Abstract
Blood-brain barrier (BBB) permeability can be measured by the ratio of albumin in cerebrospinal fluid (CSF) and blood and by dynamic contrast-enhanced MRI (DCEMRI). Albumin is a large molecule measured in CSF and blood to form the albumin index (Qalb), which is a global measure of BBB permeability, while the smaller Gadolinium molecule measures regional transfer (Ktrans); few studies have directly compared them in the same patients. We used both methods as part of a study of mechanisms of white matter injury in patients with different forms of dementia. In addition, we also measured biomarkers for inflammation, including proteases, angiogenic growth factors, and cytokines, and correlated them with the BBB results. We found that there was no correlation between Qalb and Ktrans. The Qalb was associated with the matrix metalloproteinases (MMP-2, MMP-3, and MMP-10), the angiogenic factors (VEGF-C and PlGF), and the cytokines (IL-6, IL-8 and TNF-α). On the other hand, Ktrans was associated with the diffusion measures, mean free water and PSMD, which indicate white matter injury. Our results show that the Qalb and Ktrans measure different aspects of BBB permeability, with albumin being a measure of inflammatory BBB opening and Ktrans indicating white matter injury.
Keywords: Blood-brain barrier, permeability, albumin index, dynamic contrast-enhanced MRI, inflammation
Introduction
The worldwide increase in the prevalence of dementia due to aging and improved treatment of cancer and cardiac disease has accelerated attempts to understand the multiple causes and to develop novel treatments based on the pathophysiology.1,2 One of the main theories is that the blood-brain barrier (BBB) is disrupted, contributing to the molecular cascade that leads to cell death. This theory is based on studies of vascular permeability using two main methods: one is a contrast-based MRI method called dynamic contrast-enhanced MRI (DCEMRI), and the other is the albumin index (Qalb) formed by albumin in the CSF divided by the amount in the plasma. Opening of the BBB has been shown in Alzheimer's disease (AD) and in vascular cognitive impairment and dementia (VCID). Early studies of BBB in dementia using Qalb showed the opening in vascular dementia, and only in Alzheimer’s disease when there was injury to the white matter suggestive of mixed dementia.3,4 More recent studies done with DCEMRI to quantify transport across the blood vessels (Ktrans) indicate that the BBB is compromised in AD and VCID.5–9 Pathological studies indicate that the most common form of dementia is a combination of AD and VCID, referred to as mixed dementia (MX).10,11 Although there are many essential differences between the two methods to measure BBB permeability, there is little information comparing them. Albumin is a large molecule of over 66 kDa that is present in low concentration in CSF and increases when there is global leakage. On the contrary, Gadolinium (Gd) is a small molecule with a molecular weight of 157 Da that can show regional permeability on MRI. Although both are thought to measure similar features of vascular permeability, there is little information comparing the two methods. Our group has collected permeability data on participants using both methods for over ten years as part of studies to understand the role of BBB in white matter damage in VCID. In addition, since many of these patients with white matter injury on MRI were suspected of having multiple sclerosis (MS), those who consented underwent a lumbar puncture to measure MS proteins in CSF, which included Qalb. 12 Therefore, we had a unique patient population with measurements of both Qalb and Ktrans. As a site in the NIH Biomarkers in VCID consortium (MarkVCID), we also measured proteases, angiogenic growth factors, and cytokines in CSF and blood. 13 With this additional data, we were able to correlate biomarkers with each of the permeability measures to give a more complete picture of the pathophysiology of the white matter injury. We tested two hypotheses: 1) Qalb and Ktrans in white matter gave similar results for BBB permeability, and 2) BBB disruption is associated with inflammatory biomarkers.
Materials and methods
Participant information
The study was approved by the University of New Mexico Human Research Review Committee. All patients gave informed consent to study procedures, including lumbar puncture in a subset of participants. Patients were recruited from the Neurology clinic and the Center for Memory and Aging clinic at the University of New Mexico Hospital and the memory clinic at the Albuquerque Veterans Administration Hospital. Patients underwent neurological examinations, neuropsychological tests, a lumbar puncture to collect CSF and venipuncture for blood, and MRI. All subjects were at least 50 years old. ApoE genotyping was not performed.
Participants included 136 patients. Patient diagnoses were assigned by a consensus of the three study neurologists into four groups: AD, VCID, MX, or leukoaraiosis (LA). The AD diagnosis was made according to published criteria; 14 when available, we also used the results of CSF amyloid and tau measurements. 15 VCID was diagnosed as described in a consensus paper. 16 MX was diagnosed by combining the AD diagnosis, including CSF AD proteins when available, with white matter injury as indicated by diffusion tensor imaging (DTI). LA designated participants with significant white matter hyperintensities (WMHs) on FLAIR MRI but with minimal cognitive or neurological impairment. 17 Patients with multiple or single strategic strokes were excluded.
Cognitive assessments
Participants underwent a battery of in-person neuropsychological tests by trained research coordinators. The tests covered five cognitive domains: memory (Craft story delayed recall, Benson complex figure recall, HVLT delayed recall), executive function (trails B time to finish, digit span backward, and f-words), language (MINT and semantic fluency), attention (digit span forward and trails A), and processing speed (symbol digit and symbol search). Each test was scored, and an age-adjusted t-score was calculated. To calculate the composite score for each domain, the t-scores of all tests in each domain were averaged. For a score of overall cognition, all five domain scores were averaged.
Blood and CSF studies
Phosphorylated tau and amyloid-β
Several biomarkers were measured in CSF and plasma. CSF biomarkers were obtained by lumbar puncture performed in the morning after fasting by one of the authors (JCA). Blood draws were performed during the same patient visit. Samples were centrifuged, aliquoted, and stored at −80°C for later analysis.
Levels of CSF Tau protein phosphorylated at threonine position 181 (pTau181) were measured using Innotest Phospho-Tau181 ELISA (Fujirebio US; Malvern PA). Prior to analysis, all CSF underwent one freeze-thaw cycle. Assays were performed according to manufacturer protocols and were read with a Bio-Tek multimodal plate reader with absorbance at 450 nm. The output data were used to quantify the concentrations based on the manufacturer in-assay standard curve. We measured Amyloid β1-42 (Aβ1-42) and Amyloid β1-40 (Aβ1-40) to calculate the Aβ1-42/Aβ1-40 ratio (V-PLEX Aβ Peptide Panel 1–6E10; MesoScale Discovery MSD, Rockville, Maryland). The output data quantify the concentrations based on the 2-fold sample dilution and the supplied in-assay standard curve. All data were expressed as pg/mL, though the ratio is unitless.
Matrix metalloproteinase, angiogenesis, and proinflammatory assays
We used MesoScale Discovery (MSD) multiplex assay kits selected by the MarkVCID consortium to measure matrix metalloproteinases (MMP-1, MMP-2, MMP-3, MMP-9, and MMP-10); these were measured with two ELISA kits (MSD; MMP 2-Plex and MMP 3-Plex). Angiogenic growth factors were measured by ELISA (MSD; Angiogenesis Panel 1) were vascular endothelial growth factor-c (VEGF-C) and placental growth factor (PlGF). Similarly, multiple pro-inflammatory cytokines were measured with the Proinflammatory Panel 1 (MSD). The cytokines, included interleukins (IL) and tumor necrosis factor (TNF). All CSF samples were run undiluted for these assays while all plasma samples were diluted 2-fold except for the MMP 3-Plex, in which case the plasma samples were diluted 10-fold. All data were expressed as pg/mL.
Assays were performed using established protocols on an MSD Quickplex SQ 120 plate reader, followed by analysis performed in the MSD Discovery Workbench 4.0 software used to quantify analyte concentrations, and all data were expressed as pg/mL. Protein markers measured with MSD assays were subjected to intra-plate variability tests to calculate the coefficient of variation (CV), as determined by duplicate runs for each sample. Samples with a CV ≥15% were removed from further analysis. Another assessment involved two CSF, and two plasma pooled control samples run in duplicate on the same plate in all assays. These control samples were held to the same intra-plate CV (≥15%) and assessed for plate-to-plate variability.
MRI studies
To obtain information on the integrity of the white matter, we used MRI scans that were performed on a Siemens 3T scanner (Prisma). Initial scans were performed on a 12-channel radio frequency (RF) coil, and later scans were acquired with a 32-channel RF coil. The imaging parameters with the two RF coils were closely matched. The 3D MPRAGE sequence had TR = 2530 ms, four echoes, and TI = 1200 ms with an acquisition time of 6.5 minutes. The 3D FLAIR sequence had a TR = 6000 ms, TE = 427 ms, and TI = 2000 ms. The diffusion data were collected with a FOV = 224, 2 mm isotropic resolution, and 72 slices for both RF coils. On the 12-channel coil, the diffusion protocol had a single-shell of b-value =800 s/mm2 with 30 volumes collected with different gradient directions and five volumes with b = 0. On the 32-channel coil, three shell diffusion data with a multi-band sequence were collected. A single b-value shell with 55 gradient directions of either b-value = 800 s/mm2 or b-value = 1000 s/mm2 was extracted for this study.
White matter hyperintensity (WMH) volume was calculated from T1 and FLAIR images using a fully automated WMH detection and quantification script (idealab.ucdavis.edu) selected by the MarkVCID consortium. The diffusion images were corrected for motion, distortion, and mean diffusivity (MD), and fractional anisotropy (FA) were calculated (www.fmrib.ox.ac.uk). The peak width of skeletonized mean diffusivity (PSMD) and mean free water was calculated based on the method adopted by the MarkVCID consortium, and all three scripts are available (www.markvcid.org).
The details of the blood-brain permeability method have been described previously.5,9 Eight axial slices, 4 mm thick and 2 mm in-plane resolution were imaged. The 3.2 cm imaging slab was place above the corpus callosum. Rapid inversion-recovery T1 measurements were made, with one T1 measurement before Magnevist (Gd-DTPA) injection and eight measurements afterwards, with each measurement being 2.5 minutes apart for a total imaging time of 22.5 minutes.
Statistical methods
Numeric data were log2(X + 1) transformed as needed to reduce the undue influence from right skewness. Analyses were done using the subset of participants with all necessary data. A Type-I error rate of 0.05 was used, and p-values were not adjusted except when making multiple comparisons.
The relationship between MRI-measured permeability (Ktrans) and albumin index (Qalb) was assessed by the Spearman correlation. Differences in permeability by diagnoses were evaluated using a Kruskal-Wallis test followed by a Dunn test with Holm adjustment to identify group differences.
Multiple linear regression was used to identify associations between each of the two measures of BBB permeability, Ktrans, and Qalb, with biomarkers from fluids and MRI. We used each permeability measure as the response variable and each biomarker as an explanatory variable in separate models, all models including covariates of age, sex (Female is the reference group), education, and their interactions with each biomarker. Final models were determined using best subset selection based on the minimum Bayesian Information Criterion (BIC); that is, any combination of covariates with each biomarker is considered.
For the relationship between BBB permeability and cognition, a similar model used each cognition variable as the response variable and a permeability measure as an explanatory variable. All effect sizes were put on the standard deviation scale by z-scoring all numeric variables to simplify comparisons. All analyses are exploratory and p-values have to be interpreted cautiously since no adjustment for multiple testing was done.
Results
Demographics
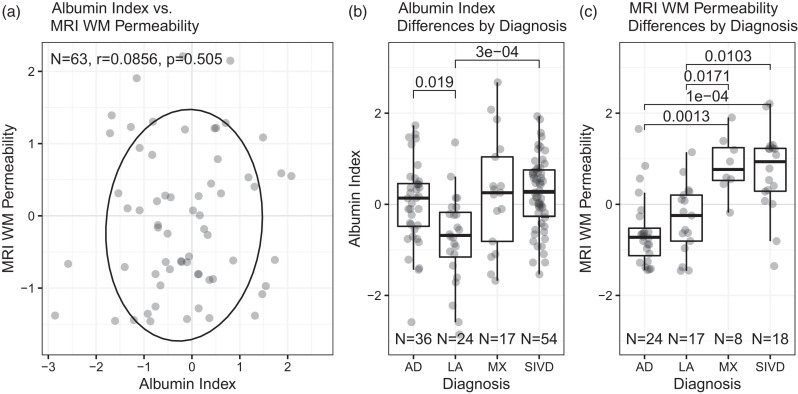
The sample had 136 patients, including 66 women and 70 men (Table 1). The LA group were primarily women, and the MX patients were primarily male, while the AD and VCID groups were balanced. Mean ages differed between diagnosis groups; the youngest patients were LA (63), with increasing ages for VCID (67), AD (70), and MX (76). Education levels did not differ significantly between the diagnostic groups and were high, with the average participant holding a college degree. A Spearman correlation test showed no relationship between Ktrans and Qalb (Figure 1(a)). The Kruskal-Wallis test revealed significant differences in both Ktrans and Qalb between the diagnostic groups. Dunn's test showed Qalb was lower in cognitively intact LA patients than in AD or VCID patients but showed no differentiation between AD, VCID, and MX diagnoses (Figure 1(b)). In contrast, patients diagnosed with VCID or MX have higher total Ktrans than LA or AD (Figure 1(c)).
Table 1.
Demographic and permeability characteristics overall and by diagnostic category.
| Characteristics | N | Total | AD | LA | MX | VCID | |
|---|---|---|---|---|---|---|---|
| (N = 39) | (N = 25) | (N = 17) | (N = 55) | p-value | |||
| Age | 136 | 68 [62; 75] | 70 [66; 76] | 63 [55; 68] | 76 [74; 79] | 67 [61; 71.5] | <0.0005 |
| Sex | 136 | 0.063 | |||||
| Female | 66 (48.5%) | 17 (43.6%) | 18 (72.0%) | 6 (35.3%) | 25 (45.5%) | ||
| Male | 70 (51.5%) | 22 (56.4%) | 7 (28.0%) | 11 (64.7%) | 30 (54.5%) | ||
| Years of education | 101 | 16 [14; 18] | 16 [14; 18] | 16 [12.5; 18] | 15 [13; 18] | 16 [13; 18] | 0.575 |
| Albumin Index | 131 | 6.40 [4.85; 7.95] | 6.69 [5.21; 7.60] | 4.85 [3.90; 6.02] | 7.00 [4.60; 9.40] | 7.05 [5.67; 8.50] | 0.001 |
| MRI WM Permeability | 67 | 4.15 [1.13; 10.51] | 1.33 [0.44; 2.23] | 2.91 [1.13; 5.36] | 10.92 [7.78; 19.88] | 13.27 [5.92; 18.39] | <0.0005 |
Table values: Median [Q1; Q3] for numeric values and n (%) for categorical values. IQR bounds [Q1, Q3] are 25th and 75th percentiles. AD: Alzheimer's disease; LA: leukoaraiosis; MX: mixed dementia; vascular cognitive impairment and dementia: vascular cognitive impairment and dementia.
P-values reported from the Kruskal-Wallis test for continuous data, and from the chi-square test with continuity correction for categorical data.
Figure 1.
(a) Scatterplot of Qalb and Ktrans with Spearman correlation test and 80% confidence ellipse. (b) Qalb distribution by diagnosis with Dunn's pairwise tests. AD is Alzheimer’s disease, LA is leukoaraiosis, MX is mixed, SIVD is subcortical ischemic vascular disease, which is the subcortical form of VCID and (c) Ktrans distribution by diagnosis with Dunn's pairwise tests.
MRI and albumin relationships with CSF inflammatory biomarkers
Albumin index
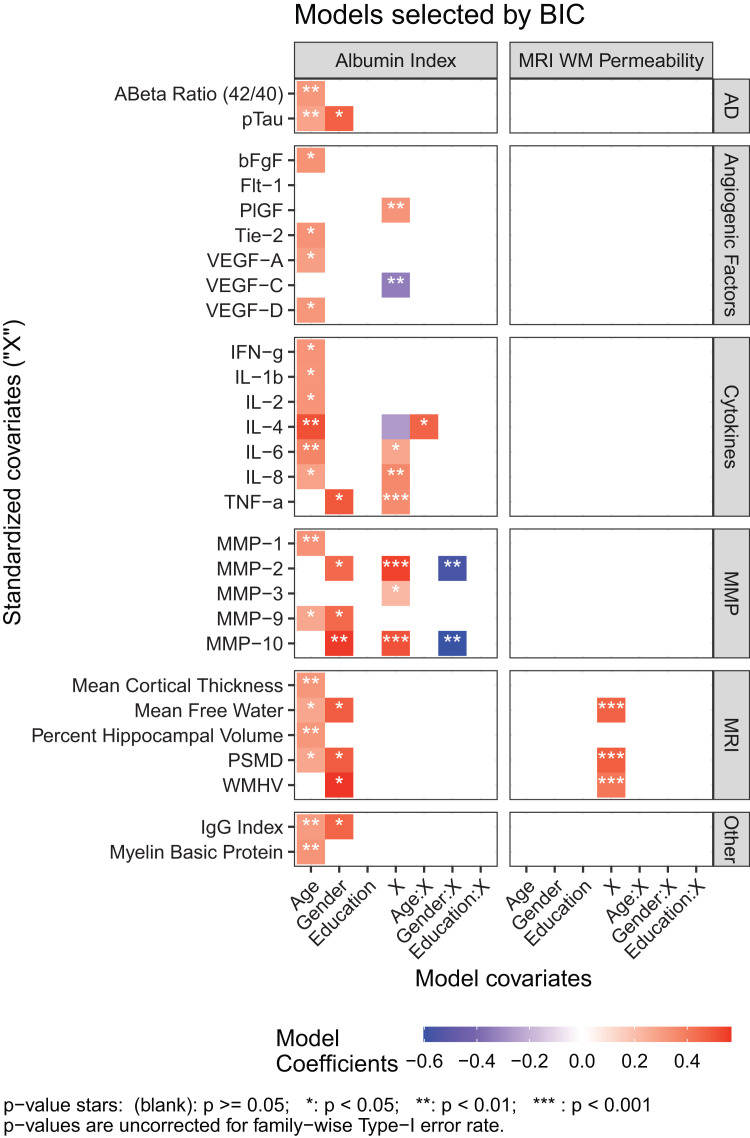
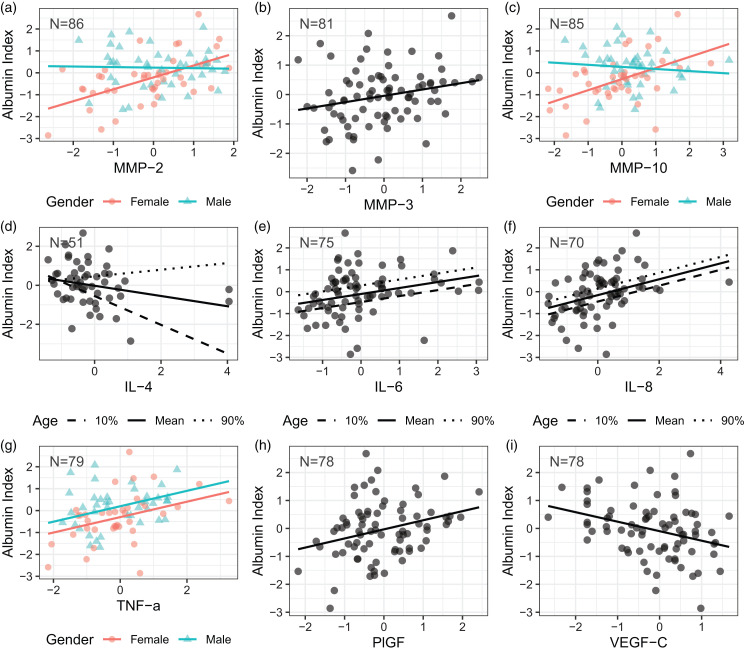
The Qalb correlated with several CSF biomarkers (Figure 2; parameter estimates in Supplemental Table A 1). Three of the proteases, MMP-2 (Figure 3(a)), MMP-3 (Figure 3(b)), and MMP-10 (Figure 3(c)), were positively associated with albumin index in females, but only MMP-3 also showed that relationship for males. Higher Qalb was associated with decreased levels of cytokine IL-4 (Figure 3(d)), but increased levels of cytokines IL-6 (Figure 3(e)), IL-8 (Figure 3(f)), and TNF-α (Figure 3(g)). Furthermore, IL-6 and IL-8 are all associated with age. IL-6 and IL-8 are higher in older patients, while IL-4 interacts with age, with the negative association between IL-4 and albumin strongest in our youngest patients. This relationship weakens with increasing age. For TNF-α, males had higher Qalb than females. In addition, angiogenic growth factors correlated with Qalb, PlGF positively (Figure 3(h)), and VEGF-C negatively (Figure 3(i)). There were no significant relationships between Qalb and plasma measures of proteases, angiogenic growth factors, or cytokines (data not shown), in spite of several plasma and CSF measures being positively correlated (MMP-3, MMP-10, VEGF-D, PlGF, and IL-1B). 18
Figure 2.
Albumin index and MRI WM permeability associations with fluid and MRI biomarkers adjusted for age, gender, and education. Coefficients for models where the covariate (“X”) was retained after model selection for Qalb (left panel) and Ktrans (right panel) are of primary interest.
Figure 3.
Model fit associations of Albumin index with MMPs, cytokines, and angiogenic factors. Where Age is a covariate, the mean value is plotted as well as the 10th and 90th percentiles of Age.
MRI WM permeability
No significant relationships were observed between Ktrans and CSF biomarkers (Figure 2, right panel). There were no significant relationships between Ktrans and plasma measures of proteases, angiogenic growth factors, or cytokines (data not shown).
MRI and albumin relationship with MRI measures
Albumin index
Albumin index is not related to MRI measures of cortical thickness, hippocampal volume, white matter hyperintensities, and diffusion imaging measures (Figure 2, right panel).
MRI WM permeability
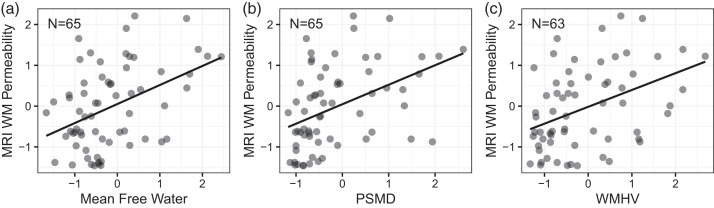
Mean free water (Figure 4(a)), PSMD (Figure 4(b)), and white matter hyperintensity volume (Figure 4(c)) were positively correlated to Ktrans measurements (Figure 2, right panel). Demographic variables had no effect on PSMD or WMH volume. For mean free water, age was negatively associated with Ktrans with older patients showing lower permeability. No relationship was found between either BBB measure and CSF pTau or Aβ42/40 ratio.
Figure 4.
Model fit associations of MRI WM Permeability with imaging biomarkers of white matter damage. Where Age is a covariate, the mean value is plotted as well as the 10th and 90th percentiles of Age.
Cognitive performance by domain relationship with MRI and albumin
No relationships between albumin index or MRI WM permeability with cognitive performance measures were observed.
Discussion
Our results show that Qalb and Ktrans, the two main measures of BBB permeability in humans, are not correlated, suggesting that they measure different aspects of permeability. Qalb is a global measure that indicates the movement of a 66 kDa albumin molecule into CSF, while Ktrans shows a regional transfer of the smaller gadolinium molecule across blood vessels. By directly comparing the measurement of BBB permeability by the QaIb and Ktrans methods, we disproved the first hypothesis. However, the association of each measure with biomarkers indicated that Qalb showed inflammatory disruption of the BBB, supporting the second hypothesis.
The difficulty of measuring low values of BBB permeability has been documented previously.19,20 The differences exist both because of different acquisition protocols and data analysis methods used. 21 The long experimental time (> 20 min) does not help, because image registration needed to correct for head motion leads to biases. Although, there are whole brain, Gd-contrast based BBB measurement methods currently available, 20 our focus was primarily on white matter. Only cortical gray matter was included in the measurement slab. Ktrans values in the cortical gray matter were not consistent across our control group, possibly because it is a thin layer contiguous to CSF. Other groups have been successful in measuring Ktrans in deep gray matter structures, such as hippocampus. 8
Our results not only showed the lack of correlation of the BBB measurements by the two methods, but more importantly, we indicated which method was associated with each biomarker. Measurements with albumin as the tracer were associated with biomarkers associated with inflammation, specifically the proteases (MMP-2, MMP-3, and MMP-10), the angiogenic growth factor (VEGF-C and PlGF), and the cytokines (IL-6, IL-8, and TNF-α). The anti-inflammatory cytokine, IL-4, was negatively associated with Qalb. These results are consistent with the second hypothesis that inflammation contributes to the disruption of the BBB. Our results were observed primarily in the CSF rather than the plasma. Our results are derived from white matter and in line with prior studies, measurement of Ktrans by the DCEMRI method correlated best with MRI-related biomarkers of the white matter, including mean free water, PSMD, and white matter hyperintensity volume.22,23
Albumin is a large molecule typically found in low concentrations in the CSF, making its detection in the CSF an indicator of increased permeability. Its role as a marker of BBB permeability was initially recognized during studies of CSF proteins related to MS and subsequently expanded into studies in dementia;3,12 it was initially used in MS to determine the leakage of proteins from the blood to the brain to eliminate the systemic origin of the proteins detected in CSF. When Qalb was measured in AD and vascular dementia, it was mainly increased in vascular dementia. 3
It is not surprising that the two measures show different aspects of BBB permeability in patients with VCID and AD. In earlier studies, AD patients with higher Qalb showed evidence of white matter damage on neuroimaging, suggesting that they had mixed dementia. 24 With the increased availability of MRI to diagnose dementia, fewer lumbar punctures were done. This trend has been reversed by using CSF to diagnose AD in research studies. 15 However, our study is one of the few to have measured Ktrans in white matter and global Qalb in the same individual, making our study unique.
For the past five years, we have been one of the sites in the MarkVCID consortium, which has collected data on many biomarkers derived from blood, CSF, and MRI.13,25 The fluid biomarkers were selected because they could be measured by validated, commercially available multiplex ELISA assays. The consortium selected to measure MMPs, angiogenic growth factors, and cytokines in blood and CSF. We found that MMP-2, MMP-3 and MMP-10, correlated with Qalb but not with Ktrans. In addition, Qalb correlated with two angiogenic factors, VEGF-C and PlGF, and three cytokines, IL-6, IL-8, and TNF-α. It is noteworthy that all of the biomarkers that correlated positively with Qalb are considered inflammatory.
Cells in the neurovascular unit produce several MMPs, which have been implicated in opening the BBB. 26 MMP-2 is present in astrocytic foot processes surrounding the blood vessels, where it has a direct action on the extracellular matrix and the tight junction proteins. 27 While little is known about the role of MMP-10 (stromelysin-2) in the brain, it is in the same family as MMP-3 (stromelysin-1), which is in pericytes, macrophages, and microglia, making it a potential candidate for disruption of the BBB. Our results are highly suggestive that MMP-2, MMP-3, and MMP-10 are involved in BBB opening; however, since very little is known about MMP-10, further work will be needed to understand its role. Furthermore, we found gender differences in MMP-2 and MMP-10 measures that will require further exploration.
We found higher Qalb values in the males for all of the biomarkers measured, which was shown by others in a large number of patients between 1 and 90 years of age that revealed a significant sex difference in all age groups; our observation is consistent with lower integrity of the brain barriers in males.28,29
Angiogenic growth factors are potential disrupters of the BBB. 30 They induce sprouting to increase the delivery of oxygenated blood to the brain; VEGF has been identified at autopsy in AD. 31 Newly formed vessel sprouting induce both MMPs to clear away the extracellular matrix and VEGF to stimulate vessel growth; these sprouting vessels have increased permeability. 32 Our results showed that VEGF-C and PlGF correlated with Qalb. Other angiogenic growth factors that could disrupt the BBB were measured, including VEGF-D, flt-1, and tie-2 but these did not reach statistical significance in our cohort. Three cytokines that were positively correlated with Qalb, IL-6, IL-8, and TNF-α, are implicated in inflammation, while IL-4, which is anti-inflammatory, was negatively correlated. Cytokines are known to augment brain inflammatory responses as they are secreted by several resident brain cells, particularly microglia and macrophages. Most fluid biomarkers were found at detectable levels only in the CSF. 18
We separated patients into subgroups (AD, VCID, MX, and LA) using the double dichotomy method with CSF to detect AD proteins and MRI to evaluate white matter injury. 33 The AD group had the lowest values for Ktrans compared to VCID and MX. Our DCEMRI WM permeability measurements were made in white matter, while others have measured permeability in the gray matter; a prior study that measured hippocampal permeability found increases in aging and mild cognitive impairment. 8 Another reason for differences in our BBB results is that we separated patients with MX from AD. Other studies may have included MX in their AD patient groups since 70% of AD patients are MX at autopsy. 11 Further studies with removal of AD patients with vascular disease will be necessary to resolve the impact of vascular permeability on AD.
Several biomarkers have been used to indicate injury to the white matter. The DTI measurements, PSMD, and mean Free Water were associated with Ktrans. Gd has a much smaller molecular weight and size than albumin. In acute inflammatory diseases, such as MS attacks, the extensive extravasation of Gd suggests disruption of the tight junctions, which would allow the smaller-sized Gd to cross through an open tight junction, while the larger albumin molecule would be blocked from this route. Further evidence for transport related to molecular size comes from MS studies; in acute MS, there is extensive extravasation of Gd on MRI, while albumin is generally not elevated. 12 In contrast, vascular dementia patients have low levels of Gd leakage with DCEMRI, while albumin is increased.3,20 This suggests that different pathological mechanisms could be involved in permeability to Gd versus albumin.
Animal studies in acute hypertension using electron microscopy with the large electron-dense molecule, lanthanum, showed pinocytosis in arterioles was the main transport route across the vessels when the blood pressure was acutely increased, 34 which was also found in spontaneously hypertensive rats. 35 A similar finding was observed in Ang II-induced hypertension where engagement of AT1R in cerebral endothelial cells led to increased transcytosis and TJ remodeling. 36 This suggests that albumin is too large to cross through a disrupted endothelial cell tight junction, but it could cross the endothelial cell by pinocytosis. Typically, brain blood vessels show very few pinocytotic vessels compared to vessels in the heart and muscle. Electron microscopic studies with the large horseradish peroxidase (HRP) molecule showed the presence of tight junctions that blocked its movement across the vessels but indicated the presence of micropinocytotic vesicles as another route for the large HRP molecule. 37 Pinocytosis is consistent with the high incidence of hypertension in vascular dementia. Interestingly, arterioles, the primary site of pathology in VCID, are also the site of pinocytosis.
In a recent study of 1015 individuals with various forms of dementia, Qalb was increased compared to controls in vascular and frontotemporal dementia. The investigators failed to find a relationship between APOEε4 and amyloid PET to Qalb, leaving unresolved the question of the BBB in AD. Moreover, in agreement with our results, they found an increase in BBB permeability related to angiogenesis biomarkers. 38
There are limitations to our results. The study was done in a small group of patients from a single center. Gadolinium-based permeability measures restrict use of DCEMRI to individuals without renal dysfunction. Diffusion-prepared pseudo-continuous arterial spin labeling obviates the requirement for Gd and could be applied to a broader range of subjects (Shao et al. 2020). While we had many subjects with either DCEMRI or albumin index alone, only a subgroup had both measurements in the same subjects. In addition, the DCEMRI was done in white matter and not in gray matter. Another limitation is that the measured biomarkers were those used in the MarkVCID consortium study and were measured by the MSD assay system. Using newer methods, particularly the ultrasensitive assay platforms, such as Quanterix SIMOA and Olink, may have shown that other biomarkers were involved.
Despite the limitations, our results provide unique information about the currently used methods to measure the BBB permeability. Our dataset has several significant advantages: it directly compared Qalb and Ktrans and it associated both with a large set of biomarkers derived from CSF and MRI. Another important aspect is that this is the first study to follow the recent suggestion to incorporate a vascular factor, V, into the ATN framework (“ATNV”) to classify AD, MX, and VCID patients during life. 39
In conclusion, Qalb and Ktrans are not correlated, indicating they show different aspects of BBB injury. In addition, the correlation of Qalb with CSF biomarkers derived from three different classes provides evidence for inflammatory disruption of the BBB. The MRI measure of permeability by DCEMRI correlated with biomarkers of diffusion in the white matter, mean free water, and PSMD, as well as the volume of white matter hyperintensities, while Qalb correlates with inflammatory biomarkers. We propose that inflammation is driving the disruption of the BBB as measured by the global biomarker, Qalb. Although still speculative, our data suggest that albumin leakage may be occurring by a transendothelial pinocytosis involving the arterioles. The regional DCEMRI method measures changes in the smaller vessels that impact MRI diffusion. These results suggest that the albumin index and DCEMRI measurements of BBB are complementary and show different aspects of the pathophysiology involved in BBB disruption.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221146127 for Blood-brain barrier disruption measured by albumin index correlates with inflammatory fluid biomarkers by Laura Hillmer, Erik B Erhardt, Arvind Caprihan, John C Adair, Janice E Knoefel, Jill Prestopnik, Jeffrey Thompson, Sasha Hobson and Gary A Rosenberg in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for these studies came from NIH grants to GAR: UH3 NS100598 (MarkVCID), RO1 NS052305 and RO1 NS068048. This project was supported by an award from the National Center for Advancing Translational Sciences, National Institutes of Health under grant number UL1TR001449. The PRISMA 3T upgrade at The Mind Research Network was supported by NIH award S10OD025313.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
L.H. and E.B.E. did the statistical analysis. A.C. performed and analyzed the MRI studies. J.C.A., J.K and G.R. performed the clinical assessments. J.P. did the neuropsychological studies. J.T. and S.H. performed the biochemical analyses. G.A.R. designed the study and obtained the research funding. All the authors participated in the writing of the manuscript.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including alzheimer's disease. Alzheimers Dement 2015; 11: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallin A, Blennow K, Fredman P, et al. Blood brain barrier function in vascular dementia. Acta Neurol Scand 1990; 81: 318–322. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Wallin A, Fredman P, et al. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol Scand 1990; 81: 323–326. [DOI] [PubMed] [Google Scholar]
- 5.Taheri S, Gasparovic C, Huisa BN, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke 2011; 42: 2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlokovic BV, Gottesman RF, Bernstein KE, et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 national heart, lung, and blood institute and national institute of neurological disorders and stroke workshop. Alzheimers Dement 2020; 16: 1714–1733. [DOI] [PubMed] [Google Scholar]
- 7.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huisa BN, Caprihan A, Thompson J, et al. Long-term blood-brain barrier permeability changes in Binswanger disease. Stroke 2015; 46: 2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197–2204. [DOI] [PubMed] [Google Scholar]
- 11.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the national Alzheimer's coordinating centre. Brain 2013; 136: 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tourtellotte WW, Tavolato B, Parker JA, et al. Cerebrospinal fluid electroimmunodiffusion. An easy, rapid, sensitive, reliable, and valid method for the simultaneous determination of immunoglobulin-G and albumin. Arch Neurol 1971; 25: 345–350. [DOI] [PubMed] [Google Scholar]
- 13.Wilcock D, Jicha G, Blacker D, et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement 2021; 17: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR, Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018; 14: 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 2016; 36: 6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hachinski VC, Potter P, Merskey H.Leuko-araiosis. Arch Neurol 1987; 44: 21–23. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt EB, Adair JC, Knoefel JE, et al. Inflammatory biomarkers aid in diagnosis of dementia. Front Aging Neurosci 2021; 13: 717344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning C, Stringer M, Dickie B, et al. Sources of systematic error in DCE-MRI estimation of low-level blood-brain barrier leakage. Magn Reson Med 2021; 86: 1888–1903. [DOI] [PubMed] [Google Scholar]
- 20.Thrippleton MJ, Backes WH, Sourbron S, et al. Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement 2019; 15: 840–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringer MS, Heye AK, Armitage PA, et al. Tracer kinetic assessment of blood-brain barrier leakage and blood volume in cerebral small vessel disease: associations with disease burden and vascular risk factors. Neuroimage Clin 2021; 32: 102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao X, Jann K, Ma SJ, et al. Comparison between blood-brain barrier water exchange rate and permeability to gadolinium-based contrast agent in an elderly cohort. Front Neurosci 2020; 14: 571480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Li M, Zuo L, et al. Compromised blood-brain barrier integrity is associated with total magnetic resonance imaging burden of cerebral small vessel disease. Front Neurol 2018; 9: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blennow K, Wallin A, Uhlemann C, et al. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol Scand 1991; 83: 187–193. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Kashani AH, Arfanakis K, et al. MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols. Alzheimers Dement 2021; 17: 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg GA.Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 2009; 8: 205–216. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg GA, Cunningham LA, Wallace J, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res 2001; 893: 104–112. [DOI] [PubMed] [Google Scholar]
- 28.Parrado-Fernández C, Blennow K, Hansson M, et al. Evidence for sex difference in the CSF/plasma albumin ratio in ∼20 000 patients and 335 healthy volunteers. J Cell Mol Med 2018; 22: 5151–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber CM, Clyne AM.Sex differences in the blood-brain barrier and neurodegenerative diseases. APL Bioeng 2021; 5: 011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg DA, Jin K.From angiogenesis to neuropathology. Nature 2005; 438: 954–959. [DOI] [PubMed] [Google Scholar]
- 31.Harris R, Miners JS, Allen S, et al. VEGFR1 and VEGFR2 in Alzheimer's disease. J Alzheimers Dis 2018; 61: 741–752. [DOI] [PubMed] [Google Scholar]
- 32.Rundhaug JE.Matrix metalloproteinases and angiogenesis. J Cell Mol Med 2005; 9: 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caprihan A, Raja R, Hillmer LJ, et al. A double-dichotomy clustering of dual pathology dementia patients. Cereb Circ Cogn Behav 2021; 2: 20210402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nag S, Robertson DM, Dinsdale HB.Quantitative estimate of pinocytosis in experimental acute hypertension. Acta Neuropathol 1979; 46: 107–116. [DOI] [PubMed] [Google Scholar]
- 35.Nag S, Robertson DM, Dinsdale HB.Morphological changes in spontaneously hypertensive rats. Acta Neuropathol 1980; 52: 27–34. [DOI] [PubMed] [Google Scholar]
- 36.Santisteban MM, Ahn SJ, Lane D, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension 2020; 76: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reese TS, Karnovsky MJ.Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 1967; 34: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janelidze S, Hertze J, Nägga K, et al. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging 2017; 51: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampel H, Cummings J, Blennow K, et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol 2021; 17: 580–589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221146127 for Blood-brain barrier disruption measured by albumin index correlates with inflammatory fluid biomarkers by Laura Hillmer, Erik B Erhardt, Arvind Caprihan, John C Adair, Janice E Knoefel, Jill Prestopnik, Jeffrey Thompson, Sasha Hobson and Gary A Rosenberg in Journal of Cerebral Blood Flow & Metabolism