Abstract
Post-stroke depression exacerbates neurologic deficits and quality of life. Depression after ischemic stroke is known to some extent. However, depression after intracerebral hemorrhage (ICH) is relatively unknown. Increasing evidence shows that exposure to an enriched environment (EE) after cerebral ischemia/reperfusion injury has neuroprotective effects in animal models, but its impact after ICH is unknown. In this study, we investigated the effect of EE on long-term functional outcomes in mice subjected to collagenase-induced striatal ICH. Mice were subjected to ICH with the standard environment (SE) or ICH with EE for 6 h/day (8:00 am–2:00 pm). Depressive, anxiety-like behaviors and cognitive tests were evaluated on day 28 with the sucrose preference test, tail suspension test, forced swim test, light-dark transition experiment, morris water maze, and novel object recognition test. Exposure to EE improved neurologic function, attenuated depressive and anxiety-like behaviors, and promoted spatial learning and memory. These changes were associated with increased expression of transcription factor Nrf2 and brain-derived neurotrophic factor (BDNF) and inhibited glutaminase activity in the perihematomal tissue. However, EE did not change the above behavioral outcomes in Nrf2−/− mice on day 28. Furthermore, exposure to EE did not increase BDNF expression compared to exposure to SE in Nrf2−/− mice on day 28 after ICH. These findings indicate that EE improves long-term outcomes in sensorimotor, emotional, and cognitive behavior after ICH and that the underlying mechanism involves the Nrf2/BDNF/glutaminase pathway.
Keywords: Anxiety behaviors, enriched environment, intracerebral hemorrhage, nuclear regulatory factor 2, post-stroke depression
Introduction
Post-stroke depression (PSD) is a common and chronic problem that affects 30–35% of patients in the first year after stroke. 1 It affects neurologic and cognitive function, 2 quality of life, 3 and mortality.4,5 More than 20% of patients who survive intracerebral hemorrhage (ICH) have a depressed mood at 3 months. However, clinical, preclinical, and translational research on ICH-induced depression is scarce. Furthermore, its pathophysiology is poorly understood. Therefore, identifying promising therapies for tackling depression and understanding the specific pathogenesis of depression after ICH is vital to improving clinical outcomes.
Environmental enrichment in housing can improve the mental and physical health of laboratory mice. 5 Striking evidence suggests that an enriched environment (EE) promotes neuronal growth, restructuring, and recovery after brain injury.6,7 However, although exposure to EE provides neuroprotection after ischemic stroke, its effects on post-ICH depression and the underlying mechanisms are unknown.
The interaction between depression and stroke is complex. 8 Both conditions involve inflammation, oxidative stress, and various forms of cell death.2,9–13 Six main pathways are believed to be dysregulated in depression, including inflammatory cell-mediated immune, oxidative, mitochondrial, antioxidant (lowered glutathione levels), and neuroprogressive pathways. 14 With marked effects on the six pathways, the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway could be involved in the development of PSD. 14 In fact, we have reported previously that the volume of injury is significantly larger in Nrf2−/− mice than in wild-type controls after collagenase-induced striatal ICH.15,16 Our new study showed that downregulation of the Nrf2-brain-derived neurotrophic factor (BDNF) signaling pathway contributes to the development of cortical hemorrhage-induced depression. 17 BDNF, which has been implicated in the pathophysiology of depressive disorders, 18 is known to regulate neurogenesis, synaptogenesis, and angiogenesis and promote neuronal survival. 19 In particular, BDNF is an Nrf2 target gene, and Nrf2/BDNF signaling is involved in the antidepressant-like effect of agmatine and fluoxetine. 20 In the chronic social defeat stress model, behavioral abnormalities are associated with altered glutamate signaling. 21 Glutaminase, the enzyme that produces glutamate from glutamine, has a vital role in generating toxic glutamate in the brain. 22 Although BDNF and glutaminase expression changes might be associated with altered depression-like behaviors in other disease models, their role in post-ICH depression has not been reported.
Therefore, in this study, using a mouse model of ICH, we evaluated whether an EE attenuates neurologic deficits, cognitive deficits, and depressive and anxiety-like behavior, and whether the Nrf2/BDNF/glutaminase signaling pathway is involved.
Materials and methods
Mice
In our study, a total of 228 10-month-old C57BL/6 male mice (30–35 g) and 90 Nrf2−/− mice (3-month-old, 20–25 g) were used. Mice were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (NO.11400700282075) and housed in a specific pathogen-free environment with a 12 h light-dark cycle and controlled temperature (24 ± 1°C) and relative humidity (60 ± 10%). Nrf2−/− mice were generated by Dr. Masayuki Yamamoto (on a C57BL/6 background). Genotype was determined by PCR amplification of genomic DNA extracted from the tail snips of the mice. Three to five mice per cage were housed with free access to standard food and water. We used male mice only in this study because there are sex differences in behavior between males and females; therefore, mixed male and female mice will cause data variability. All animal protocols were approved by the Animal Ethics Committee of Zhengzhou University (ZZUIRB 2022-31). All experiments were performed and reported according to the Guide for the Care and Use of Laboratory Animals, 8th edition (2011) and the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines). All mice were randomly allocated into different groups using the randomizer form at http://www.randomization.com.16,23,24
ICH mouse model
Mice were anesthetized with isoflurane (70% N2O and 30% O2; 4% induction, 2% maintenance) and placed in a stereotaxic frame (RWD Life Science, China) for aseptic surgery. A 1-cm-long incision was made on the midline of the scalp. A mouse adaptor was used to drill a borehole 2 mm left and 0.5 mm anterior to the bregma, with a depth of 3.1 mm. ICH was induced by injecting collagenase VII-S (sterile filtered, relatively endotoxin-free, 0.05 U in 0.5 μL sterile saline, Sigma, St. Louis, MO) into the left striatum as previously described.25–27 Collagenase was infused with a microinfusion pump at a constant rate of 0.1 μL/min. Body temperature was maintained at 37 ± 0.5°C during surgery. After surgery, the mice were returned to their home cages. The sham control group underwent a surgical incision but was infused with saline. Animals that died or were sacrificed within 24 h after surgery were excluded from the sample size. Otherwise, all animals proceeded to the final analysis.
EE conditions
On day 1 after surgery, mice in the ICH + EE group were housed in a box with an enriched environment that we made ourselves (120 cm × 90 cm × 76 cm) according to a published protocol. 28 The box contained two mouse cages with food and water, running wheels, igloos with saucer wheels, plastic tubing, and other toys (Supplementary Fig. 1). Six to eight mice were housed in an EE box for 6 h/day (8:00 am to 2:00 pm) until day 28 after ICH (Supplemental video 1). 29 The devices were rearranged and renewed every day to stimulate the exploratory behavior of the animals and to maintain the novelty of the environment. ICH + EE control mice and sham mice were housed in groups of 3 to 5 in standard cages (30 cm × 45 cm × 20 cm) without toys, defined as a standard environment (SE). All animals had free access to food and water. The elements used in the EE included: 1) two cross pipes (interface diameter: 5.5 cm); 2) eight straight pipes (interface diameter: 5.5 cm); 3) six frisbee running wheels (diameter: 18 cm, height: 11 cm); 4) six windmill running wheels (diameter: 13 cm, height: 14.5 cm, width: 7.5 cm); and 5) the large cage (120 cm × 90 cm × 76 cm) with an EE made of aluminum alloy panel (Zhengzhou Welding Factory, Zhengzhou, China). An illustration of the EE condition with toys is shown in Supplementary Fig. 1 and Supplementary video 1.
Brain lesion volume
On day 3 after ICH, the mice were anesthetized with 3% isoflurane and perfused through the left ventricle with saline, followed by 4% paraformaldehyde. Then the brain was removed. Coronal sections through the entire striatum were stained with Cresyl violet acetate (CV, for Nissl bodies) and Luxol fast blue (for myelin). ImageJ software was used to quantify hemorrhagic injury volume. Sections were analyzed by an investigator blind to the experimental cohort as previously described.25,30
Brain edema measurement
On day 3 after ICH, the mice were sacrificed under deep anesthesia with 3% isoflurane and their brains were harvested and dissected along the sagittal fissure into the ipsilateral and contralateral hemispheres and cerebellum. Brain tissue was immediately weighed to obtain the wet weight and then heated to 100°C in a drying oven for 72 h to obtain the dry weight. We determined brain edema by calculating brain water content as follows: brain water content (%) = (wet weight – dry weight)/wet weight × 100%.25,30
Sensorimotor tests
Neurologic deficit score
We tested each mouse for neurologic deficit scores on days 1, 3, 5, 7, 14, and 21 after ICH according to a previously published method. 26 Scores were summed on six subtests: body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling. Each test was graded from 0 to 4, establishing a maximum deficit score of 24.
Forelimb and hind limb placing test
On days 3, 5, 7, 14, and 21 after ICH, forelimb, and hindlimb placement tests were performed based on our previous studies.26,31,32 Mice were placed facing the edge of a desktop, and the contralateral hindlimb was pulled. The ability of the mouse to put the hind limb back on the desktop was quantified as follows: immediate and complete pullback of limb = 0, delayed pullback (>2 s) = 1, inability to pull back = 2. The placement was determined in 10 consecutive trials. The video of the forelimb and hindlimb placing tests was displayed in supplementary videos 2, 3.
Corner turn test
On days 3, 5, 7, 14, and 21 after ICH, the mouse was directed into a 30° corner as described previously. 23 The mouse had the option to turn either right or left to exit the corner. The number of turns in each direction in 10 repeated tests was recorded. Then the percentage of right turns was calculated. Normal mice exhibit approximately 50% of turns in each direction. 33
Cognitive tests
Morris water maze (MWM)
The MWM test evaluated rodent spatial learning and memory abilities based on an established standard procedure. 34 The system used consisted of a black circular swimming pool (120 cm in diameter), an escape platform (10 cm in diameter) submerged 0.5 cm below the water surface in the center of one of four imaginary quadrants, and a SMART 3.0 animal behavior analysis system (Panlab, Spain). Mice were randomly released into the pool facing the wall and swam to find the submerged platform. In the training phase, the mice underwent 1 trial/day over 4 consecutive days from day 24. On day 28, spatial memory was estimated by trajectory and navigation parameters in the testing phase.
Novel object recognition test
On days 27 and 28 after ICH, the novel object recognition test was performed to measure recognition memory according to an established protocol. 11 In short, mice were allowed to explore two identical novel objects (violet cubes, 4 × 4 × 3 cm) in an open field (47 × 26 × 20 cm) for 10 min on the first day (day 27). The following day (day 28), the mice were exposed to a novel object (white ball, 5 cm in diameter) and a familiar object (violet cube) in the field for 5 min. The behaviors displayed by each mouse were recorded on video. The discrimination index was calculated to assess cognitive ability: Discrimination index (%) = (total time devoted to a new object/total time spent exploring objects) × 100%. Exploring the object was identified as direct contact with the paw, nose, or mouth, or the nose directed at the object at <0.5 cm.
Depression-like behavior
Forced swim test (FST)
The FST, also known as the behavioral despair test, was conducted to measure depression-like behavior according to the protocol described previously. 11 The swimming apparatus was a glass cylinder (20 cm high × 22 cm in diameter) containing 10 cm of water at 24 ± 1°C. On day 28 after ICH, mice were forced to swim for 6 min, and the duration of immobility in the last 4 min was video recorded. Mice were defined as immobile when they were motionless or made only slight movements to maintain their head above the water.
Tail suspension test (TST)
As previously described, TST was carried out to measure behavioral despair or depression-like behavior and learned helplessness on day 28 after ICH.35,36 Each mouse was placed in the testing room for a period of acclimatization (generally at least 1 h) before the behavioral procedure. Mice were individually suspended by the tail from a bar 55 cm above the floor with a piece of adhesive tape (17 cm long, 2 cm from the tip of the tail). A polycarbonate tube (4 cm in length, 1 cm in diameter, 1.5 g) was placed around the tail to prevent mice from climbing their tails. A camera was used to record the movement of the mice for 6 min, and the duration of immobility was calculated.
Sucrose preference test (SPT)
The SPT evaluated anhedonia based on an established protocol. 11 On day 25, mice were placed in separate cages with two bottles, one containing water and the other a 1% sucrose solution. Bottles were weighed at the start of the test, and their positions in the cage were changed daily. On day 28, the two bottles were reweighed, and the amount of liquid consumed was measured. The sucrose preference was calculated as a percentage of the sucrose solution consumed relative to the total amount of liquid consumed: sucrose preference (%) = sucrose consumption (g)/[water consumption (g) + sucrose consumption (g)].
Light/dark transition test
On day 28, a light/dark transition test was performed to evaluate anxiety-like behavior as described. 37 The apparatus consisted of a cage (100 cm) divided into two chambers of equal size by a barrier with a door. One chamber was light and the other dark. The mice were placed in the dark chamber and allowed to freely move between the two chambers through the door for 10 min. The total number of times the mouse traversed and the time spent in each chamber (seconds) were analyzed using a SMART 3.0 animal behavior analysis system.
Anxiety-like behavior
Open field test
On day 28, the open field test was used to assess locomotor activity and anxiety-like behavior. 38 Each mouse was allowed to acclimate to the testing room for 2 h prior to the test. Then it was placed in the center of the open field box to explore freely for 5 min. After each test, we used alcohol to clean the box to prevent smells from influencing the behavior of the next mouse. Smart Video Tracking Software was used to track and analyze each mouse movement. The time spent and the distance traveled in the center, and the periphery of the box was examined. 39
Elevated plus-Maze (EPM)
The EPM is one of the most widely used tests to assess anxiety-like behavior in mice.39,40 The apparatus consists of a pair of open arms (50 cm × 5 cm) perpendicular to a pair of arms with walls but no ceiling (50 cm × 10 cm × 40 cm) and a central area connected (10 cm × 10 cm). The maze is located 50 cm above the ground. The mouse was placed in the center of the maze facing one of the closed arms, and allowed to explore for 5 min. A computer recorded the number of times the mice entered open and closed arms and the time spent in open and closed arms. An arm entry was defined as a mouse having all four legs in one arm of the maze.
Western blot analysis
On day 28, Western blotting was performed to evaluate Nrf2 and BDNF protein expression levels in the mouse brain around the injured area.34,41 Brain tissues collected around the injured area were lysed on ice in ice-cold lysis buffer (RIPA: PMSF = 100:1) with a protease inhibitor cocktail. A bicinchoninic acid (BCA) assay kit (PC0020, Solarbio Science& Technology Co, Ltd. Beijing) was used to measure protein concentration. We separated 35 μg of protein from each sample by SDS-PAGE and transferred the proteins to nitrocellulose membranes. After being blocked with nonfat milk for 2 h, the membranes were incubated with primary antibodies against Nrf2 (1:1000, Abcam, Cambridge, MA), BDNF (1:500, Abcam) and tubulin (1:250, Abcam) at 4 °C overnight. Subsequently, the membranes were incubated with HRP-labeled anti-mouse or anti-rabbit secondary antibodies (1:10,000, Santa Cruz, Dallas, TX) at room temperature for 2 h. The immunoglobulins were then detected with a FluorChem imaging system (San Jose, CA) with the enhanced chemiluminescence (ECL) technique. ImageJ software was used to normalize the intensities of the target bands to those of the corresponding loading control.
Immunofluorescence staining
Immunofluorescence was performed as described previously. 42 Mice in each group were anesthetized with an overdose of 10% chloral hydrate and transcardially perfused through the left ventricle with saline, followed by 4% paraformaldehyde on day 28. The brains were dissected, post-fixed in 4% paraformaldehyde for 24 h, and then dehydrated with 20% and 30% sucrose successively. Brain tissues were serially sectioned into 20-µm-thick sections with a freezing microtome (Leica CM1950, Germany). Sections were blocked with 5% goat serum in phosphate-buffered saline with 0.1% Triton-X 100 for 2 h at room temperature. Slices were incubated with rabbit anti-BDNF (1:500, Abcam) primary antibody at 4°C overnight, followed by Cy3-conjugated goat anti-rabbit IgG secondary antibody (1:100, Boster Biological Technology, Wu Han) for 2 h at 37°C. Labeled tissues were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; D8417; 1:1000, Sigma). The sections were examined and photographed with a fluorescence microscope (Olympus Corporation, Tokyo, Japan). Four fields from each section were selected, and five consecutive brain sections were used. The numbers of positive cells were determined in each field, and the mean values of 20 fields were calculated to represent BDNF-positive cells in a mouse.
High-Performance liquid chromatography (HPLC) analysis
Ultra-high performance liquid chromatography-tandem triple four-stage mass spectrometry (UPLC-MS) was used to measure glutamate concentration in brain tissue around the hematoma on day 28 after ICH. 43 Brain tissue collected around the hematoma (about 3 mm thick) was lysed in methanol solution (7 g/ml) on ice. It was homogenized with a tissue grinder (XIN-M48, Shanghai Xinwen Scientific Instrument Co., Ltd, Shanghai, China) at a speed of 9424 g for 1 min. The homogenate was then centrifuged at a rate of 15078 g for 10 min, and the supernatant was diluted 50 times with 0.5% formic acid. The mixture was then dried using nitrogen evaporators (N-EVAP, Organomation, MA, USA) at 35°C, and a 100 μL initial mobile phase complex (2.5 mol/L ammonium acetate and a 0.1% formic acid) was added as the sampling solution. Glutamate purchased from Sigma-Aldrich was used to create the standard curve. We injected 2 μL of the sampling solution into a Porosell 120 EC-C1 column (Phenomenex, St. Louis, Missouri) in the HTEC-500. We eluted it with 2.5 mmol/L ammonium acetate buffer with 0.1% formic acid solution and acetonitrile with a 0.1% formic acid solution. The peak of neurotransmitter chromatograms was identified by the retention time in the standard solution. The concentration was calculated according to the peak area in the standard solution. Data were analyzed using Chemstation software (Agilent, Santa Clara, USA).
Glutaminase activity
The tissue around the injured area of the brain was collected on day 28 after ICH; Glutaminase activity was measured with commercial glutaminase activity kits according to the manufacturer’s instructions (Institute of Nanjing Jiancheng Bioengineering, Nanjing, China).
Statistical analysis
Data are expressed as means ± standard deviation (SD), dot plots, or bar graphs. The Shapiro-Wilk test assessed the normal distribution before choosing the statistical analysis. Mortality was analyzed using a chi-square test. The difference between the two groups was analyzed with a two-tailed Student's t-test if the data were normally distributed. We used a one-way or two-way ANOVA, and a Bonferroni post hoc test to compare differences between multiple groups. A p < 0.05 was considered statistically significant. All analyzes were performed with GraphPad Software (GraphPad Prism 5.0; GraphPad Software, Inc., La Jolla, CA). The outlying data points were defined with statistical software assuming a normal distribution (the threshold was set as 2.0-fold SD from the mean).
Results
Effect of EE on mortality after ICH
The mortality of the ICH + EE mice (5 out of 76) was not different from that of the ICH + SE mice (5 out of 79). Mortality of Nrf2−/− ICH + EE mice (2 out of 33) did not differ from that of Nrf2−/− ICH + SE mice (2 out of 30). No WT (n = 73) or Nrf2−/− mice (n = 27) died in the sham group (Supplementary Fig. 2).
EE does not decrease acute brain injury after ICH
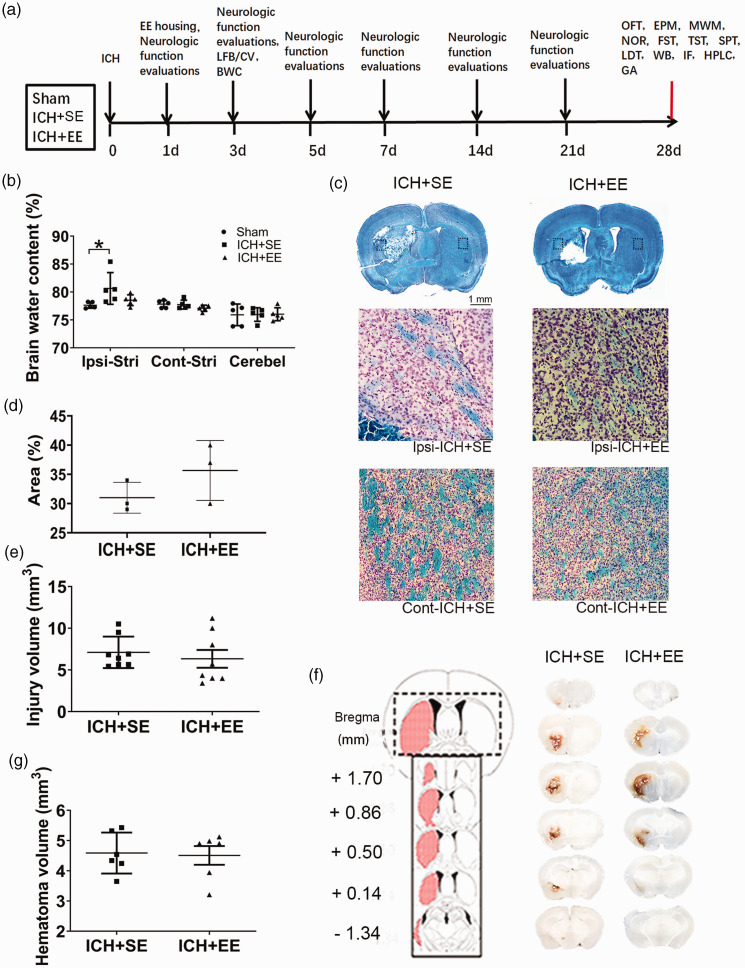
We illustrated the sequence and timeline of the experimental procedures in Figure 1(a). The brain water content of the ipsilateral hemisphere tended to be lower in the ICH + EE group than in the ICH + SE group on day 3 after ICH, but the difference was not significant between the two groups (78.19 ± 0.70% vs. 80.63 ± 2.85%; n = 5 mice/group, F = 3.825, p = 0.0519; Figure 1(b)). In the ICH + SE and ICH + EE groups, myelin showed substantial damage on the ipsilateral side compared to that on the contralateral side (Figure 1(c)). However, EE did not increase the area with normal myelin around the hematoma, as assessed by Luxol fast blue staining (35.67 ± 5.132% vs. 31 ± 2.646%; n = 3 mice/group; Figure 1(d)). The hemorrhage was located predominantly within the striatum, without extending to other areas (Figure 1(f)). Images of fresh brain slices and CV/Luxol fast blue-stained slices showed that lesion and hematoma volume did not differ significantly between the ICH + SE and ICH + EE groups on day 3 (7.104 ± 0.6679 mm3 vs. 6.329 ± 1.068 mm3; 4.587 ± 0.6796 mm3 vs. 4.508 ± 0.7632 mm3; n = 6–8 mice/group, p > 0.05; Figure 1(e) and (g)).
Figure 1.
Enriched environment (EE) has no effect on acute brain injury after ICH. (a) Experimental design. The timeline shows the sequence of all behavioral tests and experimental procedures, beginning on the day of surgery (day 0). LFB: Luxol fast blue; CV: Cresyl violet; BWC: Brain water content; OFT: open field test; EPM: elevated plus-maze; MWM: Morris Water Maze; NOR: novel object recognition; FST: forced swim test; TST: tail suspension test; SPT: sucrose preference test; LDT: light/dark transition test; WB: western blot; IF: immunofluorescence; HPLC: high-performance liquid chromatography; GA: glutaminase activity. (b) On day 3 after ICH, mice exposed to EE did not reduce brain water content in the ipsilateral hemisphere compared to mice that received ICH and standard conditions (ICH + SE). n = 5 mice/group. p > 0.05 vs. the sham group (one-way ANOVA followed by Bonferroni’s post hoc test). Cont-Stri, contralateral striatum; Ipsi-Stri, ipsilateral striatum; Cerebel, cerebellum. (c) Top: Representative Luxol fast blue/crystal violet-stained brain sections of the ICH + SE and ICH + EE groups (scale bar = 1 mm). Bottom: High magnifications of the dashed areas in the top photograph on the ipsilateral and contralateral side (Scale bar= 50 μm) (d) The plot graph shows EE did not increase the percentage of areas with normal myelin after ICH on day 3. n = 3 mice/group (t-test). (e) Quantification analysis did not show differences in brain injury volume between the ICH + SE and ICH + EE groups. n = 8 mice/group (t-test). (f) Left: Schematic illustration of the spatial extent of the hemorrhagic lesion 72 h after microinjection of collagenase VII into the left striatum. Right: Representative fresh brain sections show hematomas (red areas) in mice exposed to standard conditions or EE and (g) Quantification analysis did not show differences in hematoma volume between the ICH + SE and ICH + EE groups. n = 6 mice/group (t-test). Data are expressed as mean ± SD.
EE mitigates neurologic deficits after ICH
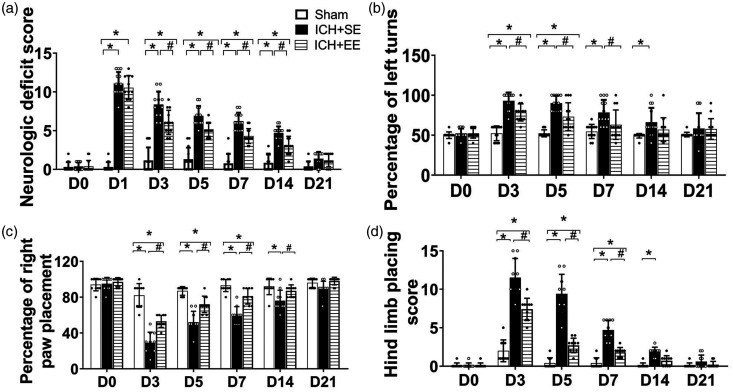
The neurologic deficit scores of mice were significantly elevated on day 1 after ICH and then gradually decreased in the two ICH groups with time. Compared to ICH + SE mice, those in the ICH + EE group had significantly lower neurologic deficit scores on days 3, 5, 7, and 14 after ICH (p > 0.9999 on day 1, p < 0.001 on day 3, p = 0.003 on day 5, p < 0.001 on day 7, p = 0.0011 on day 14, p > 0.9999 on day 21; n = 10–13 mice/group; Figure 2(a)). EE also increased the percentage of left turns on the corner turn test on days 3, 5, and 7 after ICH (p > 0.9999 on day 1, p = 0.0061 on day 3, p = 0.0011 on day 5, p = 0.0036 on day 7, p = 0.1499 on day 14, p > 0.9999 on day 21; n = 13 mice/group; Figure 2(b)). Furthermore, EE significantly improved motor performance in the forelimb on days 3, 5, 7 and 14 and hindlimb placement tests on days 3, 5, 7 after ICH (p < 0.0001 on day 3, p < 0.0001 on day 5, p < 0.0001 on day 7, p = 0.0149 on day 14, p = 0.0632 on day 21; n = 10 mice/group; Figure 2(c); n = 12 per group; p < 0.0001 on day 3, p < 0.0001 on day 5, p < 0.0001 on day 7, p = 0.055 on day 14, p > 0.9999 on day 21; n = 10 mice/group; Figure 2(d)).
Figure 2.
An enriched environment (EE) mitigates neurologic deficits and motor function after ICH. (a) Accommodation in an EE decreased the neurologic deficit scores of mice on days 3, 5, 7, and 14 after ICH. n = 10–13 mice/group. *p < 0.05 vs. sham group; #p < 0.05 vs. ICH group(repeated measures ANOVA followed by Bonferroni's post hoc test). (b) ICH mice exposed to EE performed better in the corner turn test than ICH mice exposed to the standard environment (SE) on days 3, 5, and 7 after ICH. n = 13 mice/group. *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (repeated measures ANOVA followed by Bonferroni's post hoc test). (c) ICH mice exposed to EE performed better in the forelimb placement test than ICH mice exposed to SE on days 3, 5, 7 and 14. n = 10 mice/group. *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (repeated measures ANOVA followed by Bonferroni’s post hoc test) and (d) ICH mice exposed to EE had a better motor performance on the hindlimb placement test than ICH mice exposed to SE on days 3, 5, and 7. n = 10 mice/group. *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (repeated measures ANOVA followed by Bonferroni’s post hoc test). Values are mean ± SD.
EE mitigates depression-like behaviors after ICH
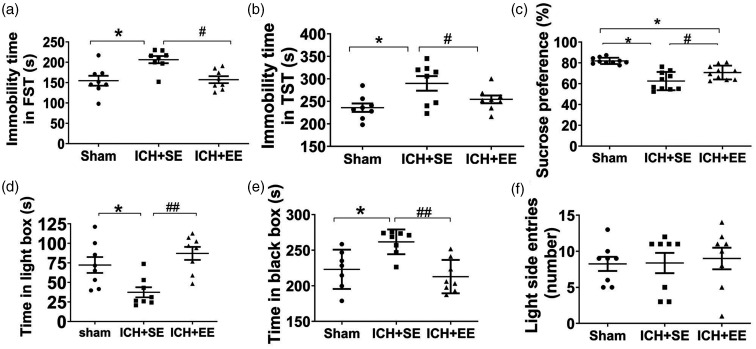
No difference exists in FST, light/dark transition test, open field test, and novel object recognition test on day 28 among the control group, the sham group, and the control + EE group (Supplementary Fig. 3). In both the FST and the TST, the immobility time in the ICH + EE group was significantly shorter than that of the ICH + SE group on day 28 (177.9 ± 11.01 s vs. 225 ± 44.56 s in the FST; 254.4 ± 24.48 s vs. 289.9 ± 45.88 s in the TST; F = 7.47 and 5.28, respectively; both p < 0.05; n = 8 mice/group; Figure 3(a) and (b)). Furthermore, in the SPT, ICH + EE mice consumed more sucrose-sweetened water (70.72 ± 6.66%) than those ICH + SE mice (62.5 ± 8.76%; F = 21.85, p < 0.05; n = 10 mice/group; Figure 3(c)), although the total liquid consumed did not differ between the two ICH groups (Supplementary Fig. 4). In the light/dark transition test, mice in the ICH + EE group spent significantly more time in the light compartment and less time in the dark box compared to ICH + SE mice (87.44 ± 23.44 s vs 38.32 ± 17.42 s in the lightbox; 212.9 ± 23.44 s vs. 261.7 ± 17.42 s in the dark box) on day 28 (F = 9.194 and 8.954, respectively, p < 0.05; n = 7–8 mice/group; Figure 3(d) and (e)). However, the number of entries in the lightbox did not differ significantly among the ICH + EE, ICH + SE, and sham groups (F = 0.09439, p > 0.05; n = 8 mice/group; Figure 3(f)).
Figure 3.
Enriched environment (EE) mitigates depression-like behaviors in mice after ICH. (a and b) Mice in the ICH + EE group exhibited less immobility time in the forced swim test (FST, a) and the tail suspension test (TST, b) on day 28 than ICH mice housed in a standard environment (SE). n = 8 mice/group. F = 7.47, 5.28, respectively; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (c) Mice in the ICH + EE group consumed more sucrose-sweetened water in the sucrose preference test than ICH mice in the standard environment on day 28. n = 10 mice/group. F = 21.85, *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (d and e) In the light/dark transition test, the mice in the ICH + EE group spent more time in the light box (d) and less time in the dark box (e) than those in the ICH + SE group. n = 7–8. F = 8.954, 8.954, *p < 0.05 vs. sham group; ##p < 0.01 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test) and (f) The number of entries in the lightbox was not statistically different between the ICH + SE and ICH + EE groups. n = 8 mice/group. F = 0.09439, Values are mean ± SD.
EE reduces anxiety-like behaviors after ICH
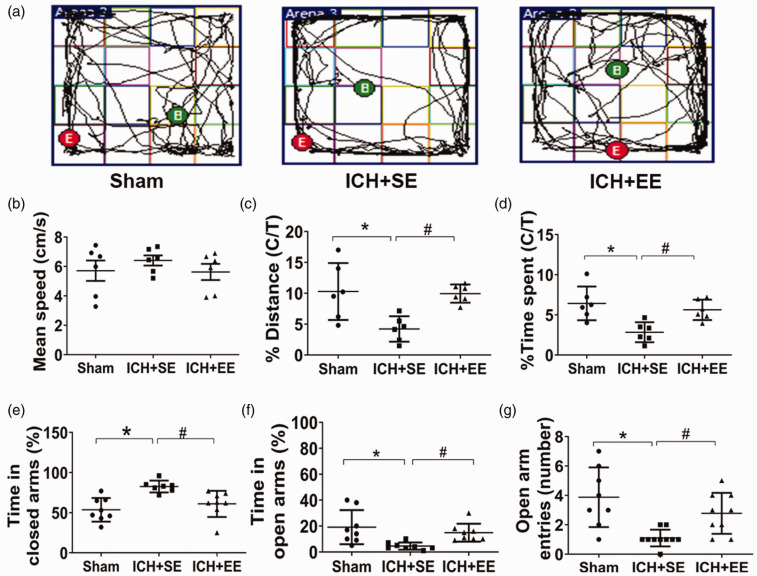
Anxiety-like behaviors improved in mice in the ICH + EE group. In the open field test, mice from the ICH + SE group exhibited less activity in the center of the field than mice from the ICH + EE group (Figure 4(a)), although the mean speed did not differ (p > 0.05; n = 6 mice/group; Figure 4(b)). The percentages of distance traveled and time in the center area were greater for mice in the ICH + EE group than for mice in the ICH + SE group (9.94 ± 1.47% vs. 4.21 ± 2.05% and 5.62 ± 1.27% vs. 2.83 ± 1.24%, respectively) on day 28 after ICH (F = 1.33 and 7.57, respectively; both p < 0.05; n = 6 mice/group; Figure 4(c) and (d)). Similarly, mice in the ICH + EE group spent more time in the open arms of the EPM and less in the closed arms compared to mice in the ICH + SE group (14.88 ± 6.93% vs. 4.5 ± 2.82% in the open arms; 61 ± 16.37% vs. 82.71 ± 7.34% in the closed arms) on day 28 after ICH (F = 5.93 and 9.10, respectively; both p < 0.05; n = 7–8 mice/group; Figure 4(f) and (e)). Mice in the ICH + EE group also made more entries in the open arms than mice in the ICH + SE group (2.78 ± 1.39 times vs. 1.10 ± 0.57 times; F = 6.126, p < 0.05; n = 8–10 mice/group; Figure 4(g)).
Figure 4.
An enriched environment (EE) mitigates anxiety-like behaviors in mice after ICH. (a) Trajectories of mice from the three groups in the open field test on day 28. B, begin; E, end. (b) The mean speeds of the mice did not differ in the open-field test. n = 6 mice/group. (c and d) Mice in the ICH + EE group traveled a significantly longer distance in the center area of the open field (c) and spent more time in the open field (d) than ICH mice exposed to the standard environment (ICH + SE) group. C/T, center area/total area; n = 6 mice/group. F = 1.33, 7.57, respectively; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (e–g) In the elevated plus-maze test, mice in the ICH + EE group spent less time in closed arms (e) and more time in open arms than in the ICH + SE group (f). (g) Furthermore, they entered the open arms more times than mice in the ICH + SE group. n = 7–10 mice/group. F = 5.93, 9.10, and 6.126, respectively; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). Values are mean ± SD.
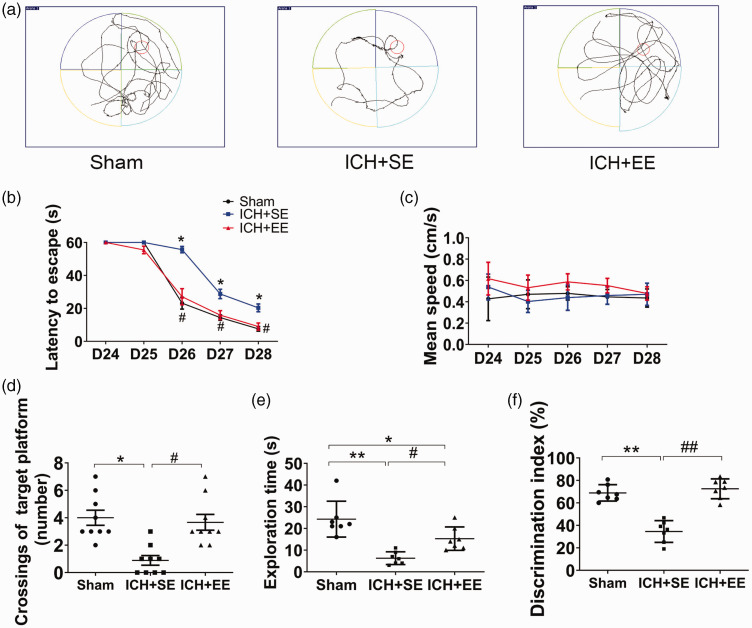
EE improves spatial learning and memory function after ICH
Representative trajectories in the MWM test for a mouse from each group are shown in Figure 5(a). Mice in the ICH + EE group required significantly less time to find the hidden platform than mice in the ICH + SE group on days 26, 27, and 28 post-ICH (day 25: 55.5 ± 2.3 s vs. 60 ± 0 s [p > 0.05]; day 26: 27.1 ± 4.9 s vs. 55.7 ± 1.9 s [p < 0.05]; day 27: 15.9 ± 2.7 s vs. 28.8 ± 2.8 s [p < 0.05]; day 28: 8.9 ± 2.1 s vs.20.3 ± 2.4 s [p < 0.05]; n = 9 mice/group; Figure 5(b)) but the swimming speed did not differ significantly among the three groups during the training period (p > 0.05; n = 9–10 mice/group; Figure 5(c)). Mice exposed to EE conditions also exhibited a higher frequency of crossings of the target platform (3.5 ± 1.7 times) than those housed in SE after ICH (1.0 ± 1.1 times) on day 28 (F = 9.20, p < 0.05; n = 9 mice/group; Figure 5(d)). In the novel object recognition test, mice in the ICH + EE and sham groups spent significantly more time exploring the novel object than the old object and had a more significant discrimination index than ICH mice exposed to SE (15.29 ± 2.044 s vs. 6.286 ± 1.107 s; F = 0.4307; 72.55 ± 8.80% vs. 34.55 ± 9.70%; F = 4.532; n = 7 mice/group; p < 0.05; Figure 5(e) and (f)).
Figure 5.
An enriched environment (EE) improves spatial learning and memory function in mice after ICH. (a) Swimming trajectories of mice from each group in the Morris water maze (MWM) test on day 28. (b) In the MWM test, EE exposure decreased escape latency on days 26, 27, and 28 compared to standard environment (SE) exposure; n = 9–10 mice/group, *p < 0.05 vs. sham group, #p < 0.05 vs. ICH + SE group (repeated measures ANOVA followed by Bonferroni’s post hoc test). (c) Exposure to EE did not affect the mean swim speed of the mice in the MWM test on days 25, 26, 27, or 28. P > 0.05 vs. the ICH + SE group (repeated measures ANOVA followed by Bonferroni’s post hoc test, F = 1.373). (d) Mice in the ICH + EE group crossed the target platform more than mice in the ICH + SE group on day 28. n = 9 mice/group. *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test) and (e) In the novel object recognition (NOR) test, mice in the ICH + EE group spent more time exploring the novel object than mice in the ICH + SE group. n = 7 mice/group. **p < 0.01 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (f) Exposure to an EE increased the discrimination index in the NOR test compared to exposure to an SE. n = 7 mice/group. **p < 0.01 vs. sham group; ##p < 0.01 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). Values are mean ± SD.
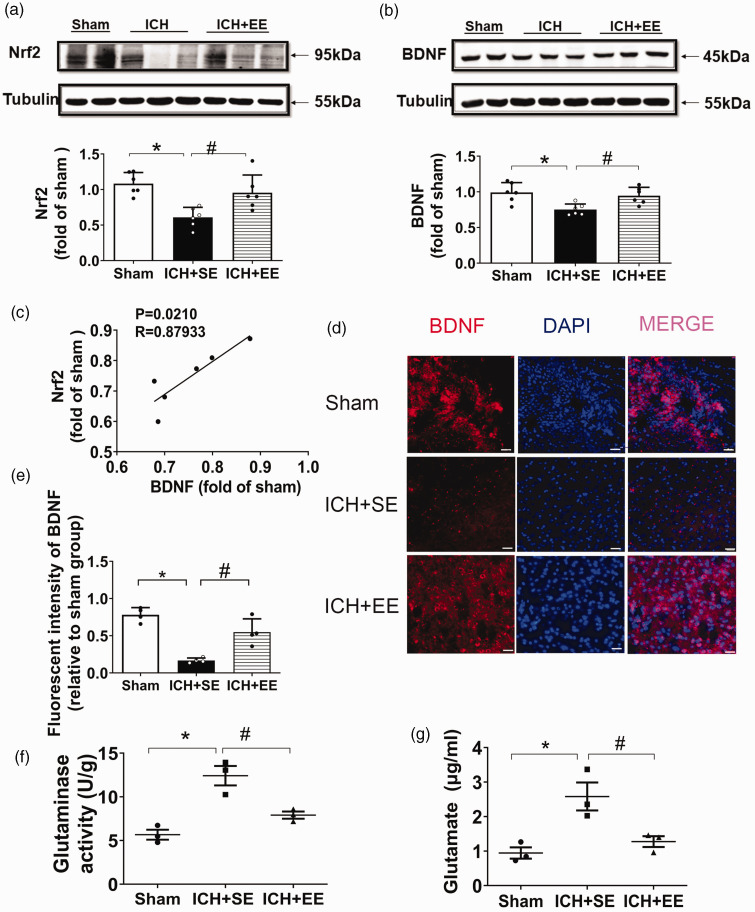
EE increases the expression of Nrf2 and BDNF and inhibits glutaminase activity after ICH
Western blot analysis revealed that exposure to EE significantly increased Nrf2 and BDNF protein expression compared to exposure to SE on day 28 (F = 7.979 and 6.846, respectively, n = 6 mice/group; p < 0.05; Figure 6(a) and (b)). Furthermore, Nrf2 expression was significantly correlated with BDNF (R = 0.87933; Figure 6(c)). Immunofluorescence staining (Figure 6(d)) indicated that BDNF expression decreased after ICH + SE but increased with exposure to an EE (F = 27.16, n = 4 mice/group; p < 0.05; Figure 6(e)). Furthermore, EE significantly inhibited glutaminase activity in brain tissue (F = 20.45; Figure 6(f)) and striatal glutamate expression, as evaluated by HPLC (1.28 ± 0.28 μg/mL vs. 2.58 ± 0.70 μg/mL; F = 10.51; Figure 6(g)) after ICH (n = 3 mice/group; both p < 0.05).
Figure 6.
An enriched environment (EE) restores Nrf2 and BDNF expression and blocks glutaminase activity in mice after ICH. (a and b) Western blot analysis of Nrf2 and BDNF expression in the perihematomal tissue on day 28 after ICH. Exposure to EE significantly increased Nrf2 and BDNF expression compared to exposure to a standard environment (SE). n = 6 mice/group. F = 10.58, 14.14, respectively; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (c) Correlation between the expression of Nrf2 and BDNF. n = 6 mice/group, Spearman test. R = 0.87933. (d) Representative immunofluorescence staining of BDNF in perihematomal tissue on day 28. Scale bar = 100 μm. (e) Quantification of BDNF fluorescence in the perihematomal area. n = 4 mice/group. F = 31.57, *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (f) Glutaminase activity in the perihematomal brain tissue on day 28. n = 3 mice/group. F = 20.45, *p < 0.05 vs. sham group; #p < 0.05 vs. ICH group (one-way ANOVA followed by Bonferroni’s post hoc test) and (g) Exposure to EE reduced glutamate levels in mice after ICH. n = 3 mice/group. F = 10.51; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH group (one-way ANOVA followed by Bonferroni’s post hoc test).
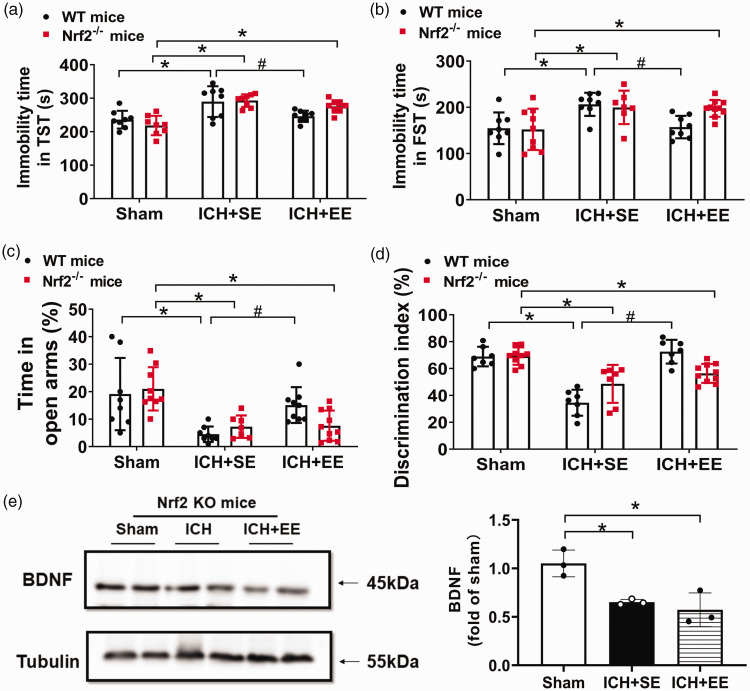
EE does not mitigate depression or anxiety-like behaviors or memory impairment of recognition in Nrf2 − /− mice with ICH
To determine whether EE mitigates depression-like behaviors via the Nrf2 signaling pathway, we first subjected Nrf2−/− mice to ICH and then exposed those mice to EE or SE and analyzed FST, TST, OFT, EPM, and a novel object recognition test 28 days after ICH. The immobility time of FST and TST did not differ between Nrf2−/− ICH mice exposed to EE or SE (F = 23.82 and 5.399, respectively, both p > 0.05, n = 8–10 mice/group, Figure 7(a) and (b)). Furthermore, in the EPM test, Nrf2−/− mice in the ICH + EE group did not show differences from the ICH + SE group in percentages of time in open arms on day 28 after ICH (F = 14.33, p > 0.05, n = 8–10 mice/group, Figure 7(c)). In the novel object recognition test, Nrf2−/− mice in the ICH + EE group tended to have a higher discrimination index than Nrf2−/− mice in the ICH + SE group, without significant differences between them (F = 10.33, p < 0.05, n = 8–10 mice/group, Figure 7(d)). In the open field test, Nrf2−/− mice in the ICH + EE group did not show differences from the ICH + SE group in percentages of distance traveled in the center area, nor the difference between the ICH + EE group and the sham group (p > 0.05, n = 8–10 mice/group, supplementary Fig. 5A). Furthermore, Nrf2−/− mice in the ICH groups (SE and EE) did not show differences in time spent in the center area on day 28 (p > 0.05, n = 8–10 mice/group, supplementary Fig. 5B). Western blot analysis revealed that Nrf2 expression was absent in Nrf2−/− mice (supplementary Fig. 6) and exposure to EE did not increase the expression of the BDNF protein compared to exposure to SE in Nrf2−/− mice on day 28 after ICH (F = 11.82, p > 0.05, n = 3 mice/group, Figure 7(e)). These results indicate that the Nrf2/BDNF pathway could be involved in improving depression- or anxiety- like behaviors or memory impairment of recognition after ICH.
Figure 7.
An enriched environment (EE) does not mitigate depression- or anxiety-like behaviors or recognition memory impairment in Nrf2−/− mice with ICH. (a, b) The immobility time in TST and FST did not differ between Nrf2−/− ICH mice exposed to EE or standard environment (SE). n = 7–10 mice/group. F = 23.82, p = 0.4247, for A between ICH + SE and ICH + EE group in Nrf2−/− mice, F = 5.399, p = 0.9157, for B between ICH + SE and ICH + EE group in Nrf2−/− mice; *p < 0.05 vs. the sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (c) Exposure of Nrf2−/− mice to EE did not increase percentages of time in open arms in the elevated plus-maze test compared to exposure to an SE. n = 8–10 mice/group. F = 14.33, p > 0.05 between ICH + SE and ICH + EE group in Nrf2−/− mice; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (d) Exposure of Nrf2−/− mice to an EE did not increase the discrimination index after ICH in the novel object recognition (NOR) test compared to exposure to an SE. n = 8–10 mice/group. F = 10.35, p = 0.3060 between ICH + SE and ICH + EE group in Nrf2−/− mice; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test). (e) Western blot analysis of BDNF expression in perihematomal tissue on day 28 after ICH of Nrf2−/− mice. BDNF expression did not differ between exposure to EE and exposure to SE. n = 3 mice/group. F = 11.82, p > 0.05 between ICH + SE and ICH + EE group in Nrf2−/− mice; *p < 0.05 vs. sham group; #p < 0.05 vs. ICH + SE group (one-way ANOVA followed by Bonferroni’s post hoc test).
Discussion
In this study, we evaluated the benefits of an EE on long-term functional outcomes after ICH. We showed that exposure to EE did not affect lesion volume or brain edema after ICH at 72 h. However, EE exposure improved neurologic function and attenuated depression- and anxiety-like behaviors after ICH. Furthermore, it promoted spatial learning and memory. In particular, the expression of glutamate in the mouse striatum was increased after ICH but reversed by exposure to EE. EE increased Nrf2 and BDNF protein expression and inhibited glutaminase activity in perihematomal brain tissues. However, EE did not improve the behavioral outcomes detected in Nrf2−/− mice. These novel findings indicate that EE improves long-term sensorimotor, emotion, and cognition after ICH. The mechanisms that underlie EE stimulation may involve the Nrf2/BDNF/glutaminase pathway.
In this study, we used a collagenase-induced ICH model to produce acute cerebrovascular injury, similar to clinical ICH.40,44–46 This model has a reproducible hematoma in the desired region of the brain. We evaluated tasks that can reflect the severity of PSD to improve the innovation of the project. We chose striatal ICH as the PSD model in our study because the incidence of depression in ICH patients is closely related to the location of the hematoma.47,48 Data also indicate that striatal hemorrhage causes severe damage to dopaminergic neurons, 49 thus inducing depression-like behavior in animals.33,50 EE is a powerful tool to counteract cognitive and somatosensory deficits. Studies have shown that exposure of rats to EE has several beneficial effects in common with antidepressant administration. 51 Using TST, SPT, and FST, we showed that the collagenase-induced striatal ICH model produced marked depression-like behavior in mice on day 28. It is well known that an increase in immobility time in the TST and FST, a reduced struggle to escape, or decreased sucrose intake can indicate a depressive state. We showed that exposure to EE mitigated emotional dysfunction in ICH mice, characterized by a reduced immobility time in FST, an increased struggle in TST, and an increased consumption of sweetened water.
The pathogenesis of PSD is complex, and its neurobiological mechanisms differ from those of other depression subtypes. In mammals, Nrf2 upregulates antioxidant genes to help reduce inflammation and reactive oxygen species.41,52–54 Nrf2 signaling decreases the production of proinflammatory cytokines and chemokine releasing factors,55,56 decreases MMP2/9 activity, and decreases the production of other proinflammatory mediators, for example, COX-2 and iNOS. 57 It also regulates the NF-KB and MAPK pathways. 58 We reported the protective effect of Nrf2 after ICH in 2007. 15 Nrf2 is also involved in the antidepressant-like effects of agmatine. 59 A recent study showed the protective effects of EE against cerebral ischemia-induced cognitive impairment through activation of the Nrf2-ARE pathway. 60 Another study showed that EE exposure stimulates the brain to release neural growth factors. 61 We examined Nrf2 and its downstream target gene BDNF. We found that Nrf2 levels in the brain decreased after ICH, similar to other reports, and exposure to EE increased Nrf2 and BDNF expression.
Depression is an incapacitating psychiatric disorder associated with decreased monoamines and neurotrophic factors. 62 BDNF is known to regulate neurogenesis, synaptogenesis, and angiogenesis and promote neuronal survival. 63 It is also involved in the pathophysiology of depressive disorders. Its role in the effect of antidepressants began to be recognized years ago. 64 In our study, the level of expression of BDNF in the perihematomal tissue decreased compared to that of sham mice, but was reversed by EE. Others have reported that serum BDNF levels are lower in patients with PSD than in healthy controls and therefore could predict the risk of PSD clinically.65,66 Previous studies showed that daily exposure to an EE increased BDNF mRNA and protein expression levels and improved functional outcomes.66,67 However, few studies addressed changes in brain BDNF levels in ICH patients or animal models of ICH-induced PSD. A study reported that housing rats in an EE after occlusion of the middle cerebral artery enhances sensorimotor recovery by decreasing BDNF mRNA and protein levels, 68 which is different from the previous report and our current findings. 69 We suggest that the discrepancy may be related to the animal models used and the measurement protocol. Heme oxygenase-1 (HO-1) and the BDNF-mediated pathway are involved in the pathophysiology of depression. 70 Several studies have shown that the Nrf2 downstream molecule HO-1 and its heme metabolites may have anti-inflammatory effects.70–73 We have demonstrated previously that HO-1 activity exacerbates early brain injury probably through controlling brain iron deposition.16,74 Therefore, in our future study, we will take advantage of HO-1 knockout mice to investigate whether the Nrf2/HO-1 signaling pathway is involved in the pathophysiology of PSD and whether EE can activate or inhibit it. 74
Glutamine-mediated oxidative stress can affect the extent of cell swelling and lead to other CNS diseases such as multiple sclerosis and brain edema.11,75 Glutaminase siRNA can protect against focal ischemia, suggesting that glutaminase may be a therapeutic target. 76 In patients with ICH, perihematomal glutamate levels increase, associated with an increase in brain water content. 77 In our study, we observed elevated glutamate levels in the hemorrhagic brain of mice by HPLC on day 28, but exposure to EE returned glutamate to control levels. This result is consistent with previous studies that showed that depression and poor neurologic outcomes are related to increased plasma glutamate concentrations after ICH.77–79 Therefore, our data indicate that the Nrf2/BDNF/glutaminase pathway could be involved in the improvement related to EE after ICH. Next, we subjected Nrf2−/− mice to ICH and determined whether EE mitigates PSD via the Nrf2 signaling pathway. We showed that EE does not mitigate depression or anxiety-like behaviors or memory impairment of recognition in Nrf2−/− mice with ICH. This result suggests that EE does not mitigate depression-like behaviors after ICH when Nrf2 is globally eliminated, and further confirms the primary role of Nrf2 in EE exposure.
An EE provides multiple sensorimotor stimuli and opportunities for training and learning, including social interaction, space exploration, and spontaneous physical activities.68,69,80,81 Similarly, EE improved learning skills and recognition ability in mice with ICH in our study. EE has also been shown to restore normal behavior by providing cytoskeletal restoration and synaptic changes in the hippocampus of rats exposed to an experimental model of depression. 82
Previous studies have shown that an EE can improve brain plasticity after focal brain ischemic injury.83,84 Here, we showed that daily exposure to an EE after ICH had a beneficial effect on motor function of mice on days 3, 5, 7 and 14. This finding supports the results of previous studies on focal ischemia in rodents,84,85 indicating that EE stimulation can improve lesion-induced plasticity during the first weeks and months of recovery after stroke.86,87
In our study, only male mice were used. However, there is a sexual dimorphism in cerebrovascular structure and functions, response to stroke or traumatic brain injury, and post-stroke brain remodeling.46,88–92 Thus, we will test in our future research whether female mice react differently to the EE housing against depression-like behaviors after ICH compared to male mice.
In our study, ICH mice developed a high incidence of depression-like behaviors than control mice (about 53% vs. 2%). Although PSD has been studied clinically for over a decade, an ideal PSD model is required to investigate the underlying pathomechanism. Developing an appropriate PSD model is a pressing and challenging task in this young research field.
Conclusion
Together, we speculate that motor and emotional dysfunction after ICH is due to an imbalance of neurotransmitters and neurobiological changes and that EE could promote functional recovery by reducing oxidative damage. In conclusion, exposure to EE improves sensorimotor function, depression- and anxiety-like behaviors, and cognitive function after ICH. The underlying mechanisms may involve the Nrf2/BDNF/glutaminase pathway. Therefore, providing EE could be a potential therapeutic strategy to treat post-ICH depression.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221135419 for An enriched environment improves long-term functional outcomes in mice after intracerebral hemorrhage by mechanisms that involve the Nrf2/BDNF/glutaminase pathway by Peijun Jia, Junmin Wang, Xiuhua Ren, Jinxin He, Shaoshuai Wang, Yinpei Xing, Danyang Chen, Xinling Zhang, Siqi Zhou, Xi Liu, Shangchen Yu, Zefu Li, Chao Jiang, Weidong Zang, Xuemei Chen and Jian Wang in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the Key Project of Science and Technology of the department of Science and Technology of Henan Province (grant number 212102310220 to XC).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Xuemei Chen and Jian Wang conceived the study. Xuemei Chen and Peijun Jia performed the experiments and analyzed data. Peijun Jia and Jian Wang wrote and revised the manuscript. Junmin Wang, Xiuhua Ren, Jinxin He and Yinpei Xing performed molecular experiments. Danyang Chen, Xinling Zhang, Siqi Zhou, Xi Liu, Shangchen Yu, Yinpei Xing, Shaoshuai Wang, Zefu Li, Chao Jiang and Weidong Zang performed animal behavior tests or analyzed data. All the co-authors commented, edited, and critically revised the manuscript.
Data availability
The raw data of this manuscript have been deposited in the Mendeley database (DOI: 10.17632/v57x3t925v.1). The datasets from this study and its supplementary files are available from the corresponding authors.
ORCID iD
Jian Wang https://orcid.org/0000-0003-2291-640X
Supplementary material
Supplemental material for this article is available online.
References
- 1.Villa RF, Ferrari F, Moretti A.Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol Ther 2018; 184: 131–144. [DOI] [PubMed] [Google Scholar]
- 2.Feng P, Huang C.Phospholipase D-mTOR signaling is compromised in a rat model of depression. J Psychiatr Res 2013; 47: 579–585. [DOI] [PubMed] [Google Scholar]
- 3.Feng C, Fang M, Liu XY.The neurobiological pathogenesis of poststroke depression. ScientificWorldJournal 2014; 2014: 521349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoli F, Di Brita C, Crocamo C, et al. Early post-stroke depression and mortality: meta-analysis and meta-regression. Front Psychiatry 2018; 9: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Xu D, Qi H, et al. Enriched environment promotes post-stroke neurogenesis through NF-kappaB-mediated secretion of IL-17A from astrocytes. Brain Res 2018; 1687: 20–31. [DOI] [PubMed] [Google Scholar]
- 6.Johansson BB, Ohlsson AL.Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp Neurol 1996; 139: 322–327. [DOI] [PubMed] [Google Scholar]
- 7.Paban V, Chambon C, Manrique C, et al. Neurotrophic signaling molecules associated with cholinergic damage in young and aged rats: environmental enrichment as potential therapeutic agent. Neurobiol Aging 2011; 32: 470–485. [DOI] [PubMed] [Google Scholar]
- 8.Loubinoux I, Kronenberg G, Endres M, et al. Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med 2012; 16: 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan J, Ren H, Wang J.Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol 2019; 4: 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D, Xu X, Pan L, et al. Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J Neuroinflammation 2017; 14: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Gao Y, Wan J, et al. Changes in motor function, cognition, and emotion-related behavior after right hemispheric intracerebral hemorrhage in various brain regions of mouse. Brain Behav Immun 2018; 69: 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhang Z, Lu H, et al. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol 2017; 54: 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strekalova T, Evans M, Costa-Nunes J, et al. Tlr4 upregulation in the brain accompanies depression- and anxiety-like behaviors induced by a high-cholesterol diet. Brain Behav Immun 2015; 48: 42–47. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Fisar Z, Medina M, et al. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates–Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 2012; 20: 127–150. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Fields J, Zhao C, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 2007; 43: 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CF, Cho S, Wang J.(−)-epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol 2014; 1: 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren H, Han R, Liu X, et al. Nrf2-BDNF-TrkB pathway contributes to cortical hemorrhage-induced depression, but not sex differences. J Cereb Blood Flow Metab 2021; 41: 3288–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Audet MC, Anisman H.Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front Cell Neurosci 2013; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kronenberg G, Gertz K, Heinz A, et al. Of mice and men: modelling post-stroke depression experimentally. Br J Pharmacol 2014; 171: 4673–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez-David I, Tritschler L, Ali ZE, et al. Nrf2-signaling and BDNF: a new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci Lett 2015; 597: 121–126. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Nedelcovych MT, Thomas AG, et al. JHU-083 selectively blocks glutaminase activity in brain CD11b(+) cells and prevents depression-associated behaviors induced by chronic social defeat stress. Neuropsychopharmacology 2019; 44: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maezawa I, Jin LW.Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci 2010; 30: 5346–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Lan X, Li Q, et al. Inhibition of prostaglandin E2 receptor EP3 mitigates thrombin-induced brain injury. J Cereb Blood Flow Metab 2016; 36: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Han X, Lan X, et al. Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol Dis 2017; 108: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Wu T, Chang CF, et al. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun 2015; 46: 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Han X, Lan X, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017; 2: e90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Wu T, Han X, et al. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. J Cereb Blood Flow Metab 2017; 37: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrinda M, Sasidharan A, Aparna S, et al. Enriched environment attenuates behavioral seizures and depression in chronic temporal lobe epilepsy. Epilepsia 2017; 58: 1148–1158. [DOI] [PubMed] [Google Scholar]
- 29.Slater AM, Cao L.A protocol for housing mice in an enriched environment. JoVE 2015; June 8: e52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Wan J, Lan X, et al. Neuroprotection of brain-permeable iron chelator VK-28 against intracerebral hemorrhage in mice. J Cereb Blood Flow Metab 2017; 37: 3110–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-Román J, Varela-Aguilar JM, Bravo-Ferrer J, et al. Idiopathic orbital myositis: treatment with cyclosporin . Ann Rheum Dis 1993; 52: 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia P, He J, Li Z, et al. Profiling of Blood-Brain barrier disruption in mouse intracerebral hemorrhage models: collagenase injection vs. Front Cell Neurosci 2021; 15: 699736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi X, Bai H, Wang J, et al. Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Front Neurol 2021; 12: 667511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Jiang C, Zhang K, et al. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic Biol Med 2019; 131: 345–355. [DOI] [PubMed] [Google Scholar]
- 35.Carlson GC, Lin RE, Chen Y, et al. Dexras1 a unique ras-GTPase interacts with NMDA receptor activity and provides a novel dissociation between anxiety, working memory and sensory gating. Neuroscience 2016; 322: 408–415. [DOI] [PubMed] [Google Scholar]
- 36.Can A, Dao DT, Terrillion CE, et al. The tail suspension test. JoVE 2011; Jan 28: e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirata N, Hattori S, Shoji H, et al. Comprehensive behavioral analysis of indoleamine 2,3-dioxygenase knockout mice. Neuropsychopharmacol Rep 2018; 38: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong MX, Li CM, Shen P, et al. Recombinant tissue plasminogen activator induces long-term anxiety-like behaviors via the ERK1/2-GAD1-GABA Cascade in the hippocampus of a rat model. Neuropharmacology 2018; 128: 119–131. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Wang J, Huang L, et al. The pros and cons of motor, memory, and emotion-related behavioral tests in the mouse traumatic brain injury model. Neurol Res 2022; 44: 65–89. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Wang J, Xing Y, et al. Behavioral assessment of post-stroke depression and anxiety in rodents. Brain Hemorrhages 2020; 1: 105–111. [Google Scholar]
- 41.Lan X, Han X, Li Q, et al. (-)-epicatechin, a natural flavonoid compound, protects astrocytes against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways. Mol Neurobiol 2017; 54: 7898–7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T, Wu H, Wang J, et al. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation 2011; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Wan P, Wang W, et al. Dopamine in the hippocampal dentate gyrus modulates spatial learning via D1-like receptors. Brain Res Bull 2019; 144: 101–107. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Wang J.Animal models: cerebral hemorrhage. Primer on Cerebrovascular Diseases 2017; 306–311. [Google Scholar]
- 45.Wang J, Dore S.Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 2007; 27: 894–908. [DOI] [PubMed] [Google Scholar]
- 46.Boltze J, Aronowski JA, Badaut J, et al. New mechanistic insights, novel treatment paradigms, and clinical progress in cerebrovascular diseases. Front Aging Neurosci 2021; 13: 623751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern-Nezer S, Eyngorn I, Mlynash M, et al. Depression one year after hemorrhagic stroke is associated with late worsening of outcomes. NeuroRehabilitation 2017; 41: 179–187. [DOI] [PubMed] [Google Scholar]
- 48.Christensen MC, Mayer SA, Ferran JM, et al. Depressed mood after intracerebral hemorrhage: the FAST trial. Cerebrovasc Dis 2009; 27: 353–360. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Zhang K, Zhong J, et al. Stably maintained microtubules protect dopamine neurons and alleviate depression-like behavior after intracerebral hemorrhage. Sci Rep 2018; 8: 12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu W, Gao Y, Chang CF, et al. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One 2014; 9: e97423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ickes BR, Pham TM, Sanders LA, et al. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol 2000; 164: 45–52. [DOI] [PubMed] [Google Scholar]
- 52.Bakunina N, Pariante CM, Zunszain PA.Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015; 144: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Wu Q, Lu Y, et al. Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radic Biol Med 2018; 124: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hua W, Chen X, Wang J, et al. Mechanisms and potential therapeutic targets for spontaneous intracerebral hemorrhage. Brain Hemorrhages 2020; 1: 99–104. [Google Scholar]
- 55.Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 2016; 7: 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu H, Wang Z, Yu J, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol 2019; 178: 101610. [DOI] [PubMed] [Google Scholar]
- 57.Benedict AL, Mountney A, Hurtado A, et al. Neuroprotective effects of sulforaphane after contusive spinal cord injury. J Neurotrauma 2012; 29: 2576–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed SM, Luo L, Namani A, et al. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 585–597. [DOI] [PubMed] [Google Scholar]
- 59.Freitas AE, Egea J, Buendia I, et al. Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol Neurobiol 2016; 53: 3030–3045. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Yuan M, Yang S, et al. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway. Int J Neurosci 2021; 131: 641–649. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Cao M, Pu T, et al. Enriched physical environment attenuates spatial and social memory impairments of aged socially isolated mice. Int J Neuropsychopharmacol 2018; 21: 1114–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duman RS.Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med 2004; 5: 11–25. [DOI] [PubMed] [Google Scholar]
- 63.Miao H, Li R, Han C, et al. Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J Neurophysiol 2018; 120: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 64.Shirayama Y, Chen AC, Nakagawa S, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002; 22: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Zhao YD, Zeng JW, et al. Serum brain-derived neurotrophic factor levels in post-stroke depression. J Affect Disord 2014; 168: 373–379. [DOI] [PubMed] [Google Scholar]
- 66.Zhao LR, Risedal A, Wojcik A, et al. Enriched environment influences brain-derived neurotrophic factor levels in rat forebrain after focal stroke. Neurosci Lett 2001; 305: 169–172. [DOI] [PubMed] [Google Scholar]
- 67.Dahlqvist P, Ronnback A, Risedal A, et al. Effects of postischemic environment on transcription factor and serotonin receptor expression after permanent focal cortical ischemia in rats. Neuroscience 2003; 119: 643–652. [DOI] [PubMed] [Google Scholar]
- 68.Nygren J, Kokaia M, Wieloch T.Decreased expression of brain-derived neurotrophic factor in BDNF(+/−) mice is associated with enhanced recovery of motor performance and increased neuroblast number following experimental stroke. J Neurosci Res 2006; 84: 626–631. [DOI] [PubMed] [Google Scholar]
- 69.Mansour AG, Xiao R, Bergin SM, et al. Enriched environment enhances NK cell maturation through hypothalamic BDNF in male mice. Eur J Immunol 2021; 51: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manosso LM, Moretti M, Rosa JM, et al. Evidence for the involvement of heme oxygenase-1 in the antidepressant-like effect of zinc. Pharmacol Rep 2017; 69: 497–503. [DOI] [PubMed] [Google Scholar]
- 71.Chen-Roetling J, Regan RF.Targeting the Nrf2-Heme oxygenase-1 axis after intracerebral hemorrhage. Curr Pharm Des 2017; 23: 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Song Y, Zhang Z, et al. Distinct role of heme oxygenase-1 in early- and late-stage intracerebral hemorrhage in 12-month-old mice. J Cereb Blood Flow Metab 2017; 37: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Doré S.Heme oxygenase 2 deficiency increases brain swelling and inflammation after intracerebral hemorrhage. Neuroscience 2008; 155: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Doré S.Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 2007; 130: 1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayakumar AR, Rao KV, Murthy CR, et al. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int 2006; 48: 623–628. [DOI] [PubMed] [Google Scholar]
- 76.Thomas AG, O'Driscoll CM, Bressler J, et al. Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem Biophys Res Commun 2014; 443: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu G, Li S, Wang L, et al. The perihematomal glutamate level is associated with the outcome of patients with basal ganglia hematomas treated by minimally invasive procedures. Neurol Res 2013; 35: 829–836. [DOI] [PubMed] [Google Scholar]
- 78.Castillo J, Davalos A, Alvarez-Sabin J, et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology 2002; 58: 624–629. [DOI] [PubMed] [Google Scholar]
- 79.McCarthy DJ, Alexander R, Smith MA, et al. Glutamate-based depression GBD. Med Hypotheses 2012; 78: 675–681. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y, Zhang M, Kang X, et al. Impaired adult hippocampal neurogenesis and cognitive ability in a mouse model of intrastriatal hemorrhage. Neurosci Lett 2015; 599: 133–139. [DOI] [PubMed] [Google Scholar]
- 81.Fan D, Li J, Zheng B, et al. Enriched environment attenuates Surgery-Induced impairment of learning, memory, and neurogenesis possibly by preserving BDNF expression. Mol Neurobiol 2016; 53: 344–354. [DOI] [PubMed] [Google Scholar]
- 82.Sifonios L, Trinchero M, Cereseto M, et al. An enriched environment restores normal behavior while providing cytoskeletal restoration and synaptic changes in the hippocampus of rats exposed to an experimental model of depression. Neuroscience 2009; 164: 929–940. [DOI] [PubMed] [Google Scholar]
- 83.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996; 272: 1791–1794. [DOI] [PubMed] [Google Scholar]
- 84.Ohlsson AL, Johansson BB.Environment influences functional outcome of cerebral infarction in rats. Stroke 1995; 26: 644–649. [DOI] [PubMed] [Google Scholar]
- 85.Nygren J, Wieloch T.Enriched environment enhances recovery of motor function after focal ischemia in mice, and downregulates the transcription factor NGFI-A. J Cereb Blood Flow Metab 2005; 25: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 86.Witte OW.Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol 1998; 11: 655–662. [DOI] [PubMed] [Google Scholar]
- 87.Kreisel SH, Bazner H, Hennerici MG.Pathophysiology of stroke rehabilitation: temporal aspects of neuro-functional recovery. Cerebrovasc Dis 2006; 21: 6–17. [DOI] [PubMed] [Google Scholar]
- 88.Gu Y, Dong Y, Wan J, et al. Interleukin-10 deficiency aggravates traumatic brain injury in male but not female mice. Exp Neurol 2022; 355: 114125. [DOI] [PubMed] [Google Scholar]
- 89.Ren H, Han R, Chen X, et al. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: an update. J Cereb Blood Flow Metab 2020; 40: 1752–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chandra PK, Cikic S, Baddoo MC, et al. Transcriptome analysis reveals sexual disparities in gene expression in rat brain microvessels. J Cereb Blood Flow Metab 2021; 41: 2311–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang R, Oh JM, Motovylyak A, et al. Impact of sex and APOE ε4 on age-related cerebral perfusion trajectories in cognitively asymptomatic Middle-aged and older adults: a longitudinal study. J Cereb Blood Flow Metab 2021; 41: 3016–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cirillo C, Brihmat N, Castel-Lacanal E, et al. Post-stroke remodeling processes in animal models and humans. J Cereb Blood Flow Metab 2020; 40: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221135419 for An enriched environment improves long-term functional outcomes in mice after intracerebral hemorrhage by mechanisms that involve the Nrf2/BDNF/glutaminase pathway by Peijun Jia, Junmin Wang, Xiuhua Ren, Jinxin He, Shaoshuai Wang, Yinpei Xing, Danyang Chen, Xinling Zhang, Siqi Zhou, Xi Liu, Shangchen Yu, Zefu Li, Chao Jiang, Weidong Zang, Xuemei Chen and Jian Wang in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
The raw data of this manuscript have been deposited in the Mendeley database (DOI: 10.17632/v57x3t925v.1). The datasets from this study and its supplementary files are available from the corresponding authors.