Abstract
Background
Chagas disease is a well-known cause of cardiomyopathy in Latin America; however, 300 000 individuals are estimated to have Chagas disease in the United States. This study examined the prevalence and impact of Chagas cardiomyopathy (CCM) in a US population. We hypothesized that patients with CCM would have increased morbidity and mortality when compared with patients with non-CCM.
Methods and Results
This is a single-center, prospective cohort study. Enrollment criteria were new diagnosis of nonischemic cardiomyopathy (left ventricular ejection fraction ≤40%) and previous residence in Latin America for at least 12 months. Serological testing for Trypanosoma cruzi was performed at enrollment. The primary end point was all-cause mortality or heart transplantation. The secondary end point was heart failure–related hospitalization. A total of 135 patients were enrolled, with a median of 43 months of follow-up. Chagas disease was diagnosed in 25 (19%) patients. The primary end point occurred in 9 patients (36%) in the CCM group and in 11 patients (10%) in the non-CCM group (hazard ratio [HR], 4.46; 95% confidence interval, 1.8–10.8; P=0.001). The secondary end point occurred in 13 patients (52%) in the CCM group and in 35 patients (32%) in the non-CCM group (HR, 2.22; 95% confidence interval, 1.2–4.2; P=0.01).
Conclusions
There is a high prevalence of Chagas disease among Latin American immigrants diagnosed with nonischemic cardiomyopathy in Los Angeles. Advanced CCM portends a poor prognosis and is associated with increased all-cause mortality/heart transplantation and heart failure–related hospitalization.
Keywords: cardiomyopathies, Chagas cardiomyopathy, heart failure, mortality, prognosis
Chagas disease (CD), first described in 1909 by the Brazilian physician Carlos Chagas, is caused by the protozoan Trypanosoma cruzi.1 The disease is primarily transmitted to humans via triatomine insects, also known as kissing bugs, although other potential routes of transmission include blood transfusion, organ transplantation, contaminated food ingestion, laboratory accidents, or vertically from mother to fetus. CD is chronic and systemic, with approximately one third of patients developing cardiac manifestations ranging from conduction abnormalities and dysrhythmias to apical aneurysms and dilated cardiomyopathy.2
The World Health Organization estimates that 7 to 8 million people worldwide are infected with T cruzi.3 Although most infected persons live in Latin America where the disease is endemic, immigration patterns have made CD an important health issue in the United States where >300 000 individuals are estimated to be infected.4 California has >5 million residents who were born in Latin America, and 40% of those live in Los Angeles County alone.5,6 T cruzi has been detected in Los Angeles since 1984,7 and a small cohort of patients with Chagas heart disease from a Los Angeles hospital were reported in 1991.8 Los Angeles blood banks have estimated seroprevalence rates among all blood donors to be as high as 1 in 3800,9 and 2 cases of CD transmitted from heart transplantation in Los Angeles were reported in 2006.10
The prevalence of heart failure (HF) in the United States continues to rise: there are currently 5.1 million Americans with HF and it is estimated that by 2030 the prevalence of HF will increase by 25%. The total costs of HF are significant: the current estimate of $32 billion is expected to increase nearly 120% to $70 billion by 2030.11 Using available immigration trends and existing prevalence rates, 1 conservative estimate places the number of Chagas cardiomyopathy (CCM) cases in the United States at 30 000 to 45 000.12 These prevalence estimates, combined with available cost information for HF, imply a total cost of at least $200 to $300 million for CCM in the United States.
Despite a growing appreciation for CD in the United States, the burden of disease remains poorly defined.13 The purpose of this study is to estimate the prevalence and impact of CD in a population of Latin American immigrants diagnosed with nonischemic cardiomyopathy in a Los Angeles county hospital. On the basis of previous studies, we hypothesized that CCM would be associated with increased mortality and hospitalization compared with non-CCM.
Methods
Ethics Statement
This study was approved by the Institutional Review Board of the Olive View-UCLA Education and Research Institute. All participants provided written informed consent before study enrollment.
Study Location
Olive View-UCLA Medical center is a 377-bed Los Angeles county hospital, which serves a population of 1 584 000 adults (≥18) covering 1123 m2. This population is 43% white, 38% Latino, 14% Asian, 3% black, and 2% other. Thirty-one percent of adults have an income of <200% of the federal poverty level, and 28% of residents (aged 18–64 years) are uninsured all or part of the year. These statistics were not significantly different when compared with the entire population of Los Angeles County.14
Study Population
All Latin American immigrant patients with newly diagnosed non-ischemic cardiomyopathy at Olive View-UCLA Medical center were asked to participate in this study. From May 2007 to October 2011, 135 patients were prospectively enrolled into the study and followed until June 2012. Inclusion criteria were cardiomyopathy with left ventricular ejection fraction (LVEF) of ≤40% as documented by either echocardiography, gated single-photon emission computed tomography, or left ventriculography during cardiac catheterization, aged ≥18 years, and previous residence in Latin America (Mexico, Central, and South America) for at least 1 year. Exclusion criteria were coronary artery disease (evidence of ischemia or infarct on non-invasive stress testing or >70% stenosis on coronary angiography), severe valvular disease, history of any substance abuse, thyroid disease, severe uncontrolled hypertension, or a history of uncontrolled tachyarrhythmias. There was no compensation for participation. No information was collected on individuals who refused to participate, although only 1 individual refused to participate. Once enrolled, no participant was lost to follow-up or withdrew from the study.
Demographic Variables
At the time of study enrollment, participants were interviewed to assess basic demographic information, previous and current living situations, medical history, and current medication use. Because of low counts, response categories for country of origin were collapsed into 4 categories: Mexico, El Salvador, Guatemala, and other (Honduras, Nicaragua, Argentina, and Colombia). Similarly, response categories for type of Latin American house were collapsed into 3 categories: concrete, adobe, and other (brick, thatched, and wood).
Objective Data
At the time of study enrollment, all patients underwent a standard 12-lead ECG and transthoracic echocardiogram. Electrocardiographic abnormalities were classified according to the Minnesota Code Manual of Electrocardiographic Findings.15 Echocardiography was used to determine LVEF by visual estimation and left ventricular end-diastolic diameter (LVEDD) from the standard parasternal long axis view.16 ECGs and echocardiograms were interpreted by 2 board-certified cardiologists blinded to the study; discrepancies were resolved by a third board-certified cardiologist with consensus opinion.
Diagnosis of CD
Serological testing was performed on study enrollment by the Centers for Disease Control and Prevention, Parasitic Diseases Laboratory using immunofluorescence assay, and ELISA (Chagatest ELISA recombinate v. 3.0; Wiener Laboratorios, Rosario, Argentina) tests. Participants were considered seropositive only if both the tests were positive.
End Points
The primary end point was all-cause mortality or heart transplantation. Death or heart transplantation was confirmed by reviewing medical records, contacting patients/family members, and accessing social security databases. The secondary end point was HF-related hospitalization. HF-related hospitalizations were confirmed by reviewing medical records or contacting patients/family members. For the purposes of this study, the diagnosis of HF was made when the terms CHF, CHF exacerbation, and fluid/volume overload were documented as discharge diagnoses on the discharge summary. One investigator with a medical background ascertained and interpreted the information about end points with the assistance of nonmedical study personnel. The time from study enrollment until the primary or secondary end points occurred was used for survival analysis.
Statistical Analysis
Categorical variable frequencies and proportions (%) are reported, whereas continuous variables are reported with medians and quartiles (25%, 75%). The P value for comparing baseline differences was determined by the Fisher exact test for categorical data or Wilcoxon rank-sum test for continuous data.
Survival curves for the primary and secondary end points were estimated by the Kaplan–Meier method, and the P values for differences in survival curves between groups were computed by the log-rank test. The crude mortality rate is the number of deaths for all patients in that group divided by the total number of person-months of follow-up for all patients in that group. The crude hospitalization rate is similar, with the numerator representing the total number of hospitalizations for that group.
Univariate Cox proportional-hazard models were used to compute the hazard rate ratio for comparing time-dependent primary and secondary end point event rates between groups. The proportional hazard assumption was examined in all Cox models using the Supremum test. There was no evidence that the proportional hazard assumption was violated. There were insufficient events to perform valid multivariate Cox proportional-hazard models. All P values are 2-sided, with P<0.05 considered significant for all analyses. Analyses were conducted with SAS software, version 9.4 (SAS Inc, Cary, NC).
Results
A total of 135 participants were followed for a median of 43 months (3.6 years), with CD diagnosed in 25 (19%) patients. The baseline characteristics of the study population are shown in Table 1, categorized by the presence or absence of CD. As a group, 57% of participants were men. The median age of the overall group was 57 years. The prevalence of CCM, in decreasing order, was 38% for El Salvador, 25% for Guatemala, and 8% for Mexico. There were no differences in location (rural versus urban) or type of house (concrete, adobe, or other) among the CCM or non-CCM groups; however, those with CCM spent more time in their native country (41 versus 26 years; P=0.002) and less time in the United States (13 versus 24 years, P=0.01). Clinical variables such as the prevalence of hypertension, hyperlipidemia, tobacco use, and alcohol use were not clinically or significantly different between CCM and non-CCM groups, but diabetes mellitus was less prevalent in the CCM group (4% versus 34%; P=0.002). Therapeutic variables, including the use of β-blockers, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, aldosterone-antagonists, or HMG-CoA reductase inhibitors (statins), were no different between CCM and non-CCM groups, but amiodarone (40% versus 6%; P<0.001) and implantable cardioverter-defibrillator use (36% versus 7%; P=0.001) was more common in the CCM group.
Table 1.
Baseline Characteristics
| Characteristic | CCM (n=25) | Non-CCM (n=110) | All (n=135)* | P Value† |
|---|---|---|---|---|
| Demographic | ||||
| Male sex, n (%) | 12 (48) | 65 (59) | 77 (57) | 0.37 |
| Median age, y (25%, 75%) | 58 (50, 66) | 56 (46, 66) | 57 (47, 66) | 0.04 |
| Country of origin | ||||
| Mexico, n (%) | 6 (25) | 65 (59) | 71 (53) | 0.005 |
| El Salvador, n (%) | 13 (54) | 21 (19) | 34 (25) | 0.001 |
| Guatemala, n (%) | 5 (21) | 15 (14) | 20 (15) | 0.36 |
| Other, n (%) | 0 (0) | 9 (8) | 9 (7) | 0.36 |
| Location | ||||
| Rural, n (%) | 10 (40) | 56 (52) | 66 (50) | 0.38 |
| Urban, n (%) | 15 (60) | 52 (48) | 67 (50) | 0.38 |
| Type of house | ||||
| Concrete, n (%) | 11 (46) | 48 (44) | 59 (45) | 1.00 |
| Adobe, n (%) | 9 (38) | 44 (41) | 53 (40) | 0.95 |
| Other, n (%) | 4 (17) | 16 (15) | 20 (15) | 0.76 |
| Median time in native country, y (25%, 75%) | 41 (26, 56) | 26 (16, 35) | 29 (16, 42) | 0.002 |
| Median time in United States, y (25%, 75%) | 13 (11, 34) | 24 (16, 31) | 22 (13, 31) | 0.01 |
| Clinical | ||||
| Hypertension, n (%) | 10 (40) | 47 (43) | 57 (42) | 0.83 |
| Hyperlipidemia, n (%) | 4 (16) | 16 (15) | 20 (15) | 0.77 |
| Diabetes mellitus, n (%) | 1 (4) | 37 (34) | 38 (28) | 0.002 |
| Tobacco use, n (%) | 8 (32) | 21 (19) | 29 (22) | 0.18 |
| Alcohol use, n (%) | 6 (24) | 26 (24) | 32 (24) | 1.00 |
| Therapeutic | ||||
| β-blocker, n (%) | 22 (88) | 103 (94) | 125 (93) | 0.39 |
| ACEI/ARB, n (%) | 23 (92) | 98 (89) | 121 (90) | 1.00 |
| Aldosterone-antagonist, n (%) | 7 (28) | 32 (29) | 39 (29) | 1.00 |
| Statin, n (%) | 9 (36) | 56 (51) | 65 (48) | 0.19 |
| Amiodarone, n (%) | 10 (40) | 6 (6) | 16 (12) | < 0.001 |
| ICD device, n (%) | 9 (36) | 8 (7) | 17 (13) | 0.001 |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCM, Chagas cardiomyopathy; and ICD, implantable cardioverter-defibrillator.
The following variables had a different number of total patients: country of origin (CCM [n=24], Non-CCM [n=110]), location (CCM [n=25], Non-CCM [n=108]), type of house (CCM [n=24], Non-CCM [n=108]), tobacco use (CCM [n=25], Non-CCM [n=109]), and alcohol use (CCM [n=25], Non-CCM [n=109]).
Fisher exact test (categorical variables) or t test (continuous variables).
Electrocardiographic and echocardiographic characteristics of the study group are shown in Table 2. The presence of right-bundle branch block (20% versus 1%; P<0.001) was more common in the CCM group, but all other electrocardiographic variables were similar between the 2 groups. As a study group, the mean LVEDD was mildly dilated at 62 mm, whereas the median LVEF was severely depressed at 25%. There were no differences in LVEDD or LVEF among CCM or non-CCM groups.
Table 2.
Electrocardiographic and Echocardiographic Characteristics
| Characteristic | CCM (n=25) | Non-CCM (n=103)* | All (n=128) | P Value† |
|---|---|---|---|---|
| Electrocardiographic | ||||
| Rhythm | ||||
| NSR, n (%) | 21 (84) | 79 (77) | 100 (78) | 0.60 |
| Atrial fibrillation/flutter, n (%) | 1 (4) | 16 (16) | 17 (13) | 0.19 |
| Paced, n (%) | 3 (12) | 6 (6) | 9 (7) | 0.38 |
| Conduction disease | ||||
| LBBB, n (%) | 2 (8) | 26 (25) | 28 (22) | 0.11 |
| RBBB, n (%) | 5 (20) | 1 (1) | 6 (5) | < 0.001 |
| LAFB, n (%) | 1 (4) | 11 (11) | 12 (9) | 0.46 |
| IVCD, n (%) | 6 (24) | 9 (9) | 15 (11) | 0.08 |
| Other | ||||
| Q waves, n (%) | 4 (16) | 8 (8) | 12 (9) | 0.25 |
| LVH, n (%) | 6 (24) | 27 (26) | 33 (26) | 1.00 |
| Echocardiographic | ||||
| Median LVEDD, mm (25%, 75%) | 66 (59, 73) | 62 (56, 69) | 62 (55, 69) | 0.15 |
| Median LVEF, % (25%, 75%) | 20 (8, 33) | 25 (18, 33) | 25 (18, 32) | 0.24 |
CCM indicates Chagas cardiomyopathy; IVCD, intraventricular conduction delay; LAFB, left anterior fascicular block; LBBB, left bundle-branch block; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NSR, normal sinus rhythm; and RBBB, right bundle-branch block..
7 EKGs were missing from the non-CCM group.
Fisher exact test (categorical variables) or t test (continuous variables).
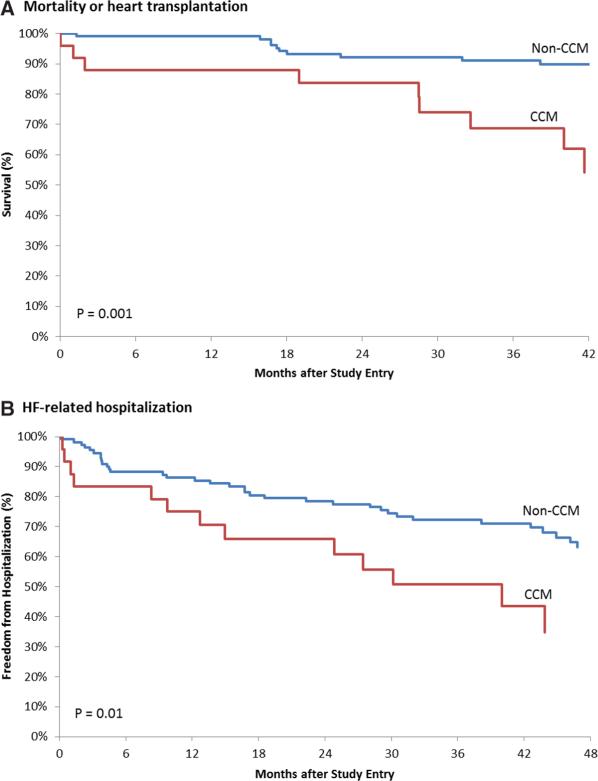
Table 3 displays the results of the unadjusted crude outcomes. All-cause mortality or heart transplantation occurred in 9 patients (36%) in the CCM group and in 11 patients (10%) in the non-CCM group (hazard ratio [HR], 4.46; 95% confidence interval, 1.8–10.8; P=0.001). The primary end point was driven almost entirely by all-cause mortality, because only 1 heart transplantation occurred in a patient with CCM. HF-related hospitalization occurred in 13 patients (52%) in the CCM group and in 35 patients (32%) in the non-CCM group (HR, 2.22; 95% confidence interval, 1.2–4.2; P=0.01). Kaplan–Meier survival curves for all-cause mortality/heart transplantation and HF-related hospitalization are shown in Figure 1.
Table 3.
Unadjusted Outcomes
| Outcome | CCM (n=25) | Non-CCM (n=110) | P Value* | Hazard Ratio (95% CI)† |
|---|---|---|---|---|
| Mortality or heart transplant, n (%) | 9 (36) | 11 (10) | 0.001 | 4.46 (1.8–10.8) |
| Hazard rates per 1000 person-months, % (95% CI) | 11.2 (3.9–18.5) | 2.4 (1.0–3.8) | ||
| Heart failure–related hospitalization, n (%) | 13 (52) | 35 (32) | 0.01 | 2.22 (1.2–4.2) |
| Hazard rates per 1000 person-months, % (95% CI) | 21.1 (9.6–32.5) | 9.0 (6.0–12.0) |
CCM indicates Chagas cardiomyopathy; and CI, confidence interval.
Log-rank test (Mantel-Cox).
Univariate Cox proportional-hazard models.
Figure 1.
Kaplan–Meier survival curves for the primary and secondary end points. Kaplan–Meier estimates are shown for all-cause mortality/heart transplantation (A) and HF-related hospitalization (B). Differences in the CCM and non-CCM groups were assessed using the log-rank test. CCM indicates Chagas cardiomyopathy; and HF, heart failure.
Discussion
This is the first and largest prospective study of patients with CCM in the United States. We followed a group of 135 Latin American immigrants diagnosed with advanced nonischemic cardiomyopathy (median LVEF, 25%) at a Los Angeles county hospital for a median of 43 months. CD was diagnosed by positive immunofluorescence assay and ELISA tests in 25 patients, yielding a 19% prevalence of advanced CCM (at least intermediate risk by the Rassi criteria17). This prevalence estimate is similar to the 13% prevalence found in a smaller study of Latin American immigrants diagnosed with dilated cardiomyopathy in New York City.18 This finding cannot be emphasized enough because multiple investigators have documented an increased mortality among patients with various stages of CCM when compared with those with non-CCM. Our data corroborate these findings, as CCM was associated with a primary end point of mortality or heart transplantation that was 4.46× higher than non-CCM (Figure 1A), despite similar LVEDD and LVEF. This HR estimate is in accordance with previous estimates of 3.29 to 6.09 for CCM-related mortality.19–21
In addition to increased mortality/heart transplantation, our study demonstrated an increased burden of HF-related hospitalization in patients with CCM. HF-related hospitalization was 2.22× higher in CCM compared with non-CCM (Figure 1B), despite similar LVEDD and LVEF. These findings are of paramount importance in the era of Medicare's Hospital Readmission Reduction Program, in which hospital admissions and readmissions for HF are increasingly scrutinized and tied to reimbursement.22 Our study provides the first insight into HF-related hospitalization for CCM in a US population.
Identifying CD in a patient with cardiomyopathy not only helps with prognostication but also aids in clinical treatment decisions. For instance, the antiarrhythmic amiodarone has been shown to have direct anti-T cruzi effects by disrupting calcium homeostasis in vitro,23 with a case report of decreased parasitemia and improved LVEF.24,25 Our center uses amiodarone liberally in patients with CCM, thus explaining the significant difference in amiodarone use observed in our study between the CCM and non-CCM groups (40% versus 6%; P<0.001). The high burden of malignant ventricular arrhythmias, which can involve the epicardium, right ventricle, or apical aneurysm, are important considerations when contemplating implantable cardioverter-defibrillator therapy or radiofrequency ablation in patients with CD.26–29 Finally, it is now recognized that survival in patients with CCM after heart transplant may be better than those patients with other forms of nonischemic cardiomyopathy; however, reactivation rates as high as 26.5% to 42.9% have been reported and require a unique post-transplant surveillance process to monitor for reactivation and provide prompt antiparasitic treatment.30,31
Many baseline characteristics were found to be associated with CD in this study. Although the majority of participants originated from Mexico (53%), those diagnosed with CCM were predominantly from El Salvador (54%). These findings may be because of sampling error as the heterogeneous geographical distribution of CD in Mexico makes it possible that the Mexican immigrants included in our study were not from highly endemic areas within Mexico.32,33 However, 1 small study of patients with Chagas heart disease in Los Angeles also reported the highest prevalence among those from El Salvador.8 The fact that El Salvador has the highest prevalence of CD in Central America likely also plays a role in these findings.2 We also found that participants who lived in their native country longer were more likely to have acquired CD, presumably because of increased exposure to the causative agent. Finally, the finding of right-bundle branch block on EKG was associated with CCM as has been previously reported.2
Despite our prospective cohort of patients with CCM representing the largest in the United States, one of the major limitations of our study was the small sample size. Because CD is not endemic to the United States, our sample of CCM was relatively small compared with similar studies conducted in Brazil, which have study populations of CCM exceeding 200 patients.19–21 However, as our entire CCM cohort originated from Mexico, El Salvador, or Guatemala, this cohort represents an area of Latin America not previously studied with respect to risk factors and outcomes. Furthermore, Central America and Brazil are populated with different strains of T cruzi, which may affect the virulence and clinical course of CD.2,34,35 Another limitation of the study is the assumption that seropositivity for CD meant that the parasitic infection had caused cardiomyopathy. To address this issue, we excluded patients with other known causes of nonischemic cardiomyopathy. Because it is entirely possible that CD and another cause of nonischemic cardiomyopathy may coexist in the same person, the prevalence of CD in our study group may not accurately reflect the true prevalence of CCM among Latin American patients with cardiomyopathy in Los Angeles. Moreover, because only advanced cases of CCM were enrolled in this study, our results should not be misconstrued to represent outcomes of all patients with CD. Previous research has shown that seropositivity for CD with little or no cardiac involvement is associated with a more indolent disease course.2,17
In conclusion, this is the first and largest prospective study following a group of Latin American immigrants with CCM in the United States. Although somewhat limited by sample size, the data suggest that ≈1 in 5 Latin American immigrants in Los Angeles with nonischemic cardiomyopathy may have CD. Immigrants from El Salvador and those who have lived in their native country >40 years were more likely to have CCM. Survival analysis showed that advanced CCM is clearly associated with increased mortality/heart transplantation and hospitalization compared with non-CCM, despite similar LVEDD and LVEF. On the basis of the results of this and other studies, healthcare facilities providing care for Latin American immigrants diagnosed with nonischemic cardiomyopathy in the United States should consider evaluating for CD as a possible cause of cardiomyopathy.18
CLINICAL PERSPECTIVE.
There is a growing concern for Chagas disease in the United States; however, little data have been published on this subject. Our work represents the first and largest prospective study following a group of Latin American immigrants evaluating Chagas cardiomyopathy (CCM) in the United States. The data suggest that ≈1 in 5 Latin American immigrants in Los Angeles with nonischemic cardiomyopathy have Chagas disease. Despite similar cardiac risk factors, medical management, and ejection fraction, the diagnosis of CCM was associated with an over 4× higher rate of mortality or heart transplantation than non-CCM. Furthermore, heart failure–related hospitalization was more than twice as high in CCM compared with non-CCM. The practicing clinician, particularly in areas of the United States with large Latin American immigrant populations, should be cognizant of these findings, as the data suggest that nonischemic cardiomyopathy in Latin American immigrants may be because of Chagas disease, which portends a poorer prognosis than other forms of cardiomyopathy. The diagnosis of CCM may also influence several other management decisions.
Acknowledgments
We thank Dr Shah, MD, Olive View-UCLA Medical Center, for the acquisition and analysis of EKG data and Jeffrey Gornbien, MS, DrPH, UCLA, for his statistical expertise and compensated assistance with statistical analysis.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control.
Disclosures
None.
References
- 1.Chagas C. Nova tripanozomiase humana. Estudos sobre a morfolojía e o ciclo evolutivo de Schizotrypanum cruzi n. gen., n. sp., ajente etiolójico de nova entidade morbida do homen. Mem Inst Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- 2.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Fact sheet no. 340: Chagas disease (American trypanosomiasis) [March 31, 2015]. http://www.who.int/mediacentre/factsheets/fs340/en.
- 4.Centers for Disease Control and Prevention American Trypanosomiasis (also known as Chagas Disease) [March 31, 2015]. http://www.cdc.gov/parasites/chagas/index.html.
- 5.US Census Bureau Selected social characteristics in the United States: 2006–2010 American Community Survey 5-year estimates. [March 31, 2015]. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk.
- 6.Pew Hispanic Center Statistical portrait of the foreign-born population in the United States, 2011. [March 31, 2015]. http://www.pewhispanic.org/2013/01/29/statistical-portrait-of-the-foreign-born-population-in-the-united-states-2011.
- 7.Schiffler RJ, Mansur GP, Navin TR, Limpakarnjanarat K. Indigenous Chagas’ disease (American trypanosomiasis) in California. JAMA. 1984;251:2983–2984. [PubMed] [Google Scholar]
- 8.Hagar JM, Rahimtoola SH. Chagas’ heart disease in the United States. N Engl J Med. 1991;325:763–768. doi: 10.1056/NEJM199109123251103. doi: 10.1056/NEJM199109123251103. [DOI] [PubMed] [Google Scholar]
- 9.Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21:476–482. doi: 10.1097/QCO.0b013e32830ef5b6. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- 10.Kun H, Moore A, Mascola L, Steurer F, Lawrence G, Kubak B, Radhakrishna S, Leiby D, Herron R, Mone T, Hunter R, Kuehnert M, Chagas Disease in Transplant Recipients Investigation Team Transmission of Trypanosoma cruzi by heart transplantation. Clin Infect Dis. 2009;48:1534–1540. doi: 10.1086/598931. doi: 10.1086/598931. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–e54. doi: 10.1086/605091. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 13.Hotez PJ, Dumonteil E, Betancourt Cravioto M, Bottazzi ME, Tapia-Conyer R, Meymandi S, Karunakara U, Ribeiro I, Cohen RM, Pecoul B. An unfolding tragedy of Chagas disease in North America. PLoS Negl Trop Dis. 2013;7:e2300. doi: 10.1371/journal.pntd.0002300. doi: 10.1371/journal.pntd.0002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UCLA Center for Health Policy Research Los Angeles County Service Planning Area 2: San Fernando Valley. [March 31, 2015]. http://healthpolicy.ucla.edu/health-profiles/adults/Documents/2011-2012/SPA/SanFernandoValley.pdf.
- 15.Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. 2nd ed. Springer-Verlag; London, UK: 2010. [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher-Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 18.Kapelusznik L, Varela D, Montgomery SP, Shah AN, Steurer FJ, Rubinstein D, Caplivski D, Pinney SP, Turker D, Factor SH. Chagas disease in Latin American immigrants with dilated cardiomyopathy in New York City. Clin Infect Dis. 2013;57:e7. doi: 10.1093/cid/cit199. doi: 10.1093/cid/cit199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestetti RB, Muccillo G. Clinical course of Chagas’ heart disease: a comparison with dilated cardiomyopathy. Int J Cardiol. 1997;60:187–193. doi: 10.1016/s0167-5273(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 20.Pereira Nunes Mdo C, Barbosa MM, Ribeiro AL, Amorim Fenelon LM, Rocha MO. Predictors of mortality in patients with dilated cardiomyopathy: relevance of Chagas disease as an etiological factor. Rev Esp Cardiol. 2010;63:788–797. doi: 10.1016/s1885-5857(10)70163-8. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa AP, Cardinalli Neto A, Otaviano AP, Rocha BF, Bestetti RB. Comparison of outcome between Chagas cardiomyopathy and idiopathic dilated cardiomyopathy. Arq Bras Cardiol. 2011;97:517–525. doi: 10.1590/s0066-782x2011005000112. [DOI] [PubMed] [Google Scholar]
- 22.James J. Health policy brief: Medicare hospital readmissions reduction program. [March 31, 2015]. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102.
- 23.Benaim G, Sanders JM, Garcia-Marchán Y, Colina C, Lira R, Caldera AR, Payares G, Sanoja C, Burgos JM, Leon-Rossell A, Concepcion JL, Schijman AG, Levin M, Oldfield E, Urbina JA. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem. 2006;49:892–899. doi: 10.1021/jm050691f. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 24.Paniz-Mondolfi AE, Pérez-Alvarez AM, Lanza G, Márquez E, Concepción JL. Amiodarone and itraconazole: a rational therapeutic approach for the treatment of chronic Chagas’ disease. Chemotherapy. 2009;55:228–233. doi: 10.1159/000219436. doi: 10.1159/000219436. [DOI] [PubMed] [Google Scholar]
- 25.Benaim G, Paniz Mondolfi AE. The emerging role of amiodarone and dronedarone in Chagas disease. Nat Rev Cardiol. 2012;9:605–609. doi: 10.1038/nrcardio.2012.108. doi: 10.1038/nrcardio.2012.108. [DOI] [PubMed] [Google Scholar]
- 26.Cardinalli-Neto A, Greco OT, Bestetti RB. Automatic implantable cardioverter-defibrillators in Chagas’ heart disease patients with malignant ventricular arrhythmias. Pacing Clin Electrophysiol. 2006;29:467–470. doi: 10.1111/j.1540-8159.2006.00377.x. doi: 10.1111/j.1540-8159.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 27.Milei J, Pesce R, Valero E, Muratore C, Beigelman R, Ferrans VJ. Electrophysiologic-structural correlations in chagasic aneurysms causing malignant arrhythmias. Int J Cardiol. 1991;32:65–73. doi: 10.1016/0167-5273(91)90045-q. [DOI] [PubMed] [Google Scholar]
- 28.Sarabanda AV, Sosa E, Simões MV, Figueiredo GL, Pintya AO, Marin-Neto JA. Ventricular tachycardia in Chagas’ disease: a comparison of clinical, angiographic, electrophysiologic and myocardial perfusion disturbances between patients presenting with either sustained or nonsustained forms. Int J Cardiol. 2005;102:9–19. doi: 10.1016/j.ijcard.2004.03.087. doi: 10.1016/j.ijcard.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 29.Hsia HH, Marchlinski FE. Electrophysiology studies in patients with dilated cardiomyopathies. Card Electrophysiol Rev. 2002;6:472–481. doi: 10.1023/a:1021109130276. [DOI] [PubMed] [Google Scholar]
- 30.Casadei D, Chagas’ Disease Argentine Collaborative Transplant Consortium Chagas’ disease and solid organ transplantation. Transplant Proc. 2010;42:3354–3359. doi: 10.1016/j.transproceed.2010.09.019. doi: 10.1016/j.transproceed.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Kransdorf EP, Czer LS, Luthringer DJ, Patel JK, Montgomery SP, Velleca A, Mirocha J, Zakowski PC, Zabner R, Gaultier CR, Qvarnstrom Y, Benedict T, Steurer F, Bosserman E, Paddock CD, Rafiei M, Kobashigawa JA. Heart transplantation for Chagas cardiomyopathy in the United States. Am J Transplant. 2013;13:3262–3268. doi: 10.1111/ajt.12507. doi: 10.1111/ajt.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzmán-Bracho C. Epidemiology of Chagas disease in Mexico: an update. Trends Parasitol. 2001;17:372–376. doi: 10.1016/s1471-4922(01)01952-3. [DOI] [PubMed] [Google Scholar]
- 33.Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years–a review. Mem Inst Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 34.Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- 35.Manoel-Caetano Fda S, Silva AE. Implications of genetic variability of Trypanosoma cruzi for the pathogenesis of Chagas disease. Cad Saude Publica. 2007;23:2263–2274. doi: 10.1590/s0102-311x2007001000002. [DOI] [PubMed] [Google Scholar]