Abstract
Current data suggests that coronavirus disease 2019 (COVID-19) survivors experience long-lasting problems. It is not yet understood how long these symptoms last. The goal of this study was to compile all the data that was currently available to evaluate COVID-19's long-term effects at 12 months and above. We looked for studies published by December 15, 2022, in PubMed and Embase that discussed follow-up findings for COVID-19 survivors who had been alive for at least a year. A random-effect model was carried out to determine the combined prevalence of different long-COVID symptoms. The Joanna Briggs Institute tool was used to assess the risk of bias for the included studies, and the I2 statistics were used to evaluate the heterogeneity. After reviewing 3,209 studies, 46 were deemed admissible, with an aggregate COVID-19 population of 17976. At 12 months and above, 57% of patients reported a minimum of one symptom, and the five most prevalent symptoms were: dyspnea on exertion (34%, 95% CI 0.2; 0.94); difficulty in concentration (32%, 95% CI 0.16; 0.52); fatigue (31%, 95% CI 0.22; 0.40); frailty (31%, 95% CI 0.06; 0.78); and arthromyalgia (28%, 95% CI 0.09; 0.6). The findings of the present study showed that at 12 months and beyond, a sizable fraction of COVID-19 survivors still have lasting symptoms that impair several body systems. Long-COVID patients require an urgent understanding of pathophysiological processes and the development of tailored treatments.
Keywords: a systematic review, symptoms, prevalence, meta-analysis, long term covid-19
Introduction and background
Presently, the biggest threat to global public health is the coronavirus disease 2019 (COVID-19) pandemic. The World Health Organization (WHO) recorded approximately 668 million COVID-19 confirmed cases as of January 8, 2023, with over six million fatalities [1].
After recovering from an acute COVID-19 infection, a sizable proportion of patients report continuing symptoms that interfere with daily activities after the initial acute stage. A condition referred to as "Long COVID-19," "post-acute COVID-19," "persistent COVID-19 symptoms," "chronic COVID-19," "long-term sequelae," or "long-haulers" has been used to describe these post-COVID-19 patients. More recently, "post-acute sequelae of COVID-19" or "post-acute COVID-19 Syndrome (PACS)" has also been used to describe these individuals [2,3]. The World Health Organization (WHO) published a clinical case description for the post-COVID-19 condition using a Delphi consensus method on October 6, 2021: “the condition affects people who have a history of probable or confirmed SARS-CoV-2 infection, typically three months after the onset, and has symptoms that last for at least two months and cannot be accounted for by any other diagnosis.” Another review suggested long COVID-19 when symptoms last longer than four weeks after onset and/or problems develop months or years later [3,4].
Evidence is mounting that COVID-19 survivors may continue to have symptoms that involve several organ systems following the acute stage of the illness (also known as long-COVID) [5]. In multiple earlier systematic reviews and meta-analyses on long-COVID-19 infections, the prevalence of short- and medium-term persistent cardiac, respiratory, neurological, integumentary, musculoskeletal, and gastrointestinal symptoms was evaluated [6-9]. A meta-analysis [7] of 33 articles reported that 63.2%, 71.9%, and 45.9% of COVID-19 survivors had a minimum of one persistent symptom 30, 60, and 90 days after hospitalization or onset, and fatigue, difficulty breathing, cough, anosmia, ageusia, and joint pain are the most common symptoms. The most frequently reported symptoms, according to another meta-analysis [8] of 39 studies with seven months of follow-up, were weakness, fatigue, impaired focus, and shortness of breath. Another meta-analysis [6] of 15 publications found that COVID-19 survivors experienced over 50 persisting symptoms between 14 and 110 days following infection.
For longer-term persistent symptoms of COVID-19, it is unclear how long these various groups of symptoms will last. In view of the growing body of information on longer-duration follow-up of COVID-19 survivors, it is imperative to determine whether the pattern of persistent symptoms is different from the previously documented short- or medium-term symptoms. To offer a better picture of the long-term effects of COVID-19 based on scientific data, we sought to synthesize the evidence by pooling related studies related to the long-term effects of COVID-19 at 12 months and beyond. It will assist with the management and monitoring of COVID-19 survivors as well as the development of public health policies for medical services.
Review
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses statement was followed by this systematic review and meta-analysis [10]. The registration number for this study with PROSPERO is CRD42022355069.
Search strategy
We carried out a thorough search of the PubMed and Embase databases for articles that presented results for people who survived COVID-19 at 12-month or longer follow-ups, published after January 31, 2021, and in English only. The following search keywords yielded results: “(COVID-19 OR SARS-CoV-2 OR coronavirus OR long COVID-19 OR post-COVID-19)” AND “(long-term effect OR sequelae OR consequences)” AND “(cohort OR follow-up OR retrospective OR prospective)”. The searches merged free-text words with Medical Subject Headings (MeSH) phrases.
Study selection
The following criteria were met for articles to be considered for inclusion: (a) Cohort, cross-sectional, or case-control peer-reviewed studies reporting the prevalence of long-term symptoms among COVID-19 survivors; (b) articles published in the English language; (c) including a minimum of 50 COVID-19 survivors; (d) at least a follow-up period of 12 months after symptom onset; (e) patient inclusion of only those with laboratory-confirmed COVID-19. The following studies were left out: (a) studies where SARS-CoV-2 infection was not used as the exposure; (b) studies that did not have enough data to figure out what the long-term COVID-19 symptoms are; (c) duplicate studies or studies with the same participants; (d) editorials, reviews, case reports/series, conference papers, secondary analysis, or animal studies; and (e) qualitative designs.
Using the previously stated specific eligibility criteria, four reviewers independently looked at the titles along with the abstracts of the articles in pairs. Following that, each article was subjected to a full-text review to ensure that it met the eligibility criteria. The fifth reviewer addressed and resolved disagreements on the inclusion of a full-text article.
Data extraction
Four reviewers simultaneously extracted data in duplicate into a predetermined data collection form, and any inconsistencies were settled through consensus or collaboration with a fifth reviewer's input. Data were gathered in the following domains: i) basic study information, such as first author, publication year, and country; ii) study design; iii) follow-up duration and method; and iv) baseline patient demographics, such as the number of participants, median age, gender, and length of stay (LOS) in the hospital and intensive care unit (ICU).
Study quality
The "Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Studies Reporting Prevalence Data" [11] was used to assess the study quality of eligible papers. There are four possibilities for each of the nine items on this checklist. A qualitative assessment of the nine items was done, and we rated each paper's overall quality as excellent, moderate, or low. Two investigators independently assessed the quality, and disagreements were resolved through consensus or collaboration with a third investigator.
Statistical analyses
This meta-analysis was done to assess the Pooled Prevalence (PP) and its 95% confidence interval (CI) of COVID-19's long-term effects at 12 months and beyond. Random-effects or fixed-effects models were used, depending on the heterogeneity of the estimates (I2). Fixed-effects models were applied when studies had I2 ≤ 50%, and if studies showed significant heterogeneity (I2 ≥ 50%), random-effects models were applied. Only symptoms reported by three or more studies were included in the meta-analyses. A funnel plot for prevalence estimates following logit transformation was constructed for each symptom with more than 10 studies to visually detect publication bias. The R studio “version 4.1.2 (2021-11-01)” and the “Meta package (Schwarzer, 2016)” were used to conduct all the analyses. The significance level (0.05) for each two-sided statistical test was set.
Results
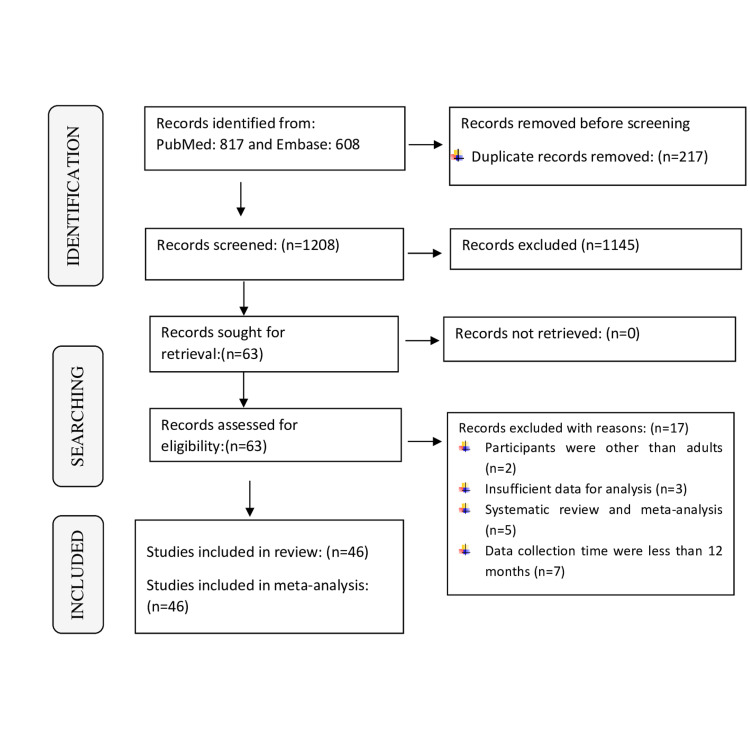
In total, 1425 records were found during the search of PubMed and Embase. After the abstract and full-text screening, this systematic review and meta-analysis included 46 eligible studies with a total of 17976 COVID-19 survivors. The PRISMA flowchart provided details on the procedure for evaluating the literature (Figure 1).
Figure 1. PRISMA flowchart.
Table 1 provides a summary of the 46 included studies’ characteristics (12-57). Most of these investigations were conducted in China (n = 13), Italy (n = 11), Spain (n = 7), Germany (n = 3), and Switzerland (n = 2), with a median follow-up length of 685 days from the initial SARS-CoV-2 positive swab being the longest. The number of participants for the listed studies ranges from 51 to 2433. More than half of the included research (n=22) used an in-person method of follow-up, and the preponderance of the included studies (n = 30) were single-center studies.
Table 1. Characteristics of included studies.
F: Female; ICU: Intensive care unit; LOS: Length of stay; M: Male; NR: Not reported; * Data in Median (Inter quartile range); ** Data in mean± Standard deviation
| Authors (Year of Publication) | Country | Study setting | Number of participants | Gender M: F | Hospitalization (LOS days) | ICU (LOS days) | Follow-up time | Method of follow-up |
| Aranda et al. (2022) [12] | Spain | Multicentre | 150 | 87: 63 | 9.0 (6.0–14.0) * | 30.5 (15.8–36.8) * | 395 days (384–417) * | In person |
| Becker et al. (2021) [13] | Switzerland | Bicentric | 90 | 56:34 | 9.44±6.77 ** | 15 NR | At 12 Month | NR |
| Bellan et al. (2021) [14] | Italy | NR | 200 | 122:78 | 9 (5–16) * | 9 (5–16) * | 366 days (363–369) * | In person |
| Boscolo-Rizzo et al. (2021) [15] | Italy | Single centre | 304 | 119:185 | Home isolation | Home isolation | At 12 Month | Telephone |
| Brien et al. (2022) [16] | Ireland | Single centre | 61 | 35:26 | 13.1(5.8-18.1) * | 16(26) NR | 430 days (398-458) * | In person |
| Catalán et al. (2021) [17] | Spain | Single centre | 76 | 67:09 | NR | NR | 1 year | Telephone |
| Chai et al. (2021) [18] | China | Multi centre | 432 | 246:252 | 21 (11–28) * | NR | 12.2 months (12.1–12.6) * | Telephone & in person |
| Fang et al. (2021) [19] | China | Multi centre | 1233 | 591:642 | 15(10–22) * | NR | 363(357–371) * days | NR |
| Fernández‑de‑las‑Peñas et al. (2021) [20] | Spain | Multi centre | 1950 | 1035:915 | 11.4 ±11.2** | 13 ±14** | 11.2±0.5 months ** | Telephone |
| Ferrucci et al. (2022) [21] | Italy | Single centre | 53 | 38:15 | 11.94 (7.07) * | NR | 11.92 ± 1.46 months ** | Telephone |
| Gamberini et al. (2021) [22] | Italy | Multi centre | 178 | 129:49 | NR | 23 (15–35) * | 1Year | Electronic |
| Huang et al. (2021) [23] | China | Single centre | 1276 | 681:595 | 14·0 (10·0–20·0) * | 18·0 (7·0–30·0) * | 12 Months | In person |
| Huang et al. (2022) [24] | China | Single centre | 1192 | 641:551 | 14·0 (10·0–20·0) * | 18·0 (6·0–30·0) * | 685 days (675-698) * | In person |
| Hussain et al. (2022) [25] | Sweden | Single centre | 105 | 80:25 | NR | 15 (7.5–26.5) * | 365 days (351–379) * | In person |
| Izquierdo et al. (2022) [26] | Spain | Multi centric | 453 | 260:193 | 15+13.5 ** | 48 ±10.6 ** | At 12 Month | Telephone |
| Kim et al. (2022) [27] | Korea | Single centre | 241 | 77:164 | NR | NR | 454 days (451- 458) * | Telephone |
| Latronico et al. (2021) [28] | Italy | Single centre | 51 | NR | NR | NR | At 12 Months | Telephone |
| Li et al. (2022) [29] | China | Single centre | 230 | 116:114 | 21 (16–29) * | 12 (5.7–9.5) * | At 1 year | In person |
| Li Z et al. (2022) [30] | China | Single centre | 535 | 216:319 | NR | NR | At 1 year | In person |
| Liao T et al. (2022) [31] | China | Single centre | 303 | 59:244 | 15 (9–26) * | NR | 395 days (382–408) * | In person |
| Lim RK et al. (2022) [32] | Canada | Single centre | 62 | 27:35 | NR | NR | 387 days (251–402) * | Telephone |
| Liu T et al. (2022) [33] | China | Single centre | 486 | 225:261 | NR | NR | At 12 months | In person |
| Liu Y et al. (2022) [34] | China | Single centre | 1438 | 691:747 | 20 (15-25) * | 72 NR | At 12 Months | Telephone |
| Lombardo MDM et al. (2021) [35] | Italy | Single centre | 303 | 138:165 | NR | NR | At 12 Months | Telephone |
| Maestre-Muñiz MM et al. (2021) [36] | Spain | Single centre | 543 | 275:268 | NR | NR | During 1year follow-up | Telephone |
| Maestrini V et al. (2021) [37] | Italy | Single centre | 118 | 67:51 | NR | NR | At 1year follow-up | Telephone |
| Martino GP et al. (2022) [38] | Italy | Single centre | 64 | 41:23 | NR | NR | At 12 months | Telephone |
| Mazza MG et al. (2022)[39] | Italy | Single centre | 192 | 131:61 | NR | NR | 387.39 ± 23.67 days** | NR |
| Noujaim et al. (2022) [40] | France | Multi centre | 120 | 65:55 | NR | NR | 12 months | In person |
| Ovrebotten et al. (2022) [41] | Norway | Multi centric | 178 | 105:73 | 6(3-11) * | NR | 389 days (289-462) * | In person |
| PHOSP COVID collaborative group (2022) [42] | United Kingdom | Multi centre | 924 | 572:319 | 17.0+24.7 ** | NR | 13 months (12-13) * | In person |
| Rank et al. (2021) [43] | Germany | Single centre | 83 | 63:20 | NR | NR | At 12 Month | In person |
| Rass et al. (2021) [44] | Austria | Multi centre | 81 | 48:33 | 8(0-18) * | 31(24-49) * | 416 days (401-437) * | In person |
| Rigoni et al. (2022) [45] | Italy | Single centre | 471 | 301:170 | 10(6-16) * | NR | 12 months | In person & Telephone |
| Seeble et al. (2021) [46] | Germany | NR | 96 | 53:43 | 31±32.3 ** | NR | 12 months | In person |
| Serra et al. (2022) [47] | Rome | Single centre | 109 | 63:46 | 15 (9-24) * | NR | 12 months | Telephone |
| Tarraso et al. (2022) [48] | Spain | Multi centre | 284 | 157:127 | NR | NR | 1 year | in person |
| Tessitore et al. (2021) [49] | Switzerland | Single centre | 184 | 114:70 | 11.1 + 9.1 * | NR | 1 year + 15 days * | Telephone |
| Tortajada et al. (2022) [50] | Spain | Single centre | 68 | 49:19 | 17(15-19) * | NR | 12 months | Telephone |
| Weihe et al. (2022) [51] | Denmark | Single centre | 110. | 77:33 | NR | 13.5(8-21) * | 12 months | Telephone |
| Wu et al. (2021) [52] | China | Single centre | 83 | 47:36 | 29(25-35) * | NR | 348 days (341-359) * | In person |
| Zangrillo et al. (2022) [53] | Italy | Single centre | 56 | 50:06 | 30(23-44) * | 13(9-21) * | 349 days (343-356) * | |
| Zhan et al. (2021) [54] | China | Single centre | 121 | 50:71 | 24(19-30) * | NR | 316 days (311-321) * | In person |
| Zhang (2021) [55] | China | Multi centre | 2433 | 1205:1228 | 14(9-20) * | NR | 364 days (357-371) * | Telephone |
| Zhao et al. (2021) [56] | China | Multi centre | 94 | 54:40 | 15.1 ± 5.71 ** | NR | 345 days (333- 349) * | In person |
| Zuschlag (2022) [57] | Germany | Single centre | 162 | 88:74 | 9.6+9.2 ** | NR | 1 year | Telephone |
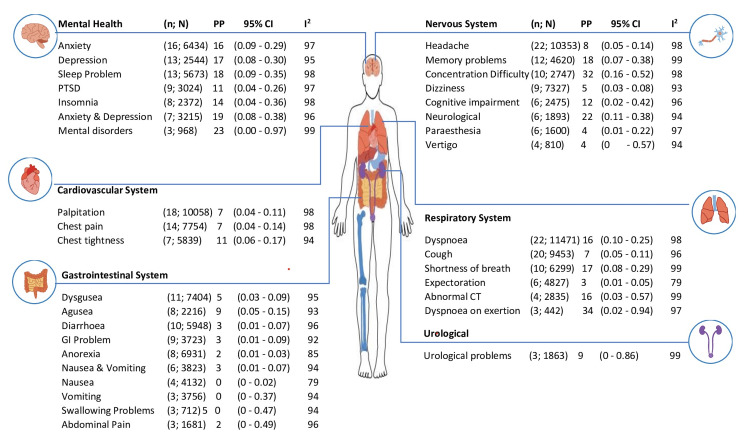
In the present review, we found 66 long-term health-related issues associated with COVID-19 (Figures 2, 3), which were reported by a minimum of three studies. The majority of participants experienced several or at least one persistent symptom (57%, 95% confidence interval [CI]: 0.47 to 0.66; n = 24; the number of participants: 12882). The five most prevalent symptoms were: dyspnea on exertion (34%, 95% CI 0.2; 0.94, n = 3, number of participants = 442); difficulty in concentration (32%, 95% CI 0.16; 0.52; n = 10, number of participants = 2747); fatigue (31%, 95% CI 0.22; 0.40, n =24, number of participants = 10866); frailty (31%, 95% CI 0.06; 0.78, n = 3, number of participants = 534); and arthomyalgia (28%, 95% CI 0.09; 0.6, n = 5, number of participants = 642).
Figure 2. Long COVID-19 symptoms at 12 months and above follow-up periods.
n: Number of studies; N: Total number of participants; PP: Pooled prevalence; CI: Confidence interval; I2: Heterogeneity in percentage
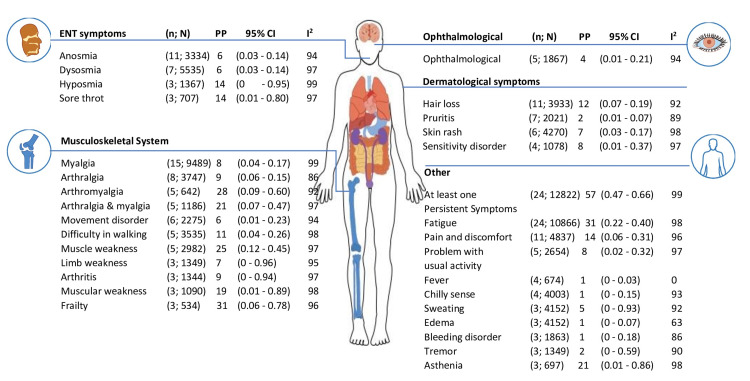
Figure 3. Long COVID-19 symptoms at 12 months and above follow-up periods.
n: Number of studies; N: Total number of participants; PP: Pooled prevalence; CI: Confidence interval; I2: Heterogeneity in percentage
In meta-analyses of at least one persistent symptom, including anxiety, depression, fatigue, dyspnea, sleep disturbance, headache, memory problems, chest pain, cough, palpitation, dysgeusia, myalgia, pain, and discomfort, and hair loss (when the number of studies was > 10), there was no indication of publication bias, according to the funnel plots and Egger's tests (p > 0.10).
The study quality of 36 publications was considered good, followed by eight studies of moderate quality and two of low quality. The reasons for the lower quality of these studies include lack of representation (20 single-center studies), small participant numbers, low response rates, the absence of a universal scale for the evaluation of symptoms, and the lack of standardized and trustworthy follow-up techniques, which were sources of possible bias (especially for phone interviews) (Table 2).
Table 2. Quality assessment by the Joanna Briggs Institute’s (JBI) critical appraisal tool for prevalence studies.
| Study | Was the sample frame appropriate to address the target population? | Were study participants sampled in an appropriate way? | Was the sample size adequate? | Were the study subjects and the setting described in detail? | Was the data analysis conducted with sufficient coverage of the identified sample? | Were valid methods used for the identification of the condition? | Was the condition measured in a standard, reliable way for all participants? | Was there appropriate statistical analysis? | Was the response rate adequate, and if not, was the low response rate managed appropriately? | Overall |
| Aranda et al., 2022 [12] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Becker et al., 2021 [13] | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Moderate |
| Bellan et al., 2021 [14] | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Unclear | Moderate |
| Boscolo-Rizzo et al., 2021 [15] | Yes | Yes | Yes | No | Yes | Yes | Unclear | Yes | Yes | High |
| Brien et al., 2022 [16] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Unclear | Moderate |
| Catalán et al., 2021 [17] | No | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear | Low |
| Chai et al., 2021 [18] | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| Fang et al., 2021 [19] | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | High |
| Fernández‑de‑las‑Peñas et al., 2021 [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Ferrucci et al., 2022 [21] | Unclear | Yes | No | Yes | No | Yes | Yes | Yes | No | Low |
| Gamberini et al., 2021 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Huang et al., 2021 [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Huang et al., 2022 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Hussain et al., 2022 [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Izquierdo et al., 2022 [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Kim et al., 2022 [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Latronico et al., 2021 [28] | No | Yes | No | Yes | Yes | No | Yes | Yes | No | Low |
| Li et al., 2022 [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Li Z et al., 2022 [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear | High |
| Liao T et al., 2022 [31] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Lim RK et al., 2022 [32] | No | No | No | No | No | Yes | No | No | Unclear | Low |
| Liu T et al., 2022 [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Liu Y et al., 2022 [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Lombardo MDM et al., 2021 [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Maestre-Muñiz MM et al., 2021 [36] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Maestrini V et al., 2021 [37] | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Unclear | Moderate |
| Martino GP et al., 2022 [38] | No | No | No | Yes | Yes | Yes | Yes | Unclear | Unclear | Low |
| Mazza MG et al., 2022 [39] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Noujaim et al., 2022 [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Ovrebotten et al., 2022 [41] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Moderate |
| PHOSP COVID collaborative group, 2022 [42] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Rank et al., 2021 [43] | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Low |
| Rass et al., 2021 [44] | Unclear | Unclear | No | Yes | No | Unclear | Yes | Yes | Unclear | Low |
| Rigoni et al., 2022 [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Seeble et al., 2021 [46] | Yes | Unclear | No | Yes | Unclear | Yes | Yes | Yes | Unclear | Moderate |
| Serra et al., 2022 [47] | Yes | Unclear | No | No | Yes | Yes | Yes | Yes | Unclear | Low |
| Tarraso et al., 2022 [48] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Tessitore et al., 2021 [49] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Tortajada et al., 2022 [50] | Yes | Yes | No | Yes | Unclear | Yes | Yes | Yes | Yes | High |
| Weihe et al., 2022 [51] | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear | Moderate |
| Wu et al., 2021 [52] | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| Zangrillo et al., 2022 [53] | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| Zhan et al., 2021 [54] | No | No | No | No | Yes | Yes | Yes | No | No | Low |
| Zhang et al., 2021 [55] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Zhao et al., 2021 [56] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Unclear | Moderate |
| Zuschlag et al., 2022 [57] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
Discussion
This systematic review and meta-analysis contribute to the sparse literature available on the health impacts of post-COVID-19 disease, particularly sudden cardiac death, prolonged arthralgia, neurological problems, and autoimmune diseases. There is a very limited amount of information available on such impacts, and none of them have evaluated these symptoms for as long as 12 months, to the best of our knowledge. Developing effective interventions, treatments, and vaccine strategies requires a comprehension of the long-term effects of COVID-19. In earlier research, the COVID-19 effects were examined after three months, six months, nine months, or longer.
The studies that investigated the health effects of adult patients who had recovered from COVID-19 and were included in our study had the longest follow-up periods. In this review, we presented the pooled prevalence (PP) of long-term effects of COVID-19 at 12 months and above based on a meta-analysis of 46 investigations encompassing 17976 cases of COVID-19. These findings suggested that COVID-19 patients may experience a range of short-, mid-, and long-term consequences even if they fully recover.
At the 12-month follow-up, 57% of patients exhibited at least one symptom. The results were consistent with research that indicated 53% of patients had at least one symptom after 12 months of COVID-19 infection [15]. In Lombardo's study [35], the proportion was larger (over 80%), whereas in, Bellan et al.’s [14] study, a smaller proportion (about 40%) was reported. The inconsistent findings of the 12-month follow-up studies show that additional original research is required to determine the long-term effects of COVID-19. This suggests that COVID-19 may have long-lasting effects on organs.
We report a prevalence of fatigue of 31% at 12 months or more, which is comparable to Michelen et al.’s [8] findings (30.1%), but lower than the studies reported by Iqbal et al. [58] (37%), and the meta-analysis by Alkodaymi et al. [59] (41%). It has already been noted that different viral and bacterial infections can cause persistent fatigue that lasts for up to six months, but the processes causing this symptom are yet unknown. These might be brought on by changes in immune system functioning, which have been linked to potential post-viral fatigue [60].
In terms of persistent dyspnea, Fernandez de las Peas et al. [20] (23.3%) and Huang et al. [24] (30%) reported lower percentages than we did (34%), although Aranda et al. [12] (62%), and Gamberini et al. (58.4%), reported a greater prevalence of dyspnea. This phenomenon may be explained by the mixed population of critical and non-critical COVID-19 patients or by the fact that both hospitalized and out-of-hospital patients were included in the study's population [22].
This is also true for arthromyalgia, with Gamberini et al. [22] (34.8% prevalence) and Catalán et al. [17] (37.5% prevalence) reporting a higher prevalence than this meta-analysis (28%). This discrepancy may be due to the subjective nature of this symptom, population differences, and the use of different assessment scales.
The greatest strategy to lessen the effects of COVID-19 is to prevent infection, for which vaccination is crucial because the disease's long-term implications are still unknown. Additionally, enhancing COVID-19 testing can aid in the earliest possible diagnosis and management. Furthermore, studies can be conducted on the use of artificial intelligence in all areas of COVID-19 [61].
Limitation and strength
To date, our work is one of the largest and most comprehensive systematic reviews of symptoms that persist after an acute COVID-19 infection. The included studies and their designs' inherent limitations, however, are present in the study. First, the literature we reviewed lacked unified terminology, standardized recording methods, and the categorization of various symptoms under general headings. We were unable to compare the incidence and prevalence of these symptoms between studies as a result. Second, numerous studies did not describe the severity of the disease, and the results were only given to the entire cohort. This results in incorrect estimations of symptom frequencies when all symptoms of different disease severity are combined. Lastly, the significant statistical heterogeneity constrains the interpretation of the pooled frequencies.
Conclusions
This comprehensive review and meta-analysis showed that a significant fraction of COVID-19 survivors experience a variety of physical, cognitive, and behavioral health problems that last for at least a year. Understanding the underlying pathophysiologic mechanisms and conducting intervention studies to treat or stop the persistence of these long-term consequences are urgently needed. However, the studies available on long-term COVID-19 are extremely diverse. Future research must include comparators, standardized symptom criteria, and an extended follow-up period.
The authors have declared that no competing interests exist.
References
- 1.World Health Organization: WHO coronavirus (COVID-19) dashboard. [ Nov; 2022 ]. 2021. https://covid19.who.int. https://covid19.who.int.
- 2.Long COVID and post-infective fatigue syndrome: a review. Sandler CX, Wyller VB, Moss-Morris R, et al. Open Forum Infect Dis. 2021;8:0. doi: 10.1093/ofid/ofab440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Post-acute COVID-19 syndrome. Nalbandian A, Sehgal K, Gupta A, et al. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A clinical case definition of post-COVID-19 condition by a Delphi consensus. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. Lancet Infect Dis. 2022;22:0–7. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Int J Clin Pract. 2021;75:0. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anxiety, depression and quality of life (QOL) related to COVID-19 among frontline health care professionals: A multicentric cross-sectional survey. Sharma SK, Mudgal SK, Thakur K, Parihar A, Chundawat DS, Joshi J. J Family Med Prim Care. 2021;10:1383–1389. doi: 10.4103/jfmpc.jfmpc_2129_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Characterising long COVID: a living systematic review. Michelen M, Manoharan L, Elkheir N, et al. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID- 19: Guidance outlines on infection prevention and control for health care workers. Sharma SK, Mudgal S, Panda P, et al. Indian J Community Health. 202032:8–14. [Google Scholar]
- 10.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Int J Evid Based Healthc. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 12.Persistent COVID-19 symptoms 1 year after hospital discharge: A prospective multicenter study. Aranda J, Oriol I, Feria L, et al. PLoS One. 2022;17:0. doi: 10.1371/journal.pone.0275615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long COVID 1 year after hospitalisation for COVID-19: a prospective bicentric cohort study. Becker C, Beck K, Zumbrunn S, et al. Swiss Med Wkly. 2021;151:0. doi: 10.4414/smw.2021.w30091. [DOI] [PubMed] [Google Scholar]
- 14.Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Bellan M, Baricich A, Patrucco F, et al. Sci Rep. 2021;11:22666. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19) Boscolo-Rizzo P, Guida F, Polesel J, et al. Int Forum Allergy Rhinol. 2021;11:1685–1688. doi: 10.1002/alr.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long COVID and episodic disability: advancing the conceptualisation, measurement and knowledge of episodic disability among people living with Long COVID - protocol for a mixed-methods study. O'Brien KK, Brown DA, Bergin C, et al. BMJ Open. 2022;12:0. doi: 10.1136/bmjopen-2022-060826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. Catalán IP, Martí CR, Sota DP, et al. J Med Virol. 2022;94:205–210. doi: 10.1002/jmv.27296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.One-year mortality and consequences of COVID-19 in cancer patients: A cohort study. Chai C, Feng X, Lu M, et al. IUBMB Life. 2021;73:1244–1256. doi: 10.1002/iub.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post-sequelae one year after hospital discharge among older COVID-19 patients: A multi-center prospective cohort study. Fang X, Ming C, Cen Y, et al. J Infect. 2022;84:179–186. doi: 10.1016/j.jinf.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: A multicenter study. Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V, Torres-Macho J. Lung. 2021;199:249–253. doi: 10.1007/s00408-021-00450-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.One-year cognitive follow-up of COVID-19 hospitalized patients. Ferrucci R, Dini M, Rosci C, et al. Eur J Neurol. 2022;29:2006–2014. doi: 10.1111/ene.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Gamberini L, Mazzoli CA, Prediletto I, et al. Respir Med. 2021;189:106665. doi: 10.1016/j.rmed.2021.106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Huang L, Yao Q, Gu X, et al. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Huang L, Li X, Gu X, et al. Lancet Respir Med. 2022;10:863–876. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prevalence of fatigue at one-year follow-up from the Gothenburg recovery and rehabilitation after COVID-19 and intensive care unit study. Hussain N, Samuelsson CM, Drummond A, Persson CU. Sci Rep. 2022;12:11501. doi: 10.1038/s41598-022-14787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long COVID at Different Altitudes: A Countrywide Epidemiological Analysis. Izquierdo-Condoy JS, Fernandez-Naranjo R, Vasconez-González E, et al. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph192214673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. Kim Y, Bitna-Ha Bitna-Ha, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. BMC Infect Dis. 2022;22:93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Latronico N, Peli E, Calza S, et al. Thorax. 2022;77:300–303. doi: 10.1136/thoraxjnl-2021-218064. [DOI] [PubMed] [Google Scholar]
- 29.Clinical status of patients 1 year after hospital discharge following recovery from COVID-19: a prospective cohort study. Li D, Liao X, Ma Z, et al. Ann Intensive Care. 2022;12:64. doi: 10.1186/s13613-022-01034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A cross-sectional study on the mental health of patients with COVID-19 1 year after discharge in Huanggang, China. Li Z, He J, Wang Y, et al. Eur Arch Psychiatry Clin Neurosci. 2022:1–10. doi: 10.1007/s00406-022-01484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long-term effects of COVID-19 on health care workers 1-year post-discharge in Wuhan. Liao T, Meng D, Xiong L, et al. Infect Dis Ther. 2022;11:145–163. doi: 10.1007/s40121-021-00553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quality of life, respiratory symptoms, and health care utilization 1 year following outpatient management of COVID-19: a prospective cohort study. Lim RK, Rosentreter R, Chen Y, et al. Sci Rep. 2022;12:12988. doi: 10.1038/s41598-022-17243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twelve-month systemic consequences of coronavirus disease 2019 (COVID-19) in patients discharged from hospital: a prospective cohort study in Wuhan, China. Liu T, Wu D, Yan W, et al. Clin Infect Dis. 2022;74:1953–1965. doi: 10.1093/cid/ciab703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. Liu YH, Chen Y, Wang QH, et al. JAMA Neurol. 2022;79:509–517. doi: 10.1001/jamaneurol.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long-term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Lombardo MD, Foppiani A, Peretti GM, et al. Open Forum Infect Dis. 2021;8:0. doi: 10.1093/ofid/ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, et al. J Clin Med. 2021;10 doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardiac involvement in consecutive unselected hospitalized COVID-19 population: In-hospital evaluation and one-year follow-up. Maestrini V, Birtolo LI, Francone M, et al. Int J Cardiol. 2021;339:235–242. doi: 10.1016/j.ijcard.2021.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.6 and 12 month outcomes in patients following COVID-19-related hospitalization: a prospective monocentric study. Martino GP, Benfaremo D, Bitti G, et al. Intern Emerg Med. 2022;17:1641–1649. doi: 10.1007/s11739-022-02979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.One-year mental health outcomes in a cohort of COVID-19 survivors. Mazza MG, Palladini M, De Lorenzo R, et al. J Psychiatr Res. 2021;145:118–124. doi: 10.1016/j.jpsychires.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fatigue and quality-of-life in the year following SARS-Cov2 infection. Noujaim PJ, Jolly D, Coutureau C, Kanagaratnam L. BMC Infect Dis. 2022;22:541. doi: 10.1186/s12879-022-07517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Changes in cardiac structure and function from 3 to 12 months after hospitalization for COVID-19. Øvrebotten T, Myhre P, Grimsmo J, et al. Clin Cardiol. 2022;45:1044–1052. doi: 10.1002/clc.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. https://pubmed.ncbi.nlm.nih.gov/35472304/ Lancet Respir Med. 2022;10:761–775. doi: 10.1016/S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.One year after mild COVID-19: the majority of patients maintain specific immunity, but one in four still suffer from long-term symptoms. Rank A, Tzortzini A, Kling E, et al. J Clin Med. 2021;10 doi: 10.3390/jcm10153305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neurological outcomes 1 year after COVID-19 diagnosis: A prospective longitudinal cohort study. Rass V, Beer R, Schiefecker AJ, et al. Eur J Neurol. 2022;29:1685–1696. doi: 10.1111/ene.15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45."Long COVID" results after hospitalization for SARS-CoV-2 infection. Rigoni M, Torri E, Nollo G, et al. Sci Rep. 2022;12:9581. doi: 10.1038/s41598-022-13077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Seeßle J, Waterboer T, Hippchen T, et al. Clin Infect Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Post-traumatic stress disorder trajectories the year after COVID-19 hospitalization. Serra R, Borrazzo C, Vassalini P, et al. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph19148452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lung function and radiological findings 1 year after COVID-19: a prospective follow-up. Tarraso J, Safont B, Carbonell-Asins JA, et al. Respir Res. 2022;23:242. doi: 10.1186/s12931-022-02166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Symptoms and quality of life at 1-year follow up of patients discharged after an acute COVID-19 episode. Tessitore E, Handgraaf S, Poncet A, et al. Swiss Med Wkly. 2021;151:0. doi: 10.4414/smw.2021.w30093. [DOI] [PubMed] [Google Scholar]
- 50.Prevalence and duration of symptoms among moderate and severe COVID-19 patients 12 months after discharge. Tortajada C, Navarro A, Andreu-Ballester JC, Mayor A, Añón S, Flores J. Intern Emerg Med. 2022;17:929–934. doi: 10.1007/s11739-021-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long-term cognitive and functional status in Danish ICU patients with COVID-19. Weihe S, Mortensen CB, Haase N, et al. Acta Anaesthesiol Scand. 2022;66:978–986. doi: 10.1111/aas.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Wu X, Liu X, Zhou Y, et al. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.One-year multidisciplinary follow-up of patients with COVID-19 requiring invasive mechanical ventilation. Zangrillo A, Belletti A, Palumbo D, et al. J Cardiothorac Vasc Anesth. 2022;36:1354–1363. doi: 10.1053/j.jvca.2021.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SARS-CoV-2 immunity and functional recovery of COVID-19 patients 1-year after infection. Zhan Y, Zhu Y, Wang S, et al. Signal Transduct Target Ther. 2021;6:368. doi: 10.1038/s41392-021-00777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. Zhang X, Wang F, Shen Y, et al. JAMA Netw Open. 2021;4:0. doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Follow-up study on COVID-19 survivors one year after discharge from hospital. Zhao Y, Yang C, An X, et al. Int J Infect Dis. 2021;112:173–182. doi: 10.1016/j.ijid.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spontaneously reported persistent symptoms related to coronavirus disease 2019 one year after hospital discharge : A retrospective cohort single-center study. Zuschlag D, Grandt D, Custodis F, Braun C, Häuser W. Schmerz. 2022;36:315–325. doi: 10.1007/s00482-022-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. EClinicalMedicine. 2021;36:100899. doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Alkodaymi MS, Omrani OA, Fawzy NA, et al. Clin Microbiol Infect. 2022;28:657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chronic fatigue syndrome, the immune system and viral infection. Bansal AS, Bradley AS, Bishop KN, Kiani-Alikhan S, Ford B. Brain Behav Immun. 2012;26:24–31. doi: 10.1016/j.bbi.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Real-world application, challenges and implication of artificial intelligence in healthcare: an essay. Mudgal SK, Agarwal R, Chaturvedi J, Gaur R, Ranjan N. Pan Afr Med J. 2022;43:3. doi: 10.11604/pamj.2022.43.3.33384. [DOI] [PMC free article] [PubMed] [Google Scholar]