Abstract
A homogeneous photocatalytic recyclable system for the selective radical–radical cross-coupling of N-substituted amines and indoles has been established. This system could conduct in water or acetonitrile, featuring the reuse of uranyl nitrate as the recyclable photocatalyst via a simple extraction. With this mild strategy in hand, good to excellent yields of cross-coupling products could be achieved even under the irradiation of sunlight, including 26 natural product derivatives and 16 natural product inspired re-engineered compounds. A radical–radical cross-coupling mechanism was newly proposed based on experimental evidence and reported literature. This strategy has been also applied to a gram scale synthesis to demonstrate its practical utility.
A homogeneous photocatalytic recyclable system for the selective radical–radical cross-coupling of N-substituted amines and indoles has been established.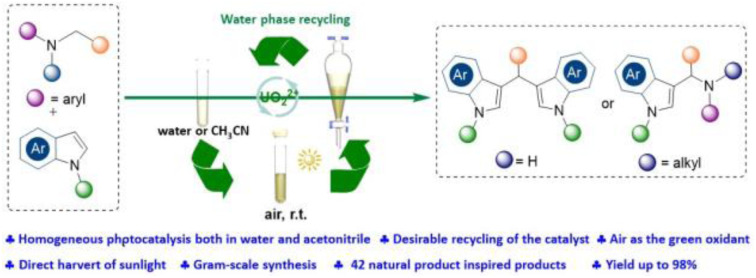
Introduction
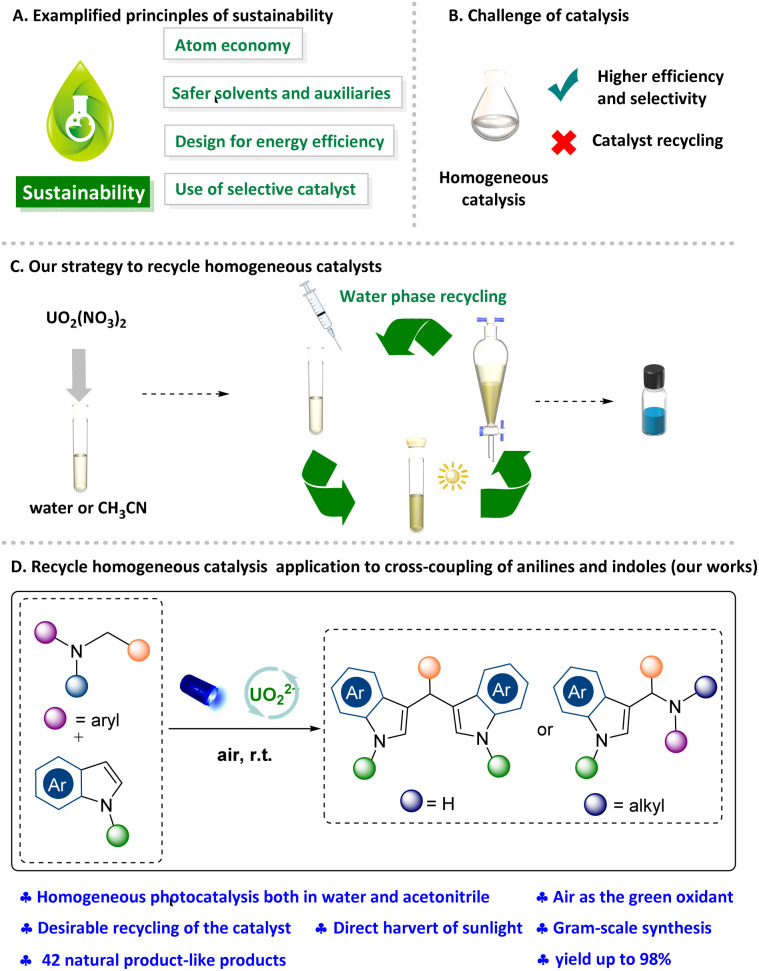
In the past decades, great effort has been devoted to accessing sustainability in organic syntheses, such as by using metal-free strategies, green solvents, recyclable catalysts, photocatalysis, etc (Fig. 1A).1 Among them, recycling and reusing the catalyst is a highly interesting alternative to achieve the goal of green synthesis. Generally, homogeneous catalysts have better activities, while they are rarely recycled because of the difficulty of separation from the solvent,2 thereby resulting in the waste of catalysts and solvents in most homogeneous catalysis (Fig. 1B). To overcome these, several elegant strategies have been reported to achieve this goal, all these methods can be summed up in two main solutions, homogeneous catalysis and heterogeneous separation, and heterogenized (immobilized) homogeneous catalysts.3 The most popular strategy is the water-based recycling catalysis system,4 such as water-soluble catalysts in an aqueous/organic biphasic system,5,6 even in switchable water.7 However, application to diverse organic transformations are still underexplored.
Fig. 1. Homogeneous photocatalyzed chemical transformation via a recyclable uranyl nitrate in water or CH3CN.
Photoredox reactions in water have elicited increasing interest.8–11 However, only a few examples regarding recycling catalysts in water have been reported and mainly concentrated on micellar catalysis or heterogeneous catalysts in water.12–15 Uranyl cations (238UO22+) were recently emerging as an ideal water-soluble photoredox catalysts.16,17 It should be noted that uranyl cations have obvious solubility difference in solvents especially the excellent solubility in water and acetonitrile. However, it showed strongly limited solubility in many solvents such as dichloromethane and ethyl acetate. Although a few excellent organic transformations using uranyl cations were reported recently,18–22 it is still untapped to recycle and reuse it in organic synthesis. Moreover, uranyl cations, activated by visible-light irradiation through ligand-to-metal charge transfer (LMCT), achieves the unique characteristic of excellent oxidizing ability (E° = +2.36 V vs. SCE), were successfully used to selectively oxidation of sulfides,18 the photocatalyzed hydrolysis of diaryl ethers,19 activate the C–N bond of protogenetic anilines, tertiary anilines to form phenols via a single electron transfer (SET) process.20
Single electron oxidation of amines provides an efficient way to access synthetically useful α-aminoalkyl radicals as reactive intermediates. The generated α-aminoalkyl radicals has been utilized in a variety of reaction systems to form several important bonds such as C–C, C–N, C–O, and C–P.23,24 The presence of amines in numerous alkaloids, pharmaceuticals, and agrochemicals lends credence to the potential utility of this chemistry in construction of functional molecular libraries.25 Inspired by these, we aim to develop an elegant and mild strategy enabling reuse the uranyl nitrate based on its specific solubleness and photochemical characteristics (Fig. 1C). Herein, two efficient radical cross-coupling reactions between anilines and indoles were developed using the uranyl nitrate as photocatalyst for multiple times with maintaining performance, which different from the previous works in term of mechanism (Fig. 1D).
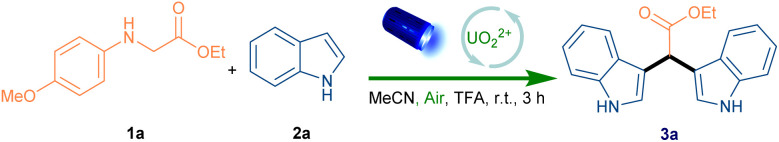
Our investigation into this new recyclable homogeneous photoredox catalysis began with SET oxidative cross-coupling of secondary anilines with indoles. We commenced our studies with secondary anilines N-aryl glycine ethyl ester (1a) and indole (2a) as model substrates (Table 1). Uranyl cation was used as the photocatalyst under the irradiation of blue LEDs (λmax = 456 nm), in which CH3CN was added as the homogeneous phase. To our delight, we found that uranyl nitrate hexahydrate in CH3CN under air atmosphere with TFA exhibited the best reactivity, and afforded desired product 3a in 96% isolated yield (entry 1). Replacing the photocatalyst resulted in diminished efficiency (57% yield, entry 2). We also attended to changing the solvent into the water, however, only 20% desired product was detected (entry 3). Of note, the catalyst was recycled from water phase during the work-up, which exhibited comparable reactivity (entry 4). Moreover, the absence of the oxygen and the photocatalyst, visible light or acid, exhibited lower reactivity, respectively (entries 5–8). These control experiments indicated that photocatalyst, air, visible light and acid are essential for this reaction.
Optimization of SET oxidative cross-coupling of secondary anilines with indolesa.
| ||
|---|---|---|
| Entry | Conditions | Yield (%) |
| 1 | UO2(NO3)2·6H2O | 96 |
| 2 | UO2(OAc)2·2H2O | 57 |
| 3 | H2O instead of MeCN | 20 |
| 4 | Recycled catalystb | 91 |
| 5 | Under Ar | 22 |
| 6 | Without UO2(NO3)2·6H2O | 0 |
| 7 | Without light | 26 |
| 8 | Without TFA | 18 |
Reaction onditions: 1a (1.0 equiv.), 2a (2.1 equiv.), photocatalyst (4 mol%), TFA (2.0 equiv.), MeCN (0.05 M), room temperature, Blue LEDs(456 nm 40 W), 3 h.
Obtained by lyophilization of the water phase from extraction.
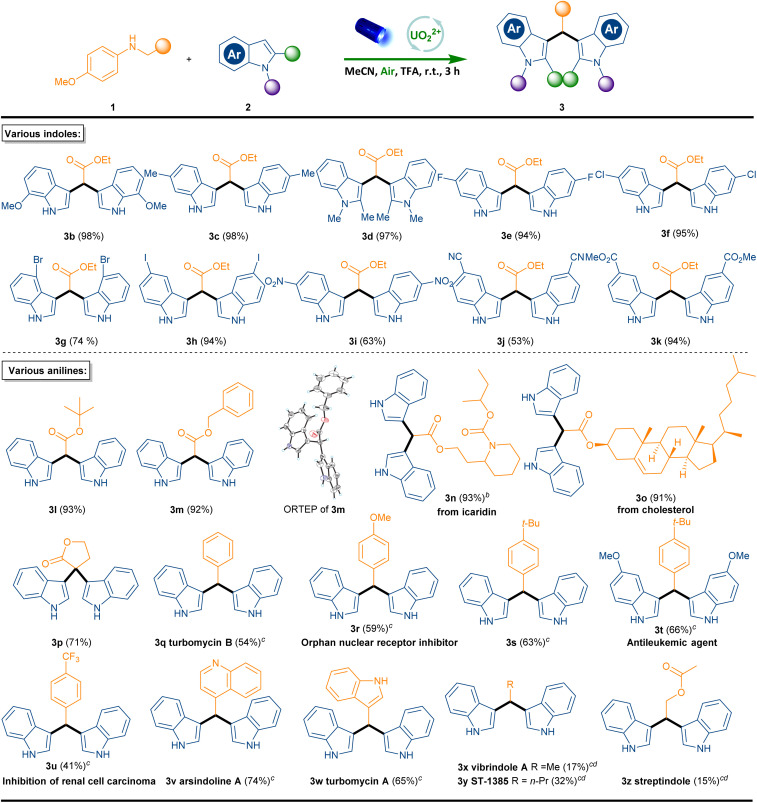
With the best-optimized conditions in hand, the scope was further explored to determine the generality (Scheme 1). Initially, we explored the scope of indoles in this tandem reaction. Various substrates bearing functional groups at the indoles motif (1b–1k) underwent smoothly and delivered expected disindoles products (3b–3k) in good to excellent yields. Specifically, electrically neutral or electron-rich indoles (2b–2d) performed well (3b–3d, 97–99% yields). It is worth noting that, halogen substituents on indoles, even a photosensitive iodine moiety (3e–3h, 74–95% yields) were well tolerated under the standard conditions. Notably the efficiency of the reaction was not impeded by strong electron-deficient substituents such as nitro (3i), cyano (3j) and ester (3k) (53–94% yields). Subsequently, the substrate scope of anilines for this photocatalytic coupling reaction was also evaluated. Firstly, a range of esters, including t-butyl ester, benzyl ester, icariidin ester, cholesteryl ester and 1,4-butyrolactone can be readily employed in this reaction to afford the products with good to excellent yield (3l–3p, 71–93% yields), and the structure of 3m was further confirmed by a single crystal X-ray analysis. Perhaps most importantly, this transformation is not limited to glycine derivatives.26 For example, N-benzyl or N-alkyl substituted anilines were found to be suitable substrates. The reaction of unsubstituted indole 2a with N-benzyl-4-methoxyaniline (1q) selectively afforded 3,3’-(phenylmethylene)bis(1H-indole) (3q), which acts as an antibiotic natural product named turbomycin B in 54% isolated yield.27 In addition, N-benzylic anilines bearing an electron-donating substituent such as methoxy and t-butyl groups or electron-deficient trifluoromethyl group could also be incorporated (3r–3u 41–66% yields). It is noteworthy that product 3r is an orphan nuclear receptor inhibitor28 and product 3t acts as antitileukemic agent.293u shows an inhibition of renal cell carcinoma.30 Moreover, heteroaromatic N-benzylic anilines also reacted efficiently with 2a to afford the arsindoline A (3v) and turbomycin A (3w) (74% and 65% yields respectively). Pleasingly, N-alkyl-anilines such as ethyl, n-butyl, 1-pentanol were also reactive and afforded vibrindole A (3x), ST-1385 (3y) and streptindole (3z) in 17%, 32% and 15% yields.
Scheme 1. Scope of indoles and anilines.a Reaction conditions: 1 (1.0 equiv.), 2 (2.1 equiv.), photocatalyst (4 mol%), TFA (2.0 equiv.), MeCN (0.05 M), room temperature, blue LEDs (456 nm, 40 W) for 3 h.b For 10 h.c For 16 h.d Photocatalyst (8 mol%).
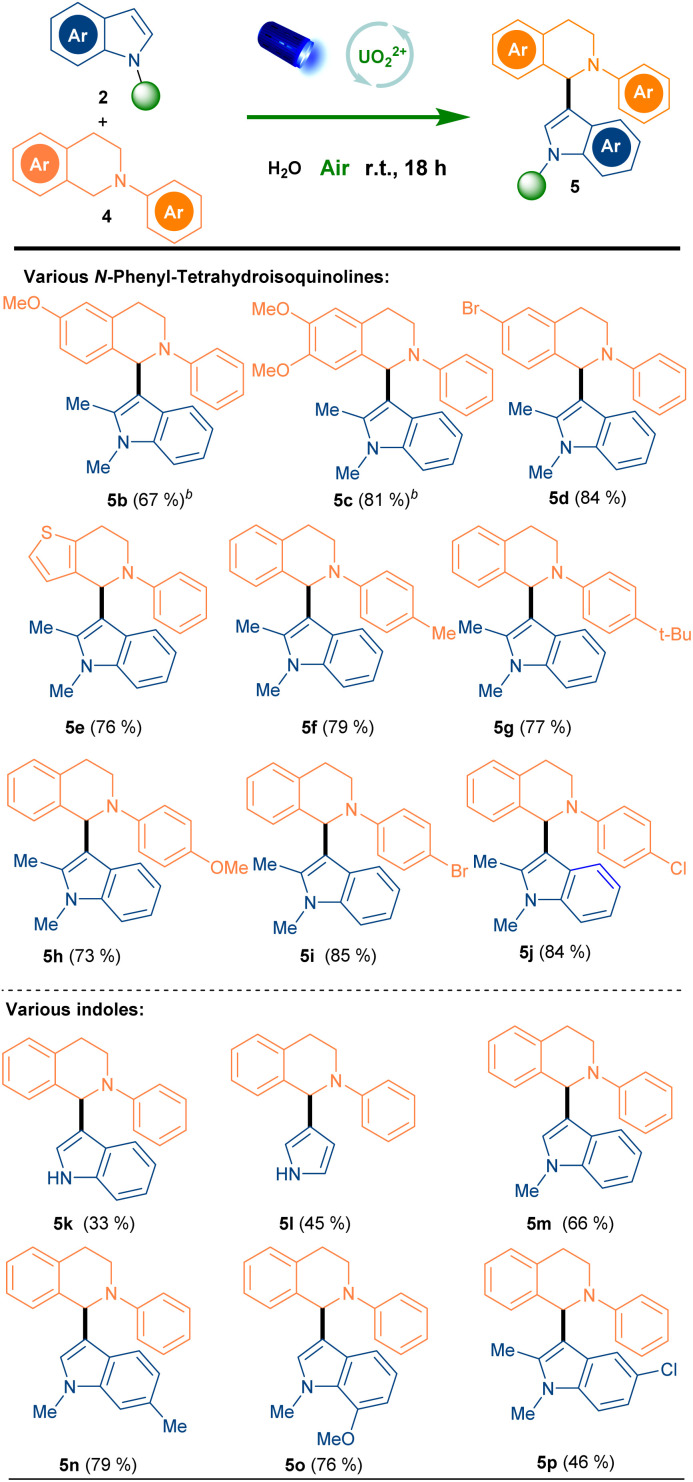
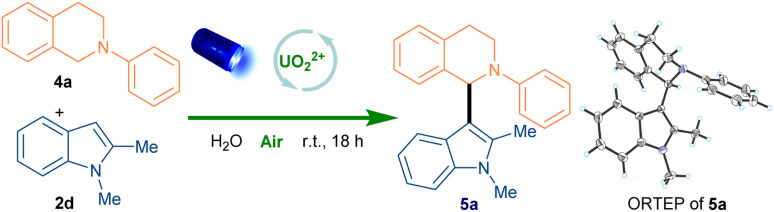
After the oxidative deaminative dimerization of second anilines and indoles, we further examined the SET oxidation of tertiary anilines with indoles.31–33 We initialed our studies with N-phenyl-tetrahydroisoquinoline (4a) and 1,2-dimethyl-1H-indole (2d) as model substrates (Table 2). Under the same condition, the cross-coupling product 5a was obtained in 96% yield within 6 h and the TFA was found to be useless (entry 1). The structure of 5a was further confirmed by a single crystal X-ray analysis. To our delight, this transformation could be conducted in the water with same yield (entry 2). Therefore, the recycled water phase from extraction could be directly reused for the next reaction with same reactivity (entry 3). Later, the absence of oxygen and the photocatalyst exhibited lower reactivity, respectively (entries 4, 5). No formation of the product in the dark strongly proved the necessity of the light (entry 6). Furthermore, reducing the amount of 4a or photocatalyst slightly decreased the yield (entries 7, 8). With the best-optimized conditions in hand, the scope was further explored to determine the generality (Scheme 2).
Optimization of the reaction conditionsa.
| ||
|---|---|---|
| Entry | Variations | Yield (%) |
| 1 | Acetonitrile as solvent, 6 h | 95 |
| 2 | — | 96 |
| 3 | Recycled water phaseb | 93 |
| 4 | Under Ar | 11 |
| 5 | Without UO2(NO3)2·6H2O | 23 |
| 6 | Dark at 40 °C | 0 |
| 7 | 4a (1.5 equiv.) | 81 |
| 8 | UO2(NO3)2·6H2O (4 mol%) | 69 |
Reaction conditions: 1a (2.0 equiv.), 2a (1.0 equiv.), UO2(NO3)2·6H2O (8 mol%) and H2O (1.0 mL), blue LEDs (456 nm, 40 W), 18 h.
Water phase from extraction was directly used.
Scheme 2. Scope of tetrahydroisoquinolines and indoles.a Reaction conditions: 4 (2.0 equiv.), 2 (1.0 equiv.), photocatalyst (8 mol%), H2O (1.0 mL), room temperature, blue LEDs (456 nm, 40 W) for 18 h.b4 was added via a solution in MeCN (50 μL) (these tetrahydroisoquinolines need to be dispersed into water by MeCN).
Firstly, various substrates bearing functional groups at the tetrahydroisoquinoline motif 4b–4d underwent smoothly standard conditions and delivered expected cross-coupling products 5b–5d in good to excellent yields. It was noteworthy that N-substituted 4,5,6,7-tetrahydrothieno [3,2-c] pyridine, a core structure of popular medicines such as (S)-clopidogrel34 and prasugrel,35 could afford the corresponding product in 76% yield (5e). Afterwards, a variety of N-aryl-tetrahydroisoquinolines containing diverse substituted aromatic rings was tolerated satisfactorily (5f–5j). Notably, the electronic properties of 4-substituted groups on the phenyl ring have an unobvious effect on the reaction performance. Interestingly, those products were highly potential intermediates for further functionalization. Meanwhile, various substituted indoles were examined under standard conditions. Among them, the indole and pyrrole without protection suppressed the reactivity greatly (5k, 5l), probably due to the stability of indole radical.36 However, indoles containing electron-donating groups proceeded efficiently (5m–5o). Indoles containing weak electron-withdrawing groups exhibited reactivity (5p). Moreover, stronger electron-withdrawing groups such as acyl group, nitro group at the 5 or 6 positions of indoles lead to no reaction at all (5q–5s).
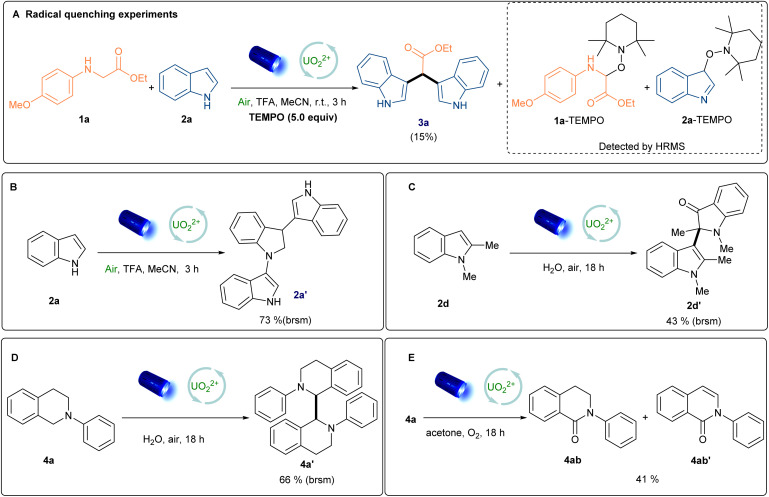
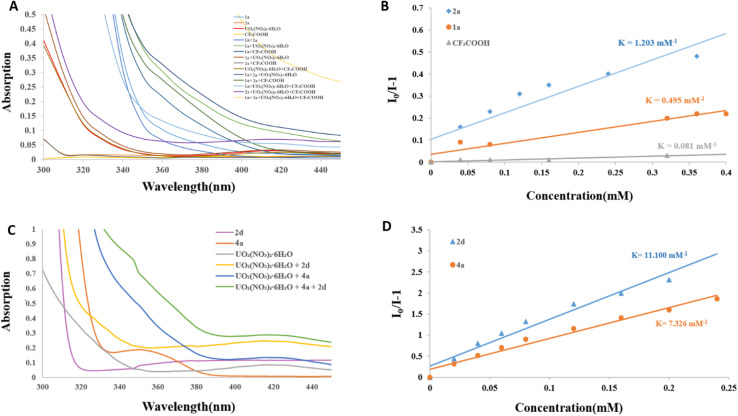
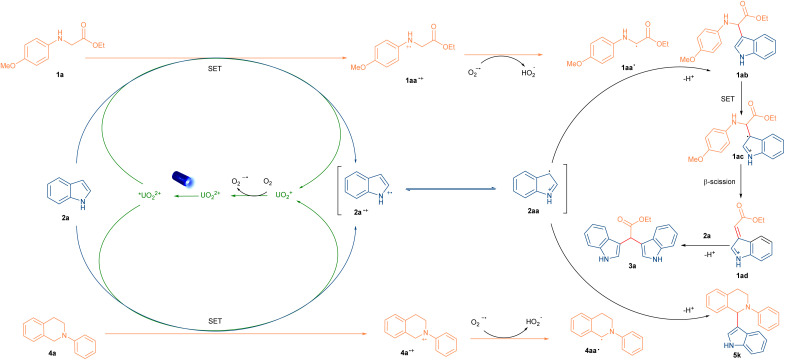
To shed light on the mechanism and uncover the unique photooxidation properties of excited uranyl cation, radical quenching experiments with 2,2,6,6-tetramethyl-1-piperinedinyloxy (TEMPO) as the radical quencher were carried out. Indeed, both reactions were suppressed and the TEMPO-trapped anilines and indole radical were detected by HRMS analysis, which indicated that the radical process was possible involved in our reaction (Scheme 3A and S2†). Furthermore, the uranyl cation was approved to serve as a photosensor via UV-vis absorption at 420 nm (Fig. 2A and C). Stern–Volmer analysis was further performed to uncover the species of active uranyl cation was quenched by 1a, 2a, 2d and 4a (Fig. 2B and D). Indole 2a and 2d have been widely shown to act as quenchers in visible-light mediated reactions, involved in the reaction in the form of allyl radicals.36,37 Combining all of results, it was suggested that the reaction was initiated with excited uranyl cation. Most of reported literature32 involved cross-coupling between the indoles and anilines gave a formal Mannich process, and in their system, the indole radical cannot form for the low excited state oxidation potential of photocatalysts (E° = +1.16 V vs. SCE, for indole).38 So alternative mechanistic pathways need to be considerd. To further confirm thepossible pathway, control experiments were carried out. The trimer of indole 2a′, the dimer of tetrahydroisoquinoline (4a′) and 2-indole-substituted 3-oxindole (2d′) were obtained under standard reaction conditions (CH3CN or water as solvent, respectively) in the absence of a coupling partner, respectively.32a,37c–e,39 (Scheme 3B–D) The mixture of 4ab and 4ab′ could be isolated under O2 atmosphere,32a,39 even in the presence of a solvent amount of nucleophile (acetone), and formal Mannich product was not detected.32f,40 (Scheme 3E). Although cross coupling reaction involved indole radical is rarely reported,26a,41 In our uranyl nitrate system, the radical–radical coupling process was preferred. Moreover, the Manich type reaction between unactivated indoles and imines need very harsh condition.37c,42 On the basis of the above results and reported literature,26a,41 we proposed a plausible radical–radical coupling mechanism as shown in Scheme 4. Firstly, the excited *UO22+ ion is generated upon the irradiation of blue light. In this process, excited *UO22+ promotes the SET oxidation of indole 2a and 2°, 3° anilines (1a and 4a) to generate corresponding radical cations. Meanwhile, UO2+ intermediate reacted with O2 to produce the superoxide radical anion (O2˙−) and further regenerate UO22+. The α-aminoalkyl radicals 1aa˙ and 4aa˙ were subsequently formed after the deprotonation from HOO˙ radical. Rradical 4aa˙ could further coupled with intermediate 2aa to generate desirable product 5a. For reaction with 2o anilines, 1aa˙ will further react with 2aa to form the intermediate the 1ab, which undergoes an SET oxidative and β-scission to generate the 1ad. Then it will be trapped by the purposely added nucleophile indole 2a to afford desired product 3a after deprotonation. However, alternative mechanistic pathways should not be fully excluded.
Scheme 3. Radical quenching experiments and control experiments.
Fig. 2. UV-vis experiments and Stern–Volmer plot of fluorescence quenching experiment.
Scheme 4. Proposed mechanism of cross coupling reactions of N-substituted amines and indoles.
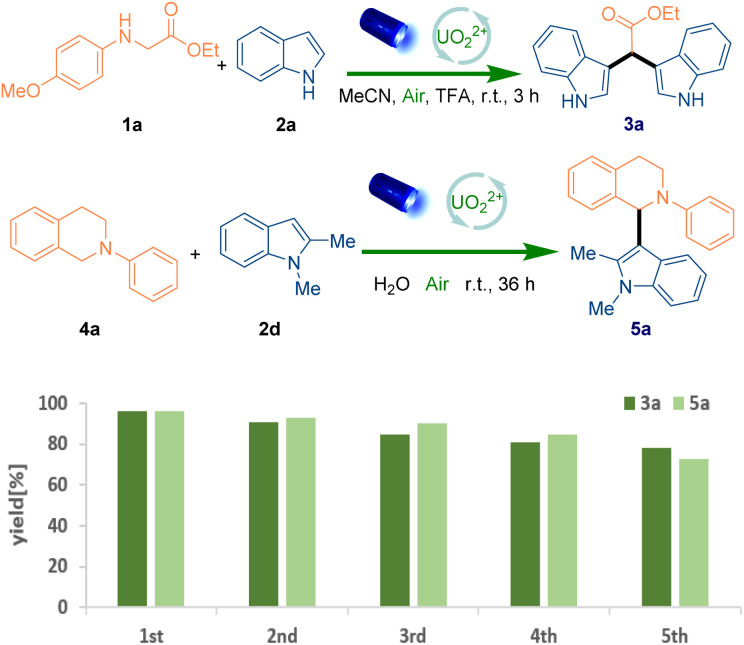
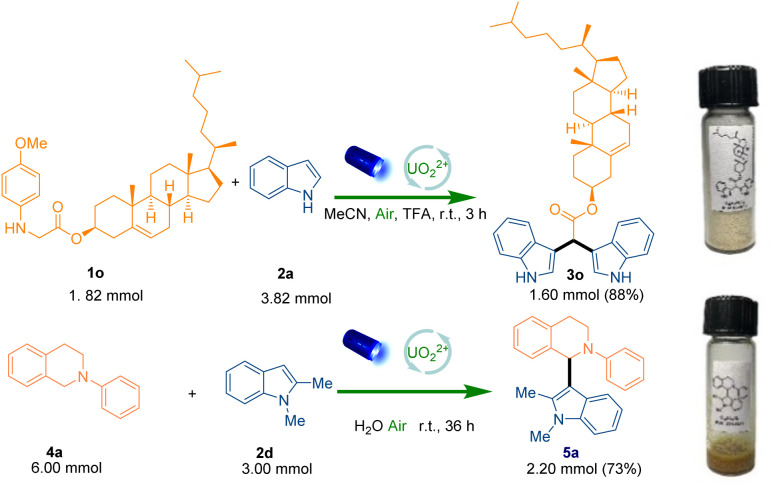
After the understanding of the reaction mechanism, the recyclability of UO22+ in the system was further investigated and a convenient recycle process was showcased. The simple work-up (extraction with water and dichloromethane three times) was carried out to remove the organic reagents after the completion of reaction, and then the water phase containing the water-soluble photocatalyst could be reused for the next reaction cycle. Delightedly, five recycling cycles were performed which showed the stable performance of recycled homogeneous photocatalytic system (Fig. 3). To further exploit other characteristics of sustainability, the reactions were also conducted in excellent yield via harvesting the sunlight (95% and 94% yield respectively) (Scheme S6 and S7†). Finally, gram-scale (3.0 mmol) reactions (Scheme 5) were carried out, which afforded the target product in 88% and 73% yield respectively.
Fig. 3. Recyclability of UO22+ in the reactions for the generation of 3a and 5a.
Scheme 5. Gram-scale synthesis 3o and 5a.
Conclusions
In summary, we have developed a highly mild and environmentally benign strategy for the construction of two types of natural product-like libraries by the selective cross-coupling of anilines and indoles. The homogeneous catalysis system is keeping comparable performance after 5 recycling cycles via using water (acetonitrile)-soluble depleted uranium as the recycled photocatalyst. Although cross coupling reaction involved indole radical is rarely reported, in our uranyl nitrate system, the radical–radical coupling process was preferred based on experimental evidences and reported literature. Moreover, the reaction via harvesting the sunlight and gram-scale reaction strongly proved the practicability of this system. We believe this method enables the development of uranium enrichment catalysis and represents a lower-cost, milder organic transformation process.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 82003624, 82141203, 82004003, 82004215), Science and Technology Commission of Shanghai Municipality (No. 20YF1458700, 20YF1459000), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTDD-202004) and Shanghai Frontiers Science Center of TCM Chemical Biology. We are also grateful to Dr Lu Lu for carrying out the NMR experiments.
Electronic supplementary information (ESI) available. CCDC 21248302220117. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra01037f
Notes and references
- Kar S. Sanderson H. Roy K. Benfenati E. Leszczynski J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2022;122:3637–3710. doi: 10.1021/acs.chemrev.1c00631. [DOI] [PubMed] [Google Scholar]
- Szőllősi G. Asymmetric one-pot reactions using heterogeneous chemical catalysis: recent steps towards sustainable processes. Catal. Sci. Technol. 2018;8:389–422. [Google Scholar]
- (a) de Vries J. G. Jackson S. D. Homogeneous and heterogeneous catalysis in industry. Catal. Sci. Technol. 2012;2:2009. [Google Scholar]; (b) Xia J.-D. Deng G.-B. Zhou M.-B. Liu W. Xie P. Li J.-H. Reusable Visible Light Photoredox Catalysts; Catalyzed Benzylic C(sp3)–H Functionalization/Carbocyclization Reactions. Synlett. 2012;23:2707–2713. [Google Scholar]
- Wang W. Cui L. Sun P. Shi L. Yue C. Li F. Reusable N-heterocyclic carbene complex catalysts and beyond: a perspective on recycling strategies. Chem. Rev. 2018;118:9843–9929. doi: 10.1021/acs.chemrev.8b00057. [DOI] [PubMed] [Google Scholar]
- Rösler T. Faßbach T. A. Schrimpf M. Vorholt A. J. Leitner W. Toward Water-based recycling techniques: methodologies for homogeneous catalyst recycling in liquid/liquid multiphase media and their implementation in continuous processes. Ind. Eng. Chem. Res. 2019;58:2421–2436. [Google Scholar]
- Kohlpaintner C. W. Fischer R. W. Cornils B. Aqueous biphasic catalysis: Ruhrchemie/Rhône-Poulenc oxo process. Appl. Catal., A. 2001;221:219–225. [Google Scholar]
- Mercer S. M. Robert T. Dixon D. V. Jessop P. G. Recycling of a homogeneous catalyst using switchable water. Catal. Sci. Technol. 2012;2:1315–1318. [Google Scholar]
- Steinbauer J. Longwitz L. Frank M. Epping J. Kragl U. Werner T. Immobilized bifunctional phosphonium salts as recyclable organocatalysts in the cycloaddition of CO2 and epoxides. Green Chem. 2017;19:4435–4445. [Google Scholar]
- Barata-Vallejo S. Yerien D. E. Postigo A. Advances in photocatalytic organic synthetic transformations in water and aqueous media. ACS Sustainable Chem. Eng. 2021;9:10016–10047. [Google Scholar]
- Bu M.-J. Cai C. Gallou F. Lipshutz B. H. PQS-enabled visible-light iridium photoredox catalysis in water at room temperature. Green Chem. 2018;20:1233–1237. [Google Scholar]
- Sun K. Lv Q.-Y. Chen X.-L. Qu L.-B. Yu B. Recent advances in visible-light-mediated organic transformations in water. Green Chem. 2021;23:232–248. [Google Scholar]
- Chen D. Liu J. Zhang X. Jiang H. Li J. Recent advances in aqueous phase visible light catalytic reactions. Chin. J. Org. Chem. 2019;39:3353–3362. [Google Scholar]
- Long B. Ding Z. Wang X. Carbon nitride for the selective oxidation of aromatic alcohols in water under visible light. ChemSusChem. 2013;6:2074–2078. doi: 10.1002/cssc.201300360. [DOI] [PubMed] [Google Scholar]
- Xu Z.-Y. Luo Y. Zhang D.-W. Wang H. Sun X.-W. Li Z.-T. Iridium complex-linked porous organic polymers for recyclable, broad-scope photocatalysis of organic transformations. Green Chem. 2020;22:136–143. [Google Scholar]
- Cui J.-W. Ma S. Rao C.-H. Jia M.-Z. Yao X.-R. Zhang J. Reusable homogeneous metal- and additive-free photocatalyst for high-performance aerobic oxidation of alcohols to carboxylic acids. Appl. Catal., B. 2022;305:121028. [Google Scholar]
- Hua D. Q. Jiang X. F. Perspectives for uranyl photoredox catalysis. Synlett. 2021;32:1330–1342. [Google Scholar]
- Cowie B. E. Purkis J. M. Austin J. Jason B. Love J. B. Arnold P. L. Thermal and photochemical reduction and functionalization chemistry of the uranyl dication, [UVIO2]2+ Chem. Rev. 2019;119:10595–10637. doi: 10.1021/acs.chemrev.9b00048. [DOI] [PubMed] [Google Scholar]
- Li Y. Rizvi S. A.-e.-A. Hu D. Sun D. Gao A. Zhou Y. Li J. Jiang X. Selective Late-Stage Oxygenation of Sulfides with Ground-State Oxygen by Uranyl Photocatalysis. Angew. Chem., Int. Ed. 2019;58:13499–13506. doi: 10.1002/anie.201906080. [DOI] [PubMed] [Google Scholar]
- Hu D. Zhou Y. Jiang X. From aniline to phenol: carbon-nitrogen bond activation via uranyl photoredox catalysis. Nat. Sci. Rev. 2021:nwab156. doi: 10.1093/nsr/nwab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y. Liu Y. Yu L. Ni S. Pan Y. Uranyl-catalysed C–H alkynylation and olefination. Org. Chem. Front. 2021;8:5968–5974. [Google Scholar]
- Zhou Y. Hu D. Li D. Jiang X. Uranyl-photocatalyzed hydrolysis of diaryl ethers at ambient environment for the directional degradation of 4-O-5 lignin. J. Am. Chem. Soc. 2021;1:1141–1146. doi: 10.1021/jacsau.1c00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D. Jiang X. Stepwise Benzylic Oxygenation via Uranyl-Photocatalysis. Green Chem. 2022;24:124–129. [Google Scholar]
- Beatty J. W. Stephenson C. R. J. Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015;48:1474–1484. doi: 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K. Miyake Y. Nishibayashi Y. Synthetic Utilization of α-Aminoalkyl Radicals and Related Species in Visible Light Photoredox Catalysis. Acc. Chem. Res. 2016;49:1946–1956. doi: 10.1021/acs.accounts.6b00251. [DOI] [PubMed] [Google Scholar]
- (a) Karageorgis G. Foley D. J. Laraia L. Brakmann S. Waldmann H. Pseudo Natural Products—Chemical Evolution of Natural Product Structure. Angew. Chem., Int. Ed. 2021;60:15705–15723. doi: 10.1002/anie.202016575. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Grigalunas M. Brakmann S. Waldmann H. Chemical Evolution of Natural Product Structure. J. Am. Chem. Soc. 2022;144:3314–3329. doi: 10.1021/jacs.1c11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) D'Auria M. Photochemical synthesis of diindolylmethanes. Tetrahedron. 1991;41:9225–9230. [Google Scholar]; (b) Jadhav S. D. Bakshi D. Singh A. Visible Light Mediated Organocatalytic Activation of Ethyl Bromofluoroacetate: Coupling with Indoles and Anilines. J. Org. Chem. 2015;80:10187–10196. doi: 10.1021/acs.joc.5b01736. [DOI] [PubMed] [Google Scholar]; (c) Zhang Y. Yang X. Zhou H. Li S. Zhu Y. Li Y. Visible light-induced aerobic oxidative cross-coupling of glycine derivatives with indoles: a facile access to 3,3' bisindolylmethanes. Org. Chem. Front. 2018;5:2120–2125. [Google Scholar]; (d) Yang T. Lu H. Shu Y. Ou Y. Hong L. Au C. T. Qiu R. CF3SO2Na-Mediated, UV-Light-Induced Friedel–Crafts Alkylation of Indoles with Ketones/Aldehydes and Bioactivities of Products. Org. Lett. 2020;22:827–831. doi: 10.1021/acs.orglett.9b04272. [DOI] [PubMed] [Google Scholar]; (e) Stanek F. Pawlowski R. Morawska P. Bujok R. Stodulski M. Dehydrogenation and α-functionalization of secondary amines by visible-light-mediated catalysis. Org. Biomol. Chem. 2020;18:2103–2112. doi: 10.1039/c9ob02699a. [DOI] [PubMed] [Google Scholar]; (f) Zhu Y. Li S. Yang X. Wang S. Zhang Y. Direct Synthesis of Triphenylamine-based Ordered Mesoporous Polymers for Metal-Free Photocatalytic Aerobic Oxidation. J. Mater. Chem. A. 2022;10:13978–13986. [Google Scholar]
- Gillespie D. E. Brady S. F. Bettermann A. D. Cianciotto N. P. Liles M. R. Rondon M. R. Clardy J. Goodman R. M. Handelsman J. Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl. Environ. Microbiol. 2002;68:4301–4306. doi: 10.1128/AEM.68.9.4301-4306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S. Burghardt R. Papineni S. Ramaiah S. Yoon K. Safe S. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- Contractor R. Samudio I. J. Estrov Z. Harris D. McCubrey J. A. Safe S. H. Andreeff M. Konopleva M. A novel ring-substituted diindolylmethane,1,1-bis[3'-(5-methoxyindolyl)]-1-(p-t-butylphenyl) methane, inhibits extracellular signal-regulated kinase activation and induces apoptosis in acute myelogenous leukemia. Cancer Res. 2005;65:2890–2898. doi: 10.1158/0008-5472.CAN-04-3781. [DOI] [PubMed] [Google Scholar]
- York M. Abdelrahim M. Chintharlapalli S. Lucero S. D. Safe S. 1,1-Bis(3'-Indolyl)-1-(p-Substitutedphenyl)methanes Induce Apoptosis and Inhibit Renal Cell Carcinoma Growth. Clin. Cancer Res. 2007;13:6743–6752. doi: 10.1158/1078-0432.CCR-07-0967. [DOI] [PubMed] [Google Scholar]
- For selected examples using chemical oxidants, see: ; (a) Wu J.-C. Song R.-J. Wang Z.-Q. Huang X.-C. Xie Y.-X. Li J.-H. Copper-Catalyzed C–H Oxidation/Cross-Coupling of α-Amino Carbonyl Compounds. Angew. Chem., Int. Ed. 2012;51:3453–3457. doi: 10.1002/anie.201109027. [DOI] [PubMed] [Google Scholar]; (b) Wei W.-T. Song R.-J. Li J.-H. Copper-Catalyzed Oxidative α-Alkylation of α-Amino Carbonyl Compounds with Ethers via Dual C(sp3)-H Oxidative Cross-Coupling. Adv. Synth. Catal. 2014;356:1703–1707. [Google Scholar]; (c) Huo C. Xie H. Wu M. Jia X. Wang X. Chen F. Tang J. CBr4-mediated cross-dehydrogenative coupling reaction of amines. Chem. – Eur. J. 2015;21:5723–5726. doi: 10.1002/chem.201500453. [DOI] [PubMed] [Google Scholar]; (d) Ho H. E. Ishikawa Y. Asao N. Yamamoto Y. Jin T. Highly efficient heterogeneous aerobic cross-dehydrogenative coupling via C–H functionalization of tertiary amines using a nanoporous gold skeleton catalyst. Chem. Commun. 2015;51:12764–12767. doi: 10.1039/c5cc04856g. [DOI] [PubMed] [Google Scholar]; (e) Dutta B. Sharma V. Sassu N. Dang Y. Weerakkody C. Macharia J. Miao R. Howell A. R. Suib S. L. Cross dehydrogenative coupling of N-aryltetrahydroisoquinolines (sp3 C–H) with indoles (sp2 C–H) using a heterogeneous mesoporous manganese oxide catalyst. Green Chem. 2017;19:5350–5355. [Google Scholar]; (f) Liu Y. Wang C. Xue D. Xiao M. Li C. Xiao J. Reactions Catalysed by a Binuclear Copper Complex: Aerobic Cross Dehydrogenative Coupling of N-Aryl Tetrahydroisoquinolines. Chem. – Eur. J. 2017;23:3051–3061. doi: 10.1002/chem.201604749. [DOI] [PubMed] [Google Scholar]; (g) Liang W. W. Zhang T. Liu Y. F. Huang Y. X. Liu Z. P. Liu Y. Z. Yang B. Zhou X. C. Zhang J. M. Polydimethylsiloxane sponge-supported nanometer gold: highly efficient recyclable catalyst for cross-dehydrogenative coupling in water. ChemSusChem. 2018;11:3586–3590. doi: 10.1002/cssc.201801180. [DOI] [PubMed] [Google Scholar]
- For selected examples using visible-light photoredox catalysis, see: ; (a) Freeman D. B. Furst L. Condie A. G. Stephenson C. R. J. Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light. Org. Lett. 2012;14:94–97. doi: 10.1021/ol202883v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang Z.-Q. Hu M. Huang X.-C. Gong L.-B. Xie Y.-X. Li J.-H. Direct α-Arylation of α-Amino Carbonyl Compounds with Indoles Using Visible Light Photoredox Catalysis. J. Org. Chem. 2012;77:8705–8711. doi: 10.1021/jo301691h. [DOI] [PubMed] [Google Scholar]; (c) Meng Q. Y. Zhong J. J. Liu Q. Gao X. W. Zhang H. H. Lei T. Li Z. J. Feng K. Chen B. Tung C. H. Wu L. Z. A cascade cross-coupling hydrogen evolution reaction by visible light catalysis. J. Am. Chem. Soc. 2013;135:19052–19055. doi: 10.1021/ja408486v. [DOI] [PubMed] [Google Scholar]; (d) Wu C. J. Zhong J. J. Meng Q. Y. Lei T. Gao X. W. Tung C. H. Wu L. Z. Cobalt-catalyzed cross-dehydrogenative coupling reaction in water by visible light. Org. Lett. 2015;17:884–887. doi: 10.1021/ol503744a. [DOI] [PubMed] [Google Scholar]; (e) Gao X.-W. Meng Q.-Y. Li J.-X. Zhong J.-J. Lei T. Li X.-B. Tung C.-H. Wu L.-Z. Visible Light Catalysis Assisted Site-Specific Functionalization of Amino Acid Derivatives by C–H Bond Activation without Oxidant: Cross-Coupling Hydrogen Evolution Reaction. ACS Catal. 2015;5:2391–2396. [Google Scholar]; (f) Guo X. Shao B. R. Jiang W. F. Shi L. The photocatalyst-free cross-dehydrogenative coupling reaction enabled by visible-light direct excitation of substrate. J. Org. Chem. 2021;86:15743–15752. doi: 10.1021/acs.joc.1c01775. [DOI] [PubMed] [Google Scholar]
- For selected examples using electrochemical oxidation, see: ; (a) Wang P. Tang S. Huang P. Lei A. Electrocatalytic oxidant-free dehydrogenative C−H/S−H cross-coupling. Angew. Chem., Int. Ed. 2017;56:3009–3013. doi: 10.1002/anie.201700012. [DOI] [PubMed] [Google Scholar]; (b) Wang J. H. Li X. B. Li J. Lei T. Wu H. L. Nan X. L. Tung C. H. Wu L. Z. Photo-electrochemical cell for P−H/C−H cross-coupling with hydrogen evolution. Chem. Commun. 2019;55:10376–10379. doi: 10.1039/c9cc05375a. [DOI] [PubMed] [Google Scholar]; (c) Xie W. X. Liu N. Gong B. Ning S. L. Che X. Cui L. L. Xiang J. B. Electrochemical cross-dehydrogenative coupling of N-aryl-tetrahydroisoquinolines with phosphites and indole. Eur. J. Org. Chem. 2019;2019:2498–2501. [Google Scholar]
- Saeed A. Shahzad D. Faisal M. Larik F. A. El-Seedi H. R. Channar P. A. Developments in the synthesis of the antiplatelet and antithrombotic drug (S)-clopidogrel. Chirality. 2017;29:684–707. doi: 10.1002/chir.22742. [DOI] [PubMed] [Google Scholar]
- Aalla S. Gilla G. Metil D. S. Anumula R. R. Vummenthala P. R. Padi P. R. Process improvements of prasugrel hydrochloride: an adenosine diphosphate receptor antagonist. Org. Process Res. Dev. 2012;16:240–243. [Google Scholar]
- Festa A. A. Voskressensky L. G. Van der Eycken E. V. Visible light-mediated chemistry of indoles and related heterocycles. Chem. Soc. Rev. 2019;48:4401–4423. doi: 10.1039/c8cs00790j. [DOI] [PubMed] [Google Scholar]
- For selected examples: ; (a) Hopkinson M. N. Gómez-Suárez A. Teders M. Sahoo B. Glorius F. Accelerated Discovery in Photocatalysis Using a Mechanism-Based Screening Method. Angew. Chem., Int. Ed. 2016;55:4361–4366. doi: 10.1002/anie.201600995. [DOI] [PubMed] [Google Scholar]; (b) Gieseler A. Steckhan E. Wiest O. Knoch F. Photochemically Induced Radical-Cation Diels-Alder Reaction of Indole and Electron-Rich Dienes. J. Org. Chem. 1991;56:1405–1411. [Google Scholar]; (c) Lerch S. Unkel L.-N. Brasholz M. Tandem Organocatalysis and Photocatalysis: An Anthraquinone-Catalyzed Indole-C3-Alkylation/Photooxidation/1,2-Shift Sequence. Angew. Chem., Int. Ed. 2014;53:6558–6562. doi: 10.1002/anie.201402920. [DOI] [PubMed] [Google Scholar]; (d) Zhang C. Li S. Bureš F. Lee R. Ye X. Jiang Z. Visible light photocatalytic aerobic oxygenation of indoles and pH as a chemoselective switch. ACS Catal. 2016;6:6853–6860. [Google Scholar]; (e) Schilling W. Zhang Y. Riemer D. Das S. Visible-light-mediated dearomatisation of indoles and pyrroles to pharmaceuticals and pesticides. Chem. – Eur. J. 2020;26:390–395. doi: 10.1002/chem.201904168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H. G. Romero N. A. Nicewicz D. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett. 2016;27:714–723. [Google Scholar]
- Kohls P. Jadhav D. Pandey G. Reiser O. Visible light photoredox catalysis: generation and addition of N-aryltetrahydroisoquinoline-derived α-amino radicals to Michael acceptors. Org. Lett. 2012;14:672–675. doi: 10.1021/ol202857t. [DOI] [PubMed] [Google Scholar]
- For selected examples ; (a) Liang W. Zhang T. Liu Y. Huang Y. Liu Z. Liu Y. Yang B. Zhou X. Zhang J. Polydimethylsiloxane Sponge-Supported Nanometer Gold: Highly Efficient Recyclable Catalyst for Cross-Dehydrogenative Coupling in Water. ChemSusChem. 2018;11:3586–3590. doi: 10.1002/cssc.201801180. [DOI] [PubMed] [Google Scholar]; (b) Kumar G. Solanki P. Nazish M. Neogi S. Kureshy R. I. Khan N. H. Covalently Hooked EOSIN-Y in a Zr(IV) Framework as Visible-Light Mediated, Heterogeneous Photocatalyst for Efficient C–H Functionalization of Tertiary Amines. J. Catal. 2019;371:298–304. [Google Scholar]; (c) Kumar G. Dash S. R. Neogi S. Dual-Catalyst Engineered Porous Organic Framework for Visible-Light Triggered, Metal-free and Aerobic sp3 C–H Activation in Highly Synergistic and Recyclable Fashion. J. Catal. 2021;394:40–49. [Google Scholar]
- (a) Baran P. S. Richter J. M. Direct Coupling of Indoles with Carbonyl Compounds: Short, Enantioselective, Gram-Scale Synthetic Entry into the Hapalindole and Fischerindole Alkaloid Families. J. Am. Chem. Soc. 2004;126:7450–7451. doi: 10.1021/ja047874w. [DOI] [PubMed] [Google Scholar]; (b) Baran P. S. Richter J. M. Enantioselective Total Syntheses of Welwitindolinone A and Fischerindoles I and G. J. Am. Chem. Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. [DOI] [PubMed] [Google Scholar]; (c) Baran P. S. Richter J. M. Enantiospecific Total Synthesis of the Hapalindoles, Fischerindoles, and Welwitindolinones via a Redox Economic Approach. J. Am. Chem. Soc. 2008;130:17938–17954. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Baran P. S. Maimone T. J. Richter J. M. Total Synthesis of Marine Natural Products without Using Protecting Groups. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- Imm S. Bähn S. Tillack A. Mevius K. Neubert L. Beller M. Selective Ruthenium-Catalyzed Alkylation of Indoles by Using Amines. Chem. – Eur. J. 2010;16:2705–2709. doi: 10.1002/chem.200903261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.