Abstract
Background
Physiological changes brought about by pain may contribute to the development of morbidity in neonates. Clinical studies have shown reduction in changes in physiological parameters and pain score measurements following pre‐emptive analgesic administration in situations where the neonate is experiencing pain or stress. Non‐pharmacological measures (such as holding, swaddling and breastfeeding) and pharmacological measures (such as acetaminophen, sucrose and opioids) have been used for this purpose.
Objectives
The primary objective was to evaluate the effectiveness of breastfeeding or supplemental breast milk in reducing procedural pain in neonates. The secondary objective was to conduct subgroup analyses based on the type of control intervention, gestational age and the amount of supplemental breast milk given.
Search methods
We performed a literature search using the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 10), MEDLINE (1966 to February 2011), EMBASE (1980 to February 2011), CINAHL (1982 to February 2011), abstracts from the annual meetings of the Society for Pediatric Research (1994 to 2011), and major paediatric pain conference proceedings. We did not apply any language restrictions.
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs of breastfeeding or supplemental breast milk versus no treatment/other measures in neonates were eligible for inclusion in this review. The study must have reported on either physiologic markers of pain or validated pain scores.
Data collection and analysis
We assessed the methodological quality of the trials using the information provided in the studies and by personal communication with the authors. We extracted data on relevant outcomes, estimated the effect size and reported this as a risk ratio (RR), risk difference (RD) and weighted mean difference (MD) as appropriate.
Main results
Of twenty eligible studies, ten evaluated breastfeeding and ten evaluated supplemental breast milk. Sixteen studies analysed used heel lance and four used venepuncture as procedure. We noted marked heterogeneity in control intervention and pain assessment measures among the studies. Neonates in the breastfeeding group had statistically a significantly lower increase in heart rate, reduced proportion of crying time and reduced duration of first cry and total crying time compared to positioning (swaddled and placed in a crib), holding by mother, placebo, pacifier use, no intervention or oral sucrose group, or both.
Premature Infant Pain Profile (PIPP) scores were significantly lower in the breastfeeding group compared to positioning, placebo or oral sucrose group, or both. However, there was no statistically significant difference in PIPP scores when compared to no intervention. Douleur Aigue Nouveau‐ne scores (DAN) were significantly lower in the breastfeeding group compared to the placebo group and the group held in mother's arms, but not when compared to the glucose group. Neonatal Infant Pain Scale (NIPS) was significantly lower in the breastfeeding group compared to the no intervention group, but there was no difference when compared to the oral sucrose group. The Neonatal Facial Coding System (NFCS) was significantly lower in the breastfeeding group when compared to oral glucose, pacifier use, holding by mother and no intervention, but no difference was found when compared to formula feeding.
Supplemental breast milk yielded variable results. Neonates in the supplemental breast milk group had a significantly lower increase in heart rate, a reduction in duration of crying and a lower NFCS compared to the placebo group. Neonates in the supplemental breast milk group had a significantly higher increase in heart rate changes when compared to the sucrose group. Sucrose (in any concentration, i.e. 12.5%, 20%, 25%) was found to reduce the duration of cry when compared to breast milk, as did glycine, pacifier use, rocking, or no intervention. Breast milk was found not to be effective in reducing validated and non‐validated pain scores such as NIPS, NFCS, and DAN; only being significantly better when compared to placebo (water) or massage. We did not identify any study that has evaluated safety/effectiveness of repeated administration of breastfeeding or supplemental breast milk for pain relief.
Authors' conclusions
If available, breastfeeding or breast milk should be used to alleviate procedural pain in neonates undergoing a single painful procedure rather than placebo, positioning or no intervention. Administration of glucose/sucrose had similar effectiveness as breastfeeding for reducing pain. The effectiveness of breast milk for painful procedure should be studied in the preterm population, as there are currently a limited number of studies in the literature that have assessed it's effectiveness in this population.
Plain language summary
Breastfeeding or breast milk for procedural pain in neonates
Breastfeeding provides pain relief for newborn babies undergoing painful procedures. Medicine for pain relief is commonly given for major painful procedures, but may not be given for minor painful procedures such as blood sampling (by heel prick or venepuncture). There are different forms of non‐pharmacological strategies that may be used to reduce pain in babies, such as holding, swaddling them, sucking on a pacifier, or giving sweet solutions (such as sucrose or glucose). Different studies done in babies have shown that breastfeeding is a good way to reduce the pain babies feel when subjected to minor painful procedures. These studies have been done in full‐term babies and they have shown that breastfeeding is effective by demonstrating that it reduces babies' crying time and reduces different pain scores that have been validated for babies. Breast milk given by syringe has not shown the same efficacy as breastfeeding itself. No studies have been done in premature babies, and so new studies are needed to determine if the use of supplemental breast milk in these small babies is effective in reducing their pain.
Summary of findings
for the main comparison.
| Breastfeeding compared with control for procedural pain relief | ||||||
|
Patient or population: Healthy full‐term newborns Settings: Neonatal ward Intervention: Breastfeeding or supplemental breast milk Comparison: Control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Breastfeeding or supplemental breast milk | |||||
|
Percentage of time crying (Breastfeeding versus control) |
The mean percentage of time crying ranged across control groups from 43% to 65.6% | The mean percentage of time crying in the intervention groups was lower and ranged from 4% to 33% | 227 | ⊕⊕⊕⊝ moderate | Three studies evaluated this outcome, and overall there was a statistically significant reduction in percentage of time crying in the breastfeeding group | |
|
Duration of crying (seconds) (Breastfeeding versus control) |
The mean duration of crying ranged across control groups from 5 to 184 seconds | The mean duration of crying in the intervention groups was lower and ranged from 8.8 to 75.8 seconds | 539 | ⊕⊕⊕⊝ moderate | There seems to be a tendency towards a reduction in duration of crying in the breastfeeding group, except when it was compared with formula feeding | |
|
Neonatal Infant Pain Scale (NIPS) (Breastfeeding versus control) |
The mean NIPS ranged across control groups from 0.3 to 5.6 | The mean NIPS in the intervention groups was lower, with a mean of 0.9 | 102 | ⊕⊕⊕⊝ moderate | Only one study evaluated this outcome and there was no statistically significant difference between breastfeeding and sucrose, but there was a decrease in NIPS when compared to no intervention | |
|
Neonatal Facial Coding Score (NFCS) (Breastfeeding versus control) |
The mean NFCS ranged across control groups from 0.94 to 7.1 | The mean NFCS in the intervention groups was lower, ranging from 0.62 to 2.9 | 240 | ⊕⊕⊕⊝ moderate | Two studies evaluated NFCS.,Breastfeeding reduced NFCS, except when compared with formula feeding (where there was no statistically significant difference) | |
|
Percentage of time crying (Supplemental breast milk versus control) |
The mean percentage of time crying ranged across control groups from 76% to 90% | The mean percentage of time crying in the intervention groups was higher, with a mean of 91% | 80 | ⊕⊕⊕ moderate | Only one study evaluated this outcome, and there was no statistically significant difference between supplemental breast milk and control | |
|
Duration of crying (seconds) (Supplemental breast milk versus control) |
The mean duration of crying ranged across control groups from 9.13 to 157.05 seconds | The mean duration of crying in the intervention groups was similar to control group, ranging from 22.04 to 151.34 seconds | 730 | ⊕⊕⊕⊝ moderate | Seven studies evaluated this outcome, and there was no statistically significant difference between supplemental breast milk and control | |
|
Neonatal Infant Pain Scale (NIPS) (Supplemental breast milk versus control) |
The mean NIPS ranged across control groups from 2.6 to 5.1 | The mean NIPS in the intervention groups was similar with a mean score of 4.8 | 120 | ⊕⊕⊝⊝ moderate | Only one study evaluated this outcome. It did not show any statistically significant difference between supplemental breast milk and control groups, but it was a low quality study | |
|
Neonatal Facial Coding Score (NFCS) at 3 minutes (Supplemental breast milk versus control) |
The mean NFCS at 3 minutes ranged across control groups from 2.6 to 3.54 | The mean NFCS at 3 minutes in the intervention groups was similar, ranging from 0.6 to 3.08 | 223 | ⊕⊕⊕⊝ moderate | No statistically significant difference was shown between supplemental breast milk and control | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Pain is "an unpleasant sensory and emotional experience associated with actual or potential tissue damage" (AAP 2000). Evaluation of pain in neonates is difficult due to the subjective nature of pain and the inability of neonates to verbally express pain. Surrogate measures used to describe pain in neonates include motor responses (Marshall 1980; Craig 1993), facial expressions (Grunau 1987; Stevens 1993), cry (Grunau 1987; Johnston 1993) and changes in physiologic parameters like heart rate, blood pressure, oxygen saturation and respiratory rate. Various changes have been compiled to create various scores (Abu‐Saad 1998). Validated scores for the assessment of pain include the Neonatal Facial Coding System (NFCS) (Craig 1994), Neonatal Infant Pain Scale (NIPS) (Lawrence 1993) or Premature Infant Pain Profile (PIPP) (Stevens 1996). These reactions to pain may contribute to the development of hypoxia, hypercarbia, acidosis, ventilator asynchrony, pneumothoraces, reperfusion injury and venous congestion and subsequent late intraventricular haemorrhage or late extension of early intraventricular haemorrhage and periventricular leukomalacia (Abdel‐Rahman 1994; Anand 1998). These behavioural changes may also disrupt postnatal adaptation, parent‐infant bonding and feeding schedules.
Description of the intervention
Clinical studies have shown beneficial effects of pre‐emptive analgesic administration in reducing neonatal pain and stress (Anand 1989). Pharmacological interventions include acetaminophen, sucrose and opioid analgesics. Non‐pharmacological interventions include reduction of noxious stimuli (Schechter 1997), implementation of neurobehaviorally supportive relationship‐based care (Gunnar 1984; Corff 1995), limitation of the number of painful procedures (Anand 2001) and breastfeeding during the actual procedure.
How the intervention might work
There are several potential mechanisms by which breast milk or breast feeding might provide an analgesic effect. Components of breast feeding that may be analgesic include presence of a comforting person (mother) (Blass 1995), physical sensation (skin‐to‐skin contact with comforting person) (Blass 1995), diversion of attention (Gunnar 1984) and sweetness of breast milk (presence of lactose or other ingredients present in the breast milk) (Blass 1997). Compared to artificial formulas, breast milk contains a higher concentration of tryptophan (Heine 1999), a precursor of melatonin. Melatonin is shown to increase the concentration of beta endorphins (Barrett 2000) and could possibly be one of the mechanisms for the nociceptive effects of breast milk. Preterm neonates incapable of direct breastfeeding from the mother may benefit from placement of breast milk on the tongue or administering breast milk via the naso/orogastric route (supplemental breast milk) through some of the mechanisms listed above. Among the analgesics studied for neonatal pain, breastfeeding/breast milk is a natural, easily available, easy to use and potentially risk free (Schollin 2004) intervention. It is an intervention that could be easily adopted from the perspectives of health care providers and parents. No adverse effects of breastfeeding apart from rare transmission of micro‐organisms have been reported.
In a systematic review, 24% sucrose was found to be effective in alleviating procedural pain in neonates (Stevens 2010). Both opioid and non‐opioid mechanisms were suggested for its effectiveness. Breast milk contains only 7% lactose and may not be as effective as sucrose. On the other hand, interventions like pacifiers or positioning may result in an effect similar to breastfeeding or supplemental breast milk without interruption of the regular breastfeeding schedule.
Why it is important to do this review
To our knowledge, the topic of breastfeeding or breast milk for procedural pain in neonates has not been systematically evaluated.
Objectives
The overall objective was to evaluate the effect of breastfeeding or supplemental breast milk on procedural pain in neonates as assessed by physiological (heart rate, respiratory rate, oxygen saturation and blood pressure) or behavioural (cry duration, proportion of time crying, facial actions) pain indicators, or both, and physiological or validated composite pain scores, or both.
Primary
Compare breastfeeding with control (placebo, no treatment, sucrose, glucose, non‐nutritive sucking, holding by mother or research assistant, or positioning).
Compare supplemental breast milk with control (placebo, no treatment, sucrose, glucose, non‐nutritive sucking, massaging, rocking, or positioning).
Secondary
Within each comparison, to conduct subgroup analyses according to:
types of control intervention: placebo, no treatment, sucrose, glucose, pacifiers and positioning;
type of painful procedure: heel lance and venepuncture; and
gestational age: preterm (< 37 weeks) and full‐term (> 37 weeks).
Within the group of supplemental breast milk, we planned to carry out subgroup analysis based on the amount of breast milk if data were available.
Methods
Criteria for considering studies for this review
Types of studies
RCTs or quasi‐RCTs of breastfeeding/supplemental breast milk (given via naso/orogastric tube or orally) to alleviate procedural pain in neonates.
Types of participants
Both term (> 37 completed weeks postmenstrual age) and preterm infants (< 37 completed weeks postmenstrual age) up to maximum of 44 weeks postmenstrual age, undergoing heel lance or venepuncture for diagnostic and/or therapeutic procedures.
Types of interventions
Breastfeeding or supplemental breast milk (breast milk placed on the tongue or given through naso/oro gastric tube) prior to or during the painful procedure versus placebo or no treatment or sucrose or glucose or pacifiers or positioning.
Types of outcome measures
Primary outcomes
Pain as assessed by (at least one of the following).
-
Physiological parameters:
changes in heart rate;
changes in respiratory rate;
changes in oxygen saturation; or
changes in blood pressure.
-
Cry variables:
percentage time crying;
duration of crying (in seconds); or
duration of first cry (in seconds).
-
Validated pain measures:
Neonatal Infant Pain Scale (NIPS) (Lawrence 1993);
Premature Infant Pain Profile (PIPP) (Stevens 1996);
Neonatal Facial Coding System (NFCS) (Craig 1994); or
other pain scores as reported. (We identified during this review that authors had reported other non‐validated scores such as the Douleur Aigue Nouveau‐né score (DAN) (Carbajal 2003), Composite Score (Shendurnikar 2005), Body Pain Score (Bucher 2000), and Visual Analogue Scale (VAS) (Gradin 2004) and we have reported them also in this review).
Secondary outcomes
Any clinically important outcome reported by authors (not prespecified).
Any harmful effects reported by any author.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 10), MEDLINE (1948 to September 16, 2011), EMBASE (1980 to 2011 Week 36); CINAHL (1982 to June 13, 2011). We searched the reference lists of identified trials, abstracts from the annual meetings of the Society for Pediatric Research, American Pediatric Society and Pediatric Academic Societies (published in Pediatric Research (2007 to 2011).
We did not apply language restrictions. We also searched Clinical Trials Registry (ClinicalTrials.gov ) (September 20, 2011) to identify ongoing or recently completed trials.
We excluded the following types of articles: letters (which do not contain original data), editorials, reviews, lectures and commentaries.
Data collection and analysis
We followed the recommendations of the Cochrane Handbook and the Cochrane Neonatal Review Group.
Selection of studies
LA and PS assessed all published articles identified as potentially relevant by the literature search for inclusion in the review. LA and PS obtained data from the authors where published data provided inadequate information for the review or where relevant data could not be abstracted.
Data extraction and management
In the first version of the review LA and PS independently assessed the retrieved articles and abstracted data, and in the second version of the review CH and PS carried out these tasks (with VS rechecking in the case of any discrepancy). We resolved discrepancy regarding inclusion/exclusion of the studies by consensus.
Assessment of risk of bias in included studies
All review authors independently evaluated the quality of included trials using the following criteria in the first version of the review.
Masking of randomisation.
Masking of intervention.
Completeness of follow‐up.
Masking of outcome assessment.
There were three potential answers to these questions ‐ yes, no and cannot tell.
In this review update, we used the 'Risk of bias' assessment tool suggested by the Cochrane Collaboration and reassessed all included studies (Higgins 2011).
-
Sequence generation: Was the allocation sequence adequately generated? For each included study, we described the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
unclear.
-
Allocation concealment: Was allocation adequately concealed? For each included study, we described the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
-
Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment? For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as:
low, high or unclear risk for participants;
low, high or unclear risk for study personnel;
low, high or unclear risk for outcome assessors and specific outcomes assessed.
-
Incomplete outcome data: Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We addressed whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
unclear.
-
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting? For each included study, we assessed the possibility of selective outcome reporting bias as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to be reported);
or unclear.
-
Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias? For each included study, we noted any important concerns regarding other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We planned to assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear.
Measures of treatment effect
We used Review Manager 5.1 (RevMan 2011) for statistical analysis. Statistical parameters included risk ratio (RR), risk difference (RD), number needed to treat to benefit (NNTB), number needed to treat to harm (NNTH) and weighted mean difference (WMD) when appropriate. We reported 95% confidence intervals (CIs) for estimates of treatment effects.
Assessment of heterogeneity
We applied tests for between‐study heterogeneity, including the I2 statistic to assess the appropriateness of combining studies.
Data synthesis
We used Review Manager 5.1 (RevMan 2011) for statistical analysis. Statistical parameters included risk ratio (RR), risk difference (RD), number needed to treat to benefit (NNTB), number needed to treat to harm (NNTH) and weighted mean difference (WMD) when appropriate. We reported 95% confidence intervals (CIs) for estimates of treatment effects. We used a fixed‐effect model for meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We compared the data for the outcomes outlined in the previous section as follows (planned primary and subgroup analyses).
Breastfeeding versus control (the infant must be actually feeding from the breast at the time of intervention) (Comparison 1)
Category 1: Type of control intervention Subgroup A: Breastfeeding versus placebo Subgroup B: Breastfeeding versus no treatment Subgroup C: Breastfeeding versus sucrose or glucose Subgroup D: Breastfeeding versus pacifiers Subgroup E: Breastfeeding versus positioning
Category 2: Type of procedure Subgroup 1: Heel lance Subgroup 2: Venepuncture
Category 3: Gestational age Subgroup 1: Preterm (< 37 weeks gestational age) Subgroup 2: Term (> 37 weeks gestational age)
Supplemental breast milk versus control (the infant may be receiving breast milk via oral or nasogastric tube in the intervention group) (Comparison 2)
Category 1: Type of control intervention Subgroup A: Supplemental breast milk versus placebo Subgroup B: Supplemental breast milk versus no treatment Subgroup C: Supplemental breast milk versus sucrose or glucose Subgroup D: Supplemental breast milk versus pacifiers Subgroup E: Supplemental breast milk versus positioning
Category 2: Type of procedure Subgroup 1: Heel lance Subgroup 2: Venepuncture
Category 3: Gestational age Subgroup 1: Preterm (< 37 weeks gestational age) Subgroup 2: Term (> 37 weeks gestational age)
We added posthoc subgroups for comparison when we identified that comparisons of breastfeeding or supplemental breast milk had been reported with artificial sweetener and glycine.
Results
Description of studies
Results of the search
This is an update of our previous review (Shah 2006).
We identified a total of twenty studies eligible for inclusion (Figure 1). Ten studies evaluated breastfeeding and ten studies evaluated supplemental breast milk. Sixteen studies analysed used heel lance and four studies used venepuncture as procedure. Clinical details regarding the participants, interventions and outcomes are given in the table 'Characteristics of included studies'.
1.
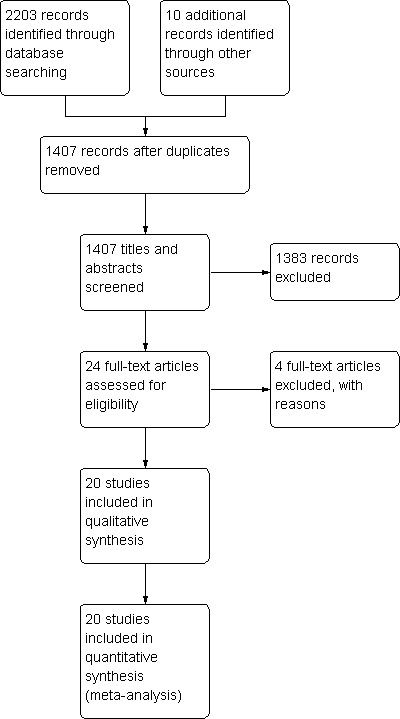
Study flow diagram.
We identified five studies on ClinicalTrials.gov (www.clinicaltrials.gov/) as either ongoing or completed, but they are not yet published (NCT01355640; NCT00175409; NCT00414258; NCT01276366; NCT00908401).
Included studies
Blass 2001 compared the effects of supplemental breast milk (colostrum) to water and sucrose. This was a quasi‐RCT of 60 full‐term infants. The infants were randomly assigned to one of the following groups (10 neonates in each group).
Group 1: water via syringe.
Group 2: colostrum via syringe.
Group 3: sucrose via syringe.
Group 4: water on a pacifier.
Group 5: colostrum on a pacifier.
Group 6: sucrose on a pacifier.
The infants were between 30 to 55 hours of age at the time of blood collection for routine neonatal screening using the heel lance procedure. 2 ml of the allocated solution was given either by slow administration via syringe over a span of two minutes or by allowing the infant to suck a pacifier dipped in the solution every 30 seconds for two minutes. Prior to the procedure, baseline data were obtained for 60 seconds and continuous monitoring was done throughout and after the procedure during the recovery time. The blood collection was done by an experienced phlebotomist for 49 of the 60 infants. The outcomes measured were reduction in the percentage crying and grimacing times during the procedure;,the mean crying time following the procedure, and the mean heart rate change during and following the procedure. Despite repeated requests, we were unable to obtain data regarding individual groups from the authors.
Bucher 2000 compared the effects of commercially available artificial sweetener (containing 10 parts cyclamate and one part saccharin) to glycine (sweet amino acid), expressed breast milk and sterile water. This was a RCT of 80 full‐term infants. The infants were randomly assigned to one of the following groups (20 neonates in each group).
Group 1: 2 ml of artificial sweetener via syringe.
Group 2: 2 ml of glycine via syringe.
Group 3: 2 ml of breast milk via syringe.
Group 4: 2 ml of sterile water via syringe.
The infants were studied on postnatal day four at the time of blood collection for routine neonatal screening using the heel lance procedure. 2 ml of the allocated solution was given via syringe on the anterior part of the tongue by a nurse not involved in the study. Prior to the procedure, baseline data were obtained and continuous monitoring was done throughout and after the procedure during the recovery time. The blood collection was performed two minutes after administration of solution by a research nurse. The procedure was video taped and evaluated by two independent observers unaware of allocation. The outcomes measured were heart rate change, percentage time crying, body pain score, facial pain score (five components of NFCS) and body pain score (torso movements 1 = one side, 2 = both sides; head movements = 1; arm movements 1 = one arm, 2 = both arms; hand movements 1 = one hand, 2 = both hands; bringing hands to face (mouth) = 1 point; maximum score was 8 points, minimum score was 0 points) during and after blood collection. The data were presented in graphical format. We obtained numerical data by contacting the author.
Carbajal 2003 compared the effects of breastfeeding to positioning, sterile water and 30% glucose. This was a RCT of 180 term neonates. The infants were randomised to one of the following four groups.
Group 1: breastfeeding (n = 44).
Group 2: held in mother's arms without breastfeeding (n = 45).
Group 3: sterile water without pacifier (n = 45).
Group 4: 30% glucose followed by a pacifier (n = 45).
In Group 1 and 2, the interventions were started two minutes before the procedure and continued throughout the procedure. In groups 3 and 4 the intervention was commenced two minutes prior to the procedure. Venepuncture was performed when infants were at least 24 hours of age and had not been fed for the previous 30 minutes. The primary outcome measure was the DAN scale (Carbajal 1997), a behavioural scale developed to rate acute pain in term and preterm neonates. The score comprised of three items namely facial expressions, limb movements, and vocal expression with values in each ranging from zero (no pain) to 10 (maximum pain). The secondary outcome measure was the PIPP score. Mothers were interviewed 48 to 72 hours after the study by standardised questionnaires to assess any change in the sucking behaviour. One infant was excluded from the analysis as the outcome measure could not be assessed properly due to the mother's head partially covering her infant's face. Data from all four groups were used in their respective appropriate comparisons.
Codipietro 2008 compared the efficacy of breastfeeding versus orally administered 25% sucrose solution. It was a RCT including 101 healthy term newborns.
Group 1: breastfeeding (n = 51).
Group 2: received 1 ml of 25% sucrose (n = 50).
Infants underwent heel lance for routine newborn screening. Infants in group 1 were held by mother and breastfed until there was a continuous active suction prior to heel lance. Group 2 infants were laid on a changing table and a bolus of 1 ml of 25% sucrose solution was administered through a syringe in the mouth two minutes before the heel lance. The outcomes measured were the PIPP scale, changes in heart rate and saturation 30 seconds after the procedure, duration of first cry, and percentage of crying in the first two minutes after the procedure. The procedure was taped (audio) and the tape recording was evaluated by two assistants (who were blinded to the groups) to assess cry behaviour.
Efe 2007 compared breastfeeding and 25% sucrose solutions to reduce pain due to venepuncture in term neonates. They included 102 term neonates in a quasi‐randomised trial:
Group 1: breastfeeding (n = 34).
Group 2: 25% sucrose solution (n = 34).
Group 3: control, no intervention (n = 34).
Infants underwent venepuncture for routine screening of phenylketonuria and hyperbilirubinaemia. Infants in Group 1 were held in skin‐to‐skin contact with their mothers during the entire procedure. Three minutes after the first jaw movements were observed, the venous blood sample was taken. Infants continued to breastfeed during and after the venepuncture. Group 2 infants received 2 ml of 25% sucrose solution dipped into pacifiers. The infants started to suck the pacifier with sucrose three minutes before the venepuncture and continued to suck during and after sampling. The control group infants were wrapped in a blanket with only the hand that would be used for sampling outside the blanket. The mother stayed next to the infant trying to soothe him verbally. After the sample was collected, the infant was cuddled by the mother and could be given a pacifier. The outcomes measured were NIPS, heart rate, oxygen saturation levels and crying time. Crying time was assessed by audio tapes.
Gradin 2004 compared the effects of breastfeeding to sterile water and 30% glucose. This was a RCT of 120 full‐term neonates. The infants were randomised to four groups.
Group 1: breastfeeding and 1 ml of sterile water (n = 27).
Group 2: breastfeeding and 1 ml of 30% glucose (n = 29).
Group 3: fasting and 1 ml of sterile water (n = 26).
Group 4: fasting and 1 ml of 30% glucose (n = 29).
Infants underwent a routine neonatal screening procedure using venepuncture at three to five days of age. The data from Group 3 were not used for this review. For the breastfed group, the infants were allowed breastfeeding ad libitum 45 minutes prior to blood sampling, while infants in the fasting group had blood sampling performed at least two hours after the last feeding. 1 ml of either sterile water or 30% glucose was administered through a syringe into the infants mouth, and one minute later the blood sampling was performed. After sampling, the infants were left undisturbed for three minutes during recovery phase. The outcomes measured were the PIPP score and mean crying time. Parents were asked to assess pain using a Visual Analogue Scale (VAS). The agreement between the parental assessment of pain and the PIPP score and crying time was determined. The primary author provided missing data. Nine infants were excluded from the study by the authors mostly due to technical problems with the video recordings (n = 6) and maternal choice to withdraw their infants from the study (n = 3). Data from groups 1, 3 and 4 were used for this review as the combination of breastfeeding and glucose was not planned to be compared a priori.
Gray 2002 compared the effects of breastfeeding to positioning. This was a RCT of 30 full‐term neonates. The infants were randomised to two groups (15 neonates in each group).
Group 1: breastfed and cuddled with full body skin‐to‐skin contact.
Group 2: swaddled and placed on their side in the crib.
All infants underwent heel lance for routine neonatal screening procedure. Mean postnatal age at procedure was 46 hours in Group 1 and 40 hours in Group 2. The outcomes measured were differences in crying, grimacing and heart rate between the two groups before, during and after blood collection. The primary author provided additional information.
Jatana 2003 compared the effects of breast milk versus different solutions of glucose. The authors say it was a RCT of 125 term infants, who were randomised to 5 groups.
Group 1: control, received 1 ml of sterile water (n = 25).
Group 2: 1 ml of glucose 10% solution (n = 25).
Group 3: 1 ml of glucose 25% solution (n = 25).
Group 4: 1 ml of glucose 50% solution (n = 25).
Group 5: 1 ml of expressed breast milk (EBM) (n = 25).
All infants underwent heel lance for blood sampling. The solution tested was administered slowly over a period of 30 seconds by means of a syringe placed in the mouth. Two minutes after giving the oral solution, the heel lancing was performed. The outcomes assessed were duration of crying (first cry and total duration), change in heart rate, change in oxygen saturation and facial action score.
Leite 2009 compared the effects of breastfeeding versus maternal holding in a RCT including 60 healthy term newborns.
Group 1: breastfeeding (n = 31).
Group 2: held by mother (n = 29).
Infants underwent heel lance for routine newborn screening. Infants in Group 1 were held by the mother and were breastfeeding with effective sucking movements five minutes prior to the procedure. Group 2 infants were held by the mother for the same length of time. The outcomes measured were NFCS and change in heart rate. Data were provided as mean and SD at baseline, during blood collection and recovery.
Mathai 2006 compared the effects of breast milk with 20% sucrose solution, distilled water, non‐nutritive sucking, massaging and rocking. It was a RCT on 104 term neonates. The infants were randomised to one of the following groups.
Group 1: expressed breast milk (n = 18).
Group 2: 20% sucrose solution (n = 17).
Group 3: distilled water (n = 15).
Group 4: non‐nutritive sucking (n = 20).
Group 5: massaging (n = 17).
Group 6: rocking (n = 17).
Infants underwent heel prick at more than 24 hours of age for collection of blood for bilirubin estimation. 2 ml of expressed breast milk, 20% sucrose or distilled water were administered in the baby's mouth with a dropper. In the non‐nutritive sucking group, a sterile pacifier was held gently in the baby's mouth and the palate was tickled to stimulate sucking. This was continued during and for two minutes after the heel prick. In the massaging group, neonates were subjected to firm, gentle stroking with bare fingers in a rhythmical manner starting from the forehead and going down to the chest, arms and legs, during and for two minutes after the heel prick. In the rocking group, newborns were rocked by lifting the baby's head off the cot on the palm of the hand (without lifting the body off the cot) and making rocking movements in a gentle rhythmic manner for two minutes after the heel prick. The outcomes measured were duration of first cry, total crying time and DAN score at 30 seconds, one minute, two minutes and four minutes after the prick. For the purpose of this review, we analysed the DAN score at two minutes. Other outcome variables were heart rate increase and saturation reduction; however, results were not shown, but they commented that there were no significant differences.
Okan 2010 compared skin‐to‐skin contact and breastfeeding with only skin‐to‐skin contact and no intervention (lying on the table). It was a RCT of 107 healthy, full‐term neonates between 24 and 48 hours of age. Infants were randomised into 3 groups.
Group 1: breastfeeding with skin‐to‐skin contact (n = 35).
Group 2: held by mother with skin‐to‐skin contact (n = 36).
Group 3: lying on the table (n = 36).
Heel lancing was done for the purpose of metabolic newborn screening. Mothers and infants from Groups 1 and 2 were left alone for 15 minutes to allow them to rest comfortably in skin‐to‐skin contact position. Mothers in Group 1 were asked to begin to breastfeed their infants during this time. In the no‐contact group, tests were performed with the infants lying on an examination table in a silent nursery. Infants were wrapped in blankets and placed supine on the examination table. The outcomes measured were heart rate and saturation changes, total time of crying and NFCS in Groups 2 and 3 (calculated at the moment of heel lance, and after one, two, three, four and five minutes).
Ors 1999 compared the effects of supplemental breast milk to water and 25% sucrose. This was a RCT of 102 healthy term neonates. The infants were randomised to three groups.
Group 1: received 2 ml of 25% sucrose (n = 35).
Group 2: received 2 ml of human milk (n = 33).
Group 3: received 2 ml of sterile water (n = 34).
All infants underwent heel lance blood sampling by a single performer. The allocated solution was given by syringe into the baby's mouth over one minute. The heel prick was performed two minutes after administration of the solution. Crying duration and heart rate at three minutes were recorded from the time of the heel prick. The outcomes measured were crying time, percentage change in heart rate and recovery time for the heart rate. The primary author provided additional information. Data from all three groups were used for this review in their respective appropriate comparisons.
Ozdogan 2010 compared the effects of breast milk to sterile water and 12.5% sucrose solution. It was a RCT that included 142 healthy newborns. The infants were randomised to one of the six following groups.
Group 1: single‐dose of breast milk (n = 18).
Group 3: received 2 ml of sterile water (n = 34).
Group 3: single‐dose of 12.5% sucrose solution (n = 25).
Group 4: two doses of breast milk (n = 23).
Group 5: two doses of sterile water (n = 26).
Group 6: two doses of 12.5% sucrose solution (n = 23).
Infants underwent routine neonatal screening through heel lance. In all the groups, babies received 2 ml of the test solutions through syringe onto the anterior part of the tongue, and they were not allowed to suck the syringe tip. In the single‐dose groups, the test solution was given two minutes before the heel prick and in the repeated‐dose groups the dose was repeated just prior to heel prick. The outcomes measured were total crying time and NFCS at 0, one, two, and three minutes. For the purpose of this review, we analysed the NFCS values at two minutes.
Phillips 2005 compared the effects of breastfeeding in three groups in a RCT of 96 healthy term neonates.
Group 1: breastfeeding (n = 32).
Group 2: neonates held by mother holding pacifier in infant's mouth (n = 39).
Group 3: neonates held by research assistant holding pacifier in infant's mouth (n = 25).
All infants underwent heel lance blood sampling by a single performer. Mothers held babies in their bed while giving pacifier (Group 2) while research assistant held infants in bedside chairs (Group 3). The outcomes measured were crying duration, percentage of infants crying, changes in the heart rate, blood pressure and oxygen saturation. The primary author provided additional information. The purpose of studying three groups was to assess the differences in outcome measures caused by one of the components of the act of breastfeeding (maternal contact).
Shendurnikar 2005 compared the effects of breastfeeding to positioning (swaddling). The authors provided details about the study as it was published as a letter to the editor. This was a RCT of 100 full‐term neonates. The infants were randomised to two groups (50 neonates in each group).
Group 1: breastfeeding group.
Group 2: swaddled and placed in a cradle.
Infants in Group 1 were breastfed for 15 minutes prior to the heel prick. All infants underwent a heel lance procedure for clinical indication such as measurement of packed cell volume or bilirubin. The outcomes measured were behavioural (state of arousal, cry, facial expression, body movements); physiological (breathing pattern, heart rate) and composite score (non‐validated) between the two groups before, during and after blood collection. The primary author provided additional information. The composite score was calculated using the following criteria.
Heart rate (0 = < 120/minute; 1 = 120 to 160/minute and 2 = > 160/minute).
Breathing (0 = relaxed; 1 = changed).
Facial expression (0 = relaxed; 1 = grimaced).
Body movements (0 = relaxed; 1 = no gross movement; 2 = gross body movement).
State of arousal (0 = sleepy; 1 = awake; 2 = fussy).
Cry (0 = no; 1 = whimper; 2 = vigorous) and combining the score.
The minimum score was 0 and maximum score was 10. This study was published as a letter to the editor and authors provided additional data.
Skogsdal 1997 compared the effects of no intervention to 30% oral glucose, 10% oral glucose and breast milk. This was a RCT of 120 neonates (66 preterm neonates between 30 to 37 weeks and 54 term neonates). The infants were randomly assigned to one of the following groups (30 neonates in each group).
Group 1: no intervention.
Group 2: 1 ml of 30% glucose via syringe.
Group 3: 1 ml of 10% glucose via syringe.
Group 4: 1 ml of breast milk via syringe.
The infants were studied on mean (SD) postnatal day five at the time of blood collection for their routine care using the heel lance procedure. 1 ml of the allocated solution was given via syringe by a nurse not aware of allocation. Prior to the procedure, baseline data were obtained and continuous monitoring was done throughout and after the procedure during the recovery time. The blood collection was performed two minutes after administration of solution. The outcomes measured were heart rate change and duration of crying. The data were presented in graphical format, however, the contact author provided the data necessary for the review. As this study had two comparative groups with different concentrations of glucose, for the purpose of analyses we combined the group who received 30% glucose with data from the Ors 1999 study where they used 25% sucrose in one group (presuming very minimal difference in pain responses between 25% and 30% sugar solution). We compared the group who received 10% glucose with the breast milk group separately.
Upadhyay 2004 compared the effects of supplemental breast milk to sterile water. This was a RCT of 87 full‐term neonates. The infants were randomised to two groups.
Group 1: received 5 ml of expressed breast milk (n = 40).
Group 2: received 5 ml of distilled water (n = 41) prior to venepuncture.
Venepuncture was performed based on clinical indications. Three babies from each group were excluded from the study by the authors due to venepuncture failure and failure to attain state 3 or 4 of wakefulness. Data from 81 infants were analysed. The primary outcome was the duration of the cry after the venepuncture. The secondary outcomes included changes in physiological parameters, namely heart rate and oxygen saturation from baseline to one and three minutes after venepuncture and the modified NFCS. Only five easily recordable parameters of the NFCS (out of ten) were assessed by the investigators. Data on heart rate and oxygen saturation were provided as mean and SD at baseline and three minutes. We contacted authors to provide data on mean changes in these parameters, but no response was obtained. We calculated the MD and SD of the difference assuming 50% correlation between baseline and subsequent findings.
Uyan 2005 compared the effects of supplemental breast milk (two groups foremilk and hindmilk) to water. This was a quasi‐RCT of 62 healthy term neonates. The infants were randomised to three groups.
Group 1: received 2 ml of foremilk (n = 20).
Group 2: received 2 ml of hindmilk (n = 21).
Group 3: received 2 ml of sterile water (n = 21).
All infants underwent heel lance blood sampling by a single performer. The allocated solution was given by syringe into the baby's mouth. The heel prick was performed two minutes after administration of the solution. Crying duration and heart rate changes at one, two, and three minutes were recorded from the time of the heel prick. The outcomes measured were crying time, percentage change in heart rate and NFCS at one, two and three minutes. The data from Group 1 and 2 were combined for the analyses. Authors provided data on combined groups.
Weissman 2009 compared breastfeeding, formula feeding, a 30% glucose solution, holding by mother, and non‐nutritive sucking with a control group in a total of 180 term newborn infants in a quasi‐RCT.
Group 1: breastfeeding (n = 31).
Group 2: formula feeding (n = 30).
Group 3: 2 ml of 30% glucose solution (n = 31).
Group 4: infants were held by their mothers (n = 29).
Group 5: non‐nutritive sucking with pacifier (n = 30).
Group 6: control (no intervention) (n = 29).
All infants underwent heel lance for routine neonatal screening. They were assigned to the six groups according to the mothers' preference. For infants in Group 3, the solution was given orally two minutes before the procedure, infants in Group 2 were fed formula while in their cribs. The outcomes assessed were NFCS, through video recording, duration of cry and heart rate increase. The intervention was not blinded.
Yilmaz 2011 compared the effects of supplemental breast milk to 20% sucrose, pacifier and a control group with no intervention. It was a RCT of 120 healthy term newborns. The infants were randomised to four groups.
Group 1: 2 ml of breast milk (n = 30).
Group 2: 2 ml of 20% sucrose (n = 30).
Group 3: infants were given a pacifier (n = 30).
Group 4: control group with no intervention (n = 30).
All infants underwent heel lance blood sampling. The allocated solution was given by syringe into the baby's mouth two minutes prior to the heel prick, avoiding contact of the syringe with the mouth and lips. Heart rate, respiratory rate, body temperature and saturation changes were measured. The infants were video taped to assess the behavioural responses through the NIPS.
Excluded studies
We excluded Bilgen 2001 from the review because it is a duplicate publication of the same data reported by Ors 1999. We excluded two reports because they were not RCTs (Osinaike 2007; Iturriaga 2009) and excluded a further report because it studied infants between two and four months of age (Efe 2007a).
Risk of bias in included studies
The methodological quality of the reviewed studies is given in the table 'Characteristics of included studies' and Figure 2 and Figure 3. We extracted the information from the published paper and by contacting the primary authors.
2.
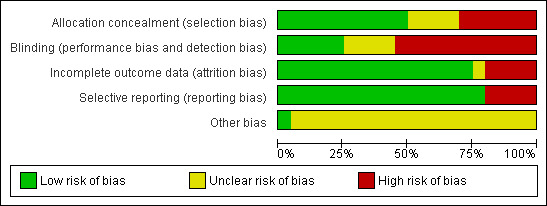
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
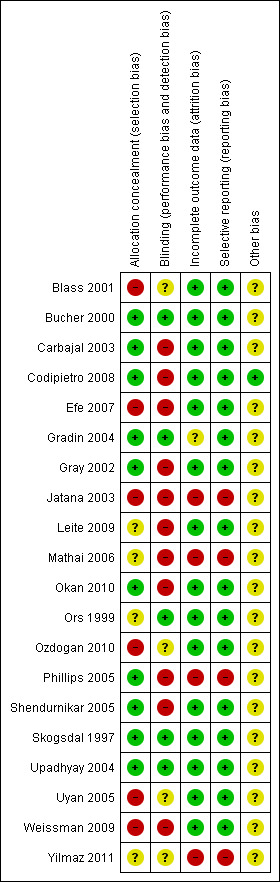
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blass 2001: The infants were initially assessed to determine whether they were successfully breastfed or not and then randomised into the colostrum groups and non‐colostrum groups. Investigators initially planned the assignment of the infants based on a table of random numbers. Group assignment needed to be adjusted because some mothers were unable to obtain sufficient colostrum. After passing the exclusion criteria, investigators assessed mother's success regarding breastfeeding. If the mother was unsuccessful, she was assigned to groups that didn't involve breast milk (Groups 1, 3, 4 and 6). If breastfeeding was established the infant was assigned to Groups 2 or 5. The phlebotomist who performed the heel lance was unaware of allocation, study purpose or hypotheses. The authors did not define what constituted successful breastfeeding. The data collection for sucrose, water and pacifier groups was completed in June 1998, while colostrum data collection ended in March 1999.
Although the phlebotomist and the person who rated the video data were unaware of treatment allocation, this could have introduced a degree of bias. Masking of intervention was not possible in this study since it involved the use of a pacifier and a liquid (colostrum) that differed in colour from two other solutions. Masking of outcome assessment was possible with crying time and heart rate changes, but not so when assessing grimacing since the intervention involved the use of a pacifier. A number of infants in the water and colostrum groups were excused (data collection not continued and infant allowed to be comforted in other ways) after 90 seconds of recovery period due to excessive crying, although all infants were included in the final analysis with the assumption that these behaviours would have continued at the same level for the rest of the recovery period.
Bucher 2000: The randomisation was done through sealed envelopes. One nurse administered the solution in the absence of investigators and was not involved in heel prick or data collection. Masking of outcome assessment was done by blinding observer as to the assignments to the study group.
Carbajal 2003: The randomisation was done by a research assistant using numbered envelopes. Allocation was concealed from the investigators. Masking of the intervention was not possible in this study since it involved breastfeeding, the use of a pacifier and cuddling before and throughout the procedure. The outcome assessment was masked as the observers who assessed the outcome measures were not aware as to the purpose and hypothesis of the study. However, personal bias on the part of the outcome observer could not be excluded.
Codipietro 2008: Randomisation was done by using a computer random number generator. Allocation was concealed using opaque, sealed envelopes, which were opened sequentially by the paediatric nurse who performed blood sampling. Masking of intervention was not possible since it involved breastfeeding before and throughout the procedure. However, assessment of one of the outcomes (cry behaviour) was masked as it was assessed by two assistants who listened to tape recordings. All infants were accounted for in the analysis of outcomes.
Efe 2007: It was a quasi‐randomised trial as allocation was done according to the mothers' preferences. There was no allocation of concealment or blinding of the intervention. All patients were accounted for in the analysis of outcomes.
Gradin 2004: The randomisation was done through sealed envelopes. The intervention involved the use of placebo to mask the solution in question. Masking of outcome assessment was done by blinding the observer as to the assignments to the study group.
Gray 2002: The randomisation was done through sealed envelopes. The masking of the intervention was not possible since it involved breastfeeding before and throughout the procedure. Masking of outcome assessment was also not possible. All participants were accounted for in the analysis of outcomes.
Jatana 2003: There is no comment on how the randomisation was done, although the authors comment that the groups were matched for gestational age, birth weight and sex distribution. There are also no comment on whether the intervention was masked or not, which could have been possible, given that all the solutions were administered in the same way. No comments on whether the outcome assessment was masked or not. One of the outcomes, neonatal facial scoring, was not published in the results.
Leite 2009: Randomisation was done by a computer random number generator. Masking of the intervention was not possible since it involved breastfeeding before and throughout the procedure. Two digital cameras were used to record the newborns' behaviour, one focused on the newborns' face and the second camera on the neonates' body. Analysis of facial actions was carried out by a person who was blinded to the phase of the procedure (blood collection, compression or recovery). It was not possible to blind for group assignment as the information about breastfeeding was easily determined in both body and face videos. All participants were accounted for in the analysis of outcomes.
Mathai 2006: Randomisation was done through a random number table. Masking of the intervention was not possible since some of the participants took oral solutions while others were held or rocked in different ways. Masking of cry behaviour was possible, as one of the investigators stood behind a screen during the assignment of the infant and during the procedure (this observer assessed the total duration of cry). Not all of the study's prespecified outcomes were reported, no data were available for two of the outcomes i.e. heart rate and saturations, although the authors commented that there was no significant difference between the groups. All infants were accounted for in the analysis of outcomes.
Okan 2010: Randomisation was done through a random number table. Masking of the intervention was not possible since it involved breastfeeding, skin‐to‐skin contact, and no contact at all. Authors commented that NFCS was not assessed in breastfed infants, as facial actions of these babies could not be evaluated. All infants were accounted for in the analysis.
Ors 1999: The manner of randomisation was not discussed by the authors. Masking of the intervention was made possible by using a placebo and by performing the heel prick one minute after giving the solutions. The two investigators who analysed the data were unaware of the treatment intervention, hence, the outcome measure analysis was blinded. All infants were accounted for in the analysis.
Ozdogan 2010: This was a quasi‐randomised trial. Participants were consecutively allocated to the different groups by order of admission. There is insufficient information to know if this study was blinded or not, as the authors do not comment on whether the syringe was wrapped or covered and whether the person watching the video tape could see the contents of it or not. The first three groups were probably masked (as the intervention occurred before the video was taken), but we do not know if the intervention was masked for the two‐dose groups. All infants are accounted for in the analysis.
Phillips 2005: The randomisation was done through envelopes containing allocation cards. Masking of intervention was not possible since it involved breastfeeding before and throughout the procedure. Masking of outcome assessment (from video recordings) was not done; however, data from monitors (heart rate, saturation and blood pressure) were analysed in a masked manner. All participants were accounted for in the analysis of outcomes; however, for some analyses complete data were not available from all patients.
Shendurnikar 2005: The primary author provided this information. The randomisation was done by the primary author asking the mother to choose from a collection of randomisation cards. The masking of the intervention was not possible since it involved breastfeeding before and throughout the procedure. Masking of outcome assessment was not done and the primary author collecting the data was aware of the allocation and hypothesis of the study. All participants were accounted for in the analysis of outcomes.
Skogsdal 1997: The randomisation was done through a random digit table. The heel prick and administration of allocated solution was done by the same nurse. Outcome data collection was done by a different nurse who was unaware of allocation. All participants were accounted for in the analysis of outcomes.
Upadhyay 2004: The randomisation was performed using computer generated numbers. Allocation was adequately concealed. The observers were blinded as to the intervention given to the infants. The data of the 81 subjects were available for analysis because in six infants, either there was a technical problem or the infants were not fully awake.
Uyan 2005: The authors provided further information on the method of randomisation, indicating that it was quasi‐randomised (based on number or day of the procedure). According to the authors the intervention was masked. The two investigators who analysed the data and the person who recorded the video for the NFCS coding were unaware of the treatment allocation; hence, the outcome measure analysis was blinded. All infants were accounted for in the analysis.
Weissman 2009: This was a quasi‐randomised trial, given that the allocation was done by mothers' preferences. There was no blinding of the interventions. All infants are accounted for in the analysis.
Yilmaz 2011: The authors state that this was a RCT, but no information is given in regards to method of randomisation, allocation concealment or blinding. We do not have information on whether the investigators analysing the video tapes for the NIPS were blinded to the infants' intervention. There was plan to assess saturation changes, which are reported as "no difference" without providing data. All infants were accounted for in the analysis.
Effects of interventions
See: Table 1
Primary outcome
Breastfeeding versus control (Comparison 1)
Ten studies reported on this comparison (Gray 2002; Carbajal 2003; Gradin 2004; Phillips 2005; Shendurnikar 2005; Efe 2007; Codipietro 2008; Leite 2009; Weissman 2009; Okan 2010).
1. Physiological parameters
a. Heart rate change (beats per minute) (Analysis 1.1)
1.1. Analysis.
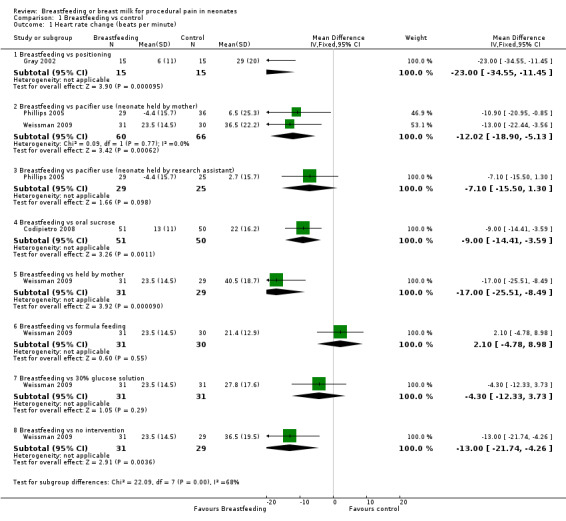
Comparison 1 Breastfeeding vs control, Outcome 1 Heart rate change (beats per minute).
Seven studies (Gray 2002; Efe 2007; Phillips 2005; Codipietro 2008; Leite 2009; Weissman 2009; Okan 2010) reported on the heart rate change during heel lance. The heart rate tended to increase in every group during the procedure, but the increase was significantly lower in the breastfeeding group compared to the positioning (swaddled and laid in a crib) group (Gray 2002) (mean difference (MD) ‐23; 95% confidence interval (CI) ‐35 to ‐11), and group of infants held by mother holding a pacifier in the infant's mouth (Phillips 2005; Weissman 2009) (MD ‐12; 95% CI ‐19 to ‐5). Codipietro 2008 reported that the increase in heart rate was significantly lower in the breastfeeding group compared to the 20% sucrose group of infants (MD ‐9; 95% CI ‐14 to ‐4) who were held by their mothers (MD ‐17; 95% CI ‐26 to ‐8) and the no intervention group (MD‐13; 95% CI ‐22 to ‐4) (Weissman 2009). There was no statistically significant difference between the breastfeeding group and group of infants held by the research assistant along with the use of a pacifier (MD ‐7; 95% CI ‐15 to 1) (Phillips 2005). There was also no significant difference between the breastfeeding and formula feeding groups (Weissman 2009) (MD 2; 95% CI ‐5 to 9), or between breastfeeding and 30% glucose (Weissman 2009) (MD ‐4; 95% CI ‐12 to 4). Efe 2007, Leite 2009 and Okan 2010 did evaluate heart rate changes, but did not report on SD of change, and therefore, we were not able to meta‐analyse those results; however, overall there was a reduction in heart rate change in the breastfeeding group compared to the control group.
b. Changes in the respiratory rate
None of the studies included in this review reported on this outcome.
c. Oxygen saturation change (Analysis 1.2)
1.2. Analysis.
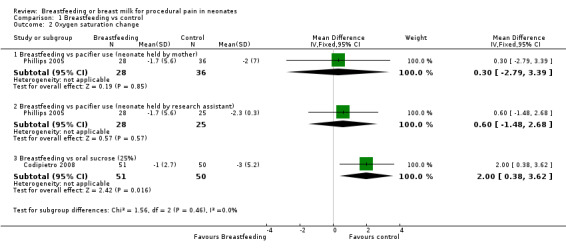
Comparison 1 Breastfeeding vs control, Outcome 2 Oxygen saturation change.
Four studies (Phillips 2005; Efe 2007; Codipietro 2008; Okan 2010) reported on the oxygen saturation change during heel lance. There was no difference in oxygen saturation change between the breastfeeding group and the group of infants held by the mother holding a pacifier in the infant's mouth (Phillips 2005) (MD 0.3; 95% CI ‐2.8 to 3.4) and group of infants held by the research assistant holding a pacifier in the infant's mouth (MD 0.6; 95% CI ‐1.5 to 2.7). The study that compared breastfeeding and 20% sucrose (Codipietro 2008) reported a statistical significant difference (MD 2.0; 95% CI 0.4 to 3.6) in favour of oral sucrose. Efe 2007 and Okan 2010 reported on oxygen saturation changes, but there was no information on the SD of the change in oxygen saturation and therefore we could not meta‐analyse that data; however, overall there was no difference in the change in oxygen saturation between the breastfeeding and control groups.
d. Blood pressure changes (Analysis 1.3)
1.3. Analysis.
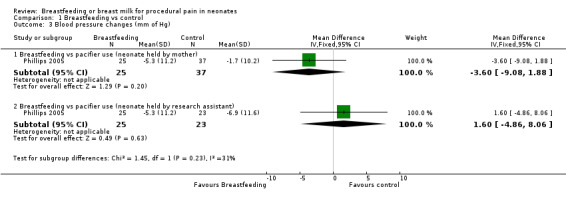
Comparison 1 Breastfeeding vs control, Outcome 3 Blood pressure changes (mm of Hg).
One study (Phillips 2005) reported on the blood pressure change during heel lance. There was no difference in blood pressure change between the breastfeeding group and the group of infants held by the mother holding a pacifier in the infant's mouth (MD ‐4; 95% CI ‐9 to 2) and the breastfeeding group and the group of infants held by the research assistant holding a pacifier in the infant's mouth (MD 2; 95% CI ‐5 to 8).
2. Cry variables
a. Percentage of time crying (Analysis 1.4)
1.4. Analysis.
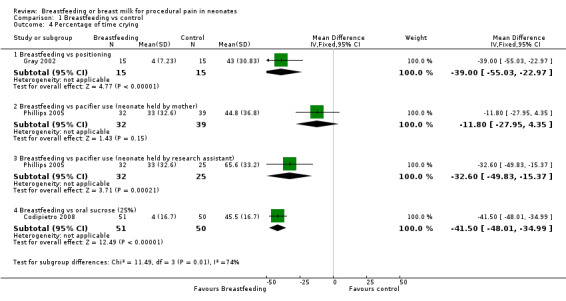
Comparison 1 Breastfeeding vs control, Outcome 4 Percentage of time crying.
Three studies (Gray 2002; Phillips 2005; Codipietro 2008) reported percentage of time crying during heel lance. There was a statistically significant reduction in the percentage time crying among infants in the breastfeeding group compared to the positioning group (Gray 2002) (MD ‐39; 95% CI ‐55 to ‐23), the group of infants held by the research assistant holding a pacifier in the infant's mouth (Phillips 2005) (MD ‐33; 95% CI ‐50 to ‐15) and infants who received sucrose (Codipietro 2008) (MD ‐42; 95% CI ‐48 to ‐35). There was no statistically significant reduction in the percentage time crying between the breastfeeding group and the group of infants held by mothers holding a pacifier in the infant's mouth (Phillips 2005) (MD ‐12; 95% CI ‐28 to 4).
b. Duration of crying in seconds (Analysis 1.5)
1.5. Analysis.
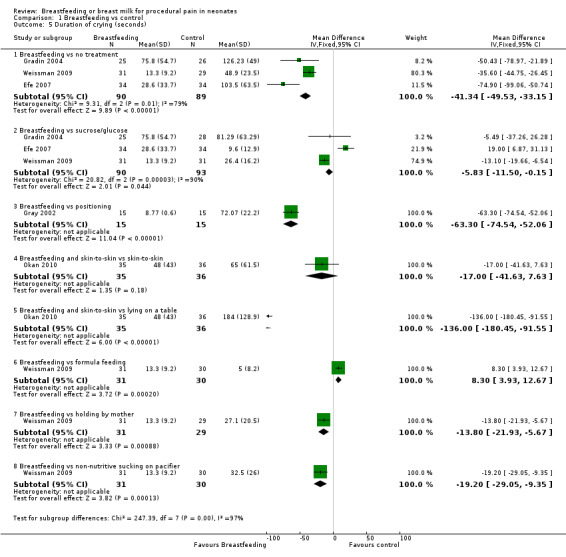
Comparison 1 Breastfeeding vs control, Outcome 5 Duration of crying (seconds).
Five studies (Gray 2002; Gradin 2004; Efe 2007; Weissman 2009; Okan 2010) reported on the duration of crying. Infants in the breastfeeding group compared to the no intervention group had a significant reduction in the duration of crying (Gradin 2004; Efe 2007; Weissman 2009) (MD ‐41; 95% CI ‐50 to ‐33), as well as infants in the breastfeeding group compared to the group given glucose (Gradin 2004; Efe 2007; Weissman 2009) (MD ‐6; 95% CI ‐12 to ‐0.2). Duration of crying was reduced in the breastfeeding group compared to the positioning group during heel lance (Gray 2002) (MD ‐63; 95% CI ‐75 to ‐52), when compared to those infants held by their mothers (Weissman 2009) (MD ‐14; 95% CI ‐22 to ‐6), and when compared to non‐nutritive sucking (on a pacifier) (Weissman 2009) (MD ‐19; 95% CI ‐29 to ‐9). Infants in the breastfeeding and skin‐to‐skin group compared to the group of infants lying on an examination table had a significant reduction in the duration of crying during heel lance (Okan 2010) (MD ‐136; 95% CI ‐180 to ‐92), while no statistically significant difference was found when compared to the skin‐to‐skin group (MD ‐17; 95% CI ‐42 to 8). When the breastfeeding group of infants were compared with the formula feeding group of infants (Weissman 2009), there was a statistically significant difference in favour of formula feeding (MD 8; 95% CI 4 to 13).
Phillips 2005 reported that 69% of infants in the breastfeeding group cried during the procedure compared to 81% of the infants in the group held by mothers with pacifier use and 100% of infants in the group held by a research assistant with use of a pacifier (P < 0.01).
c. Duration of first cry in seconds (Analysis 1.6)
1.6. Analysis.
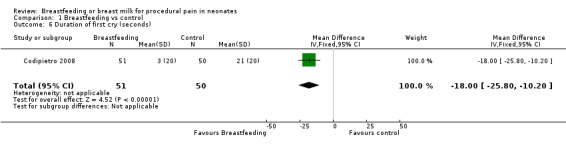
Comparison 1 Breastfeeding vs control, Outcome 6 Duration of first cry (seconds).
Only one study (Codipietro 2008) reported on the duration of first cry, which was reduced in the breastfeeding group compared to the sucrose group (MD ‐18; 95% CI ‐26 to ‐10).
3. Validated pain measures
a. Neonatal Infant Pain Score (NIPS) (Analysis 1.7)
1.7. Analysis.
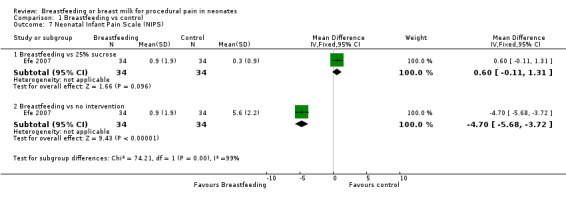
Comparison 1 Breastfeeding vs control, Outcome 7 Neonatal Infant Pain Scale (NIPS).
One study (Efe 2007) reported on this outcome. The NIPS in the breastfeeding group was significantly lower compared to the no intervention group (MD ‐4.7; 95% CI ‐5.7 to ‐3.7), while there was no statistical significant difference when compared to 25% sucrose solution (MD 0.6; 95% CI ‐0.1 to 1.3).
b. Premature Infant Pain Profile (PIPP) Score (Analysis 1.8)
1.8. Analysis.
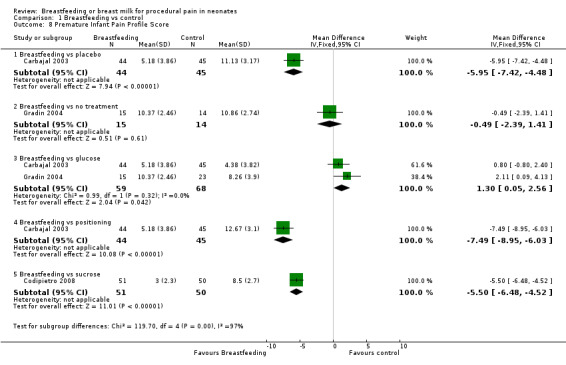
Comparison 1 Breastfeeding vs control, Outcome 8 Premature Infant Pain Profile Score.
Three studies reported on the PIPP scores (Carbajal 2003; Gradin 2004; Codipietro 2008). The PIPP scores in the breastfeeding group were significantly lower compared to the placebo group (Carbajal 2003) (MD ‐6; 95% CI ‐7 to ‐4) or the positioning in mother's arms group (MD ‐7; 95% CI ‐9 to ‐6). The PIPP score between breastfeeding and no treatment group (Gradin 2004) was not statistically significantly different (MD 0; 95% CI ‐2 to 1). The PIPP score was statistically significantly higher in the glucose group compared to the breastfeeding group (Carbajal 2003; Gradin 2004; Codipietro 2008) (MD 1.3; 95% CI 0.05 to‐ 2.6) but lower in the sucrose group compared to the breastfeeding group (MD ‐5.5; 95% CI ‐6.5 to ‐4.5).
c. Neonatal Facial Coding System (NFCS) (Analysis 1.9) (Figure 4)
1.9. Analysis.
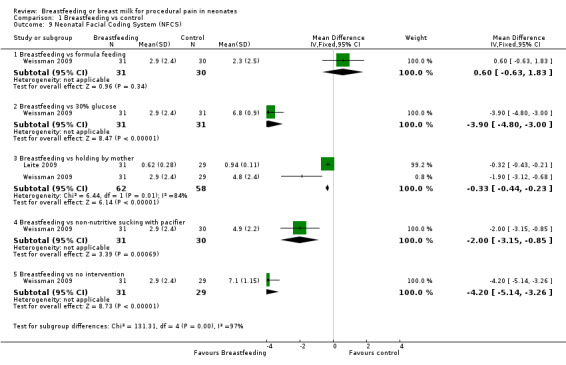
Comparison 1 Breastfeeding vs control, Outcome 9 Neonatal Facial Coding System (NFCS).
4.
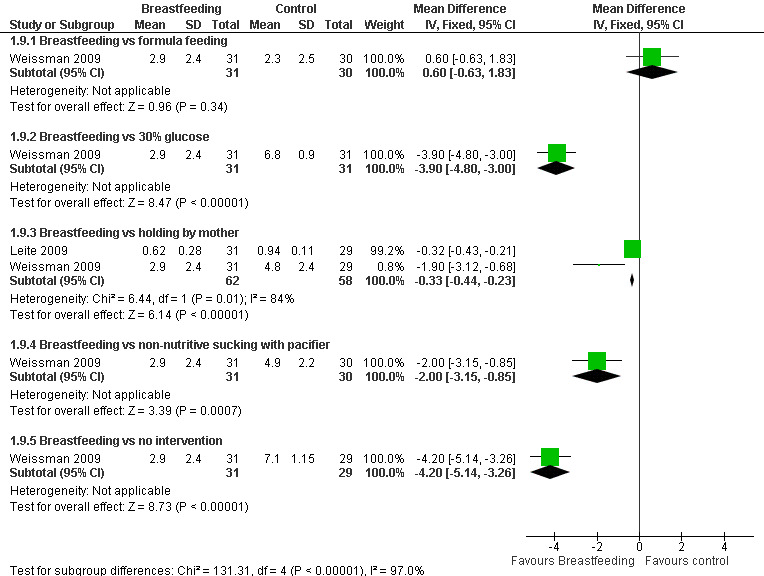
Forest plot of comparison: 1 Breastfeeding vs control, outcome: 1.9 Neonatal Facial Coding Score (NFCS).
Two studies reported on the NFCS score (Leite 2009; Weissman 2009). The scores were statistically significantly lower in the breastfeeding group compared to the infants held by their mother (Leite 2009; Weissman 2009) (MD ‐0.3; 95% CI ‐0.4 to ‐0.2), compared to (Weissman 2009) infants who received a 30% glucose solution (MD ‐4; 95% CI ‐5 to ‐3), infants who had non‐nutritive sucking on a pacifier (MD ‐2; 95% CI ‐3 to ‐1), and the group of infants who had no intervention (MD ‐4; 95% CI ‐5 to ‐3). When breastfeeding was compared to formula feeding, no statistical significant difference was found (MD 0.6; 95% CI ‐0.6 to 1.8) (Figure 4).
d. Other pain scores as reported (non‐validated)
Douleur Aigue Nouveau‐né score (DAN) Scale (Analysis 1.10)
1.10. Analysis.
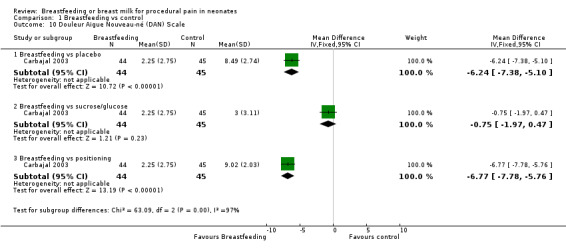
Comparison 1 Breastfeeding vs control, Outcome 10 Douleur Aigue Nouveau‐né (DAN) Scale.
Only one study reported on the DAN score (Carbajal 2003). The DAN scores in the breastfeeding group compared to the placebo group (MD ‐6; 95% CI ‐7 to ‐5) and breastfeeding group compared to positioning in mother's arms group (MD ‐7; 95% CI ‐8 to ‐6) were statistically significantly lower. The DAN score between the breastfeeding group and glucose group was not statistically significantly different (MD ‐0.8; 95% CI ‐2.0 to 0.5).
Composite score (Analysis 1.11)
1.11. Analysis.
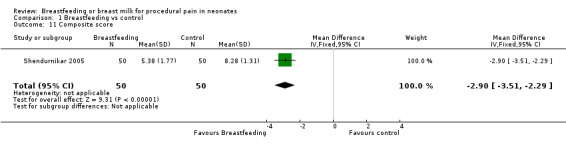
Comparison 1 Breastfeeding vs control, Outcome 11 Composite score.
Shendurnikar 2005 calculated the Composite score. The Composite score was calculated using the following criteria.
Heart rate (0 = < 120/minute; 1 = 120 to 160/minute and 2 = > 160/minute).
Breathing (0 = relaxed; 1 = changed).
Facial expression (0 = relaxed; 1 = grimaced).
Body movements (0 = relaxed; 1 = no gross movement; 2 = gross body movement).
State of arousal (0 = sleepy; 1 = awake; 2 = fussy).
Cry (0 = no; 1 = whimper; 2 = vigorous) and combining the score.
There was a statistically significant decrease in the Composite score in the breastfeeding group compared to the swaddled group (MD ‐3; 95% CI ‐4 to ‐2).
Supplemental breast milk versus control (Comparison 2)
Ten studies reported on this comparison (Skogsdal 1997; Ors 1999; Bucher 2000; Blass 2001; Jatana 2003; Upadhyay 2004; Uyan 2005; Mathai 2006; Ozdogan 2010; Yilmaz 2011 )
1. Physiological parameters
a. Heart rate change (beats per minute) (Analysis 2.1)
2.1. Analysis.
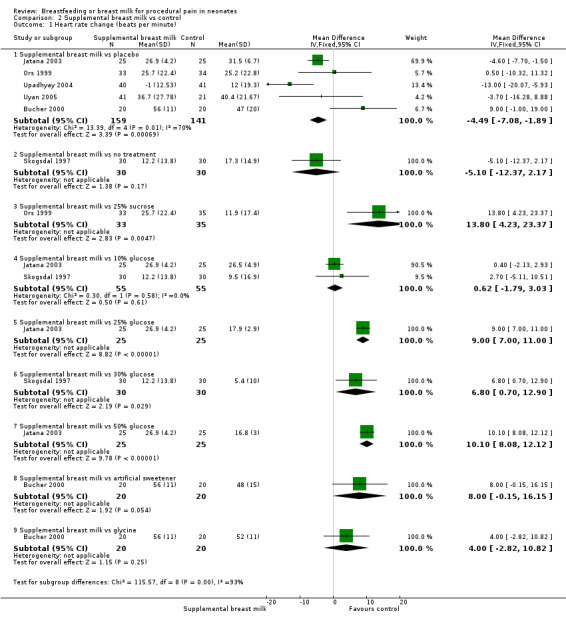
Comparison 2 Supplemental breast milk vs control, Outcome 1 Heart rate change (beats per minute).
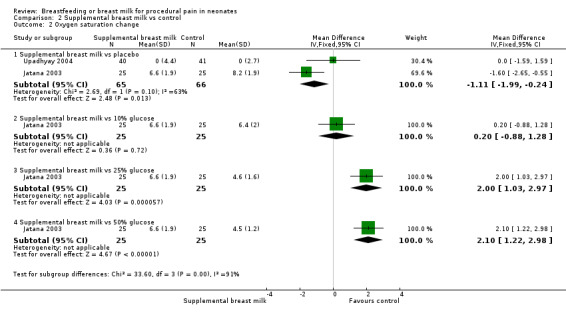
Eight studies reported on changes in the heart rate (Skogsdal 1997; Ors 1999; Bucher 2000; Blass 2001; Jatana 2003; Upadhyay 2004; Uyan 2005; Yilmaz 2011). The heart rate tended to increase in both groups during the procedure. There was no statistically significant difference in the heart rate change between the supplemental breast milk group and the placebo group (MD ‐4; 95% CI ‐9 to 1; P = 0.08, I2 = 78%); supplemental breast milk and no treatment group (Skogsdal 1997) (MD ‐5; 95% CI ‐12 to 2 ); supplemental breast milk and 10% glucose group (Skogsdal 1997; Jatana 2003) (MD 0.6; 95% CI ‐2 to 3 ); supplemental breast milk and artificial sweetener group (MD 8; 95% CI 0 to 16 ; P = 0.05) and supplemental breast milk and glycine group (MD 4; 95% CI ‐3 to 11 ). We identified statistical heterogeneity (Higgins 2003) when pooling data from breast milk versus placebo studies (I2 = 78%; P = 0.0004) which is concordant with clinical heterogeneity observed between studies (population and dose of breast milk). Blass 2001 reported on mean heart rate change during and following the heel lance in the form of a bar graph. The mean heart rate change in the group given colostrum via a pacifier and the groups given sucrose, either via syringe or pacifier, were significantly less than the group given water, either by syringe or pacifier, and the group given colostrum via syringe. Ors 1999 reported a significantly higher increase in the heart rate change in the supplemental breast milk group compared to the 25% sucrose group (MD 14; 95% CI 4 to 23) as did Jatana 2003 when comparing supplemental breast milk to 25% glucose (MD 9.0; 95% CI 7 to 11). Skogsdal 1997 also reported a significantly higher increase in heart rate change in the supplemental breast milk group compared to the 30% glucose group (MD 7; 95% CI 1 to 13) as did Jatana 2003 when comparing the supplemental breast milk group to the 50% glucose (MD 10; 95% CI 8 to 12). Yilmaz 2011 also reported heart rate changes and showed no difference, but no measure of dispersion was provided and so we were unable to meta‐analyse the data. This study presented the results showing heart rate before, during and after the procedure; heart rate increased in the four groups, although the authors comment that there was no statistically significant differences between them.
b. Respiratory rate change
One study reported on oxygen saturation change (Yilmaz 2011), but did not provide the measure of dispersion. The authors reported the mean respiratory rate before and after the procedure, and there was an increase in all four groups.
c. Oxygen saturation change (Analysis 2.2)
2.2. Analysis.
Comparison 2 Supplemental breast milk vs control, Outcome 2 Oxygen saturation change.
One study reported on the change in oxygen saturation (Upadhyay 2004). The infants in the supplemental breast milk group compared to the placebo group had no statistically significant difference in the change in oxygen saturation at three minutes (MD 0; 95% CI ‐2 to 2). Yilmaz 2011 mentioned that they assessed for saturation differences, and did not find any significant differences, but no actual numbers were provided in the article.
d. Changes in blood pressure
None of the studies included in this review reported on this outcome.
2. Cry variables
a. Percentage of time crying (Analysis 2.3)
2.3. Analysis.
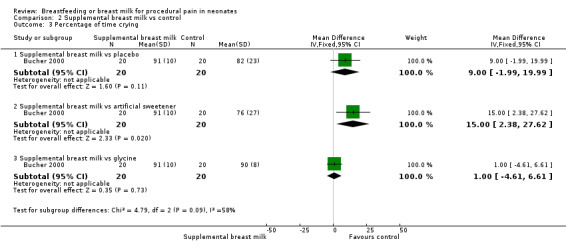
Comparison 2 Supplemental breast milk vs control, Outcome 3 Percentage of time crying.
Blass 2001 reported the mean time spent crying during the recovery period in the form of a linear graph. This study identified a statistically significant reduction in the proportion of time crying in the group given sucrose (via syringe or pacifier) compared to the control group and the group given colostrum (via syringe or pacifier) (P < 0.0015). There was no statistically significant difference between the colostrum group and the control group. It was not possible to abstract data from the graphs. Bucher 2000 reported a statistically significant reduction in the percentage time crying in the artificial sweetener group compared to the supplemental breast milk group (MD 15; 95% CI 2 to 28), but no statistically significant reduction between the supplemental breast milk group and the placebo group (MD 9; 95% CI 2 to 20) and the supplemental breast milk group and the glycine group (MD 1; 95% CI ‐5 to 7).
b. Duration of crying (in seconds) (Analysis 2.4)
2.4. Analysis.
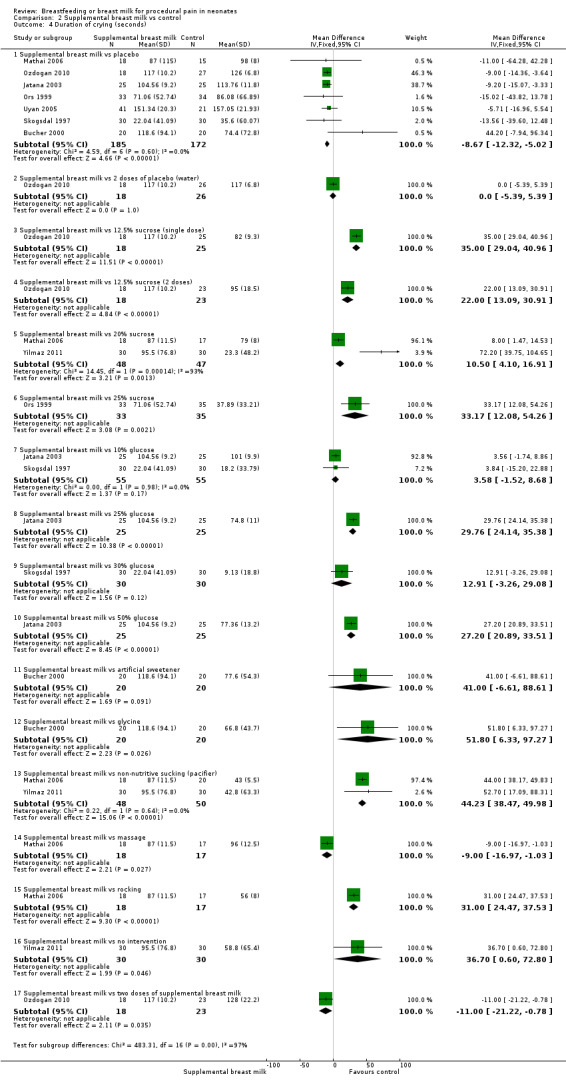
Comparison 2 Supplemental breast milk vs control, Outcome 4 Duration of crying (seconds).
All ten studies (Skogsdal 1997; Ors 1999; Bucher 2000; Blass 2001; Jatana 2003; Upadhyay 2004; Uyan 2005; Mathai 2006; Ozdogan 2010; Yilmaz 2011) reported on the duration of crying. Blass 2001 reported reduction in crying time, however, the data were not in a format that could be abstracted. Upadhyay 2004 reported a statistically significant reduction in the duration of crying among infants fed breast milk compared to placebo (MD 71 ; 95% CI 37 to 105 ); comparative data could not be abstracted. Combining the data from seven studies (Skogsdal 1997; Ors 1999; Bucher 2000; Jatana 2003; Uyan 2005; Mathai 2006; Ozdogan 2010) revealed a statistically significant reduction in the duration of crying between the supplemental milk and the placebo group (MD ‐9; 95% CI ‐12 to ‐5). Mathai 2006 compared the supplemental breast milk group and the massage group and reported a reduction in duration of crying (MD‐9; 95% CI ‐17 to ‐1) and the comparison of one dose of supplemental breast milk versus two doses of supplemental breast milk revealed a reduction with the single dose (MD ‐11; 95% CI ‐21 to ‐1). When comparing supplemental breast milk with two doses of placebo (Ozdogan 2010) no statistical difference was identified (MD 0; 95% CI ‐5 to 5). Ozdogan 2010 observed a statistically significant increase in the duration of crying comparing the supplemental breast milk versus 12.5% sucrose single dose (MD 35; 95% CI 29 to 41 sec) or versus 12.5% sucrose (two doses) (MD 22; 95% CI 13 to 31). Ors 1999 compared the supplemental breast milk group to the 25% glucose group and reported an increase in duration of crying in the supplemental breast milk group (MD 33; 95% CI 12 to 54). When supplemental breast milk was compared with 20% sucrose (Mathai 2006; Yilmaz 2011) there was an increase in the duration of crying in the breast milk group (MD 11; 95% CI 4 to 17).There was a statistically significant increase in the duration of crying in the supplemental breast milk group compared to the glycine group (MD 52; 95% CI 6 to 97), non‐nutritive sucking group (MD 44; 95% CI 38 to 50), with rocking group (MD 31; 95% CI 24 to 38), the 25% glucose group (MD 30; 95% CI 24 to 35), 50% glucose group (MD 27; 95% CI 21 to 34) and no intervention group (MD 37; 95% CI 1 to 73). There was no statistically significant difference in the duration of crying between the supplemental breast milk and the 30% glucose group (MD 13; 95% CI ‐3 to 29); the supplemental breast milk group and the 10% glucose group (MD 4; 95% CI ‐2 to 9), and the supplemental breast milk group and the artificial sweetener group (MD 41; 95% CI ‐7 to 89).
c. Duration of first cry (in seconds) (Analysis 2.5)
2.5. Analysis.
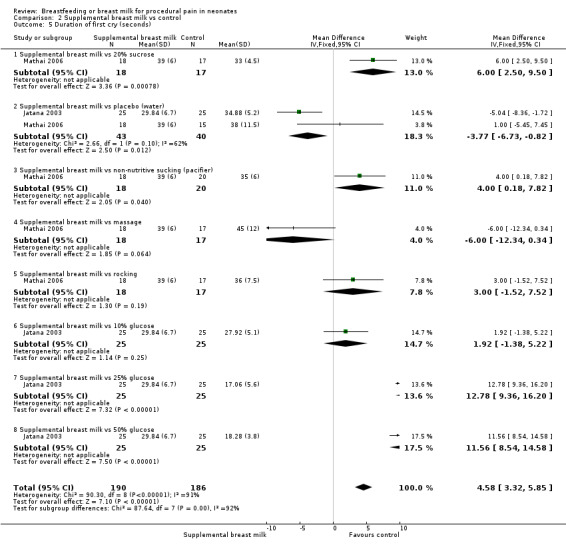
Comparison 2 Supplemental breast milk vs control, Outcome 5 Duration of first cry (seconds).
Only two studies (Jatana 2003; Mathai 2006) assessed the duration of first cry. A significant reduction in duration of first cry was found when comparing supplemental breast milk with placebo (water) (MD ‐4; 95% CI ‐7 to ‐1. No significant difference was found when comparing supplemental breast milk with massage (MD ‐6; 95% CI ‐12 to 0.3), with rocking (MD 3; 95% CI ‐2 to 8), or with the 10% glucose group (MD 2; 95% CI ‐1 to 5). The duration of first cry was significantly higher in the supplemental breast milk group when compared to 20% sucrose (MD 6; 95% 3 to 10), non‐nutritive sucking (pacifier) (MD 4; 95% 0.2 to 8), 25% glucose (MD 13; 95% CI 9 to 16), or 50% glucose (MD 12; 95% CI 8 to 15).
3. Validated pain measures
a. Neonatal infant pain score (NIPS) (Analysis 2.6)
2.6. Analysis.
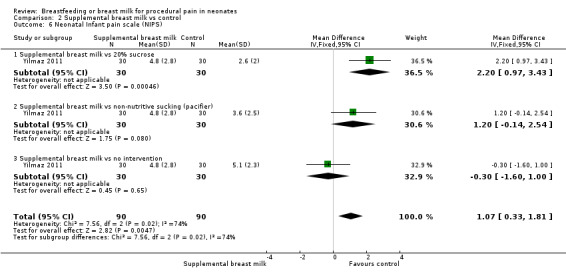
Comparison 2 Supplemental breast milk vs control, Outcome 6 Neonatal Infant pain scale (NIPS).
One study (Yilmaz 2011) reported on this outcome. The NIPS was significantly higher in the breast milk group when compared to 20% sucrose (MD 2; 95% CI 1 to 3). No statistical difference was found when supplemental breast milk was compared with no intervention (MD ‐0.3; 95% CI ‐2 to 1) and to non‐nutritive sucking (pacifier) (MD 1.2; 95% CI ‐0.1 to 2.5).
b. Premature Infant Pain Profile (PIPP)
None of the studies included in this review reported on this outcome.
c. Neonatal Facial Coding Score (NFCS) at three minutes (Analysis 2.7)
2.7. Analysis.
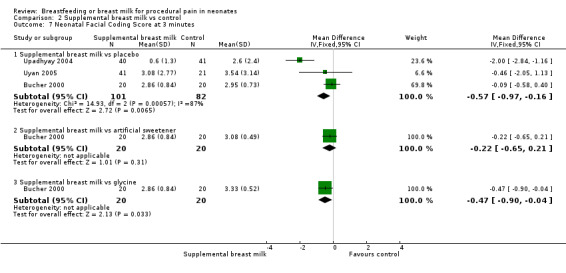
Comparison 2 Supplemental breast milk vs control, Outcome 7 Neonatal Facial Coding Score at 3 minutes.
Three studies (Bucher 2000; Upadhyay 2004; Uyan 2005) reported on NFCS. Bucher 2000 used five components of NFCS and Upadhyay 2004 modified the score and collected data on only part of the components. Bucher 2000 reported no statistically significant difference between the supplemental breast milk and the placebo group (MD ‐0.1; 95% CI ‐0.6 to 0.4). Upadhyay 2004 reported a statistically significant reduction in the NFCS in the supplemental breast milk group compared to the placebo group (MD ‐2.0; 95% CI ‐3 to ‐1). Uyan 2005 reported no statistically significant difference between the supplemental breast milk and the placebo group (MD ‐0.5; 95% CI ‐2.0 to 1.1). There was marked heterogeneity in the data collection for NFCS. The data were not combined statistically due to this marked clinical heterogeneity. Bucher 2000 reported no statistically significant reduction in NFCS between the supplemental breast milk group and the artificial sweetener group (MD ‐0.2; 95% CI ‐0.7 to 0.2). However, a statistically significant reduction in NFCS was noted in the supplemental breast milk group compared to the glycine group (MD ‐0.5; 95% CI ‐0.9 to ‐0.04).
d. Neonatal Facial Coding Score (NFCS) at two minutes (Analysis 2.8) (Figure 5)
2.8. Analysis.
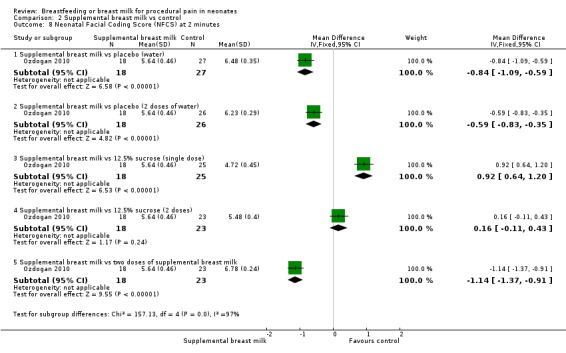
Comparison 2 Supplemental breast milk vs control, Outcome 8 Neonatal Facial Coding Score (NFCS) at 2 minutes.
5.
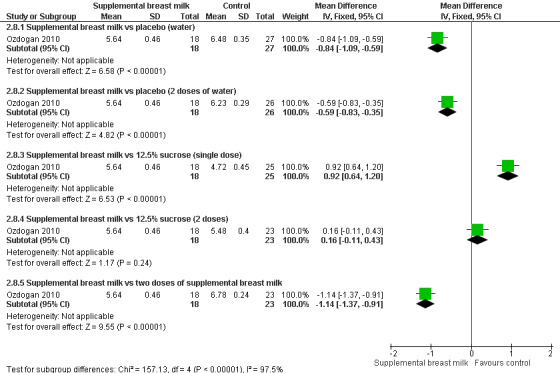
Forest plot of comparison: 2 Supplemental breast milk vs control, outcome: 2.8 Neonatal Facial Coding Score (NFCS) at 2 minutes.
One study (Ozdogan 2010) reported on NFCS at two minutes. There was a statistically significant reduction in the NFCS in the supplemental breast milk group when compared to placebo (water) (MD ‐0.8; 95% CI ‐1.1 to ‐0.6), to two doses of water (MD ‐0.6; 95% CI ‐0.8 to ‐0.4) and to two doses of supplemental breast milk (MD ‐1.1; 95% CI ‐1.4 to ‐0.9). The NFCS scores were significantly higher in the breast milk group when compared to 12.5% sucrose single dose (MD 0.9; 95% CI 0.6 to 1.2) and there was no significant statistical difference when breast milk was compared to a double dose of 12.5% sucrose (MD 0.2; 95% CI ‐0.1 to 0.4) (Figure 5).
e. Other pain scores as reported (non‐validated)
Douleur aigue du nouveau‐ne (DAN) at two minutes (Analysis 2.9) (Figure 6)
2.9. Analysis.
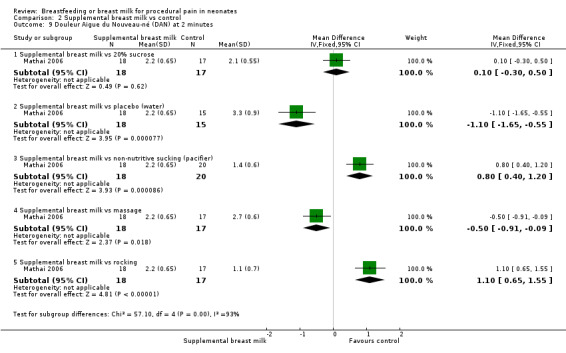
Comparison 2 Supplemental breast milk vs control, Outcome 9 Douleur Aigue du Nouveau‐né (DAN) at 2 minutes.
6.
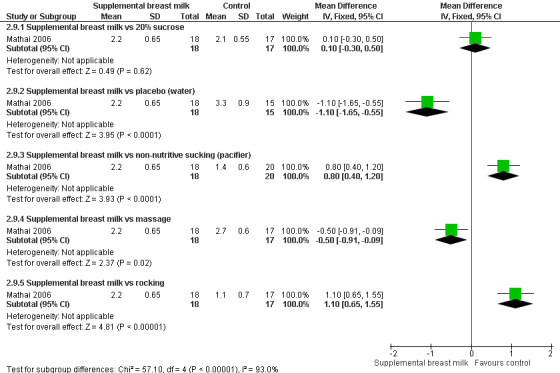
Forest plot of comparison: 2 Supplemental breast milk vs control, outcome: 2.9 Douleur Aigue du Nouveau‐né (DAN) at 2 minutes.
One study reported on DAN scores at two minutes (Mathai 2006). There was a statistically significant reduction in the DAN scores in the supplemental breast milk group when compared to placebo (water) (MD ‐1.1; 95% CI‐1.6 to ‐0.6) and when compared to the massage group (MD ‐0.5; 95% CI ‐0.9 to ‐0.1). There was no significant statistical difference between the breast milk group and 20% sucrose group (MD 0.1; 95% CI ‐0.3 to 0.5), the non‐nutritive sucking group (MD 0.8; 95% CI 0.4 to 1.2) and the massage group (MD ‐0.5; 95% CI ‐0.9 to ‐0.1). The DAN scores were significantly higher in the breast milk group when compared to the rocking group (MD 1.1; 95% CI 0.7 to 1.6).
Body pain score (Analysis 2.10)
2.10. Analysis.
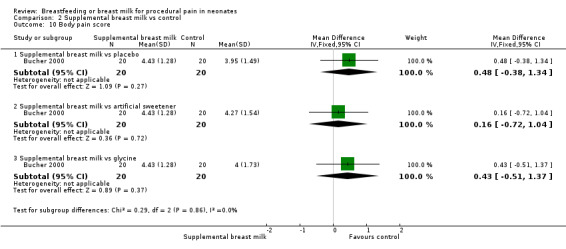
Comparison 2 Supplemental breast milk vs control, Outcome 10 Body pain score.
One study (Bucher 2000) reported on the body pain score outcome (maximum score was 8 and minimum score was 0). There was no statistically significant reduction in body pain score between the supplemental breast milk and the placebo group (MD 0.5; 95% CI ‐0.4 to 1.3), the supplemental breast milk group and the artificial sweetener group (MD 0.2; 95% CI ‐0.7 to 1.0) and the supplemental breast milk group and the glycine group (MD 0.4; 95% CI ‐0.5 to 1.4).
Secondary outcome
Carbajal 2003 gathered information on infants' sucking behaviour 48 to 72 hours after venepuncture by interviewing mothers. There was no difference in the number of infants in whom the suck was the same or more effective among four groups (P = 0.14). The authors reported that infants who underwent venepuncture while they were being breastfed did not suck less effectively after the procedure.
We did not perform the planned subgroup analyses according to gestational age groups in this version of the review because, with the exception of one study (Skogsdal 1997), all other studies included only term infants. We did not perform other planned subgroup analyses according to type of intervention and type of procedure because subdividing the current data in these subgroups will have one, and at the most two studies, for comparison between groups at this point. However, in future updates of this review we plan to evaluate this subgroup analysis.
Discussion
Summary of main results
Breastfeeding was associated with a reduction in changes in heart rate, percentage of time crying, duration of crying and duration of first cry, validated and non‐validated pain measures such as the Neonatal Infant Pain Score (NIPS), Premature Infant Pain Profile (PIPP), and Douleur Aigue Nouveau‐né Scale (DAN), when compared to positioning, held by mother, placebo, pacifier use, no intervention or oral sucrose, or both. However no statistical difference was found in heart rate change when compared to formula feeding or high concentrations of glucose (30%). Breastfeeding was not found to be advantageous in duration of crying when compared to formula feeding, and there was no statistical difference when compared to skin‐to‐skin contact. No difference was found in other physiological parameters such as oxygen saturation change and blood pressure changes when comparing breastfeeding with pacifier use or oral sucrose. In terms of pain scores, there was no statistically significant difference in NIPS when compared to sucrose, in PIPP scores when compared to no treatment, in NFCS scores when compared to formula feeding and in DAN scores when compared to sucrose/glucose. One study developed a pain Composite score, and breastfeeding was found to be effective in reducing this score.
Supplemental breast milk yielded variable results. No statistical difference was found in changes in heart rate when compared to placebo, no treatment, 10% glucose, artificial sweetener and glycine. Higher concentrations of glucose (25% sucrose and 30% glucose) were associated with a reduction in heart rate when compared to breast milk. No statistical difference was found in terms of oxygen saturation change when compared to placebo. No statistical difference was found between breast milk and placebo or glycine in terms of percentage of time crying, while artificial sweetener reduced it. Breast milk reduced duration of crying when compared to placebo and massage, however there was no statistical difference when compared to two doses of placebo, 10% glucose and 30% glucose, or artificial sweetener. Sucrose (in any concentration, i.e. 12.5%, 20%, 25%) was found to reduce the duration of cry when compared to breast milk, as did glycine, pacifier use, rocking, and no intervention. Breast milk was found not to have an effect in duration of first cry; it was either non‐statistically significant or favoured the control (in 20% sucrose and pacifier use). Breast milk was found not to be effective in reducing validated and non‐validated pain scores such as NIPS, NFCS, and DAN; only being significantly better when compared to placebo (water) or massage. Sucrose, use of pacifier and rocking of the infant were shown to reduce the scores significantly when compared to breast milk. Body pain score was reported in one study and there was no statistically significant difference between breast milk and placebo, artificial sweetener or glycine.
Overall completeness and applicability of evidence
All studies evaluated in this review assessed the effects of breastfeeding or supplemental breast milk on single painful procedure only. Based on the available results of these studies we can conclude that neonates undergoing single painful procedure should be provided breastfeeding for analgesia when possible, compared to positioning/pacifier/holding and swaddling. If it is not available or feasible to give breastfeeding, alternatives such as glucose or sucrose should be considered. It appears that none of these agents completely eliminate the pain. On the other hand, the efficacy of supplemental breast milk on physiological or pain scores was not convincing. However, provision of breastfeeding or supplemental breast milk for painful procedures may further encourage mothers to breastfeed their infants, facilitate bonding, and provide psychological advantage for the mother in terms of her involvement in the care of her infant without any additional cost to the health care system.
The results of these studies are applicable to a large population, i.e. term infants requiring heel lance or venepuncture in their first days of life. Currently, in many countries around the world, all neonates are subjected to heel lance in the first week of life for metabolic screening, and even in those countries where metabolic screening is not yet available, healthy full‐term neonates frequently require heel lance for simple tests such as glucose or bilirubin testing; therefore, the results of these studies are easily applicable to hospitals with obstetric wards.
The majority of the studies included in this review have included healthy term neonates or stable late preterm neonates. A different population of interest who are subjected to a significantly higher number of interventions include preterm or sick full‐term neonates who are subjected to repeated painful procedures during hospitalisation; for them, the ideal analgesic has not yet been identified. Johnston 2002 evaluated effects of repeated administration of sucrose prior to painful procedures in infants < 31 weeks postmenstrual age. Use of sucrose was associated with reduced scores on motor development, vigour, alertness and orientation at 36 weeks; affected motor development and vigour at 40 weeks and had higher Neuro‐Biological Risk Score at two weeks postnatal age. Although unproven, breast milk may be an effective and safe alternative to sucrose, even for repeated use. Placing a small amount of solution in the oral cavity of small preterm infants was only associated with minor complications such as transient desaturation or transient choking, which did not require any intervention. As breast milk is the most natural/physiological substance available for oral stimulation, repeated exposure is not perceived to be associated with complications of oral aversion or repeated tongue thrusting. However, this needs to be studied. Though the reasons for the effectiveness of breastfeeding over simple measures such as positioning or no intervention are unclear, it is perceived to be due to psychological or chemical properties of breast milk, or both.
Several methodological challenges were apparent during this review. First, assessment of pain varied between studies. This has been a problem encountered in a previous review of sucrose for procedural pain in neonates (Stevens 2010). Behavioural and physiological parameters of pain or validated pain measures, or both were used to assess pain at random in various studies. Standardisation of utilising only validated pain scales should be the framework of further research. Future studies of adequate sample size should only include validated measures of pain as outcomes. Second, all studies explored effects of breastfeeding or breast milk following a single painful procedure. Future studies should include preterm or term neonates who require repeated painful stimuli to assess side effects of repeated oral administration of breast milk. Additionally, it should also measure the future success of breastfeeding as an outcome, as repeated conditioning may prime infants to refuse breastfeeding at a later stage. This is an important consideration, particularly for preterm neonates. Only one study that evaluated maternal perception regarding sucking after single venepuncture while breastfeeding found no changes; however, the effect of repeated exposure has not been studied. Finally, it must be recognised that there was marked heterogeneity between studies in terms of control intervention, amount/time of prior exposure to breastfeeding or breast milk, and the time interval between this exposure and the type of painful procedure.
Quality of the evidence
Quality of evidence in these studies is high, given that we have only included randomised or quasi‐randomised controlled studies. However, the majority of studies lost points on "other bias", as for many studies we were not able to find the protocol with the completed study. Additionally, due to inherent limitations, masking is not possible for the breastfeeding group. Some investigators have utilised independent assessors to score pain scales from video recordings; however, this may not be always feasible.
Potential biases in the review process
There are no potential biases in the review process, given that we performed an extensive literature search, and applied no language restrictions. Also, we reviewed the references in the articles included to make sure that we were not missing any other studies.
Agreements and disagreements with other studies or reviews
There are multiple studies in the literature that have studied the effect of sucrose as a means to provide procedural pain relief in newborns. The Cochrane review of sucrose (Stevens 2010) included 44 studies and they concluded that sucrose is also a safe and effective way of significantly reducing pain from a single painful procedure in newborns. Other studies have shown the benefits of other methods for procedural pain in newborns. In a meta‐analysis Warnock 2010 reported that maternal kangaroo care significantly reduced pain from a single pain procedure in term infants and stable preterm infants (of more than 26 weeks of gestational age). Therefore, pharmacological and non‐pharmacological interventions available for procedural pain in neonates have increased significantly. It is important that non‐pharmacological measures are given first preference, especially when there is a need for repeated analgesia.
Authors' conclusions
Implications for practice.
If available, breastfeeding or breast milk should be used to alleviate procedural pain in neonates undergoing a single painful procedure compared to placebo or positioning or no intervention. When repeated painful procedures are needed, the safety or effectiveness of breastfeeding or supplemental breast milk is not established.
Implications for research.
Further randomised controlled studies are needed to assess the efficacy and effectiveness of breastfeeding and breast milk for repeated painful procedures in neonates, especially preterm neonates.
What's new
| Date | Event | Description |
|---|---|---|
| 11 October 2011 | New citation required but conclusions have not changed | This review has been updated to include new studies. No change to conclusions. |
| 11 October 2011 | New search has been performed | This updates the review 'Breastfeeding or breast milk for procedural pain in neonates' (Shah 2006). |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to acknowledge Prof. Arne Ohlsson for reviewing the protocol and for expert advice. We would like to thank authors Dr Ulrich, Dr Gray, Dr Gradin, Dr Ors, Dr Shendurnikar, Dr Chantry and Dr Schollin for providing data from their studies. We would also like to acknowledge Ms Elizabeth Uleryk for providing support to develop and execute the literature search for this review.
Appendices
Appendix 1. Search strategy
We ran searches using the OVID search platforms in the following databases: MEDLINE, EMBASE, CINAHL and CCTR. We retrieved a total of 2203 references from all 3 databases.
The following tables and text record the search strategies and terms used.
MEDLINE:
The search strategy for MEDLINE (1948 to September 16, 2011) retrieved 699 references. We used a combination of MeSH and free text terms for
| Set | History | Results | Comments |
| 1 | Infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, premature/ or exp Infant, Newborn, Diseases/ or pregnancy, high‐risk/ or quadruplets/ or quintuplets/ or superfetation/ or triplets/ or twins/ or twins, dizygotic/ or twins, monozygotic/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw).ti,ab. or ((intrauterine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab. | 809582 | Infant age group Terms |
| 2 | Breast Feeding/ or Milk, Human/ or (breastfeed* or (breast adj2 milk) or breastmilk or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp. | 41519 | Breast Feeding terms |
| 3 | Pain Measurement/ or exp pain/ or Antibody Formation/ or Crying/ or anxiety/ or fear/ or panic/ or (adverse adj2 effect:).ti,ab. or (side adj2 effect:).ti,ab. or (skin adj2 reaction:).ti,ab. or (distress* or discomfort* or fright* or anxious).ti,ab. | 698718 | Pain terms |
| 4 | 1 and 2 and 3 |
1283 | Base clinical set 1 |
| 5 | needles/ or (needle: adj2 (gauge: or length: or thick: or angle: or size:)).ti,ab. or injections/ or injections, intramuscular/ or injections, subcutaneous/ or injections, intradermal/ or injections, jet/ or biolistics/ or ((needle: or inject: or vaccinat:) adj2 (technique: or techinc: or aspirat: or angle: or speed: or slow: or fast: or order:)).ti,ab. or punctures/ or blood specimen collection/ or phlebotomy/ or infusions, parenteral/ or infusions, intravenous/ or injections, intravenous/ or catheterization/ or catheterization, central venous/ or catheterization, peripheral/ or cannula*.mp. or ((intravenous or intravascular) adj2 access).ti,ab. or venipuncture*.mp. or (painf* adj2 procedur*).ti,ab. | 338692 | Procedure terms |
| 6 | 2 and 5 | 359 | Base clinical set 2 |
| 7 | 4 or 6 | 1579 | Base Results |
| 8 | ("clinical trial, all" or clinical trial).pt. or clinical trials as topic/ or clinical trial, phase i.pt. or clinical trials, phase i as topic/ or clinical trial, phase iii.pt. or clinical trials, phase iii as topic/ or clinical trial, phase iv.pt. or clinical trials, phase iv as topic/ or controlled clinical trial.pt. or controlled clinical trials as topic/ or meta‐analysis.pt. or meta‐analysis as topic/ or multicenter study.pt. or multicenter studies as topic/ or randomized controlled trial.pt. or randomized controlled trials as topic/ or evaluation studies as topic/ or validation studies as topic/ or evaluation study.pt. or validation study.pt. or double‐blind method/ or random allocation/ or single‐blind method/ or (guideline* or cochrane or medline or cinahl or embase or CCTR or scopus or "web of science" or lilacs or (systematic* adj2 review*)).mp. or comparative study/ or (random* or (doubl* adj2 dummy) or ((singl* or doubl* or tripl* or trebl*) adj25 (mask* or blind*)) or rct or rcts or (control adj25 trial*) or multicent* or placebo* or metaanalys* or (meta adj5 analys*) or sham or effectiveness or efficacy or compar*).ti,ab. or (guideline* or cochrane or medline or cinahl or embase or CCTR or scopus or "web of science" or lilacs or (systematic* adj2 review*)).mp. or comparative study/ | 4754353 | Study Design/Methodology terms |
| 9 | 7 and 8 | 699 | Final results |
EMBASE
The search strategy for EMBASE (1980 to 2011 Week 36) retrieved 1212 references. We used a combination of EMBASE and free text terms for
| Set | History | Results | Comments |
| 1 | newborn/ or newborn period/ or low birth weight/ or extremely low birth weight/ or small for date infant/ or very low birth weight/ or Prematurity/ or exp newborn disease/ or multiple pregnancy/ or twin pregnancy/ or twins/ or dizygotic twins/ or monozygotic twins/ or human triplets/ or intrauterine growth retardation/ or small for date infant/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw or (intrautrine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab. | 1397165 | Infant age group Terms |
| 2 | breast feeding/ or breast milk/ or (breastfeed* or (breast adj2 milk) or breastmilk or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp. | 48144 | Breast Feeding terms |
| 3 | Pain Assessment/ or pain/ or injection pain/ or vaccination reaction/ or exp application site reaction/ or exp injection site reaction/ or antibody production/ or crying/ or facial expression/ or gesture/ or fear/ or anticipatory anxiety/ or anxiety/ or (adverse adj2 effect:).ti,ab. or (side adj2 effect:).ti,ab. or (skin adj2 reaction:).ti,ab. or (distress* or discomfort* or fright* or anxious).ti,ab. | 726581 | Pain terms |
| 4 | 1 and 2 and 3 | 1466 | Base clinical set 1 |
| 5 | needle/ or exp Injection/ or intradermal drug administration/ or intramuscular drug administration/ or intraosseous drug administration/ or subcutaneous drug administration/ or transdermal drug administration/ or (needle: adj2 (gauge: or length: or thick: or angle: or size:)).ti,ab. or injections/ or injections, intramuscular/ or injections, subcutaneous/ or injections, intradermal/ or injections, jet/ or biolistics/ or ((needle: or inject: or vaccinat:) adj2 (technique: or techinc: or aspirat: or angle: or speed: or slow: or fast: or order:)).ti,ab. or cannula*.mp. or ((intravenous or intravascular) adj2 access).ti,ab. or venipuncture*.mp. or (painf* adj2 procedur*).ti,ab. or puncture/ or blood sampling/ or vein puncture/ or phlebotomy/ or intravenous drug administration/ or parenteral drug administration/ or intravenous drug administration/ or catheterization/ or blood vessel catheterization/ or central venous catheter/ or "catheters and tubes"/ or cannula/ or ((intravenous or intravascular) adj2 access).ti,ab. or venipuncture*.mp. | 693172 | Procedure terms |
| 6 | 2 and 5 | 1163 | Base clinical set 2 |
| 7 | 4 or 6 | 2514 | Base Results |
| 8 | ct.fs. or clinical trial/ or controlled clinical trial/ or multicenter study/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or cohort analysis/ or double blind procedure/ or single blind procedure/ or triple blind procedure/ or meta analysis/ or randomized controlled trial/ or "systematic review"/ or case control study/ or longitudinal study/ or prospective study/ or retrospective study/ or multicenter study/ or validation study/ or (((evaluation or validation) adj2 study) or ((evaluation or validation) adj2 studies)).ti,ab. or double‐blind method/ or random allocation/ or single‐blind method/ or (guideline* or cochrane or medline or cinahl or embase or CCTR or scopus or "web of science" or lilacs or (systematic* adj2 review*)).mp. or comparative study/ or (random* or (doubl* adj2 dummy) or ((singl* or doubl* or tripl* or trebl*) adj25 (mask* or blind*)) or rct or rcts or (control adj25 trial*) or multicent* or placebo* or metaanalys* or (meta adj5 analys*) or sham or effectiveness or efficacy or compar*).ti,ab. | 5294788 | Study Design/Methodology terms |
| 9 | 7 or 8 | 1212 | Final results |
EBM Reviews ‐ Cochrane Central Register of Controlled Trials
The search strategy for CCTR (3rdQuarter 2011) retrieved 174 references. This database consists exclusively of RCTs, no study design terms were used. We used a combination of primarily MeSH and free text terms for
| Set | History | Results | Comments |
| 1 | Infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, premature/ or exp Infant, Newborn, Diseases/ or pregnancy, high‐risk/ or quadruplets/ or quintuplets/ or superfetation/ or triplets/ or twins/ or twins, dizygotic/ or twins, monozygotic/ or newborn/ or newborn period/ or low birth weight/ or extremely low birth weight/ or small for date infant/ or very low birth weight/ or Prematurity/ or exp newborn disease/ or multiple pregnancy/ or twin pregnancy/ or dizygotic twins/ or monozygotic twins/ or human triplets/ or intrauterine growth retardation/ or small for date infant/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw).ti,ab. or ((intrauterine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab. | 25542 | Infant age group Terms |
| 2 | Breast Feeding/ or Milk, Human/ or breast milk/ or (breastfeed* or (breast adj2 milk) or breastmilk or breastfed or (breast adj2 feed*) or (breast adj2 fed)).mp. | 2349 | Breast Feeding terms |
| 3 | Pain Measurement/ or exp pain/ or Antibody Formation/ or Crying/ or anxiety/ or fear/ or panic/ or Pain Assessment/ or injection pain/ or vaccination reaction/ or exp application site reaction/ or exp injection site reaction/ or antibody production/ or facial expression/ or gesture/ or anticipatory anxiety/ or anxiety/ or (adverse adj2 effect:).ti,ab. or (side adj2 effect:).ti,ab. or (skin adj2 reaction:).ti,ab. or (distress* or discomfort* or fright* or anxious).ti,ab. | 80214 | Pain terms |
| 4 | 1 and 2 and 3 | 146 | Base clinical set 1 |
| 5 | needle/ or exp Injection/ or intradermal drug administration/ or intramuscular drug administration/ or intraosseous drug administration/ or subcutaneous drug administration/ or transdermal drug administration/ or injections/ or injections, intramuscular/ or injections, subcutaneous/ or injections, intradermal/ or injections, jet/ or biolistics/ or puncture/ or blood sampling/ or vein puncture/ or phlebotomy/ or intravenous drug administration/ or parenteral drug administration/ or intravenous drug administration/ or catheterization/ or blood vessel catheterization/ or central venous catheter/ or "catheters and tubes"/ or cannula/ or needles/ or (needle: adj2 (gauge: or length: or thick: or angle: or size:)).ti,ab. or injections/ or ((needle: or inject: or vaccinat:) adj2 (technique: or techinc: or aspirat: or angle: or speed: or slow: or fast: or order:)).ti,ab. or punctures/ or blood specimen collection/ or phlebotomy/ or infusions, parenteral/ or infusions, intravenous/ or injections, intravenous/ or catheterization, central venous/ or catheterization, peripheral/ or cannula*.mp. or ((intravenous or intravascular) adj2 access).ti,ab. or venipuncture*.mp. or (painf* adj2 procedur*).ti,ab. | 28461 | Procedure terms |
| 6 | 2 and 5 | 49 | Base clinical set 2 |
| 7 | 4 or 6 | 174 | Final results |
CINAHL
CINAHL as of October 4, 2011
S1 (MH "Infant, Newborn") OR (MH "Infant, Low Birth Weight") OR (MH "Infant, Small for Gestational Age") OR (MH "Infant, Very Low Birth Weight") OR (MH "Infant, Premature") OR (MH "Infant, Newborn, Diseases+") OR (MH "Pregnancy, High Risk") OR (MH "Pregnancy, Multiple") OR (MH "Twins") OR (MH "Childbirth, Premature") OR (MH "Multiple Offspring") OR (MH "Fetal Growth Retardation") OR (TX quadruplet* OR quintuplet* OR superfetation OR triplet* OR twin* OR infan* OR neonat* OR newborn* OR prematur* OR iugr OR sga OR vlbw OR lbw OR elbw) OR (TX (intrauterine N2 growth N2 restrict*)) OR (TX (intrauterine N2 growth N2 retard*))
S2 (MH "Breast Feeding") OR (MH "Breast Feeding Positions") OR (MH "Latching, Breastfeeding") OR (MH "Milk, Human") OR (TX breastfeed* OR breastmilk or breastfed) OR (TX (breast N2 milk) OR (TX breast N2 feed*) OR (TX breast N2 fed))
S3 (MH "Pain+") OR (MH "Treatment Related Pain") OR (MH "Antibody Formation") OR (MH "Crying") OR (MH "Facial Expression") OR (MH "Anxiety") OR (MH "Anticipatory Anxiety") OR (MH "Fear") OR (MH "Suffering") OR (TX panic OR distress* OR discomfort* OR fright* OR anxious) OR (TX (adverse N2 effect*)) OR (TX (side N2 effect*)) OR (TX (skin N2 reaction*))
S4 S1 and S2 and S3
S5 (MH "Needles") OR (MH "Injections") OR (MH "Injection Sites") OR (MH "Injections, Intradermal") OR (MH "Injections, Intramuscular+") OR (MH "Injections, Intravenous") OR (MH "Injections, Subcutaneous+") OR (MH "Administration, Intravenous+") OR (MH "Infusions, Parenteral+") OR (MH "Punctures") OR (MH "Arterial Puncture") OR (MH "Venipuncture") OR (MH "Phlebotomy") OR (MH "Blood Specimen Collection") OR (MH "Catheterization") OR (MH "Catheterization, Central Venous+") OR (MH "Catheterization, Peripheral+") OR (MH "Catheterization, Umbilical Vessels") OR (MH "Venous Cutdown")OR (TX (needle* N2 gauge*)) OR (TX (needle* N2 length*)) OR (TX (needle* N2 thick*)) OR (TX (needle* N2 angle*)) OR (TX (needle* N2 size*)) OR (TX (needle* N2 techni*)) or (TX (Needle* N2 aspirat*)) OR (TX (needle* N2 angle*)) OR (TX (needle* N2 speed)) OR (TX (needle N2 slow*)) OR (TX (needle* N2 fast*)) OR (TX (needle N2 order*)) OR (TX (inject* N2 techni*)) or (TX (inject* N2 aspirat*)) OR (TX (inject* N2 angle*)) OR (TX (inject* N2 speed)) OR (TX (inject* N2 slow*)) OR (TX (inject* N2 fast*)) OR (TX (inject* N2 order*)) OR (TX (vaccinat* N2 techni*)) or (TX (vaccinat* N2 aspirat*)) OR (TX (vaccinat* N2 angle*)) OR (TX (vaccinat* N2 speed)) OR (TX (vaccinat* N2 slow*)) OR (TX (vaccinat* N2 fast*)) OR (TX (vaccinat*t N2 order*)) OR (TX cannula*) OR (TX venipunctur*) OR (TX (pain* N2 procedure*))
S6 S2 and S5
S7 S4 or S6
S8 (MH "Triple‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Randomized Controlled Trials") OR (MH "Double‐Blind Studies") OR (TX (cochrane OR medline OR cinahl OR embase OR CCTR OR scopus OR lilacs)) OR (TX (systematic* N2 review*)) OR (TX (web N2 science)) Results 118 references
Data and analyses
Comparison 1. Breastfeeding vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate change (beats per minute) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Breastfeeding vs positioning | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐23.0 [‐34.55, ‐11.45] |
| 1.2 Breastfeeding vs pacifier use (neonate held by mother) | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐12.02 [‐18.90, ‐5.13] |
| 1.3 Breastfeeding vs pacifier use (neonate held by research assistant) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐15.50, 1.30] |
| 1.4 Breastfeeding vs oral sucrose | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐14.41, ‐3.59] |
| 1.5 Breastfeeding vs held by mother | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐17.0 [‐25.51, ‐8.49] |
| 1.6 Breastfeeding vs formula feeding | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐4.78, 8.98] |
| 1.7 Breastfeeding vs 30% glucose solution | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐12.33, 3.73] |
| 1.8 Breastfeeding vs no intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐21.74, ‐4.26] |
| 2 Oxygen saturation change | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Breastfeeding vs pacifier use (neonate held by mother) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.79, 3.39] |
| 2.2 Breastfeeding vs pacifier use (neonate held by research assistant) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.48, 2.68] |
| 2.3 Breastfeeding vs oral sucrose (25%) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.38, 3.62] |
| 3 Blood pressure changes (mm of Hg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Breastfeeding vs pacifier use (neonate held by mother) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐9.08, 1.88] |
| 3.2 Breastfeeding vs pacifier use (neonate held by research assistant) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐4.86, 8.06] |
| 4 Percentage of time crying | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Breastfeeding vs positioning | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐39.0 [‐55.03, ‐22.97] |
| 4.2 Breastfeeding vs pacifier use (neonate held by mother) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐11.80 [‐27.95, 4.35] |
| 4.3 Breastfeeding vs pacifier use (neonate held by research assistant) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐32.60 [‐49.83, ‐15.37] |
| 4.4 Breastfeeding vs oral sucrose (25%) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐41.5 [‐48.01, ‐34.99] |
| 5 Duration of crying (seconds) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Breastfeeding vs no treatment | 3 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐41.34 [‐49.53, ‐33.15] |
| 5.2 Breastfeeding vs sucrose/glucose | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐5.83 [‐11.50, ‐0.15] |
| 5.3 Breastfeeding vs positioning | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐63.3 [‐74.54, ‐52.06] |
| 5.4 Breastfeeding and skin‐to‐skin vs skin‐to‐skin | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐17.0 [‐41.63, 7.63] |
| 5.5 Breastfeeding and skin‐to‐skin vs lying on a table | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐136.0 [‐180.45, ‐91.55] |
| 5.6 Breastfeeding vs formula feeding | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 8.3 [3.93, 12.67] |
| 5.7 Breastfeeding vs holding by mother | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐13.80 [‐21.93, ‐5.67] |
| 5.8 Breastfeeding vs non‐nutritive sucking on pacifier | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐19.2 [‐29.05, ‐9.35] |
| 6 Duration of first cry (seconds) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐25.80, ‐10.20] |
| 7 Neonatal Infant Pain Scale (NIPS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Breastfeeding vs 25% sucrose | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.11, 1.31] |
| 7.2 Breastfeeding vs no intervention | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐5.68, ‐3.72] |
| 8 Premature Infant Pain Profile Score | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Breastfeeding vs placebo | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐5.95 [‐7.42, ‐4.48] |
| 8.2 Breastfeeding vs no treatment | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐2.39, 1.41] |
| 8.3 Breastfeeding vs glucose | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.05, 2.56] |
| 8.4 Breastfeeding vs positioning | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐7.49 [‐8.95, ‐6.03] |
| 8.5 Breastfeeding vs sucrose | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐6.48, ‐4.52] |
| 9 Neonatal Facial Coding System (NFCS) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Breastfeeding vs formula feeding | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.63, 1.83] |
| 9.2 Breastfeeding vs 30% glucose | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐3.9 [‐4.80, ‐1.00] |
| 9.3 Breastfeeding vs holding by mother | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.44, ‐0.23] |
| 9.4 Breastfeeding vs non‐nutritive sucking with pacifier | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐3.15, ‐0.85] |
| 9.5 Breastfeeding vs no intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐5.14, ‐3.26] |
| 10 Douleur Aigue Nouveau‐né (DAN) Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Breastfeeding vs placebo | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐6.24 [‐7.38, ‐5.10] |
| 10.2 Breastfeeding vs sucrose/glucose | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.97, 0.47] |
| 10.3 Breastfeeding vs positioning | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐6.77 [‐7.78, ‐5.76] |
| 11 Composite score | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐3.51, ‐2.29] |
Comparison 2. Supplemental breast milk vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate change (beats per minute) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Supplemental breast milk vs placebo | 5 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐4.49 [‐7.08, ‐1.89] |
| 1.2 Supplemental breast milk vs no treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐12.37, 2.17] |
| 1.3 Supplemental breast milk vs 25% sucrose | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 13.80 [4.23, 23.37] |
| 1.4 Supplemental breast milk vs 10% glucose | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐1.79, 3.03] |
| 1.5 Supplemental breast milk vs 25% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 9.00 [7.00, 11.00] |
| 1.6 Supplemental breast milk vs 30% glucose | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 6.80 [0.70, 12.90] |
| 1.7 Supplemental breast milk vs 50% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [8.08, 12.12] |
| 1.8 Supplemental breast milk vs artificial sweetener | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.15, 16.15] |
| 1.9 Supplemental breast milk vs glycine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐2.82, 10.82] |
| 2 Oxygen saturation change | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Supplemental breast milk vs placebo | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐1.99, ‐0.24] |
| 2.2 Supplemental breast milk vs 10% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.88, 1.28] |
| 2.3 Supplemental breast milk vs 25% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [1.03, 2.97] |
| 2.4 Supplemental breast milk vs 50% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.22, 2.98] |
| 3 Percentage of time crying | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Supplemental breast milk vs placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐1.99, 19.99] |
| 3.2 Supplemental breast milk vs artificial sweetener | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [2.38, 27.62] |
| 3.3 Supplemental breast milk vs glycine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.61, 6.61] |
| 4 Duration of crying (seconds) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Supplemental breast milk vs placebo | 7 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐8.67 [‐12.32, ‐5.02] |
| 4.2 Supplemental breast milk vs 2 doses of placebo (water) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐5.39, 5.39] |
| 4.3 Supplemental breast milk vs 12.5% sucrose (single dose) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 35.0 [29.04, 40.96] |
| 4.4 Supplemental breast milk vs 12.5% sucrose (2 doses) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 22.0 [13.09, 30.91] |
| 4.5 Supplemental breast milk vs 20% sucrose | 2 | 95 | Mean Difference (IV, Fixed, 95% CI) | 10.50 [4.10, 16.91] |
| 4.6 Supplemental breast milk vs 25% sucrose | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 33.17 [12.08, 54.26] |
| 4.7 Supplemental breast milk vs 10% glucose | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [‐1.52, 8.68] |
| 4.8 Supplemental breast milk vs 25% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 29.76 [24.14, 35.38] |
| 4.9 Supplemental breast milk vs 30% glucose | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 12.91 [‐3.26, 29.08] |
| 4.10 Supplemental breast milk vs 50% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 27.2 [20.89, 33.51] |
| 4.11 Supplemental breast milk vs artificial sweetener | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 41.0 [‐6.61, 88.61] |
| 4.12 Supplemental breast milk vs glycine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 51.8 [6.33, 97.27] |
| 4.13 Supplemental breast milk vs non‐nutritive sucking (pacifier) | 2 | 98 | Mean Difference (IV, Fixed, 95% CI) | 44.23 [38.47, 49.98] |
| 4.14 Supplemental breast milk vs massage | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐16.97, ‐1.03] |
| 4.15 Supplemental breast milk vs rocking | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 31.0 [24.47, 37.53] |
| 4.16 Supplemental breast milk vs no intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 36.7 [0.60, 72.80] |
| 4.17 Supplemental breast milk vs two doses of supplemental breast milk | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐21.22, ‐0.78] |
| 5 Duration of first cry (seconds) | 2 | 376 | Mean Difference (IV, Fixed, 95% CI) | 4.58 [3.32, 5.85] |
| 5.1 Supplemental breast milk vs 20% sucrose | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [2.50, 9.50] |
| 5.2 Supplemental breast milk vs placebo (water) | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐3.77 [‐6.73, ‐0.82] |
| 5.3 Supplemental breast milk vs non‐nutritive sucking (pacifier) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [0.18, 7.82] |
| 5.4 Supplemental breast milk vs massage | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐12.34, 0.34] |
| 5.5 Supplemental breast milk vs rocking | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐1.52, 7.52] |
| 5.6 Supplemental breast milk vs 10% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.92 [‐1.38, 5.22] |
| 5.7 Supplemental breast milk vs 25% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 12.78 [9.36, 16.20] |
| 5.8 Supplemental breast milk vs 50% glucose | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 11.56 [8.54, 14.58] |
| 6 Neonatal Infant pain scale (NIPS) | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [0.33, 1.81] |
| 6.1 Supplemental breast milk vs 20% sucrose | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [0.97, 3.43] |
| 6.2 Supplemental breast milk vs non‐nutritive sucking (pacifier) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.14, 2.54] |
| 6.3 Supplemental breast milk vs no intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.60, 1.00] |
| 7 Neonatal Facial Coding Score at 3 minutes | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Supplemental breast milk vs placebo | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐0.97, ‐0.16] |
| 7.2 Supplemental breast milk vs artificial sweetener | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.65, 0.21] |
| 7.3 Supplemental breast milk vs glycine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.90, ‐0.04] |
| 8 Neonatal Facial Coding Score (NFCS) at 2 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Supplemental breast milk vs placebo (water) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.09, ‐0.59] |
| 8.2 Supplemental breast milk vs placebo (2 doses of water) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.83, ‐0.35] |
| 8.3 Supplemental breast milk vs 12.5% sucrose (single dose) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [0.64, 1.20] |
| 8.4 Supplemental breast milk vs 12.5% sucrose (2 doses) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.11, 0.43] |
| 8.5 Supplemental breast milk vs two doses of supplemental breast milk | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.37, ‐0.91] |
| 9 Douleur Aigue du Nouveau‐né (DAN) at 2 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Supplemental breast milk vs 20% sucrose | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.30, 0.50] |
| 9.2 Supplemental breast milk vs placebo (water) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.65, ‐0.55] |
| 9.3 Supplemental breast milk vs non‐nutritive sucking (pacifier) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.40, 1.20] |
| 9.4 Supplemental breast milk vs massage | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.91, ‐0.09] |
| 9.5 Supplemental breast milk vs rocking | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.65, 1.55] |
| 10 Body pain score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Supplemental breast milk vs placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.38, 1.34] |
| 10.2 Supplemental breast milk vs artificial sweetener | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.72, 1.04] |
| 10.3 Supplemental breast milk vs glycine | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.51, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blass 2001.
| Methods | Quasi‐randomised controlled trial I. Masking of randomisation ‐ cannot tell unclear II. Masking of intervention ‐ no high risk III. Masking of outcome assessment ‐ no high risk IV. Completeness of follow‐up ‐ yes low risk | |
| Participants | 60 stable full‐term newborn infants undergoing routine newborn screening (heel lance) between 30 and 55 hours of age were randomly assigned to one of the 6 treatment groups (10 neonates in each group) Mean (range) BW ‐ 3200 (2400‐4200) grams Male: Female ‐ 27:33 | |
| Interventions | Group 1: 2 ml water given over 2 minutes via syringe Group 2: 2 ml colostrum given over 2 minutes via syringe Group 3: 2 ml of 12% sucrose given over 2 minutes via syringe Group 4: 2 ml water given on a pacifier dipped in water every 30 seconds for 2 minutes Group 5: 2 ml of colostrum given on a pacifier dipped in colostrum every 30 seconds for 2 minutes Group 6: 2 ml of sucrose given on a pacifier dipped in sucrose every 30 seconds for 2 minutes | |
| Outcomes | Percentage of time crying during the procedure in relation to control Percentage of time grimacing during the procedure Mean crying time during the recovery phase Mean changes in heart rate during and following the procedure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The phlebotomist who performed the heel‐lance and the person who rated video data were unaware of allocation. Masking of intervention was not possible in this study since it involved the use of a pacifier and a liquid (colostrum) that differed in colour from two other solutions. Masking of outcome assessment was possible with crying time and heart rate changes, but not so when assessing the grimacing since the intervention involved the use of a pacifier |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were included |
| Selective reporting (reporting bias) | Low risk | All infants were included in the final analysis |
| Other bias | Unclear risk | Protocol not available to compare |
Bucher 2000.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ low risk III. Masking of outcome assessment ‐ low risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 80 stable full‐term newborn infants undergoing routine newborn screening (heel lance) on postnatal day 3 were randomly assigned to one of the 4 treatment groups Group 1: 20 neonates Mean (range) BW ‐ 3420 (2650 to 5000) grams Male: Female ‐ 10: 10 Group 2: 20 neonates Mean (range) BW ‐ 3430 (2640 to 3960) grams Male: Female ‐ 10:10 Group 3: 20 neonates Mean (range) BW ‐ 3350 (2720 to 4200) grams Male: Female ‐ 8:12 Group 4: 20 neonates Mean (range) BW ‐ 3410 (2740 to 4170) grams Male: Female ‐ 9:11 | |
| Interventions | Group 1: 2 ml of artificial sweetener Group 2: 2 ml of glycine Group 3: 2 ml of breast milk Group 4: 2 ml of sterile water | |
| Outcomes | Heart rate change Percentage time crying Body pain score Facial pain score Combined pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Randomisation was done through sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A nurse administered the solution in the absence of investigators and was not involved in heel prick or data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Masking of outcome assessment was done by blinding observer as to the assignments to the study group |
| Selective reporting (reporting bias) | Low risk | All infants were included in final analysis |
| Other bias | Unclear risk | Protocol not available to compare |
Carbajal 2003.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ low risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 179 healthy term neonates Inclusion criteria: healthy term (≥ 37 weeks GA) undergoing venepuncture for diagnostic evaluation Exclusion criteria: medical instability, received naloxone in the last 24 hours, received sedative or major analgesic in the last 48 hours Group 1: 44 neonates Mean GA ‐ 39.7 (1.15) weeks Mean BW ‐ 3306 (382.8) grams Group 2: 45 neonates Mean GA ‐ 39.8 (1.23) weeks Mean BW ‐ 3304 (483.0) grams Group 3: 45 neonates Mean GA ‐ 40.0 (1.14) weeks Mean BW ‐ 3420 (418.8) grams Group 4: 45 neonates Mean GA ‐ 39.6 (1.20) weeks Mean BW ‐ 3313 (401.2) grams | |
| Interventions | Group 1: Breastfeeding 2 minutes before and throughout the procedure Group 2: Cuddled in mother's arms without breastfeeding starting 2 minutes prior to procedure Group 3: One ml of placebo (sterile water) without pacifier 2 minutes before the procedure while lying supine on the table Group 4: One ml of 30% glucose followed by pacifier 2 minutes prior to venepuncture while lying supine on the table | |
| Outcomes | DAN rating scale for pain in neonates PIPP Standardised questionnaires to mothers to determine the effect of venepuncture on breastfeeding at 48 to 72 hours after the venepuncture | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Randomisation was done by using numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking was not possible since it involved breastfeeding, the use of a pacifier and cuddling before and throughout the procedure. Personal bias on the part of outcome observer cannot be excluded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors comment that each group has 45 patients but they only show characteristics and results for 44 patients in the breastfeeding group |
| Selective reporting (reporting bias) | Low risk | All of the outcomes mentioned by the authors were reported on |
| Other bias | Unclear risk | Protocol not available to compare |
Codipietro 2008.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention high risk III. Masking of outcome assessment ‐ low risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 101 full‐term infants at more than 60 hours of age undergoing heel lance for metabolic screening Exclusion criteria: at‐risk pregnancy, medical instability, birth in general anaesthesia, maternal use of opioids, administration of naloxone or phenobarbital in the previous 48 hours and artificial feeding Group 1: 51 neonates Mean (range) GA ‐ 39.3 (+/‐ 1.2) weeks Mean (range) BW ‐ 3318 (+/‐ 402) grams Group 2: 50 neonates Mean (range) GA ‐ 39.4 (+/‐ 1.1) weeks Mean (range) BW ‐ 3308 (+/‐ 430) grams | |
| Interventions | Group 1: Breastfeeding Group 2: 1ml of 25% sucrose Infants in group 1 were held by mother and breast fed until there was a continuous active suction prior to heel lance. Group 2 infants were laid on a changing table and a bolus of 1 ml of 25% sucrose solution was administered through syringe in the mouth 2 minutes before the heel lance |
|
| Outcomes | PIPP scale, changes in heart rate and saturation 30 seconds after the procedure, duration of first cry, percentage of crying in the first 2 minutes after the procedure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by using a computer random number generator. Allocation was concealed using opaque, sealed envelopes, which were opened sequentially by the paediatric nurse who performed blood sampling |
| Blinding (performance bias and detection bias) All outcomes | High risk | Incomplete blinding as breastfeeding group was held by mother while group 2 infants were laid on a changing table. Assessment of one of the outcomes (cry behaviour) was masked as it was assessed by 2 assistants who listened to tape recordings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were accounted for in the analysis of outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported on |
| Other bias | Low risk | Protocol available. All prespecified outcomes addressed |
Efe 2007.
| Methods | Quasi‐randomised controlled trial I. Masking of randomisation ‐ high risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ high risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 102 full‐term infants undergoing venepuncture for metabolic screening or hyperbilirubinaemia
Group 1: 34 neonates
Mean GA ‐ 38.9 (0.9) weeks
Mean BW ‐ 3327.5 (409) grams Group 2: 34 neonates Mean GA ‐ 38.9 (1.1) weeks Mean BW ‐ 3202.5 (360) grams Group 3: 34 neonates Mean GA ‐ 39.2 (1.1) weeks Mean BW ‐ 3381.6 (434.3) grams |
|
| Interventions | Infants in group 1 (breastfeeding group) were held in skin‐to‐skin contact with their mothers during the entire procedure. Three minutes after the first jaw movements were observed, the venous blood sample was taken. Infants continued to breastfeed during and after the venepuncture. Group 2 infants received 2 ml of 25% sucrose solution put into pacifiers. The infants started to suck the pacifier with sucrose 3 minutes before the venepuncture and continued to suck during and after sampling. The control group infants were wrapped in a blanket with only the hand that would be used for sampling outside the blanket. The mother stayed next to the infant trying to soothe him verbally. After the sample was collected, the infant was cuddled by the mother and could be given a pacifier | |
| Outcomes | NIPS, heart rate, saturation levels and crying time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | The allocation was done according to mothers' preference |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The article includes all the outcomes that were prespecified |
| Other bias | Unclear risk | Protocol not available to compare |
Gradin 2004.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ low risk III. Masking of outcome assessment ‐ low risk IV. Completeness of follow‐up ‐ high risk | |
| Participants | 120 full‐term infants at 3 to 5 days of age undergoing venepuncture for metabolic screening Exclusion criteria: feeding problems or suspicion of illness Group 1: 27 neonates Mean (range) GA ‐ 39.4 (37 to 42) weeks Mean (range) BW ‐ 3638 (2325 to 4425) grams Group 2: 29 neonates Mean (range) GA ‐ 39.5 (37 to 42) weeks Mean (range) BW ‐ 3637 (2700 to 4830) grams Group 3: 26 neonates Mean (range) GA ‐ 39.4 (37 to 42) weeks Mean (range) BW ‐ 3442 (2185 to 4560) grams Group 4: 29 neonates Mean (range) GA ‐ 39.4 (37 to 42) weeks Mean (range) BW ‐ 3660 (3025 to 4950) grams | |
| Interventions | Group 1: Breastfeeding and 1 ml sterile water
Group 2: Breastfeeding and 1 ml 30% glucose
Group 3: Fasting and 1 ml sterile water
Group 4: Fasting and 1 ml 30% glucose For breastfed group, breastfeeding was allowed for as long as the infant wanted within 45 minutes prior to blood sampling For the fasting group, blood sampling performed at least 2 hours after the last feed Venepuncture was done 1 minute after giving 30% glucose or sterile water |
|
| Outcomes | PIPP Visual Analogue Scale Median crying time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking was done by blinding the observer as to the assignments to study groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some infants had to be excluded from the analysis because of missing data (problems with video tapes) |
| Selective reporting (reporting bias) | Low risk | All patients were accounted for |
| Other bias | Unclear risk | Protocol not available to compare |
Gray 2002.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ high risk IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 30 term neonates Inclusion criteria: Healthy full‐term neonates delivered by normal spontaneous vaginal delivery undergoing heel lance for newborn screening Exclusion criteria: Patients with evidence of congenital abnormalities, medical complications, drug exposure, history of oxygen administration or ventilatory support Group 1: 15 neonates Mean GA ‐ 39.8 weeks Mean BW ‐ 3480 grams Group 2: 15 neonates Mean GA ‐ 39.9 weeks Mean BW ‐ 3524 grams | |
| Interventions | Group 1: Breastfeeding during procedure Group 2: Swaddled in the bassinet during procedure | |
| Outcomes | Changes in facial grimacing, crying time and heart rate before, during and after blood collection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible since it involved breastfeeding before and throughout the procedure. Masking of outcome assessment not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported |
| Other bias | Unclear risk | Protocol not available to compare |
Jatana 2003.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ not clear II. Masking of intervention ‐ not clear III. Masking of outcome assessment ‐ not clear IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 125 term neonates
Inclusion criteria: Healthy full‐term neonates between 1 and 7 days old, undergoing heel lance for blood sampling
Exclusion criteria: Age below 24 hours or above 7 days, GA below 37 weeks, Apgar score of less than 7 or 8 at 1 and 5 minutes respectively, neonates with oxygen requirement of more than 40%, analgesic or sedative drug given within 5 days, neurological symptoms like seizures, listlessness, altered sensorium, etc There is no report on the mean GA or weight in each group, although the authors say that the groups were matched for GA, BW and gender |
|
| Interventions | Group 1: Control group, 1 ml of sterile water
Group 2: 1ml of 10% glucose Group 3: 1ml of 25% glucose Group 4: 1ml of 50% glucose Group 5: 1ml of EBM |
|
| Outcomes | Crying time (total and first cry), change in heart rate, change in oxygen saturation and facial action score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | No comment on how it was done |
| Blinding (performance bias and detection bias) All outcomes | High risk | No comment on how it was done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are no results for one of the outcomes that was supposed to be evaluated (facial action score) |
| Selective reporting (reporting bias) | High risk | There are no results for one of the outcomes that was supposed to be evaluated (facial action score) |
| Other bias | Unclear risk | Protocol not available to compare |
Leite 2009.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ low risk IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 60 term neonates Inclusion criteria: Healthy full‐term neonates being exclusively breastfed, GA at least 37 weeks, Apgar ≥7 at 5 minutes after birth, and postnatal age not more than 7 days Exclusion criteria: Congenital diseases of the nervous system, malformation or neurologic damage, stomatognathic disorders that would interfere with sucking mechanics, use of analgesics interfering in nociceptive responses in infants, use postdelivery analgesics for the mothers and newborns being admitted to the neonatal intensive care unit (NICU) Group 1: 31 neonates Mean BW ‐ 3168 (± 517) grams Group 2: 29 neonates Mean BW ‐ 3300 (±478) grams | |
| Interventions | Group 1: Breastfeeding
Group 2: Held by mother Infants underwent heel lance for routine newborn screening. Infants in group 1 were held by mother and were breastfeeding with effective sucking movements 5 minutes prior to procedure. Group 2 infants were held by the mother for the same length of time |
|
| Outcomes | NFCS and change in heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done by a computer random number generator, but there is insufficient information to decide whether allocation concealment was effective |
| Blinding (performance bias and detection bias) All outcomes | High risk | It was not possible to blind for group assignment as the information about breastfeeding was easily determined in both body and face videos |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for in the analysis of outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the article |
| Other bias | Unclear risk | Protocol not available to compare |
Mathai 2006.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ low risk IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 104 term neonates
Inclusion criteria: Healthy full‐term neonates who underwent heel prick at more than 24 hours of age for collection of blood for bilirubin estimation
Exclusion criteria: Patients with BW less than 2 kg, sick babies with unstable vitals or on intravenous fluids, oxygen or any drugs requiring resuscitation at birth, with neurological abnormalities or having major congenital defects
Group 1: 18 neonates
Mean BW ‐ 2992 (+/‐ 312.2) grams
Group 2: 17 neonates
Mean BW ‐ 2953 (+/‐ 289) grams Group 3: 15 neonates Mean BW ‐ 3027 (+/‐ 302) grams Group 4: 20 neonates Mean BW ‐ 2994 (+/‐ 290) grams Group 5: 17 neonates Mean BW ‐ 3123 (+/‐ 302) grams Group 6: 17 neonates Mean BW ‐ 2995 (+/‐ 300) grams |
|
| Interventions | Group 1: 2 ml of EBM
Group 2: 2ml of 20% sucrose
Group 3: 2 ml of distilled water
Group 4: Non‐nutritive sucking Group 5: Massage Group 6: Rocking For groups 1 to 3, the solution was administered in the infants' mouth with a dropper. In the NNS group, a sterile pacifier was held gently in the babies' mouth and the palate tickled to stimulate sucking. This was continued during and up until 2 minutes after the heel prick. In the massaging group, neonates were subjected to firm, gentle stroking with bare fingers in a rhythmical manner starting from the forehead and going down to the chest, arms and legs, during and up until 2 minutes after the heel prick. In the rocking group, newborns were rocked by lifting the infants' head off the cot on the palm of the hand (without lifting the body off the cot) and making rocking movements in a gentle rhythmic manner up until 2 minutes after the heel prick |
|
| Outcomes | Duration of first cry, total crying time and DAN at 30 sec, 1 min, 2 min and 4 min after the prick. For the purpose of this review, we analysed DAN at 2 minutes. Other outcome variables were heart rate increase and saturation decrease | |
| Notes | Results for heart rate increase and saturation decrease were not shown, but the study authors commented that there was no significant differences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done with a random number table, but the method of allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Partially blinded. The person assessing cry variables was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No actual data on heart rate and saturations were given |
| Selective reporting (reporting bias) | High risk | Not all the outcomes were reported completely |
| Other bias | Unclear risk | Protocol not available to compare |
Okan 2010.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ high risk IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 107 term neonates
Inclusion criteria: Healthy full‐term neonates between 24 and 48 hours of age undergoing heel lance for metabolic newborn screening
Exclusion criteria: Patients with evidence of congenital abnormalities, perinatal asphyxia, medical complications or drug exposure, history of oxygen or ventilatory support
Group 1: 35 neonates
Mean GA ‐ 40 (+/‐ 0.7) weeks
Mean BW ‐ 3350 (+/‐ 360) grams
Group 2: 36 neonates
Mean GA ‐ 39.5 (+/‐ 0.5) weeks
Mean BW ‐ 3300 (+/‐ 285) grams Group 3: 36 neonates Mean GA‐ 39.9 (+/‐ 0.7) weeks Mean BW‐ 3317 (+/‐ 235) grams |
|
| Interventions | Group 1: Breastfeeding with skin‐to‐skin contact Group 2: Held by mother with skin‐to‐skin contact Group 3: Lying on the table Mothers and infants from groups 1 and 2 were left alone for 15 minutes to allow them to rest comfortably in skin‐to‐skin contact position. Mothers in group 1 were asked to begin to breastfeed their infants during this time. In the no‐contact group, tests were performed with the infants lying on an examination table in a silent nursery. Infants were wrapped in blankets and placed supine on the examination table |
|
| Outcomes | Heart rate and saturation changes, total time of crying and NFCS in group 2 and 3 (done at the moment of heel lance, 1, 2, 3, 4 and 5 minutes) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation was done through random number table and allocation concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | NFCS was not done in breastfed infants as it was difficult to evaluate the facial actions of babies while breastfeeding |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported on, but NFCS was not done in breastfed infants as it was difficult to evaluate the facial actions of babies while breastfeeding |
| Other bias | Unclear risk | Protocol not available to compare |
Ors 1999.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ unclear II. Masking of intervention ‐ unclear III. Masking of outcome assessment ‐ low risk IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 102 healthy term infants at median age of 1.6 days undergoing routine heel lance blood sampling Exclusion criteria: Infants < 24 hours of age, Apgar score < 7 at 1 minute and on any medication were excluded Group 1: 35 neonates Median (range) GA ‐ 40.0 (37 to 42) weeks Median (range) BW ‐ 3220 (2445 to 4210) grams Group 2: 33 neonates Median (range) GA ‐ 39.5 (37 to 42) weeks Median (range) BW ‐ 3200 (2390 to 4200) grams Group 3: 34 neonates Median (range) GA ‐ 39.0 (37 to 42) weeks Median (range) BW ‐ 3380 (2450 to 4300) grams | |
| Interventions | All infants were fed 1 hour before the procedure Group 1: 2 ml of 25 % sucrose Group 2: 2 ml of human milk Group 3: 2 ml of sterile water The solutions were administered by syringe over 1 minute Heel lance was performed 2 minutes after administration of the solution | |
| Outcomes | Recovery time Percentage change in heart rate at 1, 2 and 3 minutes Median crying time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Manner of randomisation was not discussed by the authors |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking was possible using placebo and performing the heel prick one minute after giving the solutions. The investigators who analysed the data were unaware of the treatment intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported on |
| Other bias | Unclear risk | Protocol not available to compare |
Ozdogan 2010.
| Methods | Quasi randomised controlled trial I. Masking of randomisation ‐ high risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ unclear IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 142 healthy term infants undergoing routine heel lance blood sampling for screening tests.
Exclusion criteria: Infants born before the gestational age of 37 weeks, birth weight below 2500 g, Apgar score < 7 at 5 minutes, infants younger than 48 hours, ill or on any medication were excluded
Group 1: 18 neonates
Median (range) GA ‐ 39 (38 to 41) weeks
Median (range) BW ‐ 3210 (3120 to 3400) grams
Group 2: 27 neonates
Median (range) GA ‐ 39 (38 to 41) weeks
Median (range) BW ‐ 3372 (3100 to 3726) grams
Group 3: 25 neonates
Median (range) GA ‐ 39.0 (38 to 41) weeks
Median (range) BW ‐ 3444 (3010 to 3512) grams Group 4: 23 neonates Median (range) GA ‐ 39.4 (38 to 41) weeks Median (range) BW ‐ 3523 (3328 to 3722) grams Group 5: 26 neonates Median (range) GA ‐ 38.7 (38 to 41) weeks Median (range) BW ‐ 3225 (3162 to 3388) grams Group 6: 23 neonates Median (range) GA ‐ 39 (38 to 41) weeks Median (range) BW ‐ 3227 (3212 to 3430) grams |
|
| Interventions | All infants were fed 1 hour before the procedure
Group 1: single‐dose breast milk
Group 2: single‐dose sterile water
Group 3: single‐dose 12.5% sucrose solution Group 4: Two doses of breast milk Group 5: Two doses of sterile water Group 6: Two doses of 12.5% sucrose solution Infants underwent routine neonatal screening through heel lance. In all the groups, babies received two ml of the test solutions through syringe onto the anterior part of the tongue, and they were not allowed to suck the syringe tip. In the single‐dose groups, the test solution was given 2 minutes before the heel prick and in the repeated‐dose groups the dose was repeated just prior to heel prick |
|
| Outcomes | Total crying time NFCS at 0, 1, 2, 3 minutes. For the purpose of this review, we analysed the NFCS at 2 minutes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Participants were consecutively allocated to the different groups by order of admission |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors do not comment on if the syringe was wrapped or covered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All prespecified outcome data was reported on |
| Other bias | Unclear risk | Protocol not available to compare |
Phillips 2005.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ not done for outcome recorded on video camera (cry duration, percentage time crying), outcomes on monitors (heart rate, saturation, blood pressure) were masked IV. Completeness of follow‐up ‐ low risk | |
| Participants | 96 stable full‐term newborn infants undergoing routine newborn screening (heel lance) were randomly assigned to one of the 3 treatment groups Group 1: 32 neonates Mean (range) age at procedure ‐ 37 (9) hours Male: Female ‐ 13: 19 Group 2: 39 neonates Mean (range) age at procedure 36 (8) hours Male: Female ‐ 13:26 Group 3: 25 neonates Mean (range) age at procedure 38 (14) hours Male: Female ‐ 12:13 | |
| Interventions | Group 1: Breastfeeding Group 2: Held by mother with use of pacifier Group 3: Held by research assistant with the use of pacifier | |
| Outcomes | Percentage of infants cried Proportion of cry time Heart rate, blood pressure and oxygen saturation change before and after the procedure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Randomisation was done by blindly drawing a card from an envelope containing equal numbers of cards with letters representing each group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention was not possible since it involved breastfeeding throughout the procedure. Masking of outcome assessment was not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Blood pressure measurements were not obtained in all infants, the authors comment that this was due to occasional malfunction of blood pressure equipment |
| Selective reporting (reporting bias) | High risk | Heart rate and oxygen saturation were secondary outcomes that were not reported on. Authors comment that there was no significant differences amongst the groups, but no data is given. Also, five babies were dropped from the study, according to the authors due to either excessive difficulties with equipment or 2 of them due to excessive physiologic instability |
| Other bias | Unclear risk | Protocol not available to compare |
Shendurnikar 2005.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ high risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 100 full‐term newborn infants who underwent heel lance were randomly assigned to one of the 2 treatment groups Group 1: 50 neonates GA: 38.2 weeks Male: Female = 22:28 Group 2: 50 neonates GA: 38.6 weeks BW: 2865 grams Male: Female = 31:19 Postnatal age 3.4 days BW: 2910 grams Postnatal age 3.1 days Inclusion criteria: Full‐term neonates > 2500 g BW Exclusion criteria: Septicemia, birth asphyxia, major congenital malformation | |
| Interventions | Group 1: Breastfeeding group Group 2: Swaddled group | |
| Outcomes | Behavioural (state of arousal, cry, facial expression, body movements) Physiological (heart rate, breathing pattern) Composite score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. The randomisation was done by the primary author asking mother to choose from a collection of randomisation cards |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention was not possible since it involved breastfeeding during the procedure Masking of outcome assessment was not done. Primary author was aware of the allocation and hypothesis of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for in the analysis of outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were given |
| Other bias | Unclear risk | Protocol not available to compare |
Skogsdal 1997.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ low risk III. Masking of outcome assessment ‐ low risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 120 stable newborn infants (66 preterm and 54 full‐term) undergoing heel lance for blood collection for their care between 1 to 30 days of age were randomly assigned to one of the 4 treatment groups (30 neonates in each group) Exclusion criteria: age < 24 hours, analgesic or sedative drug given within last 5 days, gestational age < 30 weeks, ventilator or CPAP treatment, oxygen requirement > 40%, neurological symptoms, antibiotic therapy and age > 1 month Mean (SD) GA ‐ 35.5 (2.3) weeks Mean (SD) age at testing ‐ 5.4 (4.9) days | |
| Interventions | Group 1: no treatment group Group 2: 1 ml of 30% glucose Group 3: 1 ml of 10% glucose Group 4: 1 ml of breast milk | |
| Outcomes | Crying time Heart rate change | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. The randomisation was done through random digit table |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The administration of the allocated solution and the heel‐prick was done by the same nurse, who did not participate in recording the outcomes. The study personnel involved in assessing for the outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for in the analysis of outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | Protocol not available to compare |
Upadhyay 2004.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ low risk II. Masking of intervention ‐ low risk III. Masking of outcome assessment ‐ low risk IV. Completeness of follow‐up ‐ unclear | |
| Participants | 81 neonates requiring venepuncture for clinical indication Inclusion criteria: GA of 37 to 41weeks who were ≤ 4 weeks of postnatal age and required venepuncture for clinical indication Exclusion criteria: Perinatal asphyxia (Apgar score < 7 at 1 min), major congenital malformations, admission to neonatal intensive care unit, maternal anaesthesia, opiates administration before delivery or within 48 hours of sampling, babies given naloxone or phenobarbitone Group 1: 40 neonates Mean (SD) GA ‐ 38 (0.9) weeks Mean (SD) BW ‐ 2600 (300) grams Group 2: 41 neonates Mean (SD) GA ‐ 38 (0.8) weeks Mean (SD) BW ‐ 2900 (300) grams | |
| Interventions | Group 1: 5 ml of expressed breast milk Group 2: 5 ml of distilled water The solutions were administered over 2 minutes prior to venepuncture | |
| Outcomes | Duration of crying after venepuncture NFCS at 1 and 3 minutes after the venepuncture Changes in heart rate and oxygen saturation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. The randomisation was performed using computer generated numbers. Allocation was adequately concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A single independent investigator who was not involved in the observations and analysis administered the solution. The outcome observers were blinded to the groups. For the NFCS, the two independent observers came in the room after the intervention had been completed, therefore they were blinded to the solution given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data of 81 subjects was available for analysis. The authors explain why six infants were excluded |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported |
| Other bias | Unclear risk | Protocol not available to compare |
Uyan 2005.
| Methods | Quasi‐randomised controlled trial I. Masking of randomisation ‐ high risk II. Masking of intervention ‐ low risk III. Masking of outcome assessment ‐ low risk IV. Completeness of the follow‐up ‐ low risk | |
| Participants | 62 term infants undergoing heel lance blood sampling for screening tests Exclusion criteria: preterm neonates, neonates with Apgar score < 7 at 5 minutes, neonates with low birth weight, sick neonates and neonates on any medication Group 1: 20 neonates Median (range) GA ‐ 39 (38 to 41) weeks Median (range) BW ‐ 3300 (2800 to 4260) grams Group 2: 21 neonates Median (range) GA ‐ 39 (38 to 41) weeks Median (range) BW ‐ 3510 (2750 to 4030) grams Group 3: 21 neonates Median (range) GA ‐ 40 (38 to 41) weeks Median (range) BW ‐ 3300 (2800 to 4500) grams | |
| Interventions | All infants were fed 1 hour before the procedure Group 1: 2 ml of foremilk Group 2: 2 ml of hindmilk Group 3: 2 ml of sterile water The solutions were administered by syringe Heel lance was performed 2 minutes after administration of the solution | |
| Outcomes | Crying time Duration of first cry Percentage change in heart rate at 1, 2 and 3 minutes NFCS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. The authors provided further information on method of randomisation indicating that it was quasi‐randomised (based on number or day of the procedure) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors do not comment on how the investigators were blinded, but they say the intervention was masked. The two investigators who analysed the data and the person who recorded video for the NFCS coding were unaware of the treatment allocation; hence, the outcome measure analysis was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported |
| Other bias | Unclear risk | Protocol not available to compare |
Weissman 2009.
| Methods | Quasi‐randomised controlled trial I. Masking of randomisation ‐ high risk II. Masking of intervention ‐ high risk III. Masking of outcome assessment ‐ high risk IV. Completeness of follow‐up ‐ low risk | |
| Participants | 180 term infants undergoing heel lance blood sampling for routine screening tests
Group 1: 31 neonates
Mean (SD) GA ‐ 39.7 (1.2) weeks
Mean (SD) BW ‐ 3398 (428) grams
Group 2: 30 neonates
Mean (SD) GA ‐ 39.1 (1.4) weeks
Mean (SD) BW ‐ 3227 (417) grams Group 3: 31 neonates Mean (SD) GA ‐ 39.5 (1.3) weeks Mean (SD) BW ‐ 3157 (397) grams Group 4: 29 neonates Mean (SD) GA ‐ 39.4 (1.1) weeks Mean (SD) BW ‐ 3390 (356) grams Group 5: 30 neonates Mean (SD) GA ‐ 39.6 (1.2) weeks Mean (SD) BW ‐ 3364 (460) grams Group 6: 29 neonates Mean (SD) GA ‐ 39.8 (1.5) weeks Mean (SD) BW ‐ 3368 (382) grams |
|
| Interventions | All infants underwent heel lancing for routine neonatal screening. Infants in group 1 were breastfed, infants in group 2 were fed formula while in their cribs. For infants in group 3, the solution was given orally 2 minutes before the procedure. Infants in group 4 were held by their mothers (mothers were free to choose how to hold their infant with no specific recommendations given) and infants in group 5 had non‐nutritive sucking with a pacifier during heel lancing. Infants in group 6 (control group) had no pain relief | |
| Outcomes | NFCS, through video recording, duration of cry and heart rate increase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised trial; allocation was done according to mothers' preference |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding of the interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants are accounted for in the analysis. No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes |
| Other bias | Unclear risk | Protocol not available to compare |
Yilmaz 2011.
| Methods | Randomised controlled trial I. Masking of randomisation ‐ unclear II. Masking of intervention ‐ unclear III. Masking of outcome assessment ‐ unclear IV. Completeness of follow‐up ‐ low risk | |
| Participants | 120 term infants undergoing heel lance blood sampling for screening tests
Exclusion criteria: preterm neonates, neonates with Apgar score < 7 at 1 and 5 minutes, neonates with low birth weight (< 2500 g), sick neonates, newborns with congenital anomalies and newborns born by vaginal delivery
Group 1: 30 neonates
Mean (SD) GA ‐ 39.1 (+/‐ 1.03) weeks
Mean (SD) BW ‐ 3363 (+/‐ 391) grams
Group 2: 30 neonates
Mean (SD) GA ‐ 39.1 (+/‐0.71) weeks
Mean (SD) BW ‐ 3400 (+/‐ 353) grams
Group 3: 30 neonates
Mean (SD) GA ‐ 39.2 (+/‐ 0.93) weeks
Mean (SD) BW ‐ 3298 (+/‐ 406) grams Group 4: 30 neonates Mean (SD) GA‐ 39.7 (+/‐ 0.8) weeks Mean (SD) BW ‐ 3391 (+/‐ 383) grams |
|
| Interventions | All infants were fed half an hour before the procedure
Group 1: 2 ml of breast milk
Group 2: 2 ml of 20% sucrose
Group 3: non‐nutritive sucking (pacifier) Group 4: control (no intervention) The solutions were administered by syringe avoiding contact of the syringe with the mouth and lips Heel lance was performed 2 minutes after administration of the solution |
|
| Outcomes | NIPS Change in heart rate, saturation of oxygen, respiratory rate and body temperature |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information in terms of the randomisation and allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information is given regarding blinding. We do not have information either on whether the investigators analysing the video tapes for the NIPS were blinded to the infants' intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors mention saturation of oxygen as one of the outcomes, and they say there was no significant difference amongst the groups, but they do not show the data |
| Selective reporting (reporting bias) | High risk | There is no data for one of the prespecified outcomes, oxygen saturation |
| Other bias | Unclear risk | Protocol not available to compare |
BW: birth weight DAN: Douleur Aigue Nouveau‐ne GA: gestational age NFCS: Neonatal Facial Coding Score NIPS: Neonatal Infant Pain Score NNS: non‐nutritive sucking PIPP: Premature Infant Pain Profile SD: standard deviation
EBM: expressed breast milk CPAP: continuous positive airway pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bilgen 2001 | Article is retracted by the journal We have not used any of the data from this report because the data were previously reported by Ors 1999 |
| Efe 2007a | Excluded because the age of patients studied was 2 to 4 months, not newborns |
| Iturriaga 2009 | Not a RCT |
| Osinaike 2007 | Not a RCT |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00175409.
| Trial name or title | The Holding Study: feeding analgesia in preterm infants |
| Methods | Between subject RCT |
| Participants | Infants born between 30 and 35 weeks of GA |
| Interventions | For the standard care condition, infants will remain in their isolettes and will be positioned in prone and given a pacifier to suck on throughout the blood collection. For the holding condition, infants will be held skin‐to‐skin by their mothers and given breast milk using a soother trainer during the blood collection |
| Outcomes | Pain assessed by Neonatal Facial Coding System |
| Starting date | September 2006 |
| Contact information | Dr. Liisa Holsti, University of British Columbia |
| Notes |
NCT00414258.
| Trial name or title | Breastfeeding analgesia in preterm infants |
| Methods | Cross‐over RCT |
| Participants | Infants between 30 and 36 weeks GA |
| Interventions | For the standard care condition, infants will remain in their isolettes and will be positioned in prone and given a pacifier to suck on throughout the blood collection For the feeding condition, infants will be held and then breastfed by their mother during the blood collection |
| Outcomes | Behavioural Indicators of Infant Pain (BIIP) ‐ total score |
| Starting date | January 2008 |
| Contact information | Dr. Liisa Holsti, University of British Columbia |
| Notes |
NCT00908401.
| Trial name or title | Analgesic effect of breastmilk for procedural pain in preterm infants (BMoS) |
| Methods | RCT |
| Participants | Preterm neonates between 27 and 29 + 6 weeks gestation |
| Interventions | Sucrose 0.2 ml or breast milk 0.2 ml |
| Outcomes | Pain evaluated by DAN score |
| Starting date | April 2009 |
| Contact information | Elodie Zana, MD ‐ Centre Hospitalier Intercommunal Creteil |
| Notes |
NCT01276366.
| Trial name or title | Amphia premature infant pain study (APIP) |
| Methods | RCT |
| Participants | Infants born between 32 and 36 + 6 weeks GA |
| Interventions | Sucrose versus supplemental breast milk versus breastfeeding |
| Outcomes | Pain assessed by COMFORT neo score |
| Starting date | January 2010 |
| Contact information | Dr. R.H.T. van Beek, Amphia Hospital, Paediatric Department |
| Notes |
NCT01355640.
| Trial name or title | Two methods of analgesia for Chinese term infants receiving heel lance |
| Methods | RCT |
| Participants | Term infants |
| Interventions | Breastfeeding versus non‐nutritive sucking versus no Intervention |
| Outcomes | Changes in heart rate value and oxygen saturation value, time length of grimacing and crying |
| Starting date | April 2008 |
| Contact information | Jingli Chen, Master‐ Peking Union Medical College |
| Notes |
DAN: Douleur Aigue Nouveau‐ne GA: gestational age RCT: randomised controlled trial
Differences between protocol and review
None.
Contributions of authors
Prakeshkumar Shah (PS): protocol development; editing the protocol; identification of trials; writing the review; editing the review; collecting and entering data in Revman; and revision of the review.
Cecilia Herbozo (CH): review updating; identification of new studies; entering data in RevMan; and revision of the review.
Lucia Aliwalas (LA): protocol writing; review writing; identification of earlier studies; entering data in RevMan; and revision of the review.
Vibhuti Shah (VS): protocol editing; review editing; checking the search for trials; identification of studies; and checking the data in Revman.
Sources of support
Internal sources
Shared Fellowship Program in Neonatal Perinatal Medicine, University of Toronto, Canada.
Mount Sinai Hospital, University of Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Blass 2001 {published data only}
- Blass EM, Miller LW. Effects of colostrum in newborn humans: dissociation between analgesic and cardiac effects. Journal of Developmental and Behavioral Pediatrics 2001;22:385‐90. [DOI] [PubMed] [Google Scholar]
Bucher 2000 {published and unpublished data}
- Bucher HU, Baumgartner R, Bucher N, Seiler M, Fauchere JC. Artificial sweetener reduces nociceptive reaction in term newborn infants. Early Human Development 2000;59:51‐60. [DOI] [PubMed] [Google Scholar]
Carbajal 2003 {published data only}
- Carbajal R, Veerapen S, Couderc S, Jugie M, Ville Y. Analgesic effect of breast feeding in term neonates: randomised controlled trial. BMJ 2003;326:13‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Codipietro 2008 {published data only}
- Codipietro L, Ceccarelli M, Ponzone A. Breastfeeding or oral sucrose solution in term neonates receiving heel lance: a randomized controlled trial. Pediatrics 2008;122:e716‐21. [DOI] [PubMed] [Google Scholar]
Efe 2007 {published data only}
- Efe E, Savaser S. The effect of two different methods used during peripheral venous blood collection on pain reduction in neonates. Agri 2007;19:49‐56. [PubMed] [Google Scholar]
Gradin 2004 {published data only}
- Gradin M, Finnstrom O, Schollin J. Feeding and oral glucose‐additive effects on pain reduction in newborns. Early Human Development 2004;77:57‐65. [DOI] [PubMed] [Google Scholar]
Gray 2002 {published data only}
- Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics 2002;109:590‐3. [DOI] [PubMed] [Google Scholar]
Jatana 2003 {published data only}
- Jatana Lt Col SK, Dalal Sqn Ldr SS, Wilson Col CG. Analgesic effect of oral sucrose in neonates. Medical Journal of Armed Forces India 2003;59:100‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leite 2009 {published data only}
- Leite AM, Linhares MB, Lander J, Castral TC, dos Santos CB, Silvan Scochi CG. Effects of breastfeeding on pain relief in full‐term newborns. Clinical Journal of Pain 2009;25:827‐32. [DOI] [PubMed] [Google Scholar]
Mathai 2006 {published data only}
- Mathai S, Natrajan N, Rajalakshmi NR. A comparative study of nonpharmacological methods to reduce pain in neonates. Indian Pediatrics 2006;43:1070‐5. [PubMed] [Google Scholar]
Okan 2010 {published data only}
- Okan F, Ozdil A, Bulbul A, Yapici Z, Nuhoglu A. Analgesic effects of skin‐to‐skin contact and breastfeeding in procedural pain in healthy term neonates. Annals of Tropical Paediatrics 2010;30:119‐28. [DOI] [PubMed] [Google Scholar]
Ors 1999 {published data only}
- Ors R, Ozek E, Baysoy G, Cebeci D, Bilgen H, Turkuner M, et al. Comparison of sucrose and human milk on pain response in newborns. European Journal of Pediatrics 1999;158:63‐6. [DOI] [PubMed] [Google Scholar]
Ozdogan 2010 {published data only}
- Ozdogan T, Akman I, Cebeci D, Bilgen H, Ozek E. Comparison of two doses of breast milk and sucrose during neonatal heel prick. Pediatrics International 2010;52:175‐9. [DOI] [PubMed] [Google Scholar]
Phillips 2005 {published data only}
- Philips RM, Chantry CJ, Gallagher MP. Analgesic effects of breast‐feeding or pacifier use with maternal holding in term infants. Ambulatory Pediatrics 2005;5:359‐64. [DOI] [PubMed] [Google Scholar]
Shendurnikar 2005 {published and unpublished data}
- Shendurnikar N, Gandhi K. Analgesic effects of breastfeeding on heel lancing. Indian Pediatrics 2005;42:730‐2. [PubMed] [Google Scholar]
Skogsdal 1997 {published and unpublished data}
- Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatrica 1997;86:217‐20. [DOI] [PubMed] [Google Scholar]
Upadhyay 2004 {published data only}
- Upadhyay A, Aggarwal R, Narayan S, Joshi M, Paul VK, Deorari AK. Analgesic effect of expressed breast milk in procedural pain in term neonates: a randomized, placebo‐controlled, double‐blind trial. Acta Paediatrica 2004;93:518‐22. [DOI] [PubMed] [Google Scholar]
Uyan 2005 {published data only}
- Uyan ZS, Ozek E, Bilgen H, Cebeci D, Akman I. Effect of foremilk and hindmilk on simple procedural pain in newborns. Pediatrics International 2005;47:252‐7. [DOI] [PubMed] [Google Scholar]
Weissman 2009 {published data only}
- Weissman A, Aranovitch M, Blazer S, Zimmer EZ. Heel‐lancing in newborns: behavioral and spectral analysis assessment of pain control methods. Pediatrics 2009;124(5):e921‐6. [DOI] [PubMed] [Google Scholar]
Yilmaz 2011 {published data only}
- Yilmaz F, Arikan D. The effects of various interventions to newborns on pain and duration of crying. Journal of Clinical Nursing 2011;20:1008‐17. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bilgen 2001 {published data only}
- Bilgen H, Ozek E, Cebeci D, Ors R. Comparison of sucrose, expressed breast milk, and breast‐feeding on the neonatal response to heel prick. Journal of Pain 2001;2:301‐5. [DOI] [PubMed] [Google Scholar]
Efe 2007a {published data only}
- Efe E, Ozer ZC. The use of breast‐feeding for pain relief during neonatal immunization injections. Applied Nursing Research 2007;20:10‐6. [DOI] [PubMed] [Google Scholar]
Iturriaga 2009 {published data only}
- Iturriaga GS, Unceta‐Barrenechea AA, Zárate KS, Olaechea IZ, Núñez AR, Rivero MM. Analgesic effect of breastfeeding when taking blood by heel‐prick in newborns [Efecto analgesico de la lactancia materna en la toma sanguinea del talón en el recien nacido]. Anales de Pediatria 2009;71:310‐3. [DOI] [PubMed] [Google Scholar]
Osinaike 2007 {published data only}
- Osinaike BB, Oyedeji AO, Adeoye OT, Dairo MD, Aderinto DA. Effect of breastfeeding during venepuncture in neonates. Annals of Tropical Paediatrics 2007;27:201‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00175409 {unpublished data only}
- NCT00175409. Breast feeding analgesia in preterm infants. www.clinicaltrials.gov/ct2/show/NCT00175409?term=breastfeeding&cond=pain&age=0&rank=1 (accessed 11 April 2011).
NCT00414258 {unpublished data only}
- NCT00414258. The Holding Study: feeding analgesia in preterm infants. www.clinicaltrials.gov/ct2/show/NCT00414258?term=breastfeeding&cond=pain&age=0&rank=6 (accessed 12 April 2011).
NCT00908401 {unpublished data only}
- NCT00908401. Analgesic effect of breastmilk for procedural pain in preterm infants (BMoS). www.clinicaltrials.gov/ct2/show/NCT00908401?term=breast+milk&cond=pain&age=0&rank=1 (accessed 22 May 2009).
NCT01276366 {unpublished data only}
- NCT01276366. Amphia premature infant pain study (APIP). www.clinicaltrials.gov/ct2/show/NCT01276366?term=breast+milk&cond=pain&age=0&rank=2 (accessed 23 April 2010).
NCT01355640 {unpublished data only}
- NCT01355640. Two methods of analgesia for Chinese term infants receiving heel lance. www.clinicaltrials.gov/ct2/show/NCT01355640?term=breastfeeding&cond=pain&age=0&rank=2 (accessed 1 June 2011).
Additional references
AAP 2000
- American Academy of Pediatrics. Prevention and management of pain and stress in the neonate. Pediatrics 2000;105:454‐61. [PubMed] [Google Scholar]
Abdel‐Rahman 1994
- Abdel‐Rahman AM, Rosenberg AA. Prevention of intraventricular hemorrhage in the premature infant. Clinics in Perinatology 1994;21:505‐21. [PubMed] [Google Scholar]
Abu‐Saad 1998
- Abu‐Saad HH, Bours GJ, Stevens B, Hamers JP. Assessment of pain in the neonate. Seminars in Perinatology 1998;22:402‐16. [DOI] [PubMed] [Google Scholar]
Anand 1989
- Anand KJ, Carr DB. The neuroanatomy, neurophysiology and neurochemistry of pain, stress and analgesia in newborns and children. Pediatric Clinics of North America 1989;36:795‐822. [DOI] [PubMed] [Google Scholar]
Anand 1998
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biology of the Neonate 1998;73:1‐9. [DOI] [PubMed] [Google Scholar]
Anand 2001
- Anand KJ, International Evidence‐Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Archives of Pediatric and Adolescent Medicine 2001;155:173‐80. [DOI] [PubMed] [Google Scholar]
Barrett 2000
- Barrett T, Kent S, Voudoris N. Does melatonin modulate beta‐endorphin, corticosterone, and pain threshold?. Life Sciences 2000;66:467‐76. [DOI] [PubMed] [Google Scholar]
Blass 1995
- Blass EM, Shide DJ. Mother as a shield: differential effects of contact and nursing on pain responsivity in infant rats ‐ evidence for nonopioid mediation. Behavioral Neuroscience 1995;109:342‐53. [DOI] [PubMed] [Google Scholar]
Blass 1997
- Blass EM. Milk‐induced hypoalgesia in human newborns. Pediatrics 1997;99:825‐9. [DOI] [PubMed] [Google Scholar]
Carbajal 1997
- Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier‐Martin M. APH: evaluation of behavioral scale of acute pain in newborn infants [Douleur Aigue Nouveau‐ne: une echelle comportementale d'evaluation de la douleur aigue du nouveau‐ne]. Archives of Pediatrics 1997;4:623‐8. [DOI] [PubMed] [Google Scholar]
Corff 1995
- Corff K, Seideman R, Venkataraman P, Lutes L, Yates B. Facilitated tucking: a nonpharmacologic comfort measure for pain in preterm infants. Journal of Obstetrics and Gynecological and Neonatal Nursing 1995;24:143‐7. [DOI] [PubMed] [Google Scholar]
Craig 1993
- Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioral and physiological indices. Pain 1993;52:287‐99. [DOI] [PubMed] [Google Scholar]
Craig 1994
- Craig KD, Hadjistavropoulos HD, Grunau RVE, Whitfield RF. A comparison of two measures of facial activity during pain in the newborn child. Journal of Pediatric Psychology 1994;19:305‐18. [DOI] [PubMed] [Google Scholar]
Grunau 1987
- Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28:395‐410. [DOI] [PubMed] [Google Scholar]
Gunnar 1984
- Gunnar M. The effects of a pacifying stimulus on behavioral and adrenocortical responses to circumcision in the newborn. Journal of the American Academy of Child and Adolescent Psychiatry 1984;23:34‐8. [DOI] [PubMed] [Google Scholar]
Heine 1999
- Heine WE. The significance of tryptophan in infant nutrition. Advances in Experimental Medicine and Biology 1999;467:705‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnston 1993
- Johnston CC, Stevens B, Craig RD, Grunau RV. Developmental changes in pain expression in premature, full term, two‐ and four‐months‐old infants. Pain 1993;52:201‐8. [DOI] [PubMed] [Google Scholar]
Johnston 2002
- Johnston CC, Filion F, Snider L, Majnemer A, Limperopoulos C, Walker CD, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks' postconceptional age. Pediatrics 2002;110:523‐8. [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12:59‐66. [PubMed] [Google Scholar]
Marshall 1980
- Marshall RE, Stratton WC, Moore JA, Boxerman SB. Circumcision I: Effects upon newborn behavior. Infant Behaviour and Development 1980;3:1‐14. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schechter 1997
- Schechter NL, Blankson V, Pachter LM, Sullivan CM, Costa L. The ouchless place: no pain, children's gain. Pediatrics 1997;99:890‐4. [DOI] [PubMed] [Google Scholar]
Schollin 2004
- Schollin J. Analgesic effect of expressed breast milk in procedural pain in neonates. Acta Paediatrica 2004;93:453‐5. [DOI] [PubMed] [Google Scholar]
Stevens 1993
- Stevens BJ, Johnston CC, Horton L. Multidimensional pain assessment in premature neonates: a pilot study. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1993;22:531‐41. [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnson C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12:13‐22. [DOI] [PubMed] [Google Scholar]
Stevens 2010
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001069.pub3] [DOI] [PubMed] [Google Scholar]
Warnock 2010
- Warnock FF, Castral TC, Brant R, Sekilian M, Leite AM, Owens Sde L, et al. Brief report: Maternal kangaroo care for neonatal pain relief: a systematic narrative review. Journal of Pediatric Psychology 2010;35:975‐84. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shah 2006
- Shah PS, Aliwalas L, Shah V. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004950.pub2] [DOI] [PubMed] [Google Scholar]


