | Postoperative haemorrhage/haematoma | 10 | Neurological complications | 14 | |||
| Uterine necrosis | 1 | Depression/anxiety | 1 | Cardiorespiratory / venous thromboembolism complication | 4 | |||
| Cerclage problems | 6 | Loss of sexual desire/sensation | 1 | Time/duration for need of regular analgesia | 1 | |||
| Use of vaginal dilators | 2 | Sleep disturbance | 1 | Time from surgery to out of bed | 2 | |||
| Vulval oedema | 8 | Back pain | 2 | Time to return of menses | 13 | |||
| Wound dehiscence | 1 | Abdominal pain | 2 | Length of hospital stay | 38 | |||
| 30‐day readmission | 3 | Hernia formation | 2 | Time to return to normal bladder function | 12 | |||
| Return to theatre | 15 | Peritoneal inclusion cyst/pseudocyst | 2 | |||||
| Fistula formation | 4 | Self‐catheterisation | 1 | |||||
TABLE 2.
Reported fertility and oncological outcomes
| Outcomes | Number of trials | Outcomes | Number of trials | Outcomes | Number of trials | |||
|---|---|---|---|---|---|---|---|---|
| Fertility | Attempt conception | 47 | Fertility | Mode of delivery | 41 | Fertility | Abnormal placental attachment | 5 |
| Time to conception | 6 | Gestation of delivery | 29 | Gestational diabetes | 1 | |||
| Need for assisted conception | 36 | Live birth | 30 | Hypertensive disorders of pregnancy | 3 | |||
| Use of surrogate | 1 | Fetal loss | 3 | Multiple pregnancy | 8 | |||
| Premature ovarian failure post‐chemotherapy | 1 | Recurrence of cancer in pregnancy | 1 | Non‐cephalic presentation | 2 | |||
| First‐trimester miscarriage | 38 | Cervical length in pregnancy | 2 | Growth restriction/oligohydramnios | 1 | |||
| Second‐trimester miscarriage | 33 | Preterm prelabour rupture of membranes | 29 | Neonatal death | 5 | |||
| Termination of pregnancy | 21 | Chorioamnionitis/ infection | 14 | Birthweight | 4 | |||
| Ectopic pregnancy | 11 | First‐trimester bleeding | 1 | Apgar score | 2 | |||
| (Ongoing) Pregnancy | 15 | Rescue cervical cerclage | 6 | Cause‐specific perinatal morbidity | 3 | |||
| Number of pregnancies per woman | 11 | Antepartum haemorrhage | 1 | Congenital malformation/syndrome | 3 | |||
| Oncological | Number of lymph nodes (LN) sampled | 38 | Oncological | Disease‐related death | 23 | Oncological | Cervical length resected | 13 |
| LN status | 39 | Non‐cancer‐related death | 2 | Remaining cervical length | 1 | |||
| Sentinel LN status | 6 | Overall survival | 4 | Hysterectomy during follow‐up (f/u) period | 22 | |||
| Number of sentinel LN sampled | 2 | Disease‐free survival | 2 | Interval from conisation to hysterectomy | 1 | |||
| Adjuvant therapy | 46 | Overall survival rate | 4 | Re‐conisation during f/u period | 4 | |||
| Recurrence site | 33 | Overall mortality rate | 0 | Additional surgery during f/u period | 1 | |||
| Time to recurrence | 10 | Specimen margin status | 32 | Interval from initial surgery to second surgery | 1 | |||
| Treatment for recurrence | 33 | Stromal invasion | 4 | Smear/cytology status during f/u | 8 | |||
| Interval from recurrence to death | 2 | Lymphovascular space invasion | 38 | HPV status during f/u | 2 | |||
3.3. Intra‐operative outcomes
Of the intra‐operative outcomes reported, the commonest variables recorded were blood loss (49/72, 68%), complications (45/72, 63%), duration of the procedure (55/72, 76%), peri‐operative blood transfusion (38/72, 53%) and conversion to hysterectomy (31/72, 43%). Most documentation of blood loss did not specify a measurement tool; however, estimated blood loss was the most standard way to record blood loss (14/49, 29%). Other methods included ‘amount recorded from the suction tube’ and ‘the difference in haemoglobin before and after surgery’. Twenty‐three (51%) trials that recorded intra‐operative complications did not specify the type of complication. Of the complications listed, vascular injury (28/46, 61%) was most common, followed closely by urological issues (26, 57%). Nine studies reported the number of cases that were initially performed with minimally invasive techniques but were converted to laparotomy. Thirty‐one (43%) of the 72 studies reported the need to convert to a radical hysterectomy. A comprehensive breakdown of all intra‐operative outcomes is detailed in Table S3.1.
3.4. Postoperative outcomes
Commonly recorded postoperative variables included early and late complications (67/74, 91%), length of stay in hospital (38/74, 51%), time taken for the return of bladder function (12/74, 16%) and duration required for return of menses (13/74, 18%). Other outcomes recorded included duration of need for regular analgesia (1/74, 1%), readmission to hospital (3/74, 4%) and interval from surgery to passing flatus (2/74, 3%). Of the complications recorded, the commonest were either gynaecological or lymphatic in nature. Forty‐two trials (57%) recorded patients with cervical stenosis/haematometra requiring dilatation. Menstrual disorder (12, 18%), abnormal bleeding (5, 7%), and amenorrhoea (12, 18%) were also common complaints following surgery. Thirty studies (41%) reported the incidence of lymphocysts requiring drainage. Fifteen (45%) trials documented cases of lower limb oedema/lymphoedema, and 15 (45%) trials reported women who returned to theatre during the peri‐operative period. The number of women requiring emergency hysterectomy in the postoperative period was reported by three studies. Urological issues were also recorded, with ten (14%) studies reporting bladder hypotonia or dysfunction following fertility‐sparing surgery, five (7%) recording urinary retention following treatment and two (3%) citing long‐term bladder dysfunction. Four studies (5%) reported paralytic ileus and three (4%) noted either partial or complete bowel obstruction following surgery. A comprehensive breakdown of all postoperative outcomes is detailed in Table S3.2.
3.5. Fertility and pregnancy outcomes
Fertility and pregnancy outcomes were typical findings in this review, with 47 papers (55%) specifying the inclusion of participants attempting to conceive, and 55 papers (64%) noting women who successfully conceived without fertility intervention. Other reported outcomes were incidence of miscarriage (60/86, 70%) and termination (21/86, 24%), live birth (30/86, 35%), mode of delivery (41/86, 48%), and gestational age at birth (29/86, 34%). Obstetric complications were also reported, with preterm prelabour rupture of membranes (29/86, 34%) and chorioamnionitis (14/86, 16%) the most common. A comprehensive breakdown of all fertility and pregnancy outcomes is detailed in Table S3.3.
3.6. Oncological outcomes
Of the 97 studies that recorded oncological outcomes, the commonest variables were survival (any form of survival outcome 39/97, 40%), recurrence (69, 71%), utilisation of adjuvant therapy (49, 51%), lymph node status (39, 40%), lymphovascular space invasion status (38, 39%) and specimen margin status (32, 33%). Survival outcomes were reported in a variety of ways, including ‘disease‐related death’ (23/39, 59%), ‘overall survival’ (4, 10%), ‘disease‐free status’ (3, 8%) and ‘5‐year recurrence‐free survival rate’ (3, 8%). The number of lymph nodes resected was recorded in 38 studies (39%). Sixty‐four studies (66%) published data relating to recurrence during the follow‐up period, with 33 studies (52%) specifying the site of recurrence as well as the type of treatment provided. Ten studies (10%) highlighted the interval between the initial surgical therapy and confirmation of recurrence of the disease. Several publications (27, 28%) reported the number of women having a hysterectomy within the study follow‐up period. Seven of the 97 studies (7%) recorded cytology findings, with two (2%) also highlighting the HPV status during the follow‐up period. A comprehensive breakdown of all oncological outcomes is detailed in Table S3.4.
3.7. Quality of life outcomes
Quality of life data was less studied, with functional assessment (1/5, 20%), 50 symptom scales (2/5, 40%) and concerns (2/5, 40%) being frequently investigated themes. A comprehensive breakdown of all outcomes relating to quality of life is detailed in Table S3.5.
3.8. Other outcomes
Miscellaneous data that did not apply to those mentioned earlier included those related to neoadjuvant chemotherapy (7/12, 58%) and non‐disease‐related surgeries (1/12, 8%).
Of the studies reporting neonatal outcomes, five reported neonatal deaths, four recorded birth weight, and three reported on neonatal ward admission. As this review included studies that conducted neoadjuvant chemotherapy before surgery, complications arising from chemotherapy toxicity and response to chemotherapy was also documented. All miscellaneous outcomes are detailed in Table S3.6.
3.9. Outcome measurements
Few studies documented the tools used to measure the reported outcomes. Standard measurement tools were those used for documenting survival and mortality rates, such as 5‐year overall survival 4 and 5‐year recurrence‐free survival rates. 3 Three studies referenced the Clavien–Dindo classification system when grading complications. One study applied Bailey's scale of infant development to assessment childhood development, 21 and different quality of life questionnaires were used in various studies, including QLQ‐C30, 1 , 50 QLQ‐CX24, 1 , 50 and FACT. 1 , 68 A variety of clinical and radiological assessments were used to survey remission during follow up, including Papanicolaou testing, 2 annual magnetic resonance imaging of pelvis, 1 internal examination 1 and colposcopic assessment. 1 The different types of measurement tools used are recorded in Table S4.
As there were no RCT in this review, the Newcastle–Ottawa Scale was applied to assess the quality of the studies in the systematic review. Of which 13 (12%) were judged as good quality, one (1%) was deemed of fair quality, and 91 (87%) were of poor quality. The breakdown of the Newcastle–Ottawa Scale assessment can be found in Table S5. Table S6 is included detailing all abbreviations used in this paper.
4. DISCUSSION
4.1. Main findings
Our systematic review shows international interest in assessing the outcomes of women who undergo fertility‐sparing surgery for cervical cancer. Oncological outcomes were the most commonly reported topic in most studies, followed by fertility outcomes. Over half of the studies did not specify primary and secondary outcomes. However, this can be explained by there being no randomised controlled trials eligible for this review. Our data highlight wide heterogeneity in outcomes, limited standardisation in outcome measures, and the existing small proportion of good‐quality studies. There was heterogeneity in assessing outcomes such as pregnancy losses, survival rate, blood loss, infections and more. Definitions for outcomes were often either lacking or varied, such as preterm delivery, first‐ or second‐trimester miscarriage and postoperative infection. This makes drawing comparisons between studies challenging. Many of the studies included within this systematic review described a broad range of outcomes, and a small proportion of studies were set to study more specific outcomes relating to fertility‐sparing surgery following a cervical cancer diagnosis; these studies predominantly focused on quality‐of‐life impacts or neonatal effects. The deficiency of the methodology used to describe the reported outcomes is also a concern.
4.2. Strength and limitations
This is the first systematic review that seeks to report all relevant outcomes reported in the literature for studies assessing fertility‐sparing surgery for cervical carcinoma. A robust methodology was used throughout this review. Imposing no language restrictions allowed us to capture a diverse group of participants to inform this review with 12 studies published in non‐English journals. The major limiting factor for this review was that most studies were observational studies, of which only 12% were deemed to be of good quality. We acknowledge that 24% of the studies recorded within this review did not appear during our literature search but were included from other systematic reviews. However, because of the ‘saturation’ of outcomes reported, we can be confident that we are unlikely to have missed any other significant outcomes.
4.3. Interpretation
Outcomes described in this systematic review mainly represent the outcomes that several researchers and clinicians have chosen to investigate and report globally. This has been the norm with other systematic reviews that aimed to describe outcomes for benign gynaecological conditions. 141 As a result, most studies report predominantly on oncological or fertility‐related outcomes. Nevertheless, despite the presence of a dominating theme of outcomes reported, the majority of studies report on a wide range of outcomes with an overall significant variation in reported outcome measures. This is not surprising because several other systematic reviews in other areas of gynaecology report the same findings. 142 , 143 , 144 , 145 This poses a significant burden when interpreting study findings, essentially limiting those studies' international amplitude and clinical applicability.
More importantly, forming policies, implementing robust guidelines, and describing reference standard practice is predominantly based on the ability of researchers and clinicians to synthesise available evidence effectively. Delivering high‐quality systematic reviews and data synthesis can only be possible if reported outcomes are harmonised. 146 Additionally, one can argue that initiation of large‐scale high‐quality trials may be based on robust systematic reviews that successfully demonstrate a need for further research. In our case, variation of reported outcomes directly prohibits robust evidence synthesis and perhaps creates an unfavourable ground to design or undertake a high‐quality RCT or well‐designed studies targeted to provide answers for knowledge gaps that arise from current studies. Undoubtedly, the observed lack of RCT can be secondary to ethical challenges; however, lack of available high‐quality evidence may lead to a vicious cycle.
From the public and patient's perspective, a patient can only make a properly informed decision if clinicians and researchers are able to provide strong evidence confidently. Lack of harmonised outcomes results in knowledge gaps that would essentially pose a significant burden in standardising evidence‐based clinical practice. Subsequently, clinicians may at times be less confident to offer fertility‐sparing surgery, and patients may feel nervous about opting for a fertility‐sparing option when this perhaps is available and safe; or a corollary may be deciding to opt for fertility‐sparing surgery that is ill‐informed and in retrospect may be regretted. Further to this, our primary search failed to demonstrate patient‐centred outcomes, and quality of life was reported in only five studies. Many of the outcomes most frequently reported were those that are easy to collect and not very meaningful to patients. This emphasises the need for active patient and public involvement in developing COS. Fertility‐sparing treatment must be offered on the basis of patients' wishes. Any effort to develop and identify COS should incorporate patients' in the process and represent their views as one of the important components. We speculate that a final COS is likely to include outcomes like overall survival, progression‐free survival, cancer‐specific mortality, recurrence, surgical complications, live birth rate, fetal loss, quality of life and patient satisfaction.
Overall, this underlines the necessity of agreeing to design, disseminate and implement COS for fertility‐sparing surgery in cervical cancer. This will facilitate an international consensus in reporting outcomes following fertility‐sparing interventions, and therefore allow interpretation of each study on a global scale. It will also act as a catalyst to bring experts and stakeholders from international institutions, societies and patient groups together, to agree on establishing robust guidelines as to when fertility‐sparing surgery is indicated, its oncological safety profile, contraindications, surgical morbidity, potential impact and effect on quality of life, as well as success in pregnancy‐related outcomes after treatment. Well‐established evidence‐based guidelines make clinicians confident to counsel women effectively and to use the option of fertility‐sparing surgery wisely when this is indicated, as well as helping patients make informed decisions on whether to opt for the intervention.
5. CONCLUSION
We recommend the development of COS for fertility‐sparing surgery in cervical cancer. This will prevent unnecessary duplication of research time and provide key stakeholders including patients, clinicians, nurses, researchers and allied health professionals as well as professional societies, with the opportunity to identify outcome sets prospectively while designing their study. This can also facilitate ethics committee's approval of novel trial protocols as it provides a form of standardised approach. 30 , 147 Delivering COS will facilitate a global approach towards providing high‐quality evidence in the field of fertility‐sparing surgery for cervical cancer.
Our data highlight heterogeneity in the reporting of outcomes used in studies of fertility‐sparing surgery for cervical carcinoma. A defined set of agreed core outcomes is critical to facilitate future studies, for research studies to be meaningfully compared to advise clinical practice and drive forward management change and informed decision‐making. The decision to proceed with fertility‐sparing surgery is predominantly patient‐centred. It is essential that patients and public stakeholders be involved in the development of COS and that the final COS also reflect outcomes that are important to them. This systematic review will inform the development of a COS by forming the basis of a broad‐based Delphi survey, with the addition of data from qualitative work with patients.
AUTHOR CONTRIBUTIONS
NC and KK developed the methodology, and secured funding and ethical approval. RM refined the protocol. NY and CB performed the systematic search, and NY wrote the initial draft of the paper. RM, MS and SI refined and finalised the manuscript. AT, MS, and RM provided insight regarding cervical cancer and staging. All authors edited and accepted the manuscript prior to submission.
FUNDING INFORMATION
This study is funded by the British Medical Association's Strutt and Harper Grant. The funders have no involvement in any stage of this systematic review.
CONFLICT OF INTERESTS
NC, KK, and RM have received grant funding from Cancer Research UK (CRUK) to develop core outcome sets for endometrial cancer and atypical endometrial hyperplasia. NC has received a starter grant from the Academy of Medical Sciences to develop a core outcome set for heavy menstrual bleeding. The remaining authors have no competing interest to disclose. Completed disclosure of interests form available to view online as supporting information.
ETHICS APPROVAL
Although ethical approval is not required for a systematic review, the core outcome set project needed ethical approval for the second part of the process, which involves patients. Therefore, the project as a whole was reviewed, and East Midlands granted ethical approval – Nottingham 1 Research Ethics Committee on 14 December 2015, REC reference ID 15/EM/0565.
Supporting information
Appendix S1
Table S1
Table S2
Table S3.1
Table S3.2
Table S3.3
Table S3.4
Table S3.5
Table S3.6
Table S4
Table S5
Table S6
Data S1
Data S2
Data S3
Data S4
Data S5
Data S6
Data S7
Data S8
Data S9
Yong N, Cooper N, Yorke S, Baran C, Khan K, Tan A, et al. Variation in outcome reporting in studies of fertility‐sparing surgery for cervical cancer: A systematic review. BJOG. 2022;130(2):163–175. 10.1111/1471-0528.17342
Natalie Cooper, Michail Sideris, Stamatina Iliodromiti and Ranjit Manchanda contributed equally to this study.
 This article includes Author Insights, a video abstract available at: https://vimeo.com/771508530
This article includes Author Insights, a video abstract available at: https://vimeo.com/771508530
DATA AVAILABILITY STATEMENT
Data available on reasonable request from the corresponding author.
REFERENCES
- 1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dappa E, Elger T, Hasenburg A, Düber C, Battista MJ, Hötker AM. The value of advanced MRI techniques in the assessment of cervical cancer: a review. Insights Imaging. 2017;8(5):471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pannu HK, Corl FM, Fishman EK. CT evaluation of cervical cancer: spectrum of disease. Radiographics. 2001;21(5):1155–68. [DOI] [PubMed] [Google Scholar]
- 4. Salib MY, Russell JHB, Stewart VR, Sudderuddin SA, Barwick TD, Rockall AG, et al. 2018 FIGO staging classification for cervical cancer: added benefits of imaging. Radiographics. 2020;40(6):1807–22. [DOI] [PubMed] [Google Scholar]
- 5. The British Association of Gynaecological Pathologists . 2018 FIGO staging system for cervical cancer: summary and comparison with 2009 FIGO staging system. 2021. [cited 2 May 2022]. Available from: https://www.thebagp.org/wp‐content/uploads/download‐manager‐files/1642607060wpdm_BAGP%202018%20FIGO%20Cervix%20Ca%20staging%20v1.5.pdf
- 6. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet. 2009;105(2):103–4. [DOI] [PubMed] [Google Scholar]
- 7. Corrigendum to “Revised FIGO staging for carcinoma of the cervix uteri” [Int J Gynecol Obstet 145(2019) 129–135]. Int J Gynecol Obstet. 2019;147(2):279–80. [DOI] [PubMed] [Google Scholar]
- 8. Roque DR, Wysham WZ, Soper JT. The surgical management of cervical cancer: an overview and literature review. Obstet Gynecol Surv. 2014;69(7):426–41. [DOI] [PubMed] [Google Scholar]
- 9. Reed N, Balega J, Barwick T, Buckley L, Burton K, Eminowicz G, et al. British Gynaecological Cancer Society (BGCS) cervical cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2021;256:433–65. [DOI] [PubMed] [Google Scholar]
- 10. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154–61. [DOI] [PubMed] [Google Scholar]
- 11. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para‐aortic radiation for high‐risk cervical cancer. N Engl J Med. 1999;340(15):1137–43. [DOI] [PubMed] [Google Scholar]
- 12. Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–13. [DOI] [PubMed] [Google Scholar]
- 13. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–53. [DOI] [PubMed] [Google Scholar]
- 14. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler JWC, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB‐IVA carcinoma of the cervix with negative para‐aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group Study. J Clin Oncol. 1999;17(5):1339–48. [DOI] [PubMed] [Google Scholar]
- 15. Willows K, Lennox G, Covens A. Fertility‐sparing management in cervical cancer: balancing oncologic outcomes with reproductive success. Gynecol Oncol Res Pract. 2016;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter J, Rowland K, Chi D, Brown C, Abu‐Rustum N, Castiel M, et al. Gynecologic cancer treatment and the impact of cancer‐related infertility. Gynecol Oncol. 2005;97(1):90–5. [DOI] [PubMed] [Google Scholar]
- 17. Wenzel L, DeAlba I, Habbal R, Kluhsman BC, Fairclough D, Krebs LU, et al. Quality of life in long‐term cervical cancer survivors. Gynecol Oncol. 2005;97(2):310–7. [DOI] [PubMed] [Google Scholar]
- 18. Bhatla N, Berek JS, Fredes MC, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145(1):129–35. [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y, Chen C, Li L. Comparison of cold‐knife conization versus loop electrosurgical excision for cervical adenocarcinoma in situ (ACIS): a systematic review and meta‐analysis. PLoS ONE. 2017;12(1):e0170587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentivegna E, Maulard A, Pautier P, Chargari C, Gouy S, Morice P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literature. Fertil Steril. 2016;106(5):1195–211. [DOI] [PubMed] [Google Scholar]
- 21. Van Der Velden J, Mom CH. Tailoring radicality in early cervical cancer: how far can we go? J Gynecol Oncol. 2018;30(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pareja R, Rendón GJ, Sanz‐Lomana CM, Monzón O, Ramirez PT. Surgical, oncological, and obstetrical outcomes after abdominal radical trachelectomy—a systematic literature review. Gynecol Oncol. 2013;131(1):77–82. [DOI] [PubMed] [Google Scholar]
- 23. Carter J, Sonoda Y, Baser RE, Raviv L, Chi DS, Barakat RR, et al. A 2‐year prospective study assessing the emotional, sexual, and quality of life concerns of women undergoing radical trachelectomy versus radical hysterectomy for treatment of early‐stage cervical cancer. Gynecol Oncol. 2010;119(2):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shepherd JH, Spencer C, Herod J, Ind TEJ. Radical vaginal trachelectomy as a fertility‐sparing procedure in women with early‐stage cervical cancer—cumulative pregnancy rate in a series of 123 women. BJOG. 2006;113(6):719–24. [DOI] [PubMed] [Google Scholar]
- 25. Salihi R, Leunen K, Van Limbergen E, Moerman P, Neven P, Vergote I. Neoadjuvant chemotherapy followed by large cone resection as fertility‐sparing therapy in stage IB cervical cancer. Gynecol Oncol. 2015;139(3):447–51. [DOI] [PubMed] [Google Scholar]
- 26. Lanowska M, Mangler M, Speiser D, Bockholdt C, Schneider A, Köhler C, et al. Radical vaginal trachelectomy after laparoscopic staging and neoadjuvant chemotherapy in women with early‐stage cervical cancer over 2 cm: oncologic, fertility, and neonatal outcome in a series of 20 patients. Int J Gynecol Cancer. 2014;24(3):586–93. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt KLT, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow‐up of ovarian function post‐chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20(12):3539–46. [DOI] [PubMed] [Google Scholar]
- 28. CROWN . Core outcomes in women's and newborn health. [cited 1 Mach 2022]. Available from: http://www.crown‐initiative.org/
- 29. Khan K, on behalf of Chief Editors of Journals Participating in The CIlateota . The CROWN Initiative: journal editors invite researchers to develop core outcomes in women's health. Fertil Res Pract. 2015;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duffy JMN, Rolph R, Gale C, Hirsch M, Khan KS, Ziebland S, et al. Core outcome sets in women's and newborn health: a systematic review. BJOG. 2017;124(10):1481–9. [DOI] [PubMed] [Google Scholar]
- 31. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chien PFW, Khan KS, Siassakos D. Registration of systematic reviews: PROSPERO. BJOG. 2012;119(8):903–5. [DOI] [PubMed] [Google Scholar]
- 33. Gorst SL, Gargon E, Clarke M, Blazeby JM, Altman DG, Williamson PR. Choosing important health outcomes for comparative effectiveness research: an updated review and user survey. PLoS ONE. 2016;11(1):e0146444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gargon E, Gurung B, Medley N, Altman DG, Blazeby JM, Clarke M, et al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLoS ONE. 2014;9(6):e99111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 36. Harman NL, Bruce IA, Callery P, Tierney S, Sharif MO, O'Brien K, et al. MOMENT–management of otitis media with effusion in cleft palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials. 2013;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. 2000.
- 38. Covens A, Shaw P, Murphy J, DePetrillo D, Lickrish G, Laframboise S, et al. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA–B carcinoma of the cervix? Cancer. 1999;86(11):2273–9. [DOI] [PubMed] [Google Scholar]
- 39. Diaz JP, Sonoda Y, Leitao MM, Zivanovic O, Brown CL, Chi DS, et al. Oncologic outcome of fertility‐sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol Oncol. 2008;111(2):255–60. [DOI] [PubMed] [Google Scholar]
- 40. Li X, Li J, Wen H, Ju X, Chen X, Xia L, et al. The survival rate and surgical morbidity of abdominal radical trachelectomy versus abdominal radical hysterectomy for stage IB1 cervical cancer. Ann Surg Oncol. 2016;23(9):2953–8. [DOI] [PubMed] [Google Scholar]
- 41. Muraji M, Sudo T, Nakagawa E, Ueno S, Wakahashi S, Kanayama S, et al. Type II versus type III fertility‐sparing abdominal radical trachelectomy for early‐stage cervical cancer: a comparison of feasibility of surgical outcomes. Int J Gynecol Cancer. 2012;22(3):479–83. [DOI] [PubMed] [Google Scholar]
- 42. Li J, Wu X, Li X, Ju X. Abdominal radical trachelectomy: is it safe for IB1 cervical cancer with tumors ≥ 2 cm? Gynecol Oncol. 2013;131(1):87–92. [DOI] [PubMed] [Google Scholar]
- 43. He Y, Wu Y‐M, Zhao Q, Wang T, Wang Y, Kong W‐M, et al. Clinical value of cold knife conization as conservative management in patients with microinvasive cervical squamous cell cancer (stage IA1). Int J Gynecol Cancer. 2014;24(7):1306–11. [DOI] [PubMed] [Google Scholar]
- 44. Basta PB, Jach R, Laskowicz Ł, Kotlarz A, Schwarz J. Konizacja i radykalna pochwowa trachelektomia z laparoskopową limfadenektomią w leczeniu chirurgicznym kobiet z rakiem szyjki macicy pozwalajacym na zachowanie płodności. Ginekol Pol. 2015;86(8):590–7. [DOI] [PubMed] [Google Scholar]
- 45. Shepherd JH, Milliken DA. Conservative surgery for carcinoma of the cervix. Clin Oncol. 2008;20(6):395–400. [DOI] [PubMed] [Google Scholar]
- 46. Speiser D, Mangler M, Köhler C, Hasenbein K, Hertel H, Chiantera V, et al. Fertility outcome after radical vaginal trachelectomy: a prospective study of 212 patients. Int J Gynecol Cancer. 2011;21(9):1635–9. [DOI] [PubMed] [Google Scholar]
- 47. Abu‐Rustum NR, Sonoda Y. Fertility‐sparing surgery in early‐stage cervical cancer: indications and applications. J Natl Compr Canc Netw. 2010;8(12):1435–8. [DOI] [PubMed] [Google Scholar]
- 48. Sonoda Y, Chi DS, Carter J, Barakat RR, Abu‐Rustum NR. Initial experience with Dargent's operation: the radical vaginal trachelectomy. Gynecol Oncol. 2008;108(1):214–9. [DOI] [PubMed] [Google Scholar]
- 49. Mathevet P, de Kaszon EL, Dargent D. La préservation de la fertilité dans les cancers du col utérin de stade précoce. Gynecol Obstet Fertil. 2003;31(9):706–12. [DOI] [PubMed] [Google Scholar]
- 50. Park JY, Joo WD, Chang SJ, Kim DY, Kim JH, Kim YM, et al. Long‐term outcomes after fertility‐sparing laparoscopic radical trachelectomy in young women with early‐stage cervical cancer: an Asan Gynecologic Cancer Group (AGCG) study. J Surg Oncol. 2014;110(3):252–7. [DOI] [PubMed] [Google Scholar]
- 51. Lai JC‐Y, Chen H‐H, Chu K‐H, Weng C‐S, Chou Y‐J, Huang N, et al. Nationwide trends and in‐hospital complications of trachelectomy for surgically resectable cervical cancer in Taiwanese women: a population‐based study, 1998–2013. Taiwan J Obstet Gynecol. 2017;56(4):449–55. [DOI] [PubMed] [Google Scholar]
- 52. Mangler M, Speiser D, Nguyen BD, Cremer M, Koehler C, Schneider A, et al. Neonatal outcome in infants of patients with radical vaginal trachelectomy. J Perinat Med. 2012;40(5):503–9. [DOI] [PubMed] [Google Scholar]
- 53. Ebisawa K, Takano M, Fukuda M, Fujiwara K, Hada T, Ota Y, et al. Obstetric outcomes of patients undergoing total laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol Oncol. 2013;131(1):83–6. [DOI] [PubMed] [Google Scholar]
- 54. Mangler M, Lanowska M, Köhler C, Vercellino F, Schneider A, Speiser D. Pattern of cancer recurrence in 320 patients after radical vaginal trachelectomy. Int J Gynecol Cancer. 2014;24(1):130–4. [DOI] [PubMed] [Google Scholar]
- 55. Speiser D, Köhler C, Schneider A, Mangler M. Radical vaginal trachelectomy: a fertility‐preserving procedure in early cervical cancer in young women. Dtsch Arztebl Int. 2013;110(17):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johansen G, Lönnerfors C, Falconer H, Persson J. Reproductive and oncologic outcome following robot‐assisted laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol Oncol. 2016;141(1):160–5. [DOI] [PubMed] [Google Scholar]
- 57. Park J‐Y, Kim D‐Y, Suh D‐S, Kim J‐H, Kim Y‐M, Kim Y‐T, et al. Reproductive outcomes after laparoscopic radical trachelectomy for early‐stage cervical cancer. J Gynecol Oncol. 2014;25(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Slama J, Cerny A, Dusek L, Fischerova D, Zikan M, Kocian R, et al. Results of less radical fertility‐sparing procedures with omitted parametrectomy for cervical cancer: 5 years of experience. Gynecol Oncol. 2016;142(3):401–4. [DOI] [PubMed] [Google Scholar]
- 59. Zusterzeel PLM, Pol FJM, van Ham M, Zweemer RP, Bekkers RLM, Massuger LFAG, et al. Vaginal radical trachelectomy for early‐stage cervical cancer: increased recurrence risk for adenocarcinoma. Int J Gynecol Cancer. 2016;26(7):1293–9. [DOI] [PubMed] [Google Scholar]
- 60. Plante M, Renaud M‐C, Hoskins IA, Roy M. Vaginal radical trachelectomy: a valuable fertility‐preserving option in the management of early‐stage cervical cancer. A series of 50 pregnancies and review of the literature. Gynecol Oncol. 2005;98(1):3–10. [DOI] [PubMed] [Google Scholar]
- 61. Chen Y, Xu H, Zhang Q, Li Y, Wang D, Liang Z. A fertility‐preserving option in early cervical carcinoma: laparoscopy‐assisted vaginal radical trachelectomy and pelvic lymphadenectomy. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):90–3. [DOI] [PubMed] [Google Scholar]
- 62. Rob L, Pluta M, Strnad P, Hrehorcak M, Chmel R, Skapa P, et al. A less radical treatment option to the fertility‐sparing radical trachelectomy in patients with stage I cervical cancer. Gynecol Oncol. 2008;111(2):S116–20. [DOI] [PubMed] [Google Scholar]
- 63. Nishio H, Fujii T, Kameyama K, Susumu N, Nakamura M, Iwata T, et al. Abdominal radical trachelectomy as a fertility‐sparing procedure in women with early‐stage cervical cancer in a series of 61 women. Gynecol Oncol. 2009;115(1):51–5. [DOI] [PubMed] [Google Scholar]
- 64. Deng X, Zhang Y, Li D, Zhang X, Guo H, Wang F, et al. Abdominal radical trachelectomy guided by sentinel lymph node biopsy for stage IB1 cervical cancer with tumors >2 cm. Oncotarget. 2017;8(2):3422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cibula D, Sláma J, Svárovský J, Fischerova D, Freitag P, Zikán M, et al. Abdominal radical trachelectomy in fertility‐sparing treatment of early‐stage cervical cancer. Int J Gynecol Cancer. 2009;19(8):1407–11. [DOI] [PubMed] [Google Scholar]
- 66. Căpîlna ME, Ioanid N, Scripcariu V, Gavrilescu MM, Szabo B. Abdominal radical trachelectomy: a Romanian series. Int J Gynecol Cancer. 2014;24(3):615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Testa R, Ramirez PT, Ferreyra H, Saadi J, Franco G, Goldsman M, et al. Abdominal radical trachelectomy: a safe and feasible option for fertility preservation in developing countries. J Low Genit Tract Dis. 2013;17(4):378–84. [DOI] [PubMed] [Google Scholar]
- 68. Tomao F, Maruccio M, Preti EP, Boveri S, Ricciardi E, Zanagnolo V, et al. Conization in early stage cervical cancer: pattern of recurrence in a 10‐year single‐institution experience. Int J Gynecol Cancer. 2017;27(5):1001–8. [DOI] [PubMed] [Google Scholar]
- 69. Wethington SL, Sonoda Y, Park KJ, Alektiar KM, Tew WP, Chi DS, et al. Expanding the indications for radical trachelectomy: a report on 29 patients with stage IB1 tumors measuring 2 to 4 centimeters. Int J Gynecol Cancer. 2013;23(6):1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hertel H, Köhler C, Hillemanns P, Possover M, Grund D, Michels W, et al. Fertilitätserhaltung bei Frauen mit frühem Zervixkarzinom. Onkologe. 2006;12(9):895–900. [Google Scholar]
- 71. Kim JH, Park JY, Kim DY, Kim YM, Kim YT, Nam JH. Fertility‐sparing laparoscopic radical trachelectomy for young women with early stage cervical cancer. BJOG. 2010;117(3):340–7. [DOI] [PubMed] [Google Scholar]
- 72. Abu‐Rustum NR, Sonoda Y, Black D, Levine DA, Chi DS, Barakat RR. Fertility‐sparing radical abdominal trachelectomy for cervical carcinoma: technique and review of the literature. Gynecol Oncol. 2006;103(3):807–13. [DOI] [PubMed] [Google Scholar]
- 73. Raju SK, Papadopoulos AJ, Montalto SA, Coutts M, Culora G, Kodampur M, et al. Fertility‐sparing surgery for early cervical cancer—approach to less radical surgery. Int J Gynecol Cancer. 2012;22(2):311–7. [DOI] [PubMed] [Google Scholar]
- 74. Ditto A, Martinelli F, Bogani G, Fischetti M, Di Donato V, Lorusso D, et al. Fertility‐sparing surgery in early‐stage cervical cancer patients: oncologic and reproductive outcomes. Int J Gynecol Cancer. 2015;25(3):493–7. [DOI] [PubMed] [Google Scholar]
- 75. Roy M, Plante M. La trachelectomie vaginale élargie pour cancer invasif du col utérin. J Gynécol Obstet Biol Reprod. 2000;29(3):279–81. [PubMed] [Google Scholar]
- 76. Vercellino GF, Piek JMJ, Schneider A, Köhler C, Mangler M, Speiser D, et al. Laparoscopic lymph node dissection should be performed before fertility preserving treatment of patients with cervical cancer. Gynecol Oncol. 2012;126(3):325–9. [DOI] [PubMed] [Google Scholar]
- 77. Martin A, Torrent A. Laparoscopic nerve‐sparing radical trachelectomy: surgical technique and outcome. J Minim Invasive Gynecol. 2010;17(1):37–41. [DOI] [PubMed] [Google Scholar]
- 78. Kucukmetin A, Biliatis I, Ratnavelu N, Patel A, Cameron I, Ralte A, et al. Laparoscopic radical trachelectomy is an alternative to laparotomy with improved perioperative outcomes in patients with early‐stage cervical cancer. Int J Gynecol Cancer. 2014;24(1):135–40. [DOI] [PubMed] [Google Scholar]
- 79. Saadi JM, Perrotta M, Orti R, Salvo G, Giavedoni ME, Gogorza S, et al. Laparoscopic radical trachelectomy: technique, feasibility, and outcomes. JSLS. 2015;19(1):e2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rob L, Charvat M, Robova H, Pluta M, Strnad P, Hrehorcak M, et al. Less radical fertility‐sparing surgery than radical trachelectomy in early cervical cancer. Int J Gynecol Cancer. 2007;17(1):304–10. [DOI] [PubMed] [Google Scholar]
- 81. Malmsten C, Hellberg P, Bergmark K, Dahm‐Kähler P. Long‐term fertility, oncological, and quality‐of‐life outcomes after trachelectomy in early stage cervical cancer. Arch Gynecol Obstet. 2019;299(4):1033–41. [DOI] [PubMed] [Google Scholar]
- 82. Marchiole P, Tigaud J‐D, Costantini S, Mammoliti S, Buenerd A, Moran E, et al. Neoadjuvant chemotherapy and vaginal radical trachelectomy for fertility‐sparing treatment in women affected by cervical cancer (FIGO stage IB–IIA1). Gynecol Oncol. 2011;122(3):484–90. [DOI] [PubMed] [Google Scholar]
- 83. Tamauchi S, Kajiyama H, Sakata J, Sekiya R, Suzuki S, Mizuno M, et al. Oncologic and obstetric outcomes of early stage cervical cancer with abdominal radical trachelectomy: single‐institution experience. J Obstet Gynaecol Res. 2016;42(12):1796–801. [DOI] [PubMed] [Google Scholar]
- 84. Ayhan A, Tohma YA, Sahin H, Kocaman E, Tunc M, Haberal AN. Oncological and obstetric outcomes after fertility‐sparing radical abdominal trachelectomy for early stage cervical cancer: a tertiary centre's 10 years' experience. J Obstet Gynaecol. 2019;39(2):248–52. [DOI] [PubMed] [Google Scholar]
- 85. Robova H, Halaska MJ, Pluta M, Skapa P, Matecha J, Lisy J, et al. Oncological and pregnancy outcomes after high‐dose density neoadjuvant chemotherapy and fertility‐sparing surgery in cervical cancer. Gynecol Oncol. 2014;135(2):213–6. [DOI] [PubMed] [Google Scholar]
- 86. Yao YY, Wang Y, Wang JL, Zhao C, Wei LH. Outcomes of fertility and pregnancy in patients with early‐stage cervical cancer after undergoing neoadjuvant chemotherapy. Eur J Gynaecol Oncol. 2016;37(1):109–12. [PubMed] [Google Scholar]
- 87. Ma LK, Cao DY, Yang JX, Liu JT, Shen K, Lang JH. Pregnancy outcome and obstetric management after vaginal radical trachelectomy. Eur Rev Med Pharmacol Sci. 2014;18(20):3019–24. [PubMed] [Google Scholar]
- 88. Estevez JP, Hequet D, Dubot C, Fourchotte V, Rouge TDLM, Becette V, et al. Préservation de la fertilité chez les patientes atteintes d'un cancer du col de plus de 2 cm. Bull Cancer. 2016;103(2):173–9. [DOI] [PubMed] [Google Scholar]
- 89. Schlaerth JB, Spirtos NM, Schlaerth AC. Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer. Am J Obstet Gynecol. 2003;188(1):29–34. [DOI] [PubMed] [Google Scholar]
- 90. Wu C‐J, Chang W‐C, Chen C‐H, Chen C‐A, Huang S‐C, Sheu B‐C. Radical trachelectomy for early stage cervical cancer: a case series and literature review. Taiwan J Obstet Gynecol. 2017;56(2):143–6. [DOI] [PubMed] [Google Scholar]
- 91. Shepherd JH, Crawford RAF, Oram DH. Radical trachelectomy: a way to preserve fertility in the treatment of early cervical cancer. BJOG. 1998;105(8):912–6. [DOI] [PubMed] [Google Scholar]
- 92. Burnett AF, Roman LD, O'Meara AT, Morrow CP. Radical vaginal trachelectomy and pelvic lymphadenectomy for preservation of fertility in early cervical carcinoma. Gynecol Oncol. 2003;88(3):419–23. [DOI] [PubMed] [Google Scholar]
- 93. Beiner ME, Hauspy J, Rosen B, Murphy J, Laframboise S, Nofech‐Mozes S, et al. Radical vaginal trachelectomy vs. radical hysterectomy for small early stage cervical cancer: a matched case–control study. Gynecol Oncol. 2008;110(2):168–71. [DOI] [PubMed] [Google Scholar]
- 94. Einstein MH, Park KJ, Sonoda Y, Carter J, Chi DS, Barakat RR, et al. Radical vaginal versus abdominal trachelectomy for stage IB1 cervical cancer: a comparison of surgical and pathologic outcomes. Gynecol Oncol. 2009;112(1):73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Carter J, Raviv L, Sonoda Y, Chi DS, Abu‐Rustum NR. Recovery issues of fertility‐preserving surgery in patients with early‐stage cervical cancer and a model for survivorship: the physician checklist. Int J Gynecol Cancer. 2011;21(1):106–16. [DOI] [PubMed] [Google Scholar]
- 96. Komatsu H, Yagasaki K, Shoda R, Chung Y, Iwata T, Sugiyama J, et al. Repair of the threatened feminine identity: experience of women with cervical cancer undergoing fertility preservation surgery. Cancer Nurs. 2014;37(1):75–82. [DOI] [PubMed] [Google Scholar]
- 97. Nishio H, Fujii T, Sugiyama J, Kuji N, Tanaka M, Hamatani T, et al. Reproductive and obstetric outcomes after radical abdominal trachelectomy for early‐stage cervical cancer in a series of 31 pregnancies. Hum Reprod. 2013;28(7):1793–8. [DOI] [PubMed] [Google Scholar]
- 98. Carter J, Sonoda Y, Abu‐Rustum NR. Reproductive concerns of women treated with radical trachelectomy for cervical cancer. Gynecol Oncol. 2007;105(1):13–6. [DOI] [PubMed] [Google Scholar]
- 99. Ramirez PT, Schmeler KM, Malpica A, Soliman PT. Safety and feasibility of robotic radical trachelectomy in patients with early‐stage cervical cancer. Gynecol Oncol. 2010;116(3):512–5. [DOI] [PubMed] [Google Scholar]
- 100. Fanfani F, Landoni F, Gagliardi ML, Fagotti A, Preti E, Moruzzi MC, et al. Sexual and reproductive outcomes in early stage cervical cancer patients after excisional cone as a fertility‐sparing surgery: an Italian experience. J Reprod Infertil. 2014;15(1):29–34. [PMC free article] [PubMed] [Google Scholar]
- 101. Demirkiran F, Kahramanoglu I, Bese T, Turan H, Meseci E, Arvas M. Simple vaginal trachelectomy for early stage cervical cancer: a tertiary cancer center experience. Ginekol Pol. 2018;89(9):475–80. [DOI] [PubMed] [Google Scholar]
- 102. Abu‐Rustum NR, Neubauer N, Sonoda Y, Park KJ, Gemignani M, Alektiar KM, et al. Surgical and pathologic outcomes of fertility‐sparing radical abdominal trachelectomy for FIGO stage IB1 cervical cancer. Gynecol Oncol. 2008;111(2):261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sopracordevole F, Chiossi G, Barbero M, Cristoforoni P, Ghiringhello B, Frega A, et al. Surgical approach and long‐term clinical outcome in women with microinvasive cervical cancer. Anticancer Res. 2014;34(8):4345–9. [PubMed] [Google Scholar]
- 104. Yao T, Mo S, Lin Z. The functional reconstruction of fertility‐sparing radical abdominal trachelectomy for early stage cervical carcinoma. Eur J Obstet Gynecol Reprod Biol. 2010;151(1):77–81. [DOI] [PubMed] [Google Scholar]
- 105. Cui RR, Chen L, Tergas AI, Hou JY, St Clair CM, Neugut AI, et al. Trends in use and survival associated with fertility‐sparing trachelectomy for young women with early‐stage cervical cancer. Obstet Gynecol. 2018;131(6):1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pahisa J, Alonso I, Torné A. Vaginal approaches to fertility‐sparing surgery in invasive cervical cancer. Gynecol Oncol. 2008;110(3):S29–32. [DOI] [PubMed] [Google Scholar]
- 107. Liang Z‐Q, Xu H‐C, Chen Y, Li Y‐Y, Xiong G‐W, Shi C‐X. [Role of radical vaginal trachelectomy and laparoscopic pelvic lymphadenectomy in treating early cervical carcinoma]. Zhonghua Fu Chan Ke Za Zhi. 2004;39(5):305–7. [PubMed] [Google Scholar]
- 108. Hertel H, Possover M, Krause N, Kühne‐Heid R, Schneider A. Fertilität nach radikaler Trachelektomie bei Patientinnen mit frühem Zervixkarzinom. Geburtsh Frauenheilk. 2001;61:117–20. [Google Scholar]
- 109. Brătilă E, Brătilă CP, Coroleuca CB. Radical vaginal trachelectomy with laparoscopic pelvic lymphadenectomy for fertility preservation in young women with early‐stage cervical cancer. Indian J Surg. 2016;78(4):265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu K, Liu Q, Han N‐N, Wang J, Li P‐Q, Ru M‐F. Short term clinical outcomes of laparoscopic fertility preserving radical hysterectomy in the management of early stage cervical cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011;33:436–9. [DOI] [PubMed] [Google Scholar]
- 111. Sun YX, Liu Q, Liu KJ, Li PQ, Hu ZJ. [A retrospective study on the outcomes of the oncology, fertility and pregnancy in patients with early‐stage cervical cancer after undergoing the fertility‐sparing treatments]. Zhonghua Fu Chan Ke Za Zhi. 2016;51(6):442–7. [DOI] [PubMed] [Google Scholar]
- 112. Cao D, Yang J, Xiang Y, Wu M, Pan L, Huang H, et al. [Oncologic and fertility outcomes of young patients with early stage of cervical cancer treated by vaginal radical trachelectomy]. Zhonghua Fu Chan Ke Za Zhi. 2014;49(4):249–53. [PubMed] [Google Scholar]
- 113. Roy M, Plante M. Pregnancies after radical vaginal trachelectomy for early‐stage cervical cancer. Am J Obstet Gynecol. 1998;179(6):1491–6. [DOI] [PubMed] [Google Scholar]
- 114. Rob L, Charvát M, Robova H, Pluta M, Strnad P, Hrehorcák M, et al. Fertility sparing surgery in early cervical cancer today and tomorrow. Ceská Gynekol. 2006;71:302–7. [PubMed] [Google Scholar]
- 115. Shen K, Lang J‐H, Yang J‐X, Chen Y‐L, Xiang Y, Hua K‐Q, et al. [Analysis of 16 patients with early cervical cancer treated by laparoscopic vaginal radical trachelectomy]. Zhonghua Fu Chan Ke Za Zhi. 2006;41:222–5. [PubMed] [Google Scholar]
- 116. Guo J, Zhang Y, Chen X, Sun L, Chen K, Sheng X. Surgical and oncologic outcomes of radical abdominal trachelectomy versus hysterectomy for stage IA2‐IB1 cervical cancer. J Minim Invasive Gynecol. 2019;26(3):484–91. [DOI] [PubMed] [Google Scholar]
- 117. Alexander‐Sefre F, Chee N, Spencer C, Menon U, Shepherd JH. Surgical morbidity associated with radical trachelectomy and radical hysterectomy. Gynecol Oncol. 2006;101(3):450–4. [DOI] [PubMed] [Google Scholar]
- 118. Persson J, Imboden S, Reynisson P, Andersson B, Borgfeldt C, Bossmar T. Reproducibility and accuracy of robot‐assisted laparoscopic fertility sparing radical trachelectomy. Gynecol Oncol. 2012;127(3):484–8. [DOI] [PubMed] [Google Scholar]
- 119. Cao DY, Yang JX, Wu XH, Chen YL, Li L, Liu KJ, et al. Comparisons of vaginal and abdominal radical trachelectomy for early‐stage cervical cancer: preliminary results of a multi‐center research in China. Br J Cancer. 2013;109(11):2778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yoon A, Choi CH, Lee Y‐Y, Kim T‐J, Lee J‐W, Kim B‐G, et al. Perioperative outcomes of radical trachelectomy in early‐stage cervical cancer: vaginal versus laparoscopic approaches. Int J Gynecol Cancer. 2015;25(6):1051–7. [DOI] [PubMed] [Google Scholar]
- 121. Vieira MA, Rendón GJ, Munsell M, Echeverri L, Frumovitz M, Schmeler KM, et al. Radical trachelectomy in early‐stage cervical cancer: a comparison of laparotomy and minimally invasive surgery. Gynecol Oncol. 2015;138(3):585–9. [DOI] [PubMed] [Google Scholar]
- 122. Bernardini M, Barrett J, Seaward G, Covens A. Pregnancy outcomes in patients after radical trachelectomy. Am J Obstet Gynecol. 2003;189(5):1378–82. [DOI] [PubMed] [Google Scholar]
- 123. Plante M, Gregoire J, Renaud M‐C, Roy M. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol Oncol. 2011;121(2):290–7. [DOI] [PubMed] [Google Scholar]
- 124. Lanowska M, Mangler M, Spek A, Grittner U, Hasenbein K, Chiantera V, et al. Radical vaginal trachelectomy (RVT) combined with laparoscopic lymphadenectomy: prospective study of 225 patients with early‐stage cervical cancer. Int J Gynecol Cancer. 2011;21(8):1458–64. [DOI] [PubMed] [Google Scholar]
- 125. Maneo A, Chiari S, Bonazzi C, Mangioni C. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecol Oncol. 2008;111(3):438–43. [DOI] [PubMed] [Google Scholar]
- 126. Pareja R, Ramirez PT, Borrero M. Abdominal radical trachelectomy for invasive cervical cancer: a case series and literature review. Gynecol Oncol. 2008;111(3):555–60. [DOI] [PubMed] [Google Scholar]
- 127. Olawaiye A, Del Carmen M, Tambouret R, Goodman A, Fuller A, Duska LR. Abdominal radical trachelectomy: success and pitfalls in a general gynecologic oncology practice. Gynecol Oncol. 2009;112(3):506–10. [DOI] [PubMed] [Google Scholar]
- 128. Landoni F, Parma G, Peiretti M, Zanagnolo V, Sideri M, Colombo N, et al. Chemo‐conization in early cervical cancer. Gynecol Oncol. 2007;107(1):S125–6. [DOI] [PubMed] [Google Scholar]
- 129. Maneo A, Sideri M, Scambia G, Boveri S, Dell'Anna T, Villa M, et al. Simple conization and lymphadenectomy for the conservative treatment of stage IB1 cervical cancer. An Italian experience. Gynecol Oncol. 2011;123(3):557–60. [DOI] [PubMed] [Google Scholar]
- 130. Palaia I, Musella A, Bellati F, Marchetti C, Di Donato V, Perniola G, et al. Simple extrafascial trachelectomy and pelvic bilateral lymphadenectomy in early stage cervical cancer. Gynecol Oncol. 2012;126(1):78–81. [DOI] [PubMed] [Google Scholar]
- 131. Lee SW, Kim YM, Son WS, You HJ, Kim DY, Kim JH, et al. The efficacy of conservative management after conization in patients with stage IA1 microinvasive cervical carcinoma. Acta Obstet Gynecol Scand. 2009;88(2):209–15. [DOI] [PubMed] [Google Scholar]
- 132. Shepherd JH, Mould T, Oram DH. Radical trachelectomy in early stage carcinoma of the cervix: outcome as judged by recurrence and fertility rates. BJOG. 2001;108(8):882–5. [DOI] [PubMed] [Google Scholar]
- 133. Tokunaga H, Watanabe Y, Niikura H, Nagase S, Toyoshima M, Shiro R, et al. Outcomes of abdominal radical trachelectomy: results of a multicenter prospective cohort study in a Tohoku Gynecologic Cancer Unit. Int J Clin Oncol. 2015;20(4):776–80. [DOI] [PubMed] [Google Scholar]
- 134. Lu Q, Zhang Y, Liu C, Wang S, Guo S, Zhang Z. Total laparoscopic radical trachelectomy in the treatment of early squamous cell cervical cancer: a retrospective study with 8‐year follow‐up. Gynecol Oncol. 2013;130(2):275–9. [DOI] [PubMed] [Google Scholar]
- 135. Lu Q, Zhang Y, Wang S, Guo S, Guo H, Zhang Z, et al. Neoadjuvant intra‐arterial chemotherapy followed by total laparoscopic radical trachelectomy in stage IB1 cervical cancer. Fertil Steril. 2014;101(3):812–7. [DOI] [PubMed] [Google Scholar]
- 136. Biliatis I, Kucukmetin A, Patel A, Ratnavelu N, Cross P, Chattopadhyay S, et al. Small volume stage 1B1 cervical cancer: is radical surgery still necessary? Gynecol Oncol. 2012;126(1):73–7. [DOI] [PubMed] [Google Scholar]
- 137. Lee S‐J, Kim WY, Lee J‐W, Kim HS, Choi Y‐L, Ahn GH, et al. Conization using electrosurgical conization and cold coagulation for International Federation of Gynecology and Obstetrics stage IA1 squamous cell carcinomas of the uterine cervix. Int J Gynecol Cancer. 2009;19(3):407–11. [DOI] [PubMed] [Google Scholar]
- 138. Jeremic K, Petkovic S, Stefanovic A, Stojnic J, Maksimovic M, Likic I, et al. Radical abdominal trachelectomy in managing early cervical invasion. Eur J Gynaecol Oncol. 2009;30(3):309–12. [PubMed] [Google Scholar]
- 139. Matsuo K, Machida H, Mandelbaum RS, Mikami M, Enomoto T, Roman LD, et al. Trachelectomy for stage IB1 cervical cancer with tumor size >2 cm: trends and characteristics in the United States. J Gynecol Oncol. 2018;29(6):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Estevez JP, Hequet D, Dubot C, Fourchotte V, De La Motte RT, Becette V, et al. Préservation de la fertilité chez les patientes atteintes d'un cancer du col de plus de 2cm. Bull Cancer. 2016;103(2):173–9. [DOI] [PubMed] [Google Scholar]
- 141. Tellum T, Omtvedt M, Naftalin J, Hirsch M, Jurkovic D. A systematic review of outcome reporting and outcome measures in studies investigating uterine‐sparing treatment for adenomyosis. Hum Reprod Open. 2021;2021(3):hoab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ghai V, Subramanian V, Jan H, Pergialiotis V, Thakar R, Doumouchtsis SK, et al. A systematic review on reported outcomes and outcome measures in female idiopathic chronic pelvic pain for the development of a core outcome set. BJOG. 2021;128(4):628–34. [DOI] [PubMed] [Google Scholar]
- 143. Doumouchtsis SK, Pookarnjanamorakot P, Durnea C, Zini M, Elfituri A, Haddad JM, et al. A systematic review on outcome reporting in randomised controlled trials on surgical interventions for female stress urinary incontinence: a call to develop a core outcome set. BJOG. 2019;126(12):1417–22. [DOI] [PubMed] [Google Scholar]
- 144. de Mattos Lourenco TR, Pergialiotis V, Duffy JMN, Durnea C, Elfituri A, Haddad JM, et al. A systematic review on reporting outcomes and outcome measures in trials on synthetic mesh procedures for pelvic organ prolapse: urgent action is needed to improve quality of research. Neurourol Urodyn. 2019;38(2):509–24. [DOI] [PubMed] [Google Scholar]
- 145. Hirsch M, Duffy JMN, Kusznir JO, Davis CJ, Plana MN, Khan KS, et al. Variation in outcome reporting in endometriosis trials: a systematic review. Am J Obstet Gynecol. 2016;214(4):452–64. [DOI] [PubMed] [Google Scholar]
- 146. Kirkham JJ, Gargon E, Clarke M, Williamson PR. Can a core outcome set improve the quality of systematic reviews? – a survey of the co‐ordinating editors of Cochrane review groups. Trials. 2013;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dickersin K, Rennie D. Registering clinical trials. JAMA. 2003;290(4):516–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1
Table S2
Table S3.1
Table S3.2
Table S3.3
Table S3.4
Table S3.5
Table S3.6
Table S4
Table S5
Table S6
Data S1
Data S2
Data S3
Data S4
Data S5
Data S6
Data S7
Data S8
Data S9
Data Availability Statement
Data available on reasonable request from the corresponding author.


