Abstract
Decreased cerebral blood flow (CBF) may be an important mechanism associated with depression. In this study we aimed to determine if the association of CBF and depression is dependent on current level of depression or the tendency to experience depression over time (trait depression), and if CBF is influenced by depression-related factors such as stressful life experiences and antidepressant medication use. CBF was measured in 254 participants from the Amish Connectome Project (age 18–76, 99 men and 154 women) using arterial spin labeling. All participants underwent assessment of symptoms of depression measured with the Beck Depression Inventory and Maryland Trait and State Depression scales. Individuals diagnosed with a unipolar depressive disorder had significantly lower average gray matter CBF compared to individuals with no history of depression or to individuals with a history of depression that was in remission at time of study. Trait depression was significantly associated with lower CBF, with the associations strongest in cingulate gyrus and frontal white matter. Use of antidepressant medication and more stressful life experiences were also associated with significantly lower CBF. Resting CBF in specific brain regions is associated with trait depression, experience of stressful life events, and current antidepressant use, and may provide a valuable biomarker for further studies.
Keywords: Antidepressants, arterial spin labeling, cingulate gyrus, major depressive disorder, stressful life events
Introduction
Recent population surveys in the United States indicate that 7.2% of adults report having experienced a major depressive episode in the past year, 1 and 13.2% of adults report having taken antidepressant medications within the past 30 days. 2 Depression represents a massive clinical burden directly and is associated with a greater risk for cognitive decline and cardiovascular problems in later life.3–9 The need to better understand the neurobiology of depression as well as the mechanisms by which depression is related to cerebrovascular disease has motivated investigation of cerebral blood flow (CBF) in depression. 10
Previous studies of CBF in depression have yielded mixed findings. Although there is consistent evidence for decreased CBF, particularly in the anterior cingulate and prefrontal cortex,11–13 the findings in other regions are less consistent, with some studies reporting increased CBF in white matter and various cortical and subcortical structures, the caudate in particular (see Supplementary Table 1 for literature review). Differences in clinical samples (i.e., late life depression versus adolescent-onset depression) and imaging methods (positron emission tomography versus arterial spin labeling, ASL) could account for some variability in findings. As depressive mood is highly state-dependent and tends to fluctuate, it is also unclear whether CBF changes associated with depression are associated with the state-dependent aspects of depression or with the lifespan predisposition to experience depression, here termed ‘trait depression.’ CBF is sensitive to acute changes in neural activity, but test-retest reliability across one week has been found to be moderate to good for key gray matter regions involved in depression, 14 likely due to relatively stable factors in vascular physiology.
In this study we examine the role of CBF in depression among individuals of Old Order Amish and Mennonite (OOA/M) heritage. Compared to the general American population, the OOA/M have a more homogenous lifestyle based on agricultural work, including similar levels of education, diet and occupations, and have low rates of substance misuse.15,16 This may allow identification of altered regional patterns of CBF in depression that could be difficult to detect in more heterogeneous samples. Although this is a cross-sectional study, we approach depression from a lifespan perspective, first by including individuals who have had a major depressive episode in the past but are in remission at time of study, second by assessing severity of depression symptoms both at time of study and across adulthood, and including participants from a broad age range. In addition, we examined the influence of experience of stressful life events and use of antidepressants at time of study on CBF. Stressful life experiences are well-known as a risk factor for depression, 17 and traumatic experiences in childhood have been associated with lower CBF. 18 Further variability in CBF can be introduced by use of antidepressant medications, as changes in CBF have been observed following short-term challenges with medications such as citalopram and paroxetine.19–21 In this manner we aim to determine if there is a trait-like relationship between depression and CBF, accounting for the confounding effects of age, stress, and antidepressant treatment.
Methods
Participants
This study included 254 participants from the Amish Connectome Project over the age of 18 without bipolar or psychotic disorder (age range 18–76, 39% males). The Structured Clinical Interview (SCID) for DSM-IV or 5 was used to identify current or past psychiatric disorders. Individuals with seizure disorders, history of traumatic brain injury or cerebrovascular accident, intellectual disability, or unstable medical conditions were excluded. The sample included 44 participants with history of a unipolar depressive disorder that was in remission at time of study; 36 participants with current unipolar depression not in remission; and 174 individuals without a history of depression or other serious mental illness. Sex ratio was significantly different between these groups, with women being overrepresented in both depression groups compared to the control group. Further demographic information including antidepressant medication use is provided in Table 1. Participants gave written informed consent approved by the University of Maryland Baltimore IRB. All study procedures were conducted in accord with ethical guidelines as expressed in the Belmont Report.
Table 1.
Demographic and imaging differences between individuals without history of depression, individuals with active unipolar depressive disorder, and individuals with history of depression that was in remission at time of study.
| Full sample (n = 254) | No depression (n = 174) | Current depression (n = 36) | Past depression (n = 44) | Test-statistic | p-value | |
|---|---|---|---|---|---|---|
| Age (years) | 41.6 ± 14.9 | 41.6 ± 15.4 | 40.9 ± 15.3 | 42.2 ± 12.9 | F = .08 | .98 |
| Sex (M/F), (%M) | 99/155 (39.0%) | 79/95 (45.4%) | 10/26 (27.8%) | 10/34 (22.7%) | χ2 = 9.80 | .007 |
| Current smoker, n | 21 (8.3%) | 16 (9.2%) | 3 (8.3%) | 2 (4.5%) | χ2 = 1.00 | .61 |
| On antidepressant, n (%) | 38 (15.0%) | 6 (3.4%)a | 20 (55.6%) | 12 (27.3%) | χ2 = 70.0 | <.001 |
| – SSRI | 24 (9.4%) | 5 (2.9%) | 13 (36.1%) | 6 (13.6%) | ||
| – SNRI | 6 (2.4%) | 1 (0.6%) | 3 (8.3%) | 2 (4.5%) | ||
| – tricyclic | 1 (0.4%) | 0 | 0 | 1 (2.3%) | ||
| – multiple | 6 (2.4) | 0 | 3 (8.3%) | 3 (6.8%) | ||
| BDI | 4.1 ± 4.3 | 14.5 ± 8.1 | 6.5 ± 6.1 | F = 54.0b | <.001 | |
| MTSD-State | 0.12 ± 0.18 | 0.84 ± 0.73 | 0.18 ± 0.28 | F = 67.5b | <.001 | |
| MTSD-Trait | 0.30 ± 0.37 | 1.25 ± 0.61 | 1.01 ± 0.79 | F = 68.4b | <.001 | |
| Stressful life events | 2.8 ± 1.6 | 3.7 ± 2.0 | 3.5 ± 1.9 | F = 4.66b | .010 | |
| Total CBF | 55.4 ± 9.8 | 53.2 ± 10.0 | 57.7 ± 11.3 | F = 3.45b | .032 | |
| Average GM CBF | 59.1 ± 10.6 | 56.7 ± 10.9 | 61.5 ± 12.3 | F = 3.49b | .032 | |
| Average WM CBF | 23.2 ± 4.6 | 22.1 ± 4.1 | 23.8 ± 4.0 | F = 2.25b | .11 |
Variance provided as standard deviation. BDI: Beck Depression Inventory; MTSD: Maryland Trait and State Depression scales; CBF: cerebral blood flow.
aTaking antidepressants for anxiety disorders in remission at time of study.
bAge, sex and smoking status included as covariates.
Clinical assessments
Symptoms of depression were assessed quantitatively with the Beck Depression Inventory (BDI 22 ) and the Maryland Trait and State Depression scales (MTSD). The MTSD is a self-report questionnaire containing two scales assessing depressive symptoms experienced over the past week (state) and over the course of adult life (trait). The MTSD was initially validated in a sample of patients with schizophrenia and community comparison controls. 23 Further validation data for the MTSD in the OOA/M population was included in a more recent manuscript. 24 Experience of stressful life events was assessed using a version of the Life Stressor Checklist–Revised 25 adapted for use in the OOA/M community. The range for this scale is 0 to 15, based on how many items that participants indicate they have experienced. Current stress levels were assessed with the Perceived Stress Scale. 26
Imaging
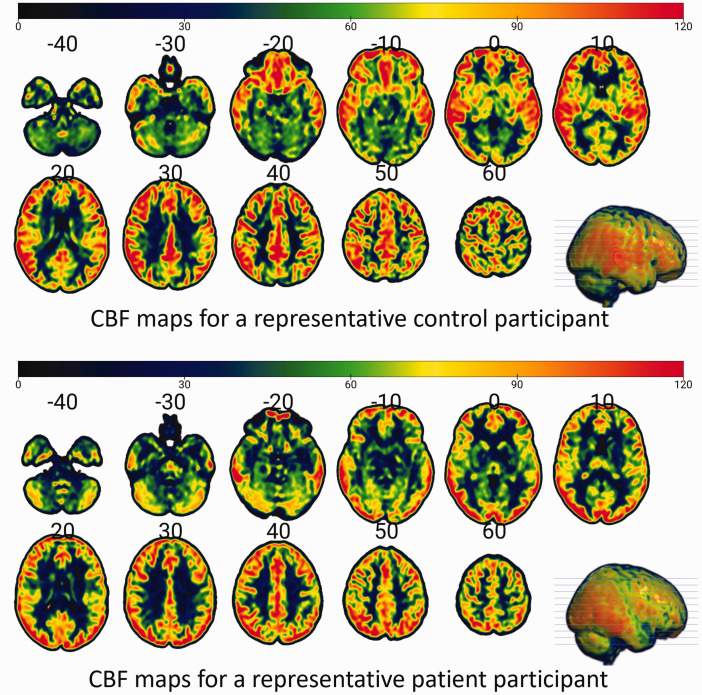
All imaging was performed on a 3 T Siemens Prisma scanner with a 64-channel head coil. Resting cerebral blood flow (CBF) data were acquired using a three-dimensional (3 D) pseudo-continuous ASL (pCASL) with background suppressed gradient and spin-echo sequence consisting of 13 pairs of labeled and control scans. The acquisition parameters were: spatial resolution = 2.5 mm × 2.5 mm × 2.6 mm, matrix size = 96 × 96 with 58 axial slices, repetition time/echo time (TR/TE) = 4000/37 ms, field of view (FoV) read = 220 mm, FoV phase = 100%, post-label delay = 1700 ms, labeling duration = 1650 ms, number of segments = 5. Total scan time was approximately 10 minutes. A 3D T1-weighted image with the following parameters: TR = 2400 ms, TE = 2.22 ms, TI = 1000 ms, flip angle = 8°, matrix = 320 × 320, slices per slab = 208, 0.8 mm × 0.8 mm × 0.8 mm spatial resolution with slice thickness = 0.80 mm was acquired for anatomical reference. A volume of M0 image was also acquired without background suppression to normalize the control-label difference for CBF quantification. The M0 image was smoothed with a 5 mm Gaussian-kernel to suppress effects of noise. 27 CBF data analysis was performed with the FSL software package; perfusion was estimated by using a standard single compartment ASL model; partial volume effects correction was performed with a spatially regularized method. 28 Spatial regularization, motion correction and partial volume corrections were performed in FSL v6.0.1. T1w image processing and registration to ASL images were performed using the FSL commands fsl_anat Oxford_asl. The high-resolution structural image provides partial volume estimates (PVE) for the different tissue types (gray matter (GM), white matter (WM), and cerebrospinal fluid). Partial volume corrected CBF maps were used to extract the regional CBF signals from parcellated GM and WM structures, separately for both hemispheres, resulting in 107 regions of interest (ROIs) based on the brain template ‘Everything Parcellation Map in Eve Space’ atlas- also called the ‘EvePM’ atlas; 29 we refer to this as the JHU-MNI atlas from now on. The ROI values were used in the statistical analyses. Example CBF maps are shown in Figure 1.
Figure 1.
CBF maps for representative participants from the control and current depression cohorts.
Statistical analyses
Comparisons of CBF and clinical measures between individuals with depression, history of depression, and no history of depression were conducted with ANCOVA tests using age and sex as covariates. Normality of variables was tested with Kolmogorov-Smirnov tests. Depression (MTSD-trait, MTSD-state, BDI) and depression-related variables (antidepressant use, stressful life experiences) measures were each correlated with regional CBF in linear regression models covarying for age, sex, and current smoking status. For regional analyses, results from these analyses were considered significant if they passed Benjamini-Hochberg correction for multiple comparisons of 107 regions with false discovery rate q set at .05. 30 Secondary analyses were based on the significant results of the primary analyses and aimed to determine potential confounding and interaction effects, including the role of hematocrit.
Results
Group differences
Whole-brain average CBF differed significantly among individuals with no lifetime history of depression (controls), individuals with a diagnosis of depression in full remission, and individuals with current depression (F(2,248) = 3.45, p = .032). Post-hoc contrasts showed that a diagnosis of current depression was associated with lower CBF compared to both controls (p = .014) and depression in remission (p = .020). Similar results were found for CBF averaged across gray matter regions (F(2,248) = 3.49, p = .032), with current depression associated with lower gray matter CBF compared to controls (p = .014) and participants with depression in remission (p = .020). CBF averaged across white matter regions did not differ significantly among groups (F(2,248) = 2.25, p = .11). For regional analysis, after controlling for multiple comparisons, there was no individual region in which a significant difference in CBF was found between the 3 groups.
Relationship of depression symptoms to regional CBF
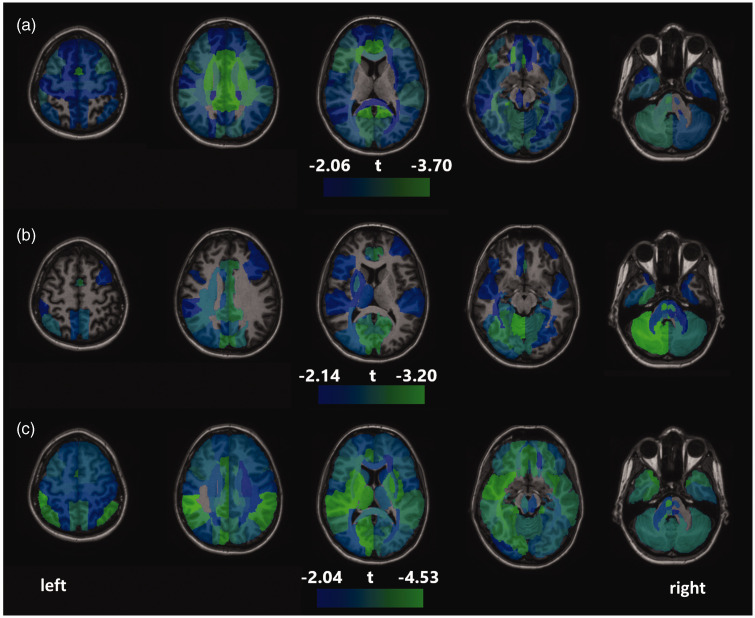
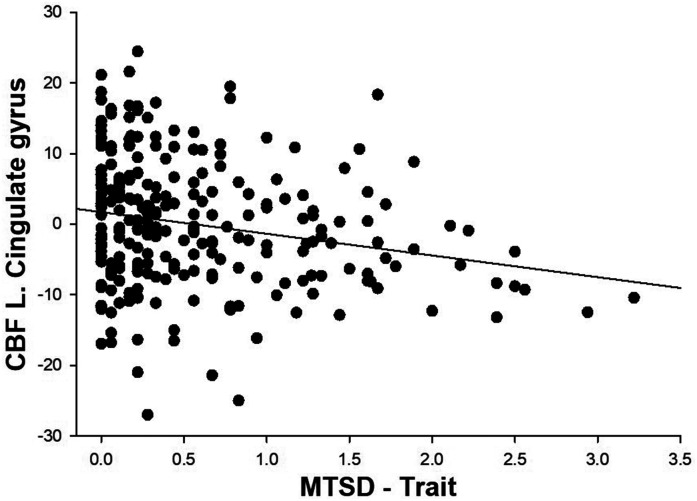
Whole-brain average CBF was not significantly associated with BDI (β = −.04, p = .44), but was negatively associated with MTSD-state (β = −.11, p = .045) and MTSD-trait (β = −.16, p = .003). No brain region showed significant correlation between current depression symptoms as measured by BDI and CBF following correction for multiple comparisons. Similarly, MTSD-state depression was not significantly correlated with CBF in any regions after correction of multiple comparisons. In comparison, MTSD-trait depression was significantly and negatively correlated with CBF in 88 regions following Benjamini-Hochberg corrections (see Figure 2(a)). The strongest correlations were in the cingulate gyrus both in right (β = −.195, p = .00027) and left (β = −.191, p = .00042) hemispheres (see Figure 3 for scatterplot for left cingulate gyrus).
Figure 2.
Areas of significant negative correlations between regional CBF and (a) trait depression; (b) stressful life events and (c) antidepressant use at time of study, based on linear regression models covarying for age, sex and smoking. No significant positive associations between CBF and the above variables were found. The range for colored regions is based on t score for the regression coefficients, with the lower limit based on the minimum t score significant after correction for multiple comparisons, and the upper limit based on the highest t score found in the set of analyses.
Figure 3.
Scatterplot showing relationship of MTSD-trait to CBF in left cingulate gyrus (residual after regressing for age, sex and smoking status).
Relationship of stress and antidepressants to regional CBF
Greater number of past stressful life events was significantly associated with lower whole-brain average CBF (β = −.14, p = .011) and lower CBF in 36 regions (Figure 2(b)), after correction for multiple comparisons. These regions included the cingulate gyrus, hippocampus, cerebellum, pons, parahippocampal gyrus, superior occipital gyrus, and cuneus (both hemispheres for all) as well as several white matter tracts and other mostly occipital and parietal gray matter regions. Levels of recent perceived stress were not significantly associated with CBF in any region. Antidepressant use was significantly associated with lower CBF in 105 regions after correction for multiple comparisons (Figure 2(c)).
Secondary analyses
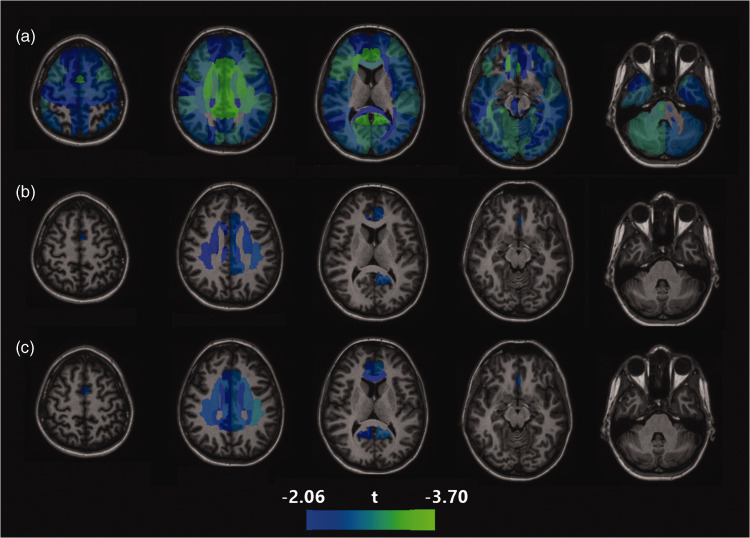
To clarify whether trait depression was associated with regional CBF without the confounding effects of antidepressants, we examined the relationship of MTSD-trait to CBF in the sample after removing participants taking an antidepressant at time of study (n = 38, leaving n = 216 for the analysis), covarying for age, sex, and smoking. There were no significant associations between trait depression and CBF that survived correction for multiple comparisons, though of the 88 regions where this association was found above, the association remained nominally significant (p < .05 without correction) for 5 regions (Figure 4(b)). We then repeated this analysis in the sample excluding both participants taking antidepressants and participants with current depression (leaving n = 200). Again, no significant association was found after correction for multiple comparisons, but nominally significant associations were found for trait depression and CBF in 13 regions, primarily cingulate gyrus and frontal white matter (Figure 4(c)). For instance, the relationship of trait depression to CBF of the right cingulate cortex was β = −.195 in the full sample but was reduced to β = −.147 (p = .013) in the sample not taking antidepressants or β = −.161 (p = .010) in the sample not taking antidepressants or diagnosed with active depression. Similarly, in the full sample with the addition of antidepressant use as a covariate, this relationship between trait depression and CBF of the right cingulate cortex remained nominally significant at β = −.155 (p = .007). These secondary analyses suggest that controlling for antidepressant use at time of study only partially attenuates the relationship between CBF and trait depression.
Figure 4.
Areas of negative correlations between regional CBF and trait depression in (a) full sample; (b) sample with participants on antidepressants removed and (c) sample with participants on antidepressants or experiencing current depressive episode removed. The range for colored regions is based on t score for the regression coefficients for analysis A, with the lower limit based on the minimum t score significant after correction for multiple comparisons, and the upper limit based on the highest t score found in the set of analyses.
We also examined the correlation of fluoxetine equivalent dosage of current antidepressant medication 31 to CBF in the subset of participants taking antidepressants (n = 38). However, these analyses showed no correlation between antidepressant dose and CBF that was significant for whole-brain average CBF (p = .68) or for any region in regional analysis after correction for multiple comparisons.
To test if hematocrit levels could have confounded results from the primary analyses, we first examined diagnostic group differences for hematocrit, which showed a non-significant trend for hematocrit levels to be lower in the current and remitted depression groups compared to the control group (F = 2.63, p = .074). We then repeated a linear regression analysis including both hematocrit and MTSD-trait as predictors. Consistent with previous studies, hematocrit was negatively associated with whole brain CBF (β = −.54, p < .001), and the association between MTSD-trait and CBF remained significant (β = −.12, p = .015). Taking a similar approach to determine if hematocrit confounded the relationship between antidepressant treatment and CBF, we found that participants taking an antidepressant had significantly higher hematocrit than participants not on an antidepressant (F = 5.23, p = .023). Linear regression showed that antidepressant was still negatively associated with total CBF even with hematocrit added as a covariate (β = −.14, p = .003).
To test for interactions between trait depression and either age or sex we repeated linear regression analyses for whole brain CBF with the addition of interaction terms. There were no significant age × MTSD-trait or sex × MTSD-trait interactions.
Discussion
The primary findings of the study are that more severe depression symptoms experienced across adulthood was significantly associated with reduced whole-brain average CBF, and also with reduced CBF in a majority of the brain regions, especially in the cingulate gyrus and frontal white matter. Although present diagnosis of a unipolar depressive disorder was associated with lower average gray matter CBF compared to controls or individuals with a history of depressive disorder in remission, severity of depressive symptoms at time of study was not significantly associated with CBF. However, we also found evidence that use of antidepressant medications and greater exposure to lifetime stressful events were also significantly associated with decreased CBF, and when controlling for these variables the relationship between trait depression symptoms and CBF was largely attenuated.
Importantly, our study assessed symptoms both at the time of study (state) and across adult life (trait). This allowed us to address the question if there are changes in CBF associated with the acute manifestation of depression, or the tendency to experience depression over time. As CBF was related primarily to trait depression, and not state depression or to the diagnosis of depression itself, our findings suggest that decreased CBF is more likely related to the underlying neurovascular predisposition or longitudinal sequelae of depression as opposed to a state-dependent change in cerebrovascular biology or neurobiology. However, as this is a cross-sectional study this conclusion must be considered tentative and should be tested with a longitudinal design.
The strongest regional association of trait depression was with lower CBF in the cingulate cortex, consistent with the findings of multiple other studies11–13 (see also Supplementary Table 1). This replication despite considerable differences in clinical cohorts and methods of measuring CBF is reassuring. Subregions of the cingulate cortex have been strongly implicated in research on depression. The subcallosal cingulate is one of the major targets for deep brain stimulation treatment for depression. 32 Multiple studies have identified hyperactivation or increased glucose metabolism of this region in individuals with depression,33,34 even in euthymic periods. 35
The association of both greater trait depression and antidepressant use with lower CBF raises questions regarding the interpretation of CBF changes. Several previous studies of acute challenge with citalopram found decreased CBF in multiple regions of interest, including hippocampus, amygdala, orbitofrontal cortex, and occipital cortex.19,21 Treatment with paroxetine for 7 days was also associated with decreased CBF across multiple regions, most prominently frontal and temporal cortex, as well as caudate; in contrast bupropion had only modest effects on decreasing CBF. 20 A study in rats also found an acute decrease in CBF following citalopram administration in multiple brain regions, with concomitant decrease in glucose consumption; the effects were most pronounced in occipital cortex. 36 In this study, use of an antidepressant at time of study was strongly associated with decreased average whole-brain CBF. Although we cannot discount the possibility that participants taking antidepressants represented the most severe cases of depression, our secondary analyses indicated that both antidepressants and trait depression were associated with lower CBF when both were included as covariates in linear models. Therefore, antidepressant use and trait depression are both, paradoxically, associated with lower CBF. Further complicating interpretation of these seemingly contradictory findings, there have been several recent findings of an inverse association between resting CBF and cardiovascular fitness.37,38 In adolescents lower resting CBF was associated with greater performance on a battery of cognitive tests, leading to the counter-intuitive hypothesis that in some contexts lower resting CBF could indicate greater metabolic efficiency and dynamic potential. 39 Increased activity of the cingulate cortex has been implicated in rumination and negative self-referential thought,40,41 and decreased resting CBF could potentially indicate greater efficiency or capacity for engagement of the cingulate cortex in these behaviors. Extending this line of reasoning, lower CBF with use of antidepressants could represent a therapeutic downregulation of neural activity related to heightened perception and attentiveness to negative stimuli and memories. Longitudinal studies tracking both symptom changes and CBF changes are needed to further explain these findings. An additional consideration is the potential for antidepressant effects on CBF to be drug-specific, though the sample size was not sufficient to examine the relative effect of different types of antidepressants. We also did not have data on past use of antidepressants or duration of use. Regardless, our findings demonstrate that antidepressant use should be considered an important confound in future studies of risk factors on changes in CBF.
Experience of more stressful life events was negatively associated with CBF in multiple regions, including hippocampus, cerebellum, and occipital and temporal cortical areas. We should note that trait depression and past stressful life events are significantly correlated (r = .41, p < .001). However, the regional pattern of the stressful life events – CBF associations indicates a potential effect specific to the posterior circulation of the brain, including the vascular territories of the basilar and posterior cerebral arteries, and not entirely overlapping with the trait depression – CBF association pattern. A study of people with Cushing’s disease, characterized by chronic hypercortisolism, found reduced CBF compared to healthy controls in regions across the cerebral cortex, including occipital, temporal and frontal regions as well as cingulate gyrus, 42 consistent with the association of stressful life events and pattern of reduced CBF in cingulate, temporal and occipital regions in this study. However, the study of Cushing’s patients also found increased CBF compared to controls in subcortical regions, including hippocampus and parahippocampal gyrus. 42 Glucocorticoids may interfere with vasodilating signaling pathways that allow increased vascular supply in response to increased neuronal activity.43,44 Our results are also consistent with previous findings of an association between childhood trauma and lower CBF. 18 The association of lower CBF with more stressful life events may also reflect some level of pathology induced by stress-induced inflammation, possibly via endothelial damage. 45
Our study included individuals with depression in remission at time of study as well as individuals with active depression to further parse past vs. current depression effects on CBF. However, one limitation of our study is that we also included a wide age range of participants, without consideration to age of onset of the depressive disorder. This approach may miss important biological and clinical nuances specific to child/adolescent onset or late life depression. On the other hand, the finding of significant associations between CBF and depression after covarying age in a sample with a wide age range may indicate that vascular effects in depression may not be primarily dependent on age of onset, though this must be directly tested in future studies. A second limitation is that our study focused on the OOA/M population. Rates of medical conditions that likely influence CBF such as diabetes, hypertension and hyperlipidemia are lower in the OOA/M population compared to the general US population, due in part to lower rates of smoking and greater levels of physical exercise. 46 In the current study we did not have data on participants’ exercise habits or physical activity, though in general the OOA/M are accustomed to high levels of physical labor. The generalizability of the current results to the other populations should be considered with caution. However, it is reassuring that our study replicated the finding between depression and reduced CBF and also between antidepressant use and reduced CBF. Another limitation of this study is that we excluded participants with serious mental illnesses other than depression. This was due to inadequate power to compare depression to bipolar disorder and schizophrenia, and the potential that these illnesses may involve different pathophysiology related to CBF. This remains an important direction for future research. In this study we examined left and right brain regions separately to be comprehensive, though this added to the burden of multiple comparisons. Although we did not observe right-left asymmetry in CBF, this may be worth closer investigation in further studies. Another valuable direction for future research that was not addressed in the current work is the comparison of changes in CBF and structural volume of corresponding regions, which may provide further insight into the nature of structural brain changes in depression.
In conclusion, experiencing more depression over one’s adult life was found to be significantly associated with reduced whole-brain average CBF, but in a complex manner that was influenced by lifetime exposure to stressful life events as well as use of antidepressant medications. Further studies incorporating longitudinal follow-up and measures of cerebrovascular reactivity will be necessary to advance our understanding of the role of cerebrovascular abnormalities in depression and its treatment.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221148979 for Depression, stress, and regional cerebral blood flow by Joshua Chiappelli, Bhim M Adhikari, Mark D Kvarta, Heather A Bruce, Eric L Goldwaser, Yizhou Ma, Shuo Chen, Seth Ament, Alan R Shuldiner, Braxton D Mitchell, Peter Kochunov, Danny JJ Wang and L Elliot Hong in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We thank the members of the Old Order Amish and Mennonite communities for their support and participation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support was received from the National Institutes of Health (K23MH112010, U01MH108148, R01NS114628).
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Hong has received or plans to receive research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho Pharmaceutical, Luye Pharma, Sound Pharma, IGC Pharma, Takeda, Heptares, Pfizer and Regeneron. Dr. Shuldiner is an employee of Regeneron Pharmaceuticals, Inc. and receives compensation for his employment, and is co-inventor of multiple patents. Dr. Kvarta is the co-inventor of a patent pending for the use of GABA-related compounds as fast-acting antidepressants. All other authors declare no conflict of interest.
Authors’ contributions
JC: analysis and interpretation of data, drafting of manuscript; BMA: acquisition and analysis of data, drafting of manuscript; MDK, HAB, ELG, YM, SA, DJW: interpretation of data and critical revision; SC: analysis of data and critical revision; ALS, BDM: conception and design of work, critical revision; PK: acquisition, processing, and interpretation of data, critical revision; EH: conception and design of work, data interpretation, critical revision
ORCID iD
Joshua Chiappelli https://orcid.org/0000-0001-6121-7439
Supplemental material
Supplemental material for this article is available online.
References
- 1.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf (2019, accessed 21 October 2021).
- 2.Brody DJ, Gu Q.Antidepressant use among adults: United States, 2015–2018. NCHS Data Brief, no 377. Hyattsville, MD: National Center for Health Statistics, 2020. [PubMed]
- 3.Barlinn K, Kepplinger J, Puetz V, et al. Exploring the risk-factor association between depression and incident stroke: a systematic review and meta-analysis. Neuropsychiatr Dis Treat 2015; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byers AL, Yaffe K.Depression and risk of developing dementia. Nat Rev Neurol 2011; 7: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harshfield EL, Pennells L, Schwartz JE, et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA 2020; 324: 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ly M, Karim HT, Becker JT, et al. Late-life depression and increased risk of dementia: a longitudinal cohort study. Transl Psychiatry 2021; 11: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Kling JM.Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine (Baltimore) 2016; 95: e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Li X, Pan KY, et al. Association of life-course depression with the risk of dementia in late life: a nationwide twin study. Alzheimers Dement 2021; 17: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 9.Ryan MC, Hong LE, Hatch KS, et al. The additive impact of cardio-metabolic disorders and psychiatric illnesses on accelerated brain aging. Hum Brain Mapp 2022; 43: 1997–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chithiramohan T, Parekh JN, Kronenberg G, et al. Investigating the association between depression and cerebral haemodynamics – a systematic review and meta-analysis. J Affect Disord 2022; 299: 144–158. [DOI] [PubMed] [Google Scholar]
- 11.Monkul ES, Silva LA, Narayana S, et al. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: a (15)O-H(2)O PET study. Hum Brain Mapp 2012; 33: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasic N, Wolf ND, Grön G, et al. Baseline brain perfusion and brain structure in patients with major depression: a multimodal magnetic resonance imaging study. J Psychiatry Neurosci 2015; 40: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Yang Y, Zhu Y, et al. Towards characterizing the regional cerebral perfusion in evaluating the severity of major depression disorder with SPECT/CT. BMC Psychiatry 2018; 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida JRC, Greenberg T, Lu H, et al. Test-retest reliability of cerebral blood flow in healthy individuals using arterial spin labeling: findings from the EMBARC study. Magn Reson Imaging 2018; 45: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs JA, Levinson RM, Stoddard RR, et al. Health risk factors among the Amish: results of a survey. Health Educ Q 1990; 17: 197–211. [DOI] [PubMed] [Google Scholar]
- 16.Nugent KL, Million-Mrkva A, Backman J, et al. Familial aggregation of tobacco use behaviors among Amish men. Nicotine Tob Res 2014; 16: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendler KS, Karkowski LM, Prescott CA.Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999; 156: 837–841. [DOI] [PubMed] [Google Scholar]
- 18.Gur RE, Moore TM, Rosen AFG, et al. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry 2019; 76: 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wan HI, O'Reardon JP, et al. Quantification of cerebral blood flow as biomarker of drug effect: arterial spin labeling phMRI after a single dose of oral citalopram. Clin Pharmacol Ther 2011; 89: 251–258. [DOI] [PubMed] [Google Scholar]
- 20.Viviani R, Abler B, Seeringer A, et al. Effect of paroxetine and bupropion on human resting brain perfusion: an arterial spin labeling study. Neuroimage 2012; 61: 773–779. [DOI] [PubMed] [Google Scholar]
- 21.Solleveld MM, Schrantee A, Homberg JR, et al. The influence of age-of-onset of antidepressant use on the acute CBF response to a citalopram challenge; a pharmacological MRI study. Psychiatry Res Neuroimaging 2020; 303: 111126. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Carbin MG.Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8: 77–100. [Google Scholar]
- 23.Chiappelli J, Nugent KL, Thangavelu K, et al. Assessment of trait and state aspects of depression in schizophrenia. Schizophr Bull 2014; 40: 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiappelli J, Kvarta M, Bruce H, et al. Stressful life events and openness to experience: relevance to depression. J Affect Disord 2021; 295: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe J, Kimberling R, Brown P, et al. Life Stressor Checklist-Revised (LSC-R), www.ptsd.va.gov/professional/assessment/te-measures/lsc-r.asp1997 (accessed 16 January 2020).
- 26.Cohen S, Kamarck T, Mermelstein R.A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396. [PubMed] [Google Scholar]
- 27.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European Consortium for ASL in Dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chappell MA, Groves AR, MacIntosh BJ, et al. Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn Reson Med 2011; 65: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 29.Lim IA, Faria AV, Li X, et al. Human brain atlas for automated region of interest selection in quantitative susceptibility mapping: application to determine iron content in deep gray matter structures. Neuroimage 2013; 82: 449–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y.Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodological) 1995; 57: 289–300. [Google Scholar]
- 31.Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 2015; 180: 179–184. [DOI] [PubMed] [Google Scholar]
- 32.Hamani C, Mayberg H, Stone S, et al. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry 2011; 69: 301–308. [DOI] [PubMed] [Google Scholar]
- 33.Sacher J, Neumann J, Fünfstück T, et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord 2012; 140: 142–148. [DOI] [PubMed] [Google Scholar]
- 34.Miller CH, Hamilton JP, Sacchet MD, et al. Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatry 2015; 72: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 35.Schöning S, Zwitserlood P, Engelien A, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp 2009; 30: 2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBean DE, Ritchie IM, Olverman HJ, et al. Effects of the specific serotonin reuptake inhibitor, citalopram, upon local cerebral blood flow and glucose utilisation in the rat. Brain Res 1999; 847: 80–84. [DOI] [PubMed] [Google Scholar]
- 37.Foster C, Steventon JJ, Helme D, et al. Assessment of the effects of aerobic fitness on cerebrovascular function in young adults using multiple inversion time arterial spin labeling MRI. Front Physiol 2020; 11: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furby HV, Warnert EA, Marley CJ, et al. Cardiorespiratory fitness is associated with increased middle cerebral arterial compliance and decreased cerebral blood flow in young healthy adults: a pulsed ASL MRI study. J Cereb Blood Flow Metab 2020; 40: 1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gur RC, Butler ER, Moore TM, et al. Structural and functional brain parameters related to cognitive performance across development: Replication and extension of the parieto-frontal integration theory in a single sample. Cereb Cortex 2021; 31: 1444–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makovac E, Fagioli S, Rae CL, et al. Can't get it off my brain: meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Res Neuroimaging 2020; 295: 111020. [DOI] [PubMed] [Google Scholar]
- 41.Preuss A, Bolliger B, Schicho W, et al. SSRI treatment response prediction in depression based on. Front Psychiatry 2020; 11: 538393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, Gao L, Hou B, et al. Reversibility of cerebral blood flow in patients with Cushing's disease after surgery treatment. Metabolism 2020; 104: 154050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burrage E, Marshall KL, Santanam N, et al. Cerebrovascular dysfunction with stress and depression. Brain Circ 2018; 4: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longden TA, Dabertrand F, Hill-Eubanks DC, et al. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A 2014; 111: 7462–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massardo T, Quintana JC, Jaimovich R, et al. Regional brain perfusion is associated with endothelial dysfunction markers in major depressive disorder. Neuropsychobiology 2021; 80: 214–224. [DOI] [PubMed] [Google Scholar]
- 46.He S, Ryan KA, Streeten EA, et al. Prevalence, control, and treatment of diabetes, hypertension, and high cholesterol in the Amish. BMJ Open Diabetes Res Care 2020; 8: e000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221148979 for Depression, stress, and regional cerebral blood flow by Joshua Chiappelli, Bhim M Adhikari, Mark D Kvarta, Heather A Bruce, Eric L Goldwaser, Yizhou Ma, Shuo Chen, Seth Ament, Alan R Shuldiner, Braxton D Mitchell, Peter Kochunov, Danny JJ Wang and L Elliot Hong in Journal of Cerebral Blood Flow & Metabolism