Abstract
Objective
The aim of this study was to investigate the influence of morning versus evening exercise on weight loss, cardiometabolic health, and components of energy balance.
Methods
A total of 100 inactive adults with overweight or obesity were randomized to morning exercise (AMEx; 06:00–09:00), evening exercise (PMEx; 16:00–19:00), or wait‐list control (CON). AMEx and PMEx were prescribed 250 min·wk−1 of self‐paced aerobic exercise for 12 weeks. Anthropometry and body composition, physical activity, and dietary intake were assessed at baseline, 6 weeks, and 12 weeks. Cardiorespiratory fitness (V̇O2peak), resting metabolic rate, and blood markers were assessed at baseline and 12 weeks. Body composition and V̇O2peak were also measured at 3‐ and 6‐month follow‐up.
Results
AMEx and PMEx lost weight during the intervention (mean [SD], AMEx, −2.7 [2.5] kg, p < 0.001; PMEx, −3.1 [3.4] kg, p < 0.001). V̇O2peak significantly increased in both intervention groups, and these changes were different from CON (AMEx, +4.7 mL·kg−1·min−1, p = 0.034; PMEx, +4.2 mL·kg−1·min−1, p = 0.045). There were no between‐group differences for resting metabolic rate or physical activity. At 12 weeks, total energy intake was significantly reduced in both AMEx and PMEx versus CON (AMEx, −3974 kJ, p < 0.001; PMEx, −3165 kJ, p = 0.001).
Conclusions
Adults with overweight and obesity experience modest weight loss in response to an exercise program, but there does not appear to be an optimal time to exercise.
Study Importance.
What is already known?
Exercise plays an important role in weight management. Morning and evening are key windows of opportunity to incorporate exercise.
There is evidence to suggest exercising at the same time each day is important for long‐term maintenance.
Randomizing inactive adults with overweight or obesity to morning or evening exercise is considered feasible and acceptable.
What does this study add?
Compliance and adherence to the program and self‐selected exercise intensity were similar between the morning (AMEx) and evening (PMEx) exercise groups.
There were no significant differences in cardiometabolic health risk factors between AMEx and PMEx; however, there was a greater proportion of participants in PMEx who achieved clinically meaningful weight loss (≥ 5% starting 5% body weight; 33% of participants randomized to PMEx) and clinically meaningful improvements in V̇O2peak (≥3.5 mL·kg−1·min−1; 75% of participants randomized to PMEx), compared with participants in AMEx (19% and 69%, respectively).
How might these results change the direction of research?
Trial descriptors of exercise interventions are often suboptimal and incompletely described in study reports. Consistent reporting of time of day of exercise interventions among high‐quality studies would significantly contribute to the literature and provide critical insight into the relative importance (or lack thereof) of prescribing exercise at a particular time of day.
INTRODUCTION
The Australian Institute of Health and Welfare added “obesity” to the national health priority areas list in 2008 [1]. Despite considerable efforts to promote a healthy lifestyle, rates of overweight and obesity are continuing to rise worldwide, and obesity is now considered a pandemic [2]. Although dietary intervention is the most effective lifestyle intervention for weight loss, exercise plays an important role in weight management and high volumes of exercise (> 250 min·wk−1) are recommended for individuals to elicit clinically significant weight loss (> 5%) [3].
Morning and evening are key windows of opportunity to incorporate exercise [4, 5]. However, the “time” dimension of exercise prescription components (FITT: frequency, intensity, time, and type) tends to be interpreted as the duration of exercise rather than the time of day [6]. The circadian system plays a significant role in regulating several physiological processes that influence appetite, sleep/wake cycles, and exercise performance [7, 8]. Therefore, it is reasonable to suggest that physiological responses to exercise could be different in the morning compared with the evening. Although exercise timing is prominent in athletic performance literature [7], the influence and efficacy of exercise timing on weight loss are relatively understudied areas. Often, research studies investigating exercise and weight loss are incompletely described in research reports, hampering evaluation of results and replication and implementation into practice [9]. Indeed, the time of day at which exercise is prescribed or undertaken is often unreported in study protocol descriptions or not controlled. Rather, participants often select when to exercise, and the time may change day to day and be incorporated around competing demands.
There is evidence to suggest exercising at the same time each day (i.e., “time‐structured”) versus different times of the day (i.e., “sporadic”) is important for long‐term exercise adherence [10, 11]. Therefore, investigating the efficacy of exercising at different times of day is essential. Indeed, this is of interest to the public; “when is the best time to exercise for weight loss” is a question commonly featured in mainstream media. Among a small sample of recreationally active adults, we have also established that most individuals (> 75%) would be willing to alter the time of day they exercised if there was evidence to indicate they would gain greater benefit [5]. Understanding this relationship is necessary to provide evidence for recommendations for exercise timing in real‐world settings and clinical practice.
The feasibility and acceptability of randomizing insufficiently active individuals with overweight and obesity to complete aerobic exercise in the morning versus the evening have been explored in small sample sizes [12, 13]. Both studies report high rates of adherence (> 80%) to the intervention and conclude that both morning and evening time periods are acceptable and feasible. The few studies that have investigated the influence of exercise time of day on weight loss and components of energy balance report mixed findings [4, 14, 15, 16, 17, 18]. Although there is agreement that regular exercise plays an important role in improving general health and maintaining energy balance, there remains a distinct lack of evidence regarding an optimal time of day for exercise to maximize efficacy. In addition to small sample sizes, all the research to date has had methodological limitations including (1) not being specifically designed to detect differences between time of day of exercise (such as secondary analyses); (2) not being specifically designed to target weight loss (e.g., prescribing doses of exercise intended to meet standards derived to improve general health and fitness [ranging from 60 to 180 min·wk−1] rather than for weight loss [≥ 250 min·wk−1]); or (3) lacking objective measures of intervention compliance and components of energy balance. In addition, most studies allowed participants to self‐select the time of day of exercise. In some instances, the time of day of exercise may not be conducive to regular work/social schedules, such as those chosen by Bilski et al. (morning exercise trial, 11:00; evening exercise trial, 23:00) [19]. These limitations make the generalizability of findings and comparisons between studies difficult, and whether there is an optimal time of day to exercise for weight loss remains unknown.
These (lack of) findings highlight the need for a well‐designed, large‐scale randomized controlled trial to understand whether there is any effect of exercise timing on weight loss. Therefore, the purpose of this study was to investigate the influence of a 12‐week exercise program, performed in either the morning or evening, on weight loss, cardiometabolic health risk factors, and components of energy balance in a sufficiently powered sample of inactive adults with overweight and obesity. To determine the impact and residual effect of the intervention, weight change was also assessed following a period of complete withdrawal of contact with participants, at 3‐ and 6‐month follow‐ups.
METHODS
This study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12616000457448p) and approved by the Bellberry Human Research Ethics Committee (HREC2016‐02‐130). Informed consent was obtained from all individual participants included in this study. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Study design
This study used a three‐armed randomized controlled trial design. Following baseline testing, participants were randomized to one of three groups—morning exercise (AMEx), evening exercise (PMEx), or wait‐list control (CON) —at a 2:2:1 ratio using permuted block randomization with multiple randomized block sizes. Participants allocated to CON were asked to continue with their usual day‐to‐day activities and were offered the exercise program after all formal testing was completed.
Participants
Participants were recruited from the local community and metropolitan universities via electronic media and print advertising. Interested individuals were screened for eligibility by web‐based survey, which included stage one of the Adult Pre‐Exercise Screening System [20]. To be included in the study, individuals were (1) insufficiently active (< 150 minutes of moderate/vigorous physical activity per week), (2) classified as having overweight or obesity (body mass index [BMI] ≥ 25 kg·m2), and (3) weight stable (± 3 kg) in the previous 3 months. Individuals were considered ineligible if they (1) were pregnant or had plans to become pregnant over the course of the study, (2) participated in shift work, (3) were currently participating in a weight loss program, or (4) were using any medication or supplements that would affect weight loss. Individuals deemed eligible were invited to attend the laboratory for their baseline assessment.
Intervention
Participants allocated to the two intervention conditions were prescribed 250 min·wk−1 of self‐paced aerobic (treadmill‐based) exercise for 12 weeks, the dose of exercise recommended by the American College of Sports Medicine to elicit clinically significant weight loss [3]. Participants exercised between 06:00 and 09:00 (AMEx) or 16:00 and 19:00 (PMEx). The time periods were chosen to coincide with diurnal hormone patterns and, for convenience, were based on when most people could accommodate exercise (i.e., before or after work) [5]. The exercise program included both supervised and unsupervised aerobic exercise sessions, designed to create an exercise habit [10] (Table 1). All supervised exercise sessions were conducted at the School of Human Movement and Nutrition Sciences at The University of Queensland, St. Lucia, Australia. Food intake was not standardized prior to exercise sessions.
TABLE 1.
Schedule of supervision for the exercise intervention
| Intervention weeks | Prescribed supervised sessions (n, min·wk−1) | Expected unsupervised exercise volume (min·wk−1) |
|---|---|---|
| 1–4 | 5, 250 | ‐ |
| 5–6 | 4, 200 | 50 |
| 7–8 | 3, 150 | 100 |
| 9–12 | 2, 100 | 150 |
Compliance with exercise sessions was measured by the total number of supervised sessions attended (out of the maximum 42). To measure adherence, participants were asked to keep an exercise diary to record information about unsupervised sessions, including the type, mode, time of day, and duration of the activity, and these were collected weekly. Participants wore a heart rate monitor (Polar RCX3, Polar, Kempele, Finland) during supervised training sessions to measure exercise intensity, calculated as a percentage of peak heart rate, established during their prior V̇O2peak test using the Swain equation [21]. Ratings of perceived exertion (RPE) were recorded using Borg's original 6‐ to 20‐point scale [22]. The shortened version of the Physical Activity Enjoyment scale was used to assess and compare participant enjoyment between the exercise intervention groups, measured in weeks 1 and 12. Individuals' chronotype was also assessed using Horne and Östberg's Morningness‐Eveningness Questionnaire, a 19‐item self‐rated questionnaire that uses Likert‐type responses to evaluate whether a person is a morning or evening type based on factors of sleep/wake patterns and preferred physical and mental activity times [23]. For participants < 40 years of age, data were scored as per Horne and Östberg; for participants aged 40 and over, data were scored using cut points proposed by Taillard et al. [24].
The secondary component of the intervention involved several constituents of theoretical approaches to motivate behavior change and promote weight loss, which are especially important for long‐term adherence. Informational and behavioral approaches were the focus of the intervention. Strategies used to enhance behavior change are outlined in Supporting Information Table S1.
Follow‐up
Following the intervention, participants assigned to the intervention were encouraged to continue to exercise for ≥ 250 min·wk−1; however, there was a complete withdrawal of contact from all participants, other than to schedule follow‐up assessment appointments. This design of the program was to determine the impact and residual effect of the intervention arms [25].
Outcome measures
Participants underwent a battery of testing at baseline (< 2 weeks before the intervention began), mid‐intervention (weeks 5–6), post‐intervention (week 12), and at 3‐ and 6‐month follow‐up (Supporting Information Table S2). Except for cardiorespiratory fitness, physical activity, and dietary assessment, all measures were assessed when participants were in a fasted state.
Anthropometry and body composition
Standing height (SECA 217–172‐1009, Hamburg, Germany) was measured to the nearest 0.1 cm at baseline and body mass (A&D Mercury Load Cell Digitizer; A&D Weighting, Melbourne, Australia) was measured to the nearest 0.05 kg at all time points. Measures were taken in accordance with the International Society for the Advancement of Kinathropometry data collection procedures [26]. Fat mass (kg), fat‐free mass (kg), and percent fat mass (%) were estimated by dual‐energy x‐ray absorptiometry (DXA; Hologic Discovery W, Waltham, Massachusetts). Scans were analyzed using the APEX system software version 4.5.3.
Cardiometabolic health
Cardiorespiratory fitness was assessed via indirect calorimetry at baseline, week 12, and 3‐ and 6‐month follow‐up. Participants were required to walk/run on a treadmill, following the Modified Bruce protocol, until volitional fatigue. Peak oxygen uptake (V̇O2peak; TrueOne 2400 Metabolic Measurement System, ParvoMedics, Sandy, Utah) was recorded as the highest mean value attained during two 30‐second periods, before volitional exhaustion [27]. Participants were asked to avoid exercise and any stimulants (such as caffeine and tobacco) and alcohol in the 24 hours prior to testing and to avoid eating in the 2 hours prior to the test.
Blood pressure, lipid profiles, and blood glucose were sampled at baseline and 12 weeks in a fasted state. Samples of capillary blood were extracted via a contact‐activated lancet and processed using the CardioChek PA (Polymer Technology Services, Inc., Indianapolis, Indiana) and AccuChek Performa (Model NC; Roche, Mannheim, Germany) analyzers, handheld, battery‐operated reflectance spectrophotometers to measure blood lipids (triglycerides, high‐density lipoprotein [HDL], low‐density lipoprotein [LDL], total cholesterol) and blood glucose, respectively.
Components of energy balance
Indirect calorimetry was used to measure resting metabolic rate (RMR) via a metabolic cart (TrueOne 2400 Metabolic Measurement System) at baseline and week 12. Participants rested quietly for a 15‐minute equilibration period, after which they were placed under a ventilated plastic hood for 30 minutes to assess VO2 and VCO2. During the first 10 minutes of measurement, the dilution pump flow rate was adjusted (approximate body weight [kg] divided by 3) until steady state was reached (≤ 10% coefficient of variation for VO2 and VCO2). Resting energy expenditure (kcal/d) was calculated with the system software (TrueOne 32 RMR, version 4.3.4), using the modified Weir equation (5.616 × VO2 + 1.584 × VCO2). Following best practice guidelines, RMR (kcal/d) was defined as the average of the final 20 minutes of measurement and converted to kilojoules (kJ) using a conversion factor of 4.18 [28].
Participants were provided with accelerometers (GENEActiv; Activinsights Ltd., Cambridgeshire, UK]) to wear on their nondominant wrist continuously for seven consecutive days to objectively measure physical activity at baseline, 6 weeks, and 12 weeks (including during exercise sessions, where applicable). The accelerometers were sampled at 30 Hz, and the raw.bin files were converted to 60‐second epoch.csv files. Accelerometry profiles were cleaned and checked for valid days prior to any data analysis. As in other studies, a valid day was defined as minimum wear time of ≥ 16 hours, from at least 4 of the 7 days [29]. Predefined acceleration cut points established by Esliger et al. [30] were used to classify each 60‐second epoch of waking wear time into one of four physical activity levels: sedentary, light, moderate, or vigorous.
Energy intake was estimated using a five‐step, multiple‐pass 24‐hour dietary interview at baseline, 6 weeks, and 12 weeks. At each time point, food recalls were administered on two occasions approximately 1 week apart, recalling the previous day's food and beverage intake. Data were entered into a computerized nutrition database (FoodWorks Premium Version 8, Xyris software, Brisbane, Australia) for analysis.
Statistical analysis
All data were analyzed using SPSS Statistics version 25 (IBM Corp., Armonk, New York). Group characteristics at baseline were summarized but not tested for differences, as per the 2010 CONSORT statement [31]. Repeated measures ANOVA was used to compare change over time and test for differences between groups for measures of compliance, adherence, self‐selected exercise intensity, and exercise enjoyment. For other outcomes, linear mixed modeling with fixed and random effects was used to assess changes over time and differences among groups, estimated by a restricted maximum likelihood algorithm. Group (AMEx, PMEx, CON), time (0, 6, 12), and group × time interactions were treated as fixed factors; participants were treated as a random factor with individual intercepts. Model residuals were formally assessed for normality by use of the Shapiro–Wilk test and visual inspection of histogram plots. Statistical significance was set at p < 0.05. All results are reported as mean (standard deviation), unless specified otherwise. Fisher least significant difference test was used for post hoc analyzes to compare mean changes in the outcome variables between groups at each assessment period. An advantage of the linear mixed modeling approach is it allows for unbalanced, unequally spaced observations over time, making it ideal to analyze longitudinal data [32].
An a priori power calculation at 5% α level and 80% power determined that a total sample of 69 participants would be sufficient to detect small effect sizes (Cohen d = 0.3) for within‐ and between‐group differences with five measurement occasions (G*Power software, version 3.1.9.2; University of Düsseldorf, Düsseldorf, Germany). To detect the same effect size with equivalent power, a 2:1 allocation sequence requires 12% more participants than the typical 1:1 [33, 34, 35]. Therefore, the required total sample size would be increased to 78 participants. Because of the documented dropout rates among exercise and lifestyle interventions [36, 37, 38] and the high participant burden of this protocol, an attrition rate of 20% was estimated. Therefore, a target sample size of 95 participants was set for recruitment: n = 19 in the control group and n = 38 in each intervention group.
RESULTS
Participants
A total of 100 participants were randomly allocated to morning exercise (AMEx; n = 40), evening exercise (PMEx; n = 40), or wait‐list control (CON; n = 20) groups. A participant flow diagram is presented in Figure 1. A total of 82 participants completed the intervention. By the final assessment (6‐month follow‐up), 45 participants had withdrawn from the study. Reasons for dropout were due to personal, work, or family reasons (n = 12); being unable to make the time commitment (n = 6); medical reasons (n = 4); lost interest (n = 4); or being lost to follow‐up (n = 19). Participants were recruited on a rolling basis from June 2016 to May 2017. Follow‐up testing was completed in April 2018.
FIGURE 1.
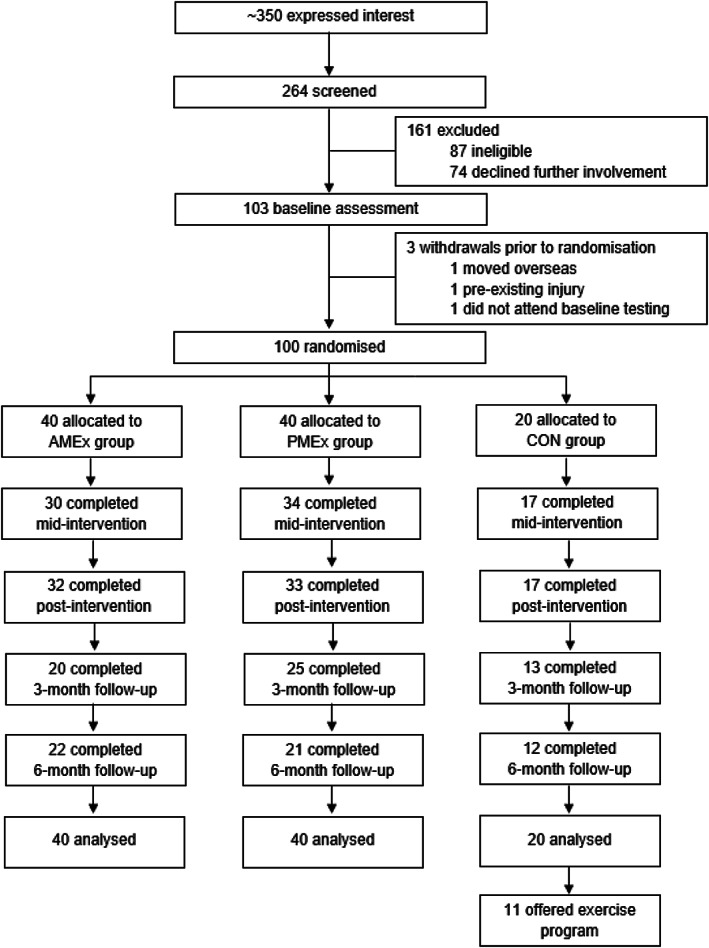
CONSORT (Consolidated Standards of Reporting Trials) flow diagram of participant progression through the study. AMEx, morning exercise; CON, control; PMEx, evening exercise
The sociodemographic characteristics of enrolled participants (Table 2) were typical of studies using volunteer recruitment and convenience sampling [39]; the sample consisted mainly of middle‐aged and socioeconomically advantaged females. Regarding chronotype, most (46%) participants were characterized as neither types, 30% were considered morning types, and 23% as evening types. The distribution of chronotype by group is presented in Table 2.
TABLE 2.
Baseline anthropometric and sociodemographic characteristics
| AMEx | PMEx | CON | |
|---|---|---|---|
| n | 40 | 40 | 20 |
| Female (%) | 73 | 75 | 85 |
| Age, ya | 41 (12) | 38 (11) | 38 (10) |
| Weight, kga | 88.47 (11.6) | 90.86 (18.6) | 84.80 (12.6) |
| BMI, kg·m−2a | 31.06 (4.3) | 31.99 (5.9) | 29.29 (3.6) |
| Education level (n) | |||
| Did not complete school | 1 | 1 | 1 |
| High school | 1 | 3 | 0 |
| Vocational qualification | 5 | 4 | 1 |
| University degree | 33 | 32 | 18 |
| Employment status (n) | |||
| Full‐time | 31 | 32 | 14 |
| Part‐time | 6 | 2 | 5 |
| Casual | 1 | 5 | 0 |
| Retired/unemployed | 2 | 1 | 1 |
| Marital status (n) | |||
| Married/de facto | 30 | 26 | 13 |
| Single/widowed | 10 | 14 | 7 |
| Dependents (n) | |||
| Yes | 18 | 14 | 9 |
| No | 22 | 26 | 11 |
Abbreviations: AMEx, morning exercise; CON, control; PMEx, evening exercise.
aData are presented as mean (standard deviation).
Intervention characteristics
There were no significant between‐group differences for measures of compliance, adherence, exercise‐induced energy expenditure, ratings of perceived exertion, or enjoyment (all p > 0.05; Table 3). There was a statistically significant group × time interaction of RPE (F[2] = 3.92, p = 0.02). Simple main effects analysis showed that RPE significantly increased during the exercise sessions (from warm‐up to mid‐ and end‐session) in both groups, but there were no differences between AMEx and PMEx.
TABLE 3.
Characteristics specific to exercise sessions
| AMEx | PMEx | p value a | |
|---|---|---|---|
| Exercise session compliance | |||
| n (attended)/n (prescribed) | 31/42 | 33/42 | |
| % | 74 (28) | 78 (20) | 0.41 |
| Intervention adherence (intended dose 250 min·wk−1) | |||
| min·wk−1 | 168 (76) | 173 (54) | 0.79 |
| % | 67 (30) | 69 (22) | |
| Exercise intensity | |||
| Mean HR (bpm) | 137 (14) | 139 (13) | 0.22 |
| Mean HR (as %HRpeak) | 78 (7) | 77 (7) | 0.09 |
| % V̇O2peak (derived from %HRpeak) | 64 (11) | 64 (10) | 0.09 |
| Warm‐up RPE | 7 (1) | 7 (1) | 0.27 |
| Mid‐session RPE | 13 (1) | 14 (2) | 0.09 |
| End‐session RPE | 14 (2) | 15 (2) | 0.10 |
| Exercise‐induced energy expenditure | |||
| Estimated average energy expenditure per session (kJ) | 1688 (451) | 1645 (440) | 0.14 |
| Enjoyment rating (%) | |||
| Week 1 | 71 (19) | 63 (18) | 0.11 |
| Week 12 | 69 (21) | 65 (18) | 0.54 |
Note: Data presented as mean (standard deviation).
Abbreviation: AMEx, morning exercise; bpm, beats per minute; HR, heart rate; PMEx, evening exercise; RPE, ratings of perceived exertion; %HRpeak, percent of peak heart rate.
Result presented from independent samples t test.
Anthropometry and body composition
Weight change during the intervention period varied from −12.7 to +6.6 kg across the three groups. There was a significant time effect; body mass significantly decreased during the intervention in both AMEx (−2.7 kg, p < 0.001) and PMEx (−3.1 kg, p < 0.001). There was a significant group × time interaction (p = 0.012), but these differences were no longer significant after conducting post hoc analyses (Supporting Information Tables S3 and S4). Some participants in the intervention groups achieved clinically meaningful weight loss (≥ 5% body mass) during the intervention period (AMEx = 19%; PMEx = 33%; CON = 6%). Relative to baseline, participants in AMEx and PMEx continued to lose weight after the intervention, as assessed at 3‐month follow‐up (AMEx, −3.9 kg, p = 0.010; PMEx, −4.5 kg, p < 0.001) and at 6‐month assessment in PMEx only (−4.7 kg, p = 0.003). There were no differences in body mass for CON during the study period (Table 4).
TABLE 4.
Summary of changes in anthropometry and body composition presented by group across the study period
| Outcome | Time | AMEx | PMEx | CON | Group | Time | Group × Time |
|---|---|---|---|---|---|---|---|
| Body mass (kg) | 0.535 | <0.001 | 0.012 | ||||
| Baseline | 88.5 (11.6) | 90.9 (18.6) | 84.8 (12.6) | ||||
| Mid | 87.1 (11.7) | 89.5 (18.5) | 83.6 (13.2) | ||||
| Post | 85.9 (11.4) | 88.8 (19.2) | 84.7 (13.7) | ||||
| 3‐month | 84.6 (11.0) | 86.4 (20.7) | 84.8 (13.3) | ||||
| 6‐month | 86.3 (9.5) | 86.2 (18.3) | 83.8 (13.9) | ||||
| Δ Body mass (kg) | |||||||
| Mid | −1.9 (1.9) | −2.3 (2.3) | −0.1 (1.7) | ||||
| Post | −2.7 (2.5) | −3.1 (3.4) | −0.1 (2.6) | ||||
| 3‐month | −2.0 (3.3) | −2.7 (3.1) | −1.3 (4.2) | ||||
| 6‐month | −1.3 (3.3) | −2.2 (3.8) | −1.4 (5.0) | ||||
| Body fat % | 0.868 | <0.001 | 0.141 | ||||
| Baseline | 41.3 (7.4) | 42.7 (7.3) | 41.6 (6.1) | ||||
| Mid | 41.3 (7.7) | 41.6 (8.0) | 40.7 (6.1) | ||||
| Post | 40.6 (8.1) | 41.6 (7.6) | 41.1 (5.9) | ||||
| 3‐month | 39.6 (8.5) | 41.1 (6.9) | 40.6 (6.4) | ||||
| 6‐month | 39.3 (7.9) | 42.4 (6.9) | 42.4 (6.6) | ||||
| Δ Body fat (%) | |||||||
| Mid | −0.8 (1.1) | −1.1 (1.5) | −0.3 (1.3) | ||||
| Post | −0.7 (1.3) | −1.0 (1.5) | −0.4 (1.6) | ||||
| 3‐month | −0.2 (2.2) | −1.8 (2.1) | −1.2 (2.1) | ||||
| 6‐month | +0.3 (1.8) | +0.1 (2.3) | −0.5 (2.6) | ||||
| Fat mass (kg) | 0.563 | <0.001 | 0.005 | ||||
| Baseline | 36.1 (9.1) | 38.78 (13.0) | 34.96 (8.9) | ||||
| Mid | 35.7 (9.4) | 37.3 (13.1) | 33.8 (9.1) | ||||
| Post | 34.6 (9.5) | 36.9 (12.9) | 34.4 (8.7) | ||||
| 3‐month | 33.3 (9.7) | 35.7 (12.7) | 34.3 (9.0) | ||||
| 6‐month | 33.5 (7.5) | 37.3 (11.8) | 35.4 (9.1) | ||||
| Δ Fat mass (kg) | |||||||
| Mid | −1.3 (1.3) | −1.8 (1.8) | −0.2 (1.4) | ||||
| Post | −1.6 (1.8) | −2.2 (2.2) | −0.4 (2.2) | ||||
| 3‐month | −0.8 (2.6) | −2.5 (2.8) | −1.4 (3.6) | ||||
| 6‐month | 0.0 (2.6) | −0.4 (2.9) | −1.0 (4.3) | ||||
| Fat‐free mass (kg) | 0.693 | 0.016 | 0.135 | ||||
| Baseline | 48.4 (8.0) | 48.3 (8.9) | 46.1 (6.8) | ||||
| Mid | 47.8 (7.9) | 48.6 (9.6) | 46.3 (6.8) | ||||
| Post | 47.6 (7.9) | 48.2 (9.8) | 46.4 (7.6) | ||||
| 3‐month | 47.4 (7.4) | 47.3 (10.0) | 46.9 (6.9) | ||||
| 6‐month | 49.3 (8.5) | 46.9 (8.6) | 45.1 (7.8) | ||||
| Δ Fat‐free mass (kg) | |||||||
| Mid | −0.2 (1.3) | −0.2 (1.9) | +0.2 (1.5) | ||||
| Post | −0.9 (1.2) | −0.8 (2.4) | +0.2 (1.8) | ||||
| 3‐month | −1.2 (2.1) | +0.1 (1.9) | +0.3 (1.4) | ||||
| 6‐month | −0.8 (1.4) | −1.1 (1.9) | −0.1 (1.7) |
Note: Data presented as mean (standard deviation). Significant differences are indicated in bold.
Abbreviations: AMEX, morning exercise; CON, control; PMEx, evening exercise.
There were no significant group × time interactions for change in per cent body fat (p = 0.141) or fat‐free mass (p = 0.135). There was a significant group × time interaction for change in fat mass (p = 0.005), but these differences were no longer significant after conducting post hoc analyses (Supporting Information Tables S3 and S4). There were changes in body composition within the intervention groups; both fat mass and fat‐free mass significantly decreased in AMEx and PMEx at 12 weeks (relative to baseline). Subsequently, there was a significant reduction in body fat percentage in AMEx (−0.7%, p = 0.005) and PMEx (−1.1%, p < 0.001) during the intervention. Percent body fat continued to decline in PMEx after the intervention at 3‐month follow‐up and was significantly different from baseline (−1.6%, p < 0.001). There were no significant changes in fat mass or fat‐free mass in CON, but there was a reduction in percent body fat at the 3‐month assessment (−0.9%, p = 0.045). The magnitude of change in body composition, relative to baseline, across the study period is illustrated in Figure 2.
FIGURE 2.
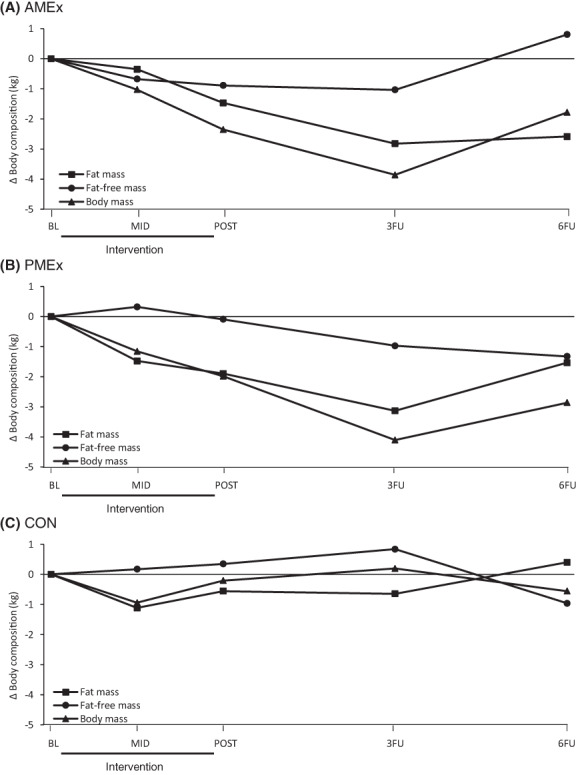
Magnitude of change in body composition across the study period, relative to baseline: (A) morning exercise group (AMEx); (B) evening exercise group (PMEx); and (C) control group (CON). Change in body mass calculated as the sum of change in fat mass and fat‐free mass, measured by dual‐energy x‐ray absorptiometry. BL, baseline; MID, mid‐intervention; POST, post‐intervention; 3FU, 3‐month follow‐up; 6FU, 6‐month follow‐up
Cardiometabolic health
One participant (PMEx) was unable to tolerate the mouthpiece fitting to facilitate the measurement of V̇O2peak, and as such, they were excluded from the analysis of cardiorespiratory fitness. Changes in cardiorespiratory fitness during the intervention varied from −6.4 to +15.1 mL·kg−1·min−1. Most participants in the intervention groups achieved clinically meaningful improvements (≥3.5 mL·kg−1·min−1) [40] in V̇O2peak (AMEx = 69%; PMEx = 75%; CON = 6%) following the intervention. There was a significant group × time interaction for change in V̇O2peak (p = 0.002; Table 5). V̇O2peak significantly increased from baseline in both intervention groups, and these changes were different from CON (AMEx, +4.7 mL·kg−1·min−1, p = 0.034; PMEx, +4.2 mL·kg−1·min−1, p = 0.045). There was no significant difference in the magnitude of improvement in V̇O2peak between AMEx and PMEx (p = 0.820). V̇O2peak values were significantly different from baseline at 3‐month follow‐up in both intervention groups and at 6‐month follow‐up for AMEx. There were no differences in V̇O2peak for CON during the study period (Supporting Information Tables S5 and S6).
TABLE 5.
Summary of changes in cardiometabolic health outcomes presented by group across the study period
| Outcome | Time | AMEx | PMEx | CON | Group | Time | Group × Time |
|---|---|---|---|---|---|---|---|
| V̇O2peak (mL·kg−1·min−1) | 0.42 | <0.001 | 0.002 | ||||
| Baseline | 29.10 (6.3) | 28.01 (7.5) | 28.59 (6.7) | ||||
| Post | 33.61 (8.2) | 33.21 (8.6) | 29.91 (6.4) | ||||
| 3‐month | 34.36 (7.0) | 32.79 (8.8) | 28.06 (7.7) | ||||
| 6‐month | 34.88 (8.5) | 31.15 (9.5) | 29.36 (6.5) | ||||
| Δ V̇O2peak | |||||||
| Post | +4.8 (3.9) | +5.4 (3.9) | +0.5 (2.3) | ||||
| 3‐month | +3.0 (4.9) | +3.4 (2.5) | −0.2 (3.3) | ||||
| 6‐month | +2.7 (6.2) | +1.7 (5.1) | +0.4 (3.4) | ||||
| BGL (mmol·L−1) | 0.679 | 0.085 | 0.410 | ||||
| Baseline | 5.53 (0.5) | 5.73 (0.5) | 5.44 (0.4) | ||||
| Post | 5.48 (0.6) | 5.56 (0.5) | 5.48 (0.4) | ||||
| Δ BGL | −0.1 (0.5) | −0.2 (0.5) | −0.1 (0.5) | ||||
| TC (mmol·L−1) | 0.205 | 0.051 | 0.023 | ||||
| Baseline | 4.94 (0.9) | 4.58 (0.8) | 5.09 (1.0) | ||||
| Post | 4.13 (1.2) | 4.72 (1.0) | 5.12 (0.8) | ||||
| Δ TC | −0.8 (1.5) | +0.0 (0.9) | −0.4 (1.2) | ||||
| HDL (mmol·L−1) | 0.167 | 0.585 | 0.874 | ||||
| Baseline | 1.43 (0.5) | 1.31 (0.4) | 1.42 (0.4) | ||||
| Post | 1.40 (0.4) | 1.32 (0.4) | 1.48 (0.4) | ||||
| Δ HDL | +0.1 (0.3) | +0.05 (0.2) | +0.0 (0.2) | ||||
| LDL (mmol·L−1) | 0.381 | 0.439 | 0.510 | ||||
| Baseline | 2.89 (0.8) | 2.65 (0.7) | 2.94 (0.7) | ||||
| Post | 2.56 (0.7) | 2.80 (0.9) | 2.97 (0.8) | ||||
| Δ LDL | −0.2 (0.6) | +0.2 (0.7) | −0.1 (0.5) | ||||
| TC:HDL | 0.902 | 0.145 | 0.654 | ||||
| Baseline | 3.79 (1.2) | 3.72 (1.0) | 3.84 (1.2) | ||||
| Post | 3.53 (1.0) | 3.92 (1.1) | 3.67 (1.0) | ||||
| Δ TC:HDL | −0.2 (0.6) | +0.1 (0.8) | −0.2 (0.5) | ||||
| Triglycerides (mmol·L−1) | 0.402 | 0.465 | 0.907 | ||||
| Baseline | 1.32 (0.7) | 1.36 (0.6) | 1.60 (0.7) | ||||
| Post | 1.32 (0.7) | 1.38 (0.8) | 1.49 (0.7) | ||||
| Δ Triglycerides | −0.0 (0.7) | +0.0 (0.8) | −0.1 (0.6) | ||||
| SBP (mmHg) | 0.526 | 0.006 | 0.249 | ||||
| Baseline | 120.3 (16) | 125.8 (16) | 119.7 (20) | ||||
| Post | 118.2 (15) | 120.2 (10) | 118.0 (15) | ||||
| Δ SBP | −3.7 (8.2) | −6.7 (9.3) | −2.0 (10.9) | ||||
| DBP (mmHg) | 0.984 | 0.001 | 0.076 | ||||
| Baseline | 84.8 (11) | 85.1 (11) | 83.1 (12) | ||||
| Post | 81.3 (11) | 81.5 (9) | 83.1 (10) | ||||
| Δ DBP | −3.4 (5.2) | −5.0 (5.5) | +1.2 (4.1) |
Note: Data presented as mean (standard deviation). Significant differences are indicated in bold.
Abbreviations: AMEX, morning exercise; BGL, blood glucose; CON, control; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PMEx, evening exercise; SBP, systolic blood pressure; TC, total cholesterol; V̇O2peak, peak oxygen uptake.
Diastolic blood pressure significantly decreased after the intervention in both intervention groups (AMEx, p = 0.002; PMEx, p < 0.001) but not in CON (p = 0.952). In PMEx, systolic blood pressure also decreased significantly (p < 0.001). There were no between‐group differences for either systolic or diastolic blood pressure. Blood biochemistry results showed some modest improvements and were similar between groups and over time (Table 5). At 12 weeks, fasting blood glucose levels decreased in PMEx (from baseline), and total cholesterol decreased in AMEx and was significantly different from CON. There were no within‐group changes or between‐group differences for measures of HDL, LDL, total cholesterol:HDL, or triglycerides.
Components of energy balance
Because of intolerance to RMR testing (feelings of claustrophobia; n = 4) and equipment malfunction (n = 8), baseline data for RMR were available for 88 individuals. There were no significant between‐ or within‐group differences (group or time effects) or group × time interactions (Table 6). Relative to baseline values, moderate/vigorous physical activity increased during the intervention; significant increases in moderate/vigorous physical activity were observed in AMEx at 6 and 12 weeks (by 45 and 19 min·d−1, respectively) and at 6 weeks for PMEx (+25 min·d−1). These differences were no longer significant at follow‐up and were approaching baseline levels. There were no significant changes over time for CON.
TABLE 6.
Summary of changes in components of energy balance presented by group across the study period
| Outcome | Time | AMEx | PMEx | CON | Group | Time | Group × Time |
|---|---|---|---|---|---|---|---|
| RMR (kJ·d−1) | 0.900 | 0.990 | 0.951 | ||||
| Baseline | 6305 (845) | 6403 (1104) | 6285 (992) | ||||
| Post | 6257 (883) | 6457 (1043) | 6167 (1225) | ||||
| Δ RMR | +45 (525) | −32 (715) | +2 (691) | ||||
| MVPA (min·d−1) | 0.334 | 0.001 | 0.793 | ||||
| Baseline | 95 (38) | 102 (47) | 88 (32) | ||||
| Mid | 140 (37) | 127 (45) | 100 (35) | ||||
| Post | 114 (46) | 110 (42) | 103 (30) | ||||
| Δ MVPA | |||||||
| Mid | +28.4 (28.6) | +25.0 (28.6) | +17.4 (56) | ||||
| Post | +23.1 (55.4) | +9.8 (25.7) | +16.3 (24.6) | ||||
| Light PA (min·d−1) | 0.459 | 0.263 | 0.698 | ||||
| Baseline | 229 (74) | 225 (81) | 238 (89) | ||||
| Mid | 234 (30) | 238 (100) | 230 (62) | ||||
| Post | 233 (94) | 209 (65) | 256 (94) | ||||
| Δ Light PA | |||||||
| Mid | +2.2 (48.5) | +19.0 (66.6) | −28.4 (92.2) | ||||
| Post | +7.8 (114.2) | −20.8 (60.9) | +35.0 (103.6) | ||||
| Sedentary (min·d−1) | 0.497 | 0.148 | 0.862 | ||||
| Baseline | 625 (153) | 635 (108) | 608 (100) | ||||
| Mid | 608 (78) | 602 (108) | 639 (73) | ||||
| Post | 603 (109) | 637 (88) | 628 (209) | ||||
| Δ Sedentary activity | |||||||
| Mid | −11.0 (77.5) | −56.5 (96.4) | +57.2 (137.6) | ||||
| Post | −25.9 (115.6) | −4.7 (81.3) | +1.8 (185.6) | ||||
| Energy (kJ) | 0.024 | <0.001 | 0.016 | ||||
| Baseline | 9028 (3010) | 9471 (3101) | 9974 (2943) | ||||
| Mid | 7136 (2299) | 7723 (2803) | 7200 (2435) | ||||
| Post | 6464 (2059) | 7237 (2696) | 10,312 (3183) | ||||
| Protein (g) | 0.113 | 0.010 | 0.128 | ||||
| Baseline | 93 (31) | 97 (34) | 91 (26) | ||||
| Mid | 75 (27) | 82 (27) | 76 (33) | ||||
| Post | 70 (22) | 83 (31) | 102 (40) | ||||
| Total fat (g) | 0.027 | <0.001 | 0.006 | ||||
| Baseline | 92 (38) | 95 (37) | 102 (34) | ||||
| Mid | 71 (34) | 81 (37) | 65 (27) | ||||
| Post | 60 (29) | 68 (34) | 111 (39) | ||||
| Total carbohydrate (g) | 0.182 | <0.001 | 0.327 | ||||
| Baseline | 222 (89) | 234 (91) | 245 (103) | ||||
| Mid | 175 (74) | 186 (75) | 174 (87) | ||||
| Post | 160 (44) | 192 (71) | 230 (62) |
Note: Data presented as mean (standard deviation). Significant differences are indicated in bold.
Abbreviations: AMEX, morning exercise; CON, control; MVPA, moderate/vigorous physical activity; PA, physical activity; PMEx, evening exercise; RMR, resting metabolic rate.
There was a significant group, time, and group × time interaction for energy intake; both AMEx and PMEx reported reductions in total energy intake, which were also significantly different from CON (AMEx, −3974 kJ, p < 0.001; PMEx, −3165 kJ, p = 0.001). For AMEx, total fat and protein intakes measured at mid‐ and post‐intervention had also declined significantly from baseline. For PMEx, a significant decrease in protein was observed at mid‐intervention and in total fat at post‐intervention, as compared with baseline. Significant changes observed in the CON group at mid‐intervention all returned to baseline levels at post‐intervention assessment (Supporting Information Tables S7 and S8).
DISCUSSION
The purpose of this study was to investigate the influence of time of day of exercise on weight loss, cardiometabolic health risk factors, and components of energy balance in a sufficiently powered sample of inactive adults with overweight and obesity. After rigorous analyses, we found no compelling evidence to support or encourage exercise exclusively at a particular time of day for weight loss. We did, however, observe improvements in cardiometabolic health, such as weight reduction and increased cardiorespiratory fitness, increased levels of physical activity, and positive changes to dietary intake in both intervention groups. Following the intervention, both exercise groups continued to lose weight, and the improvements in cardiorespiratory fitness were sustained.
Studies comparing the effect of morning and evening exercise on cardiometabolic health and components of energy balance are limited. Three chronic experimental studies [4, 14, 15] have explored the influence of time of day of exercise on weight loss and findings are mixed. After 6 weeks of supervised aerobic exercise training 3 d·wk−1 with a target heart rate on the ventilatory threshold, Alizadeh et al. found participants who were randomized to morning training lost significantly more weight than those randomized to evening training (−1.55 vs. −0.6 kg, respectively). Similarly, in their secondary analysis, Willis et al. observed significantly greater weight loss in the self‐selected “early‐exercise” group (−7.2%) compared with “late‐exercisers” (−2.1%) in their 10‐month trial. In contrast, in their 3‐month partially supervised walking intervention, Di Blasio et al. found evening exercisers reduced fat mass to a greater extent than morning exercisers (−1.71 kg vs. −0.24 kg, respectively), despite similar rates of adherence between groups (83% vs. 87% for morning and evening, respectively) [4]. Participants (n = 29) were asked to walk for 50 minutes, 4 d·wk−1, at an intensity of 55% heart rate reserve in their chosen “walking hour” either in the morning between 07:00 and 09:00 (after breakfast), or evening (18:00–20:00, after dinner). Based on these preliminary and conflicting findings, there is insufficient evidence to encourage exercise participation at a specific time of day to elicit greater reductions in weight loss.
Body weight regulation is multifactorial and complex. In this study, there was a mean reduction in total energy intake of approximately 2000 kJ·d−1 during the intervention in conjunction with the exercise‐induced energy expenditure (approximately 1600 kJ per session; data not shown) and no significant changes in RMR or non‐exercise physical activity. This energy deficit of over 3500 kJ·d−1 could be considered clinically meaningful. Health professionals encourage patients to make modest lifestyle changes to support their weight‐loss endeavors. Previously, guidance to create an energy deficit of 3500 kcal to lose 1 pound of body weight has been suggested, the equivalent of a daily energy deficit of approximately 2100 kJ for 0.5 kg of weight loss per week. However, the “3500 kcal per pound” rule is often misapplied to predict weight change, falsely giving the impression and expectations of a linear change in body weight [41]. Indeed, based on these data, an average overall weight loss of more than 6 kg should be expected over the course of the 12‐week intervention; however, we observed only modest changes in the intervention groups (AMEx, −2.6; PMEx, −2.1 kg). The difference between expected and actual weight loss is well‐documented in the literature and may be related to other physiological or behavioral compensatory adaptive responses to oppose the energy deficit [42]. However, we found no evidence of compensatory adjustments to the exercise stimulus that could explain the discrepancy based on the measures we collected.
Despite the strengths of this research, including the randomized design and sufficiently powered sample size, some limitations must be acknowledged. The rate of attrition during the intervention period (18%) was typical (and acceptable) of exercise trials. However, by the 6‐month follow‐up, the rate of attrition was high (45%) and may have introduced bias to the results. Although the measurement tools used in our study were purposefully selected, and in some cases, gold‐standard, their limitations must be considered, especially in a free‐living environment. The difference between expected and actual weight loss in this study may be due to inaccuracies associated with self‐report energy intake. In their review, Hill and Davies found individuals with obesity underreport their usual dietary intake by an average of 41% (range 25%–50%). To assess dietary intake, we used 24‐hour multiple‐pass recalls on two separate occasions. Using a multiple‐pass 24‐hour recall on multiple days, compared with a single day, has been shown to reduce underreporting from 30% to 11% [43]. Risk of underreporting could have been even further reduced with the inclusion of a third day [43]; however, this was not feasible due to resource constraints in our study and also measurement burden. Finally, measures were only assessed on limited occasions during the intervention and study period, so it is unknown how individuals may have adjusted their energy intake and expenditure outside of the assessment periods. For example, it is possible that participants were motivated by the accelerometers to exercise more [44], or make short‐term dietary changes, enabling them to report a more favorable (“socially desirable”) dietary intakes [45].
CONCLUSION
Participation in regular exercise has many well‐documented health benefits, and experts agree that exercise should be incorporated as part of individuals' daily routine. The findings from the research support the message of Dr. Michael Joyner of the Mayo Clinic who suggests, “the ‘do something’ message is far more important than the ‘do something at a specific time of the day’ message.” Although it was not the purpose of our study to specifically investigate the compensatory response to the exercise‐induced energy deficit, components of energy balance were measured in an attempt to explain any observed differences in cardiometabolic health risk factors. There were no statistically significant differences in cardiometabolic health risk factors between AMEx and PMEx; however, there was a greater proportion of participants randomized to PMEx who achieved clinically meaningful weight loss (33%) and clinically meaningful improvements in V̇O2peak (75%), compared with participants randomized to AMEx (19% and 69%, respectively). This finding warrants further examination. Trial descriptors of exercise interventions are often suboptimal and incompletely described in study reports. Consistent reporting of time of day of exercise interventions among high‐quality studies would significantly contribute to the literature and provide critical insight into the relative importance (or lack thereof) of prescribing exercise at a particular time of day.
AUTHOR CONTRIBUTIONS
Paige G. Brooker was responsible for conceptualization and design of the study, the acquisition, analysis and interpretation of data, and manuscript writing. Sjaan R. Gomersall, Neil A. King, and Michael D. Leveritt contributed to design of the study, data interpretation, and critical revision of the manuscript.
FUNDING INFORMATION
PGB was supported by an Australian Government Research Training Program scholarship (living stipend).
CONFLICT OF INTEREST
The authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
Australian New Zealand Clinical Trials Registry ACTRN12616000457448p.
Supporting information
TABLE S1 Behavior change techniques incorporated into the intervention
TABLE S2 Overview of outcome measures and administration of measurement tools across the study period
TABLE S3 Anthropometric and body composition changes across the intervention: within‐group changes
TABLE S4 Anthropometric and body composition measures across the intervention: between‐group differences
TABLE S5 Cardiorespiratory fitness changes across the intervention: within‐group changes
TABLE S6 Cardiorespiratory fitness changes across the intervention: between‐group differences
TABLE S7 Changes in components of energy balance across the intervention: within‐group changes
TABLE S8 Changes in components of energy balance across the intervention: between‐group differences
ACKNOWLEDGMENTS
The data that support the findings of this study are available from the corresponding author on reasonable request. Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Brooker PG, Gomersall SR, King NA, Leveritt MD. The efficacy of morning versus evening exercise for weight loss: A randomized controlled trial. Obesity (Silver Spring). 2023;31(1):83‐95. doi: 10.1002/oby.23605
REFERENCES
- 1. Australian Institute of Health and Welfare . Overweight and obesity. Updated August 16, 2022. Accessed September 10, 2021. http://www.aihw.gov.au/overweight-and-obesity/
- 2. Hand GA, Blair SN. Energy flux and its role in obesity and metabolic disease. US Endocrinol. 2014;10(1):59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459‐471. [DOI] [PubMed] [Google Scholar]
- 4. Di Blasio A, Di Donato F, Mastrodicasa M, et al. Effects of the time of day of walking on dietary behaviour, body composition and aerobic fitness in post‐menopausal women. J Sports Med Phys Fitness. 2010;50:196‐201. [PubMed] [Google Scholar]
- 5. Brooker PG, Jung ME, Kelly‐Bowers D, et al. Does the time‐of‐day of exercise influence the total volume of exercise? A cross‐sectional analysis of objectively monitored physical activity among active individuals. J Phys Act Health. 2021;18(9):1029‐1036. [DOI] [PubMed] [Google Scholar]
- 6. Barisic A, Leatherdale ST, Kreiger N. Importance of frequency, intensity, time and type (FITT) in physical activity assessment for epidemiological research. Can J Public Health. 2011;102:174‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thun E, Bjorvatn B, Flo E, Harris A, Pallesen S. Sleep, circadian rhythms, and athletic performance. Sleep Med Rev. 2015;23:1‐9. [DOI] [PubMed] [Google Scholar]
- 8. Froy O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol. 2007;28:61‐71. [DOI] [PubMed] [Google Scholar]
- 9. Slade SC, Dionne CE, Underwood M, et al. Consensus on Exercise Reporting Template (CERT): modified Delphi study. Phys Ther. 2016;96:1514‐1524. [DOI] [PubMed] [Google Scholar]
- 10. Kaushal N, Rhodes RE. Exercise habit formation in new gym members: a longitudinal study. J Behav Med. 2015;38:652‐663. [DOI] [PubMed] [Google Scholar]
- 11. Schumacher LM, Thomas JG, Raynor HA, et al. Relationship of consistency in timing of exercise performance and exercise levels among successful weight loss maintainers. Obesity (Silver Spring). 2019;27:1285‐1291. [DOI] [PubMed] [Google Scholar]
- 12. Brooker PG, Gomersall SR, King NA, Leveritt MD. The feasibility and acceptability of morning versus evening exercise for overweight and obese adults: a randomized controlled trial. Contemp Clin Trials Commun. 2019;14:100320. doi:10.1016/j.conctc.2019.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Creasy SA, Wayland L, Panter SL, et al. Effect of morning and evening exercise on energy balance: a pilot study. Nutrients. 2022;14:816. doi:10.3390/nu14040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alizadeh Z, Younespour S, Tabesh MR, Haghravan S. Comparison between the effect of 6 weeks of morning or evening aerobic exercise on appetite and anthropometric indices: a randomized controlled trial. Clin Obes. 2017;7:157‐165. [DOI] [PubMed] [Google Scholar]
- 15. Willis EA, Creasy SA, Honas JJ, Melanson EL, Donnelly JE. The effects of exercise session timing on weight loss and components of energy balance: midwest exercise trial 2. Int J Obes (Lond). 2020;44:114‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teo SYM, Kanaley JA, Guelfi KJ, Marston KJ, Fairchild TJ. The effect of exercise timing on glycemic control: a randomized clinical trial. Med Sci Sports Exerc. 2020;52:323‐334. [DOI] [PubMed] [Google Scholar]
- 17. Burn N, Norton LH, Drummond C, Norton KI. Changes in physical activity behaviour and health risk factors following a randomised controlled pilot workplace exercise intervention. Am J Public Health. 2017;4:189‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teo SYM, Kanaley JA, Guelfi KJ, Dimmock JA, Jackson B, Fairchild TJ. Effects of diurnal exercise timing on appetite, energy intake and body composition: a parallel randomized trial. Appetite. 2021;167:105600. doi:10.1016/j.appet.2021.105600. [DOI] [PubMed] [Google Scholar]
- 19. Bilski J, Jaworek J, Pokorski J, et al. Effects of time of day and the Wingate test on appetite perceptions, food intake and plasma levels of adipokines. J Physiol Pharmacol. 2016;67:667‐676. [PubMed] [Google Scholar]
- 20. Exercise and Sports Science Australia . Adult Pre‐Exercise Screening System (APSS). Accessed October 10, 2022. https://www.essa.org.au/pre-exercise-screening-system
- 21. Swain DP, Abernathy KS, Smith CS, Lee SJ, Bunn SA. Target heart rates for the development of cardiorespiratory fitness. Med Sci Sports Exerc. 1994;26:112‐116. [PubMed] [Google Scholar]
- 22. Borg G. An Introduction to Borg's RPE‐Scale. Mouvement Publications; 1985. [Google Scholar]
- 23. Horne JA, Ostberg O. A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97‐110. [PubMed] [Google Scholar]
- 24. Taillard J, Philip P, Chastang JF, Bioulac B. Validation of horne and Ostberg morningness‐eveningness questionnaire in a middle‐aged population of French workers. J Biol Rhythms. 2004;19:76‐86. [DOI] [PubMed] [Google Scholar]
- 25. Norton LH, Norton KI, Lewis N, Dollman J. A comparison of two short‐term intensive physical activity interventions: methodological considerations. Int J Behav Nutr Phys Act. 2011;8:133. doi:10.1186/1479‐5868‐8‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart AD, Marfell‐Jones M, Olds T, de Ridder H. International Standards for Anthropometric Assessment. International Society for the Advancement of Kinanthropometry; 2011.
- 27. Robergs RA, Dwyer D, Astorino T. Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Med. 2010;40:95‐111. [DOI] [PubMed] [Google Scholar]
- 28. Compher C, Frankenfield D, Keim N, Roth‐Yousey L. Evidence analysis working, best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881‐903. [DOI] [PubMed] [Google Scholar]
- 29. van Hees VT, Renström F, Wright A, et al. Estimation of daily energy expenditure in pregnant and non‐pregnant women using a wrist‐worn tri‐axial accelerometer. PLoS One. 2011;6:e22922 doi:10.1371/journal.pone.0022922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA accelerometer. Med Sci Sports Exerc. 2011;43:1085‐1093. [DOI] [PubMed] [Google Scholar]
- 31. de Boer MR, Waterlander WE, Kuijper LDJ, Steenhuis IHM, Twisk JWR. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. 2015;12:4. doi:10.1186/s12966‐015‐0162‐z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shek DTL, Ma CMS. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. ScientificWorldJournal. 2011;11:42‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hey SP, Kimmelman J. The questionable use of unequal allocation in confirmatory trials. Neurology. 2014;82:77‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meinert CL, Tonascia S. Clinical Trials: Design, Conduct, and Analysis. Oxford University Press; 1986. [Google Scholar]
- 35. Dibao‐Dina C, Caille A, Sautenet B, Chazelle E, Giraudeau B. Rationale for unequal randomization in clinical trials is rarely reported: a systematic review. J Clin Epidemiol. 2014;67:1070‐1075. [DOI] [PubMed] [Google Scholar]
- 36. Mutsaerts MAQ, Kuchenbecker WKH, Mol BW, Land JA, Hoek A. Dropout is a problem in lifestyle intervention programs for overweight and obese infertile women: a systematic review. Hum Reprod. 2013;28:979‐986. [DOI] [PubMed] [Google Scholar]
- 37. Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl IH, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281:327‐334. [DOI] [PubMed] [Google Scholar]
- 38. Groeneveld IF, Proper KI, van der Beek AJ, Hildebrandt VH, van Mechelen W. Factors associated with non‐participation and drop‐out in a lifestyle intervention for workers with an elevated risk of cardiovascular disease. Int J Behav Nutr Phys Act. 2009;6:80. doi: 10.1186/1479-5868-6-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ganguli M, Lytle ME, Reynolds MD, Dodge HH. Random versus volunteer selection for a community‐based study. J Gerontol A Biol Sci Med Sci. 1998;53:M39‐M46. [DOI] [PubMed] [Google Scholar]
- 40. Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024‐2035. [DOI] [PubMed] [Google Scholar]
- 41. Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95:989‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise‐induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc. 2013;45:1600‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma Y, Olendzki BC, Pagoto SL, et al. Number of 24‐hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19:553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coughlin SS, Stewart J. Use of consumer wearable devices to promote physical activity: a review of health intervention studies. J Environ Sci Health. 2016;2(6). doi: 10.15436/2378-6841.16.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shim J‐S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. doi:10.4178/epih/e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Behavior change techniques incorporated into the intervention
TABLE S2 Overview of outcome measures and administration of measurement tools across the study period
TABLE S3 Anthropometric and body composition changes across the intervention: within‐group changes
TABLE S4 Anthropometric and body composition measures across the intervention: between‐group differences
TABLE S5 Cardiorespiratory fitness changes across the intervention: within‐group changes
TABLE S6 Cardiorespiratory fitness changes across the intervention: between‐group differences
TABLE S7 Changes in components of energy balance across the intervention: within‐group changes
TABLE S8 Changes in components of energy balance across the intervention: between‐group differences


