SUMMARY
Hemp (Cannabis sativa) is a highly versatile crop with a multitude of applications, from textiles, biofuel and building material to high‐value food products for consumer markets. Furthermore, non‐hallucinogenic cannabinoids like cannabidiol (CBD), which can be extracted from female hemp flowers, are potentially valuable pharmacological compounds. In addition, hemp has high carbon sequestration potential associated with its rapid growth rate. Therefore, the hemp industry is gaining more traction and breeding hemp cultivars adapted to local climate conditions or bred for specific applications is becoming increasingly important. Here, we present a method for the rapid generation cycling (speed breeding) of hemp. The speed breeding protocol makes use of the photoperiod sensitivity of Cannabis. It encompasses vegetative growth of the plants for 2 weeks under continuous light, followed by 4 weeks under short‐day conditions, during which flower induction, pollination and seed development proceed, and finally a seed ripening phase under continuous light and water stress. With the protocol described here, a generation time of under 9 weeks (61 days) from seed to seed can be achieved. Furthermore, our method synchronises the flowering time of different hemp cultivars, thus facilitating crosses between cultivars. The extremely short generation time will enable hemp researchers and breeders to perform crosses in a time‐efficient way and generate new hemp cultivars with defined genetic characteristics over a short period of time.
Keywords: speed breeding, plant breeding, rapid generation cycling, Cannabis sativa, hemp, sustainability, cannabinoids, technical advance
Significance Statement
Shortening crop generation times accelerates plant breeding and research. Here we present a speed breeding protocol for hemp (Cannabis sativa) that enables the growth of five generations in 1 year.
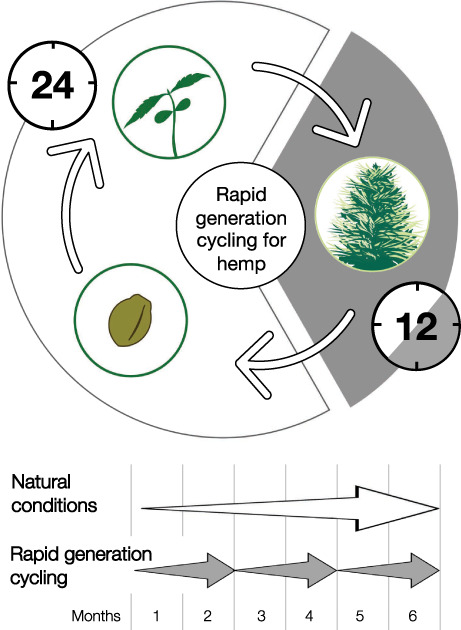
INTRODUCTION
Hemp (Cannabis sativa) is a highly versatile crop with dozens of different applications, ranging from the fibres that can be manufactured into textiles and ropes to the highly nutritious seeds that can be processed into oil for human consumption. In addition, hemp has high carbon sequestration potential when used as insulation material (Ingrao et al., 2015; Schilling et al., 2021), and when utilized as a biofuel, hemp can act as a source of green carbon and renewable energy (Ahmed et al., 2022). The beneficial effects of non‐hallucinogenic cannabinoids like cannabidiol (CBD) are being analysed for the treatment of various diseases, such as anxiety and depression, Parkinson's disease, epilepsy and different types of cancers (Schluttenhofer & Yuan, 2017). Therefore, after previously being banned for decades in many countries, hemp farming and breeding are gaining more traction, with the need for cultivars adapted to the local climate or for a specific application steadily increasing.
Rapid generation cycling (speed breeding) is a technique that involves the optimization of different environmental factors to achieve a short generation time for a given species (Ghosh et al., 2018; Watson et al., 2018). Speed breeding has recently been established for several crops, including wheat (Triticum aestivum), canola (Brassica napus) and chickpea (Cicer arietinum) (Ghosh et al., 2018; Watson et al., 2018). These are photoperiod‐sensitive species that require long days (16 h and above) to flower, and hence they are referred to as long‐day plants (Andrés & Coupland, 2012). One primary measure to achieve short generation times (rapid cycling) for long‐day plants is growing them under 22–24 h of light per day in order to transition them from the vegetative to the reproductive stage of the life cycle as quickly as possible (Ghosh et al., 2018; Watson et al., 2018).
In contrast, species that require the day length to drop below a certain critical threshold to induce flowering are termed short‐day plants. Speed breeding has been established for short‐day plants like soybean (Glycine max); however, this requires more complex protocols, involving, for example, the modification of day length, CO2 concentration or light quality (Fang et al., 2021; Jähne et al., 2020; Nagatoshi & Fujita, 2019).
Hemp was among the first plants for which day length was described as a critical denominator for flowering in the scientific literature (Tournois 1912; as cited in Heslop‐Harrison, 1957; Kobayashi & Weigel, 2007). It is well established that hemp is a short‐day plant, and that flowering is induced by day lengths shorter than 11–15 h, depending on the cultivar (Moher et al., 2021; Zhang et al., 2021). This results in long generation times in the field: the flowering time of hemp grown in middle latitudes, such as northern Europe or northern parts of North America, can range from 60 to more than 100 days (Faux et al., 2013; Stack et al., 2021), and the generation time in the field is often longer than 120 days (Faux et al., 2013). This, in turn, delays breeding new hemp varieties as only one generation can be grown in the field per year.
Here, we describe a method for achieving a shortened generation time for hemp of under 9 weeks (61 days). Ten different hemp cultivars with variable flowering times under field conditions were subjected to speed breeding conditions, and flowering time could be decreased to about 30 days for all of them. Plants cultivated using our method showed a seed set and germination rate sufficient for crosses and single seed descent lines. Our method enables rapid generation cycling for hemp, facilitating both research and breeding of this high‐value crop.
RESULTS
Flowering time of photoperiod‐sensitive hemp cultivars can be shortened by a combination of continuous light and short‐day treatments
To identify the conditions best suited to accelerate flowering in hemp, the photoperiod‐sensitive cultivars ‘Fedora 17’ and ‘Felina 32’ and the photoperiod‐insensitive cultivar ‘Finola’ were grown under artificial light in a temperature‐controlled environment for a complete life cycle, from seed to seed (Figures 1 and S1). Seeds were germinated and plants were initially cultivated for 2 weeks under continuous light to facilitate vegetative development and support the accumulation of biomass sufficient to support robust flowering. Subsequently, plants were subjected to different light treatments, to simulate ultra‐short days (8 h of light and 16 h of dark, 8:16), short days (12:12), long days (16:8) and continuous light (24:0), to assess how different light regimes affect flowering. Additionally, hemp plants were grown under natural light in a glasshouse to simulate a field season.
Figure 1.

Vegetative and reproductive stages of hemp plants grown under speed breeding conditions. The sex of diecious plants cannot be morphologically determined before flowering, as male (a) and female (b) plants look identical. The inflorescence of a monoecious plant becomes visible about 5 days before flowering (c). After flowering, female (d) and male (e) flowers are easily distinguishable, with female flowers displaying characteristic white stigmata (arrow) and male flowers containing five stamens per flower (arrowhead). A hemp plant grown under speed breeding conditions (f) shows ripening seeds after approximately 60 days (g). Ripe hemp seeds harvested 61 days after sowing (h) are able to germinate, with seedlings showing their first true leaves after 1 week (j). Scale bars: (f) 5 cm; (h, j) 0.5 cm.
Alterations in flowering times were observed for different day lengths. Short days (12:12) led to a significant reduction in flowering time compared with glasshouse conditions or continuous light for both ‘Fedora 17’ and ‘Felina 32’ cultivars (Figure 2a,b). At the same time, there was no difference in flowering time under different day lengths for the photoperiod‐insensitive ‘Finola’ cultivar (Figure 2c). A further reduction of the day length to ultra‐short days (8:16) did result in later flowering compared with short‐day treatment for ‘Fedora 17’ (Figure 2a). Photoperiod‐sensitive plants grown under short‐day conditions flowered approximately 44 and 70 days earlier compared with glasshouse conditions (‘Fedora 17’ and ‘Felina 32’, respectively; Table 1).
Figure 2.
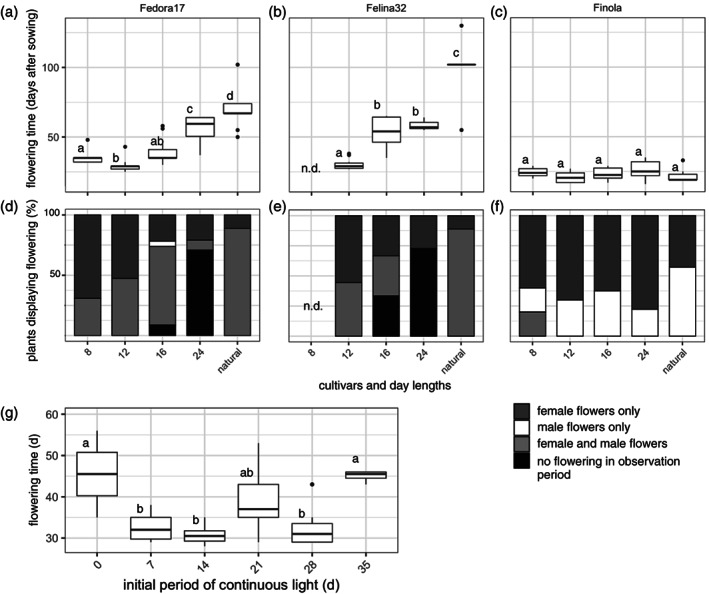
Photoperiod‐sensitive hemp cultivars respond to changes in day length with alterations in flowering time and sex expression. The photoperiod‐sensitive hemp cultivars ‘Fedora 17’ (a) and ‘Felina 32’ (b) show different flowering times under 8 h of light and 16 h of dark (8) (not determined for ‘Felina 32’), 12 h of light and 12 h of dark (12), 16 h of light and 8 h of dark (16) and continuous light (24). Flowering times in a glasshouse with natural lighting (natural) are also shown (plants grown between May and September 2019). In the photoperiod‐insensitive cultivar ‘Finola’ (c), the flowering time did not change in response to different light regimes. Significance levels (P < 0.05) are indicated with letters a–d. With different day lengths, the percentage of plants flowering varied for ‘Fedora 17’ (d) and ‘Felina 32’ (e), but not for ‘Finola’ (f). Plants showed either female‐only flowers (dark grey) or male‐only flowers (white) or both male and female flowers (light grey). Percentage of plants flowering was highest at 8 and 12 h of light, and declined with 16 h and continuous light (24 h light), where a high percentage of photoperiod‐sensitive individuals did not show any flowering during the 60‐day observation period (black). Under ultra‐short days, male and female flowers could be observed on the same plant in the usually dioecious ‘Finola’. The initial vegetative growth period influences flowering time (g). Hemp plants of the cultivar ‘Fedora 17’ were grown with no initial continuous light period (0) or with different ascending periods of an initial continuous period of light of 7, 14, 21, 28 and 35 days. Significance levels (P < 0.05) are indicated with letters; n.d., not determined.
Table 1.
Average flowering time of hemp cultivars grown under different light regimes
| Flowering time (days ± SD) | Light condition (h light:h dark) | ||||
|---|---|---|---|---|---|
| 8:16 | 12:12 | 16:8 | 24:0 | Natural | |
| Finola | 24.4 ± 3.6 | 20.8 ± 3.8 | 23.3 ± 4.7 | 25.1 ± 6.1 | 21.3 ± 4.1 |
| Fedora 17 | 33.5 ± 1.6 | 29.2 ± 3.7 | 39.1 ± 8.8 | 55.0 ± 12.7 | 73.1 ± 18.2 |
| Felina 32 | nd | 29.9 ± 3.2 | 53.3 ± 11.6 | 58.7 ± 4.7 | 99.9 ± 19.2 |
| Futura 75 | nd | 30.4 ± 2.3 | 39.0 ± 2.8 | nd | 116.0 ± 16.2 |
| Santhica 27 | nd | 33.3 ± 2.2 | 44.0 ± 10.8 | nd | 102.0 ± 15.0 |
| GEORGIEN | nd | 33.2 ± 3.3 | nd | nd | 73.0 ± 23.7 |
| KOREA | nd | 32.0 ± 2.7 | nd | nd | 127.7 ± 8.1 |
| Futura | nd | 32.2 ± 4.3 | nd | nd | 96.8 ± 20.7 |
| Kompolti.a | nd | 32.3 ± 3.8 | nd | nd | 130.0 ± 0.0 |
| Kompolti.b | nd | 30.6 ± 2.0 | nd | nd | 87.1 ± 25.4 |
| Average of flowering time of photoperiod‐sensitive cultivars | na | 31.5 ± 3.0 | 43.8 ± 8.5 | 56.8 ± 8.7 | 100.6 ± 16.3 |
The majority of photoperiod‐sensitive hemp plants grown under continuous light did not flower during the 60‐day observation period (74% and 80% of ‘Fedora 17’ and ‘Felina 32’, respectively), and equally some individuals grown under long‐day conditions (16:8) did not set flowers within the 60‐day observation period (Figure 2d,e), whereas all ‘Finola’ plants flowered, irrespective of day length (Figure 2f). Photoperiod‐sensitive hemp plants that did flower under continuous light appeared to have a lower number of flowers compared with plants grown under short‐day conditions. Further, the biomass, height and overall stability of plants were impacted by growth conditions, with plants grown under ultra‐short days being much shorter and feebler than plants grown under short days, long days and continuous light (Figure S2).
In an attempt to further shorten the flowering time, the initial period of growth under continuous light to support vegetative growth was altered. However, no significantly earlier flowering was observed if plants were moved to short‐day conditions after 7 instead of 14 days (Figure 2g). Eliminating the initial vegetative growth phase resulted in significantly delayed flowering (P = 0.017). Similarly, an extension of the phase with continuous lighting to 35 days instead of 14 days delayed the flowering times (P < 0.01).
Subsequently, we tested whether reducing the soil volume from 1 L to 0.2 L would accelerate flowering time. In contrast to our expectations, smaller pots led to a significant increase in flowering time when compared with larger pots (Figure S3).
Flowering time of hemp cultivars can be synchronized using short‐day artificial lighting
To verify the applicability of the developed protocol for hemp cultivars other than ‘Felina 32’ and ‘Fedora 17’, the flowering time of seven additional photoperiod‐sensitive hemp cultivars was tested under short‐day conditions and in the glasshouse. For all cultivars, flowering time under artificial lighting and short‐day conditions was strongly accelerated, compared with glasshouse conditions (Figure 3). The mean flowering time under short‐day conditions was about 31.5 days for all photoperiod‐sensitive cultivars (Table 1). Intriguingly, the flowering times of all cultivars under short‐day conditions were extremely similar to each other, ranging from 29.2 to 33.3 days on average, with an average standard deviation of 3 days (Figure 3; Table 1). In contrast, flowering time under natural day lengths varied enormously between individuals and cultivars, ranging from 50 to 130 days for photoperiod‐sensitive cultivars (Figure 3; Table 1).
Figure 3.
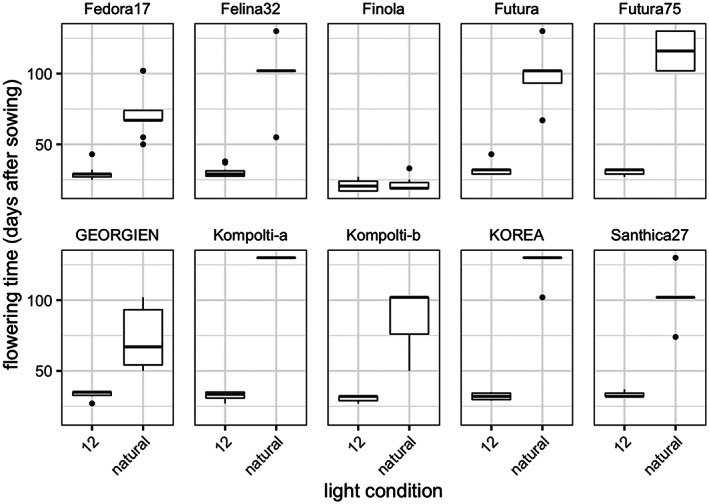
Flowering times of photoperiod‐sensitive hemp cultivars are lower under speed breeding conditions. Nine photoperiod‐sensitive hemp cultivars, as well as the photoperiod‐insensitive hemp cultivar ‘Finola’, were grown under 12 h of light after an initial 14 days of 24 h of light (12). For comparison, the same cultivars were grown in a glasshouse with natural lighting (natural). Flowering times are significantly different between treatments for all cultivars (P < 0.01) except ‘Finola’.
Viable seeds were obtained from plants grown under speed breeding conditions
Beyond achieving a short flowering time, obtaining viable seeds is an essential part of any speed breeding protocol. After seed set, plants were grown under continuous light and watering was stopped 1 week later to accelerate seed ripening. For the photoperiod‐sensitive cultivar ‘Fedora 17’, the number of seeds obtained per plant varied, with the highest number of seeds observed for plants grown under short days but with no significant differences between ultra‐short, short and long‐day treatments (Figure 4a). ‘Fedora 17’ plants grown under continuous light did not set seeds (Figure 4a). For photoperiod‐insensitive ‘Finola’ hemp plants, no significant differences in seed number were observed between day lengths, including the continuous light treatment, which overall yielded the highest number of seeds per plant (Figure 4b). All other photoperiod‐sensitive cultivars tested set seeds under short‐day conditions, with no significant differences between cultivars (Figure 4c).
Figure 4.
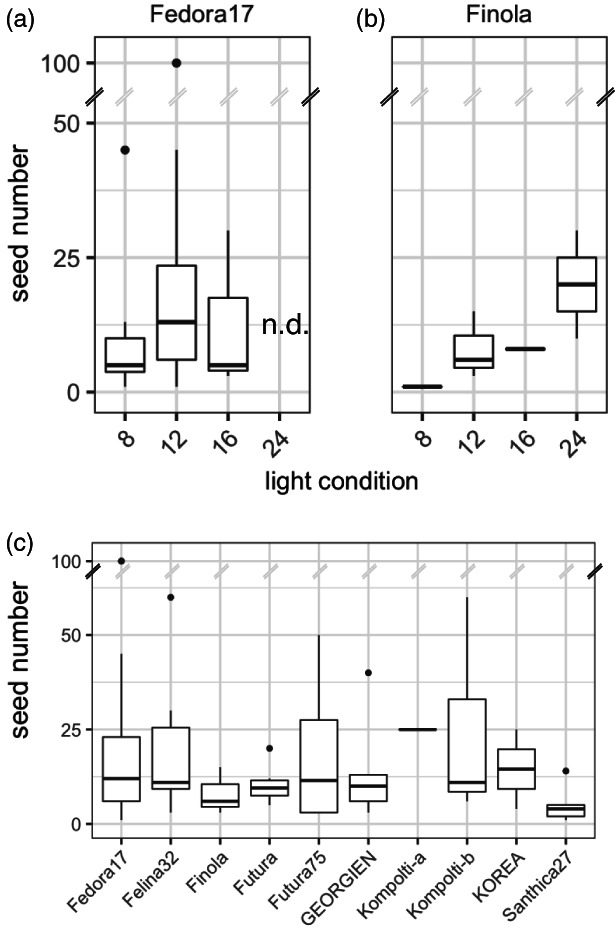
Seed yield from plants grown under speed breeding conditions. The photoperiod‐sensitive cultivar ‘Fedora 17’ (a) produced most seeds under short‐day conditions (12), with lower yields under ultra‐short (8) and long days (16). No seeds set when plants were cultivated under continuous light (24). The photoperiod‐insensitive cultivar ‘Finola’ produced most seeds under continuous light (b). For both ‘Fedora 17’ and ‘Finola’, no significant differences were detected in seed numbers between conditions. Different photoperiod‐sensitive hemp cultivars grown under short‐day conditions produced an average of 13.2 seeds per individual, with no significant differences between cultivars (c).
To assess germination rate, seeds generated from plants grown under short‐day conditions were placed directly on soil for germination with no prior treatment. Germination rates on average were 55% and 86% for ‘Fedora 17’ and ‘Felina 32’ seeds, respectively (Figure S4). The fastest generation time achieved, from seed to seed, was 61 days.
Male flowers can be induced by the application of silver nitrate
Hemp is primarily dioecious, i.e. male and female flowers develop on separate individuals. Some cultivars are monoecious, with male and female flowers developing on the same plant.
Surprisingly, we found that for the monoecious cultivars ‘Felina 32’ and ‘Fedora 17’, about 50% of the plants developed only female flowers when grown under short‐day conditions. In contrast, more than 80% of the plants grown under natural light in the glasshouse developed male as well as female flowers, indicating that sex expression is affected by the specific conditions of cultivation (Figure 2d,e).
For some research or breeding applications, however, it is important to self pollinate plants. We therefore tested whether we can induce male flowers on female plants grown under speed breeding conditions. Silver nitrate in combination with sodium thiosulphate has been described previously for male flower induction in dioecious females (Lubell & Brand, 2018). Repeated direct application of an aqueous silver nitrate sodium thiosulphate solution to developing inflorescences of both monoecious as well as dioecious female plants resulted in the induction of male flowers approximately 14 days after the first treatment (Figure S5).
DISCUSSION
A protocol to synchronize and accelerate flowering in photoperiod‐sensitive hemp cultivars
Hemp (C. sativa) is a short‐day plant and will often only flower 2–4 months after sowing in field conditions in Europe and North America (Faux et al., 2013; Stack et al., 2021). Long generation times can hinder genetic research on hemp and slow down the generation of new hemp cultivars.
Here, we describe a method of rapid generation cycling (speed breeding, Figure 5) for photoperiod‐sensitive hemp cultivars. The combination of an initial phase of continuous light, followed by short‐day conditions (12 h of light and 12 h of dark), provides a robust and quick transition from vegetative to reproductive development in all hemp varieties tested (Figure 1). The flowering time for photoperiod‐sensitive hemp cultivars decreased dramatically under short‐day conditions compared with the other conditions tested (Figures 2 and 3).
Figure 5.
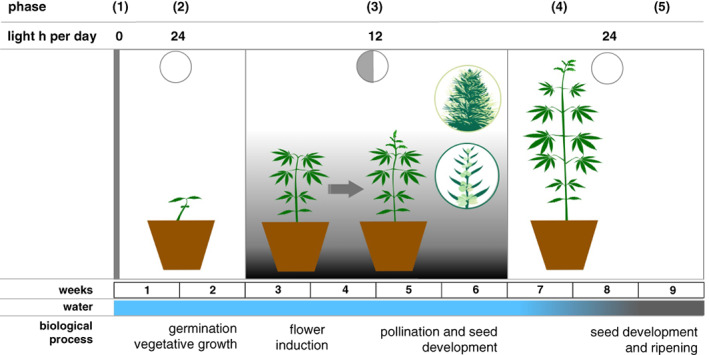
Overview of rapid generation cycling (speed breeding) protocol for hemp (Cannabis sativa). After sowing, seeds are incubated for 2 days in darkness (phase 1). Seedlings are then grown for 2 weeks under continuous light to facilitate vegetative growth (phase 2). Subsequently, hemp plants are grown under short‐day conditions (12 h light per day) for 4 weeks to induce flowering, pollination and seed setting (phase 3). After 4 weeks the plants are grown under continuous light to limit further flowering and accelerate seed ripening (phase 4). Water stress is introduced in week 8 to accelerate seed ripening (phase 5).
Hence, we propose the following protocol for rapid generation cycling for photoperiod‐sensitive hemp cultivars (Figure 5):
Seeds are placed on soil and germinated in darkness for 2 days.
To facilitate the rapid vegetative growth of the plants, the seedlings are subjected to 2 weeks of continuous light.
To induce flowering and facilitate pollination and seed setting, plants are grown under short‐day conditions (12 h of light per day for approximately 4 weeks or until the first seeds have set).
To facilitate seed ripening, the plants are subjected to continuous light. Additionally, water stress is induced once seeds have reached their full size in order to accelerate seed ripening.
Seeds are harvested approximately 30–35 days after flowering, when they turn brown.
With the method described here, a generation time of under 9 weeks (61 days) from seed to seed can be achieved. This very short generation time can enable hemp researchers as well as breeders to perform crosses in a time‐efficient way. Hence, this method can be used to create mapping populations for genetic studies and generate new hemp cultivars with defined genetic characteristics in a short period of time.
Short‐day treatment synchronizes the flowering time of different hemp varieties
By growing plants under short‐day conditions, the flowering of all hemp cultivars could be induced after a period of about 13–17 days under short‐day conditions, even though flowering under natural light was highly variable between and even within cultivars (Figure 3; Table 1). This synchronization enables the efficient crossing of different hemp cultivars, even if these have very distinct flowering times under natural light conditions.
It has been reported for Arabidopsis thaliana and Boechera stricta that the canalization of developmental patterns can depend on environmental conditions (Hall et al., 2007; Lee et al., 2014). It will be interesting to study what the genetic basis of this synchronization in hemp is. Given that hemp is a short‐day plant, the variation of flowering time under natural (long‐day) light conditions may reflect variation in the genetic background of different cultivars. Under inductive short‐day conditions, the activation of the photoperiod pathway, which presumably is similar between cultivars, may then outweigh subtle genetic differences in other flowering‐time pathways, leading to similar flowering times between cultivars.
Hemp undergoes an initial vegetative period of at least 2 weeks before competence to flower is acquired
The transition from vegetative to reproductive development in hemp can be divided into three phases: a basic vegetative phase, a photoperiod‐induced phase and a flower development phase (Lisson et al., 2000a).
The basic vegetative phase is the juvenile phase, during which flowering does not occur even if the day length is potentially inductive. This phase has been reported to last approximately 3 weeks or longer, depending on the cultivar (Amaducci et al., 2008; Lisson et al., 2000a). Our data agree with those observations and show that a period of continuous light during the first 2 weeks of growth does not delay flowering time (Figure 2g), likely because the plants have not acquired the developmental competence to flower yet. However, this initial continuous light period benefits plant growth, as it generally improves vegetative development.
The photoperiod‐induced phase follows the basic vegetative phase and is day‐length dependent. Under optimal light conditions, flowering initiation is assumed to be instantaneous once the plants have entered the photoperiod‐induced phase (Amaducci et al., 2008; Lisson et al., 2000a). The optimal day length for floral induction varies for different hemp cultivars but has been reported to be between 11 and 15 h for many cultivars (Zhang et al., 2021). In agreement with this we observed that flowering is readily induced with a day length of 12 h, and is delayed with a day length of 16 h. Ultra‐short days do not provide an additional advantage, as flowering time does not decrease further when compared with short‐day conditions (Figure 2). Under ultra‐short days, the plants accumulate too little biomass and are less durable than under short days (Figure S2). It is also noteworthy that under treatment with continuous light, some plants from both photoperiod‐sensitive cultivars tested flowered eventually (Figure 2), confirming earlier reports that hemp is a facultative short‐day plant (Salentijn et al., 2019).
Taken together, our data and the available literature (Amaducci et al., 2008; Lisson et al., 2000a; Zhang et al., 2021) suggest that the light conditions suggested above are close to optimal for the rapid induction of flowering in hemp. Seed yield is comparatively low under these conditions but should be sufficient for various breeding and research purposes, such as single seed descent lines and crosses.
Interestingly, we found that short‐day treatments increased the number of plants developing only female and no male flowers (Figure 2d). An effect of day length on hemp sex expression has been documented (Heslop‐Harrison, 1957), but has received comparatively little attention so far. Male flowers could efficiently be induced with the ethylene inhibitor silver nitrate (Figure S5). It will be interesting to study how photoperiod and hormonal pathways interact to influence sex expression in hemp.
Beyond photoperiod, growth temperature has a major effect on hemp growth, with a temperature range between 26°C and 29°C being described as optimal (Amaducci et al., 2008; Lisson et al., 2000b). In our experiments, temperatures were around 25°C (Figure S1). Thus, slightly higher cultivation temperatures may yield a slight further acceleration of hemp development and flowering time.
CONCLUSION
We show that speed breeding in hemp is inexpensive, can be achieved with relatively affordable equipment, is space efficient and facilitates crossing between cultivars that diverge in flowering time under non‐inductive conditions. It can serve as an efficient method to facilitate hemp breeding and research.
EXPERIMENTAL PROCEDURES
Plant material
Hemp seeds were obtained from GreenLight Medicines(Ireland) (‘Finola’, ‘Fedora 17’, ‘Felina 32’, ‘Santhica 27’ and ‘Futura 75’) and from the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) seed bank (https://www.ipk‐gatersleben.de; KOREA (CAN22), GEORGIEN (CAN23), Kompolti‐a (CAN56), Kompolti‐b (CAN70), Futura (CAN69)).
Sowing and hemp plant cultivation
Plants were grown in 1‐L pots in a soil mixture (1:1:1, John Innes No. 2:vermiculite:perlite) to allow for soil drainage with simultaneous moisture retention. Seeds were sown directly onto the soil and placed in the dark for 2 days at ambient temperature (20–25°C). Subsequently, plants were grown for 2 weeks under continuous light (red–blue LEDs, light intensity approximately 500 μmol sec−1 at approximately 15 cm distance from the light source; HORTILED MULTI, 44 W; HORTILUX, https://www.hortilux.com) in a growth chamber at 22–30°C (temperature and humidity log in Figure S1). In parallel, plants were germinated and grown under natural day length in glasshouse conditions between May and September 2019 in Dublin, Ireland. Per cultivar and light treatment, between 4 and 51 plants were observed. Plants were fertilized with a commercial fertilizer (NPK 25‐3‐14, diluted in water according to manufacturer's instructions; ScottsMiracle‐Gro, https://scottsmiraclegro.com) once a week except for the period of water stress during the ripening stage.
Day‐length cycling for optimizing flower induction, seed set and ripening
After cultivation under continuous light for 2 weeks, plants were subjected to different conditions: either short‐day conditions with 12 h of light and 12 h of dark (12:12), ultra‐short‐day conditions with 8 h of light and 16 h of dark (8:16), long‐day conditions with 16 h of light and 8 h of dark (16:8) or continuous light (24:0). Plants were kept under those conditions until they flowered and had set seed. Thereafter, plants were exposed to continuous light for the remainder of the cultivation. After 1 week of continuous light, watering was suspended to put the plants under water stress to facilitate rapid seed ripening, as described before for other crops (Ghosh et al., 2018).
Flowering time, pollination and seed ripening
Plants were designated as flowering if either stigmata were visible (in female flowers) or single flowers were visible (in male flowers). Plants cultivated under artificial light were assessed for evidence of flowering every 1–3 days over the course of 60 days. For plants cultivated in the glasshouse (natural light) flowering was observed between two and four times a week up to day 74; afterwards, flowering was observed on days 102 and 130. All phenotyping data are available in Table S1.
Pollen was dispersed by shaking plants bearing male flowers after anthesis or by plucking male open flowers and bringing their anthers in direct contact with female flowers. Seeds were harvested once they were hardened and the colour had changed from green to brown. Harvested seeds were directly sown out onto soil without prior treatment.
Silver nitrate treatment for induction of male flowers
Male flowers on female plants were induced for some plants grown under short‐day conditions (12:12) using an aqueous solution of 1.5 mm silver nitrate and 6 mm sodium thiosulphate, as described previously (Lubell & Brand, 2018). The silver nitrate solution was applied using a pipette on individual inflorescence primordia (50 μL). Once plants had started the short‐day treatments (12 h of light), silver nitrate treatment was conducted between two and five times with 3‐day intervals and suspended as soon as male flowers were visible.
CONFLICT OF INTEREST
This research was partially funded by GreenLight Medicines (Ireland); however, the company was not involved in the study design and analysis.
Supporting information
Figure S1. Temperature and humidity records.
Figure S2. Hemp plants grown under different light conditions differ in size.
Figure S3. Pot size influences flowering time.
Figure S4. Germination rate of seeds generated with the speed breeding protocol.
Figure S5. Treatment of monoecious and dioecious hemp plants with aqueous silver nitrate induces the development of male flowers.
Table S1. Phenotyping data of plants grown in this study.
ACKNOWLEDGEMENTS
SS was supported by a postdoctoral scholarship of the Irish Research Council and GreenLight Medicines (Ireland) under the Enterprise Partnership Scheme (grant no. EPSPD/2019/220, Enterprise Partner GreenLight Medicines). CAD is supported by an Irish Research Council–Environmental Protection Agency Government of Ireland Postgraduate Scholarship (grant no. GOIPG/2019/1987). JS is supported by a Chinese Research Council Postgraduate Scholarship (grant no. CSC 201908300031). We thank GreenLight Medicines for providing seeds. We are grateful to James Linden and Le Roy Dowey from GreenLight Medicines for support and stimulating discussions. Open access funding provided by IReL.
Linked article: This paper is the subject of a Research Highlight article. To view this Research Highlight article visit https://doi.org/10.1111/tpj.16108.
DATA AVAILABILITY STATEMENT
All relevant data can be found within the article and its supporting materials.
REFERENCES
- Ahmed, A.T.M.F. , Islam, M.Z. , Mahmud, M.S. , Sarker, M.E. , & Islam, M.R. (2022). Hemp as a potential raw material toward a sustainable world: A review. Heliyon, 8, e08753. 10.1016/j.heliyon.2022.e08753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaducci, S. , Colauzzi, M. , Bellocchi, G. & Venturi, G. (2008) Modelling post‐emergent hemp phenology (Cannabis sativa L.): theory and evaluation. European Journal of Agronomy, 28, 90–102. Available from: 10.1016/j.eja.2007.05.006 [DOI] [Google Scholar]
- Andrés, F. & Coupland, G. (2012) The genetic basis of flowering responses to seasonal cues. Nature Reviews. Genetics, 13, 627–639. Available from: 10.1038/nrg3291 [DOI] [PubMed] [Google Scholar]
- Fang, Y. , Wang, L. , Sapey, E. , Fu, S. , Wu, T. , Zeng, H. et al. (2021) Speed‐breeding system in soybean: integrating off‐site generation advancement, fresh seeding, and marker‐assisted selection. Frontiers in Plant Science, 12, 717077. Available from: 10.3389/fpls.2021.717077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux, A.‐M. , Draye, X. , Lambert, R. , d'Andrimont, R. , Raulier, P. & Bertin, P. (2013) The relationship of stem and seed yields to flowering phenology and sex expression in monoecious hemp (Cannabis sativa L.). European Journal of Agronomy, 47, 11–22. Available from: 10.1016/j.eja.2013.01.006 [DOI] [Google Scholar]
- Ghosh, S. , Watson, A. , Gonzalez‐Navarro, O.E. , Ramirez‐Gonzalez, R.H. , Yanes, L. , Mendoza‐Suárez, M. et al. (2018) Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nature Protocols, 13, 2944–2963. Available from: 10.1038/s41596-018-0072-z [DOI] [PubMed] [Google Scholar]
- Hall, M.C. , Dworkin, I. , Ungerer, M.C. & Purugganan, M. (2007) Genetics of microenvironmental canalization in Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America, 104, 13717–13722. Available from: 10.1073/pnas.0701936104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop‐Harrison, J. (1957) The experimental modification of sex expression in flowering plants. Biological Reviews, 32, 38–90. Available from: 10.1111/j.1469-185X.1957.tb01576.x [DOI] [Google Scholar]
- Ingrao, C. , Lo Giudice, A. , Bacenetti, J. , Tricase, C. , Dotelli, G. , Fiala, M. et al. (2015) Energy and environmental assessment of industrial hemp for building applications: a review. Renewable and Sustainable Energy Reviews, 51, 29–42. Available from: 10.1016/j.rser.2015.06.002 [DOI] [Google Scholar]
- Jähne, F. , Hahn, V. , Würschum, T. & Leiser, W.L. (2020) Speed breeding short‐day crops by LED‐controlled light schemes. Theoretical and Applied Genetics, 133, 2335–2342. Available from: 10.1007/s00122-020-03601-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, Y. & Weigel, D. (2007) Move on up, it's time for change mobile signals controlling photoperiod‐dependent flowering. Genes and Development, 21, 2371–2384. Available from: 10.1101/gad.1589007 [DOI] [PubMed] [Google Scholar]
- Lee, C.‐R. , Anderson, J.T. & Mitchell‐Olds, T. (2014) Unifying genetic canalization, genetic constraint, and genotype‐by‐environment interaction: QTL by genomic background by environment interaction of flowering time in Boechera stricta. PLoS Genetics, 10, e1004727. Available from: 10.1371/journal.pgen.1004727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisson, S.N. , Mendham, N.J. & Carberry, P.S. (2000a) Development of a hemp (Cannabis sativa L.) simulation model 2.The flowering response of two hemp cultivars to photoperiod. Australian Journal of Experimental Agriculture, 40, 413. Available from: 10.1071/EA99059 [DOI] [Google Scholar]
- Lisson, S.N. , Mendham, N.J. & Carberry, P.S. (2000b) Development of a hemp (Cannabis sativa L.) simulation model 1.General introduction and the effect of temperature on the pre‐emergent development of hemp. Australian Journal of Experimental Agriculture, 40, 405. Available from: 10.1071/EA99058 [DOI] [Google Scholar]
- Lubell, J.D. & Brand, M.H. (2018) Foliar sprays of silver thiosulfate produce male flowers on female hemp plants. HortTechnology, 28, 743–747. Available from: 10.21273/HORTTECH04188-18 [DOI] [Google Scholar]
- Moher, M. , Jones, M. & Zheng, Y. (2021) Photoperiodic response of In vitro Cannabis sativa plants. HortScience, 56, 108–113. Available from: 10.21273/HORTSCI15452-20 [DOI] [Google Scholar]
- Nagatoshi, Y. & Fujita, Y. (2019) Accelerating soybean breeding in a CO2‐supplemented growth chamber. Plant & Cell Physiology, 60, 77–84. Available from: 10.1093/pcp/pcy189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salentijn, E.M.J. , Petit, J. & Trindade, L.M. (2019) The complex interactions between flowering behavior and fiber quality in hemp. Frontiers in Plant Science, 10, 614. Available from: 10.3389/fpls.2019.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling, S. , Dowling, C.A. , Shi, J. , Hunt, D. , O'Reilly, E. , Perry, A.S. et al. (2021) The cream of the crop: biology, breeding, and applications of Cannabis sativa. Annual Plant Reviews, 4, 471–528. Available from: 10.1002/9781119312994 [DOI] [Google Scholar]
- Schluttenhofer, C. & Yuan, L. (2017) Challenges towards revitalizing hemp: a multifaceted crop. Trends in Plant Science, 22, 917–929. Available from: 10.1016/j.tplants.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Stack, G.M. , Toth, J.A. , Carlson, C.H. , Cala, A.R. , Marrero‐González, M.I. , Wilk, R.L. et al. (2021) Season‐long characterization of high‐cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy, 13, 546–561. Available from: 10.1111/gcbb.12793 [DOI] [Google Scholar]
- Watson, A. , Ghosh, S. , Williams, M.J. , Cuddy, W.S. , Simmonds, J. , Rey, M.‐D. et al. (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants, 4, 23–29. Available from: 10.1038/s41477-017-0083-8 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Anderson, S.L. , Brym, Z.T. & Pearson, B.J. (2021) Photoperiodic flowering response of essential oil, grain, and fiber hemp (Cannabis sativa L.) cultivars. Frontiers in Plant Science, 12, 694153. Available from: 10.3389/fpls.2021.694153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Temperature and humidity records.
Figure S2. Hemp plants grown under different light conditions differ in size.
Figure S3. Pot size influences flowering time.
Figure S4. Germination rate of seeds generated with the speed breeding protocol.
Figure S5. Treatment of monoecious and dioecious hemp plants with aqueous silver nitrate induces the development of male flowers.
Table S1. Phenotyping data of plants grown in this study.
Data Availability Statement
All relevant data can be found within the article and its supporting materials.


