Abstract
Transcatheter electrosurgery describes the ability to cut and traverse tissue, at a distance, without an open surgical field and is possible using either purpose-built or off-the-shelf devices. Tissue traversal requires focused delivery of radiofrequency energy to a guidewire tip. Initially employed to cross atretic pulmonary valves, tissue traversal has enabled transcaval aortic access, recanalization of arterial and venous occlusions, trans-septal access and many other techniques. To cut tissue, the selectively denuded inner curvature of a kinked guidewire (the “Flying V”) or a single loop snare is energized during traction. Adjunctive techniques may compliment or enable contemporary transcatheter procedures, whereas myocardial slicing or excision of ectopic masses may offer definitive therapy. In this contemporary review we discuss the principles of transcatheter electrosurgery, and through exemplary clinical applications highlight the range of therapeutic options offered by this versatile family of procedures.
Keywords: Transcatheter electrosurgery, Transcatheter aortic valve replacement, Transcatheter mitral valve replacement, Left ventricular outflow obstruction, BASILICA, LAMPOON, SESAME, Catheter-Based coronary and Valvular Interventions, Aortic Valve Replacement/Transcatheter Aortic Valve Implantation
INTRODUCTION
Transcatheter electrosurgery is a newer component of the interventional catheterization armamentarium. Remarkably, electrosurgery can be applied not just in an open operative field insulated by air, but even within blood spaces, which short-circuit intended electrical pathways. Despite the challenge, targeted tissue vaporization is accomplished with simple bedside modification of interventional guidewires and other commercially available electrically conductive equipment. Using these tools, operators can traverse blood vessels and cardiac chambers, and can lacerate heart valve leaflets and even heart muscle without surgery. In this review we discuss the principles of transcatheter electrosurgery and exemplary clinical applications[figure 1].
Fig 1.
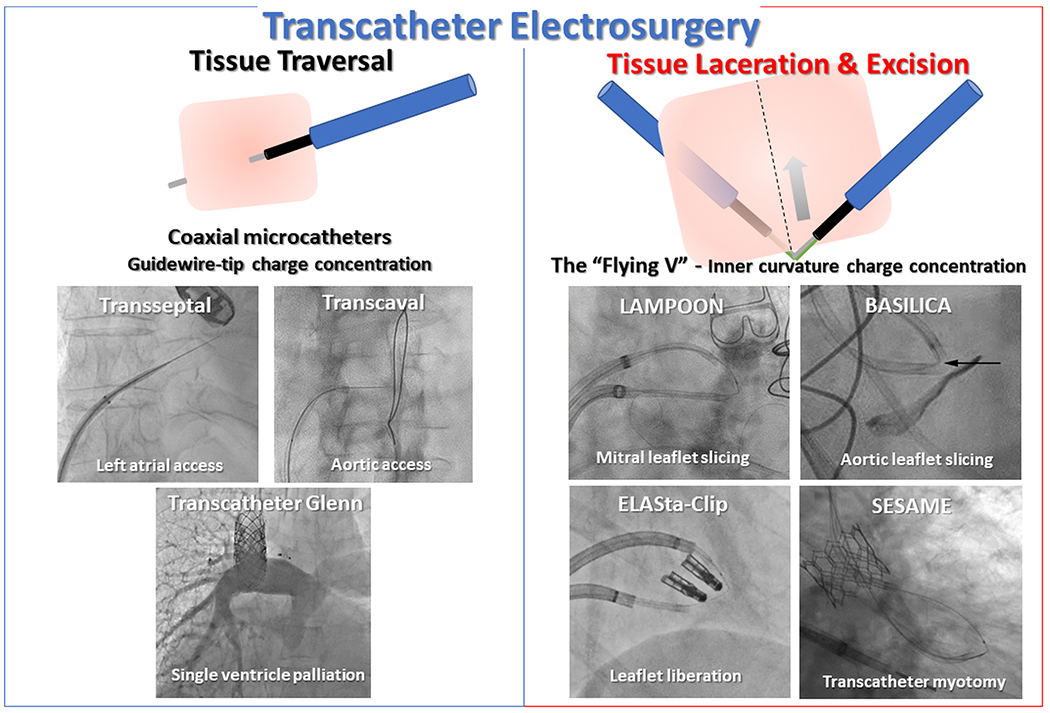
Clinical applications of transcatheter electrosurgery.
Electrosurgical Principles
Basic physics of electrosurgery
Electrosurgery refers to delivery of high (‘radio’) frequency (240-470kHz) alternating current to cut or coagulate tissue. Electrosurgery may be performed in monopolar or bipolar1 configurations with monopolar being the most common for transcatheter electrosurgery2. In monopolar mode, current flows in a circuit from a conductive object (an active electrode) to a target tissue, through the patient’s body and back to the electrosurgical radiofrequency generator via a dispersive electrode on the patient’s skin[figure 2]. Equal current flows through the active and dispersive electrodes, but the contact surface area of the active electrode is dramatically smaller and therefore concentrates energy. Tissue adjacent to the active electrode imparts resistance to current flow, converting electrical energy to heat. Continuous radiofrequency energy delivery (100% duty cycle ‘on’ [figure 2B]) causes rapid, focal heating above 100°C at the point of maximum current density, adjacent to the active electrode. Cells vaporize which therefore ‘cuts’ the tissue.
Fig 2. Monopolar electrosurgery circuit and radiofrequncy waveforms.
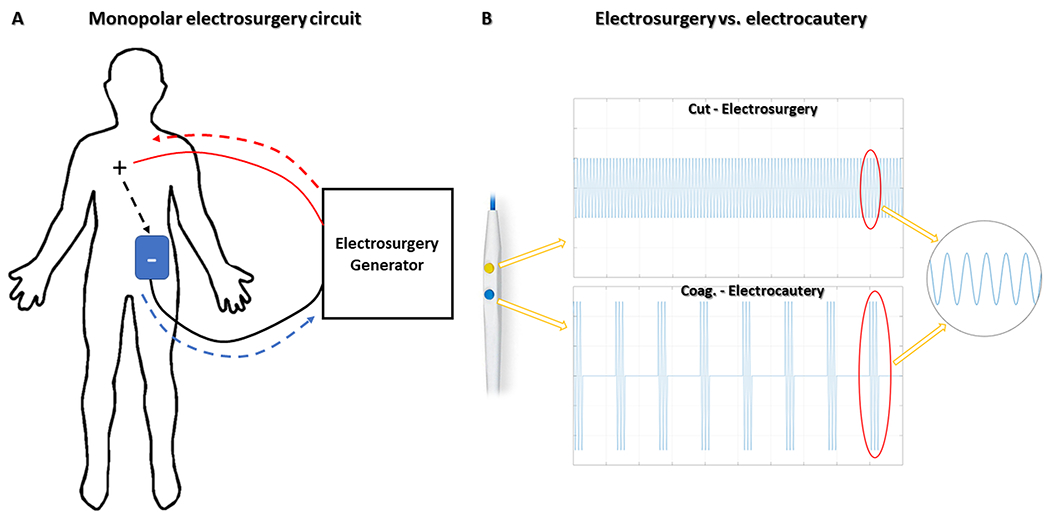
Monopolar electrosurgical circuits (A) consist of an active electrode(+) that receives current from an electrosurgery generator (red interrupted-arrow) and conducts through the body (black interrupted-arrow) to a dispersive electrode(blue patch), placed on the patient’s skin, and thereafter back to the generator (blue interrupted-arrow). (B) Continuous versus intermittent radiofrequency application (“duty-cycle”) creates different electrosurgical effects. Continuous (100% ‘on’) radiofrequency energy (top panel) vaporizes cells and cuts tissue at the point of maximum current density adjacent to the active electrode. In (“low duty cycle”) electrocautery(bottom panel), interrupted radiofrequency energy causes tissue heating, protein denaturation, and blood coagulation. Reprinted from Khan et al. JACC, 20202.
Electrosurgery is distinct from electrocautery, in which tissue is heated to induce coagulation and escharification. Electrocautery employs “coagulation” mode, which relies on interrupted current delivery (low duty cycle ‘on’) that induces heating with attendant denaturation (electrocoagulation), dehydration (electrodessication), or spark-spraying (electrofulguration) without vaporization.
Adaptations for transcatheter electrosurgery
Surgeons traditionally perform electrosurgery using hand-held active electrodes (such as electrosurgery pencils), under direct visualization, and within an insulating medium (air). By contrast, transcatheter electrosurgery vaporizes targets at a distance from the operator, internally and without visualization, and inside conductive blood or tissue media. Conductive metallic guidewires are the most common active transcatheter electrosurgical devices.
Guidewire denudation
Most commercial guidewires have polymer coating, such as polytetrafluoroethylene (PTFE) to improve mechanical properties such as lubricity and biocompatibility. Serendipitously these coatings are electrical insulators. Bedside-modification consists mostly of selectively stripping insulative coating —distally to expose/create the active electrode, and proximally to create contact points with connectors. The exposed proximal contact point is carefully clamped to electrosurgery accessories (such as ‘Bovie” electrosurgery pencils) to minimize energy loss when connecting to electrosurgery generators[Figure 3].
Fig 3. Guidewire modifications that enable transcatheter electrosurgery.
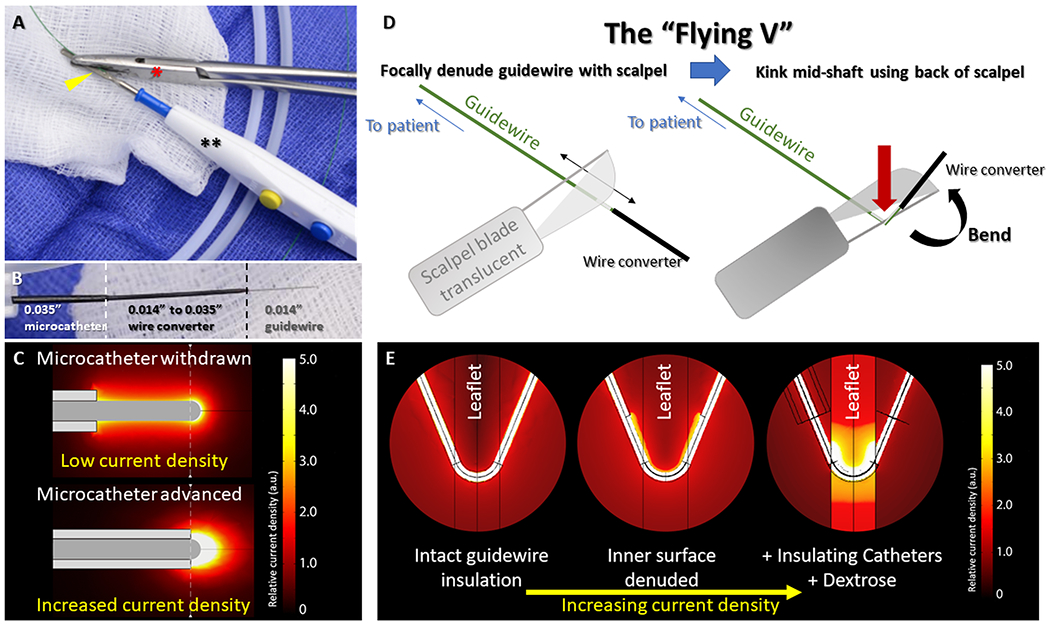
(A) The denuded back-end of a guidewire(yellow arrowhead) is clamped to an electrosurgery pencil(**) by hemostatic forceps(red*). (B) A ‘crossing-system’ for electrosurgical tissue traversal consists of an 0.014” guidewire inside a hubless-locking wire-converter, inside a 0.035” microcatheter. (C) Microcatheters increase current density at the guidewire tip increasing electrosurgical efficiency. Focal denudation and kinking the mid-shaft of the guidewire (D) create the “Flying V”. When placed at the target tissue, inner curvature denudation focuses charge and increases current density (E). Microcatheter insulation and dextrose infusion further enhance charge concentration. Reprinted from Khan et al. JACC, 20202.
Electrosurgical tissue cutting is usually accomplished using the kinked shaft of a guidewire that traverses the tissue, and during traction[Figure 3]. The active electrode is the inner curve of the kinked guidewire shaft where it contacts the target tissue. However, electrosurgical charge tends to concentrate at convexities such as the outer curve of the kinked guidewire shaft. To resist this well-known phenomenon, insulation is selectively denuded from the inner curve and retained along the outer curve in order to concentrate electrosurgical charge at the tissue laceration target. The resulting “Flying-V” configuration[Figure 3, Video S1] self-orients when appropriately positioned across valve leaflets, and assures energy is appropriately targeted.
Coaxial catheter insulation of guidewires
Electrosurgical tissue traversal employs the tips of guidewires as active electrodes. Most unaltered commercial off-the-shelf guidewires have long uninsulated distal metallic tips having excessively large conductive surface area prone to current dispersal and inadequate charge concentration.
To counteract this phenomenon, a fundamental technique of transcatheter electrosurgery is to insulate all-but-the-tip of the guidewire, or all-but-the Flying-V of the guidewire, with a combination of microcatheters and guiding catheters. By exposing only small portions of the metallic guidewire beyond microcatheters and guiding catheters, conductive surfaces are minimized and charge is concentrated[figure 3].
Infusion of non-ionic solution such as dextrose/iodinated contrast
Blood is a highly conductive medium. When performing transcatheter electrosurgery, the active electrode is nearly always in contact with blood, allowing for charge dispersal through undesirable alternative current paths. By infusing a non-ionic solution of dextrose or iodinated contrast simultaneous with the application of radiofrequency current, blood is displaced and charge is concentrated in the target tissue[figure 3]. Blood displacement also minimizes electrode carbonization and local blood thromboembolism.
Clinical Applications
TISSUE TRAVERSAL
Pulmonary atresia
The first reported application of transcatheter electrosurgery was in pediatrics for pulmonary valve atresia with intact ventricular septum, using purpose-built electrosurgery guidewires3. Thereafter, electrosurgery-assisted pulmonary valvulotomy has become widely applied to treat pulmonary atresia with4–6 and without7 intact ventricular septa.
Transcaval aortic access
Transcaval aortic access describes electrosurgical entry to the abdominal aorta from the adjacent inferior vena cava[figure 4]8. First described in 2013 as an alternative access for TAVR in otherwise ineligible patients9, ‘transcaval’ has now been performed in thousands of patients worldwide and in many experienced centers is a routine option for large-bore arterial access to the aorta when iliofemoral arteries are small or diseased.
Fig 4. Clinical applications – Tissue Traversal.
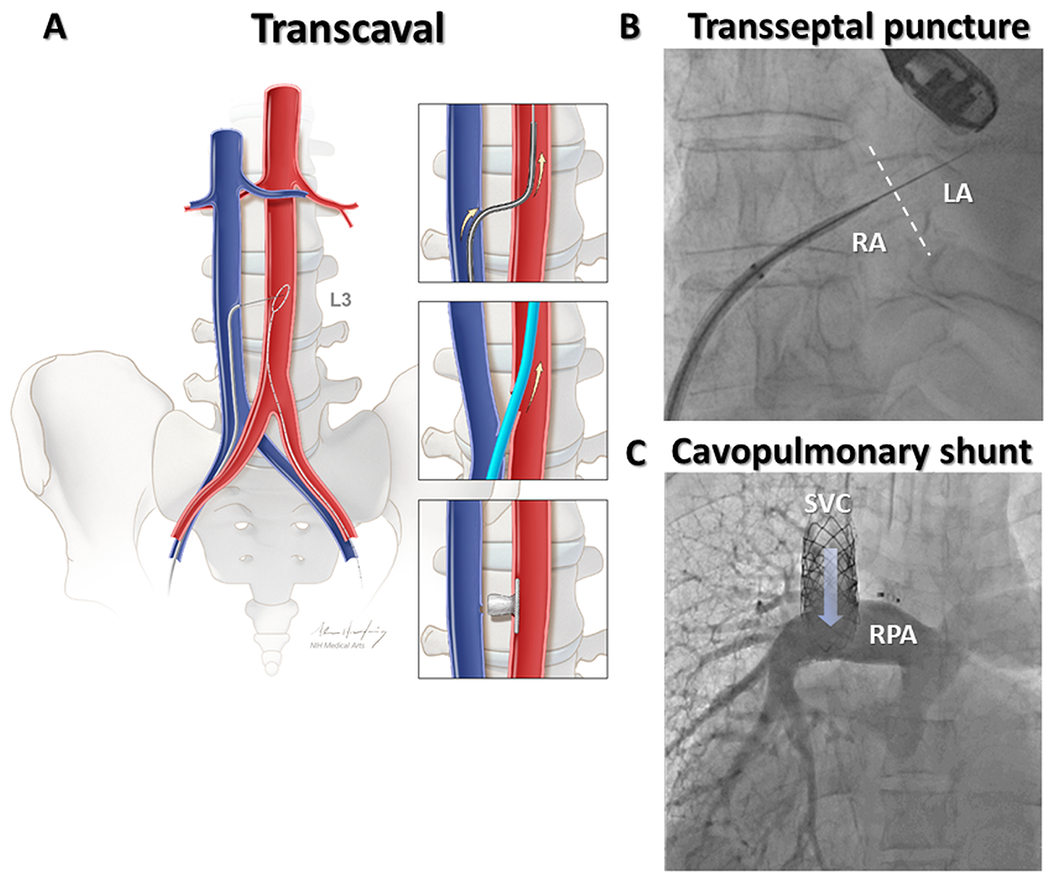
In transcaval aortic access (A), the electrified guidewire is advanced through the walls of inferior vena cava and abdominal aorta where it is ensnared. Exchange for a stiff guidewire permits large bore access to the aorta in patients with unsuitable femoral arteries. The transcaval tract is closed with a nitinol vascular occluder. (B) Electrosurgical transseptal puncture using the dilator of a deflectable sheath for insulation. (C) A transcatheter superior cavopulmonary shunt was following electrosurgical traversal from superior vena cava to right pulmonary artery. RA=Right atrium; LA=Left atrium; SVC=Superior vena cava; RPA=Right pulmonary artery. Reprinted from Greenbaum et al. JACC, 20178.
Technical overview
A suitable, calcium free crossing target is identified from a pre-procedure CT of the abdominal aorta10. Orthogonal fluoroscopic projection angles are planned, and the desired crossing site is related to anatomic fiducials, for example the superior margin of the iliac crests and nearest lumbar vertebrae which are then used for procedural co-registration. Safety and bailout options are planned by evaluating proximity of the crossing target to the renal vessels and aortoiliac bifurcation (minimum distance 15mm), presence of interposed structures such as small bowel, and femoral access for bailout covered stent delivery.
Transcaval access offers superior ergonomics and greater operator distance from X-ray scatter compared with transaxillary and transcarotid approaches. A coaxial system comprising CTO-indicated angioplasty guidewire (Astato XS20, Asahi-Intecc), hubless locking 0.014”−0.035” microcatheter (Piggyback, Teleflex) or alternative 0.014” 130-150cm microcatheter, 0.035” x 90cm microcatheter (e.g. NaviCross, Terumo) and 6-7Fr renal length guiding catheter (IM or RDC1) are inserted from the right femoral vein to the crossing target and directed towards a single loop snare draped along the aortic target from a femoral artery10. The guidewire is electrified with a short burst of 30-50W ‘pure’ cut monopolar energy and advanced from vena cava into the awaiting aortic snare. Following guidewire ensnarement and advancement to the aortic arch, the coaxial catheters are advanced to the aorta to permit exchange for a stiff 0.035” guidewire (e.g. Lunderquist) over which the intended large-bore sheath is advanced into the aorta to perform TAVR or percutaneous LVAD delivery otherwise per standard practice. On completion, heparin anticoagulation is reversed with protamine, the system is withdrawn and the transcaval access tract is closed with a nitinol cardiac occluder (Amplatzer Duct Occluder-1, Abbott). Completion aortography typically demonstrates complete occlusion or residual aortocaval fistulae. Extravasation usually responds to balloon aortic tamponade, but otherwise covered stents are required in approximately 1-5% of cases.
Clinical experience and outcomes
Early transcaval access and closure was systematically studied with CT in 100 patients in a prospective, multi-center, single-arm, core-lab adjudicated, investigational device exemption (IDE) Trial of patients with no other access options for TAVR8,11. In this extreme risk cohort (STS predicted mortality 9.6±6.3%) access and closure was successful in 99 of 100 attempts, covered stent was required for hemostasis in 1, and there were no deaths attributable to transcaval access8. Systematic post-procedure CT identified bleeding complications not otherwise ascertained on other TAVR studies. At 12-months there were no post-discharge vascular complications despite universal implantation of permeable vascular nitinol occluders11. Results were similar in an early European12 and Israeli13 reports.
In a more contemporary report, 238 patients undergoing transcaval TAVR were compared with 106 undergoing transaxillary access. Transcaval access conferred bleeding and vascular complications similar to transaxillary access, but lower “femoral-like” rates of stroke and discharge directly to home14. Selection bias does not appear to account for the different outcomes, before or after inverse-propensity weighting of baseline characteristics. Sites included a mix of operators who preferentially employed one route or the other (3 transaxillary vs 5 transcaval), and 3 that abandoned the transaxillary approach before the study period. Overall these findings suggest transcaval TAVR is at least as safe as transaxillary.
Applications
Transcaval access has been used extensively for TAVR in native vessels 8,9,14,15, including through aneurysmal segments 16 and surgical abdominal aortic grafts17,18. In the setting of resistant aortic walls, transcaval crossing can be accomplished using angioplasty balloons or with laser atherectomy 19. Recent creative applications employ transcaval access for large bore hemodynamic support devices 20,21, extracorporeal membrane oxygenation22, for treatment of congenital heart disease 23 including subaortic stenosis 24, for congenital syndromes at prohibitive operative risk 25, and for thoracic aortic endovascular aneurysm repair 26.
Arterial occlusion
Pediatric patients with chronically occluded27 or atretic28 pulmonary arteries have been successfully recanalized following electrosurgical traversal, as has an iatrogenic endograft occlusion of the right pulmonary artery during transcatheter pulmonary valve replacement29. Aorto-ostial coronary artery occlusions are challenging due to a lack of antegrade options. In cases where conventional retrograde approaches failed, electrosurgical traversal (E-CART) has enabled successful coronary artery recanalization 30. In aortic coarctation, electrosurgery has enabled stent placement in uncrossable lesions31,32 and in late stent thrombosis33.
Venous occlusion
Electrosurgical traversal is possible in complex central and peripheral venous occlusions when mechanical approaches fail. Electrosurgery has been applied for retrograde pulmonary vein recanalization in complex congenital heart disease34, in longstanding left subclavian vein occlusion related to chronic hemodialysis catheters35, to enable pacemaker upgrades36, and for a chronically occluded portal vein, recanalized to treat recurrent, variceal, upper-gastrointestinal bleeding37. In a single-center experience of 20 central, otherwise-uncrossable, chronic venous occlusions, electrosurgical recanalization was successful in 80% with only one major, conservatively managed, complication38.
Non-anatomic bypass
Electrosurgical traversal between superior vena cava and right pulmonary artery enabled a transcatheter Glenn Shunt to successfully palliate an adult patient with uncorrected functional single ventricle39. By connecting descending aorta and main pulmonary artery, transcatheter reverse Potts shunts have alleviated symptoms in adult and pediatric patients with supra-systemic pulmonary arterial hypertension40,41.
Trans-septal access
Electrosurgical atrial transseptal access is straightforward using purpose-built needles and guidewires (NRG and VersaCross, Bayliss Medical)42,43 and off-label angioplasty guidewires44 for both structural heart and electrophysiology procedures. Electrosurgery reduces time to cross and number of crossing attempts compared to mechanical puncture42,43,45, allows for more precise transseptal access by eliminating the forward force required for puncture using transseptal needles, and may reduce unintended “back-wall” left atrial injury.
Electrosurgical fenestration
Electrosurgical fenestration of chronic Type-B aortic dissection46,47 creates communication between true and false aortic lumens, allowing access to or perfusion of visceral vascular branches.
In situ fenestration has allowed rescue of unintended pulmonary artery branch obstruction after transcatheter pulmonary valve implantation 29. More important, electrosurgical fenestration has allowed in situ construction of complex endograft landing zones in patients with pulmonary arteries otherwise too large for commercial or investigational transcatheter pulmonary valve devices 48.
Electrophysiologic applications
Left ventricular access has been established by electrosurgical crossing from the right atrium, creating an iatrogenic Gerbode defect, to perform VT ablation in patients with mechanical aortic and mitral valves49 and across the interventricular septum to enable direct LV endocardial pacing50.
TISSUE LACERATION
Procedures discussed to this point have concentrated charge at guidewire tips, to traverse through or between structures. Eccentric denudation and kinking the mid-shaft of an angioplasty guidewire to create the “Flying-V” (described above) was a major advancement to slice tissues in a variety of settings[Video S1].
CARDIAC VALVE LEAFLET SLICING
LAMPOON (intentional Laceration of the Anterior Mitral valve leaflet to Prevent left ventricular Outflow ObstructioN)
Technical overview
LAMPOON is a transcatheter mimic of surgical anterior leaflet resection, commonly performed during mitral valve replacement to prevent left ventricular outflow obstruction51[figure 5]. In addition to fixed LVOT obstruction, long anterior mitral valve leaflets can cause dynamic LVOT obstruction following TMVR despite capacious outflow tracts. Moreover, TMVR-displaced overhanging leaflets occasionally create flow patterns that disrupt normal TMVR leaflet coaptation52 or create dynamic LVOT obstruction. All can be averted using LAMPOON.
Fig 5. Clinical applications – Tissue laceration; Mitral leaflet modification.
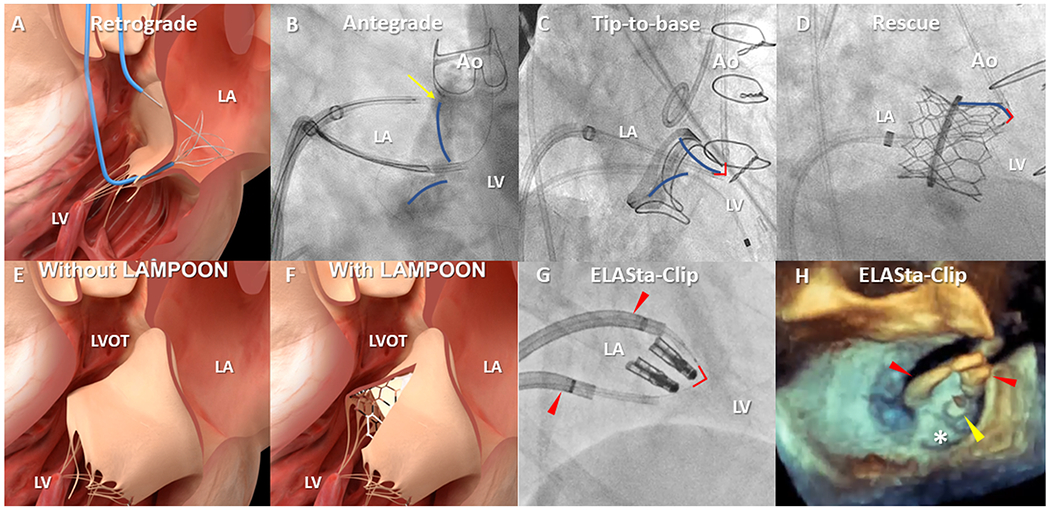
The original, retrograde LAMPOON (A) crossed the base of the A2 mitral scallop across the aortic valve. Technical refinements include an antegrade approach (B) across the interventricular septum. One catheter is positioned on the atrial surface at the base (yellow arrow) of the A2 mitral leaflet (blue overlay) from where the guidewire is electrified towards a snare in the LVOT. Tip-to-base LAMPOON (C) lacerates the mitral leaflet (blue overlay) in reverse, without leaflet traversal, in patients with a suitable backstop such as a surgical prosthesis. The flying V (red-outline) is positioned on the tip and withdrawn until it meets a hard-stop. Rescue LAMPOON (D) similarly lacerates from tip backwards to slice long, overhanging leaflets causing dynamic LVOT obstruction after transcatheter mitral valve replacement. LAMPOON techniques uncover cells otherwise draped with anterior mitral valve leaflet tissue (E&F). (G) A pair of deflectable sheaths(red arrowheads) for ELASta-Clip to liberate MitraClips from the anterior mitral leaflet to enable TMVR. (H) Positioning of sheaths(red arrowheads) anterior to the MitraClip(yellow arrowhead) allows TMVR to pin the posterior leaflet(white asterisk) harmlessly. Ao=Aorta; LA=Left atrium; LV=Left ventricle; LVOT=Left ventricular outflow tract.
LAMPOON works by creating a midline incision in the anterior mitral valve leaflet that subsequently splays when displaced by TMVR, exposing THV cells otherwise covered by the anterior mitral leaflet. Serendipitously, chordal attachments to the papillary muscles remain intact, preserving ventricular function, and pulling the sliced leaflet halves safely outwards.
LAMPOON is not helpful when TMVR devices have lengthwise fabric skirts preventing blood flow across their stent frames. In this circumstance the risk of LVOT obstruction may be mitigated by septal reduction therapy (see SESAME, below).
Base-to-tip variants
In retrograde LAMPOON (“Classique”)53, a guide catheter engages the base of the A2 mitral leaflet scallop from a percutaneous retrograde aortic approach. A 0.014” guidewire electrosurgically traverses the leaflet into to an awaiting multi-loop snare in the left atrium. The “Flying-V” is positioned at the base of the leaflet. Continuous, gentle, guide catheter traction during dextrose infusion and application of 70W “pure cut” electrosurgical energy creates a midline incision from base to tip. Because the retrograde aortic catheters are intrinsically aligned with the LVOT, retrograde LAMPOON remains the most reliable approach to true midline splitting of the A2 anterior mitral valve cusp.
Positioning of the retrograde aortic catheters can be technically demanding and can induce or exacerbate valvular regurgitation, and led to the development of an antegrade LAMPOON approach, which is now usually preferred for base-to-tip cases54[figure 5]. Parallel deflectable sheaths are placed trans-septally in the left atrium to direct a pair of guide catheters; one snare catheter through the mitral valve orifice in the left ventricular outflow, the other delivering the coaxial electrosurgery crossing system to the atrial surface at the base of the anterior mitral valve leaflet. Following echocardiographic confirmation, leaflet traversal and snaring are accomplished. The key to successful midline laceration is creation of a central fulcrum in the left atrium using the deflectable sheaths; otherwise lacerations tend to be oriented obliquely towards the interatrial septum.
Tip-to-base variants
“Reverse,” or “tip-to-base” LAMPOON, is a further simplification for patients in whom either a prosthetic surgical ring55 or valve replacement56 creates a backstop to excessive laceration, protecting the aorto-mitral curtain and aortic valve. Because there is no leaflet traversal, the procedure is straightforward for newcomers. The procedure entails a balloon-wedge end-hole catheter floated from left atrium to LVOT to aorta to assure a chord-free trajectory. This catheter delivers a guidewire, pre-prepared with “Flying-V”, to be ensnared by a retrograde aortic snare. Traction is applied to guide catheters in the aorta and left atrium with the Flying-V straddling the leaflet tip. In resistant leaflets, laceration can be repeated. It is important during tip-to-base LAMPOON to assure no electrification near the aortic leaflets and to assure ring annuloplasty “backstops” protect the aortomitral curtain and transverse sinus, both evident on CT.
Rescue LAMPOON57 describes a variation of tip-to-base LAMPOON in which the tip of a long anterior mitral valve leaflet that is causing TMVR leaflet dysfunction or dynamic LVOT obstruction is lacerated back to the THV frame.
Clinical experience and outcomes
In the prospective NHLBI LAMPOON IDE trial, 30 patients at prohibitive risk of LVOT obstruction were successfully treated using retrograde LAMPOON58. No procedural deaths were recorded in this extreme risk cohort (STS PROM 10.2±6.2) and 30-day survival was 93%. LAMPOON is most commonly performed in the setting of TMVR using balloon expandable THVs created for the aortic position, but has also enabled implantation of dedicated transcatheter mitral valves when overhanging anterior mitral valve leaflet would otherwise cause obstruction59. When implanting dedicated TMVR systems without uncovered cells LAMPOON may be combined with septal reduction techniques to synergistically prevent LVOT obstruction. After guidewire traversal through a non-calcified target, LAMPOON laceration is usually successful despite heavy anterior mitral leaflet calcification.
Liberation of double orifice mitral valve
In patients with prior surgical Alfieri stitch (ELASTIC; Electrosurgical Laceration of Alfieri STItCh 60) or transcatheter edge-to-edge repair (ELASta-Clip; Electrosurgical Laceration And Stabilization of failed MitraClip(s) 61,62), positioning of the “Flying-V” on the anterior mitral valve leaflet anteriorly, adjacent to the suture- or leaflet-bridge, liberates the leaflets upon electrosurgical laceration[figure 5]. This procedural adjunct enables TMVR devices to pin the suture or clip harmlessly and posteriorly along with the posterior mitral leaflet. As in all such electrosurgery procedures, the lacerating guidewire is used “off-label.”
BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction)
Technical overview
LAMPOON was adapted to aortic valve therapy as BASILICA63. Briefly, CT planning64 assesses mechanisms and likelihood of threatened coronary obstruction (sinus sequestration or direct coronary ostial obstruction), and identifies procedural fluoroscopic projections. An appropriately sized coronary guide catheter engages the hinge-point of the target aortic leaflet; typically an oversized AL shape for the left and a JR4 or multipurpose for the right. Catheter shape is fine-tuned using rigid 0.035” guidewire back-ends during positioning. A co-axial traversal system comprising guidewire (Astato XS20) and microcatheter (e.g. PiggyBack) is advanced to the crossing target, clamped to the electrosurgical generator and, during brief application of 30-50W pure-cut energy, is advanced to a single-loop snare pre-positioned in the LVOT through a separate guiding catheter through the major orifice of the aortic valve[figure 6]. The Flying-V65 is created and passed to the base of the leaflet during guidewire externalization. Guide catheters are advanced and slack removed from the system with gentle traction. Catheters are withdrawn towards the ascending aorta during application of 70W energy and with continuous dextrose infusion through both.
Fig 6. Clinical applications – Tissue laceration; BASILICA.
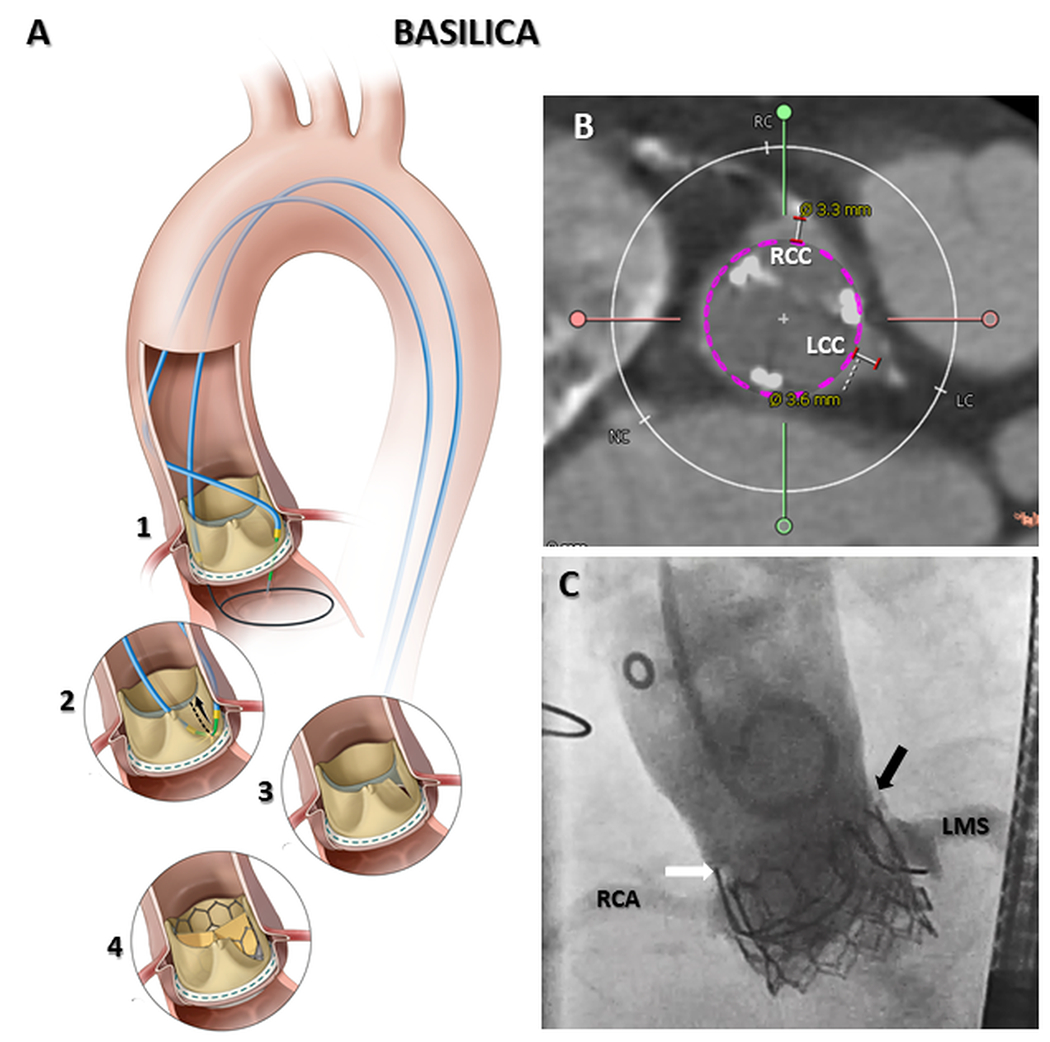
Tissue traversal at the base of the offending aortic leaflet (A1), followed by tissue laceration (A2) creates a midline slice in target aortic leaflet (A3). BASILICA enables leaflet splay following transcatheter heart valve implantation, preventing coronary artery obstruction (A4). In cases where both right and left coronary ostia are at risk (B) BASILICA can be performed on both leaflets in a ‘doppio’ procedure. In (C) brisk coronary flow remains following TAVR despite surgical valve leaflets being visible above left (black arrow) and right (white arrow) aortic sinuses. RCA=Right coronary artery; RCC=Right coronary cusp; LCC=Left coronary cusp; LMS=Left main stem. Reprinted from Khan et al. JACC: Cardiovascular Interventions, 201863.
Careful electrosurgical technique, including the dextrose flushing, is essential to allow electrosurgical leaflet slicing rather than mechanical avulsion. Importantly, appropriately sliced leaflets continue to coapt sufficiently to prevent hemodynamic deterioration in the period between laceration and TAVR. Following TAVR, the midline leaflet incision preserves coronary artery flow through the open cells of the THV. If both arteries are at risk of obstruction a “doppio” procedure can be performed without hemodynamic decompensation[figure 6].
Clinical experience and outcomes
Approaches to predict iatrogenic coronary artery obstruction are sensitive but non-specific. Several high-risk features have been defined, including a coronary ostial height <12mm, virtual-valve-to-coronary (VTC) distance <4mm in bioprosthetic or <3mm in native valves, virtual-valve-to-sinotubular-junction distance <2mm and the presence of externally mounted prosthetic leaflets66. The BASILICA Trial, a prospective, investigator-led, IDE trial included 30 patients at risk for coronary obstruction. 30-day67 and 1-year68 outcomes demonstrated procedural efficacy and safety in native and bioprosthetic valves. The Multicenter International BASILICA registry reported real-world results from 214 patients at 25 centers in North America and Europe69. Procedural success was high, with laceration in 94% of cases. Importantly, stroke (2.8%) and disabling stroke (0.5%) events were low and comparable to that reported in patients who do not undergo BASILICA70.
Adaptations and future directions
Subsets of patients carry a higher risk for coronary artery obstruction, including those planned for TAVR-in-TAVR71. Balloon-augmented BASILICA (BA-BASILICA) may increase leaflet splay by expanding the traversal centrifugally closer to the bioprosthetic valve ring or native annulus, through balloon dilatation of the leaflet crossing point prior to laceration72,73. Purpose-built Pachyderm guide catheters74 and custom guidewires are expected further to simplify the procedure.
CARDIAC VALVE LEAFLET REMOVAL
CATHEDRAL (CATHeter Electrosurgical Debulking and RemovAL)
A proportion of patients may experience coronary obstruction despite successful BASILICA laceration, such as from a prolapsing leaflet73. The CATHEDRAL procedure75 uses transcatheter electrosurgery to energize a single-loop snare to cut and excise the aortic cusp. Further enhancements may prove important in the management of obstructive leaflets, especially for TAVR-in-TAVR.
Technical overview
Similar to BASILICA, the target aortic leaflet is electrosurgically crossed at its base, ensnared and externalized. In contrast, the kinked guidewire shaft is not denuded, allowing the leaflet to be grasped and minimizing injurious electrosurgical coupling in subsequent steps. A single loop snare is positioned over the V, at the base of the leaflet, tightened and energized whilst flooding the field with dextrose. Gentle guidewire countertraction assists excision and retrieves the excised leaflet.
MODIFICATION OF OTHER CARDIOVASCULAR TISSUE
SESAME (SEptal Scoring Along Midline Endocardium)
LVOT obstruction may still occur from TMVR when the THV fabric skirt obstructs outflow despite successful LAMPOON76. The SESAME procedure77,78, still under development, is a transcatheter septal myotomy that increases LVOT area in patients with, or at risk for, LVOT obstruction from TMVR or hypertrophic cardiomyopathy[figure 7].
Fig 7. Clinical applications – Myocardial slicing.
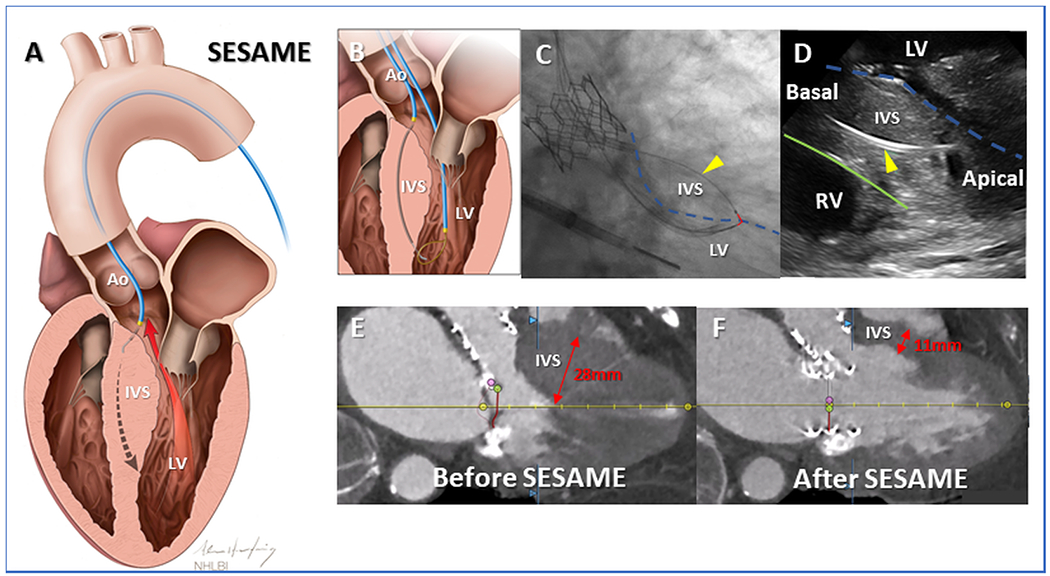
(A) In SESAME transcatheter septal myotomy, a guidewire traverses the interventricular septum, defining length and depth of subsequent myotomy. Once ensnared (B) the flying V (C, red overlay) is positioned on the left ventricular endocardium(broken blue-outline). Fluoroscopic (C) and intracardiac echocardiographic (D) appearance of catheters, one wholly within septal myocardium(yellow arrowhead), safely deep to right ventricular endocardium (green outline), positioned ready to cut muscle during guidewire electrification. SESAME creates space in the left ventricular outflow tract to enable TMVR (E&F). Ao=Aorta; IVS=Interventricular septum; LV=Left ventricle; RV=Right ventricle. Reprinted from Khan et al. Circulation: Cardiovascular Interventions, 202278.
Technical overview
A retrograde aortic guiding catheter is engaged to the basal most interventricular septum, underneath the aortic valve, between the nadir of the right coronary cusp and right-left commissure to avoid conduction tissue. Using Intramyocardial Guidewire Navigation, a CTO-tipped angioplasty guidewire (Astato XS20)79 traverses the septum and exits to a pre-positioned snare in the left ventricle. This trajectory lies away from the conduction system and defines the length and depth of subsequent myotomy. A modified “Flying-V” is fashioned with eccentric denudation of the mid-point of the guidewire, increasing the lacerating surface in contact with myocardium78. Electrosurgical laceration is performed under traction and 70W pure cut energy. The resultant myotomy immediately reduces LVOT gradient and splays further over 30 days77. In early experience there have been no major conduction disturbances and few anatomic exclusions, in contrast to transcoronary alcohol septal ablation80.
PASTA (Pledget-Assisted Suture Tricuspid Annuloplasty)
In combination with other novel techniques, including guidewire-assisted suture and pledget delivery, electrosurgery has enabled tricuspid annuloplasty with the PASTA procedure81,82.
Excision and removal of ectopic structures
Electrosurgery performed by energizing single-loop snares has resulted in successful resection of an aortic valve fibroelastoma83, ruptured mitral papillary muscle84 and most recently a right atrial myxoma; SEATTLE (Simplified Extraction of Atrial Tumor with Targeted Loop Electricity) procedure [personal correspondence, James M. McCabe]. A stray central venous catheter was removed from the superior vena cava of a post operative patient using a modified electrosurgical guidewire “lasso”85.47
Troubleshooting
When faced with failure to traverse or cut, a few simple remedies usually suffice.
Connections and dispersive electrode
When guidewires are connected to electrosurgery generators via electrosurgery pencils, contact points should be examined to assure appropriate denudation of insulation. Avoid electrosurgery pencils having insulating (‘Edge’) coating, or otherwise strip the insulation.
The dispersive gel electrode should be in good (moist) condition and have good contact with clean and dry skin. We have experienced electrosurgical failure from mal-applied dispersive electrodes even when the generator contact quality indicator is illuminated. We recommend a low threshold to replace dispersive electrodes.
Catheter positioning
Successful tissue traversal requires orthogonal catheter positioning, which is analogous to guide catheter backup support in endovascular interventions. Calcification resists electrosurgical traversal, which succeeds only through calcium-free gaps, however small. If the guidewire is seen deflecting away from targets during electrosurgical traversal attempts, it is advisable to withdraw and reposition the guide and coaxial microcatheters, if only minutely, before further attempts.
Carbonization
The active electrode easily becomes coated with insulating organic materials (“carbonized”) when energized in the presence of blood or tissue. If other maneuvers do not correct electrosurgical failure, inspection and replacement of the guidewire may be required. This is part of the rationale for dextrose flushing during electrification, which reduces carbonization.
Power
Up-titration of applied power may aid electrosurgery. Using bedside-modified guidewires, we usually first attempt tissue traversal at 30W and increase to 50W as needed; we initiate tissue laceration at 50-70W and cautiously increase to 90W as needed.
How to adopt electrosurgery techniques into practice
Industry-sponsored proctorship is not generally available to physicians using significant-risk medical devices “off-label.” NHLBI has begun investigation of a dedicated BASILICA electrosurgical guidewire (TELLTALE, Transmural Systems, Andover, MA). Until commercial availability of such devices, new users can adopt electrosurgical techniques into their practices by combining study of medical literature and live or online video demonstrations; observing expert operators; and bringing patients to care alongside expert operators. On-site proctorship and even video-proctorship86 are options, but proctors may require institutional indemnification against tort claims.
In our experience, transcaval TAVR is a good “entry-level” electrosurgical technique, followed by solo (single-leaflet) BASILICA. We recommend new users achieve proficiency in these techniques before tackling more complex ones such as multi-leaflet BASILICA and LAMPOON.
Conclusion
Transcatheter electrosurgery is a versatile tool that continues to inspire novel, innovative therapies for patients with complex anatomic challenges. Dedicated transcatheter electrosurgery devices will further simplify techniques and encourage routine adoption of these broadly applicable transcatheter electrosurgical procedures.
Supplementary Material
Sources of Funding
Supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health USA, Z01-HL006040 and Z01-HL006041 (to RJL).
Disclosures
CGB, JMK, TR and RJL are co-inventors on patents, assigned to NIH, for transcatheter electrosurgical devices.
TR is a consultant and physician proctor for Edwards Lifesciences, Medtronic and Boston Scientific; is a Medtronic advisory board member; and has an equity interest in Transmural Systems.
VCB and ABG receive institutional research support from Abbott Vascular, Ancora Heart, Edwards Lifesciences, Gore Medical, Jena Valve, Medtronic, Polares Medical, Transmural Systems, 4C Medical; receive consulting fees from Abbott Vascular, Edwards Lifesciences, and Medtronic; and have equity interest in Transmural Systems. ABG also is an advisor to, and has equity in Excision Medical.
DKY has no relevant disclosures.
Abbreviations
- BASILICA
Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction
- HCM
Hypertrophic Cardiomyopathy
- IDE
Investigational Device Exemption
- LAMPOON
Intentional Laceration of the Anterior Mitral leaflet to Prevent left ventricular Outflow ObstructioN
- LVOT
Left ventricular outflow tract
- SESAME
Septal Scoring Along Midline Endocardium
- AVR
Transcatheter Aortic Valve Replacement
- TMVR
Transcatheter Mitral Valve Replacement
References
- 1.Khan JM, Lederman RJ. Transcatheter electrosurgery in bipolar or monopolar modes. Catheter Cardiovasc Interv. 2018;91:1052–1053. doi: 10.1002/ccd.27620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan JM, Rogers T, Greenbaum AB, Babaliaros VC, Yildirim DK, Bruce CG, Herzka DA, Schenke WH, Ratnayaka K, Lederman RJ. Transcatheter Electrosurgery: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1455–1470. doi: 10.1016/j.jacc.2020.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal E, Qureshi SA, Chan KC, Martin RP, Skehan DJ, Jordan SC, Tynan M. Radiofrequency-assisted balloon dilatation in patients with pulmonary valve atresia and an intact ventricular septum. Br Heart J. 1993;69:347–351. doi: 10.1136/hrt.69.4.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akagi T, Hashino K, Maeno Y, Ishii M, Sugimura T, Kawano T, Kato H. Balloon dilatation of the pulmonary valve in a patient with pulmonary atresia and intact ventricular septum using a commercially available radiofrequency catheter. Pediatr Cardiol. 1997;18:61–63. doi: 10.1007/s002469900112 [DOI] [PubMed] [Google Scholar]
- 5.Alwi M, Geetha K, Bilkis AA, Lim MK, Hasri S, Haifa AL, Sallehudin A, Zambahari R. Pulmonary atresia with intact ventricular septum percutaneous radiofrequency-assisted valvotomy and balloon dilation versus surgical valvotomy and Blalock Taussig shunt. J Am Coll Cardiol. 2000;35:468–476. doi: 10.1016/s0735-1097(99)00549-5 [DOI] [PubMed] [Google Scholar]
- 6.Lee ML, Tsao LY, Chiu HY, Chen M, Chiu IS. Outcomes in neonates with pulmonary atresia and intact ventricular septum underwent pulmonary valvulotomy and valvuloplasty using a flexible 2-French radiofrequency catheter. Yonsei Med J. 2009;50:245–251. doi: 10.3349/ymj.2009.50.2.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan AA, Mullen MJ, Magee AG. Radiofrequency perforation of the pulmonary valve in an adult with tetralogy of Fallot and pulmonary atresia. Cardiol Young. 2009;19:517–518. doi: 10.1017/s104795110999076x [DOI] [PubMed] [Google Scholar]
- 8.Greenbaum AB, Babaliaros VC, Chen MY, Stine AM, Rogers T, O’Neill WW, Paone G, Thourani VH, Muhammad KI, Leonardi RA, et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J Am Coll Cardiol. 2017;69:511–521. doi: 10.1016/j.jacc.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenbaum AB, O’Neill WW, Paone G, Guerrero ME, Wyman JF, Cooper RL, Lederman RJ. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol. 2014;63:2795–2804. doi: 10.1016/j.jacc.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lederman RJ, Greenbaum AB, Rogers T, Khan JM, Fusari M, Chen MY. Anatomic Suitability for Transcaval Access Based on Computed Tomography. JACC Cardiovasc Interv. 2017;10:1–10. doi: 10.1016/j.jcin.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederman RJ, Babaliaros VC, Rogers T, Stine AM, Chen MY, Muhammad KI, Leonardi RA, Paone G, Khan JM, Leshnower BG, et al. The Fate of Transcaval Access Tracts: 12-Month Results of the Prospective NHLBI Transcaval Transcatheter Aortic Valve Replacement Study. JACC Cardiovasc Interv. 2019;12:448–456. doi: 10.1016/j.jcin.2018.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa G, De Backer O, Pilgrim T, Kasel M, Redwood S, Aminian A, Lanz J, Michel J, Patterson T, Windecker S, et al. Feasibility and safety of transcaval transcatheter aortic valve implantation: a multicentre European registry. EuroIntervention. 2020;15:e1319–e1324. doi: 10.4244/EIJ-D-19-00797 [DOI] [PubMed] [Google Scholar]
- 13.Barbash IM, Segev A, Berkovitch A, Fefer P, Maor E, Elian D, Regev E, Guetta V. Clinical Outcome and Safety of Transcaval Access for Transcatheter Aortic Valve Replacement as Compared to Other Alternative Approaches. Front Cardiovasc Med. 2021;8:731639. doi: 10.3389/fcvm.2021.731639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederman RJ, Babaliaros VC, Lisko JC, Rogers T, Mahoney P, Foerst JR, Depta JP, Muhammad KI, McCabe JM, Pop A, et al. Transcaval Versus Transaxillary TAVR in Contemporary Practice: A Propensity-Weighted Analysis. JACC Cardiovasc Interv. 2022;15:965–975. doi: 10.1016/j.jcin.2022.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers T, Greenbaum AB, Babaliaros VC, Stine AM, Khan JM, Schenke WH, Eng MH, Paone G, Leshnower BG, Satler LF, et al. Dedicated Closure Device for Transcaval Access Closure: From Concept to First-in-Human Testing. JACC Cardiovasc Interv. 2019;12:2198–2206. doi: 10.1016/j.jcin.2019.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piayda K, Veulemans V, Kelm M, Zeus T. Transcaval aortic valve implantation through a partially thrombosed infrarenal aortic aneurysm. Eur Heart J. 2020;41:974. doi: 10.1093/eurheartj/ehz164 [DOI] [PubMed] [Google Scholar]
- 17.Lederman RJ, O’Neill WW, Greenbaum AB. Transcaval access for TAVR across a polyester aortic graft. Catheter Cardiovasc Interv. 2015;85:1270–1273. doi: 10.1002/ccd.25781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanz J, Pilgrim T, Greenbaum AB, Lederman RJ, Windecker S. Sheathless Transcaval Transcatheter Aortic Valve Implantation Through an Abdominal Aortic Graft. Can J Cardiol. 2018;34:1688 e1617–1688 e1619. doi: 10.1016/j.cjca.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers T, Waksman R, Slack M, Satler L. Laser-Assisted Transcaval Access for Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:e3–e4. doi: 10.1016/j.jcin.2017.10.046 [DOI] [PubMed] [Google Scholar]
- 20.Afana M, Altawil M, Basir M, Alqarqaz M, Alaswad K, Eng M, O’Neill WW, Lederman RJ, Greenbaum AB. Transcaval access for the emergency delivery of 5.0 liters per minute mechanical circulatory support in cardiogenic shock. Catheter Cardiovasc Interv. 2021;97:555–564. doi: 10.1002/ccd.29235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamioka N, Patel A, Burke MA, Greenbaum A, Babaliaros V. Biventricular Impella placement via complete venous access. Catheter Cardiovasc Interv. 2019;93:E343–E345. doi: 10.1002/ccd.27103 [DOI] [PubMed] [Google Scholar]
- 22.Chiang M, Gonzalez PE, Basir MB, O’Neill BP, Lee J, Frisoli T, Wang DD, O’Neill WW, Villablanca PA. Modified Transcaval Left Atrial Venoarterial Extracorporeal Membrane Oxygenation Without Preplanning Contrast CT: Step-by-Step Guide. JACC Cardiovasc Interv. 2022;15:e181–e185. doi: 10.1016/j.jcin.2022.05.033 [DOI] [PubMed] [Google Scholar]
- 23.Heyden CM, Moore JW, Ryan JR, Lederman RJ, El-Said HG, Ratnayaka K. Alternative Access in Congenital Heart Disease. JACC Case Rep. 2020;2:1734–1735. doi: 10.1016/j.jaccas.2020.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamioka N, Patel A, Lerakis S, Parastatidis I, Forcillo J, Corrigan F, Thourani V, Block P, Babaliaros V. Transcatheter Treatment of Subaortic Stenosis Via Transcaval Access. JACC Cardiovasc Interv. 2017;10:740–741. doi: 10.1016/j.jcin.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Jain A, Mehta Y, Chandra P. Transcaval aortic valve implantation in a patient with Larsen syndrome: technical and anesthetic challenges. Indian J Thorac Cardiovasc Surg. 2021;37:434–437. doi: 10.1007/s12055-020-01107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uflacker A, Lim S, Ragosta M, Haskal ZJ, Lederman RJ, Kern J, Upchurch G, Huber T, Angle JF, Ailawadi G. Transcaval Aortic Access for Percutaneous Thoracic Aortic Aneurysm Repair: Initial Human Experience. J Vasc Interv Radiol. 2015;26:1437–1441. doi: 10.1016/j.jvir.2015.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink C, Peuster M, Bertram H, Hausdorf G. Transcatheter recanalization of the left main pulmonary artery after four years of complete occlusion. Catheter Cardiovasc Interv. 2001;53:81–84. doi: 10.1002/ccd.1135 [DOI] [PubMed] [Google Scholar]
- 28.Pedra CA, Filho RM, Arrieta RS, Tellez R, Fontes VF. Recanalization of a discrete atretic right pulmonary artery segment with a new radiofrequency system. Catheter Cardiovasc Interv. 2003;60:82–87. doi: 10.1002/ccd.10602 [DOI] [PubMed] [Google Scholar]
- 29.Nageotte SJ, Lee JW, El-Said HG, Moore JW, Ratnayaka K. Transcatheter Electrosurgery Rescue: Jailed Pulmonary Artery Following Melody Pulmonary Valve Replacement. JACC Cardiovasc Interv. 2020;13:e21–e22. doi: 10.1016/j.jcin.2019.08.051 [DOI] [PubMed] [Google Scholar]
- 30.Nicholson W, Harvey J, Dhawan R. E-CART (ElectroCautery-Assisted Re-enTry) of an Aorto-Ostial Right Coronary Artery Chronic Total Occlusion: First-in-Man. JACC Cardiovasc Interv. 2016;9:2356–2358. doi: 10.1016/j.jcin.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 31.Farjat Pasos JI, Ontiveros Mercado H, Marroquin Donday LA, Jimenez Rodriguez GM, Jimenez Santos M, Arias Sanchez EA, Damas de Los Santos F. Electro-Cut Assisted Crossing Technique for Noncrossable Extreme Aortic Coarctation: First-in-Man. JACC Cardiovasc Interv. 2020;13:e111–e115. doi: 10.1016/j.jcin.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 32.Almashham Y, Dahdah N, Miro J. Use of radiofrequency then stent implantation for recanalization of complete aorta coarctation. Pediatr Cardiol. 2008;29:207–209. doi: 10.1007/s00246-007-9090-2 [DOI] [PubMed] [Google Scholar]
- 33.Aguirre-Molina CA, Garcia-Montes JA, Bialkowski J. Application of radiofrequency perforation to recanalization late stent thrombosis of aortic coarctation. Catheter Cardiovasc Interv. 2011;78:428–431. doi: 10.1002/ccd.23103 [DOI] [PubMed] [Google Scholar]
- 34.Almasarweh SI, Kuo JA, Bauser-Heaton HD, Babaliaros VC, Kim DW. Retrograde Pulmonary Vein Recanalization Using Transcatheter Electrosurgery. JACC Case Rep. 2022;4:592–595. doi: 10.1016/j.jaccas.2022.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baerlocher MO, Asch MR, Myers A. Successful recanalization of a longstanding complete left subclavian vein occlusion by radiofrequency perforation with use of a radiofrequency guide wire. J Vasc Interv Radiol. 2006;17:1703–1706. doi: 10.1097/01.RVI.0000243637.23923.A7 [DOI] [PubMed] [Google Scholar]
- 36.Foerst JR, Kim D, May TP. Percutaneous electrosurgical technique for treatment of subclavian vein occlusion: Application of transcaval techniques. HeartRhythm Case Rep. 2017;3:551–554. doi: 10.1016/j.hrcr.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferris MC, Cherniak DV, Wong JK, Herget EJ. Successful Radiofrequency Guidewire Recanalization of a Chronic Portal Vein Occlusion That Failed Conventional Therapy. Cardiovasc Intervent Radiol. 2015;38:1343–1345. doi: 10.1007/s00270-015-1176-2 [DOI] [PubMed] [Google Scholar]
- 38.Keller EJ, Gupta SA, Bondarev S, Sato KT, Vogelzang RL, Resnick SA. Single-Center Retrospective Review of Radiofrequency Wire Recanalization of Refractory Central Venous Occlusions. J Vasc Interv Radiol. 2018;29:1571–1577. doi: 10.1016/j.jvir.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 39.Ratnayaka K, Moore JW, Rios R, Lederman RJ, Hegde SR, El-Said HG. First-in-Human Closed-Chest Transcatheter Superior Cavopulmonary Anastomosis. J Am Coll Cardiol. 2017;70:745–752. doi: 10.1016/j.jacc.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson JH, Cabalka AK, Frantz RP, Cajigas HR, Taggart NW. Transcatheter Nonductal Reverse Potts Shunt Creation in Pulmonary Arterial Hypertension. Circ Cardiovasc Interv. 2022;15:e011315. doi: 10.1161/CIRCINTERVENTIONS.121.011315 [DOI] [PubMed] [Google Scholar]
- 41.Boudjemline Y, Sizarov A, Malekzadeh-Milani S, Mirabile C, Lenoir M, Khraiche D, Levy M, Bonnet D. Safety and Feasibility of the Transcatheter Approach to Create a Reverse Potts Shunt in Children With Idiopathic Pulmonary Arterial Hypertension. Can J Cardiol. 2017;33:1188–1196. doi: 10.1016/j.cjca.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 42.Andrade JG, Macle L, Bennett MT, Hawkins NM, Essebag V, Champagne J, Roux JF, Makanjee B, Tang A, Skanes A, et al. Randomized trial of conventional versus radiofrequency needle transseptal puncture for cryoballoon ablation: the CRYO-LATS trial. J Interv Card Electrophysiol. 2022;65:481–489. doi: 10.1007/s10840-022-01277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu JC, Badhwar N, Gerstenfeld EP, Lee RJ, Mandyam MC, Dewland TA, Imburgia KE, Hoffmayer KS, Vedantham V, Lee BK, et al. Randomized trial of conventional transseptal needle versus radiofrequency energy needle puncture for left atrial access (the TRAVERSE-LA study). J Am Heart Assoc. 2013;2:e000428. doi: 10.1161/JAHA.113.000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan JM, Rogers T, Eng MH, Lederman RJ, Greenbaum AB. Guidewire electrosurgery-assisted trans-septal puncture. Catheter Cardiovasc Interv. 2018;91:1164–1170. doi: 10.1002/ccd.27311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inohara T, Gilhofer T, Luong C, Tsang M, Saw J. VersaCross radiofrequency system reduces time to left atrial access versus conventional mechanical needle. J Interv Card Electrophysiol. 2022;63:9–12. doi: 10.1007/s10840-020-00931-7 [DOI] [PubMed] [Google Scholar]
- 46.Regazzoli D, Leone PP, Sanz-Sanchez J, Poletto G, Civilini E, Pedicini V, Reimers B. The Growing Role of Transcatheter Electrosurgery: Peripheral Procedures. JACC Cardiovasc Interv. 2021;14:704–705. doi: 10.1016/j.jcin.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 47.Gandet T, Alushi K, Westermann D, Schirmer J, Rohlffs F, Panuccio G, Kolbel T. “Powered Cheese Wire Technique” for Septal Fenestration in Complex Endovascular Repair for Chronic Thoracoabdominal Aortic Dissection. J Endovasc Ther. 2021;28:676–681. doi: 10.1177/15266028211007474 [DOI] [PubMed] [Google Scholar]
- 48.Kamioka N, Babaliaros VC, Lisko JC, Sahu A, Shashidharan S, Carazo MR, Jokhadar M, Rodriguez FH 3rd, Book WM, Gleason PT, et al. Single-Barrel, Double-Barrel, and Fenestrated Endografts to Facilitate Transcatheter Pulmonary Valve Replacement in Large RVOT. JACC Cardiovasc Interv. 2020;13:2755–2765. doi: 10.1016/j.jcin.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santangeli P, Hyman MC, Muser D, Callans DJ, Shivkumar K, Marchlinski FE. Outcomes of Percutaneous Trans-Right Atrial Access to the Left Ventricle for Catheter Ablation of Ventricular Tachycardia in Patients With Mechanical Aortic and Mitral Valves. JAMA Cardiol. 2020;6:1–6. doi: 10.1001/jamacardio.2020.4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamble JHP, Herring N, Ginks MR, Rajappan K, Bashir Y, Betts TR. Endocardial left ventricular pacing across the interventricular septum for cardiac resynchronization therapy: Clinical results of a pilot study. Heart Rhythm. 2018;15:1017–1022. doi: 10.1016/j.hrthm.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 51.Case BC, Lisko JC, Babaliaros VC, Greenbaum AB, Satler L, Ben-Dor I, Forrestal BJ, Yerasi C, Kamioka N, Rogers T, et al. LAMPOON techniques to prevent or manage left ventricular outflow tract obstruction in transcatheter mitral valve replacement. Ann Cardiothorac Surg. 2021;10:172–179. doi: 10.21037/acs-2020-mv-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenbaum AB, Condado JF, Eng M, Lerakis S, Wang DD, Kim DW, Lederman RJ, Paone G, Neill WWO, Thourani VH, et al. Long or redundant leaflet complicating transcatheter mitral valve replacement: Case vignettes that advocate for removal or reduction of the anterior mitral leaflet. Catheter Cardiovasc Interv. 2018;92:627–632. doi: 10.1002/ccd.27054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babaliaros VC, Greenbaum AB, Khan JM, Rogers T, Wang DD, Eng MH, O’Neill WW, Paone G, Thourani VH, Lerakis S, et al. Intentional Percutaneous Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction During Transcatheter Mitral Valve Replacement: First-in-Human Experience. JACC Cardiovasc Interv. 2017;10:798–809. doi: 10.1016/j.jcin.2017.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lisko JC, Greenbaum AB, Khan JM, Kamioka N, Gleason PT, Byku I, Condado JF, Jadue A, Paone G, Grubb KJ, et al. Antegrade Intentional Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction: A Simplified Technique From Bench to Bedside. Circ Cardiovasc Interv. 2020;13:e008903. doi: 10.1161/CIRCINTERVENTIONS.119.008903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lisko JC, Babaliaros VC, Khan JM, Kamioka N, Gleason PT, Paone G, Byku I, Tiwana J, McCabe JM, Cherukuri K, et al. Tip-to-Base LAMPOON for Transcatheter Mitral Valve Replacement With a Protected Mitral Annulus. JACC Cardiovasc Interv. 2021;14:541–550. doi: 10.1016/j.jcin.2020.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Case BC, Khan JM, Satler LF, Ben-Dor I, Lederman RJ, Babaliaros VC, Greenbaum AB, Waksman R, Rogers T. Tip-to-Base LAMPOON to Prevent Left Ventricular Outflow Tract Obstruction in Valve-in-Valve Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2020;13:1126–1128. doi: 10.1016/j.jcin.2020.01.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan JM, Trivedi U, Gomes A, Lederman RJ, Hildick-Smith D. “Rescue” LAMPOON to Treat Transcatheter Mitral Valve Replacement-Associated Left Ventricular Outflow Tract Obstruction. JACC Cardiovasc Interv. 2019;12:1283–1284. doi: 10.1016/j.jcin.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J Am Coll Cardiol. 2019;73:2521–2534. doi: 10.1016/j.jacc.2019.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan JM, Lederman RJ, Devireddy CM, Clements SD Jr., Kamioka N, Yousef A, Gleason PT, Guyton RA, Babaliaros VC. LAMPOON to Facilitate Tendyne Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2018;11:2014–2017. doi: 10.1016/j.jcin.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan JM, Lederman RJ, Sanon S, Leshnower BG, Yousef A, Gleason P, Lerakis S, Rogers T, Greenbaum AB, Babaliaros VC. Transcatheter Mitral Valve Replacement After Transcatheter Electrosurgical Laceration of Alfieri STItCh (ELASTIC): First-in-Human Report. JACC Cardiovasc Interv. 2018;11:808–811. doi: 10.1016/j.jcin.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lisko JC, Greenbaum AB, Guyton RA, Kamioka N, Grubb KJ, Gleason PT, Byku I, Condado JF, Jadue A, Paone G, et al. Electrosurgical Detachment of MitraClips From the Anterior Mitral Leaflet Prior to Transcatheter Mitral Valve Implantation. JACC Cardiovasc Interv. 2020;13:2361–2370. doi: 10.1016/j.jcin.2020.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inci EK, Greenbaum AB, Lederman RJ, Kohli K, Lisko JC, Byku I, Gleason PT, Xie JX, Shekiladze N, Babaliaros VC. Transcatheter Electrosurgical Laceration and Stabilization of Failed MitraClip[s]/SAPIEN M3 for Treatment of Failed MitraClip. Circ Cardiovasc Interv. 2022;15:e012014. doi: 10.1161/CIRCINTERVENTIONS.122.012014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan JM, Dvir D, Greenbaum AB, Babaliaros VC, Rogers T, Aldea G, Reisman M, Mackensen GB, Eng MHK, Paone G, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc Interv. 2018;11:677–689. doi: 10.1016/j.jcin.2018.01.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lederman RJ, Babaliaros VC, Rogers T, Khan JM, Kamioka N, Dvir D, Greenbaum AB. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv. 2019;12:1197–1216. doi: 10.1016/j.jcin.2019.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruce CG, Greenbaum AB, Babaliaros VC, Rogers T, Lederman RJ, Khan JM. Safeguards and pitfalls for Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction during transcatheter aortic valve replacement-the BASILICA technique. Ann Cardiothorac Surg. 2021;10:700–707. doi: 10.21037/acs-2021-tviv-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ribeiro HB, Rodes-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, Makkar R, Simonato M, Barbanti M, Schofer J, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018;39:687–695. doi: 10.1093/eurheartj/ehx455 [DOI] [PubMed] [Google Scholar]
- 67.Khan JM, Greenbaum AB, Babaliaros VC, Rogers T, Eng MH, Paone G, Leshnower BG, Reisman M, Satler L, Waksman R, et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv. 2019;12:1240–1252. doi: 10.1016/j.jcin.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan JM, Greenbaum AB, Babaliaros VC, Dvir D, Reisman M, McCabe JM, Satler L, Waksman R, Eng MH, Paone G, et al. BASILICA Trial: One-Year Outcomes of Transcatheter Electrosurgical Leaflet Laceration to Prevent TAVR Coronary Obstruction. Circ Cardiovasc Interv. 2021;14:e010238. doi: 10.1161/CIRCINTERVENTIONS.120.010238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan JM, Babaliaros VC, Greenbaum AB, Spies C, Daniels D, Depta JP, Oldemeyer JB, Whisenant B, McCabe JM, Muhammad KI, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: Results From the Multicenter International BASILICA Registry. JACC Cardiovasc Interv. 2021;14:941–948. doi: 10.1016/j.jcin.2021.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. Ann Thorac Surg. 2021;111:701–722. doi: 10.1016/j.athoracsur.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 71.Khan JM, Bruce CG, Babaliaros VC, Greenbaum AB, Rogers T, Lederman RJ. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. 2020;13:787–789. doi: 10.1016/j.jcin.2019.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenbaum AB, Kamioka N, Vavalle JP, Lisko JC, Gleason PT, Paone G, Grubb KJ, Bruce CG, Lederman RJ, Babaliaros VC. Balloon-Assisted BASILICA to Facilitate Redo TAVR. JACC Cardiovasc Interv. 2021;14:578–580. doi: 10.1016/j.jcin.2020.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perdoncin E, Bruce CG, Babaliaros VC, Yildirim DK, Depta JP, McCabe JM, Gleason PT, Xie J, Grubb KJ, Paone G, et al. Balloon-Augmented Leaflet Modification With Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction and Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction: Benchtop Validation and First In-Man Experience. Circ Cardiovasc Interv. 2021;14:e011028. doi: 10.1161/CIRCINTERVENTIONS.121.011028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lisko JC, Babaliaros VC, Lederman RJ, Khan JM, Rogers T, Greenbaum AB. Pachyderm-Shape Guiding Catheters to Simplify BASILICA Leaflet Traversal. Cardiovasc Revasc Med. 2019;20:782–785. doi: 10.1016/j.carrev.2019.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Babaliaros VC, Gleason PT, Xie JX, Khan JM, Bruce CG, Byku I, Grubb K, Paone G, Rogers T, Lederman RJ, et al. Toward Transcatheter Leaflet Removal With the CATHEDRAL Procedure: CATHeter Electrosurgical Debulking and RemovAL. JACC Cardiovasc Interv. 2022;15:1678–1680. doi: 10.1016/j.jcin.2022.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan JM, Rogers T, Babaliaros VC, Fusari M, Greenbaum AB, Lederman RJ. Predicting Left Ventricular Outflow Tract Obstruction Despite Anterior Mitral Leaflet Resection: The “Skirt NeoLVOT”. JACC Cardiovasc Imaging. 2018;11:1356–1359. doi: 10.1016/j.jcmg.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greenbaum AB, Khan JM, Bruce CG, Hanzel GS, Gleason PT, Kohli K, Inci EK, Guyton RA, Paone G, Rogers T, et al. Transcatheter Myotomy to Treat Hypertrophic Cardiomyopathy and Enable Transcatheter Mitral Valve Replacement: First-in-Human Report of Septal Scoring Along the Midline Endocardium. Circ Cardiovasc Interv. 2022;15:e012106. doi: 10.1161/CIRCINTERVENTIONS.122.012106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan JM, Bruce CG, Greenbaum AB, Babaliaros VC, Jaimes AE, Schenke WH, Ramasawmy R, Seemann F, Herzka DA, Rogers T, et al. Transcatheter Myotomy to Relieve Left Ventricular Outflow Tract Obstruction: The Septal Scoring Along the Midline Endocardium Procedure in Animals. Circ Cardiovasc Interv. 2022;15:e011686. doi: 10.1161/CIRCINTERVENTIONS.121.011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruce CG, Khan JM, Rogers T, Yildirim DK, Jaimes AE, Seemann F, Chen MY, O’Brien K, Herzka DA, Schenke WH, et al. Reshaping the Ventricle From Within. JACC: Basic to Translational Science. 2022;0. doi: doi: 10.1016/j.jacbts.2022.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elhadi M, Guerrero M, Collins JD, Rihal CS, Eleid MF. Safety and Outcomes of Alcohol Septal Ablation Prior to Transcatheter Mitral Valve Replacement. Journal of the Society for Cardiovascular Angiography & Interventions. 2022;1:100396. doi: 10.1016/j.jscai.2022.100396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenbaum AB, Khan JM, Rogers T, Babaliaros VC, Eng MHK, Wang DD, Paone G, Lederman RJ. First-in-human transcatheter pledget-assisted suture tricuspid annuloplasty for severe tricuspid insufficiency. Catheter Cardiovasc Interv. 2021;97:E130–E134. doi: 10.1002/ccd.28955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan JM, Rogers T, Schenke WH, Greenbaum AB, Babaliaros VC, Paone G, Ramasawmy R, Chen MY, Herzka DA, Lederman RJ. Transcatheter pledget-assisted suture tricuspid annuloplasty (PASTA) to create a double-orifice valve. Catheter Cardiovasc Interv. 2018;92:E175–E184. doi: 10.1002/ccd.27531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fredi JL, Louka BF, Mehmood SA, Master RG, Sogomonian R, Singh I. Transcatheter Electrosurgical Removal of an Aortic Valve Fibroelastoma. JACC Cardiovasc Interv. 2022;15:e141–e143. doi: 10.1016/j.jcin.2022.03.033 [DOI] [PubMed] [Google Scholar]
- 84.Bode MF, Mintz AJ, Shaikh N, Alhajri F, Baron SJ, Gadey G, Labib SB, Piemonte TC. Retrograde-Antegrade Snaring With Electrosurgery and Removal of a Ruptured Papillary Muscle. Circ Cardiovasc Interv. 2020;13:e009016. doi: 10.1161/CIRCINTERVENTIONS.120.009016 [DOI] [PubMed] [Google Scholar]
- 85.Arunothayaraj S, Tanseco K, Koerling AL, Hill A, Hyde J, Michail M, Cockburn J, Hildick-Smith D. Electrosurgical Removal of a Central Venous Catheter Inadvertently Sutured Into the Superior Vena Cava. JACC Cardiovasc Interv. 2021;14:e279–e280. doi: 10.1016/j.jcin.2021.07.036 [DOI] [PubMed] [Google Scholar]
- 86.Goel SS, Greenbaum AB, Patel A, Little SH, Parikh R, Wyler von Ballmoos MC, Lumsden AB, Reardon MJ, Kleiman NS. Role of Teleproctoring in Challenging and Innovative Structural Interventions Amid the COVID-19 Pandemic and Beyond. JACC Cardiovasc Interv. 2020;13:1945–1948. doi: 10.1016/j.jcin.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


